2
Neuroscience, Biomechanics, and Risks of Concussion in the Developing Brain
Over the past 30 years, a number of experimental models of traumatic brain injury (TBI) have been developed to study various aspects of TBI in humans. This area of research originally focused on adult animal models of moderate to severe brain injuries, and these models have significantly contributed to our understanding of the biomechanics and neurochemical changes that occur after TBI. More recently, research efforts have focused on animal models of mild TBI (mTBI) and concussions, but only a few studies have addressed age and sex differences in injuries of this severity. TBI of any severity in the developing brain is complicated by ongoing cerebral maturation. The goal of this chapter is to (1) summarize the normal changes that occur with brain maturation; (2) explain the biomechanics involved in generating brain injuries of a range of severities; (3) summarize what is known about the neurochemical and metabolic changes that occur after concussions; and (4) describe risk factors for concussions in youth. In the section on the biomechanics of concussion, the committee responds to the portion of its charge concerning physical and biological thresholds for concussive injury.
The human brain is a complex system of connections that continues to be refined and reshaped throughout an individual’s lifespan. During development, rapid changes in synapses, myelination, and metabolism occur, with the brain achieving adult-like connections by the mid-20s (see Figure 2-1). The changes in both the structural architecture and the func-
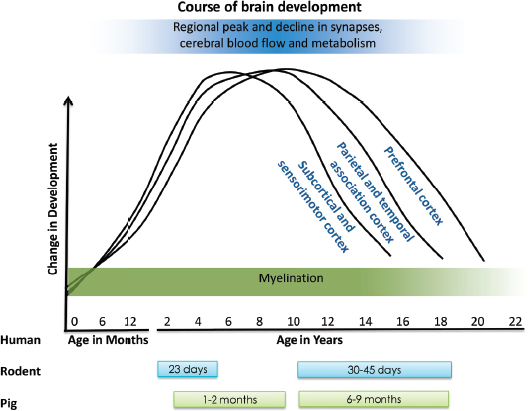
FIGURE 2-1 Profiles of parameters of human brain development and the estimated age ranges for research animal models. This figure illustrates the timing of changes in the number of brain synapses, cerebral blood flow, and metabolism (blue) and in brain myelination (green). The colored bars below the figure reflect the approximated human age equivalent of the rat (blue) and pig (green) based on species specific developmental profiles.
SOURCE: Adapted from Casey et al., 2005.
tional organization of the brain reflect a dynamic interplay of progressive and regressive events that occur simultaneously as the developing individual interacts with the environment. Although total brain size is about 90 percent of adult size by age 6 years, the brain continues to undergo dynamic changes throughout adolescence and into young adulthood (Yakovlev and Lecours, 1967). Current neuroimaging methods do not have the resolution to delineate the processes that underlie the observed developmental changes beyond observations of the brain’s gray and white matter subcomponents. The techniques do, however, allow for assessment of functional sequelae of concussions and, together with animal models, suggest that the developing brain responds differently to concussion than does the mature brain (Choe et al., 2012; Shrey et al., 2011).
Synaptic Density
Brain cells communicate with each other through synapses, which are dynamic points of contact between cells where chemicals called neurotransmitters are transferred. During brain development there are dramatic changes in the number of synapses in the brain, with different regions of the brain developing at different rates and times (Bourgeois et al., 1994; Huttenlocher and Dabholkar, 1997). There is a dramatic increase in the number of synapses during the first few years of life. This production of synapses is followed by a prolonged period of synaptic pruning that, based on an individual’s experiences, eliminates weaker synaptic contacts while retaining and strengthening the stronger connections. This pruning process occurs earlier in the auditory and visual cortex (by approximately 12 years of age), than in areas of the prefrontal cortex, which plays a role in executive functions such as problem solving and decision making (by approximately 18 years of age) (Bourgeois et al., 1994; Huttenlocher and Dabholkar, 1997).
Gray and White Matter
Gray and white matter are two major components of the central nervous system (CNS), which includes the brain and the spinal cord. Gray matter is associated with processing and cognition, while white matter is involved in coordinating communication between different brain regions. Longitudinal studies using magnetic resonance imaging (MRI) to map the developmental time-course of structural changes in the normal brain indicate that increases in white matter are linear throughout childhood and adolescence and continue well into young adulthood. In contrast, the growth in gray matter volume shows an inverted U-shaped course, first increasing during the first few years of life and then decreasing during adolescence (Giedd, 2004; Giedd et al., 1999, 2012; Gogtay et al., 2004; Sowell et al., 2004). These changes do not occur uniformly throughout an individual’s development. The primary sensorimotor regions mature during early adolescence, while parietal and prefrontal regions, which are important for attention and working memory, have a more protracted development, lasting into young adulthood (Gogtay et al., 2004; Sowell et al., 2004). In general, regions of cortical gray matter (parietal, frontal, and temporal) develop earlier in females than in males during adolescence (Giedd et al., 2012). Concussions and other head injuries can result in changes to the integrity of gray and white matter.
Cerebral Blood Flow and Glucose Metabolism
In parallel with the structural changes that occur with brain development, there are changes in cerebral glucose metabolism and cerebral blood flow (CBF), which refers to the blood supply to the brain. Normal CBF at birth is lower than the adult rate of 50ml/min/100g (Chiron et al., 1992). Blood flow rates increase rapidly during the first year of life and are 50 to 80 percent higher than flow rates in adults by the time a child is 5 to 6 years of age. CBF rates then decrease gradually during childhood and adolescence, reaching adult values when an individual is between 15 and 19 years of age. CBF varies with age and sex (Tontisirin et al., 2007; Udomphorn et al., 2008; Vavilala et al., 2005), with adolescents and adult females showing greater middle cerebral artery flow rates than male adults (Bakker et al., 2004; Vavilala et al., 2002a).
Given that cerebral substrate supply and utilization are linked, it is not surprising that the developmental profile of cerebral glucose uptake mirrors that of CBF in both animals and humans (Nehlig et al., 1989). Positron emission tomography (PET) has been used to image the regional changes in glucose uptake of the normal developing brain. This research shows that the overall cerebral metabolic rate of glucose consumption (CMRglc) at birth are 30 percent lower than they are for adults and that glucose uptake increases sharply after birth to peak at approximately 4 years of age (Chugani, 1998). CMRglc plateaus at 50-60 μmol/min/100g between 4 and 10 years, followed by a decline in glucose uptake until the uptake reaches adult rates by the age of 16 to 18 years.
Changes in CBF, cerebral metabolic rates for oxygen, and CMRglc mirror one another as they peak during early childhood and gradually decrease to adult levels (Udomphorn et al., 2008). CBF is coupled to glucose metabolism, partial pressure of carbon dioxide, partial pressure of oxygen, and blood viscosity (Len and Neary, 2011). Several studies have demonstrated that females from 4 to 8 years of age and between 10 and 16 years of age show greater middle cerebral artery and basilar artery blood flow velocities relative to males of the same age (Tontisirin et al., 2007; Vavilala et al., 2005). Compared with adults, youth ages 12 to 17 years show lower autoregulation of blood flow and higher blood flow velocities (Vavilala et al., 2002b). While evidence in the literature sufficiently demonstrates the existence of age and sex differences in the maturation of these couplings, it is also clear that much more research needs to be done to understand the extent and significance of these differences in regard to how they affect cerebral response to concussions and other brain injuries.
Behavioral Changes
Sleep patterns change dramatically during brain maturation. The average sleep duration for infants is 12.7 hours and for toddlers is 11.9 hours (Galland et al., 2012). Sleep duration is influenced by the start of school attendance, which begins around 5 or 6 years of age. The average sleep duration gradually decreases from approximately 10.5 hours during elementary school (5- to 10-year-olds) to about 9.3 hours during middle school (11- to 13-year-olds) (Iglowstein et al., 2003). During adolescence, biological sleep patterns shift toward later times for both sleeping and waking (Malone, 2011). Although the optimal sleep duration for adolescents is about 9 hours each night (Carskadon et al., 2004), the average high schooler (14- to 18-year-olds) gets between 6 and 8 hours of sleep each night, largely due to early school start times and wake-inducing activities (e.g., social and academic activities) that interfere with sleep. This reduction in sleep coincides with the period of adolescent cortical synaptic pruning. The possibility of interactive factors at work, with changes in sleep altering cognitive, linguistic, and emotional behaviors, should be considered when identifying sleep and cognitive impairments in youths following a concussion.
Adolescence is also a period of brain development that is marked by an increase in risk behaviors and addiction. The prefrontal cortex is important in executive decision making, the regulation of emotions, and the assessment of risk and reward (Bechara et al., 2000; Kelley et al., 2004; Romer, 2010; Spear, 2010). Protracted development of the prefrontal cortex may contribute to an increase in risk-taking behaviors in adolescence (Bava and Tapert, 2010; Casey and Jones, 2010; Dayan et al., 2010; Pharo et al., 2011). Such behaviors may include those that result in increased risk of injury. Compared with previous generations, today’s youth, due to an earlier average age of puberty (Biro et al., 2010), may have a greater mismatch between the propensity to engage in risk taking (which arises with the onset of puberty) and behavioral inhibition (which is associated with development of the prefrontal cortex).
The biomechanics of TBI (including concussions) is defined broadly as the interrelationships among the forces experienced during impact, head and neck movements, stiffness of the tissue that composes the head/neck complex, deformation of structures at the macroscopic and microscopic level, and the biological responses to the various loading conditions imposed on the head. The biological responses may be structural (torn vessels and axons) or functional (changes in blood flow or neurological status), and they may be immediate or delayed.
Understanding the biomechanics of sports-related concussions in youth requires knowledge about what head and neck movements and applied forces occur in an array of sporting environments, how the developing head and neck mechanical and biological properties change with age and gender, how mechanical responses change during development, and how tissue deformations or changes in physiology (e.g., CBF, metabolism) produced by these motions and forces may directly or indirectly cause a concussion. This information plays a pivotal role in understanding risk factors and designing practices and equipment to reduce the incidence of concussions in sports. The studies employed for biomechanics investigations typically include direct measurements of loading conditions and responses in humans, animals, and anthropomorphic surrogates (i.e., crash test dummies); visualization of tissue responses to prescribed loads in order to characterize the responses of complex geometries or composite structures; mechanical property testing of individual components in order to identify changes with age; computational models to predict how tissues will deform during impact or rapid head rotations; and identification of the time-course of cell or tissue responses to specified deformations in order to define thresholds associated with various types of injuries. Note that blast injuries are typically associated with exposure to extremely rapid pressure waves that cause rapid expansion and contraction of brain tissue as they pass through the brain; the biomechanics and characteristics of these types of brain injuries are distinct from most brain injuries that occur in sporting environments (Holbourn, 1945; Ommaya et al., 1971), and they will not be discussed further in this section. Non-blast-related impacts to the head that occur in the military setting may be governed by similar mechanics as those on the athletic field, however, and the concepts discussed in the following section are applicable.
Understanding the relationships between biomechanics and concussions in the developing brain is an essential step in creating protective devices to reduce the incidence of sports-related concussion and in developing rule changes aimed at reducing individuals’ exposure to hazardous conditions. This section summarizes the range of experimental platforms used to study brain injury, what has been learned from these studies about how brain injuries occur, and the many gaps in our understanding of how concussions occur in a youth sports environment.
Overview of Common Experimental Platforms for Understanding the Biomechanics of Traumatic Brain Injury
Investigators can use human data obtained in the field during sporting events to help understand what scenarios cause concussion. Typically, a sensor affixed to a helmet or a mouthpiece is used to measure the magnitude,
direction, and type (i.e., linear, rotational, centroidal, or non-centroidal) of head motion (see, for example, Camarillo et al., 2013; Crisco et al., 2010; Daniel et al., 2012; Rowson and Duma, 2013; Rowson et al., 2012). These sensors do not usually measure head impact forces directly (for an exception, see Ouckama and Pearsall, 2011), but rather they measure the head’s movement (acceleration or velocity) in response to an impact. The mechanical data recorded by the sensors are correlated with clinical assessments of injuries sustained on the sport field. However, such human data are influenced by equipment design limitations. For example, some devices are unable to measure actual head movements because of slipping between head and sensor, some cannot measure linear motions independently from angular motions or only report motion in a single plane of motion or a composite, and some sensors have errors or measurement variability that exceed reasonable standards. The human data may also be limited by inaccuracies in self-reports of concussions and difficulties in adequately adjusting for varying histories of previous impact exposures. Furthermore, the lack of control over the direction, extent, and number of head movements each subject experiences impedes the ability to determine whether the person-to-person variability in outcome measures is attributable to differences in impact forces or head motions or to differences in sex, age, and previous history of head injuries. As the current obstacles related to biomechanical measurements are addressed and objective clinical measures of concussion are improved, human data collected from on-field settings will play an increasingly valuable role in understanding what biomechanical conditions or predisposing factors contribute to concussions.
In 2012 the U.S. Army began using sensors to collect data on the effects of blasts on the body, including the mechanisms that lead to concussions and other traumatic brain injury in soldiers. Results are not yet published (Hoffman, 2012).
To obtain kinematic information in more controlled settings, humanlike anthropomorphic surrogates (i.e., crash test dummies) and laboratory-based studies are used to reenact film and witness accounts of sports-related events in order to estimate the forces of impact and head movements (kinematics). Surrogates and humans are also used to document the kinematics associated with non-injurious activities (Feng et al., 2010; Funk et al., 2011; Lloyd et al., 2011), which are important for identifying both tolerable and injurious kinematic conditions. It is important to note that surrogates measure only kinematic responses and that at this time, in the absence of accepted tolerance values, surrogates cannot be used to predict or measure concussions or tissue distortions. Instead, results obtained using surrogates must be correlated with animal studies, autopsy reports, and patient records to infer biological responses to kinematic loading conditions or with computational models to infer tissue deformations resulting from a head
rotation or impact. A final issue is that there are few surrogates for youth and no surrogate has been validated as representative of human responses in sports settings, where kinematic conditions are often considerably lower than in car crashes.
Computational models are used to estimate the tissue distortions and stresses that may result from the kinematics of a rapid head motion or head impact, but they are valuable for understanding mechanics only when they use life-like tissue stiffnesses. Brain and skull tissue stiffnesses are available for young children (infants and toddlers) and adults (Coats and Margulies, 2006; Elkin et al., 2010; Kaster et al., 2011; Prange and Margulies, 2002; Prevost et al., 2011), but there are few data for older youth. As is the case with surrogates, computational models cannot be used to directly measure concussion or axonal injury, skull fracture, or vessel rupture; instead, predicted deformations or stresses from the model must be compared to published tissue-specific thresholds in order to infer injury. An important point is that early data have demonstrated that the brain tissue distortions and stresses in the skull that are associated, respectively, with axonal injury and skull fracture are smaller in young children than in adults (Coats and Margulies, 2006; Ibrahim et al., 2010; Raghupathi and Margulies, 2002; Robbins and Wood, 1969), but there are no concussion tissue threshold data for older youth.
It is important to note that, typically, biomechanical thresholds of injury correspond to the risk of acute injury. Rarely is biomechanics used to develop injury thresholds for long-term consequences, repeated exposures, or predisposing biological conditions. The physiological response to an initial injury may continue for days or weeks (see discussion on the neurochemistry of concussion later in this chapter), potentially creating a prolonged period when the brain may respond differently to a second event (discussed in Chapter 5). It is unknown whether deformation injury thresholds for previously injured tissue, which may be hypoxic or metabolically compromised, are lower than for normally functioning tissue. Research at the intersection of biomechanics and physiology is required before investigators can predict whether a subsequent head rotation or impact after a concussion may be more damaging than a single event.
Another common application of computational models is to simulate the head response to an impact and, if human data for that event are known, to use the model’s estimates of tissue distortions to infer tissue deformations that may be associated with brain injury (Kimpara and Iwamoto, 2012; Kleiven, 2007; Takhounts et al., 2008). This indirect prediction of brain injury is hampered by the drawbacks described above and, given that brain injury is now understood to be heterogeneous, by the challenge of defining the critical deformations associated with various types and severity of the specific brain injury of interest (Saatman et al., 2008). Previous
biomechanics studies have linked tissue deformations to a spectrum of brain injuries and have demonstrated that deformation thresholds are age- and injury-specific and that the magnitude and rate of the distortion required to rupture a blood vessel are different from those required to injure an axon or cause a concussion (Coats et al., 2012; Monson et al., 2003; Smith et al., 1999; Zhu et al., 2006). It is widely accepted that smaller deformations may be associated with brief functional changes (deficits in synaptic transmission, signaling pathways, and membrane permeability; see Meaney and Smith, 2011) and that larger deformations may cause permanent structural changes (Cater et al., 2006; Elkin and Morrison, 2007). Thus, tissue distortions and the rates of tissue deformation associated with concussion (with no lingering neural or vascular structural changes visible in radiological imaging or pathology) are probably lower than those for more severe brain injuries (Gennarelli et al., 2003), so it is inappropriate to rely on a single threshold for all head injuries. Moreover, it is unknown whether a concussion is produced when a critical proportion of the entire brain experiences deformation above a threshold level or whether only certain brain locations must be exposed to deformation (King et al., 2003; Ommaya and Gennarelli, 1974). Furthermore, the research community has not reached a consensus regarding whether the most appropriate injury thresholds should be defined as an average value, a threshold associated with zero injury risk, or one associated with some modest acceptable chance of injury. For comparison, in automotive safety, vehicles and restraint systems are designed so that body regions experience mechanical loads below a threshold value associated with a chance of moderate to serious injury of anywhere from 15 to 50 percent (Kleinberger et al., 1998). Each of these gaps in knowledge about concussion thresholds limits the ability of computational models to predict whether a concussion would occur from a specific head rotation or impact. However, when appropriate and acceptable tissue thresholds specific to concussions in youth are determined in the future, computational models of the human head will be powerful platforms to identify dominant and secondary factors that contribute to the biomechanics of concussion, to integrate future data regarding synergistic effects of biology and biomechanics, and to develop rational guidelines for protective equipment.
Presently, because human data and computational models are limited, researchers use alternative idealized experimental preparations such as animals, tissues, and isolated cells to create controllable settings with similar predisposing conditions among subjects and reproducible mechanical loads. Animal models are useful for measuring physiological responses (e.g., reflexes, blood flow, tissue oxygen content, metabolic derangements); injuries to the vessels, axons, and neural cell bodies; and changes in motor, memory, learning, and behavioral aptitudes at prescribed time-points after injury.
Although they are the best substitute for humans, there are four chal-
lenges in using animal models to understand sports-related concussion youth. First, human concussion includes changes in mental status without a loss of consciousness, and no metrics have been developed to assess these subtle alterations in animals immediately after injury. Thus, the definition of brain injury in an animal model includes loss of consciousness, often accompanied with demonstrable changes in axon structure or function, appearance of hemorrhages, or longer-term alterations in neurological function. Because concussions may not be associated with axonal injury, hemorrhage, and loss of consciousness, animal models, even those of mTBI, most commonly involve more severe brain injuries than concussion. Second, informative injury models need to mimic the injuries seen in sporting environments (Wall and Shani, 2008), yet most brain injury models create focal hemorrhagic cortical lesions caused by direct impact to the skull or exposed brain (Xiong et al., 2013), while the human concussed brain is more commonly associated with distributed white matter alterations (Benson et al., 2007; Kraus et al., 2007). Third, most models use adult animals, and, given the developmental differences described earlier in this chapter, extensions to youth should be made with caution. Fourth, animal models most commonly involve mice and rats but have also included ovine, porcine, and nonhuman primate models (Browne et al., 2011; Durham et al., 2000; Finnie et al., 2012; Gennarelli et al., 1981, 1982; Viano et al., 2012). Recent reports indicate that rodents have limited fidelity to human genomic and proteomic responses, injury time-courses, and gray and white brain matter distribution (Duhaime et al., 2006; Seok et al., 2013), which implies that there are challenges in applying what is learned about injury in the developing rodent brain to the human child. Despite these four substantial challenges, animal models are a valuable tool for understanding how head impacts and sudden head movements translate to brain deformations and how brain deformations result in a spectrum of brain injuries, from mild to severe.
What Has Been Learned About How Traumatic Brain Injuries Occur
Using the tools described above, researchers have determined that with or without a helmet, when the head contacts a stationary or moving object there is a rapid change in velocity and a possible deformation of the skull. Skull deformation may produce a local contusion or hemorrhage if the deformations of the tissues exceed their injury thresholds. When the properties of the contact surfaces are softer or allow sliding or deformation, the rate of velocity change (acceleration or deceleration, depending on whether the velocity is increasing or decreasing) is lower. Similarly, if there is no head contact but only body contact (e.g., in a tackle), the deceleration of the moving head is usually lower than when the head is contacted directly.
After the initial rapid change in velocity caused by impact to the head
or body, the subsequent motion of the head is influenced by the location of that initial point of contact and the interaction between the head, neck, and body. There are three possible kinematic responses to head contact. First, if the contact is directed through the center of mass of the brain (i.e., is centroidal), there may be linear motion and no rotation of the head (e.g., a weight dropping down onto the top of the head or a blow to the back of the head that moves the ears and nose forward without neck flexion or extension). Animal studies have shown that these purely linear motions produce little brain motion or distortion and no concussion (Hardy et al., 2001; Ommaya and Gennarelli, 1974; Ommaya et al., 1971). However, most often the contact force is not directed centroidally through the brain, a situation that is referred to as a non-centroidal impact. After a non-centroidal contact, the head may rotate without a linear motion (e.g., shaking the head “no”). This purely rotational motion produces a distortion of the brain’s neural and vascular structures within the skull because the brain is softer than the skull and loosely coupled to the skull. More commonly, though, a head impact produces a change in head velocity that is associated with both linear acceleration and rotation of the head. This combined rotational and linear motion may occur because the contact is glancing (further away from the rotation center) or the body continues to move after the head is restrained by the contact surface or the head bounces or rebounds after contact. For these combined rotation/linear head responses, computational simulations have illuminated the relationship between the location of the head impact, the kinematic responses of the head (linear and rotational accelerations), and the predicted brain tissue deformations (Aare et al., 2004; Kleiven, 2007; Post et al., 2011). Specifically, for those unusual instances when the head impact is through the center of mass of the head, linear acceleration correlates with the rotational acceleration response and the average deformation response in the brain. In these situations, linear acceleration is a reasonable surrogate for the brain tissue distortion response. By contrast, in the more common non-centroidal head impacts, linear and rotational accelerations are not correlated significantly, and the rotational acceleration component of the head response correlates most strongly with the average and peak brain deformations. Thus, for the most common head contact events, the linear acceleration component does not describe all of the brain’s deformation response, and therefore when used alone, is not a robust predictor of injury risk.
Internal structures of the head, such as the falx cerebri and tentorium, influence how the brain moves within the skull and may cause local brain regions with very high deformations only in certain directions of head rotation, so that sagittal and coronal rotations may produce more severe injuries in primates at lower accelerations and velocities (Gennarelli et al., 1982). In addition, animal and human studies have shown a general
trend that higher rotational velocities and accelerations—rather than linear accelerations—can cause larger diffuse brain deformations and worse diffuse brain injuries (Gennarelli et al., 2003, Kimpara and Iwamoto, 2012; Ommaya et al., 1971) and that head injuries depend on the direction of head motion as well as on the magnitude of rotational kinematics (Eucker et al., 2011, Gennarelli et al., 1981).
Previous research attempted to tease out if it is the change in rotational velocity (“delta v”), or the rate of change in rotational velocity (rotational acceleration or deceleration), or the acceleration duration that best predicts injury. Kimpara and Iwamoto (2012) demonstrated that head impact scenarios have shorter durations and higher accelerations than non-head contact events. Ommaya and colleagues showed that unconsciousness occurred more readily in adult nonhuman primates when rapid head rotations were achieved via direct blows to the head than when they were achieved via a non-head contact whiplash motion (Ommaya et al., 1973); other research showed that unconsciousness occurred less frequently for a given change in velocity when a cervical collar was used to decrease rotation of the head (Ommaya, 1985). Similarly, animal models that allow more head rotation after impact or enhanced head or brain movement produce more severe brain injuries (Foda and Marmarou, 1994; Marmarou et al., 1994). It is difficult to determine whether change in rotational velocity and acceleration contribute independently to concussion because no studies have used high and low rotational accelerations for the same change in rotational velocity to determine the relative importance of these kinematic variables on injury risk.
Animal studies have indicated that it is important to limit the duration of exposure to acceleration, as research has shown that concussions occur when the duration of rotational acceleration is increased (Ommaya et al., 1966). Furthermore, animal studies have demonstrated that the location of brain deformation may affect the resulting injury, suggesting that even a concussion-specific brain deformation threshold may vary with region (Cater et al., 2006; Elkin and Morrison, 2007; Vink et al., 2001; Yoshino et al., 1991).
In summary, further research is needed to define the direction-specific and brain-region-specific thresholds for linear and rotational accelerations associated with concussions in youth. These thresholds may differ between youth and adults and may vary across the pediatric age spectrum. Finally, as mentioned above, the deformations associated with functional and structural impairment may differ, and tools need to be developed to accurately assess functional derangement. The physio-mechanical link between multiple impacts must be defined. Until these foundational biomechanics are defined, it will be premature to make predictions of concussions based
upon computational model simulations and head kinematic measurements in humans and surrogates.
Damage to brain tissue resulting from movement of the brain within the skull, as may occur with a head impact, initiates a cascade of molecular events that disrupt normal brain cell function. In this section the molecular processes that have been shown to characterize brain injuries are discussed in the context of age and sex. It should be noted that much of what is currently known about these processes is drawn from research involving animals and subjects sustaining moderate to severe brain injuries. Box 2-1 describes some of the experimental models that were used in the research described in this section to study the physiological response to brain injury in animal models.
Ionic Flux and Neurotransmitter Release
TBI induces immediate changes in brain neurochemistry (see Figure 2-2, Steps 1-3). Normally, significant cellular energy is used to keep ions distributed across the plasma membranes in such a way so as to maintain a membrane potential between –40 and –80 millivolts (mVs). Studies primarily involving rodents show that an indiscriminate efflux of potassium and glutamate (Katayama et al., 1990) and an influx of calcium follow immediately after a concussive fluid percussion brain injury (Enerson and Drewes, 2003; Osteen et al., 2001). The magnitude of the potassium rise in the extracellular space increases with injury severity (Katayama et al., 1990), with as much as a fivefold increase observed at 1.5 minutes post injury. These rapid increases resolve after approximately 2.5 minutes in mild injuries and within 6 minutes after more severe injuries. Increases in extracellular glutamate concentrations showed a similar time-course following TBI in the adult rat. The release of excitatory amino acids following severe brain injury in humans has been observed with microdialysis probes (Bullock et al., 1995, 1998; Persson and Hillered, 1992; Vespa et al., 1998). Research involving humans also provides evidence for neurotransmitter release after concussive injuries. In a study involving 12 concussed athletes (mean age 22.5 years) and 12 non-concussed athletes, Henry and colleagues used (1)H magnetic resonance spectroscopy ((1)H-MR) to noninvasively study acute metabolic changes post injury. The concussed athletes showed more symptoms and a significant decrease in N-acetylaspartylglutamic acid (NAA) in the primary motor cortex between 1 and 6 days post injury as compared with the non-concussed controls (Henry et al., 2010). The findings of decreased glutamate are in contrast to the changes seen in studies
BOX 2-1
Overview of Commonly Used Experimental Models of Traumatic Brain Injury
The fluid percussion device is commonly used to produce general brain movement injuries. Saline is injected rapidly into the epidural space of the brain of an experimental animal through a craniotomy upon release of a pendulum that generates a fluid wave pulse in a saline-filled cylinder (Lindgren and Rinder, 1965; Stålhammar, 1990). Various injury severities can be generated with this model, and the location of the impact site has been shown to determine histopathology (Vink et al., 2001). Lateral mild injuries produce general brain tissue deformation that produces metabolic derangements with no overt cell loss (Fineman et al., 1993; Prins et al., 1996). In contrast, mid-lateral injuries do generate a developing contusion core (Vink et al., 2001). This model has been used in numerous age groups to generate diffuse brain injuries with measurable cognitive deficits, and mild fluid percussion injuries mimic numerous aspects of concussive injuries (Prins and Hovda, 1998, 2001; Prins et al., 1996).
The weight drop injury model involves the release of a known weight onto either the unrestrained exposed skull to produce diffuse injury (Marmarou model) or directly onto the brain through a craniotomy to produce a focal contusion (Feeney model) (see Feeney et al., 1981). In both cases injury severity can be adjusted by the amount of weight and the height from which the weight is released. The Marmarou weight drop injury has been applied to the developing brain, both are well characterized in the adult brain, and milder injuries can replicate aspects of concussive injuries (Kane et al., 2012; Milman et al., 2005).
The controlled cortical impact (CCI) model uses a pneumatic piston that is driven a known depth and velocity into the exposed brain to produce an evolving contusion (Lighthall, 1988), or it can be applied to the closed skull to produce “concussive” types of injuries (Huh et al., 2007; Laurer et al., 2001; Prins et al., 2010). As with the other models, the depth of penetration and velocity can be varied to produce different injury severities in different age groups.
Fluid percussion, weight drop, and CCI injuries have been applied to various animal species, including mice, rats, cats, and piglets. Rotational injuries have been applied to rats (Davidsson and Risling, 2011; Li et al., 2010) as well as to piglets and primates (Browne et al., 2011; Ståhlhammer, 1986; Sullivan et al., 2013).
involving animals and more severe TBI, but the time-course of changes was not examined in this study. Scans collected between 1 and 6 days post injury were averaged and may not reflect the same time-course of changes observed in other studies. The animal studies were conducted immediately after impact, and the clinical studies were conducted 1 to 6 days post injury. There is currently little research that examines the time-course of these changes over longer periods and at different injury severities.
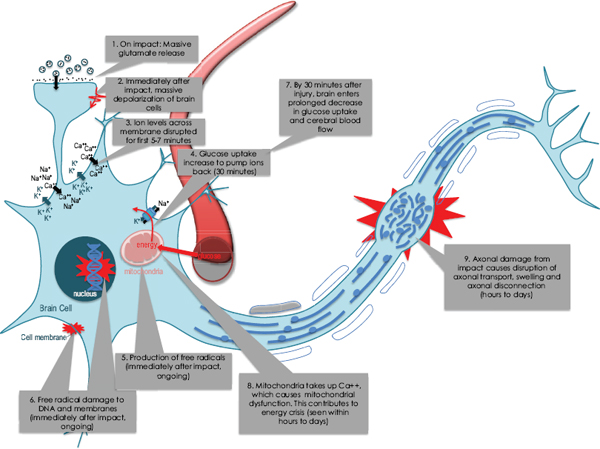
FIGURE 2-2 Neurochemical cascade observed after moderate traumatic brain injuries. Many of these events are believed to be involved to a lesser extent following mild and concussive brain injuries. Step 1 shows the indiscriminate release of glutamate and other neurotransmitters immediately after impact. These neurotransmitters bind to the post-synaptic membrane, causing rapid large-scale depolarization of brain cells (Step 2). The depolarization opens ionic channels, allowing many ions to flow down their concentration gradients and disrupting cell membrane potential (Step 3). Na+/K+ pumps work harder to reestablish the normal ionic gradient, but this requires cellular energy in the form of adenosine triphosphate, or ATP (Step 4). Glucose uptake into the brain transiently increases shortly after injury (Step 4), but within 30 minutes enters a prolonged stage of decreased glucose uptake (Step 7). During these early metabolic changes there are also increases in free radical production by the mitochondria (Step 5), which are increasingly compromised by their attempt to buffer intracellular calcium (Step 8). The free radicals contribute to protein, DNA, and lipid damage (Step 6). Additionally, the mechanical disruption of the impact can cause damage to microtubules, leading to axonal swelling, disruption of axonal transport, and eventual disconnection (Step 9).
Collectively, the animal studies of moderate brain injuries suggest that such injuries cause a release of signaling molecules which results in a disruption of the ionic balance across the cell membrane immediately after the injury event. Unless these ions are pumped back to the correct side of the cell membranes, the brain cells will not be able to be activated again. While it is thought that these events occur to a lesser degree or for a shorter duration following milder or concussive injuries, human studies examining acute neurochemical changes following concussive injuries in youth as well as adults or that address sex differences in response are lacking.
Metabolic Cascade After Traumatic Brain Injury: Glucose and Cerebral Blood Flow
The energy required to resolve the disruption of the neurochemical environment seen after injury triggers an immediate increase in brain glucose uptake or CMRglc. The immediate release of glutamate after impact plays a significant role in this transient increase in glucose uptake (Kawamata et al., 1992). In an adult rat, CMRglc rates increase 30 to 46 percent immediately after moderate fluid percussion impact and remain elevated for 30 minutes (Yoshino et al., 1991) (see Figure 2-2, Step 4). The early increase in cerebral glucose uptake following brain injury is thought to reflect the increased energy demands associated with reestablishing ionic homeostasis and maintaining neuronal membrane potential (Hovda et al., 1994; Sutton et al., 1994; Yoshino et al., 1991). While [18F]-fluorodeoxyglucose positron emission tomography (FDG-PET) studies have shown increased glucose uptake following severe TBI in humans (Bergsneider et al., 1997), no studies have examined this immediate increase in glucose uptake after mild or concussive brain injuries.
TBI-induced increases in cerebral glucose uptake are followed by a reduction of adenosine triphosphate (ATP) levels and prolonged CMRglc depression (see Figure 2-2, Step 7). This post-injury decrease in CMRglc has been established as a hallmark response after TBI. It is a response that is observed in animals following experimental fluid percussion that results in concussive injuries and cortical contusion (Andersen and Marmarou, 1992; Buczek et al., 2002; Chen et al., 2004; Kawamata et al., 1992; Richards et al., 2001; Yoshino et al., 1991, 1992) and that is also observed in humans who have sustained severe TBI (Bergsneider et al., 1997; O’Connell et al., 2005). On the other hand, there is no evidence of these changes from studies involving humans who have sustained concussions.
Relationship Between Injury Severity and CMRglc Depression
Research involving adult rats has found glucose metabolic depression to remain for 5, 10, or 14 days after experimental mild, moderate, or severe fluid percussion injury, respectively (Hovda et al., 1994). Focal injuries from controlled cortical impact (CCI) appear to produce a more profound and longer-lasting depression than the more diffuse fluid percussion injury does (Prins and Hovda, 2009; Sutton et al., 1994). TBIs that do not cause overt cell death or gross pathology can still produce metabolic dysfunction. Studies involving humans show that mTBI, which may involve axonal damage or dysfunction as detected by diffusion tensor imaging, is associated with measurable decreases in CMRglc (Gross et al., 1996; Humayun et al., 1989). The decreases in CMRglc in mTBI patients are correlated with persistent post-concussive symptoms (Gross et al., 1996; Umile et al., 2002). PET studies in 14 human TBI patients (mean age 36 years) with a mean Glasgow coma score scale of 6.5 (mild-severe) showed CMRglc depression between 5 and 28 days for all levels of injury severity (Bergsneider et al., 2001). This duration of CMRglc depression has been estimated to be five to six times longer in humans than in rats. Initial injury severity in adult human TBI patients does not appear to correlate with the magnitude or time-course of cortical metabolic recovery (Bergsneider et al., 2001). However, the CMRglc rates for thalamus, brain stem, and cerebellum correlate significantly with the level of consciousness at the time of PET imaging in adult TBI patients (Hattori et al., 2003). This pattern may suggest that concussions would result in a shorter duration of decreased metabolic depression than would moderate and severe brain injuries.
Relationship Between Age, Sex, and CMRglc Depression
Animal research suggests that the magnitude and duration of CMRglc depression following a brain injury increases with age. For example, 17-day-old rats (analogous to human newborns) that were injured via fluid percussion showed glucose metabolic recovery to age-matched shams within 3 days (Thomas et al., 2000), whereas adult (70-day-old) rats took 10 days for recovery. After CCI injury, 35-day-old rats (human adolescent) showed recovery of metabolic rates of subcortical structures within 3 days, as compared with 7 days in 90-day-old rats and also exhibited better metabolic recovery in the cortex by day 7 (Prins and Hovda, 2009). It is important to note that these are metabolic recoveries observed in animal models and that pediatric CMRglc after various severities of TBI has not been measured. While these studies all modeled injuries that are more severe than concussions, there was one experimental study that measured CMRglc after concussive-like injuries in the adolescent rat; in this case CMRglc
depression was observed at 24 hours after a closed-head concussive injury, and recovery was observed in all structures by 5 days (Prins et al., 2013).
There have been no studies in humans on age and sex differences in the metabolic alterations that occur after a concussion. Some research has used PET imaging of TBI in youth populations as a way of measuring metabolic activity, but these studies did not evaluate whether there were differences in the findings by age (Gross et al., 1996; Roberts et al., 1995; Worley et al., 1995). In one study, abnormal CMRglc rates were positively correlated with the number of clinical symptoms reported and with neuropsychological test results following mTBI in patients 12 to 59 years old at 3.5 years post injury (Gross et al., 1996). Worley and colleagues (1995) demonstrated that abnormalities on PET images obtained within 12 weeks following severe TBI were associated with poorer clinical outcomes in children 4 months to 19 years of age. The limited data from research involving human subjects suggests that younger and female youth may take longer to become symptom-free following a concussion and that younger youth may take longer than older youth to return to baseline on neurocognitive measures (Berz et al., 2013; Zuckerman et al., 2012). Future clinical studies comparing the time-course between children and adults after similar types of injuries may give insight into whether the experimental evidence translates to the human condition.
Changes in Cerebral Blood Flow After TBI
There have been hundreds of studies on changes in CBF after TBI, but relatively few of them have focused on CBF changes in pediatric populations, and even fewer have addressed changes following a concussion or mTBI. Only a couple of studies have evaluated sex differences in changes in CBF following TBI. As with CMRglc responses, CBF decreases immediately following both moderate to severe TBIs and mTBIs, and it can remain depressed for extended durations (Bonne et al., 2003; Giza and Hovda, 2001; Golding et al., 1999; Grindel, 2003; McQuire et al., 1998; Werner and Engelhard, 2007). Younger TBI patients can show a different response to injury than do older individuals, with acute hyperemia followed by decreases in CBF (Mandera et al., 2002). This pattern has also been observed in experimental studies with 3.5- to 4.5-week-old rats (human adolescent), which demonstrated age differences in CBF response to weight drop injury, with young adult animals showing a greater reduction in blood flow at the injury site 2 hours post injury (Grundl et al., 1994). Subsequent study found that both 3- to 4-week-old and 2- to 3-month-old (young adult) rats show hyperemia between 24 and 48 hours post injury (Biagas et al., 1996). Dramatic decreases in CBF were also measured within 1 hour after rapid head rotations that caused diffuse white matter injury in 3- to 5-day-old
infant piglets and 4-week-old toddler piglets (Eucker et al., 2011; Friess et al., 2011; Zhou et al., 2009), with significant reductions even 24 hours after diffuse brain injury (Kilbaugh et al., 2011). Decreases were also detected in 1- and 4-month-olds at 3 hours after CCI injury (Durham et al., 2000). Differing ages, injury severity, and injury type may also result in different CBF responses. Fluid percussion injury in 1- to 3-day-old piglets showed greater reductions in CBF than did the same injury in juvenile (3- to 4-week-old) pigs (Armstead, 1999). The different CBF age responses among piglets of different ages versus among rats of different ages may be related to differences in the types and severities of the injuries as well as differences in the time that the CBF was measured. There are fewer studies examining the effects of mild or concussive injuries on CBF in the younger brain. A recent study of 12 concussed children ages 11 to 15 showed that CBF, as measured by phase-contrast magnetic resonance angiography, was decreased relative to controls immediately after injury and that this decrease persisted beyond 30 days after injury even after symptoms resolved (Maugans et al., 2012).
In addition to causing changes in CBF, TBI can also affect cerebral autoregulation. Autoregulation is the ability of the brain to maintain a constant CBF in response to changes in blood pressure between 50 and 150 mmHg. TBI can directly affect the brain’s ability to maintain appropriate blood flow. Age and severity-dependent impairment in autoregulation has been observed. Mild brain injury has been shown to impair autoregulation and vascular reactivity in children (Becelewski and Pierzchala, 2003; Choe et al., 2012; Junger et al., 1997; Vavilala et al., 2004). Clinical studies examining changes in autoregulation after pediatric TBI have shown that the prevalence of impaired autoregulation increases with injury severity (Vavilala et al., 2004). Sex differences in CBF or autoregulation following TBI of any injury severity have not been addressed in the literature. Given that children and adolescents have higher normal blood flow rates, age-dependent definitions for “hyperemia” and “hypoperfusion” have been established for patient management (Vavilala et al., 2004). The risks for secondary ischemia versus elevations in intracranial pressure must be established by age and sex following all injury severities.
In summary, changes in brain glucose metabolism and blood flow after brain injury have been shown to be important biomarkers of TBI pathophysiology in all age groups and injury severities. However, there is a lack of data on changes in blood flow and glucose metabolism following mTBI and concussions in both males and females and at different ages during childhood and adolescence.
Oxidative Damage
In addition to TBI-induced metabolic crisis, there are also various pathways that result in oxidative damage that are activated acutely after TBI. It is unclear whether oxidative damage occurs after concussion, but given the potentially additive nature of multiple concussions it is important to discuss this common finding in animal models. Free radicals are molecules with unpaired electrons that will react with surrounding compounds to gain electrons. TBI-induced increases in reactive oxygen species (ROS) and reactive nitrogen species can generate hydroxyl radicals (•OH) and peroxynitrite (ONOO-). While normal mitochondrial metabolism produces ROS, they are managed by cellular antioxidant systems. Studies have shown that trauma to the brain and CNS can result in greater production of ROS, resulting in oxidative damage to proteins, lipids, and DNA (Hall and Braughler, 1993; Kerr et al., 1996; O’Connell and Littleton-Kearney, 2013) (see Figure 2-2, Steps 5 and 6).
It is difficult to quantify the level of the short-lived free radicals precisely, but the level has been shown to be elevated shortly after experimental brain injury. Hydroxyl radical (•OH) production has been observed to increase 60 percent within the first minute, peak within 30 minutes, and then subsequently decrease after a weight drop resulting in a concussive-like injury in adult mice (Althaus et al., 1993; Hall et al., 1993). CCI injury in adult rats produced a 250 percent increase in •OH production, which was sustained for 90 minutes (Marklund et al., 2001; Sen et al., 1993). Focal CCI injury produced a rapid increase in nitrate levels, with the peak at 5 minutes and a return to baseline by 6 hours post injury (Rao et al., 1998). Longer-lasting changes in cortical levels of the inducible isoform of nitric oxide synthase (iNOS) were observed by immunostaining 3 to 7 days following adult concussive fluid percussion injury (Wada et al., 1998). Fluid percussion injury induces iNOS expression after 24 to 28 hours in immature vascular smooth muscle cells and neutrophils (Clark et al., 1996). Until recently, it was not possible to measure free radicals in human TBI patients. Biomarkers for oxidative damage in human severe head injuries have been explored in adults and children through microdialysis sampling (Bayir et al., 2009; Clausen et al., 2012; Cristofori et al., 2005), which remains too invasive for concussion patients. Free radical production has not been quantified after concussive injuries in any age group.
Laboratory measurement of free radicals is difficult because their persistence in the body is generally short-lived. Thus, researchers have focused on the measurement of damage produced by free radicals by quantifying the lipid peroxidation, protein nitration, and DNA oxidation that occurs after moderate to severe TBI. In rodent models, increases in lipid peroxidation were observed 1 to 24 hours after weight drop injury (severity not always
noted) (Hall et al., 2004; Hsiang et al., 1997; Léwen and Hillered, 1998; Marmarou et al., 1994; Tyurin et al., 2000; Vagnozzi et al., 1999), after CCI (Singh et al., 2006), and after moderate fluid percussion injury (Praticò et al., 2002). Similarly, protein oxidation or nitration products were significantly elevated 30 minutes after CCI injury in adult mice and returned to baseline within 12 hours (Singh et al., 2006). DNA damage as measured by poly-ADP ribose polymerase (PARP) activity has also been shown to be elevated between 1 and 21 days after CCI injury in adult rodents (Satchell et al., 2003). Although there are a limited number of studies directly measuring PARP, research shows the inhibition of PARP to improve lesion volume after moderate fluid percussion injury (LaPlaca et al., 2001) and shows preserved cellular NAD+ concentrations with improved functional performance in the Morris water maze1 (Satchell et al., 2003) in rodents.
While significant strides have been made toward understanding the effects of free radicals after more severe TBI, far fewer studies have addressed oxidative stress after mild or concussive injuries. Mild TBI produced decreases in cytochrome oxidase activity in the cortex and hippocampus 1 to 10 days after fluid percussion injury in adult rats (Hovda et al., 1991). A threefold increase in protein carbonyls was seen immediately after mild weight drop injury, the protein carbonyls peaked at 3 hours, and they remained elevated at 12 hours (Petronilho et al., 2010). In this study the magnitude and duration of protein oxidative damage was inversely related to the severity of the injury following weight drop injury in adult rats. Another injury severity study with weight drop showed that mild injury did not produce histopathology, astrogliosis, or evidence of acute oxidative stress (Schwarzbold et al., 2010). In this study the degree of pathology and oxidative damage increased with injury severity.
While experimental studies have been used to address oxidative injury in various injury models and for various severities in adult animals, there are very few studies quantifying age- or sex-related differences in oxidative response after brain injury, including concussions. Currently there is only one study that examined the consequences of oxidative injury in the developing brain, and this was done in animals. Tsuru-Aoyagi and colleagues (2009) showed that 21-day-old mice have increases in protein nitration after CCI injury prior to contusion development. Other studies have addressed age-related differences in the antioxidant defense systems after injury. Despite increased thiobarbituric acid reactive substances after CCI, no acute compensatory increase in superoxide dismutase or glutathione
____________________________________
1The Morris water maze is a procedure used to measure spatial learning and memory in rodents. Rodents are placed into a pool of water from which they may escape onto a platform. Comparison of the performance of normal and injured animals on this test is a way of examining the effects of a brain injury (Morris, 1984).
peroxidase is observed in 7-day-old (Ozdemir et al., 2005) or 21-day-old (Fan et al., 2003) mice. This lack of increase in antioxidant response in the younger brain may increase vulnerability to oxidative damage after injury.
Both clinical and experimental evidence demonstrates that moderate and severe TBI increases oxidative injuries to proteins, membranes, and DNA in both the younger and the adult brain. However, there are currently no studies addressing oxidative injury specifically in concussive injuries with regard to sex or age.
Axonal Injury
In experimental models, axonal damage has been found to be present acutely after TBI of all severities, with the regional distribution and the number of damaged axons increasing with injury severity. Originally, this microscopic pathology was thought to be caused by a mechanical shearing of the axons during impact that resulted in the formation of an axoplasm swelling and axonal disconnection (Strich, 1956; Strich and Oxon, 1961). However, experimental studies have revealed that TBI of any severity can induce disruptions in axonal transport that cause swellings during the first 6 to 24 hours (Povlishock and Christman, 1995; Povlishock et al., 1983) (see Figure 2-2, Step 9). Calcium entry through the compromised plasma membrane has been shown to activate proteases (calpain, calcineurin), resulting in neurofilament degradation, disruption of axonal transport, and functional failure (Schlaepfer, 1974, 1987). Both mechanical disruption of cytoskeletal elements and neurochemical changes contribute to acute and long-term secondary axotomy.
Evidence of axonal damage has been well documented in various experimental adult models and at varying severities of TBI. The disruption of axonal transport of amyloid precursor protein (APP) after TBI led to the use of APP as a marker of axonal injury. Positive APP labeling has been shown in adult animal models after mild fluid percussion injury (Hoshino et al., 2003; Hylin et al., 2013; Shultz et al., 2011), after weight drop (Creed et al., 2011), after CCI injury (Chen et al., 2004; Dunn-Meynell and Levin, 1997; Itoh et al., 2009), and after rotational injury (Browne et al., 2011).
In contrast to the numerous studies on axonal injury in the adult brain, most of which have involved animals, few studies have been conducted in the developing brain, and only a couple of studies have examined mild injuries. Models of greater injury severities in P11-P21 age ranges show axonal bulbs and varicosities with APP staining between 6 hours and 1 week (Adelson et al., 2001; Huh et al., 2006, 2007, 2008; Tong et al., 2002). Studies of mTBI also show positive APP staining. Mild CCI in the P7 mouse produced significant APP labeling in the cingulum/external capsule between 30 minutes and 24 hours post injury, with decreased labeling
at 48 hours (Dikranian et al., 2008). While the “mild” impact depth used in these experiments did not produce cortical cavitation, it did produce increases in caspase 3 in the posterior cingulate cortex and anterior thalamus between 16 and 48 hours. Because age was not incorporated into the study design, it remains unclear if these findings suggest a specific vulnerability of the “infant” brain to injury. Studies examining rotational injuries in the neonatal pig have also shown axonal injuries. However, studies examining rotational injuries in the neonatal and toddler pig have shown that a single rapid rotational injury without impact in the 3- to 5-day-old piglet produced more axonal swellings and disconnections at 6 hours post injury than in the adult pig (Raghupathi and Margulies, 2002), and the damage was similar to that seen in the toddler pig (Ibrahim et al., 2010), despite the infant pig’s mechanically protective much smaller brain, indicating a vulnerability in the very young brain. Even single mild concussive injury in the infant piglet (Kilbaugh et al., 2011) and adult 35-day-old rat showed significant axonal injury 24 hours after injury (Prins et al., 2010).
In summary, experimental studies have demonstrated damage to neuronal “cables” or axons in mild injuries in both adult and infant age groups. There is not yet enough known to compare regional and time-course differences in axonal pathology across the pediatric age range. There are currently no studies addressing whether sex differences in axonal pathology exist in concussions.
Plasticity and Synaptic Changes
TBI leads to changes in the connectivity of the brain and alterations in the intimate communication between synapses. Experimental models with adult moderate fluid percussion injury have revealed that synaptophysin (a protein within the synapse) labeling increased in the cortex and white matter in a manner that was dependent on the severity of the injury. Western blot analysis also showed that the accumulation of synaptophysin was long lasting (30 days), suggesting that the transportation of synaptic vesicles may be disrupted (Shojo and Kibayashi, 2006). In this same model, decreases in dendritic spine densities were observed 24 hours after injury, but increases above controls were observed 7 days later (Campbell et al., 2012b). These acute synaptic changes are thought to reflect synaptic instability and degeneration (Campbell et al., 2012a) and may have significant implications for the initiation of rehabilitation. Griesbach and colleagues (2004) showed that exercise initiated immediately after a mild fluid percussion injury decreases synaptic plasticity molecules (including phosphorylated CREB [cAMP response element-binding protein], synapsin I, and mitogen activated protein kinase) and worsens spatial learning and memory tasks in the adult rat. By contrast, when exercise was initiated 2 weeks after mild
fluid percussion injury, the expression of plasticity proteins (BDNF [brainderived neurotrophic factor], phosphorylated CREB, synapsin I) increased (Griesbach et al., 2007).
Environmental experiences can induce significant alterations in the brain during normal cerebral maturation. These types of “learning” or “good plasticity” have been shown to produce long-lasting connectivity changes in the brain following environmental enrichment (Greenough et al., 1973). Age differences in this type of plasticity response have been observed following TBI induced by fluid percussion. The brains of young rats raised in an enriched environment (EE) (consisting of toys, tunnels, and ladders placed in a two-level cage) show increased dendritic branching, increased cortical thickness, and improved cognitive performance (Ip et al., 2002). However, when 17- to 21-day-old rats were placed in an EE immediately after mild to moderate injury, their brains failed to show the EE-related plasticity and improved cognitive performance (Fineman et al., 2000; Ip et al., 2002). Recovery of this plasticity response was observed when immature rats were placed in EE 2 weeks after mild fluid percussion injury (Giza et al., 2005). Examination of the molecular signals involved in brain enrichment revealed changes in the N-methyl-D-aspartate (NMDA) receptor after injury. The NR2A subunit of the NMDA receptor increases during normal brain development and also in response to stimulation. Following this concussive-like brain injury, the decrease in NR2A expression in the younger brain is thought to contribute to the failure to show EE plasticity acutely (Giza et al., 2006). Collectively these research findings provide evidence that mTBI results in early synaptic changes that can affect experience-dependent plasticity in the developing brain. More research is needed to further understand the effects of concussive injury on neuroplasticity at different ages and in males and females.
Hormonal and Pituitary Changes
The pituitary gland is responsible for hormonal production essential for normal reproductive, cognitive, social, and emotional maturation. Among adults with mild, moderate, and severe TBI, between 28 and 69 percent showed pituitary dysfunction between 3 months and 23 years post injury (Agha et al., 2004; Bondanelli et al., 2004; Kelly et al., 2000; Lieberman et al., 2001). Case studies of children with moderate to severe brain injuries and clinical studies of such children have reported acute and persistent pituitary dysfunction (Acerini and Tasker, 2007; Ives et al., 2007; Niederland et al., 2007; Norwood et al., 2010). Among pediatric patients with mild to moderate TBI, 42 percent were found to have significantly lower than normal growth hormone levels, with the effect seen predominantly in boys. While deficiency in growth hormone is common after TBI, children can also
experience deficiency in adrenocorticotropic hormone,2 diabetes insipidus, hypothyroidism, and increases in prolactin (Rose and Auble, 2012). Cortisol deficiencies have also been observed in children several months post injury (Niederland et al., 2007). The injury severities in this study ranged from concussions to severe injuries, and the authors report that pituitary dysfunction was not severity-dependent, although these data were not shown. There is evidence for pituitary dysfunction after mild to severe TBI in children (Casano-Sancho et al., 2013; Einaudi and Bondone, 2007; Khadr et al., 2010; Niederland et al., 2007) that may result in acute and long-term disruption of normal growth, but changes that occur specifically after concussions have not been examined. There are currently no experimental models quantifying hormonal deficiencies after concussions by age or sex. Undetected hormonal changes may have effects on memory, attention, executive function, mood, fatigue, and sleep and may thereby increase the duration of recovery.
Window of Vulnerability
The time period during which the brain remains vulnerable to another injury is the time that athletes should remain out of play. Although there are currently consensus-derived return-to-play guidelines for professional and pediatric sports, there are no biological markers of this window of vulnerability. A measurable biological marker would allow physicians to assess the extent of recovery from a concussion.
Although there are no studies addressing biomarkers for increased risk of subsequent concussions, there is evidence to support the idea that cerebral vulnerability increases immediately following a concussion. An experimental study by Meehan and colleagues (2012) showed that as the interval between injuries decreased, the duration of cognitive impairments increased. Adult mice were given five repeat weight drop injuries at 1-, 7-, or 30-day intervals and then assessed in the Morris water maze (MWM) at 1 month and 1 year post last injury. Animals that received daily or weekly injuries showed significant cognitive impairments that remained at 1 year post injury. Animals injured at 1-month intervals showed no significant MWM impairments. The injury model produced no significant edema, hippocampal cell loss, change in cerebral volume, or axonal injury relative to shams, thus indicating the mild nature of the injury. The cumulative effect of cognitive dysfunction when injuries occur at shorter intervals suggests that the impact interval reflects the duration of cerebral vulnerability.
These experimental findings were subsequently tested in human pa-
____________________________________
2Adrenocorticotropic hormone is released from the pituitary gland in the brain. It helps to modulate the secretion of cortisol and other hormones in the body.
tients using (1)H-MR spectroscopy. The clinical study, which involved 13 concussed athletes, found that NAA/Cr ratios had decreased by 18.5 percent at 3 days post injury and that they had returned to control levels by 30 days. Three of these patients who resumed normal activities shortly thereafter sustained a second concussion, which resulted in more prolonged deficits in NAA/Cr ratios with delayed recovery at 45 days (Vagnozzi et al., 2008). The length of time it takes to recover from the metabolic alterations following a concussion appears to correspond to the duration of adult cerebral vulnerability. Similar metabolic and structural changes have been documented in small groups of concussed athletes who showed no significant changes in neuropsychological test scores (see Henry et al., 2010, 2011).
RISK FACTORS FOR CONCUSSION IN THE DEVELOPING BRAIN
The concussion literature has identified several factors that appear to modify an individual’s risk of sustaining a concussion. In this section, the risk factors for concussion as they pertain to the developing brain, including sex, age, genetics, and history of prior concussions, are addressed. A discussion of factors that influence concussion outcomes and recovery appears in Chapter 4.
Sex
As discussed in Chapter 1, data from sports played by both males and females show higher reported rates of concussions for females. Cross-sex comparisons of the mechanics of concussion have included heading in soccer, which is one of the few sports in which the rules, protective equipment, and playing style are similar for the two sexes. Tierney and colleagues (2005, 2008) found that female soccer players exhibited greater head-neck segment peak acceleration and angular displacement than males did when exposed to a similar force. Reduced neck musculature and larger ball-to-head size ratios in females relative to males may explain this difference, but the literature is not clear on the influence of cervical muscle strength on kinematic measures such as head acceleration (Garces et al., 2002; Mansell et al., 2005; Queen et al., 2003; Schneider and Zernicke, 1988; Tierney et al., 2008). The data from the soccer studies were collected under controlled loading conditions such that the force applied to male and female participants was equivalent. In order to translate these laboratory-based data to the playing field, one must take into account that males and females may experience different loading conditions while playing sports. For example, two studies in which head acceleration data were collected under normal play conditions in ice hockey and boxing demonstrated that males experienced greater accelerations on average than did females (Brainard et al.,
2012; Stojsih et al., 2010). Furthermore, from a mechanics perspective, although player-to-player contact appears to be a primary mechanism for sustaining a concussion overall, player contact is responsible for a major proportion of concussions in male athletes (65 to 81 percent), while contact with the playing surface or the ball is a more common cause of concussions in female athletes (Dick, 2009). However, no details exist concerning whether impacts with the playing surface impart more energy to the female head than do impacts with other players. Further research on the biomechanics of concussions in male and female athletes is warranted, especially in sports in which males and females have similar rules, equipment, and environment. It is important that comparisons across the sexes be made in circumstances with similar loading environments. Finally, some of the difference in concussion rates between males and females may be explained by a lower rate of reporting of concussion symptoms by male athletes (Daneshvar et al., 2011; Granite and Carroll, 2002; Harmon et al., 2013), although there is no definitive evidence that this is the case.
Age
There is currently a lack of data to determine whether there are variations in concussion rates across the pediatric age spectrum. Most studies comparing concussion incidence in high school and collegiate athletes have focused on football, with less attention being given to other sports, including those played by females (Gessel et al., 2007). Few studies of sports-related concussions have focused on pre-high-school-age youth. Immaturity of the developing CNS, a larger head-to-body ratio, thinner cranial bones, reduced development of neck and shoulder musculature, a larger subarachnoid space in which the brain can move, and differences in cerebral blood volume have been proposed as possible sources of increased susceptibility to concussions for youth relative to adults (Karlin, 2011; Meehan et al., 2011). In addition, it has been speculated that the gains in weight and mass that occur during the adolescent growth spurt may increase force and momentum during collisions without corollary increases in neck strength (Buzzini and Guskiewicz, 2006; Karlin, 2011). Incomplete myelination of the brain tissue may put the developing brain at greater risk for shear injury (Cook et al., 2006; Kieslich et al., 2002; Ommaya et al., 2002). Relative to adults, children demonstrate more widespread and prolonged cerebral swelling and increased metabolic sensitivities following a head injury, and these physiological changes may result in more apparent (i.e., more severe and persistent) symptoms (Biagas et al., 1996; Field et al., 2003; Giza and Hovda, 2001; Karlin et al., 2011; Reddy et al., 2008).
Genetics
There has been little research done on how genetic factors influence susceptibility to concussion. In a retrospective survey of collegiate male football players (n=163) and female soccer players (n=33), those with three alleles (E2, E4, and promoter) of the apolipoprotein E (APOE) gene (four participants) were nearly 10 times more likely to self-report that they had experienced a concussion in the past than were individuals who did not carry these alleles. Furthermore, athletes possessing the promoter allele (nine participants) were 8.4 times more likely to report a history of multiple concussions (Tierney et al., 2010). An earlier retrospective study of intercollegiate football and soccer players (n=195) showed that those with the APOE promoter genotype were nearly three times as likely to have a history of concussion, after adjusting for age, sport, school, and years in their primary sport (Terrell et al., 2008). On the other hand, a few prospective studies of high school and collegiate athletes found no significant differences in the frequency of concussions between individuals with or without the APOE or tau genotype, although these studies were limited by relatively small sample sizes (Kristman et al., 2008; Terrell et al., 2012, 2013). Genetic studies beyond APOE are lacking. Prospective research with larger samples sizes that control for athletic exposure, prior concussion history, and other predisposing factors may help to clarify the role that genetics play in risk for and recovery from concussions in youth populations (Harmon et al., 2013).
History of Prior Concussion
A history of previous concussions is a relatively well-established predictor of increased risk for future concussions. (See Chapter 5 for a more complete discussion of multiple concussions.) Studies of high school and college athletes suggest that, compared with those who have never had a concussion, individuals with a history of prior concussion are 2 to 5.8 times more likely to sustain a (subsequent) concussion (Collins et al., 2002; Guskiewicz et al., 2000, 2003; Harmon et al., 2013; Hollis et al., 2009; Schulz et al., 2004; Zemper, 2003). It is not known the extent to which the increased risk is due to changes in the brain after an initial concussion that may make it more vulnerable to future impacts as opposed to factors (e.g., playing style, position, musculature) that might make an individual more susceptible to concussion in general. A prospective study of 2,905 college football players found a dose-response relationship between number of self-reported previous concussions and incident concussions after controlling for division of play, playing position, years of participation in organized football, academic year in school, and body mass index (Guskiewicz et al., 2003). Brain injury models indicate that brain function may be altered for several days to weeks
after brain injury, including concussion. Additional research is needed to elucidate how physiological factors, including enduring changes in the brain that may occur after an individual appears to have clinically recovered from a concussion, may influence future concussion risk.
The committee offers the following findings on the neuroscience, biomechanics, and risks of concussion in the developing brain:
- There are normal changes in brain structure, blood flow, and metabolism that occur with brain development that may influence susceptibility to and prognosis following concussions in youth.
- Research primarily involving animals and individuals with more severe head injury has provided a limited framework for understanding the neuroscience of concussion. This research indicates that there are a series of molecular and functional changes that take place in the brain following head injury, some of which may serve as important biomarkers for the pathophysiology of concussion. However, little research has been conducted specifically on changes in the brain following concussions in youth or has attempted to evaluate the differences in such changes between female and male youth. Research using newer noninvasive imaging techniques in the first hours and days following injury may help to improve understanding of the neurobiology of concussion.
- Existing studies of head injury biomechanics have limited applicability to youth and thus are inadequate to define the direction- and age-related thresholds for linear and rotational acceleration specifically associated with concussions in youth.
- Available data indicate that female youth athletes and youth with a history of prior concussion have higher rates of reported sports-related concussions. The extent to which these findings are due to physiological, biomechanical, and other factors (e.g., possible differences between males and females in the reporting of concussion symptoms or player aggressiveness) is not yet well understood.
- While it has been suggested that physiological and biomechanical risks for concussion may differ between younger children and older youth and adults, there is currently a lack of epidemiologic data from a variety of sports to calculate and compare rates of sports-related concussions across the age spectrum.
- The findings of studies examining associations between genetic variation and risk for concussion have been mixed and are limited by small sample sizes.
Aare, M., S. Kleiven, and P. Halldin. 2004. Injury tolerances for oblique impact helmet testing. International Journal of Crashworthiness 9(1):15-23.
Acerini, C. L., and R. C. Tasker. 2007. Traumatic brain injury induced hypothalamic-pituitary dysfunction: A paediatric perspective. Pituitary 10(4):373-380.
Adelson, P. D., L. W. Jenkins, R. L. Hamilton, P. Robichaud, M. P. Tran, and P. M. Kochanek. 2001. Histopathologic response of the immature rat to diffuse traumatic brain injury. Journal of Neurotrauma 18(10):967-976.
Agha, A., B. Rogers, M. Sherlock, P. O’Kelly, W. Tormey, J. Phillips, and C. J. Thompson. 2004. Anterior pituitary dysfunction in survivors of traumatic brain injury. Journal of Clinical Endocrinology and Metabolism 89(1):4929-4936.
Althaus, J. S., P. K. Andrus, C. M. Williams, P. F. Vonvoigtlander, A. R. Cazers, and E. D. Hall. 1993. The use of salicylate hydroxylation to detect hydroxyl radical generation in ischemic and traumatic brain injury. Reversal by tirilazad mesylate (U-74006F). Molecular and Chemical Neuropathology 20(2):147-162.
Andersen, B. J., and A. Marmarou. 1992. Post-traumatic selective stimulation of glycolysis. Brain Research 585(1-2):184-189.
Armstead, W. M. 1999. Role of endothelin-1 in age dependent cerebrovascular hypotensive responses after brain injury. American Journal of Physiology 274(5 Pt 2): H1884-H1894.
Bakker, S. L., F. E. de Leeuw, T. den Heijer, P. J. Koudstaal, A. Hofman, and M. M. Breteler. 2004. Cerebral haemodynamics in the elderly: The Rotterdam study. Neuroepidemiology 23(4):178-184.
Bava, S., and S. F. Tapert. 2010. Adolescent brain development and the risk for alcohol and other drug problems. Neuropsychology Review 20(4):398-413.
Bayir, H., P. D. Adelson, S. R. Wisniewski, P. M. Shore, Y. C. Lai, S. D. Brown, K. L. Janeski-Feldman, V. E. Kagan, and P. M. Kochanek. 2009. Therapeutic hypothermia preserves antioxidant defenses after severe traumatic brain injury in infants and children. Critical Care Medicine 37(2):689-695.
Becelewski, J., and K. Pierzchala. 2003. Cerebrovascular reactivity in patients with mild head injury. Polish Journal of Neurology and Neurosurgery 37(2):339-350.
Bechara, A., H. Damasio, and A. R. Damasio. 2000. Emotion, decision making and the orbitofrontal cortex. Cerebral Cortex 10(3):295-307.
Benson, R. R., S. A. Meda, S. Vasudevan, Z. Kou, K. A. Govindarajan, R. A. Hanks, S. R. Millis, M. Makki, Z. Latif, W. Coolin, J. Meythaler, and E. M. Haacke. 2007. Global white matter analysis of diffusion tensor images is predictive of injury severity in traumatic brain injury. Journal of Neurotrauma 24(3):446-459.
Bergsneider, M., D. A. Hovda, E. Shalmon, D. F. Kelly, P. M. Vespa, N. A. Martin, M. E. Phelps, D. L. McArthur, M. J. Caron, J. F. Kraus, and D. P. Becker. 1997. Cerebral hyperglycolysis following severe traumatic brain injury in humans: A positron emission tomography study. Journal of Neurosurgery 86(2):241-251.
Bergsneider, M., D. A. Hovda, D. L. McArthur, M. Etchepare, S. C. Huang, N. Sehati, P. Satz, M. E. Phelps, and D. P. Becker. 2001. Metabolic recovery following human traumatic brain injury based on FDG-PET: Time course and relationship to neurological disability. Journal of Head Trauma Rehabilitation 16(2):135-148.
Berz, K., J. Divine, K. B. Foss, R. Heyl, K. R. Ford, and G. D. Myer. 2013. Sex-specific differences in the severity of symptoms and recovery rate following sports-related concussions in young athletes. Physician and Sports Medicine 41(2):64-69.
Biagas, K. V., P. D. Grundl, P. M. Kochanek, J. K. Schiding, and E. M. Nemoto. 1996. Posttraumatic hyperemia in immature, mature, and aged rats: Autoradiographic determination of cerebral blood flow. Journal of Neurotrauma 13(4):189-200.
Biro, F. M., M. P. Galvez, L. C. Greenspan, P. A. Succop, N. Vangeepuram, S. M. Piney, S. Teitelbaum, G. C. Windham, L. H. Kushi, and M. S. Wolff. 2010. Pubertal assessment method and baseline characteristics in a mixed longitudinal study of girls. Pediatrics 126(3):e583-e590.
Bondanelli, M., M. L. De, M. R. Ambrosio, M. Monesi, D. Valle, M. C. Zatelli, A. Fusco, A. Bianchi, M. Farneti, and E. C. degli Uberti. 2004. Occurrence of pituitary dysfunction following traumatic brain injury. Journal of Neurotrauma 21(6):685-696.
Bonne, O., A. Gilboa, Y. Louzoun, O. Kempf-Sherf, M. Katz, Y. Fishman, Y. Z. Ben-Nahum, Y. Krausz, M. Bocher, H. Lester, R. Chisin, and B. Lerer. 2003. Cerebral blood flow in chronic symptomatic mild traumatic brain injury. Psychiatry Research 124(3):141-152.
Bourgeois, J. P., P. S. Goldman-Rakic, and P. Rakic. 1994. Synaptogenesis in the prefrontal cortex of rhesus monkeys. Cerebral Cortex 4(1):78-96.
Brainard, L. L., J. G. Beckwith, J. J. Chu, J. J. Crisco, T. W. McAllister, A. C. Duhaime, A. C. Maerlender, and R. M. Greenwald. 2012. Gender differences in head impacts sustained by collegiate ice hockey players. Medicine and Science in Sports and Exercise 44(2):297-304.
Browne, K. D., X. H. Chen, D. F. Meaney, and D. H. Smith. 2011. Mild traumatic brain injury and diffuse axonal injury in swine. Journal of Neurotrauma 28(9):1747-1755.
Buczek, M., J. Alvarez, J. Azhar, Y. Zhou, W. D. Lust, W. R. Selman, and R. A. Ratcheson. 2002. Delayed changes in regional brain energy metabolism following cerebral concussion in rats. Metabolic Brain Disease 17(3):153-167.
Bullock, R., A. Zauner, J. Woodward, and H. F. Young. 1995. Massive persistent release of excitatory amino acids following human occlusive stroke. Stroke 26(11):2187-2189.
Bullock, R., A. Zauner, J. Woodward, J. Myseros, S. C. Choi, J. D. Ward, A. Marmarou, and H. F. Young. 1998. Factors affecting excitatory amino acid release following severe human head injury. Journal of Neurosurgery 89(4):507-518.
Buzzini, S. R., and K. M. Guskiewicz. 2006. Sport-related concussion in the young athlete. Current Opinion in Pediatrics 18(4):376-382.
Camarillo, D. B., P. B. Shull, J. Mattson, R. Shultz, and D. Garza. 2013. An instrumented mouthguard for measuring linear and angular head impact kinematics in American football. Annals of Biomedical Engineering 41(9):1939-1949.
Campbell, J. N., B. Low, J. E. Kurz, S. S. Patel, M. T. Young, and S. B. Churn. 2012a. Mechanisms of dendritic spine remodeling in a rat model of traumatic brain injury. Journal of Neurotrauma 29(2):218-234.
Campbell, J. N., D. Register, and S. B. Churn. 2012b. Traumatic brain injury causes an FK506-sensitive loss and an overgrowth of dendritic spines in rat forebrain. Journal of Neurotrauma 29(2):201-217.
Carskadon, M. S., C. Acebo, and O. G. Jenni. 2004. Regulation of adolescent sleep: Implications for behavior. Annals of the New York Academy of Sciences 1021:276-291.
Casano-Sancho, P., L. Suarez, L. Ibanez, G. Garcia-Fructuoso, J. Medina, and A. Febrer. 2013. Pituitary dysfunction after traumatic brain injury in children: Is there a need for ongoing endocrine assessment? Clinical Endocrinology 79(6):853-858.
Casey, B. J., and R. M. Jones. 2010. Neurobiology of the adolescent brain and behavior: Implications for substance use disorders. Journal of the American Academy of Child and Adolescent Psychiatry 49(12):1189-1285.
Casey, B. J., N. Tottenham, C. Liston, and S. Durston. 2005. Imaging the developing brain: What have we learned about cognitive development? Trends in Cognitive Sciences 9(3): 104-110.
Cater, H. L., L. E. Sundstrom, and B. Morrison. 2006. Temporal development of hippocampal cell death is dependent on tissue strain but not strain rate. Journal of Biomechanics 39(15):2810-2818.
Chen, J. R., Y. J. Wang, and G. F. Tseng. 2004. The effects of decompression and exogenous NGF on compressed cerebral cortex. Journal of Neurotrauma 21(11):1640-1651.
Chiron, C., C. Raynaud, B. Maziere, M. Zilbovicius, L. Laflamme, M. C. Masure, O. Dulac, and M. Bourguignon. 1992. Changes in regional cerebral blood flow during activation during brain maturation in children and adolescents. Journal of Nuclear Medicine 33(5):696-703.
Choe, M. C., T. Babikian. J. DiFiori, D. A. Hovda, and C. C. Giza. 2012. A pediatric perspective on concussion pathophysiology. Current Opinion in Pediatrics 24(6):689-695.
Chugani, H. T. 1998. A critical period of brain development: Studies of cerebral glucose utilization with PET. Preventive Medicine 27(2):184-188.
Clark, R. S., P. M. Kochanek, W. D. Obrist, H. R. Wong, R. R. Billiar, S. R. Wisniewski, and D.W. Marion. 1996. Cerebrospinal fluid and plasma nitrite and nitrate concentrations after head injury in humans. Critical Care Medicine 24(7):1243-1251.
Clausen, F., N. Marklund, A. Lewén, P. Enblad, S. Basu, and L. Hillered. 2012. Interstitial F(2)-isoprostane 8-iso-PGF(2α) as a biomarker of oxidative stress after severe human traumatic brain injury. Journal of Neurotrauma 29(5):766-775.
Coats, B., and S. S. Margulies. 2006. Material properties of human infant skull and suture at high rates. Journal of Neurotrauma 23(8):1222-1232.
Coats, B., S. A. Eucker, S. Sullivan, and S. S. Margulies. 2012. Finite element model predictions of intracranial hemorrhage from non-impact, rapid head rotations in the piglet. International Journal of Developmental Neuroscience 30(3):191-200.
Collins, M. W., M. R. Lovell, G. L. Iverson, R. Cantu, J. Maroon, and M. Field. 2002. Cumulative effects of concussion in high school athletes. Neurosurgery 51(5):1175-1179.
Cook, R. S., L. Schweer, K. F. Shebesta, K. Hartjes, and R. A. Falcone. 2006. Mild traumatic brain injury in children: Just another bump on the head? Journal of Trauma Nursing 13(2):58-65.
Creed, J. A., A. M. DiLeonardi, D. P. Fox, A. R. Tessler, and R. Raghupathi. 2011. Concussive brain trauma in the mouse results in acute cognitive deficits and sustained impairment of axonal function. Journal of Neurotrauma 28(4):547-563.
Crisco, J. J., R. Fiore, J. G. Beckwith, J. J. Chu, P. G. Brolinson, S. Duma, T. W. McAllister, A. C. Duhaime, and R. M. Greenwald. 2010. Frequency and location of head impact exposures in individual collegiate football players. Journal of Athletic Training 45(6):549-559.
Cristofori, L., B. Tavazzi, R. Gambin, R. Vagnozzi, S. Signoretti, A. M. Amorini, G. Fazzina, and G. Lazzarino. 2005. Biochemical analysis of the cerebrospinal fluid: Evidence for catastrophic energy failure and oxidative damage preceding brain death in severe head injury: A case report. Clinical Biochemistry 38(1):97-100.
Daneshvar, D. H., C. J. Nowinski, A. C. McKee, and R. C. Cantu. 2011. The epidemiology of sport-related concussion. Clinical Journal of Sports Medicine 30(1):1-17.
Daniel, R. W., S. Rowson, and S. M. Duma. 2012. Head impact exposure in youth football. Annals of Biomedical Engineering 40(4):976-981.
Davidsson, J., and M. Risling. 2011. A new model to produce sagittal plane rotational induced diffuse axonal injuries. Frontiers in Neurology 2:41.
Dayan, J., A. Bernard, B. Olliac, A. S. Mailhes, and S. Kermarrec. 2010. Adolescent brain development, risk-taking and vulnerability to addiction. Journal of Physiology, Paris 104(5):279-286.
Dick, R. W. 2009. Is there a gender difference in concussion incidence and outcomes? British Journal of Sports Medicine 43(Suppl 1):I46-I50.
Dikranian, K., R. Cohen, C. Mac Donald, Y. Pan, D. Brakefield, P. Bayly, and A. Parsadanian. 2008. Mild traumatic brain injury to the infant mouse causes robust white matter axonal degeneration which precedes apoptotic death of cortical and thalamic neurons. Experimental Neurology 211(2):551-560.
Duhaime, A. C., A. J. Saykin, B. C. McDonald, C. P. Dodge, C. J. Eskey, T. M. Darcey, L. L. Grate, and P. Tomashosky. 2006. Functional magnetic resonance imaging of the primary somatosensory cortex in piglets. Journal of Neurosurgery 104(4 Suppl):259-264.
Dunn-Meynell, A. A., and B. E. Levin. 1997. Histological markers of neuronal, axonal and astrocytic changes after lateral rigid impact traumatic brain injury. Brain Research 761(1):25-41.
Durham, S. R., R. Raghupathi, M. A. Helfaer, S. Marwaha, and A. C. Duhaime. 2000. Age-related differences in acute physiologic response to focal traumatic brain injury in piglets. Pediatric Neurosurgery 33(2):76-82.
Einaudi, S., and C. Bondone. 2007. The effects of head trauma on hypothalamic-pituitary function in children and adolescents. Current Opinion in Pediatrics 19(4):465-470.
Elkin, B. S., and B. Morrison. 2007. Region-specific tolerance criteria for the living brain. Stapp Car Crash Journal 51:127-138.
Elkin, B. S., A. Ilankovan, and B. Morrison. 2010. Age-dependent regional mechanical properties of the rat hippocampus and cortex. Journal of Biomechanical Engineering 132(1): 011010.
Enerson, B. E., and L. R. Drewes. 2003. Molecular features, regulation and function of monocarboxylate transporters: Implications for drug delivery. Journal of Pharmaceutical Science 92(8):1531-1544.
Eucker, S. A., C. Smith, J. Ralston, S. H. Friess, and S. S. Margulies. 2011. Physiological and histopathological responses following closed rotational head injury depend on direction of head motion. Experimental Neurology 227(1):559-564.
Fan, P., T. Yamauchi, L. J. Noble, and D. M. Ferriero. 2003. Age-dependent differences in glutathione peroxidase activity after traumatic brain injury. Journal of Neurotrauma 20(5):437-445.
Feeney, D. M., M. G. Boyeson, R. T. Linn, H. M. Murray, and W. G. Dail. 1981. Response to cortical injury: I. Methodology and local effects of contusions in the rat. Brain Research 211(1):67-77.
Feng, Y., T. M. Abney, R. J. Okamoto, R. B. Pless, G. M. Genin, and P. V. Bayly. 2010. Relative brain displacement and deformation during constrained mild frontal head impact. Journal of the Royal Society 7(53):1677-1688.
Field, M., M. W. Collins, M. R. Lovell, and J. Maroon. 2003. Does age play a role in recovery from sports-related concussion? A comparison of high school and collegiate athletes. Journal of Pediatrics 142(5):546-553.
Fineman, I., D. A. Hovda, M. Smith, A. Yoshino, and D. P. Becker. 1993. Concussive brain injury is associated with a prolonged accumulation of calcium: A 45CA autoradiographic study. Brain Research 624(1-2):94-102.
Fineman, I., C. C. Giza, B. V. Nahed, S. M. Lee, and D. A. Hovda. 2000. Inhibition of neocortical plasticity during development by a moderate concussive brain injury. Journal of Neurotrauma 17(9):739-749.
Finnie, J. W., P. C. Blumbergs, J. Manavis, R. J. Turner, S. Helps, R. Vink, R. W. Byard, G. Chidlow, B. Sandoz, J. Dutschke, and R. W. Anderson. 2012. Neuropathological changes in a lamb model of non-accidental head injury (the shaken baby syndrome). Journal of Clinical Neuroscience 19(8):1159-1164.
Foda, M. A., and A. Marmarou. 1994. A new model of diffuse brain injury in rats. Part II: Morphological characterization. Journal of Neurosurgery 80(2):301-313.
Friess, S. H., J. Ralston, S. A. Eucker, M. A. Helfaer, C. Smith, and S. S. Margulies. 2011. Neurocritical care monitoring correlates with neuropathology in a swine model of pediatric traumatic brain injury. Neurosurgery 69(5):1139-1147.
Funk, J. R., J. M. Cormier, C. E. Bain, H. Guzman, E. Bonugli, and S. J. Manoogian. 2011. Head and neck loading in everyday and vigorous activities. Annals of Biomedical Engineering 39(2):766-776.
Galland, B. C., B. J. Taylor, D. E. Elder, and P. Herbison. 2012. Normal sleep patterns in infants and children: A systematic review of observational studies. Sleep Medicine Reviews 16(3):213-222.
Garces, G. L., D. Medina, L. Milutinovic, P. Garavote, and E. Guerado. 2002. Normative database of isometric cervical strength in a healthy population. Medicine and Science in Sports and Exercise 34(3):464-470.
Gennarelli, T. A., J. H. Adams, and D. I. Graham. 1981. Acceleration induced head injury in the monkey. I. The model, its mechanical and physiological correlates. Acta Neuropathologica Supplementum 7:23-25.
Gennarelli, T. A., L. E. Thibault, J. H. Adams, D. I. Graham, C. J. Thompson, and R. P. Marcincin. 1982. Diffuse axonal injury and traumatic coma in the primate. Annals of Neurology 12(6):564-574.
Gennarelli, T. A., F. A. Pintar, and N. Yoganandan. 2003. Biomechanical tolerances for diffuse brain injury and a hypothesis for genotypic variability in response to trauma. Annual Proceedings from the Association of Advancement in Automotive Medicine 47:624-628.
Gessel, L. M., S. K. Fields, C. L. Collins, R. W. Dick, and R. D. Comstock. 2007. Concussions among United States high school and collegiate athletes. Journal of Athletic Training 42(4):495-503.
Giedd, J. N. 2004. Structural magnetic resonance imaging of the adolescent brain. Annals of the New York Academy of Sciences 1021:77-85.
Giedd, J. N., J. Blumenthal, N. O. Jeffries, F. X. Castellanos, H. Liu, A. Zijdenbos, T. Paus, A. C. Evans, and J. L. Rapoport. 1999. Brain development during childhood and adolescence: A longitudinal MRI study. Nature Neuroscience 2(10):861-863.
Giedd, J. N., A. Raznahan, K. L. Mills, and R. K. Lenroot. 2012. Review: Magnetic resonance imaging of male/female differences in human adolescent brain anatomy. Biological Sex Differences 3(1):19.
Giza, C. C., and D. A. Hovda. 2001. The neurometabolic cascade of concussion. Journal of Athletic Training 36(3):228-235.
Giza, C. C., G. S. Griesbach, and D. A. Hovda. 2005. Experience-dependent behavioral plasticity is disturbed following traumatic injury to the immature brain. Behavioural Brain Research 157(1):11-22.
Giza, C. C., N. S. Maria, and D. A. Hovda. 2006. N-methyl-D-aspartate receptor subunit changes after traumatic injury to the developing brain. Journal of Neurotrauma 23(6):950-961.
Gogtay, N., J. N. Giedd, L. Lusk, K. M. Hayashi, D. Greenstein, A. C. Vaituzis, T. F. Nugent, D. H. Herman, L. S. Clasen, A. W. Toga, J. L. Rapoport, and P. M. Thompson. 2004. Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences of the United States of America 101(21):8174-8179.
Golding, E. M., C. S. Robertson, and R. M. Bryan. 1999. The consequences of traumatic brain injury on cerebral blood flow and autoregulation: A review. Clinical and Experimental Hypertension 21(4):299-332.
Granite, V., and J. Carroll. 2002. Psychological response to athletic injury: Sex differences. Journal of Sport Behavior 25(3):243-259.
Greenough, W. T., F. R. Volkmar, and J. M. Juraska. 1973. Effects of rearing complexity on dendritic branching in frontolateral and temporal cortex of the rat. Experimental Neurology 41(2):371-378.
Griesbach, G. S., D. A. Hovda, R. Molteni, A. Wu, and F. Gomez-Pinilla. 2004. Voluntary exercise following traumatic brain injury: Brain-derived neurotrophic factor upregulation and recovery of function. Neuroscience 125(1):129-139.
Griesbach, G. S., F. Gómez-Pinilla, and D. A. Hovda. 2007. Time window for voluntary exercise-induced increases in hippocampal neuroplasticity molecules after traumatic brain injury is severity dependent. Neurotrauma 24(7):1161-1171.
Grindel, S. H. 2003. Epidemiology and pathophysiology of minor traumatic brain injury. Current Sports Medicine Reports 2(1):18-23.
Gross, H., A. Kling, G. Henry, C. Herndon, and H. Lavretsky. 1996. Local cerebral glucose metabolism in patients with long-term behavioral and cognitive deficits following mild traumatic brain injury. Journal of Neuropsychiatry and Clinical Neurosciences 8(3):324-334.
Grundl, P. D., K. V. Biagas, P. M. Kochanek, J. K. Schiding, M. A. Barmada, and E. M. Nemoto. 1994. Early cerebrovascular response to head injury in immature and mature rats. Journal of Neurotrauma 11(2):135-148.
Guskiewicz, K. M., N. L. Weaver, D. A. Padua, and W. E. Garrett. 2000. Epidemiology of concussion in collegiate and high school football players. American Journal of Sports Medicine 28(5):643-650.
Guskiewicz, K. M., M. McCrea, S. W. Marshall, R. C. Cantu, C. Randolph, J. A. Onate, and J. P. Kelly. 2003. Cumulative effects associated with recurrent concussion in collegiate football players: The NCAA Concussion Study. JAMA 290(19):2549-2555.
Hall, E. D., and J. M. Braughler. 1993. Free radicals in CNS injury. Research Publications—Association for Research on Nervous in Mental Disease 71:81-105.
Hall, E. D., P. K. Andrus, and P. A. Yonkers. 1993. Brain hydroxyl radical generation in acute experimental head injury. Journal of Neurochemistry 60(2):588-594.
Hall, E. D., M. R. Detloff, K. Johnson, and N. C. Kupina. 2004. Peroxynitrite-mediated protein nitration and lipid peroxidation in a mouse model of traumatic brain injury. Journal of Neurotrauma 21(1):9-20.
Hardy, W. N., C. D. Foster, M. J. Mason, K. H. Yang, A. I. King, and S. Tashman. 2001. Investigation of head injury mechanisms using neutral density technology and high-speed biplanar X-ray. Stapp Car Crash Journal 45:337-368.
Harmon, K., J. Drezner, M. Gammons, K. M. Guskiewicz, M. Halstead, S. A. Herring, J. S. Kutcher, A. Pana, M. Putukian, and W. O. Roberts. 2013. American Medical Society for Sports Medicine position statement: Concussion in sport. British Journal of Sports Medicine 47(1):15-26.
Hattori, N., S. C. Huang, H. M. Wu, E. Yeh, T. C. Glenn, P. M. Vespa, D. McArthur, M. E. Phelps, D. A. Hovda, and M. Bergsneider. 2003. Correlation of regional metabolic rates of glucose with Glasgow coma scale after traumatic brain injury. Journal of Nuclear Medicine 44(11):1709-1716.
Henry, L. C., S. Tremblay, Y. Boulanger, D. Ellemberg, and M. Lassonde. 2010. Neurometabolic changes in the acute phase after sports concussions correlate with symptom severity. Journal of Neurotrauma 27(1):65-76.
Henry, L. C., J. Tremblay, S. Tremblay, A. Lee, C. Brun, N. Lepore, H. Theoret, D. Ellemberg, and M. Lassonde. 2011. Acute and chronic changes in diffusivity measures after sports concussion. Journal of Neurotrauma 28(20):2049-2059.
Hoffman, M. 2012. Army ships next-gen blast sensors. http://www.military.com/dailynews/2012/07/27/army-ships-out-next-gen-blast-sensors.html (accessed October 3, 2013).
Holbourn, A. H. S. 1945. The mechanics of brain injuries. British Medical Bulletin 3(6): 147-149.
Hollis, S. J., M. R. Stevenson, A. S. McIntosh, E. A. Shores, M. W. Collins, and C. B. Taylor. 2009. Incidence, risk, and protective factors of mild traumatic brain injury in a cohort of Australian nonprofessional male rugby players. American Journal of Sports Medicine 37(12):2328-2333.
Hoshino, S., S. Kobayashi, T. Furukawa, T. Asakura, and A. Teramoto. 2003. Multiple immunostaining methods to detect traumatic axonal injury in the rat fluid-percussion brain injury model. Neurologia Medico-Chirurgica (Tokyo) 43(4):165-173.
Hovda, D. A., A. Yoshino, T. Kawamata, Y. Katayama, and D. P. Becker. 1991. Diffuse prolonged depression of cerebral oxidative metabolism following concussive brain injury in the rat: A cytochrome oxidase histochemistry study. Brain Research 567(1):1-10.
Hovda, D. A., K. Fu, H. Badie, A. Samii, P. Pinanong, and D. P. Becker. 1994. Administration of an omega-conopeptide one hour following traumatic brain injury reduces 45calcium accumulation. Acta Neurochirurgica 60 (Suppl):521-523.
Hsiang, J. N., J. Y. Wang, S. M. Ip, H. K. Ng, A. Stadlin, A. L. Yu, and W. S. Poon. 1997. The time course and regional variations of lipid peroxidation after diffuse brain injury in rats. Acta Neurochirurgica (Wien) 139(5):464-468.
Huh, J. W., M. A. Franklin, A. G. Widing, and R. Raghupathi. 2006. Regionally distinct patterns of calpain activation and traumatic axonal injury following contusive brain injury in immature rats. Developmental Neuroscience 28(4-5):466-476.
Huh, J. W., A. G. Widing, and R. Raghupathi. 2007. Basic science–Repetitive mild noncontusive brain trauma in immature rats exacerbates traumatic axonal injury and axonal calpain activation: A preliminary report. Journal of Neurotrauma 24(1):15-27.
Huh, J. W., A. G. Widing, and R. Raghupathi. 2008. Midline brain injury in the immaturer at induces sustained cognitive deficits, bihemispheric axonal injury and neurodegeneration. Experimental Neurology 213(1):84-92.
Humayun, M. S., S. K. Presty, N. D. Lafrance, H. H. Holcomb, H. Loats, D. M. Long, H. N. Wagner, and B. Gordon. 1989. Local cerebral glucose abnormalities in mild closed head injured patients with cognitive impairments. Nuclear Medicine Communications 10(5):335-344.
Huttenlocher, P. R., and A. S. Dabholkar. 1997. Regional differences in synaptogenesis in human cerebral cortex. Journal of Comparative Neurology 387(2):167-178.
Hylin, M. J., S. A. Orsi, J. Zhao, K. Bockhorst, A. Perez, A. N. Moore, and P. K. Dash. 2013. Behavioral and histopathological alterations resulting from mild fluid percussion injury. Journal of Neurotrauma 30(9):702-715.
Ibrahim, N. G., J. Ralston, C. Smith, and S. S. Margulies. 2010. Physiological and pathological responses to head rotations in toddler piglets. Journal of Neurotrauma 27(6):1021-1035.
Iglowstein, I., O. G. Jenni, L. Molinari, and R. H. Largo. 2003. Sleep duration from infancy to adolescence: Reference values and generational trends. Pediatrics 111(2):302-307.
Ip, E. Y., C. C. Giza, G. S. Griesbach, and D. A. Hovda. 2002. Effects of enriched environment and fluid percussion injury on dendritic arborization within the cerebral cortex of the developing rat. Journal of Neurotrauma 19(5):573-585.
Itoh, T., T. Satou, S. Nishida, M. Tsubaki, S. Hashimoto, and H. Ito. 2009. Expression of amyloid precursor protein after rat traumatic brain injury. Neurological Research 31(1):103-109.
Ives, J. C., M. Alderman, and S. E. Stred. 2007. Hypopituitarism after multiple concussions: A retrospective case study in an adolescent male. Journal of Athletic Training 42(3):431-439.
Junger, E. C., D. W. Newell, G. A. Grant, A. M. Avellino, S. Ghatham, C. M. Dauville, A. M. Lam, R. Aaslid, and H. R. Winn. 1997. Cerebral autoregulation following minor head injury. Journal of Neurosurgery 86(3):425-532.
Kane, M. J., M. Angoa-Perez, D. I. Briggs, D. C. Viano, C. W. Kreipke, and D. M. Kuhn. 2012. A mouse model of human repetitive mild traumatic brain injury. Journal of Neuroscience Methods 203(1):41-49.
Karlin, A. M. 2011. Concussion in the pediatric and adolescent population: Different population, different concerns. Physical Medicine and Rehabilitation 3(Suppl):S369-S379.
Kaster, T., I. Sack, and A. Samani. 2011. Measurement of the hyperelastic properties of ex vivo brain tissue slices. Journal of Biomechanics 44(6):1158-1163.
Katayama, Y., D. P. Becker, T. Tamura, and D. A. Hovda. 1990. Massive increases in extracellular potassium and the indiscriminate release of glutamate following concussive brain injury. Journal of Neurosurgery 73(6):889-900.
Kawamata, T., Y. Katayama, D. A. Hovda, A. Yohino, and D. P. Becker. 1992. Administration of excitatory amino acid antagonists via microdialysis attenuates the increase in glucose utilization seen following concussive brain injury. Journal of Cerebral Blood Flow and Metabolism 12(1):12-24.
Kelley, A. E., T. Schochet, and C. F. Landry. 2004. Risk taking and novelty seeking in adolescence: Introduction to part 1. Annals of the New York Academy of Sciences 1021:27-32.
Kelly, D. F., I. T. Gonzalo, P. Cohan, N. Berman, R. Swerdloff, and C. Wang. 2000. Hypopituitarism following traumatic brain injury and aneurysmal subarachnoid hemorrhage: A preliminary report. Journal of Neurosurgery 93(5):743-752.
Kerr, M. E., C. M. Bender, and E. J. Monti. 1996. An introduction to oxygen free radicals. Heart and Lung: The Journal of Critical Care 25(3):200-208.
Khadr, S. N., P. M. Crofton, P. A. Jones, B. Wardhaugh, J. Roach, A. J. Drake, R. A. Minns, and C. J. Kelnar. 2010. Evaluation of pituitary function after traumatic brain injury in children. Clinical Endocrinology 73(5):637-643.
Kieslich, M., A. Fielder, C. Heller, W. Kreuz, and G. Jacobi. 2002. Minor head injury as cause and co-factor in the aetiology of stroke in children: A report of eight cases. Journal of Neurology, Neurosurgery, and Psychiatry 7(1)3:13-16.
Kilbaugh, T. J., S. Bhandare, D. H. Lorom, M. Saraswati, C. L. Robertson, and S. S. Margulies. 2011. Cyclosporin A preserves mitochondrial function after traumatic brain injury in the immature rat and piglet. Journal of Neurotrauma 29(5):763-774.
Kimpara, H., and M. Iwamoto. 2012. Mild traumatic brain injury predictors based on angular accelerations during impacts. Annals of Biomedical Engineering 40(1):114-126.
King, A. I., K. H. Yang, L. Zhang, W. Hardy, and D. C. Viano. 2003. Is head injury caused by linear or angular acceleration? Proceedings of the 2003 International Research Conference on the Biomechanics of Impact. http://www.smf.org/docs/articles/hic/King_IRCOBI_2003.pdf (accessed July 1, 2013).
Kleinberger, M., E. Sun, R. H. Eppinger, S. Kuppa, and R. Saul. 1998. Development of improved injury criteria for the assessment of automotive restraint systems. National Highway Traffic Safety Administration, NHTSA Docket No. NHTSA-98-4405. September.
Kleiven, S. 2007. Predictors for traumatic brain injuries evaluated through accident reconstructions. Stapp Car Crash Journal 51:81-114.
Kraus, M. F., T. Susmaras, B. P. Caughlin, C. J. Walker, J. A. Sweeney, and D. M. Little. 2007. White matter integrity and cognition in chronic traumatic brain injury: A diffusion tensor imaging study. Brain 130(Part 10):2508-2519.
Kristman, V. L., C. H. Tator, N. Kreiger, D. Richards, L. Mainwaring, S. Jaglal, G. Tomlinson, and P. Comper. 2008. Does the apolipoprotein epsilon 4 allele predispose varsity athletes to concussion? A prospective cohort study. Clinical Journal of Sport Medicine 18(4):322-328.
LaPlaca, M. C., J. Zhang, R. Raghupathi, J. H. Li, F. Smith, F. M. Bareyre, S. H. Snyder, D. I. Graham, and T. K. McIntosh. 2001. Pharmacologic inhibition of poly (ADP-ribose) polymerase is neuroprotective following traumatic brain injury in rats. Journal of Neurotrauma 18(4):369-376.
Laurer, H., F. M. Bareyre, V. M. Lee, J. Q. Trojanowski, L. Longhi, R. Hoover, K. E. Saatman, R. Raghupathi, S. Hoshino, M. S. Grady, and T. K. McIntosh. 2001. Mild head injury increases the brain’s vulnerability to a second traumatic impact. Journal of Neurosurgery 95(5):859-870.
Len, T. K., and J. P. Neary. 2011. Cerebrovascular pathophysiology following mild traumatic brain injury. Clinical Physiology and Functional Imaging 31(2):85-93.
Lewén, A., and L. Hillered. 1998. Involvement of reactive oxygen species in membrane phospholipid breakdown and energy perturbation after traumatic brain injury in the rat. Journal of Neurotrauma 15(7):521-530.
Li, X. Y., J. Li, D. F. Feng, and L. Gu. 2010. Diffuse axonal injury induced by simultaneous moderate linear and angular head accelerations in rats. Neuroscience 169(1):357-369.
Lieberman, S. A., A. L. Oberoi, C. R. Gilkison, B. E. Masel, and R. J. Urban. 2001. Prevalence of neuroendocrine dysfunction in patients recovering from traumatic brain injury. Journal of Clinical Endocrinology and Metabolism 86(6):2752-2756.
Lighthall, J. W. 1988. Controlled cortical impact: A new experimental brain injury model. Journal of Neurotrauma 5(1):1-15.
Lindgren, S., and L. Rinder. 1965. Experimental studies of head injury. I. Some factors influencing results of model experiments. Biophysik 2(5):320-329.
Lloyd, R., B. Parr, S. Davies, and C. Cooke. 2011. A kinetic comparison of back-loading and head-loading in Xhosa women. Ergonomics 54(4):380-391.
Malone, S. K. 2011. Early to bed, early to rise?: An exploration of adolescent sleep hygiene practices. Journal of School Nursing 27(5):348-354.
Mandera, M., D. Larysz, and M. Wojtacha. 2002. Changes in cerebral hemodynamics assessed by transcranial Doppler ultrasonography in children after head injury. Child’s Nervous System 18(3-4):124-128.
Mansell, J., R. T. Tierney, M. R. Sitler, K. A. Swanik, and D. Stearne. 2005. Resistance training and head-neck segment dynamic stabilization in male and female collegiate soccer players. Journal of Athletic Training 40(4):310-319.
Marklund, N., T. Lewander, F. Clausen, and L. Hillered. 2001. Monitoring of reactive oxygen species production after traumatic brain injury in rats with microdialysis and the 4-hydroxybenzoic acid trapping method. Journal of Neurotrauma 18(11):1217-1227.
Marmarou, A., M. A. Foda, W. van den Brink, J. Campbell, H. Kita, and K. Demetriadou. 1994. A new model of diffuse brain injury in rats. Part I: Pathophysiology and biomechanics. Journal of Neurosurgery 80(2):291-300.
Maugans, T. A., C. Farley, M. Altaye, J. Leach, and K. M. Cecil. 2012. Pediatric sports-related concussion produces cerebral blood flow alterations. Pediatrics 129(1):28-37.
McQuire, J. C., J. C. Sutcliffe, and T. J. Coats. 1998. Early changes in middle cerebral artery blood flow velocity after head injury. Journal of Neurosurgery 89(4):526-532.
Meaney, D. F., and D. H. Smith. 2011. Biomechanics of concussion. Clinics in Sports Medicine 30(1):19-31.
Meehan, W. P., A. M. Taylor, and M. Proctor. 2011. The pediatric athlete: Younger athletes with sport-related concussion. Clinics in Sports Medicine 30(1):133-144.
Meehan, W. P., J. Zhang, R. Mannix, and M. J. Whalen. 2012. Increasing recovery time between injuries improves cognitive outcome after repetitive mild concussive brain injuries in mice. Neurosurgery 71(4):885-891.
Milman, A., A. Rosenberg, R. Weizman, and C. G. Pick. 2005. Mild traumatic brain injury induces persistent cognitive deficits and behavioral disturbances in mice. Journal of Neurotrauma 22(9):1003-1010.
Monson, K. L., W. Goldsmith, N. M. Barbaro, and G. T. Manley. 2003. Axial mechanical properties of fresh human cerebral blood vessels. Journal of Biomechanical Engineering 125 (2):288-294.
Morris, R. 1984. Developments of a water maze procedure for studying spatial learning in the rat. Journal of Neuroscience Methods 11(1):47-60.
Nehlig, A., A. Pereira de Vasconcelos, and S. Boyet. 1989. Postnatal changes in local cerebral blood flow measured by the quantitative autoradiographic [14C]iodoantipyrine technique in freely moving rats. Journal of Cerebral Blood Flow and Metabolism 9(5):579-588.
Niederland, T., H. Makovi, V. Gál, B. Andréka, C. S. Ábrahám, and J. Kovács. 2007. Abnormalities of pituitary function after traumatic brain injury in children. Journal of Neurotrauma 24(1):119-127.
Norwood, K. W., M. D. Deboer, M. J. Gurka, M. N. Kuperminc, A. D. Rogol, J. A. Blackman, J. B. Wamstad, M. L. Buck, and P. D. Patrick. 2010. Traumatic brain injury in children and adolescents: Surveillance for pituitary dysfunction. Clinical Pediatrics (Phila) 49(11):1044-1049.
O’Connell, K. M., and M. T. Littleton-Kearney. 2013. The role of free radicals in traumatic brain injury. Biological Research for Nursing 15(3):253-263.
O’Connell, M. T., A. Seal, J. Nortje, P. G. Al-Rawi, J. P. Coles, T. D. Fryer, D. K. Menon, J. D. Pickard, and P. J. Hutchinson. 2005. Glucose metabolism in traumatic brain injury: A combined microdialysis and [18F]-2-fluoro-2-deoxy-d-glucose-positron emission tomography (FDG-PET) study. Acta Neurochirurgica Supplement 95:165-168.
Ommaya, A. K. 1985. Biomechanics of head injury: Experimental aspects. In Biomechanics of Trauma, edited by A. M. Nahum and J. W. Melvin. Prentice-Hall, Pp. 245-269.
Ommaya, A. K., and T. A. Gennarelli. 1974. Cerebral concussion and traumatic unconsciousness. Correlation of experimental and clinical observations of blunt head injuries. Brain 97(4):633-654.
Ommaya, A. K., A. E. Hirsch, and J. L. Martinez. 1966. The role of whiplash in cerebral concussion. 10th Stapp Car Crash Conference, SAE, 10:314-324.
Ommaya, A. K., R. L. Grubb, and R. A. Naumann. 1971. Coup and contre-coup injury: Observations on the mechanics of visible brain injuries in the rhesus monkey. Journal of Neurosurgery 35(5):503-516.
Ommaya, A. K., P. Corrao, and F. S. Letcher. 1973. Head injury in the chimpanzee. Part 1: Biodynamics of traumatic unconsciousness. Journal of Neurosurgery 39(2):152-166.
Ommaya, A. K., W. Goldsmith, and L. Thibault. 2002. Biomechanics and neuropathology of adult and paediatric head injury. British Journal of Neurosurgery 16(3):220-242.
Osteen, C. L., A. H. Moore, M. L. Prins, and D. A. Hovda. 2001. Age-dependency of 45calcium accumulation following lateral fluid percussion: Acute and delayed patterns. Journal of Neurotrauma 18(2):141-162.
Ouckama, R., and D. J. Pearsall. 2011. Evaluation of a flexible force sensor for measurement of helmet foam impact performance. Journal of Biomechanics 44(5):904-909.
Ozdemir, D., N. Uysal, S. Gonenc, O. Acikgoz, A. Sonmez, A. Topcu, N. Ozdemir, M. Duman, I. Semin, and H. Ozkan. 2005. Effect of melatonin on brain oxidative damage induced by traumatic brain injury in immature rats. Physiological Research 54(6):631-637.
Persson, L., and L. Hillered. 1992. Chemical monitoring of neurosurgical intensive care patients using intracerebral microdialysis. Journal of Neurosurgery 76(1):72-80.
Petronilho, F., G. Feier, B. de Souza, C. Guglielmi, L. S. Constantino, R. Walz, J. Quevedo, and R. Dal-Pizzol. 2010. Oxidative stress in brain according to traumatic brain injury intensity. Journal of Surgical Research 164(2):316-320.
Pharo, H., C. Sim, M. Graham, J. Gross, and H. Hayne. 2011. Risky business: Executive function, personality, and reckless behavior during adolescence and emerging adulthood. Behavioral Neuroscience 125(6):970-978.
Post, A., E. S. Walsh, T. B. Hoshizaki, and M. Gilchrist. 2011. Analysis of loading curve characteristics on the production of brain deformation metrics. International Union of Theoretical and Applied Mechanics Symposium on Impact Biomechanics in Sport, Dublin, Ireland. http://www.ucd.ie/t4cms/proceedings_IUTAM.pdf (accessed July 1, 2013).
Povlishock, J. T., and C. W. Christman. 1995. The pathobiology of traumatically induced axonal injury in animals and humans: A review of current thoughts. Journal of Neurotrauma 12(4):555-564.
Povlishock, J. T., D. P. Becker, C. L. Cheng, and G. W. Vaughan. 1983. Axonal change in minor head injury. Journal of Neuropathology and Experimental Neurology 42(3):225-242.
Prange, M. T., and S. S. Margulies. 2002. Regional, directional, and age-dependent properties of the brain undergoing large deformation. Journal of Biomechanical Engineering 124(2):244-252.
Praticò, D., P. Reiss, L. X. Tang, S. Sung, J. Rokach, and T. K. McIntosh. 2002. Local and systemic increase in lipid peroxidation after moderate experimental traumatic brain injury. Journal of Neurochemistry 80(5):894-898.
Prevost, T. P., G. Jin, M. A. de Moya, H. B. Alam, S. Suresh, and S. Socrate. 2011. Dynamic mechanical response of brain tissue indentation in vivo, in situ, and in vitro. Acta Biomaterialia 7(12):4090-4101.
Prins, M. L., and D. A. Hovda. 1998. Traumatic brain injury in the developing rat: Effects of maturation on Morris water maze acquisition. Journal of Neurotrauma 15(10):799-811.
Prins, M. L., and D. A. Hovda. 2001. Mapping cerebral glucose metabolism during spatial learning: Interactions of development and traumatic brain injury. Journal of Neurotrauma 18(1):31-46.
Prins, M. L., and D. A. Hovda. 2009. The effects of age and ketogenic diet on local cerebral metabolic rates of glucose after controlled cortical impact injury in rats. Journal of Neurotrauma 26(7):1083-1093.
Prins, M. L., S. M. Lee, C. L. Y. Cheng, D. P. Becker, and D. A. Hovda. 1996. Fluid percussion brain injury in the developing and adult rat: A comparative study of mortality, morphology, intracranial pressure and mean arterial blood pressure. Developmental Brain Research 95(2):272-282.
Prins, M. L., A. Hales, M. Reger, C. C. Giza, and D. A. Hovda. 2010. Repeat traumatic brain injury in the juvenile rat is associated with increased axonal injury and cognitive impairments. Developmental Neuroscience 32(5-6):510-518.
Prins, M. L., D. Alexander, C. C. Giza, and D. A. Hovda. 2013. Repeated mild traumatic brain injury: Mechanisms of cerebral vulnerability. Journal of Neurotrauma 30(1):30-38.
Queen, R. M., P. Weinhold, D. T. Kirkendall, and B. Yu. 2003. Theoretical study of the effect of ball properties on impact force in soccer heading. Medicine and Science in Sports Exercise 35(12):2069-2076.
Raghupathi, R., and S. S. Margulies. 2002. Traumatic axonal injury after closed head injury in the neonatal pig. Journal of Neurotrauma 19(7):843-853.
Rao, A. M., A. Dogan, J. F. Hatcher, and R. J. Dempsey. 1998. Fluorometric assay of nitrite and nitrate in brain tissue after traumatic brain injury and cerebral ischemia. Brain Research 793(1-2):265-270.
Reddy, C. C., M. W. Collins, and G. A. Gioia. 2008. Adolescent sports concussion. Physical Medicine and Rehabilitation Clinics of North America 19(2):247-269.
Richards, H. K., S. Simac, S. Piechnik, and J. D. Pickard. 2001. Uncoupling of cerebral blood flow and metabolism after cerebral contusion in the rat. Journal of Cerebral Blood Flow and Metabolism 21(7):779-781.
Robbins, D. H., and J. L. Wood. 1969. Determination of the mechanical properties of bones of the skull. Experimental Mechanics 9(5):236-240.
Roberts, M. A., F. F. Manshadi, D. L. Bushnell, and M. E. Hines. 1995. Neurobehavioural dysfunction following mild traumatic brain injury in childhood: A case report with positive findings on positron emission tomography (PET). Brain Injury 9(5):427-436.
Romer, D. 2010. Adolescent risk taking, impulsivity, and brain development: Implications for prevention. Developmental Psychobiology 52(3):263-276.
Rose, S. R., and B. A. Auble. 2012. Endocrine changes after pediatric traumatic brain injury. Pituitary 15(3):267-275.
Rowson, S., and S. M. Duma. 2013. Brain injury prediction: Assessing the combined probability of concussion using linear and rotational head acceleration. Annals of Biomedical Engineering 41(5):873-882.
Rowson, S., S. M. Duma, J. G. Beckwith, J. J. Chu, R. M. Greenwald, J. J. Crisco, P. G. Brolinson, A. C. Duhaime, T. W. McAllister, and A. C. Maerlender. 2012. Rotational head kinematics in football impacts: An injury risk function for concussion. Annals of Biomedical Engineering 40(1):1-13.
Saatman, K. E., A. C. Duhaime, R. Bullock, A. I. Maas, A. Valadka, and G. T. Manley. 2008. Classification of traumatic brain injury for targeted therapies. Journal of Neurotrauma 25(7):719-738.
Satchell, M. A., X. Zhang, P. M. Kochanek, C. E. Dixon, L. W. Jenkins, J. Melick, C. Szabo, and R. S. Clark. 2003. A dual role for poly-ADP-ribosylation in spatial memory acquisition after traumatic brain injury in mice involving NAD+depletion and ribosylation of 14-3-3gamma. Journal of Neurochemistry 85(3):697-708.
Schlaepfer, W. W. 1974. Calcium-induced degeneration of axoplasm in isolated segments of rat peripheral nerve. Brain Research 69(2):203-215.
Schlaepfer, W. W. 1987. Neurofilaments: Structure, metabolism and implications in disease. Journal of Neuropathology and Experimental Neurology 46(2):117-129.
Schneider, K., and R. F. Zernicke. 1988. Computer simulation of head impact: Estimation of head-injury risk during soccer heading. International Journal of Sport Biomechanics 4(4):358-371.
Schulz, M. R., S. W. Marshall, F. O. Mueller, J. Yang, N. L. Weaver, W. D. Kalsbeek, and J. M. Bowling. 2004. Incidence and risk factors for concussion in high school athletes, North Carolina, 1996–1999. American Journal of Epidemiology 160(10):937-944.
Schwarzbold, M. L., D. Rial, T. De Bem, D. G. Machado, M. P. Cunha, A. dos Santos, D. P. dos Santos, C. P. Figueiredo, M. Farina, E. M. Goldfeder, A. L. Rodrigues, R. D. Prediger, and R. Walz. 2010. Effects of traumatic brain injury of different severities on emotional, cognitive, and oxidative stress-related parameters in mice. Journal of Neurotrauma 27(10):1883-1893.
Sen, S., H. Goldman, M. Morehead, S. Murphy, and J. W. Phillis. 1993. Oxypurinol inhibits free radical release from the cerebral cortex of closed head injured rats. Neuroscience Letters 162(1-2):117-120.
Seok, J., H. S. Warren, A. G. Cuenca, M. N. Mindrinos, H. V. Baker, W. Xu, D. R. Richards, G. P. McDonald-Smith, H. Gao, L. Hennessy, C. C. Finnerty, C. M. Lopez, S. Honari, E. E. Moore, J. P. Minei, J. Cuschieri, P. E. Bankey, J. L. Johnson, J. Sperry, A. B. Nathens, T. B. Billiar, M. A. West, M. G. Jeschke, M. B. Klein, R. L. Gamell, N. S. Gibran, B. H. Brownstein, C. Miller-Graziano, S. E. Calvano, P. H. Mason, J. P. Cobb, L. G. Rahme, S. F. Lowry, R. V. Maier, L. L. Moldawer, D. N. Herndon, R. W. Davis, W. Xiao, R. G. Tompkins, and the Inflammation and Host Response to Injury, Large Scale Collaborative Research Program. 2013. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proceedings of the National Academy of Sciences of the United States of America 110(9):3507-3512.
Shojo, H., and K. Kibayashi. 2006. Changes in localization of synaptophysin following fluid percussion injury in the rat brain. Brain Research 1078(1):198-211.
Shrey, D. W., G. S. Griesbach, and C.C. Giza. 2011. The pathophysiology of concussions in youth. Physical Medicine and Rehabilitationl Clinics of North America 22(4):577-602.
Shultz, S. R., F. Bao, V. Omana, C. Chiu, A. Brown, and D. P. Cain. 2011. Repeated mild lateral fluid percussion brain injury in the rat causes cumulative long-term behavioral impairments, neuroinflammation, and cortical loss in an animal model of repeated concussion. Journal of Neurotrauma 29(2):281-294.
Singh, I. N., P. G. Sullivan, Y. Deng, L. H. Mbye, and E. D. Hall. 2006. Time course of posttraumatic mitochondrial oxidative damage and dysfunction in a mouse model of focal traumatic brain injury: Implications for neuroprotective therapy. Journal of Cerebral Blood Flow and Metabolism 26(11):1407-1418.
Smith, D. H., J. A. Wolf, T. A. Lusardi, V. M. Lee, and D. F. Meaney. 1999. High tolerance and delayed elastic response of cultured axons to dynamic stretch injury. Journal of Neuroscience 19(11):4263-4269.
Sowell, E. R., P. M. Thompson, and A. W. Toga. 2004. Mapping changes in the human cortex throughout the span of life. Neuroscientist 10(4):372-392.
Spear, L. P. 2010. The Behavioral Neuroscience of Adolescence. New York: Norton.
Stålhammar, D. 1986. Experimental models of head injury. Acta Neurochirurgica Supplementum (Wien) 36:33-46.
Stålhammar, D. A. 1990. The mechanism of brain injuries. In Handbook of Clinical Neurology, Vol. 13(57): Head Injury, edited by R. Braakman. New York: Elsevier Science Publishers. Pp. 17-41.
Stojsih, S., M. Boitano, M. Wilhelm, and C. Bir. 2010. A prospective study of punch biomechanics and cognitive function for amateur boxers. British Journal of Sports Medicine 44(10):725-730.
Strich, S. J. 1956. Diffuse degeneration of the cerebral white matter in severe dementia following head injury. Journal of Neurology, Neurosurgery, and Psychiatry 19(3):163-185.
Strich, S. J., and D. M. Oxon. 1961. Shearing of nerve fibers as a cause of brain damage due to head injury: A pathological study of twenty cases. Lancet 278(7200):443-448.
Sullivan, S., S. H. Friess, J. Ralston, C. Smith, K. J. Propert, P. E. Rapp, and S. S. Margulies. 2013. Behavioral deficits and axonal injury persistence after rotational head injury are direction dependent. Journal of Neurotrauma 30(7):520-534.
Sutton, R. L., D. A. Hovda, P. D. Adelson, E. C. Benzel, and D. P. Becker. 1994. Metabolic changes following cortical contusion: Relationships to edema and morphological changes. Acta Neurochirurgica 60(Suppl):446-448.
Takhounts, E. G., S. A. Ridella, V. Hasiia, R. E. Tannous, J. Q. Campbell, D. Malone, K. Danelson, J. Stitzel, S. Rowson, and S. Duma. 2008. Investigation of traumatic brain injuries using the next generation of simulated injury monitor (SIMon) finite element head model. Stapp Car Crash Journal 52:1-31.
Terrell, T., R. Bostick, R. Abramson, D. Xie, W. Barfield, R. Cantu, M. Stanek, and T. Ewing. 2008. APOE, APOE promoter, and tau genotypes and risk for concussion in college athletes. Clinical Journal of Sports Medicine 18(1):10-17.
Terrell, T. R., R. M. Bostick, and J. T. Barth. 2012. Prospective cohort study of the association of genetic polymorphisms and concussion risk and postconcussion neurocognitive deficits in college athletes. Clinical Journal of Sports Medicine 22(2):172-208.
Terrell, T. R., R. M. Bostick, J. Barth, D. McKeag, R. C. Cantu, R. Sloane, L. Galloway, D. Erlanger, V. Valentine, and K. Bielak. 2013. Genetic polymorphisms, concussion risk, and post concussion neurocognitive deficits in college and high school athletes. British Journal of Sports Medicine 47(5):e1.
Thomas, S., M. L. Prins, M. Samii, and D. A. Hovda. 2000. Cerebral metabolic response to traumatic brain injury sustained early in development: A 2-deoxy-D-glucose autoradiographic study. Journal of Neurotrauma 17(8):649-665.
Tierney, R. T., M. R. Sitler, B. Swanik, K. Swanik, M. Higgins, and J. Torg. 2005. Gender differences in head-neck segment dynamic stabilization during head acceleration. Medicine and Science in Sports and Exercise 37(2):272-279.
Tierney, R. T., M. Higgins, S. V. Caswell, J. Brady, K. McHardy, J. B. Driban, and K. Davish. 2008. Sex differences in head acceleration during heading while wearing soccer headgear. Journal of Athletic Training 43(6):578-584.
Tierney, R. T., J. L. Mansell, M. Higgins, J. T. McDevitt, N. Toone, J. P. Gaughan, A. Mishra, and E. Krynetskiy. 2010. Apolipoprotein E genotype and concussion in college athletes. Clinical Journal of Sport Medicine 20(6):464-468.
Tong, W., T. Igarashi, D. M. Ferriero, and L. J. Noble. 2002. Traumatic brain injury in the immature mouse brain: Characterization of regional vulnerability. Experimental Neurology 176(1):105-116.
Tontisirin, N., S. Muangman, P. Suz, C. Pihoker, D. Fisk, A. Moore, A. M. Lam, and M. S. Vivilala. 2007. Early childhood gender differences in anterior and posterior cerebral blood flow velocity and autoregulation. Pediatrics 119(3):610-615.
Tsuru-Aoyagi, K., M. B. Potts, A. Trivedi, T. Pfankuch, J. Raber, M. Wendland, C. P. Claus, S. E. Koh, D. Ferriero, and L. J. Noble-Haeusslein. 2009. Glutathione peroxidase activity modulates recovery in the injured immature brain. Annals of Neurology 65(5):540-549.
Tyurin, V. A., Y. Y. Tyurina, G. G. Borisenko, T. V. Sokolova, V. B. Ritov, P. J. Quinn, M. Rose, P. Kochanek, S. H. Graham, and V. E. Kagan. 2000. Oxidative stress following traumatic brain injury in rats: Quantitation of biomarkers and detection of free radical intermediates. Journal of Neurochemistry 75(5):2178-2189.
Udomphorn, Y., W. M. Armstead, and M. S. Vavilala. 2008. Cerebral blood flow and autoregulation after pediatric traumatic brain injury. Pediatric Neurology 38(4):225-234.
Umile, E., M. Sandel, A. Alavi, C. Terry, and R. Plotkin. 2002. Dynamic imaging in mild traumatic brain injury: Support for the theory of medial temporal vulnerability. Archive of Physical Medical Rehabilitation 83(11):1506-1513.
Vagnozzi, R., A. Marmarou, B. Tavazzi, S. Signoretti, D. Di Pierro, F. del Bolgia, A. M. Amorini, G. Fazzina, S. Sherkat, and G. Lazzarino. 1999. Changes of cerebral energy metabolism and lipid peroxidation in rats leading to mitochondrial dysfunction after diffuse brain injury. Journal of Neurotrauma 16(10):903-913.
Vagnozzi, R., S. Signoretti, B. Tavazzi, A. Lucovici, S. Marziali, G. Tarascio, A. M. Amorini, V. DiPietro, R. Delfini, and G. Lazzorino. 2008. Temporal window of metabolic brain vulnerability to concussion: A pilot 1H-magnetic resonance spectropic study in concussed athletes—Part III. Neurosurgery 62(6):1286-1295.
Vavilala, M. S., L. A. Lee, and A. M. Lam. 2002a. Cerebral blood flow and vascular physiology. Anesthesiology Clinics of North America 20(2):247-264.
Vavilala, M. S., D. W. Newell, E. Junger, C. M. Douville, R. Aaslid, F. P. Rivara, and A. M. Lam. 2002b. Dynamic cerebral autoregulation in healthy adolescents. Acta Anaesthesiologica Scandinavica 46(4):393-397.
Vavilala, M. S., L. A. Lee, K. Boddu, E. Visco, D. W. Newell, J. J. Zimmerman, and A. M. Lam. 2004. Cerebral autoregulation in pediatric traumatic brain injury. Pediatric Critical Care Medicine 5(3):257-263.
Vavilala, M., M. Kincaid, S. Muangman, P. Suz, I. Rozet, and A. Lam. 2005. Gender differences in cerebral blood flow velocity and autoregulation between the anterior and posterior circulations in healthy children. Pediatric Research 58(3):574-578.
Vespa, P., M. Prins, E. Ronne-Engstrom, M. Caron, E. Shalmon, D. A. Hovda, N. A. Martin, and D. P. Becker. 1998. Increase in extracellular glutamate caused by reduced cerebral perfusion pressure and seizures after human traumatic brain injury: A microdialysis study. Journal of Neurosurgery 89(6):971-982.
Viano, D. C., A. Hamberger, H. Bolouri, and A. Saljo. 2012. Evaluation of three animal models for concussion and serious brain injury. Annals of Biomedical Engineering 40(1): 212-226.
Vink, R., P. G. Mullins, M. D. Temple, W. Bao, and A. I. Faden. 2001. Small shifts in craniotomy position in the lateral fluid percussion injury model are associated with differential lesion development. Journal of Neurotrauma 18(8):839-847.
Wada, K., K. Chatzipanteli, S. Kraydieh, R. Busto, and W. D. Dietrich. 1998. Inducible nitric oxide synthase expression after traumatic brain injury and neuroprotection with amino-guanidine treatment in rats. Neurosurgery 43(6):1427-1436.
Wall, W. J., and M. Shani. 2008. Are animal models as good as we think? Theriogenology 69(1):2-9.
Werner, C., and K. Engelhard. 2007. Pathophysiology of traumatic brain injury. British Journal of Anaesthesia 99(1):4-9.
Worley, G., J. M. Hoffman, S. S. Paine, S. L. Kalman, S. J. Claerhout, O. B. Boyko, R. S. Kandt, C. C. Santos, M. W. Hanson, and W. J. Oakes. 1995. 18-Flurodeoxyglucose positron emission tomography in children and adolescents with traumatic brain injury. Developmental Medicine and Child Neurology 37(3):213-220.
Xiong, Y., A. Mahmood, and M. Chopp. 2013. Animal models of traumatic brain injury. Nature Reviews Neuroscience 14(2):128-142.
Yakovlev, P. I., and A. R. Lecours. 1967. The myelogenetic cycles of regional maturation of the brain. In Regional Development of the Brain in Early Life, edited by A. Minkowski. Oxford: Blackwell Scientific. Pp. 3-70.
Yoshino, A., D. A. Hovda, T. Kawamata, Y. Katayama, and D. P. Becker. 1991. Dynamic changes in local cerebral glucose utilization following cerebral conclusion in rats: Evidence of a hyper- and subsequent hypometabolic state. Brain Research 561(1):106-119.
Yoshino, A., D. A. Hovda, T. Kawamata, Y. Katayama, and D. P. Becker. 1992. Hippocampal CA3 lesion prevents postconcussive metabolic dysfunction in CA1. Journal of Cerebral Blood Flow and Metabolism 12(6):996-1006.
Zemper, E. D. 2003. Two-year prospective study of relative risk of a second cerebral concussion. American Journal of Physical Medicine and Rehabilitation 82(9):653-659.
Zhou, C., S. A. Eucker, T. Durduran, G. Yu, J. Ralston, S. H. Friess, R. N. Ichord, S. S. Margulies, and A. G. Yodh. 2009. Diffuse optical monitoring of hemodynamic changes in piglet brain with closed head injury. Journal of Biomedical Optics 14(3):034015.
Zhu, Q., M. Prange, and S. Margulies. 2006. Predicting unconsciousness from a pediatric brain injury threshold. Developmental Neuroscience 28(4-5):388-395.
Zuckerman, S. L., Y. M. Lee, M. J. Odom, G. S. Solomon, J. A. Forbes, and A. K. Sills. 2012. Recovery from sports-related concussion: Days to return to neurocognitive baseline in adolescents versus young adults. Surgical Neurology International 3:130.












































