2
A Vision for the CTSA Program in a Changing Landscape
The Clinical and Translational Science Awards (CTSA) Program does not exist in isolation; it is part of a larger clinical and translational research ecosystem that plays a vital role in an increasingly complex and dynamic U.S. health care system. The individual CTSAs were originally designed as a set of academic focal points (or academic “homes”) for facilitating clinical and translational research. To better determine whether the CTSA Program’s mission and goals remain appropriate, as requested in its statement of task, the Institute of Medicine (IOM) committee examined how the changing U.S. health care landscape affects the relationship between the CTSA Program and the larger clinical and translational research ecosystem.
This chapter begins by exploring some of these large-scale changes and their impact on clinical and translational research and concludes by describing a new vision for the CTSA Program and opportunities for the National Center for Advancing Translational Sciences (NCATS) to fulfill this vision.
THE CURRENT U.S. HEALTH CARE RESEARCH LANDSCAPE
Decades of innovation and technological advances have led to progress in biomedical sciences, medicine, and public health, contributing to increased life expectancy and improved individual and population health. National initiatives and an emphasis on public reporting of quality measures have improved some specific health outcomes and the management of chronic diseases (Commonwealth Fund, 2011). For example,
in recent decades there have been strides in controlling high blood pressure and in improving factors associated with diabetes control (i.e., A1C, blood pressure, and cholesterol) (Casagrande et al., 2013; Commonwealth Fund, 2011). Another significant change is that medical screening and diagnostics continue to move to less expensive settings—from hospitals, to physician offices, to retail clinics, to homes. For example, rapid advances in screening tools led the Food and Drug Administration (FDA) to approve the first in-home testing kit for HIV in 2012 (Chappel et al., 2009; FDA, 2012). At the same time, sophisticated laboratory techniques, combined with a growing data infrastructure and new analytic tools, are reshaping research. For example, researchers and entrepreneurs now have online access to an ever-expanding library of human genome sequence data generated by the 1000 Genomes Project, provided at no cost through the Amazon Web Services cloud (EMBL-EBI, 2013; NIH, 2012a).
The accelerating pace of scientific discoveries has a downside as well. The complexity of the U.S. health care system has increased, contributing to inconsistent health care quality, escalating costs, inequities in access, and shortcomings in improvement in population health outcomes (IOM, 2013a). The IOM has reported that systematic underuse, misuse, and overuse of medical treatments significantly and negatively affect the overall quality and safety of health care and put individual patients at risk (IOM, 1998, 2001, 2013a). In response to these persistent challenges, diverse stakeholders (e.g., policy makers, payers, health care professionals, researchers, industry representatives, community advocacy groups, and individual patients) have called for dramatic changes in health care research and delivery. Efforts are under way to increase accountability for the effectiveness and efficiency of the U.S. health care system by aligning stakeholder interests around the concept of value (Public Law 111-148) (Porter, 2010; Porter and Teisberg, 2006). Across the United States, momentum is growing in support of a learning health care system that promotes novel partnerships and collaborations around research networks and clinical and delivery system innovations to continually improve health care value.
A learning health care system is founded on the concept of continuous improvement and the imperative to translate “what we know” into “what we do.” Such a system fuels greater value in health care by harnessing the promise of new technological capabilities, market opportunities, and policies across the health care landscape (IOM, 2013a). Leaders in the public and private sectors are generating and using real-
time knowledge to improve outcomes; engaging patients, families, and communities in decisions related to health and health care; and promoting a new culture of care committed to sustained improvement in human health and health care efficiency (IOM, 2013a).
Clinical and translational research are integral to a learning health care system, which relies on an “iterative innovation process designed to generate and apply the best evidence for the collaborative health care choices of each patient and provider; to drive the process of discovery as a natural outgrowth of patient care; and to ensure innovation, quality, safety, and value in health care” (Kemp, 2012, p. 1).
As Figure 2-1 illustrates, the interconnected forces of clinical research and practice fuel a learning health care system. Researchers and health care providers design and implement care, evaluations, or research based on needs of specific communities and populations, which are identified through needs assessments (external scans) or on the basis of observations of researchers and health care providers (internal scans). The resulting data and analyses measure the effectiveness of particular health care goods, services, and processes. These findings are disseminated to inform clinical practice and research models to improve health. This cyclical relationship allows a learning health care system to stay relevant in an ever-changing health care landscape.
The concepts of a learning health care system and translational research both rely on successful integration of clinical research and practice, which has significant implications for conventional notions of bioethics. The traditional paradigm draws a “sharp distinction between clinical research and practice” (Faden et al., 2013, p. s16). Human subject research, unlike clinical practice, is subject to strict federal regulations that protect research participants,1 and overall health care system improvement activities must act accordingly (Faden et al., 2013). Regulatory oversight burdens can threaten the health of patients and populations by delaying or obstructing potentially beneficial changes to clinical practice (Millum and Menikoff, 2010), especially if concerns about research oversight limit research utility, analytic rigor, or dissemination of quality improvement data (Faden et al., 2013).
CTSAs can play a crucial role in recalibrating the longtime ethical divide between research and clinical care. The Ethics Consultation
_____________________
1 See, for example, 45 C.F.R. 46.
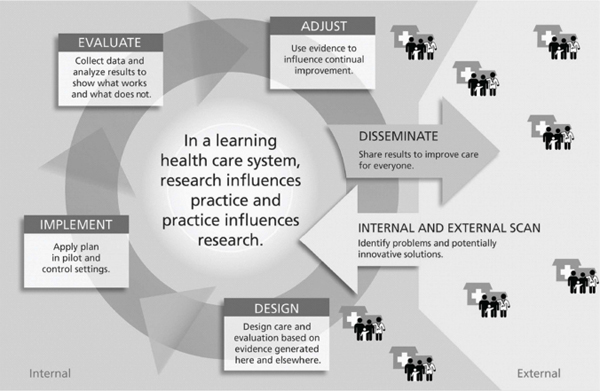
FIGURE 2-1 The learning health care system.
SOURCE: Larson et al., 2013. Reprinted with permission from Radcliffe Publishing.
Working Group, which includes members from 40 CTSAs, “creat[es] a professional community to share strategies, policies, practices, approaches, and information” on this topic (NCATS, 2012, p. 20). By serving as best-practices laboratories in this new ethics environment and by disseminating lessons learned, CTSAs have the potential to advance a new regulatory framework in which the interests in improving health care for patients and protecting individual research participants converge. In addition to the CTSAs’ efforts in advancing this new regulatory framework, ongoing collaboration with key research and regulatory agencies, such as the FDA, the National Institutes of Health (NIH), and the Department of Health and Human Services’ (HHS’s) Office for Human Research Protections, will be necessary.
AN EVOLVING CLINICAL AND TRANSLATIONAL RESEARCH ECOSYSTEM
The clinical and translational research ecosystem currently involves researchers, funders, health care systems, research networks, health
professionals, regulators, industry, community stakeholders, and individuals working in varied settings—laboratories, hospitals, academic health centers, community clinics, private practices, and other places patients receive care. The CTSA Program facilitates interactions between stakeholders within the ecosystem and across settings to accelerate progress in clinical and translational research.
The diversity of stakeholders’ interests makes the research ecosystem susceptible to a wide range of transformative forces. In the 1970s, for example, clinical and basic research began to diverge; basic biomedical research developed as a distinct discipline, with a separate training and career trajectory (Butler, 2008), a divide that translational research is now trying to address.
Other broader social concerns also affect researchers. With the proliferation of health information systems and technologies, including the use of electronic health records (EHRs) and databases, concerns about individual privacy and the security of health information have intensified, especially around data not directly relevant to health care goods and services (IOM, 2010). Major shifts toward enhanced patient confidentiality (e.g., the Health Insurance Portability and Accountability Act of 1996) have led to greater protection of personal information, but also present challenges in the conduct of efficient and clinical and translational research by “restrict[ing] the manner in which health care providers may use and disclose health information for health research” (Pritts, 2008). These challenges will likely increase as research expands within the context of the learning health care system, with traditional distinctions between clinical care and research blurring and with the role of research participant protections requiring new analysis, clarification, or even revision.
Although advances in biomedical sciences and informatics have vastly expanded opportunities for research, a number of persistent data challenges will need to be overcome in order to realize the full potential of clinical and translational science. For example, interoperability and connectivity issues among data sources, along with privacy concerns, cultural barriers, and lack of incentives, impede data sharing among researchers and across sectors (e.g., academia, industry). Nevertheless, a recent IOM workshop report noted that “research advances derived from data pooling and analysis could improve public health, enhance patient safety, and spur drug development” (IOM, 2013c, p. 1). Fragmentation, lack of standardization/heterogeneity, and the uneven accuracy and
quality of available health data are additional challenges for researchers (IOM, 2010, 2013c).
Finally, funding limits are forcing stakeholders to set priorities, share resources, and target their investments. Today, industry’s focus has shifted away from the full range of bench-to-bedside discoveries toward investments in potential products that have already completed the early phases of research (Butler, 2008; Reed et al., 2012). Resource scarcity has focused attention on research value, and increasingly stakeholders, including research funders, the public, and Congress, are demanding evidence of returns on investments and greater accountability (Austin, 2013; Reed et al., 2012; Shuster, 2012).
Although “measuring the outcomes of translational research is notoriously difficult” (Butler, 2008, p. 842) for many reasons (e.g., significant time lapses between original clinical trials and measurable impacts on population health or clinical practice), tracking research outcomes is necessary in order to improve accountability and efficiency of translational research, as well as overall health care system performance.
Responding to a Changing Ecosystem
In response to this shifting health care landscape, the clinical and translational research ecosystem has begun to reinvent and realign itself. These adaptations include enhanced collaboration, emerging data and technology, streamlined institutional review board (IRB) processes and enhanced patient protections, broader research participant recruitment efforts, and development of a dynamic research workforce.
Enhanced Collaboration
A growing number of private and public institutions are collaborating to share limited resources for clinical and translational research activities. The resulting coordination is creating broader research networks, enhancing facilitation of investigator-initiated projects, and improving the validity of patient-centered research outcomes.2 It also has
_____________________
2 For example, the NIH initiative PROMIS (Patient Reported Outcomes Measurement Information System) was designed to provide flexible yet valid, precise, and responsive assessment tools to measure self-reported health status (Fries et al., 2011; NIH PROMIS, 2013).
the potential to accelerate the translation of research findings into clinical practice (Lieu et al., 2011; Main et al., 2012; Marantz et al., 2011; Melese et al., 2009). These partnerships build on the strength of the stakeholders, expand the stakeholders’ reach and capacity, and push boundaries on roles in translational science.
Diverse collaborations and novel roles and partnerships among academic, industry, and nonprofit organizations are emerging. Pfizer’s Centers for Therapeutic Innovations are partnering with academic institutions through jointly staffed laboratories and shared access to compound libraries and screening technologies (Pfizer, 2013). Disease advocacy organizations have spun off drug discovery and development entities (e.g., Cystic Fibrosis Foundation Therapeutics, Inc.) (CFF, 2013).
Academic health centers, which once served as the exclusive “home” for clinical investigation, now share the field with other types of research organizations. Health maintenance organizations (HMOs), hospital networks, health care systems, community health centers, and practice-based research networks (PBRNs) have established their own research networks to conduct and facilitate clinical studies (Calmbach et al., 2012; Lieu et al., 2011). The conduct of translational research in primary care settings can lead to improvements in patient care by directly evaluating the feasibility of an intervention or protocol (Calmbach et al., 2012; Fulda et al., 2011). For example, the North Texas Primary Care Practice-Based Research Network (NorTex), housed within the Primary Care Research Center at the University of North Texas Health Science Center, is engaged in a range of studies involving more than 300 physicians at 135 clinics (Fulda et al., 2011). CTSAs provide an opportunity to further strengthen collaborative programs with HMOs and PBRNs in order to accelerate patient-focused research initiatives (also see Chapter 3). The NIH has established the Common Fund’s Health Care Systems (HCS) Research Collaboratory, which encourages partnerships between health care delivery systems rather than relying on single research centers (Matthews, 2012; NIH, 2012b). The Collaboratory enhances “the national capacity to implement cost-effective large-scale research studies” (NIH, 2012b). In 2010 the Patient-Centered Outcomes Research Institute was established to provide the best available evidence to help patients and their health care providers make more informed decisions (Burns, 2012; PCORI, 2013). These new entities and collaborations are bridging gaps between research and practice in ways that could expedite the translational process.
Community engagement is becoming an established principle in facilitating and strengthening clinical research (Task Force on the Principles of Community Engagement, 2011; Zerhouni, 2005). Government, patients, families, and advocacy groups are increasingly recognizing the value of community engagement. As a result, patient advocacy groups, health care providers, and community organizations are assuming more active roles in processes related to peer review, research protocol design, recruitment and retention of participants in clinical research, and translation of findings back to the community. Studies of broader community involvement could strengthen evidence of the value of community participation and provide best practices of community engagement across all phases of clinical and translational research.
Emerging Data and Technology
The public- and private-sectors are further increasing access to new sources of health-related data and forging new partnerships to revolutionize the way this information is disseminated and used. For example, the Health Data Initiative, launched in 2010 by HHS and the IOM, “is a public–private collaboration that encourages innovators to utilize health data to develop applications to raise awareness of health and health system performance and spark community action to improve health” (IOM, 2013b).
Advances in computational abilities and connectivity, as well as implementation of EHRs, are facilitating many clinical and translational research projects. Each year, the capacity to share information rises by approximately 30 percent (Hilbert and López, 2011; IOM, 2011). New developments in bioinformatics allow researchers to store, retrieve, organize, protect, and analyze vast amounts of data, resulting in larger and more efficient clinical research and trials (CTSA PIs, 2012). This capacity is enhanced by developing technologies, such as “mobile and social computing with advanced analytics to enable fact-based decision-making” (Cognizant, 2012b, p. 5). These rapidly changing technologies hold great promise for drug development and first-in-human studies (Melese et al., 2009; Waldman and Terzic, 2010).
Data and computational capabilities are “expanding the reach of knowledge, increasing access to clinical information when and where needed, and assisting patients and providers in managing chronic diseases” (IOM, 2013a, p. 15). Health information technology also has
the potential to improve the quality and efficiency of the care that patients receive, through improved diagnostic practices and personalized medicine (Friedman et al., 2010). For example, in Arkansas, Blue Health Intelligence is applying predictive analytics to claims databases in order to reduce costs by improving the care of patients with diabetes (Rosenbush, 2012). EHRs, now used by more than half of all physicians in the United States (Jamoom et al., 2012), enable HMOs, PBRNs, and other research networks to serve as venues and partners for patientoriented research (e.g., comparative-effectiveness research) (Elliott, 2012; Miriovsky et al., 2012). In addition, studies are currently investigating the potential usefulness of EHRs in improving patient participation in their care and overall outcomes (University of Illinois at Urbana-Champaign, 2011).
Streamlined IRB Review Processes and Enhanced Patient Protections
Increased coordination between a growing number of private and public institutions has led to streamlined research oversight while enhancing patient protections. In compliance with federal regulations governing human subject research,3 a number of multisite studies are choosing to use single IRBs to coordinate joint, multi-institution IRB review of research protocols. For example, the Central Institutional Review Board Initiative performs a single review of some National Cancer Institute–sponsored multicenter protocols, allowing the local IRB to focus solely on ethical issues unique to local conditions (Millum and Menikoff, 2010). The CTSA Consortium’s IRBshare system facilitates multisite studies by using shared review documents that are supported by a centralized secure Web portal and an IRBshare Master Agreement (Vanderbilt University, 2013).
New approaches to clinical trial monitoring also strengthen research participant protections. In response to increasingly variable investigator experience, ethical oversight, site infrastructure, health care standards, treatment choices, and the globalization of clinical research (Glickman et al., 2009), many study sponsors are capitalizing on new technologies and strategies to enhance the efficiency and effectiveness of monitoring activities. For example, centralized monitoring allows sponsor organizations to assess data trends and access data remotely to identify deviations or problems more efficiently (Bhatt, 2011; Cognizant, 2012a; FDA,
_____________________
3 45 C.F.R. 46.
2011). Moreover, risk-based monitoring verifies completion of critical study parameters that protect human research participants while maintaining study integrity (FDA, 2011).
Broader Research Participant Recruitment
Research participant recruitment efforts have increased and diversified in response to persistent barriers to enrollment and retention of research participants. Research participants now include individuals in randomized trials, patients and their records within health care networks, and collaborating institutions and community organizations. As noted by Harris and colleagues (2012), however, the general public has little knowledge about clinical research or how to participate, despite an interest in clinical trials. ResearchMatch, a CTSA-developed tool, was created to overcome recruitment challenges by connecting volunteers and researchers. In its first 19 months, approximately half of the studies that used ResearchMatch were clinical trials. Other types of research using ResearchMatch include behavioral and psychosocial studies, observational studies, and community-based research (Harris et al., 2012). Collaborations between CTSAs and organizations such as PBRNs, are also working to engage a wide range of community members across the entire clinical research process, including clinical research participant recruitment (NCATS, 2012).
Development of a Dynamic Research Workforce
The continued success of clinical and translational research depends on an adaptable, well-trained, and diverse workforce. As the field of translational science continues to evolve, so too must programs continue to update the skills of the existing workforce as well as prepare the next generation of scientists and clinicians. Demographic shifts in the U.S. population require a strong commitment to achieving greater participation of underrepresented groups in clinical and translational research careers at both the investigator and patient care levels. Effective clinical and translational research requires teams of researchers that can traverse the divides between basic and clinical sciences and health care practice. Key members of these teams will be clinician-scientists. The proportion of physicians involved in research has declined steadily in recent decades, however, and innovative solutions and incentives are needed to reverse this trend (Roberts et al., 2012). Investments in interdisciplinary
training and mentoring programs continue to be a core function of the CTSA Program, offering opportunities to bolster the research workforce. Continuous innovations are necessary to encourage multidisciplinary, team-based science—a core CTSA principle (Kroenke et al., 2010; Pienta et al., 2011).
Despite the accelerating pace of scientific discovery, the current clinical research enterprise does not adequately tackle pressing clinical and methodological questions relevant to health and health care improvement. CTSAs can play a substantial role in facilitating efforts to remediate limitations in the clinical and translational research ecosystem such as the following: challenges associated with first-in-human studies; limited recruitment and retention in clinical trials; the identification and measurement of health outcomes to assess intervention effectiveness; barriers to increasing awareness about research resources and potential research partnerships at the investigator and community levels; lack of incentives for team-based science; policy and regulatory challenges in developing full and substantive collaborations with industry and other partners; and ethical concerns (including related regulatory requirements) associated with the interplay between clinical research and practice.
The CTSA Program has been successful in establishing CTSAs as academic focal points for clinical and translational research. Its 61 awardees can have a broad impact on research practices and informatics and, ultimately, on patient care and individual health outcomes. The CTSAs continue to provide funding and infrastructure to investigators in their local environments, facilitating the conduct of clinical and translational research and the training of the translational science workforce. In addition, the CTSA Program has begun to foster interactions with the community and outside partners, such as the pharmaceutical industry.
Yet, more progress is needed in order to assure a proactive research environment responsive to the demands of a continually evolving health care landscape. As the new home for the CTSA Program, NCATS has described a commitment to building on the program’s initial accomplishments with further advances. NCATS has called the next phase of the program CTSA 2.0 (Briggs and Austin, 2012), a term the IOM committee has adopted and uses in this report. The challenge for
this next phase of the CTSA Program will be to set the goals and create the incentives for these 61 sites to function as the core of a national network that initiates and sustains collaborations both inside and outside their home institutions, across NIH institutes and centers, and with community, industry, and research network partners.
The IOM committee envisions a transformation of the CTSA Program from its current, loosely organized structure into a more tightly integrated network, with all the sites, committees, and coordinating center working collectively to enhance the transit of therapeutics, diagnostics, and preventive interventions along the developmental pipeline; disseminate innovative translational research methods and best practices; and provide leadership in informatics standards and policy development to promote shared resources. By providing infrastructure and innovations to accelerate clinical and translational research, an increasingly networked CTSA Program will increase its benefit to research and researchers across diseases, health conditions, age ranges, and health care delivery systems.
To reach its potential in an ever-changing environment, the CTSA Program must build on its core strengths and successes and transform CTSAs from academic research homes to active hubs in a fully integrated network of clinical and translational research. On the basis of its findings, the IOM committee identified four key opportunities for action to guide efforts to strengthen the CTSA Program and ensure future success:
• Adopt and sustain active program leadership—NCATS should increase its leadership presence in the overall program, consistent with the cooperative agreement model under which the CTSAs are funded. A centralized leadership model that includes participation by NCATS, leaders of individual CTSAs, community partners, and other stakeholders will increase overall program efficiency, enable mechanisms for maximizing accountability, and provide the direction needed to develop and nurture substantive partnerships.
• Engage in substantive and productive collaborations—The CTSA Program needs to capitalize on the collaborations developed within and among individual CTSAs and continue to initiate and forge true partnerships with other NIH institutes and centers and with entities external to the program, including
patient groups, communities, health care providers, industry, and regulatory organizations.
• Develop and widely disseminate innovative research resources—Fully developing the role of the CTSA Program as a facilitator and accelerator of clinical and translational research will require enhanced efforts to engage and support researchers and other stakeholders as they develop, refine, widely disseminate, and implement novel research and health informatics tools, methodologies, policies, and other resources.
• Build on initial successes in training and education, community engagement, and child health research—The CTSA Program needs to continue its strong efforts in each of these areas. A robust and diverse workforce that is well trained in team science is critically important. Ensuring an emphasis on community involvement across the research spectrum will bring a range of much-needed perspectives and innovations along with increased public support for research. Program efforts can also help overcome the paucity of research specific to child health.
Austin, C. P. 2013. National Center for Advancing Translational Sciences: Catalyzing translational innovation. PowerPoint presented at Meeting 3: IOM Committee to Review the CTSA Program at NCATS, Washington, DC, January 24. http://www.iom.edu/~/media/Files/Activity%20Files/Research/CTSAReview/2013-JAN-24/Chris%20Austin.pdf (accessed February 13, 2013).
Bhatt, A. 2011. Quality of clinical trials: A moving target. Perspectives in Clinical Research 2(4):115–150.
Briggs, J., and C. P. Austin. 2012. NCATS and the evolution of the Clinical and Translational Science Award (CTSA) Program. PowerPoint presented at Meeting 1: IOM Committee to Review the CTSA Program at NCATS, Washington, DC, October 29. http://www.iom.edu/~/media/Files/Activity%20Files/Research/CTSAReview/2012-OCT-29/IOM%20Briggs-Austin%20102912.pdf (accessed February 13, 2013).
Burns, J. 2012. What works best for patients? PCORI hopes to provide answers. Managed Care, December: 36–39. http://www.managedcaremag.com/archives/1212/1212.pcori.html (accessed February 13, 2013).
Butler, D. 2008. Translational research: Crossing the valley of death. Nature 453(12):840–842.
Calmbach, W. L., J. G. Ryan, L.-M. Baldwin, and L. Knox. 2012. Practice-Based Research Networks (PBRNs): Meeting the challenges of the future. Journal of the American Board of Family Medicine 25(5):572–576.
Casagrande, S. S., J. E. Fradkin, S. H. Saydah, K. F. Rust, and C. C. Cowie. 2013. The prevalence of meeting A1C, blood pressure, and LDL goals among people with diabetes, 1988–2010. Diabetes Care, February 15. http://care.diabetesjournals.org/content/early/2013/02/07/dc12-2258.full.pdf+html (accessed April 3, 2013).
CFF (Cystic Fibrosis Foundation). 2013. Cystic Fibrosis Foundation therapeutics. http://www.cff.org/research/cfft (accessed May 6, 2013).
Chappel, R. J., K. M. Wilson, and E. M. Dax. 2009. Immunoassays for the diagnosis of HIV: Meeting future needs by enhancing the quality of testing. Future Microbiology 4(8):963–982.
Cognizant. 2012a. Cognizant’s clinical transformation: More than a solution, it’s a new way to work. http://www.cognizant.com/OurApproach/Cognizants-Clinical-Transformation-More%20-Than-Solution-Its-a-New-Way-to-Work.pdf (accessed April 10, 2013).
———. 2012b. A vision for U.S. healthcare’s radical makeover. http://www.cognizant.com/InsightsWhitepapers/A-Vision-for-US-Health-Radical-Makeover.pdf (accessed March 28, 2013).
Commonwealth Fund. 2011. Why not the best? Results from the National Scorecard on U.S. Health System Performance, 2011. Washington, DC: Commonwealth Fund. http://www.commonwealthfund.org/~/media/Files/Publications/Fund%20Report/2011/Oct/1500_WNTB_Natl_Scorecard_2011_web.pdf (accessedMarch 26, 2013).
CTSA PIs (Clinical and Translational Science Awards Principal Investigators). 2012. Preparedness of the CTSA’s structural and scientific assets to support the mission of the National Center for Advancing Translational Sciences (NCATS). Clinical and Translational Science 5(2):121–129.
Elliott, V. S. 2012. Practices can use EMRs to join more clinical trials. AMA News. http://www.ama-assn.org/amednews/2012/03/12/bica0312.htm (accessed February 13, 2013).
EMBL-EBI (European Molecular Biology Laboratory-European Bioinformatics Institute). 2013. 1000 Genomes Project. http://www.1000genomes.org/data (accessed May 7, 2013).
Faden, R. R., N. E. Kass, S. N. Goodman, P. Pronovost, S. Tunis, and T. L. Beauchamp. 2013. An ethics framework for a learning health care system: A departure from traditional research ethics and clinical ethics. Hastings Center Special Report 43(1):s16–s27.
FDA (Food and Drug Administration). 2011. Guidance for industry: Oversight of clinical investigations—a risk-based approach to monitoring: Draft guidance. Rockville, MD: FDA. http://www.fda.gov/downloads/Drugs/.../Guidances/UCM269919.pdf (accessed April 10, 2013).
———. 2012. FDA approves first over-the-counter home-use rapid HIV test. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm310542.htm (accessed April 3, 2013).
Friedman, C. P., A. K. Wong, and D. Blumenthal. 2010. Achieving a nationwide learning health system. Science Translational Medicine 2(57):1–3.
Fries, J., M. Rose, and E. Krishnan. 2011. The PROMIS of better outcome assessment: Responsiveness, floor and ceiling effects, and Internet administration. Journal of Rheumatology 38(8):1759–1764.
Fulda, K. G., K. A. Hahn, R. A. Young, J. D. Marshall, B. J. Moore, A. M. Espinoza, N. M. Beltran, P. McFadden, A. D. Crim, and R. Cardarelli. 2011. Recruiting Practice-based Research Network (PBRN) physicians to be research participants: Lessons learned from the North Texas (NorTex) needs assessment study. Journal of the American Board of Family Medicine 24(5):610–615.
Glickman, S. W., J. G. McHutchison, E. D. Peterson, C. B. Cairns, R. A. Harrington, R. M. Califf, and K. A. Schulman. 2009. Ethical and scientific implications of the globalization of clinical research. New England Journal of Medicine 360(8):816–823.
Harris, P. A., K. W. Scott, L. Lebo, N. Hassan, C. Lightner, and J. Pulley. 2012. ResearchMatch: A national registry to recruit volunteers for clinical research. Academic Medicine 87(1):66–73.
Hilbert, M., and P. López. 2011. The world’s technological capacity to store, communicate, and compute information. Science 332(6025):60–65.
IOM (Institute of Medicine). 1998. Statement on quality of care. Washington, DC: National Academy Press.
———. 2001. Crossing the quality chasm: A new health system for the 21st century. Washington, DC: National Academy Press.
———. 2010. Clinical data as the basic staple of health learning: Creating and protecting a public good: Workshop summary. Washington, DC: The National Academies Press.
———. 2011. Visioning perspectives on the digital health utility. Chapter 2. In Digital infrastructure for the learning health system: The foundation for continuous improvement in health and health care: Workshop series summary. Washington, DC: The National Academies Press.
———. 2013a. Best care at lower cost: The path to continuously learning health care in America. Washington, DC: The National Academies Press.
———. 2013b. The health data initiative. http://www.iom.edu/Activities/PublicHealth/HealthData.aspx (accessed March 28, 2013).
———. 2013c. Sharing clinical research data: Workshop summary. Washington, DC: The National Academies Press.
Jamoom, E., P. Beatty, A. Bercovitz, D. Woodwell, K. Palso, and E. Rechtsteiner. 2012. Physician adoption of electronic health record systems: United States, 2011. Hyattsville, MD: National Center for Health Statistics. http://www.cdc.gov/nchs/data/databriefs/db98.htm (accessed April 3, 2013).
Kemp, K. B. 2012. Research insights: Using evidence to build a learning health care system. Washington, DC: AcademyHealth. http://www.academyhealth.org/files/publications/AHUsingEvidence2012.pdf (accessed April 10, 2013).
Kroenke, K., W. Kapoor, M. Helfand, D. O. Meltzer, M. A. McDonald, H. Selker, and CTSA Consortium Strategic Goal Committee on Comparative Effectiveness Research: Workgroup on Workforce Development. 2010. Training and career development for comparative effectiveness research workforce development. Clinical and Translational Science 3(5):258–262.
Larson, E. B., C. Tachibana, and E. H. Wagner. 2013. Sparking and sustaining the essential fuctions of research: What supports translation of research into health care? Answers from the Group Health experience. In Enhancing the professional culture of academic health science centers: The organizational environment and its impact on research, edited by T. S. Inui and R. M. Frankel. London, UK: Radcliffe.
Lieu, T. A., V. L. Hinrichsen, A. Moreira, and R. Platt. 2011. Collaborations in population-based health research. Clinical Medicine and Research 9(3/4):137–140.
Main, D. S., M. C. Felzien, D. J. Magid, B. N. Calonge, R. A. O’Brien, A. Kempe, and K. Nearing. 2012. A community translational research pilot grants program to facilitate community-academic partnerships: Lessons from Colorado's Clinical Translational Science Awards. Progress in Community Health Partnerships: Research, Education, and Action 6(3):381–387.
Marantz, P. R., A. H. Strelnick, B. Currie, R. Bhalla, A. E. Blank, P. Meissner, P. A. Selwyn, E. A. Walker, D. T. Hsu, and H. Shamoon. 2011. Developing a multidisciplinary model of comparative effectiveness research within a Clinical and Translational Science Award. Academic Medicine 86(6):712–717.
Matthews, S. 2012. New NIH effort seeks to find ways to make trials run smoother. Nature Medicine 18(11):1598.
Melese, T., S. M. Lin, J. L. Chang, and N. H. Cohen. 2009. Open innovation networks between academia and industry: An imperative for breakthrough therapies. Nature Medicine 15(5):502–507.
Millum, J., and J. Menikoff. 2010. Streamlining ethical review. Annals of Internal Medicine 153(10):655–657.
Miriovsky, B. J., L. N. Shulman, and A. P. Abernethy. 2012. Importance of health information technology, electronic health records, and continuously aggregating data to comparative effectiveness research and learning health care. Journal of Clinical Oncology 30(34):4243–4248.
NCATS (National Center for Advancing Translational Sciences). 2012. Request for information: Enhancing the Clinical and Translational Science Awards Program. http://www.ncats.nih.gov/files/report-ctsa-rfi.pdf (accessed April 8, 2013).
NIH (National Institutes of Health). 2012a. 1000 Genomes Project available on Amazon Cloud. http://www.nih.gov/news/health/mar2012/nhgri-29.htm (accessed April 3, 2013).
———. 2012b. NIH funds will strengthen national capacity for cost-effective, large-scale clinical studies. NIH News. hhtp://www.nih.gov/news/health/sep2012/nccam-25.htm (accessed February 13, 2013).
NIH PROMIS (Patient Reported Outcomes Measurement Information Systems). 2013. PROMIS: Patient Reported Outcomes Measurement Information Systems. http://www.nihpromis.org (accessed February 13, 2013).
PCORI (Patient-Centered Outcomes Research Institute). 2013. Patient-Centered Outcomes Research Institute (PCORI): About us. http://www.pcori.org/about-us (accessed February 13, 2013).
Pfizer. 2013. Centers for Therapeutic Innovation. http://www.pfizer.com/research/rd_works/centers_for_therapeutic_innovation.jsp (accessed May 6, 2013).
Pienta, K. J., J. Scheske, and A. L. Spork. 2011. The Clinical and Translational Science Awards (CTSAs) are transforming the way academic medical institutions approach translational research: The University of Michigan experience. Clinical and Translational Science 4(4):233–235.
Porter, M. E. 2010. What is value in health care? New England Journal of Medicine 363(26):2477–2481.
Porter, M. E., and E. O. Teisberg. 2006. Redefining health care: Creating valuebased competition on results. Cambridge, MA: Harvard Business School Press.
Pritts, J. L. 2008. The importance and value of protecting the privacy of health information: The roles of the HIPAA Privacy Rule and the Common Rule in health research. Commissioned by the Committee on Health Research and the Privacy of Health Information, Washington, DC: Institute of Medicine. http://www.iom.edu/~/media/Files/Activity%20Files/Research/HIPAAandResearch/PrittsPrivacyFinalDraftweb.ashx (accessed May 7, 2013).
Reed, J. C., E. L. White, J. Aube, C. Lindsley, M. Li, L. Sklar, and S. Schreiber. 2012. The NIH’s role in accelerating translational sciences. Nature Biotechnology 30(1):16–19.
Roberts, S. F., M. A. Fischhoff, S. A. Sakowski, and E. L. Feldman. 2012. Perspective: Transforming science into medicine: How clinician-scientists can build bridges across research’s “valley of death.” Academic Medicine 87(3):266–270.
Rosenbush, S. 2012. Blue Cross expects costs savings from big data dive. CIO Journal, March 30, 2012. http://mobile.blogs.wsj.com/cio/2012/03/30/blue-cross-expects-cost-savings-from-big-data-dive (accessed May 7, 2013).
Shuster, J. J. 2012. U.S. government mandates for clinical and translational research. Clinical and Translational Science 5(1):83–84.
Task Force on the Principles of Community Engagement (Clinical and Translational Science Awards Consortium Community Engagement Key Function Committee Task Force on the Principles of Community
Engagement). 2011. Principles of community engagement: Second edition. NIH Publication No. 11-7782. http://www.atsdr.cdc.gov/communityengagement/pdf/PCE_Report_508_FINAL.pdf (accessed April 2, 2013).
University of Illinois at Urbana-Champaign. 2011. Medtable: An electronic medical record strategy to promote patient medication understanding and use, NCT01296633. http://www.clinicaltrials.gov/ct2/show/NCT01296633?term=medtable&rank=1 (accessed February 13, 2013).
Vanderbilt University. 2013. IRBshare. https://www.irbshare.org (accessed February 13, 2013).
Waldman, S. A., and A. Terzic. 2010. Molecular therapy drives patient-centric healthcare paradigms. Clinical and Translational Science 3(4):170–171.
Zerhouni, E. A. 2005. Translational and clinical science—time for a new vision. New England Journal of Medicine 353(15):1621–1623.


















