10
Intergenerational Transfers, Social Arrangements, Life Histories, and the Elderly
Ronald Lee
Because of the historical emphasis on energy flow within individuals rather than among the members of a family, intra-individual tradeoffs have played a much larger role to date than intergenerational tradeoffs…. (Stearns, 1992, p. 80)
INTRODUCTION
A transfer is a gift with no quid pro quo. It is not reciprocal; it is not an exchange. An intergenerational transfer is a gift of food or assistance by one generation to another, typically a descendant. The descendant, when mature, then herself makes a similar transfer to her offspring or grandoffspring. The chain of transfers continues downward from one generation to the next, never returning to earlier generations.
Reproduction is an intergenerational transfer. Building an offspring requires energy. Helping it survive and grow takes additional energy, provided in a seed or egg, or as a capital bequest (as when a dung beetle lays its egg on a dung ball or a wasp lays its egg in prey) or through progressive feeding as the offspring grows over an extended period (as in mammalian lactation or a bird feeding its chick). In addition, the parent incurs risks in reproduction and may provide services requiring energy expenditure such as building nests or burrows, guarding, ventilating, fanning, warming, and training the offspring. While reproduction involves costly transfers, it lies at the very heart of evolution and natural selection, and does not require explanation through kin selection or inclusive fitness.
Yet many questions arise about the intergenerational transfers associated with reproduction. How many offspring versus how much investment in each (quantity versus quality)? For how long does an offspring receive transfers, and how are these transfers related to growth and to the age of sexual and economic maturity? Is duration of offspring dependency related to adult longevity? What factors favor the co-evolution of transfers and longevity? How does the marginal fitness value of energy vary across the life span, and how is this age pattern related to transfers and fertility? How are transfers related to sexual dimorphism? Could upward transfers from adult child to older parent evolve? Are intergenerational transfers a form of cooperative behavior? Is menopause related to transfer behavior?
As Stearns (1992) suggested in his classic work, life history theory has focused mainly on intra-individual tradeoffs (the allocation of energy among growth, maintenance, survival, and reproduction) and paid less attention to intergenerational tradeoffs. Yet intergenerational transfers can profoundly alter the energetic budget constraint faced by the individual organism over its lifecourse, since an offspring receiving transfers can consume more than it acquires through its own efforts, in which case adults must consume less than they acquire.
My own research, much of which is joint with Cyrus Chu, and sometimes also with Hung-Ken Chien or Carl Boe, has focused on the role of intergenerational transfers in life history theory. Here I will discuss this topic with heavy emphasis on my own research. In a nutshell, parents invest in the quality of their offspring when such investments yield a higher fitness rate of return than would parental consumption of those resources. Parental transfers permit offspring to grow faster for a longer time to a greater size or complexity (e.g., the brain or body armor) or to mature earlier. The balance between quantity and quality of offspring evolves as well, with orchids and oysters close to the “corner” of maximum number of seeds with minimum size of each, and humans and dung beetles at a low fertility interior optimum with great investment in each offspring.
When evolution raises or prolongs such investments, the age at maturity is postponed and nutritional dependency is extended to older ages. In this case parental death entails the wasteful death of offspring in whom much has already been invested, so selection favors longer adult lifespan, perhaps including post-reproductive lifespan.
When there is long offspring dependency, there is also a fitness reward for cooperative breeding as a form of life insurance, with other adults available to substitute for parents who die. Similarly, there is a fitness reward for cooperative food sharing because it eases the lifecycle squeeze when there are multiple dependent offspring at the same time, a situation that is particularly important for humans (Lee and Kramer, 2002). Thus, investments in offspring quality through substantial and prolonged intergenerational
transfers create conditions that favor the evolution of cooperative breeding. Cooperative breeding itself likewise alters the forces of selection on levels and age patterns of fertility and mortality, and provides some evolutionary opportunities for free-riding.
These basic ingredients provide a foundation for considering the evolution of menopause and post-reproductive survival, time preference, specialization and division of labor between younger and older adults, sexual dimorphism, and some incentives for reproductive cooperation.
SENSITIVITY AND SELECTION
My personal starting point was Hamilton’s (1966) analysis of how natural selection molds senescence. Hamilton’s study used sensitivity analysis, asking how much a perturbation in fertility or mortality at a given age would affect reproductive fitness measured by the intrinsic rate of natural increase (stable population growth rate). Hamilton assumed that deleterious mutations occur at some rate at birth, with each one raising mortality at a specific age. (He thought this approach was less applicable to fertility since it ignored tradeoffs.) If fitness at that age is highly sensitive to mortality, then such mutations would be deselected from the population rapidly. In the balance reached between arrival of new mutations raising mortality at that age and selection removing them, fewer mutations would be present in the genome, so mortality at that particular age would be lower than otherwise. The accumulation of mutations affecting mortality at a given age is inversely proportional to the strength of selection at that age (see Charlesworth, 1994). The mechanism at work is negative selection on deleterious mutations. Because the sensitivities are calculated based on the observed values of fertility and mortality at each age, which are taken as given, there is an element of circularity in the theory. Furthermore, it has now been shown that the linearity assumption underlying most such calculations leads to incorrect conclusions (Wachter, this volume).
Hamilton’s analysis implies that the force of selection against mutations that raise mortality at a given age is inversely proportional to expected remaining fertility at that age, and predicts a very rapid increase in mortality at ages after reproduction ceases (menopause in human females). The long post-reproductive survival of humans is a puzzle for the theory, although it was understood that post-birth parental care probably played a role. Similarly, the theory predicts that mortality will be low and constant from birth until the age of start of reproduction, and the actual pattern, in which it declines following birth, is a puzzle for the theory.
In Lee (2003), I developed a model that added food and intergenerational transfers to the purely demographic model of Hamilton. In my model, fertility is a positive function of lifetime consumption and mortality
is negatively related to it. Foraging productivity is also positively related to higher lifetime consumption levels, since they would lead to bigger body size, better health, and perhaps better brains. Individuals of all ages live in food-sharing groups in which population age distributions are on average stable and depend on the levels of fertility and mortality determined by the model. Foraging success depends also on overall population density relative to a given resource, so population equilibrates eventually at a particular density, foraging productivity, fertility, mortality, and age distribution. At young ages, individuals may receive transfers from adults and, therefore, consume more than they produce. The overall population age distribution is stable and the analysis is comparatively static.
I then find an expression for sensitivities to perturbations in mortality and fertility in the neighborhood of the equilibrium, drawing conclusions about age patterns of mortality using arguments similar to Hamilton’s. In this model, higher survival at post-reproductive ages contributes to reproductive fitness if older individuals make net transfers of food to young members of the population (perhaps offspring or grandoffspring). The force of selection against mortality starts at a low level at birth and rises up to an age between reproductive and economic maturity, so mortality is high following birth, declines until maturity, and then rises throughout adulthood but with substantial post-reproductive survival.
The general conclusion is that the force of selection against higher mortality at a given age is proportional to a weighted average of the share of lifetime net fertility remaining above that age (as in Hamilton) and the share of remaining lifetime net transfers to others above that age. If the species in question makes no transfers after birth, then the weight on remaining fertility is unity and the weight on remaining transfers is zero, giving the classical result of Hamilton (1966). If the species in question makes transfers to the point where the tradeoff between quantity and quality of offspring is optimal, then the remaining-fertility component gets zero weight and the force of selection depends entirely on remaining transfers, which get a weight of unity. Intermediate weights can also occur according to explicit expressions in the analysis.
Based on observed fertility, mortality, and patterns of intergenerational transfers for some hunter-gatherer groups, Figure 10-1 plots actual age-specific mortality, the mortality predicted by the Hamilton analysis, and the mortality predicted by the theory of Lee (2003) incorporating intergenerational transfers. The agreement with the transfer theory is quite striking, but I view this as in part coincidental. There are many empirical difficulties with the estimates of food transfers, and food transfers are only one aspect of the ways older humans help younger ones, and surely many other forces are at play besides those incorporated in the model. Nonetheless, the figure is encouraging.
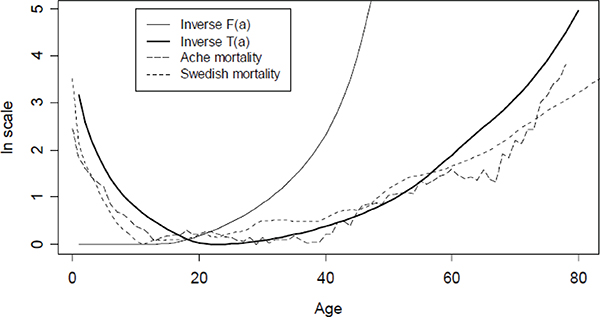
FIGURE 10-1 Comparison of actual mortality schedules for Aché and historical Sweden (1750-1754) with those predicted by force of selection for Hamilton theory and theory including transfers.
NOTE: F(a) is the share of lifetime net maternity remaining at age a, ranging from 1.0 until the age of sexual maturity to 0 at the first age at which fertility reaches 0, at some time before menopause. T(a) is the share of lifetime net transfers to others remaining at age a. At birth (or conception) this zero, since transfers made and received over the remainder of life are equal. From zero this share rises until it peaks at the age when the average child becomes nutritionally self-sufficient and then begins to make net transfers to others, which happens around age 18 or 20 for hunter-gatherers. After this, the share remaining declines with age until death.
SOURCES: Swedish mortality from Human Mortality Database. Aché mortality and fertility from Hill and Hurtado (1996). Transfers T(a) calculated from Kaplan (1994). See Lee (2003).
MICROSIMULATION OF THE TRANSFER MODEL WITH EXPLICIT FOOD-SHARING GROUPS AND RULES
The theory and analysis just described, in Lee (2003), raises questions: How could the populations of sharing groups be stable when individual families would have been tiny, perhaps with only one or two or three members? How could the catastrophic effects of a parental death be reflected in the analysis when in stable populations all ages are always present? How could comparative analysis of outcomes for gene lines be appropriate in the context of a mutation accumulation mechanism that would mean that every individual had a different mix of deleterious genes? These questions motivated a microsimulation approach (Lee, 2008). While Lee (2003) applies to any species, the microsimulation analysis is calibrated for human hunter-gatherers.
The simulations are single sex. Individuals inherit the genome of their mothers, but experience random mutations at birth. They live together in small food-sharing groups within which intergenerational transfers take place. Group membership is built up from membership in “matriarchies” consisting of all the living descendants of a single living female. Different rules for membership in sharing groups are considered, for example: (a) sharing only within matriarchies, which are therefore strongly kin-based or (b) sharing within groups containing up to third cousins, and therefore within bigger groups with weaker kin ties or (c) sharing within groups of 8 to 25 members that contain several distinct third-cousin groups, consistent with hunter-gatherer ethnographies (Lee, 2008). When group sizes fall below 8 members then two groups fuse, and when they rise above 25 members, they fission; or (d) sharing within groups like (c), but with the constituent up-to-third-cousin groups reshuffled every five cycles (25 years) to reflect the actual fluidity of group membership; or (e) sharing at the level of the total population, an unrealistic specification that should mimic the Hamilton results. Within the (b) through (e) type groups, the completeness of sharing among versus within the constituent matriarchies or third-cousin groups can be specified, but typically half of food acquired by a kin group is consumed by it directly, and half is shared with other kin groups. The general setup is guided by descriptions such as Hill and Hurtado (2009): “food provisioning is ubiquitous, generally biased in favour of helping families with large dependency loads and not limited to kin assistance.”
Food consumption and the transfers implicit in these sharing arrangements affect reproductive fitness because each individual’s fertility and mortality are related to its food consumption. These relationships are calibrated on estimated relations in historical demographic data (Lee, 2008).
Age distributions within sharing groups reflect stochastic births and deaths. Mutations occur randomly at each birth so there is genetic heterogeneity within groups and among kin. Catastrophic effects of maternal death may occur, or the consequences of maternal death may be buffered by the presence of other adults in the sharing groups. In these ways the simulations avoid the questions raised above, but do not avoid a degree of circularity. They can assess the underlying coherence and consistency of an age-patterned set of behaviors—fertility, mortality, and transfers. However, they are based on the observed age shapes of fertility and productivity as inputs.
The basic result is that starting from a flat and very low mortality schedule, after 75,000 years of simulated evolution along the lines described above, the age-specific mortality schedule assumes a shape much like that derived analytically in Lee (2003) and shown in Figure 10-1. The simulated mortality schedules are shown in Figure 10-2, each corresponding to a different assumed social-sharing rule. The schedule labeled “population level
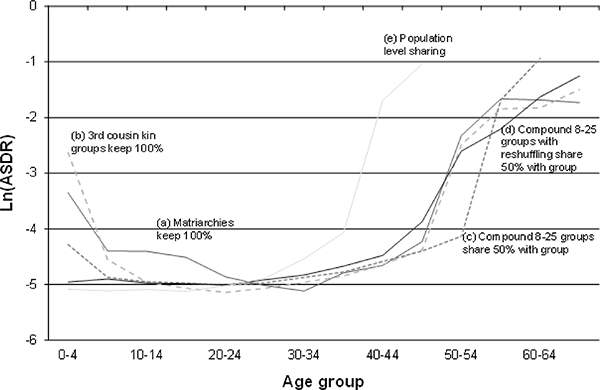
FIGURE 10-2 Simulated evolution of mortality by social arrangement after 75,000 years.
SOURCE: Author’s simulations as described in text and Lee (2008) and Lee and Boe (2007).
sharing” assumes that all food is pooled and shared at the level of the total population. In this case, there is no selective advantage to post-reproductive life since sharing is not at all kin-based. In this case, evolved adult mortality conforms to Hamilton’s theory: It is shaped solely by remaining lifetime fertility. It rises sharply in the 40s and is completely flat up to sexual maturity.
Schedule (e) mimics the Hamilton results as it should, because here only fertility matters for reproductive fitness, while the transfers that allow the young to survive and grow come from the population at large whether relatives live or die. Schedule (a) assumes food is pooled and shared within matriarchal groups, as defined earlier. In this case, mortality declines after birth and post-reproductive survival emerges. The bulge in mortality from age 10 to 20 reflects child death following maternal death. Schedule (b) assumes all sharing is within third-cousin groups and none between. Here maternal deaths are buffered by help from relatives so the 10-20 mortality bulge disappears, while post-reproductive survival is still selected. Schedules (c) and (d) come closer to actual hunter-gatherer arrangements, and they look quite similar to one another and to the theoretical schedules in Figure 10-1. The different levels and age patterns of mortality above age 45 are largely explained by different probabilities that a surviving adult has
an orphaned grandchild, as shown by a separate analysis of the simulation results.
It is interesting that infant and child mortality is substantially higher in the more closely related groups with 100 percent sharing than in those with less sharing. This happens because with 100 percent sharing within a kin group, when an infant dies, all the foregone future costs of rearing that child are recaptured and reabsorbed by kin and can be used to invest more in siblings or to better nourish the adults. When 50 percent of resources are shared outside the group and received from the other groups, only 50 percent of the foregone future costs of rearing a young child can be recovered by the kin if the child dies. There is therefore greater selective pressure to avoid the child death. Fertility would be expected to be higher when there is sharing outside the kin group, because the costs of raising an incremental child are partially born by non-kin.
WHICH SOCIAL ARRANGEMENTS HAVE THE HIGHEST FITNESS?
In Lee and Boe (2007), we simulated co-existing subpopulations with different social arrangements that interacted only through the influence of total population density (for the sum of subpopulations with all social arrangements) on foraging efficiency. The subpopulations remained totally separate and competed only through their abilities, due to the sharing arrangements, to reproduce and grow at a given density. Other than the sharing arrangements, each evolved over time subject to identical functional constraints and mutation rates. There were three Matriarchal subpopulations, three subpopulations with food sharing within groups with relatedness up to Third Cousin, and three subpopulations with larger sharing groups of 8 to 25 individuals composed of several unrelated Third Cousin groups, in which these groups pooled half of their food and kept half to share with their up-to-Third-Cousin kin. The simulations were run for 15,000 years. Inclusion of three groups of each kind allowed us to assess the role of randomness in the success of the social arrangement.
The clear results for the first 3,000 years are shown in Figure 10-3. The social arrangements in which groups had the fewest sharing adults, that is the Matriarchies with an average of 1.8 members including offspring, went extinct first. The arrangements with Third Cousin groups that had an average of 4.8 members went extinct next. The arrangements with larger sharing groups of 8 to 25 members and an average of 15 members from an average of three Third Cousin subgroups did best and were positively selected. Presumably sharing raised fitness through maternal life insurance and through smoothing of dependency burdens. Social arrangements with broader sharing are able to reproduce successfully at higher densities.
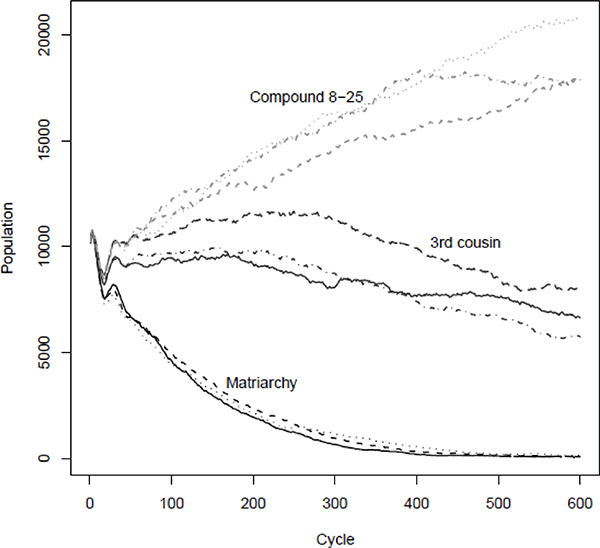
FIGURE 10-3 Evolutionary competition through density pressure on foraging among three types of sharing groups: Matriarchal, Third Cousins, and Compound Sharing Groups with Third Cousin components sharing 50-50 in groups of 8-25.
NOTE: Three subpopulations of each kind are simulated, for a total of nine, over 3000 cycles (15,000 years). Foraging efficiency depends on density of total population of the nine groups, and the kind of group that is able maintain itself at the highest density is selected, while the others go extinct.
SOURCE: Author’s simulations as described in text and Lee (2008) and Lee and Boe (2007).
That said, the effect of sharing is to smooth over the demographic variance. In some circumstances, variance might promote fitness rather than inhibit it through inefficiencies. In the simulations shown in Figure 10-3, consumption in childhood was specified to have no effect on productivity in adulthood. In other simulations in which childhood consumption was specified to raise adult foraging productivity, the outcome was the opposite of Figure 10-3: The large sharing groups went extinct first, the intermediate Third Cousin groups went extinct next, and the smallest and most variable
Matriarchal groups won out. The lesson is that variance can be useful, particularly when there is positive feedback. Matriarchal offspring have high variance in consumption while young. For example, high consumption could arise when demographic randomness generates a low dependency ratio due to only one offspring and both a mother and grandmother surviving, and perhaps an aunt. A high-consumption child in the model grows up, by assumption, to be more successful at foraging, and therefore its offspring will themselves consume more and become more successful adults. To quote Clutton-Brock (1991, p. 255): “… the effects of variation in parental investment are rarely confined to a single stage of the offspring’s life history or a single component of its fitness and can frequently be identified throughout its entire breeding career. Some studies show that the benefits … can be transferred across generations, influencing the phenotype and fitness of grandchildren….” Such intergenerational transmission happens in the simulation model (with appropriate settings) and can be strong enough to reward the higher variance in offspring consumption in the Matriarchal units.
The message is that social smoothing of risks can indeed raise fitness, but it can also ensure mediocrity by smoothing out both high success and dismal failure and, in some circumstances, mediocrity may lose.
OPTIMAL LIFE HISTORIES WITH INTERGENERATIONAL TRANSFERS
To get at the more fundamental forces shaping the observed age patterns, I turn to a different approach: finding the optimal life history using analytic methods. This approach avoids the circularity of the mutation accumulation and sensitivity analysis approach. However, one must begin by assuming underlying biological constraints and relationships, and then consider how the organism acquires energy and allocates it among growth, survival, reproduction, and transfers over its lifetime. Here, deleterious mutations are ignored, and the implicit assumption is that a species can evolve to the optimal life history by positive selection on a sufficient variety of beneficial mutations. Because evolution through positive selection of beneficial mutations is implicitly assumed and the analysis is atemporal, the strength of selection does not matter. Only the sign, and not the magnitude of sensitivities, matters. Furthermore, selection is always forward-looking and is therefore conditional on survival to a given age, whereas sensitivities are assessed at birth, as in Hamilton’s (1966) analysis. Some baseline conditions for the optimization must be assumed, such as that the organism has a deterministic growth pattern (first growth with no reproduction and then reproduction with no growth) or that the organism has oocytes that tend to decay with age and therefore faces rising costs of fertility with age.
This optimal life history approach is taken in my work with Chu and Chien. While the earlier literature on optimal life histories assumes optimization is carried out over an individual life history, with an organism constrained to consume no more than it produces at each moment, our approach is distinctive in relaxing this constraint by permitting intergenerational transfers. Despite the very different theoretical approaches and assumptions relative to the transfer model described earlier, there are many formal similarities in the results, and the central elements that emerge from the sensitivity approach occur again in the optimal life history approach.
Here are some basic results on the age-shape of mortality across the life span (Chu et al., 2008). If a linear energy budget constraint is assumed for energy expenditures on survival, fertility, and growth, then the optimal life history involves growth without fertility up to some age of maturity, and fertility without growth thereafter, as has been known for some time. This is known as determinate growth, which is characteristic of mammals and birds among others, in contrast to most fish, reptiles, and plants. The results described below are also true of a model with non-linear budget constraints, provided these entail determinate growth.
Consider mortality from birth until maturity. We find that survival from age a to a + 1, pa, will be proportional to an expression that has in the numerator the weighted sum of two terms. The first term is expected remaining lifetime fertility at age x, the Hamilton effect, which is constant across all juvenile ages. The second term is expected remaining lifetime transfers to others at age x, the Transfer effect, which rises with age since adult transfers to be made remain unchanged while fewer future transfers will be received as a juvenile approaches maturity. This weighted average is very close to the sensitivity analysis result of Lee (2003). However, there is additionally the possibility of investing in growth before maturity, which is in the denominator of the expression. An investment in growth has a compounded effect because size enables increased energy through foraging and that increased energy is then optimally expended, perhaps on further growth. The compounding is greater for expenditures made at younger ages, with more time for compounding before adulthood. Thus, the denominator declines with age. The rising numerator divided by the falling denominator implies rising survival with age and, therefore, mortality declines from a high level at birth up to maturity.
Now consider adult mortality. The numerator is exactly the same, a weighted sum of the Hamilton effect that declines to zero when fertility stops and the Transfer effect that gradually declines but remains positive throughout adult life (unless there are net transfers from younger to older in old age). Thus the numerator declines with age but remains positive after fertility ceases. This points to continued survival after completion of fertility as older people raise, through transfers, the fertility and survival of their
descendants and thereby raise their own fitness. By itself, this would imply that survival rates at each adult age were declining and mortality rising.
However, in optimal life history analysis. optimization is forward-looking from each given age, unlike sensitivity analysis, which evaluates a perturbation’s impact at birth. For this reason, impact sensitivities for mortality perturbations late in life tend to be small, since relatively few survive to be affected by the perturbation. By contrast, the optimization expression has the probability of survival from birth to age a in the denominator, and this survival declines with age. The declining denominator tends to offset the declining Hamilton and Transfer effects in the numerator, postponing the increase in mortality following maturity and slowing the pace of mortality increase. While the U-shape of mortality remains a prediction of the model, the upswing may start later than maturity. The age schedule of optimal mortality may have a bathtub shape rather than a J-shape, with flat mortality from, perhaps, age 20 to 40, as often found for contemporary hunter-gatherer populations (Gurven and Kaplan, 2007). This flatness could also result from a violation of our determinate growth assumption. Foraging productivity may continue to rise during adult years due to accumulating skills and experience, rather than being fixed at maturity. For example, the hunting productivity of male hunter-gatherers has been found to rise to a peak at age 40.
THE EVOLUTION OF TRANSFERS AND LONGEVITY
In Chu and Lee (2006), preconditions favoring the evolution of transfers include (a) the initial ratio of adults to juveniles is high (and therefore life expectancy is high relative to age of maturity), (b) greater productive efficiency of adults relative to juveniles per unit of body size (and therefore it is efficient when young receive transfers of food that is more easily acquired by adults), and (c) greater efficiency of juveniles in converting energy into body size. Initial low mortality and long life favors the selection of transfers from old to young through (a), but lower mortality itself evolves farther when transfers are greater as discussed earlier. Therefore, longevity and transfers co-evolve in a mutually reinforcing way, as argued in Lee (2003).
THE EVOLUTION OF MENOPAUSE AND INTER-ADULT TRANSFERS
In Chu and Lee (2013), we consider the comparative advantages of younger women and older women, and the efficient division of labor between them. Suppose that for young women, the ratio of productivity in foraging to that in childcare is greater than the corresponding ratio for older women such as their mothers. (If the opposite is true, slightly dif-
ferent results follow, but the principles involved are the same.) Then, in a hypothetical state before the evolution of menopause, it would be efficient if the grandmother spent more time in camp rearing the children and reduced time spent foraging for herself and her children, while the younger mother increased her foraging time and left her children to the care of the grandmother. This efficient division of labor would raise the fitness of both grandmother and her adult daughter, and is the first stage of specialization.
However, the grandmother is less efficient in childbearing as well, with higher costs per birth or lower quality due to the declining quality of oocytes with age. Her continuing childbearing also limits her specialization in providing childcare for her daughter’s children. There could be fitness gains for both grandmother and mother if the older woman reduced her fertility and increased her childcare, with the younger mother providing more food. We show that evolution could move the human life history in this direction of further specialization, eventually reaching the reproductive “corner” outcome where the older woman has zero fertility (menopause) and the younger woman has higher fertility, shorter birth intervals, and greater reliance on the older woman’s assistance to cope with the increased dependency burden. The older woman would continue to forage for her own subsistence and have no dependent children of her own to feed. This is the second stage of specialization.
However, there is still room for further efficiency gain and increased fitness if the grandmother reduces her foraging even more and relies on food provided by the younger woman, in the limit ceasing to forage altogether and subsisting entirely on upward transfers of food from the younger woman. This is the third stage of specialization.
We show in Chu and Lee (2013) that such a life history pattern could yield higher fitness. Furthermore, under certain parameter restrictions in a two-sex population, the mutations causing this behavior could successfully invade the population. That is, we show that individual selection and not just group selection could lead to this outcome. In this way, menopause, age-specific division of labor, and intergenerational transfers among adults could evolve together. But has this ever in fact occurred?
Ethnographic evidence reviewed in Chu and Lee (2013) suggests that at younger old ages, grandmothers may specialize in foraging rather than childcare, but at older old ages a pattern like the one described above does occur for both men and women. Some species of toothed whales appear to have menopause and extensive post-reproductive survival. The older adults may guard the young while the parents dive to hunt. The adults bring up food to the surface for their young and if some were consumed by the grandparents (I know of no evidence on this point), this would be consistent with the scenario described for humans. However, although such behaviors appear possible and not inconsistent with known facts (for example, for
pilot whales, Orcas, or Sperm whales), they are very far from established, and research on whale life histories is very difficult. Life histories of some other species, such as African hunting dogs or naked mole rats, share some elements of the theory sketched above, although in these the division of labor and reproductive specialization are not based on age but on dominance that may be transitory or by size. There are also some similarities to the reproductive and foraging specialization in eusocial insects.
INTERGENERATIONAL TRANSFERS AND SEXUAL DIMORPHISM
The classic theory of sexual dimorphism, dating to Darwin, begins with the observation that females invest much more heavily in reproduction than do males, starting with the trivial cost of sperm compared to the female egg, and continuing with the costs of pregnancy for viviparous species and birds, and then post-birth care, mammalian lactation, and transfers (although transfers are sometimes shared with males). Consequently, fitness is constrained by the female’s resources while the male has resources to spare. Here, the evolutionary path forks. In one direction lie pair bonding, relative monogamy, greater paternity certainty, and male investment of his surplus resources in rearing the offspring, as in the case of humans and many other species.
Down the other fork, the males’ surplus energy is devoted to competition for reproductive access to females. Female preferences shape the form of male-male competition, which may be combat based on size, teeth, and horns; sperm competition with large testes and spermicidal weapons; competition by display of colors, claws, tails, dances, or noises; and so on. In this case the male seeks to mate with multiple females, and paternity is highly uncertain. Down the monogamous first fork, sexual dimorphism is slight. Down the second fork, dimorphism flourishes. In Chu and Lee (2012), we develop this classic theory in mathematical form, focusing on the second dimorphic fork, and draw out some new implications. Because successful males father many offspring while most males father none, and because of high paternity uncertainty, the father’s transfers would have small fitness value to him, and he has greater incentives to compete for more females, to guard access to the females he already has, and perhaps to protect his purported offspring from lethal assault by other males.
TIME PREFERENCE, TRANSFERS, AND THE MARGINAL VALUE OF ENERGY
Organisms must frequently make decisions about tradeoffs across time, or intertemporal allocations. Most of these are developmental and physiological, and have evolved, but others are strategic and a matter of choice
and decision. Eat a nut now or store it for later? Wait to grow bigger or start reproduction now? Disperse now to breed or postpone this step to remain as a helper at the nest? Search longer for a mate or accept this one now? Bear another litter this year or wait until next?
In general, an incremental unit of energy may have different incremental fitness value at different ages over the lifespan. Often it would have greater fitness impact early in life when it can enhance survival and growth than it would in later adult life when energy is more easily acquired and the perils of early life have already been avoided. Time preference between ages i and j is measured by the number of incremental units of energy at age j an organism could trade for one unit of energy at age i, with no change in fitness (along a fitness isoquant).
Intergenerational transfers evolve in species that have a high rate of time preference between early and adult years. The transfer of resources from the adult to the offspring raises the marginal value of energy to the giver and reduces it for the receiver, tending to equalize them and to move the rate of time preference towards unity across all ages. Paternity uncertainty and the possibility of parental death would likely prevent complete equalization. Chu et al. (2010) develop these ideas in a formal analysis. This general line of theory was pioneered by Alan Rogers and followed by Sozou and Robson.
INTERGENERATIONAL TRANSFERS AND COOPERATION
Individuals in many species cooperate with one another, and their behavior is a mixture of competition and collaboration. A sizeable literature in various disciplines (Bowles and Gintis, 2011; Nowak, 2011) discusses how cooperation could evolve given that defection would often seem to give an advantage, at least in the short term. Cooperation is advantageous because it makes production more efficient, because risk-sharing shifts some resources from those for whom they have a lower marginal value to those for whom it is higher, and because it aids in defense and in acquisition of territory.
The literature appears to be largely about cooperation among adults. An adult who cooperates will on average experience a net individual gain as a consequence. By contrast, an individual adult who makes an intergenerational transfer of food or care to her offspring is permanently worse off as an individual as a consequence. Of course, she raises her reproductive fitness thereby and is therefore better off indirectly, which is why the behavior of transferring to her offspring was selected. But her transfer is not an effort to improve her individual situation. Her recipient offspring will not return the favor to her parent, but will instead herself grow and mature and later make similar transfers to her own offspring. To the extent that the parent
and offspring do help one another reciprocally, the behavior is cooperative or an exchange, not a transfer.
Cooperation resembles a mutually advantageous exchange more than an altruistic act or transfer. Unlike market exchanges, the terms of the exchange are not enforced by rules of the market, police, and a judicial and penal system. Instead, reciprocal behavior develops in the context of repeated interactions that are maintained through mutual benefits, an evolved sense of fairness, and occasional punishment of offenders.
Nowak (2011) discusses five mechanisms through which cooperation could evolve: reciprocity, indirect reciprocity, spatial selection, multilevel selection, and kin selection. Bowles and Gintis (2011, p. 2) define cooperation as “… engaging with others in a mutually beneficial activity.” As an example, consider a pair of unrelated foragers with families to feed, who are each disabled by illness or injury on 20 percent of the potential foraging days (as estimated in Hill and Hurtado, 2009). They might agree to provide food for one another’s family on these days of disability, which would be an example of reciprocity, a form of cooperation that could evolve for foragers who were repeatedly in contact over a long time. (A similar outcome could be achieved in a different institutional setting through formal insurance markets or through money.) Both the foragers and their families are better off with this cooperative arrangement than without.
Intergenerational transfers benefit the recipient but not the giver—other than indirectly through reproductive fitness. Intergenerational transfers are never reciprocal, since by definition there is no quid pro quo. For example, young birds sometimes remain at their parents’ breeding site rather than dispersing and assist their parents in rearing their younger siblings as so-called “helpers at the nest.” It appears that they do this in exchange for being allowed to avoid risky dispersal by staying at the breeding site, although they may gain a bit through inclusive fitness as well. This is not a true transfer.
In the literature on cooperation, I find very little discussion of fertility, survival, or reproductive fitness (Bowles and Gintis, 2011; Nowak, 2011). To understand the evolution of life histories, in the sense of age-patterning of fertility, mortality, growth, investment in offspring, and behavior over the life span, the evolution of intergenerational relations must be considered. There is remarkably little overlap between this topic and the evolution of cooperation.
COOPERATION AND INTERGENERATIONAL TRANSFERS AS REALLOCATION SYSTEMS
At a general and abstract level, we can consider the possible ways of shifting resources in a population across age and over time. There are only three ways (Bommier and Lee, 2003: (1) saving, accumulation, and then dis-
saving; (2) borrowing, lending, repaying—that is, intertemporal exchange; and (3) transfers across ages or generations. The first, accumulation, can shift resources only forward in time: accumulation (of nuts, berries, a nest or beaver dam, or 401K account) necessarily precedes consumption of the asset or its services. This cannot help a juvenile to grow. The second, intertemporal exchange, is limited in its ability to shift resources from one age to another. It could shift resources to the young only if they repaid those resources at a later age to their elderly parents, which is inconsistent with maximization of reproductive fitness and does not occur in nature, although it does occur in agricultural and industrial human societies. The third possibility, intergenerational transfers, is the only mechanism capable of shifting resources to children to promote their survival and development. A parent may accumulate an asset or capital good, of course, and then give it as a lump-sum transfer to an offspring at birth or at laying, for example a dung ball or paralyzed prey on which an egg is laid. This nonetheless remains an intergenerational transfer.
Cooperation is based on reciprocity. One consequence is that at any instant, the average age of all those contributing and all those benefiting from a cooperative situation are equal. This is obviously true when every participant also simultaneously benefits, since the ages of the contributor and beneficiary are then one and the same. Consider the less obvious case of sharing the risk of disability through food sharing as described above. Suppose hunter X is age Ax, and Y is age Ay. On one day X gives food to Y, and d days later Y gives equal food to X. The average age of givers is [Ax + (Ay + d)]/2. The average age of receiving food is [Ay + (Ax + d)]/2. Clearly these are equal, and this will be so no matter how circuitous and indirect is the path by which X eventually receives the reciprocal gift. (I call these “gifts” here, but clearly they are not gifts; they are essentially loans to be repaid, directly or indirectly.)
Contrast the case of intergenerational transfers. Suppose each generation is g years older than the next. A parent in generation X makes a transfer to her offspring in generation Y who eventually makes a transfer to her offspring in generation Z. The transfer is always made to someone g years younger than the donor parent. The average age of donors is g years greater than the average age of recipients.
The average participant in the cooperative arrangement, looking to the future, expects to contribute and receive equal amounts. In the language of reallocation systems, there is a zero aggregate credit balance in this system (Lee, 1994; Bommier and Lee, 2002). Individuals who have contributed more in the past than they received, and therefore who expect to receive more in the future than they will contribute, are exactly balanced by those in the opposite situation. The aggregate credit balance is proportional to the difference in average ages of receiving and contributing, which is zero.
The situation is different in the case of intergenerational transfers. Juveniles have already received transfers from their parents, and even if they expect to receive more in the future, at every age after birth these expectations will be outweighed by the transfers they expect to make later when they are themselves parents. For this reason the young have a negative credit balance. As for adults, they expect to continue to make transfers themselves for the rest of their lives so they also have a negative credit balance. In fact, every member of the population expects to make more transfers in the future than will be received. The aggregate credit balance is therefore negative, and indeed is proportional to the difference in average ages of receiving and donating, which is g—one generation. The system of intergenerational transfers enables what would be impossible through exchange, cooperation, and reciprocity. It enables the young to consume more than they produce through their own foraging efforts and therefore to grow faster, larger, and more complex than otherwise. As adults they will never repay their parents, since they received a transfer with no quid pro quo. Instead they will enable their own children in the same was as they were earlier enabled themselves. These points are established in mathematical models and analyses in Lee (1994) and elsewhere.
And what about cooperative breeding? The part of cooperative breeding that involves relatives of the parents and offspring is intergenerational transfers, not cooperation. But in some species, including humans (Gurven 2004; Hrdy 2009), African hunting dogs (Creel and Creel, 2002), and acorn woodpeckers (Koenig and Mumme, 1987), for example, non-kin may assist in provisioning and caring for the young of others. This is a form of cooperative behavior in which the non-kin will expect to receive similar support when they are in similar circumstances, although not necessarily from the same adults that they assisted. In human hunter-gatherer sharing groups, for example, families with higher dependency ratios (more children relative to adults) often receive extra help from others including non-kin (Gurven, 2004).
Lee and Mason (2011, p. 88) report differences between average ages of consuming and producing in hunter-gatherer groups of 11 years and 10 years, based on bio-anthropological studies of contemporary groups in the Amazon Basin (Aché, Macheguenga, and Piro: Kaplan 1994) and the Kalahari (!Kung: Howell, 2010). Multiplying these by one over the aggregate share of transfers in consumption, or by roughly a factor of two, gives the corresponding difference between the average ages of receiving and giving transfers, which would then be 22 and 20 years, respectively. Lee and Mason (2011, p. 92) give estimates of these age differences for private transfers in 17 countries ranging from low-income developing countries to high-income industrial nations, and all are quite similar to these results for hunter-gatherers. Once public transfers are taken into account, however,
the results are dramatically different. Now the average age differences are much shorter, particularly in the rich nations. In two cases (Austria and Hungary), the direction is reversed, with average transfers flowing upwards from young to old rather than the reverse (Lee and Mason, 2011, p. 101). In most rich nations, the average age of consumption now exceeds the average age of earning labor income, and as populations age, these trends will be reinforced.
DIRECT AND INDIRECT GENETIC EFFECTS AND INTERGENERATIONAL TRANSFERS
Intergenerational transfers are all instances of indirect genetic effects, to the extent that the transfer behavior is genetically influenced and therefore heritable. Review articles on indirect gene effects often give maternal care of offspring, the most basic kind of intergenerational transfer, as a lead example of an indirect genetic effect (Wolf et al, 1998; Cheverud, 2003). Certainly the quality and quantity of this care influences the survival, growth, and development of the offspring, in addition to the direct genetic effects operating in the offspring to influence height, speed of growth, body mass, and so on. Later in life, the continued survival and presence of the mother influences her daughters’ fertility and the survival and growth of the mother’s grandoffspring (Sear and Mace, 2008; Coall and Hertwig, 2011). When the effects of the presence of the mother or grandmother on the survival, growth, development, and reproduction of the offspring are not additive but involve interactions, then there are gene-on-gene (GxG) epigenetic effects that arise through intergenerational transfers. In this case, selection acts on both genes, for example, of mother and offspring, through the offspring trait in question. Therefore the evolution of intergenerational transfer behavior will act through both direct and indirect genetic effects.
HYPOTHESES
- Longer nutritional and other dependence of offspring on one or both parents is associated across species with longer post-reproductive survival. The length of dependence must be substantial in relation to the lifespan of the mother or parents. In the case of humans, years of dependence for a child, say 18-20 years, is about half of total life expectancy of individuals surviving to maturity. When offspring care is limited to a short time followed by more reproduction, it will be difficult or impossible to separate its effects on longevity from the effects of fertility itself.
- The male or female parent that is more heavily involved in care and transfers to offspring (if either) will have longer life expectancy,
-
with due allowance for mortality risks incurred through care and foraging, and for mortality risks incurred in intra-specific competition for access to the other sex. For example, when only the female bird cares for the chicks, she thereby exposes herself to risks of predation while obtaining food for chicks and while attempting to defend the nest against predators. Whether or not she has evolved a lower level of senescent mortality, she typically has higher overall mortality. Unfortunately, it will typically be impossible to make “due allowance” (although analysis of animals in captivity may help), so it may be impossible to test this hypothesis. A study of sex differences in longevity of apes did find that the sex mainly responsible for offspring care did live longer.
- The period of time in which mortality falls following birth should be associated with the duration of dependency. Unfortunately, it will be difficult to distinguish this hypothesis from the hypothesis that mortality is lowest when reproductive value is highest.
- In species in which mothers or parents are fully responsible for provisioning and caring for their offspring, in contrast to otherwise similar species in which provisioning and care are provided cooperatively, infant and juvenile mortality will be intrinsically lower in the cooperative groups, by which I mean that mortality will be genetically lower and, therefore, lower than the sharing of food and care could itself explain.
- Mortality follows a U-shaped pattern, declining from a high level at birth to a low point at sexual/economic maturity, rising to early adulthood and either continuing to rise throughout the rest of life or possibly having a flatter segment for a while following maturity. The flatness may arise in an optimal life history because even in species with deterministic growth like mammals, the accumulation of skills and knowledge may continue after somatic growth has ceased, so the opportunity set may continue to expand (Chu et al., 2008, and references therein). This is not relevant for late life plateaus in mortality.
- Intergenerational transfers are more likely to evolve in species that (a) have higher ratios of life table person years lived in adulthood to juvenile person years lived; (b) have higher ratios of adult foraging productivity to juvenile productivity; (c) have more efficient conversion of calories received through transfers into somatic growth.
EMPIRICAL ANALYSIS
Consider conspecifics of varying ages and sex, living in a group. They may compete for breeding sites and for food, and members of some age-
sex groups may tend to kill members of other age-sex groups. Offspring in a family may compete with one another for parental care. Some members may assist all others as sentinels or in combating other groups, or defending the group from predators. Some age-sex members may store information and use it to guide group decisions. Others may lead the group. Some may provide food or care for others. It would be impossible to account for all the relevant pathways through which one individual affects the survival and fertility of another. However, the interaction of all these different influences will be a net effect of an incremental member of age x and sex s on the fertility or mortality or fitness of the average member of age a and sex j. A matrix can be formed with all these net effect parameters, for example, for the case of fertility f, where the i,j element is df(i)/dN(j), where N(j) is the number or proportion of group members of age j and f(i) is the average fertility of females age i. This describes an effect evaluated at some given number or population share by age and sex, but the effect may vary depending on the absolute and relative sizes of the cells of the matrix, so we may need more elaborate functions as elements of the matrix. Information on kinship could be added as well, leading to a three-dimensional matrix. In principle, such a matrix could be estimated from data, although there are many pitfalls. A matrix like this expresses the sensitivities described earlier. These sensitivities might then be argued to shape the evolution of life histories.
A matrix of effects from theory combined with focused experiments or field studies could be developed, as has been done in a partial way focusing on optimal clutch size for birds, as well as an attempt to estimate the matrix of effects empirically from observations on a group over time. A coefficient matrix of this sort is what economists would call a “reduced form.” It does not incorporate theoretical insights. The virtue of this approach is that it is comprehensive. It measures the net outcome of all ways in which the size of one subgroup affects the other. Instead of simple derivatives in each cell, the cells could contain quadratic or other functions, and population shares or ratios instead of absolute numbers could be used to compute sensitivities.
There is a substantial literature for humans on the effect of the presence of specific kin on the survival or fertility of a reference individual in historical and anthropological populations, as surveyed by Coall and Hertwig (2011) and Sear and Mace (2008). The consistent finding is that the mother has a large beneficial effect on outcomes for children while the father does not; for grandparents, the ranking by size of beneficial effect is maternal grandmother, maternal grandfather, paternal grandmother, and paternal grandfather, generally interpreted as reflecting degrees of certainty about relatedness. Some years ago, I estimated matrices for acorn woodpeckers based on a large longitudinal dataset created by Walter Koenig covering many breeding sites and individuals, to which he generously gave
me access. However, there are serious issues of identification in studies of this sort—shared genes, shared environment, and so on.
This sketch of population dynamics as it is influenced by numbers in different age-sex bins is very general. There can be a focus on particular theories and accounting frameworks. The Leslie matrix incorporates only demographic accounting identities, on the assumption that vital rates are independent of the numbers in the bins. This approach is based on Lotka’s equation and its precursors. It was used by Hamilton (1966) to develop his theory of how natural selection molds senescence, based on the idea that the force of selection against deleterious mutations would be proportional to the sensitivity of the intrinsic rate of natural increase to a perturbation in the vital rate affected by the mutation. The optimal clutch size analysis would go a step beyond this Hamilton-Lotka approach by taking into account the inter-sibling competition for parental resources and quality of average offspring as a function of the number of offspring. But there are many other kinds of effects that could be incorporated, such as those arising from density dependence if the territory is bounded.
Earlier I described the model of Lee (2003, 2008), which spelled out a structural theoretical model in which incremental individuals affected others not only through the classic demographic accounting identities, but also through density dependence in food acquisition and dependency in kin groups and broader sharing groups. This dependence indirectly affected fertility and mortality of all members of the broader population, as well as had stronger and more focused effects on kin and sharing group members.
Based on these models, a specific matrix of sensitivities could be constructed. Unlike the Leslie matrix, it would have no zeros because it would be filled by indirect effects operating through density, inter-age transfers, and competition for transferred resources. In this setup, genes have both direct and indirect genetic effects on reproductive fitness (Cheverud, 2003; Wolf, 2003), unlike the Lotka setup used by Hamilton in which only direct effects occur. However, other theories could also be considered, each bringing its own set of constraints on the elements of the matrix. It is possible that an empirical approach of this sort would have sufficient power to distinguish between different theories.
ACKNOWLEDGMENTS
My research for this paper was funded by NIA grant R37-AG025247. I am grateful to my collaborators on optimal life history work, Cyrus Chu and H-K Chien, and my collaborator on the work on social arrangements, Carl Boe, and have benefitted from valuable suggestions from reviewers at various stages of the preparation of this paper.
REFERENCES
Bommier, A., and Lee, R. (2003). Overlapping generations models with realistic demography. Journal of Population Economics, 16(1), 135-160.
Bowles, S., and Gintis, H. (2011). A Cooperative Species: Human Reciprocity and Its Evolution. Princeton, NJ: Princeton University Press.
Charlesworth, B. (1994). Evolution in Age-Structured Populations. Cambridge, MA: Cambridge University Press.
Cheverud, J.M. (2003). Evolution in a genetically heritable social environment. Proceedings of the National Academy of Sciences of the United States of America, 100(8), 4357-4359.
Chu, C.Y.C., and Lee, R.D. (2006). The coevolution of intergenerational transfers and longevity: An optimal life history approach. Theoretical Population Biology, 69, 193-201.
Chu, C.Y.C., and Lee, R.D. (2012). Sexual dimorphism and sexual selection: A unified economic analysis. Theoretical Population Biology, 82(4), 355-363.
Chu, C.Y.C., and Lee, R.D. (2013). On the evolution of intergenerational division of labor, menopause and transfers among adults and offspring. Journal of Theoretical Biology, 332, 171-180.
Chu, C.Y.C., Chien, H-k, and Lee, R. (2008). Explaining the optimality of u-shaped age-specific mortality. Theoretical Population Biology, 73, 171-180.
Chu, C.Y.C., Chien, H-k, and Lee, R. (2010). The evolutionary theory of time preferences and intergenerational transfers. Journal of Economic Behavior & Organization, 76(3), 451-464.
Clutton-Brock, T.H. (1991). The Evolution of Parental Care. Princeton, NJ: Princeton University Press.
Coall, D.A., and Hertwig, R. (2009). Grandparental investment: Past, present, and future. Behavioral and Brain Sciences, 33(1), 1-19.
Creel, S., and Creel, N.M. (2002). The African Wild Dog. Princeton, NJ: Princeton University Press.
Gurven, M. (2004). To give and to give not: The behavioral ecology of human food transfers. Behavioral and Brain Sciences, 27(4), 543-583.
Gurven, M., and Kaplan, H. (2007). Longevity among hunter-gatherers: A cross-cultural examination. Population and Development Review, 33(2), 321-365.
Hamilton, W.E. (1966). The molding of senescence by natural selection. Theoretical Population Biology, 12, 12-45.
Hill, K., and Hurtado, M.A. (1996). Ache Life History. New York: Aldine de Gruyter.
Hill, K., and Hurtado, M.A. (2009). Cooperative breeding in South American hunter-gatherers. Proceedings of the Royal Society B: Biological Sciences, 276(1674), 3863-3870.
Hrdy, S.B. (2009). Mothers and Others. Cambridge, MA: Harvard University Press.
Howell, N. (2010). Life histories of the Dobe !Kung. Oakland: University of California Press.
Kaplan, H.S. (1994). Evolutionary and wealth flows theories of fertility: Empirical tests and new models. Population and Development Review, 20(4), 753-791.
Koenig, W.D., and Mumme, R.L. (1987). Population Ecology of the Cooperatively Breeding Woodpecker. Princeton, NJ: Princeton University Press.
Lee, R. (1994). The formal demography of population aging, transfers, and the economic life cycle. In L. Martin and S. Preston (Eds.), The Demography of Aging (pp. 8-49). Committee on Population, Commission of Behavioral and Social Sciences and Education, National Research Council. Washington, DC: National Academy Press.
Lee, R. (2003). Rethinking the evolutionary theory of aging: Transfers, not births, shape senescence in social species. Proceedings of the National Academy of Sciences of the United States of America, 100(16), 9637-9642.
Lee, R. (2008). Sociality, selection and survival: Simulated evolution of mortality with intergenerational transfers and food sharing. Proceedings of the National Academy of Sciences of the United States of America, 105(20), 7124-7128.
Lee, R., and Boe, C. (2007). Intergenerational Transfers, Life Histories and the Evolution of Sociality. Paper presented at the Workshop on Sociality and Longevity, Azores. Department of Demography, University of California, Berkeley.
Lee, R., and Kramer, K. (2002). Children’s economic roles in the Maya family life cycle: Cain, Caldwell, and Chayanov revisited. Population and Development Review, 28(3), 475-499.
Lee, R., and Mason, A. (2011). Population Aging and the Generational Economy: A Global Perspective. Cheltenham, UK: Edward Elgar.
Nowak, M.A. (2011). Super Cooperators: Altruism, Evolution, and Why We Need Each Other to Succeed. New York: Free Press, Simon and Schuster.
Sear, R., and Mace, R. (2008). Who keeps children alive? A review of the effects of kin on child survival. Evolution and Human Behavior, 29, 1-18.
Stearns, S.C. (1992). The Evolution of Life Histories. New York: Oxford University Press.
Wolf, J.B. (2003). Genetic architecture and evolutionary constraint when the environment contains genes. Proceedings of the National Academy of Sciences of the United States of America, 100(8), 4655-4660.
Wolf, J.B., Brodie, E., Cheverud, J., Moore, A., and Wade, M. (1998). Evolutionary consequences of indirect genetic effects. Trends in Ecology & Evolution, 13(2), 64-69.
























