Below is the uncorrected machine-read text of this chapter, intended to provide our own search engines and external engines with highly rich, chapter-representative searchable text of each book. Because it is UNCORRECTED material, please consider the following text as a useful but insufficient proxy for the authoritative book pages.
gamma rays will be small. The usual scintillator for counting is ZnS, activated by Ag. Although the light output is high, ZnS(Ag) is only available as a multicrystalline powder whose light transmission is poor; therefore, it is not used for measuring energy spectra. The scintillator is usually deposited ' by allowing ZnS(Ag) particles, ~ 20 u in size, to settle from a water or alcohol suspension onto a glass or plastic disk which will serve as a light guide. Typical scintillators made by this technique have a surface density of 5-25 mg/cm2. When the deposit is dry, it is usually sprayed with clear plastic or covered with a thin plastic film. Aluminizing the covering film inproves the light collection efficiency and may be used to protect the phototube from ambient light. The total thick- ness of material covering the scintillator proper should be < 1 mg/cm2. Scintillator assemblies very similar to the above may be obtained from several manufacturers. The scintillator assembly is mounted by using silicon oil or grease as an opti- cal coupling between the photomultiplier faceplate and the uncoated side of the glass or plastic light guide. When it is desirable to use a scintillation device for determination of charged-particle energies, some inorganic scintillator other than ZnS(Ag) must be used. Thin clear disks of Nal(Tl) or Csl(Tl) are often employed. The resolution of these devices cannot compare with the resolution obtained with a gas-ionization or semiconductor detector, and so are not often used except in special situations. Further information 4 5 on this subject may be found in recent reviews. ' III. IONIZATION CHAMBERS* 1. lonization in Gases Most of the experimental information about the stopping of charged particles in matter has been obtained from a study of the ionization produced. A very useful class of counters makes use of the ionization produced in a gas by collecting either the electron which is formed, or the ion pair, i.e., the electron and positive ion. *See references (37), (38), (39), (40), and (41). 45
It will be recalled that the specific ionization of a charged particle in ion pairs per cm of path, d<S/dx, increases slowly to a maximum value a few mm from the end of the particle range, and then drops sharply. Specific ionization is related to the stopping power -dE/dx by dVdx = - (1/w) dE/dx (7) where w is the average energy to produce one ion pair ("total ionization"). The total ionization is of considerable practi- cal importance, because the appropriate value of w can be used to predict whether a particular energy loss will render detection possible. The value of w varies for different stopping materials, but is remarkably constant for gases. Some typical values of w in gases are listed in Table 3 for electrons as primaries. It will be noted that w is always greater than the first ioni- TABLE 3. SINGLE IONIZATION POTENTIALS <50 COMPARED WITH AVERAGE VALUES OF w FOR ELECTRONS IRRADIATING VARIOUS GASES Gas *., eva w, ev b H2 15 .6 36.9 N2 15 .7 34.9 02 12 .5 31.3 He 24 .6 41.3 Ne 21 .6 35.9 Ar 15 .8 26.3 Kr 14 .0 24.4 Xe 12 .1 22.1 C02 13 .7 32.7 Air â â 34.2 CH4 13 .1 28.1 42 Single ionization potentials of monatomic gases, from Dieke; data on other gases from Craggs and Massey.4-* Average of values from recent literature, summarized in a review by Fulbright. ° 46
zation potential. This probably happens because the electrons produced in primary ionization frequently have sufficient energy to cause further, or secondary ionization; also energy may be absorbed which is lost by excitation and dissociation. The energy loss per ion pair is very nearly independent of particle energy and particle type. This immediately suggests that the integrated ionization S. produced when an energy E is transferred, is given by 5 = E/w. Although in most cases this proportionality can be assumed, there is some evidence that a nonlinear relationship between jl and E exists for alpha 3 8 particles having energies less than about 0.1 Mev. Once free electrons and positive ions are formed, their behavior depends upon the nature of the gas and the electric field present. An electron makes many collisions with gas molecules, and although its direction of motion is randomized by such collisions, there is a net drift in an electric field along a direction parallel to the field lines. The drift velocity depends on the type of gas, its pressure, and the electric field strength. Positive and negative ions move much more slowly through gases than do electrons. Further, ionic mobilities are rela- tively insensitive to changes in the applied electric field strength and the gas pressure. Therefore, in the interest of fast response, pulse ionization chambers are almost invariably arranged for electron collection. Electrons may form negative ions by attaching themselves to neutral atoms or moleculesâthis effect is especially harmful in pulse ionization chambers using fast electron collection. Of the common gases, the halogens, oxygen, and water vapor are the most serious offenders. The rare gases, hydrogen, nitrogen, carbon dioxide, and methane have attachment coefficients 103 times smaller than the halogens, and are considered acceptable filling gases for ionization detectors. 2. Current Chambers The essential parte of a gas ionization chamber are two electrodes insulated from each other, defining a gas-filled space between them. A parallel-plate ionization chamber oper- ated as a current chamber is sketched in Fig. 20. The figure shows idealized current-voltage curves for a low- and a high- 47
s .- J- z UJ a: tr. ID o o I- N Ion Pairs Along Particle Track Radioactive Source .Collecting Electrode -/_ ' High-Voltage/1 J. VGOS Electrode -S i/ -=- VP -=r R To Electrometer 1 HIGH INTENSITY SOURCE >SATU RATION CURRENT LOW INTENSITY SOURCE APPLIED POTENTIAL VP Fig. 20. Illustration of ionization chamber operation. Typical current-voltage curves are shown for different source intensities. The insert shows how a parallel-plate chamber is arranged for current measurement by the "IR-drop" method; for measurements by the "rate-of-drift" technique, both switches St and S2 must be opened (see text). 48
intensity source. At low applied voltage, there is a loss of charge through recombination of electrons and positive ions. As the potential is increased, the current flowing through resistance R from the collection of charge rises until it reaches a limiting value, the saturation current. At very high potential the current begins to rise again, due to the onset of gas multiplication (see Section V.I.). The number of ion pairs formed per second n may be calcu- lated from N, the rate at which particles are absorbed in the chamber, the average energy per particle E, and w: (8) w The steady-state saturation current I is obtained by multiplying by the electronic charge e (1.60 x 10~19 coulomb): I = en. Thus, if sources having identical energy spectra (i.e., the same E) are compared, the saturation current is proportional to the source strength N. This is the basis of the many ioni- zation chamber instruments used for monitoring and assay purposes. As shown in Fig. 20, the current is always measured in terms of a voltage, using an electrometer. For this reason, the method just described is called the "IR-drop" method, because the voltage across R is given by the product IR. The currents of interest lie in a range of about 10~8 to 10~14 amp. The IR-drop method requires very high resistances for high sensitivity; however, in most cases it is not advisable to use resistors larger than 1012 ohms if special techniques are to be avoided. When the rate N is very low, the statisti- cal variations in the measured voltage require careful analysis if high accuracy is required. For these reasons the rate-of- drift method is used for small currents (< 10'12 amp.). In the rate-of-drift method, the load resistance R is removed by opening Sa (see Fig. 20). The collecting electrode is grounded by closing S2; thus the voltage across C is zero. At the start of the measurement S2 is opened, and the voltage after a time t is given by V = 1/C f Idt = It/C . (9) â¢r A 49
The value used for C must include the combined capacitance of the chamber, leads, and electrometer input, and it typically lies in the range of 10-30 picofarads; therefore, the sensi- tivity is very high. Further, because the rate-of-drift makes available to the electrometer all the charge produced in the time t, this method is fundamentally more sensitive than the IR-drop method, in which charge is continuously consumed by the load. The theory and design of electrometers and the properties of insulators suitable for ionization chambers have been reviewed by Fairstein. Helpful suggestions on these tech- niques as applied to the determination of radioactive gases 15 are given by Tolbert and Siri. 3. Pulse-Type Chambers When the rate of arrival of ionizing pulses is too low for convenient dc measurements, or when it is necessary to deter- mine the energy distribution of particles stopped in the gas, the ionization chamber is operated as a pulse instrument. Here, the details of the collection process and the transient response of the ancillary equipment are both very important, since the complicated signal from the chamber is always observed distorted by the measuring system. Consider a parallel-plate ionization chamber in which a single ion pair has just been formed (cf., Fig. 21). If the product RC is very large, the current through R can be neg- lected during the ion collection, and V(t) = q(t)/C, where q(t) is the net charge collected and C is the total circuit capaci- tance. The ion pair influences the net charge not only by being collected, but also by electrostatic induction. At time t a iter the pair is formed, a charge -q (t) and -q-(t) is induced o J3 the collecting electrode by the positive ion and the electro*! respectively. The potential is q.(t) + q (t) V(t) = -t â^ (10) At the time of formation, the ion and electron are both at x.o, and induce equal charges of opposite sign; therefore, V(O) = O. The electron moves rapidly toward the collecting electrode, 50
f d I Fig. 21. Schematic representation of a parallel-plate ionization chamber in which one ion pair has just been formed. causing a linear increase in q-(t) until the electron is col- lected. During this interval the effect of the positive ion is negligible, as its transit time is about 103 that of the electron. The potential now is V(t) = q_(t) - e (11) The important fact to note is that the expected final potential of -e/C is not attained when the electron is collected, but only when the positive ion ceases to induce a charge, i.e., when the ion strikes the high-voltage electrode. The collector potential for the process just described is sketched in Fig. 22. The pulse profile shown makes the simplifying assumption that n ion pairs were formed at a point x0 ; actually the ionization is produced along a track, and the qualitative pulse shape in the figure will be distorted by the spacial distribution of ion pairs. Electron diffusion will tend to obscure the sharp changes in slope. From the dis- cussion of ionization in gases it will be apparent that the presence of an electronegative gas such as oxygen will seriously distort the pulse. 51
TIME, / Fig. 22. Idealized voltage pulse in a parallel-plate ionization chamber with plate spacing d, after production of n ion pairs a distance x0 from the collecting electrode. The electrons are collected at t- and the positive ions at t+. Note that the time scale is distorted to show the initial rise. As seen in Fig. 22 the potential due to electron col- lection depends on the location of the ion pair at t = 0. This is not especially important if only counting is required, for the pulse must only be large enough to be recorded. However, some of the important applications for ionization chambers require a pulse whose height is proportional to the number of ion pairs. At first glance it may seem that one should amplify the pulse corresponding to the total ionization, i.e., V of Fig. 22. Although this approach has been used very success- fully, the amplifier required for broad, slow-rising pulses is prone to be rather noisy and is very sensitive to microphonics 52
and power-supply hum. The tolerable rates are only a few per second, because of the danger that the pulses can "pile up." The slow rise time makes timing very uncertain, so that coinci- dence techniques are not very applicable (see discussion of electronic equipment in Section VI). To avoid some of the difficulties encountered when total ionization pulses are collected, only the portion of the pulse due to electron collection is employed. Figure 22 shows that the electrons are collected in a much shorter time; it now remains to avoid the variation in pulse height with position of the ionized track. Two methods are used: either the collecting electrode is made very small, or it can be shielded by a grid. The addition of a grid to a parallel-plate chamber is the most desirable technique for removing the effect of positive- 38 41 44 ion induction. Such an arrangement is shown in Fig. 23. The sample is placed on the high-voltage electrode; the gas pressure and geometry are so arranged that all of the ioni- zation is produced in the region between the grid and the high- voltage electrode. The grid shields the collecting electrode from the influence of the positive charges, but the electrons are accelerated toward the collector. Then the charge at the collector is equal to the total ionization induced by the primary particle. Collecting Electrode Grid -1/2 High-Voltagey Electrode R Fig. 23, chamber. Schematic diagram of a gridded ionization 53
4. Design Considerations Because complete saturation can be attained with rather modest electrical fields, it is possible to design an ioni- zation chamber to suit almost any experimental arrangement. For current chambers the parallel-plate geometry is preferred, because it is the easiest design to analyze mathematically. Coaxial cylinder chambers are very easy to construct, and most of the ionization chambers in radiation survey instruments are of this type. Pulse-type chambers present a less critical design problem, because regions of weak field are of less con- cern than for current chambers. In current chambers the placement and construction of the insulators are matters of the greatest importance, since the current flowing through the insulator should be negligibly small compared with the current flowing through the conductor it supports. Even materials of high resistivity may develop serious leakage currents if the surfaces are permitted to acquire a charge from mechanical stresses; from rubbing one surface against the other; or from the electric field, which can induce an image charge or cause ions from the active volume to be collected on the insulating surfaces. On the other hand, ordinary surface leakage is not very important in fast pulse chambers at voltages of 1 or 2 kv. Small leakage currents can be tolerated, since the electronic system sees only fast transient signals; naturally, any corona discharge or other source of erratically changing leakage will cause bursts of spurious counts to be recorded. Current chambers should always use guard rings. As shown in Fig. 24, the guard ring serves two purposes: (a) When the guard ring is grounded, any high voltage leakage is passed to ground instead of to the collector. Since only a small potential difference appears across the collecting electrode insulator, the collector leakage current is greatly reduced; (b) the active volume of the chamber is defined by the guard ring. Guard rings are not always required in pulse chambers and in some cases may lead to spurious counts. For example, the sensitive volume of a current chamber may be defined by a guard ring; but if the same chamber is operated in the pulse mode, ionization produced in the volume between the guard ring 54
High-Voltage Electrode Collecting Electrode (a) Guard-Ring Insulator Guard Ring ion (b) Fig. 24. Importance of guard rings in a cylindrical ioni- zation chamber. (a) Without a guard ring, the measured current through load resistance R is the sum of the ionization current and the leakage current. (b) A grounded guard electrode ensures that the high-voltage leakage current will not pass through the load resistor, so the current through R will be due only to ionization. and high-voltage electrode can induce on the collector a pulse of detectable amplitude. 5. Counting and Assay Applications Ionization chambers are widely used for measuring the strengths of sources of heavy charged particles. Very simple detectors can be made for routine alpha counting if the energy 55
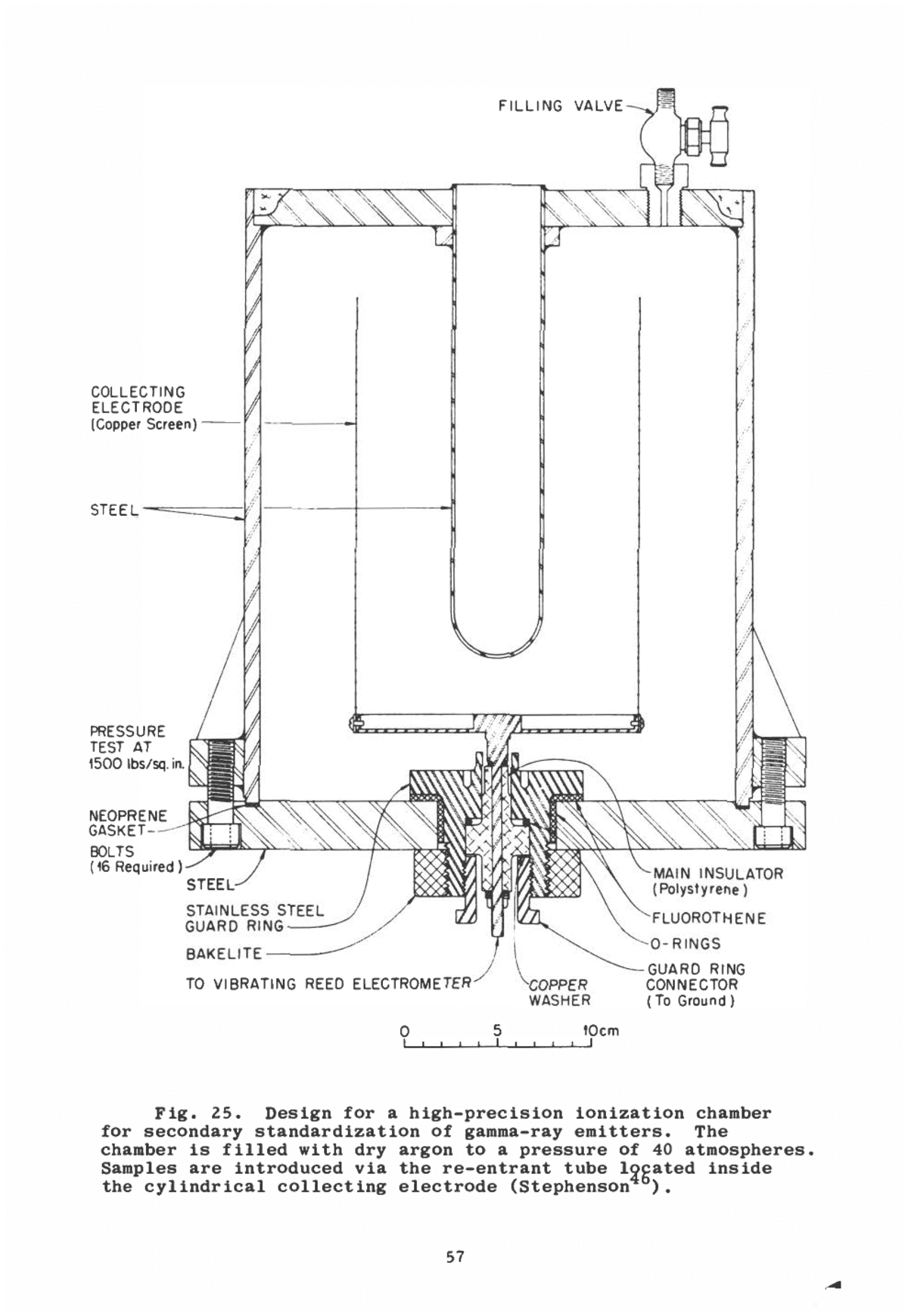
distribution is not required. Usually a spherical collecting electrode concentric with a cylindrical high-voltage electrode will suffice. To avoid random summing of low-amplitude pulses when a high beta- or gamma-ray activity is present in the alpha-particle source, a short clipping time is needed. A use- ful shortening of the detector rise time can be obtained by increasing electron drift velocity; in argon, a common filling gas, the rise time is improved by the addition of 5% C02 . The energy released in fission is nearly forty times that for a 5-Mev alpha particle, and so fissions may be counted to the exclusion of other events. Parallel-plate pulse chambers are widely used for measuring fission cross sections and for intercomparison of fissile sources. If the number of alpha particles per fission event is very high, then the "pileup" of alpha pulses will cause a troublesome background; in such cases it would be advisable to use a detector with a more rapid response, such as, for example, a gas scintillation counter or a semiconductor radiation detector (Section IV., below). lonization chambers also may be used for neutron detection. Fission chambers containing UZ3S are widely used as neutron- sensitive devices in reactor control and personnel protection. In some applications, chambers are filled with BF3 gas, or lined with boron or lithium. The ionization is produced by the alpha particles and recoil nuclei from the (n,a) reaction on B1 ° or Li6 . Low-energy beta emitters may be introduced as gases into a calibrated chamber for quantitative assay (Section IX.5.). lonization chambers may be used for relative assay of either gas or solid samples, even if the particles are not completely stopped in the gas. ' In this application the chamber must be calibrated for the particular beta activity and type of source mount ing. Current ionization chambers are particularly well suited to the assay of gamma-ray emitters. A chamber designed with the proper regard for insulator considerations and mechanical rigidity should retain its calibration to within a fraction of a per cent for years. Vibrating-reed electrometers are capable of measuring the saturation current with high precision (-0.05%). If standard gamma sources of known disintegration rate are used for efficiency calibration, the chamber may be used as a precise secondary standard. Figure 25 shows the 56
FILLING VALVE- COLLECTING ELECTRODE [Copper Screen) STEEL PRESSURE TEST AT 1500 Ibs/sq.in. NEOPRENE GASKET- BOLTS (16 Required MAIN INSULATOR (Polystyrene) STAINLESS STEEL GUARD RING BAKELITE TO VIBRATING REED ELECTROMETER COPPER WASHER FLUOROTHENE 0-RINGS -GUARD RING CONNECTOR (To Ground ) 10cm Fig. 25. Design for a high-precision ionization chamber for secondary standardization of gamma-ray emitters. The chamber is filled with dry argon to a pressure of 40 atmospheres. Samples are introduced via the re-entrant tube located inside the cylindrical collecting electrode (Stephenson ). 57