Peter T. Ellison and Mary Ann Ottinger
INTRODUCTION
The relationships between reproductive aging, reproductive cessation, the emergence of extended, post-reproductive life, and social behavior in humans continue to be topics of both theoretical and empirical interest. Debate continues over the physiological processes underlying human reproductive aging (Downs and Wise, 2009; Perheentupa and Huhtaniemi, 2009; Ferrell et al., 2012), the uniqueness of human menopause (Packer et al., 1998; Herndon and Walker, 2010; Levitis et al., 2013), and the evolutionary forces that may have shaped late life and reproduction in humans (Johnstone and Cant, 2010; Kaplan et al., 2010; Hawkes et al., 2011; Mittledorf and Goodnight, 2012; Chu and Lee, 2013). However, from a comparative perspective, it is critical to recognize the existence of highly conserved mechanisms in these lifelong processes and to identify similarities as well as unique differences across vertebrates. Follicular depletion is widely considered to be the primary cause of the phenomenon of human menopause, as well as of cessation of ovarian function in other birds and mammals (Edson et al., 2009; Perheentupa and Huhtaniemi, 2009; Finch, 2013), but the degree to which this imposes a constraint on evolution is not clear. There is also disagreement over whether the trajectory of follicular depletion in humans displays evidence of significant acceleration prior to menopause (Richardson et al., 1987; Faddy and Gosden, 1995; Hansen et al., 2008; Coxworth and Hawkes, 2010;), as well as over recent evidence
for follicular renewal throughout life (Johnson et al., 2004; Eggan et al., 2006; Faddy and Gosden, 2007; Begum et al., 2008; Kerr et al., 2012).
Other components of reproductive aging, including gonadal senescence in males and changes in hypothalamic-pituitary function in both sexes, are often neglected in these debates. There is also debate over the relative frequency of significant periods of post-reproductive survival in nature and its phylogenetic distribution (Cohen, 2004; Pollycove et al., 2011). Finally, there are theoretical debates regarding the evolutionary origin of the currently observed human pattern of long, regularly occurring post-reproductive life and its relationship to human social behavior, ranging from those who see it as a consequence of intergenerational conflict to those who see it as a consequence of intergenerational cooperation (Mace, 2000; Hawkes, 2003; Cant and Johnstone, 2008; Johnstone and Cant, 2010; Chu and Lee, 2013).
In this paper, we will attempt to bring some of these debates into comparative perspective, focusing particularly on vertebrates. We will first consider the mechanisms of reproductive aging and cessation in vertebrates and particularly in birds and mammals, then the phenomenological distribution of post-reproductive life in captivity and in the wild. In addition, we will consider the insights provided by laboratory or captive populations in order to compare and contrast conserved mechanisms as opposed to unique adaptations in some populations. Finally, we will return to questions of the evolutionary origins of human post-reproductive life in particular.
MECHANISMS OF VERTEBRATE REPRODUCTIVE AGING
Follicular Depletion in Females
In all vertebrates, gonads develop embryologically from the genital ridge mesoderm and are populated by migrating primordial germ cells that give rise to mitotically competent oogonia and spermatogonia in females and males respectively. Spermatogonia are present in the testes throughout life in males of all vertebrate species and respond mitotically to gonadotropin stimulation. In females, however, there are two contrasting patterns. In fish (with a few exceptions noted below), amphibians, and reptiles, mitotically active oogonia remain present in the ovary throughout life, whereas in birds and mammals, they either largely or completely disappear before birth or hatching (Aranzàbal, 2011; Flament et al., 2011; Johnson, 2011; Jones, 2011; Norris and Lopez, 2011; Urbatzka et al., 2011). In birds and mammals, a period of clonal proliferation during embryonic development produces a large supply of daughter cells that become surrounded by a single layer of granulosa cells and commence meiosis I, becoming arrested in prophase where they remain until just before ovulation. At this stage
the cells are known as oocytes and the follicles are referred to as primordial follicles. The only notable exceptions to the phylogenetic distribution of these two modes of oocyte production are certain viviparous species of chondrichthyes, which appear to have a finite oocyte supply from early in development, like mammals and birds (Franchi et al., 1962).
There is some recent evidence that in mice and humans, some stem cells may persist in the ovary capable of generating new oogonia and oocytes long after birth (Johnson et al., 2004; Eggan et al., 2006; Woods et al., 2013), though there is no evidence of clonal proliferation capable of repopulating a depleted ovary with functional primordial follicles (Faddy and Gosden, 2007; Begum et al., 2008; Kerr et al., 2012). This intriguing observation of persistent germinal stem cells may have important medical applications in the domain of assisted reproduction, but does not appear to have any effect on processes of reproductive aging or follicular depletion.
Although birds and mammals are temporally limited in oocyte production, the initial supply of primordial follicles in the ovary at birth is very large relative to the number of potential ovulations over a female’s lifespan (see Figure 14-1). In humans, for example, where a female may
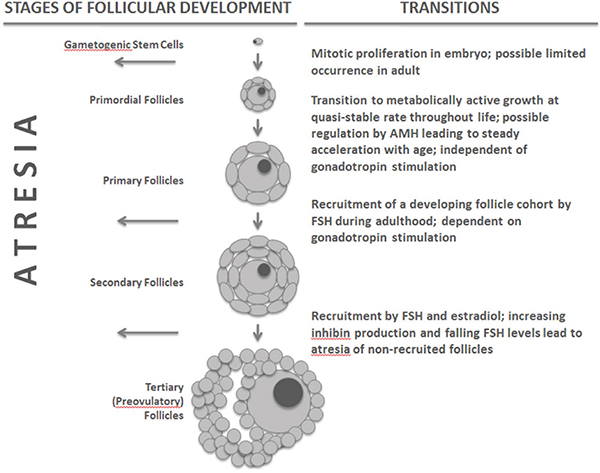
FIGURE 14-1 Stages and transitions in follicular development in birds and mammals.
NOTE: AMH = anti-Müllerian hormone, FSH = follicle stimulating hormone.
expect to ovulate a maximum of 500 or so times, follicular supply at birth is typically four orders of magnitude greater. However, from the moment of initial oocyte arrest in meiosis, primordial follicles emerge from a state of metabolic quiescence at a low, quasi-stable rate to become metabolically active, growing follicles known as primary follicles (Fortune et al., 2000). In humans, the annual probability of such a transition has been estimated at 0.118 per follicle. Once past this initial transition, a follicle and its contained oocyte have only two possible fates: ovulation or atresia (regression of the follicle and apoptosis of the oocyte) (Depalo et al., 2003). A second transition, from primary to secondary follicle, depends on appropriate gonadotropin stimulation and recruits a cohort of follicles from the active primary pool in conjunction with mature ovarian cycling (Edson et al., 2009; McGee and Hsueh, 2000). Because this second transition is sensitive to gonadotropin stimulation, it can be affected by neuroendocrine changes in the hypothalamic-pituitary-ovarian (HPO) axis that may accompany aging. The rate of the initial transition from a quiescent primordial follicle to a metabolic active primary follicle appears, however, to be constant or increasing throughout life irrespective of reproductive state. Thus, the vast majority of primary follicles are lost through atresia without ever being recruited for potential ovulation. It is important to note that this loss is independent of the recruitment of follicles during mature ovarian cycling and is unaffected by variation in the timing, frequency, or occurrence of ovarian cycling or ovulation. In humans, approximately 1 percent of the remaining follicular pool is lost each month from birth on. The stages and transitions of follicular development are summarized in Figure 14-1.
There is no evidence that the rate of primordial to primary follicle transition ever abates, but there is some evidence that it may progressively accelerate with age (Gougeon et al., 1994). In humans, evidence of this acceleration was initially misinterpreted as reflecting an age threshold at which the rate of disappearance of primordial follicles shifted in a discontinuous fashion (Richardson et al., 1987; Faddy and Gosden, 1995; Hansen et al., 2008). More sophisticated analyses of the available data suggest that slow but steady acceleration of loss is more likely (Coxworth and Hawkes, 2010). There is evidence as well that the rate of the primordial to primary follicle transition may be subject to feedback regulation from the primary pool via anti-Müllerian hormone (AMH), perhaps as a mechanism to help buffer the size of the available primary pool from the attrition of the primordial stock (Durlinger et al., 2002).
Given the finite supply of primordial follicles and the inexorable rate of attrition in that stock, the follicular supply in a bird or mammal that lives long enough will eventually drop below a threshold level necessary to supply a sufficient cohort of primary follicles for recruitment, and estrogen production by the primary follicles will drop below the levels necessary for
cyclic ovarian function. Data from cows indicate that female fecundity is positively correlated with the size of the secondary follicular pool in a given cycle, which in turn is positively related to the size of the available primary follicular pool (Ireland et al., 2011). Because the primary follicular pool declines in size with age in an inexorable fashion due to the dwindling size of the primordial follicle stock, female fecundity in most species of birds and mammals begins to decline prior to the end of reproductive life (Holmes et al., 2003; Cohen, 2004; Finch and Holmes, 2010).
Neuroendocrine Aging in Females
Characteristic age-related changes occur at all levels of HPO axis regulation in females that interact with declining follicular supply in the ovary. These changes have been well reviewed elsewhere (Brann and Mahesh, 2005; Downs and Wise, 2009; Perheentupa and Huhtaniemi, 2009) and are summarized in Figure 14-2. As noted above, declining numbers of actively growing primary and secondary follicles lead to diminished endocrine feedback to the hypothalamus and pituitary via estrogen and inhibin,
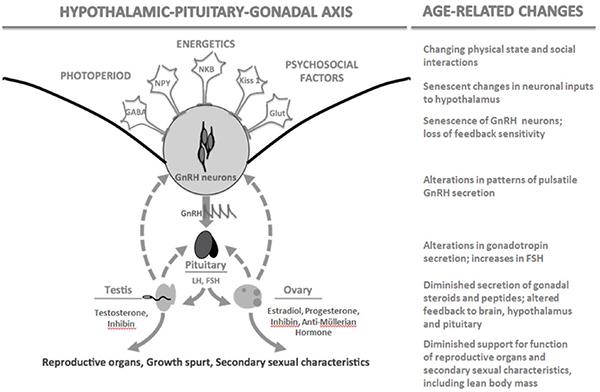
FIGURE 14-2 The hypothalamic-pituitary-gonadal axis in birds and mammals and associated age-related changes.
NOTE: FSH = follicle stimulating hormone, GABA = gamma-aminobutyric acid, Glut = glutamine, GnRH = gonadotropin-releasing hormone, Kiss 1 = kisspeptin q, LH = luteinizing hormone, NKB = neuokinin B, NPY = neuropeptide Y.
resulting in elevated FSH, as well as lower production of AMH. All of these hormones can be used as biomarkers of female reproductive aging. Hypothalamic sensitivity to ovarian steroid feedback may diminish with age as well, either as a consequence of lower steroid stimulation steroid receptor maintenance in the brain or as an independent consequence of neural senescence (Downs and Wise, 2009).
Fitness Value of Mechanisms of Female Reproductive Aging
In most species of birds and mammals, the combination of initial primordial follicle supply, rate of follicular depletion, and balance of neuroendocrine feedback results in a reproductive lifespan in the wild that is at least as long as the natural lifespan. It also provides a system that can be acted upon by natural selection to shape age-specific patterns of female fecundity in response to ecology and other elements of life history. Many, perhaps most, female mammal and bird species show demographic evidence of fertility decline with advanced age, evidenced as increasingly long interbirth or inter-clutch intervals, smaller brood or clutch sizes, and an increasing frequency of failure to reproduce at all. However, there is considerable variability, both between individuals within species and between species, in the trajectory and pace of this decline (Holmes and Ottinger, 2003; Finch and Holmes, 2010). At the species level, more rapid decline in fertility may result from selection to increase fertility earlier in the life history, even at the expense of late-age fertility, by increasing the rate of primordial to primary follicle transition in females. Conversely, sustained fertility late in life may reflect selection for late-age fertility even at the expense of early-age fertility. It is notable, for example, that there is some evidence that fertility in wild chimpanzee females may be sustained in late life to a greater degree than is commonly observed in humans, but that human fertility early in life is on the order of twice as high as that observed in wild chimpanzees (Emery Thompson et al., 2007).
It remains somewhat mysterious, from an evolutionary point of view, why birds and mammals have evolved such a seemingly strange pattern of female gamete production, departing from the ancestral pattern of lifelong gamete renewal followed by females of other vertebrate orders and males of all vertebrate orders. The answer may lie in the reduction in mutational load that results from restricting the number of mitotic generations leading to each ovulated oocyte. Mutational load increases steadily in male gametes due to continuous mitosis. Chromosomal disjunction errors may increase with age in female gametes (though it is not clear whether this is due to the aging of the oocyte itself or to some selective process in follicular recruitment that results in an accumulation of defective oocytes in the ovaries of older individuals), but mutational load does not. This leads to a question
of mutational load in non-mammalian/avian vertebrates and if there would be a prediction of increasing mutational load in aging females. Given current technologies, this hypothesis could be tested using cross-species comparisons. With the necessary restrictions in female fecundity imposed increased investment per reproductive attempt, mechanisms that favor and preserve gamete quality may have been subject to positive selection.
Mechanisms of Male Reproductive Aging
Reproductive aging in human males does not manifest in the same way as in human females. It appears to occur gradually from an early age and without any necessary outer limit imposed anatomically by constraints on gamete supply. Reproductive aging in males is a consequence of age-related changes in neuroendocrine function of the hypothalamic-pituitary-gonadal axis together with senescent loss of function by Leydig and Sertoli cells in the testis (Figure 14-2). Hallmarks of male aging in most birds and mammals include documented declines in circulating gonadal hormones, notably testosterone and 5α dihydrotestosterone (DHT), the peripherally active form of testosterone, as well as decreased hypothalamic secretion of GnRH, and altered pituitary release of gonadotropins (Harman et al., 2001; Moffat et al., 2002; Ottinger, 1998; Veldhuis et al., 2009). Data from primates indicate age-related changes in pituitary function, including circadian production of hormones (Sitzmann et al., 2008, 2010). Testosterone decline can result in weakened muscle function, decreased bone density, and degradation of other physiological parameters associated with aging and potentially important in male reproductive success (Harman et al., 2001; Moffat et al., 2002). Declines in other aspects of male fecundity are also apparent, including mating competence, sperm production, and sperm quality.
Age-related reproductive alterations become apparent in human males after the 5th decade in men, though they may begin much earlier, and include decreasing testosterone levels, loss of potency, increasing sperm abnormalities, and the potential for an increase in birth defects attributable to paternal age. Observations from the Massachusetts Male Aging Study show that total testosterone decreases 0.4-0.8 percent annually, while biologically active free testosterone levels decrease by 1.2-1.7 percent per year starting at the age of 50 (Plas et al., 2000; Henkel et al., 2005). Cross-cultural studies demonstrate a nearly linear decline in salivary testosterone (which parallels free serum testosterone) starting as early as age 30 in a wide range of human ecologies (Ellison et al., 2002). The rate of decline is gradual and in many studies, the variation has led to conflicting conclusions about the age-related loss in circulating gonadal steroid (Ottinger, 1998). Testosterone supports Sertoli cell function and spermatogenesis and is primarily metabolized into biologically active 5-α dihydrotesterone (5-α)
in the periphery and estradiol in the brain, with some aromatization of testosterone into estradiol in the periphery. These steroids are critical for negative-feedback regulation of gonadotropin secretion, adult reproductive function, sexual behavior, metabolism, immune function, bone health, and accessory sex structures. Consequently, diminishing T levels precipitate a cascade that impacts the entire reproductive system, as well as impacting muscle function, bone density, and steroid hormone target tissues.
Although declining testosterone levels do promote some increase in gonadotropin production, this change is not sufficient to prevent the age-related decline in reproductive function. There is evidence for diminishing hypothalamic sensitivity to feedback by gonadal steroids in the aging male and loss of inhibin production by the Sertoli cells (Ottinger, 1998; Zirkin and Chen, 2000; Hardy and Schlegel, 2004; Veldhuis et al., 2009). Reduced Leydig cell steroidogenesis impacts both circulating and intra-testicular testosterone levels and are reflected in altered timing of pulsatile release of hormones and reduced amplitude of these pulses (Syntin and Robaire, 2001; Black and Lane, 2002; Chen et al., 2002; Ellison et al., 2002; Uchida et al., 2006).
Assessing reproductive function and fertility in elderly men has traditionally focused on semen analysis. Based on conventional spermiogram measures, the weight of scientific evidence suggests that increased male age is associated with undesirable changes such as decreased semen volume (Henkel et al., 2005; Kidd et al., 2001), increased DNA fragmentation (Evenson and Wixon, 2006), lower sperm motility (Zubkova and Robaire, 2006), and increased frequency of sperm abnormalities (Kidd et al., 2001; Zubkova and Robaire, 2006), all of which negatively affect male fecundity. In general, however, most measures of male reproductive health exhibit no evidence of a relative age fertility “threshold,” but rather display gradual changes over time.
Based on studies in animals, an intriguing aspect of the age-related demise of reproduction in females appears to be a functional loss of pacemakers residing in the hypothalamus and pituitary gland, resulting in diminished amplitude in normal cycles of circulating hormones (Sitzmann et al., 2010). There is evidence that the male brain is steroid hormone responsive, and the age-related testosterone depletion creates a vulnerability to various senescence-related effects of androgen loss (Ottinger, 1998). Women experience a higher incidence of neurodegenerative disease than men during aging and especially following menopause. This raises the question of the utility of specific estrogen receptor modulators (SERMS) as a potential intervention for both disease and for cognitive function.
Fitness Value of Mechanisms of Male Reproductive Aging
Most life history theory is based on one-sex, “female only” demographic models, and human evolutionary biologists are often focused on female menopause as an evolutionary “problem.” Male reproductive aging is usually assumed to simply reflect senescence and the decreasing fitness value of investment in the maintenance of reproductive function with age. However, there is no reason to expect that male reproductive aging would not be shaped by ecology in ways similar to female reproductive aging (for instance, changing in response to changes in extrinsic mortality). In species with significant and long-term bi-parental investment, such as humans, it might also be reasonable to expect age-specific patterns of male fecundity to evolve in response to age-specific shifts in the benefit-cost ratios of reproductive effort versus parenting effort. These considerations raise the intriguing possibility that patterns of male reproductive aging may reflect adaptation and not merely constraint. It may serve the male’s evolutionary fitness to reduce mating effort with age, even when fecundity is non-zero. Thus, although there is no evidence of abrupt termination of male fecundity in a manner similar to menopause or follicular exhaustion, the rate of age-related reproductive decline may be species and life-history specific.
HOW UNUSUAL IS POST-REPRODUCTIVE LIFE?
The empirical study of post-reproductive lifespan and its taxonomic distribution is complicated by a number of factors, among them a frequent confusion of terms and concepts. Reproductive termination in females can, for example, be defined anatomically, as follicular depletion below some threshold; endocrinologically, as a level of estrogen production insufficient to promote endometrial proliferation or to suppress gonadotropin production to a normal range; phenomenologically, as a cessation of menses, sexual swellings, estrus behavior, or other outward markers of ovarian cyclicity; or demographically, as a cessation of conceptions or births. Of these, only the anatomical definition can be applied in the present, but it is, of course, the most onerous, requiring histological examination of ovarian tissue. All the others only apply retrospectively: that is, only longitudinal data can reveal whether a given menses, birth, etc., was the last in a female’s life and even then, only after her death. Nor are these indices of reproductive termination necessarily highly correlated. For example, using the endocrinological or phenomenological definitions, one might conclude that reproductive termination in humans is sensitive to energetic conditions, since energetic conditions independently influence ovarian steroid production. Women under energetic stress may produce less estrogen from a given cohort of follicles than other women and thus fall below the level
required to support endometrial proliferation and menstruation, while still sustaining a significant follicular reserve. Reproductive termination by the demographic definition also may be highly sensitive to energetics, as well as breastfeeding practices, cultural norms, and individual motivations, and thus be quite decoupled from the anatomical and physiological determinants of reproductive cessation.
Reproductive termination in males can similarly be indexed in a number of different, not necessarily correlated ways. Anatomical cessation occurs when the production of viable sperm ceases; endocrinological cessation may occur when testosterone levels are insufficient to support gametogenesis; phenomenological cessation occurs when potency is lost or mating effort ceases; and demographic cessation is marked by the last offspring fathered or pregnancy engendered. In males, none of these indices is usually available outside of a clinical or laboratory context, and inferences from female fertility, mating behavior, or sheer conjecture take their place.
In animals, difficulty in collecting longitudinal data in the wild is also a formidable difficulty, leading to a bias in the available data toward captive samples. Generally, there is an assumption in the literature that data from captivity cannot be used to make inferences about reproductive cessation in the wild, but such data can speak to the existence and degree of phenotypic plasticity in reproductive and total lifespan. Because anatomical, endocrinological, or even phenomenological markers of reproductive termination are rarely available, most animal studies use age at last reproduction (e.g., egg laying, pregnancy, live birth) as the relevant datum. Note that since this datum can only be determined retrospectively, all female animals in a study population must necessarily experience post-reproductive life according to the phenomenological definition, unless they die in the act of parturition or egg-laying. Thus there is an additional layer of complexity in determining whether a given female has died in a state of positive or zero fecundity; that is, did she die physiologically unable to have additional offspring, or merely during a long but potentially closed interbirth interval? The usual approach is to compare the duration of life since the last birth (or hatching) with the average interval between births. However, because there is evidence that female fecundity declines significantly prior to death in many, perhaps most, vertebrate species, even this operational approach is problematic. The final interbirth interval may be significantly longer than the average interval. Nevertheless, this approach does provide some basis for controlled comparison.
Post-Reproductive Life in Mammals
There is no systematic survey or sufficient body of data describing the distribution of reproductive cessation or post-reproductive life in mammals.
The best summary available is by Cohen (2004), although some important data have been published since (Alberts et al., 2013). Those data that do exist are spotty, not randomly distributed, and pertain almost exclusively to females.
Other than humans, long periods of post-reproductive life (> 10 years) occurring in a large percentage (> 25%) of females in a wild population have only been attributed to some toothed whale species (Odontoceti), including short-fined pilot whales (Globicephala macrorhynchus: Kasuya and Marsh, 1984) and killer whales (Orcinus orcus: Olesiuk and Ellis, 1990). Shorter and/or less prevalent post-reproductive lifespans have been reported for wild populations of lions (Panthera leo: Packer et al., 1998), polar bears (Usrsus maritimus: Ramsay and Stirling, 1988), olive baboons (Papio cynocephalus: Packer et al., 1998), and African elephants (Loxodonta Africana: Laws et al., 1975), though the claim for reproductive cessation in the female olive baboon and African elephant have been disputed more recently (Moss, 2001; Alberts et al., 2013). Alberts et al. (2013) have recently presented the most complete comparative study of female post-reproductive life in any mammalian group, based on longitudinal data for seven species of nonhuman primates: sifakas (Propithecus verreauxi), muriquis (Brachyteles hypoxanthus), capuchins (Cebus capucinus), olive baboons (Papio cynocephalus), blue monkeys (Cercopithecus mitis), chimpanzees (Pan troglodytes), and gorillas (Gorilla beringei). Very few individuals survived significantly beyond their last birth in any of the species studied, and statistical modeling of the rate of senescence in survivorship and fertility indicated that lifespan and reproductive lifespan were essentially coterminous in all seven species.
The data from captivity, including free-ranging, managed populations of animals, stand in some contrast to the data from wild populations and include many examples of species with a significant prevalence of post-reproductive life among females. Females of laboratory species, including mice (Mus musculus: vom Saal et al., 1994) and Chinese hamsters (Chrisetelus griseus: Parkening, 1982), regularly live for a significant period after reproductive cessation, even by the strictest anatomical definition, and are often used as models of follicular depletion. Females of familiar domestic species, such as cattle (vom Saal et al., 1994), dogs (Anderson, 1965), rabbits (vom Saal et al., 1994), and horses (Comfort, 1979) and more recently domesticated species such as red deer (Cervus elaphus: Fisher et al., 1966), also regularly live well beyond the end of reproduction. Post-reproductive life is particularly frequent and well-documented in captive female primates, including marmosets (Callithrix jacchus: Caro et al., 1995); tamarins (Leontopithecus rosalia: Caro et al., 1995, and Saguinus spp.: Tardif and Ziegler, 1992); squirrel monkeys (Saimiri sciurus: Caro et al., 1995); various lemurs (Lemur spp.: Caro et al., 1995); many macaques
(Macaca radiata: Caro et al. 1995; M. fuscata: Fedigan, 1991; Nozaki et al., 1995; Takahata et al., 1995; M. mulatta: Dyke et al., 1986; Johnson and Kapsalis, 1995, 1998; Tigges et al., 1988; Walker, 1995; M. nemestrina: Caro et al., 1995; Ha et al., 2000; M. sylvanus: Paul et al., 1993); Hanuman langurs (Presbytis entellus: Borries et al., 1991); olive baboons (Papio cynocephalus: Caro et al., 1995); chimpanzees (Pan troglodytes: Caro et al., 1995); orangutans (Pongo pygmaeus: Caro et al., 1995); and gorillas (Gorilla gorilla: Caro et al., 1995). In captivity, the general observation is that females enjoy longer lifespans than their wild counterparts, not shorter reproductive spans. Many of these captive primate colonies serve as important models for research on human post-menopausal physiology and disease and have been documented to undergo both endocrinological and anatomical cessation of reproduction in addition to phenomenological and demographic cessation. Thus, it appears that conditions that produce low adult mortality and extended lifespan will very often result in a significant proportion of female mammals living a significant period after their last reproduction and, in primates at least, experiencing both follicular exhaustion and endocrinological menopause.
Post-Reproductive Life in Birds
There are few examples of post-reproductive lifespan in wild birds, though relevant data are sparse and difficult to obtain. In captivity and in domestic species, post-reproductive life is more common. For example, American kestrels maintained in outdoor pens show reproductive decline to the point of cessation at about 10 years of age (Holmes and Ottinger, 2003). Domestic fowl also commonly cease egg-laying well before natural death (Holmes et al., 2003).
Holmes et al. (2003) describe different “themes” or patterns of reproductive aging in birds (cf. Figure 14-3). One pattern, typical of most galliformes (quail, pheasant, chicken, etc.), includes shorter lifespans, rapid decline in age-specific fertility, and relatively common post-reproductive life in captivity and domesticity. A second pattern, typical of songbirds, small raptors, and parrots, includes medium to long lifespan, an initial rise in age-specific fertility after maturity followed by steady decline, and not infrequent post-reproductive life in captivity. A third pattern, typical of coastal and pelagic seabirds, includes very long lifespans and negligible or slow declines in age-specific fertility. A similar pattern of age-specific fertility can be observed across mammals (Cohen, 2004).
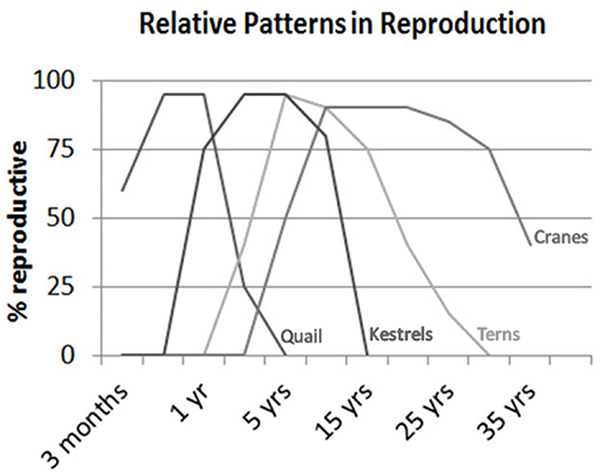
FIGURE 14-3 Variation in age-specific fertility among different groups of birds varying in average lifespan.
NOTE: Similar patterns of variation are observed in mammals.
SOURCE: Ottinger (2007).
Socioecological Correlates of Extended Post-Reproductive Life
Because the only species with well-documented, extended, female post-reproductive life in the “wild” are humans and a few toothed whales, some researchers have postulated that cooperative group living and the opportunity for older females to contribute behaviorally to the reproductive success of their offspring is an important correlate of extended female post-reproductive life (McAuliffe and Whitehead, 2005; Johnstone and Cant, 2010). This suggestion is difficult to test, however, given the paucity of examples of extended post-reproductive life in the wild. Certainly there are many cooperative breeding species of both birds and mammals in which extended post-reproductive life does not occur, as well as large, long-lived, highly social, matriarchal species such as baboons and African elephants (Moss, 2001; Alberts et al., 2013).
The more notable socioecological correlates are captivity and domesticity, which are regularly associated with significant post-reproductive life across a broad range of birds and mammals. Domesticity often alters natural life history in two important ways: It lowers age-specific rates of mortality and, especially in those species that are bred for reproductive output, it increases age-specific rates of fertility. Captivity often results in lower age-specific mortality. These changes in life history pattern almost certainly contribute to the regular appearance of significant periods of post-reproductive life. Lower mortality increases the likelihood that an individual female will outlive her finite supply of primordial follicles. And breeding for increased fecundity may increase the rate of transition from primordial to primary follicles, leading to earlier follicular exhaustion.
It is more onerous to speak of male post-reproductive life, since there is no necessary, physiological terminus to male fecundity. However, there is every reason to believe that male fecundity progressively declines to levels that are demographically insignificant in long-lived species such as humans.
Summary of Observations on the Phylogenetic Distribution of Post-Reproductive Life
The conserved mechanisms of reproductive aging in the females of birds and mammals make post-reproductive life a possibility. The ineluctable decline in primordial follicle supply makes reproductive cessation inevitable if an individual lives long enough. The dynamics of follicular stocking and depletion are subject to natural selection, however, and have led to female reproductive lifespans that are at least co-terminous with natural lifespans in the vast majority of birds and mammals. The only notable exceptions in the wild are certain toothed whales. However, many, perhaps most, birds and mammals evidence regular post-reproductive life in captivity and domesticity. These conditions are associated with lowered mortality and (in many domestic species) increased fecundity.
It is interesting to consider the human case against this background. Some researchers are drawn to parallels of social organization between toothed whales and humans as a potential common context in which female post-reproductive life appears. But there are also intriguing parallels between human ecology and captivity and domesticity. In particular, human life histories diverge from those of chimpanzees and other hominoids in two important respects: lower age-specific mortality and higher age-specific fecundity. Both of these traits may be related to the particular nature of human socioecology, including the sharing of food and work, the complementary division of labor among age and sex classes, and the emergence of “pooled energy budgets” to support individual physiology (Reiches et al., 2009; Kramer and Ellison, 2010). These are characteristics of socio-
ecology that are not shared by toothed whales, or perhaps any other vertebrate. The consequences of this socioecology for mortality and fertility patterns are similar to those seen in captive and domestic species. The “self-domestication” of humans may lie at the root of the phenomenon of extended post-reproductive life.
HUMAN SOCIALITY AND THE EVOLUTION OF POST-REPRODUCTIVE LIFE
In most wild vertebrates, and in particular in wild primates, including humans’ closest relatives, the chimpanzee, female lifespans and female reproductive spans are closely coterminous. Although it is not unprecedented among wild mammals, humans display a significant and lengthy gap between the average age at death and the average age at last reproduction. A similar gap can often be observed in captive and domestic species. Both in human and in captive and domestic species, this gap appears to be a function of lower adult mortality. With respect to chimpanzees, humans, and wild populations in both captivity and domesticated, it is possible that increased fecundity at younger ages also reflects a more rapid depletion of the primordial follicular supply. Rates of human follicular depletion and ages at reproductive cessation are nevertheless quite close to those of chimpanzees and therefore presumably to the two species’ last common ancestor (Jones et al., 2007). Adult mortality rates display considerable environmental plasticity, while rates of follicular depletion that ultimately relate to reproductive cessation in human females do not. The interim conclusions from the comparative perspective adopted in this paper provide the groundwork for any hypothesis construction relative to the evolutionary origins of long post-reproductive life in human females.
Most contemporary hypotheses for human menopause/female post-reproductive life compare the fitness of competing life history alternatives, given various assumptions about relevant costs and benefits. In terms of the adaptive landscape metaphor, this approach is basically a comparison of the height of various adaptive peaks, but does not take account of the available paths to those peaks. That is, little attention is given to the context in which this novel life history pattern would emerge from that of humans’ last common ancestor with chimpanzees. It is one thing to posit a fitness advantage to the provisioning behavior of post-reproductive females, for example, but that behavior cannot be used to explain the existence of post-reproductive females capable of doing that provisioning, nor can putative effects of this behavior on their post-reproductive survival explain the existence of a significant cohort of extant individuals at the average age of menopause. Only changes in the rate of adult survival before reproductive cessation can account for the initial existence of a significant number
of post-reproductive individuals. This also presumes that there is some heritable phenotypic variation in the age at provisioning and continuing this function into old age, which could depend on increasing the somatic longevity of those individuals. One set of hypotheses, developed by Chu and Lee (2013), does adopt an incremental approach, conceiving of the evolution of the current pattern through a series of intermediate steps, the first of which is the emergence of a division of labor based on age. But the Chu and Lee hypotheses assume that the evolved characteristic in humans is an early termination of reproduction relative to its ancestral state, whereas it seems clear that the evolved characteristic is prevalent and extended post-reproductive life, not premature reproductive cessation.
It seems to us that the most appropriate starting point to posit for the current life history pattern would be the regular appearance of a phenotypic gap between female lifespan and reproductive cessation, a gap similar to that observed in contemporary captive populations of primates (Ellison, 2010). Such a gap would occur as a natural expression of phenotypic plasticity when proto-human socioecology led to a reduced adult mortality rate without (yet) involving any change in the genetic basis of senescence or mortality. It is quite possible that such a change would have occurred with the intensification of social cooperation that led to regular food sharing and division of labor based on age and sex, all of which imply a model of cooperative energy pooling. An intensification of social cooperation of this kind features in virtually every scenario of human evolution. Given the prevalence of environmental plasticity in mortality and the lack of it in reproductive senescence, the opening of a phenotypic gap between female lifespan and reproductive cessation would be inevitable.
Once such a phenotypic gap emerged, it would have immediately imposed new selective pressures, since post-reproductive adults of zero reproductive value would be competing for shared resources with reproductive adults and immatures with positive reproductive value. From this formative location on the fitness landscape of human life history evolution, three paths lead to higher ground. That is, there are three directions in genetic change that could lead to greater fitness in the new socioecology. The three paths might be termed the “path of accelerated somatic senescence,” the “path of extended reproductive life,” and “the path of indirect reproductive effort.” Natural selection takes the path with the steepest initial ascent in fitness, not the one that necessarily leads to the highest adaptive peak.
The “path of accelerated somatic senescence” would increase fitness by increasing the rate of adult mortality through genetic change, shifting the norm of reaction so that lifespan and reproductive span are once again coterminous. This would eliminate the “parasitism” of post-reproductive individuals. The degree of fitness increase achieved via this path would depend on the seriousness of the parasitism, since it would only restore whatever fitness
had been sacrificed with the appearance of post-reproductive individuals. The phenotypic gap would be closed by genetically shortening lifespan.
The “path of extended reproductive life” would increase fitness by generating positive reproductive value for the post-reproductive individuals through genetic change. These changes would either have to increase the initial supply of primordial follicles or slow the rate of follicular depletion so that a sufficient follicular reserve would exist to support reproduction beyond the ordinary age of follicular exhaustion. For many evolutionary theorists, it seems clear that this path should lead to the highest adaptive peak, but it is not clear that its initial slope would be very steep. Because the rate of follicular depletion is exponential, changes in initial follicular supply would need to be prodigious to significantly extend reproductive life. The equivalent of two additional ovaries would be needed, for example, to delay follicular depletion by 3–4 years. In this context, it is notable that the African elephant, the only land mammal whose reproductive lifespan is thought to exceed the human by more than a decade, has an ovary that is an order of magnitude larger, while the human ovary remains approximately the same size as a chimpanzee’s. It would be interesting to have a clear comparative dataset that relates ovary size to longevity as well as to predicted number of offspring typical for that species. Changes in the rate of follicular depletion would potentially have a much stronger effect in shifting the age at follicular exhaustion, but their effect on fitness would be complicated by a countervailing decrease in fertility before that age caused by a consequent reduction in the size of the pool of growing follicles. Thus, although the path of extended reproductive life might eventually lead to the highest adaptive peak, its initial rate of ascent might be quite slow.
The “path of indirect reproductive effort” closes the phenotypic gap by selecting for behavior on the part of post-reproductive individuals that increases their inclusive fitness by increasing the reproductive success of relatives, either through direct contributions of time and energy, or through contributions to the common pool that result in benefits to their relatives as well as others. Because this “reproductive effort” is indirect, it must be discounted by effective degree of relatedness relative to potential direct reproductive effort. But the opportunity for contributions of this kind would likely be readily available, generated by the same shifts in socioecology that opened the phenotypic gap in the first place. Note, however, that in contrast to the standard formulation of the “grandmother hypothesis,” in this scenario it is the phenotypic occurrence of post-reproductive life that creates selection pressure for old age contributions to the reproductive fitness of younger relatives, not the other way around. Given the presence of post-reproductive individuals arising due to the phenotypic gap between lifespan and reproductive cessation, those who engage in indirect reproductive effort will contribute more copies of their genes to future generations than those
who do not. Once begun, this path is then likely to be reinforced by positive feedback, since as positive reproductive value accrues to post-reproductive individuals, weak selection in favor of delayed somatic senescence will be generated, extending lifespan even further.
The most important aspects of this analysis in comparison to previous treatments are (1) a foundation in the comparative biology of reproductive cessation and post-reproductive life that leads to an appreciation of the likelihood of a phenotypic gap appearing between female lifespan and reproductive cessation, and (2) an attention to the selective pressures generated by this phenotypic gap and the potential for initial increases in fitness due to different responses rather than a comparison of ultimate fitness peaks. We conclude that the path of indirect reproductive effort may have provided the path of steepest initial ascent, and that the path of extended reproductive life may be compromised by insensitivity to changes in initial follicular supply and fitness tradeoffs associated with decreases in the rate of follicular depletion. The result is that the derived life history trait in humans is extended post-reproductive life, supported by indirect reproductive effort, not any change in the trajectory of follicular depletion.
Our analysis is compatible, though derived differently, with hypotheses that suggest that menopause was “uncovered” by an evolved extension of lifespan and not a novel feature of ovarian physiology. It is also compatible with data used to support the “grandmother hypothesis,” though as noted, our analysis suggests that post-reproductive life selects for “grandmotherly” behavior, not the other way around. A notable corollary is that indirect reproductive effort need not be confined to post-reproductive individuals. Immature, “pre-reproductive” individuals might also engage in indirect reproductive effort by contributing time and energy to the reproductive success of relatives. Another anomalous feature of human life histories is that the rate of reproduction has increased since the last common ancestor with chimpanzees, while the rate of maturation has decreased. Ordinarily across mammalian taxa, rate of growth during immaturity and rate of reproduction once mature are highly correlated, representing the same metabolic effort net of survival and maintenance. In humans this is not the case. This anomaly may be partly explained by immature individuals diverting some metabolic effort to indirect reproductive effort in advance of their own reproductive maturation. They may, in effect, be “grandparents before their time,” performing menial, unskilled tasks that leverage the skilled, more productive efforts of older individuals.
CONCLUSION
The physiological mechanisms that underlie reproductive aging in vertebrates are highly conserved. Prominent among these mechanisms in birds
and mammals are the sexually dimorphic patterns of gamete production and the integrated nature of the neuroendocrine control of reproduction. The phylogenetically ancient pattern of temporally restricted production of a finite pool of primordial follicles in birds and mammals is particularly notable and evolutionarily curious. But since this pattern of female gamete production has evolved, no mammal or bird species is known to have lost it. Hence its fitness value must be high and tightly bound to the reproductive biology of birds and mammals, or it must be a pattern that is very difficult to change due to developmental constraint, or both.
This pattern of female gamete production, involving both a finite primordial follicle supply and an ineluctable attrition in that supply with age, makes female post-reproductive life possible and even predictable, should a female live long enough. Post-reproductive lifespan appears to be rare in wild birds and mammals, though the necessary data are difficult to obtain and not widely available. Ecologies associated with reduced mortality rates, such as captivity and domesticity, commonly result in significant rates and durations of post-reproductive life. No other socioecological correlates manifest such a regular association with post-reproductive lifespan.
Human females do manifest regular and lengthy post-reproductive life. They also manifest lower age-specific mortality and higher age-specific fertility than expected for a hominoid of their size. Hence humans fit the socioecological pattern established by captive and domestic birds and mammals. In order to understand how this life history pattern may have emerged from that of a chimpanzee-like hominoid ancestor, it is necessary to consider what might have led to changes in age-specific mortality that would have created a “phenotypic gap” between reproductive and total lifespan in females, and then what the novel selective forces resulting from that “phenotypic gap” would have been. We argue that the pathway of steepest initial increase in fitness (as distinct from the pathway leading to the greatest ultimate level of fitness) would have favored “indirect reproductive effort” on the part of the newly emerged age-sex class of post-reproductive individuals. Thus, extended lifespan would have selected for indirect reproductive effort, not the other way around.
We also suggest that, although male gamete production does not lead ineluctably to age-related sterility as in the female case, age-related decline in male fecundity may not simply be a matter of senescence. It may be adaptive in its own right, especially in a long-lived, social species with bi-parental care and extended parental investment, like humans. Selection for reduced mating effort with age in human males may lead to “effective” post-reproductive life under the same socioecological conditions that generate it in females. If so, post-reproductive males would also be subject to selection for indirect reproductive effort, although perhaps to a lesser degree than females, due to the continued possibility, at least theoretically, for direct reproductive effort.
REFERENCES
Alberts, S.C., Altmann, J., Brockman, D.K., Cords, M., Fedigan, L.M., Pusey, A., Stoinski, T.S., Strier, K.B., Morris, W.F., and Bronikowski, A.M. (2013). Reproductive aging patterns in primates reveal that humans are distinct. Proceedings of the National Academy of Sciences of the United States of America, 110(33), 13440-13445.
Anderson, A.C. (1965). Reproductive ability of female beagles in relation to advancing age. Experimental Gerontology, 1, 189-192.
Aranzàbal, M.C.U. (2011). Hormones and the female reproductive system of amphibians. In D.O. Norris and K.H. Lopez (Eds.), Hormones and Reprodution of the Vertebrates (Vol. 2, pp. 55-82). Amsterdam: Elsevier.
Begum, S., Papaioannou, V.E., and Gosden, R.G. (2008). The oocyte population is not renewed in transplanted or irradiated adult ovaries. Human Reproduction, 23(10), 2326-2330.
Black, A., and Lane, M.A. (2002). Nonhuman primate models of skeletal and reproductive aging. Gerontology, 48(2), 72-80.
Borries, C., Sommer, V., and Srivastava, A. (1991). Dominance, age, and reproductive success in female Hanuman langurs (Presbtis entellus). International Journal of Primatology, 12, 231-257.
Brann, D.W., and Mahesh, V.B. (2005). The aging reproductive neuroendocrine axis. Steroids, 70(4), 273-283.
Cant, M.A., and Johnstone, R.A. (2008). Reproductive conflict and the separation of reproductive generations in humans. Proceedings of the National Academy of Sciences of the United States of America, 105(14), 5332-5336.
Caro, T.M., Sellen, D.W., Parish, A., Frank, R., Brown, D.M., Voland, E., and Borgerhoff Mulder, M. (1995). Termination of reproduction in nonhuman and human female primates. International Journal of Primatology, 16, 205-220.
Chen, H., Hardy, M.P., and Zirkin, B.R. (2002). Age-related decreases in Leydig cell testosterone production are not restored by exposure to LH in vitro. Endocrinology, 143(5), 1637-1642.
Chu, C.Y.C., and Lee, R.D. (2013). On the evolution of intergenerational division of labor, menopause, and transfers among adults and offspring. Journal of Theoretical Biology, 332, 171-180.
Cohen, A.A. (2004). Female post-reproductive lifespan: A general mammalian trait. Biological Reviews of the Cambridge Philosophical Society, 79(4), 733-750.
Comfort, A. (1979). The Biology of Senescence. New York: Elsevier.
Coxworth, J.E., and Hawkes, K. (2010). Ovarian follicle loss in humans and mice: Lessons from statistical model comparison. Human Reproduction, 25(7), 1796-1805.
Depalo, R., Nappi, L., Loverro, G., Bettocchi, S., Caruso, M.L., Valentini, A.M., and Selvaggi, L. (2003). Evidence of apoptosis in human primordial and primary follicles. Human Reproduction, 18(12), 2678-2682.
Downs, J.L., and Wise, P.M. (2009). The role of the brain in female reproductive aging. Molecular and Cellular Endocrinology, 299(1), 32-38.
Durlinger, A.L., Gruijters, M.J., Kramer, P., Karels, B., Ingraham, H.A., Nachtigal, M.W., Uilenbroek, J.T., Grootegoed, J.A., and Themmen, AP. (2002). Anti-Müllerian hormone inhibits initiation of primordial follicle growth in the mouse ovary. Endocrinology, 143(3), 1076-1084.
Dyke, B., Gage, T.B., Mamelka, P.M., Goy, R.W., and Stone, W.H. (1986). A demographic analysis of the Wisconsin USA Regional Primate Research Center rhesus colony 1962-1982. American Journal of Primatology, 10, 257-270.
Edson, M.A., Nagaraja, A.K., and Matzuk, M.M. (2009). The mammalian ovary from genesis to revelation. Endocrine Reviews, 30(6), 624-712.
Eggan, K., Jurga, S., Gosden, R., Min, I.M., and Wagers, A.J. (2006). Ovulated oocytes in adult mice derive from non-circulating germ cells. Nature, 441(7097), 1109-1114.
Ellison, P.T. (2010). Life historical perspectives on human reproductive aging. Annals of the New York Academy of Sciences, 1204, 11-20.
Ellison, P.T., Bribiescas, R.G., Bentley, G.R., Campbell, B.C., Lipson, S.F., Panter-Brick, C., and Hill, K. (2002). Population variation in age-related decline in male salivary testosterone. Human Reproduction, 17(12), 3251-3253.
Emery Thompson, M., Jones, J.H., Pusey, A.E., Brewer-Marsden, S., Goodall, J., Marsden, D., and Wrangham, R.W. (2007). Aging and fertility patterns in wild chimpanzees provide insights into the evolution of menopause. Current Biology, 17(24), 2150-2156.
Evenson, D.P., and Wixon, R. (2006). Clinical aspects of sperm DNA fragmentation detection and male infertility. Theriogenology, 65(5), 979-991.
Faddy, M.J., and Gosden, R.G. (1995). A mathematical model of follicle dynamics in the human ovary. Human Reproduction, 10(4), 770-775.
Faddy, M.J., and Gosden, R.G. (2007). Numbers of ovarian follicles and testing germ line renewal in the postnatal ovary: Facts and fallacies. Cell Cycle, 6(15), 1951-1952.
Fedigan, L. (1991). Life span and reproductin in Japanese macaque females. In L.M. Fedigan and P.J. Asquith (Eds.), The Monkeys of Arashiyama: Thirty-five Years of Research in Japan and the West (pp. 140-154). New York: State University of New York Press.
Ferrell, R.J., Rodriguez, G., Holman, D., O’Connor, K., Wood, J.W., and Weinstein, M. (2012). Hypoestrogenic “inactive phases” at the start of the menstrual cycle: Changes with age and reproductive stage, and relationship to follicular depletion. Fertility and Sterility, 98(5), 1246-1253.
Finch, C.E. (2013). The menopause and aging, a comparative perspective. The Journal of Steroid Biochemistry and Molecular Biology, 142C, 132-141.
Finch, C.E., and Holmes, D.J. (2010). Ovarian aging in developmental and evolutionary contexts. Annals of the New York Academy of Sciences, 1204, 82-94.
Fisher, M.W., McLeod, B.J., Mockett, B.G., Moore, G.H., and Drew, K.R. (1966). Reproductive senescence in aged red deer hinds. Proceedings of the New Zealand Society of Animal Production, 56, 344-346.
Flament, S., Chardard, D., Chesnel, A., and Dumond, H. (2011). Sex determination and sexual differentiation in amphibians. In D.O. Norris and K.H. Lopez (Eds.), Hormones and Reproduction of Vertebrates. (Vol. 2, pp. 1-20). Amsterdam: Elsevier.
Fortune, J.E., Cushman, R.A., Wahl, C.M., and Kito, S. (2000). The primordial to primary follicle transition. Molecular and Cellular Endocrinology, 163(1-2), 53-60.
Franchi, L.L., Mandl, A.M., and Zuckerman, S. (1962). The development of the ovary and the process of oögenesis. In S. Zuckerman (Ed.), The Ovary (Vol. 1, pp. 1-88). New York: Academic Press.
Gougeon, A., Ecochard, R., and Thalabard, J.C. (1994). Age-related changes of the population of human ovarian follicles: Increase in the disappearance rate of non-growing and early-growing follicles in aging women. Biology of Reproduction, 50(3), 653-663.
Ha, J.C., Robinette, R.L., and Sackett, G.P. (2000). Demographic analysis of the Washington Regional Primate Research Center pigtailed macaque colony, 1967-1996. American Journal of Primatology, 52, 187-198.
Hansen, K.R., Knowlton, N.S., Thyer, A.C., Charleston, J.S., Soules, M.R., and Klein, N.A. (2008). A new model of reproductive aging: The decline in ovarian non-growing follicle number from birth to menopause. Human Reproduction, 23(3), 699-708.
Hardy, M.P., and Schlegel, P.N. (2004). Testosterone production in the aging male: Where does the slowdown occur? Endocrinology, 145(10), 4439-4440.
Harman, S.M., Metter, E.J., Tobin, J.D., Pearson, J., and Blackman, M.R. (2001). Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. The Journal of Clinical Endocrinology & Metabolism, 86(2), 724-731.
Hawkes, K. (2003). Grandmothers and the evolution of human longevity. American Journal of Human Biology, 15(3), 380-400.
Hawkes, K., Kim, P.S., Kennedy, B., Bohlender, R., and Hawks, J. (2011). A reappraisal of grandmothering and natural selection. Proceedings of the Royal Society B: Biological Sciences, 278(1714), 1936-1938; discussion 1939-1941.
Henkel, R., Maass, G., Schuppe, H.C., Jung, A., Schubert, J., and Schill, W.B. (2005). Molecular aspects of declining sperm motility in older men. Fertility and Sterility, 84(5), 1430-1437.
Herndon, J.G., and Walker, L.C. (2010). The grandmother effect and the uniqueness of the human aging phenotype. Gerontology, 56(2), 217-219.
Holmes, D.J., and Ottinger, M.A. (2003). Birds as long-lived animal models for the study of aging. Experimental Gerontology, 38(11-12), 1365-1375.
Holmes, D.J., Thomson, S.L., Wu, J., and Ottinger, M.A. (2003). Reproductive aging in female birds. Experimental Gerontology, 38(7), 751-756.
Ireland, J.J., Smith, G.W., Scheetz, D., Jimenez-Krassel, F., Folger, J. K., Ireland, J. L., Mossa, F., Lonergan, P., and Evans, A.C. (2011). Does size matter in females? An overview of the impact of the high variation in the ovarian reserve on ovarian function and fertility, utility of anti- Müllerian hormone as a diagnostic marker for fertility and causes of variation in the ovarian reserve in cattle. Reproduction, Fertility and Development, 23(1), 1-14.
Johnson, A.L. (2011). Organization and functional dynamics of the avian ovary. In D.O. Norris and K.H. Lopez (Eds.), Hormones and Reprodution of the Vertebrates (Vol. 4, pp. 71-90). Amsterdam: Elsevier.
Johnson, R.L., and Kapsalis, E. (1995). Ageing, infecundity and reproductive senescence in free-ranging female rhesus monkeys. Journal of Reproduction and Fertility, 105, 271-278.
Johnson, R.L., and Kapsalis, E. (1998). Menopause in free-ranging rhesus macaques: Estimated incidence, relatin to body condition, and adaptive significance. International Journal of Primatology, 19, 751-765.
Johnson, J., Canning, J., Kaneko, T., Pru, J.K., and Tilly, J.L. (2004). Germline stem cells and follicular renewal in the postnatal mammalian ovary. Nature, 428(6979), 145-150.
Johnstone, R.A., and Cant, M.A. (2010). The evolution of menopause in cetaceans and humans: The role of demography. Proceedings of the Royal Society B: Biological Sciences, 277(1701), 3765-3771.
Jones, S.M. (2011). Hormonal regulation of ovarian function in reptiles. In D.O. Norris and K.H. Lopez (Eds.), Hormones and Reproduction of Vertebrates. (Vol. 3, pp. 89-116). Amsterdam: Elsevier.
Jones, K.P., Walker, L.C., Anderson, D., Lacreuse, A., Robson, S.L., and Hawkes, K. (2007). Depletion of ovarian follicles with age in chimpanzees: Similarities to humans. Biological Reproduction, 77(2), 247-251.
Kaplan, H., Gurven, M., Winking, J., Hooper, P.L., and Stieglitz, J. (2010). Learning, menopause, and the human adaptive complex. Annals of the New York Academy of Sciences, 1204, 30-42.
Kasuya, T., and Marsh, H. (1984). Life history and reproductive biology of the short-fined pilot whale, Gloicephala macrorhyncus, off the Pacific coast of Japan. Reports of the International Whaling Commision, Special Issue 6, 259-310.
Kerr, J.B., Brogan, L., Myers, M., Hutt, K. J., Mladenovska, T., Ricardo, S., Hamza, K., Scott, C.L., Strasser, A., and Findlay, J.K. (2012). The primordial follicle reserve is not renewed after chemical or gamma-irradiation mediated depletion. Reproduction, 143(4), 469-476.
Kidd, S.A., Eskenazi, B., and Wyrobek, A.J. (2001). Effects of male age on semen quality and fertility: A review of the literature. Fertility and Sterility, 75(2), 237-248.
Kramer, K.L., and Ellison, P.T. (2010). Pooled energy budgets: Resituating human energy allocation trade-offs. Evolutionary Anthropology, 19, 136-147.
Laws, R.M., Parker, I.S.C., and Johnstone, R.C.B. (1975). Elephants and their Habits: The Ecology of Elephants in North Bunyoro, Uganda. Oxford, UK: Clarendon Pess.
Levitis, D.A., Burger, O., and Lackey, L.B. (2013). The human post-fertile lifespan in comparative evolutionary context. Evolutionary Anthropology, 22(2), 66-79.
Mace, R. (2000). Evolutionary ecology of human life history. Animal Behaviour, 59(1), 1-10.
McAuliffe, K., and Whitehead, H. (2005). Eusociality, menopause and information in matrilineal whales. Trends in Ecology & Evolution, 20(12), 650.
McGee, E.A., and Hsueh, A.J. (2000). Initial and cyclic recruitment of ovarian follicles. Endocrine Reviews, 21(2), 200-214.
Mittledorf, J., and Goodnight, C. (2012). Post-reproductive life span and demographic stability. Oikos, 121, 1370-1378.
Moffat, S.D., Zonderman, A.B., Metter, E.J., Blackman, M.R., Harman, S.M., and Resnick, S.M. (2002). Longitudinal assessment of serum free testosterone concentration predicts memory performance and cognitive status in elderly men. The Journal of Clinical Endocrinology & Metabolism, 87(11), 5001-5007.
Moss, C.J. (2001). The demography of an African elephant (Loxodonta africana) population in Amboseli, Kenya. Journal of Zoology, 255, 145-156.
Norris, D.O., and Lopez, K.H. (2011). The endocrinology of the mammalian ovary. In D.O. Norris and K.H. Lopez (Eds.), Hormones and Reproduction of Vertebrates. (Vol. 5, pp. 59-72). Amsterdam: Elsevier.
Nozaki, M., Mitsunaga, F., and Shimizu, K. (1995). Reproductive senescence in female Japanese monkeys (Macaca fuscata): Age- and season-related changes in hypothalamic-pituitary-ovarian functions and fecundity rates. Biology of Reproduction, 52, 1250-1257.
Olesiuk, P.F., Bigg, M.A., and Ellis, G.M. (1990). Life history and populatin dynamics of resident killer whales (Orcinus orca) in the coast waters of British Columbia and Washington State. Reports of the International Whaling Comision, Special Issue 12, 209-243.
Ottinger, M.A. (1998). Male reproductin: Testosterone, gonadotropins, and aging. In C.V. Mobbs and P.R. Hof (Eds.), Interdisciplinary Topics in Gerontology: Functional Endocrinology of Aging. New York: S. Karger AG.
Ottinger, M.A. (2007). Neuroendocrine aging in birds: Comparing lifespan differences and conserved mechanisms. Ageing Research Reviews 6, 46-53.
Packer, C., Tatar, M., and Collins, A. (1998). Reproductive cessation in female mammals. Nature, 392(6678), 807-811.
Parkening, T.A. (1982). Reproductive senescence in the Chinese hamster (Cricetulus griseus). Journal of Gerontology, 37, 283-287.
Paul, A., Keuster, J., and Podzuweit, D. (1993). Reproductive senesscence and terminal investment in female Barbary macaques (Macaca sylvanus) at Salem. International Journal of Primatology, 14, 105-124.
Perheentupa, A., and Huhtaniemi, I. (2009). Aging of the human ovary and testis. Molecular and Cellular Endocrinology, 299(1), 2-13.
Plas, E., Berger, P., Hermann, M., and Pfluger, H. (2000). Effects of aging on male fertility? Experimental Gerontology, 35(5), 543-551.
Pollycove, R., Naftolin, F., and Simon, J.A. (2011). The evolutionary origin and significance of menopause. Menopause, 18(3), 336-342.
Ramsay, M.A., and Stirling, I. (1988). Reproductive biology and eology of female polar bears Ursus maritimus. Journal of Zoology (London), 214, 601-634.
Reiches, M.W., Ellison, P.T., Lipson, S.F., Sharrock, K.C., Gardiner, E., and Duncan, L.G. (2009). Pooled energy budget and human life history. American Journal of Human Biology, 21(4), 421-429.
Richardson, S.J., Senikas, V., and Nelson, J.F. (1987). Follicular depletion during the menopausal transition: Evidence for accelerated loss and ultimate exhaustion. The Journal of Clinical Endocrinology & Metabolism, 65(6), 1231-1237.
Sitzmann, B.D., Urbanski, H.F., and Ottinger, M.A. (2008). Aging in male primates: Reproductive decline, effects of calorie restriction and future research potential. Age, 30(2-3), 157-168.
Sitzmann, B.D., Leone, E.H., Mattison, J.A., Ingram, D.K., Roth, G.S., Urbanski, H.F., Zelinski, M.B., and Ottinger, M.A. (2010). Effects of moderate calorie restriction on testosterone production and semen characteristics in young rhesus macaques (Macaca mulatta). Biology of Reproduction, 83(4), 635-640.
Syntin, P., and Robaire, B. (2001). Sperm structural and motility changes during aging in the Brown Norway rat. Journal of Andrology, 22(2), 235-244.
Takahata, Y., Koyama, N., and Suzuki, S. (1995). Do the old aged females experience a long post-reproductive life span? The cases of Japanese macaques and chimpanzees. Primates, 36, 169-180.
Tardif, S.D., and Ziegler, T.E. (1992). Features of female reproductive senescence in tamarins (Saguinus spp.), a New World primate. Journal of Reproduction and Fertiity, 94(2), 411-421.
Tigges, J., Gordon, T.P., McClure, H.M., Hall, E.C., and Peters, A. (1988). Survival rates and life span of rhesus monkeys at the Yerkes Regional Primate Research Center, Atlanta, Georgia, USA. American Journal of Primatology, 15, 163-174.
Uchida, A., Bribiescas, R.G., Ellison, P.T., Kanamori, M., Ando, J., Hirose, N., and Ono, Y. (2006). Age related variation of salivary testosterone values in healthy Japanese males. Aging Male, 9(4), 207-213.
Urbatzka, R., Rocha, M.J., and Rocha, E. (2011). Regulation of ovarian development and function in teleosts. In D.O. Norris and K.H. Lopez (Eds.), Hormones and Reproduction of Vertebrates. (Vol. 1, pp. 65-82). Amsterdam: Elsevier.
Veldhuis, J.D., Keenan, D.M., Liu, P.Y., Iranmanesh, A., Takahashi, P.Y., and Nehra, A.X. (2009). The aging male hypothalamic-pituitary-gonadal axis: Pulsatility and feedback. Molecular and Cellular Endocrinology, 299(1), 14-22.
vom Saal, F.S., Finch, C.E., and Nelson, J.F. (1994). Natural history and mechanisms of reproductive aging in humans, laboratory rodents, and other selected vertebrates. In E. Knobil and J.D. Neill (Eds.), The Physiology of Reproduction (pp. 1213-1314). New York: Raven Press.
Walker, M.L. (1995). Menopause in female rhesus monkeys. American Journal of Primatology, 35, 59-71.
Woods, D.C., White, Y.A., and Tilly, J.L. (2013). Purification of oogonial stem cells from adult mouse and human ovaries: An assessment of the literature and a view toward the future. Reproductive Sciences, 20(1), 7-15.
Zirkin, B.R., and Chen, H. (2000). Regulation of Leydig cell steroidogenic function during aging. Biology of Reproduction, 63(4), 977-981.
Zubkova, E.V., and Robaire, B. (2006). Effects of ageing on spermatozoal chromatin and its sensitivity to in vivo and in vitro oxidative challenge in the Brown Norway rat. Human Reproduction, 21(11), 2901-2910.
























