David A. W. Miller, Fredric J. Janzen, Gary M. Fellers, Patrick M. Kleeman, and Anne M. Bronikowski
INTRODUCTION
Evolution acts to shape aging rates within the set of ecological and organismal constraints that individual species experience. Biodemography seeks to understand the shape of age-dependent reproductive and mortality patterns and how they are impacted by these constraints (Carey and Vaupel, 2006). Although model laboratory organisms have been crucial to understanding patterns and causes of aging, there is great value in also studying the underlying evolutionary and ecological forces that shape rates of aging in the environment in which they evolved. In this paper we examine the biodemography of wild populations of three species (a turtle, a frog, and a snake). All are ectotherms, an understudied subset of the vertebrate taxa for understanding aging. Low metabolic rates coupled with a tendency for indeterminate growth, and thus increasing fecundity with age, lead to the prediction that general patterns of aging should be slower in these taxa. We focus on age-dependent differences in mortality (i.e., the shape or trajectory of mortality), describing basic patterns and determining how early-life and late-life exposure to poor environmental conditions shape these patterns.
Classic evolutionary theory of aging predicts that the declining power of natural selection with advancing age will mold the age-dependent trajectory of mortality for a species, or the rate of aging (Hamilton, 1966; Promislow and Bronikowski, 2006). This occurs because fewer individuals are expected to survive to later age-classes due to extrinsic sources of death, and thus alleles that are deleterious at advanced ages become fixed (Medawar,
1946), as do alleles that confer fitness advantages earlier in life despite possible deleterious effects later in life (Williams, 1957). Within this paradigm, a fundamental prediction is the inevitability of senescence as organisms age (Medawar, 1946; Williams, 1957), while relative rates of aging are expected to evolve when the strength of selection is altered across the lifespan (Hamilton, 1966; Promislow and Bronikowski, 2006).
This prediction of universal senescence has been called into question due to problematic assumptions: lack of consideration of the sources of mortality and the role of phenotypic plasticity, and a need for additional mathematical rigor (Abrams, 1993; Lee, 2003; Williams and Day, 2003; Vaupel et al., 2004; Baudisch, 2005; Nussey et al., 2013). At the same time, empirically determined mortality trajectories, such as species-specific Gompertz curves, and notions that animals do not display significant senescence in the wild have been challenged by larger datasets and more sophisticated analyses in many species, including humans (Brunet-Rossinni and Austad, 2006; Carey and Vaupel, 2006; Bronikowski et al., 2011; Jones et al., 2014). Moreover, both the diversity among species and variation within species in the shapes of mortality trajectories have been relatively understudied and remain major unsolved biological questions that have relevance for understanding the evolution of diverse life histories and lifespans (Munch and Mangel, 2006). Taken together, these criticisms and insights suggest that broadening an understanding of the evolution of senescence in diverse taxa, and incorporating analyses that include sex-specific and environment-specific differences, are important for a comprehensive understanding of the constraints governing aging.
General patterns of mortality may differ both among and within species. Important sources of within-species variation are environmental drivers that lead to differences among individuals in age-dependent mortality and reproductive probabilities (Le Galliard et al., 2010; Peterson et al., 2010). The effect of the environment on demography can manifest as immediate impacts on reproduction and survival of individuals and through long-term ontogenetic carry-over effects that may be realized long after the period of exposure (Madsen and Shine, 2000a). In both cases, the environment in which an individual occurs may shape the pattern of mortality senescence. The literature on compensatory growth in vertebrates has numerous examples of the negative effects of early nutritional stress on growth, reproduction, and survival (Metcalfe and Monaghan, 2001; Hector et al., 2012), as well as the converse notion of a “silver spoon” effect of abundant resource availability during the juvenile stage (Madsen and Shine, 2000b; Mangel and Munch, 2005). For example, recent intriguing examples of an early-age nutritional effect is reported in the lizard Zootoco vivipara (Massot and Aragón, 2013), where the first meal of one’s life shapes rates of reproduction and survival months to several years later.
Within populations, individuals born in the same year may share the same early life conditions, which can generate common effects on age-dependent mortality for individuals within these cohorts. Cohort effects may either shift the average mortality rate throughout life or interact with age to affect the rate of senescence (Massot et al., 2011). The effects of common environmental conditions on mortality are not limited to early life, but occur throughout life (Eskew et al., 2010). These again may lead to common shifts in mortality across all ages or affect individuals differently based on age-dependent vulnerability to environmental stress (Wikelski and Thom, 2000). In addition, cohort and later-life effects may interact, so that individuals exposed to poor conditions early in life may respond better or worse to later stressors, depending on whether the early exposure has a priming or a weakening effect. Studying factors related to cohort and annual effects in wild populations provides an opportunity to test the magnitude of environmental influences in shaping age-dependent mortality and thereby clarifies the nuances of evolutionary senescence theory.
Natural selection over evolutionary time periods has led to vertebrate species that vary in lifespan by orders of magnitude (< 1yr – 100+ years) (Austad, 2010), whereas the greatest difference achieved via artifical selection in lab mice has been little more than 2-fold (Bartke et al., 2013). Thus, studies seeking to understand how variable lifespans and senescence evolves need to utilize this natural variation. Ectotherm (cold-blooded) vertebrates, including reptiles and amphibians, demonstrate such extensive variation in lifespan among major lineages and species, and include reports of negligible aging (e.g., Congdon et al., 2003; Jones et al., 2014). Amphibians are best known for their transition from aquatic larvae to semi-terrestrial adults accompanied by transformation of the morphology, gut, and respiration systems. In wild populations of reptiles, the oldest adults are often the most fecund and robust (Paitz et al., 2007; Sparkman et al., 2007) in contrast to most mammals (e.g., Alberts et al., 2013); this is a trait that commonly distinguishes species with indeterminate versus determinate growth. Taken together, low metabolic rates relative to mammals (White et al., 2006), coupled with indeterminate growth and indeterminate fecundity, suggest that aging rates, measured as the shape of age-specific mortality, will be slower in reptiles and amphibians than in mammals and birds (reviewed in Schwartz and Bronikowski, 2010). The extensive variation in ectothermic vertebrates in lifespan indicates that forging new links with biodemography holds exciting promise to illuminate evolutionary mechanisms for the origin and modulation of vertebrate aging patterns.
We examined age-specific mortality in populations of three species that have been studied for at least several generations: the Sierra Nevada yellow-legged frog (hereafter, yellow-legged frog; Rana sierrae), western terrestrial garter snakes (hereafter, garter snake; Thamnophis elegans), and painted
turtles (Chrysemys picta). Data for each study derive from long-term mark-recapture studies where environmental factors influencing demography are well characterized and monitored (Miller et al., 2011; Schwanz et al., 2011 Fellers et al., 2013). For each species, we first examine the general age-dependent mortality trajectory. Then we examine how environmental variation influences age-dependent mortality, by focusing on how shared cohort and annual environmental conditions act and interact on mortality patterns. Our goal is to expand the comparative perspective into aging in nonmodel species.
METHODS
Data Collection
Data for yellow-legged frogs were collected from the Summit Meadow population in Yosemite National Park (Fellers et al., 2013). This species has been studied at that site from 2003-present. Animals were marked during 10-15 days each summer where observers conducted systematic searches of the wetland and captured and marked all animals that were at least 40 mm in length. We conditioned aging analyses on the age of maturation (~6 years in this population; Fellers et al., 2013). Based on extensive analysis of growth rates, which included estimating the distribution of individual size at maturity, we classified all individuals that were less than 51 mm at the time of first capture as known-age individuals that matured in the current year and larger individuals as unknown age. Previous work has shown that water availability (as measured by relative precipitation two years previous) has a strong correlation with population size fluctuations (Fellers et al., 2013). To evaluate the role of precipitation on mortality trajectories, we classified individuals exposed to less than average precipitation during the 2 years prior to maturation as coming from “poor” quality cohorts and above average as being from “good” quality cohorts. Similarly, we classified a year as being a “poor” year when precipitation was less than the long-term average and a “good” year as having above-average precipitation. In this way, animals could be either from poor or good cohorts and poor or good recapture years.
Mark/recapture data for the garter snake are from a long-term study in the northern Sierra Nevada (Lassen County, California), in the Eagle Lake basin and surrounding meadows. These populations have been the object of extensive studies from 1976-present. Previous work has shown strong evidence of life-history divergence among populations along a fast-to-slow pace of life continuum, with low-elevation populations characterized by individuals that grow fast, mature early, and have both higher fecundity and shorter median lifespan than higher elevation populations (Bronikowski
and Vleck, 2010; Robert and Bronikowski, 2010), hereafter referred to as “short-lived” and “long-lived” ecotypes of garter snakes. Although observed maximum life spans do not differ (ca. 18-20 years), the age structure is markedly different with low-elevation populations skewed towards younger individuals. For our analyses, we focused on two populations of the long-lived ecotype and one population of the short-lived ecotype for which we had sufficient power to estimate age-specific mortality. Capture effort varied among years, but substantial effort occurred for all populations from 1979–1988 and 1994–1996; thus we use these years for our analyses. Known-age individuals were defined by age/size relationships. Individuals less than 280 mm in long-lived and 350 mm in short-lived populations were considered young of the year and treated as known-age (1-year-olds), and all individuals initially caught at larger sizes were treated as unknown-age. Previous work showed a strong tie between food availability and demography, with precipitation and its effect on anuran prey and water availability being the major driver of annual variation (Bronikowski and Arnold, 1999). We defined “poor” and “good” years following criteria developed by Miller et al. (2011) as years with less than or greater than 500 mm precipitation in the preceding year. “Poor” cohorts were defined to be cohorts that experienced a “poor” first year and “good” cohorts were the opposite. Like the frogs, animals could be either from poor or good cohorts and poor or good recapture years.
Our painted turtle data are from long-term studies of a nesting population of painted turtles on the Mississippi River between Iowa and Illinois (Whiteside County, Illinois). This area has been monitored since 1988, with mark-recapture beginning in earnest in 1995 (Schwanz et al., 2010; Jergenson et al., 2014; Warner et al., 2014). There are two sources of data: females are primarily captured when they move onto land to construct nests or when they are trapped aquatically in fyke and hoop nets. Males are caught almost exclusively in aquatic traps, with the occasional rare male hand-captured on land. Known-age individuals are identified based on pectoral scute annuli when they first return to the breeding grounds (6-8 years of age for females, 3-5 years of age for males). Less is known about what environmental factors drive annual variation in survival beyond the first year of life, in part because adult survival remains fairly stable across years (Jergenson et al., 2014; Warner et al., 2014). Our primary analyses for this painted turtle population do not consider “good” and “bad” recapture years, although adverse winter conditions likely impact individual survival via anoxia. However, previous work (Schwanz et al., 2010) has detected high variability among annual cohorts in hatching success, which is tied to above-average thermal conditions during embryogenesis. Based on this criterion, we assigned individuals from years where less than two-thirds of intact eggs hatched as “poor” cohorts,
with “good” cohorts in years where greater than two-thirds of intact eggs hatched (Schwanz et al., 2010).
Analyses
We estimated age-specific mortality probabilities using standard mark-recapture methodologies to account for detection uncertainty common to monitoring of wild populations (Lebreton et al., 1992; Williams et al., 2002). We first estimated the age-specific mortality schedule from all individuals pooled within each population (i.e., one population of yellow-legged frogs, three populations of garter snakes, one population of painted turtles), with the yellow-legged frog data partitioned further into males and females. Secondly, using only animals of known age, we related mortality to the covariates of cohort quality (“good” vs. “poor”) and quality of year in which the animal was recaptured (“good” vs. “poor”). Although the effect of sex was not estimable due to statistical power of our sample size in this second analysis, we were able to test for age-specific cohort and year effects (and their interaction) on known-age individuals. Findings from this latter analysis specifically address the question of whether early-life experiences leave a signature on mortality trajectories, and indeed whether this signature manifests differently among animals that experienced “good” vs. “poor” recapture years.
For the first analysis, we estimated age-specific mortality on data pooled over years for each species (i.e., ignoring variation due to cohort and annual effects, but testing for an effect of advancing chronological age) using the program BaSTA (Colchero and Clark, 2012; Colchero et al., 2012). This program fits models using a Bayesian approach that includes both known and unknown age individuals, as well as accounting for incomplete detection. Models were fit using MCMC. For each population, we fit a Gompertz model of accelerating death rate to the data, reasoning that more parameterized models were not justified given the sparse data. A Gompertz model is defined by ux = Aebx where ux is the mortality hazard rate, A is the initial mortality rate (IMR), and b is rate of increasing death probability with advancing age (referred to hereafter as “aging rate”). We fit models using the complete data sets (i.e., that included both known-age and unknown-age individuals). This approach worked well for the yellow-legged frog and painted turtle datasets, and for the long-lived garter snake populations. We were unable to fit models to short-lived snake populations, likely because of the limited capability of BaSTA to deal with detection heterogeneity and the shorter median lifespan and higher mortality of animals in this population. Thus, we could not estimate mortality trajectories for this population. For yellow-legged frogs we were able to estimate separate mortality functions for males and females, while for the painted turtles,
our data were limited to females. For garter snakes, a large proportion of animals of known age-at-first-encounter (i.e., neonates) were of unknown sex. Thus, we could not test for a difference between the sexes for garter snakes in age-specific mortality.
While powerful, the BaSTA approach has limitations, including an inability to deal with cohort and annual effects on survival, and known-age animals that enter a population at later ages. In addition, the approach makes strong assumptions about a lack of emigration and immigration, no relationship between age and detection variation, and no individual heterogeneity. Therefore, to examine cohort and annual effects, we fit Cormack-Jolly-Seber models (Cormack, 1964; Jolly, 1965; Seber, 1965) for known-age individuals using Program MARK (White and Burnham, 1999). For these analyses, we were limited to the known-age component of the dataset. We again were unable to examine sex-related differences in the painted turtles and garter snakes. Due to data constraints, we also did not consider sex-specific differences for yellow-legged frogs. Other analyses show a high degree of congruence in female and male mortality patterns (Fellers et al., 2013; BaSTA analysis in this study). Our ability to fit complex models for age-specific mortality probabilities was limited when simultaneously considering cohort and annual effects; thus, we grouped data into several age-classes rather than using annual age for each species. For yellow-legged frogs, the age-classes were the first year after reaching maturity (i.e., 6 years of age) and greater than 1 year after reaching maturity (> 6 years of age). Following our previous studies (Bronikowski and Arnold, 1999; Miller et al., 2011), we divided garter snakes from the long-lived ecotype into 1-year-olds (neonates), 2-4 year olds (juveniles), and greater than 4 years of age (adults). Likewise for the short-lived ecotype of garter snakes, we divided garter snakes into 1-year-olds (neonates), 2-year-olds (juveniles), and greater than 2 years of age (adults). Finally, adult female painted turtles were divided into less than 2 years after first breeding (young adult, i.e., 6-8 years of age), 2-4 years after first breeding (i.e., 8-10 years of age), and greater than 4 years after first breeding (i.e., 10+ years of age).
We fit survival models that allowed for variation among age-classes, poor and good cohorts, and poor and good years, along with all two-way interactions among these effects. Our global model took the form of
logit(Survival) = Age-Class + Cohort + Year-Quality +
(Age × Cohort) + (Age × Year-Quality) + (Cohort × Year)
where all explanatory variables were categorical. Age-classes for each species are as explained above. Cohort and year effects are divided into poor and good based on criteria defined in the “Data Collection” section above.
For yellow-legged frogs and garter snakes, we were able to include all the factors in the models. Because we were unable to define poor and good years for adult painted turtles based on an environmental variable, we did not test for an effect of “Year” quality, but did test for effects of age and cohort for this species. We fit models for all possible combinations of two-way interactions and main effects and selected among models using AIC. We present model-averaged estimates of parameters to account for uncertainty in the model selection procedures (Burnham and Anderson, 2002).
RESULTS
We successfully fit age-specific Gompertz mortality models to male and female yellow-legged frogs, female painted turtles, and two populations of long-lived garter snakes of both sexes (see Table 13-1, Figure 13-1). Overall mortality was lower and rates of aging slower for painted turtles than for yellow-legged frogs and garter snakes. Mortality trajectories were similar for both sexes in yellow-legged frogs, with the only difference being slightly higher mortality for males than females. Mortality acceleration was similar in both populations of long-lived garter snakes. One difference between these two populations was that the best model for Population 1 included an age-independent (constant) mortality, i.e., a Makeham term, which is additive with the standard Gompertz model (Table 13-1.) Notwithstanding, rates of aging were quite similar among garter snakes and yellow-legged frogs (range: 0.16-0.22), which corresponds to a mortality rate doubling time (MRDT) of 3.2-4.3 years. The MRDT for female painted turtles was 6.8 years, which is significantly slower than for frogs and snakes (Table 13-1).
When fitting models to determine cohort and annual survival effects, with data limited to only known-age individuals, all populations showed differences in mortality among age-classes similar to the Gompertz analysis (see Table 13-2). In addition, whereas long-lived garter snakes and yellow-legged frogs showed strong evidence of cohort and annual effects on mortality, painted turtles were more resilient and were seemingly buffered against years of poor cohort quality and environmental variance (see Figure 13-2). Overall, mortality of yellow-legged frogs decreased strongly in the first year post-maturity. This was pronounced and consistent for animals from good quality cohorts, regardless of whether their recapture year was of poor or good quality. For individuals who were in cohorts from poor quality years, mortality increased significantly when these poor cohorts experienced poor resource years (Figure 13-2). Growth is still rapid during this first year post-maturation, perhaps contributing to the added mortality in this latter group (Fellers et al., 2013).
TABLE 13-1 Information for Three Species of Ectothermic Vertebrates for Age-Specific Mortality Analyses Including Sample Sizes and Gompertz Model Parameters
| Species | Study Years | Group | N | Gompertz Model Parameters (ux = Aebx) | Oldest Observed Age | Lifespan (yrs) (given survival to maturation) | ||
| A (IMR /yr) (CI) | b (CI) | Mean | 90% | |||||
| Sierra Nevada Yellow-legged Frog (Rana sierra) | 2003-2013 | Male | 543 | 0.153 (0.106, 0.210) | 0.22 (0.14, 0.30) | 16 | 8 | 11 |
| Female | 464 | 0.130 (0.092, 0.176) | 0.21 (0.13, 0.28) | 16 | 9 | 12 | ||
| Western Terrestrial Garter Snake (Thamnophis elegans) | 1978-1996 | Short-lived: Male & Female | 2176 | * | * | 15 | 5 | 6 |
| Long-lived: Pop 1 Male & Female | 1655 | 0.25 (0.16, 0.38) | 0.16 (0.12, 0.20) | 18 | 9 | 15 | ||
| Long-lived: Pop 2 Male & Female | 449 | 0.10 (0.04, 0.21) | 0.20 (0.13, 0.29) | 17 | 9 | 13 | ||
| Painted Turtle (Chrysemys picta) | 1996-2012 | Female | 1031 | 0.102 (0.090, 0.116) | 0.05 (0.03, 0.07) | 23 | 11 | 19 |
NOTES: N is sample size for the Gompertz analysis and includes all individuals of known and unknown age. Gompertz parameters were estimated using a Bayesian mark-recapture estimator. A (the Initial Mortality Rate, IMR) is estimated at age of maturation for frogs and turtles, and age 0 for snakes. Mean and 90% lifespan are calculated conditioned on attainment of the age-at-maturity. Thus, mean lifespan is the average number of years individuals survive given that they have reached maturity (maturity occurs in the sixth year for frogs, third year for short-lived snakes, fifth year for long-lived snakes, and sixth year for turtles). The 90% lifespan is the number of years until 90% of individuals have died. Lifespan values are calculated from the Gompertz parameters except in the case of short-lived snakes, for which we were unable to fit a Gompertz model and instead estimated mean lifespan from the known-age mark-recapture model. For population 1 of the long-lived snakes, a Makeham term improved the fit: c = 0.23 with CI (0.15, 0.32). Oldest age data observed are from Fellers et al. (2013), Sparkman et al. (2007), and Janzen (unpublished).
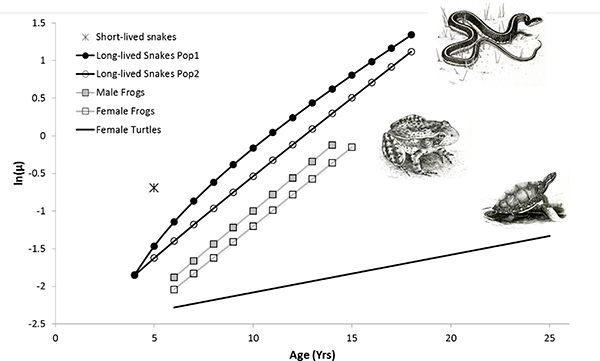
FIGURE 13-1 Age-specific mortality rates for two populations of long-lived western terrestrial garter snakes (Thamnophis elegans), male and female Sierra Nevada yellow-legged frogs (Rana sierrae), and female painted turtles (Chrysemys picta).
NOTES: Estimates are for a Gompertz mortality model (see Table 13-1 for parameters), excepting Population 1 of the long-lived snakes, for which an additive age-independent mortality term (i.e., a Makeham term) was included for improved model selection. For the populations of short-lived garter snakes, we were unable to fit a Gompertz model; the value indicated on the graph corresponds to the point estimate for adult mortality from our analysis of mark-recapture data.
SOURCE: Illustrations by Shawna Snyder, Iowa State University. Used with permission.
Long-lived snakes and yellow-legged frogs exhibited a similar interaction between cohort and annual effects. Mortality for long-lived snakes initially decreased after their first year and then began to increase again once they reached maturity. Individuals from poor cohorts began to exhibit higher mortality starting in their second year and the magnitude of the difference increased for adults. This suggests that poor developmental conditions during the first year of life may lead to greater rates of senescence. In addition, deleterious effects of poor conditions later in life only occurred for individuals from poor cohorts, indicating that early-life stress may reduce the ability of individuals to cope with poor conditions later in life (Figure 13-2).
The model with the strongest support for both painted turtles and short-lived garter snakes only included effects of age (Table 13-2, Figure 13-2).
TABLE 13-2 Mortality Estimates for the Indicated Age-Classes from Cormack-Jolly-Seber Analysis of Mark-Recapture Data
| Species | N | Cohort Quality/Capture Year Quality | Age-specific survival (yrs of age) | |||
| 0-6 | 6-7 | 7+ | ||||
| Sierra Nevada | 366 | Good cohort/Good year | 0.523 | 0.952 | ||
| Yellow-legged | Good cohort/Poor year | 0.291 | 0.749 | |||
| Frog | Poor cohort/Good year | 0.774 | 0.813 | |||
| Poor cohort/Poor year | 0.576 | 0.411 | ||||
| 0-1 | 1-4 | 4+ | ||||
| Western | 1080 | Good cohort/Good year | 0.366 | 0.948 | 0.942 | |
| Terrestrial | Good cohort/Poor year | 0.976 | 0.926 | |||
| Garter Snake | Poor cohort/Good year | 0.880 | 0.872 | |||
| Long-lived | Poor cohort/Poor year | 0.395 | 0.858 | 0.663 | ||
| 0-1 | 1-2 | 2+ | ||||
| Western | 341 | Good cohort/Good year | 0.316 | 0.707 | 0.627 | |
| Terrestrial | Good cohort/Poor year | 0.716 | 0.648 | |||
| Garter Snake | Poor cohort/Good year | 0.706 | 0.629 | |||
| Short-lived | Poor cohort/Poor year | 0.323 | 0.722 | 0.659 | ||
| 0-6 | 6-8 | 8-10 | 10+ | |||
| Painted Turtle | 603 | Good cohort | 0.811 | 0.924 | 0.904 | |
| Poor cohort | 0.815 | 0.922 | 0.899 | |||
NOTES: See Table 13-1 for years studied. Point estimates are from model-averaging.
Overall, short-lived garter snakes showed a similar pattern of age-specific mortality to long-lived garter snakes, with the lowest mortality occurring after the first year of life and prior to maturation. Unlike the long-lived garter snakes, whether cohorts were of poor or good quality did not influence this pattern. Interestingly, the painted turtles had higher mortality in the first 2 years after maturation, decreasing in the next 2 years before starting to increase again in later life, suggestive of a signature of senescence even within these broad age-classes.
DISCUSSION
Evolutionary senescence theory, underpinned by clear expectations from population genetic principles, has guided much of the research in compara-
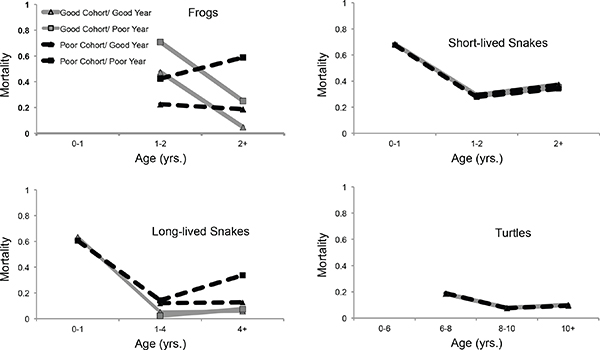
FIGURE 13-2 Tests for effect of early life experience and environmental variation on late life mortality for short-lived and long-lived ecotypes of the western terrestrial garter snakes (Thamnophis elegans), Sierra Nevada yellow-legged frogs (Rana sierrae), and painted turtles (C. picta).
NOTES: Model averaged estimates of mortality are from analysis of mark-recapture models accounting for age, cohort, and annual effects on survival. For yellow-legged frogs and long-lived garter snakes, we found effects of both cohort and year, along with their interaction suggesting that poor developmental conditions are associated with both greater mortality later in life and greater vulnerability to poor conditions at later ages.
tive biodemography for nearly 50 years. Even so, it has become apparent that variation in mortality trajectories demands a deeper understanding of mechanisms, particularly as such variation relates to potential constraints, be they environmental, sex-specific, or otherwise (Austad, 2010). Employing a comparative perspective by examining wild populations of relatively long-lived ectothermic vertebrates, we found that (1) across all three species, there was strong evidence for mortality senescence and (2) environmental factors, including stress, influence age-specific patterns of mortality both in current and later years and therefore produces plastic variation in the shapes of mortality trajectories.
We observed increased mortality with age in populations of all three species—yellow-legged frogs (R. sierrae), garter snakes (T. elegans), and painted turtles (C. picta). Painted turtles aged at a much slower rate than yellow-legged frogs and garter snakes, in agreement with a handful of aging
studies on turtles. However, the results for female painted turtles suggest that contrary to other turtle reports (Congdon et al., 2001; Miller, 2001; Jones et al., 2014), there was measurable aging, though this rate of aging is much slower than in many other taxa (reported here and see Finch et al., 1990; Finch, 2009; Bronikowski et al., 2011).
We found similarities with mammalian studies in the observed rate of aging for yellow-legged frogs and garter snakes. This was surprising, because indeterminate growth and indeterminate fecundity have been predicted to offset declines in the power of natural selection to remove deleterious mutations with late-age phenotypes (Reznick et al., 2004; Bronikowski and Promislow, 2005). The lack of uniformity in aging among our three ectothermic species suggests metabolism and indeterminate growth may not explain evolved differences in aging rates. Future work should evaluate other aspects of comparative aging, including sex-specific dynamics and other mechanisms underlying increasing mortality with advancing age. Such studies should include external sources of mortality (e.g., predation, drought, food availability) and internal mechanisms such as immunosenescence and accumulation of damaged cell components. Such a comparative perspective on biodemography and its underlying mechanisms will provide much needed insights into vertebrate aging.
Our results validate previous observations for the garter snakes, the one species where we had data on multiple populations. Interestingly, long-lived ecotypic individuals are generally smaller bodied and live on average twice as long as the larger-bodied, short-lived ecotypes (Bronikowski and Vleck, 2010). This is consistent with the pattern typically seen in lab rodents and dogs where smaller-bodied genotypes are generally characterized by longer lifespans than larger bodied (e.g., Miller and Austad, 2006).
Due to the limitations of our data, we were only able to examine sex-specific differences in mortality trajectories for the yellow-legged frogs. Not only did males and females in this population exhibit very similar mortality rates, but also the rate at which mortality increased with age was nearly identical between the sexes (Table 13-1). In the case of painted turtles, long-term data were only available for females, while for garter snakes, sex was unknown for the youngest age-classes and could only be determined for individuals recaptured at older ages. Thus, analyzing data for only known-sex individuals would rely on a non-random sample with respect to realized mortality schedules (Miller et al., 2011).
Work is currently under way to characterize survival patterns in male painted turtles, and sex-specific comparisons in this species will be especially interesting. Painted turtles have temperature-dependent sex determination (TSD), where temperatures experienced during a limited period of embryonic development determine the sex of the individual. Thus, since sex-linked genes do not exist in this species, differences between the sexes in
their biology, including their mortality patterns, must derive from environmental contexts or interactions between the environment and sex-specific physiology once gender has been determined.
Many studies on ectothermic vertebrates have demonstrated that environmental conditions in early life, especially during embryogenesis, influence subsequent individual phenotypes (Deeming, 2004), including behavior, physiology, life history, learning, and memory. Our study shows that environmental variation can affect mortality patterns (Figure 13-2). In both yellow-legged frogs and long-lived garter snakes, this is seen as a relationship between environmental quality during early life and an individual’s ability to tolerate poor environmental conditions later on. That is, individuals that experienced poor environmental conditions as embryos or neonates had higher mortality when exposed to poor environmental conditions later in life. This pattern suggests that animals stressed early in life senesce more quickly in response to later life stressors. Further work to understand the physiological underpinnings of this relationship could shed light on the mechanisms leading to this pattern. Such an effect was not seen in painted turtles and short-lived garter snakes (Figure 13-2). The apparent buffering of female painted turtles against detrimental effects of early stress may be due to the consistency of food availability that characterizes their habitats and their resilience to extreme environmental perturbations such as flooding (Jergenson et al., 2014). Similarly, short-lived garter snakes reside in close proximity to continuous water and food availability (fish) in contrast to the long-lived ecotype. Thus, the susceptibility to low precipitation may be more pronounced in habitats inhabited by the long-lived ecotype where snakes rely on less reliable breeding anurans for food (Robert and Bronikowski, 2010).
All three species exhibited mortality senescence—increasing mortality with advancing adult age. However, Darwinian fitness is measured not in survival, but as lifetime reproductive success. For a complete understanding of how early life stressors relate to mortality, reproduction, and ultimately fitness, we would need information on how reproductive output changes with age and hence how lifetime reproduction varies with early-life environmental conditions. In the painted turtle population, female reproductive output increases with age until the late age-classes, at which point reproduction falls off quickly (D.A. Warner and F.J. Janzen, personal communication)—a pattern that suggests delayed but measurable reproductive senescence. However, the garter snakes—both the short- and long- lived ecotypes—continue to increase reproductive output with age, although much more rapidly in the short-lived than long-lived ecotype (Sparkman et al., 2007). We have no data for yellow-legged frog reproductive output as they age. However, novel environmental stressors such as pesticide exposure (Sparling and Fellers, 2007; Sparling and Fellers, 2009; Fellers et al.,
2014) and chytrid fungus (Fellers et al., 2001) are likely influencing lifetime reproduction and longevity more than traditional environmental variation.
Finally, we suggest that ectothermic tetrapods, are an underutilized comparative system for understanding the evolutionary forces that shape variation in age-dependent mortality relative to mammals. Ectothermic reptiles may have different mortality and reproduction trajectories across the adult lifespan than seen in many mammals (Lutz et al., 2003; Paitz et al., 2007; Bronikowski, 2008). Evolutionary theory posits alterations in traits that protect the organism from mortality as an ultimate source of variation in species-specific rates-of-aging and lifespan (Bronikowski and Promislow, 2005), and reptiles and amphibians have a number of such evolutionarily novel traits (Schwartz and Bronikowski, 2010). For example, crocodilians, turtles, tuatara, lizards, snakes, and amphibians have evolved the following: an external ribcage (turtles); venom (snakes); toxic secretions (anurans); limblessness (snakes, some lizards, caecilians); extended metabolic shutdown (all); starvation resistance, including remodeling of the digestive tract (snakes); supercooling, freeze tolerance, and heat tolerance (turtles, crocodilians, and some frogs); and extended hypoxia resistance (turtles, crocodilians, lizards) (Robert et al., 2007; Owerkowicz et al., 2009). Thus, mammalian aging and its limits may be best understood by studying variation across all tetrapods.
ACKNOWLEDGMENTS
The authors acknowledge the states of California and Illinois and Yosemite National Park for research permits during the course of this study. We also thank the various field crews who worked with us, the National Science Foundation for funding the painted turtle and garter snake research, and the USGS Amphibian Research and Monitoring Initiative for funding the Sierra Nevada yellow-legged frog research. Original illustrations in Figure 13-1 were drawn by Shawna Snyder (Biological and Premedical Illustration Program, Iowa State University). Any use of trade, product, or firm names is for descriptive purposes only and does not imply endorsement by the U.S. Government. This is contribution 471 of the USGS Amphibian Research and Monitoring Initiative.
REFERENCES
Abrams, P.A. (1993). Does increased mortality favor the evolution of more rapid senescence? Evolution, 47(3), 877-887.
Alberts, S.C., Altmann, J., Brockman, D.K., Cords, M., Fedigan, L.M., Pusey, A., Stoinski, T.S., Strier, K.B., Morris, W.F., and Bronikowski, A.M. (2013). Reproductive aging patterns in primates reveal that humans are distinct. Proceedings of the National Academy of Sciences of the United States of America, 110(33), 13440-13445.
Austad, S.N. (2010). Methusaleh’s Zoo: How nature provides us with clues for extending human health span. Journal of Comparative Pathology, 142(Suppl. 1), S10-S21.
Bartke, A., Sun, L.Y., and Longo, V. (2013). Somatotropic signaling: Trade-offs between growth, reproductive development, and longevity. Physiological Reviews, 93(2), 571-598.
Baudisch, A. (2005). Hamilton’s indicators of the force of selection. Proceedings of the National Academy of Sciences of the United States of America, 102(23), 8263-8268.
Bronikowski, A.M. (2008). The evolution of aging phenotypes in snakes: A review and synthesis with new data. Age, 30(2-3), 169-176.
Bronikowski, A.M., and Arnold, S.J. (1999). The evolutionary ecology of life history variation in the garter snake Thamnophis elegans. Ecology, 80(7), 2314-2325.
Bronikowski, A.M., and Promislow, D.E.L. (2005). Testing evolutionary theories of aging in wild populations. Trends in Ecology & Evolution, 20(6), 271-273.
Bronikowski, A.M., and Vleck, D. (2010). Metabolism, body size and life span: A case study in evolutionarily divergent populations of the garter snake (Thamnophis elegans). Integrative and Comparative Biology, 50(5), 880-887.
Bronikowski, A.M., Altmann, J., Brockman, D. K., Cords, M., Fedigan, L.M., Pusey, A., Stoinski, T., Morris, W.F., Strier, K.B., and Alberts, S.C. (2011). Aging in the natural world: Comparative data reveal similar mortality patterns across primates. Science, 331(6022), 1325-1328.
Brunet-Rossinni, A.K., and Austad, S.N. (2006). Senescence in wild populations of mammals and birds. In E.J. Masoro and S.N. Austad (Eds.), Handbook of the Biology of Aging (pp. 243-266). Amsterdam: Elsevier.
Burnham, K.P., and Anderson, D.R. (2002). Model Selection and Multimodel Inference. New York: Springer.
Carey, J.R., and Vaupel, J.W. (2006). Biodemography. In D.L. Poston and M. Micklin (Eds.), Handbook of Population (pp. 625-658). New York: Springer.
Colchero, F., and Clark, J.S. (2012). Bayesian inference on age-specific survival for censored and truncated data. Journal of Animal Ecology, 81(1), 139-149.
Colchero, F., Jones, O.R., and Rebke, M. (2012). BaSTA: An R package for Bayesian estimation of age-specific survival from incomplete mark-recapture/recovery data with covariates. Methods in Ecology and Evolution, 3(3), 466-470.
Congdon, J.D., Nagle, R.D., Kinney, O.M., and Sels, R.C.V. (2001). Hypotheses of aging in a long-lived vertebrate, Blanding’s turtle (Emydoidea blandingii). Experimental Gerontology, 36(4-6), 813-827.
Congdon, J.D., Nagle, R.D., Kinney, O.M., and Sels, R.C.V. (2003). Testing hypotheses of aging in long-lived painted turtles (Chrysemys picta). Experimental Gerontology, 38(7), 765-772.
Cormack, R. (1964). Estimates of survival from the sighting of marked animals. Biometrika, 51, 429-438.
Deeming, D.C. (2004). Reptilian Incubation: Environment, Evolution and Behaviour. Nottingham, UK: Nottingham University Press.
Eskew, E.A., Price, S.J., and Dorcas, M.E. (2010). Survivorship and population densities of painted turtles (Chrysemys picta) in recently modified suburban landscapes. Chelonian Conservation and Biology, 9(2), 244-249.
Fellers, G.M., Green, D.E., and Joyce E. Longcore. (2001). Oral chytridiomycosis in mountain yellow-legged frogs (Rana muscosa). Copeia, 2001(4), 945-953.
Fellers, G.M., Kleeman, P.M., Miller, D.A.W., Halstead, B.J., and Link, W.A. (2013). Population size, survival, growth, and movements of Rana sierrae. Herpetologica, 69(2), 147-162.
Fellers, G.M., McConnell, L.L., Sparling, D.W., and Kleeman, P. (2014). Pesticides in amphibian habitats of northern and central California. In L.L. McConnell (Ed.), Occurrence, Fate and Impact of Atmospheric Pollutants on Environmental and Human Health (pp. 128-150). Washington, DC: American Chemical Society Books.
Finch, C.E. (2009). The evolution of slow aging and negligible senescence. Gerontologist, 49, 508-508.
Finch, C.E., Pike, M.C., and Witten, M. (1990). Slow mortality rate accelerations during aging in some animals approximate that of humans. Science, 249, 902-905.
Hamilton, W.D. (1966). Moulding of senescence by natural selection. Journal of Theoretical Biology, 12(1), 12-45.
Hector, K.L., Bishop, P.J., and Nakagawa, S. (2012). Consequences of compensatory growth in an amphibian. Journal of Zoology, 286(2), 93-101.
Jergenson, A.M., Miller, D.A.W., Neuman-Lee, L.A., Warner, D.A., and Janzen, F.J. (2014). Swimming against the tide: Resilience of a riverine turtle to extreme environmental events. Biology Letters, 10(3), 20130782.
Jolly, G. (1965). Explicit estimates from capture-recapture data with both death and immigration-stochastic model. Biometrika, 52, 225-247.
Jones, O.R., Scheuerlein, A., Salguero-Gomez, R., Camarda, C.G., Schaible, R., Casper, B.B., Dahlgren, J.P., Ehrlen, J., Garcia, M.B., Menges, E.S., Quintana-Ascencio, P.F., Caswell, H., Baudisch, A., and Vaupel, J.W. (2014). Diversity of ageing across the tree of life. Nature, 505(7482), 169-173.
Le Galliard, J.F., Marquis, O., and Massot, M. (2010). Cohort variation, climate effects and population dynamics in a short-lived lizard. Journal of Animal Ecology, 79(6), 1296-1307.
Lebreton, J.D., Burnham, K.P., Clobert, J., and Anderson, D.R. (1992). Modeling survival and testing biological hypotheses using marked animals—a unified approach with case-studies. Ecological Monographs, 62(1), 67-118.
Lee, R.D. (2003). Rethinking the evolutionary theory of aging: Transfers, not births, shape social species. Proceedings of the National Academy of Sciences of the United States of America, 100(16), 9637-9642.
Lutz, P.L., Prentice, H.M., and Milton, S.L. (2003). Is turtle longevity linked to enhanced mechanisms for surviving brain anoxia and reoxygenation? Experimental Gerontology, 38(7), 797-800.
Madsen, T., and Shine, R. (2000a). Rain, fish and snakes: Climatically driven population dynamics of Arafura filesnakes in tropical Australia. Oecologia, 124(2), 208-215.
Madsen, T., and Shine, R. (2000b). Silver spoons and snake body sizes: Prey availability early in life influences long-term growth rates of free-ranging pythons. Journal of Animal Ecology, 69(6), 952-958.
Mangel, M., and Munch, S.B. (2005). A life-history perspective on short- and long-term consequences of compensatory growth. American Naturalist, 166(6), E155-E176.
Massot, M., and Aragón, P. (2013). Phenotypic resonance from a single meal in an insectivorous lizard. Current Biology, 23, 1320-1323.
Massot, M., Clobert, J., Montes-Poloni, L., Haussy, C., Cubo, J., and Meylan, S. (2011). An integrative study of ageing in a wild population of common lizards. Functional Ecology, 25(4), 848-858.
Medawar, P.B. (1946). Old age and natural death. Modern Quarterly, 2, 30-49.
Metcalfe, N.B., and Monaghan, P. (2001). Compensation for a bad start: Grow now, pay later? Trends in Ecology & Evolution, 16(5), 254-260.
Miller, J.K. (2001). Escaping senescence: Demographic data from the three-toed box turtle (Terrapene carolina triunguis). Experimental Gerontology, 36(4-6), 829-832.
Miller, R.A., and Austad, S.N. (2006). Growth and Aging: Why do big dogs die young? In E.J. Masoro and S.N. Austad (Eds.), Handbook of the Biology of Aging (pp. 512-533). San Diego, CA: Academic Press.
Miller, D.A., Clark, W.R., Arnold, S.J., and Bronikowski, A.M. (2011). Stochastic population dynamics in populations of western terrestrial garter snakes with divergent life histories. Ecology, 92(8), 1658-1671.
Munch, S.B., and Mangel, M. (2006). Evaluation of mortality trajectories in evolutionary biodemography. Proceedings of the National Academy of Sciences of the United States of America, 103(44), 16604-16607.
Nussey, D.H., Froy, H., Lemaitre, J.-F., Gaillard, J.-M., and Austad, S.N. (2013). Senescence in natural populations of animals: Widespread evidence and its implications for bio-gerontology. Ageing Research Reviews, 12(1), 214-225.
Owerkowicz, T., Elsey, R.M., and Hicks, J.W. (2009). Atmospheric oxygen level affects growth trajectory, cardiopulmonary allometry and metabolic rate in the American alligator (Alligator mississippiensis). Journal of Experimental Biology, 212(9), 1237-1247.
Paitz, R.T., Harms, H.K., Bowden, R.M., and Janzen, F.J. (2007). Experience pays: Offspring survival increases with female age. Biology Letters, 3(1), 44-46.
Peterson, R.O., Vucetich, J.A., Fenton, G., Drummer, T.D., and Larsen, C.S. (2010). Ecology of arthritis. Ecology Letters, 13(9), 1124-1128.
Promislow, D.E.L., and Bronikowski, A.M. (2006). The evolutionary genetics of senescence. In J. Wolf and C. Fox (Eds.), Evolutionary Genetics: Concepts and Case Studies (pp. 464-481). Oxford, U.K.: Oxford University Press.
Reznick, D.N., Bryant, M.J., Roff, D., Ghalambor, C.K., and Ghalambor, D.E. (2004). Effect of extrinsic mortality on the evolution of senescence in guppies. Nature, 431(7012), 1095-1099.
Robert, K.A., and Bronikowski, A.M. (2010). Evolution of senescence in nature: Physiological evolution in populations of garter snake with divergent life histories. American Naturalist, 175(2), 147-159.
Robert, K.A., Brunet-Rossinni, A., and Bronikowski, A.M. (2007). Testing the “free radical theory of aging” hypothesis: Physiological differences in long-lived and short-lived colubrid snakes. Aging Cell, 6(3), 395-404.
Schwanz, L.E., Spencer, R.J., Bowden, R.M., and Janzen, F.J. (2010). Climate and predation dominate juvenile and adult recruitment in a turtle with temperature-dependent sex determination. Ecology, 91(10), 3016-3026.
Schwanz, L., Warner, D.A., McGaugh, S., Di Terlizzi, R., and Bronikowski, A. (2011). State-dependent physiological maintenance in a long-lived ectotherm, the painted turtle (Chrysemys picta). Journal of Experimental Biology, 214(1), 88-97.
Schwartz, T.S., and Bronikowski, A.M. (2010). Molecular stress pathways and the evolution of life histories in reptiles. In T. Flatt and A. Heyland (Eds.), Molecular Mechanisms of Life History Evolution (pp. 193-209). Oxford, UK: Oxford University Press.
Seber, G.A.F. (1965). A note on multiple recapture census. Biometrika, 52, 249-259.
Sparkman, A.M., Arnold, S.J., and Bronikowski, A.M. (2007). An empirical test of evolutionary theories for reproductive senescence and reproductive effort in the garter snake Thamnophis elegans. Proceedings of the Royal Society B: Biological Sciences, 274(1612), 943-950.
Sparling, D.W., and Fellers, G. (2007). Comparative toxicity of chlorpyrifos, diazinon, malathion and their oxon derivatives to larval Rana boylii. Environmental Pollution, 147(3), 535-539.
Sparling, D.W., and Fellers, G.M. (2009). Toxicity of two insecticides to California, USA, anurans and its relevance to declining amphibian populations. Environmental Toxicology and Chemistry, 28(8), 1696-1703.
Vaupel, J.W., Baudisch, A., Dolling, M., Roach, D.A., and Gampe, J. (2004). The case for negative senescence. Theoretical Population Biology, 65(4), 339-351.
White, G.C., and Burnham, K.P. (1999). Program MARK: Survival estimation from populations of marked animals. Bird Study, 46, S120-S139.
White, C.R., Phillips, N.F., and Seymour, R.S. (2006). The scaling and temperature dependence of vertebrate metabolism. Biology Letters, 2(1), 125-127.
Wikelski, M., and Thom, C. (2000). Marine iguanas shrink to survive El Niño. Nature, 403(6765), 37-38.
Williams, G.C. (1957). Pleiotropy, natural selection, and the evolution of senescence. Evolution, 11(4), 398-411.
Williams, B.K., Nichols, J.D., and Conroy, M.J. (2002). Analysis and Management of Animal Populations: Modeling, Estimation, and Decision Making. New York: Academic Press.
Williams, P.D., and Day, T. (2003). Antagonistic pleiotropy, mortality source interactions, and the evolutionary theory of senescence. Evolution, 57(7), 1478-1488.




















