Artificial Solar Fuel Generators
MIGUEL A. MODESTINO
École Polytechnique Fédérale de Lausanne
RACHEL A. SEGALMAN
University of California, Berkeley Lawrence Berkeley National Laboratories
There has been significant interest in increasing the share of renewable energy sources in the world energy landscape (Chu and Majumdar 2012). Associated technologies support the generation or capture of energy from carbon-neutral sources, storage so that the energy can be used when and where needed, and more efficient use. In discussions of alternatives available for power generation from renewable sources, solar energy conversion is prominent, given the vast amount of energy it can yield (peak irradiation of 7.5 kWh/m2/day, mean annual global irradiation of 8,372 terawatt hours [TWh]/yr), making it a potentially important candidate for a sizable portion of US energy.
This potential contrasts with its current relatively small portion of the global energy portfolio: 0.06 percent (IEA 2012; Zhang and Shen 2012). While economic factors account for a substantial part of the barrier to implementation, considerable technological challenges stem from the inherently intermittent nature of the solar generation process. Adoption of solar power generation will entail significant changes in operation of the power grid, as classical power generation plants will need to respond not only to changes in consumer demand but also to noncontrollable variations in energy generation.
BACKGROUND
One option to mitigate the intermittency of solar energy generation is the incorporation of energy storage capacity into the grid, so that fluctuations in energy generation are buffered and do not affect the operation of the electricity distribution channels. But large-scale implementation of energy storage faces both technological and economic hurdles requiring significant research and development.
Alternatively, one could take inspiration from nature, where energy is stored in the form of chemical bonds. In the case of artificial photosynthesis, this means the generation of fuels directly from solar energy.
Types of Solar Fuel Generators
Integrated energy capture and storage solutions such as solar fuel generators have the potential to increase the fraction of renewables in the mix of energy sources, and can apply to all sectors of energy consumption (industrial, commercial, residential, and transportation) (Bard and Fox 1995; Chu and Majumdar 2012; Concepcion et al. 2012; Faunce et al. 2013; Lewis and Nocera 2006; Nocera 2012). Integrated solar fuel generators are photoelectrochemical (PEC) cells that can capture solar energy and catalytically convert low-energy reactants into energy-dense fuels.
One category of solar fuel generators, water-splitting systems, take water as a feed and produce hydrogen fuel and oxygen as byproduct. A general representation of these systems is shown in Figure 1. Practical systems take water and solar energy as inputs and produce output streams of hydrogen and oxygen in a safe and scalable manner. In this way pure fuel streams can be collected and used in electrochemical energy conversion devices (i.e., fuel cells) or in chemical processes to synthesize or enhance the energy content of liquid fuels (e.g., the Fischer-Tropsch process).
The concept of solar fuel generation can be extended to the electrochemical reduction of CO2, which can yield carbon-containing fuels but represents a closed cycle from the carbon perspective, making these new fuels truly carbon neutral. Solar-driven CO2 reduction poses greater technical challenges because the number of electron transfer steps in the reactions is higher, the concentration of CO2 in electrolytes is generally low, and the diversity of products makes the necessary separation more difficult.
Mechanics of Solar Fuel Generators
As shown in Figure 1, solar fuel generation begins with the absorption of light to form charges that are used to drive oxidation and reduction reactions. These three processes can be done in separate units—for example, using a photovoltaic cell to generate electricity that powers an electrolyzer, which incorporates the catalysts—or in a fully integrated device. Practical comparisons between these two scenarios are largely dependent on possible gains in terms of economics and flexibility of deployment.
A fully integrated water-splitting or solar hydrogen generator, as shown in Figure 1, would consist of interconnected photovoltaic and catalytic units. Ideally, the oxidation and reduction sites are physically separated so that the product (H2 in the case of water splitting) is generated in a space different from the byproduct
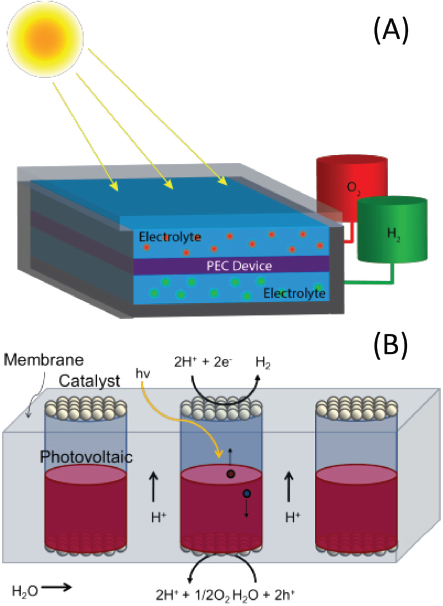
FIGURE 1 (A) Solar fuel generators are composed of photoelectrochemical (PEC) devices that can generate separate streams of fuels directly from sunlight (H2 and O2 in the case of water splitting). (B) A PEC device contains photovoltaic units that absorb light and move charges to catalytic centers, where electrochemical hydrogen and oxygen evolution reactions take place. In the diagram, the membrane component is used as the matrix for PEC units and allows for both ion conduction (i.e., H+) and gas separation.
(O2 in this case). The size of the photovoltaic unit is generally set by the solar absorption depth of the material and is on the order of microns to millimeters.
For a number of reasons it makes sense to have an array of photovoltaic units held together by a mechanically robust membrane that separates the product gases and shuttles ions from one catalyst site to the other. Under acidic conditions, water will be dissociated into O2 and protons on the oxidation side of the membrane. The protons will then be transported through the membrane to the reduction side, where H2 will be evolved. In this way oxidation and reduction products will be generated in separate regions of the membrane, preventing the need for further separation.
In the case of operation under basic electrolytes, the processes are analogous and the ionic current in the system is carried by OH- ions at steady state. The incorporation of ion-conducting membranes is crucial for this type of operation, as they provide transport pathways for charged intermediaries between the oxidation and reduction sites and at the same time serve as a barrier for gas diffusion, allowing the production of fuel in its pure form. Achieving this configuration can be simple for macroscopic units, but for micrometer- to nanometer-scale (i.e., mesoscale) systems significant advances are required in terms of both membrane and PEC unit self-assembly.
Progress on these technological options depends on research and development currently under way in academic, government, and industrial laboratories. In this article, we discuss aspects of an integrated system that are the focus of current exploration and development. The sections below touch on some of the advances and challenges in achieving practical solar fuel generators, implications for mesoscale assemblies and for membranes used in these systems, and overall system design considerations. Throughout, we describe current research as well as specific areas that require further study to enable progress in this area.
SOLAR FUEL GENERATION SYSTEMS
Since the first demonstration of solar-driven water splitting by Fujishima and Honda (1972), the prospect of using PEC cells for solar fuel generation has motivated the quest for components and integrated systems that can continuously and robustly produce hydrogen fuels directly from sunlight. Over the past 40 years many studies have attempted to tackle parts of the problem, and fuel-generating systems have reached solar hydrogen generation efficiencies of up to 18 percent (Peharz et al. 2007).
But solar hydrogen generation units fall short in satisfying stability and cost-effectiveness requirements. Some high-efficiency systems rely on III-V multijunction photovoltaic components that have prohibitively high costs and serious photocorrosion challenges at the interface between the semiconductor and the electrolyte (Khaselev and Turner 1998; Khaselev et al. 2001; Peharz et al. 2007).
Other systems, based on silicon light-absorbing components, including earth-abundant catalysts, face significant stability problems when operated under basic or acidic electrolytes. Recently, however, Nocera’s group at the Massachusetts Institute of Technology demonstrated integrated systems that incorporate earth-abundant components that can stably operate under buffered electrolytes at moderate pH (Reece et al. 2011). This promising demonstration can open avenues for the implementation of cost-effective solar hydrogen generators, but important challenges for the management of ion and mass transport remain, largely based on the need to separate the gaseous products while providing pathways for steady-state ion conduction (Haussener et al. 2012; Hernandez-Pagan et al. 2012).
When systems are operated at moderate pH regimes, the low concentration of proton or hydroxide conduction results in high solution resistance for these ions, and most of the ionic current is carried by supporting ions present in the solution (i.e., ions dissociated from buffer molecules). Under these circumstances, as the conducting ions are not part of the electrode reactions, concentration gradients will evolve and the overall system will not be able to operate continuously.
Efficient solar hydrogen generation would represent a large step to increase the share of renewable fuel sources but implementation would be challenging as current infrastructure is based on liquid carbon–based fuels.
An alternative to solar water splitting lies in the direct reduction of CO2 for the generation of liquid carbon–containing fuels (Gattrell et al. 2007; Kondratenko et al. 2013; Lewis and Nocera 2006; Olah et al. 2008). Notwithstanding considerable research in this field, challenges persist because requirements for catalyst selectivity, CO2 absorption, and product separation are quite stringent.
Last, the technoeconomic aspects of solar fuel generators are crucial for the achievement of deployable systems. The US Department of Energy has set the price of hydrogen produced at less than $4/kg, which imposes bounds on the material systems and configurations that are implementable (Saur and Ainscough 2011). Few reports have tackled these aspects or provided guidance to achieve this price point (e.g., James et al. 2009; Pinaud et al. 2013).
As both the scientific and engineering aspects of artificial photosynthesis devices mature, a better understanding of the challenges to fabricate cost-effective solar fuel generators will be critical for their deployment.
MESOSCALE BUILDING BLOCKS FOR ARTIFICIAL PHOTOSYNTHESIS SYSTEMS
The examples cited above represent initial attempts at developing integrated devices that can produce hydrogen fuels directly from the sun, and they all rely on macroscopic PEC units arranged such that ion transport involves a liquid electrolyte. Under concentrated electrolyte conditions (~1 M), ion transport does not provide significant resistance if the ionic pathway is less than a few centimeters
(Haussener et al. 2012). Furthermore, if the ionic conductivity of electrolyte is lowered, or for operation of systems under water vapor (Spurgeon and Lewis 2011), it is highly desirable to develop PEC units with dimensions in the micro- or nanometer range so that ions have to migrate only small distances.
Several mesoscale building blocks for PEC units have been developed. Complex nanocrystal structures (e.g., nanorods, nanowires) can be synthesized in solution (Amirav and Alivisatos 2010; Dukovic et al. 2008; Sun et al. 2011, 2013) and have shown promising performance in terms of hydrogen evolution. Methods for arranging these nanostructures into architectures that enable oxidation and reduction reactions to occur at separate locations depend on the shape, dimensions, and self-assembly characteristics of the particles.
For long semiconducting nanowire systems, large surface area mats permit a percolated network of wires to act as a self-standing water-splitting membrane (Sun et al. 2011). And for nanorod-based systems, self-assembly techniques are required to achieve architectures resembling that shown in Figure 1 (Baker et al. 2010; Baranov et al. 2010; Gupta et al. 2006; Ryan et al. 2006).
Although these self-assembly techniques have demonstrated the fabrication of large-scale vertically aligned nanorod arrays from solution, it is not clear how to obtain preferential directionality of the ends of asymmetric water-splitting nanorods (Amirav and Alivisatos 2010).
As an alternative to solution-based methods, photocatalytic units can be directly grown via vapor-liquid-solid deposition methods so that the resulting arrays have the desired directionality. The development of silicon-based microwire arrays is an example of such a strategy and can lead to large-area coverage of the photoactive components that can then be incorporated into ion-conducting membranes (Boettcher et al. 2010; Maiolo et al. 2007; Plass et al. 2009; Spurgeon et al. 2011). These systems have many advantages over planar PEC devices because they can absorb nearly all the incident light with only a small fraction of areal coverage (Kelzenberg et al. 2010) and each microwire in the arrays acts as an independent unit, largely alleviating stability constraints.
The incorporation of mesoscale PEC units into fully functional solar hydrogen generators represents a promising alternative to overcome the technological challenges that prevent deployment, and so a great deal of research is being conducted in this area.
MEMBRANE MATERIALS FOR ARTIFICIAL PHOTOSYNTHESIS
Membranes in solar hydrogen generators serve two basic functions: to provide pathways for ion conduction and to keep gaseous products separated (as shown in Figure 2). Ion-conducting membranes, which have been investigated for several decades, are important components not only in artificial photosynthesis applications but also in a variety of energy conversion devices (Walter et al. 2010; Zhang and Shen 2012). The fundamental similarities in membrane requirements
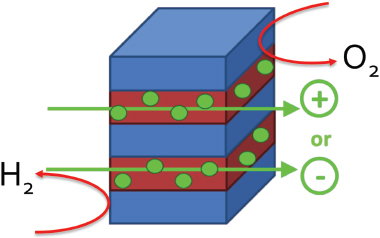
FIGURE 2 Diagram of membrane material used for solar water splitting. These materials contain conductive domains capable of transporting ions across the membrane (positive or negative), while preventing crossover of the gases produced.
between solar fuel devices, hydrogen fuel cells, and electrolyzers suggest an existing set of candidate materials.
In the case of artificial photosynthesis applications, the operating current density is dictated by the solar absorption rate and is relatively low when compared to the requirements for other similar devices, but very sensitive to crossover due to the relatively small quantity of product. Moreover, the presence of a large number of interfaces between the polymer and inorganic PEC components can severely affect the structure and transport properties of common nanostructured fuel cell membrane materials. Perfluorosulfonic acid (PFSA) ionomer membranes (e.g., Nafion®) are the most prominent alternatives for proton conduction, given their high ionic conductivity and remarkable chemical and structural stability.
With artificial photosynthesis membranes, high levels of conductivity are not required and the emphasis should instead be on the balance between the ionic and gas transport properties of materials. The development of ion-conducting block copolymers (BCPs) represents a promising route to decouple these two properties, as different blocks can be designed to provide complementary structural and gas barrier properties as well as ionic conductivity.
Furthermore, properties of BCP systems can be easily tuned and optimized by altering the molecular weight and volume fraction of each phase (Peckham and Holdcroft 2010). BCP membranes based on blends with ionic liquids (ILs) and polymerized ILs (PILs) are characterized by good ionic conductivity and tenability (Bara et al. 2008, 2009; Gu and Lodge 2011; Gwee et al. 2010; Hoarfrost and Segalman 2011, 2012; Lu et al. 2009; Mecerreyes 2011; Simone and Lodge 2009). Recent work has demonstrated the potential of PIL BCP materials for tuning
transport properties in membranes used for solar fuel applications (Schneider et al. 2013; Sudre et al. 2013).
SYSTEM DESIGN CONSIDERATIONS
All the components of a solar fuel generator system need to operate stably and perform efficiently under the same conditions (i.e., temperature, electrolyte selection). Additionally, the photovoltage generated by the light-absorbing units needs to be sufficient to support the water-splitting reaction (1.23 V), the catalyst overpotential requirement, the ohmic drop associated with transporting both electrons and ions across the device, and any additional overpotential that may arise from chemical potential differences (i.e., concentration overpotential). Furthermore, all the transport processes in the system need to occur in parallel so that the electronic current matches the ionic current across reaction sites.
Several electrochemical modeling studies provide some guidance on the optimal arrangements and dimensions of each of the components in an integrated solar hydrogen generator (Berger and Newman 2013; Haussener et al. 2012; Surendranath et al. 2012; Winkler et al. 2013). The output from the photovoltaic component must match the electrochemical load from the catalytic and ion transport components of the device. By controlling the dimensions and component architecture, it is possible to optimize the performance of the device so that it operates at near maximum possible efficiency (Jacobsson et al. 2013; Peharz et al. 2007; Winkler et al. 2013).
LOOKING AHEAD
Optimizing the topology of the components in a device can help overcome some stability limitations, achieve operations under a wide range of conditions, and increase overall efficiency. As new components become available, significant work in this design area is necessary to understand what shape and form will lead to optimization of cost, efficiency, and stability.
ACKNOWLEDGMENTS
This material is based on work performed at the Joint Center for Artificial Photosynthesis, a DOE Energy Innovation Hub, through the US Department of Energy Office of Science under Award No. DE-SC0004993.
REFERENCES
Amirav L, Alivisatos AP. 2010. Photocatalytic hydrogen production with tunable nanorod hetero-structures. Journal of Physical Chemistry Letters 1(7):1051–1054.
Baker JL, Widmer-Cooper A, Toney MF, Geissler PL, Alivisatos AP. 2010. Device-scale perpendicular alignment of colloidal nanorods. Nano Letters 10(1):195–201.
Bara JE, Hatakeyama ES, Gin DL, Noble RD. 2008. Improving CO2 permeability in polymerized room-temperature ionic liquid gas separation membranes through the formation of a solid composite with a room-temperature ionic liquid. Polymers for Advanced Technologies 19(10):1415–1420.
Bara JE, Carlisle TK, Gabriel CJ, Camper D, Finotello A, Gin DL, Noble RD. 2009. Guide to CO2 separations in imidazolium-based room-temperature ionic liquids. Industrial and Engineering Chemistry Research 48(6):2739–2751.
Baranov D, Fiore A, van Huis M, Giannini C, Falqui A, Lafont U, Zandbergen H, Zanella M, Cingolani R, Manna L. 2010. Assembly of colloidal semiconductor nanorods in solution by depletion attraction. Nano Letters 10(2):743–749.
Bard AJ, Fox MA. 1995. Artificial photosynthesis: Solar splitting of water to hydrogen and oxygen. Accounts of Chemical Research 28(3):141–145.
Berger A, Newman JS. 2013. Photoelectrochemical modeling of a water-splitting membrane. In preparation for Journal of Electrochemical Society.
Boettcher SW, Spurgeon JM, Putnam MC, Warren EL, Turner-Evans DB, Kelzenberg MD, Maiolo JR, Atwater HA, Lewis NS. 2010. Energy-conversion properties of vapor-liquid-solid-grown silicon wire-array photocathodes. Science 327(5962):185–187.
Chu S, Majumdar A. 2012. Opportunities and challenges for a sustainable energy future. Nature 488(7411):294–303.
Concepcion JJ, House RL, Papanikolas JM, Meyer TJ. 2012. Chemical approaches to artificial photosynthesis. Proceedings of the National Academy of Sciences U S A 109(39):15560–15564.
Dukovic G, Merkle MG, Nelson JH, Hughes SM, Alivisatos AP. 2008. Photodeposition of Pt on colloidal CdS and CdSe/CdS semiconductor nanostructures. Advanced Materials 20(22):4306–4311.
Faunce TA, Lubitz W, Rutherford AW, MacFarlane D, Moore GF, Yang P, Nocera DG, Moore TA, Gregory DH, Fukuzumi S, Yoon KB, Armstrong FA, Wasielewski MR, Styring S. 2013. Energy and environment policy case for a global project on artificial photosynthesis. Energy and Environmental Science 6(3):695–698.
Fujishima A, Honda K. 1972. Electrochemical photolysis of water at a semiconductor electrode. Nature 238(5358):37–38.
Gattrell M, Gupta N, Co A. 2007. Electrochemical reduction of CO2 to hydrocarbons to store renewable electrical energy and upgrade biogas. Energy Conversion and Management 48(4):1255–1265.
Gu Y, Lodge TP. 2011. Synthesis and gas separation performance of triblock copolymer ion gels with a polymerized ionic liquid mid-block. Macromolecules 44(7):1732–1736.
Gupta S, Zhang Q, Emrick T, Russell TP. 2006. “Self-corralling” nanorods under an applied electric field. Nano Letters 6(9):2066–2069.
Gwee L, Choi JH, Winey KI, Elabd YA. 2010. Block copolymer/ionic liquid films: The effect of ionic liquid composition on morphology and ion conduction. Polymer 51(23):5516–5524.
Haussener S, Xiang C, Spurgeon JM, Ardo S, Lewis NS, Weber AZ. 2012. Modeling, simulation, and design criteria for photoelectrochemical water-splitting systems. Energy and Environmental Science 5(12):9922–9935.
Hernandez-Pagan EA, Vargas-Barbosa NM, Wang T, Zhao Y, Smotkin ES, Mallouk TE. 2012. Resistance and polarization losses in aqueous buffer-membrane electrolytes for water-splitting photoelectrochemical cells. Energy and Environmental Science 5(6):7582–7589.
Hoarfrost ML, Segalman RA. 2011. Ionic conductivity of nanostructured block copolymer/ionic liquid membranes. Macromolecules 44(13):5281–5288.
Hoarfrost ML, Segalman RA. 2012. Conductivity scaling relationships for nanostructured block copolymer/ionic liquid membranes. ACS Macro Letters 1(8):937–943.
IEA (International Energy Agency). 2012. Key World Energy Statistics. Paris. Available online at www.iea.org/publications/freepublications/publication/kwes.pdf.
Jacobsson JT, Fjallstrom V, Sahlberg M, Edoff M, Edvinsson T. 2013. A monolithic device for solar water splitting based on series interconnected thin film absorbers reaching over 10% solar-to-hydrogen efficiency. Energy and Environmental Science, doi: 10.1039/C3EE42519C.
James BD, Baum GN, Perez J, Baum KN. 2009. Technoeconomic Analysis of Photoelectrochemical (PEC) Hydrogen Production. Arlington VA: Directed Technologies.
Kelzenberg MD, Boettcher SW, Petykiewicz JA, Turner-Evans DB, Putnam MC, Warren EL, Spurgeon JM, Briggs RM, Lewis NS, Atwater HA. 2010. Enhanced absorption and carrier collection in Si wire arrays for photovoltaic applications. Nature Materials 9(3):239–244.
Khaselev O, Turner JA. 1998. A monolithic photovoltaic-photoelectrochemical device for hydrogen production via water splitting. Science 280(5362):425–427.
Khaselev O, Bansal A, Turner JA. 2001. High-efficiency integrated multijunction photovoltaic/ electrolysis systems for hydrogen production. International Journal of Hydrogen Energy 26(2):127–132.
Kondratenko EV, Mul G, Baltrusaitis J, Larrazabal GO, Perez-Ramirez J. 2013. Status and perspectives of CO2 conversion into fuels and chemicals by catalytic, photocatalytic and electrocatalytic processes. Energy and Environmental Science 6:3112–3135.
Lewis NS, Nocera DG. 2006. Powering the planet: Chemical challenges in solar energy utilization. Proceedings of the National Academy of Sciences U S A 103(43):15729–15735.
Lu J, Yan F, Text J. 2009. Advanced applications of ionic liquids in polymer science. Progress in Polymer Science 34(5):431–448.
Maiolo JR, Kayes BM, Filler MA, Putnam MC, Kelzenberg MD, Atwater HA, Lewis NS. 2007. High aspect ratio silicon wire array photoelectrochemical cells. Journal of the American Chemical Society 129(41):12346–12347.
Mecerreyes D. 2011. Polymeric ionic liquids: Broadening the properties and applications of poly-electrolytes. Progress in Polymer Science 36(12):1629–1648.
Nocera DG. 2012. The artificial leaf. Accounts of Chemical Research 45(5):767–776.
Olah GA, Goeppert A, Prakash GKS. 2008. Chemical recycling of carbon dioxide to methanol and dimethyl ether: From greenhouse gas to renewable, environmentally carbon neutral fuels and synthetic hydrocarbons. Journal of Organic Chemistry 74(2):487–498.
Peckham TJ, Holdcroft S. 2010. Structure-morphology-property relationships of non-perfluorinated proton-conducting membranes. Advanced Materials 22(42):4667–4690.
Peharz G, Dimroth F, Wittstadt U. 2007. Solar hydrogen production by water splitting with a conversion efficiency of 18%. International Journal of Hydrogen Energy 32(15):3248–3252.
Pinaud BA, Benck JD, Seitz LC, Forman AJ, Chen Z, Deutsch TG, James BD, Baum KN, Baum GN, Ardo S, Wang H, Miller E, Jaramillo TF. 2013. Technical and economic feasibility of centralized facilities for solar hydrogen production via photocatalysis and photoelectrochemistry. Energy and Environmental Science 6(7):1983–2002.
Plass KE, Filler MA, Spurgeon JM, Kayes BM, Maldonado S, Brunschwig BS, Atwater HA, Lewis NS. 2009. Flexible polymer-embedded Si wire arrays. Advanced Materials 21(3):325–328.
Reece SY, Hamel JA, Sung K, Jarvi TD, Esswein AJ, Pijpers JJH, Nocera DG. 2011. Wireless solar water splitting using silicon-based semiconductors and earth-abundant catalysts. Science 334(6056):645–648.
Ryan KM, Mastroianni A, Stancil KA, Liu H, Alivisatos AP. 2006. Electric-field-assisted assembly of perpendicularly oriented nanorod superlattices. Nano Letters 6(7):1479–1482.
Saur G, Ainscough C. 2011. US Geographic Analysis of the Cost of Hydrogen from Electrolysis. Technical Report NREL/TP-5600-52640. Golden CO: National Renewable Energy Laboratory, US Department of Energy. Available at www.nrel.gov/hydrogen/pdfs/52640.pdf.
Schneider Y, Modestino MA, McCulloch BL, Hoarfrost ML, Hess RW, Segalman RA. 2013. Ionic conduction in nanostructured membranes based on polymerized protic ionic liquids. Macromolecules 46(4):1543–1548.
Simone PM, Lodge TP. 2009. Phase behavior and ionic conductivity of concentrated solutions of polystyrene-poly(ethylene oxide) diblock copolymers in an ionic liquid. ACS Applied Materials and Interfaces 1(12):2812–2820.
Spurgeon JM, Lewis NS. 2011. Proton exchange membrane electrolysis sustained by water vapor. Energy and Environmental Science 4(8):2993–2998.
Spurgeon JM, Walter MG, Zhou J, Kohl PA, Lewis NS. 2011. Electrical conductivity, ionic conductivity, optical absorption, and gas separation properties of ionically conductive polymer membranes embedded with Si microwire arrays. Energy and Environmental Science 4(5):1772–1780.
Sudre G, Inceoglu S, Cotanda P, Balsara NP. 2013. Influence of bound ion on the morphology and conductivity of anion-conducting block copolymers. Macromolecules 46(4):1519–1527.
Sun J, Liu C, Yang P. 2011. Surfactant-free, large-scale, solution-liquid-solid growth of gallium phosphide nanowires and their use for visible-light-driven hydrogen production from water reduction. Journal of the American Chemical Society 133(48):19306–19309.
Sun Y, Sun J, Long JR, Yang P, Chang CJ. 2013. Photocatalytic generation of hydrogen from water using a cobalt pentapyridine complex in combination with molecular and semiconductor nanowire photosensitizers. Chemical Science 4(1):118–124.
Surendranath Y, Bediako DK, Nocera DG. 2012. Interplay of oxygen-evolution kinetics and photovoltaic power curves on the construction of artificial leaves. Proceedings of the National Academy of Sciences U S A 109(39):15617–15621.
Walter MG, Warren EL, McKone JR, Boettcher SW, Mi Q, Santori EA, Lewis NS. 2010. Solar water splitting cells. Chemical Reviews 110:6446–6473.
Winkler MT, Cox CR, Nocera DG, Buonassisi T. 2013. Modeling integrated photovoltaic-electrochemical devices using steady-state equivalent circuits. Proceedings of the National Academy of Sciences U S A 110(12):E1076–E1082.
Zhang H, Shen PK. 2012. Advances in the high-performance polymer electrolyte membranes for fuel cells. Chemical Society Reviews 41:2382–2394.
This page intentionally left blank.












