Informed chemical use in modern society should consider a variety of factors, including performance, costs, potential adverse effects to human health and the environment, and societal impacts. Chemical alternatives assessments are designed to facilitate consideration of these factors by assisting users in identifying alternative chemicals or approaches that are safer and have reduced environmental impact. The Committee on the Design and Evaluation of Safer Chemical Substitutions—A Framework to Inform Government and Industry Decisions was given the task1 of developing a framework for assessing potentially safer substitute chemicals in terms of human health and ecological risks and demonstrating how the framework could be used. This report presents the committee’s consideration of select existing frameworks, the committee’s framework, and recommendations for implementation and future research needs.
STATE OF THE ART OF EXISTING FRAMEWORKS FOR ALTERNATIVE ANALYSIS
Alternatives assessment is a process for comparing alternatives, usually to a chemical of concern and identifying those that are safer. It is different from a safety assessment, where the primary goal is to ensure that exposure is below a prescribed standard; different from risk assessment, where risk associated with a given level of exposure is calculated; and different from a sustainability assessment, which considers all aspects of a chemical’s life cycle, including energy and material use. The goal of an alternatives assessment is to facilitate an informed consideration of the advantages and disadvantages of alternatives to a chemical of concern, resulting in the identification of safer alternatives.
The development of this committee’s framework built upon the work of regulatory agencies, academic institutions, and others who have developed alternatives assessment frameworks. The committee considered ten frameworks and approaches.2 These frameworks share many common elements, such as assessing human health and ecological hazards, evaluating critical physicochemical properties, performing life cycle analyses, performance, and social assessments. Across these frameworks, assessments of human health hazards evaluate an array of health-related end points, including carcinogenicity, mutagenicity, reproductive and developmental toxicity, endocrine disruption, acute and chronic or repeat dose toxicity, dermal and eye irritation, and dermal and respiratory sensitization. Most frameworks also include some consideration of ecotoxicity, but the focus tends to be primarily on aquatic toxicity. Many existing frameworks compare chemicals of concern and alternatives against a series of mammalian and ecotoxicity metrics. These frameworks often use tools like the United Nations Globally Harmonized System (GHS) for the Classification and Labelling of Chemicals (GHS 2013) and the GreenScreen® for Safer Chemicals hazard assessment tool (Heine and Franjevic 2013) to classify hazards.3
The committee identified several elements that were often missing from existing frameworks. For example, despite the known importance of exposure, many frameworks downplay it and focus on inherent hazards of chemicals. This approach assumes that chemical alternatives would result in similar exposure levels to people, animals, and the environment and is in contrast to an approach that addresses both inherent hazard and exposure.
_____________
1 Official Statement of Task is in Chapter 1.
2 Frameworks and approaches considered by the committee included BizNGO Alternatives Assessment Protocol, California Safer Consumer Products Regulation, EPA’s Design for the Environment (DfE) Program Alternatives Assessments, German Guide on Sustainable Chemicals, Interstate Chemicals Clearinghouse (IC2) Alternatives Assessment Guide, Lowell Center Alternatives Assessment Framework, REACH Guidance on the Preparation of an Application for Authorisation, TURI Alternatives Assessment Process Guidance, UCLA Multi-Criteria Decision Analysis, and UNEP Persistent Organic Pollutants Review Committee General Guidance on Alternatives.
3 Classification (or benchmarking) tools provide threshold values for toxicological end points of interest, for evaluating data about effects of chemicals. These tools often result in assignment of a score (e.g., low, medium, high) that can be used to compare alternatives.
Many frameworks also do not consider the decision-making process or decision rules used for resolving trade-offs among different categories of toxicity and other factors (e.g., social impact), or the values that underlie such trade-offs. Also absent from several frameworks is the use of novel toxicity data streams, in silico computational models, and methods to estimate physicochemical information. In addition, a lack of consistency is seen in that existing frameworks provide users with a wide range of options on implementation and minimum data sets. Lack of consistency among frameworks is not unexpected given that their development is often motivated by different factors, such as regulatory pressures, industry concerns, and organizational or stakeholder drivers, which understandably affect the variables and elements considered by the author or authoring organization. Because of both gaps in framework elements within existing frameworks and lack of consistency across frameworks, the committee identified no “ideal” framework from the existing set. The existing frameworks that the committee examined, however, helped to inform the development of the framework offered in this study.
THE COMMITTEE’S ALTERNATIVES ASSESSMENT FRAMEWORK
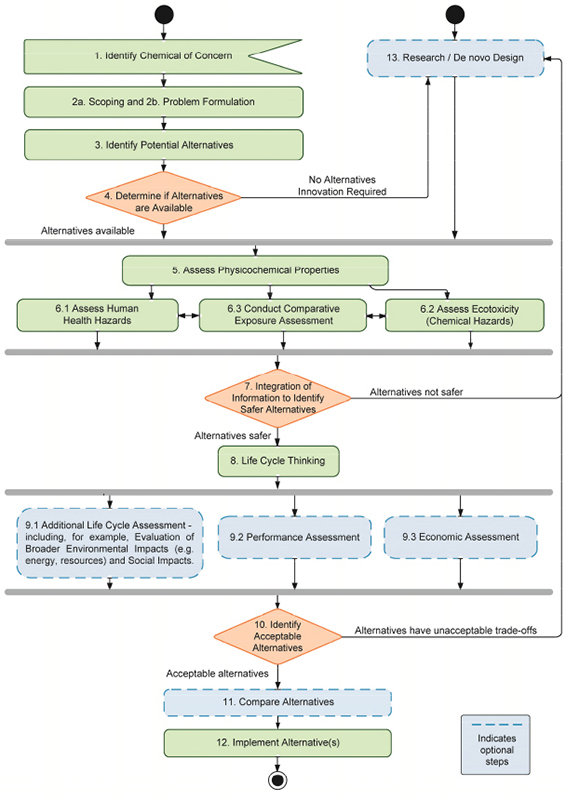
This report provides a description of the committee’s 13-step framework (Figure S-1), which is structured to support decision-making about alternatives to chemicals of concern. The framework is flexible enough for an assessor to use a hybrid approach, in which certain steps are completed sequentially, in parallel, or iteratively, providing an opportunity for fit-for-purpose decision making. Wherever possible, the committee’s detailed guidance on the implementation of its framework is intended to provide users with that flexibility. To that end, some steps or sub-steps are considered optional, as indicated in Figure S-1. Whether or not assessments lacking certain parts of the committee’s framework are acceptable will depend on the type of decision made.
Users of the Committee’s Framework
The committee identified multiple audiences and users for this report, all of which would benefit from a unified approach to this challenge and a common understanding of the different processes involved in chemical alternatives assessment:
- regulatory agencies at the federal, state, local, and international level;
- industry, including small, medium, and large businesses;
- organizations encouraging the adoption of safer chemicals; and
- developers of chemicals and chemical processes.
The framework is intended to be used by a multidisciplinary team with training and expertise in toxicology (human health and ecotoxicology), chemistry, materials science, exposure assessment, and life cycle assessment. Additional expertise in engineering, social sciences, economics, and cost analysis also might be required. Assessors without such expertise, such as and small- and medium-sized firms, may need user-friendly assessment tools or technical support to carry out parts of the assessment. Examples of such tools are given throughout the report.
Summary of the Committee’s Framework
The committee’s alternatives assessment framework has the following main activities, including the asterisked optional activities:
- Step 1: Identify Chemical of Concern
- Step 2: Scoping and Problem Formulation
- Step 3: Identify Potential Alternatives
- Step 4: Initial Screening of Identified Alternatives
- Step 5: Assess Physicochemical Properties
- Step 6-1: Assess Human Health Hazards
- Step 6-2: Assess Ecotoxicity
- Step 6-3: Conduct Comparative Exposure Assessment
- Step 7: Integration of Information to Identify Safer Alternatives
- Step 8: Life Cycle Thinking
- Step 9-1: Additional Life Cycle Assessment*
- Step 9-2: Performance Assessment*
- Step 9-3: Economic Assessment*
- Step 10: Integrate Data and Identify Acceptable Alternatives
- Step 11: Compare Alternatives*
- Step 12: Implement Alternatives
- Step 13: Research and Innovation*
Individuals who currently use other frameworks will quickly recognize familiar elements in the committee’s framework. Thus, in many ways, the committee’s framework is not a revolutionary new approach; rather, it incorporates ideas from existing approaches into a flexible, inclusive framework.
Additionally, this framework includes several important unique elements or advancements, such as:
- a focus on scoping and problem formulation;
- an increased emphasis on comparative exposure assessment;
- increased use of physicochemical properties4 to
- assess human health and ecotoxicity hazards;
- a two-tiered approach to evaluating chemical alternatives that includes health and ecotoxicity, followed by a consideration of broader impacts; and
- recognition of the need for research and innovation.
The following sections explain each of these elements in more detail.
A Focus on Scoping and Problem Formulation
An often neglected, but very important, step is that of scoping and problem formulation (Step 2). This step defines and documents the goals, principles, and decision rules that will guide all of the following steps in the assessment and thus, the outcome of the assessment. Many decisions about the selection of alternatives are not purely technical, but rather are value-driven or context-dependent. It is important to explicitly articulate and document those assumptions and constraints—which often take the form of decision rules that flow from an organization’s goals and principles. The inclusion of a problem formulation and scoping step in the committee’s framework is consistent with recent National Research Council (NRC) reports that have recommended similar efforts in other types of assessments (NRC 2014). Specifically, the preferences of the decision maker need to be made explicit in the form of decision rules or algorithms to be applied to resolve trade-offs across different attribute domains (e.g., toxicity, material and energy use, and cost) and address uncertainty. The committee anticipates that the chemical of interest and its alternatives will often present different hazards both across domains (e.g., ecological vs. human health hazards) as well as within domains (e.g., neurotoxicity vs. respiratory sensitization). Prioritization of alternatives will require the integration of data and consideration of trade-offs and associated uncertainties. How these trade-offs are resolved is inevitably shaped by applying goals, principles, and decision rules defined in Step 2—aspects that are not scientific judgments. The user should also describe the decision rules used to identify a “safer” alternative. This important description of what constitutes “safer” comes into play when considering trade-offs, as described in Chapter 9. When the alternatives assessment is striving to improve the safety of a specific end point (because, for example, the chemical is on a carcinogen list), the alternative will, for pragmatic reasons, need to be an improvement over, or no worse than, the original chemical of concern in the domain that initiated the alternatives assessment. However, a focus on a key end point does not eliminate the need for an assessment of the full range of human health hazard end points and ecotoxicity, or consideration of the life cycle of alternatives. To not include these important elements could lead to the transfer of risks to other parties (burden shifting) and other types of regrettable substitutions. Safer could also be defined in many other ways, including beneficial incremental improvements in one or more domains of interest, or an overall improvement in human health and/or ecotoxicity. What is deemed safer is ultimately context-dependent and also reflects a set of personal, corporate, legislative, or other values.
The problem formulation step (Step 2) also defines the bounds of the assessment, including identifying specific hazards of interest, and establishes the set of steps that will be required to complete the alternatives assessment. At a minimum, the committee recommends consideration of physicochemical properties, comparative exposure, ecotoxicity, human health hazards, and Life Cycle Thinking. Whether or not broader environmental impacts, such as resource use and impact on climate, are within the scope of the assessment should be decided in the problem formulation step. Consideration of economic, performance, and social impact are also optional steps that many assessors will want to consider. Problem formulation also
_____________
4 For the purpose of this report, physicochemical properties are broadly defined as physical properties, solvation properties related to interactions with different media and properties or molecular attributes that define intrinsic chemical reactivity.
defines when and how novel data streams will be used to inform the assessment.
Within problem formulation, the committee found that characterization of function and performance requirements are often undervalued parts of alternatives assessment frameworks but are essential for successful prioritization and adoption of alternatives. Characterizing the function of a chemical of concern at the beginning of an alternatives assessment process can help focus the assessment on those functions provided by the chemical of concern. It can also support the identification of a broad range of viable chemical and non-chemical alternatives that meet the functional requirement of that chemical in a particular process or product. A focus on function changes the lens by which chemicals of concern are viewed, from avoidance of such chemicals to identifying the safest, most viable options to meet a particular function in a particular application.
Another crucial item that is embedded in the scoping step is defining the role of stakeholders. Stakeholder engagement helps ensure that the assessment will address a broad range of concerns, improves understanding and support of the assessment outcomes, and provides additional review of technical information, analytical methods, and data, improving the overall quality and accuracy of the assessment.
An Increased Emphasis on Comparative Exposure Assessment
The committee recommends an increased emphasis on comparative exposure assessment (Step 6.3). The committee found that most of the existing assessment frameworks it studied focus on reducing inherent hazards, with only minor considerations of exposure. The committee believes that consideration of inherent hazard can be a useful initial step for identifying safer alternatives and streamlining assessment. However, an approach that focuses on inherent hazard should only be used when a comparative exposure assessment indicates that the expected routes and amount of exposure are not expected to be substantially different between a chemical of concern and its alternatives. Thus, the committee recommends that the potential for differential exposure (in the absence of exposure-mitigating protection) between the chemical of concern and alternatives be explicitly considered rather than assuming equivalent exposure.
The committee’s increased emphasis on exposure should not be interpreted as a recommendation for more comprehensive risk assessment. The committee concludes that simplified exposure estimates without elaborate exposure modeling can meet the needs of many alternatives assessments. The committee’s approach allows for the use of either available exposure models or comparison of critical physicochemical properties as a way to determine the relative exposure potential of alternatives.
Elevating the Role of Physicochemical Property Evaluation
The committee’s framework elevates the role of evaluation of physicochemical properties (Step 5) in the alternatives assessment process. The committee broadens the consideration of physicochemical properties beyond the current practice of evaluating physical hazards such as explosivity and corrosivity. This increased emphasis is consistent with the growing body of literature showing that a number of physicochemical properties are often predictive of ecological and human health hazards and can be used to inform data gaps and guide the chemical design process. Moreover, low-cost and reliable state-of-the-art in silico methods, which are a good source of physicochemical property data, are available to support alternatives assessments. These data also can be obtained experimentally. The physicochemical property data emphasized by the committee’s framework can be used to:
- determine the environmental compartment(s) into which the chemicals will partition;
- estimate the potential for bioconcentration and bioavailability;
- estimate the likely route(s) of mammalian exposure and bioavailability; and
- estimate the likelihood for high aquatic toxicity.
A Two-Tiered Approach to Integrating Data
A two-tiered approach to integrating data on chemical alternatives (Steps 7 and 10) is described in the committee’s framework. Step 7 primarily focuses on information about comparative exposure, human health, ecotoxicity, and physicochemical properties, with the goal of identifying alternatives that warrant further data gathering and analysis. In most cases, Step 7 is best considered a triage activity rather than
a final ranking and selection process because it is followed by further life cycle considerations described in Step 8.
In Step 10, the entire data set for a chemical of concern and its alternatives is considered, including data from optional analyses such as environmental impact, cost, performance and social impact—factors that may require further trade-offs. All this information is added to the mix of data obtained through Step 8. The consideration of trade-offs and uncertainties may impact the identification of suitable alternatives. This process may range from being extremely simple to very challenging. Because of this complexity, as well as the value and context-dependent nature of this process, the committee does not provide a step-by-step algorithm for the completion of Step 10; rather, the committee emphasizes the need to apply the decision rules for resolving trade-offs and uncertainty that were established in Step 2. Similarly, the committee calls for thorough documentation of the assessment methods, results, and decisions.
The Need for Research and Innovation
The committee stressed the need for research and innovation in its framework (Step 13). Two types of innovation are important: the design of new chemical alternatives and the identification of ways to meet the ultimate needs of industry and the consumer using approaches other than direct chemical substitutions. In cases where no known chemical substitutions are identified, the design of new chemical alternatives by synthetic chemists and other scientists may be part of the solution. While in chemical design, it is current practice to focus on designing new molecules with better performance, the committee recommends that safety and ecological considerations also be an integral part of early chemical design. The committee provides specific suggestions for how to do this in Chapter 13.
SCIENTIFIC INFORMATION AND TOOLS REQUIRED TO SUPPORT THE COMMITTEE’S FRAMEWORK
Information that can be used to assess end points of interest (e.g., human health and ecological hazards) includes, but is not limited to, traditional data streams, such as measurement of physicochemical properties, human epidemiologic data, and the results of animal toxicity or ecotoxicity studies. Evaluation of results derived from traditional data streams is often supported by a variety of classification tools (e.g., GreenScreen® and the Globally Harmonized System of Classification and Labelling of Chemicals [GHS]), which categorize the available data into different levels of concern (e.g., low, moderate, high). The committee supports the use of harmonized GHS classification schemes, but suggests short-term refinements in how they are used, such as supplementing them with additional guidance. The committee recommends more aspirational refinements as well, such as the use of novel in vitro and in silico data. More information about the scientific information and tools is found throughout the report, as follows:
Human Health
Specifically, in the discussion of human health data (Chapter 8), the committee recommends
- Use of GHS-tied criteria with a few refinements, including using health hazard assessment guidance to classify chemicals for end points where GHS criteria require expert judgment.
- Moving beyond relying solely on traditional types of data associated with GHS or other benchmarking approaches and towards using data from novel high throughput and in silico approaches, for users with adequate scientific resources to do so. The committee specifically emphasizes greater use of available scientific information to fill data gaps when appropriate.
- The eventual development of a well-accepted classification scheme for novel types of data and in silico modeling, analogous to the GHS system, to enhance the use of this information.
Ecotoxicity
In the discussion of ecotoxicity data in Chapter 7, the committee recommends the following refinements:
- Using physicochemical data to determine which environmental compartments a chemical will partition into, and compiling ecotoxicity for these compartments.
- Using relevant high throughput data produced for human health assessment.
Incorporation of High Throughput Data
Developments in toxicity testing have changed dramatically during the past 10 years. Publication of the NRC report entitled Toxicity Testing in the 21st Century (TT21C): A Vision and a Strategy (NRC 2007) has spurred new approaches and thinking about chemical hazard assessment. Similarly, advances in chemistry, material sciences, and toxicology will lead to future changes in the conduct of alternatives assessments. It is critical that the scientific community embrace the challenge and advantages of using novel data streams in the alternatives assessment process. This report provides the committee’s thinking on how these novel in vitro data streams and in silico modeling approaches can be used. In keeping with the spirit of the NRC TT21C report, the committee strived not to provide detailed guidance that could restrict future thinking, but rather to demonstrate how these data could be used to support informed decision making. The pharmaceutical industry’s experience with integrating novel data and tools early in the product development pipelines serves as an important blueprint for this activity.
Future efforts are needed to develop principles or tools that support the benchmarking and integration of high throughput data on chemical effects, especially in the context of different regulatory requirements. This effort is needed for two types of interrelated activities; first, to address how novel data streams could be used as primary data in human health and ecotoxicity hazard assessments (e.g., the use of in vitro mutagenicity data for DNA reactive chemicals) and second, to address how these data can be used to fill data gaps across a broad range of domains, including health, ecotoxicity, exposure assessment, and physicochemistry.
The committee anticipates that, unlike benchmarking of animal and ecotoxicity data, which have a manageable range of end points and outcomes, the approaches used for novel data streams, especially the broad range of end points provided by high throughput assays, may be less amenable to a formal, endpoint-driven GHS-like classification scheme. Instead, user-defined decision rules and principles will likely guide incorporation of these data into the alternatives assessment process. As a result, the expert, judgment-guided discussions with regulatory bodies may not follow an identical template for all types of chemical alternatives assessments.
Other Considerations
In keeping with the theme of transparency and documentation described earlier, the committee notes the importance of tools to improve communication of assessment methods and information to all stakeholders. Tools that transparently capture how data are considered and integrated into the assessment process, as well as tools to help visualize new types of data, will be critical to facilitating communication of the complex information on chemical alternatives.
The committee’s framework is designed to accommodate the advances in tools, including those developed for mixtures and high throughput data, that surely lay ahead, and to allow for the integration of information from a variety of scientific disciplines. The case studies described in Chapter 12 demonstrate how high throughput data and other computational approaches can be used to complete certain steps in the committee’s framework. The committee recognizes that the application of these methods may be beyond the scientific capacities of some users, particularly small- and medium-sized companies. Thus, the committee recognizes the importance of developing new tools, education, and technical support networks to assist entities with less capability in implementing novel data streams into the alternatives assessment process.