Susan C. Alberts,* Elizabeth A. Archie,* Laurence R. Gesquiere, Jeanne Altmann, James W. Vaupel, and Kaare Christensen
INTRODUCTION
The male-female health-survival paradox—the phenomenon observed in modern human societies in which women experience greater longevity and yet higher rates of disability and poor health than men—has far-reaching economic, sociological, and medical implications. Prevailing evidence indicates that men die at younger ages than women, despite better health, because of both biological and environmental differences that include behavioral, cultural, and social factors (Wingard, 1984; Verbrugge, 1985, 1989; Kinsella and Gist, 1998; Kinsella, 2000; Case and Paxson, 2005; Oksuzyan et al., 2008; Lindahl-Jacobsen et al., 2013). The male-female health-survival paradox is very well documented in late 20th century high-income countries (Crimmins et al., 2011; Thorslund et al., 2013; Oksuzyan et al., 2014). For instance, cross-national comparisons between the United States, Europe (Denmark), and Japan found consistent but opposite sex differences in survival and health: Men had higher mortality rates at all ages in all three countries, but men also exhibited a substantial advantage in handgrip strength and in activity of daily living at older ages—phenotypes that in both sexes are positively correlated with survival (Oksuzyan et al., 2010).
The mortality part of the paradox, the female survival advantage, has been well documented earlier than the 20th century. In fact, in the very first
_______________
*Co-first authors.
lifetables that were categorized by sex, estimated by Struyck (1740) and Deparcieux (1746), female life expectancy exceeded that of males. More than 250 years later, Thorslund et al. (2013) reported on life expectancy data at age 65 for 16 Western countries and Japan, covering various parts of the period from 1751 to 2007. During the 19th century in Western societies, women generally had a constant, higher life expectancy than men at age 65, although the difference was less than 1 year. The 20th century saw rapid country-specific rises in life expectancy, increasing the male-female gap to approximately 4 years, but with variance across countries. During the last three decades, however, all 16 countries experienced a simultaneous narrowing of the gap to 0.5-1 years. This suggests that country-specific factors may have driven the rise in female advantage in life expectancy, whereas factors shared by all countries may underlie the simultaneous fall (Thorslund et al., 2013). There is general agreement that changes in cigarette smoking is the largest identifiable factor in explaining changes in the sex gap in mortality in the developed countries (Pampel, 2003; Payne, 2004; Preston and Wang, 2006; Jacobsen et al., 2008; Leon, 2011; Lindahl-Jacobsen et al., 2013).
With regard to the health part of the paradox, the female disadvantages in health and functioning, research on contemporary populations generally suggests that men are physically stronger, report fewer diseases, and have fewer limitations in the activities of daily living at older ages than women. However, the issue of sex differences in morbidity is more complex than the pattern in activities of daily living and physical performance tests because of variation in the definitions of diseases, diagnostic procedures, and age-related change in incidence and prevalence of many diseases. For example, the incidence of coronary heart disease starts to rise earlier for men than for women, but the sex difference in heart disease is small at the oldest ages. Women generally have a significantly higher mean number of reported disabling, nonlethal conditions than men (Hjertestatistik, 2004; Crimmins et al., 2011). Hence, sex differences in morbidity depend on disease definitions, the measure of severity, and age trajectories of the particular diseases.
It is generally not clear whether sex differences in health also occur in populations that experience living conditions and cultures very different from contemporary Western societies. For instance, historical populations with very different cultural practices, such as low-risk male behavior combined with high fertility (and hence high risk of female mortality), might have experienced much less of a male-female health-survival paradox than modern human populations, which are characterized by high-risk male behavior but relatively low fertility. As another example, in human populations with extremely high male mortality relative to female mortality, male health might also be more compromised than it is in high-income Western societies.
It is even less clear whether the male-female health-survival paradox is preserved across species: Somewhat surprisingly, no systematic investigations of the paradox exist for nonhuman animals. Research on aging in wild or semi-natural vertebrate populations has generally focused on demographic senescence alone (increases in mortality rates with age), rather than on declines in health or functioning with age (Brunet-Rossinni and Austad, 2006). Research on aging in insects has focused on the molecular basis of aging variation across species and between males and females (reviewed in Keller and Jemielty, 2006) and more recently on how the social environment influences aging, particularly in honey bees (Amdam, 2011). In spite of the significant advances made by these various studies on vertebrates and invertebrates, much remains unknown about the evolutionary significance and proximate mechanisms underlying male-female differences in lifespan. Studies of mortality in animal populations suggest that males experience higher mortality than females in many species, particularly mammals (Promislow and Harvey, 1990; Forsyth et al., 2004; Clutton-Brock and Isvaran, 2007), but they also suggest that this may not be a general rule in either vertebrates or invertebrates (McDonald, 1993; Allman et al., 1998; Carey, 2003). Data regarding the second element of the paradox, sex differences in health, are sparser than mortality data. Some data have arisen from animal models of particular human traits or conditions (e.g., menopause: Bellino and Wise, 2003; memory loss: Picq, 2007; Parkinson disease: Smith and Cass, 2007), but such studies rarely involve systematic investigations of sex differences in these health measures with age.
A relatively recent evolutionary framework predicts that, in many species, males will tend to have worse health than females of the same age, as well as shorter lifespans, because in many cases the most important component of male fitness is mating success rather than investment in health maintenance (Rolff, 2002; Zuk and Stoehr, 2002; Stoehr and Kokko, 2006). This framework thus posits an explicit tradeoff between investment in mating activity and investment in somatic maintenance. Furthermore, males in many species gain substantial fitness benefits from seeking additional mates while females generally do not (Bateman, 1948). The energetic demands of obtaining additional mates will often require the sacrifice of somatic maintenance in general and immune function in particular. The consequence is that males are predicted to show compromised immune function and health relative to females, while females maximize fitness by investing in immune function and thus enhancing longevity. Importantly, this framework, sometimes called “Bateman’s Principle for Immunity,” predicts no health-survival paradox, but instead predicts that females in many vertebrate species will experience both greater health and greater longevity than males. Nonetheless, it represents one of the few well-developed evolutionary frameworks for predictions about male-female differences in
health, and has received some empirical support; for instance, Nunn and colleagues (2009) found a positive association between sex differences in a measure of immune function and sex differences in investment in mating. However, very few data on health and functioning over the lifespan exist for animals of either sex in any species.
OBJECTIVES
Here we provide a comparative perspective on the male-female health-survival paradox. First, we examine health and survival patterns in humans living in unusual demographic circumstances to determine whether they show a non-paradoxical pattern. Specifically, we summarize recent evidence on the health-survival paradox in a 20th century Russian population and on female survival advantages in the late 19th and early 20th century Mormon population and other historic and prehistoric populations.
Second, we examine age-specific changes in health-related measures in a nonhuman primate in which male life expectancy is short relative to females, to determine whether they conform to the paradoxical pattern described in humans. Specifically, we provide a detailed analysis of age-related declines in health and physical functioning in a wild baboon population in southern Kenya. Baboons are a good choice of species from a comparative evolutionary perspective because baboons, like humans, are diurnal, ecologically flexible omnivores that evolved in a savannah environment. Males in our study population experience both a higher initial mortality rate than females at the beginning of adulthood and a faster acceleration in age-specific mortality with increasing age (Alberts and Altmann, 2003; Bronikowski et al., 2011). By comparing the health trajectories of males and females, we examine whether baboons, like many human societies, experience a health-survival paradox.
RESULTS: HUMAN STUDIES
At the beginning of the 21st century, it is well established that females, on average, outlive men in all countries around the globe (Barford et al., 2006). In high-income countries, they generally do so despite more disabilities and worse self-reported health. In this section, we explore patterns of all-cause mortality in four sets of populations, working our way backwards in time to shed light on whether:
- the male health advantage is present in a contemporary Russian population with extreme excess male mortality;
- the female survival advantage was present in the late 19th and early 20th century Mormon population living in Utah, in which
-
male risk-taking behavior was minimized by societal norms and fertility was high;
- the female survival advantage was present in other 19th and 20th century populations; and
- the female survival advantage was present prior to the 18th century using preliminary paleodemographic data.
The Male-Female Health-Survival Pattern in a Contemporary Russian Population
Life expectancy in Russia is lagging behind that in the United States and Europe, and this difference has been very pronounced since the 1960s (Shkolnikov and Meslé, 1996; Meslé, 2004; Oksuzyan et al., 2014). In Russia in 2009, life expectancy was 74.7 years for women and 62.7 years for men. The female-male gap in life expectancy in Russia increased from 8.3 years in 1953 to the maximum level of 13.6 years in 2005 with a decline in 1986-1987 (to 9.4 years) in connection with Gorbachev’s anti-alcohol campaign and a steeper reduction in male than female mortality (Human Mortality Database; Field, 2000). Although a narrowing of the sex difference in life expectancy in Russia has occurred since 2006, the sex gap of 11.9 years in 2009 was second only to Kazakhstan as the highest in the world.
In Russia, the main contributors to the declining life expectancy for younger and middle-aged adults from 1988 to 2000 were deaths due to cardiovascular diseases, violence, accidents, and alcohol-related causes (Meslé, 2004; Zaridze et al., 2014). Also, higher mortality rates were observed in Russia at older ages than in other European countries, suggesting worse health in Russia than in old-aged populations elsewhere. A study conducted in the 1990s showed that middle-aged Russians and Swedes had similar prevalence of poor self-rated health and disability, but after about age 45, the prevalence of good general health and the level of physical functioning were substantially lower in Russia compared to Sweden (Bobak et al., 2004). Another study of Russian men and women in the 1990s showed a much steeper decline with age in the probability of being healthy, in comparison not only to the populations in Western Europe, but also to the former communist Eastern European countries (Andreev et al., 2003).
Recently, we have studied sex gaps in mortality rates in Denmark, Russia, and Moscow, as well as sex differences in several health outcomes in Denmark and Moscow among individuals aged 55 to 89 years (Oksuzyan et al., 2014). Pronounced male excess mortality in Russia led us to expect smaller male advantages in selected health domains in Russia compared to Denmark.
The Human Mortality Database and the Russian Fertility and Mortality Database were used to examine sex differences in all-cause death rates in Denmark, Russia, and Moscow in 2007-2008. Self-reported health data were obtained from the Study of Middle-Aged Danish Twins (n = 4,314), the Longitudinal Study of Aging Danish Twins (n = 4,731), and the study of Stress, Aging, and Health in Russia (n = 1,800). In both Moscow and Denmark there was a consistent female advantage in survival at ages 55-89 years and a male advantage in self-rated health, physical ability, and depression symptomatology. Only on cognitive tests did men perform similarly to, or worse than, women. In other words, on the large majority of health indicators, Muscovite males performed better than females. This occurred despite Muscovite men having twice the mortality of Muscovite women at ages 55-69 years, a male-female ratio almost twice as large as that seen in Denmark. Hence, the male-female health-survival paradox is very pronounced in this contemporary Russian population.
Sex Differences in Survival in the Late 19th and Early 20th Century Utah Population
Behavioral factors have been proposed as a key source of female-male differences in mortality, with risk-taking behaviors—including cigarette smoking and alcohol consumption— occurring more frequently among men than among women. Cigarette smoking is the largest identifiable factor in explaining changing sex gaps in mortality, but it is well known that cigarette smoking alone cannot explain the sex difference in mortality; for instance, male non-smokers have higher mortality than female non-smokers (Wang and Preston, 2009).
With this background, we hypothesized that the late 19th and early 20th century Utah male-female survival difference should be among the lowest observed and smaller than that in Denmark and Sweden (Lindahl-Jacobsen et al., 2013). This hypothesis is based on the fact that many residents in Utah in this period were active in the Mormon Church, which proscribes the use of alcohol and tobacco, and whose members would therefore have a healthier lifestyle than the general population with regard to typical male risk factors. This lifestyle was common among members of the Church during the early settlement years, though it was not enforced until the 1860s (Alexander, 1981) and was not institutionalized until 1906 with the Word of Wisdom (Bush, 1993; Alexander, 1996). Females, on the other hand, had a very high fertility level, which was associated with increased maternal mortality risks (Skolnick et al., 1978). We anticipated that the female longevity advantage would grow over the last half of the 19th and early part of the 20th centuries, as their elevated fertility declined during the demographic transition. Denmark and Sweden were chosen as com-
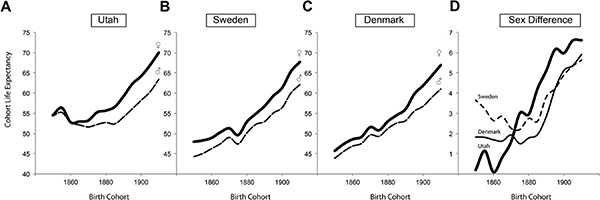
FIGURE 15-1 Cohort life expectancy in Utah (A), Sweden (B), and Denmark (C) and the sex differences in each population (D).
SOURCE: Lindahl-Jacobsen et al. (2013).
parison countries because many descendants of both nations were widely represented among the early migrants to Utah and because these countries have high-quality cohort mortality data spanning back as early as 1850.
As seen in Figure 15-1 and contrary to our expectation, the sex difference in cohort life expectancy was similar or larger in Utah than in Denmark and Sweden, except during the early frontier settlement era (1850-1870), which was distinguished by a series of food shortages and hardships associated with migration and the vagaries of establishing communities (Skolnick et al., 1978). Active male Mormons had longer life expectancy than other groups in Utah (approximately 2 years at age 50), while the difference was minimal for females, suggesting that male Mormons benefitted from a healthy lifestyle. Still, sex differences in cohort life expectancy at the age of 50 years were similar for individuals actively affiliated with the Mormon Church and for individuals living in the general population in Denmark and Sweden. This comparison confirms that even under the particular circumstances found in Utah during the historical period, women had a survival advantage similar to that seen in European populations at that time.
The Female Survival Advantage Was Present in Other 19th and 20th Century Populations
The male-female life-expectancy gap was smaller in the past than it is today, as indicated for time periods in Figure 15-2a for France, and as illustrated for cohorts in Figure 15-1d. In the 1850s, the male-female gap in eo (life expectancy at birth, a measure of mortality conditions in a given year of birth; see Figure 15-2) was 1.6 years for France and only 0.4 years for Belgium. The gap was 1.8 years in the Netherlands and 2 years in
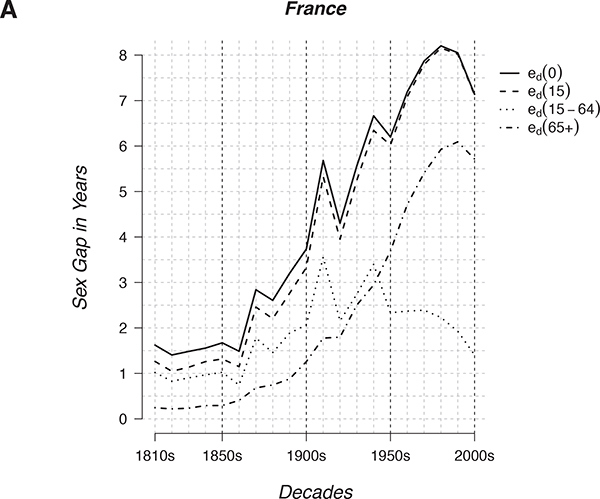
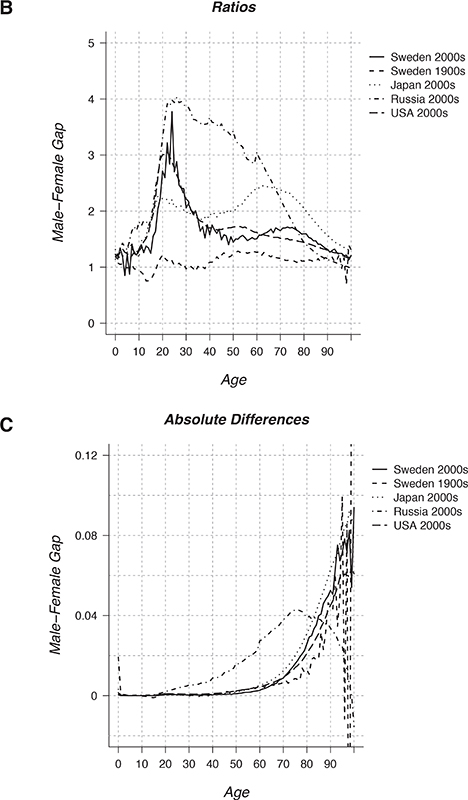
FIGURE 15-2 Male vs. female life expectancy over time (A) and male vs. female death rates over age (B and C).
NOTES: In A, for the French population, the difference plotted is female minus male life expectancy (eo for females - eo for males = ed) at ages 0 (ed(0)), 15 (ed(15)) and 65 (ed(65+)) as well as the difference in partial life expectancy between age 15 and 65 (ed(15-64)). Each point pertains to a decade of data: for example, the point for 1850 pertains to 1850-9. In B the ratio of male to female death rates is plotted for five populations. In C the difference between male and female death rates is plotted for the same five populations. Note that A, B, and C all pertain to mortality conditions in the specified decades, whereas the graphs in Figure 15-1 pertain to cohorts followed from birth through time. In contrast to the cohort life expectancy values in Figure 15-1, which reflect the lifespans of people born in various years, the period life expectancy values in Figure 15-2 are measures of mortality conditions in the specified decade.
England and Wales. The gap in Sweden was higher, 4.1 years, but it fell to 2.3 years by the 1920s. Preliminary analysis suggests that when eo started to rise in European countries in the 19th century, the female eo tended to increase faster than the male eo, widening the initially small gap. As shown in Figure 15-2a, the eo gap in the 19th century was largely due to the gap in remaining life expectancy at age 15. The rise in the gap in the second half of the 19th century was fueled in roughly equal measure by a rise in the gap at ages 15-64 and the gap in remaining life expectancy at age 65.
The Swedish gap of 2.3 years in the 1920s rose to almost 6 years in the 1980s, followed by a fall to less than 4 years in 2010-2012. In France, as shown in Figure 15-2a, the gap rose to more than 8 years in the 1980s and then fell to 7.2 years in the first decade of this century—with the rise in the gap entirely due to the rise in the gap in e65. The radical rise and recent fall in the gap can be seen in nearly all the countries in the Human Mortality Database. The main underlying factor is almost certainly the rise of male cigarette smoking followed by the more recent rise in female smoking (National Research Council, 2011a, 2011b).
Most of the research to date on discrepancies in age-specific male vs. female death rates has focused on the ratio of male to female rates, as shown in Figure 15-2b for five populations. Several features merit discussion. The age-specific ratios are close to 1 for Swedes in the first decade of the 20th century—this is also true for Swedes earlier and for other countries in the 18th and early 19th centuries. Indeed at some ages and for some countries (e.g., for Swedes in 1900-1909 at ages 8 to 17), it is males that experience lower mortality than females. The low values of the ratios strongly contrast with the much higher values shown in Figure 15-2b for the first decade of the 21st century, a pattern that also held in the mid- and late-20th century and for other countries.
The rise in the ratio starting around puberty, the very high peak in Russia as opposed to Japan, and the secondary maximum for Japan and Sweden in 2000-2009 among older adults (probably due to smoking) are noteworthy, as is the dramatic decline in the Russian ratio from a peak of 4 to a value under 1 at age 100. This Russian pattern may be partly due to mortality selection in a heterogeneous population: The few Russian males who survive to advanced old age may be exceptionally robust. The pattern, however, may partially be an artifact of smoothing algorithms, based on the Kannisto mortality model, that are used by the Human Mortality Database.
It is uncommon to study age-specific differences in male-female death rates, as shown in Figure 15-2c. Hence, for many researchers, including some experienced demographers, it may come as a surprise that the ratio of male to female death rates declines toward 1 but the difference increases exponentially (Wisser and Vaupel, 2014)—except for Russia at older ages, when the impact of heterogeneity and of data artifacts may be dominant.
Also worth noting in Figure 15-2c is the high level of the difference between male and female infant mortality for Sweden in 1900-1909. To put this difference into perspective, the gap arises because the male infant mortality rate was 10.2 percent compared with a female infant mortality rate of 8.3 percent
The Female Survival Advantage Was Present Prior to the 18th Century Using Preliminary Paleodemographic Data
There is general agreement that life was short in the prehistoric past (e.g., Hassan, 1981). Given the deficiencies of published paleodemographic lifetables (Hoppa and Vaupel, 2002), it is not clear for particular populations whether life expectancy at birth was around 20, 25, or 30. The biases in published lifetables may affect males and females in a roughly similar manner. If so, it may be possible to use the available data to assess whether female life expectancy was higher than for males. Jesper Boldsen has done so, based on data from careful studies of single-site mortality patterns (Milner et al., 1989) and reanalysis of archived data for many populations (Boldsen and Paine, 1995). Boldsen’s hypothesis (described in Boldsen and Paine, 1995) is that male and female life expectancies were approximately equal for hunter-gatherers prior to and during the Mesolithic period. In the Neolithic period, many people lived in more permanent villages and relied heavily on agriculture to supplement hunting. In the Bronze and Iron Ages reliance on agriculture intensified. As humans began to settle down, fertility increased, which resulted in higher female mortality from complications of pregnancy and childbearing. Furthermore, infectious disease mortality increased, which dramatically increased death rates, especially for older children. Women, who spent almost all their time in the villages, suffered more from this than men, who frequently left the villages for hunting. Hence, Boldsen believes that male life expectancy during the Mesolithic and Neolithic remained roughly constant, but that female life expectancy fell. Starting some 1,500 to 2,000 years ago in Northern Europe, in the Iron Age and thereafter, with increasing levels of trade and of manufacturing, Boldsen thinks that female survival improved more than male survival. Hence, he hypothesizes, the male life-expectancy advantage gradually diminished, with female life expectancy reaching male levels about 500 years ago in Northern Europe.
Until better skeletal data become available, Boldsen’s hypothesis must be viewed as an unproven conjecture that is only partly consistent with data on contemporary hunter-gatherers. The Aché of Paraguay during the “forest period” (Hill and Hurtado, 1996) show similar life expectancies for men and women, with men experiencing higher mortality than women only in middle to old age. However, the Hiwi of Venezuela show substantially
higher male than female mortality among middle-aged and older men (Hill et al., 2007), and among the Hadza of Tanzania, women have substantially greater life expectancy than men (Marlowe, 2010).
RESULTS: BABOON STUDIES
In the Amboseli baboons, males experience both higher initial adult mortality and more rapid acceleration of mortality during adulthood than females, resulting in greater longevity in female baboons than in males (Alberts and Altmann, 2003; Bronikowski et al., 2011). However, while the prediction that females live longer than males is supported in this population, the other component of the health survival paradox—better health among males than among females during aging—does not seem to be well supported. To establish this result, we examined several indicators of health and functioning in the Amboseli baboons and compared changes in these indicators with age for males and females. We found that, unlike humans, age-related declines in health and functioning tended to either be similar in both sexes or more extreme in males than in females. This pattern was also true for two measures of individuals’ social circumstances that are linked to health in human populations: social status and social connectedness (Holt-Lunstad et al., 2010). Male baboons experienced more rapid age-related declines than females in both of these measures, with potentially negative effects on health during aging. Below we describe our results for each of our measures of health and social circumstances.
Health Indicators for Which Females and Males Experienced Similar Declines with Age
Physical Condition
In humans, very low body mass index is a known risk factor for mortality, particularly among the elderly (Harris et al., 1988; Wilson, 2001; Corrada et al., 2006). We tested for age-related changes in body mass index (BMI), a mass-for-stature measure calculated as a baboon’s body mass divided by the square of its crown-rump length. We collect both of these morphological values when we anesthetize animals for blood draws, which we do with only a subset of the population; consequently, these one-time measures yield cross-sectional rather than longitudinal analyses (Altmann et al., 2010). In a linear model, with a quadratic term for age, males and females both exhibited a decline in BMI with age, after reaching a peak BMI in early adulthood (R2adj. = 0.68, p = 2.2×10–16, b = 2.204 for age, b = –0.080 for age2: N = 83 measures for adult females and 99 measures for adult males; Figure 15-3a). As expected, males had a significantly higher
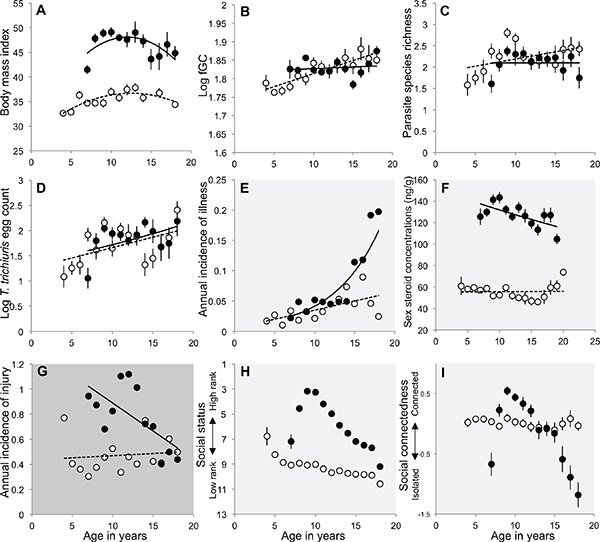
FIGURE 15-3 Changes in health indicators and social circumstances as a function of age.
NOTES: Presented for male (filled circles, solid line) and female (open circles, dashed line) baboons, including: (A) body mass index (BMI), (B) concentrations of fecal glucocoticoid hormones, (C) richness of gastrointestinal parasite species, (D) burden of whipworm parasites (log-transformed eggs per gram), (E) incidence of observed illnesses, (F) concentrations of fecal sex steroid hormones, testosterone and estrogen, (G) incidence of injuries, (H) social status (i.e. dominance rank), and (I) social connectedness. Plots with white backgrounds indicate traits for which males and females experienced similar changes with age; light gray backgrounds indicate traits for which males declined more rapidly than females, and dark gray backgrounds indicate traits for which females declined more rapidly than males.
BMI than females (b = 9.8 for sex; p < 0.001), but we found no significant sex-by-age interaction, indicating that males and females did not differ in their pattern of decline in BMI with age (b = –0.001, p = 0.8 for the sex-by-age interaction term in the linear model).
Glucocorticoid Concentrations
Like humans, male and female baboons exhibit age-related hypercortisolism; for instance, Sapolsky and Altmann (1991) reported age-related increases in both serum cortisol concentrations and dexamethasone resistance in Amboseli baboons that had been temporarily immobilized for blood draws. Here we report changes in glucocorticoid hormones measured via fecal metabolites of glucocorticoids (e.g., Khan et al., 2002; Altmann et al., 2004; Gesquiere et al., 2005). Fecal samples are collected on an opportunistic basis from known individuals, and we typically collect several samples per individual throughout the year. In the baboons, we found that both sexes exhibited significant increases with age in concentrations of fecal glucocorticoids (fGC), and we found no evidence of sex differences in the age-related fGC increase. Specifically, in a general linear mixed model (GLMM) including individual identity as a random effect, we identify a significant age term (b = 0.012, p < 0.0001) but no significant sex-by-age interaction (p = 0.498; N = 1,941 monthly values for 165 adult females and 3,602 monthly values for 161 adult males; Figure 15-3b).
Gastrointestinal Parasitism
Like most wild animals, the Amboseli baboons are infected with gastrointestinal macroparasites; such infections are a widely recognized measure of health in wild animal populations (Gulland, 1992; Craig et al., 2006; Gillespie, 2006; Hillegass et al., 2010). We used standard methods for fecal flotation and sedimentation to count eggs of different parasite species (Gillespie, 2006; Bowman, 2009). These data were used to examine two measures of the severity of parasite infection: (1) the number of different pathogenic parasite species infecting an individual, including Trichuris trichiura, Abbreviata caucasica, and the strongyle-type parasites Trichostrongylus axei and Oesophagostomum bifurcum, and (2) the burden (as eggs per g of feces) of the especially common and pathogenic parasite T. trichiura (whipworm). Both males and females exhibited increased evidence of parasitism with age. Specifically, older animals were infected with a greater number of species with known pathogenic effects (GLMM including individual as a random factor; b for age effect = 0.032, p for age effect < 0.001; N = 232 samples from 90 adult females and 209 samples from
68 adult males; Figure 15-3c). In addition, older animals had increased burdens of T. trichiura (GLMM including individual as a random factor; b for age effect = 0.052; p for age effect < 0.01; 232 samples from 90 adult females and 209 samples from 68 adult males; Figure 15-3d). However, contrary to the expectations of the health-survival paradox, we found no evidence for sex-differences in rates of increase with age; that is, there was no significant sex-by-age interaction for either parasite richness (p = 0.728) or T. trichiura burden (p = 0.135).
Health Indicators for Which Males Declined More Rapidly than Females with Age
Incidence of Observed Illness
Records of illness in the Amboseli baboons are collected as part of our long-term records and are based on visually observed signs of illness, including diarrhea, vomiting, cachexia, lethargy, respiratory problems, and other pathologies. These data are not clinical diagnoses, and the dataset is necessarily small; however, these data present an intriguing picture of greater vulnerability in older males than in older females—a pattern quite different from that seen in humans. Specifically, while both sexes exhibit increasing incidence of illness with age (b = 0.113, p < 0.001 in a GLMM of the annual incidence of illness, including age, sex, and individual as a random effect; N = 59 illnesses in 244 adult females and 48 illnesses in 246 adult males; Figure 15-3e), we also observed a marginal sex-by-age interaction (p = 0.07), with males experiencing a sharper increase in the incidence of illness in old age relative to females (Figure 15-3f).
Sex Steroid Hormones
Patterns of decline in sex steroid concentrations (measured as fecal metabolites of testosterone in males and estrogen in females) are quite different in the two sexes. Males show a marked decline in T concentrations with age (b = –0.023, p < 0.001 in a GLMM including age as a fixed effect and individual identity as a random factor; N = 3,602 monthly values in 161 adult males; Figure 15-3f). In contrast, estrogen levels are relatively stable in females as they age (b = –0.001, p = 0.504 in a GLMM including age as a fixed effect and individual identity as a random factor; N = 1,941 monthly values for 165 adult females; Figure 15-3f). These sex differences correspond with a marked decline in male reproductive activity with age, but the maintenance of relatively high female fertility well into old age.
Health Indicators for Which Females Deteriorate More Rapidly Than Males with Age
Incidence of Injury
The only health measure for which females appear to experience more rapid age-related deterioration than males with age is the rate of injury. For both sexes, most injuries occur in the context of conflict with conspecifics. In males, injuries actually declined markedly in old age, perhaps because older males are less engaged in conflicts over reproductive opportunities than young males. In contrast, for females, the risk of injury increased as they aged (GLMM including individual as a random factor: b = 0.028, p = 0.01 for age, b = 1.093, p = 0.0001 for sex difference (males versus females), b = –0.040, p = 0.053 for age-by-sex interaction; N = 625 injuries in 241 adult females, 568 injuries in 234 adult males; Figure 15-3g). However, the increase in risk that females experienced brought them to the same level of risk as their male age-mates, indicating that even though females experienced a more detrimental change with age than males, they did not experience a more detrimental level of injury risk in old age.
Social Conditions Deteriorate More Rapidly During Aging for Males than Females
In addition to the health indicators described above, we also analyzed two important measures of an individual’s social circumstances that are likely to influence changes in health with age: social status (i.e., dominance rank) and social connectedness to group members. In humans, low social status and social isolation both pose considerable health risks (Berkman and Glass, 2000; Blazer, 1982; Holt-Lunstad et al., 2010; Marmot, 2004; Marmot et al., 1991; Olsen et al., 1991; Uchino, 2004, 2006). In baboons, we found that both of these features declined with age, and that they deteriorated more rapidly for males than for females.
Social Status
Social status is a particularly important trait for both males and females of many species of primates, with consequences for access to food, mating opportunities, and other resources. Consequently, social status is linked to several key life history outcomes (Sapolsky, 2004; Alberts, 2012; Pusey, 2012;). Social status (specifically, social dominance rank) depends partly upon fighting ability and physical strength. In Amboseli, both sexes experience a decline in social status with age, but we saw a much steeper decline with age in male social status than in female social status (GLMM including
individual identity as a random factor: (b = –10.42, p < 0.0001 for sex difference, b = 0.20, p < 0.0001 for age, and b = 0.52, p < 0.0001 for sex-by-age interaction; N = 8,279 monthly rank values for 373 adult males and 19,410 values for 357 females; Figure 15-3h). This steeper decline in male social status may arise, in part, from the fact that female social status, for baboons and other cercopithecine primates, has strong familial influences. Hence, support from relatives may keep females from the precipitous declines in social status during old age that males experience. Moreover, the decline in social status with age likely carries more severe health consequences for males than females. This is because social status is a strong predictor of stress hormone levels (particularly glucocorticoids) for male baboons (Gesquiere et al., 2011), but not for females (Weingrill et al., 2004; Engh et al., 2006). Low-ranking male baboons also experience slower wound healing than high-ranking males (Archie et al., 2012), a pattern that is, again, not true for females (social status has no effect on wound healing in female baboons; Archie et al., 2014). Hence the precipitous decline in male social status with age, in combination with the negative health effects of low social status for males, may result in a more precipitous health decline for aging males than for aging females.
Social Connectedness
We measured social connectedness in the Amboseli baboons as an individual’s age-specific frequency of grooming with group mates, relative to all other same-sex adults present in the population in the same year. Grooming is a major social activity in many species of social mammals, and grooming in primates appears to be key for establishing and maintaining affiliative relationships and reducing tension and aggression between individuals (reviewed in Aureli et al., 2012; Lonsdorf and Ross, 2012; Silk, 2012). Relative grooming frequency is commonly used as a quantitative measure of the strength of dyadic social relationships (Lazaro-Perea et al., 2004; Silk et al., 2006). Like social dominance rank, social connectedness also declined more rapidly with age for male baboons than for females in Amboseli (Figure 15-3i). While both sexes experienced a decline in social connectedness with age, males exhibited a much steeper decline than females (GLMM including individual as a random factor: (b = –0.011, p < 0.035 for age effect, (b = 1.66, p < 0.0001 for sex difference, and (b = –0.14, p < 0.0001 for the sex-by-age interaction; N = 8,279 monthly rank values for 373 adult males and 19,410 value for 357 females; Figure 15-3i). As with social rank, the steeper decline in social connectedness for Amboseli males may put males at greater risk for health declines during aging.
CONCLUSIONS
Our objective was to investigate the male-female health-survival paradox with two new approaches. First, we examined male-female mortality and health differences in human populations that experience living conditions and cultures very different from contemporary Western societies, and the female survival advantage since the 19th century. Second, we examined components of the health-survival paradox in a natural population of nonhuman primates. In doing so, we hoped to achieve two goals. First, we wished to probe the historical limits and extent of the paradox in human populations, by asking how universal is the female survival advantage—one fundamental component of the paradox—in historical and modern human populations. Second, we wished to provide an evolutionary context by asking whether components of the paradox occur in wild nonhuman primates.
Our first approach yielded strong evidence that a female survival advantage is a typical feature of nearly all human populations for which serviceable vital-statistics data are available. Indeed, with the exception of a few ages in a few cohorts and periods (as illustrated here by ages 8-18 in Sweden in the period 1900-1909), females had lower mortality than males across all ages and all populations in the Human Mortality Database, which includes several countries since the mid-1800s and Sweden since 1751. Even in unusual demographic circumstances, our evidence suggests that both the female survival advantage and the health-survival paradox persist. The 20th century Russian population was characterized by exceptionally high male mortality (largely driven by cardiovascular disease and alcohol-related deaths), and yet women reported poorer health than men and showed poorer performance on physical tests, conforming to the paradox seen in more typical Western societies. The late 19th and early 20th century Mormon population, in contrast, was characterized by social proscription of alcohol and tobacco use; yet even in the absence of these male-biased risk-taking behaviors, women had a survival advantage over men.
Prehistorical periods are less clear, because data on age-at-death are difficult to acquire from skeletal data, but Boldsen and Paine (1995) have proposed that women lost ground relative to men during the early agricultural era, when fertility and density-related disease epidemics were presumably both on the rise and negatively affected women more than men. In contrast, they propose that men and women had similar life expectancies during the hunter-gatherer period. Existing skeletal data are too problematic to conclusively test this hypothesis, and the idea is only partly supported by data from the few modern hunter-gatherers for which mortality is known. Hence, whether females tended to live longer than males over most of human existence up to a few hundred years ago is uncertain. Importantly, chimpanzees, the closest nonhuman primate relative of humans, are charac-
terized by greater female than male longevity, as are most other nonhuman primates living in the wild (Bronikowski et al., 2011).
Thus, most existing data point to a long evolutionary history of a human (and nonhuman primate) female survival advantage, albeit with periods and places in which the female survival advantage waned. Concomitant health data are not available for most human populations living before modern times, or for that matter for nonhuman primate populations. However, based on the ubiquity of the health-survival paradox in the contemporary West and the evidence that it was equally robust in the unusual demographic circumstances in Russia in the 20th century, we predicted that we would see a health-survival paradox in baboons, with females experiencing more marked declines in health with age than males. This prediction was informed not only by the human data, but also by our data indicating that the energetic costs of reproduction, which female baboons bear throughout their lives in a constant cycle of conception, birth, and lactation, are substantial. After birth, females bear the double burden of milk production and of carrying infants, which they do almost constantly for the first two months of life and then intermittently until the infant weighs 15 percent of the mother’s body mass, over average daily distances of 8–10 km (Altmann and Samuels, 1992). The costs of lactation and infant-carrying appear to be partly met by metabolizing body tissues, which in turn delays future reproduction: Lactating females weigh less than cycling females, and lactating females with the lowest body masses take longer to achieve their next conception (Bercovitch, 1987). Finally, wounds heal more slowly for a female baboon when she is lactating than at other times (Archie et al., 2014). Male baboons, in contrast, bear reproductive costs primarily in the form of transient, rather than sustained, energetic demands associated with fighting and with mate guarding when females are in estrous (Alberts et al., 1996).
Our prediction was not supported. Not only do male baboons experience higher mortality than females (Alberts and Altmann, 2003; Bronikowski et al., 2011), but also they experienced a greater increase in the incidence of illness with age than females, and a steeper decline in sex steroid hormones with age (Figure 15-3). On several other measures—an age-related decline in body mass index and age-related increases in glucocorticoid concentrations and vulnerability to parasites—males and females were similar. On only one measure—incidence of wounding—did females experience a greater deterioriation than males, but this deterioration put them at a level of wounding equivalent to, not greater than, males in old age. In other words, we found no evidence of a male-female health-survival paradox in this nonhuman primate species.
Hence, our data suggest that while the female survival advantage has a long evolutionary history, the male health advantage that contributes to
the health-survival paradox does not. Across many human and nonhuman primate populations, females experience lower mortality than males, but we find no evidence, at least in baboons, of a male health advantage. We propose two possible explanations for this. First, age-related changes in sex steroid concentrations are marked and abrupt in women over the age of 50, but are strikingly absent in female baboons, who maintain high sex steroid concentrations and reproduce well into old age (Altmann et al., 2010). This difference may contribute to human-primate differences in whether males or females experience a health advantage in old age. Second, a number of the measures used to document the male health advantage in humans—specifically, measures of physical strength (e.g., grip strength and functioning ability) and self-reported measures of health—are impossible to collect in wild primate populations. Our results call for comparative data on a broad, multifaceted set of noninvasive health measures that are strictly parallel in both humans and nonhuman primates, in order to more clearly understand the evolutionary history of the male-female health-survival paradox.
ACKNOWLEDGMENTS
All analyses presented here received major support from the National Institute on Aging through NIA P01-AG031719, and we thank Oliver Wisser at the Max Planck Institute for Demographic Research for producing Figure 15-2. In addition, the Amboseli Baboon Research Project gratefully acknowledges the support of the National Science Foundation (including, in the past decade, IOS 1053461, IBN 0322613, IBN 0322781, BCS 0323553, BCS 0323596, DEB 0846286, DEB 0846532 and DEB 0919200), the National Institute on Aging (R01AG034513), the Princeton Center for the Demography of Aging (P30AG024361), the Chicago Zoological Society, and the Max Planck Institute for Demographic Research. We also thank the Kenya Wildlife Services, Institute of Primate Research, National Museums of Kenya, the members of the Amboseli-Longido pastoralist communities in Kenya, and K. Pinc, R.S. Mututua, S. Sayialel, J.K. Warutere, V. Somen and T. Wango for their untiring efforts on behalf of the baboon research.
REFERENCES
Alberts, S.C. (2012). Magnitude and sources of variation in male reproductive performance. In J.C. Mitani, J. Call, P. Kappeler, R. Palombit, J.B. Silk (Eds.), Evolution of Primate Societies (pp. 343-366). Chicago, IL: University of Chicago Press.
Alberts, S.C., and Altmann, J. (2003). Matrix models for primate life history analysis. In P. Kappeler and M. Pereira (Eds.), Primate Life Histories and Socioecology (pp. 66-102). Chicago, IL: University of Chicago Press.
Alberts, S.C., Altmann, J., and Wilson, M.L. (1996). Mate guarding constrains foraging activity of male baboons. Animal Behaviour, 51, 1269-1277.
Alexander, T.G. (1981). The word of wisdom: From principle to requirement. Dialogue, 14, 78-88.
Alexander, T.G. (1996). Mormonism in Transition: A History of the Latter-Day Saints 1890-1930. Urbana and Chicago, IL: University of Illinois Press.
Allman, J., Rosin, A., Kumar, R., and Hasenstaub, A. (1998). Parenting and survival in anthropoid primates: Caretakers live longer. Proceedings of the National Academy of Sciences of the United States of America, 95, 6866-6869.
Altmann, J., and Samuels, A. (1992). Costs of maternal care: Infant-carrying in baboons. Behavioral Ecology and Sociobiology, 29, 391-398.
Altmann, J., Lynch, J.W., Nguyen, N., Alberts, S.C., and Gesquiere, L.R. (2004). Life-history correlates of steroid concentraions in wild peripartum baboons. American Journal of Primatology, 64, 95-106.
Altmann, J., Gesquiere, L., Galbany, J., Onyango, P.O., and Alberts, S.C. (2010). Life history context of reproductive aging in a wild primate model. Annals of the New York Academy of Sciences, 1204, 127-138.
Amdam, G.V. (2011). Social context, stress, and plasticity of aging. Aging Cell, 10, 18-27.
Andreev, E.M., McKee, M., and Shkolnikov, V.M. (2003). Health expectancy in the Russian Federation: A new perspective on the health divide in Europe. Bulletin of the World Health Organization, 81, 778-787.
Archie, E.A., Altmann, J., and Alberts, S.C. (2012). Social status predicts wound healing in wild baboons. Proceedings of the National Academy of Sciences of the United States of America, 109, 9017-9022.
Archie, E.A., Altmann, J., and Alberts, S.C. (2014). Costs of reproduction in a long-lived female primate: Injury risk and wound healing. Behavioral Ecology and Sociobiology, 68, 1183-1193.
Aureli, F., Fraser, O.N., Schaffner, C.M., and Schino, G. (2012). The regulation of social relationships. In J.C. Mitani, J. Call, P. Kappeler, R. Palombit, and J.B. Silk (Eds.), Evolution of Primate Societies (pp. 531-551). Chicago, IL: University of Chicago Press.
Barford, A., Dorling, D., Smith, G., and Shaw, M. (2006). Life expectancy: Women now on top everywhere. British Medical Journal, 332, 808.
Bateman, A.J. (1948). Intra-sexual selection in Drosophila. Heredity, 2, 349-368.
Bellino, F.L., and Wise, P.M. (2003). Nonhuman primate models of menopause workshop. Biology of Reproduction, 68(1), 10-18.
Bercovitch, F.B. (1987). Female weight and reproductive condition in a population of olive baboons (Papio anubis). American Journal of Primatology, 12, 189-195.
Berkman, L.F., and Glass, T. (2000). Social integration, social networks, social support, and health. In L.F. Berkman and I. Kawachi (Eds.), Social Epidemiology (pp. 137-173). New York: Oxford University Press.
Blazer, D.G. (1982). Social support and mortality in an elderly community population. American Journal of Epidemiology, 115, 684-694.
Bobak, M., Kristenson, M., Pikhart, H., and Marmot, M. (2004). Life span and disability: A cross sectional comparison of Russian and Swedish community based data. British Medical Journal, 329, 767.
Boldsen, J.L., and Paine, R.R. (1995). The evolution of human longevity from the Mesolithic to the Middle Ages: An analysis based on skeletal data. In B. Jeune and J.W. Vaupel (Eds.), Exceptional Longevity: From Prehistory to the Present (pp. 25-36). Odense, Denmark: Odense University Press.
Bowman, D.D. (2009). Georgis’ Parasitology for Veterinarians (9th ed.). St. Louis, MO: Saunders, Elsevier.
Bronikowski, A.M., Altmann, J., Brockman, D.K., Cords, M., Fedigan, L.M., Pusey, A., Stoinski, T.S., Morris, W.F., Strier, K.B., and Alberts, S.C. (2011). Aging in the natural world: Comparative data reveal similar mortality patterns across primates. Science, 331, 1325-1328.
Brunet-Rossinni, A.K., and Austad, S.N. (2006). Senescence in wild populations of mammals and birds. In E.J. Masoro and S.N. Austad (Eds.), Handbook of the Biology of Aging (pp. 243-266). Amsterdam: Elsevier.
Bush, L.E. (1993). Health and Medicine among the Latter-Day Saints. New York: Crossroad.
Carey, J.R. (2003). Longevity: The Biology and Demography of Life Span. Princeton, NJ: Princeton University Press.
Case, A., and Paxson, C. (2005). Sex differences in morbidity and mortality. Demography, 42, 189-214.
Clutton-Brock, T.H., and Isvaran, K. (2007). Sex differences in ageing in natural populations of vertebrates. Proceedings of the Royal Society B: Biological Sciences, 274, 3097-3104.
Corrada, M.M., Kawas, C.H., Mozaffar, F., and Paganini-Hill, A. (2006). Association of body mass index and weight change with all-cause mortality in the elderly. American Journal of Epidemiology, 163, 938-949.
Craig, B.H., Pilkington, J.G., and Pemberton, J.M. (2006). Gastrointestinal nematode species burdens and host mortality in a feral sheep population. Parasitology, 133, 485-496.
Crimmins, E.M., Kim, J.K., and Solé-Auró, A. (2011). Gender differences in health: Results from SHARE, ELSA and HRS. European Journal of Public Health, 21, 81-91.
Deparcieux, A. (1746). Essai sur les probabilite’s de la dure’e de la vie humaine. D’ou lton de’duit la maniere de de’terminer les rentes viageres, tant simples qu ‘en Tontines. Pre’ce’de’ d ‘une courte explication sur les rentes a terme, ou annuite’s. Paris, France: Freres Guerin.
Engh, A.L., Beehner, J.C., Bergman, T.J., Whitten, P.L., Hoffmeier, R.R., Seyfarth, R.M., and Cheney, D.L. (2006). Female hierarchy instability, male immigration and infanticide increase glucocoticoid levels in female chacma baboons. Animal Behavior, 71, 1227-1237.
Field, M. (2000). Gender gaps in mortality. Dissimilarities in mortality rates: Analysis of standard data. In V.M. Shkolnikov, E. Andreev, and T. Maleva (Eds.), Inequality and Mortality in Russia (pp. 20-23). Moscow, Russia: Moscow Carnegie Center.
Forsyth, D.M., Tustin, K.G., Gaillard, J.M., and Loison, A. (2004). Fetal sex ratio variation in the highly polygynous Himalayan tahr: Evidence for differential male mortality. Behavioral Ecology, 15, 572-578.
Gesquiere, L.R., Learn, N.H., Simao, M.C.M., Onyango, P.O., Alberts, S.C., and Altmann, J. (2011). Life at the top: Energetic and psychological stress in wild male primates. Science, 333, 357-360.
Gesquiere, L.R., Altmann, J., Khan, M.Z., Couret, J., Yu, J.C., Endres, C.S., Lynch, J.W., Ogola, P., Fox, E.A., Alberts, S.C., and Wango, E.O. (2005). Coming of age: Steroid hormones of wild immature baboons (Papio cynocephalus). American Journal of Primatology, 67, 83-100.
Gillespie, T.R. (2006). Noninvasive assessment of gastrointestinal parasite infections in free-ranging primates. International Journal of Primatology, 27, 1129-1143.
Gulland, F.M.D. (1992). The role of nematode parasites in soay sheep (Ovis aries) mortality during a population crash. Parasitology, 105, 493-503.
Harris, T., Cook, E.F., Garrison, R., Higgins, M., Kannel, W., and Goldman, L. (1988). Body mass index and mortality among nonsmoking older persons. Journal of the American Medical Association, 259, 1520-1524.
Hassan, F.A. (1981). Demographic Archaeology. New York: Academic Press.
Hill, K., and Hurtado, A.M. (1996). Ache Life History: The Ecology and Demography of a Foraging People. New York: Aldine de Gruyter, Hawthorne.
Hill, K., Hurtado, A.M., and Walker, R.S. (2007). High adult mortality among Hiwi hunter-gatherers: Implications for human evolution. Journal of Human Evolution, 52, 443-454.
Hillegass, M.A., Waterman, J.M., and Roth, J.D. (2010). Parasite removal increases reproductive success in a social African ground squirrel. Behavioral Ecology, 21, 696-700.
Hjertestatistik. (2004). Hjertestatistik 2004: Heart Statistics 2004. Copenhagen: The Danish Heart Foundation and the Danish National Institute of Public Health.
Holt-Lunstad, J., Smith, T.B., and Layton, J.B. (2010). Social relationships and mortality risk: A meta-analytic review. PLOS Medicine, 7.
Hoppa, R.D., and Vaupel, J.W. (2002). Paleodemography. Age Distributions from Skeletal Samples. Cambridge, UK: Cambridge University Press.
Jacobsen, R., Oksuzyan, A., Engberg, H., Jeune, B., Vaupel, J.W., and Christensen, K. (2008). Sex differential in mortality trends of old-aged Danes: A nation wide study of age, period and cohort effects. European Journal of Epidemiology, 23, 723-730.
Keller, L., and Jemielty, S. (2006). Social insects as a model to study the molecular basis of aging. Experimental Gerontology, 41, 553-556.
Khan, M.Z., Altmann, J., Isani, S.S., and Yu, J. (2002). A matter of time: Evaluating storage of fecal samples for steroid analysis. General and Comparative Endocrinology, 128, 57-64.
Kinsella, K. (2000). Demographic dimensions of global aging. Journal of Family Issues, 21, 541-558.
Kinsella, K., and Gist, Y.J. (1998). Gender and aging: Mortality and health. International Brief—Gender and Aging: Mortality and Health, IB/98-2, 1-7.
Lazaro-Perea, C., Arruda, M.F., and Snowdon, C.T. (2004). Grooming as a reward? Social function of grooming between females in cooperatively breeding marmosets. Animal Behaviour, 67, 627-636.
Leon, D.A. (2011). Trends in European life expectancy: A salutary view. International Journal of Epidemiology, 40, 271-277.
Lindahl-Jacobsen, R., Hanson, H.A., Oksuzyan, A., Mineau, G.P., Christensen, K., and Smith, K.R. (2013). The male-female health-survival paradox and sex differences in cohort life expectancy in Utah, Denmark, and Sweden 1850-1910. Annals of Epidemiology, 23, 161-166.
Lonsdorf, E.V., and Ross, S.R. (2012). Socialization and development of behavior. In J. Mitani, J. Call, P. Kappeler, R. Palombit, and J. Silk (Eds.), Evolution of Primate Societies (pp. 245-268). Chicago, IL: University of Chicago Press.
Marlowe, F.W. (2010). The Hadza: Hunter-Gatherers of Tanzania. Berkeley: University of California Press.
Marmot, M.G. (2004). The Status Syndrome. New York: Henry Holt and Company.
Marmot, M.G., Smith, G.D., Stansfeld, S., Patel, C., North, F., Head, J., White, I., Brunner, E., and Feeney, A. (1991). Health inequalities among British civil servants: The Whitehall II study. The Lancet, 337(8754), 1387-1393.
McDonald, D.B. (1993). Demographic consequences of sexual selection in the long-tailed manakin. Behavioral Ecology, 4, 297-309.
Meslé, F. (2004). Mortality in Central and Eastern Europe: Long-term trends and recent upturns. Demographic Research Special Collection, 2, 45-70.
Milner, G.R., Humpf, D.A., and Harpending, H.C. (1989). Pattern matching of age-at-death distributions in paleodemographic analysis. American Journal of Physical Anthropology, 80, 49-58.
National Research Council. (2011a). Explaining Divergent Levels of Longevity in High-Income Countries. Panel on Understanding Divergent Trends in Longevity in High-Income Countries, E.M. Crimmins, S.H. Preston, and B. Cohen (Eds.).Committee on Population, Division of Behavioral and Social Sciences and Education. Washington, DC: The National Academies Press.
National Research Council. (2011b). International Differences in Mortality at Older Ages: Dimensions and Sources. Panel on Understanding Divergent Trends in Longevity in High-Income Countries, E.M. Crimmins, S.H. Preston, and B. Cohen (Eds.). Committee on Population, Division of Behavioral and Social Sciences and Education. Washington, DC: The National Academies Press.
Nunn, C.L., Lindenfors, P., Pursall, E.R., and Rolff, J. (2009). On sexual dimorphism in immune function. Philosophical Transactions of the Royal Society, 364, 61-69.
Oksuzyan, A., Juel, K., Vaupel, J.W., and Christensen, K. (2008). Men: Good health and high mortality. Sex differences in health and aging. Aging Clinical and Experimental Research, 20, 91-102.
Oksuzyan, A., Crimmins, E., Saito, Y., O’Rand, A., Vaupel, J.W., and Christensen, K. (2010). Cross-national comparison of sex differences in health and mortality in Denmark, Japan and the US. European Journal of Epidemiology, 25, 471-480.
Oksuzyan, A., Shkolnikova, M., Vaupel, J.W., Christensen, K., and Shkolnikov, V.M. (2014). Sex differences in health and mortality in Moscow and Denmark. European Journal of Epidemiology, 29(4), 243-252.
Olsen, R.B., Olsen, J., Gunnersvensson, F., and Waldstrom, B. (1991). Social networks and longevity: A 14 year follow-up-study among elderly in Denmark. Social Science & Medicine, 33, 1189-1195.
Pampel, F.C. (2003). Declining sex differences in mortality from lung cancer in high-income nations. Demography, 40, 45-65.
Payne, S. (2004). Gender in Lung Cancer and Smoking Research. Geneva, Switzerland: World Health Organization.
Picq, J.L. (2007). Aging affects executive functions and memory in mouse lemur primates. Experimental Gerontology, 42, 223-232.
Preston, S.H., and Wang, H. (2006). Sex mortality differences in the United States: The role of cohort smoking patterns. Demography, 43, 631-646.
Promislow, D.E.L., and Harvey, P.H. (1990). Living fast and dying young: A comparative analysis of life-history variation among mammals. Journal of Zoology, 220, 417-437.
Pusey, A. (2012). Magnitude and sources of variation in female reproductive performance. In J.C. Mitani, J. Call, P. Kappeler, R. Palombit, and J.B. Silk (Eds.), Evolution of Primate Societies (pp. 343-366). Chicago, IL: University of Chicago Press.
Rolff, J. (2002). Bateman’s principle and immunity. Proceedings of the Royal Society B: Biological Sciences, 269, 867-872.
Sapolsky, R.M. (2004). Social status and health in humans and other animals. Annual Review of Anthropology, 33, 393-418.
Sapolsky, R.J., and Altmann, J. (1991). Incidences of hypercortisolism and dexamethasone resistance increase with age among wild baboons. Biological Psychiatry, 30, 1008-1016.
Shkolnikov, V.M., and Meslé, F. (1996). The Russian epidemiological crisis as mirrored by mortality trends. In J. Da Vanzo (Ed.), Russia’s Demographic “Crisis” (pp. 113-161). Santa Monica, CA: RAND Center for Russian and Eurasian Studies.
Silk, J.B. (2012). The adaptive value of sociality. In J.C. Mitani, J. Call, P. Kappeler, R. Palombit, and J.B. Silk (Eds.), Evolution of Primate Societies (pp. 552-564). Chicago, IL: University of Chicago Press.
Silk, J.B., Altmann, J., and Alberts, S.C. (2006). Social relationships among adult female baboons (papio cynocephalus) I. Variation in the strength of social bonds. Behavioral Ecology and Sociobiology, 61, 183-195.
Skolnick, M., Bean, L., May, D., Arbon, V., De Nevers, K., and Cartwright, P. (1978). Mormon demographic history I. Nuptiality and fertility of once-married couples. Population Studies, 32, 5-19.
Smith, M.P., and Cass, W.A. (2007). Oxidative stress and dopamine depletion in an intrastriatal 6-hydroxydopamine model of Parkinson’s disease. Neuroscience, 144, 1057-1066.
Stoehr, A.M., and Kokko, H. (2006). Sexual dimorphism in immunocompetence: What does life-history theory predict? Behavioral Ecology, 17, 751-756.
Struyck, N. (1740). Inleiding tot de Algemeene Geographie, benevens eenige sterrekundige en andere Verhandelingen. Amsterdam: Issak Tirion.
Thorslund, M., Wastesson, J.W., Agahi, N., Lagergren, M., and Parker, M.G. (2013). The rise and fall of women’s advantage: A comparison of national trends in life expectancy at age 65 years. European Journal of Ageing, 10, 271-277.
Uchino, B.N. (2004). Social Support and Physical Health. New Haven, CT: Yale University Press.
Uchino, B.N. (2006). Social support and health: A review of physiological processes potentially underlying links to disease outcomes. Journal of Behavioral Medicine, 29, 377-387.
Verbrugge, L.M. (1985). Gender and health: An update on hypotheses and evidence. Journal of Health and Social Behavior, 26, 156-182.
Verbrugge, L.M. (1989). The twain meet: Empirical explanations of sex-differences in health and mortality. Journal of Health and Social Behavior, 30, 282-304.
Wang, H., and Preston, S.H. (2009). Forecasting United States mortality using cohort smoking histories. Proceedings of the National Academy of Sciences of the United States of America, 106, 393-398.
Weingrill, T., Gray, D., Barrett, L., and Henzi, S.P. (2004). Fecal cortisol in free-ranging female chacma baboons: Relationship to dominance, reproductive state, and environmental factors. Hormones and Behavior, 45, 259-269.
Wilson, M.M. (2001). Bitter-sweet memories: Truth and fiction. The Journals of Gerontology. Series A: Biological Sciences, 56, M196-M199.
Wingard, D.L. (1984). The sex differential in morbidity, mortality and lifestyle. Annual Review of Public Health, 5, 433-458.
Wisser, O., and Vaupel, J.W. (2014). The Sex Differential in Mortality: A Historical Comparison of the Adult-Age pattern of the Ratio and the Difference. Working Paper. Max Planck Institute for Demographic Research, Rostock, Germany.
Zaridze, D., Lewington, S., Boroda, A., Scélo, G., Karpov, R., Lazarev, A., Konobeevskaya, I., Igitov, V., Terechova, T., Boffetta, P., Sherliker, P., Kong, X., Whitlock, G., Boreham, J., Brennan, P., and Peto, R. (2014). Alcohol and mortality in Russia: Prospective observational study of 151 000 adults. The Lancet, 383(9927), 1465-1473.
Zuk, M., and Stoehr, A.M. (2002). Immune defense and host life history. American Naturalist, 160, S9-S22.