3
Pathophysiology of Blast Injury and Overview of Experimental Data
This chapter reviews what is known about the mechanisms of blast injury. It begins with an explanation of blast physics. Next is a discussion of how blast waves interact with the body directly and indirectly and how exposure to blast can affect multiple systems in the body and can cause systemic effects on the autonomic nervous, vascular, and immune systems. That discussion is followed by a description of models used to study blast-injury mechanisms and the challenges involved in using models. The chapter ends with a summary of the results of experimental studies conducted in blast-exposed animal models. The committee used the information presented here to understand the mechanisms of blast injury, to discern clues about possible long-term health effects in humans, and to help to identify data gaps in the evidence base.
This section is taken from the Institute of Medicine report Gulf War and Health, Volume 7: Long-Term Consequences of Traumatic Brain Injury (IOM, 2009). A blast wave generated by an explosion starts with a single pulse of increased air pressure that lasts a few milliseconds. The negative pressure or suction of the blast wave follows the positive wave immediately (Owen-Smith, 1981). The duration of the blast wave—that is, the time that an object in the path of the shock wave is subjected to the pressure effects—depends on the type of explosive and the distance from the point of detonation (Clemedson, 1956). Table 3-1 summarizes the safety zones—that is, the standoff distances—for various types of bomb explosions.
TABLE 3-1 Safety Recommendations for Standoff Distances from Different Types of Exploding Bombs
| Container or Vehicle Description | Maximum Explosives Capacity | Lethal Air-Blast Range | Maximum Evacuation Distance | Falling-Glass Hazard |
| Pipe 2 × 12 in | 5–6 lb | 850 ft (259 m) | ||
| Pipe 4 × 12 in | 20 lb | |||
| Pipe 8 × 24 in | 120 lb | |||
| Bottle 2 L | 10 lb | |||
| Bottle 2 gal | 30 lb | |||
| Bottle 5 gal | 70 lb | |||
| Boxes or shoebox | 30 lb | |||
| Briefcase or satchel bomb | 50 lb | 1,850 ft (564 m) | 1,250 ft (381 m) | |
| 1-ft3 box | 100 lb | |||
| Suitcase | 225 lb | 1,850 ft (564 m) | 1,250 ft (381 m) | |
| Compact sedan | 500 lb in trunk | 100 ft (30 m) | 1,500 ft (457 m) | 1,250 ft (381 m) |
| Full-size sedan | 1,000 lb in trunk | 125 ft (38 m) | 1,750 ft (534 m) | 1,750 ft (534 m) |
| Passenger van or cargo van | 4,000 lb | 200 ft (61 m) | 2,750 ft (838 m) | 2,750 ft (838 m) |
| Small box van | 10,000 lb | 300 ft (91 m) | 3,750 ft (1,143 m) | 3,750 ft (1,143 m) |
| Box van or water or fuel truck | 30,000 lb | 450 ft (137 m) | 6,500 ft (1,982 m) | 6,500 ft (1,982 m) |
| Semitrailer | 60,000 lb | 600 ft (183 m) | 7,000 ft (2,134 m) | 7,000 ft (2,134 m) |
NOTE: Table compiled from several publications of the Advanced Technical Group for Blast Mitigation and Technical Support Working Group.
SOURCE: Reprinted with permission from Charles Stewart, MD, EMDM, MPH(Stewart, 2014).
The blast wave progresses from the source of the explosion as a sphere of compressed and rapidly expanding gases, which displaces an equal volume of air at a high velocity (Rossle, 1950). The velocity of the blast wave in air may be extremely high, depending on the type and amount of the explosive used. The blast wave is the main determinant of the primary blast injury and consists of the front of high pressure that compresses the surrounding air and falls rapidly to negative pressure. It travels faster than sound and in few milliseconds damages the surrounding structures. The blast wind following the wave is generated by the mass displacement of air by expanding gases; it may accelerate to hurricane proportions and is responsible for disintegration, evisceration, and traumatic amputation of body parts. Thus, a person exposed to an explosion will be subjected not only to a blast wave but to the high-velocity wind traveling directly behind the shock front of the blast wave (Rossle, 1950). A hurricane-force wind traveling about 200 km/h exerts overpressure of only 1.72 kilopascal (kPa) (0.25 psi), but a blast-induced overpressure of 690 kPa (100 psi) that causes substantial lung damage and might be lethal travels at about 2,414 km/h (Owen-Smith, 1981).
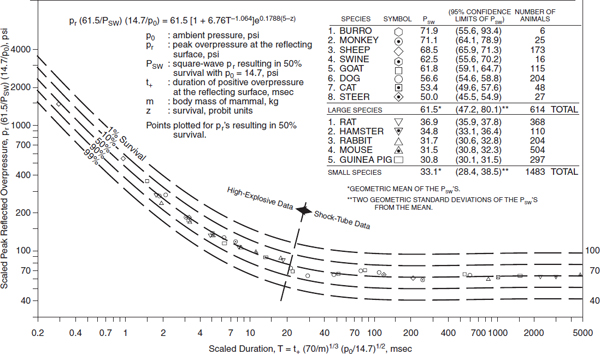
The magnitude of damage due to the blast wave depends on the peak of the initial positive-pressure wave (an overpressure of 414–552 kPa or 60–80 psi is considered potentially lethal), the duration of the overpressure, the medium of the explosion, the distance from the incident blast wave, and the degree of focusing due to a confined area or walls. For example, explosions near or within hard solid surfaces become amplified 2–9 times because of shock-wave reflection (Rice and Heck, 2000). Moreover, victims positioned between the blast and a building often suffer 2–3 times the degree of injury of a person in an open space. Indeed, people exposed to explosion rarely experience the idealized pressure-wave form, known as the Friedlander wave. Even in open-field conditions, the blast wave reflects from the ground, generating reflective waves that interact with the primary wave and thus changing its characteristics. In a closed environment (such as a building, an urban setting, or a vehicle), the blast wave interacts with surrounding structures and creates multiple wave reflections, which, interacting with the primary wave and between each other, generate a complex wave (Ben-Dor et al., 2001; Mainiero and Sapko, 1996) (see Figure 3-1). Table 3-2 summarizes the effects of different levels of overpressure on material surrounding the explosion and unprotected persons exposed to blast.
Previous attempts to define the mechanisms of blast injury suggested the involvement of spalling, implosion, and inertial effects as major physical components of the blast-body interaction and later tissue damage (Benzinger, 1950). Spallation is the disruption that occurs at the boundary between two media of different densities; it occurs when a compression wave in the denser medium is reflected at the interface. Implosion occurs
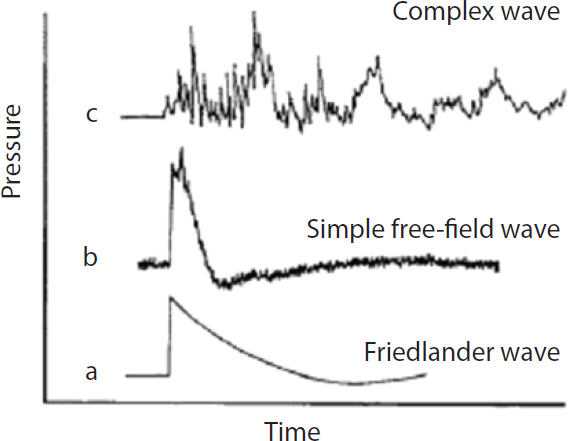
FIGURE 3-1 Explosion-induced shock waves: (a) idealized representation of pressure-time history of an explosion in air; (b) shock wave in open air; (c) complex shock-wave features in closed or urban environment.
SOURCE: Mayorga, 1997. Reprinted with permission from Elsevier Science, Ltd. 2008.
when the shock wave compresses a gas bubble in a liquid medium, raising the pressure in the bubble much higher than the shock pressure; as the pressure wave passes, the bubbles can re-expand explosively and damage surrounding tissue (Benzinger, 1950; Chiffelle, 1966; Phillips, 1986). Inertial effects occur at the interface of the different densities: the lighter object will be accelerated more than the heavier one, so there will be a large stress at the boundary. Recent results suggest that there is a frequency dependence of the blast effects: high-frequency (0.5–1.5 kHz) low-amplitude stress waves target mostly organs that contain abrupt density changes from one medium to another (for example, the air–blood interface in the lungs or the blood–parenchyma interface in the brain), and low-frequency (<0.5 kHz) high-amplitude shear waves disrupt tissue by generating local motions that overcome natural tissue elasticity (for example, at the contact of gray and white brain matter).
TABLE 3-2 Overpressure Effects on Surrounding Materials and Unprotected Persons
| Pressure, kPa (psi) | Effects on Material | Pressure, kPa (psi) | Effects on Unprotected Person |
| 0.69–34.47 (0.1–5) | Shatter single-strength glass | 34.47 (5) | Slight chance of eardrum rupture |
| 6.89–13.79 (1–2) | Crack plaster walls, shatter asbestos sheet, buckle steel sheet, failure of wood wall | 103.42 (15) | 50% chance of eardrum rupture |
| 13.79–20.68 (2–3) | Crack cinder-block wall, crack concrete block wall | 206.84–275.79 (30–40) | Slight chance of lung damage |
| 13.79–55.16 (2–8) | Crack brick wall | 551.58 (80) | 50% chance of severe lung damage |
| 34.47–68.95 (5–10) | Shatter car safety glass | 689.48 (100) | Slight chance of death |
| 896.32–1,241.06 (130–180) | 50% chance of death | ||
| 1,378.95–1,723.69 (200–250) | Death usual | ||
SOURCE: Reproduced from Journal of the Royal Army Medical Corps, Hunterian lecture 1980: A computerized data retrieval system for the wounds for war: The Northern Ireland casualties. Owen-Smith, M. S., 127(1):31–54, Copyright 1981, with permission from BMJ Publishing Group Ltd.
ACUTE BLAST–BODY AND BLAST–BRAIN INTERACTIONS
Explosive blast may have five distinct acute effects on the body (see Figure 3-2): The primary blast mechanism causes injuries as sole consequences of the shock wave–body interaction; the secondary blast mechanism is due to the propulsion of fragments of debris by the explosion and their connection with the body, which causes penetrating or blunt injuries; the tertiary blast mechanism is due to the acceleration and deceleration of the body or a part of the body when the energy released by the explosion propels the body or body part (acceleration phase) and then the body or body part stops suddenly on hitting the ground or a surrounding object; the quaternary blast mechanism (not depicted in Figure 3-2) includes flash burns caused by the transient but intense heat of the explosion (Mellor, 1988); and the quinary blast mechanism (not depicted in Figure 3-2) is caused by post-detonation environmental contaminants, such as tissue reactions to fuel,
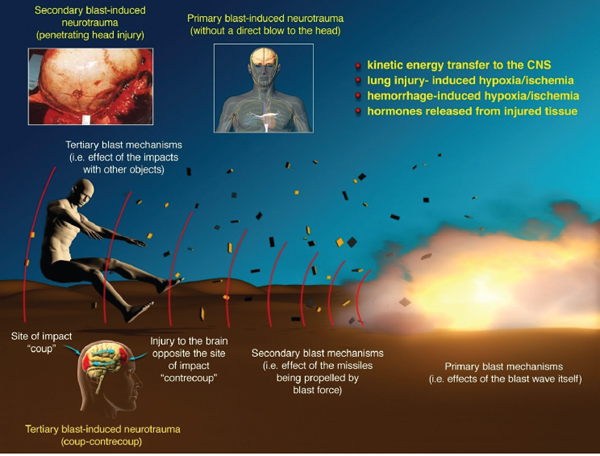
FIGURE 3-2 Complex injurious environment due to blast.
NOTES: Primary blast effects are caused by the blast wave itself (excludes penetrating and blunt-force injury); secondary blast effects are caused by particles propelled by the blast (penetrating or blunt-force injury); tertiary blast effects caused by acceleration and deceleration of the body and its impact with other objects (penetrating or blunt-force [including “coup-contrecoup”] injury). Quaternary and quinary blast effects are not depicted in this figure but are described in the text.
SOURCE: Reprinted with permission from Macmillan Publishers Ltd: Journal of Cerebral Blood Flow and Metabolism, Cernak and Noble-Haeusslein, copyright 2010.
metals, and dusts or to bacteria and radiation in dirty bombs (Kluger et al., 2007). Often, especially in the case of moderate to severe blast injuries, the multiple blast effects interact with the body simultaneously; such an injurious environment and related injuries are sometimes called blast-plus (Moss et al., 2009).
When an explosive shock wave strikes a living body, a fraction of the shock wave is reflected and another fraction is absorbed and propagates through the body as a tissue-transmitted shock wave (Clemedson and Criborn, 1955). Different organ and body structures differ in their reactions, but two main general types of tissue response are observed: One is
caused by the impulse of the shock wave and is of longer duration, and the other is caused by the pressure variations of the shock wave and is in the form of oscillations or pressure deflections of shorter duration (Clemedson and Pettersson, 1956). For example, Clemedson and colleagues demonstrated that in rabbits exposed to blast, abdominal organs and costal interspaces (that is, spaces between ribs) responded to the impulse of the shock wave, whereas the rib’s and the hind leg’s response was induced by the pressure variations of the shock wave (Clemedson et al., 1969).
During the interaction between the blast shock wave (the primary blast) and a medium—which could be solid, liquid, gas, or plasma—the energy of the shock wave is absorbed or transformed into the kinetic energy of the medium (Tümer et al., 2013). The kinetic energy, in turn, moves and accelerates the elements of the medium from their resting state with a rate that depends on the density of the medium; this leads to rapid physical movement, displacement, deformation, or rupture of the medium (Chu et al., 2005). Consequently, the main mechanisms of the blast–body interaction and later tissue damage include spallation, implosion, and inertial effects (Richmond et al., 1967). Spallation is a phenomenon that occurs at the boundary between two media of different densities where a compression wave in the denser medium is reflected at the interface. Implosion occurs in a liquid medium that contains a dissolved gas. As the shock wave passes through such a medium, it compresses the gas bubbles, and this leaves the pressure in the bubbles much higher than the shock pressure; after the passage of the pressure wave, the bubbles can re-expand explosively and damage surrounding tissue (Cooper et al., 1991; Richmond et al., 1967, 1968). Inertial effects also occur at the interface of media of different densities: the lighter object will be accelerated more than the heavier one, so there will be a large stress at the boundary (Lu and Wilson, 2003).
In addition to the consequences of the kinetic-energy transfer, recent results suggest that the primary blast effects depend on frequency: High-frequency (0.5–1.5 kHz), low-amplitude stress waves target mostly organs that contain media with contrasting densities (for example, the air–blood interface in the lungs or the blood–parenchyma interface in the brain), and low-frequency (<0.5 kHz), high-amplitude shear waves disrupt tissue by generating local motions that overcome natural tissue elasticity (Cooper et al., 1991; Gorbunov et al., 2004) (for example, at the interface between gray and white brain matter).
MODIFYING POTENTIAL OF SYSTEMIC CHANGES CAUSED BY BLAST
Because of the complexity of the injurious environment—that is, multiple blast effects that may interact with the body—blast injuries often
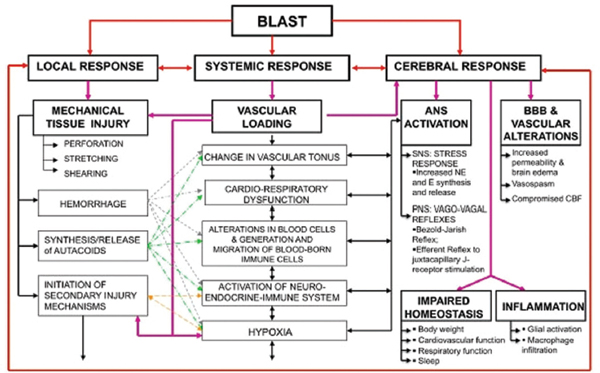
FIGURE 3-3 Simultaneous activation of systemic, local, and cerebral responses to blast exposure and interactive mechanisms that cause or contribute to the blast-induced neurotrauma.
NOTES: ANS = autonomous nervous system; BBB = blood brain barrier; CBF = cerebral blood flow; E = epinephrine; NE = norepinephrine; PNS = parasympathetic nervous system; SNS = sympathetic nervous system.
SOURCE: Cernak, 2010.
involve interwoven mechanisms of systemic, local, and cerebral responses to blast exposure (Cernak et al., 1991, 1996b) (see Figure 3-3). Even when multiorgan responses are mild, systemic changes substantially modify the original organ damage and influence its severity and outcome. Air emboli, activation of the autonomic nervous system (ANS), vascular mechanisms, and systemic inflammation are among the most important systemic alterations that could modify initial injuries due to blast.
Air Emboli
Air emboli develop as a consequence of the shock wave’s passing through media in the body that have different densities: gas, such as air; fluid, such as blood; and solid, such as parenchyma. Experimental studies published by Mason et al. (1971) and Nevison et al. (1971) used an ultrasonic Doppler blood-flow detector in dogs subjected to blast in a shock tube and showed air emboli passing through the carotid artery. The embolus
detector showed cyclic release of air emboli: Release occurred over the first 10 seconds after the blast, ceased for a time, and was then noted about 2 minutes and 12 minutes after the blast.
It is noteworthy that the air-emboli release occurred in parallel with a drastic decrease in blood-flow velocity and with seizure that was probably due to hypoxia or anoxia. Similar experimental findings have been described by others (Chu et al., 2005; Clemedson and Hultman, 1954; Kirkman and Watts, 2011) and have been noted in clinical studies (Freund et al., 1980; Tsokos et al., 2003a,b). Indeed, a massive compressed-air embolism of the aorta and multiple air spaces in the interstitium compressing the collecting tubules in the kidneys (Freund et al., 1980) and venous air embolism in the lungs (Tsokos et al., 2003a) were reported in victims of severe blast injuries. It is expected that the rate of the air-emboli release is dependent on the intensity of blast, and the subsequent changes in blood flow and oxygenation concentration are also graded (that is, when the rate of the air-emboli release increases with the increase of blast intensity, the intensity of the pathological changes in blood flow and oxygenation concentration also increases).
Activation of the Autonomic Nervous System
When the incident overpressure wave (the initial shock wave that brings a sudden increase in atmospheric pressure) is transmitted through the body, it increases the pressure in organs (Clemedson and Pettersson, 1956). The later sudden hyperinflation of the lungs (Cernak et al., 1996b; Zuckerman, 1940) stimulates the juxtacapillary (J) receptors that are located in the alveolar interstitium and innervated by vagal fibers (Paintal, 1969). The resulting vagovagal reflex leads to apnea followed by rapid breathing, bradycardia, and hypotension, which are frequently observed immediately after blast exposure. Moreover, hypoxia and ischemia due to damaged alveoli, air emboli, or a triggered pulmonary vagal reflex can activate a cardiovascular decompressor Bezold-Jarish-reflex, which involves a marked increase in vagal (parasympathetic) efferent discharge to the heart (Zucker, 1986). That effect causes a slowing of the heart (bradycardia), dilation of the peripheral blood vessels, and an ensuing drop in blood pressure, which could contribute further to cerebral hypoxia (Cernak et al., 1996a,b). Axelsson and colleagues (2000) showed in pigs that the blast-induced brief apnea correlated with flattening of the electric activity of the brain. Other experimental studies demonstrated the importance of vagally mediated cerebral effects of blast (Cernak et al., 1996b; Irwin et al., 1999; Ohnishi et al., 2001).
The environment in which an explosion occurs is dramatic and may initiate endocrine mechanisms of the classic flight-and-fight stress response
(Selye, 1976). For example, recent study (Tümer et al., 2013) showed increased expression of the catecholamine-biosynthesizing enzymes tyrosine hydroxylase and dopamine hydroxylase in the rat adrenal medulla and increased plasma concentrations of norepinephrine 6 hours after blast injury. Accumulating experimental and clinical evidence suggests that blast induces alterations in ANS activity: instantaneous triggering of the parasympathetic reflexes followed by neuroendocrine changes due to the activation of the sympathetic nervous system.
Vascular Mechanisms
One of the most important media for a shock wave’s energy transfer is blood. Veins contain about 70% of total human blood volume (including the splanchnic system, which accounts for about 20% of that total), compared with 18% in arteries and only 3% in terminal arteries and arterioles (Gelman, 2008). In general, veins are 30 times more compliant than arteries; splanchnic and cutaneous veins are the most compliant veins and constitute the largest blood reservoirs in the body. Figure 3-4 is a schematic
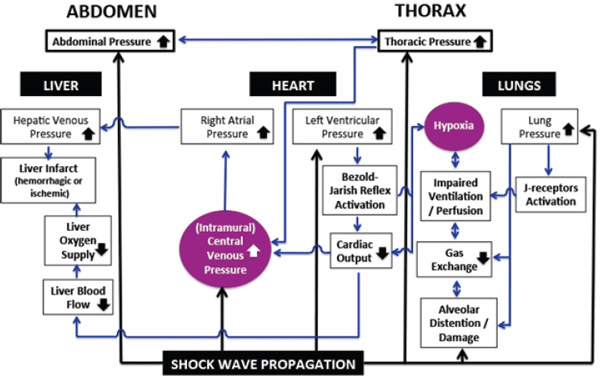
FIGURE 3-4 Overview of vascular mechanisms that are activated by shock-wave propagation through the body, lead to alterations in functions of multiple organs and organ systems, and substantially influence the brain’s response to blast.
SOURCE: Created by Ibolja Cernak for the Committee on Gulf War and Health: Long-Term Effects of Blast Exposures.
representation of the consequences of blast-induced pressure changes and their extremely complex interactions, which form several interconnected loops. The transfer of the shock wave’s energy to the body not only leads to a sudden increase in both abdominal pressure (AbdP) and thoracic pressure (ThorP) but causes an increase in intramural central venous pressure (CVP). Hypoxia caused by alveolar damage and later by reduced surface for gas exchange, impaired ventilation and perfusion caused by J-receptor activation, or decreased cardiac output due to activation of the Bezold-Jarish reflex all increase pulmonary arterial resistance, which might increase ThorP (Gelman, 2008). An increase in ThorP amplifies the increase in CVP.
Venoconstriction and the mobilization of blood volume depend mainly on the splanchnic circulation, which has a high population of α1- and α2-adrenergic receptors and hence a high sensitivity to adrenergic stimulation (Pang, 2001; Rutlen et al., 1979). Thus, it is likely that the initial sudden drop in systemic arterial pressure caused by blast-induced vagovagal reflexes and the accompanying reduction in the inhibitory influences of the baroreceptors of the carotid sinus and aortic area on the vasomotor center initiate a compensatory increase in sympathetic outflow. The increased sympathetic stimuli constrict venous smooth muscle and lead to mobilization of blood from the splanchnic vasculature toward the heart (Rutlen et al., 1979).
Spasm of the cerebral vasculature has frequently been found in moderate or severe blast-induced traumatic brain injury (TBI)—more often than in patients who have TBI of other origins (for example, impact, fall, or acceleration) (Armonda et al., 2006; Ling et al., 2009). It can develop early, often within 48 hours of injury, and can also be manifested later, typically 10–14 days after exposure. It is noteworthy that although cerebral vasospasm is usually prompted by subarachnoid hemorrhage, that is not required for vasospasm in blast-induced TBI (Magnuson et al., 2012). A recent experimental study of theoretical and in vitro models demonstrated that a single rapid mechanical insult is capable of inducing vascular hypercontractility and remodeling, which are indicative of vasospasm initiation (Alford et al., 2011). Alford and colleagues used in vitro engineered arterial lamellae exposed to high-velocity acute uniaxial stretch to reproduce blast-induced stretch of arterial blood vessels and test whether blast forces can lead to phenotypic switch in vascular smooth muscle cells (VSMCs). The authors measured protein and mRNA expression of two primary markers of contractile VSMCs, smooth muscle myosin heavy chain (SM-MHC) and smoothelin, 24 hours after the injury induction. The results showed that severe (10%) strain decreased expression of smoothelin and decreased mRNA expression of both smoothelin and SM-MHC, suggesting that acute mechanical injury can potentiate a switch away from the contractile phenotype in VSMCs. The findings support a hypothetical scenario in which the
shock wave passing through the vasculature interacts with cellular elements of vascular wall (endothelium and vascular smooth muscle) and stimulates synthesis and release of different mediators and modulators. The released biologically active molecules, in turn, cause hypercontractility and later a phenotype switching that potentiates vascular remodeling and cerebral vasospasm (Alford et al., 2011).
Systemic Inflammation
Blast exposure can activate multiple inflammatory mechanisms (Cernak, 2010). Tissue disruption stimulates synthesis and release of autacoids, biologic factors that act briefly like local hormones near the site of their synthesis. Increased concentrations of prostaglandins, leukotrienes, and cytokines have been found in the blood of blast casualties (Cernak et al., 1999a,b; Surbatovic et al., 2007). The autacoids directly affect a number of stages of immunity and act as feedback modifiers in connecting the early and late phases of the immune response (Melmon et al., 1981). They can stimulate selected migration of cells to an injury site and directly or indirectly modify the turnover of T and B lymphocytes, the production or release of lymphokines, and the activity of T-helper or T-suppressor cells (Khan and Melmon, 1985; Melmon et al., 1981). It has been suggested that inflammatory cells of systemic origin induced by shock-wave propagation through the body contribute substantially to blast-induced inflammation in the brain and related neurodegeneration (Cernak, 2010); the suggestion was supported by experimental data from preclinical models (Valiyaveettil et al., 2013).
Blast exposures have been reported to cause alterations in the neuroendocrine system that involve multiple hypothalamopituitary end axes (Cernak et al., 1999c; Wilkinson et al., 2012). The importance of the immune-neuroendocrine network in injury response and inflammation control is well established (Besedovsky and DelRey, 1996; Chrousos, 1995). It is likely that blast exposure, through multiple interwoven mechanisms, causes a massive perturbation of the central nervous system (CNS) with broad consequences for all aspects of vital functions.
REQUIREMENTS FOR MODELS OF BLAST-INDUCED INJURY
Regardless of the research questions to be addressed, clinically and militarily relevant blast-injury models should satisfy the following criteria (Cernak and Noble-Haeusslein, 2010):
- the injurious component of the blast is clearly identified and reproduced in a controlled and quantifiable manner.
- the inflicted injury is reproducible and quantifiable and mimics components of human blast injuries.
- the injury outcome—on the basis of morphologic, physiologic, biochemical, and behavioral measures—is related to the chosen injurious component of the blast.
- the mechanical properties—intensity, complexity of blast signature, and duration—of the injurious factor predicts outcome severity.
Compared with the injuries caused by an impact or acceleration– deceleration force, the mechanistic factors underlying blast injuries are extremely complex. Hence, an appropriate and clinically relevant blast-injury model should be based on sufficient knowledge of shock-wave physics and on the characteristics of the injurious environment generated by an explosion and clinical manifestations of resulting injuries. Substantial interspecies differences in responses to blast exposure across different mammalian species make it imperative that research studies of blast effects and the mechanism by which they are produced consider the possible advantages of using species similar in size to humans, and caution should be exercised in extrapolating to humans observations made in rodents and isolated cells and tissues.
Choice of Models
The design and choice of a specific model depend on the goal of research and the component of clinical CNS injury that one wishes to simulate (Cernak, 2005; Risling and Davidsson, 2012). Given the complex nature of blast injuries, it is obvious that the conditions used in a model to reproduce some aspects of blast injuries should be defined with rigor; otherwise, the results obtained will lack military and clinical relevance and can be dangerously misleading. Indeed, despite the growing literature on experimental blast injuries, the results of studies are difficult to compare because of vast differences in methods and experimental conditions (Panzer et al., 2012). Figure 3-5 is a schematic depiction of the decision-making steps in the process of choosing a model for blast research. First, the researcher should clarify the blast effects to be reproduced. If the choice is primary blast, the researcher should ensure that the animals are fixed so that there will be no blast-induced acceleration of the body and head during the exposure.
In a situation in which the body or head is allowed to move, the injury mechanisms involve both primary and tertiary blast effects, which could introduce difficulties in the proper interpretation of results. Next, a decision should be made about the biologic complexity of the research study because this will dictate the choice of research environment, the means of
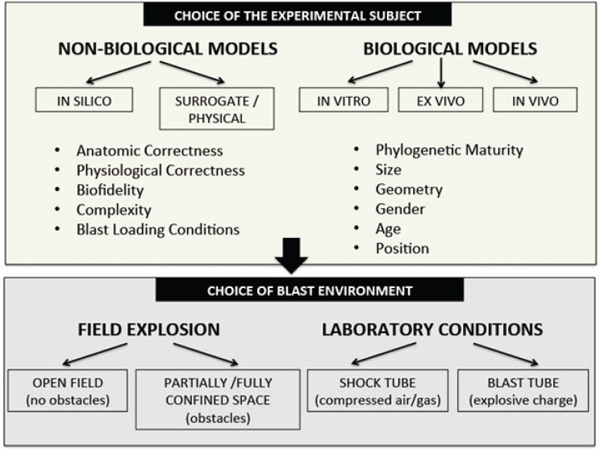
FIGURE 3-5 Factors that influence the choice of blast-injury and blast-induced-neurotrauma models.
SOURCE: Created by Ibolja Cernak for the Committee on Gulf War and Health: Long-Term Effects of Blast Exposures.
generating a shock wave, the choices of models and their positioning, and the length of the experiment. Thus, on the basis of the research question and the complexity, a choice is made between nonbiologic models and biologic models. The nonbiologic models provide an experimental platform for analyzing interactions between blast loading and different types of materials; the information gained is extrapolated to biologic materials at different levels of scaling. The nonbiologic models can be computer simulations and surrogate physical models. Biofidelic models (mechanical models with computerized sensors that mimic particular human characteristics) are helpful for characterizing the physics of the blast-induced mechanical changes in the brain or head. They are made from synthetic materials, such as glass and epoxy or polyurethane. Multiple displacement and pressure sensors molded into the organs’ material are used to record biomechanical measures, such as linear and angular acceleration, velocity, displacement,
force, torque, and pressure (Desmoulin and Dionne, 2009; Ganpule et al., 2012; Roberts et al., 2012).
Nonbiologic models can be useful in recording biomechanical alterations induced by blast load and suggesting potential consequences, but they are incapable of providing insight into the mechanisms of later physiologic alterations; hence the need for biologic models. The latter models use biologic systems of differing complexity and include in vitro, ex vivo, and in vivo models. In vitro models based on cell cultures can be useful for characterizing cell responses to blast loading in a highly controlled experimental environment (Effgen et al., 2012; Panzer et al., 2012). Ex vivo models use an organ or a segment of a specific tissue, such as brain or spinal cord, taken from the organism and placed in an artificial environment that is more controlled than is possible with in vivo experiments. As with all blast-injury models, applying operationally relevant loading histories is critical for the in vitro and ex vivo models. Only if blast-loading conditions that are realistic and that mimic what would happen at the cellular or tissue level in a person exposed to a militarily relevant blast environment are used can the mechanisms of the energy transfer to the tissue and the resulting biologic response be reliably analyzed (Effgen et al., 2012).
The success of a research study that uses biologic models, especially at the whole-animal level, depends on rigorous selection of the species to be used as experimental models. The choice of animal species depends on the focus of the study (Cernak, 2005). Many investigators have accepted rodent models as the most suitable choice for trauma research. The relatively small size and low cost of rodents permit repetitive measurements of morphologic, biochemical, cellular, and behavioral characteristics that require relatively large numbers of animals; for ethical, technical, and financial reasons, such measurements are less achievable in phylogenetically higher species (Cernak, 2005). However, because of substantially anatomic and physiologic differences, especially in the circulatory and nervous systems, it has been suggested that rodents should not be the sole choice in blast-injury research.
Extensive studies conducted in Albuquerque, New Mexico, and confirmed by British, German, and Swedish findings demonstrated substantial differences in blast tolerance among 15 mammals (Bowen et al., 1968a; Richmond et al., 1967, 1968). Body size–dependent differences in blast tolerance have been explained on the basis of lung density: the lung density in larger species—including humans, monkeys, cats, and dogs—is only about one-half that in smaller species, such as rodents (see Figure 3-6). In contrast, the lung volumes relative to body mass are three times greater in large species than in smaller animals (White et al., 1965). The body size of the animal model is an important consideration for extrapolating to humans; however, size is only one factor to be considered when validating
a model. In addition, substantial interspecies differences in body geometry influence blast–body and blast–head interactions (Bass et al., 2012). The body position of the animal also has an important effect on blast-injury severity. Animals facing an incoming shock-wave front with their chest and abdomen (that is, in the supine position with the shock wave coming from above) provide the most efficient conditions for the shock wave’s energy transfer and thus sustain the highest mortality and the most severe injuries (Cernak et al., 2011). In blast-injury modeling, especially when acceleration is included as one of the mechanistic factors, the basic principles of scaling laws should be carefully considered (Bass et al., 2008, 2012). For example, a given blast–head scenario, calculation of the net loading scales for a cross-sectional area of the skull, even if other measures are identical, shows that a specimen 20 times as large would experience one-twentieth the acceleration. However, there are other important anatomic differences between human and animal heads, such as bone volume fraction, trabecular separation, trabecular number, and connectivity density (Bauman et al., 2009; Holzer et al., 2012). Interspecies differences in the structure and arrangement of blood vessels (Vriese, 1904) should also be taken into account in choosing models to reproduce blast injury. For example, the internal carotid artery in lower vertebrates directs the blood to the brain parenchyma through the posterior branch without a contribution from the basilar artery, whereas the two posterior branches in higher vertebrates stem from a single, central branch at the basilar artery (Casals et al., 2011). This anatomical difference could significantly influence the shock-wave propagation through the cerebral vasculature.
It has been shown that phylogenetic maturity has a decisive role in the brain’s response to a high-pressure environment (Brauer et al., 1979), and this should be taken into account in planning blast-induced neurotrauma (BINT) experiments. Characterization of basic molecular and gene injury mechanisms that have persisted through evolution might use phylogenetically lower species such as rodents, whereas establishing the pathogenesis of impaired higher brain functions would require larger animals that have a gyrencephalic brain (one that has convolutions).
A short overview of experimental results of biologic models, mainly at the whole-animal level, provides information on mechanisms that potentially underlie long-term functional deficits or organ failure.
Experimental Environment of Blast Generation
Experimental studies of primary blast-induced biologic responses are performed either in an open environment or in laboratory conditions. In open-field exposure studies, animals are exposed to a blast wave that is generated by detonation of an explosive (Axelsson et al., 2000; Bauman
et al., 2009; Lu et al., 2012; Richmond, 1991; Saljo and Hamberger, 2004; Savic et al., 1991). Such an experimental setting is comparable with in-theater conditions, but the physical characteristics (such as homogeneity of the blast wave) are less controllable, so a broader array of biologic response should be expected.
Experiments performed in laboratory conditions use shock tubes (in which compressed air or gas generates a shock wave) or blast tubes (in which explosive charges generate a shock wave) (Nishida, 2001; Robey, 2001). The tubes focus the blast-wave energy from the source to the subject; this maximizes the blast energy (Reneer et al., 2011) without the exponential decay of the shock wave’s velocity and pressure that is seen in free-field explosions (Celander et al., 1955).
The induction system routinely used in blast-exposure models consists of a cylindric metal tube divided by a plastic or metal diaphragm into two main sections: driver and driven. The anesthetized animals are fixed individually in holders that prevent movement of their bodies in response to the blast. The high pressure in the driver section is generated by an explosive charge or compressed gas and ruptures the diaphragm when it reaches the material’s tolerance to pressure. After the diaphragm ruptures, the shock wave travels along the driven section with supersonic velocity and interacts with the animal. The blast overpressure duration can be varied by changing the size of the high-pressure chamber (Celander et al., 1955). The compressed atmospheric air in the tube fails to expand as quickly as would an ideal gas when the membrane is ruptured and also fails to generate a broad range of overpressure peaks. Use of a light gas, such as helium, improves the performance of the shock tubes because of the increased speed of sound in such types of gas (Celander et al., 1955; Lu and Wilson, 2003).
Although shock and blast tubes are convenient means of generating shock waves, they lack the ability to generate other factors of the blast environment, such as acoustic, thermal, optical, and electromagnetic components (Ling et al., 2009). A wide range of blast overpressure sustained for various durations has been used in single-exposure experimental studies. In most studies, the animals are subjected to a shock or blast wave that has a mean peak overpressure of 52–340 kPa (7.54–49.31 psi) on the nearest surface of an animal’s body (Cernak et al., 2001b; Chavko et al., 2007; Clemedson et al., 1969; Saljo et al., 2000).
Most experiments used rodents (mice and rats) (Cernak et al., 2001a; Long et al., 2009), but some have used rabbits (Cernak et al., 1997), sheep (Savic et al., 1991), pigs (Bauman et al., 2009), or nonhuman primates (Bogo et al., 1971; Damon et al., 1968; Lu et al., 2012; Richmond et al., 1967).
EXPERIMENTAL MODELS OF MULTIORGAN RESPONSES TO BLAST
Respiratory System
Pathologic Changes
In the lung, damage related to spalling is exemplified by alveolar hemorrhages, whereas implosion produces air embolism from the alveoli into the pulmonary circulation (Yeh and Schecter, 2012). Blast overpressure results in primary injury to the lungs owing in part to compression of alveolar septa and capillary walls (DePalma et al., 2005). Such compression culminates in rupture of these structures, which produce interstitial edema and hemorrhage (Kirkman and Watts, 2011). The edema results in pronounced abnormal physiologic characteristics that reflect reduced gas exchange, including bradycardia, hypotension, and apnea. Brown et al. (1993) reported the first ultrastructural findings of blast lung (see Figure 3-7). They recognized the possibility that blast injury would be progres-
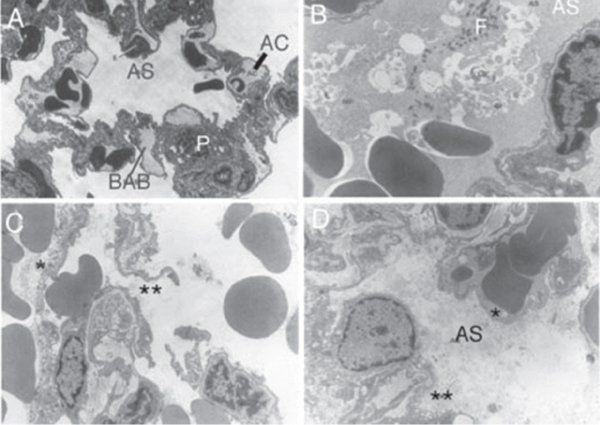
FIGURE 3-7 A. Control lung. B. Right lung 30 minutes after blast. C. Left lung 24 hours after blast. D. Right lung 24 hours after blast.
NOTES: AC = aveolar capillary; AS = alveolar space; BAB = blood–air barrier; F = fibrin clot; P = type II pneumocyte.
SOURCE: Reprinted from Brown et al. (1993). Copyright 1993, with permission from Blackwell Publishing Ltd.
sive, that is, that areas of the lung would initially appear intact but become hemorrhagic and show other signs of tissue damage. To test that, they exposed female rats to a blast wave to the right lateral surface of the lung and studied them within 30 minutes or 24 hours. Within 30 minutes, there were gross and light-microscopic distinctions between the right and left lungs. Extensive hemorrhages were restricted to the right lung. By 24 hours, however, both lungs were hemorrhagic and showed various degrees of congestion.
On electron microscopy, the left lung showed discrete changes in type I epithelial cells and endothelial cells by 30 minutes after the blast. The changes consisted of pinocytosis, ballooning, and rupture of the cells. The vasculature, bronchiolar epithelial cells, and related interstitium were unremarkable, and type II pneumocytes generally appeared normal in structure. These findings contrasted with those in the right lung, where two distinct conditions were evident 30 minutes after the blast. In the first, alveolar spaces were filled with erythrocytes, and there was little evidence of a fibrin clot. The interstitium of the alveolar walls, capillaries, and type II pneumocytes appeared intact, but there was extensive ballooning of the endothelium, and type I epithelial cells showed increased pinocytosis. The second was characterized by alveolar spaces that were filled with erythrocytes and by evidence of fibrin clotting. Alveolar walls showed interstitial disruption and capillary rupture. Intact endothelial cells exhibited ballooning, pinocytosis, and necrosis, but there was only isolated evidence of structural damage in type II pneumocytes. By 24 hours, the pathologic conditions had expanded markedly and, although the right lung was more affected than the left, both showed interstitial and intra-alveolar edema and isolated hemorrhage. Microemboli were evident in the lumina of both arterioles and venules, and the inter-alveolar septa showed more pronounced damage, with overt interstitial and intra-alveolar edema and hemorrhage. In summary, the early ultrastructural study provided confirmation that hemorrhages, present soon after blast injury, are progressive and associated with the emergence of interstitial and intra-alveolar edema, microemboli, and damage to the alveolar blood–air barrier. Collectively, those events probably compromise gas exchange and contribute to increased pulmonary vascular resistance and reduced lung compliance.
The initial study by Brown and colleagues (1993) has served as a platform for validation of blast-induced changes in the lungs in other animal models. In general, the studies reveal common pathologic features that support the earlier ultrastructural findings, namely, damage to the alveolar septa, pulmonary hemorrhage, and edema (Chavko et al., 2006; Elsayed and Gorbunov, 2007; Koliatsos et al., 2011). Koliatsos et al. (2011) confirmed the vulnerability of the lungs to blast injury and reported the following findings: intra-alveolar hemorrhages, edema, atelectasis, and inflamma-
tory cell infiltrates. Similar findings of hemorrhages and atelectasis were reported by Zhang et al. (2011), who exposed male New Zealand rabbits to chest–abdomen blast injuries produced by explosives that were suspended above the xiphoid process. Repeated low-level blast overpressure exposures of rodents also revealed hemorrhages and ruptured alveolar walls, but the number of blast exposures did not appear to alter the magnitude of pulmonary injury; that is, the pathologic picture after repeated exposures was similar to that after a single exposure and in all cases pathologic changes increased over time (Elsayed and Gorbunov, 2007).
A proinflammatory response (that is, the activation of macrophages, lymphocytes, and neutrophils) begins within hours after blast injury. Chavko et al. (2006) evaluated inflammatory responses in male, Sprague-Dawley rats that were positioned in a shock tube and subjected to a blast wave driven by compressed air. A pronounced inflammatory response was characterized by increased expression of myeloperoxidase (MPO), an enzyme found in neutrophils, and a number of cytokines and chemokines. There is also evidence of remote damage to the lungs when blast trauma is limited to a hind limb in rodents (Ning et al., 2012). In this case, the pathologic effects over the course of 6 hours consisted of alveolar congestion and disruption, hemorrhage, and leukocyte infiltration.
Oxidative Stress and Inflammation
Blast injury commonly results in a triad of events in the lungs (Elsayed et al., 1997): damage to structures that include the alveolar or capillary barrier and resulting hemorrhage and edema, formation of an air embolism that leads to impaired circulation and ischemia, and inflammation. Oxidative stress arises from an imbalance between oxidants and antioxidants and typically evolves as a consequence of the formation of reactive species that exceeds the capacity of antioxidant systems (see Figure 3-8). (For a more detailed review of this subject, see Elsayed and Gorbunov, 2003.) Oxidative stress may evolve as a consequence of each of those components of the triad. Pulmonary hemorrhage and the resulting accumulation of free hemoglobin trigger free-radical reactions that produce oxidative damage and support a proinflammatory state (Kirkman and Watts, 2011). Here, we address two major cascades that are triggered in part by hemorrhage and emerge within minutes to hours after injury in the lungs: oxidative stress and inflammation. Oxidative stress may lead to oxidative damage to cellular constituents, including lipids, proteins, and DNA. A number of sources may contribute to oxidative stress and injury in the lungs, including hemoglobin, which can generate reactive oxygen species (Regan and Panter, 1993), and inflammatory cells, including leukocytes and macrophages, which are key sources of reactive oxygen species (Chavko et al., 2009). Pro-oxidants
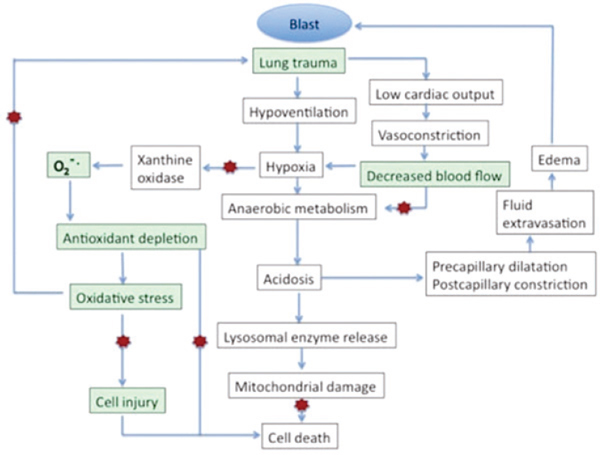
FIGURE 3-8 Secondary pathogenesis after blast-induced lung injury.
NOTE: Red stars indicate pathways whereby antioxidants may exert protection.
SOURCE: Adapted from Elsayed and Gorbunov, 2003. Toxicology, Vol. 189, Nos. 1–2, N. M. Elsayed and N. V. Gorbunov, Interplay between high energy impulse noise (blast) and antioxidants in the lung, pages 63–74, Copyright 2003, with permission of Elsevier.
include both radicals (such as superoxide anion radical, hydroxyl radical, nitrogen dioxide, nitric oxides, and ethyl radicals) and nonradical reactive species (such as hydrogen peroxide, lipid hydroperoxide, hypochloric acid, and iron–oxygen complexes). Free radicals have the capacity to interact with one another to form more potent compounds, such as peroxynitrite radical resulting from the reaction of the superoxide anion radical with nitric oxide. Involvement of radicals and nonradical reactive species is complicated by the fact that although some are toxic, such as iron–oxide complexes, others may be beneficial by warding off infection or in defined contexts may act as antioxidants.
Oxidative stress and inflammation have been shown to be related key components of blast-induced damage in the lungs in a variety of experimental models (rats, rabbits, and sheep) that used low-level blast waves and blasts restricted to a limb (Chavko et al., 2006; Elsayed and Gorbunov, 2003, 2007; Gorbunov et al., 1997; Ning et al., 2012). In rats exposed
to low-level blast overpressure, oxidative stress is evidenced by a 3.5-fold decrease in total antioxidant reserves and depletion of the water-soluble antioxidants ascorbate and glutathione by 50–75% and of the lipid-soluble antioxidant vitamin E by 30%. Those reductions are accompanied by lipid peroxidation and increased methemoglobin content without degradation of hemoglobin (see Table 3-3) (Gorbunov et al., 1997). Repeated low-level blast exposures result in decreases in vitamins C and E (by 20–60% and 25–40%, respectively) that are concomitant with an increase in lipid peroxidation (by 25–50%) (Elsayed and Gorbunov, 2007). Those biochemical changes are the same whether produced by a single exposure or by multiple exposures.
Such findings suggest that repeated exposures do not compound the effects of the initial exposure. Chavko et al. (2006) evaluated the progression of inflammatory and oxidative events in the lungs after exposure to medium-intensity blast overpressure. The proinflammatory response was characterized by an increase in MPO that peaked at 24 hours and increases in chemokines CINC-1 and ICAM-1 that peaked at 2–24 hours and 24–48 hours, respectively. It is thought that the early increase in CINC-1 contributes to the early influx of neutrophils (MPO+). Indexes of oxidative stress (protein oxidation and nitration) and of MnSOD and heme oxygenase-1, which have antioxidative and anti-inflammatory properties, respectively, were also evaluated. Protein oxidation and nitration peaked at 2 hours. The early rise corresponded to early activation of inflammation (CINC-1) and thus implicates oxidative stress in the activation of the chemokines. Finally, the induction of MnSOD and heme oxygenase-1 at 24–48 hours after injury may not only contribute to the observed reduction in oxidative and nitrative damage that occurred later than 2 hours after injury but also facilitate the resolution of inflammation.
Pharmacologic Manipulations of Oxidative Stress and Early Inflammation
Pharmacologic strategies provide useful means of confirming mechanisms of action and establishing efficacy. N-acetylcysteine amide (NACA) and hemin, an inducer of heme oxygenase-1, are candidate therapeutics whose use has reinforced the involvement of oxidative stress and inflammation in the lung after a blast. Chavko et al. (2009) evaluated NACA, focusing on blast-induced pulmonary inflammation. NACA is a novel derivative of N-acetylcysteine that has hydrophobic and membrane-permeable properties. It has been shown to protect against hemoglobin oxidation and to reduce inflammation in a murine model of asthma (Bahat-Stroomza et al., 2005; Grinberg et al., 2005; Lee et al., 2007). Rats were given NACA 30 minutes to 24 hours after a moderate blast overpressure. It reduced infil-
TABLE 3-3 Animal Models
| Description of Blast | Species, Sexa | Times Studied |
| Compressed-air-driven shock tube | Sprague-Dawley rats | 5, 60 min |
| Compressed-air-driven shock tube | Sprague-Dawley rats | 1, 3, 6, 12, 24 hr |
| Compressed-air-driven shock tube | Sprague-Dawley rats | 1, 3, 6, 12, 24 hr |
| Compressed-air-driven shock tube | Sprague-Dawley rats | 15 min, 1, 3, 6, 12, 24, 34, 56 hr |
| Blast to hind limb by commercial detonator | Sprague-Dawley rats, male | 0.5, 1, 3, 6 hr |
| Compressed-air-driven shock tube | Sprague-Dawley rats, male | 1, 6, 24 hr |
| Compressed-air-driven shock tube | Sprague-Dawley rats, male | 2–192 hr |
NOTES: 3NTyr = 3-nitrotyrosine; BAL = bronchoalveolar lavage; CINC-1 = cytokine chemoattractant-1; HO-1 = heme oxygeanse-1; ICAM-1 = intercellular adhesion molecule-1; IL = interleukin; iNOS = inducible nitric oxide synthase; MCP-1 = monocyte chemoattractant protein-1; MIP-2 = macrophage inhibitory protein-2; MnSOD = manganese superoxide dismutase; MPO = myeloperoxidase; SOD = superoxide dismutase; TNF = tumor necrosis factor.
aSome studies do not report gender.
| Indexes of Oxidative Stress or Inflammation | Structural Changes | References | ||
| Decrease in total antioxidant reserves; depletion of ascorbate, glutathione, and vitamin E; increase in lipid peroxidation and methemoglobin | Gorbunov et al., 1997 | |||
| In blood, decrease in iron–transferrin complexes and increase in neutrophils; in BAL fluid and blood, cytokine release (IL-1, IL-6, MCP-1, MIP-2) | Gorbunov et al., 2005 | |||
| Increase in MIP-2 in plasma from 1 to 6 hr; increase in neutrophils in blood from 1 to 3 hr and decrease thereafter; no change in CD11b; transferrin-bound iron sequestration by 3 hr | Bilateral diffuse hemorrhage of alveolar septal capillaries, infiltration of neutrophils by 3 hr | Gorbunov et al., 2004 | ||
| Up-regulation of HO-1, MPO, 3NTyr, and SOD at 1–6 hr | Hemorrhage, deposition of hemoglobin in entire thickness of lobe; neutrophils (MPO+, CD11b+, VE-cadherin+), damage to endothelium, epithelial cell necrosis, deposition of free iron | Gorbunov et al., 2006 | ||
| Increase in TNF-alpha; decrease in SOD, cystathionine gamma-lyase, hydrogen sulfide | Alveolar congestion, neutrophil infiltration, hemorrhagic lesions | Ning et al., 2012 | ||
| Vitamins C and E decreased after initial blast by 20–60%; lipid peroxidation increased by 25–60% | After single blast, multifocal minimal to mild hemorrhage throughout lobes; by 24 hr, increased intra-alveolar hemorrhage, ruptured alveolar walls | Elsayed and Gorbunov, 2007 | ||
| Increases in MPO, CINC1, ICAM-1, iNOS, protein oxidation and nitration, hemeoxygenase-1, MnSOD | Chavko et al., 2006 | |||
tration of neutrophils and blocked the activation of CC chemokines, macrophage inflammatory protein-1, monocyte chemotactic peptide-1, CXC chemokine, and cytokine-induced neutrophil chemoattractant 2 and 8 days after injury. Although NACA reduced expression of heme-oxygenase-1, it had no substantial effect on MnSOD or glutathione reductase; this finding only partially supports its antioxidant effect.
Heme-oxygenase-1 is an enzyme that is responsible for the breakdown of the pro-oxidant heme to biliverdin, carbon monoxide, and ferrous iron. The free iron that is released is bound to ferritin, and this reduces its capacity to induce oxidative stress. After trauma, hemorrhage and the resulting free hemoglobin induce vasoconstriction, which results in hypoperfusion of the lung. Moreover, free iron and heme are generated from auto-oxidation of oxyhemoglobin, both of which can cause oxidative damage. Strategies to induce heme-oxygenase-1 have been shown to have therapeutic effects in different diseases. The unusual broad spectrum of its beneficial effects probably reflects its ability to function as an anti-inflammatory and cytoprotectant (via carbon monoxide) and its antioxidant properties (biliverdin and bilirubin) (Abraham and Kappas, 2005; Soares and Bach, 2009). Chavko et al. (2008) evaluated hemin, a known inducer of heme-oxygenase-1. Adult Sprague-Dawley rats were treated with hemin (treatment group) or saline (vehicle group) 20 hours before blast exposure, and euthanized 30 minutes after the blast. The blast induced heme-oxygenase-1 mRNA and protein in the lungs, but there were no differences between the treated and vehicle groups, although there was a significant difference in survival rates between the two groups: 35% survival in vehicle controls and 68% in the hemin-treated group. The authors did not speculate on the absence of differences in heme-oxygenase-1 expression between the vehicle and control (saline-treated, unexposed to blast) groups. Because the activity of the enzyme was not measured, it is conceivable that heme-oxygenase-1 activity increased in response to hemin without changes in either mRNA or protein.
Acute Respiratory Distress Syndrome: Parallels to the Pathobiology of Blast Lung
Despite its association with a number of risk factors and inciting insults, acute respiratory distress syndrome, first described in 1967, is characterized by common mechanisms of pathogenesis as blast lung (Fanelli et al., 2013). The mechanisms include dysregulated inflammation, uncontrolled coagulation pathways, and disruption of the alveolar endothelial and epithelial barriers, which can lead to alveolar edema (Matthay et al., 2012). Leukocyte-directed proteolytic activity, oxidative stress, and excessive production of chemokines and cytokines contribute to acute lung damage. Many of those features—most notably inflammation, alveolar edema, and
oxidative stress—are also signatures of blast lung. Researchers can only speculate on why that is the case. One possibility may be related to constituents of the lung that are most vulnerable to injury, namely, the alveolar endothelial and epithelial barriers that may become overwhelmed by a local proinflammatory state and the ensuing oxidative stress that results from inadequate endogenous antioxidant reserves. Considerable progress has been made in understanding the pathophysiology of acute respiratory distress syndrome. Such a foundation may prove useful to researchers who are only beginning to decipher the complex pathophysiology of blast lung and its long-term consequences.
Abdominal Organs
The most popular explanation of primary blast-induced injuries to the abdominal organs involves the transmission of a shock wave’s kinetic energy (Clemedson and Criborn, 1955) and the later generation of two main types of waves during its propagation: stress waves and shear waves. Stress waves are longitudinal pressure forces that move at supersonic speeds and create a spalling effect at the air–tissue interfaces, which results in microvascular damage and tissue disruption. Shear waves are transverse waves that cause asynchronous movement of the tissue and possible disruption at the interfaces. Stress waves cause injuries mainly to hollow organs, and shear waves, mainly to solid organs (Wightman and Gladish, 2001).
Hollow Abdominal Organs
The most frequent primary blast-induced pathologic changes confirmed in experiments on small (Tatic et al., 1996) and large (Cripps and Cooper, 1997; Holzer et al., 2012; Savic et al., 1991) experimental animals are: widespread petechiae and localized ecchymosis in mucosa and serosa, rarely with small ulcerations. In the most severe cases, perforation of hollow gastrointestinal organs causes accumulation of air in the abdominal cavity and peritonitis. Lacerations and perforations of the diaphragm are extremely rare. Exposure to an extreme blast environment, in which primary and secondary effects act in parallel, produces immediate abdominal laceration or perforation, involving mainly the large and small bowel (Bala et al., 2008). However, contusions and intramural hematomas have been shown to predominate in nonfatal blast exposures (Cripps and Cooper, 1997); these lesions, which are morphologically and histologically similar to those caused by blunt abdominal trauma, are subject to late perforation (Cripps and Cooper, 1997; Ignjatovic et al., 1991; Paran et al., 1996). It has been suggested (Guy and Cripps, 2011) that the combined effect of stress waves and shear waves damages the mucosa and the serosa, in which the contu-
sions at highest risk for late perforation are the ones that show evidence of subserosal bleeding rather than hemorrhage confined to the mucosa and submucosa.
Cripps and Cooper (1997) developed a blast-injury model in large white pigs that weighed 49–66 kg and had accelerometers mounted on their chests to measure the transfer of kinetic energy to the torso. Anesthetized animals were fixed to an animal holder and exposed to blast generated by detonating spherical plastic explosive charges that weighed 2.0–3.6 kg and were 1.8–3.0 m from the animal’s chest wall. The animals were sacrificed after 24 hr and subjected to postmortem examination and abdominal-organ analyses. The authors provided a histologic classification of the spectrum of intestinal injury (see Table 3-4), which is consistent with injury directed from mucosa to serosa, that is, injury caused by the release of energy in the mucosa and, if energy transfer is sufficient, progressive disruption of the submucosal, muscular, and serosal layers. They concluded that serosal injury is the de facto evidence of transmural injury. Although the usual interval for perforation after blast exposure is 1–14 days, their study identified patterns of injury and a classification in which no difference was observed in the distribution of histologic grades at the two times. Thus, the authors made
TABLE 3-4 Histologic Classification of Primary Intestinal Blast Injuries
| Grade of Injury | Mucosal Appearance | Muscular Appearance | Serosal Appearance | |
| Ia | Normal or diffuse bleeding only | Normal | Normal | |
| II | Mucosal hematoma without glandular disruption | Mild edema only | Normal | |
| III | Marked hemorrhage with glandular disruption | Marked edema or minor hemorrhage only | Normal | |
| IV | Gross glandular or mucosal disruption; muscularis mucosae may be disrupted | Muscular disruption or major hematoma | Subserosal hemorrhage | |
| V | Complete mucosal laceration | Muscular laceration or disruption | Serosal laceration | |
aIncludes contusions in which submucosal hematoma is evident without other evidence of mural injury.
SOURCE: Reprinted from Cripps and Cooper, 1997. Copyright 1997, with permission from Blackwell Science Ltd.
their final recommendations based on the histological features of the lesions without regard to the age of the contusion (see Table 3-5). It has been suggested that the appearance of necrosis and inflammatory cell infiltrates and the size of early pathologic changes could be predictive of perforation. If these experimental guidelines were adopted and small-bowel contusions less than 15 mm in diameter and colonic contusions smaller than 20 mm were left alone, the number of small-bowel contusions requiring excision would be reduced by one-fourth and colonic contusions by two-thirds (see Table 3-5) (Cripps and Cooper, 1997).
Solid Organs
Pathologic changes in solid abdominal organs (liver, spleen, pancreas, kidneys, and adrenal glands) that result from blast exposure range from subcapsular hemorrhage and small foci of parenchymal hemorrhage, to primary or secondary rupture of capsules and parenchymal laceration (Vriese, 1904). Recent experimental data clearly showed the importance of body position relative to the shock-wave front not only for injury severity but for the pattern of multi-organ damage (Koliatsos et al., 2011). Lungs, heart, and kidneys are damaged more frequently and severely when the body is supine, and the liver and spleen are injured more frequently and severely when the body is prone (see Table 3-6). In a recently published mouse blast-injury model (Koliatsos et al., 2011), liver damage was observed in a little over 40% of the cases. The pathologic changes included congestion, mottling, and white discoloration adjacent to apparently hemorrhagic sites. White infarcts were the lesion most reliably observed at the microscopic level, usually next to a distended, hemorrhaging branch of the portal vein (see Figure 3-9, panels A and B), whereas tissue ischemia was most marked around portal veins (see Figure 3-9, panel C). In contrast with lung injuries, prone position caused more severe lesions than supine position. In the prone position but not the supine position, there was some association between blast severity and infarct rate. In more severe injuries, in sheep exposed to open field blast (Savic et al., 1991; Tatic et al., 1991a; Vriese, 1904), liver laceration and subcapsular liver rupture have been reported with a lesion of the extrahepatic biliary duct and gallbladder hematoma.
The spectrum of pathologic alterations seen in pancreas, kidneys, and adrenal glands of sheep exposed to open-field blast (Savic et al., 1991; Tatic et al., 1991a; Vriese, 1904) includes petechiae, ecchymosis, and small foci of parenchymal hemorrhage. The experimental findings were comparable with findings in victims of industrial blast (Tatic et al., 1991b). In the mouse model developed by Cernak et al. (2011), the most consistent macroscopic and microscopic findings in the spleen and kidney were red infarcts. Hemorrhagic kidney infarcts occurred almost exclusively in the
TABLE 3-5 Distribution of Histologic Grades Between Contusion Groups of Different External Appearance
| Contusion Measure | Histologic Grade | Significance | ||||
| I and II | III | IV and V | χ2 | d.f. | p | |
| Size (mm) | ||||||
| Small bowel | ||||||
| ≤15 | 30 | 5 | 3 | 9.09 | 2 | 0.01 |
| >15 | 28 | 17 | 13 | |||
| Colon | ||||||
| ≤20 | 60 | 4 | 3 | 14.95 | 2 | 0.0006 |
| >20 | 14 | 3 | 8 | |||
| Position | ||||||
| Small bowel | ||||||
| Mesenteric | 18 | 12 | 11 | 7.5 | 2 | 0.02 |
| Antimesenteric | 39 | 10 | 6 | |||
| Colon | ||||||
| Mesenteric | 8 | 1 | 1 | 0.12 | 2 | 0.94 |
| Antimesenteric | 66 | 6 | 10 | |||
| Circumferential extenta | ||||||
| Small bowel | ||||||
| One-half or less | 47 | 13 | 6 | 14.79 | 2 | 0.0006 |
| More than one-half | 10 | 9 | 11 | |||
| Confluence | ||||||
| Small bowel | ||||||
| Confluent | 22 | 11 | 12 | 5.49 | 2 | 0.06 |
| Diffuse | 35 | 11 | 5 | |||
| Colon | ||||||
| Confluent | 26 | 4 | 8 | 6.37 | 2 | 0.04 |
| Diffuse | 48 | 3 | 3 | |||
NOTE: d.f. = degrees of freedom.
aThe relationship between grade and circumferential extent is not shown for the colon because only two contusions were larger than half the circumference—a feature more to do with colonic size than contusion size.
SOURCE: Reprinted from Cripps and Cooper, 1997. Copyright 1997, with permission from Blackwell Science Ltd.
TABLE 3-6 Type, Severity, and Frequency of Microscopic Lesions in Key Thoracic and Abdominal Viscera
| Severe, Supine (%) | Severe, Prone (%) | Moderate, Supine (%) | Moderate, Prone (%) | Mild, Supine (%) | Mild, Prone (%) | Total (%)a | |
| Lungs | |||||||
| Superficial | 0.0 | 0.0 | 5.3 | 50.0 | 33.3 | 25.0 | 20.7 |
| hemorrhages | |||||||
| Hematomas | 0.0 | 33.3 | 10.5 | 0.0 | 22.2 | 25.0 | 12.1 |
| Hemorrhagic | 100.0 | 66.6 | 84.2 | 50.0 | 44.4 | 37.5 | 65.5 |
| consolidation | |||||||
| Total | 98.3 | ||||||
| Liver | |||||||
| Infarcts | 14.3 | 100.0 | 52.6 | 50.0 | 0.0 | 50.0 | 41.4 |
| Heart | |||||||
| Right ventricle | 57.1 | 33.3 | 21.0 | 0.0 | 11.1 | 0.0 | 17.2 |
| dilation | |||||||
| Left ventricle | 28.6 | 33.3 | 21.0 | 0.0 | 11.1 | 0.0 | 12.0 |
| dilation | |||||||
| Total | 29.3 | ||||||
| Spleen | |||||||
| Red infarcts | 28.6 | 0.0 | 10.5 | 16.6 | 0.0 | 12.5 | 12.1 |
| Kidney | |||||||
| Red infarcts | 14.3 | 0.0 | 26.3 | 8.3 | 0.0 | 0.0 | 12.1 |
aPercentages are rates of cases with indicated lesion out of the total number of cases in first group.
SOURCE: Reprinted with permission from Koliatsos et al., 2011(http://links.lww.com/NEN/A233 [accessed March 27, 2014]).
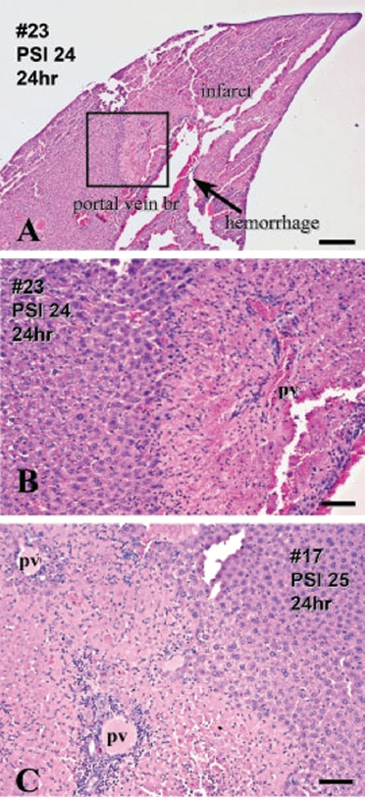
FIGURE 3-9 Patterns of blast injury to liver with settings used in Koliatsos et al. (2011). Hematoxylin and eosin.
NOTES: Panels A and B illustrate site of white infarct distal to a dilated or hemorrhaging branch of portal vein. Panel B is enlargement of framed area in panel A and shows further detail of hemorrhagic smaller portal vein branch (pv) and sharp border between normal and ischemic tissue. Panel C shows severe tissue hypoxia around portal triads from another case. Normal liver parenchyma is shown on left of panels A and B and right of panel C. Scale bars: A, 500 ìm; B and C, 100 ìm.
SOURCE: Reprinted with permission from Koliatsos et al., 2011 (http://links.lww.com/NEN/A235 [accessed March 27, 2014]).
medullary zone (see Figure 3-10, panel A). In spleens, a dilated vessel filled with blood was seen in the trabeculae proximal to the infarct in nearly all cases (see Figure 3-10, panel B). Potential chronic consequences of blast exposure for morphologic and functional integrity of kidneys were analyzed in sheep exposed to blast (Casals et al., 2011). Substantial atrophy of parenchyma and infarction sequelae were found 30 days after exposure to a high-energy explosive-produced overpressure of 166 psi with a 3.0-ms duration (near the LD50). It has been hypothesized that kidney contusion aided by endothelial damage of glomerular blood vessels and compromised circulation due to severed arterioles and venules may cause chronic renal insufficiency (Casals et al., 2011; Vriese, 1904). The resulting impairment
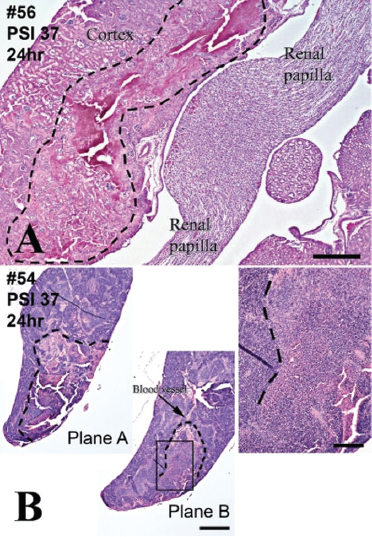
FIGURE 3-10 Pathologic signatures of blast injury to kidney (panel A) and spleen (panel B). Hematoxylin and eosin.
NOTES: A: hemorrhagic infarct in medullary zone delineated with broken line. Normal kidney parenchyma is outside broken line. B: spleen infarct associated with dilated hemorrhaging blood vessel in two planes (planes A and B). Illustration on right panel is magnification of framed area in plane B. Normal spleen parenchyma is shown on left and top. Scale bars: 500 μm.
SOURCE: Reprinted from Cernak et al., 2011, with permission from Elsevier.
of the renin–angiotensin system could lead to chronic hypertension, and this may offer a rational explanation for the hypertension noted in survivors of the Texas City explosions (Blocker and Blocker, 1949; Ruskin et al., 1948).
Cardiovascular System
The main vascular mechanisms are described in the section on systemic changes that modify individual organ responses to blast. This section describes microvessel injury from exposure to blast in experimental models.
Zhang et al. (2011) used New Zealand rabbits exposed to explosive-generated blast in laboratory conditions to show that increased permeability, measured by 125I-albumin leakage, and microvessel injury in the lungs and kidneys are among the key outcomes of blast overpressure. Damage to the microvessels led to leakage of albumin and caused hemoconcentration in the absence of active bleeding after a blast. The resulting increase in blood viscosity and hematocrit can aggravate a blast-induced oxygen deficit and decrease vasodilation function. The microvascular endothelium is essential in maintaining circulatory homeostasis and normal physiologic function of organs. Its impairment has a broad array of implications: Diffuse microvascular hyperpermeability and later plasma extravasation may result in hypovolemic shock, pulmonary edema, abdominal compartment syndrome, and generalized tissue malperfusion (Garner et al., 2009; Lamb et al., 2010; Zhang et al., 2011).
In the mouse blast-injury model (Cernak et al., 2011), the most pronounced pathologic changes in the heart, as in the lungs, were found in animals exposed in a supine position (Koliatsos et al., 2011) (see Table 3-6). Although the animals were positioned so that their torsos were parallel to the front of the shock wave, pathologic findings were more frequent in the right side of the heart compared to the left side. The macroscopic and microscopic observations in the heart include dilation of ventricles and atria (Koliatsos et al., 2011). Venous congestion was the most frequent finding in animals exposed to mild blast, whereas dilation-filling (“congestion”) of ventricles was seen with increased blast-injury severity. Hemorrhagic infarct of ventricular walls was found only in the most severe injuries. A broad array of dose-dependent pathologic changes has been reported in the hearts of sheep exposed to blast generated by detonating an aerosol bomb in an open field (Savic et al., 1991; Tatic et al., 1991a; Vriese, 1904). The findings included petechiae and ecchymosis in the pericardium, epicardium, and endocardium; hemorrhage in the myocardium; and rare endocardial hematoma.
Chronic effects of blast exposure have been demonstrated in experiments in sheep that were exposed to a high-energy explosive-produced overpressure of 164 psi with a 3.3-ms duration (near the LD50) and sacri-
ficed 30 days later (Casals et al., 2011). The findings included infarction sequelae in the heart in a form of multiple scars in the myocardial walls, which implied reduced cardiac contractility and compliance.
Sensory Organs
Fronto-Orbital Fractures
It has been posited that bone structures that provide the least resistance to transferred kinetic energy are prone to fractures when exposed to blast (Lu and Wilson, 2003). In a study that exposed 115 dogs in a shock tube to blast waves with rise times of 12–155 ms, peak overpressures of 52–231 psi, and durations of 0.4–20 s, 11 blow-out fractures (fracture of the walls or floor of the orbit) were seen in 9 animals (Guitton and Dudai, 2007). The time to and magnitude of the maximal overpressure have been shown to be critical for orbital fracture in the nearby paranasal sinuses. Orbital fractures and related eye signs have been noted in rhesus monkeys exposed to a high-explosive-produced, fast-rising overpressure of 325 psi with a duration of 3.5 ms (Casals et al., 2011). Although midface fractures in recent wars have been reported to have high complication rates (Kittle et al., 2012), there are no quantitative data that would permit an assessment of blast conditions that can be expected to produce lesions in the human orbit or make it possible to know whether the lesions are likely to be seen in survivors exposed to a combat-relevant blast environment.
Auditory Blast
Auditory dysfunction due to blast is among the most frequent service-connected disabilities in veterans; compensation totals more than $1 billion a year (Fausti et al., 2009). Accumulating evidence demonstrates peripheral hearing loss, central auditory processing deficits, vestibular impairment, and tinnitus as the most prevalent impairments caused by blast (Fausti et al., 2009; Mehlenbacher et al., 2012; Nageris et al., 2008). The clinical manifestations of auditory blast injuries are well documented (Patterson and Hamernik, 1997), and experimental studies have provided useful information about the mechanisms underlying them.
Eardrum rupture has been posited as one of the hallmarks of blast injuries (Hirsch, 1968; White et al., 1970), but recent data indicate that the status of the tympanic membrane after exposure to a blast does not obviate further investigations to discern a primary blast injury (Peters, 2011). Early experimental data showed that tympanic membrane rupture opens the middle ear, mastoid air cells, and Eustachian tube to the invasion of pathogens and other foreign materials via the external auditory meatus. A
damaged eardrum also means compromised protection of the ossicles and inner ear from overload when a single exposure to pressure variations due to blast occurs. Finally, the eardrum plays a role in energy transfer through the oval window to the organ of Corti via the ossicles and the endolymph when repetitive exposure to blast and high noise levels occurs (White et al., 1970). Using human cadavers, Zalewski (1906) demonstrated characteristic variability of the eardrum’s response to overpressure that depends on age, previous scarring, calcification, infection thickening (fibrosis), unusual thinning of the tympanum, and the presence of any material in the external auditory meatus. White et al. (1970) analyzed a large series of blast experiments involving dogs, guinea pigs, goats, and rabbits and concluded that, because of the wide tolerance limits of the tympanic membrane, rupture of the eardrum or lack thereof cannot be considered a reliable clinical sign for judging the severity of a blast injury. Notably, the eardrum often remains intact when exposure pressures produce serious lung injury, but may rupture at pressures well below generally hazardous ones.
Mao et al. (2012) used a rat blast-injury model to investigate the underlying mechanisms of blast-induced tinnitus, hearing loss, and associated TBI. Briefly, anesthetized rats placed on supportive netting were subjected to a single blast exposure with a custom-designed shock tube (Leonardi et al., 2011). The 10 ms blast exposure was estimated to be in a wide range of frequencies with an average energy of under 10 kHz measured at 14 psi, which was translated to a sound pressure around 95 kPa or 194 dB above the standard reference sound pressure in air. Blast exposure induced early onset of tinnitus and central hearing impairments at a broad frequency range but showed a tendency to shift toward high frequencies over time. The immediate increase in the hearing threshold measured with auditory brainstem responses was followed by recovery on day 14; behavioral changes showed a comparable temporal profile. Diffusion-tensor magnetic resonance imaging results demonstrated substantial damage and compensatory plastic changes in some auditory brain regions; most of the changes occurred in the inferior colliculus and medial geniculate body. The authors hypothesized that the lack of important microstructural changes in the corpus callosum could be explained on the grounds that the blast exposure in their experimental setting exerted effects mainly through the auditory pathways rather than through direct impact on the brain parenchyma. Several experiments focused on mechanisms of cochlear pathology in guinea pigs (Fang, 1988; Hu, 1991; Liu, 1992a,b; Yokoi and Yanagita, 1984; Yuan, 1993; Zhai, 1991; Zhai et al., 1997), rats (Guitton and Dudai, 2007; Kirkegaard et al., 2006), and chinchillas, pigs, and sheep (Roberto et al., 1989). Effects of impulse noise exposure (25 impulses with a peak level of 165 dB SPL) on endocochlear potentials (EPs) and compound action potentials (CAPs) were investigated in guinea pigs (Fang, 1988; Zheng,
1992). The positive EPs returned to normal values 7 days after exposure but the negative EPs and the CAP threshold did not; this implied that the stria vascularis was damaged in addition to the organ of Corti. Indeed, impaired cochlear microcirculation and increased exudation of vascular stria early after impulse noise have been found by others (Kellerhals, 1972; Liu, 1992a) and suggest a potential linkage between altered microcirculation, later impaired oxygen delivery, and oxidative stress in leading to functional and morphologic impairments (Branis and Burda, 1988). A more recent study showed a smaller extent of damage to the cochlea of mice on the basis of imaging techniques newer than were used in earlier studies (Cho et al., 2013). However, although it did not show gross cochlear membrane damage, it did reveal hair-cell death and spiral ganglion neuron loss that were consistent with past studies. It has been suggested that hair-cell death could underlie chronic hearing impairments due to blast.
Visual System
A recent mouse model of primary ocular blast injury (Hines-Beard et al., 2012) used a device that applied a localized overpressure to the eyes of experimental animals. The overpressure was generated by a device that consisted of a pressurized air tank attached to a regulated paintball gun with a machined barrel and a chamber that protected the mouse from direct injury and recoil while the eye was exposed. The experimental setting enabled analysis of the localized effects of a focused overpressure wave, but it did not reproduce a field condition in which the entire body is exposed to a blast environment. Mice were exposed to one of three blast pressures (23.6, 26.4, or 30.4 psi), and gross pathologic effects, intraocular pressure, optical coherence tomography, and visual acuity were assessed 0, 3, 7, 14, and 28 days after exposure. Focally delivered shock wave caused corneal edema, corneal abrasions, optic nerve avulsion, and retinal damage.
Clinical data demonstrated that disruption of central visual pathways represents an additional cause of blast-related visual dysfunction (Dougherty et al., 2011). In the experimental model developed by Petras et al. (1997), the effect of blast overpressure on the visual system was studied in rats exposed to blast overpressure that was generated by a compressed-air-driven shock tube. Neurologic injury to brain visual pathways was observed in male rats that survived blast overpressure exposures of 104–110 kPa and 129–173 kPa. Optic nerve fibers degenerated on the same side as the blast pressure wave. The optic chiasm contained small numbers of degenerated fibers. Optic tract fiber degeneration was present bilaterally but was predominantly on the same side. Optic tract fiber degeneration was followed to nuclear groups at the level of the midbrain, midbrain–diencephalic junction, and thalamus, where degenerated fibers arborized among the neurons
of the superior colliculus, pretectal region, and lateral geniculate body. The superior colliculus contained fiber degeneration localized principally to the stratum opticum (layer III) and stratum cinereum (layer II). The pretectal area contained degenerated fibers that were widespread in the nucleus of the optic tract, olivary pretectal nucleus, anterior pretectal nucleus, and posterior pretectal nucleus. Degenerated fibers in the lateral geniculate body were not universally distributed: they appeared to arborize among neurons of the dorsal and ventral nuclei, including the ventral lateral geniculate nucleus (parvocellular and magnocellular parts) and the dorsal lateral geniculate nucleus. The study showed that blast exposure can induce permanent injury to some of the brain’s central visual pathways (retinofugal axonopathy). Considering that the nuclei of the pretectal region receive connections from the superior colliculi and emit fibers to several tegmental nuclear groups implicated in coordinated movements of the eyes, the authors suggested that the blast-induced degeneration of retino-pretectal fibers might cause substantial chronic impairments in control of the pupillary light reflex and the muscles of the ciliary body for accommodation of the lens (Petras et al., 1997). Chen et al. (2003) provided further information about potential mechanisms underlying blast-induced degeneration of visual pathways. They used rabbits exposed to blast and showed that blast exposure initiates apoptosis of retinal ganglion cells parallel with increased glutamate concentration in the corpus vitreum; this suggested a potential biologic link (Chen et al., 2003).
Heterotopic Ossification
In the recent wars in Afghanistan and Iraq, development of heterotopic ossification (HO) in residual limbs has been reported in up to 63% of people who suffered combat-related amputations (Potter et al., 2007). That was an unexpected finding in that until recent years HO has rarely been found in the residual limbs of amputees (Alfieri et al., 2012). To test the possibility that amputation of an extremity by a blast spontaneously stimulates development of HO in the residual limb, Tannous et al. (2011) developed a rat model of localized exposure of a limb to a controlled high-energy blast. The blast setup consisted of an aluminum platform placed above a water-filled steel tank. The platform contained a hole 2.5 in. in diameter and raised 1 in. above the surface of the water. A 0.75-g charge of pentaerythritol tetranitrate (PTN) was 0.5 in. beneath the surface of the water, directly beneath the center of the hole in the platform. The anesthetized rat, secured with Velcro straps onto the platform with the designated extremity positioned over the hole at the predetermined amputation level, was exposed to the kinetic energy generated by detonation of PTN submersed beneath the water surface that caused a column of water
to rise at a speed of 534 m/s through the hole in the platform. The rats tolerated an isolated extremity blast injury well as long as the amputation was a forelimb amputation or a below-the-knee hind limb amputation. In this pilot experiment, three of four hindlimb amputees formed true islands of heterotopic bone in the soft tissues surrounding the amputation stump.
Blast-Induced Neurotrauma
Blast can interact with the brain by means of (1) direct interaction with the head via direct passage of the blast wave through the skull (primary blast), which causes acceleration or rotation of the head (tertiary blast), or through the impact of particles accelerated by the energy released during the explosion (secondary blast) (Axelsson et al., 2000; Cernak et al., 1996a; Clemedson and Hultman, 1954) and (2) kinetic energy transfer of the primary blast wave to organs and organ systems, including blood in large vessels in the abdomen and chest, reaching the CNS (Irwin et al., 1999; Ohnishi et al., 2001). During the interaction with the body surface, the shock wave compresses the abdomen and chest and transfers its kinetic energy to the body’s internal structures, including blood. The resulting oscillating waves traverse the body at about the speed of sound in water and deliver the shock wave’s energy to the brain. Clemedson, on the basis of his extensive experimental work on shock-wave propagation through the body (Gelman, 2008; Selye, 1976; Tümer et al., 2013), was among the first scientists to suggest the possibility of shock-wave transmission to the CNS (Pang, 2001). Those two potential paths of interaction are not mutually exclusive (Rutlen et al., 1979). Most recent experimental data suggest both the importance of the blast’s direct interaction with the head (Armonda et al., 2006; Axelsson et al., 2000) and the role of shock-wave-induced vascular load (Cernak et al., 1996a; Ling et al., 2009) in the pathogenesis of BINT.
Most currently used experimental models of BINT use rodents exposed to a shock wave generated in laboratory conditions with a compressed-air shock tube (Baalman et al., 2013; Cernak et al., 2011; Goldstein et al., 2012; Kamnaksh et al., 2012; Pun et al., 2011; Readnower et al., 2010; Reneer et al., 2011; Svetlov et al., 2012; Valiyaveettil et al., 2012a; Vandevord et al., 2012). Experiments with larger animals involve mainly pigs (Ahmed et al., 2012; Bauman et al., 2009) or nonhuman primates (Bogo et al., 1971; Lu et al., 2012).
Accumulating evidence suggests that primary blast causes substantial behavioral impairments and cognitive deficits in multiple animal models (Bogo et al., 1971; Cernak et al., 2001a; Lu et al., 2012). The deficits and degenerative processes in the brain show a dose–response relationship with primary blast intensity. The widely varied molecular changes start early
with metabolic impairments that include altered glucose metabolism, a shift from an aerobic toward an anaerobic pathway measured as increased lactate concentration and increased lactate:pyruvate ratio (Cernak et al., 1996b), then a decline in energy reserve (Cernak et al., 1995, 1996b), development of oxidative stress (Readnower et al., 2010) in parallel with ultrastructural changes in the brainstem and hippocampus (Cernak et al., 2001b; Saljo et al., 2000) and activation of early immediate genes (Saljo et al., 2002). Later, the mechanisms include inflammation (Cernak et al., 2011; Kaur et al., 1995, 1997; Kwon et al., 2011; Readnower et al., 2010; Saljo et al., 2001), diffuse axonal injury (Garman et al., 2011; Risling et al., 2011), and apoptotic and nonapoptotic cascades that lead to neurodegeneration (Svetlov et al., 2009; Vandevord et al., 2012; Wang et al., 2010). Emerging evidence suggests that some brain structures might have a more pronounced sensitivity to blast effects either because of anatomic features and localization or because of functional properties of neuronal pathways and cells (Koliatsos et al., 2004; Valiyaveettil et al., 2012a,b). Indeed, higher sensitivity of the cerebellum and brainstem, the corticospinal system, and the optic tract has been found (Koliatsos et al., 2004) on the basis of the extent of multifocal axonal and neuronal cell degeneration. In addition, according to region-specific alterations in the activity of the enzyme acetyl-cholinesterase, the vulnerability of the frontal cortex and medulla has been observed in mice exposed to blast overpressure (Valiyaveettil et al., 2012a,b). Those changes showed a tendency toward chronicity.
The mechanisms involved in the pathobiology of BINT show some similarities with those of blunt TBI but with earlier onset of brain edema and later onset of cerebral vasospasm (see Figure 3-11) (Agoston et al., 2009). Using a pig model of blast exposure, Ahmed et al. (2012) have shown that protein biomarker concentrations in cerebrospinal fluid provide insight into the pathobiology of BINT. Their findings implicated neuronal and glial cell damage, compromised vascular permeability, and inflammation induced by blast. The early-phase biomarkers included claudin-5, vascular endothelial growth factor, and von Willebrand factor, whereas neurofilament heavy chain, neuron-specific enolase, vascular endothelial growth factor, and glial fibrillary acidic protein levels remained substantially increased compared with baseline 2 weeks after injury.
Despite the growing experimental models of BINT, there is a serious need for a well-coordinated, multidisciplinary research approach to clarify injury tolerance in animal models that are relevant to the military experience and to define the injury mechanisms that underlie acute and chronic consequences of blast exposure.
FIGURE 3-11 Schematic representation of the approximate onset and duration of selected clinical pathologic conditions after blast TBI and nonblast TBI.
NOTE: BINT = blast-induced neurotrauma.
SOURCE: Reprinted from Agoston et al., 2009, with permission from Mary Ann Liebert, Inc. Publishers.
Abraham, N. G., and A. Kappas. 2005. Heme oxygenase and the cardiovascular-renal system. Free Radical Biology & Medicine 39(1):1-25
Agoston, D. V., A. Gyorgy, O. Eidelman, and H. B. Pollard. 2009. Proteomic biomarkers for blast neurotrauma: Targeting cerebral edema, inflammation, and neuronal death cascades. Journal of Neurotrauma 26(6):901-911.
Ahmed, F., A. Gyorgy, A. Kamnaksh, G. Ling, L. Tong, S. Parks, and D. Agoston. 2012. Time dependent changes of protein biomarker levels in the cerebrospinal fluid after blast traumatic brain injury. Electrophoresis 33(24):3705.
Alfieri, K. A., J. Forsberg, and B. K. Potter. 2012. Blast injuries and heterotopic ossification. Bone and Joint Research 1(8):192-197.
Alford, P. W., B. E. Dabiri, J. A. Goss, M. A. Hemphill, M. D. Brigham, and K. K. Parker. 2011. Blast-induced phenotypic switching in cerebral vasospasm. Proceedings of the National Academy of Sciences of the United States of America 108(31):12705-12710.
Armonda, R. A., R. S. Bell, A. H. Vo, G. Ling, T. J. DeGraba, B. Crandall, J. Ecklund, and W. W. Campbell. 2006. Wartime traumatic cerebral vasospasm: Recent review of combat casualties. Neurosurgery 59(6):1215-1225.
Axelsson, H., H. Hjelmqvist, A. Medin, J. K. Persson, and A. Suneson. 2000. Physiological changes in pigs exposed to a blast wave from a detonating high-explosive charge. Military Medicine 165(2):119-126.
Baalman, K. L., R. J. Cotton, S. N. Rasband, and M. N. Rasband. 2013. Blast wave exposure impairs memory and decreases axon initial segment length. Journal of Neurotrauma 30(9):741-751.
Bahat-Stroomza, M., Y. Gilgun-Sherki, D. Offen, H. Panet, A. Saada, N. Krool-Galron, A. Barzilai, D. Atlas, and E. Melamed. 2005. A novel thiol antioxidant that crosses the blood brain barrier protects dopaminergic neurons in experimental models of Parkinson’s Disease. European Journal of Neuroscience 21(3):637-646.
Bala, M., A. I. Rivkind, G. Zamir, T. Hadar, I. Gertsenshtein, Y. Mintz, A. J. Pikarsky, D. Amar, N. Shussman, M. Abu Gazala, and G. Almogy. 2008. Abdominal trauma after terrorist bombing attacks exhibits a unique pattern of injury. Annals of Surgery 248(2):303-309.
Bass, C. R., K. A. Rafaels, and R. S. Salzar. 2008. Pulmonary injury risk assessment for short-duration blasts. Journal of Trauma: Injury, Infection, & Critical Care 65(3):604-615.
Bass, C. R., M. B. Panzer, K. A. Rafaels, G. Wood, J. Shridharani, and B. Capehart. 2012. Brain injuries from blast. Annals of Biomedical Engineering 40(1):185-202.
Bauman, R. A., G. Ling, L. Tong, A. Januszkiewicz, D. Agoston, N. Delanerolle, Y. Kim, D. Ritzel, R. Bell, J. Ecklund, R. Armonda, F. Bandak, and S. Parks. 2009. An introductory characterization of a combat-casualty-care relevant swine model of closed head injury resulting from exposure to explosive blast. Journal of Neurotrauma 26(6):841-860.
Ben-Dor, C., O. Igra, and T. Elperin. 2001. Handbook of Shock Waves. San Diego, CA: Academic Press.
Benzinger, T. 1950. Physiological effects of blast in air and water. In German Aviation Medicine, World War II. Volume 2. Washington, DC: Department of the Air Force. Pp. 1225-1229.
Besedovsky, H. O., and A. DelRey. 1996. Immune-neuro-endocrine interactions: Facts and hypotheses. Endocrine Reviews 17(1):64-102.
Blocker, V., and T. G. Blocker. 1949. The Texas City disaster: A survey of 3,000 casualties. American Journal of Surgery 78(5):756-771.
Bogo, V., R. A. Hutton, and A. Bruner. 1971. The Effects of Airblast on Discriminated Avoidance Behavior in Rhesus Monkeys. http://search.ebscohost.com/login.aspx?direct=true&db=nts&AN=AD-742+819%2fXAB&site=ehost-live (accessed October 1, 2013).
Bowen, I. G., E. R. Fletcher, D. R. Richmond, F. G. Hirsch, and C. S. White. 1968a. Biophysical mechanisms and scaling procedures applicable in assessing responses of the thorax energized by air-blast overpressures or by non-penetrating missiles. Annals of the New York Academy of Sciences 152(1):122-146.
Bowen, I. G., E. R. Fletcher, D. R. Richmond. 1968b. Estimate of Man’s Tolerance to the Direct Effects of Air Blast. Washington, DC: Defense Atomic Support Agency.
Branis, M., and H. Burda. 1988. Effect of ascorbic-acid on the numerical hair cell loss in noise exposed guinea-pigs. Hearing Research 33(2):137-140.
Brauer, R. W., R. W. Beaver, S. Lahser, R. D. McCall, and R. Venters. 1979. Comparative physiology of the high-pressure neurological syndrome: Compression rate effects. Journal of Applied Physiology 46(1):128-135.
Brown, R. F., G. J. Cooper, and R. L. Maynard. 1993. The ultrastructure of rat lung following acute primary blast injury. International Journal of Experimental Pathology 74(2):151-162.
Casals, J. B., N. C. G. Pieri, M. L. T. Feitosa, A. C. M. Ercolin, K. C. S. Roballo, R. S. N. Barreto, F. F. Bressan, D. S. Martins, M. A. Miglino, and C. E. Ambrosio. 2011. The use of animal models for stroke research: A review. Comparative Medicine 61(4):305-313.
Celander, H., C. J. Clemedson, U. A. Ericsson, and H. I. Hultman. 1955. A study on the relation between the duration of a shock wave and the severity of the blast injury produced by it. Acta Physiologica Scandinavica 33(1):14-18.
Cernak, I. 2005. Animal models of head trauma. NeuroRx 2(3):410-422.
Cernak, I. 2010. The importance of systemic response in the pathobiology of blast-induced neurotrauma. Frontiers in Neurology [electronic resource] 1:151.
Cernak, I., and L. J. Noble-Haeusslein. 2010. Traumatic brain injury: An overview of pathobiology with emphasis on military populations. Journal of Cerebral Blood Flow and Metabolism 30(2):255-266.
Cernak, I., D. Ignjatovic, G. Andelic, and J. Savic. 1991. Metabolic changes as part of the general response of the body to the effect of blast waves. Vojnosanitetski Pregled 48(6):515-522.
Cernak, I., P. Radosevic, Z. Malicevic, and J. Savic. 1995. Experimental magnesium depletion in adult rabbits caused by blast overpressure. Magnesium Research 8(3):249-259.
Cernak, I., J. Savic, Z. Malicevic, D. Djurdjevic, and V. Prokic. 1996a. The pathogenesis of pulmonary blast injury: Our point of view. Chinese Journal of Traumatology 12(3):28-31.
Cernak, I., J. Savic, Z. Malicevic, G. Zunic, P. Radosevic, I. Ivanovic, and L. Davidovic. 1996b. Involvement of the central nervous system in the general response to pulmonary blast injury. Journal of Trauma: Injury, Infection, & Critical Care 40(3 Suppl):S100-S104.
Cernak, I., Z. Malicevic, and V. Prokic. 1997. Indirect neurotrauma caused by pulmonary blast injury: Development and prognosis. International Review Armed Forces Medical Services 52(4,5,6):114-120.
Cernak, I., J. Savic, D. Ignjatovic, and M. Jevtic. 1999a. Blast injury from explosive munitions. Journal of Trauma: Injury, Infection, & Critical Care 47(1):96-103; discussion 103-104.
Cernak, I., J. Savic, G. Zunic, N. Pejnovic, O. Jovanikic, and V. Stepic. 1999b. Recognizing, scoring, and predicting blast injuries. World Journal of Surgery 23(1):44-53.
Cernak, I., V. J. Savic, A. Lazarov, M. Joksimovic, and S. Markovic. 1999c. Neuroendocrine responses following graded traumatic brain injury in male adults. Brain Injury 13(12):1005-1015.
Cernak, I., Z. Wang, J. Jiang, X. Bian, and J. Savic. 2001a. Cognitive deficits following blast injury-induced neurotrauma: Possible involvement of nitric oxide. Brain Injury 15(7):593-612.
Cernak, I., Z. Wang, J. Jiang, X. Bian, and J. Savic. 2001b. Ultrastructural and functional characteristics of blast injury-induced neurotrauma. Journal of Trauma: Injury, Infection, & Critical Care 50(4):695-706.
Cernak, I., A. C. Merkle, V. E. Koliatsos, J. M. Bilik, Q. T. Luong, T. M. Mahota, L. Xu, N. Slack, D. Windle, and F. A. Ahmed. 2011. The pathobiology of blast injuries and blast-induced neurotrauma as identified using a new experimental model of injury in mice. Neurobiology of Disease 41(2):538-551.
Chavko, M., W. K. Prusaczyk, and R. M. McCarron. 2006. Lung injury and recovery after exposure to blast overpressure. Journal of Trauma-Injury Infection & Critical Care 61(4):933-942.
Chavko, M., W. A. Koller, W. K. Prusaczyk, and R. M. McCarron. 2007. Measurement of blast wave by a miniature fiber optic pressure transducer in the rat brain. Journal of Neuroscience Methods 159(2):277-281.
Chavko, M., W. K. Prusaczyk, and R. M. McCarron. 2008. Protection against blast-induced mortality in rats by hemin. Journal of Trauma: Injury, Infection, & Critical Care 65(5):1140-1145; discussion 1145.
Chavko, M., S. Adeeb, S. T. Ahlers, and R. M. McCarron. 2009. Attenuation of pulmonary inflammation after exposure to blast overpressure by n-acetylcysteine amide. Shock 32(3):325-331.
Chen, S., Z. Huang, L. Wang, T. Jiang, B. Wu, and G. Sun. 2003. Study on retinal ganglion cell apoptosis after explosive injury of eyeballs in rabbits. Yen Ko Hsueh Pao [Eye Science] 19(3):187-190.
Chiffelle, T. L. 1966. Pathology of Direct Air-Blast Injury. http://search.ebscohost.com/login.aspx?direct=true&db=nts&AN=AD-637+212%2fXAB&site=ehost-live (accessed October 13, 2012).
Cho, S.-I., S. S. Gao, A. Xia, R. Wang, F. T. Salles, P. D. Raphael, H. Abaya, J. Wachtel, J. Baek, D. Jacobs, M. N. Rasband, and J. S. Oghalai. 2013. Mechanisms of hearing loss after blast injury to the ear. PLoS ONE [electronic resource] 8(7).
Chrousos, G. P. 1995. The hypothalamine-pituitary-adrenal axis and immune-mediated inflammation. New England Journal of Medicine 332(20):1351-1362.
Chu, S. J., T. Y. Lee, H. C. Yan, S. H. Lin, and M. H. Li. 2005. L-arginine prevents air embolism-induced acute lung injury in rats. Critical Care Medicine 33(9):2056-2060.
Clemedson, C. J. 1956. Blast injury. Physiological Reviews 36(3):336-354.
Clemedson, C. J., and C. O. Criborn. 1955. Mechanical response of different parts of a living body to a high explosive shock wave impact. American Journal of Physiology 181(3):471-476.
Clemedson, C. J., and H. I. Hultman. 1954. Air embolism and the cause of death in blast injury. Military Surgeon 114(6):424-437.
Clemedson, C. J., and H. Pettersson. 1956. Propagation of a high explosive air shock wave through different parts of an animal body. American Journal of Physiology 184(1):119-126.
Clemedson, C. J., L. Frankenberg, A. Jonsson, H. Pettersson, and A. B. Sundqvist. 1969. Dynamic response of thorax and abdomen of rabbits in partial and whole-body blast exposure. American Journal of Physiology 216(3):615-620.
Cooper, G. J., D. J. Townend, S. R. Cater, and B. P. Pearce. 1991. The role of stress waves in thoracic visceral injury from blast loading: Modification of stress transmission by foams and high-density materials. Journal of Biomechanics 24(5):273-285.
Cripps, N. P., and G. J. Cooper. 1997. Risk of late perforation in intestinal contusions caused by explosive blast. British Journal of Surgery 84(9):1298-1303.
Damon, E. G., C. S. Gaylord, J. T. Yelverton, D. R. Richmond, I. G. Bowen, R. K. Jones, and C. S. White. 1968. Effects of ambient pressure on tolerance of mammals to air blast. Aerospace Medicine 39(10):1039-1047.
DePalma, R. G., D. G. Burris, H. R. Champion, and M. J. Hodgson. 2005. Blast injuries. New England Journal of Medicine 352(13):1335-1342.
Desmoulin, G. T., and J. P. Dionne. 2009. Blast-induced neurotrauma: Surrogate use, loading mechanisms, and cellular responses. Journal of Trauma: Injury, Infection, & Critical Care 67(5):1113-1122.
Dougherty, A. L., A. J. MacGregor, P. P. Han, K. J. Heltemes, and M. R. Galarneau. 2011. Visual dysfunction following blast-related traumatic brain injury from the battlefield. Brain Injury 25(1):8-13.
Effgen, G. B., C. D. Hue, E. Vogel, M. B. Panzer, D. F. Meaney, C. D. R. Bass, and B. Morrison. 2012. A multiscale approach to blast neurotrauma modeling. Part II: Methodology for inducing blast injury to in vitro models. Frontiers in Neurology 3:23.
Elsayed, N. M., and N. V. Gorbunov. 2003. Interplay between high energy impulse noise (blast) and antioxidants in the lung. Toxicology 189(1-2):63-74.
Elsayed, N. M., and N. V. Gorbunov. 2007. Pulmonary biochemical and histological alterations after repeated low-level blast overpressure exposures. Toxicological Sciences 95(1):289-296.
Elsayed, N. M., N. V. Gorbunov, and V. E. Kagan. 1997. A proposed biochemical mechanism involving hemoglobin for blast overpressure-induced injury. Toxicology 121(1):81-90.
Fanelli, V., A. Vlachou, S. Ghannadian, U. Simonetti, A. S. Slutsky, and H. Zhang. 2013. Acute respiratory distress syndrome: New definition, current and future therapeutic options. Journal of Thoracic Disease 5(3):326-334.
Fang, Y. 1988. Changes in cochlear action potential response threshold and ache in guinea pigs after blast injury. Zhonghua Er Bi Yan Hou Ke Za Zhi 23(6):330-333, 384.
Fausti, S. A., D. J. Wilmington, F. J. Gallun, P. J. Myers, and J. A. Henry. 2009. Auditory and vestibular dysfunction associated with blast-related traumatic brain injury. Journal of Rehabilitation Research and Development 46(6):797-809.
Freund, U., J. Kopolovic, and A. L. Durst. 1980. Compressed air emboli of the aorta and renal artery in blast injury. Injury 12(1):37-38.
Ganpule, S., A. Alai, E. Plougonven, and N. Chandra. 2012. Mechanics of blast loading on the head models in the study of traumatic brain injury using experimental and computational approaches. Biomechanics and Modeling in Mechanobiology 12(3):511-531.
Garman, R. H., L. W. Jenkins, R. C. Switzer, 3rd, R. A. Bauman, L. C. Tong, P. V. Swauger, S. A. Parks, D. V. Ritzel, C. E. Dixon, R. S. Clark, H. Bayir, V. Kagan, E. K. Jackson, and P. M. Kochanek. 2011. Blast exposure in rats with body shielding is characterized primarily by diffuse axonal injury. Journal of Neurotrauma 28(6):947-959.
Garner, J. P., S. Watts, C. Parry, J. Bird, and E. Kirkman. 2009. Development of a large animal model for investigating resuscitation after blast and hemorrhage. World Journal of Surgery 33(10):2194-2202.
Gelman, S. 2008. Venous function and central venous pressure: A physiologic story. Anesthesiology 108(4):735-748.
Goldstein, L. E., A. M. Fisher, C. A. Tagge, X. L. Zhang, L. Velisek, J. A. Sullivan, C. Upreti, J. M. Kracht, M. Ericsson, M. W. Wojnarowicz, C. J. Goletiani, G. M. Maglakelidze, N. Casey, J. A. Moncaster, O. Minaeva, R. D. Moir, C. J. Nowinski, R. A. Stern, R. C. Cantu, J. Geiling, J. K. Blusztajn, B. L. Wolozin, T. Ikezu, T. D. Stein, A. E. Budson, N. W. Kowall, D. Chargin, A. Sharon, S. Saman, G. F. Hall, W. C. Moss, R. O. Cleveland, R. E. Tanzi, P. K. Stanton, and A. C. McKee. 2012. Chronic traumatic encephalopathy in blast-exposed military veterans and a blast neurotrauma mouse model. Science Translation Medicine 4(134):160.
Gorbunov, N. V., N. M. Elsayed, E. R. Kisin, A. V. Kozlov, and V. E. Kagan. 1997. Air blast-induced pulmonary oxidative stress: Interplay among hemoglobin, antioxidants, and lipid peroxidation. American Journal of Physiology 272(2 Pt 1):L320-L334.
Gorbunov, N. V., S. J. McFaul, S. Van Albert, C. Morrissette, G. M. Zaucha, and J. Nath. 2004. Assessment of inflammatory response and sequestration of blood iron transferrin complexes in a rat model of lung injury resulting from exposure to low-frequency shock waves. Critical Care Medicine 32(4):1028-1034.
Gorbunov, N. V., S. J. McFaul, A. Januszkiewicz, and J. L. Atkins. 2005. Pro-inflammatory alterations and status of blood plasma iron in a model of blast-induced lung trauma. International Journal of Immunopathology & Pharmacology 18(3):547-556.
Gorbunov, N. V., L. V. Asher, V. Ayyagari, and J. L. Atkins. 2006. Inflammatory leukocytes and iron turnover in experimental hemorrhagic lung trauma. Experimental & Molecular Pathology 80(1):11-25.
Grinberg, L., E. Fibach, J. Amer, and D. Atlas. 2005. N-acetylcysteine amide, a novel cell-permeating thiol, restores cellular glutathione and protects human red blood cells from oxidative stress. Free Radical Biology & Medicine 38(1):136-145.
Guitton, M. J., and Y. Dudai. 2007. Blackade of cochlear NMDA receptors prevents long-term tinnitus during a brief consolidation window after acoustic trauma. Neural Plasticity. Published online. Article ID 80904.
Guy, R. J., and N. P. Cripps. 2011. Abdominal trauma in primary blast injury (British Journal of Surgery 2011; 98:168-179). British Journal of Surgery 98(7):1033; author reply 1033-1034.
Hines-Beard, J., J. Marchetta, S. Gordon, E. Chaum, E. E. Geisert, and T. S. Rex. 2012. A mouse model of ocular blast injury that induces closed globe anterior and posterior pole damage. Experimental Eye Research 99:63-70.
Hirsch, F. G. 1968. Effects of overpressure on the ear: A review. Annals of the New York Academy of Sciences 152(1):147-162.
Holzer, A., M. F. Pietschmann, C. Rosl, M. Hentschel, O. Betz, M. Matsuura, V. Jansson, and P. E. Muller. 2012. The interrelation of trabecular microstructural parameters of the greater tubercle measured for different species. Journal of Orthopaedic Research 30(3):429-434.
Hu, B. 1991. Cochlear microcirculation in living guinea pigs following explosion. Zhonghua Er Bi Yan Hou Ke Za Zhi 26(1):6-9, 61.
Ignjatovic, D., G. Tasic, M. Jevtic, and D. Durdevic. 1991. Occurrence and evolution of primary non-perforating lesions in blast injuries of the abdomen. Vojnosanitetski Pregled 48(6):523-525.
IOM (Institute of Medicine). 2009. Gulf War and Health, Volume 7: Long-Term Consequences of Traumatic Brain Injury. Washington, DC: The National Academies Press.
Irwin, R. J., M. R. Lerner, J. F. Bealer, P. C. Mantor, D. J. Brackett, and D. W. Tuggle. 1999. Shock after blast wave injury is caused by a vagally mediated reflex. Journal of Trauma-Injury Infection & Critical Care 47(1):105-110.
Kamnaksh, A., S. K. Kwon, E. Kovesdi, F. Ahmed, E. S. Barry, N. E. Grunberg, J. Long, and D. Agoston. 2012. Neurobehavioral, cellular, and molecular consequences of single and multiple mild blast exposure. Electrophoresis 33(24):3680-3692.
Kaur, C., J. Singh, M. K. Lim, B. L. Ng, E. P. Yap, and E. A. Ling. 1995. The response of neurons and microglia to blast injury in the rat brain. Neuropathology & Applied Neurobiology 21(5):369-377.
Kaur, C., J. Singh, M. K. Lim, B. L. Ng, and E. A. Ling. 1997. Macrophages/microglia as “sensors” of injury in the pineal gland of rats following a non-penetrative blast. Neuroscience Research 27(4):317-322.
Kellerhals, B. 1972. Acoustic trauma and cochlear microcirculation. An experimental and clinical study on pathogenesis and treatment of inner ear lesions after acute noise exposure. Advances in Oto-Rhino-Laryngology 18:91-168.
Khan, M. M., and K. L. Melmon. 1985. Are autocoids more than theoretic modulators of immunity? Clinical Immunology Reviews 4(1):1-30.
Kirkegaard, M., N. Murai, M. Risling, A. Suneson, L. Jarlebark, and M. Ulfendahl. 2006. Differential gene expression in the rat cochlea after exposure to impulse noise. Neuroscience 142(2):425-435.
Kirkman, E., and S. Watts. 2011. Characterization of the response to primary blast injury. Philosophical Transactions of the Royal Society of London—Series B: Biological Sciences 366(1562):286-290.
Kittle, C. P., A. J. Verrett, J. Wu, D. E. Mellus, R. G. Hale, and R. K. Chan. 2012. Characterization of midface fractures incurred in recent wars. Journal of Craniofacial Surgery 23(6):1587-1591.
Kluger, Y., A. Nimrod, P. Biderman, A. Mayo, and P. Sorkin. 2007. The quinary pattern of blast injury. American Journal of Disaster Medicine 2(1):21-25.
Koliatsos, V. E., T. M. Dawson, A. Kecojevic, Y. P. Zhou, Y. F. Wang, and K. X. Huang. 2004. Cortical interneurons become activated by deafferentation and instruct the apoptosis of pyramidal neurons. Proceedings of the National Academy of Sciences of the United States of America 101(39):14264-14269.
Koliatsos, V. E., I. Cernak, L. Xu, Y. Song, A. Savonenko, B. J. Crain, C. G. Eberhart, C. E. Frangakis, T. Melnikova, H. Kim, and D. Lee. 2011. A mouse model of blast injury to brain: Initial pathological, neuropathological, and behavioral characterization. Journal of Neuropathology & Experimental Neurology 70(5):399-416.
Kwon, S. K., E. Kovesdi, A. B. Gyorgy, D. Wingo, A. Kamnaksh, J. Walker, J. B. Long, and D. V. Agoston. 2011. Stress and traumatic brain injury: A behavioral, proteomics, and histological study. Frontiers in Neurology [electronic resource] 2:12.
Lamb, C. M., J. E. Berry, W. F. DeMello, and C. Cox. 2010. Secondary abdominal compartment syndrome after military wounding. Journal of the Royal Army Medical Corps 156(2):102-103.
Lee, K. S., S. R. Kim, H. S. Park, S. J. Park, K. H. Min, K. Y. Lee, Y. H. Choe, S. H. Hong, H. J. Han, Y. R. Lee, J. S. Kim, D. Atlas, and Y. C. Lee. 2007. A novel thiol compound, n-acetylcysteine amide, attenuates allergic airway disease by regulating activation of NF-kappa-B and hypoxia-inducible factor-1-alpha. Experimental & Molecular Medicine 39(6):756-768.
Leonardi, A. D., C. A. Bir, D. V. Ritzel, and P. J. VandeVord. 2011. Intracranial pressure increases during exposure to a shock wave. Journal of Neurotrauma 28(1):85-94.
Ling, G., F. Bandak, R. Armonda, G. Grant, and J. Ecklund. 2009. Explosive blast neurotrauma. Journal of Neurotrauma 26(6):815-825.
Liu, S. 1992a. Changes in vascular stria after blast traumatized guinea pigs by colloidal lanthanum tracing technique. Chinese Journal of Otorhinolaryngology 27(3):136-137, 189.
Liu, Z. 1992b. Experimental study on the mechanism of free radical in blast trauma induced hearing loss. Chinese Journal of Otorhinolaryngology 27(1):24-26, 61.
Long, J. B., T. L. Bentley, K. A. Wessner, C. Cerone, S. Sweeney, and R. A. Bauman. 2009. Blast overpressure in rats: Recreating a battlefield injury in the laboratory. Journal of Neurotrauma 26(6):827-840.
Lu, F. K., and D. R. Wilson. 2003. Detonation driver for enhancing shock tube performance. Shock Waves 12(6):457-468.
Lu, J., K. C. Ng, G. Ling, J. Wu, D. J. Poon, E. M. Kan, M. H. Tan, Y. J. Wu, P. Li, S. Moochhala, E. Yap, L. K. Lee, M. Teo, I. B. Yeh, D. M. Sergio, F. Chua, S. D. Kumar, and E. A. Ling. 2012. Effect of blast exposure on the brain structure and cognition in macaca fascicularis. Journal of Neurotrauma 29(7):1434-1454.
Magnuson, J., F. Leonessa, and G. S. F. Ling. 2012. Neuropathology of explosive blast traumatic brain injury. Current Neurology and Neuroscience Reports 1-10.
Mainiero, R., and M. Sapko. 1996. Blast and Fire Propagation in Underground Facilities. Technical report no. DNA-TR-93-159. Alexandria, VA: Defense Nuclear Agency.
Mao, J. C., E. Pace, P. Pierozynski, Z. Kou, Y. Shen, P. Vandevord, E. M. Haacke, X. Zhang, and J. Zhang. 2012. Blast-induced tinnitus and hearing loss in rats: Behavioral and imaging assays. Journal of Neurotrauma 29(2):430-444.
Mason, W. v. H., T. G. Damon, A. R. Dickinson, and T. O. Nevison, Jr. 1971. Arterial gas emboli after blast injury. Proceedings of the Society for Experimental Biology & Medicine 136(4):1253-1255.
Matthay, M. A., L. B. Ware, and G. A. Zimmerman. 2012. The acute respiratory distress syndrome. Journal of Clinical Investigation 122(8):2731-2740.
Mayorga, M. A. 1997. The pathology of primary blast overpressure injury. Toxicology 121(1):17-28.
Mehlenbacher, A., B. Capehart, D. Bass, and J. R. Burke. 2012. Sound induced vertigo: Superior canal dehiscence resulting from blast exposure. Archives of Physical Medicine & Rehabilitation 93(4):723-724.
Mellor, S. G. 1988. The pathogenesis of blast injury and its management. British Journal of Hospital Medicine 39(6):536-539.
Melmon, K. L., R. E. Rocklin, and R. P. Rosenkranz. 1981. Autacoids as modulators of the inflammatory and immune response. American Journal of Medicine 71(1):100-106.
Moss, W. C., M. J. King, and E. G. Blackman. 2009. Skull flexure from blast waves: A mechanism for brain injury with implications for helmet design. Physical Review Letters 103(10):108702.
Nageris, B. I., J. Attias, and R. Shemesh. 2008. Otologic and audiologic lesions due to blast injury. Journal of Basic & Clinical Physiology & Pharmacology 19(3-4):185-191.
Nevison, T. O., Jr., W. V. Mason, and A. R. Dickinson. 1971. Measurement of blood velocity and detection of emboli by ultrasonic doppler technique. Paper presented at 11th Annual Symposium on Experimental Mechanics, Albuquerque, NM.
Ning, J. L., L. W. Mo, K. Z. Lu, X. N. Lai, Z. G. Wang, and D. Ma. 2012. Lung injury following lower extremity blast trauma in rats. Journal of Trauma and Acute Care Surgery 73(6):1537-1544.
Nishida, M. 2001. Shock tubes and tunnels: Facilities, instrumentation, and techniques. Shock tubes. In Handbook of Shock Waves, edited by C. Ben-Dor, O. Igra, and T. Elperin. San Diego: Academic Press. Pp. 553-585.
Ohnishi, M., E. Kirkman, R. J. Guy, and P. E. Watkins. 2001. Reflex nature of the cardiorespiratory response to primary thoracic blast injury in the anaesthetised rat. Experimental Physiology 86(3):357-364.
Owen-Smith, M. S. 1981. Hunterian lecture 1980: A computerized data retrieval system for the wounds for war: The Northern Ireland casualties. Journal of the Royal Army Medical Corps 127(1):31-54.
Paintal, A. S. 1969. Mechanism of stimulation of type J pulmonary receptors. Journal of Physiology—London 203(3):511.
Pang, C. C. Y. 2001. Autonomic control of the venous system in health and disease: Effects of drugs. Pharmacology & Therapeutics 90(2-3):179-230.
Panzer, M. B., K. A. Matthews, A. W. Yu, B. Morrison, 3rd, D. F. Meaney, and C. R. Bass. 2012. A multiscale approach to blast neurotrauma modeling. Part I: Development of novel test devices for in vivo and in vitro blast injury models. Frontiers in Neurology [electronic resource] 3:46.
Paran, H., D. Neufeld, I. Shwartz, D. Kidron, S. Susmallian, A. Mayo, K. Dayan, I. Vider, G. Sivak, and U. Freund. 1996. Perforation of the terminal ileum induced by blast injury: Delayed diagnosis or delayed perforation? Journal of Trauma: Injury, Infection, & Critical Care 40(3):472-475.
Patterson, J. H., Jr., and R. P. Hamernik. 1997. Blast overpressure induced structural and functional changes in the auditory system. Toxicology 121(1):29-40.
Peters, P. 2011. Primary blast injury: An intact tympanic membrane does not indicate the lack of a pulmonary blast injury. Military Medicine 176(1):110-114.
Petras, J. M., R. A. Bauman, and N. M. Elsayed. 1997. Visual system degeneration induced by blast overpressure. Toxicology 121(1):41-49.
Phillips, Y. Y. 1986. Primary blast injuries. Annals of Emergency Medicine 15(12):1446-1450.
Potter, B. K., T. C. Burns, A. P. Lacap, R. R. Granville, and D. A. Gajewski. 2007. Heterotopic ossification following traumatic and combat-related amputations. Prevalence, risk factors, and preliminary results of excision. Journal of Bone & Joint Surgery—American Volume 89(3):476-486.
Pun, P. B., E. M. Kan, A. Salim, Z. Li, K. C. Ng, S. M. Moochhala, E. A. Ling, M. H. Tan, and J. Lu. 2011. Low level primary blast injury in rodent brain. Frontiers in Neurology [electronic resource] 2:19.
Readnower, R. D., M. Chavko, S. Adeeb, M. D. Conroy, J. R. Pauly, R. M. McCarron, and P. G. Sullivan. 2010. Increase in blood-brain barrier permeability, oxidative stress, and activated microglia in a rat model of blast-induced traumatic brain injury. Journal of Neuroscience Research 88(16):3530-3539.
Regan, R. F., and S. S. Panter. 1993. Neurotoxicity of hemoglobin in cortical cell culture. Neuroscience Letters 153(2):219-222.
Reneer, D. V., R. D. Hisel, J. M. Hoffman, R. J. Kryscio, B. T. Lusk, and J. W. Geddes. 2011. A multi-mode shock tube for investigation of blast-induced traumatic brain injury. Journal of Neurotrauma 28(1):95-104.
Rice, D., and J. Heck. 2000. Terrorist bombings: Ballistics, patterns of blast injury and tactical emergency care. Tactical Edge Journal Summer:53-55.
Richmond, D. R. 1991. Blast criteria for open spaces and enclosures. Scandinavian Audiology Supplementum 34:49-76.
Richmond, D. R., E. G. Damon, I. G. Bowen, E. R. Fletcher, and C. S. White. 1967. Air-blast studies with eight species of mammals. Technical progress report DASA 1854. Fission Product Inhalation Project 1-44.
Richmond, D. R., E. G. Damon, E. R. Fletcher, I. G. Bowen, and C. S. White. 1968. The relationship between selected blast-wave parameters and the response of mammals exposed to air blast. Annals of the New York Academy of Sciences 152(1):103-121.
Risling, M., and J. Davidsson. 2012. Experimental animal models for studies on the mechanisms of blast-induced neurotrauma. Frontiers in Neurology [electronic resource] 3:30.
Risling, M., S. Plantman, M. Angeria, E. Rostami, B. M. Bellander, M. Kirkegaard, U. Arborelius, and J. Davidsson. 2011. Mechanisms of blast induced brain injuries, experimental studies in rats. Neuroimage 54(Suppl 1):S89-S97.
Roberto, M., R. P. Hamernik, and G. A. Turrentine. 1989. Damage of the auditory system associated with acute blast trauma. Annals of Otology, Rhinology, & Laryngology—Supplement 140:23-34.
Roberts, J. C., T. P. Harrigan, E. E. Ward, T. M. Taylor, M. S. Annett, and A. C. Merkle. 2012. Human head-neck computational model for assessing blast injury. Journal of Biomechanics 45(16):2899-2906.
Robey, R. 2001. Shock tubes and tunnels: Facilities, instrumentation, and techniques. Blast tubes. In Handbook of Shock Waves, edited by C. Ben-Dor, O. Igra, and T. Elperin. San Diego: Academic Press. Pp. 623-650.
Rossle, R. 1950. Pathology of blast effects. In German Aviation Medicine, World War II, Volume 2. Washington, DC: Department of the Air Force.
Ruskin, A., O. W. Beard, and R. L. Schaffer. 1948. Blast hypertension: Elevated arterial pressures in the victims of the Texas City disaster. American Journal of Medicine 4(2):228-236.
Rutlen, D. L., E. W. Supple, and W. J. Powell. 1979. Role of the liver in the adrenergic regulation of blood-flow from the splanchnic to the central circulation. Yale Journal of Biology and Medicine 52(1):99-106.
Saljo, A., and A. Hamberger. 2004. Intracranial sound pressure levels during impulse noise exposure. Paper presented at 7th International Neurotrauma Symposium, Adelaide, Australia.
Saljo, A., F. Bao, K. G. Haglid, and H. A. Hansson. 2000. Blast exposure causes redistribution of phosphorylated neurofilament subunits in neurons of the adult rat brain. Journal of Neurotrauma 17(8):719-726.
Saljo, A., F. Bao, and A. Hamberger. 2001. Exposure to short-lasting impulse noise causes microglial and astroglial cell activation in the adult rat brain. Pathophysiology 8(2):105-111.
Saljo, A., F. Bao, J. S. Shi, A. Hamberger, H. A. Hansson, and K. G. Haglid. 2002. Expression of c-fos and c-myc and deposition of beta-app in neurons in the adult rat brain as a result of exposure to short-lasting impulse noise. Journal of Neurotrauma 19(3):379-385.
Savic, J., V. Tatic, D. Ignjatovic, V. Mrda, D. Erdeljan, I. Cernak, S. Vujnov, M. Simovic, G. Andelic, and M. Duknic. 1991. Pathophysiologic reactions in sheep to blast waves from detonation of aerosol explosives. Vojnosanitetski Pregled 48(6):499-506.
Selye, H. 1976. 40 years of stress research: Principal remaining problems and misconceptions. Canadian Medical Association Journal 115(1):53-56.
Soares, M. P., and F. H. Bach. 2009. Heme oxygenase-1: From biology to therapeutic potential. Trends in Molecular Medicine 15(2):50-58.
Stewart, C. 2014. Blast injuries: True weapons of mass destruction. www.storysmith.net/page7/page15/files/page15_5.doc (accessed January, 14, 2014).
Surbatovic, M., N. Filipovic, S. Radakovic, N. Stankovic, and Z. Slavkovic. 2007. Immune cytokine response in combat casualties: Blast or explosive trauma with or without secondary sepsis. Military Medicine 172(2):190-195.
Svetlov, S., V. Prima, D. Kirk, J. Atkinson, H. Gutierrez, K. Curley, R. Hayes, and K. Wang. 2009. Morphological and biochemical signatures of brain injury following head-directed controlled blast overpressure impact. Journal of Neurotrauma 26(8):A75.
Svetlov, S. I., V. Prima, O. Glushakova, A. Svetlov, D. R. Kirk, H. Gutierrez, V. L. Serebruany, K. C. Curley, K. K. W. Wang, and R. L. Hayes. 2012. Neuro-glial and systemic mechanisms of pathological responses in rat models of primary blast overpressure compared to “composite” blast. Frontiers in Neurology [electronic resource] 3:15.
Tannous, O., C. Griffith, R. V. O’Toole, and V. D. Pellegrini, Jr. 2011. Heterotopic ossification after extremity blast amputation in a Sprague-Dawley rat animal model. Journal of Orthopaedic Trauma 25(8):506-510.
Tatic, V., D. Ignjatovic, V. Mrda, and J. Savic. 1991a. Morphologic changes in the tissues and organs of sheep in blast injuries caused by detonation of aerosol explosives. Vojnosanitetski Pregled 48(6):541-545.
Tatic, V., Z. Stankovic, and Z. Hadzic. 1991b. Morphologic damage in internal organs in miners caused by explosions of methane gas in mines. Vojnosanitetski Pregled 48(6):547-550.
Tatic, V., D. Ignjatovic, M. Jevtic, M. Jovanovic, M. Draskovic, and D. Durdevic. 1996. Morphologic characteristics of primary nonperforative intestinal blast injuries in rats and their evolution to secondary perforations. Journal of Trauma: Injury, Infection, & Critical Care 40(3 Suppl):S94-S99.
Tsokos, M., F. Paulsen, S. Petri, B. Madea, K. Puschel, and E. E. Turk. 2003a. Histologic, immunohistochemical, and ultrastructural findings in human blast lung injury. American Journal of Respiratory & Critical Care Medicine 168(5):549-555.
Tsokos, M., E. E. Turk, B. Madea, E. Koops, F. Longauer, M. Szabo, W. Huckenbeck, P. Gabriel, and J. Barz. 2003b. Pathologic features of suicidal deaths caused by explosives. American Journal of Forensic Medicine & Pathology 24(1):55-63.
Tümer, N., S. Svetlov, M. Whidden, N. Kirichenko, V. Prima, B. Erdos, A. Sherman, F. Kobeissy, R. Yezierski, P. J. Scarpace, C. Vierck, and K. K. W. Wang. 2013. Overpressure blast-wave induced brain injury elevates oxidative stress in the hypothalamus and catecholamine biosynthesis in the rat adrenal medulla. Neuroscience Letters 544:62-67.
Valiyaveettil, M., Y. Alamneh, S. Oguntayo, Y. Wei, Y. Wang, P. Arun, and M. P. Nambiar. 2012a. Regional specific alterations in brain acetylcholinesterase activity after repeated blast exposures in mice. Neuroscience Letters 506(1):141-145 Valiyaveettil, M., Y. A. Alamneh, S. A. Miller, R. Hammamieh, P. Arun, Y. Wang, Y. Wei, S. Oguntayo, J. B. Long, and M. P. Nambiar. 2012b. Modulation of cholinergic pathways and inflammatory mediators in blast-induced traumatic brain injury. Chemico-Biological Interactions 203(1):371-375.
Valiyaveettil, M., Y. Alamneh, Y. Wang, P. Arun, S. Oguntayo, Y. Wei, J. B. Long, and M. P. Nambiar. 2013. Contribution of systemic factors in the pathophysiology of repeated blast-induced neurotrauma. Neuroscience Letters 539:1-6.
Vandevord, P. J., R. Bolander, V. S. Sajja, K. Hay, and C. A. Bir. 2012. Mild neurotrauma indicates a range-specific pressure response to low level shock wave exposure. Annals of Biomedical Engineering 40(1):227-236.
Vriese, B. 1904. Sur la signification morfologique des artères céŕebrales. Archives de Biologie (Liege) 21:357-457.
Wang, Y., Z. Ye, X. Hu, J. Huang, and Z. Luo. 2010. Morphological changes of the neural cells after blast injury of spinal cord and neuroprotective effects of sodium beta-aescinate in rabbits. Injury 41(7):707-716.
White, C. S., I. G. Bowen, and D. R. Richmond. 1965. Biological tolerance to air blast and related biomedical criteria. Cex-65.4. Cex Reports, Civil Effects Exercise 1-239.
White, C. S., I. G. Bowen, and D. R. Richmond. 1970. The relation between eardrum failure and blast induced pressure variations. Space Life Sciences 2(2):158-205
Wightman, J. M., and S. L. Gladish. 2001. Explosions and blast injuries. Annals of Emergency Medicine 37(6):664-678.
Wilkinson, C. W., K. F. Pagulayan, E. C. Petrie, C. L. Mayer, E. A. Colasurdo, J. B. Shofer, K. L. Hart, D. Hoff, M. A. Tarabochia, and E. R. Peskind. 2012. High prevalence of chronic pituitary and target-organ hormone abnormalities after blast-related mild traumatic brain injury. Frontiers in Neurology 3:11.
Yeh, D. D., and W. P. Schecter. 2012. Primary blast injuries: An updated concise review. World Journal of Surgery 36(5):966-972.
Yokoi, H., and N. Yanagita. 1984. Blast injury to sensory hairs: A study in the guinea pig using scanning electron microscopy. Archives of Oto-Rhino-Laryngology 240(3):263-270.
Yuan, Y. G. 1993. Relationship between intracochlear oxygen tension and acoustic trauma following explosion. Chinese Journal of Otorhinolaryngology 28(4):222-224, 252.
Zalewski, T. 1906. Experimentelle untersuchungen uber die resistensfahigkeit des trommelfells. Z Ohrenheilklinik 52:109-128.
Zhai, S. 1991. Effects of explosions on cortical response threshold and enzyme activities in inner ear of guinea pigs. Chinese Journal of Otorhinolaryngology 26(2):73-75, 124.
Zhai, S., J. Cheng, and J. Wang. 1997. Treatment effects of fibroblast growth factors on blast-induced hearing loss. Chinese Journal of Otorhinolaryngology 32(6):354-356.
Zhang, B., A. Wang, W. Hu, L. Zhang, Y. Xiong, J. Chen, and J. Wang. 2011. Hemoconcentration caused by microvascular dysfunction after blast injuries to the chest and abdomen of rabbits. Journal of Trauma-Injury Infection & Critical Care 71(3):694-701.
Zheng, J. F. 1992. Effect of impulse noise exposure on the endocochlear potentials. Zhonghua Er Bi Yan Hou Ke Za Zhi 27(6):328-330, 381.
Zucker, I. H. 1986. Left-ventricular receptors: Physiological controllers or pathological curiosities. Basic Research in Cardiology 81(6):539-557.
Zuckerman, S. 1940. Experimental study of blast injuries to the lung. Lancet 2:219-224.