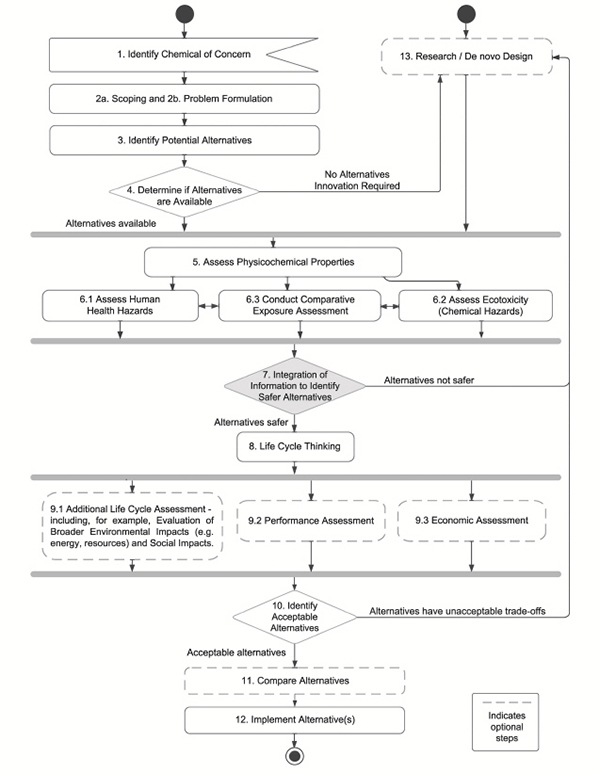
Integration of Information to Identify Safer Alternatives
Any process of choosing among alternatives either explicitly or implicitly integrates the findings from a variety of sources, including human health and ecological assessments. This process therefore requires judgment and integration of information across different hazard domains. The first integration step in the committee’s framework (Step 7) involves an initial identification of safer alternatives based on information compiled in previous framework steps. It is important to note that the decision-making process taking place during Step 7 is not expected to yield a single alternative among a set of possible alternatives. Other factors will be considered in a later integration step (Step 10). These, too, will be important, and may ultimately eliminate what appears to be a preferable alternative from a human health and ecotoxicity perspective on the basis of other valued considerations, such as its broader environmental impact, performance, cost, or technological feasibility.
Nonetheless, Step 7 represents a key transition. Most of the steps up to this point constitute activities that are traditionally considered to be aspects of risk assessment. Integrating evidence, however, also includes the application of explicit or implicit value judgments. The choices of which health end points are most important, how choices are made in the presence of uncertainty, and the relative importance of health and ecosystem end points bring societal value judgments into the alternative selection process. This suite of choices is generally considered to fall within the domain of risk management, as opposed to risk assessment or risk characterization. This chapter begins with a general overview of the data streams that will be integrated in Step 7, as illustrated in Figure 9-1. It then discusses strategies that can be used to address trade-offs and uncertainty.
The hypothetical data in Table 9-1 illustrate a number of challenges that may be expected while conducting an alternatives assessment: a) the presence of trade-offs, where one alternative is preferable with respect to one or more end points, but is less preferable for one or more other end points within a domain, such as human health or ecotoxicity; b) the presence of trade-offs between domains, where some alternatives are preferable from a human health perspective, while others are preferable from an ecotoxicity perspective; c) the presence of variable levels of uncertainty about the level of toxicity or exposure (as depicted by the colors); or d) complete absence of knowledge in that the level of toxicity cannot be determined even with caveats (as depicted by the gray entries). Depending primarily on the extent of the tradeoffs and their degree of uncertainty, the task of determining the preferred alternative ranges from extremely simple to very challenging.
As noted earlier, Step 7 represents a key transition. Most of the steps up to this point are activities traditionally considered aspects of risk assessment. The step of integrating evidence, however, includes applying explicit or implicit value judgments. Therefore, the individual conducting the alternatives assessment may need additional guidance to complete this step. For example, if that individual is not considered the decision maker, then he or she will need to have the preferences of the decision maker made explicit in the form of decision rules or algorithms that can be applied in the face of trade-offs and uncertainty. The explicit consideration and documentation of those preferences were explained in Steps 2a and 2b, Scoping and Problem Formulation, respectively (see Chapter 4).
Note: The relative uncertainty of each finding is depicted by colors (dark blue = known; light blue= limited certainty; pink=highly uncertain; gray = unknown). C= Chemical of concern.
| Human Health | Ecotoxicity | Physicochemical | ||||||||||||||||||||||||||||
| Alternatives | Acute Toxicity | Carcinogenicity | Genotoxicity | Reproductive | Developmental | Neurotoxicity | Acute Aquatic Toxicity | Chronic Aquatic Toxicity | Persistence | Bioaccumulation | ||||||||||||||||||||
| H | M | L | H | M | L | H | M | L | H | M | L | H | M | L | H | M | L | H | M | L | H | M | L | H | M | L | H | M | L | |
| C | ||||||||||||||||||||||||||||||
| A | ||||||||||||||||||||||||||||||
| B | ||||||||||||||||||||||||||||||
TABLE 9-2 Sample Results of Comparative Exposure Assessment
Note: >> indicates that the alternative may involve substantially greater exposure than the chemical of concern, ≈ indicates that exposures may be considered substantially equivalent, and << indicates that the alternative may involve lower exposures due to intrinsic properties of the chemical or the specific functional use.
| Alternatives | Human Health Exposure Routes | Eco Exposure | |||||||||||||||||||
| Oral | Dermal | Inhalation | Ocular | Water | Air | Soil | |||||||||||||||
| > | ≈ | < | > | ≈ | < | > | ≈ | < | > | ≈ | < | > | ≈ | < | > | ≈ | < | > | ≈ | < | |
| A | X | X | X | X | X | X | X | ||||||||||||||
| B | X | X | X | X | X | X | X | ||||||||||||||
INFORMATION NEEDED TO IMPLEMENT STEP 7 IN THE COMMITTEE’S FRAMEWORK
The information that the user will primarily rely on to complete Step 7 was evaluated and collated in Steps 5 (Assess Physicochemical Properties), 6.1 (Assess Human Health), 6.2 (Assess Ecotoxicity), and 6.3 (Conduct Comparative Exposure Assessment). The result of Step 6 is an assessment of human health and ecotoxicity hazards and an indication of how each alternative’s exposure is expected to compare with that of the chemical of concern. In most cases, the alternatives will present different hazards both across domains (e.g., ecotoxicity vs. human health hazards) and within an evaluated domain (e.g., neurotoxicity vs. respiratory sensitization). Tables 9-1 and 9-2 present a summary of the types of evidence that may be gathered in Steps 5 and 6.
STRATEGIES TO ADDRESS TRADEOFFS AND UNCERTAINTY
The two key underlying challenges (tradeoffs and uncertainty) inherent in data integration can be viewed as separate but potentially overlapping. Figure 9-2 shows trade-offs and uncertainty in two dimensions, along with the decision-making strategies required as a result
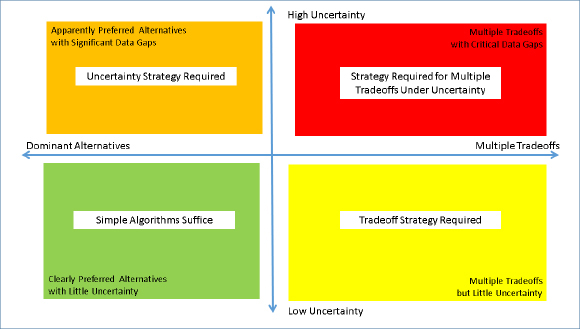
FIGURE 9-2 Strategies to address trade-offs and uncertainty in alternatives assessment. The pattern of results from Step 6 will present potential trade-offs among alternatives, as well as varying levels of certainty. In some cases, both trade-offs and uncertainty will be key challenges that will need to be addressed.
of the evidence gathered in Step 6. In the lower-left (green) quadrant, the choice among alternatives is made clear because sufficient information is available so that an alternative with no trade-offs within or among human and ecotoxicity domains can be chosen. In the upper-left (orange) quadrant, there appears to be a preferable alternative based on what is known and best estimates, but uncertainty about the findings remain, or there is a critical gap in the information available for the otherwise preferred alternatives. In the lower-right quadrant (yellow), there is adequate information available, but the pattern of findings is such that there are trade-offs within human health and ecotoxicity outcomes or between human health and ecotoxicity outcomes such that no alternatives are preferable for all end points or from all perspectives. In the upper-right quadrant (red), both challenges exist in that there are trade-offs among end points that are simultaneously affected by uncertainty.
The simplest case (green quadrant; no trade-offs, adequate and qualitatively equal levels of knowledge) can be addressed by simple algorithms that identify the preferred alternative. An example of such an algorithm is to identify alternatives that are preferable to a baseline alternative in at least one end point category, and not worse in any other.
Uncertainty Strategies (Orange Quadrant)
As discussed in Chapters 6-8, it is likely that there will be varying levels of uncertainty surrounding human or ecological toxicity and relative exposure. Box 9-1 describes types of uncertainty, and this section describes selected strategies for addressing them. With respect to variability (e.g., in exposures and in the responses of human or ecological receptors to those exposure), this is assumed to have been addressed to the extent possible in Steps 6.1, 6.2 and 6.3.
Consider the following example summarized in Table 9-3, which is a hypothetical output of Step 6. This example has been deliberately simplified such that all other end points are considered equal among the alternatives and are equally well known. Alternatives A and B are preferable (have lower or equivalent toxicity) to the chemical of concern given the best estimate available (the medium and low categories are based on the
There are numerous ways in which uncertainty may be defined and categorized. Taxonomies have been proposed that help differentiate uncertainty according to its many sources (Morgan and Henrion 1990; NRC 1994; Cullen and Frey 1999; and Krupnick et al. 2006). One key distinction that is often advocated is the conceptual separation of uncertainty and variability. The term uncertainty is most often used to describe limitations in knowledge. Uncertainty means that we do not know what the true situation is (the uncertainty can be qualitative or quantitative in nature). The term variability is used to describe real differences that exist in the world among individuals, behaviors and the natural world. When variability is described, it reflects the fact that there is no single true number that fully describes a phenomenon. In practice, it is often difficult to completely separate uncertainty and variability. This is particularly difficult when attempting to express uncertainty (or lack of knowledge) in the nature and extent of variability.
In describing uncertainty as a limitation of knowledge, scientists have found it useful to distinguish between two main types of uncertainty, parameter uncertainty and model uncertainty. When describing aspects of toxicity or exposure in quantitative terms, there will often be uncertainty with respect to specific values that need to be assigned. This type of uncertainty has often been called parameter uncertainty. Whenever there is an incomplete understanding of a causal nature (i.e., there are competing explanations for some observed phenomenon), there will necessarily be alternate mathematical models that might legitimately be used to make predictions about the level of risk. The existence of competing explanations, (and competing models), is often referred to as model uncertainty. When the model uncertainty is so great that it leads to questions about the very existence of causal relationships (as opposed to competing models based on the strength and exact nature of the relationship), it may be referred to as fundamental causal uncertainty (NRC 2009).
Examples of parameter uncertainties include the numerical thresholds for human or environmental exposure, below which no adverse effects are expected, unmeasured physicochemical properties, and estimates of the amount of exposure in a use scenario. Examples of model uncertainty include cases where the specific mode-of-action of carcinogenicity of a chemical may be unknown or debated, or where there are different interpretations of the evidence from in vitro experiments or in silico predictions, leading to alternate views of whether a specific form of toxicity should be assumed. Fundamental causal uncertainty may take the form of an unclear causal linkage between a chemical and a form of toxicity because of confounding factors and significant inconsistency in the toxicity database. Depending on the approach used to present evidence for human health and ecotoxicity, all of these types of uncertainty may need to be addressed in this step.
Note: Uncertainty in each toxicological finding is depicted by colors (dark blue = known; light blue= limited certainty; pink=highly uncertain; gray = unknown)
| Alternatives | Reproductive | Developmental | Neurotoxicity | ||||||
| H | M | L | H | M | L | H | M | L | |
| C | |||||||||
| A | |||||||||
| B | |||||||||
best judgment available after considering multiple lines of reasoning). However, Alternative A does not have any evaluation available for neurotoxicity. Although Alternative B was given a low toxicity rating, toxicity for two end points was considered “highly uncertain.”
There are a number of possible strategies for addressing the presence of uncertainty, assuming that each is presented as a separate concept (as shown in Table 9-3 or in some other qualitative or quantitative format). If the strategies were entirely embedded in the toxicological evaluation process, it is possible that toxicity would be deliberately overestimated to account for uncertainty. It is expected, however, that the toxicological evaluation process will have considered the concept of individual variability. The following section presents some strategies for addressing uncertainty.
Known best estimates basis: In this approach, only the best estimates are considered, and alternatives with unknown toxicity end points are excluded. In the example presented in Table 9-3, this strategy would prefer Alternative B to the chemical of concern. Alternative A would be excluded due to missing data. While being transparent about uncertainty, this approach does not directly apply the level of uncertainty in the algorithm, except to exclude alternatives with data gaps. According to this strategy, Alternative B would be preferred to the chemical of concern.
Uncertainty downgrade basis: In this strategy, the best-estimate toxicity value is, in some way, downgraded based on uncertainty (for example, downgraded by one level for moderate uncertainty or two levels for high uncertainty), and alternatives with missing data are excluded. According to this strategy, Alternative A would be considered unacceptable due to missing data, and Alternative B would be downgraded from {L,L,L} to {H,L,H} due to the high levels of uncertainty in the first and third end points. With this adjustment, Alternative B is no longer clearly preferred to the chemical of concern due to this uncertainty-based adjustment. This approach considers the uncertainty and “errs on the side of safety” by biasing the assessed toxicity to be greater where there is greater uncertainty in the finding. This strategy essentially “punishes” alternatives for uncertainty. The committee notes that this approach to addressing uncertainty (or any approach that excludes alternatives with limited or missing evidence on toxicity) can undermine the selection of safer alternatives and “erring on the side of safety” may be counterproductive. Depending on how it is implemented, this approach could lead to less safe alternatives consistently being preferred just by virtue of having been subject to many studies (and therefore having less uncertainty with respect to toxicity).
Quantitative uncertainty analysis: Uncertainty in toxicity values could be expressed quantitatively or illustrated graphically. This could take the form of a relatively simple expression of a range, or could be more elaborately expressed as a probability distribution, among other options. The benefit of this approach is transparency. It becomes easier to confidently conclude that one alternative is preferable to another if it is shown in a clear illustration. Therefore, this approach may be useful when the uncertainty is large enough that it presents a challenge in deciding on the preference ordering of alternatives. This approach can provide considerable insight, as illustrated by an example of a quantitative expression of uncertainty in comparing alternative chemicals (Finkel 1995).
Remaining neutral about uncertainty and missing data: A variation on the above strategies would be to note the presence of uncertainty and missing data but not exclude the alternative or otherwise demote it at this point in the selection process. The basis of temporarily treating an alternative neutrally with respect to uncertainty or missing data is to avoid prematurely removing potentially safer alternatives from the evaluation process. It may be assumed that while other assessments (economic, performance, etc.), are being conducted at later stages, the missing data can be replaced with direct or indirect evidence relating to the one or more end points for which data were missing. This approach should only be taken if the alternative appears to have sufficient merit on other grounds (e.g., safer with respect to some key end points) to warrant the effort of gathering more data. The committee considers this approach as being most compatible with the multistep consideration of alternatives recommended in the committee’s framework.
TABLE 9-4 Example of an Evidence Table Demonstrating Trade-offs with Alternatives with Adequate Information Indicated by Equal and Low Levels of Uncertainty for all End Points)
Note: Uncertainty of each finding depicted by colors (dark blue = known)
| Alternatives | Developmental Toxicity | Neurotoxicity | Aquatic Toxicity | ||||||
| H | M | L | H | M | L | H | M | L | |
| C | |||||||||
| A | |||||||||
| B | |||||||||
Trade-off Strategies (Yellow Quadrant)
Even under conditions of complete knowledge, an alternatives assessment may require the consideration of trade-offs among end points within a domain, or between health and ecotoxicity domains. This is further complicated in later stages by the consideration of other factors, such as broader environmental impacts, cost, performance, and social outcomes, which may require further consideration of trade-offs.
Table 9-4 includes a potential challenge faced in an alternatives assessment when there is no clearly preferred alternative. Alternative B is preferable from a human health perspective, but it is undesirable from the ecotoxicity perspective. While both Alternatives A and B have relatively lower ecotoxicity, Alternative B is not as safe as Alternative A from a human health perspective. And from a human health perspective, there is no clear preference between the chemical of concern and Alternative A, since it is not apparent that a rating of “medium” for developmental toxicity would necessarily be preferable to a rating of “high” for neurotoxicity. The reason that this becomes a difficult call is that the actual health consequences associated with either one could vary by many orders of magnitude in terms of severity or duration of the adverse health effect(s).
Similar to the situation for addressing uncertainty, there are a number of possible strategies for addressing the presence of tradeoffs, (assuming for the moment that the health end points are known with equivalent certainty).
Improvement on key end point: In some contexts, the alternatives assessment may be motivated by the need to improve the safety of the product with respect to a specific end point (such as the original impetus driving the alternatives assessment, another regulatory requirement, or a commercial requirement of customers). In this case, it may be appropriate to remove any alternatives from consideration that do not improve upon the toxicity with respect to the specific end point of interest. The committee acknowledges that this criterion may be appropriate or even necessary from a practical perspective, while also recognizing that this approach may prematurely eliminate an option that does not improve the toxicity with respect to the original impetus of concern, but is potentially much safer when considering many other end points. That said, it is important to note that a focus on a key end point does not eliminate the need for an appropriate level of attention to the full range of human health hazard end points and ecotoxicity or for applying broader Life Cycle Thinking. By not taking these considerations into account, the assessor runs the risk of an unacceptable transfer of risks (i.e., burden shifting) or other types of regrettable substitution.
Strict ordering of end points: In this strategy, end points are strictly ranked such that the highest-ranked end point governs the overall preference ordering. In this case, if developmental toxicity was the higher-ranked end point, then Alternative A would be preferred to the chemical of concern. If the two had been equivalent for developmental toxicity, the ranking would be based on the next
highest-rated end point. This approach requires a strict ordering of the importance of end points, which may not be justifiable on public health grounds and is not likely to be supported by all stakeholders.
Equal weighting of end points: In this strategy, each end point is considered to have equivalent importance, and the trade-off is resolved by assigning a relative weight to the high, medium, and low categories and then adding up the score. The total would indicate the preference ordering of alternatives. But this approach also has its limitations. Just as a strict ordering of end points is not necessarily appropriate, it is not necessarily preferable to treat all end points equally.
Weighted scoring of end points: In this strategy, end points are given an unequal weight, and the relative score is determined by summing up the weighted scores across the end points. This approach would also require placing a relative weight on the high, medium, and low categories or on the raw toxicity values. Weighted scoring of end points is one of the most common approaches in the discipline of Multi-Criteria Decision Analysis (MCDA). MCDA is directly applicable to the analysis of trade-offs in general. This discipline provides a diverse array of tools to use to arrive at a preference ordering of alternatives when considering multiple criteria involving trade-offs. As a general method, it can be applied either within the health and ecological considerations in this step, or later, during the final integration, when other factors ranging from costs to social impacts are considered. Or MCDA can be applied at both points. In addition to adding transparency and formality to the process of integration, MCDA tools often are implemented with software that allows for visualization of the weighting process, facilitating sensitivity analysis associated with the weights assigned to the different objectives. For example, a visualization tool like ToxPi (see Appendix C) could be used with MCDA tools to provide both transparent and formal weighting of end points in the tradeoff process. The ToxPi tool also has the benefit of helping visualize the assembled evidence and reducing the evidence to a unitless score to support expert-driven decision making.
Rule-based ranking: Rather than using weights and arithmetic to indicate preferences among alternatives, the preferences can be ordered by a series of logical statements. The GreenScreen® algorithm uses such an approach by explicitly specifying the preference ordering of all possible combinations of toxicity findings. For rule-based systems, the underlying logic represents an unequal weighting of the importance of human and ecotoxicity end points, but the weighting process may or may not be explicitly described. While this appears to avoid the challenge of assigning “weights” explicitly, an implicit relative weighting is essentially embedded in the rule-based algorithm. The basis for implicit or explicit weighting should be carefully considered before applying a rule-based system to ensure that the organization’s values with respect to the different health outcomes are appropriately represented. A key benefit associated with rule-based ranking is that the organization’s value system, once codified in the form of these rules, can be consistently applied to make the alternatives assessment process less prone to idiosyncratic judgments or manipulation of the weighting schemes toward otherwise preferred outcomes.
Eliminate the “high” rating: In this strategy, the alternative is eliminated if it scores “high” on any toxicity end point. In the example shown on Table 9-4, this approach would eliminate both Alternative A (neurotoxicity) and Alternative B (aquatic toxicity).
Exposure weighting: In this strategy, the extent of exposure that may be associated with the various toxicity end points can be included to assign weight to those endpoints. For example, if the developmental toxicity was associated only with oral exposure, while the neurotoxicity was associated only with inhalation exposure, and oral exposure was expected to be much higher or more frequent given the specific functional use for the chemicals in question, then developmental toxicity could be considered more important. This would yield a preference for Alternative A over the chemical of concern. The inclusion of exposure considerations is addressed further in the next section.
Exposure tie-breaking: If a substantial difference in exposure potential was identified among the alternatives, then the framework’s comparative exposure assessment (Step 6.3) may be used to provide a preference ordering for alternatives when they would otherwise be considered equivalent. For example, adverse
exposure potential could be used to downgrade the toxicological finding, and inherently preferred exposure potential could be used to upgrade the toxicological finding.
Relative risk assessment with disease burden estimation: This strategy involves conducting a relative risk assessment and estimating the relative frequency with which the implicated health end points (those involved in the tradeoff) might occur. If it is not possible to estimate the frequency of implicated health outcomes (e.g., due to the lack of a known dose-response relationship), surrogates for risk, such as a hazard index or margin of exposure, can be used to identify where risks to human or ecological endpoints appear to be more likely given expected exposure levels. The assessment can be further nuanced by considering the relative severity of the expected outcomes, if known, using comparative measures of burden of disease, such as Health-Adjusted Life Years (IOM 2006). To be consistent with the intent of many alternatives frameworks to avoid reliance on extrinsic exposure controls, the unmitigated exposure could be the basis of the evaluation. When considering unmitigated exposure, these relative risk estimations (or surrogate indicators) could then form the basis of focusing the attention (and weight in scoring approaches) on alternatives that appear to have reduced potential for harm. The approach to risk estimation (or at least, a more risk-based consideration of inherent toxicity) need only be done with the level of accuracy required to differentiate among the alternatives. It does not require the effort associated with a full risk assessment and health economic analysis.
Expert-manager judgment: This strategy relies on the application of expert judgment to replace all of the above algorithmic or scoring-based methods with selection by a group of presumably appropriate experts. The term “expert-manager” is used here because the expert is required to make explicit or implicit societal value judgments (e.g., the relative importance of human health and ecotoxicity among end points within each domain) in addition to applying their expertise. This approach has the benefit of using more information than is provided by the outputs of Step 6, including uncertainty and relative exposure considerations. However, this benefit comes at the cost of lower levels of transparency and idiosyncratic variability among experts, who are required by the process to impose value judgments, some of which may not be shared by the organization implementing the alternatives assessment.
List-based preference ordering:43 In response to some regulatory, commercial, or other reasons, an organization may want to, or may be required to, apply the preference ordering based on an external organization’s (for example, an important customer or an important regulatory jurisdiction) apparent preference ordering of health or ecotoxicity end points. This may be as simple as removing alternatives that appear on a list that has been designated as being “of concern.” The choices of alternatives on this basis may lead to safer alternatives, but this essentially “outsources” the value judgments to an external organization, rather than eliminating them.
These strategies are just examples of a variety of possible means to address trade-offs where available alternatives present unavoidable applications of value judgments to determine their preference ordering. The strategies range in complexity, from simple decision rules to relative risk assessment. They are not all mutually exclusive; for example, simple decision rules could be used to eliminate a few alternatives and then a more complex weighting procedure could be applied to the remaining alternatives. Key considerations in choosing the means to implement trade-off decisions include the question of who is appropriately empowered to make societal value judgments, and whether these judgments are developed in advance of the implementation of alternatives assessment or are developed during the alternatives assessment. If the latter is true, the judgments may be more likely to be adjusted in a biased fashion toward a preferred or status quo alternative.
Strategies for Multiple Trade-offs under Uncertainty (Red Quadrant)
In some cases, the alternatives assessment process may be challenged by a combination of both value-based trade-offs as well as uncertainty about one or more end points. Up
_____________
43 This strategy may have been applied in Step 2, if the number of alternatives made an initial screening necessary.
Note: Uncertainty of each finding depicted by colors (dark blue = known; light blue= limited certainty; pink=highly uncertain)
| Alternatives | Developmental Toxicity | Neurotoxicity | Aquatic Toxicity | ||||||
| H | M | L | H | M | L | H | M | L | |
| C | |||||||||
| A | |||||||||
| B | |||||||||
to this point in the discussion of integration, uncertainty and value judgments have been considered separately. In Table 9-3, trade-offs were not apparent, leaving only uncertainty. Conversely, in Table 9-4, uncertainty was eliminated as a consideration, but trade-offs were apparent.
An example of the combination of uncertainty and value-based trade-offs is shown in Table 9-5. Alternative A is preferable to the chemical of concern with respect to developmental toxicity, but appears to be less desirable from a neurotoxicity perspective. However, the neurotoxicity of the chemical of concern is highly uncertain, yielding an ambiguous preference ordering dependent upon the user’s approach to addressing the uncertainty in the neurotoxicity of the chemical of concern. Similarly, Alternative B appears to be preferable from a human health perspective; however, there remains a high level of uncertainty in the one end point that is the basis for the health-based preference, and it is clearly not preferred with respect to ecotoxicity.
This section focuses on how to consider both trade-offs and uncertainty. In cases where there are high uncertainty and apparent tradeoffs, a greater focus on de novo design to create safer options is warranted (Step 13). For analyzing the existing options, it may be useful to note that despite the separation of alternatives assessment from risk assessment, alternatives assessment does have similar goals to comparative risk assessment (supporting decisions on relative safety among decision-making options). The field of comparative risk assessment is generally associated with comparing very different risks (and therefore dealing with value-laden trade-offs), including established and emerging risks and their associated levels of uncertainty (Finkel and Golding 1995; Davies 1996; Florig et al. 2001; Morgan et al. 2001; Willis et al. 2004; Linkov et al. 2006).
The research on comparative risk assessment may provide an appropriate basis for deciding which approach to use when dealing with complex comparisons with considerable uncertainty. Approaches to comparative risk assessment, such as those studied and reported by Florig and colleagues (2001), may be appropriate for the more challenging applications of alternatives assessment, including those situations that involve both uncertainty and value-based trade-offs, because they were designed with such challenges in mind. A key component of these approaches is the parallel use of both quantitative and semi-quantitative schemes and expert consensus-based approaches to ranking risks. Quantitative (including both scoring-based and rule-based) schemes can provide more objective treatment of the evidence, and provide a degree of transparency in their conclusions by having a direct and consistent link between evidence and conclusions. Expert consensus-based approaches allow for more complete consideration of aspects of the evidence base that involve difficult and unquantifiable evaluations, such as conflicting data or conflicting valuations of outcomes.
The expert-consensus method can be augmented by the preparation of a structured summary document containing the evidence for
all alternatives, including some narrative discussion and the quantitative inputs used in the scoring approach, but leaving the final rankings aside. These parallel approaches can then be merged to consider the differences in the rankings from each process and to determine a final ranking based on consideration of the two parallel methods of ranking. This can be done by adjusting one ranking result in light of what was learned in the parallel approach.
The overall outcome of Step 7 is the identification of alternatives that are acceptable because they meet the criteria of being safer, with respect to health and ecotoxicity outcomes. Step 7 may result in some alternatives being eliminated from further consideration. Given that considerations from later steps in the framework may also eliminate some alternatives, it may be appropriate to avoid eliminating too many alternatives early-on, unless they are unambiguously unfavorable from a health or ecotoxicity perspective. When the alternatives assessment is motivated by the need to improve the safety of a specific end point (because, for example, the chemical is on a carcinogen list), the alternative chemical will, for pragmatic reasons, need to be an improvement of, or no worse than, the original chemical of concern in the domain that initiated the alternatives assessment. If several chemicals meet this minimum requirement, then the practitioners should consider whether the alternatives would lead to a reduced overall impact on human health, ecotoxicity, and/or the environment. Ultimately, the approach chosen to integrate the evidence must take into account organizational resources available for conducting the alternatives assessment. More elaborate approaches to alternatives assessment may be appropriate for major decisions. The analysis should be proportionate to the importance of the decision (e.g., the risk associated with the status quo, or to the potential benefit of finding a safer alternative given current levels of exposure to the product). Just as simple approaches are appropriate for small-scale decisions, complex and rigorous treatment is appropriate for major decisions that impact large populations and have large environmental footprints (e.g., fuel additives, energy use, common household products, products used by children or found in most homes, infrastructure and building materials, and food and agricultural uses).