Explosive blasts can cause multiple forms of damage that are more complex than those caused by other wounding agents (Champion et al., 2009). Blasts are the leading cause of death and injury on the military battlefield (Eastridge et al., 2012). Recent reports indicate that almost 80% of all combat-related injuries in US military personnel deployed to Iraq and Afghanistan have been from blasts; this is the highest proportion seen in any large-scale conflict (Murray et al., 2005; Owens et al., 2008). During the last decade, the incidence of primary blast injury and injury severity increased, and return-to-duty rates decreased. Despite increased injury severity, mortality due to explosion injuries remained low and unchanged (Kelly et al., 2008; Ritenour et al., 2010). The acute physical and psychologic human health outcomes in those who survive blast explosions can be devastating. The long-term consequences are less clear.
This chapter summarizes the committee’s evaluation of the literature on the association between exposure to blast and short- and long-term effects on the human body. The guidelines agreed on by the committee and used to determine which studies to include in the evidence review below are described in Chapter 2. The chapter is organized by health outcomes: psychologic and psychiatric, nervous system, auditory and vestibular, ocular, cardiovascular, respiratory, digestive system, genitourinary (GU), dermal, and musculoskeletal outcomes; infections; and burns. The organization generally follows that of the International Classification of Diseases, Ninth Revision. The final section, on blast protection, evaluates whether improvements in blast protection are associated with diminished blast injury.
Although the information here is presented by individual organ systems
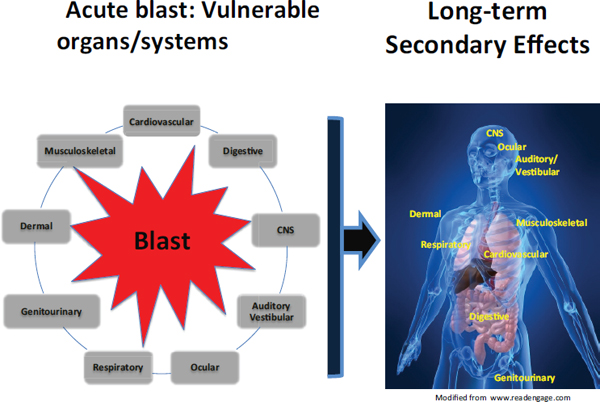
FIGURE 4-1 Blast injury may result in primary damage to a number of organ systems. Less studied are the effects that primary damage to a specified organ may have on the long-term consequences of the functioning of other organs. For example, exposure to a blast may result in air emboli that develop from damaged lungs at alveolar–pulmonary venous fistulae and cause myocardial ischemia or infarction and thus compromise long-term cardiac function. Damage to the brain may result in motor weakness; voiding dysfunction, such as an overactive (spastic) or, over time, hypoactive bladder; change in auditory processing abilities; visual symptoms; and hypogonadism caused by hypopituitarism. Damage to the cardiovascular system can affect neurologic function through ischemia, which, if sufficiently severe, can lead to permanent brain damage.
SOURCE: Created by Linda Noble-Haeusslein for the Committee on Gulf War and Health: Long-Term Effects of Blast Exposures; figure of the human body adapted from www.readengage.com.
and specific outcomes, exposure to blast often leads to polytrauma (that is, multiple traumatic injuries) and results in a multisystem response. The section of Chapter 3 titled “Modifying Potential of Systemic Changes Caused by Blast” describes how the complexity of the blast environment can lead to changes in systemic, local, and cerebral responses. Four important systemic alterations—air emboli, activation of the autonomic nervous system, vascular mechanisms, and systemic inflammation—are explained in detail there.
Figure 4-1 illustrates how damage to an organ from exposure to blast may have long-term consequences for the functioning of other organs.
Nearly all of the epidemiologic studies evaluated by the committee relied on self-reported exposure to blast, not objective measures. The mechanism of blast—primary, secondary, tertiary, quaternary, or quinary—generally was not reported in the studies. As detailed in the committee’s recommendations in Chapter 5, obtaining accurate, objective measurement of exposure to blast is essential for understanding the mechanisms of injury caused by blast.
PSYCHOLOGIC AND PSYCHIATRIC OUTCOMES
The potential relationship between blast and its psychologic and psychiatric outcomes is different from the relationships with other organ systems reviewed. Currently, it is not known whether the primary blast wave itself results in any direct physiologic or neuroanatomic changes to the nervous system that cause acute or long-term mental disorders. That knowledge is in contrast with other etiologies of traumatic brain injury (TBI) in which there is physical evidence of neurotrauma (Bazarian et al., 2013; Jorge et al., 2012). However, blast explosions often result in tremendous human carnage in the form of severe, mutilating injuries and death. During the immediate aftermath of blasts (sometimes referred to as the aftermath of battle [Stein et al., 2012]), people often remember the carnage, which can haunt them for days, months, or years. Blast explosions, such as those caused by improvised explosive devices, have been the greatest cause of death and injury in US military personnel deployed to Iraq and Afghanistan (Champion et al., 2009; Ritenour et al., 2010), and exposure to the aftermath of blasts during deployment is probably an important cause of acute and long-term psychologic and psychiatric disorders in military service members and veterans. Whether the relationship between blasts and health outcomes is primary (in which an injury is caused directly by the blast wave itself) or secondary (in which an injury is a reaction to the emotional impact of the blast) is debatable. According to the Diagnostic and Statistical Manual of Mental Disorders (APA, 2013), witnessing the devastation of a blast explosion would be considered exposure to primary trauma. For the purpose of the present review, blast was evaluated as a primary cause of behavioral health outcomes, even though in some instances it is the psychologic impact and interpretation of the aftermath of a blast that potentially result in a psychologic injury rather than the direct physical impact of the blast wave itself.
Acute Effects
Exposure to blast has a number of acute psychologic and psychiatric outcomes. Distress reactions that occur within the first 3 days after a blast exposure are considered normal and are referred to as acute stress reactions (WHO, 2010). Acute stress reactions are transient disorders in which symptoms develop within minutes of exposure to a traumatic event. The symptoms usually subside within hours or days with resumption of routine activities, when possible, and general support from friends, family, or co-workers. In most cases, professional intervention is not required.
When the signs and symptoms of acute stress reactions cluster and arrange in specific combinations and last for at least 3 days, they can lead to a diagnosis of acute stress disorder (APA, 2013). Acute stress disorder includes symptoms of intrusion, negative mood, dissociation, avoidance, and hyperarousal. The symptoms last from 3 days to a month after the trauma exposure. If they persist for more than 1 month, the diagnosis of posttraumatic stress disorder (PTSD) should be considered (APA, 2013).
Adjustment disorders are other possible acute outcomes of blast exposure in people who do not meet diagnostic criteria for acute stress disorder (APA, 2013). Adjustment disorders include the development of emotional and behavioral symptoms in response to an identifiable stressor, such as a blast, that occurs within 3 months after the event. Major depressive disorder may also be diagnosed using a separate cluster of the same symptoms at 2 weeks’ duration (APA, 2013).
Regardless of whether a person meets the diagnostic criteria for these acute outcomes, it is often only when the symptoms persist over an extended period that most psychologic and psychiatric disorders are identified.
Long-Term Effects
PTSD is the primary long-term sequela of combat-related trauma exposure, such as that experienced as a result of blasts (Peterson et al., 2011; Tanielian and Jaycox, 2008). Few studies have directly evaluated the long-term psychologic and psychiatric outcomes (for example, major depressive disorder, substance-abuse disorders, postconcussive syndrome [related to TBI], sleep disorders, marital and family discord, and suicide) of blast exposure beyond PTSD. Therefore, the present review focuses on PTSD. The long-term effects of TBI from blast are reviewed in the later section “Nervous System Outcomes.”
The lifetime prevalence of PTSD has been reported to be 8.0% in the adult US population (4.0% in males and 11.7% in females) (Kessler et al., 2012). Sex differences in population surveys are related primarily to differences in frequency and type of trauma exposure. Females are more likely
to be victims of sexual assault, and males are more likely to experience combat-related trauma (Kessler et al., 1995, 2005). However, when trauma type and frequency are controlled for, sex differences in PTSD are less likely to be found. For example, in a large sample of UK armed forces personnel, men (5.0%) and women (4.2%) reported similar rates of PTSD symptoms after deployment to Iraq (Rona et al., 2007). Similarly, US military men (2.3%) and women (2.3%) experienced the same rates of PTSD symptoms after serving in the war in Iraq (DOD, 2007). Such findings have led some to conclude that the risk of PTSD in military personnel “has more to do with the intensity and frequency of combat experience than gender” (Hoge et al., 2007, p. 328).
To evaluate the long-term psychiatric and psychologic health effects of blast exposure, the committee reviewed 40 relevant published peer-reviewed studies that involved some measure of blast injury. Only two met enough of the inclusion guidelines to be considered primary (see Table 4-1) (Bazarian et al., 2013; Polusny et al., 2011). This section details the primary studies and supportive studies of long-term psychiatric and psychologic health outcomes of blast exposure.
Primary Studies
Polusny et al. (2011) conducted a longitudinal cohort study of combat-deployed National Guard members to assess the associations between mild TBI and PTSD symptoms reported in theater and longer-term psychosocial outcomes. Participants in the study were surveyed in Iraq a month before redeploying home (time 1, during redeployment transition briefings held at military installations in the Iraq combat theater) and again a year later (time 2, with mailed surveys). The first survey included 2,677 National Guard members, and 953 completed the followup survey at time 2. The surveys incorporated the following screening tools to gather outcome measures: the PTSD Checklist–Military, the Beck Depression Inventory, the Patient Health Questionnaire, the Alcohol Use Disorders Identification Test (AUDIT), World Health Organization Quality of Life–Brief, and self-reports of blast-related mild TBI, which was defined as an injury during deployment with loss of consciousness or altered mental status.
Of the 953 participants surveyed at time 2,206 (22%) reported having a blast injury. Results for self-reported mild TBI during deployment showed 9.2% at time 1 and 22.0% at time 2. Service members who had a history of mild TBI were more likely than those who did not to report post-deployment postconcussive symptoms and poorer psychosocial outcomes. However, after adjustment for self-reported PTSD symptoms, mild TBI was not associated with post-deployment symptoms or outcomes. Time 1 PTSD symptoms predicted postdeployment PTSD and mild TBI symptoms and
TABLE 4-1 Psychiatric and Postconcussive Symptoms—Primary Studies
| Reference | Study Design | Population | Health Outcomes or Outcome Measures | |
| Bazarian et al., 2013 | Nested cohort | Parent cohort consisted of 500 OEF or OIF veterans; subset examined in study included 52 OEF or OIF combat veterans assessed 4 years after last tour of duty | Self-report of blast exposure and TBI symptoms, PTSD Checklist–Military, combat experiences survey, anatomical MRI, DTI | |
| Polusny et al., 2011 | Longitudinal cohort | 953 US National Guard brigade combat team assessed in Iraq 1 month before return (time 1) and 1 year later (time 2) | Self-report concussion or mild TBI defined as an injury during deployment with loss of consciousness (LOC) or altered mental status; PTSD checklist–military; Beck Depression Inventory; Patient Health Questionnaire (somatic symptoms); postconcussive symptoms, AUDIT (alcohol); WHO Quality of Life–Brief | |
NOTES: AUDIT = Alcohol Use Disorders Identification Test; CI = confidence interval; DTI = diffusion tensor imaging; LOC = loss of consciousness; MRI = magnetic resonance imaging; OEF = Operation Enduring Freedom; OIF = Operation Iraqi Freedom; OR = odds ratio; PTSD = posttraumatic stress disorder; SD = standard deviation; TBI = traumatic brain injury; WHO = World Health Organization.
| Results | Adjustments | Comments or Limitations | ||
| PTSD severity associated with higher 1st percentile values of mean diffusivity on DTI (regression coefficient r = 4.2, p = 0.039), abnormal MRI (r = 13.3, p = 0.046), and severity of exposure to combat events (r = 5.4, p = 0.007). PTSD severity not associated with self-report of blast exposure. Blast exposure associated with lower 1st percentile values of fractional anisotropy on DTI (OR = 0.38 per SD; 95% CI, 0.15–0.92), normal MRI (OR = 0.00, 95% likelihood ratio test CI, 0.00–0.09), and severity of exposure to traumatic events (OR = 3.64 per SD; 95% CI, 1.40–9.43). Mild TBI not significantly associated with PTSD severity. | PTSD severity, mild TBI likelihood, severity of exposure to traumatic events, time since last tour of duty, prior head injury, age, sex | |||
| Time 1: 9.2% mild TBI, 7.6% PTSD, 9.3% depression; time 2: 22% mild TBI, higher rates of PTSD and depression than time 1 (p < 0.001). Of those reporting a history of mild TBI at time 1, 30.2% had probable PTSD at time 2. Service members with a history of mild TBI were more likely than those without such symptoms to report postdeployment postconcussive symptoms and poorer psychosocial outcomes. After adjustment for PTSD symptoms, mild TBI was not associated with postdeployment postconcussive symptoms, depression, problematic drinking, nonspecific somatic complaints, social adjustment, or quality of life. | PTSD | No examination of moderate or severe TBI | ||
outcomes more strongly than did mild TBI history. The results suggest that mild TBI alone does not result in long-term health outcomes as measured in this study.
The study is limited, however, in its usefulness in determining the long-term health effects of blast exposure because there was no direct comparison of those who had a blast-related injury with those who had a non-blast injury or no injury at all. Although many of those who reported symptoms of mild TBI, PTSD, or comorbid mild TBI and PTSD had a blast injury (mild TBI, 70%; PTSD, 35.9%; comorbid mild TBI and PTSD, 80%), it cannot be determined from the data analysis in the study whether a blast injury uniquely contributed to these health outcomes. Moreover, all outcome measures were based on self-reports and could have been affected by the service members’ recall, amount of current distress, secondary gain, and so on. Because the survey participants were self-selected from a single brigade combat team and the survey had a low response rate of those who agreed to be contacted for participation in time 2 followup (50.4%), the findings may not be generalizable to all deployed military personnel. Finally, perhaps the most important limitation is that the time 1 assessment was conducted at the end of a 16-month deployment. The study would have been strengthened substantially if the time 1 assessment had been conducted before deployment so that the specific effects of deployment-related blast could be assessed (for example, concussion and mild TBI, PTSD, post-concussive symptoms, problem drinking, and depression).
Bazarian et al. (2013) conducted a nested cohort study to understand the relation of PTSD severity to mild TBI, blast exposure, and brain white matter structure. The participants were 52 Iraq and Afghanistan war veterans who served in combat areas during 2001–2008 and were studied about 4 years after their last tour of duty. Data on outcome measures were obtained from interview questions concerning blast exposure and TBI symptoms, the PTSD Checklist–Military, the Combat Experiences Scale, anatomic magnetic resonance imaging (MRI), and diffusion tensor imaging (DTI). The results of multivariate analyses demonstrated that PTSD severity was associated with higher 1st percentile values of mean diffusivity on DTI (regression coefficient r = 4.2, p = 0.039), abnormal MRI (r = 13.3, p = 0.046), and severity of exposure to combat events (r = 5.4, p = 0.007). However, PTSD severity was not associated with self-reported blast exposure. Blast exposure was associated with lower 1st percentile values of fractional anisotropy on DTI (which is an abnormal DTI associated with PTSD severity) (odds ratio [OR] = 0.38 per standard deviation [SD]; 95% confidence interval [CI], 0.15–0.92), normal MRI (only five people had abnormalities on MRI, and 47 had normal results) (OR = 0.00, 95% likelihood ratio test CI, 0.00–0.09), and severity of exposure to traumatic events
(OR = 3.64 per SD; 95% CI, 1.40–9.43). Mild TBI was not significantly associated with PTSD severity.
The findings of the study showed that PTSD severity is related to the severity of combat stress and observed structural brain changes on MRI and DTI but not related to a clinical diagnosis of mild TBI. The observed relation between blast exposure and abnormal DTI suggests that subclinical TBI may play a role in the genesis of PTSD in a combat environment. The study demonstrates that asking questions about TBI symptoms may not be a good way to determine whether a person has suffered brain damage. The study was limited by its small sample and its use of self-reports of exposure.
Supportive Studies
Four secondary studies provide some additional information on possible long-term psychological and psychiatric outcomes of blast exposure; however, each has limitations related to study design and the quantity and quality of information reported.
In a longitudinal cross-sectional and cohort study, Rona et al. (2012) conducted a questionnaire to assess the prevalence of mild TBI in UK military personnel deployed to Iraq and Afghanistan. They looked at risk factors associated with mild TBI and the association between mild TBI and postconcussive symptoms and other psychologic health outcomes. During 2007–2009, 4,620 personnel who had deployed to Iraq and Afghanistan completed the questionnaire in phase 2; 2,333 of them had been studied in 2005 (phase 1 predeployment health outcomes were observed in 2005 when the study was first established). Outcome measures included the reported incidence of mild TBI during deployment on the basis of a modified version of the Brief Traumatic Brain Injury Screen questionnaire and self-reported postconcussive symptoms that occurred in the month before the questionnaire was completed. Comorbid mental health conditions also were assessed with the PTSD checklist, General Health Questionaire–12, and AUDIT. Results showed that the overall prevalence of mild TBI was 4.4% and the prevalence in those who had a combat role, 9.5%. Having mild TBI was associated with current symptoms: PTSD (adjusted OR [AOR] = 5.2; 95% CI, 2.3–11.4), alcohol misuse (AOR = 2.3; 95% CI, 1.4–3.7), and multiple physical symptoms (AOR = 2.6; 95% CI, 1.3–5.2). Of those who had mild TBI with loss of consciousness, 46.8% reported that the mechanism of injury was blast. Of those who had mild TBI and altered mental status, 37.7% reported that the mechanism of injury was blast. No other comparison or analysis was done in this study to determine specific outcomes in blast- versus non-blast-injured people.
The study has limitations for the committee’s determination of long-term psychologic outcomes of blast because of the data that were collected
and the comparisons reported. For instance, the study did not report the average time between injury and reported health outcomes, so it is impossible to determine whether the observed outcomes were long-term consequences of injury or acute reactions. Another important limitation is that two samples were added in the phase 2 assessment because the authors were concerned that the followup sample would be too small. A separate longitudinal cohort analysis of the same samples before and after deployment would have strengthened the study.
Hoge et al. (2008) surveyed US Army infantry service members 3–4 months after their return from a year-long deployment to Iraq to compare service members who reported mild TBI with those who reported other injuries. Mild TBI was defined as a self-reported injury with loss of consciousness (LOC) or altered mental status (for example, being dazed or confused). Of 4,618 service members in two brigades who were asked to participate, 2,714 (59%) completed the questionnaire; 2,525 were then included in the study (others were screened out because of missing data or reports of head injury with no LOC or altered mental status). Findings showed that 79.0% of service members who suffered an injury with LOC were injured by blast exposure, 72.7% of those who had an injury with altered mental status were injured by blast, and only 23.2% of other reported injuries were due to blast. Service members who had mild TBI with LOC were significantly more likely to report poor general health, missed workdays, a high number of medical visits, and a high number of somatic and postconcussive symptoms than service members who had other injuries, such as moderate or severe TBI. However, after adjustment for PTSD and depression, mild TBI was no longer significantly associated with those physical health outcomes or symptoms, except for headache and heart pounding. Mild TBI was significantly associated with psychiatric symptoms such as those occurring with PTSD (more than 40% of service members who had injuries associated with LOC met criteria for PTSD). The study suggests that most of the postconcussive symptoms attributed to having previously experienced a blast-related mild TBI might actually be related to posttraumatic stress symptoms. Thus, the development of PTSD symptoms may be a long-term outcome of blast-induced mild TBI. However, analysis was not done to determine whether the blast mechanism of injury contributed uniquely to psychiatric symptoms as opposed to other mechanisms of injury. Although the study conducted surveys 3–4 months after deployment, it is impossible to know when the injuries took place and whether the reported symptoms were short-term or long-term symptoms.
As is also discussed in the section “Auditory and Vestibular Outcomes,” Vanderploeg et al. (2012) conducted a cross-sectional cohort study that was based on data collected in anonymous online surveys to determine whether there was an association between military experience and immediate and
long-term physical and psychologic health outcomes. The study also aimed to examine the effects of multiple deployment-related TBIs on health outcomes. The study included 3,098 members of the Florida National Guard (1,443 who had deployed and 1,655 who had not deployed). About 10,400 letters were mailed to solicit participation in the survey; 4,005 people completed the survey, and those who had been deployed completed it an average of 31.8 months (SD = 24.4 months, range = 0–95 months) after their deployment. ORs were calculated to assess the association between current health status and deployment-related factors, such as physical injuries, exposure to potentially traumatic deployment experiences, combat, blast exposure, and mild TBI. Demographics and predeployment experiences were controlled for as potential cofounders. The survey included a large number of questions to measure many predictors of health outcomes, such as blast exposure; of the 3,098 people in the study sample, 743 (24%) reported being exposed to blast. Results showed that deployment-related mild TBI was associated with depression, anxiety, PTSD, and postconcussive symptoms collectively and individually. There were also statistically significant increases in the frequency of depression, anxiety, PTSD, and a postconcussive symptom complex when people who had single incidents of TBI were compared with those who had multiple TBIs. A predeployment TBI did not appear to increase the likelihood of another TBI from a blast exposure. The experience of seeing others wounded or killed or experiencing the death of a fellow soldier or leader was associated with indigestion and headaches but not with depression, anxiety, or PTSD. The major limitations of this study are its cross-sectional design and its reliance on self-reported measures for all outcomes. In addition, the survey had a low response rate (41.3%), so the results shown here may not be generalizable to all deployed and nondeployed service members.
Finally, Bryant et al. (2009) conducted a longitudinal cohort study to examine the incidence of PTSD in a civilian population after nonmilitary traumatic injury in those who had mild TBI and those who had no TBI. Study participants were 1,167 survivors of traumatic injury (459 who had mild TBI and 708 who had no TBI) who were admitted to four level 1 trauma centers in Australia from April 2004 to February 2006. The subjects were assessed for PTSD symptoms and posttraumatic amnesia during hospitalization and then assessed for PTSD 3 months later. At the followup assessment, 90 (9.4%) of the 920 who were still participating met criteria for PTSD (mild TBI, 50, 11.8%; no TBI, 40, 7.5%). After controlling for injury severity, it was concluded that mild TBI patients were more likely to develop PTSD than no-TBI patients (AOR = 1.86; 95% CI, 1.78–2.94). Although the study is limited in its usefulness by its report outcomes only out to 3 months and not looking at blast injuries specifically, it adds to the evidence of a relationship between mild TBI and PTSD symptoms.
Interventions to Mitigate the Long-Term Consequences of Blast
A complete review of the treatment-outcome literature on combat-related PTSD is beyond the scope of the present report. However, a brief summary of the literature is helpful in determining the potential long-term consequences of PTSD. Historically, combat-related PTSD has been considered by many to be a chronic, lifelong condition that is difficult to treat. Indeed, combat-related PTSD in Vietnam veterans has been found to be a chronic disorder that fails to remit in almost 80% of cases evaluated decades after initial trauma exposure (Schnurr et al., 2003). Conversely, a recent long-term followup study of civilians treated with cognitive behavior therapy (cognitive processing therapy and prolonged exposure) indicated that about 80% of participants were treated to the point of remission and remained in remission for 5–10 years after participating in the study (Resick et al., 2012). Similar data on combat-related PTSD do not exist.
Additional Psychologic and Psychiatric Consequences of Blast
Scientific data on the relationship between exposure to blast and such mental disorders as major depressive disorder and substance-abuse disorders are fewer than data on the relationship between exposure to blast and PTSD. Depression and alcohol abuse are two of the most common mental-health comorbidities of PTSD. However, the relationship of depression to substance abuse separate from PTSD is not clear. The relationship of alcohol misuse to PTSD and depression symptoms was evaluated in a sample of 812 male US veterans of the Iraq war who had documented combat injuries (Heltemes et al., 2013). The results (after adjustment for age, rank, combat exposure, and mental health diagnosis before injury) indicated that veterans who had PTSD symptoms had significantly higher odds of reporting alcohol misuse than those who reported no PTSD symptoms (AOR = 4.05; 95% CI, 2.74–6.00). Veterans who had depression symptoms were also significantly more likely to have reported alcohol misuse than those who reported no depression symptoms (AOR = 4.22; 95% CI, 2.78–6.40).
Conclusions
Relationships between multiple deployment-related factors and numerous overlapping and co-occurring adverse physical and psychologic health outcomes are complex. Acute psychologic and psychiatric outcomes of exposure to blast can include anxiety, depression, addiction, and worsening of existing psychiatric disorders. Two studies, considered primary by the committee, reported an association between exposure to blast and PTSD; this finding has been corroborated by several supportive studies.
The associations between exposure to blast and other chronic mental health outcomes, such as depression and substance-use disorders, are less well understood.
The committee concludes, on the basis of its evaluation, that there is sufficient evidence of an association between exposure to blast and posttraumatic stress disorder. The association may be related to direct experience of blast or to indirect exposure, such as witnessing the aftermath of a blast or being part of a community affected by a blast.
The committee concludes, on the basis of its evaluation, that there is sufficient evidence of a substantial overlap in the symptoms of mild traumatic brain injury (TBI) and posttraumatic stress disorder (PTSD) after exposure to blast. Furthermore, the committee concludes, on the basis of its evaluation, that there is limited/suggestive evidence that most of the shared symptoms are accounted for by PTSD and are not a direct result of TBI alone.
The committee concludes, on the basis of its evaluation, that there is inadequate/insufficient evidence to assess the direct contribution of blast to depression, substance-use disorders, and chronic pain; however, the association of posttraumatic stress disorder with these disorders is well established.
TBI is the dominant blast injury that affects the nervous system. The Department of Veterans Affairs (VA) and the Department of Defense (DOD) define TBI as “traumatically induced structural injury and/or a physiological disruption of brain function as a result of an external force,” with at least one of the following manifestations: decreased level of consciousness, loss of memory immediately before or after the injury, alteration in mental state, neurological deficits, or intracranial lesions (Shively and Perl, 2012). TBI severity is generally classified into three tiers: mild, moderate, and severe. The VA and DOD shared guidelines for distinguishing TBI severity are based on the following criteria: structural imaging, LOC, alteration of consciousness (AOC), posttraumatic amnesia (PTA), and the Glasgow Coma Scale (GCS) (VA and DOD, 2009). The GCS is a severity score itself; it is aggregated from performance ratings of eye opening, motor response, and verbal response and has been the gold standard of neurologic assessment of trauma patients since its development by Teasdale and Jennett in 1974 (see Table 4-2) (IOM, 2009; Teasdale and Jennett, 1974). VA and DOD define mild TBI as presenting with one or more of the following: nor-
TABLE 4-2 Severity Scoring of the Glasgow Coma Scale
| Response | Degree of Response | Score |
| Eye opening | Spontaneous—open with blinking at baseline | 4 |
| To verbal stimuli, command, speech | 3 | |
| To pain only (not applied to face) | 2 | |
| No response | 1 | |
| Best verbal response | Oriented | 5 |
| Confused conversation, but able to answer questions | 4 | |
| Inappropriate words | 3 | |
| Incomprehensible speech | 2 | |
| No response | 1 | |
| Best motor response | Obeys commands for movement | 6 |
| Purposeful movement to painful stimulus | 5 | |
| Withdraws in response to pain | 4 | |
| Flexion response to pain (decorticate posturing) | 3 | |
| Extension response to pain (decerebrate posturing) | 2 | |
| No response | 1 | |
SOURCE: Adapted from Teasdale and Jennett (1976) with permission from Springer Science and Business Media.
mal structural imaging, LOC duration of 0–30 minutes, AOC duration of a moment to 24 hours, PTA duration less than 24 hours, and a GCS score of 13–15. Moderate TBI is defined by one or more of the following: normal or abnormal structural imaging, LOC duration of 30 minutes–24 hours, AOC duration greater than 24 hours, PTA duration of 24 hours–1 week, and a GCS score of 9–12. Severe TBI is defined by one or more of the following: normal or abnormal structural imaging, LOC and AOC lasting more than 24 hours, PTA lasting more than 1 week, and a GCS score less than 9 (VA and DOD, 2009). That schema incorporates the most widely adopted case definition of mild TBI provided by the American Congress of Rehabilitative Medicine (ACRM, 1993) and the consensus among the scientific community of the case definitions of moderate and severe TBI (Sayer, 2012).
From January 1, 2000, to August 20, 2012, a total of 253,330 US service members—of the 2.2 million deployed—had a diagnosis of TBI while serving in the Iraq and Afghanistan wars (Fischer, 2013). Most (77%) of the cases were mild. Exposure to blast can cause TBI through primary, secondary, tertiary, and quaternary mechanisms. Evidence from clinical experience and experimental neuroimaging suggests that TBI caused by blast waves (blast TBI) is distinct from TBI caused by closed head injuries due to blunt trauma and from penetrating TBI (Magnuson et al., 2012).
Studies of TBI have been conducted in nearly all the major conflicts of the 20th century, including World Wars I and II, the Korean War, and
the Vietnam War; many of the studies evaluated seizure as the outcome of interest. For example, during the Vietnam War, 12–14% of all combat casualties had a TBI (Okie, 2005). However, the populations in those studies had penetrating and severe closed head injuries and the mechanisms of injury are not typically reported. A detailed summary of studies of TBI (not specifically related to exposure to blast) in veteran populations can be found in Gulf War and Health, Volume 7: Long-Term Consequences of Traumatic Brain Injury (IOM, 2009).
Acute Effects
Mild blast TBI can cause acute headache, anxiety, vertigo, sleep disturbance, mood alteration, and a cognitive deficit that includes confusion, brief LOC, amnesia, short-term memory loss, and difficulty in concentrating (Brenner et al., 2012; Magnuson et al., 2012). Those effects may resolve in a matter of a few days; one study showed that in a civilian population exposed to mild closed-head-injury TBI, symptoms had resolved in most patients at a 1-year followup (Alexander, 1995). In rare circumstances, however, symptoms can fluctuate in severity or be unapparent immediately after the injury, only to be triggered by life stressors months later (Hicks et al., 2010). Mild blast TBI is clinically indistinguishable from other types of mild TBI at this level of severity, and the outcomes mentioned here may occur from secondary, tertiary, and quaternary effects of the blast as well. A particular danger with mild TBI is that service members may ignore or endure the milder symptoms and then expose themselves to a second blast, putting themselves at risk of second-impact syndrome. Outcomes can be more severe if someone already suffering from mild TBI is subjected to a second concussion. Second-impact syndrome can present with prolonged LOC, malignant cerebral edema, and coma. Although it is extremely rare, the risk of second-impact syndrome may be greater in patients who are exposed to blast than in those who have other types of trauma (Armonda et al., 2006). It is associated with up to 50% mortality (Magnuson et al., 2012).
Moderate to severe blast TBI can cause hemorrhage, skull fracture, cerebral edema, and parenchymal contusions which are all easily detectable with neuroimaging. Patients present with acute effects ranging from confusion and lethargy to coma and even death (Magnuson et al., 2012). Differences in diffuse axonal injury between blast TBI and closed head-injury TBI can be observed at this level of severity with advanced neuroimaging techniques (Davenport et al., 2012). Brains exposed to blast TBI can develop malignant cerebral edema faster (in less than 1 hour) than those exposed to closed-head-injury TBI (in several hours to 1 day) (Magnuson et al., 2012). Cerebral vasospasm, which may lead to secondary cerebral infarction days
after the injury, may be prolonged, with a duration after blast TBI twice that after closed-head-injury TBI (Oertel et al., 2005).
Two less understood outcomes that can occur with TBI of any type or severity are seizures and posttraumatic epilepsy. The latter is defined as the occurrence of two or more seizures more than 7 days after a blast; it is more frequent after more severe episodes of TBI (Magnuson et al., 2012). Seizures can be manifested with only mild behavioral and cognitive alterations, so in some settings it may be difficult to detect without electroencephalographic monitoring (Magnuson et al., 2012).
Of particular difficulty in determining the neurologic effects of blast TBI is that these often develop in the polytrauma setting. Patients who have TBI from blast frequently also suffer damage to neurosensory organs, such as the ears and eyes, and to solid organs (such as the heart and lungs); all these injuries can have direct and indirect influences on brain function. The Defense and Veterans Brain Injury Center found that 66% of TBI patients seen at Walter Reed Army Medical Center (WRAMC) over a 2-year period also suffered ocular trauma (Magnuson et al., 2012). Another study retrospectively evaluating 10,341 victims of blast TBI concluded that 68.5% had concomitant hearing impairment (Lew et al., 2011). It can be difficult to ascertain which neurologic symptoms are directly related to blast TBI itself and which are related to other kinds of injury from the blast that affects the nervous system secondarily.
Neurologic effects of blast to the inner ear may take the form of centrally or peripherally mediated disequilibrium, vertigo, posttraumatic Ménière disease, and sensorineural hearing loss (Magnuson et al., 2012; Scherer et al., 2007). Penetrating or severe nonpenetrating forces on the eye can cause optic nerve damage that results in impairment or loss of vision (Morley et al., 2010). Damage to the cardiovascular system caused by blast injury can compromise blood supply to the brain and cause generalized cerebral dysfunction, such as altered affect, confusion, disorientation, or focal neurologic signs from stroke, traumatic cerebral vasospasm, arterial air emboli, or arterial dissection (Magnuson et al., 2012; Phillips, 1986). Musculoskeletal injury and damage to the spinal cord and vertebrae caused by blast can result in paralysis (Eardley et al., 2012). Bone fractures and other crush injuries or compartment syndromes may result in peripheral nerve palsies or muscle damage at the site of injury (Scott et al., 1986).
Long-Term Effects
Some acute injuries to the central nervous system (CNS) will never resolve. Spinal cord injuries that result in paralysis, incontinence, or loss of ability to breathe spontaneously often will be permanent. Cerebral contusions and other structural brain injuries may lead to permanent neu-
rologic dysfunction, including posttraumatic epilepsy; in one study, 19% of patients who suffered moderate to severe TBI had epilepsy at a 10-year followup (Andelic et al., 2009).
The committee that prepared Gulf War and Health, Volume 7: Long-Term Consequences of Traumatic Brain Injury (IOM, 2009) concluded that there was sufficient evidence of a causal relationship between
- Penetrating TBI and unprovoked seizures.
- Penetrating TBI and premature death.
- Severe or moderate TBI and unprovoked seizures.
It concluded further that there was sufficient evidence of an association between
- Penetrating TBI and a decline in cognitive function.
- Penetrating TBI and long-term unemployment.
- Severe TBI and cognitive deficits.
- Severe or moderate TBI and dementia of the Alzheimer type.1
- Severe or moderate TBI and parkinsonism.
- Severe or moderate TBI and endocrine dysfunction (hypopituitarism and growth hormone deficiency).
- Severe or moderate TBI and adverse social-function outcomes.
- Severe or moderate TBI and premature death.
- All forms of TBI and depression, aggressive behaviors, and postconcussive symptoms.2
In the studies examined in the 2009 Institute of Medicine (IOM) report and other studies of TBI that have followed, both blast and non-blast mechanisms of TBI have been combined in analyses. In instances where there would be no likely difference in consequences between blast and non-blast TBI, the present committee used data from the studies to help to determine the relationship between blast exposure and long-term neurologic effects. However, given the animal and other data that suggest potentially unique, and in some cases more severe, injuries to the nervous system caused by blast exposure compared with TBI caused by other mechanisms (see Chapter 3), the committee also sought to identify studies that focused on blast-related TBI.
_________________
1Studies published after the release of the 2009 report show that the association between severe or moderate TBI and dementia is likely due to a mixture of pathologies rather than solely Alzheimer Disease.
2The association between TBI and aggressive behaviors has been shown for the severe and moderate forms of TBI.
To evaluate the long-term neurologic effects of blast exposure, the committee reviewed about 50 published peer-reviewed studies. It did not find any studies that met the inclusion guidelines for primary studies. This section details the identified supportive studies of long-term neurologic health outcomes of blast exposure.
Chronic Traumatic Encephalopathy
One recently recognized long-term effect of repeated TBI is chronic traumatic encephalopathy (CTE), a slowly progressive neurodegenerative disorder that usually does not present until 8–10 years after the exposure and is characterized pathologically by progressive accumulation of abnormal (hyperphosphorylated) deposits of tau protein in neurons and associated atrophy of brain tissue (McKee et al., 2012). Initial symptoms include irritability, impulsivity, aggression, depression, sleep disruption, memory loss, and heightened suicidality, all of which can clinically resemble common forms of dementia. CTE has been observed in professional athletes, especially American football players. However, little is known about the prevalence of CTE in the blast-injured population or even about whether it can occur after single blast TBI episodes. Goldstein et al. (2012) examined brains from three military personnel who were known to be exposed to blast and found CTE-linked neuropathologic characteristics: perivascular foci of tau-immunoreactive neurofibrillary tangles and glial tangles in the inferior frontal, dorsolateral frontal, parietal, and temporal cortices with a predilection for sulcal depths.
Headache
Headache is the most common disorder in most neurology clinics and among the three most common in general-medicine clinics. Migraine is the secondmost common type of headache; 15% of women and 6% of men in the general population experience migraine in a 1-year period (Stewart et al., 1994). Tension headache is four times as common as migraine. In the general population, 4% of adults have chronic daily headache (CDH), defined as headache on at least 15 days per month; this entity is not a single condition, and in the general population as much as 15% of cases of CDH may be attributable to some sort of head trauma (Couch et al., 2007). Headache is a common sequela of a diverse set of head-trauma mechanisms in clinical experience, so it is likely to result also from blast exposure.
The committee identified three secondary studies. In a retrospective cohort study of 91 service members from the same brigade who had chronic headache after a 1-year combat tour in Iraq, 41% had a history of head and neck trauma during their tour, and 67% of the traumas were due to
blast (Theeler and Erickson, 2009). In one-third of the patients with head and neck trauma, a new headache started after the trauma; in an additional one-third, a preexisting headache worsened after the trauma. Migraine was the most common headache type identified. Limitations of the study include the small sample, the retrospective design, and selection bias (a clinic-based population was assessed).
In a cross-sectional study of service members undergoing postdeployment health evaluation, those with a self-identified history of concussion received a specific headache questionnaire (Theeler et al., 2010). Some 20% of the 5,270 service members deployed to Iraq or Afghanistan who were studied met criteria for deployment-related concussion, and 37% experienced posttraumatic headache. Migraine was the most common phenotype seen. The study did not address the mechanism of concussion, although in many cases it probably was blast. The contribution of PTSD and psychiatric disorders was not detailed in the study. There were several limitations related to the questionnaire-based, cross-sectional design of the study, including recall error and possible misclassification of headache type.
In a cross-sectional study of 978 service members deployed to Iraq or Afghanistan who screened positive for postdeployment concussion headache, 196 were found to have CDH with a median of 27 headache days per month (Theeler et al., 2012). In 55% of the CDH patients, the headaches began within 1 week of concussion, compared with 33% of those who reported episodic headache. Two-thirds of those who had CDH met criteria for migraine. No differences were found between the CDH and episodic-headache groups in the number of blast exposures, concussions, or concussions with LOC. The CHD patients had significantly higher scores than the episodic-headache group on a PTSD checklist; this emphasizes the link between PTSD and CDH after blast exposure. The cross-sectional design of the study constitutes a limitation because reports of CDH and of blast exposure are retrospectively self-reported, and there may be recall error. In addition, it was not known whether any of the subjects experienced CDH before blast exposure.
Another study, considered tertiary by the committee, found that among 126 Iraq and Afghanistan war veterans who experienced exposure to blast and sustained a mild TBI, nearly two-thirds (80) had frequent severe headaches, usually with migrainous features, accompanied by PTSD and impaired sleep with nightmares (Ruff et al., 2008).
Endocrine Changes
Blast injury may affect the pituitary gland and thus disrupt hormonal function. Two secondary studies were identified to support that idea. Wilkinson et al. (2012) studied 26 Iraq and Afghanistan war veterans who
were exposed to blast at least 1 year before testing and compared them with 59 veterans who do not have blast exposure. Eleven of the blast group (and none of the non-blast group) had abnormal hormone concentrations in one or more pituitary axes. Half of those patients had abnormalities of anterior pituitary function, and half had posterior pituitary abnormalities. Five patients were growth hormone deficient, and three suffered from hypogonadism. Baxter et al. (2013) studied endocrine function in 19 UK service members who served in the Afghanistan war and had moderate to severe blast TBI. The service members underwent MRI, including DTI, and cognitive assessment. Control subjects were civilians who had moderate to severe nonblast TBI. Anterior pituitary dysfunction was found in 6 (32%) of the 19 service members who had blast TBI and 1 of the 39 controls (p = 0.004). The service members who had pituitary dysfunction had greater traumatic axonal injury in the cerebellum and corpus callosum, more skull and facial fractures, and worse cognitive function than the service members who did not have pituitary dysfunction.
Postconcussive Symptoms
After TBI, patients often experience a variety of persistent postconcussive symptoms including headache (discussed on page 102), difficulty in concentrating, aggressiveness, and irritability. The committee identified three secondary studies that described postconcussive symptoms after exposure to blast.
In a study of 339 veterans of the Iraq and Afghanistan wars who had a history of mild TBI, posttraumatic stress symptoms were found to be significantly worse in the blast and mixed (blast plus non-blast) groups than in the group that had only non-blast mechanisms (Lippa et al., 2010). PTSD-like symptoms accounted for 47% of the variance in the postconcussive symptoms.
In a study of 91 Iraq and Afghanistan war veterans who had a history of being within 100 m of a blast, veterans who had TBI and LOC had significantly more postconcussive symptoms than those who did not have TBI or those who had TBI without LOC (Verfaellie et al., 2013). However, after adjustment for depressive and PTSD symptoms, the result was no longer significant. The mild TBI with LOC group had greater psychosocial limitations than the other groups, and this relationship persisted even after adjustment for depressive and PTSD symptoms. The relationship between postconcussive symptoms and Axis I psychiatric disorders has been well detailed in previous studies not specific to blast injury.
In a study of structured interviews that attempt to associate blast-related TBI with neuropsychologic outcomes, 18 veterans of the Iraq and Afghanistan wars who experienced mild TBI were compared with those
who had only Axis I disorders (24 veterans), those who had mild TBI and Axis I disorders (34 veterans), and postdeployment controls; no difference was found between the mild-TBI group and the other groups (Nelson et al., 2012b). Limitations of the study include the lack of addressing the contribution of PTSD and the fact that some blast exposures reported in interviews with the veterans were minor and did not lead to concussions.
Seven additional studies were considered tertiary by the committee, but provided some further information about exposure to blast and postconcussive symptoms. Trudeau et al. (1998) reported that combat veterans who had a remote history of blast injury (27 veterans) have persistent electroencephalographic features that are consistent with TBI and attention problems. Kennedy et al. (2010) found that 130 US Iraq and Afghanistan war service members who had mild TBI but no other associated physical injuries had higher symptom ratings than those who had mild TBI and associated physical injuries (144 service members); one explanation for this result might be that patients who have physical injuries can focus on making progress toward healing and functional improvement whereas patients who have only mild TBI experience somatic neuropsychologic symptoms. Heltemes et al. (2012) found that 473 US service members who sustained blast-related mild TBI self-reported adverse changes in health 6 months after injury 5 times more often than did 656 service members who sustained other types of injuries. Scheibel et al. (2012) conducted a stimulus-response compatibility task by using functional magnetic resonance imaging (fMRI) on 15 US service personnel and veterans of the Iraq and Afghanistan wars who had blast-related mild TBI and compared them with 15 controls who did not have TBI and were not exposed to blast. The subjects who had experienced blast-related mild TBI demonstrated slower fMRI responses and increased symptoms of PTSD, depression, and somatic complaints. Another neuroimaging study used a magnetoencephalographic low-frequency source imaging method and demonstrated abnormalities in 96% of 23 blast-exposed mild TBI patients and 77% of 22 non-blast mild TBI patients (Huang et al., 2012). Walker et al. (2013) reported that 29 (33.3%) of 87 US service personnel and veterans who served in Iraq and Afghanistan reported at least one of three concrete alteration-of-consciousness items—gap in memory (17.2%), memory not continuous (13.8%), and being told by an observer that they had LOC (20.7%)—after experiencing acute effects of blast exposure within the preceeding 2 years; these results again suggest that mild TBI plays a role in the development of chronic neuropsychiatric symptoms after exposure to blast. Mendez et al. (2013) studied changes in personality in 12 US veterans who had blast-related mild TBI and 12 US veterans who experienced blunt-force mild TBI and found that, on the basis of select measures of personality, veterans
who had blast-related mild TBI had more negative personality changes than those who had blunt-force mild TBI.
Cognitive Changes
Several reported studies have many limitations and were considered tertiary by the committee but provide some information about cognitive effects related to blast exposure. Cooper et al. (2012) conducted a retrospective review to assess neurocognitive function in 32 Iraq and Afghanistan–war service members who had blast-related mild TBI and 28 who had nonblast-related mild TBI about 6 months after injury and did not find significant differences between the groups in any neurocognitive domain. Coldren et al. (2012) administered the automated neuropsychiatric assessment metric within 10 days of injury to 47 concussed and 108 non-concussed service members who served in Iraq and Afghanistan; concussed service members were more likely to have been exposed to a blast. The neurocognitive changes found with the metric were not reassessed later. Mac Donald et al. (2011) used DTI and found cerebellar abnormalities consistent with traumatic axonal injury in 18 (29%) of 63 US service members who had TBI and self-reported blast exposure; by chance alone, only two of 63 healthy subjects would be expected to have such abnormalities. Matthews et al. (2011) performed a stop-task-based fMRI on 27 US Iraq and Afghanistan– war service members and found that subjects who had a history of LOC had altered ventromedial prefrontal cortex activity more than subjects who had a history only of alteration of consciousness; this finding correlated with the severity of somatic symptoms experienced and suggested a neural correlate of impaired self-awareness after LOC. Finally, Sponheim et al. (2011) used electroencephalography phase synchronization and DTI to assess nine service members who were deployed to Iraq and Afghanistan and suffered blast-related mild TBI; they did not report cognitive deficits but observed more frequent electroencephalographic abnormalities in frontal and lateral cerebral regions and problems with structural integrity of frontal white-matter tracts than in controls, persisting after controlling for PTSD, depression, and medications.
Advanced neuroimaging (for example, DTI and fMRI) has led to several insights regarding the cognitive effects of TBI in veterans. DTI studies have revealed evidence of disruptions of CNS tracts suggestive of TBI in bodily injured service members even in the absence of reported history of TBI (Xydakis et al., 2012). That suggests that the true incidence of anatomically significant TBI may not be captured by using routine clinical and imaging criteria, especially in critically injured service members. The history typical of concussion (“seeing stars” and LOC) may not be present with blast TBI, so additional neuroimaging studies may be required for this diag-
nosis. Changes in DTI have been found in some studies of blast-associated TBI. For example, Mac Donald et al. (2011) found changes consistent with multifocal traumatic axonal injury in a group of seriously injured service members who were exposed to blast events and who had normal computed tomography scans; evolution of the changes 6–12 months later revealed persistence and some dynamic changes that were compatible with evolution of the acute lesions. DTI changes were also found with mild TBI in studies by Davenport et al. (2012), Jorge et al. (2012), and Matthews et al. (2012) but not by Levin et al. (2010). Technical differences may account for those discrepancies, and recent technical advances focusing on identifying spatially heterogeneous areas of decreased functional anisotropy (“potholes”) suggest that this method may be more sensitive in determining TBI severity and impaired executive functioning. The axonal injury that is persistent in chronic cases may create a potential surrogate identifier for a TBI event, which is especially important because the usual criteria used to recognize mild TBI may not be present or may be obscured by other bodily injuries.
Using DTI, Davenport et al. (2012) studied 25 Iraq and Afghanistan war veterans who had blast-related TBI and 33 veterans who did not have blast exposure and found global disruption of white-matter tracts in the blast-exposed veterans. No differences were found in more concentrated white-matter regions. A history of prior civilian mild TBI did not affect the results. The injury appeared to be dose dependent inasmuch as greater numbers of blast exposures were associated with a larger number of low voxels when fractional anisotropy was used. Another study of 12 Iraq war veterans who had persistent postconcussive symptoms and healthy community volunteers showed decreased metabolism in the veterans on the basis of fluorodeoxyglucose positron emission tomography in the cerebellum, pons, and medial temporal lobe; those who had mild TBI also had subtle impairments in verbal fluency, cognitive processing speed, attention, and working memory on neuropsychologic testing (Peskind et al., 2011). A limitation is that the volunteer controls were an average of 20 years older than the veterans, although the deficits identified on imaging and neuropsychologic testing in the younger veterans would be more likely to increase with age, so this potential bias is less likely to explain the results.
Spinal Injuries
The committee identified two secondary studies of spinal injuries associated with exposure to blast. Comstock et al. (2011) found that improvised explosive devices (IEDs) are more likely to cause spinal injuries than are other mechanisms, such as blunt trauma. Through the Joint Theatre Trauma Registry (JTTR) 372 Canadian Forces personnel who served in Afghanistan and were injured during the period February 7, 2006–October
14, 2009, were identified and included in the study. Of the 372, 212 (57%) were injured by IEDs, and 29 (8%) had spinal fractures. Members injured by IEDs were significantly more likely to have spinal injuries than those injured by non-blast mechanisms (10.4% vs 2.3%). A major limitation of the study is that the researchers were unable to conduct a detailed chart review of most of the patients’ medical records and were unable to ascertain details of the injuries, such as type of fracture, neurologic findings, and functional outcome.
Blair et al. (2012) used the JTTR to identify US military personnel of the Iraq and Afghanistan wars from October 2001 through December 2009 who sustained back, spinal column, and spinal cord injuries. Of 10,979 combat casualties, 598 (5.45%) sustained 2,101 injuries to the spinal column or spinal cord; 92% of these injuries were fractures. Of the 598 patients, 336 (56%) were injured by exposure to blast. Of the 104 patients who had spinal cord injuries, 38 (36.5%) were injured by exposure to blast. Limitations of the study include reliance on JTTR data, which can be incomplete, especially during the early years of the wars, and the fact that medical records of service members killed in action are not included in the JTTR.
Three additional studies, considered tertiary by the committee, provided further evidence about spinal injuries due to blast exposure. Ragel et al. (2009) conducted a retrospective analysis of North Atlantic Treaty Organization service members who sustained spine fractures when riding in vehicles attacked by IEDs. Twelve patients who had 16 thoracolumbar fractures were identified, and 6 of the fractures were flexion–distraction thoracolumbar fractures; most spine-fracture series report the prevalence of flexion–distraction thoracolumbar fractures as 1.0–2.5%, so these injuries may be characteristic of IED explosions. Bell et al. (2009) conducted a retrospective review of 513 inpatient admissions in the Iraq war from April 2003 to April 2008 that were evaluated at the National Naval Medical Center and WRAMC. Of the 513, 56% were injured by exposure to blast, and 408 had either a closed or a penetrating head injury, including 40 who also had a spinal column injury, but the number of patients who had spinal column injuries and were exposed to blast is not reported. Using the Armed Forces Medical Examiner System, Schoenfeld et al. (2013) identified 5,424 deceased military personnel who had been deployed to Iraq and Afghanistan from 2003 to 2011 and sustained a spinal injury in conjunction with wounds that resulted in death; 67% of all fatalities with spinal injury were attributed to exposure to blast.
Conclusions
Acute short-term effects of TBI were well described in Gulf War and Health, Volume 7: Long-Term Consequences of Traumatic Brain Injury (IOM, 2009). Permanent neurologic disability—including cognitive dysfunction, unprovoked seizures, and headache—is causally related to moderate or severe TBI. Those clinical syndromes result from the known pathologic conditions associated with nonpenetrating impact injuries, including fractures, hemorrhages, contusions, and brain swelling. The present committee was not able to identify primary studies that focused exclusively on acute blast-related TBI. However, inasmuch as many of the studies cited in Volume 7 included both blast and non-blast TBI, it is likely that the injuries are at least as severe in blast TBI, although further research is required to determine whether there are additional unique patterns of injury. Moreover, secondary and tertiary blast effects due to fragments of debris and acceleration and deceleration injuries, respectively, result from primary blast effects, and it is expected that the secondary and tertiary injuries will resemble missile and concussive injuries seen in other settings. Although the clinical and pathologic syndromes of blast-induced TBI and other forms of TBI probably overlap extensively, there may be some differences that could potentially produce distinctive presentations and require different therapeutic strategies. For example, typical symptoms of concussion, such as seeing stars and experiencing a transient LOC, may be absent. The limited evidence indicates that early malignant brain swelling, sometimes referred to as second-impact syndrome, may be more common in connection with blast than with other injuries (Armonda et al., 2006). In addition, numerous studies have suggested that blast TBI may confer distinctive neuroimaging patterns as measured by DTI (tractography). In blast injury, a diffuse bihemispheric pattern of disruption may occur, unlike the more focal, often frontal and occipital (coup–contracoup) pattern classically observed in acceleration–deceleration concussive injury. That pattern could potentially result in a higher frequency of global cerebral complaints involving cognitive, visual, auditory, and other sensory modalities in those exposed to blast; however, the evidence confirming these distinctive mechanisms is preliminary and insufficient to permit any firm conclusions to be drawn.
The committee concludes, on the basis of its evaluation, that there is sufficient evidence of an association between severe or moderate blast-related traumatic brain injury and endocrine dysfunction (hypopituitarism and growth hormone deficiency).
The committee concludes, on the basis of its evaluation, that there is sufficient evidence of an association between mild blast traumatic brain injury and postconcussive symptoms and persistent headache.
The committee concludes, on the basis of its evaluation, that there is limited/suggestive evidence of an association between recurrent blast traumatic brain injury and chronic traumatic encephalopathy with progressive cognitive and behavioral decline.
The committee concludes, on the basis of its evaluation, that there is limited/suggestive evidence that diffuse brain injury with swelling may be more likely after blast than in relation to other mechanisms that lead to traumatic brain injury.
The committee concludes, on the basis of its evaluation, that in other brain-injury mechanisms (non-blast traumatic brain injury [TBI]), there is sufficient evidence of an association between severe or moderate TBI and permanent neurologic disability, including cognitive dysfunction, unprovoked seizures, and headache. These associations also are known outcomes in TBI studies that included blast and non-blast mechanisms considered together. It is plausible that severe or moderate blast TBI is similarly associated with permanent neurologic disability even though studies that specifically addressed blast TBI are lacking.
AUDITORY AND VESTIBULAR OUTCOMES
The ear is typically one of the first organs to sustain damage from a blast event and is the organ most susceptible to primary blast injury (Jagade et al., 2008; Phillips and Richmond, 1991). Injury to the external ear is possible from secondary, tertiary, and quaternary blast exposure, but primary blast injury to the middle and inner ear is much more common and likely to affect auditory function. Traditionally, clinical attention has focused on tympanic membrane (TM) perforations, hearing loss, and tinnitus complaints as the primary manifestations of auditory dysfunction after blast exposure. However, those clinical outcomes do not adequately capture the array of auditory dysfunction that may be associated with acute trauma from blast. Normal auditory function requires an intact ear (especially middle and inner ear) but also relies on the complex signal transduction, transmission, and processing mechanisms that are involved in centrally translating and integrating sounds. Blast—through its effects on the microcirculation, apoptosis, shearing of neural networks, and other mechanisms—may have additional implications for the auditory system and the processing of auditory information, especially in complex environments.
Although loud noise from such exposures as gunfire may cause damage to the auditory system, the focus of this review is on blast injuries.
Acute Effects
Perforation of the TM is the most common form of injury to the middle ear (Jagade et al., 2008). The TM is extremely sensitive to pressure (its primary function is to sense vibrations in sound waves), so it is highly susceptible to blast overpressure. Some cases of TM perforation close spontaneously over weeks to months after blast exposure. Much less common in the middle ear—especially after small to medium blasts—is disruption of the ossicular chain. Patients who have sustained damage to the middle ear from primary blast injury may present with earache and conductive hearing loss, which may be temporary and resolve with the healing of the TM (Jagade et al., 2008; Walsh et al., 1995). It is possible that cholesteatoma and infection can develop from a primary blast injury to the middle ear and potentially lead to erosion and destruction of important structures of the middle ear, temporal bone, and skull casing (Jagade et al., 2008).
Primary blast injuries to the inner ear involve the disruption of the vestibular apparatus and cochlea and can result in sensorineural hearing loss due to temporary or permanent damage to the hair cells of the cochlea, which are the delicate sensory structures responsible for amplification of sound and its transduction to the auditory nerve and central auditory nervous system (Finlay et al., 2012). The inner ear can be directly affected by the blast or indirectly affected by sequelae of injury to the middle ear.
Long-Term Effects
Although the symptoms of blast ear injury often resolve spontaneously, they may also be chronic or permanent. Tinnitus and hearing loss are the two most prevalent medical disability claims in VA (2011).
To evaluate the long-term auditory and vestibular health effects of blast exposure, the committee reviewed about 80 published peer-reviewed studies. Seven met the committee’s guidelines for primary studies (see Table 4-3). Of the seven, only two reported outcomes of blast during military deployment. The others were studies of civilian exposure to blast. This section details the identified primary and supportive studies of long-term auditory and vestibular outcomes due to blast exposure.
Primary Studies
Several of the studies identified as primary by the committee involved health outcomes in survivors of the Oklahoma City bombing on April 19,
TABLE 4-3 Auditory Outcomes—Primary Studies
| Reference | Study Design | Population | Health Outcomes or Outcome Measures |
| Cohen et al., 2002 | Cohort | 17 survivors of a suicide terrorist explosion on bus in Israel, followed for 6 months; 7 males and 10 females; median age 28 years; October 1994–April 1995 | Auditory, vestibular, otoneurologic evaluations |
| Riviere et al., 2008 | Cohort | 103 blast-exposed workers at a chemical plant in France, 91.3% men, 39.9 ± 8.5 years old vs 105 “less-exposed” workers (defined by distance ≥ 1,700 m from blast), 79.1% men, 39.8 ± 8.6 years old; required routine audiometric test since 1990 and before the explosion in September 2001 | Pure-tone air conduction audiometric test, conducted 1 month–3 years after blast vs before blast |
| Shariat et al., 1999 | Cohort assembled from registry created by Oklahoma State Department of Health | 494 survivors of 1995 Oklahoma City bombing, 92% of whom had sustained physical injuries and were treated in hospital or received outpatient care | Long-term physical and emotional outcomes assessed 1.5–3 years after blast via telephone interview |
| Results | Adjustments | Comments or Limitations |
| At 6 months, 73.3% aural fullness, 71.4% dizziness, 40.0% tinnitus, 22.3% otalgia, 44.4% perforated eardrums. Hearing loss: 44.1% SNHL, 8.8% CHL, 26.4% MHL, 20.5% normal. CDP abnormal 46.1%. ENG abnormal 0%. Of 7 cases with vestibular complaints, 4 had multisensory dysfunction on CDP, 1 had vestibular loss. | None | No control group. |
| Blast wave equivalent to 3.4-magnitude earthquake. Minimum peak acoustic levels estimated to be 160–194 dB (2–100 kPa) within 1,700 m. 19.5% of exposed workers reported functional symptoms of otalgia, vertigo, tinnitus, or other. Right ear (exposed vs “less exposed”): hearing loss at 2,000 Hz (p < 0.05), 4,000 Hz (p < 0.001), borderline at 6,000 Hz (p = 0.09). Left ear: hearing loss at 2,000 Hz (p < 0.01), 6,000 Hz (p < 0.05), 8,000 Hz (p < 0.05). | Age, sex, history of ear problems, past occupational noise exposure, period between two audiograms | Precise time of audio testing relative to time of explosion not specified; could have been 1–3 years. Specificity of symptoms not reported; only total percentage with any symptoms is reported; p values in Table 1 not the same as those in abstract. |
| Auditory problems were most common health outcome. 32% of cohort reported newly diagnosed auditory problems since bombing; 44% reported “ringing/ roaring in ears”; 40% reported “trouble hearing.” Hospitalized survivors reported more hearing problems than those who had less severe injuries. 9% of uninjured or not treated patients reported newly diagnosed auditory problem; 48% of cohort used audiology services. | Self-reported data; no control group. | |
| Reference | Study Design | Population | Health Outcomes or Outcome Measures |
| Van Campen et al., 1999a | Longitudinal cohort | 83 survivors of 1995 Oklahoma City bombing; mean age 43 years, 45% female and 55% male, evaluated 4 times over 1 year vs 10 healthy subjects, 50% female and 50% male, mean age 26.1 years, evaluated twice over 6 months | Pure-tone and EHF audiometry, otoscopic inspection, immittance and speech audiometry |
| Van Campen et al., 1999b | Longitudinal cohort | 27 survivors of 1995 Oklahoma City bombing who had nonrecorded gaze abnormality or one or more episodes of vertigo or continuing imbalance, mean age 43 years, 50% female and 50% male, evaluated quarterly over 1 year | Balance questionnaire, ENG, CDP |
| Results | Adjustments | Comments or Limitations |
| Side-on incident blast estimated at 3,000 psi at 10 ft to 25 psi at 100 ft.; decibel levels estimated at 235 dB pSPL. | Age-corrected CF | Healthy subjects not age-matched to blast subjects. |
| 1 year after blast, 76% reported tinnitus, 64% loudness sensitivity, 57% otalgia; averaged across quarters, 76% had mostly sensorineural hearing loss at one or more frequencies; 63% of them were male. 24% required amplification. In CF ranges, males had poorer thresholds than females, but no sex effects for PTA. No clear relationship between location and symptoms or test results. Tympanic perforations healed by second quarter; at 1 year, poorer EHF thresholds in blast subjects with abnormal CF thresholds. | ||
| 60% with abnormal ENG mostly resolved by second quarter; 55% reported nausea with dizziness, 78% tinnitus. At 1 year, 72% said vestibular symptoms were unchanged or occurred intermittently, 67% reported that dizziness was either intermittent or same as first noted, 55% of initially abnormal CDP were normal. Averaged across quarters, SOT showed problems with vestibular (15%), surface-dependent (13%), and physiologically inconsistent (4%) patterns; motor control mostly normal; no relationship between location and tubular symptoms or test results. | No control group. Timing of postblast health outcome unspecified for several outcome measures. | |
| Reference | Study Design | Population | Health Outcomes or Outcome Measures | |
| Vanderploeg et al., 2012 | Cross-sectional cohort | 1,443 OIF or OEF deployed vs 1,655 nondeployed Florida National Guard; deployed group more likely to be male with some college education and history of psychologic trauma and TBI; subjects assessed an average of 31.8 months after deployment | Web-based survey of symptoms, predeployment trauma or TBI, symptom checklists (including 22-item Neurobehavioral Symptom Inventory), and deployment exposures; blast exposure was categorized as primary and non-primary on the basis of 4 questions | |
| Wilk et al., 2012 | Cross-sectional cohort | 3,952 Army OIF service members, 98.3% men, 66.9% less than 30 years old; assessed 3–6 months after deployment | Concussion screening and symptom reporting on Patient Health Questionnaire and question on tinnitus | |
NOTES: CDP = computerized dynamic posturography; CF = conventional frequency; CHL = conductive hearing loss; CI = confidence interval; dB pSPL = decibel re: peak sound pressure level; EHF = extended high frequency (10–20 kHz); ENG = electronystagmography; kPa = kilopascal; LOC = loss of consciousness; MHL = mixed hearing loss; NS = nonsignificant; OEF = Operation Enduring Freedom; OIF = Operation Iraqi Freedom; OR = odds ratio; PTA = pure tone audiometry; SNHL = sensorineural hearing loss; SOT = sensory organization test; TBI = traumatic brain injury.
| Results | Adjustments | Comments or Limitations | ||
| 26.3% of deployed reported primary blast, 25.2% reported non-primary blast. Primary blast exposure associated with hearing loss (OR = 2.32; 95% CI, 1.65–3.26; p < 0.001). Non-primary blast exposure associated with hearing loss (OR = 1.63; 95% CI, 1.19–2.24; p < 0.005). Primary blast exposure associated with ringing in ears (OR = 2.92; 95% CI, 2.09–4.09; p < 0.001). Non-primary blast exposure associated with ringing in ears (OR = 1.77; 95% CI, 1.29–2.41; p < 0.005). Primary blast exposure associated with dizziness (OR = 2.26; 95% CI, 1.30–3.94; p < 0.005). Non-primary blast NS for dizziness. | Demographics, predeployment psychologic trauma or TBI and deployment related factors | Low response rate (41.3%) Alpha error rate set at p < 0.01 for multiple comparisons. Assessment of blast injury developed expressly for current study and thus not previously validated. | ||
| 14.9% met criteria for concussion, 72.2% of whom reported a blast mechanism. Of 201 service members who reported concussion with LOC, blast mechanism was significantly associated with tinnitus compared with nonblast mechanism; no association was found between concussions and change in consciousness. No associations found for dizziness. | 51.5% response rate. Concussion symptoms self-reported. | |||
1995. In a cohort study, Shariat et al. (1999) followed up with survivors of the bombing to identify long-term physical and emotional health outcomes. Baseline data on blast exposure and injuries were initially collected after the bombing and recorded in the Injury Prevention Service registry of the Oklahoma State Department of Health. Some 914 survivors of the blast who were 18 years old or older were considered eligible for the study. Of those, 494 (54%) completed a telephone interview that included questions on long-term health conditions, functional status, employment, quality of life, health care use, and medical costs. The followup interview occurred 1.5–3 years after the bombing. The age range of the subjects was 21–91 years (mean = 45 years). Of the subjects, 92% reported being injured in the bombing; 13% sustained injuries that required hospitalization. Of the subjects interviewed, 156 (32%) reported newly diagnosed or treated auditory problems since the bombing. There were significant differences in rates of reports of newly diagnosed auditory problems between those who had been hospitalized and those who had been treated in an emergency department and then released (48% vs 29%; p < 0.006) and between those who had been hospitalized and those who were uninjured or not treated (48% vs 9%, p < 0.001). For tinnitus, 44% of the subjects reported experiencing ringing or roaring in their ears at some time after the bombing, and there was no significant difference between those who had been hospitalized and those who had not. Nearly half the subjects (48%) reported receiving audiology services after the bombing, and there was a significant difference found between those who had been hospitalized and those who were treated and released (72% vs 45%; p = 0.003) or those who were uninjured or not treated (72% vs 29%; p < 0.001). The study has several limitations for the committee’s determination of long-term auditory outcomes. For newly diagnosed conditions, it is not clear from the data presented whether the conditions were experienced shortly after the bombing or developed later. At the time of the followup interview, 24% of all newly diagnosed or treated conditions had resolved, but the number of resolved auditory outcomes was not reported. Similarly, at the time of the followup interview, 21% of reported symptoms experienced since the bombing had resolved, but the number of injuries to the auditory system that had resolved was not reported. Other limitations of the study include the low response rate of participation, which could mean that the results reported here are not generalizable to all survivors of the bombing. Measures of tinnitus were based on self-report (no objective measurement is available), and no objective measures of hearing function were analyzed. Self-reporting of “trouble in hearing” and other auditory problems also is a limitation because they are not objective measures.
Van Campen et al. (1999a) conducted a longitudinal cohort study of survivors of the Oklahoma City bombing to examine long-term changes
in auditory function. The subject group (83 people) was solicited to participate in the study by advertisements and by referral from physician and personal-assistance programs. Subjects were followed for 1 year during which evaluations, audiometric testing, and a survey questionnaire were completed quarterly. The numbers of subjects present for evaluation and testing at each quarter were 42 0–2 months after the bombing (Q1), 64 3–5 months after the bombing (Q2), 62 6–8 months after the bombing (Q3), and 56 12–14 months after the bombing (Q4). Only 21 (25%) of the subjects were seen at all of the time. Auditory outcomes were compared with those in a representative control group of 10 subjects with normal hearing who were not blast exposed (and not present during the bombing). The control subjects were seen twice over 6 months to document hearing-threshold stability. The results showed no significant differences in pure-tone audiometry (at 1, 2, and 4 kHz) between quarters in either group. Thresholds for high-frequency signals were poorer in blast-exposed persons than in control subjects. At 1 year, use of a hearing aid was recommended for 24% of subjects, none of whom reported hearing aid use before the blast exposure. Tinnitus was reportedly experienced within seconds to days of the blast exposure in 67% of subjects on the Q1 questionnaire. On the Q4 questionnaire, 76% of the responders reported symptoms of tinnitus. There was no clear association between location of subjects in the bombing area and symptoms or test results. Study limitations included the low response rate; only 21 subjects provided responses in each quarter, and the number who were evaluated and questioned in each quarter was variable.
In a companion study, Van Campen et al. (1999b) reported vestibular and balance outcomes in 30 survivors of the Oklahoma City bombing. Subjects were recruited from the sample of 83 included in the previous study, and all were bombing survivors from the downtown area surrounding the blast who reported gaze abnormalities and vertigo or continuing imbalance. The subjects were 25–63 years old (mean, 43 years old). Initially, all subjects completed a questionnaire on their symptoms and underwent nonrecorded gaze testing with computerized dynamic posturography (CDP). The 27 who had abnormal results on the CDP returned quarterly for full balance assessments for a year (Q1, 9 subjects; Q2, 18; Q3, 22; Q4, 24). Thirteen of the 27 (63%) reported dizziness within 48 hours of the explosion. A year after the blast, 16 of 24 (67%) of those assessed at Q4 reported that their symptoms of dizziness and imbalance were unchanged or still intermittent. Results generally showed that testing abnormalities were detected at each quarter, but the data are difficult to interpret because different subjects presented at each testing time; only five subjects were present at all four reporting times over the year. There was no association between subjects’ location relative to the blast and their reported symptoms and test results, but 97% of the subjects screened or evaluated in the study
were in buildings at the time of the blast. Given that 43% of symptomatic subjects did not report head injury, the vestibular trauma reported may be related to blast overpressure. The study has limitations similar to those of Van Campen et al. (1999a) because of the very small sample and the variability of the followup of subjects (different subjects were evaluated and questioned at each quarter).
Cohen et al. (2002) reported on the auditory and vestibular outcomes of survivors of a terrorist bus bombing in 1994. Of the 48 survivors (18–65 years old; median, 28 years old), 23 were hospitalized; of the 23, 17 continued to be followed for 6 months in an outpatient otolaryngology clinic. Patients underwent otoneurologic examinations and auditory and balance assessments. Results showed that all patients except one had at least one perforated eardrum; there were 27 perforated eardrums. Most (16) of the perforations were considered large, and gradual healing was seen during the followup period. At 6 months, perforations in 15 ears had healed, and perforations in 12 had not healed (possibly because of the number of large perforations). Hearing loss was common; hearing in only one ear was normal immediately after the blast. At 6 months, 6 of the ears had regained normal hearing, for a total of 7 ears with normal hearing. On balance tests, 6 patients had abnormal results and complained of dizziness, and 5 continued to suffer from dizziness throughout the 6 months of followup. At the time of admission to the hospital after the blast, 88% of patients who were followed for 6 months reported aural fullness and pressure; 88%, tinnitus; and 41%, dizziness. At 6 months, 73% of the patients who had reported aural fullness still complained of it, 40% of those who had reported tinnitus still had symptoms of it, and 71% of those who had reported dizziness on admission still had it. The study has limitations for the committee’s determination of evidence of long-term auditory outcomes of blast, such as the small sample and the nonmilitary blast exposure. It is unknown whether the findings reported in the study are generalizable to those exposed to blast during military combat. The study also lacked a control group for comparison and did not report outcomes beyond 6 months.
In a retrospective cohort study, Riviere et al. (2008) examined the hearing status of workers who were exposed to an industrial explosion at a chemical plant in France on September 21, 2001. During October 2001– March 2004, all 511 workers underwent audiometric testing, whether or not they were at the site of the explosion. Of the 425 subjects who participated in the study, 208 (49%) had undergone an audiometric test since 1990 but before the explosion. Therefore, the study design made it possible to compare some subjects’ pre- and post-blast measures. Subjects were divided into two groups whose outcomes were compared: 103 who were within 1,700 m of the blast and considered “exposed” and 105 who were more than 1,700 m from the blast and therefore considered “less exposed.” The
average period between preinjury and postinjury audiograms of the exposed group was 1,621 days (SD = ±1,018 days) and for the less exposed group 1,831 days (SD = ±1,121 days). Results comparing pre-blast and post-blast audiograms demonstrated that—with adjustment for age, sex, history of ear problems, and occupational noise exposure—hearing-threshold shifts were greater at 2 and 4 kHz in the right ear in those at shorter distances from the blast. Results from the left ear were different; those at shorter distances had significantly worse threshold shifts at 2, 6, and 8 kHz. In addition, 68% of the exposed group and 46% of the less exposed group had hearing loss of 10 dB or more affecting at least one ear at 2, 4, or 6 kHz (p < 0.01). The study design made it possible to compare pre-blast audiometric measures between an exposed group and a less exposed group. However, the study did not control for confounding factors, such as other exposures that may cause hearing loss. And although some subjects had followup testing more than 2 years after injury, other subjects had followup testing as little as a month after the exposure, and their results are therefore short-term results. The data were not analyzed to examine changes in hearing loss over time after the explosion.
Vanderploeg et al. (2012) conducted a cross-sectional cohort study by using data collected in anonymous online surveys to determine whether there was an association between military experience and immediate and long-term physical and psychologic health outcomes. The study also aimed to examine the effects of multiple deployment-related TBIs on health outcomes. About 10,400 letters were mailed to members of the Florida National Guard to invite participation in the survey; 4,005 people (41.3%) completed the survey, and those who had been deployed completed it an average of 31.8 months (SD = 24.4 months, range = 0–95 months) after their deployment; 3,098 subjects (1,443 who had deployed and 1,655 who had not deployed) were included in the study. ORs were calculated to assess the association between current health status and deployment-related factors, such as physical injuries, exposure to potentially traumatic deployment experiences, combat, blast exposure, and mild TBI. Demographics and predeployment experiences were controlled for as potential confounders. Many questions were included on the survey to measure predictors of health outcomes, including blast exposure. Of the 3,098 subjects in the study sample, 24% reported having been exposed to blast, but 51% of those who had deployed reported having been so exposed. Results showed a statistically significant difference in reported hearing loss between deployed subjects and non-deployed subjects (29% vs 9%, p < 0.001) and a statistically significant difference in reported tinnitus (32% vs 10%, respectively, p < 0.001). Subjects who had been exposed to blast had higher odds of hearing loss (AOR = 1.63 [95% CI, 1.59–6.65] after nonprimary blast exposure and 2.32 [95% CI, 1.65–3.26] after primary blast exposure), tin-
nitus (AOR = 1.77 [95% CI, 1.29–2.41] for nonprimary blast exposure and 2.92 [95% CI, 2.09, 4.09] for primary blast exposure), and dizziness (AOR = 2.26 [95% CI, 1.30–3.94] primary blast exposure only). The limitations of the study include a low response rate (41%), which limits the generalizability of the results to all deployed or non-deployed service members, and lack of information about other possible exposures that may have caused injury during deployment or led to auditory problems. The study also relied on self-reported measures for all outcomes and did not include any objective measures or test results.
Wilk et al. (2012) conducted a survey of three infantry brigades 3–6 months after their return from deployment to determine the extent to which a screening for blast concussion identifies people who are at higher risk for persistent postconcussive symptoms. The survey included questions about physical symptoms (including questions about auditory and vestibular symptoms), postconcussive symptoms, and mental health conditions. Of the 7,668 service members in the three brigades, 4,383 (57%) consented and completed part of the survey, and 3,952 (52%) completed the concussion questions. Most who did not participate were on leave or unavailable because of duty assignments (more than 93% of service members who attended the briefings about the study agreed to participate). Results showed that 201 people had had a concussion with LOC, 161 of whom had had a blast-related concussion. Service members who had had a blast concussion reported more tinnitus than those who had had non-blast concussion (34% vs 15%, p = 0.02). However, there was no association between blast concussion and dizziness. A limitation of the study is the subjective quality of the data, which are based exclusively on self-report. In addition, the small number of service members reporting symptoms limits the power of statistical comparisons.
Supportive Studies
Supportive studies identified by the committee have important limitations. Many had small sample or selection biases and covered only short-term followup. And many of the studies used self-report measures and self-reports of exposure to blast, so they lack objective data. St. Onge et al. (2011) was the only secondary study that used pre-blast measures. The study aimed to understand auditory and vestibular outcomes in marines enrolled in the Breacher Training Course, which includes exposure to blast explosions. Pure-tone hearing thresholds were collected before and after the training of 38 marines. Results showed significantly worse thresholds after exposure at 1, 2, and 3 kHz. Generally, the study found that hearing loss was statistically and clinically significant, whereas vestibular findings were not significant. It used short-term followup and was not meant to
study the long-term auditory and vestibular effects of the Breacher Training Course, so it does not contribute to determination of long-term outcomes of exposure to blast.
In a retrospective chart review, Lew et al. (2011) examined 36,426 patient records in the DOD Defense Manpower Data Center to determine the prevalence of self-reported auditory, visual, and dual sensory impairment in Afghanistan and Iraq war veterans who received TBI evaluations. All 12,521 subjects included in the study had screened positive in an initial TBI evaluation and then had a comprehensive second-level TBI evaluation. As part of the second-level examination, measures of self-reported blast exposure and sensory symptoms, including hearing difficulty, were collected. The subjects were compared with a control group of 9,106 who had no reported TBI. For auditory impairment, there was a significant association between history of blast exposure and severity of sensory complaint [χ2 (3) = 198.20, p < 0.0001; Cramer’s V = 0.13]. Specifically, a higher percentage of blast-exposed TBI patients than of non-blast-exposed TBI patients reported moderate to very severe impairment. Although the study had an adequate sample size, data on blast exposure were based on self-reports. In addition, although the study is analytically sophisticated, it provides a conditional analysis that is based on those who have TBI and then models the contribution of blast to self-reported sensory impairment. For example, given the presence of TBI, what is the effect of blast exposure?
Additional Long-Term Effects of Blast Injury on Auditory Function
Hearing loss as measured by increased pure-tone thresholds is an immediate and long-term effect of blast exposure and is probably due to blast-related damage to the auditory periphery (middle-ear and inner-ear structures). The changes may present as complaints related to loss of hearing acuity (that is, the ability to hear low-level sounds). With the healing of middle-ear structures and recovery of cochlear function, hearing thresholds may return to pre-blast values. However, injuries due to blast exposure can also involve the central auditory nervous system and affect accurate suprathreshold processing of the spectral and temporal properties of sounds in three-dimensional space (Gallun et al., 2012a,b; Valiyaveettil et al., 2012). Such changes may present as complaints of difficulty in conversing or localizing sound in noisy environments even though sounds are clearly audible. Precise binaural encoding of sound patterns is required for understanding speech, appreciating music, recognizing environmental sounds, and localizing sound in complex environments that have background noise, reverberation, and multiple talkers originating in different sound sources. Impairments may persist even when post-blast hearing thresholds are normal or have returned to normal, as measured by conventional audiometry.
Although the study of central auditory dysfunction in maintaining normal communication and complex sound processing is advancing, the sites of injury and underlying mechanisms responsible for specific symptoms are not known. Therefore, standardized clinical test batteries to assess the functions are not available, and there is no consensus on appropriate treatment for the communication deficits. Moreover, there is only sparse evidence on auditory effects of multiple blast exposures, medication use, and comorbid conditions, such as mild TBI and other brain injuries, PTSD, multisensory impairments, and cognitive losses related to attention and memory.
Conclusions
Blast can injure the auditory system both acutely and over the long term. There is a consensus that blast can cause perforation of the tympanic membrane and disruption of the ossicular chain and result in conductive hearing loss, which may be permanent or resolve with treatment or the spontaneous closure of the TM. The evidence needed to determine the likelihood of long-term hearing loss through this mechanism is lacking. It is common to experience an immediate loss of hearing sensitivity after blast exposure, which may not be associated with TM rupture or middle-ear damage. That loss of hearing may reflect cochlear damage from the noise exposure and improve with time; again, the literature is not adequate to estimate the risk of long-term hearing loss. Blast exposure may also affect the auditory system through inflammation, effects on microcirculation, brain edema, ototoxic side effects of medications given for other injuries, and many other mechanisms, but there is no consensus on the long-term consequences. Similarly, with respect to recurrent blast exposure that leads to cumulative changes in structure at the microscopic level, there is no consensus on whether it has long-term consequences. It is possible that blast causes dysfunction in dimensions of auditory function beyond declines in hearing acuity, which may go undetected or unnoticed in the immediate period after exposure, and there is no consensus on whether this dysfunction has long-term consequences and how long changes in auditory processing abilities may persist after hearing thresholds return to pre-blast values. Finally, there are no data with which it can be determined whether blast-related injuries increase the risk of age-related changes in hearing acuity (presbycusis) or suprathreshold auditory processing or accelerate the aging process and result in early onset of these conditions.
The committee concludes, on the basis of its evaluation, that there is limited/suggestive evidence of an association between exposure to blast and long-term effects on the tympanic membrane and auditory thresholds.
The committee concludes, on the basis of its evaluation, that there is inadequate/insufficient evidence of an association between exposure to blast and tinnitus and long-term effects on central auditory processing.
The committee concludes, on the basis of its evaluation, that there is inadequate/insufficient evidence of an association between exposure to blast and long-term balance dysfunction and vertigo.
Exposure to blast can lead to severe eye injuries, often from debris that hits the eyes and leads to blunt or penetrating injuries. Signs of ocular blast injury include bleeding, preorbital swelling or bruising, 360-degree conjunctival hemorrhage, misshapen pupil, pigmented or clear gel-like tissue outside the globe, and an abnormal shape of the anterior chamber. Symptoms are wide-ranging and include minimal discomfort, foreign body sensation, severe pain, and decreased, altered, or total loss of vision. It is important to note that there can be serious damage to the eye in the absence of vision loss or serious signs and symptoms, especially with nonpenetrating injuries.
Acute and Well-Known Long-Term Effects
Serious open-globe injuries in the form of laceration and rupture occur in 20–50% of those who have blast eye injuries (Morley et al., 2010; Peral Gutierrez De Ceballos et al., 2005; Tucker and Lettin, 1975). Such injuries are predominantly secondary blast injuries from sharp propelled particles that cause penetration, perforation, and the implantation of intraocular foreign bodies. Choroidal rupture also is possible from blunt trauma or blast wave but is less likely (Alam et al., 2012). Closed-globe injuries to the eye can result in small corneal abrasions, conjunctivitis, and superficial foreign bodies; these outcomes typically would occur when debris makes contact with the surface of the eye in a secondary blast injury. However, more serious outcomes from closed globe injuries are possible too—mainly from blunt trauma and primary blast injury (PBI)—and include hyphema, vitreous hemorrhage, commotio retinae, retinal detachment, macular holes, traumatic cataract, optic nerve damage, and orbital fracture (Alam et al., 2012; Morley et al., 2010). Blast injuries, particularly open-globe injuries, may result in loss of an eye, permanent blindness, or vision impairment.
The visual system requires more than an intact eye free of important pathology for normal function, as the information encoded by the retina must be correctly and efficiently transmitted to the visual cortex for processing and integration. In addition to overt ocular injuries, damage to the visual system caused by blast may disrupt these neural networks. Near
vision (reading) problems, light sensitivity, accommodative insufficiency, and convergence insufficiency have been reported in blast-injured patients (Magone et al., 2013). Similar visual symptoms can occur following a mild TBI.
Additional Long-Term Effects
To evaluate the long-term ocular health effects of blast exposure, the committee reviewed about 75 published peer-reviewed studies. No available studies met enough of the inclusion guidelines to be considered primary. This section details the identified supportive studies on long-term ocular health outcomes of blast exposure.
Goodrich et al. (2007), in a retrospective descriptive study, conducted a record review of patients seen in the Optometry Polytrauma Inpatient Clinic (OPTIC) at the VA Palo Alto Health Care System during December 2004–November 2006. The study aimed to assess visual function in patients who experienced deployment-related polytrauma. Data examined in the study were derived from OPTIC’s extensive examination protocol, which includes self-report questions about vision status before and after the injury, clinical measurements of distance and near visual acuity, visual fields, binocular-vision status, and other vision measures. The study compared 25 who had blast-related injuries with 25 who had other than blast injuries and found a higher rate of visual injury in the blast-injured group (13 vs 5). Blasts resulted in penetrating head injuries in 11 cases and all other causes produced penetrating head injuries in four cases. All four people who had total blindness, clearly a long-term effect, had blast-related injuries. Although this study provided good vision-examination data in addition to self-reported vision data on blast-injured patients, for several reasons it has only limited usefulness for establishing evidence of long-term ocular outcomes of blast. First, it had a small selected sample that was biased in that the subjects were patients in a polytrauma center who stayed long enough to be examined (that is, had severe injuries), and all had TBI; therefore, the results may not be generalizable to the larger population of those who suffer minor injuries from exposure to blast or those who do not have TBI. In addition, there was no statistical analysis of the differences in visual problems between blast-injured and non-blast-injured groups, and analyses did not control for confounding. In a followup study, Goodrich et al. (2013) conducted statistical analysis of group differences in ocular outcomes between the two groups from the 2007 study (blast-related and non-blast-injured); the analysis found few significant differences in visual dysfunction between the two groups.
Brahm et al. (2009) conducted another retrospective study that analyzed data from electronic examination records of vision screenings completed in
the VA Palo Alto Health Care System. Screening results were examined for 68 consecutive patients (57 were blast-injured) who were evaluated in the VA Polytrauma Rehabilitation Center (PRC) during August 2006– December 2007 and 124 consecutive patients (112 were blast-injured) who were evaluated in the VA Polytrauma Network Site (PNS) clinic during August 2006–December 2007. The patients evaluated in the PRC were seen for inpatient care and had moderate to severe TBI. Those evaluated in the PNS clinic were seen on an outpatient basis, had no life-threatening injuries, and had screened positive for mild TBI. The frequency of ocular injury, visual impairment, and visual dysfunction was examined in the two groups, and differences between blast-injured and non-blast-injured were described. The results were inconsistent in the inpatient and outpatient groups with regard to visual dysfunction and blast injury. In the inpatient group, the blast-injured were more likely to have ocular injuries than the non-blast-injured; in the outpatient group, the blast-injured were less likely to have ocular injuries than the non-blast-injured. The rate of moderate visual-acuity loss was higher in the blast-injured patients than in the non-blast-injured in the inpatient group (20 of 70 and 20 of 100) and such loss was too rare in the outpatient group to support useful conclusions. This study has limitations similar to those of Goodrich et al. (2007) in having a biased sample of only polytrauma inpatients and outpatients and no control groups. In addition, there was no statistical analysis of between-group differences in blast-injured and non-blast-injured patients. Another limitation is the lack of objective measure of blast exposure, although the study did have reasonable measures of visual dysfunction. Because the study was retrospective and did not control for confounding variables, it is impossible to determine the associated risk of visual dysfunction due specifically to blast exposure.
Coe et al. (2010) conducted a prospective study that followed combat-injured service members seen in the ophthalmology service of WRAMC during September 2003–February 2005. All 11 patients in the study had eye injuries and retained corneal foreign bodies secondary to blast trauma. They were compared with age-matched and sex-matched uninjured controls. There were numerous inclusion and exclusion criteria (for example, no other penetrating injuries and no concurrent inflammation or infection), and patients were excluded for any physical or mental impairment that would prevent them from undergoing eye examinations or providing informed consent for the study. Followup time was 1–6 months. Results showed no statistically significant differences in visual acuity with high contrast, but visual performance of injured eyes was significantly worse than that of control eyes on photopic low-contrast visual acuity (p < 0.001), mesopic low-contrast visual acuity without glare (p < 0.001), mesopic low-contrast visual acuity with glare (p = 0.0007), and contrast sensitiv-
ity (p < 0.0001). The study has limitations; the small sample and sample selection bias in which extensive inclusion and exclusion criteria were used (those with complex eye injuries were excluded from the study sample).
The results may therefore not be generalizable to the larger population of blast-exposed people. In addition, the statistical analyses did not control for potential confounders, and the followup period was short.
In a retrospective chart review Lew et al. (2011) examined 36,426 patient records in the Defense Manpower Data Center to determine the prevalence of self-reported auditory, visual, and dual sensory impairment in Afghanistan and Iraq war veterans who were evaluated for TBI. All 12,521 subjects included in the study had screened positive in an initial TBI evaluation and then had a comprehensive second-level TBI evaluation. As part of the second-level examination, measures of self-reported blast exposure and sensory symptoms were collected, including vision problems (blurring and trouble in seeing) and hearing difficulty. The subjects were compared with a control group of 9,106 who had no reported TBI (although the number of TBI cases may have been underreported). In a multiple linear regression model for visual impairment, blast exposure accounted for 0.14% of the variance. Other factors that accounted for variance were age (1.1%), sex (0.5%), auditory impairment (9.3%), and TBI (0.69%). Although the study had an adequate sample size, it reported only limited data on blast exposure. In addition, although the study was analytically sophisticated, it provided a conditional analysis based on those who have TBI and on modeling of the contribution of blast to self-reported visual impairment (for example, given the presence of TBI, what is the effect of blast exposure?).
In a case control study, Scheibel et al. (2012) examined service members and veterans to compare functional outcomes between those who had TBI and blast exposure and in those who did not. The study included 15 subjects who screened positive for TBI (all with at least one blast exposure) and 15 controls who had been similarly deployed but with no blast exposure or TBI. Results of fMRI demonstrated that the TBI group had increased activation in areas involved in visual perception, attention, and visuo-spatial functions (anterior cingulate gyrus, medial frontal cortex, and posterior cerebral areas). The study also attempted to evaluate other differences between cases and controls and reported that there were no major differences in self-reported alcohol or other drug use, psychiatric symptoms, or medication use. The study has limitations due to the biased sample of cases, 13 of which came from a TBI clinic at one VA medical center. In addition, the control group was not matched for combat exposure. With respect to understanding visual outcomes of blast exposure, the study is limited in that no direct measures of vision were used; instead, the study examined brain imaging results to quantify activation differences in visual perception between the two groups.
Capo-Aponte et al. (2012) examined the occurrence of visual dysfunction in 20 subjects who received care at WRAMC and had suffered blast-induced mild TBI within the preceding 45 days (median time between blast exposure and evaluation, 30.5 days; range, 16–45 days). A control group of 20 age-matched subjects was recruited and evaluated at the US Army Aeromedical Research Lab in Rucker, Alabama. The controls had been deployed but had no history of TBI, head concussion, or blast exposure. Results showed no statistical differences in manifest refraction and intraocular pressure between the two groups. Oculomotor function tests demonstrated that the mild-TBI group had more defective eye movements than the control group, but there was no significant difference in fixation disparity and flat fusion. The mild-TBI group had worse reading speed (p = 0.0019) and reading comprehension scores (p = 0.0106) and more reports of light sensitivity, eye strain, and reading-associated symptoms. The study has limitations due to the small sample size, and the sample may also have been biased in that case subjects were selected from a single medical center. In addition, the controls were healthy subjects whereas the cases were receiving treatment in a medical center, so between-group differences associated with blast may be overestimated. For the purposes of the committee’s review of possible long-term ocular outcomes of blast exposure, the study is limited in its usefulness owing to the short-term followup and the lack of data on longer-term outcomes. The study did document clinician records of symptoms experienced by patients.
The committee determined that 40 additional studies met the guidelines for inclusion in the committee’s review as tertiary but had little usefulness in demonstrating evidence on the long-term ocular health effects of blast exposure. The two studies that were the most useful for the committee’s review are discussed below.
Cockerham et al. (2013) conducted a prospective observational cohort study of patients who had TBI and were receiving care at a PRC from 2010 to 2012. Blast exposure was the mechanism of injury of 44 of the 53 subjects. The subjects were compared with an age-matched and sex-matched control group of 18 healthy subjects. The study provided long-term followup of some of the patients in the Goodrich et al. (2007) study described above. The reported time since injury ranged from 1 to 60 months, with a median of 6 months. Results showed that the TBI group had significantly more dry-eye disease symptoms than the control group. With respect to objective measures, 93% of the TBI group had at least one test result indicative of dry-eye disease compared with 44% of the control group (χ2 = 19.56; p < 0.001). Prevalence of abnormal dry-eye test results was generally similar in the subjects who had TBI caused by blast and those who had TBI not caused by blast. However, for the measure of tear-film breakup time, none of the non-blast TBI subjects and 33% of the blast TBI subjects
had values below 10 seconds. That difference was just short of statistical significance (χ2 = 3.2; p = 0.07). The study also found no relationship between use of particular medications (presumed to have an effect on eye function) and dry-eye disease measures. The study has limitations owing to its small sample and typical selection bias in that the TBI cases were all from one polytrauma center and probably severely injured, whereas the controls were healthy. That bias may have led to larger observed differences between cases and controls. In addition, the study authors do not specify whether blast exposure was controlled for but noted that the controls did not have TBI.
In a retrospective consecutive-case series, Blanch et al. (2011) reported injury patterns and long-term outcomes of eye injuries sustained from July 2004 through May 2008 by British armed forces personnel who were deployed to Iraq and Afghanistan. Mean followup of those who had closed-globe injuries was 245 days, and that of those who had open-globe injuries was 220 days. The assessed ocular outcomes reported in the study include final best-corrected visual acuity, rates of endophthalmitis, and proliferative vitreoretinopathy. Of the 630 people who had major traumatic injury, 63 (10%) suffered ocular injuries. Of 34 eyes with initial visual acuity ≤6/60, 18 had final visual acuity <6/60. A total of 17 eyes had a final visual acuity of 1/60 or worse, of which 6 were in three patients (bilateral). The causes were rupture or extensive disruption (nine eyes), corneal scar (two eyes), proliferative vitreoretinopathy (two eyes), traumatic optic neuropathy (one eye), subfoveal choroidal rupture (one eye), intraocular foreign-body-impacted fovea (one eye), and retinal burns from hot intraocular foreign body (one eye). Of the 56 subjects not recorded as wearing eye protection, 11 had poor visual outcomes (they could not see hand movements or worse) compared with one of the seven who wore eye protection. Although the study did not compare ocular outcomes by mechanism of injury, explosive blast injuries occurred in 54 of the 63 (86%). The study has limitations for the committee’s review of long-term ocular outcomes of blast, mainly in the case-series study design. Without a control group and without comparison of blast with non-blast injuries, it is unknown what amount and type of ocular injury is attributable specifically to blast.
Conclusions
Blast can cause both acute and long-term serious ocular injuries. Permanent ocular symptoms and structural alterations can occur in isolation or in conjunction with TBI. There is a consensus that blast can cause structural alterations that lead to long-term effects on ocular function. The structures most commonly associated with substantial visual loss are the cornea, the retina, and the optic nerve. The injuries typically need to
be bilateral to affect long-term afferent function (that is, ability to read or drive). In contrast, damage to the efferent system by definition creates a bilateral issue of dysmotility, including issues of fixation stability, diplopia, vergence, and fusion.
There is no consensus as to whether blast exposure that causes no visible change in structure but does cause symptoms has long-term consequences. The most common symptoms reported are photophobia, eyestrain, and headache.
The long-term implications of blast injury when there are known objective measures of subclinical injury are poorly understood. The implications include tear production and stability, endothelial cell count, ganglion cell loss, and fMRI alterations in the regions of the brain involved in visual function.
Finally, there are no data to determine whether blast-related ocular injuries increase the risk of such age-related conditions as cataract or macular degeneration or of early onset of presbyopia, loss of contrast sensitivity, or other visual abnormalities.
The committee concludes, on the basis of its evaluation, that there is sufficient evidence of a causal relationship between penetrating eye injuries resulting from exposure to blast and permanent blindness and visual impairment (visual acuity of 20/40 or worse).
The committee concludes, on the basis of its evaluation, that there is inadequate/insufficient evidence of an association between exposure to blast that leads to acute nonpenetrating eye injuries and long-term effects on vision.
For the purposes of this report, cardiovascular outcomes are defined as myocardial conditions, all vascular conditions, and cardiovascular death.
Acute Effects
Cardiovascular injury in blast victims can occur through many mechanisms and usually has the potential for severe and even fatal outcomes even if treatment is timely. The most severe outcome, cardiorespiratory arrest, occurs in 1–4% of all trauma patients (Tarmey et al., 2011). Blast injury to the cardiovascular system can be caused by the blast wave, penetrating projectiles, and blunt trauma (primary, secondary, tertiary, and quaternary blast injury). It can occur from direct damage to the thorax or appendages,
it can result from blast injuries to other organ systems, and blast injury to any part of the body can lead to vascular damage.
The main mechanisms of direct vascular injury caused by a blast are avulsion, perforation, and laceration of major vessels in extremities and the neck, mostly due to secondary—but also tertiary and quaternary—blast injury (Fasol et al., 1989). The two immediate outcomes of these injuries are exsanguination and ischemia. Loss of pulse occurs rapidly with exsanguination, and victims will have a poor prognosis, often death (Tarmey et al., 2011). Ischemia will result in tissue death if blood supply to deprived areas is not restored quickly, and amputation will need to be performed (Barros D’Sa et al., 1980; Fasol et al., 1989). Vascular injuries secondary to blast injuries may not be readily apparent at the time of the initial blast. The recognition that those vascular lesions can be occult has clear implications for long-term outcomes because they would be expected to be severe if vascular lesions are not immediately identified.
Direct cardiac injury to the thorax can be primary, secondary, tertiary, and quaternary blast injury and can cause myocardial contusions, arterial air emboli, valvular and cardiac-chamber rupture, pericardial injury and tamponade, conduction abnormalities, and cardiac rhythm irregularities (arrythmias) (Mayorga, 1997; Ozer et al., 2009). The more severe of those conditions, such as cardiac-chamber damage, result from the rare occurrence of tears or lacerations of the myocardium after high blast overpressure (Mayorga, 1997).
Blast trauma directly or indirectly affecting the cardiovascular system commonly causes arrhythmias. The arrythmias include bradycardia, which can eventually progress to asystole (Mayorga, 1997; Ritenour and Baskin, 2008), and various tachycardias, including such fatal forms as ventricular fibrillation. That blast trauma may induce abnormalities and dysfunction in the autonomic nervous system has potential long-term implications for cardiovascular health. Blast victims may have other hemodynamic effects that change blood concentrations and function. Acute coagulopathy, an impairment of the blood’s ability to clot, often arises with traumatic injury. Simmons et al. (2011) showed that patients injured by explosions were more coagulopathic than those who suffered gunshot wounds. Cervical dissection and carotid–cavernous fistulas associated with blast-induced craniofacial trauma and TBI have been reported (Vadivelu et al., 2010). These types of injuries may be subject to delayed presentation and detection. Diastolic hypertension was reported in persons hospitalized after an explosion in Texas in 1947 (Ruskin et al., 1948); in most cases, the patients’ blood pressure returned to normal within a few months of the explosion (Ruskin and Beard, 1948).
Additional cardiovascular effects may develop quickly from blast injuries to other organ systems. For example, air emboli that develop from
damaged lungs at alveolar–pulmonary venous fistulae can cause myocardial ischemia or infarction and are thought to pose the most immediate threat to life in blast victims (Mayorga, 1997; Phillips, 1986). Another example is the release of potentially toxic muscle cell components and electrolytes into the bloodstream that can occur with a crush injury (CDC, 2010).
Long-Term Effects
The committee considered 31 relevant studies to determine long-term cardiovascular effects of blast exposure. None of them met the committee’s inclusion guidelines for primary studies. None reported on actual long-term (longer than 6 months) cardiovascular health effects of blast, so the committee had sparse information on which to base conclusions. This section summarizes the small number of supportive studies, which may begin to inform understanding of possible long-term cardiovascular health outcomes of blast exposure.
The study of most usefulness for the committee’s review of long-term cardiovascular effects was Johnson et al. (2007b), a case-series study designed to analyze the sensitivity and specificity of physical examinations to detect vascular lesions after combat-related extremity wounds. The study found that physical examination was not a reliable means of excluding occult or delayed posttraumatic arterial lesions. Compared with arteriography, physical examination had a positive predictive value of about 85% and a negative predictive value of only 50%. For proximal injuries, physical examination had worse reliability, with a positive predictive value of 15% and a negative predictive value of 60%. However, the study has major limitations in its usefulness for the committee’s review of long-term cardiovascular outcomes of blast exposure. Of the 99 patients included in the study, although 82% had primary blast- or explosion-related injuries, there was no comparison of blast-exposed with non-blast-exposed patients. In addition, possible delayed effects of the combat-related extremity wounds were evaluated 2–14 days after injury, so the usefulness of this study in understanding long-term cardiovascular outcomes is limited.
No other studies provided the committee with evidence of possible long-term cardiovascular health outcomes of blast, but some supportive studies helped to inform the committee’s assessment. Tarmey et al. (2011) was a case-series study of 52 patients who had traumatic cardiopulmonary arrest and were admitted to a military trauma center in Afghanistan; the principal mechanism of injury was IEDs. Lower limbs were the most common sites of injury, and exsanguination was the most common cause of arrest in the patients. The study tracked the patients through hospital discharge; of the 52 included in the study, only four survived to discharge. The study reported that all four had good neurologic recovery, but long-term
cardiovascular outcomes after arrest were not reported. The study is limited in its usefulness for the committee’s review because there was no long-term followup of the survivors. In addition, there was no control group, nor was a specific population defined as blast-injured. The study is informative as to the likelihood of survival (poor but comparable with rates in other studies) of patients who had traumatic cardiopulmonary arrest and were victims of IEDs. Hilz et al. (2011) reported on long-term (5- to 43-month) cardiovascular outcomes after mild TBI and found that mild-TBI patients had less cardiovagal modulation and baroreflex sensitivity while supine than did a health control group. On standing, the mild-TBI patients still had reduced baroreflex sensitivity and did not withdraw parasympathetic or augment sympathetic modulation adequately. Although this study suggests that impaired autonomic modulation probably contributes to cardiovascular irregularities after mild TBI, the mechanism of the initial TBI was not reported, so the study does not address whether these effects would be seen in a population exposed specifically to blast.
The association between cardiovascular disease and war-related amputations has been recognized since World War II (Hrubec and Ryder, 1980). In a study of 3,890 World War II survivors who had proximal limb amputations and were followed for more than 30 years, the relative risks of total and cardiovascular mortality were 2.4 and 4.0, respectively. In those who had distal amputations, the relative risks of total and cardiovascular mortality were 1.44 and 1.45. Mechanisms by which amputation may lead to future cardiovascular disease include lack of physical activity, which leads to weight gain and obesity, and increased coagulability.
Conclusions
The committee identified no literature that assessed direct long-term effects of blast injuries on cardiovascular health. Long-term cardiovascular outcomes of blast exposure have not been studied specifically, but the committee deduced that long-term effects may be likely in patients who have severe traumatic limb injuries. The committee found that in patients who had severe traumatic limb injuries, there was a high proportion of occult vascular lesions, including arteriovenous fistulas and aneurysms. Some of those clinically silent lesions require interventions. On the basis of prior knowledge, these lesions can be said to have long-term health consequences for the limb and generally for the vascular system.
The committee concludes, on the basis of its evaluation, that there is limited/suggestive evidence of an association between major limb injuries, including amputations, resulting from exposure to blast and long-term outcomes for the affected limb and for the cardiac system.
The committee concludes, on the basis of its evaluation, that there is inadequate/insufficient evidence of an association between exposure to blast and long-term effects on cardiovascular function, such as accelerated atherosclerosis.
Exposure to blast can affect both the upper respiratory tract (the nose, the nasal cavity, paranasal sinuses, the pharynx, and the larynx) and the lower respiratory tract (the trachea, the bronchi, the bronchioles, and the alveoli). The discussion that follows focuses on the lower respiratory tract (the lungs). Other parts of the respiratory tract are susceptible to burns that result from blast; such burns are discussed later in this chapter.
Acute Effects
Blast lung injury (BLI) is the second most frequent injury in blast survivors and the most common fatal primary blast injury in initial survivors of an explosion (Finlay et al., 2012; IOM, 2009). BLI occurs when a blast wave affects the chest cavity and creates overpressure that causes alveolar contusion, tearing, and stripping of the airway epithelium (Avidan et al., 2005; CDC, 2012; Cooper et al., 1983). Contusion of the lung, in the absence of other complications, is the less severe of the acute outcomes and usually develops and resolves within a week of the exposure. Tearing of the alveolar tissue and airway epithelial damage cause hemorrhage, edema, and fistula and can lead to more serious conditions, such as hemothorax, pneumothorax, and air emboli (Argyros, 1997; Avidan et al., 2005; CDC, 2012; Cooper et al., 1983). Patients who have BLI typically present with dyspnea, chest pain, trouble in breathing, coughing, hypoxia, and respiratory following a characteristic latent period that can extend up to 48 hours after the explosion.
There are several less common acute effects of blast exposure on the lungs. Secondary, tertiary, and quaternary blast injuries to the chest—propelled shrapnel, body displacement into a fixed object, and structural collapse, respectively—can cause blunt trauma and, less commonly, penetrating damage (Caseby and Porter, 1976). Blunt trauma to the chest is pathologically similar to BLI; pulmonary contusion is the most prominent outcome. The defining difference between BLI and blunt trauma to the lung is that the latter has a much lower likelihood and severity of alveolar tear and fistula (Caseby and Porter, 1976). Burn injuries to the upper respiratory tract are not uncommon in the Iraq and Afghanistan wars and may occur from inhalation of hot air in connection with blast exposure (Eckert et al., 2006). Thermal injuries to the respiratory system are classified as
quaternary blast injuries and can have unpredictable outcomes, including permanent fibrosis of the bronchial mucosa (Krzywiecki et al., 2007).
Beyond the traditional enumeration of blast injuries, blast lung and other severe blast injuries necessitate critical care. Patients might be presumed to be at risk for the common complications of critical illness, including ventilator-associated pneumonia, line infections, and weakness acquired in the setting of an intensive care unit (ICU). Complications also can include cognitive and psychologic disorders (Desai et al., 2011; Needham et al., 2012; Schweickert and Hall, 2007). Those problems have been shown to lead to substantial new disability and cognitive impairments in patients who have severe sepsis and general critical illness (Cuthbertson et al., 2010; Herridge et al., 2011; Iwashyna et al., 2010)—sometimes termed post-intensive-care syndrome (PICS) (Needham et al., 2012)—but have not themselves been studied in survivors of BLI.
Long-Term Effects
To evaluate the long-term respiratory health effects of blast exposure, the committee reviewed about 45 published peer-reviewed studies. No available studies met enough of the inclusion guidelines to be considered primary. This section details the identified supportive studies on long-term respiratory health outcomes of blast exposure.
Two secondary studies examined the long-term respiratory outcomes of acute BLI. In a case-series study, Hirshberg et al. (1999) examined long-term lung function in 11 people who survived acute BLI after terrorist bus attacks in Jerusalem in 1996. Ten of the 11 required mechanical ventilation and ICU support. One year after injury, lung-function tests were completed on all 11 survivors; results were normal in 9, and none of the 11 had pulmonary complaints. Those findings of a small study suggest that patients follow a trajectory of complete recovery with regard to lung function; other nonpulmonary sequelae of their blast injury were not reported. The study had limitations due to the small number of subjects. In a retrospective case series, Avidan et al. (2005) looked at the long-term respiratory health outcomes in victims of terrorist bombing attacks who sustained acute BLI and survived an ICU admission during December 1983–February 2004 in a single hospital in Israel. Of the 29 patients eligible for inclusion in the study, 76% required mechanical ventilation. Of the 29, 28 survived to the time of the followup interview, 21 (75%) of whom responded to the interview and reported on their long-term outcomes. Of the 21, 16 (76%) reported no respiratory handicap, required no respiratory therapy, and were free of symptoms; the remaining 5 (24%) reported respiratory symptoms and some degree of respiratory dysfunction, but two of them had a history of asthma dating to before the blast exposure. The study showed no consistent
pattern of association between blast exposure and long-term respiratory health outcomes and lacks the clinical granularity needed to evaluate the likely trajectories of participants. Like the Hirshberg et al. (1999) study, this one was limited by its small sample and lack of a control group. The followup period was longer (6 months to 21 years), but it was limited by variable followup and the use of self-reports of symptoms gathered through telephone interviews.
Two other studies provided further information on possible long-term respiratory outcomes of a blast explosion. In a retrospective cohort study, Krzywiecki et al. (2007) reported on miners who were exposed to a methane explosion and sustained thermal injury. The study included a control group of healthy miners who had a similar period of work underground and were not exposed to the methane explosion. Long-term lung function was measured at baseline (5–7 months after the explosion) and then 6 years later. At baseline, there was no significant difference in mean pulmonary function tests (PFTs) between the exposed miners and the control group. After 6 years, PFTs showed a significantly lower (p < 0.01) forced expiratory volume in 1 second in exposed miners than in the control group. Mean absolute decreases in functional tests did not differ significantly between the exposed and control groups, except that diffusing capacity decreased more in the exposed miners and the maximal expiratory flow at 50% of vital flow capacity decreased more in the control group. The study is limited in that the exposure was not only to blast but to heat and carbon monoxide. Furthermore, the study did not adjust for different rates of smoking and different age groups between the exposed and control groups.
The final secondary study examined long-term pulmonary function after severe blunt chest trauma (Kishikawa et al., 1991). It was a case-series study that examined two groups of patients prospectively. One group had followup 6 months after injury, and some had pulmonary contusion. The other group had followup 1–4 years after injury, and all had an initial diagnosis of pulmonary contusion. Generally, patients in each group who had a diagnosis of pulmonary contusion performed worse in PFTs. The study had limited usefulness for the committee’s review because the mechanism of injury was blunt chest trauma, not specifically blast exposure. In addition, the study had a small number of subjects and no matched control group. However, the study is informative inasmuch as pulmonary contusion is a possible acute outcome of blast exposure, and it showed a difference in lung function over the long term between those who had pulmonary contusions and those who did not.
The committee determined that three additional studies met the guidelines for inclusion in its review as tertiary but had limited usefulness in presenting evidence on the long-term respiratory health effects of blast exposure. Kushelevsky (1949) reported on 25 patients who developed
emphysema and bronchial asthma after blast exposures in World War II, but the cause of injury was not solely blast but dust and inhalation. That case series is reminiscent of possible health effects in people who were near the World Trade Center collapse in 2001; the effects of exposure to the dust generated in the collapse have been studied but have not been followed up in the context of blast exposure (Aldrich et al., 2010). Leone et al. (2008) reported on long-term outcomes in 91 survivors of multiple trauma with chest trauma and pulmonary contusions. Followup after 6 months and 1 year showed reduced measures of lung function, but the mechanism of injury was not blast exposure (in 80%, it was motor vehicle collisions), and the study was unable to differentiate between outcomes of chest trauma and outcomes of other injuries. Finally, Svennevig et al. (1989) looked at long-term outcomes in 24 patients who had closed-chest injuries and were treated a mean of 4.9 years (range 2–9 years) previously; there was no clear documentation of inclusion criteria or mechanism of case-series selection, and the mechanism of injury was not discussed, so the results that showed 63% with some pulmonary complaint at followup are not very useful for the present review.
There is a further literature, not specific to blast, that suggests that respiratory complaints in general, and the specific pathologic diagnosis of constrictive bronchiolitis in particular, are more common among those who have returned from Iraq and Afghanistan (Coker et al., 1999; King et al., 2011; Smith et al., 2009). Further, blast might be an important mediator of dust exposure. For example, a common, significant, and persistent decline in pulmonary function has been found to be associated with dust exposure in firefighters at the World Trade Center site (Aldrich et al., 2010). However, the literature review did not find compelling studies examining a specific role of blast in the development of these complaints, either directly or via dust exposure.
Conclusions
Acute injuries to the respiratory tract are quite common after blasts. They range from burns in the upper airway to a classic syndrome of acute lung injury known as BLI. BLI is associated with high mortality and may require intensive care, including invasive mechanical ventilation. Blast exposure also can lead to serious acute whole-body inflammation (a risk factor for acute respiratory distress syndrome), and convalescent care for this puts patients at risk for the entire spectrum of PICS in the short and long term.
Despite the obvious acute injuries and the high plausibility of long-term sequelae, there is a striking absence of data on the long-term pulmonary outcomes of exposure to blast. That is true both of all who have been
exposed to blast and of the subset of patients who develop acute BLI that requires care. Furthermore, the possibility of other long-term pulmonary consequences of blast exposure, such as the effect of explosion-related dust exposure, and of other exposures, such as smoking, has not been adequately examined.
The available studies are of insufficient quality, validity, consistency, or statistical power to support a conclusion regarding an association between exposure to blast and long-term respiratory outcomes in humans.
The committee concludes, on the basis of its evaluation, that there is inadequate/insufficient evidence of an association between exposure to blast and long-term effects on pulmonary function, respiratory symptoms, and exercise limitation.
The committee concludes, on the basis of its evaluation, that there is inadequate/insufficient evidence of long-term effects after acute blast lung injury.
Primary blast injuries of the abdomen can cause severe damage to internal organs, typically in the absence of visible external signs of injury (Scekic et al., 1991). A recent review by Owers et al. (2011) examined the published literature on abdominal primary blast injury (PBI). According to the reviewed literature, the estimated overall incidence of abdominal PBI in hospitalized survivors of open-air blast explosions was about 3%; rates were marginally higher in those exposed to enclosed-space explosions. The incidence of abdominal PBI is generally much lower than that of PBI to the auditory and pulmonary systems (Owers et al., 2011); however, abdominal injuries may be more predominant in underwater blast explosions (Huller and Bazini, 1970). Laceration of solid organs (including abdominal organs) is rare and is associated with very high blast forces or proximity of the person to the explosion (DePalma et al., 2005) or with tertiary injury (Leibovici et al., 1996). More common, however, is PBI to gas-containing organs (Phillips and Richmond, 1991). Damage to those organs from PBI includes hemorrhage and perforations—particularly of the bowel—mesenteric shearing and hematoma, and ruptures of the hollow abdominal viscera (CDC, 2008a; Wani et al., 2009).
Acute Effects
Signs and symptoms of blast injury to the abdomen can be readily apparent or nuanced and variable. Symptoms of gastrointestinal PBI
include abdominal pain, nausea, testicular pain, tenesmus, and temporary loss of motor control in legs (CDC, 2008a; Phillips and Richmond, 1991). Signs of gastrointestinal PBI are similar to those of blunt trauma and may include abdominal and rebound tenderness, vomiting (hematemesis in rare occasions), voluntary and involuntary guarding, absence of or decrease in bowel sounds, rectal bleeding, and unexplained hypovolemia (Phillips and Richmond, 1991; Wani et al., 2009).
There is a degree of variability in the latency of the effects of PBI to the gastrointestinal tract, ranging from immediate onset to several years (Owers et al., 2011). A typical acute gastrointestinal mural hematoma can have delayed perforation up to 14 days after exposure (Owers et al., 2011). Late perforations usually occur within 2 weeks of the PBI (Scekic et al., 1991). Patients who have minor abdominal complaints may temporarily improve but then develop an abdominal crisis several weeks later (Phillips and Richmond, 1991).
Long-Term Effects
To evaluate the long-term gastrointestinal health effects of blast exposure, the committee reviewed about 75 peer-reviewed studies. None met enough of the inclusion guidelines to be considered primary. This section details supportive studies of long-term gastrointestinal health outcomes of blast exposure.
The study that met the most inclusion guidelines was a retrospective chart review by Sayer et al. (2008) of patients admitted to four PRCs during 2001–2006. It evaluated a sample of 188 blast-exposed and non-blast-exposed veterans who sustained injuries in the Iraq and Afghanistan wars. Of the 188 patients, 56% had blast-related injuries. The measures used included scores on functional tests (functional independence measure within 72 hours of admission and at discharge) and length of stay. The study looked at a variety of health outcomes (not only gastrointestinal) and sought to quantify differences in outcomes between blast-exposed and non-blast-exposed patients. The results showed no significant difference in gastrointestinal injuries or gastrointestinal impairment between the two groups. However, the study has limitations for understanding possible long-term gastrointestinal effects in that the median length of stay (observed time frame) was only 29 days. In addition, the study did not look at specific gastrointestinal outcomes but instead aggregated all gastrointestinal injuries and impairments.
A study by Yzermans et al. (2005), reported on health outcomes of victims affected by a fireworks depot explosion in the Netherlands in which 22 people died immediately and more than 1,000 were injured. This retrospective longitudinal study aimed to quantify health problems
(psychologic, medically unexplained, and gastrointestinal symptoms) and predictors of health problems. Electronic medical records from general practitioners’ offices were examined to gather pre-disaster baseline morbidity (for the 16 months before the explosion) and post-disaster data for 2.5 years. The study was able to track 89% (9,329) of people exposed to the explosion and established a control group 7,392 matched for age and sex. The exposed group was also divided into two groups—those who had to relocate after the disaster and those who did not—to test the hypothesis that relocated disaster victims would have more health complaints than nonrelocated victims. The study showed a small increase in gastrointestinal morbidity in both groups compared with pre-disaster rates and control-group rates. Gastrointestinal complaints (for example, nausea and abdominal pain) were more prevalent in relocated victims after the disaster and throughout the whole period than in the control group. The study has several limitations for the purposes of the committee’s charge to identify long-term health outcomes of blast exposure. First, the study did not gather data on exposure specific to the blast, so it is unclear what types of exposure and acute injuries the victims sustained and what mechanisms caused the injuries. Second, it reported general gastrointestinal complaints as an outcome, and this does not provide specific information about possible specific long-term gastrointestinal conditions that may result from the exposure. Third, although the study looked at socioeconomic status as a predictor of general gastrointestinal complaints, the controls were not matched for socioeconomic status.
A retrospective study by Ramasamy et al. (2012) met the guidelines only for a tertiary study but is worth mentioning because it provided long-term data on military personnel who were exposed to blast injuries. The study used a prospectively compiled combat-trauma registry of patients who sustained open pelvic blast injury during military service in Afghanistan. Of the 89 service personnel who had open pelvic fracture due to an explosion, 29 (33%) survived; 19 (66%) of the survivors had abdominal injuries. One-third of the patients had fecal diversion with a colostomy; all had either anal-sphincter disruption or severe bowel injury. The mean followup period was 20.3 months (range, 13.2–29.9). At final followup, seven patients had some continuing fecal incontinence.
Conclusions
Blast can cause substantial injury to abdominal organs—including the gastrointestinal tract, liver, and spleen—both acutely and over the long term. There is a consensus that blast can cause hematomas and perforations of the gastrointestinal tract and lacerations of the liver and spleen. Delayed perforations can occur 1–14 days after blast injury; the most frequent
period is 3–5 days. On the basis of the available literature, the committee determined that the long-term gastrointestinal effects of blast exposure have not been well studied. No studies met the committee’s guidelines to be considered primary, and few studies provided any support in determining long-term gastrointestinal health outcomes. One of the two studies that the committee could identify as secondary found a small increase in gastrointestinal morbidity during the 2.5 years after blast exposure. However, the studies presented little detail on specific gastrointestinal injury and illness. Fecal incontinence is a long-term effect in people who have blast-associated severe bowel injury and anal-sphincter disruption. It is plausible that severe acute gastrointestinal injury can have long-term complications. For example, it is well known clinically that people who have had gastrointestinal surgery can develop bowel obstructions and that perforation of the intestine can have late complications that require an ileostomy or colostomy. In addition, it is possible that blast may cause dysfunction in the gastrointestinal system and brain–gut axis (the relationship between digestive health and mental health) that may go undetected in the immediate acute period or develop in the long term and become manifested in chronic symptoms, including abdominal pain and abnormal bowel habits.
The committee concludes, on the basis of its evaluation, that there is limited/suggestive evidence of an association between exposure to blast and acute gastrointestinal perforations and hemorrhages, and solid-organ laceration, all of which can have long-term consequences.
The committee concludes, on the basis of its evaluation, that there is inadequate/insufficient evidence of an association between exposure to blast in the absence of a serious acute injury and long-term gastrointestinal outcomes.
Genitourinary (GU) injury includes injury to the upper urinary tract (kidneys and ureters), the lower urinary tract (bladder and urethra), and the external genitalia (penis, scrotum, and testicles). The literature on GU blast injuries almost exclusively describes injury to males. GU injury to female service members has not been well described in the literature and so was not a focus of this report.
Acute Effects
The committee identified several recent studies of acute GU injury that resulted from blast exposure in combat in the Iraq and Afghanistan wars.
A retrospective review by Serkin et al. (2010) found that of the 16,323 US service member trauma admissions logged into the JTTR in October 2001–January 2008, 819 (5%) involved one or more GU injuries. Explosions or blast caused 65.3% of these injuries. The scrotum was the GU organ most commonly injured, and 82.8% of scrotal injuries were caused by explosion. A smaller review of GU trauma at a combat support hospital in Iraq from April 2005 through February 2006 looked at 2,712 admissions, and 76 (2.8%) were for GU injuries, 50% of which were caused by a blast or explosive ordinance (Paquette, 2007). A retrospective review by Fleming et al. (2012) looked at trauma patients treated at the National Naval Medical Center (NNMC) who had sustained injuries in the Iraq and Afghanistan wars during September 2007–December 2010. This review focused on patients who had major-extremity amputations and examined the number and type of associated injuries. Most of the patients included in the study had blast injuries from IEDs; the injuries of 62 of the 63 patients who had multiple extremity amputations and of 37 of the 46 who had single extremity amputations were caused by blast. The authors found that multiple extremity amputations correlated with GU injuries, which included injuries to the external genitalia, the bladder, and the urethra. The 63 patients who had multiple extremity amputations had a total of 71 GU injuries (specific types of injuries were not stated, and several patients had multiple GU injuries), whereas the 46 patients who had single extremity amputations had 29 GU injuries (specific types of injuries were not stated). Another recent paper examined the UK JTTR for the Iraq and Afghanistan wars from 2003 to 2010. Of the 2,204 registered patients, 85 (3.9%) had GU injuries; the percentage who sustained GU injuries from blast was not stated (Mossadegh et al., 2012).
Andersen et al. (2012) noted that since additional troops were deployed to Afghanistan in 2010, there has been an increase in the incidence of GU trauma in US service members. The increase presumably is due in part to the nature of combat operations in Afghanistan, where terrain necessitates more patrolling on foot by dismounted troops, who are at particular risk for being injured by blast explosions from IEDs without the protection of an armored vehicle. The authors also discuss the effects of better surgical and trauma care on the battlefield, which has resulted in a greater number of survivors who have complex blast injuries, including multiple-extremity amputation patients. In prior wars, casualties with such extensive injuries more frequently failed to survive to evacuation out of theater.
A similar article by Mossadegh et al. (2012) that looked at the British experience with troops deployed to Afghanistan and Iraq from 2003 to 2010 comments on blast injuries in these conflicts as more likely to cause higher, transfemoral amputations than in other conflicts (for example, Bosnia-Herzogovena), in which explosive devices were more likely to be
land mines and more likely to cause below-knee amputations. IED blasts were also more likely to cause perineal injuries and pelvic fractures. Of the 2,204 patients registered in the UK JTTR, 118 had perineal injuries, and 85 of these patients (72%) had GU injuries. Of the 118 patients who had perineal injuries, 56 (47%) eventually died of their wounds. The combination of a perineal wound and a pelvic fracture resulted in the highest mortality (73%) in these 118 patients. A pelvic fracture alone was associated with mortality of 41% and a perineal injury with mortality of 18%. Eight of the 85 patients who had GU injuries had complete testicular loss.
Long-Term Effects
The committee reviewed about 40 peer-reviewed studies of GU health effects of blast exposure. None of the studies met the committee’s inclusion guidelines for primary studies; supportive studies are discussed below. However, the committee determined that there are expected long-term GU effects on the basis of the nature of acute injuries that have been documented. As discussed above, recent studies of combat-trauma casualties have shown that acute GU injuries are commonly caused by blast exposures such as those from IEDs (Andersen et al., 2012; Fleming et al., 2012; Mossadegh et al., 2012; Paquette, 2007; Serkin et al., 2010). Expected long-term health outcomes related to the types of acute GU injuries caused by blast exposure would be hypogonadism, infertility, erectile dysfunction, and voiding dysfunction. The committee determined that although these long-term health outcomes have not been studied in relation specifically to blast exposure, it is logical to assume that they could result from the acute GU injuries commonly incurred from exposure to blast.
The hypogonadism is caused by direct loss of testicular tissue because of a blast injury to the scrotum in addition to posttraumatic hypopituitarism from mild TBI caused by a blast injury. The anterior pituitary seems to be particularly sensitive to blast injury: there is a secondary decrease in release of follicle-stimulating hormone (FSH) and luteinizing hormone (LH) and a consequent decrease in release of testosterone from the testicles. Wilkinson et al. (2012) examined 26 service members who had mild TBI caused by a blast and compared them with a control group of 59 veterans who had similar deployment history but no history of blast injury or head trauma and normal cognitive testing. Eleven (42%) of the 26 in the mild-TBI group had some abnormality in hormones from the anterior pituitary; none in the control group had any such abnormalities. Three patients had hypogonadism as shown by serum testosterone concentrations in less than the 5th percentile of normal reference ranges, coupled with FSH and LH concentrations in less than the 10th percentile of normal reference ranges.
Low testosterone decreases sex drive and muscle mass and leads to anemia, osteoporosis, low energy, and possibly depression.
Wilkinson et al. (2012) also demonstrated a growth hormone deficiency (GHD) that occurred in five of the 26 veterans in the mild-TBI blast group and none of the control group. Growth hormone concentrations were measured indirectly on the basis of insulin-like growth factor-1 (IGF-1) concentrations; IGF-1 concentrations less than the 10th percentile of reference concentrations are thought to represent GHD. GHD decreases sex drive, strength, muscle volume, and metabolic rate. Wilkinson et al. (2012) demonstrated that those changes persist for up to 1 year. The remaining patients in the study who had hormone abnormalities had low oxytocin concentrations: two veterans had increased vasopressin concentrations (over the 95th percentile) and two had decreased vasopressin concentrations (under the 5th percentile).
Baxter et al. (2013) conducted complete endocrine evaluations of 19 UK service members for 2–48 months after they received moderate to severe blast TBI (as discussed above, neuroimaging and cognitive assessment also were conducted). They compared that group with 39 control patients. In the control non-blast-TBI group, injuries were secondary to motor vehicle collisions (43%), assaults (32%), falls (23%), and sporting injuries (2%). Six of 19 (32%) service members who had blast TBI but only 1 of 39 (2.6%) controls had anterior pituitary dysfunction (p = 0.004). Two service members who had blast TBI had hyperprolactinemia, two had GHD, one had adrenocorticotropic hormone deficiency, and one had combined GHD, adrenocorticotropic hormone deficiency, and gonadotropin deficiency. Four of the 19 patients in the blast-TBI group had hypogonadism secondary to direct blast injury to the testicles and perineum; these 4 had normal anterior pituitary function. The five patients in the blast-TBI group who had hypogonadism are examples of how direct blast injury and hypopituitarism as a result of blast affect testosterone production; all five will need lifelong testosterone replacement therapy.
Loss of testicular tissue can also result in infertility in this population of young men. The effects of low FSH and LH in the Wilkinson et al. (2012) and Baxter et al. (2013) studies discussed above also could result in infertility (sperm production depends on both hormones but primarily on FSH); however, this effect was not examined in those studies or other studies reviewed by the committee.
Direct injury to the penis from a blast can result in erectile dysfunction (ED), which can have important long-term emotional effects in this young population. The ED is caused by damage of the corporal bodies or erectile nerves to the penis and interruption of blood flow to and from the penis, which results from substantial pelvic trauma. Complex phallic reconstruction techniques are feasible in patients who have suffered blast injury to
the penis (Peker et al., 2002). Long-term studies looking at ED in patients who suffered blast injuries have not been performed.
Voiding dysfunction in patients after blast injury has not been well described, but several studies have looked at voiding dysfunction in patients who have sustained a TBI from events other than a military blast injury (usually motor vehicle collisions). The studies found a wide variation of abnormalities, from hyperactive bladder to hypoactive bladder, depending on the nature and location of and time since neurologic injury (Ersoz et al., 2011; Giannantoni et al., 2011).
Bladder and urethral injuries after a blast injury often require a series of complex reconstructive surgeries and can lead to urethral strictures and incontinence. Long-term outcomes of patients who have had urethral reconstruction after blast injuries have not been adequately described.
More than 643,000 US veterans of the Iraq and Afghanistan wars were examined to assess the association between lower urinary tract symptoms (LUTS) and PTSD. International Classification of Diseases, Ninth Revision (ICD-9) codes were used to identify LUTS and PTSD. Male veterans who had PTSD were more likely to have LUTS than those who did not have PTSD (2.9% vs 1.1%; p < 0.001) (Breyer et al., 2012). The same database was used to examine the association between PTSD and sexual dysfunction (ED and premature ejaculation). Male veterans who had PTSD were more likely than those who do not have PTSD to suffer from sexual dysfunction (9.8% vs 3.3%; p < 0.001) (Breyer et al., 2012).
Conclusions
On the basis of expert clinical knowledge, the committee knows that exposure to blast can lead acutely to complete functional and structural loss of GU organs. The committee found two studies that reported on long-term GU health outcomes of blast exposure (Baxter et al., 2013; Wilkinson et al., 2012). The studies showed persistent hypogonadism as a result of hypopituitarism in some patients up to 2 years after TBI from a blast. For example, if a soldier has an acute blast injury and loss of both testicles and trauma to the penis that results in damage to the corporal bodies and urethra, he will have permanent, long-term problems with hypogonadism, infertility, voiding dysfunction, and ED. The answer to the clinical question, How much does a partial injury to a GU organ contribute to long-term consequences from blast injuries?, is unknown because studies are lacking.
The committee concludes, on the basis of its evaluation, that there is sufficient evidence of a causal relationship between exposure to blast and some long-term effects on a genitourinary organ—such as hypogonadism, infertility, voiding dysfunction, and erectile dysfunc-
tion—associated with severe injury (defined as a complete structural and functional loss that cannot be reconstructed).
The committee concludes, on the basis of its evaluation, that there is inadequate/insufficient evidence of an association between exposure to blast and long-term effects associated with partial injury to a genitourinary organ (defined as an incomplete structural and functional loss that can be reconstructed).
Information on dermal outcomes other than direct outcomes of burns from exposure to blast is sparse. Mesquita-Guimaraes et al. (1987) reported on a patient who presented with multiple silica granulomas on the left side of his body. Fifteen years earlier, while in military service, he had stepped on a land mine that exploded, and that led to amputation of his left leg and numerous other injuries. His injuries healed, but numerous black particles remained embedded in his skin. Three years after the explosion, the patient had inflammation around the particles in his left forearm; the condition resolved with unspecified topical medication and antibiotics. X-ray spectroscopy of the particles showed them to be silica, possibly from the earth that was disrupted by the land mine. The authors believe that over the years the silica slowly converted to colloidal silica, which can cause nonallergic foreign-body reactions. In another case report, Wijekoon et al. (1995) reported a case in a 29-year-old man of sarcoid-like granulomatous skin lesions at sites that were exposed to a bomb blast 7 years earlier. The authors hypothesized that the granulomas were produced by foreign material that was embedded in the skin during the blast. In a third case report, a 14-year-old girl developed prurigo nodularis–like skin eruptions about 4 months after a bomb blast (Ghosh et al., 2009). The lesions were in the same place (her arms and face) as the original blast injury and initially consisted of multiple tiny burn injuries. The skin condition improved markedly with topical glucocorticoid and hydroxyzine use. No long-term cohort studies have reported on dermal sequelae of blast injuries not directly related to burns. However, although few studies were found, the committee concludes on the basis of expert clinical knowledge that exposure to blast can have dermal effects.
The committee concludes, on the basis of its evaluation, that there is sufficient evidence of an association between exposure to blast and long-term dermal effects, such as cutaneous granulomas.
MUSCULOSKELETAL AND REHABILITATION OUTCOMES
The musculoskeletal system and soft-tissue areas have the highest incidence of bodily injuries in blast survivors, the most extreme being traumatic amputation, which occurs in 1–3% of blast victims (CDC, 2008b). Those injuries can be manifested as primary, secondary, tertiary, or quaternary; any of them can occur alone or in combination (Covey, 2002). Musculoskeletal primary blast injuries caused by the blast wave or wind usually occur in proximity to the explosion and result primarily in traumatic amputation. That is a fairly rare outcome because of the high associated mortality, but there has been an increase in traumatic-amputation survivors owing to advances in medicine and body-armor technology (Covey, 2002; Mody et al., 2009). Secondary blast injury predominates as the most common blast injury to the musculoskeletal system and is most often the result of penetration by shrapnel and other fragments, which causes trauma ranging from minor lacerations to deep embedding of foreign bodies. Nonpenetrating contusions and bone fractures are also possible (CDC, 2008b). Tertiary and quaternary blast injuries to the musculoskeletal system are rare and mostly follow a pattern similar to that of civilian trauma from blunt-impact forces. Contusions, lacerations, and fractures are all possible, and crush injuries may result in compartment syndrome (CDC, 2008b). As of December 2012, the total number of amputations (of major limbs, such as a leg, and minor limbs, such as s finger) in military personnel serving in Iraq and Afghanistan was 1,715 (Fischer, 2013).
Acute Effects
Injuries to the musculoskeletal system are generally overt and easy to diagnose on the basis of outward signs and victim-reported symptoms; however, a few complications can arise and should be suspected with blast injuries. In many cases, soft-tissue injury extends well beyond the zone of skin and bone damage (CDC, 2008b). One of the biggest concerns in connection with blast injuries to the musculoskeletal system is wound contamination and the high risk of complicated and potentially lethal infection (CDC, 2008b; Covey, 2002). Of particular concern with regard to infection are open bone fractures (especially of long bones) and traumatic implantation of shrapnel, dirt, and biologic material, including bone fragments from other victims (Mody et al., 2009). Blast injuries contaminated with Aeromonas hydrophilia, an organism not commonly found in civilian musculoskeletal injuries, are associated with the requirement for a more proximal amputation (Penn-Barwell et al., 2012). Additional information about infections related to exposure to blast appears later in this chapter. Finally, because of the pattern of polytrauma in blast injuries, musculoskel-
etal injuries are often markers of damage to other organ systems, such as cardiovascular and neurologic damage in extremities (CDC, 2008b).
Long-Term Effects
To evaluate the long-term musculoskeletal and rehabilitation outcomes of blast exposure, the committee reviewed about 70 peer-reviewed studies. None of the studies met enough of the inclusion guidelines to be considered primary. This section details supportive studies on musculoskeletal and rehabilitation outcomes of blast exposure.
Sayer et al. (2008) is particularly informative in that it separated blast-injured and non-blast-injured patients to compare their outcomes (the study examined cognitive and motor functional gain scores and length of stay). The results showed no significant difference between blast and non-blast patients in orthopedic, fracture, amputation, and pain outcomes. In blast patients, several types of injuries were found to be more common: oral and maxillofacial (p < 0.05) and skin or soft-tissue injury (p < 0.001), including burns (p < 0.05) and wounds (p < 0.001). The study is generally informative with respect to the functional status of blast versus non-blast patients during the rehabilitation period after injury, but it has limitations in providing evidence on specific long-term musculoskeletal and rehabilitation outcomes of blast. The study did not include an objective measure of blast exposure at the time of injury and instead identified blast exposure on the basis of self-reporting in the medical chart. In addition, as mentioned previously in the review of gastrointestinal outcomes, the study has limitations for understanding possible long-term effects in that the median period of observation of patients’ functioning was just 29 days. Although the blast and non-blast groups were found not to be different at discharge in functional gain after rehabilitation, the study does not indicate whether there were long-term differences between the two groups.
Using US Army Physical Evaluation Board medical records, Rivera et al. (2012) assessed the prevalence of osteoarthritis in 1,566 combat-injured US service members who could not return to duty. Of the 1,566, 126 cases of osteoarthritis were identified. Sites of osteoarthritis were knee, elbow, ankle, shoulder, foot, wrist, spine, and hip. Eighty-one percent of the service members were exposed to blast. Only 10% of the 126 service members had osteoarthritis before deployment. A case report also describes the development of osteoarthritis in a service member who was exposed to blast (Coy and Chatfield, 1998). The soldier was exposed to blast and sustained multiple injuries, including bilateral soft-tissue shrapnel injuries to his legs and hyperextension of his knees, while serving in the Vietnam War. He developed knee pain and arthritis within 10 years of sustaining the initial injuries.
Several other studies provided information on long-term musculoskeletal and rehabilitation outcomes. The development of heterotopic ossification (the presence of bone in soft tissue where bone does not normally exist) has been reported in a residual limb in up to 63% of combat-related amputations (Potter et al., 2007); this finding is unexpected because heterotopic ossification was a rare complication of amputation before the conflicts in Iraq and Afghanistan (Alfieri et al., 2012). Forsberg et al. (2009), in a retrospective cohort study, aimed to understand the prevalence and risk factors for heterotopic ossification in combat-wounded patients who were admitted to NNMC from March 1, 2003, through December 31, 2006. The study group consisted of 157 patients who had undergone at least one orthopedic procedure on an extremity and developed heterotopic ossification; 86 patients who did not develop heterotopic ossification made up the control group. Followup of patients included in the study ranged from 2–41 months (mean, 8.4 months) for the heterotopic ossification group and from 2–36 months (mean, 7.1 months) for the control group. Mechanism of injury was specifically examined to determine whether heterotopic ossification was associated with one injury type rather than another. Results of the record review showed a relationship between blast injury and development of heterotopic ossification, but the relationship only approached significance (p = 0.06). In a univariate analysis, however, blast injury with concurrent TBI or an injury severity score (ISS) of at least 16 was predictive of heterotopic ossification (p = 0.02 and 0.04, respectively).
Dougherty (1999) carried out a record review to identify bilateral above-knee amputation patients treated at Valley Forge General Hospital during the Vietnam War and then conducted followup interviews to report on their long-term outcomes. Of the 30 identified patients, 26 (87%) had been injured by land mines or booby traps. At followup (an average of 27.5 years after the injury), the 23 patients who responded were found to have lower physical function (statistically significant with p < 0.01) than matched controls on the 36-question short-form health survey. There were no differences between groups in physical-role functioning, bodily pain, general health, vitality, social functioning, and mental health functioning. The study did not separate blast-injured and non-blast-injured patients, so any reported differences in functional status between the groups cannot be attributed specifically to blast.
A retrospective review by Cross et al. (2012) reported on 115 Army service members who sustained battle-related open tibia fractures. The study examined whether the service members returned to duty and, if they were unable to return, what disability rating they acquired. Results showed an overall return-to-duty rate of 18% among the service members and a 12.5% rate in those who had amputations. The mechanism of injury of 78% of the service members was explosion (88% of the amputees sustained
injuries that were due to explosion). Although the study indicates that amputation and medical retirement are frequent in those who have open tibia fractures caused by blast, it did not compare the long-term health outcomes between those who were blast-exposed and those who were non-blast-exposed. The study also has limitations in its usefulness for the committee’s review owing to its small sample and its use of return to duty as the main outcome examined.
A retrospective review by Tintle et al. (2012) reported on 96 patients, all US military personnel, who suffered 100 upper-extremity amputations during October 2004–April 2009. The study examined outcomes and operative complications, types of complications, and frequency of revision surgery after upper-extremity amputations as a result of combat-related trauma. Of the amputations, 87% resulted from exposure to blast and an additional 8% were due to high-velocity gunshot wounds. Nearly half the amputations—42%—were followed by at least one complication that required revision. Deep infection led to 51% of the revision procedures. Heterotopic ossification was another cause of revision procedures: It occurred in 66% of the 63 limbs that had at least 2 months of radiographic followup (not all patients had radiographic followup). Other complications were neuromas (excised in 9% of the limbs), wound dehiscence (6%), need for scar revision (5%), and need for contracture release (4%). The records indicate that all the patients had a completely healed residual limb at final followup. Continuing phantom-limb pain and residual-limb pain were reported in 37% and 51% of the patients, respectively. Pain status was not recorded for all patients.
Feldt et al. (2013) conducted a retrospective review by using data from the Joint Facial and Invasive Neck Trauma Project to assess the numbers and types of facial and penetrating neck injuries sustained by US military personnel serving in Iraq and Afghanistan from January 2003 through May 2011. The number of discrete facial and penetrating neck injuries identified was 37,523; they occurred in 7,177 service members. Exposure to blast was the mechanism of injury in 24.2% of the cases. Other mechanisms were penetrating trauma (49.1%), blunt trauma (25.7%), and other or unknown or burns (1%). The study did not report on long-term outcomes.
Another study of US military personnel serving in Iraq and Afghanistan assessed facial injuries, specifically mandibular fractures, by using JTTR data (Zachar et al., 2013). Data from October 2001 to April 2011 were analyzed, and 391 patients who had mandibular fractures were identified. The mechanisms of injury in those patients were exposure to blast (61.3%), ballistics (12.5%), and motor vehicle collisions (1%). The fracture patterns in the service members differed somewhat from those in the general population, possibly because of the mechanisms of injury: service members were exposed more to blast, whereas mechanisms of injury in civilians are typi-
cally motor vehicle collisions, interpersonal violence, and falls. The study also did not report on long-term outcomes.
A third study consisted of a retrospective review of US service members who sustained facial injuries during the Iraq war and were treated from April to October 2006 (Salinas and Faulkner, 2010). The 21 patients were identified by one of the study authors, who treated the injured service members in Iraq. The study compared outcomes in patients who were treated immediately in theater and outcomes in patients whose treatment was delayed until after transport out of theater. Overall, the mechanism of injury was exposure to blast in 57% of the patients—in 43% of the immediately treated group and 86% in the delayed-treatment group. Treatment of blast-exposed patients may have been more often delayed because their injuries were more severe and required more complex procedures that were available only in facilities that were out of theater. The complication rate was 24% (five complications in four patients). Four of the complications were infections. A limitation of the study is the small population (21 patients).
Koc et al. (2008) reported on long-term skin problems in 142 amputees in Turkey. The time between initial amputation and enrollment in the study ranged from 1 to 21 years. The results showed that amputees had a high prevalence of skin problems (73.9%), and the study also reported differences between those using soft pocket prostheses and those using silicone prostheses. Although the study documented that 80.3% of the amputations were caused by mine explosions, there was no comparison of those who suffered blast injuries with those who did not. Therefore, the study is not useful in understanding whether long-term skin problems in amputees can be attributed specifically to blast injury. Additional information about dermal effects is presented in the preceding section of this chapter.
Conclusion
Exposure to blast has several obvious outcomes, such as loss of limbs and scarring. Few studies of other outcomes related to blast exposure and the musculoskeletal system and rehabilitation were identified. There is some evidence that blast-related amputations result in a higher incidence of complications, including heterotopic ossification in those requiring amputations, and osteoarthritis.
The committee concludes, on the basis of its evaluation, that there is limited/suggestive evidence of an association between exposure to blast and long-term consequences for the musculoskeletal system, including heterotopic ossification in amputee limbs and osteoarthritis.
Infections in blast-exposed people can be caused directly by blast and can be acquired indirectly during administration of medical care after exposure to a blast. With respect to most of the studies on complications from infections described below, it was not possible for the committee to determine whether an infectious organism was introduced into a wound (during a blast or during later medical care).
Infection remains a common problem for veterans. Of 2.2 million Iraq and Afghanistan war veterans, 144,167 (0.07%) have been treated for problems related to infectious diseases (ICD-9 codes 001–139) in VA medical facilities since 2001 (VA, 2013). Pathogenic bacteria are commonly found in the wounds of military personnel who have blast injuries (Murray, 2008a,b). An infection rate of 5.5% was reported in an assessment of JTTR data on infection-related complications in combat casualties in the Iraq and Afghanistan wars (Murray et al., 2011). A retrospective chart review published in 2009 provides a summary of infections identified in Iraq and Afghanistan veterans treated in a PRC in the Palo Alto Health Care System (Dau et al., 2009). The authors reviewed patient records from the period January 2002–October 2007. During that time, 180 veterans received care in the unit, and 35 (19%) developed 137 unique infections while in the hospital. The most common isolates were from the urinary tract (26%), followed by sputum (23%), wound (18%), and blood (15%); bacteria of 21 species were recovered (see Table 4-4). Many of the veterans had polymicrobial infections, and many of the infections were trauma-related (that is, the wounds were caused by or related to high-velocity projectiles, blast devices, and burns). Gram-positive microorganisms and anaerobes predominated at the time of injury, but gram-negative organisms caused later infections in the wounds.
Fungi also have been isolated from the wounds of military personnel who served in Iraq and Afghanistan (Paolino et al., 2012; Warkentien et al., 2012). Organisms include the genera Absidia, Biopolaris, Fusarium, and Mucor and several species of Aspergillus. Nearly all the military personnel in those studies received their wounds from exposure to blast. Candidemia has been reported in people who have been exposed to blast (Wolf et al., 2000).
Infected wounds are commonly caused by explosives, such as IEDs (Murray, 2008a). The risk of infection from explosive devices can be increased by introduction of ground material and other matter added to the devices into the wounds. Injuries to the extremities are the most prevalent, followed by injuries to the head and neck, thorax, and abdomen. Burn-related infections complicate treatment of combat casualties. Burn patients are particularly susceptible to bacterial infections because of skin
|
|
|
| Culture Site (Total No. Isolates, % Total Infections) |
Organism (No. Isolatesa) |
|
|
|
| Blood (21, 15) | |
| 1 | Coagulase-negative Staphylococcus (14) |
| 2 | Candida sp. (2) |
| 3 | Pseudomonas aeruginosa (2) |
| 4–6 | Enterobacter aerogenes, Enterococcus sp., Klebsiella pneumoniae (ESBL) (1 each) |
| Sputum (31, 23) | |
| 1 | Methicillin-sensitive Staphylococcus aureus (6) |
| 2 | Pseudomonas aeruginosa (6) |
| 3 | Klebsiella pneumoniae (4, 2 ESBL) |
| 4 | Stenotrophomonas maltophilia (4) |
| 5 | Acinetobacter baumannii (3) |
| Urineb (36, 26) | |
| 1 | Pseudomonas aeruginosa (9) |
| 2 | Klebsiella pneumoniae (7, 3 ESBL) |
| 3 | Escherichia coli (6, 3 ESBL) |
| 4 | Methicillin-resistant Staphylococcus aureus (3) |
| 5 | Enterobacter cloacae (2) |
| Wound (25, 18) | |
| 1 | Methicillin-resistant Staphylococcus aureus (8) |
| 2 | Coagulase-negative Staphylococcus (4) |
| 3 | Methicillin-sensitive Staphylococcus aureus (4) |
| 4 | Pseudomonas aeruginosa (4) |
| 5 | Acinetobacter baumannii, Enterobacter cloacae, |
| Proteus mirabilis, Rhodotorula mucilaginosa, | |
| Streptococcus salivarius (1 each) | |
| Other (24, 18) | |
| 1 | Pseudomonas aeruginosa (5) |
| 2 | Coagulase-negative Staphylococcus (3) |
| 3 | Clostridium difficile (3) |
| 4 | Enterococcus sp. (3) |
| 5 | Methicillin-resistant Staphylococcus aureus (2) |
|
|
|
NOTE: ESBL = extended-spectrum-β-lactamase; sp = species.
aTop five organisms only; therefore, number may not match total number in left column.
bOne urine culture was positive for Acinetobacter.
SOURCE: Dau et al., 2009.
breakdown and the risk of nosocomial infection in the hospital environment (Dau et al., 2009). Numerous studies in military and civilian populations have reported that infection with pathogens increases the risk of death and otherwise can complicate healing in burn patients, for example, Bennett et al. (2010), Calvano et al. (2010), D’Avignon et al. (2010), Horvath et al. (2007), Murray et al. (2008), Ressner et al. (2008), and
Schofield et al. (2007). Wounded personnel can be particularly susceptible to infection because of comorbid health problems (for example, immunosuppression or subclinical conditions, such as sexually transmitted diseases or leishmaniasis).
TABLE 4-4 Top Five Isolated Organisms by Site Cultured
During the Iraq and Afghanistan wars, multidrug-resistant (MDR) bacteria have been increasingly common (Dau et al., 2009; Keen et al., 2010; Murray, 2008a; Murray et al., 2009; Sherwood et al., 2011). There have been increases in casualties’ MDR infections with such organisms as Acinetobacter baumannii-calcoaceticus complex, Pseudomonas aeruginosa, and Klebsiella pneumoniae.
The committee did not identify any studies that met its guidelines for primary studies, but did identify a number of supportive studies: a cohort study that is still going on, a case-control study, five retrospective chart reviews, and several case reports that provide information on acute and chronic complications from infections of wounds caused by blast.
Acute Effects
Wolf et al. (2000) conducted a case control study of candidemia in people who were injured by a blast while visiting an outdoor food marketplace in Israel. The cases consisted of 21 patients injured in the marketplace. To be included in the study, the cases had to be hospitalized for more than 24 hours. Two control groups were used: The first consisted of 29 patients who had similar blast injuries from a different explosion at an open-air pedestrian mall (referred to as the blast-injury controls), and the second consisted of 40 ICU patients (referred to as the ICU controls) treated at the same time as the case patients. Within 4 days after injury, four of the cases had respiratory infections from Aspergillus or Rhizopus. Between 4 and 16 days, 7 of them developed candidemia (the Candida was not isolated from the wounds before being isolated from blood). Mortality in infected cases was 43%. Quantitative air sampling was conducted, and higher concentrations of Aspergillus and Rhizopus were found in the marketplace than in the pedestrian mall and Candida was found only in the marketplace. The incidence of Candida was significantly lower in the control groups than in the case group (p = 0.001 for the ICU controls and p = 0.02 for the blast-injury controls). The cluster of cases and the early temporal pattern suggest that infection occurred at the time of the blast rather than being hospital acquired, although nosocomial infection cannot be ruled out.
Albrecht et al. (2006) assessed medical records and microbiology-laboratory data of patients admitted to a US military tertiary-care burn center from January 2003 to November 2005. Acinetobacter baumannii– calcoaceticus complex infection was found in 59 of 802 burn patients. Mortality was higher in the infected patients; however, on multivariate
analysis, infection did not independently affect mortality (p = 0.651). That result suggests that “although Acinetobacter is a marker of increased crude mortality because of its association with larger burns, it does not affect mortality independently” (Albrecht et al., 2006, p. 549). The authors did not report how many of the patients were military versus civilian (this burn center also receives civilian burn patients) or how many patients sustained their burns as a result of exposure to explosions.
Johnson et al. (2007a) reviewed infections in type III fractures (high-energy injuries typically with bone comminution or loss and lacerations of greater than 10 cm). Historically, type III fractures have a reported infection rate of 6–39% and an amputation rate of less than 10%. Thirty-five service members with type III open diaphyseal tibial fractures admitted to Brooks Army Medical Center (BAMC) during March 2003–August 2006 were identified through medical records. The mechanism of injury was an explosive device in 27 (77%) of the 35 cases. All patients had received irrigation and debridement before arriving at BAMC. During each patient’s first surgical debridement at BAMC, samples for initial cultures were taken from deep tissues. Samples were taken again later. Before arriving at BAMC, all patients received perioperative antibiotics for gram positive bacteria. The mean evacuation time from injury to arrival at BAMC was 7.4 days. Of the 35 patients, 27 had infections at admission; the most common organisms were Acinetobacter baumannii–calcoaceticus complex, Enterobacter species, and Pseudomonas aeruginosa. All 27 received antibiotics; 24 (89%) received therapy for osteomyelitis, and the remaining three for deep-wound infection. Delayed union was another complication. Thirteen patients had deep-wound infections at reassessment, most of which were staphylococcal. Five of the 35 patients ultimately required amputation, and infection was the cause in 4 of the 5 cases. A limitation of this study is the small number of patients.
The goal of Paolino et al. (2012) was to determine the incidence of invasive fungal infections at WRAMC. They reviewed electronic medical records of military personnel sent to WRAMC from March 2002 to July 2008 with suspected invasive fungal infection and found six cases (three proved and three probable). All cases had been deployed to Iraq, and five had acquired blast injuries. The fungi in the cases were Absidia, several species of Aspergillus, Biopolaris, Fusarium, and Mucor. Three of the five cases required amputation of extremities or substantial revision of previous amputation sites. No deaths were noted.
Warkentien et al. (2012) used JTTR data to study fungal wound infections in military personnel who had extremity injuries due to blast. All patients were injured in Afghanistan and admitted to WRAMC, NNMC, or BAMC via Landstuhl Regional Medical Center in Germany from June 1, 2009, to December 31, 2010. Thirty-seven patients had diagnoses of fungal
wound infections (20 proved, 4 probable, and 13 possible). The fungi found were Aspergillus, Fusarium, and Mucorales. Of the 37 patients, 33 received antifungal therapy. Twelve patients had amputations, but it is not clear whether the amputations were due specifically to the fungal infections. Five patients who had fungal infections died, and it was noted that the infections played a role in three of the deaths.
Silla et al. (2006) prospectively reviewed clinical records of 22 patients who were injured in a bombing in Bali on October 12, 2002. The patients were admitted to a burn unit in a hospital in Western Australia, and their records were compared with those of 37 burn patients in the same hospital who were not injured by blast. The incidence of primary burn-wound infection was statistically significantly higher in the Bali bombing patients (15 patients, 68%) than in the other burn patients (7 patients, 19%) (p = 0.001). Pathogens isolated from the Bali burn patients were Pseudomonas aeruginosa, Staphylococcus aureus, Bacillus cereus, Enterococcus species, MDR Acinetobacter baumannii, Chryseobacterium indologenes, Candida, Enterobacter cloacae, and Diphtheroid bacillus; the other patients were infected with Pseudomonas aeruginosa, Klebsiella pneumoniae, Serrata marcescens, and Bacillus cereus. All Bali bombing patients received antibiotic prophylaxis before arriving at the burn unit, whereas only 5 of the 37 other patients received it. The patients were not followed for a long period.
Pseudomonas putida was identified in a member of the US military who sustained injuries in an explosion in Iraq (Carpenter et al., 2008). Bilateral transtibial amputations were performed on the day of the explosion and the patient was ultimately transferred to NNMC, where after 10 days he developed leukocytosis, high fever, and purulent drainage from the right leg stump. Pseudomonas putida was cultured from the wound site, and the patient was treated with intravenous meropenem for 14 days; there were no further infectious complications.
Long-Term Effects
Tribble et al. (2011) published a preliminary report on a continuing cohort study with an observational design that is assessing short- and long-term outcomes of infections after deployment-related traumatic injury. Participants are enrolled while hospitalized at WRAMC, BAMC, or NNMC. They will be followed for 5 years via interviews, Web-based questionnaires, and review of DOD and VA electronic medical records. Patient trauma and surgical history is obtained by using JTTR data. Of 354 patients, 180 (52%) were exposed to blast, and 69 patients (31.7% of those exposed) had an infection. At 6-month followup after discharge, there were 28 incident infections (bloodstream infections, skin and soft-tissue infections,
osteomyelitis, pneumonia, sinusitis, urinary tract infection, and Clostridium difficile infection). In about 15% of cases, continuing management for the infections was needed. The study is continuing to enroll participants.
Brown et al. (2010) conducted a retrospective chart review to study infections in British service members who had sustained life-threatening and limb-threatening injuries in the Iraq and Afghanistan wars. They had undergone limb-salvage procedures for severely mangled extremity injuries, all of which resulted from blast or ballistics. The authors used JTTR data from August 12, 2003 through August 2007 (the last followup was in May 2008) to review trauma audit and clinical records. Eighty-four casualties with 85 extremity injuries were available for analysis, and 20 of the casualties had infections. The more severely injured had higher infection rates, and there were more infections in wounds of the lower extremities than of the upper extremities. No differences were found between infected and noninfected patients in ISS, time from injury to evacuation, time from injury to surgery, or time to arrival in England. More infectious complications were associated with injuries that required fasciotomy; no association was found between infectious complications and use of hemorrhage control in the field (for example, QuickClot and HemCon dressings). Bacteria initially recovered included Acinetobacter species, Pseudomonas aeruginosa, and Staphylococcus aureus. Other bacteria recovered later during the course of recovery were Aeromonas species, Bacillus species, Chryseobacterium species, Clostridium species, Enterobacter species, Escherichia coli, Klebsiella species, methicillin-resistant Staphylococcus aureus, and Stenotrophomonas species. Two of the patients’ extremities had recovery of Pseudomonas aeruginosa and Staphylococcus aureus 237 and 235 days, respectively, after injury. Those bacteria are associated with chronic complications (deep-wound infections and osteomyelitis), and it is not clear when they were introduced into the wounds. Limitations of the study include the small number of patients, the definitions available for defining extremity infections, and the fact that the JTTR has undergone changes in data collection methods that may have led to inconsistencies in the data.
Mody et al. (2009) assessed infections in 58 service members who had open or closed tibial or femoral fractures that culminated in intramedullary fixation and who were sent to WRAMC from October 2003 to June 2007. About 65% were IED-associated injuries. The authors reviewed inpatient and outpatient electronic health records and long-term followup (median, 447 days) was conducted with telephone interviews. Infectious complications occurred in 23 (40%) of the 58 patients. Osteomyelitits and hardware-associated infections were most common, occurring in 10 (43%) of 23 infected fractures (44%), or 17% of the total study populations. IED-associated injury was significantly more likely to be associated with infected fractures (21 of 23, 91%) than with noninfected fractures (17 of
35, 49%) (p = 0.005). Polymicrobial infections were common (occurring in 44% of infected fractures). Infecting bacteria were Acinetobacter baumannii, Staphylococcus species, MDR Enterobacter cloacae, and MDR Klebsiella pneumoniae.
Stevens et al. (1988) reported a case of a Vietnam veteran who sustained blast injuries from a land-mine explosion in 1967, developed gas gangrene that required amputation of his legs and several fingers, and then 17 years later had another episode of gas gangrene in a closed wound in his hand. Clostridium perfringens was cultured from the wound site. The patient was treated with penicillin, and no recurrence of infection was found at 6-month followup.
There is growing evidence that infections increase the risk of long-term cognitive decline. Shah et al. (2013) showed that there was a significant increase in the rates of cognitive impairment after pneumonia. That study of older Americans (mean age, 72.8 years) carefully controlled for patients’ preinfection cognitive function and the trajectory of that function. The result reinforces similar findings that showed that severe sepsis was associated with an absolute increase of nearly 10 percentage points in the risk of moderate to severe cognitive impairment and a similarly dramatic increase in rates of disability (Iwashyna et al., 2010). Both those studies suggest that the cognitive declines were not limited to those who were most severely ill. The data are consistent with data from other studies that showed that modest levels of systemic inflammation—such as might occur with an infection treated in the outpatient setting—are associated with more rapid decline in patients with Alzheimer disease (Holmes et al., 2009). The possibility that blast-associated infections lead to such enduring cognitive declines has not been adequately studied. All the above studies included older patients, so the generalizability of their results to the younger military population is unknown.
Conclusion
Various bacterial and fungal infections can occur after injury from exposure to blast, and multiple infections can occur in a single person. The infections can persist for days to months. Chronic infection also can lead to a state of sustained systemic inflammation with adverse long-term consequences for many organ systems. On the basis of the committee’s expert clinical knowledge, it is plausible that infections related to blast injury can have long-term outcomes, including osteomyelitis, deep-wound infection, amputation, and delayed union.
The committee concludes, on the basis of its evaluation, that there is inadequate/insufficient evidence of an association between exposure to blast and long-term effects of infections.
Although burns are frequent after exposure to blast, few studies have assessed their outcomes, particularly long-term outcomes. The outcomes most often reported are length of hospital stay, gross functional outcomes, and mortality.
Four studies reported burn-patient outcomes at the US Army Institute of Surgical Research (USAISR) in San Antonio, Texas, which is the only US military burn center. Civilians from southern Texas can be treated there as well. From March 2003 to May 2005, Kauvar et al. (2006a) tracked 171 combat casualties of the Iraq and Afghanistan wars, of whom 119 (69.6%) were burned by IEDs and another 26.7% by conventional munitions. The combat casualties were compared with 102 patients (also military personnel) who received their burns from noncombat incidents involving, for example, burning of waste, ammunition and gunpowder mishaps, and misuse of gasoline. Most patients burned in explosions had burns to the hands and face. No difference was found between combat and noncombat patients in the length of stay at the burn center. However, combat patients generally spent more days in the intensive care unit (p = 0.08) and spent significantly more days using mechanical ventilation (p = 0.05). The overall mortality rate in both groups was low, but the mortality rate was higher in the combat patients than the noncombat patients, although there were too few patients to determine statistical significance. The vast majority of both groups (90.6% of combat and 98% of noncombat patients) were discharged to their own care. Sixty-seven percent of combat patients and 55.6% of noncombat patients returned to military duty, although many of them had medical limitations that prevented them from performing certain military tasks.
Kauvar et al. (2006b) identified 274 patients admitted to USAISR from April 5, 2003, to April 23, 2005, through medical records. All patients were military personnel in the Iraq and Afghanistan wars. Of the 274 patients, 142 were exposed to blast when they were injured as a result of detonation of an explosive device. The hands and head were the most frequently burned parts of the body in these patients. The authors reported outcomes in 125 of the 142 patients (they excluded 17 of the patients from the further analysis because they were still hospitalized). Five of the 125 patients died. The length of stay of the surviving 120 patients at the burn center ranged from 2 to 154 days (median, 14 days). Nearly all (91%) of the surviving patients were discharged to their own or their families’ care. Four patients needed inpatient care after discharge from USAISR. Nearly half the patients were able to return to duty, although 10% had some duty limitations as a result of their injuries. Some 21% of the patients were released from military service, and 28% had pending decisions about their ability to return
to service. With respect to functional recovery, 109 patients (91%) were discharged at their previous level of global functioning, 8 had moderate disability but were able to care for themselves, and 3 were severely disabled and unable to care for themselves.
Wolf et al. (2006) compared records of military personnel of the Iraq and Afghanistan wars who had burn injuries with records of civilians who had burn injuries treated at the same facility. Data were collected from April 2003 to May 2005, and a total of 751 patients were included. Of the 751, 273 patients were injured during military operations; the number injured specifically by blast was not stated. When age-adjusted, the mortality was similar in the military and civilian groups. The military patients had longer stays in the hospital but no difference in length of stay between the ICU and time spent on a ventilator. Gross functional outcomes were good (that is, previous level of function in activities of daily living) in 94% of the civilian and 92% of the military patients, moderate (can care for oneself with occasional assistance) in 5% of the civilian and 6% of the military patients, and severe (needs assistance for daily living) in 1% of the civilian and 2% of the military patients.
Return to duty status was measured in 61 military personnel who were treated at USAISR for hand burns from March 2003 through June 2005 (Chapman et al., 2008). Almost all the patients (60) had thermal burns, and one had an electric burn. Impairment and disability were measured on discharge from inpatient care and during a followup outpatient visit less than 4 months later. Some 67% (41) of the patients were able to return to duty. The remaining patients were discharged from military service for medical reasons. Patients who were not able to return to duty had greater total body-surface-area burns (p < 0.001) and greater full-thickness total body-surface-area burns (p = 0.002) than the ones who could return to duty.
A fifth study assessed clinical records of patients who were admitted to 10 hospitals during 1997–2003 in Israel (Peleg et al., 2008): 219 patients who had burn injuries related to terrorist attacks, 6,546 patients who had burn injuries not related to terrorist attacks, and 2,228 patients who experienced a terrorist attack but were not burned. The patients who had burn injuries related to terrorist attacks had longer hospital stays (mean, 18.5 days) than the patients who had burn injuries only (11.1 days) and the patients who were not burned (9.5 days). ISSs showed that injuries sustained in terrorist attacks that also involved burns were more severe than injuries sustained in terrorist attacks that did not result in burns or burn injuries not related to terrorist attacks. In-hospital mortality in patients whose burn injuries were related to terrorist attacks and patients who experienced a terrorist attack but were not burned were similar (6.4% vs 6.6%, respectively). However, patients who had burn injuries not related to terrorist attacks had lower mortality (3.4%). The difference in mortality
could be due to the presence of multidimensional injuries associated with terrorist attacks.
Clinically, burns are known to lead to long-term complications. On the basis of its expert clinical knowledge, the committee does not have a reason to believe that burns from exposure to blast are different from burns from non-blast sources except that blast-related burns may have debris embedded in exposed skin. A number of long-term complications have been reported in non-blast burned patients and include scarring (Lawrence et al., 2012; Zanni, 2012), contracture (Schneider and Qu, 2011; Zanni, 2012), heterotopic ossification (Nelson et al., 2012a; Potter et al., 2007), abdominal complications (Markell et al., 2009), thromboembolic complications (Harrington et al., 2001), pruritus (Zachariah et al., 2012), loss of vision (Wisse et al., 2010), neurologic effects (Schneider and Qu, 2011), and psychologic effects (McKibben et al., 2009; Wiechman, 2011).
The committee concludes, on the basis of its evaluation, that there is limited/suggestive evidence of an association between exposure to blast and long-term complications from burns.
Protective equipment has been developed to protect military personnel from injuries caused by exposure to blast and gun shots: body armor (or vests, which can have add-on equipment, such as groin and deltoid protectors and neck collars), helmets, eye protection (spectacles and goggles), and ear protection (earplugs and earmuffs). Blast-resistant vehicles are another form of protective equipment. The committee was asked to consider whether improvements in collective and personal blast protection are associated with diminished blast injuries.
Two types of body armor are in use by the US military: soft and hard (NRC, 2012). Soft body armor is made of several compositions of aramid fibers, known as Kevlar and Twaron fibers. Vests made of aramid fibers are designed to protect against low-velocity, low-energy bullets (for example, 9-mm or .38 caliber bullets) and against shrapnel resulting from explosions. Hard body armor contains high-performance polyethylene fibers, known as Spectra or Dyneema fibers, or a ceramic composite material. It is designed to protect against high-velocity threats, such as .30 caliber and .50 caliber rifle bullets. All helmet types used by the US military are made of Kevlar. Two of the helmets, the Personnel Armored System for Ground Troops helmet and the Modular Integrated Communications helmet, are not sufficient to protect against TBI to an acceptable extent (McEntire and Whitely, 2005). The Advanced Combat Helmet met both the mean and peak standards for all impact experiments at an impact speed of 10 ft/s.
Greater detail about the composition of body armor and helmets is beyond the scope of this report, but further information can be found in Chapter 2 of the National Research Council’s (NRC’s) report Testing of Body Armor Materials: Phase III (2012).
In the past, the use of protective equipment has meant an overwhelming weight burden and the loss of dexterity and hand–eye coordination to the detriment of the render-safe mission (Bass et al., 2005). Even today, military personnel often opt not to wear protective equipment, because it can be uncomfortable and heavy, increase thermal burden, impede their ability to maneuver, and reduce situational awareness (Barwood et al., 2009; Breeze et al., 2012; Caldwell et al., 2011; Killion et al., 2011; Larsen et al., 2011; US Army, 2010). Protective equipment also may contribute to chronic health conditions, such as low-back pain (Burton et al., 1996; Konitzer et al., 2008). Indeed, the hard type of body armor can add a substantial burden of weight on military personnel. For example, the Improved Outer Tactical Vest Generation II body armor system can weigh 27.06–42.50 lb, depending on size (US Army, 2010). Given the main purpose of the body armor—to provide protection but still allow military personnel adequate mobility and flexibility—newer materials that have greater protective capabilities and are lighter are being continuously tested to improve protection.
Before being introduced in the field, protective equipment is tested to ensure that it meets military and law-enforcement standards. For gunshot and projectile injuries, the assessment methods include animal tests and “the correlation of animal chest deformation response with the response of simulant materials at velocities that are typical of rounds used to test soft body armors” (NRC, 2012, p. 34). For that type of assessment, gelatin is typically used as a tissue simulant; clay also can be used. Additional information about body armor standards and testing for gunshot and projectile injuries can be found in Chapter 3 of the NRC report mentioned above. Such a comprehensive set of guidelines does not exist for blast injuries. Nevertheless, in 2001, the North Atlantic Treaty Organization Research Technology Organization established a new task force, HFM-089/TG-024, to review how various countries test protective equipment against antipersonnel mines and their two main effects: fragmentation and blast (NATO, 2004). Among the recommendations of the task force were to use anthropomorphic mannequins to obtain good fit of protective equipment and Hybrid III anthropomorphic mannequins to perform blast tests against the upper body, including the head. The recommendations also emphasized the need for suitable instrumentation for the head, neck, and chest at a minimum and for reproducing militarily relevant conditions (using explosive charges and positioning the mannequins to mimic in-theater scenarios). Taking those recommendations into account, Bass et al. (2005) performed experiments on the effectiveness of body armor and helmets
by using a 50th-percentile male Hybrid III anthropomorphic (automobile crash-test) dummy (First Technology Safety Systems, Inc., USA) exposed to a blast generated by detonating C4 charges of differing weights. The results showed the importance of acceleration and deceleration in neck and head injury although the study was limited by the type of instrumentation, which could measure only acceleration, not pressure. The authors concluded that the larger helmet and visor frontal surface areas tend to increase the risk of head injury from IED blasts owing to increased acceleration from increased exposure to the blast flow. However, the authors also concluded that the greater helmet mass tended to decrease the risk of head injury by decreasing the acceleration of the head, helmet, and visor system. The authors discussed the implications of their findings—either decreasing the visor area or increasing the mass of the helmet visor system or some combination of both increases should be implemented to achieve better protection from blunt trauma to the head. Nevertheless, careful consideration should be given to the facts that increasing the helmet mass without regard for ergonomic factors of wearability and comfort may decrease use of the head protection and lead to chronic neck microinjuries and that decreasing the visor and helmet size may make wearers more vulnerable to penetrating fragments (Bass et al., 2005). Despite the usefulness of those findings, the data are limited to protection from secondary and tertiary blast effects, not primary blasts.
The committee identified few studies of the effectiveness of protective equipment after introduction into the field. Belmont et al. (2010) reported that the percentage of military personnel killed in action in the Iraq and Afghanistan wars is similar to percentages in previous conflicts despite improvements in personal protective equipment and blast-resistant vehicles. The widespread use of explosive weaponry, including IEDs, in Iraq and Afghanistan shifted the etiology of military injuries from gunshot-induced to blast-induced, in contrast with previous wars. Thus, although the current body armor showed great success in reducing blunt and penetrating types of injuries because of its interceptive properties, it does not protect from or substantially reduce the damaging effects of primary blasts.
Breeze et al. (2011) conducted a systematic analysis of studies to assess whether face, neck, and eye protection affects the incidence of injuries in military personnel in the 21st century. In the identified studies, facial wounds had an incidence of 8–20%, neck wounds 2–11%, and eye wounds less than 1% to 6%. Use of eye protection was associated with a reduction by one-third in the rate of eye injuries in military personnel serving in Iraq and Afghanistan. When military personnel were in 100% compliance with the use of eye protection in Iraq, the incidence of eye injuries dropped from 6% to 0.5%. Breeze et al. (2012) assessed the effectiveness of neck protection (nape protectors and neck collars) in reducing the incidence of
neck injuries. They identified, from the UK JTTR, neck wounds sustained by UK military personnel from January 1, 2006, to December 31, 2010. The incidence of neck wounds was 10% (152 of 1,528 injuries), and these wounds were more often caused by explosive events (79%) than by gun shots (21%). The records of 58% of those wounded stated whether they wore neck protection at the time of injury; all of those records indicated that they were not wearing neck protection. Of the 152 military personnel who had neck wounds, 111 (73%) died from the wounds. On the basis of the underlying pathology of the injuries and mapping the surface location of the neck injuries, the authors concluded that 16 of those deaths could have been prevented if neck collars had been worn. Eye protection was assessed in an additional study, although it is not specific for protection from exposure to blast. Thomas et al. (2009) assessed the effectiveness of combat eye protection in US service members deployed to the Iraq and Afghanistan wars. Using JTTR data on service members who entered level III hospital facilities from March 2003 to September 2006, the authors determined that eye injuries were significantly more frequent when personnel did not wear eye protection (p < 0.01).
Several studies have evaluated the effectiveness of hearing protection in military personnel, although the studies focus on noise in general and not specifically on blast. A study of 449 cases of acute acoustic trauma in a military hospital in Helsinki, Finland, from 1989 to 1993 found that 14% of the cases had been using ear protection but one-third of them had poorly fitting protectors or insufficient protection (Savolainen and Lehtomaki, 1997). The IOM evaluated hearing-conservation programs in the military and reported that there was “limited or suggestive evidence to conclude that use of hearing protection devices and the level of real-world hearing protection these devices provide have been and remain not adequate in military hearing conservation programs” (IOM, 2006, p. 12). Casali et al. (2009) field-tested three hearing-enhancement protection systems (the Combat Arms Earplug, the Communication and Enhancement Protection System, and the Peltor Comtac II headset) in cadet soldier trainees in training exercises and found that improved hearing protection would be needed to obtain adequate levels of hearing performance for user compliance in the combat environment. Another study of hearing performance in people wearing hearing protection (the Combat Arms Earplug and the Sonic II Ear valves) reported that service members subject to high-level impulse noise would not experience compromised speech understanding when using level-dependent earplugs in low-level continuous noise (Norin et al., 2011). Finally, in a controlled field experiment that compared four hearing-protection enhancement devices (Peltor Com-Trac II, Etymotic EB1 and EB15 High-Fidelity Electronic BlastPLG electronic earplugs, and the 3M Single-Ended Combat Arms passive earplug) with the unprotected ear
with ambient outdoor noise and in 82 dBA military diesel heavy-truck noise, none of the tested devices allowed normal localization performance.
Several studies have commented on whether the use of Kevlar body armor reduces the incidence of GU injury in battlefield combatants. A review of the changing patterns of wartime GU injuries in the preceding 100 years found a lower percentage of GU injuries that were abdominal in service members who wore body armor than in those service members who did not wear body armor (Hudak et al., 2005). The finding came primarily from a comparison of GU injuries in the 1991 Gulf War with those in the conflicts in Bosnia-Herzegovina. A review of 30 GU injuries in the 1991 Gulf War (body armor was worn) found that only 17% of the patients had abdominal GU injuries (Thompson et al., 1998), whereas in the Bosnian conflict (body armor was not worn) 45–53% of patients who had GU injuries had abdominal GU injuries (Hudolin and Hudolin, 2003). However, a more compressive evaluation of GU trauma of US service members who wore body armor (Serkin et al., 2010) showed that the percentage of abdominal GU trauma (kidney, ureter, and bladder) was 46.9%, which is quite similar to the percentage of abdominal GU trauma in the conflicts of Bosnia-Herzegovina. The percentage of these GU injuries in the conflicts of Bosnia-Herzegovina caused by blast (mostly from land mines and mortar and shell rounds) ranged from 52% to 70% (Vuckovic et al., 1995) and was similar to the percentage of GU injuries caused by blasts in Iraq and Afghanistan (50–63%) (Paquette, 2007; Serkin et al., 2010). However, the pattern of blast injuries in Iraq and Afghanistan caused by IEDs may be quite different from the pattern of blast injuries in Bosnia-Herzegovina caused by land mines and mortar shells, so comparing the protective effects of body armor related to GU trauma in different conflicts may have too many confounding factors. A retrospective review of GU trauma in the Iraq war found that US casualties who wore body armor had a significantly lower rate of GU injury than casualties who did not wear body armor (primarily Iraqi service members or civilians). Casualties who wore body armor had a 2.1% rate (25 of 1,216) of GU injury (upper and lower GU tract combined) versus 3.4% (51 of 1,496) in those who did not wear body armor (p = 0.037). Casualties who wore body armor had a 0.6% rate (7 of 1,216) rate of kidney injury versus 1.5% (22 of 1,496) in those who did not wear body armor (p = 0.017) (Paquette, 2007). The report seems to suggest that wearing protective body armor decreases the incidence of overall GU trauma and more specifically kidney injury. In light of the fact that most of the blast-induced GU injuries were caused by secondary blast effects (that is, shrapnel and particles of the environment generated during the explosion), the protective effects of the body armor are most probably due to its interceptive properties.
Laboratory testing can provide helpful information about the protective
effects of body armor, although the current literature contains much controversial information. Although a study comparing the protective effects of two types of vests—one a soft protective vest and the other a hard protective vest—found that the vests significantly decreased pulmonary-injury risk after blast delivered via a shock tube (Wood et al., 2012), another study had opposite findings (Phillips et al., 1988). The latter study used sheep fitted with cloth ballistic vests and exposed to blasts with peak pressures of 115, 230, 295, and 420 kPa. It showed a significantly higher lung–body weight index (p < 0.05) than in sheep without vests; this suggested substantial lung edema (Phillips et al., 1988). At the highest exposure level (420 kPa), two of the six sheep without vests died and five of the six sheep with vests died. The authors concluded that the vests increased the target surface area and thus diminished the effective loading function on the thorax. It has been suggested that even minor displacements of the body wall may produce serious injury if the body wall velocity is high (Cooper and Taylor, 1989). Cooper et al. (1991) hypothesized that the motion of the body wall generates waves that propagate within the body and transfer energy to internal sites. Wave propagation has been identified as one of the essential mechanisms in the production of injuries resulting from blast impact to the torso, and mechanistic implications for effective personal protection from the primary effects of blast overpressure have been stressed (Cooper et al., 1991). Accordingly, it has been suggested that using a body armor developed on the basis of acoustic decoupling principles might reduce the direct stress coupled into the body and thus lessen the severity of lung injury. Li et al. (2006) reported that the ear barrel and ear plug showed protective effects against blast-induced trauma in the auditory organs of guinea pigs. Sheep and pigs placed in light-armor vehicles and exposed to blast showed more middle ear damage compared to control animals; no differences were found in the respiratory or gastrointestinal tracts (Phillips et al., 1989).
Ramasamy et al. (2011) assessed the effects of modifications of armored vehicles (V-shaped hull, increased ground clearance, widened axles, heavy vehicles, and blast deflectors) used during the Rhodesian War (1972–1980) on rates of injury. Data were available on 2,212 vehicle–mine incidents involving 16,456 people. All the vehicle modifications statistically significantly reduced fatality rates and they had a cumulative effect. Except for blast deflectors, they also reduced injury rates. As in personal protective equipment, the modifications of the vehicles probably reduced the amount of energy transferred to and interacting directly with the service members’ bodies.
The importance of correctly fitting personal protective equipment cannot be overestimated. Anecdotal data indicate that body armor that is too small or too large might increase the injurious effects of a blast via enhancement of the impact-related parenchymal organ damage or via reflection of
refraction waves from the body wall and inside body armor that amplify the shock wave coupling with the body. Nevertheless, further well-designed studies that use standardized and militarily relevant models are needed to clarify the mechanisms underlying the negative side effects caused by the ill fit of personal protective equipment.
The committee concludes, on the basis of its evaluation, that there is sufficient evidence of an association between the use of personal protective equipment, including interceptive body armor and eye protection, and prevention of blunt and penetrating injuries caused by exposure to blast.
The committee concludes, on the basis of its evaluation, that there is inadequate/insufficient evidence to determine whether an association exists between the use of current personal protective equipment and prevention of primary blast-induced (non-impact-induced) injuries.
ACRM (American Congress of Rehabilitation Medicine). 1993. Definition of mild traumatic brain injury. Journal of Head Trauma Rehabilitation 8(3):86-87.
Alam, M., M. Iqbal, A. Khan, and S. A. Khan. 2012. Ocular injuries in blast victims. Journal of the Pakistan Medical Association 62(2):138-142.
Albrecht, M. C., M. E. Griffith, C. K. Murray, K. K. Chung, E. E. Horvath, J. A. Ward, D. R. Hospenthal, J. B. Holcomb, and S. E. Wolf. 2006. Impact of acinetobacter infection on the mortality of burn patients. Journal of the American College of Surgeons 203(4):546-550.
Aldrich, T. K., J. Gustave, C. B. Hall, H. W. Cohen, M. P. Webber, R. Zeig-Owens, K. Cosenza, V. Christodoulou, L. Glass, F. Al-Othman, M. D. Weiden, K. J. Kelly, and D. J. Prezant. 2010. Lung function in rescue workers at the World Trade Center after 7 years. New England Journal of Medicine 362(14):1263-1272.
Alexander, M. P. 1995. Mild traumatic brain injury: Pathophysiology, natural-history, and clinical management. Neurology 45(7):1253-1260.
Alfieri, K. A., J. Forsberg, and B. K. Potter. 2012. Blast injuries and heterotopic ossification. Bone and Joint Research 1(8):192-197.
Andelic, N., N. Hammergren, E. Bautz-Holter, U. Sveen, C. Brunborg, and C. Roe. 2009. Functional outcome and health-related quality of life 10 years after moderate-to-severe traumatic brain injury. Acta Neurologica Scandinavica 120(1):16-23.
Andersen, R. C., M. Fleming, J. A. Forsberg, W. T. Gordon, G. P. Nanos, M. T. Charlton, and J. R. Ficke. 2012. Dismounted complex blast injury. Journal of Surgical Orthopaedic Advances 21(1):2-7.
APA (American Psychiatric Association). 2013. Diagnostic and Statistical Manual for Mental Disorders. 5th ed. Washington, DC: American Psychiatric Publishing.
Argyros, G. J. 1997. Management of primary blast injury. Toxicology 121(1):105-115
Armonda, R. A., R. S. Bell, A. H. Vo, G. Ling, T. J. DeGraba, B. Crandall, J. Ecklund, and W. W. Campbell. 2006. Wartime traumatic cerebral vasospasm: Recent review of combat casualties. Neurosurgery 59(6):1215-1225.
Avidan, V., M. Hersch, Y. Armon, R. Spira, D. Aharoni, P. Reissman, and W. P. Schecter. 2005. Blast lung injury: Clinical manifestations, treatment, and outcome. American Journal of Surgery 190(6):927-931.
Barros D’Sa, A. A. B., T. H. Hassard, R. H. Livingston, and J. W. S. Irwin. 1980. Missile-induced vascular trauma. Injury 12(1):13-30.
Barwood, M. J., P. S. Newton, and M. J. Tipton. 2009. Ventilated vest and tolerance for intermittent exercise in hot, dry conditions with military clothing. Aviation Space & Environmental Medicine 80(4):353-359.
Bass, C. R., M. Davis, K. Rafaels, M. S. Rountree, R. M. Harris, E. Sanderson, W. Andrefsky, G. DiMarco, and M. Zielinski. 2005. A methodology for assessing blast protection in explosive ordnance disposal bomb suits. International Journal of Occupational Safety & Ergonomics 11(4):347-361.
Baxter, D., D. J. Sharp, C. Feeney, D. Papadopoulou, T. E. Ham, S. Jilka, P. J. Hellyer, M. C. Patel, A. N. Bennett, A. Mistlin, E. McGilloway, M. Midwinter, and A. P. Goldstone. 2013. Pituitary dysfunction after blast traumatic brain injury: UK biosap study. Annals of Neurology 74(4):527-536.
Bazarian, J. J., K. Donnelly, D. R. Peterson, G. C. Warner, T. Zhu, and J. Zhong. 2013. The relation between posttraumatic stress disorder and mild traumatic brain injury acquired during Operations Enduring Freedom and Iraqi Freedom. Journal of Head Trauma Rehabilitation 28(1):1-12.
Bell, R. S., A. H. Vo, C. J. Neal, J. Tigno, R. Roberts, C. Mossop, J. R. Dunne, and R. A. Armonda. 2009. Military traumatic brain and spinal column injury: A 5-year study of the impact blast and other military grade weaponry on the central nervous system. The Journal of Trauma 66(4 Suppl):S104-S111.
Belmont, P. J., Jr., G. P. Goodman, M. Zacchilli, M. Posner, C. Evans, and B. D. Owens. 2010. Incidence and epidemiology of combat injuries sustained during “the Surge” portion of Operation Iraqi Freedom by a U.S. Army brigade combat team. Journal of Trauma: Injury, Infection, & Critical Care 68(1):204-210.
Bennett, J. W., J. L. Robertson, D. R. Hospenthal, S. E. Wolf, K. K. Chung, K. Mende, and C. K. Murray. 2010. Impact of extended spectrum beta-lactamase producing klebsiella pneumoniae infections in severely burned patients. Journal of the American College of Surgeons 211(3):391-399.
Blair, J. A., J. C. Patzkowski, A. J. Schoenfeld, J. D. Cross Rivera, E. S. Grenier, R. A. Lehman, Jr., and J. R. Hsu. 2012. Spinal column injuries among Americans in the global war on terrorism. Journal of Bone and Joint Surgery—American Volume 94(18):e135(131-139).
Blanch, R. J., M. S. Bindra, A. S. Jacks, and R. A. H. Scott. 2011. Ophthalmic injuries in British armed forces in Iraq and Afghanistan. Eye 25(2):218-223.
Brahm, K. D., H. M. Wilgenburg, J. Kirby, S. Ingalla, C.-Y. Chang, and G. L. Goodrich. 2009. Visual impairment and dysfunction in combat-injured service members with traumatic brain injury. Optometry & Vision Science 86(7):817-825.
Breeze, J., I. Horsfall, A. Hepper, and J. Clasper. 2011. Face, neck, and eye protection: Adapting body armour to counter the changing patterns of injuries on the battlefield. British Journal of Oral and Maxillofacial Surgery 49(8):602-606.
Breeze, J., L. S. Allanson-Bailey, N. C. Hunt, R. S. Delaney, A. E. Hepper, and J. Clasper. 2012. Mortality and morbidity from combat neck injury. The Journal of Trauma and Acute Care Surgery 72(4):969-974.
Brenner, L. A., N. Bahraini, and T. D. Hernandez. 2012. Perspectives on creating clinically relevant blast models for mild traumatic brain injury and post traumatic stress disorder symptoms. Frontiers in Neurology [electronic resource] 3:31.
Breyer, B., B. Cohen, D. Berterthal, R. Rosen, T. Neylan, and K. Seal. 2012. The association of posttraumatic stress disorder with lower urinary tract symptoms in male Iraq and Afghanistan veterans. Journal of Urology 187(4):e696.
Brown, K. V., C. K. Murray, and J. C. Clasper. 2010. Infectious complications of combat-related mangled extremity injuries in the British military. Journal of Trauma: Injury, Infection, & Critical Care 69(Suppl 1):S109-S115.
Bryant, R. A., M. Creamer, M. O’Donnell, D. Silove, C. R. Clark, and A. C. McFarlane. 2009. Post-traumatic amnesia and the nature of post-traumatic stress disorder after mild traumatic brain injury. Journal of the International Neuropsychological Society 15(6):862-867.
Burton, A. K., K. M. Tillotson, T. L. Symonds, C. Burke, and T. Mathewson. 1996. Occupational risk factors for the first-onset and subsequent course of low back trouble. A study of serving police officers. Spine 21(22):2612-2620.
Caldwell, J. N., L. Engelen, C. van der Henst, M. J. Patterson, and N. A. Taylor. 2011. The interaction of body armor, low-intensity exercise, and hot-humid conditions on physiological strain and cognitive function. Military Medicine 176(5):488-493.
Calvano, T. P., D. R. Hospenthal, E. M. Renz, S. E. Wolf, and C. K. Murray. 2010. Central nervous system infections in patients with severe burns. Burns 36(5):688-691.
Capo-Aponte, J. E., T. G. Urosevich, L. A. Temme, A. K. Tarbett, and N. K. Sanghera. 2012. Visual dysfunctions and symptoms during the subacute stage of blast-induced mild traumatic brain injury. Military Medicine 177(7):804-813.
Carpenter, R. J., J. D. Hartzell, J. A. Forsberg, B. S. Babel, and A. Ganesan. 2008. Pseudomonas putida war wound infection in a US marine: A case report and review of the literature. Journal of Infection 56(4):234-240.
Casali, J. G., W. A. Ahroon, and J. A. Lancaster. 2009. A field investigation of hearing protection and hearing enhancement in one device: For soldiers whose ears and lives depend upon it. Noise Health 11(42):69-90.
Caseby, N. G., and M. F. Porter. 1976. Blast injuries to the lungs: Clinical presentation, management and course. Injury 8(1):1-12.
CDC (Centers for Disease Control and Prevention). 2008a. Blast Injuries: Abdominal Blast Injuries. http://www.bt.cdc.gov/masscasualties/blastinjury-abdominal.asp (accessed March 1, 2013).
CDC. 2008b. Blast Injuries: Blast Extremity Injuries. http://www.bt.cdc.gov/masscasualties/blastinjury-extremity.asp (accessed March 1, 2013).
CDC. 2010. Blast Injuries: Crush Injuries and Crush Syndrome. http://www.bt.cdc.gov/masscasualties/blastinjury-crush.asp (accessed March 1, 2013).
CDC. 2012. Blast Injuries: Blast Lung Injury. http://www.bt.cdc.gov/masscasualties/blastlunginjury.asp (accessed March 1, 2013).
Champion, H. R., J. B. Holcomb, and L. A. Young. 2009. Injuries from explosions: Physics, biophysics, pathology, and required research focus. Journal of Trauma: Injury, Infection, & Critical Care 66(5):1468-1477; discussion 1477.
Chapman, T. T., R. L. Richard, T. L. Hedman, E. M. Renz, S. E. Wolf, and J. B. Holcomb. 2008. Combat casualty hand burns: Evaluating impairment and disability during recovery. Journal of Hand Therapy 21(2):150-158; quiz 159.
Cockerham, G. C., S. Lemke, C. Glynn-Milley, L. Zumhagen, and K. P. Cockerham. 2013. Visual performance and the ocular surface in traumatic brain injury. The Ocular Surface 11(1):25-34.
Coe, C. D., K. S. Bower, D. B. Brooks, R. D. Stutzman, and J. B. Hammer. 2010. Effect of blast trauma and corneal foreign bodies on visual performance. Optometry & Vision Science 87(8):604-611.
Cohen, J. T., G. Ziv, J. Bloom, D. Zikk, Y. Rapoport, and M. Z. Himmelfarb. 2002. Blast injury of the ear in a confined space explosion: Auditory and vestibular evaluation. Israel Medical Association Journal 4(7):559-562.
Coker, W. J., B. M. Bhatt, N. F. Blatchley, and J. T. Graham. 1999. Clinical findings for the first 1000 Gulf War veterans in the Ministry of Defence’s medical assessment programme. British Medical Journal 318(7179):290-294.
Coldren, R. L., M. L. Russell, R. V. Parish, M. Dretsch, and M. P. Kelly. 2012. The ANAM lacks utility as a diagnostic or screening tool for concussion more than 10 days following injury. Military Medicine 177(2):179-183.
Comstock, S., D. Pannell, M. Talbot, L. Compton, N. Withers, and H. C. Tien. 2011. Spinal injuries after improvised explosive device incidents: Implications for tactical combat casualty care. Journal of Trauma: Injury, Infection, & Critical Care 71(5 Suppl 1):S413-S417.
Cooper, D. B., P. M. Chau, P. Armistead-Jehle, R. D. Vanderploeg, and A. O. Bowles. 2012. Relationship between mechanism of injury and neurocognitive functioning in OEF/OIF service members with mild traumatic brain injuries. Military Medicine 177(10):1157-1160.
Cooper, G. J., and D. E. Taylor. 1989. Biophysics of impact injury to the chest and abdomen. Journal of the Royal Army Medical Corps 135(2):58-67.
Cooper, G. J., R. L. Maynard, N. L. Cross, and J. F. Hill. 1983. Casualties from terrorist bombings. Journal of Trauma: Injury, Infection, & Critical Care 23(11):955-967.
Cooper, G. J., D. J. Townend, S. R. Cater, and B. P. Pearce. 1991. The role of stress waves in thoracic visceral injury from blast loading: Modification of stress transmission by foams and high-density materials. Journal of Biomechanics 24(5):273-285.
Couch, J. R., R. B. Lipton, W. F. Stewart, and A. I. Scher. 2007. Head or neck injury increases the risk of chronic daily headache: A population-based study. Neurology 69(11):1169-1177.
Covey, D. C. 2002. Blast and fragment injuries of the musculoskeletal system. Journal of Bone & Joint Surgery—American Volume 84(7):1221-1234.
Coy, J., and G. Chatfield. 1998. Bilateral total knee replacement for traumatic arthritis from blast injury in Vietnam. Military Medicine 163(9):651-652.
Cross, J. D., D. J. Stinner, T. C. Burns, J. C. Wenke, and J. R. Hsu. 2012. Return to duty after type III open tibia fracture. Journal of Orthopaedic Trauma 26(1):43-47.
Cuthbertson, B. H., S. Roughton, D. Jenkinson, G. Maclennan, and L. Vale. 2010. Quality of life in the five years after intensive care: A cohort study. Critical Care (London, England) 14(1):R6.
Dau, B., G. Oda, and M. Holodniy. 2009. Infectious complications in OIF/OEF veterans with traumatic brain injury. Journal of Rehabilitation Research & Development 46(6): 673-684.
Davenport, N. D., K. O. Lim, M. T. Armstrong, and S. R. Sponheim. 2012. Diffuse and spatially variable white matter disruptions are associated with blast-related mild traumatic brain injury. Neuroimage 59(3):2017-2024.
D’Avignon, L. C., B. K. Hogan, C. K. Murray, F. L. Loo, D. R. Hospenthal, L. C. Cancio, S. H. Kim, E. M. Renz, D. Barillo, J. B. Holcomb, C. E. Wade, and S. E. Wolf. 2010. Contribution of bacterial and viral infections to attributable mortality in patients with severe burns: An autopsy series. Burns 36(6):773-779.
DePalma, R. G., D. G. Burris, H. R. Champion, and M. J. Hodgson. 2005. Blast injuries. New England Journal of Medicine 352(13):1335-1342.
Desai, S. V., T. J. Law, and D. M. Needham. 2011. Long-term complications of critical care. Critical Care Medicine 39(2):371-379.
DOD (Department of Defense). 2007. Mental Health Encounters and Diagnoses Following Deployment to Iraq and/or Afghanistan, U.S. Armed forces, 2001-2006. http://www.afhsc.mil/viewMSMR?file=2007/v14_n04.pdf (accessed July 14, 2013).
Dougherty, P. J. 1999. Long-term followup study of bilateral above-the-knee amputees from the Vietnam War. Journal of Bone & Joint Surgery—American Volume 81(10):1384-1390.
Eardley, W. G. P., T. J. Bonner, I. E. Gibb, and J. C. Clasper. 2012. Spinal fractures in current military deployments. Journal of the Royal Army Medical Corps 158(2):101-105.
Eastridge, B. J., R. L. Mabry, P. Seguin, J. Cantrell, T. Tops, P. Uribe, O. Mallett, T. Zubko, L. Oetjen-Gerdes, T. E. Rasmussen, F. K. Butler, R. S. Kotwal, J. B. Holcomb, C. Wade, H. Champion, M. Lawnick, L. Moores, and L. H. Blackbourne. 2012. Death on the battlefield (2001-2011): Implications for the future of combat casualty care. Journal of Trauma and Acute Care Surgery 73:S431-S437.
Eckert, M. J., C. Clagett, M. Martin, and K. Azarow. 2006. Bronchoscopy in the blast injury patient. Archives of Surgery 141(8):806-809; discussion 806-811.
Ersoz, M., K. Kaya, S. Akkus, and S. Ozel. 2011. Urodynamic findings in patients with traumatic brain injury. Turkiye Fiziksel Tip ve Rehabilitasyon Dergisi 57(2):80-84.
Fasol, R., S. Irvine, and P. Zilla. 1989. Vascular injuries caused by anti-personnel mines. Journal of Cardiovascular Surgery (Torino) 30(3):467-472.
Feldt, B. A., N. L. Salinas, T. E. Rasmussen, and J. Brennan. 2013. The joint facial and invasive neck trauma (J-FAINT) project, Iraq and Afghanistan 2003-2011. Otolaryngology—Head & Neck Surgery 148(3):403-408.
Finlay, S. E., M. Earby, D. J. Baker, and V. S. G. Murray. 2012. Explosions and human health: The long-term effects of blast injury. Prehospital and Disaster Medicine 27(4):385-391.
Fischer, H. 2013. U.S. Military Casualty Statistics: Operation New Dawn, Operation Iraqi Freedom, and Operation Enduring Freedom. RS22452 Congressional Research Service.
Fleming, M., S. Waterman, J. Dunne, J.-C. D’Alleyrand, and R. C. Andersen. 2012. Dismounted complex blast injuries: Patterns of injuries and resource utilization associated with the multiple extremity amputee. Journal of Surgical Orthopaedic Advances 21(1):32-37.
Forsberg, J. A., J. M. Pepek, S. Wagner, K. Wilson, J. Flint, R. C. Andersen, D. Tadaki, F. A. Gage, A. Stojadinovic, and E. A. Elster. 2009. Heterotopic ossification in high-energy wartime extremity injuries: Prevalence and risk factors. Journal of Bone & Joint Surgery—American Volume 91(5):1084-1091.
Gallun, F. J., A. C. Diedesch, L. R. Kubli, T. C. Walden, R. L. Folmer, M. S. Lewis, D. J. McDermott, S. A. Fausti, and M. R. Leek. 2012a. Performance on tests of central auditory processing by individuals exposed to high-intensity blasts. Journal of Rehabilitation Research & Development 49(7):1005-1025.
Gallun, F. J., M. S. Lewis, R. L. Folmer, A. C. Diedesch, L. R. Kubli, D. J. McDermott, T. C. Walden, S. A. Fausti, H. L. Lew, and M. R. Leek. 2012b. Implications of blast exposure for central auditory function: A review. Journal of Rehabilitation Research & Development 49(7):1059-1074.
Ghosh, S. K., D. Bandyopadhyay, A. Ghosh, S. Sarkar, and R. K. Mandal. 2009. Prurigo nodularis-like skin eruptions after bomb-blast injury. Clinical and Experimental Dermatology 34(7):e471-472.
Giannantoni, A., D. Silvestro, S. Siracusano, E. Azicnuda, M. D’Ippolito, J. Rigon, U. Sabatini, V. Bini, and R. Formisano. 2011. Urologic dysfunction and neurologic outcome in coma survivors after severe traumatic brain injury in the postacute and chronic phase. Archives of Physical Medicine & Rehabilitation 92(7):1134-1138.
Goldstein, L. E., A. M. Fisher, C. A. Tagge, X. L. Zhang, L. Velisek, J. A. Sullivan, C. Upreti, J. M. Kracht, M. Ericsson, M. W. Wojnarowicz, C. J. Goletiani, G. M. Maglakelidze, N. Casey, J. A. Moncaster, O. Minaeva, R. D. Moir, C. J. Nowinski, R. A. Stern, R. C. Cantu, J. Geiling, J. K. Blusztajn, B. L. Wolozin, T. Ikezu, T. D. Stein, A. E. Budson, N. W. Kowall, D. Chargin, A. Sharon, S. Saman, G. F. Hall, W. C. Moss, R. O. Cleveland, R. E. Tanzi, P. K. Stanton, and A. C. McKee. 2012. Chronic traumatic encephalopathy in blast-exposed military veterans and a blast neurotrauma mouse model. Science Translational Medicine 4(134):1-16.
Goodrich, G. L., J. Kirby, G. Cockerham, S. P. Ingalla, and H. L. Lew. 2007. Visual function in patients of a polytrauma rehabilitation center: A descriptive study. Journal of Rehabilitation Research & Development 44(7):929-936.
Goodrich, G. L., H. M. Flyg, J. E. Kirby, C. Y. Chang, and G. L. Martinsen. 2013. Mechanisms of TBI and visual consequences in military and veteran populations. Optometry and Vision Science 90(2):105-112.
Harrington, D. T., D. W. Mozingo, L. Cancio, P. Bird, B. Jordan, and C. W. Goodwin. 2001. Thermally injured patients are at significant risk for thromboembolic complications. Journal of Trauma: Injury, Infection, & Critical Care 50(3):495-499.
Heltemes, K. J., T. L. Holbrook, A. J. Macgregor, and M. R. Galarneau. 2012. Blast-related mild traumatic brain injury is associated with a decline in self-rated health amongst US military personnel. Injury 43(12):1990-1995.
Heltemes, K. J., M. C. Clouser, A. J. Macgregor, S. B. Norman, and M. R. Galarneau. 2013. Co-occurring mental health and alcohol misuse: Dual disorder symptoms in combat injured veterans. Addictive Behaviors 39(2):392-398.
Herridge, M. S., C. M. Tansey, A. Matte, G. Tomlinson, N. Diaz-Granados, A. Cooper, C. B. Guest, C. D. Mazer, S. Mehta, T. E. Stewart, P. Kudlow, D. Cook, A. S. Slutsky, and A. M. Cheung. 2011. Functional disability 5 years after acute respiratory distress syndrome. New England Journal of Medicine 364(14):1293-1304.
Hicks, R. R., S. J. Fertig, R. E. Desrocher, W. J. Koroshetz, and J. J. Pancrazio. 2010. Neurological effects of blast injury. Journal of Trauma: Injury, Infection, & Critical Care 68(5):1257-1263.
Hilz, M. J., P. A. DeFina, S. Anders, J. Koehn, C. J. Lang, E. Pauli, S. R. Flanagan, S. Schwab, and H. Marthol. 2011. Frequency analysis unveils cardiac autonomic dysfunction after mild traumatic brain injury. Journal of Neurotrauma 28(9):1727-1738.
Hirshberg, B., A. Oppenheim-Eden, R. Pizov, M. Sklair-Levi, A. Rivkin, E. Bardach, M. Bublil, C. Sprung, and M. R. Kramer. 1999. Recovery from blast lung injury: One-year followup. Chest 116(6):1683-1688.
Hoge, C. W., J. C. Clark, and C. A. Castro. 2007. Commentary: Women in combat and the risk of post-traumatic stress disorder and depression. International Journal of Epidemiology 36(2):327-329.
Hoge, C. W., D. McGurk, J. L. Thomas, A. L. Cox, C. C. Engel, and C. A. Castro. 2008. Mild traumatic brain injury in U.S. soldiers returning from Iraq. New England Journal of Medicine 358(5):453-463.
Holmes, C., C. Cunningham, E. Zotova, J. Woolford, C. Dean, S. Kerr, D. Culliford, and V. H. Perry. 2009. Systemic inflammation and disease progression in Alzheimer disease. Neurology 73(10):768-774.
Horvath, E. E., C. K. Murray, G. M. Vaughan, K. K. Chung, D. R. Hospenthal, C. E. Wade, J. B. Holcomb, S. E. Wolf, A. D. Mason, Jr., and L. C. Cancio. 2007. Fungal wound infection (not colonization) is independently associated with mortality in burn patients. Annals of Surgery 245(6):978-985.
Hrubec, Z., and R. A. Ryder. 1980. Traumatic limb amputations and subsequent mortality from cardiovascular disease and other causes. Journal of Chronic Diseases 33(4):239-250.
Huang, M. X., S. Nichols, A. Robb, A. Angeles, A. Drake, M. Holland, S. Asmussen, J. D’Andrea, W. Chun, M. Levy, L. Cui, T. Song, D. G. Baker, P. Hammer, R. McLay, R. J. Theilmann, R. Coimbra, M. Diwakar, C. Boyd, J. Neff, T. T. Liu, J. Webb-Murphy, R. Farinpour, C. Cheung, D. L. Harrington, D. Heister, and R. R. Lee. 2012. An automatic MEG low-frequency source imaging approach for detecting injuries in mild and moderate TBI patients with blast and non-blast causes. Neuroimage 61(4):1067-1082.
Hudak, S. J., A. F. Morey, T. A. Rozanski, and C. W. Fox, Jr. 2005. Battlefield urogenital injuries: Changing patterns during the past century. Urology 65(6):1041-1046.
Hudolin, T., and I. Hudolin. 2003. Surgical management of urogenital injuries at a war hospital in Bosnia-Hrzegovina, 1992 to 1995. Journal of Urology 169(4):1357-1359.
Huller, T., and Y. Bazini. 1970. Blast injuries of the chest and abdomen. Archives of Surgery 100(1):24-30.
IOM (Institute of Medicine). 2006. Noise and Military Service: Implications for Hearing Loss and Tinnitus. Washington, DC: The National Academies Press.
IOM. 2009. Gulf War and Health, Volume 7: Long-Term Consequences of Traumatic Brain Injury. Washington, DC: The National Academies Press.
Iwashyna, T. J., E. W. Ely, D. M. Smith, and K. M. Langa. 2010. Long-term cognitive impairment and functional disability among survivors of severe sepsis. Journal of the American Medical Association 304(16):1787-1794.
Jagade, M. V., R. A. Patil, I. S. Suhail, P. Kelkar, S. Nemane, J. Mahendru, V. Kalbande, and P. Kewle. 2008. Bomb blast injury: Effect on middle and inner ear. Indian Journal of Otolaryngology and Head and Neck Surgery 60(4):324-330.
Johnson, E. N., T. C. Burns, R. A. Hayda, D. R. Hospenthal, and C. K. Murray. 2007a. Infectious complications of open type III tibial fractures among combat casualties. Clinical Infectious Diseases 45(4):409-415.
Johnson, I. O. N., C. J. Fox, P. White, E. Adams, M. Cox, N. Rich, and D. L. Gillespie. 2007b. Physical exam and occult post-traumatic vascular lesions: Implications for the evaluation and management of arterial injuries in modern warfare in the endovascular era. Journal of Cardiovascular Surgery 48(5):581-586.
Jorge, R. E., L. Acion, T. White, D. Tordesillas-Gutierrez, R. Pierson, B. Crespo-Facorro, and V. A. Magnotta. 2012. White matter abnormalities in veterans with mild traumatic brain injury. American Journal of Psychiatry 169(12):1284-1291.
Kauvar, D. S., L. C. Cancio, S. E. Wolf, C. E. Wade, and J. B. Holcomb. 2006a. Comparison of combat and non-combat burns from ongoing U.S. military operations. Journal of Surgical Research 132(2):195-200.
Kauvar, D. S., S. E. Wolf, C. E. Wade, L. C. Cancio, E. M. Renz, and J. B. Holcomb. 2006b. Burns sustained in combat explosions in Operations Iraqi and Enduring Freedom (OIF/OEF explosion burns). Burns 32(7):853-857.
Keen, E. F., 3rd, C. K. Murray, B. J. Robinson, D. R. Hospenthal, E. M. Co, and W. K. Aldous. 2010. Changes in the incidences of multidrug-resistant and extensively drug-resistant organisms isolated in a military medical center. Infection Control and Hospital Epidemiology 31(7):728-732.
Kelly, J. F., A. E. Ritenour, D. F. McLaughlin, K. A. Bagg, A. N. Apodaca, C. T. Mallak, L. Pearse, M. M. Lawnick, H. R. Champion, C. E. Wade, and J. B. Holcomb. 2008. Injury severity and causes of death from Operation Iraqi Freedom and Operation Enduring Freedom: 2003-2004 versus 2006. Journal of Trauma: Injury, Infection, & Critical Care 64(2 Suppl):S21-S26; discussion S26-S27.
Kennedy, J. E., M. A. Cullen, R. R. Amador, J. C. Huey, and F. O. Leal. 2010. Symptoms in military service members after blast mTBI with and without associated injuries. Neurorehabilitation 26(3):191-197.
Kessler, R. C., A. Sonnega, E. Bromet, M. Hughes, and C. B. Nelson. 1995. Posttraumatic-stress-disorder in the National Comorbidity Survey. Archives of General Psychiatry 52(12):1048-1060.
Kessler, R. C., P. Berglund, O. Demler, R. Jin, and E. E. Walters. 2005. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry 62(6):593-602.
Kessler, R. C., M. Petukhova, N. A. Sampson, A. M. Zaslavsky, and H.-U. Wittchen. 2012. Twelve-month and lifetime prevalence and lifetime morbid risk of anxiety and mood disorders in the United States. International Journal of Methods in Psychiatric Research 21(3):169-184.
Killion, M. C., T. Monroe, and V. Drambarean. 2011. Better protection from blasts without sacrificing situational awareness. International Journal of Audiology 50(Suppl 1): S38-S45.
King, M. S., J. E. Johnson, R. F. Miller, R. Eisenberg, J. H. Newman, J. J. Tolle, J. F. E. Harrell, H. Nian, M. Ninan, E. S. Lambright, and J. R. Sheller. 2011. Constrictive bronchiolitis in soldiers returning from Iraq and Afghanistan. New England Journal of Medicine 365(3):222-230.
Kishikawa, M., T. Yoshioka, T. Shimazu, H. Sugimoto, T. Yoshioka, and T. Sugimoto. 1991. Pulmonary contusion causes long-term respiratory dysfunction with decreased functional residual capacity. Journal of Trauma 31(9):1203-1210.
Koc, E., M. Tunca, A. Akar, A. H. Erbil, B. Demiralp, and E. Arca. 2008. Skin problems in amputees: A descriptive study. International Journal of Dermatology 47(5):463-466.
Konitzer, L. N., M. V. Fargo, T. L. Brininger, and M. Lim Reed. 2008. Association between back, neck, and upper extremity musculoskeletal pain and the individual body armor. Journal of Hand Therapy 21(2):143-148; quiz 149.
Krzywiecki, A., D. Ziora, G. Niepsuj, D. Jastrzebski, S. Dworniczak, and J. Kozielski. 2007. Late consequences of respiratory system burns. Journal of Physiology & Pharmacology 58(Suppl 5)(Pt 1):319-325.
Kushelevsky, B. P. 1949. Pulmonary emphysema combined with bronchial asthma following injury. Klinicheskaia Meditsina 27(3):3-10.
Larsen, B., K. Netto, and B. Aisbett. 2011. The effect of body armor on performance, thermal stress, and exertion: A critical review. Military Medicine 176(11):1265-1273.
Lawrence, J. W., S. T. Mason, K. Schomer, and M. B. Klein. 2012. Epidemiology and impact of scarring after burn injury: A systematic review of the literature. Journal of Burn Care & Research 33(1):136-146.
Leibovici, D., O. N. Gofrit, M. Stein, S. C. Shapira, Y. Noga, R. J. Heruti, and J. Shemer. 1996. Blast injuries: Bus versus open-air bombings: A comparative study of injuries in survivors of open-air versus confined-space explosions. Journal of Trauma: Injury, Infection, & Critical Care 41(6):1030-1035.
Leone, M., F. Brégeon, F. Antonini, K. Chaumoître, A. Charvet, L. H. Ban, Y. Jammes, J. Albanèse, and C. Martin. 2008. Long-term outcome in chest trauma. Anesthesiology 109(5):864-871.
Levin, H. S., E. Wilde, M. Troyanskaya, N. J. Petersen, R. Scheibel, M. Newsome, M. Radaideh, T. Wu, R. Yallampalli, Z. Chu, and X. Li. 2010. Diffusion tensor imaging of mild to moderate blast-related traumatic brain injury and its sequelae. Journal of Neurotrauma 27(4):683-694.
Lew, H. L., T. K. Pogoda, E. Baker, K. L. Stolzmann, M. Meterko, D. X. Cifu, J. Amara, and A. M. Hendricks. 2011. Prevalence of dual sensory impairment and its association with traumatic brain injury and blast exposure in OEF/OIF veterans. Journal of Head Trauma Rehabilitation 26(6):489-496.
Li, C.J., P.F. Zhu, Z.H. Liu, Z.G. Wang, C. Yang, H.B. Chen, X. Ning, J.H. Zhou, and J. Chen. 2006. Comparative observation of protective effects of earplug and barrel on auditory organs of guinea pigs exposed to experimental blast underpressure. Chinese Journal of Traumatology 9(4):242-245.
Lippa, S. M., N. J. Pastorek, J. F. Benge, and G. M. Thornton. 2010. Postconcussive symptoms after blast and nonblast-related mild traumatic brain injuries in Afghanistan and Iraq War veterans. Journal of the International Neuropsychological Society 16(5):856-866.
Mac Donald, C. L., A. M. Johnson, D. Cooper, E. C. Nelson, N. J. Werner, J. S. Shimony, A. Z. Snyder, M. E. Raichle, J. R. Witherow, R. Fang, S. F. Flaherty, and D. L. Brody. 2011. Detection of blast-related traumatic brain injury in U.S. military personnel. New England Journal of Medicine 364(22):2091-2100.
Magnuson, J., F. Leonessa, and G. S. F. Ling. 2012. Neuropathology of explosive blast traumatic brain injury. Current Neurology and Neuroscience Reports 12(5):570-579.
Magone, M. T., G. C. Cockerham, and S. Y. Shin. 2013. Visual dysfunction in combat related mild traumatic brain injury: A review. US Ophthalmic Review 6(1):48-51.
Markell, K. W., E. M. Renz, C. E. White, M. E. Albrecht, L. H. Blackbourne, M. S. Park, D. A. Barillo, K. K. Chung, R. A. Kozar, J. P. Minei, S. M. Cohn, D. N. Herndon, L. C. Cancio, J. B. Holcomb, and S. E. Wolf. 2009. Abdominal complications after severe burns. Journal of the American College of Surgeons 208(5):940-947; discussion 947-949.
Matthews, S., A. Simmons, and I. Strigo. 2011. The effects of loss versus alteration of consciousness on inhibition-related brain activity among individuals with a history of blast-related concussion. Psychiatry Research 191(1):76-79.
Matthews, S. C., A. D. Spadoni, J. B. Lohr, I. A. Strigo, and A. N. Simmons. 2012. Diffusion tensor imaging evidence of white matter disruption associated with loss versus alteration of consciousness in warfighters exposed to combat in Operations Enduring and Iraqi Freedom. Psychiatry Research 204(2-3):149-154.
Mayorga, M. A. 1997. The pathology of primary blast overpressure injury. Toxicology 121(1):17-28.
McEntire, B. J., and P. Whitely. 2005. Blunt Impact Performance Characteristics of the Advanced Combat Helmet and the Paratrooper and Infantry Personnel Armor System for Ground Troops Helmet. USAARL 2005-12 US Army Aeromedical Research Laboratory.
McKee, A. C., T. D. Stein, C. J. Nowinski, R. A. Stern, D. H. Daneshvar, V. E. Alvarez, H. S. Lee, G. Hall, S. M. Wojtowicz, C. M. Baugh, D. O. Riley, C. A. Kubilus, K. A. Cormier, M. A. Jacobs, B. R. Martin, C. R. Abraham, T. Ikezu, R. R. Reichard, B. L. Wolozin, A. E. Budson, L. E. Goldstein, N. W. Kowall, and R. C. Cantu. 2012. The spectrum of disease in chronic traumatic encephalopathy. Brain Jan;136(Pt 1):43-64.
McKibben, J. B., L. Ekselius, D. C. Girasek, N. F. Gould, C. Holzer, M. Rosenberg, S. Dissanaike, and A. C. Gielen. 2009. Epidemiology of burn injuries II: Psychiatric and behavioural perspectives. International Review of Psychiatry 21(6):512-521.
Mendez, M. F., E. M. Owens, E. E. Jimenez, D. Peppers, and E. A. Licht. 2013. Changes in personality after mild traumatic brain injury from primary blast vs. blunt forces. Brain Injury 27(1):10-18.
Mesquita-Guimaraes, J., F. Azevedo, and S. Aguiar. 1987. Silica granulomas secondary to the explosion of a land mine. Cutis 40(1):41-43.
Mody, R. M., M. Zapor, J. D. Hartzell, P. M. Robben, P. Waterman, R. Wood-Morris, R. Trotta, R. C. Andersen, and G. Wortmann. 2009. Infectious complications of damage control orthopedics in war trauma. Journal of Trauma: Injury, Infection, & Critical Care 67(4):758-761.
Morley, M. G., J. K. Nguyen, J. S. Heier, B. J. Shingleton, J. F. Pasternak, and K. S. Bower. 2010. Blast eye injuries: A review for first responders. Disaster Medicine & Public Health Preparedness 4(2):154-160.
Mossadegh, S., N. Tai, M. Midwinter, and P. Parker. 2012. Improvised explosive device related pelvi-perineal trauma: Anatomic injuries and surgical management. Journal of Trauma and Acute Care Surgery 73(2 Suppl 1):S24-S31.
Murray, C. K. 2008a. Epidemiology of infections associated with combat-related injuries in Iraq and Afghanistan. Journal of Trauma: Injury, Infection, & Critical Care 64(3 Suppl):S232-S238.
Murray, C. K. 2008b. Infectious disease complications of combat-related injuries. Critical Care Medicine 36(7 Suppl):S358-S364.
Murray, C. K., J. C. Reynolds, J. M. Schroeder, M. B. Harrison, O. M. Evans, and D. R. Hospenthal. 2005. Spectrum of care provided at an echelon II medical unit during Operation Iraqi Freedom. Military Medicine 170(6):516-520.
Murray, C. K., F. L. Loo, D. R. Hospenthal, L. C. Cancio, J. A. Jones, S. H. Kim, J. B. Holcomb, C. E. Wade, and S. E. Wolf. 2008. Incidence of systemic fungal infection and related mortality following severe burns. Burns 34(8):1108-1112.
Murray, C. K., H. C. Yun, M. E. Griffith, B. Thompson, H. K. Crouch, L. S. Monson, W. K. Aldous, K. Mende, and D. R. Hospenthal. 2009. Recovery of multidrug-resistant bacteria from combat personnel evacuated from Iraq and Afghanistan at a single military treatment facility. Military Medicine 174(6):598-604.
Murray, C. K., K. Wilkins, N. C. Molter, F. Li, L. Yu, M. A. Spott, B. Eastridge, L. H. Blackbourne, and D. R. Hospenthal. 2011. Infections complicating the care of combat casualties during Operations Iraqi Freedom and Enduring Freedom. Journal of Trauma: Injury, Infection, & Critical Care 71(1 Suppl):S62-S73.
NATO (North Atlantic Treaty Organization). 2004. Test Methodologies for Personal Protective Equipment Against Anti-Personnel Mine Blast. Research Technology Organization. http://ftp.rta.nato.int/public//PubFullText/RTO/TR/RTO-TR-HFM-089///TR-HFM-089-$$TOC.pdf (accessed November 13, 2013).
Needham, D. M., J. Davidson, H. Cohen, R. O. Hopkins, C. Weinert, H. Wunsch, C. Zawistowski, A. Bemis-Dougherty, S. C. Berney, O. J. Bienvenu, S. L. Brady, M. B. Brodsky, L. Denehy, D. Elliott, C. Flatley, A. L. Harabin, C. Jones, D. Louis, W. Meltzer, S. R. Muldoon, J. B. Palmer, C. Perme, M. Robinson, D. M. Schmidt, E. Scruth, G. R. Spill, C. P. Storey, M. Render, J. Votto, and M. A. Harvey. 2012. Improving long-term outcomes after discharge from intensive care unit: Report from a stakeholders’ conference. Critical Care Medicine 40(2):502-509.
Nelson, E. R., V. W. Wong, P. H. Krebsbach, S. C. Wang, and B. Levi. 2012a. Heterotopic ossification following burn injury: The role of stem cells. Journal of Burn Care & Research 33(4):463-470.
Nelson, N. W., J. B. Hoelzle, B. M. Doane, K. A. McGuire, A. G. Ferrier-Auerbach, M. J. Charlesworth, G. J. Lamberty, M. A. Polusny, P. A. Arbisi, and S. R. Sponheim. 2012b. Neuropsychological outcomes of U.S. Veterans with report of remote blast-related concussion and current psychopathology. Journal of the International Neuropsychological Society 18(5):845-855.
Norin, J. A., D. C. Emanuel, and T. R. Letowski. 2011. Speech intelligibility and passive, level-dependent earplugs. Ear Hear 32(5):642-649.
NRC (National Research Council). 2012. Testing of Body Armor Materials: Phase III. Washington, DC: The National Academies Press.
Oertel, M., W. J. Boscardin, W. D. Obrist, T. C. Glenn, D. L. McArthur, T. Gravori, J. H. Lee, and N. A. Martin. 2005. Posttraumatic vasospasm: The epidemiology, severity, and time course of an underestimated phenomenon: A prospective study performed in 299 patients. Journal of Neurosurgery 103(5):812-824.
Okie, S. 2005. Traumatic brain injury in the war zone. New England Journal of Medicine 352(20):2043-2047.
Owens, B. D., J. F. Kragh, Jr., J. C. Wenke, J. Macaitis, C. E. Wade, and J. B. Holcomb. 2008. Combat wounds in Operation Iraqi Freedom and Operation Enduring Freedom. Journal of Trauma: Injury, Infection, & Critical Care 64(2):295-299.
Owers, C., J. L. Morgan, and J. P. Garner. 2011. Abdominal trauma in primary blast injury. British Journal of Surgery 98(2):168-179.
Ozer, O., I. Sari, V. Davutoglu, and C. Yildirim. 2009. Pericardial tamponade consequent to a dynamite explosion: Blast overpressure injury without penetrating trauma. Texas Heart Institute Journal 36(3):259-260.
Paolino, K. M., J. A. Henry, D. R. Hospenthal, G. W. Wortmann, and J. D. Hartzell. 2012. Invasive fungal infections following combat-related injury. Military Medicine 177(6):681-685.
Paquette, E. L. 2007. Genitourinary trauma at a combat support hospital during Operation Iraqi Freedom: The impact of body armor. Journal of Urology 177(6):2196-2199.
Peker, A. F., I. Yildirim, S. Bedir, F. Sumer, and M. Dayanc. 2002. Penile reconstruction with prosthesis and free skin graft in a patient with land mine blast injury. Journal of Urology 167(5):2133-2134.
Peleg, K., A. Liran, A. Tessone, A. Givon, A. Orenstein, and J. Haik. 2008. Do burns increase the severity of terror injuries? Journal of Burn Care & Research 29(6):887-892.
Penn-Barwell, J. G., C. A. Fries, I. D. Sargeant, P. M. Bennett, and K. Porter. 2012. Aggressive soft tissue infections and amputation in military trauma patients. Journal of the Royal Naval Medical Service 98(2):14-18.
Peral Gutierrez De Ceballos, J., F. Turegano Fuentes, D. Perez Diaz, M. Sanz Sanchez, C. Martin Llorente, and J. E. Guerrero Sanz. 2005. Casualties treated at the closest hospital in the Madrid, March 11, terrorist bombings. Critical Care Medicine 33(Suppl 1): S107-S112.
Peskind, E. R., E. C. Petrie, D. J. Cross, K. Pagulayan, K. McCraw, D. Hoff, K. Hart, C.-E. Yu, M. A. Raskind, D. G. Cook, and S. Minoshima. 2011. Cerebrocerebellar hypometabolism associated with repetitive blast exposure mild traumatic brain injury in 12 Iraq War veterans with persistent post-concussive symptoms. Neuroimage 54(Suppl 1):S76-S82.
Peterson, A. L., C. A. Luethcke, E. V. Borah, A. M. Borah, and S. Young-McCaughan. 2011. Assessment and treatment of combat-related PTSD in returning war veterans. Journal of Clinical Psychology in Medical Settings 18(2):164-175.
Phillips, Y. Y. 1986. Primary blast injuries. Annals of Emergency Medicine 15(12):1446-1450.
Phillips, Y. Y., and D. R. Richmond. 1991. Primary blast injury and basic research: A brief history. In Conventional Warfare: Ballistic, Blast, and Burn Injuries. Washington, DC: Office of the Surgeon General at TMM Publications. Pp. 221-240.
Phillips, Y. Y., T. G. Mundie, J. T. Yelverton, and D. R. Richmond. 1988. Cloth ballistic vest alters response to blast. Journal of Trauma: Injury, Infection, & Critical Care 28(1 Suppl):S149-S152.
Phillips, Y. Y., T. G. Mundie, R. Hoyt, and K. T. Dodd. 1989. Middle ear injury in animals exposed to complex blast waves inside an armored vehicle. Annals of Otology, Rhinology, & Laryngology—Supplement 140:17-22.
Polusny, M. A., S. M. Kehle, N. W. Nelson, C. R. Erbes, P. A. Arbisi, and P. Thuras. 2011. Longitudinal effects of mild traumatic brain injury and posttraumatic stress disorder comorbidity on postdeployment outcomes in National Guard soldiers deployed to Iraq. Archives of General Psychiatry 68(1):79-89.
Potter, B. K., T. C. Burns, A. P. Lacap, R. R. Granville, and D. A. Gajewski. 2007. Heterotopic ossification following traumatic and combat-related amputations: Prevalence, risk factors, and preliminary results of excision. Journal of Bone & Joint Surgery—American Volume 89(3):476-486.
Ragel, B. T., C. D. Allred, S. Brevard, R. T. Davis, and E. H. Frank. 2009. Fractures of the thoracolumbar spine sustained by soldiers in vehicles attacked by improvised explosive devices. Spine 34(22):2400-2405.
Ramasamy, A., A. M. Hill, S. D. Masouros, F. Gordon, J. C. Clasper, and A. M. J. Bull. 2011. Evaluating the effect of vehicle modification in reducing injuries from landmine blasts: An analysis of 2212 incidents and its application for humanitarian purposes. Accident Analysis & Prevention 43(5):1878-1886.
Ramasamy, A., S. Evans, J. M. Kendrew, and J. Cooper. 2012. The open blast pelvis: The significant burden of management. Journal of Bone & Joint Surgery—British Volume 94(6):829-835.
Resick, P. A., L. F. Williams, M. K. Suvak, C. M. Monson, and J. L. Gradus. 2012. Long-term outcomes of cognitive-behavioral treatments for posttraumatic stress disorder among female rape survivors. Journal of Consulting and Clinical Psychology 80(2):201-210.
Ressner, R. A., C. K. Murray, M. E. Griffith, M. S. Rasnake, D. R. Hospenthal, and S. E. Wolf. 2008. Outcomes of bacteremia in burn patients involved in combat operations overseas. Journal of the American College of Surgeons 206(3):439-444.
Ritenour, A. E., and T. W. Baskin. 2008. Primary blast injury: Update on diagnosis and treatment. Critical Care Medicine 36(7 Suppl):S311-S317.
Ritenour, A. E., L. H. Blackbourne, J. F. Kelly, D. F. McLaughlin, L. A. Pearse, J. B. Holcomb, and C. E. Wade. 2010. Incidence of primary blast injury in US military overseas contingency operations: A retrospective study. Annals of Surgery 251(6):1140-1144.
Rivera, J. C., J. C. Wenke, J. A. Buckwalter, J. R. Ficke, and A. E. Johnson. 2012. Posttraumatic osteoarthritis caused by battlefield injuries: The primary source of disability in warriors. Journal of the American Academy of Orthopaedic Surgeons 20(Suppl 1):S64-S69.
Riviere, S., V. Schwoebel, K. Lapierre-Duval, G. Warret, M. Saturnin, P. Avan, A. Job, and T. Lang. 2008. Hearing status after an industrial explosion: Experience of the AZF explosion, 21 September 2001, France. International Archives of Occupational and Environmental Health 81(4):409-414.
Rona, R. J., N. T. Fear, L. Hull, and S. Wessely. 2007. Women in novel occupational roles: Mental health trends in the UK armed forces. International Journal of Epidemiology 36(2):319-326.
Rona, R. J., M. Jones, N. T. Fear, L. Hull, D. Murphy, L. Machell, B. Coker, A. C. Iversen, N. Jones, A. S. David, N. Greenberg, M. Hotopf, and S. Wessely. 2012. Mild traumatic brain injury in UK military personnel returning from Afghanistan and Iraq: Cohort and cross-sectional analyses. Journal of Head Trauma Rehabilitation 27(1):33-44.
Ruff, R. L., S. S. Ruff, and X. F. Wang. 2008. Headaches among Operation Iraqi Freedom/Operation Enduring Freedom veterans with mild traumatic brain injury associated with exposures to explosions. Journal of Rehabilitation Research and Development 45(7):941-952.
Ruskin, A., and O. W. Beard. 1948. The Texas City disaster: Cardiovascular studies, with followup results. Texas Reports on Biology and Medicine 6(2):234-259.
Ruskin, A., O. W. Beard, and R. L. Schaffer. 1948. Blast hypertension: Elevated arterial pressures in the victims of the Texas City disaster. American Journal of Medicine 4(2):228-236.
Salinas, N. L., and J. A. Faulkner. 2010. Facial trauma in Operation Iraqi Freedom casualties: An outcomes study of patients treated from April 2006 through October 2006. Journal of Craniofacial Surgery 21(4):967-970.
Savolainen, S., and K. M. Lehtomaki. 1997. Impulse noise and acute acoustic trauma in Finnish conscripts. Number of shots fired and safe distances. Scandinavian Audiology 26(2):122-126.
Sayer, N. A. 2012. Traumatic brain injury and its neuropsychiatric sequelae in war veterans. Annual Review of Medicine 63:405-419.
Sayer, N. A., C. E. Chiros, B. Sigford, S. Scott, B. Clothier, T. Pickett, and H. L. Lew. 2008. Characteristics and rehabilitation outcomes among patients with blast and other injuries sustained during the Global War on Terror. Archives of Physical Medicine & Rehabilitation 89(1):163-170.
Scekic, M., D. Ignjatovic, M. Duknic, and P. Janjic. 1991. Secondary perforation of the colon in a patient with blast injury of the abdomen: Case report. Vojnosanitetski Pregled 48(6):562-563.
Scheibel, R. S., M. R. Newsome, M. Troyanskaya, X. Lin, J. L. Steinberg, M. Radaideh, and H. S. Levin. 2012. Altered brain activation in military personnel with one or more traumatic brain injuries following blast. Journal of the International Neuropsychological Society 18(1):89-100.
Scherer, M., H. Burrows, R. Pinto, and E. Somrack. 2007. Characterizing self-reported dizziness and otovestibular impairment among blast-injured traumatic amputees: A pilot study. Military Medicine 172(7):731-737.
Schneider, J. C., and H. D. Qu. 2011. Neurologic and musculoskeletal complications of burn injuries. Physical Medicine in Rehabilitation Clinics of North America 22(2):261-275.
Schnurr, P. P., C. A. Lunney, A. Sengupta, and L. C. Waelde. 2003. A descriptive analysis of PTSD chronicity in Vietnam Veterans. Journal of Traumatic Stress 16(6):545-553.
Schoenfeld, A. J., R. L. Newcomb, M. P. Pallis, J. A. Serrano, J. O. Bader, and B. R. Waterman. 2013. Characterization of spinal injuries sustained by American service members killed in Iraq and Afghanistan: A study of 2,089 instances of spine trauma. Journal of Trauma & Acute Care Surgery 74(4):1112-1118.
Schofield, C. M., C. K. Murray, E. E. Horvath, L. C. Cancio, S. H. Kim, S. E. Wolf, and D. R. Hospenthal. 2007. Correlation of culture with histopathology in fungal burn wound colonization and infection. Burns 33(3):341-346.
Schweickert, W. D., and J. Hall. 2007. ICU-acquired weakness. Chest 131(5):1541-1549.
Scott, B. A., J. R. Fletcher, M. W. Pulliam, and R. D. Harris. 1986. The Beirut terrorist bombing. Neurosurgery 18(1):107-110.
Serkin, F. B., D. W. Soderdahl, J. Hernandez, M. Patterson, L. Blackbourne, and C. E. Wade. 2010. Combat urologic trauma in US military overseas contingency operations. Journal of Trauma: Injury, Infection, & Critical Care 69(Suppl 1):S175-S178.
Shah, F. A., F. Pike, K. Alvarez, D. Angus, A. B. Newman, O. Lopez, J. Tate, V. Kapur, A. Wilsdon, J. A. Krishnan, N. Hansel, D. Au, M. Avdalovic, V. S. Fan, R. G. Barr, and S. Yende. 2013. Bidirectional relationship between cognitive function and pneumonia. American Journal of Respiratory & Critical Care Medicine 188(5):586-592.
Shariat, S., S. Mallonee, E. Kruger, K. Farmer, and C. North. 1999. A prospective study of long-term health outcomes among Oklahoma City bombing survivors. Journal—Oklahoma State Medical Association 92(4):178-186.
Sherwood, J. E., S. Fraser, D. M. Citron, H. Wexler, G. Blakely, K. Jobling, and S. Patrick. 2011. Multi-drug resistant Bacteroides fragilis recovered from blood and severe leg wounds caused by an improvised explosive device (IED) in Afghanistan. Anaerobe 17(4):152-155.
Shively, S. B., and D. P. Perl. 2012. Traumatic brain injury, shell shock, and posttraumatic stress disorder in the military—past, present, and future. Journal of Head Trauma Rehabilitation 27(3):234-239.
Silla, R. C., J. Fong, J. Wright, and F. Wood. 2006. Infection in acute burn wounds following the Bali bombings: A comparative prospective audit. Burns 32(2):139-144.
Simmons, J. W., C. E. White, J. D. Ritchie, M. O. Hardin, M. A. Dubick, and L. H. Blackbourne. 2011. Mechanism of injury affects acute coagulopathy of trauma in combat casualties. Journal of Trauma: Injury, Infection, & Critical Care 71(1 Suppl):S74-S77.
Smith, B., C. A. Wong, T. C. Smith, E. J. Boyko, and G. D. Gackstetter. 2009. Newly reported respiratory symptoms and conditions among military personnel deployed to Iraq and Afghanistan: A prospective population-based study. American Journal of Epidemiology 170(11):1433.
Sponheim, S. R., K. A. McGuire, S. S. Kang, N. D. Davenport, S. Aviyente, E. M. Bernat, and K. O. Lim. 2011. Evidence of disrupted functional connectivity in the brain after combat-related blast injury. Neuroimage 54(Suppl 1):S21-S29.
St. Onge, P., D. S. McIlwain, M. E. Hill, T. J. Walilko, and L. B. Bardolf. 2011. Marine Corps breacher training study: Auditory and vestibular findings. US Army Medical Department Journal 96-106.
Stein, N. R., M. A. Mills, K. Arditte, C. Mendoza, A. M. Borah, P. A. Resick, B. T. Litz, and S. S. Consortium. 2012. A scheme for categorizing traumatic military events. Behavior Modification 36(6):787-807.
Stevens, D. L., L. L. Laposky, P. McDonald, and I. Harris. 1988. Spontaneous gas gangrene at a site of remote injury: Localization due to circulating antitoxin. Western Journal of Medicine 148(2):204-205.
Stewart, W. F., A. Schechter, and B. K. Rasmussen. 1994. Migraine prevalence: A review of population-based studies. Neurology 44(6):17-23.
Svennevig, J. L., J. Vaage, A. Westheim, G. Hafsahl, and H. E. Refsum. 1989. Late sequelae of lung contusion. Injury 20(5):253-256.
Tanielian, T., and L. H. Jaycox. 2008. Invisible Wounds of War: Psychological and Cognitive Injuries, Their Consequences, and Services to Assist Recovery. Santa Monica, CA: RAND Corporation.
Tarmey, N. T., C. L. Park, O. J. Bartels, T. C. Konig, P. F. Mahoney, and A. J. Mellor. 2011. Outcomes following military traumatic cardiorespiratory arrest: A prospective observational study. Resuscitation 82(9):1194-1197.
Teasdale, G., and B. Jennett. 1974. Assessment of coma and impaired consciousness. A practical scale. Lancet 2(7872):81-84.
Teasdale, G., and B. Jennett. 1976. Assessment and prognosis of coma after head injury. Acta Neurochirurgica 34(1-4):45-55.
Theeler, B. J., and J. C. Erickson. 2009. Mild head trauma and chronic headaches in returning US soldiers. Headache 49(4):529-534.
Theeler, B. J., F. G. Flynn, and J. C. Erickson. 2010. Headaches after concussion in US soldiers returning from Iraq or Afghanistan. Headache 50(8):1262-1272.
Theeler, B. J., F. G. Flynn, and J. C. Erickson. 2012. Chronic daily headache in US Soldiers after concussion. Headache 52(5):732-738.
Thomas, R., J. G. McManus, A. Johnson, P. Mayer, C. Wade, and J. B. Holcomb. 2009. Ocular injury reduction from ocular protection use in current combat operations. Journal of Trauma: Injury, Infection, & Critical Care 66(4 Suppl):S99-S103.
Thompson, I. M., S. F. Flaherty, and A. F. Morey. 1998. Battlefield urologic injuries: The Gulf War experience. Journal of the American College of Surgeons 187(2):139-141.
Tintle, S. M., M. F. Baechler, G. P. Nanos, J. A. Forsberg, and B. K. Potter. 2012. Reoperations following combat-related upper-extremity amputations. Journal of Bone & Joint Surgery—American Volume 94(16):e1191-e1196.
Tribble, D. R., N. G. Conger, S. Fraser, T. D. Gleeson, K. Wilkins, T. Antonille, A. Weintrob, A. Ganesan, L. J. Gaskins, P. Li, G. Grandits, M. L. Landrum, D. R. Hospenthal, E. V. Millar, L. H. Blackbourne, J. R. Dunne, D. Craft, K. Mende, G. W. Wortmann, R. Herlihy, J. McDonald, and C. K. Murray. 2011. Infection-associated clinical outcomes in hospitalized medical evacuees after traumatic injury: Trauma infectious disease outcome study. Journal of Trauma: Injury, Infection, & Critical Care 71(1 Suppl):S33-S42.
Trudeau, D. L., J. Anderson, L. M. Hansen, D. N. Shagalov, J. Schmoller, S. Nugent, and S. Barton. 1998. Findings of mild traumatic brain injury in combat veterans with PTSD and a history of blast concussion. Journal of Neuropsychiatry & Clinical Neurosciences 10(3):308-313.
Tucker, K., and A. Lettin. 1975. The Tower of London bomb explosion. British Medical Journal 3(5978):287-290.
US Army. 2010. Operator Manual for Improved Outer Tactical Vest (IOTV) and Improved Outer Tactical Vest Gen II (IOTV GEN II) Part of the Interceptor Body Armor System. TM 10-8470-208-10 US Army.
VA (Department of Veterans Affairs). 2011. Annual Benefits Report Fiscal Year 2011. http://www.publichealth.va.gov/docs/epidemiology/healthcare-utilization-report-fy2013-qtr1.pdf (accessed November 13, 2013).
VA. 2013. Analysis of VA Health Care Utilization Among Operation Enduring Freedom (OEF), Operation Iraqi Freedom (OIF), and Operation New Dawn (OND) Veterans. Washington, DC: Department of Veterans Affairs. http://www.publichealth.va.gov/docs/epidemiology/healthcare-utilization-report-fy2013-qtr1.pdf (accessed November 20, 2013).
VA and DOD. 2009. Clinical Practice Guideline: Management of Concussion/Mild Traumatic Brain Injury. http://www.healthquality.va.gov/mtbi/concussion_mtbi_full_1_0.pdf (accessed November 20, 2013).
Vadivelu, S., R. S. Bell, B. Crandall, T. DeGraba, and R. A. Armonda. 2010. Delayed detection of carotid-cavernous fistulas associated with wartime blast-induced craniofacial trauma. Neurosurgical Focus 28(5):E6.
Valiyaveettil, M., Y. Alamneh, S. A. Miller, R. Hammamieh, Y. Wang, P. Arun, Y. Wei, S. Oguntayo, and M. P. Nambiar. 2012. Preliminary studies on differential expression of auditory functional genes in the brain after repeated blast exposures. Journal of Rehabilitation Research & Development 49(7):1153-1162.
Van Campen, L. E., J. M. Dennis, R. C. Hanlin, S. B. King, and A. M. Velderman. 1999a. One-year audiologic monitoring of individuals exposed to the 1995 Oklahoma City bombing. Journal of the American Academy of Audiology 10(5):231-247.
Van Campen, L. E., J. M. Dennis, S. B. King, R. C. Hanlin, and A. M. Velderman. 1999b. One-year vestibular and balance outcomes of Oklahoma City bombing survivors. Journal of the American Academy of Audiology 10(9):467-483.
Vanderploeg, R. D., H. G. Belanger, R. D. Horner, A. M. Spehar, G. Powell-Cope, S. L. Luther, and S. G. Scott. 2012. Health outcomes associated with military deployment: Mild traumatic brain injury, blast, trauma, and combat associations in the Florida National Guard. Archives of Physical Medicine and Rehabilitation 93(11):1887-1895.
Verfaellie, M., G. Lafleche, A. Spiro, 3rd, C. Tun, and K. Bousquet. 2013. Chronic postconcussion symptoms and functional outcomes in OEF/OIF veterans with self-report of blast exposure. Journal of the International Neuropsychological Society 19(1):1-10.
Vuckovic, I., A. Tucak, J. Gotovac, B. Karlovic, I. Matos, K. Grdovic, and M. Zelic. 1995. Croatian experience in the treatment of 629 urogenital war injuries. Journal of Trauma: Injury, Infection, & Critical Care 39(4):733-736.
Walker, W. C., S. D. McDonald, J. M. Ketchum, M. Nichols, and D. X. Cifu. 2013. Identification of transient altered consciousness induced by military-related blast exposure and its relation to postconcussion symptoms. Journal of Head Trauma Rehabilitation 28(1):68-76.
Walsh, R. M., J. P. Pracy, A. M. Huggon, and M. J. Gleeson. 1995. Bomb blast injuries to the ear: The London Bridge incident series. Journal of Accident & Emergency Medicine 12(3):194-198.
Wani, I., F. Q. Parray, T. Sheikh, R. A. Wani, A. Amin, I. Gul, and M. Nazir. 2009. Spectrum of abdominal organ injury in a primary blast type. World Journal of Emergency Surgery 4:46.
Warkentien, T., C. Rodriguez, B. Lloyd, J. Wells, A. Weintrob, J. R. Dunne, A. Ganesan, P. Li, W. Bradley, L. J. Gaskins, F. Seillier-Moiseiwitsch, C. K. Murray, E. V. Millar, B. Keenan, K. Paolino, M. Fleming, D. R. Hospenthal, G. W. Wortmann, M. L. Landrum, M. G. Kortepeter, and D. R. Tribble. 2012. Invasive mold infections following combat-related injuries. Clinical Infectious Diseases 55(11):1441-1449.
WHO (World Health Organization). 2010. International Statistical Classification of Diseases and Related Health Problems. 10th ed. http://apps.who.int/classifications/icd10/browse/2010/en#/F43.0 (accessed November 20, 2013).
Wiechman, S. A. 2011. Psychosocial recovery, pain, and itch after burn injuries. Physical Medicine and Rehabilitation Clinics of North America 22(2):327-345.
Wijekoon, C. J., K. H. Weerasekera, and A. K. Weerasinghe. 1995. Sarcoid-like granulomas of the skin seven years after bomb blast injury. Ceylon Medical Journal 40(3):126-127.
Wilk, J. E., R. K. Herrell, G. H. Wynn, L. A. Riviere, and C. W. Hoge. 2012. Mild traumatic brain injury (concussion), posttraumatic stress disorder, and depression in U.S. soldiers involved in combat deployments: Association with postdeployment symptoms. Psychosomatic Medicine 74(3):249-257.
Wilkinson, C. W., K. F. Pagulayan, E. C. Petrie, C. L. Mayer, E. A. Colasurdo, J. B. Shofer, K. L. Hart, D. Hoff, M. A. Tarabochia, and E. R. Peskind. 2012. High prevalence of chronic pituitary and target-organ hormone abnormalities after blast-related mild traumatic brain injury. Frontiers in Neurology 3:11.
Wisse, R. P., W. R. Bijlsma, and J. S. Stilma. 2010. Ocular firework trauma: A systematic review on incidence, severity, outcome and prevention. British Journal of Ophthalmology 94(12):1586-1591.
Wolf, D. G., I. Polacheck, C. Block, C. L. Sprung, M. Muggia-Sullam, Y. G. Wolf, A. Oppenheim-Eden, A. Rivkind, and M. Shapiro. 2000. High rate of candidemia in patients sustaining injuries in a bomb blast at a marketplace: A possible environmental source. Clinical Infectious Diseases 31(3):712-716.
Wolf, S. E., D. S. Kauvar, C. E. Wade, L. C. Cancio, E. P. Renz, E. E. Horvath, C. E. White, M. S. Park, S. Wanek, M. A. Albrecht, L. H. Blackbourne, D. J. Barillo, and J. B. Holcomb. 2006. Comparison between civilian burns and combat burns from Operation Iraqi Freedom and Operation Enduring Freedom. Annals of Surgery 243(6):786-792.
Wood, G. W., M. B. Panzer, J. K. Shridharani, K. A. Matthews, B. P. Capehart, B. S. Myers, and C. R. Bass. 2012. Attenuation of blast pressure behind ballistic protective vests. Injury Prevention 19(1):19-25.
Xydakis, M. S., G. S. Ling, L. P. Mulligan, C. H. Olsen, and W. C. Dorlac. 2012. Epidemiologic aspects of traumatic brain injury in acute combat casualties at a major military medical center: A cohort study. Annals of Neurology 72(5):673-681.
Yzermans, C. J., G. A. Donker, J. J. Kerssens, A. J. E. Dirkzwager, R. J. H. Soeteman, and P. M. H. ten Veen. 2005. Health problems of victims before and after disaster: A longitudinal study in general practice. International Journal of Epidemiology 34(4):820-826.
Zachar, M. R., C. Labella, C. P. Kittle, P. B. Baer, R. G. Hale, and R. K. Chan. 2013. Characterization of mandibular fractures incurred from battle injuries in Iraq and Afghanistan from 2001-2010. Journal of Oral & Maxillofacial Surgery 71(4):734-742.
Zachariah, J. R., A. L. Rao, R. Prabha, A. K. Gupta, M. K. Paul, and S. Lamba. 2012. Post burn pruritus: A review of current treatment options. Burns 38(5):621-629.
Zanni, G. R. 2012. Thermal burns and scalds: Clinical complications in the elderly. The Consultant Pharmacist 27(1):16-22.




































































































