SERENA BIANCHI,*∥∥## CHERYL D. STIMPSON,*∥∥ TETYANA DUKA,* MICHAEL D. LARSEN,† WILLIAM G. M. JANSSEN,‡ ZACHARY COLLINS,* AMY L. BAUERNFEIND,* STEVEN J. SCHAPIRO,§ WALLACE B. BAZE,§ MARK J. MCARTHUR,§ WILLIAM D. HOPKINS,∥# DEREK E. WILDMAN,** LEONARD LIPOVICH,** CHRISTOPHER W. KUZAWA,†† BOB JACOBS,‡‡ PATRICK R. HOF,‡§§ AND CHET C. SHERWOOD*#
Neocortical development in humans is characterized by an extended period of synaptic proliferation that peaks in mid-childhood, with subsequent pruning through early adulthood, as well as relatively delayed maturation of neuronal arborization in the prefrontal cortex compared with sensorimotor areas. In macaque monkeys, cortical synaptogenesis peaks during early infancy and developmental changes in synapse density and dendritic spines occur synchronously across cortical regions. Thus, relatively prolonged synapse and neuronal maturation in humans might contribute to enhancement of social learning during development and transmission of cultural practices, including language. However,
_____________
*Department of Anthropology, The George Washington University, Washington, DC 20052; †Department of Statistics and Biostatistics Center, The George Washington University, Rockville, MD 20852; ‡Fishberg Department of Neuroscience and Friedman Brain Institute, Icahn School of Medicine at Mount Sinai, New York, NY 10029; §Department of Veterinary Sciences, The University of Texas MD Anderson Cancer Center, Bastrop, TX 78602; Neuroscience Institute and Language Research Center, Georgia State University, Atlanta, GA 30302; #Division of Developmental and Cognitive Neuroscience, Yerkes National Primate Research Center, Atlanta, GA 30322; **Center for Molecular Medicine and Genetics, Wayne State University, Detroit, MI 48201; ††Department of Anthropology, Northwestern University, Evanston, IL 60208; ‡‡Department of Psychology, Colorado College, Colorado Springs, CO 80903; and §§New York Consortium in Evolutionary Primatology, New York, NY 10024. Contributed equally to this work. ##To whom correspondence may be addressed. E-mail: sbianchi@gwmail.gwu.edu or sherwood@gwu.edu.
because macaques, which share a last common ancestor with humans ~25 million years ago, have served as the predominant comparative primate model in neurodevelopmental research, the paucity of data from more closely related great apes leaves unresolved when these evolutionary changes in the timing of cortical development became established in the human lineage. To address this question, we used immunohistochemistry, electron microscopy, and Golgi staining to characterize synaptic density and dendritic morphology of pyramidal neurons in primary somatosensory (area 3b), primary motor (area 4), prestriate visual (area 18), and prefrontal (area 10) cortices of developing chimpanzees (Pan troglodytes). We found that synaptogenesis occurs synchronously across cortical areas, with a peak of synapse density during the juvenile period (3–5 years). Moreover, similar to findings in humans, dendrites of prefrontal pyramidal neurons developed later than sensorimotor areas. These results suggest that evolutionary changes to neocortical development promoting greater neuronal plasticity early in postnatal life preceded the divergence of the human and chimpanzee lineages.
Among primates, humans are characterized by an especially prolonged period of postnatal brain development during which cultural traditions and practices, including language, are acquired. Because culture plays a fundamental role in the human adaptive complex (Boyd et al., 2011), the comparative examination of neural development is important to understand the origins of human sociocognitive specializations. Compared with other primates, in humans a relatively large proportion of brain size growth takes place postnatally, allowing for social and environmental factors to powerfully impact the establishment of neural connectivity (Sacher and Staffeldt, 1974; Leigh, 2004; DeSilva and Lesnik, 2008; Barton and Capellini, 2011; McFarlin et al., 2012). Whereas macaque monkeys, the primate species that has been studied most extensively as a comparative model of neurodevelopment, are born with brains that are already ~70 percent of adult mass and neonatal brain mass in great apes ranges from 36 percent to 56 percent of adult size (DeSilva, 2011; McFarlin et al., 2012), in humans only ~25 percent of adult mass is achieved at birth (Robson and Wood, 2008). Concomitantly, the postnatal refinement of cortical microstructure in humans progresses along a more protracted schedule relative to macaques. In macaques, the process of synaptogenesis, whereby new synapses are formed, peaks during infancy at around 3 months of age, and pruning of excess synapses is completed by the end of adolescence (Rakic et al., 1986; Liu et al., 2012). In contrast, in humans, peak synapse density occurs in mid-childhood around 5 years of age (Huttenlocher and Dabholkar, 1997; Liu et al., 2012), with pruning of synapses extending into the third decade of life (Petanjek et al., 2011).
Interspecific differences between macaque and human neural development have also been reported in the timing of maturation among different cortical regions. Whereas synaptogenesis occurs synchronously across the entire cerebral cortex in macaques (Rakic et al., 1986), it appears delayed in the prefrontal region in humans (Huttenlocher and Dabholkar, 1997). In macaques, moreover, densities of spines located on the dendrites of prefrontal pyramidal neurons are higher than other areas from the time of birth and throughout postnatal development (Elston et al., 2009). In humans, however, dendritic arbors of prefrontal cortex pyramidal neurons reach adult-like morphological complexity and spine density later in development than dendritic arbors in sensory and motor cortices (Travis et al., 2005). A temporally staggered, or heterochronous development of the human cerebral cortex, with association regions maturing later than sensorimotor cortices, has also been documented through imaging techniques assessing longitudinal changes in metabolic activity (Chugani et al., 1987), gray matter growth (Gogtay et al., 2004), and cortical thickness (Shaw et al., 2008).
Relatively slow development of neocortical connectivity might contribute to the emergence of uniquely human cognitive abilities. This interpretation is supported by evidence that the cortical regions that develop later in human ontogeny also underwent the greatest expansion during human brain evolution (Hill et al., 2010; Sherwood et al., 2012), suggesting that evolutionary selection to enlarge these regions was accompanied by a prolongation of their development. Among these regions, the prefrontal cortex, which shows particularly extended maturation in humans relative to macaques, has also been reported to exhibit uniquely human neuroanatomical and molecular specializations (Deacon, 1997; Elston et al., 2001; Semendeferi et al., 2001; Cáceres et al., 2007; Fu et al., 2011; Spocter et al., 2012). However, because macaques and humans shared a last common ancestor ~25 million years ago, it is currently unclear whether features that distinguish human cortical development (i.e., extended period of synaptogenesis and maturational delay of prefrontal pyramidal neurons) are unique to our lineage, or if they evolved before the divergence of modern humans and more closely related great ape species, such as chimpanzees.
Relative to macaques, chimpanzees display greater behavioral similarities with humans, including slow postnatal development during which socially learned and “culturally” transmitted behaviors, such as tool use, are acquired (Lonsdorf, 2006; Lonsdorf and Bonnie, 2010). Although chimpanzees provide one of the best animal models for comparison with which to investigate human-unique specializations, studies of cortical development in this species are lacking because of ethical and practical barriers and the rare availability of postmortem brain tissue from infants and juveniles. To date, only a few comparative studies of brain development
have included data from chimpanzees. By examining ontogenetic changes through longitudinal MRI, it has been shown that, similar to humans, the maturation of white matter volume in chimpanzees is not complete at early puberty (6 years), but in macaques it reaches adult values during earlier juvenile development (Sakai et al., 2011). Relative to chimpanzees, however, human brain development is marked by more rapid addition of white matter volume during the first year of life (Sakai et al., 2011, 2013). That white matter growth differs between humans and chimpanzees has been further demonstrated by recent histological analyses demonstrating that myelination within the cerebral cortex continues past adolescence in humans, whereas it is completed by sexual maturity in chimpanzees (Miller et al., 2012).
With regard to synaptogenesis, current data from chimpanzees are extremely limited (Liu et al., 2012). Therefore, the present study examined two developmental markers in chimpanzees, synaptogenesis and dendritic growth of pyramidal neurons, in four regions, including prestriate visual (area 18), primary motor (area 4), primary somatosensory (area 3b), and prefrontal (area 10) cortices. Previous studies of neuron morphology in adult primates have shown that these areas form a functional hierarchy whereby association cortices (i.e., area 10) that integrate input from other areas display greater potential for corticocortical connectivity than unimodal, sensory and motor regions (areas 4, 3b, and 18) (Elston et al., 2001; Jacobs et al., 2001; Bianchi et al., 2012). We predicted that, if chimpanzee cortical development is more similar to humans than to macaques, synaptogenesis and maturation of dendritic arbors of pyramidal neurons would be extended into the juvenile period, and show a more prolonged trajectory in the prefrontal cortex relative to other regions.
RESULTS
Synaptogenesis
We used immunohistochemistry against synaptophysin, a protein localized in presynaptic vesicles, to label the density of synapses in chimpanzee neocortical samples (Fig. 8.1). To assess the effect of age on synapse density during postnatal development, a cubic regression model was fitted to synaptophysin-immunoreactive puncta densities from each of the four neocortical regions. Individual cubic polynomial regression curves were significant for most cortical areas (Table 8.1), indicating the presence of developmental changes in synapse density. A steep rise was observed in early postnatal life across all regions followed by a peak at approximately 3 years, which was sustained until the age of 5 years. Synapse densities
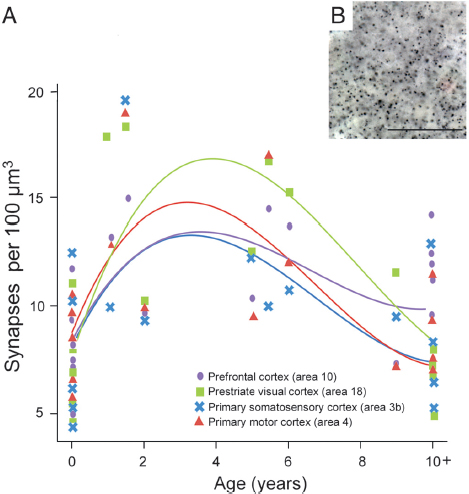
FIGURE 8.1 (A) Individual cubic polynomial regression curves fit to counts of synaptophysin-immunoreactive puncta densities for areas 3b, 4, 18, and 10. (B) Photomicrograph of synaptophysin-immunoreactive puncta from the prefrontal cortex of an 11-year-old chimpanzee. (Scale bar, 25 μm.) [NOTE: Figure can be viewed in color in the PDF version of this volume on the National Academies Press website, www.nap.edu/catalog.php?record_id18573.]
then declined gradually through late juvenile life and approached adult-like levels around puberty (~10 years of age) (Fig. 8.1).
To test for similarities in the cubic polynomial fit among the different cortical regions, we used a generalized least-squares model. This model confirmed a significant cubic polynomial regression based only on age, and showed a better Akaike information criterion value than a model including the cubic polynomial based on both age and a term for cortical region differences. The model with differences by cortical region did not fit significantly better according to a likelihood ratio test; P values were 0.55 and 0.48 for either an unstructured or a compound symmetry model, respectively. These results suggest that there is a similar developmental trajectory of synaptogenesis across cortical regions, and were further
TABLE 8.1 Regression Models for Primary Somatosensory (Area 3b), Primary Motor (Area 4), Prestriate Visual (Area 18), and Prefrontal (Area 10) Cortex
| Region | Intercept | Age | Age2 | Age3 | r2 | Root MSE | Overall Regression P Value | ||
| Area 3b | 94.3 | −11.5 | −56.5 | 48.7 | 0.26 | 33.6 | 0.22 | ||
| Area 4 | 100.5 | −25.6 | 66.2* | 59.7+ | 0.42 | 29.3 | 0.05 | ||
| Area 18 | 99.5 | −30.0 | −135.4*** | 53.0 | 0.62 | 30.9 | 0.003 | ||
| Area 10 | 106.3 | 41.1 | −41.7 | 44.3+ | 0.38 | 24.6 | 0.07 | ||
NOTE: Linear (Age), quadratic (Age2), and cubic (Age3) effects of age are shown. ***P < 0.001, *P < 0.05, +P < 0.10.
confirmed by using a cubic model including age with random effects by subjects.
Although the patterns of synaptic development were similar across cortical areas, we found regional differences in synapse density to emerge between newborns and adults (Fig. 8.1). A repeated-measures ANOVA of synapse density indicated that there were no significant differences across cortical areas in neonates (n = 6, 0- to 1-month-old; F3, 15 = 0.580, P = 0.637). However, by adulthood, regional differences in synapse density emerged (n = 5, 10+ years old; F3, 12 = 5.974, P = 0.010; after correcting for sphericity that was not assumed: P = 0.061), with the prefrontal cortex showing greater synapse density than prestriate visual cortex (P = 0.022).
Examination of synaptogenesis by electron microscopy (EM) (Fig. 8.2A) in the same regions of four of the chimpanzee brains that had also been processed for synaptophysin immunohistochemistry corroborated these findings. Agreement between counts of synaptophysin-immunoreactive puncta and synapse density by EM was demonstrated by a significant, positive relationship between both measures (Fig. 8.2B) (r = 0.63, P = 0.009, n = 16). Results from EM synapse density counts showed a pronounced developmental increase in the prefrontal cortex, which can be attributed mostly to postnatal changes in the density of excitatory asymmetric synapse subtypes.
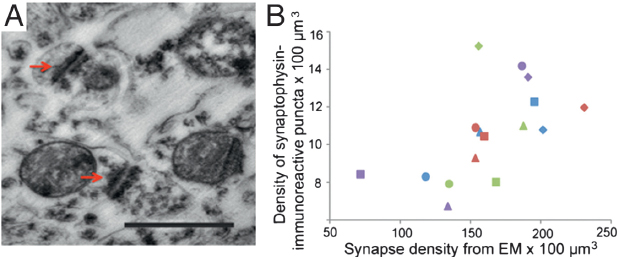
FIGURE 8.2 (A) Photomicrograph of synapses as observed under EM. Arrows indicate synaptic junctions. (Scale bar, 0.5 μm.) (B) Bivariate plot between synaptophysin-immunoreactive puncta densities and synaptic densities from EM data. Squares represent age 0, triangles age 2, diamonds age 6, and circles age 11. Color scheme for areas as in Fig. 8.1. [NOTE: Figure can be viewed in color in the PDF version of this volume on the National Academies Press website, www.nap.edu/catalog.php?record_id18573.]
Development of Pyramidal Neuron Dendritic Morphology
We analyzed the morphology of pyramidal neurons from seven infant and juvenile chimpanzee brains that were used in the immunohistochemistry and EM studies of synapse densities described above. Results indicated significant regional differences in the dendritic structure of pyramidal neurons across areas of the infant chimpanzee neocortex (n = 4, 0- to 24-month-old), as assessed by six measures of morphological complexity, including cell body size: F12, 144 = 1.86, P = 0.04; total dendritic length (TDL): F12, 144 = 3.64, P < 0.001; mean segment length (MSL): F12, 144 = 5.34, P < 0.001; dendritic segment count (DSC): F12, 144 = 2.09, P = 0.021; dendritic spine number (DSN): F12, 144 = 10.65, P < 0.001; and dendritic spine density (DSD): F12, 144 = 21.373, P < 0.001 (Table 8.2). Pairwise comparisons revealed that dendrites in prefrontal cortex (area 10) were significantly shorter than those in areas 3b (P = 0.022) and area 4 (P = 0.037). Pyramidal neurons in prefrontal area 10 also had shorter mean segment length than area 3b (P < 0.001) and area 4 (P = 0.002), as well as fewer spines (area 3b, P < 0.001; area 4, P = 0.005; area 18, P = 0.005) and lower spine density (area 3b, P < 0.001; area 4, P < 0.001; area 18, P < 0.001) than in other regions. On average, prefrontal neurons in infant chimpanzees had dendrites that were 22 percent shorter, had 44 percent fewer spines, and 30 percent lower spine density than the mean for neurons from areas 3b, 4, and 18. No differences were found in the number of dendritic segments (P = 1.000) across cortical areas (see, e.g., Fig. 8.4).
Because pyramidal neuron morphology in infant chimpanzees was analyzed with the same methodology used in a previous study of adult chimpanzees (Bianchi et al., 2012), we could compare regional variation in dendritic complexity in these two age groups (Figs. 8.3 and 8.4, and Table 8.2). It should be noted that, similar to reports in human infants (Travis et al., 2005), in the young chimpanzees, the rapid Golgi technique stained neurons predominantly in layer V, whereas neurons in layer III were typically best impregnated in adults. For this reason, only the pattern of relative differences in dendritic complexity across regions between infant and adult chimpanzees can be interpreted. Illustrations of regional variation by age are provided in Fig. 8.4. Pyramidal neurons of the prefrontal cortex continued to be the least elaborate among the cortical areas examined according to most measures of dendritic complexity through infancy and juvenile development (ages 5–6) and only began to increase in the later juvenile period (age 9), ultimately becoming the most complex neurons in adulthood (Bianchi et al., 2012).
TABLE 8.2 Measures of Pyramidal Neuron Morphological Complexity and Position (Depth from Pial Surface) in Somatosensory (Area 3b), Primary
| Morphological Measure | Area 3b | Area 4 | Area 18 | Area 10 | |||||
| Newborns and infants (n = 4) | |||||||||
| Cell soma area (μm2) | 185 ± 88 | 176 ± 45 | 152 ± 68 | 189 ± 46 | |||||
| Cell soma depth (μm) | 1,314 ± 733 | 1,040 ± 548 | 1,062 ± 468 | 1,151 ± 603 | |||||
| TDL (μm) | 879 ± 361 | 866 ± 376 | 805 ± 516 | 655 ± 293 | |||||
| MSL (μm) | 47 ± 15 | 38 ± 12 | 37 ± 12 | 30 ± 8 | |||||
| DSC (μm) | 20 ± 8 | 22 ± 8 | 21 ± 7 | 22 ± 8 | |||||
| DSN (μm) | 179 ± 105 | 139 ± 91 | 138 ± 132 | 84 ± 68 | |||||
| DSD (μm) Adults (n = 7) | 0.20 ± 0.07 | 0.15 ± 0.05 | 0.16 ± 0.07 | 0.12 ± 0.06 | |||||
| Cell soma area (μm2) | 183 ± 88 | 185 ± 62 | 167 ± 75 | 190 ± 63 | |||||
| Cell soma depth (μm) | 796 ± 209 | 707 ± 247 | 692 ± 241 | 733 ± 224 | |||||
| TDL (μm) | 894 ± 429 | 966 ± 377 | 851 ± 406 | 1,329 ± 705 | |||||
| MSL (μm) | 27 ± 8 | 47 ± 11 | 40 ± 11 | 49 ± 16 | |||||
| DSC (μm) | 21 ± 8 | 21 ± 6 | 21 ± 7 | 27 ± 10 | |||||
| DSN (μm) | 148 ± 78 | 166 ± 101 | 169 ± 84 | 401 ± 203 | |||||
| DSD (μm) | 0.16 ± 0.04 | 0.17 ± 0.07 | 0.19 ± 0.04 | 0.33 ± 0.22 | |||||
NOTE: TDL, total dendritic length; MSL, mean segment length; DSC, dendritic segment count; DSN, dendritic spine number; DSD, dendritic spine density.
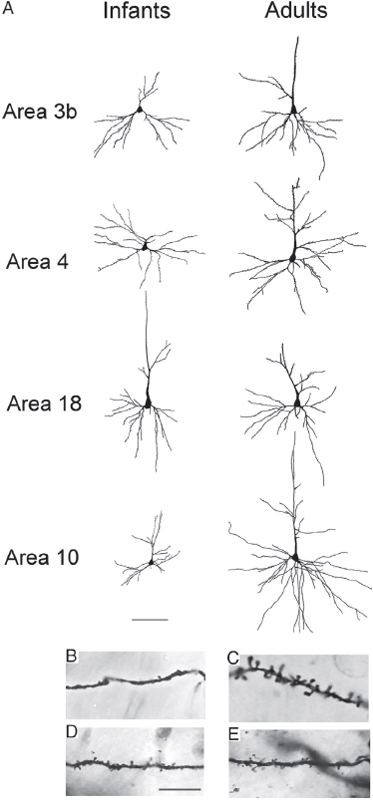
FIGURE 8.3 (A) Tracings of Golgi-stained pyramidal neurons in cortical areas 3b, 4, 18, and 10 in infant and adult chimpanzees. (Scale bar, 100 μm.) Below the tracing, closeup photomicrographs depict dendritic shafts of pyramidal neurons in (B) area 10 of a 1-year-old chimpanzee, (C) area 10 of an adult chimpanzee, (D) area 3b of a 1-year-old chimpanzee, and (E) area 3b of an adult chimpanzee. (Scale bar, 25 μm.)
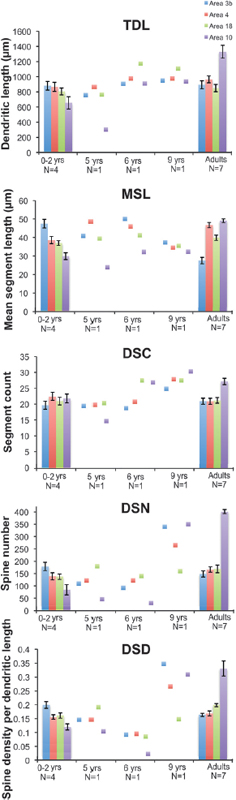
FIGURE 8.4 Regional differences in morphological measures of complexity of basilar dendrites for all cortical regions of interest between adult and infant chimpanzees, including TDL, MSL, DSC, DSN, and DSD. Data from juveniles (5–9 years), for which one individual per age group was available, are illustrated as individual data points. Error bars represent SEM. [NOTE: Figure can be viewed in color in the PDF version of this volume on the National Academies Press website, www.nap.edu/catalog.php?record_id18573.]
DISCUSSION
Similar to humans, chimpanzees have a relatively long lifespan and prolonged period of dependency during which “cultural traditions” (e.g., tool use, grooming postures) are acquired from conspecifics (Lonsdorf, 2006; Lonsdorf and Bonnie, 2010). As postnatal experience shapes cognitive and social development, changes in the timing of cortical maturation may be important for understanding the evolution of species differences in behavior. Indeed, many genetic differences between humans and other primates affect processes involved in cerebral development (Pollard et al., 2006; Dennis et al., 2012; Derrien et al., 2012). Until recently, however, little was known about the nature of microstructural changes during neural ontogeny in the cerebral cortex of nonhuman primates other than macaques, making it difficult to assess how the developmental trajectory of the human brain might be unique (Miller et al., 2012). Our current analyses demonstrate that similar to humans, synaptic proliferation in chimpanzees is prolonged through the mid-juvenile period, and development of pyramidal neurons in the prefrontal cortex is delayed relative to other cortical areas.
Prolonged Synaptogenesis
Like humans (Huttenlocher and Dabholkar, 1997; Petanjek et al., 2011), chimpanzees exhibit a substantially later peak in synapse density (between 3 and 5 years) than macaques (3 months) (Rakic et al., 1986), and a prolonged phase of synapse pruning, which extends into the late juvenile period (around 10 years). Evidence for an extended period of synaptic refinement in chimpanzees is consistent with previous findings indicating that prefrontal white matter development is prolonged until at least the mid-juvenile period in this species (Sakai et al., 2011). This finding contrasts, however, with a recent report by Liu et al. (2012), suggesting that relatively increased synapse-associated gene expression in the prefrontal cortex continues until 5 years of age in humans only, but in chimpanzees and macaques synaptic gene expression peaks within the first year of life. Because the analysis by Liu et al. (2012) included only three chimpanzee brains between 1 year of age and adulthood, however, differences in the sample sizes represented in these two studies could contribute to the divergent findings, especially because interindividual variation is pronounced. Additionally, little is still known regarding how gene expression regulates anatomical changes in synapse densities during development (Goyal and Raichle, 2013).
Prolonged synaptogenesis in chimpanzees is consistent with behavioral evidence indicating that the juvenile period is critical for social learning of group-specific behaviors acquired from conspecifics. Tool use
in chimpanzees displays regional variation to an extent that exceeds other nonhuman animals, including such varied techniques as stone hammering to access nuts, dipping or digging for ants or termites with sticks, and stabbing small vertebrates with spears (Whiten et al., 1999). Young chimpanzees require at least 5 years of postnatal development before they are able to master tool use, and the acquisition of competence in these skills depends on environmental variables, such as the time spent with the mother and her proficiency (Lonsdorf, 2006). Thus, an extended period of synaptogenesis in chimpanzees through juvenile life may be important to enhance neuronal plasticity for the experience-dependent behaviors that emerge following social exposure and learning from conspecifics.
We found no differences in the timing of peak synapse density across different regions of the chimpanzee neocortex. These findings appear to contrast with reports in humans indicating that the prefrontal cortex matures later than other cortical areas, as reflected by metabolic rate, cortical thickness, and peak synapse density (Chugani et al., 1987; Huttenlocher and Dabholkar, 1997; Giedd et al., 1999; Shaw et al., 2008; Liu et al., 2012). It should be noted, however, that prior data suggesting a relative delay of synaptogenesis in the human prefrontal cortex are based on a single study of a modest sample that lacked statistical analysis (Huttenlocher and Dabholkar, 1997). Thus, evidence of heterochronous development within the human neocortex as measured by metabolic activity and cortical thickness may reflect the combined effect of multiple microstructural changes, including neuronal dendritic branching growth (Travis et al., 2005), synapse density, glial cell numbers, and other factors.
Delayed Dendritic Growth in the Prefrontal Cortex
In addition to synaptogenesis, we examined regional changes in the development of dendritic branching of pyramidal neurons. In adult primates, remarkable variation in the morphology and complexity of pyramidal neurons has been reported (Giedd et al., 1999; Elston et al., 2001; Jacobs et al., 2001; Bianchi et al., 2012). Specifically, it has been shown that pyramidal neurons of the prefrontal cortex are characterized by more elaborate dendritic trees than other cortical areas, to support increased connectivity from integrating diverse corticocortical inputs and to orchestrate cognitively complex behaviors (Elston et al., 2001; Jacobs et al., 2001; Semendeferi et al., 2011; Bianchi et al., 2012). Although dendritic arbors are more extensive in the prefrontal cortex relative to other cortical regions in adult humans (Jacobs et al., 2001), chimpanzees (Bianchi et al., 2012), and macaques (Elston et al., 2001), the timing of development of these neuronal specializations appears to be species specific (Travis et al., 2005; Elston et al., 2009).
By demonstrating that dendritic arborization and spine density in the prefrontal cortex of infant chimpanzees is not as extensive as other cortical areas, our data indicate a delay in the maturation of pyramidal neurons in this region, a pattern that shares greater similarities with dendritic development in humans (Travis et al., 2005) than with macaques (Elston et al., 2009). Dendrites regulate the integration of inputs and provide sites for synapses on spines (Yuste and Tank, 1996; Spruston, 2008). As such, they are extremely plastic structures that undergo changes in response to experience, which is especially evident during development (Diamond and Connor, 1982; Jacobs and Scheibel, 1993; Moser et al., 1994; Anderson et al., 1995; Koenderink and Uylings, 1995; Jacobs et al., 1997; van Praag et al., 2000; Grutzendler et al., 2002; Sin et al., 2002; Radley et al., 2006; Kabaso et al., 2009; Yang et al., 2009; Bose et al., 2010; Bloss et al., 2011; Petanjek et al., 2011; Yadav et al., 2012). For this reason, a delay in the formation of dendritic trees in the prefrontal cortex may be important for processing and integrating the extensive load of information that both humans and chimpanzees acquire during development and for maintaining plasticity of executive functions. Although the present study focused on the rostral prefrontal cortex to be consistent with previous research in adult chimpanzees and humans, it is possible that delayed maturation of pyramidal neurons also characterizes association cortices in other frontal, parietal, and temporal regions.
Despite sharing these neurodevelopmental similarities, it is important to note that cognitive ontogeny in chimpanzees differs from humans in several respects. Behavioral studies suggest that the different social and environmental contexts in which humans and chimpanzee develop may have also been important in the evolution of human-specific sociocognitive abilities (Burkart et al., 2009). For example, whereas young chimpanzees do not fully wean until 4–5 years of age and remain closely attached to their mothers during the early years of development, human infants often interact with multiple caregivers and engage in joint attention (Carpenter and Tomasello, 2006). Experiments with enculturated great apes demonstrate the modulating effect of social environment on cognition; being reared in close human contact in an enriched environment is associated with improved performance on sociocognitive tasks and tool use (Buttelmann et al., 2007; Furlong et al., 2008; Lyn et al., 2010; Russell et al., 2011).
Future studies investigating whether the progression of cortical development in chimpanzees also characterizes other great apes (bonobos, gorillas, and orangutans) would help identify more precisely the evolutionary emergence of these traits. In this regard, genome sequences and brain transcriptome datasets from multiple great apes may be helpful in identifying the specific gene sequence or regulatory changes that contribute to phylogenetic variation in the timing of neocortical development.
The study of other large-brained and long-lived species, such as cetaceans and elephants, may also shed light on the evolutionary mechanisms and constraints of developing a plastic, “cultural” brain.
Altogether, our findings indicate that brain development in humans and chimpanzees are both characterized by an extended period of cortical synaptogenesis and a delay in the maturation of dendritic arbors in pyramidal neurons of the prefrontal cortex. These results suggest that several key features of human brain ontogeny for enhanced developmental plasticity emerged before the divergence of the chimpanzee and human lineages. In addition to these shared similarities of early postnatal development, later phases of human neocortical maturation appear to be more evolutionarily modified and distinct from chimpanzees, involving prolonged myelination that continues into early adulthood (Miller et al., 2012). When combined with changes to human social organization, the prolonged developmental plasticity and shift toward delayed development of the prefrontal cortex present in hominin ancestors may have increased learning potential for higher-order sociocognitive functions.
MATERIALS AND METHODS
Specimens
Formalin-fixed brain samples were obtained from the left hemisphere of 17 common chimpanzees (Pan troglodytes) (age range: 0–41 years) and one case where only the right hemisphere was available (5.3-year-old). Chimpanzees were housed according to each institution’s Animal Care and Use Committee guidelines, and died for reasons unrelated to the present study. Within 14 hours of each individual’s death, the brain was removed and immersed in 10 percent (vol/vol) formalin. After a variable period of fixation, brains were then transferred to 0.1 M PBS with 0.1 percent sodium azide solution and stored at 4°C. Blocks of tissue containing prefrontal (area 10), primary motor (area 4), primary somatosensory (area 3b), and the lateral surface of the prestriate cortex (area 18) were dissected and used for immunohistochemistry against synaptophysin protein, rapid Golgi impregnation, and quantification of synapses using electron microscopy. These regions were chosen to be consistent with previous studies in adult chimpanzees and humans. Because chimpanzees reach sexual maturity around 10–13 years (females), and 12–15 years (males), individuals up to the age of 10 years were considered subadults, age 5–9 years as juveniles, and 0–2 years as infants.
Immunohistochemistry for Synaptophysin and Stereologic Quantification
All 18 individuals were used for immunohistochemistry analyses (age range: 0–41 years). Free-floating sections of the regions of interest were stained with rabbit polyclonal IgG1 antibodies against synaptophysin, which is an acidic, homo-oligomeric integral membrane glycoprotein isolated from presynaptic vesicles (1:100 dilution, A0010; DakoCytomation). Before immunostaining, sections were rinsed thoroughly in PBS and pretreated for antigen retrieval by incubation in 10 mM sodium citrate buffer (pH 3.5) at 37°C in an oven for 30 min. Sections were rinsed and immersed in a solution of 0.75 percent hydrogen peroxide in 75 percent methanol to eliminate endogenous peroxidase activity, then incubated in the primary antiserum diluted in PBS with 2 percent normal goat serum and 0.1 percent Triton X-100 for ~24 hours on a rotator at 4°C. After rinsing in PBS, sections were incubated in biotinylated anti-rabbit IgG (1:200 dilution, BA-2000; Vector Laboratories) and processed with the avidinbiotin-peroxidase method using a Vectastain Elite ABC kit (pk-6100; Vector Laboratories). Sections were rinsed again in PBS, followed by a rinse in sodium acetate buffer. Immunoreactivity was revealed using 3,3′-diamino-benzidine and nickel enhancement according to a modification of methods described previously (Shu et al., 1988; Van der Gucht et al., 2001). Specificity of the reaction was confirmed by processing negative control sections as described, excluding the primary antibody. No immunostaining was observed in the control sections.
Quantification of the numerical density of synaptophysin-immunoreactive puncta was performed using StereoInvestigator software (v9; MBF Bioscience). Beginning at a random starting point, three equidistantly spaced sections were chosen for stereologic analysis. To quantify the density of synaptophysin-immunoreactive puncta, the area comprising the cortex, spanning layers I to VI, of each region of interest was outlined at low magnification and segmented through a set of optical disector frames (3 × 3 μm) with a square scan grid size ranging between 400 × 400 μm and 700 × 700 μm. Disector analysis was performed under Koehler illumination using a 100× oil objective (Zeiss Plan-Apochromat, N.A. 1.4). The thickness of optical disectors was set to 1 μm, with a 1-μm guard zone at the top of the section. All numerical densities of synaptic puncta derived from these optical disector counts were corrected by the number-weighted mean section thickness as described previously (Sherwood et al., 2007). All synapse counting was performed blind to the region of interest and age of the specimen. Data were analyzed by fitting cubic regression models to synapse counts from each of the four regions separately using standardized polynomials in R software (R Development Core Team, 2010). Testing for similarities in cubic polynomial fit to the regions of
interest was accomplished by using a generalized least-squares model and a linear mixed model. Results were plotted against age (0–10 years), where individuals older than 11 years were grouped together with adults to emphasize changes during growth (Fig. 8.1).
EM Counts of Synaptic Density
Sections from four individuals (age 0 year, n = 1; age 2 years, n = 1; age 6 years, n = 1; age 11 years, n = 1) that contained the regions of interest were postfixed (1 percent OsO4), and stained with 1 percent uranyl acetate. After embedding in Epon, ultrathin sections were collected on mesh copper grids. Quantification of synapse density was then measured on digital images obtained from a JEOL JEM 1200EX transmission EM at a magnification of 40,000×. Sampling fields were chosen by using the random sampling method and the number of synapses per unit volume was calculated through the following formula: NV = NA/d, where NV is the number of synapses per unit volume, NA is the number of synaptic junctions per unit area of an electron micrograph, and d is the mean length of densities associated with the synaptic junctions (Colonnier and Beaulieu, 1985). One hundred images from each cortical area were analyzed. Measures of synapse length were also obtained from 50 to 130 randomly chosen synapses in each set of micrographs for an individual cortical region. Criteria for identification of synapses included the presence of a postsynaptic density, synaptic vesicles at the presynaptic terminal, and opposing membranes between the pre- and the postsynaptic terminals. A synapse was only marked if the synaptic junction was apparent, and if at least two synaptic vesicles were seen in the presynaptic component of the synapse.
Rapid Golgi Staining
Adjacent blocks (3–5 mm in thickness) from 14 individuals were stained with a modified rapid Golgi technique (Scheibel and Scheibel, 1978) for neuronal morphology quantification. However, only seven (age range 0–9 years) yielded complete neuron staining that met criteria for tracing and quantification (see below). Blocks were sectioned on a Vibratome at 120 μm, mounted, coverslipped, and stored at 4°C. Morphological analyses of dendritic complexity were conducted on 10 neurons per region (n = 280). Criteria for neuron selection required that neurons be relatively isolated and unobstructed, located within the center of the section, and as complete as possible (Jacobs et al., 2001). Neurons were sampled from layer V, at a similar depth across regions: area 3b, 1,314 ± 733 μm; area 4, 1,040 ± 548 μm; area 18, 1,062 ± 468 μm; and area 10, 1,151 ± 603 μm.
Neurons meeting criteria were traced using a Zeiss Axioplan 2 photo-microscope equipped with a Ludl XY motorized stage (Ludl Electronics), Heidenhain z-axis encoder, and an Optronics MicroFire color videocamera coupled to a Dell PC workstation running Neurolucida software (MBF Bioscience). Tracings were performed under Koehler illumination using a 40× dry objective (Zeiss Plan-Apochromat, N.A. 0.75), and involved following dendrites along their entire length across the z-plane and manually marking all visible spines. Once tracing was complete, neuronal morphology was quantified in NeuroExplorer (MBF Bioscience), according to the following measurements of dendritic morphology, derived from Jacobs et al. (2001): (i) TDL, the sum of the individual lengths of all dendritic segments; (ii) DSC, the number of all dendritic segments; (iii) MSL, which is essentially TDL/DSC; (iv) DSN, the number of all spines marked on the dendritic arbor; and (v) DSD, the number of spines per micrometer of dendritic length. The cell body cross-sectional area was also recorded. For each variable of dendritic morphology, measurements were taken for both basilar and apical dendrites. However, because apical dendrites were often incomplete because of sectioning, quantitative comparisons of dendritic length and spine number across cortical regions were limited to basilar dendrites. All tracings were performed blind to the regions of interest by C.D.S. and S.B., who were normed with another rater (A.L.B.), and checked by C.C.S. Intra- and interrater reliability of measures were determined to be in good concordance.
Because of the small size in the juvenile and subadult category, statistical analyses were conducted only on the group including newborns and infants; data from the juveniles and subadults are illustrated as individual points (Fig. 8.4). To analyze regional differences in dendritic complexity, we used a nested ANOVA design (IBM SPSS 18.0), in which each neuron was nested within region (areas 3b, 4, 10, 18), which was nested within individual brains. Data for each variable of interest (soma area, TDL, DSC, MSL, DSN, DSD) were analyzed separately. Pairwise contrasts were then performed using a Bonferroni correction for multiple comparisons. Data for comparisons with adult chimpanzees, as shown in Fig. 8.4, were taken from a previous study that used the same methodological procedure (Bianchi et al., 2012), and included seven individuals, age 35+ years.
ACKNOWLEDGMENTS
We thank Dr. Anastas Popratiloff, director of the Center for Microscopy and Image Analysis at The George Washington University, for his advice and support in the data collection. This work was supported by National Science Foundation Grants BCS-0515484, BCS-0549117, BCS-0824531, and DGE-0801634; National Institutes of Health Grant NS-42867; and James S. McDonnell Foundation Grants 22002078 and 220020293.


















