To illustrate how the committee’s framework can be applied, two case studies are presented in this chapter. The case studies represent different users in contrasting decision contexts with diverse priorities. Case Study 1 was written from the perspective of a fictitious manufacturing company with limited expertise. Case Study 2 is intended to demonstrate how new types of data can be used by a company with sufficient scientific resources.
CASE STUDY 1: CHEMICAL SUBSTITUTION OF A RESTRICTED SUBSTANCE (decaBDE)
In Case Study 1, we present a scenario where the use of a substance, the flame retardant decabromodiphenyl ether (decaBDE), is restricted through regulation, and an alternative must be selected from available chemical and material options that have a range of trade-offs. This case study was written from the perspective of a fictitious company—KayDisplay, a small U.S. manufacturer of specialty displays for retail kiosks. In this scenario, the company wants to expand its market by selling products in the European Union (EU), but its current products contain a substance (decaBDE) that is restricted in the EU and is being phased out in the United States (EPA 2012g). This case study illustrates how a chemical alternatives assessment was conducted by a single company as part of an internal feasibility study to determine whether there are alternatives to using materials with decaBDE in order to be able to sell their products in the EU.
While considering this case study, it is important to note that:
- KayDisplay is a fictitious corporate entity, and has been envisioned as a small company headquartered in Washington State, with limited in-house expertise in chemistry, material sciences, and toxicology.
- The chemical alternatives assessment reflects the internal effort of a single company, and not the more extensive assessments that might be expected of regulators facilitating a multistakeholder review of a substance prior to regulatory action.
- Conducting a meaningful chemical alternatives assessment and implementing an informed substitution at a smaller company, like KayDisplay, can only be successful when published information is available. In this particular case, KayDisplay has access to recent multistakeholder and regulator-created alternatives assessments from which to draw.
- The use of tools or modules in this case study should not be interpreted as committee endorsement. Instead, these tools should be viewed as plausible options for an entity to use in this situation.
- The committee’s framework will be applied through Step 7 (comparative chemical hazard assessment) and context-dependent steps (Step 8 and beyond) will be described narratively.
- Alternatives to decaBDE have been studied extensively, so this scenario offers a relatively data-rich case through which to demonstrate the committee’s framework.
Steps 1- 4 of the Committee’s Framework
Step 1: Identify Chemical of Concern
The substance of interest for this assessment is the brominated flame retardant decabromodiphenyl ether (decaBDE). EU legislation restricts the use of certain hazardous substances in electrical and electronic equipment (EC 2003), including decaBDE, and KayDisplay’s kiosk displays would be regulated under Restriction of Hazardous Substances (RoHS, Directive 2002/95/EC), if the company were to place these products on the market in the EU.
Step 2: Scoping and Problem Formulation
Electronic hardware put on the market in the EU cannot contain decaBDE or other polybrominated biphenyl ethers (PBDEs) at levels in
excess of 1000 ppm in any homogenous material found in the product. As designed, the KayDisplay enclosure is made of a low- gloss blend of polyphenylene ether and high-impact polystyrene (PPE/HIPS), with 15%wt decaBDE added to meet UL V-0 flammability rating requirements.
Step 2a: Scoping
Identify Stakeholders and Determine Their Role
The IC2 includes a “Stakeholder Involvement Module,” which KayDisplay will use to consider potential stakeholders. As a small firm, KayDisplay is unable to directly contact regulators, governments, or nongovernment organizations, but will consult with key executives and technical experts within the company, relevant suppliers, and customers. Initial input from stakeholders includes:
- Company representatives: Senior leadership and executives support eliminating decaBDE to expand the company’s market to the EU. They support selecting alternatives that are not expected to be restricted in the future as long as they are technically and economically feasible. They do not need to be involved in technical or context-dependent assessments, but must approve the final decision.
- Technical experts: The primary person responsible for conducting this assessment is the mechanical designer of the enclosure because she is responsible for selecting the material for the parts. Other internal stakeholders will be consulted, including the product managers, procurement engineers, manufacturing engineers, regulatory compliance experts, and product marketing. These inputs will be noted when relevant.
- Supply chain: The direct supplier of the plastic enclosure will be consulted to identify potential alternatives and to provide input on performance and economic issues. The supplier does not want to lose KayDisplay as a customer, but the supplier is sensitive to cost and therefore not willing to acquire new capital equipment to support a change.
- Customers: KayDisplay’s products are sold to companies that assemble kiosks for retail sales (business to business). Key customers in the U.S. were consulted, along with potential EU customers. U.S. customers were most interested in maintaining fire safety and avoiding cost increases. Potential EU customers expect safe, RoHS-compliant products containing no decaBDE, and would prefer that the product qualify for an ecolabel. One ecolabel of interest to KayDisplay’s potential customers is the Total Cost of Ownership (TCO), a European sustainability certification for information technology products, including displays. Products must meet several requirements to be TCO certified, including a requirement that plastic parts weighing more than 25 grams must not contain flame retardants or plasticizers with organically bound bromine or chlorine (TCO Development AB 2012).
Goals, Principles, Decision Rules and Constraints
As a small company in a competitive market, KayDisplay is under significant cost pressure, so it must minimize cost increases. However, the company understands that the current solution is highly cost-optimized, so it may not be possible to bring in a new material or design at cost parity. If there must be a material or process cost increase to meet the new requirement, the company will favor alternatives that offer a performance or aesthetic improvement, which could potentially be used to market the product at a higher price point to compensate. KayDisplay would prefer to use the same design for both the U.S. and EU markets to minimize costs and to increase inventory flexibility.
Based on EU customers’ heightened interest in health and environmental issues, as well as executive support for reducing the risk of future regulations, the product team will attempt to include options that could meet the criteria to earn TCO Display 6.0 certification. However, if cost targets cannot be met within the ecolabel requirements, RoHS-compliant halogenated alternatives may also be considered.
KayDisplay has not conducted a formal alternatives assessment before and has no established principles or policies to guide the assessment. Through an internet search, it was able to locate several sets of principles from which to choose. The product team found a set that aligned with company values and included reducing hazard, minimizing exposure, using the best available information, requiring disclosure and transparency, resolving trade-offs, and taking action. The company will use a “missing data neutral” approach and not assume missing data would receive either the worst or best possible score for an end point or criterion.
As a small company, KayDisplay relies on guidance from outside experts to complete some of
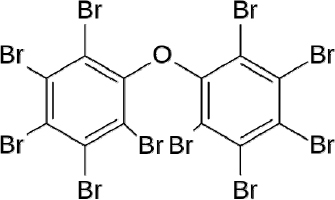
FIGURE 12-1 Chemical structure of decabromodiphenyl ether (decaBDE), CAS number 1163-19-5.
the analyses in the chemical alternatives assessment because it does not have experts on certain tools or methods on staff.
Step 2b: Problem Formulation
Gather Information on Chemical of Interest
Since KayDisplay does not have a chemist or toxicologist on staff, the company is dependent upon published information to gather information about the substance of interest. Fortunately, decaBDE has been studied extensively. The team was able to gather the following information about decaBDE:
Identifying the Chemical. DecaBDE has been identified and described in previous publications. According to Lassen et al. (2006):
- “DecaBDE is a polybrominated diphenyl ether (PBDEs), a group of aromatic brominated compounds in which one to ten hydrogens in the diphenyl oxide structure are replaced by bromine.”
- “Decabromodiphenyl ether, or Deca-BDE, as indicated by the name, has ten bromine atoms attached to the diphenyl oxide structure and a bromine content of 82%-83%. It is used as a flame retardant” (Figure 12-1).
- “The CAS No (chemical identification number) of decabromodiphenyl ether is 1163-19-5. The substance is also known as decabromodiphenyl oxide (DBDO) or bis(pentabromophenyl) ether.”
- “Three different PBDEs have been commonly commercially available. They are referred to as penta-, octa-, and decabromodiphenyl ether, but each product is, in fact, a mixture of brominated diphenyl ethers.”
- The commercial product decaBDE may contain up to 3% of other PBDEs, mostly nonabromodiphenyl ether.
Function and Application and Performance Requirements. DecaBDE is an additive flame retardant:
- Flammability rating: In the U.S., V-0 grade plastics are required for display enclosures. Although the EU has less stringent requirements, the same products will be sold in both markets, so the flammability rating for the alternative materials must be V-0 at 1/16 inch thickness (Lassen et al. 2006).
- Mechanical properties: The alternative must meet or exceed current mechanical properties and performance as listed in the datasheet for the PPE/HIPS resin (Table 12-1).
- Manufacturing: The plastic enclosure parts are injection-molded. Significantly changing the material or using another resin might require new molds. The injection molding supplier would charge KayDisplay for any significant process changes, as well as the non-recurring engineering (NRE) expense of the new molds. Information about the costs associated with mold and process changes are important and would be used for economic analysis. Table 12-2 presents characteristics of the current injection mold process.
TABLE 12-1 Mechanical Properties for the PPE/HIPS Resin Used in KayDisplay’s Kiosks.
| Mechanical | Value | Unit |
| Tensile Stress, yld, Type I, 50 mm/min | 540 | kgf/cm2 |
| Tensile Stress, brk, Type I, 50 mm/min | 490 | kgf/cm2 |
| Tensile Strain, yld, Type I, 50 mm/min | 5.1 | % |
| Tensile Strain, brk, Type I, 50 mm/min | 40 | % |
| Tensile Modulus, 5 mm/min | 24400 | kgf/cm2 |
| Flexural Stress, yld, 1.3 mm/min, 50 mm span | 860 | kgf/cm2 |
| Flexural Modulus, 1.3 mm/min, 50 mm span | 22400 | kgf/cm2 |
| Tensile Stress, yield, 50 mm/min | 51 | MPa |
| Tensile Stress, break, 50 mm/min | 48 | MPa |
| Tensile Strain, yield, 50 mm/min | 4.2 | % |
| Tensile Strain, break, 50 mm/min | 40 | % |
| Tensile Modulus, 1 mm/min | 2200 | MPa |
| Flexural Stress, yield, 2 mm/min | 77 | MPa |
| Flexural Modulus, 2 mm/min | 2200 | MPa |
| Hardness, H358/30 | 95 | MPa |
| Hardness, Rockwell R | 116 | - |
| IMPACT | Value | Unit |
| Izod Impact, notched, 23°C | 16 | cm-kgf/cm |
| Izod Impact, notched, -30°C | 11 | cm-kgf/cm |
| Instrumented Impact Total Energy, 23°C | 428 | cm-kgf |
| Izod Impact, notched 80*10*4 +23°C | 11 | kJ/m2 |
| Izod Impact, notched 80*10*4 -30°C | 7 | kJ/m2 |
| Charpy 23°C, V-notch Edgew 80*10*4 sp=62mm | 14 | kJ/m2 |
| Charpy -30°C, V-notch Edgew 80*10*4 sp=62mm | 7 | kJ/m2 |
| THERMAL | Value | Unit |
| Vicat Softening Temp, Rate B/50 | 140 | °C |
| HDT, 1.82 MPa, 3.2mm, unannealed | 117 | °C |
| CTE, -40°C to 40°C, flow | 9.2E-05 | 1/°C |
| CTE, -40°C to 40°C, xflow | 9.5E-05 | 1/°C |
| CTE, -40°C to 40°C, flow | 9.2E-05 | 1/°C |
| CTE, -40°C to 40°C, xflow | 9.5E-05 | 1/°C |
| Ball Pressure Test, 125°C +/- 2°C | Passes | - |
| Vicat Softening Temp, Rate B/50 | 139 | °C |
| Vicat Softening Temp, Rate B/120 | 142 | °C |
| HDT/Bf, 0.45 MPa Flatw 80*10*4 sp=64mm | 133 | °C |
| HDT/Af, 1.8 MPa Flatw 80*10*4 sp=64mm | 117 | °C |
| PHYSICAL | Value | Unit |
| Specific Gravity | 1.06 | - |
| Density | 1.06 | g/cm3 |
| Water Absorption, (23°C/sat) | 0.23 | % |
| Moisture Absorption (23°C / 50% RH) | 0.06 | % |
| OPTICAL | Value | Unit |
| Gloss, untextured, 60 degrees | 20 | - |
TABLE 12-2 Physical Properties for the Injection Mold Process Used by KayDisplay’s Current Supplier
| Mold Shrinkage, flow, 3.2 mm (5) | 0.5 - 0.7 | % |
| Melt Flow Rate, 280°C/5.0 kgf | 8 | g/10 min |
| Melt Volume Rate, MVR at 280°C/5.0 kg | 8 | cm3/10 min |
| Drying Temperature | 70 - 90 | °C |
| Drying Time | 2 - 3 | hrs |
| Melt Temperature | 265 - 285 | °C |
| Nozzle Temperature | 260 - 280 | °C |
| Front - Zone 3 Temperature | 260 - 285 | °C |
| Middle - Zone 2 Temperature | 240 - 260 | °C |
| Rear - Zone 1 Temperature | 200 - 220 | °C |
| Hopper Temperature | 60 - 80 | °C |
| Mold Temperature | 40 - 70 | °C |
Human Health and Environmental Effects, Exposure Pathways, and Life Cycle Segments.
- Hazards. The human health impacts, environmental impacts, and exposure pathways associated with PBDEs are well established. PBDEs are persistent, they bioaccumulate, and are of high concern to human health because they adversely affect the endocrine (e.g., thyroid) system and neurological development (de Wit 2002). Studies have demonstrated that decaBDE breaks down into more toxic PBDEs through photodegradation, microbial degradation, and metabolism (Rossi and Heine 2007). DecaBDE is an additive flame retardant (not reacted into the polymer molecule), so it can leave the material under certain conditions and enter the environment. People are exposed to PBDEs through inhalation, ingestion and dermal absorption of dust particles in the air where electronic products are installed and used (Johnson-Restrepo and Kannan 2009). Occupational exposure occurs through the same routes, but at higher concentrations at locations producing PBDEs or formulations containing PBDEs, plastic component manufacturing facilities (such as injection molders), and electronics waste recycling and disposal facilities.
- Regulations. Although this assessment is focused on decaBDE as the substance of interest, no other PBDEs can be considered as possible replacements because they are also restricted by the RoHS Directive.
Determining Assessment Methods
For this Case Study, Steps 1 through 7 will be completed in their entirety to demonstrate the framework. Actions planned for Steps 8 through 12 will only be described narratively.
- Step 3 (identify potential alternatives) will be completed through consultation with the current supplier of the plastic injection molded parts and online and offline literature searches.
- Step 5 (assess physicochemical properties) will be completed through literature searches, relying heavily on the EPA’s 2014 DfE report entitled, An Alternatives Assessment for the Flame Retardant Decabromodiphenyl Ether (DecaBDE) and in accordance with guidance provided in Chapter 5.
- Step 6 (assess human health hazards, assess ecotoxicity, and conduct comparative exposure assessment) will be completed through literature searches, relying heavily on the DfE’s DecaBDE alternatives assessment, as well as guidance presented in Chapters 6-8.
- Step 7 (identify safer alternatives) will be completed using the GreenScreen® for Safer Chemicals tool, with a preference for choosing alternatives that are Benchmark 2 or better. GreenScreen® assessments may be supplemented with additional investigations, if needed. Data gaps will be handled in accordance with the GreenScreen® guidelines.
Step 8 and beyond will not be executed as part of this case study, but to complete the exercise of
fully planning the assessment, the following steps and tools will be selected:
- Step 8 (Life Cycle Thinking) would be completed as described in the “Life Cycle Module” of the IC2. Published life cycle assessments would be used to understand the contribution of the housings to the overall environmental impacts of display products. Findings from Step 8 could trigger additional life cycle investigations (Step 9.1) and/or exposure assessments (Sub-step 6 of Step 6.3).
- Step 9.2 (performance assessment) would be completed by screening materials based on properties on their respective datasheets, by prototyping enclosure parts in the alternative materials, and subjecting the prototype parts to standard inspection and qualification tests. Flammability ratings may be verified. The “Performance Module” of the IC2 may be consulted for additional considerations.
- Step 9.3 (economic assessment) would be completed to assess the internal costs and benefits of different options, including changes in material cost, manufacturing costs and NRE charges, costs of compliance for RoHS (such as analytical testing to prove compliance), costs of certification for the TCO ecolabel, and potential market benefits from improved environmental features (such as having ecolabel certification), performance, and aesthetics. Net present value may be used to evaluate the merits of the proposal to enter the EU market, which is the driving force for eliminating decaBDE. The payback period will be calculated. Externalized costs will not be considered. The “Cost and Availability Module” of the IC2 may be consulted for additional considerations.
- Step 10 (identify acceptable alternatives) would be completed by comparing results of Step 9 to the requirements established in Step 2, and by ensuring that the alternatives had lower overall impact to the environment based on any findings in Step 8 and/or 9.1 (Life Cycle Thinking and additional life cycle assessment). Assessment methods, assumptions, data, results, and conclusions would also be documented.
- Step 11 (comparing) would be accomplished using a comparison summary matrix and weighted ranking of the performance, economic, and environmental criteria for each alternative. The best solution would be selected based on the results of Step 11.
- Step 12 (implementation) would be completed by integrating the implementation plan for the alternative solution into the overall plan for KayDisplay’s entry into the EU market. The list of stakeholders would be reviewed to determine if others needed to be consulted. The alternative would be piloted and then ramped up to volume production, addressing issues as they are identified. Finally, a milestone date would be set to review the implementation and to consider new potential alternatives prior to designing the next model.
Steps 3 and 4: Identify Potential Alternatives and Initial Screening
An extensive list of potential alternatives can be found in the literature, so the KayDisplay mechanical designer grouped the alternatives to narrow the assessment (Table 12-3).
Based on preliminary screening, KayDisplay will primarily consider PPE/HIPS with halogenated and non-halogenated flame retardants and a material change to PC/ABS with non-halogenated flame retardants.
After consulting with the injection molder and conducting online and offline literature searches, the KayDisplay mechanical designer identifies the following options:
- PPE/HIPS with a halogenated flame retardant,
- PPE/HIPS with a non-halogenated flame retardant, and
- PC/ABS with a non-halogenated flame retardant.
To identify potential halogenated and non-halogenated flame retardant alternatives, KayDisplay again refers to the DfE’s DecaBDE Alternatives Assessment (AA). KayDisplay is able to share the extended list of alternatives in the report with the injection molding supplier. After conferring with the supplier about available resins and comparing the properties in the resins’ technical datasheets to those in Table 12-4.
Therefore, the chemical alternatives to be evaluated in the assessment are:
- Decabromodiphenyl ethane [DBDPE],
- Antimony trioxide [ATO],
- Resorcinol bis-diphenylphosphate [RDP], and
- Triphenyl phosphate [TPP].
TABLE 12-3 Potential Alternatives
| Class of alternative | Comments | Alternative to be Assessed |
| PPE/HIPS with no added flame retardant | - Cannot meet U.S. flammability requirements + Meets ecolabel criteria + Material cost of PPE/HIPS is low Would require different products for U.S. and EU markets, so this option will not be considered. |
NO |
| PPE/HIPS with a halogenated flame retardanta | + Meets U.S. flammability requirements - Does not meet ecolabel criteria + Material cost of PPE/HIPS is low The advantage of low material cost (lower product cost) might offset not having the ecolabel in the EU market, so this option will be considered. |
YES |
| PPE/HIPS with a non-halogenated flame retardant | - Meeting U.S. flammability requirements with non-halogenated flame retardants in HIPS may be difficult (according to literature) + Meets ecolabel criteria + Material cost of PPE/HIPS is low If the flammability and performance targets can be met, this option offers both lower material cost than PC/ABS or metal and also the market benefit of ecolabel listing, so this option will be considered. |
YES |
| PC/ABS with a halogenated flame retardant | + Meets U.S. flammability requirements - Does not meet ecolabel criteria - Material cost of PC/ABS is significantly higher than PPE/HIPS + May get performance and aesthetic improvements This option has the combination of higher material cost and lost ecolabel market opportunity, and will not be considered. |
NO |
| PC/ABS with a non-halogenated flame retardant | + Meets U.S. flammability requirements + Meets ecolabel criteria - Material cost of PC/ABS is significantly higher than PPE/HIPS + May get performance and aesthetic improvements Although the material cost will be higher, the combination of meeting both the U.S. flammability requirements and ecolabel requirements while also potentially gaining performance and aesthetic benefits make this a viable option, and it will be considered. |
YES |
| Metal (aluminum or magnesium) | + Meets U.S. flammability requirements + Meets ecolabel criteria - Significant material cost increase - Would require changing suppliers - Would require significant design changes - Would require significant manufacturing changes Having to change suppliers combined with significant material cost increases make this option an undesirable choice, and it will not be considered. |
NO |
a The option of continuing to use decaBDE at levels below 1000ppm will not be considered because decaBDE is not effective as a flame retardant at that low level.
TABLE 12-4 Remaining Alternatives
| Class of alternative | Alternative(s) | CAS Number(s) |
| PPE/HIPS with a halogenated flame retardant | Decabromodiphenyl ethane [DBDPE] (with 5% antimony trioxide synergist) [ATO]a | 84852-53-9 [DBDPE] 1309-64-4 [ATO] |
| PPE/HIPS with a non-halogenated flame retardant | Resorcinol bis-diphenylphosphate [RDP] (with 5% triphenyl phosphate contamination) [TPP] | 125997-21-9; 57583-54-7 [RDP] 115-86-6 [TPP] |
| PC/ABS with a non-halogenated flame retardant | Resorcinol bis-diphenylphosphate [RDP] (with 5% triphenyl phosphate contamination) [TPP] | 125997-21-9; 57583-54-7 [RDP] 115-86-6 [TPP] |
a DecaBDE also requires the use of Antimony Trioxide (ATO).
TABLE 12-5 Physicochemical Properties of DecaBDE and Potential Alternatives
| Property | DecaBDE | DBDPE | ATO | RDP | TPP |
| Structure | 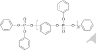 |
||||
| MW | 959.2 | 971.2 | 291.5 | 574.46 (n=1) (57583-54-7) 822.64 (n=2) (98165-92-5) | 326.29 |
Physical State of Chemical (ambient conditions)
Physical state indicates if a chemical substance is a solid, liquid, or gas under ambient conditions, and is determined from the melting and boiling points. Chemicals with a melting point more than 25°C are considered solid. Those with a melting point less than 25°C and a boiling point more than 25°C are considered liquid, and those with a boiling point less than 25°C are considered a gas.
Relevance to exposure: Physical state influences the potential for dermal and inhalation exposure. For solids, there is potential for the inhalation and ingestion of dust particles and dermal contact. For liquids, there is potential for direct dermal contact but not for direct inhalation of the liquid (except in operations that produce aerosols). In the case of these alternatives, all are solid at room temperature except for RDP, but once RDP is blended into a polymer, it has the same exposure potential as a solid, so the assessment will consider the inhalation and ingestion of dust particles and dermal contact in the solid form for all alternatives.
| Physical Form at Ambient Conditions | Solid | Solid | Solid | Liquid | Solid |
| Melting Point (°C) | 300-310 | 350 | 656 | -12 to -16 (liquid at room temperature) 300 370 (decomposes) | 50.5 |
| Boiling Point (°C) | > 320 (decomposes) | >350 (estimated) | 1425 | 245 at 11 mm Hg | |
Vapor Pressure
Relevance to exposure: Vapor pressure indicates the potential for a chemical to volatilize into the atmosphere. If a chemical has a vapor pressure leading to volatilization at room temperature or typical environmental conditions, then the chemical may evaporate and present the potential for inhalation of the gas or vapor. For a Design for the Environment (DfE) chemical alternatives assessment, inhalation exposure is assumed to occur if the vapor pressure is greater than 1 × 10-8 mm Hg. A default value of <10-8 was assigned for chemicals without data that are anticipated to be non-volatile this is based on EPA HPV assessment guidance (EPA 2011b).
| Vapor Pressure (mm Hg) | 3.5 x 10-8 at 21 °C | <7.5x10-7 | <10-8 | 1.9 x 10-5 at 20°C | 6.28 x 10-6 |
Log Kow (LogP), Water Solubility (mg/L), and dE (eV)
Relevance to bioavailability: Log Kow can be used to evaluate absorption and distribution in biological organisms, potential acute aquatic toxicity by narcosis, and potential general population exposure via ingestion. Generally, chemicals with a Log Kow < 5 are orally bioavailable to mammals; chemicals with logKow < 4 are water soluble and available to aquatic species. LogKow is linearly related to bioaccumulation factor (BAF) up to Log Kow ~ 5, where lower water solubility levels off and bioavailability becomes asymptotic.
Relevance to aquatic toxicity: LogP “usually correlates well with acute aquatic toxicity. For non-ionic organic chemicals that are toxic through narcosis, acute and chronic toxicity increases exponentially with increases in logP up to a value of about 5-7” (Voutchkova et al. 2011). Chemicals with logP <2 have higher probability of having low acute and chronic aquatic toxicity (Voutchkova et al. 2011).
Relevance to environmental transport: Chemicals with a high Log Kow also tend to bind strongly to soil and sediment.
Log Kow cannot be measured for inorganic substances, polymers, and other materials that are not soluble in either water or octanol. This is indicated in the table with “No data.”
Water solubility indicates the potential of a chemical to dissolve in water and form an aqueous solution. Water soluble chemicals present a higher potential for human exposure through the ingestion of contaminated drinking water (including well water). In general, absorption after oral ingestion of a chemical with water solubility less than 10-3 mg/L is not expected. Water soluble chemicals are more likely to be transported into groundwater, absorbed through the gastrointestinal tract or lungs, partition to aquatic compartments, and undergo atmospheric removal by rain washout. A substance with water solubility at or below 10-3 mg/L is considered insoluble.
HOMO-LUMO gap (∆E, eV): The energy separation between the highest occupied and lowest unoccupied molecular orbitals (HOMO–LUMO gap, ∆E) is related to broad chemical reactivity (Fukui et al. 1952). A molecule with a small ∆E is considered
more chemically reactive for covalent bonding than one with a larger ∆E. Chemicals with ∆E > 6.5 eV (as calculated by DFT) are much less likely to be acutely or chronically toxic to aquatic species (Kostal et al. in press; Voutchkova-Kostal et al. 2012). Conclusions:
Aquatic toxicity: DecaBDE and TPP have logP > 2 and ∆E < 6.5 eV, which puts them in the high risk category for high acute and/or chronic aquatic toxicity. DBDPE also has ∆E < 6.5 eV but its high logP value (14) suggests it is not very bioavailable to aquatic species, so is likely to be of low/moderate aquatic toxicity.
Bioaccumulation: DecaBDE and DBDPE is likely to have high tendency to bioaccumulate; TPP will likely have a lower bioaccumulation tendency due to its lower logP and higher water solubility; The likelihood of bioaccumulation for RDP will depend strongly on its dissociation to monomer units in the environment.
Environmental transport: Of the alternatives assessed, DBDPE is likely to bind most strongly to soil and sediment (highest logKow).
| Log Kow (LogP) Water Solubility (mg/L) | 6.27 < 1.00x10-4 at 25 °C | 14 (estimated) 7.2x10-4 | No data 14 at 30°C | 4.93 1.05 at 20°C | 4.59 1.9 |
| dE (eV) | 5.0 | 5.3 | No data | No data | 5.0 |
| Physical hazards | |||||
| Flammability (Flash Point) Explosivity | Not flammable Not expected to form explosive mixtures with air | Not flammable Not expected to form explosive mixtures with air | Not combustible Not expected | 302°C Not explosive | 220°C Not expected to form explosive mixtures with air |
| Metabolites, Degradates, Transformation Products | |||||
| Pyrolysis by-products are of particular importance to electronics due to improper and informal waste practices. | |||||
| Metabolites, Degradates, Transforma-tion Products | Photodegradation, anaerobic biodegradation, fish metabolism to lower brominated diphenyl ether (BDE) congeners; Pyrolysis – polybrominated dibenzofurans and polybrominated dibenzo-p-dioxins | Photodegradation —potential to form lower brominated congeners; Pyrolysis—possible polybrominated dibenzofurans and polybrominated dibenzo-p-dioxins | None | Metabolites: hydroxy-RDP, dihydroxy-RDP, resorcinol diphenyl phosphate, and hydroxyl-resorcinol diphenyl phosphate, resorcinol (108-46-3), resorcinol conjugates, resorcinyl glucuronide and resorcinyl sulfate. Environmental degradation of RDP has been demonstrated in experimental studies, but the degradates have not been identified. Degradation of RDP by sequential dephosphorylation could produce phenol, diphenyl phosphate, or resorcinol. | Diphenyl phosphate (CASRN 838-85-7) and phenol (CASRN 108-95-2) |
NOTE: Most data and text in Table 12-5 are from the DfE DecaBDE AA. However, information in this section is simulated, and presented as if it had been obtained by environmental scientists and chemists at KayDisplay’s resin formulator. All italicized text is taken from EPA 2014i.
Step 5: Assess Physicochemical Properties
The physicochemical properties of decaBDE, DBDPE, ATO, RDP, and TPP are compiled in DfE’s DecaBDE AA and presented in Table 12-5. KayDisplay does not have chemists or toxicologists on staff, so they will rely on the EPA’s DfE DecaBDE report data and conclusions.
Step 6.1: Assess Human Health (Chemical Hazards)
The human health effects of decaBDE, DBDPE, ATO, RDP, and TPP have been compiled in DfE’s DecaBDE AA. Similar to Step 5, KayDisplay will rely on the determinations published in DfE’s DecaBDE AA because the company does not have chemists or toxicologists on staff to complete comparable work (see Table 12-6).
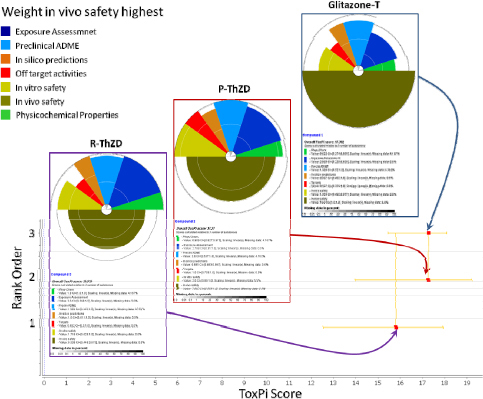
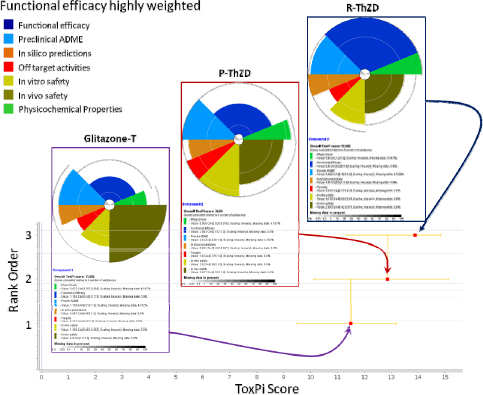
It should be noted that this tabular format is only one way of presenting summary data. There are other approaches, such as ToxPi, which are illustrated in the second case study and in Appendix C.
Step 6.2: Assess Ecotoxicity Hazards
The ecotoxicity effects of decaBDE, DBDPE, ATO, RDP, and TPP have been compiled in DfE’s DecaBDE AA. As in Step 5, KayDisplay will rely on the determinations in the EPA DfE report because the company does not have chemists or toxicologists on staff (see Table 12-7).
Although several of the alternatives under consideration (e.g., ATO, RDP) will be found primarily in sediment and soil, the DfE DecaBDE AA only evaluates aquatic toxicity because ecotoxicity data for terrestrial species was limited or completely absent for the chemicals assessed. Therefore, potential for impacts of the alternatives on high trophic level and terrestrial wildlife is unclear and could not be fully assessed.
Step 6.3: Conduct Comparative Exposure Assessment
Human and environmental exposures to decaBDE are described in Section 5.1.5 of DfE’s DecaBDE AA and the EPA report, An Exposure Assessment of Polybrominated Diphenyl Ethers (EPA 2010b). Because the manufacturing process for the enclosure part, the product-use pattern, and end-of-life hardware disposal are expected to be the same for decaBDE and its alternatives, the exposure scenarios and routes will be considered the same for alternatives as for decaBDE, which is consistent with DfE practice (Lavoie et al. 2010).
- Human exposure (occupational) from EPA 2014: “According to the U.S. EPA’s 2010 exposure assessment of polybrominated diphenyl ethers (PBDEs), individuals in occupations that would lead to higher exposures to specific congeners have higher concentrations of PBDE congeners in their blood than the general public (EPA 2010b). Workers involved in the manufacturing or recycling and disposal of products containing PBDE flame retardants have greater exposure to the chemical compared to the general population (Sjodin et al. 1999; Thomsen et al. 2001; Thuresson et al. 2006).”
- Human exposure (consumer/user) from EPA 2014: “Consumer exposure to decaBDE is possible given that it can be released from common home products and become a component in house dust (Stapleton et al. 2004; Takigamie et al. 2008). It is also possible that workers exposed to decaBDE may inadvertently carry particles containing the chemical home with them. This may lead to exposure to family members through household dust or direct contact, as has been proven with other hazardous chemicals such as pesticides and lead (Thompson et al. 2003; Minnesota Department of Health 2010). DecaBDE has been found in dust within automobiles (Lagalante et al. 2009) and automobile air (Mandalakis et al. 2008). The primary route of consumer exposure to decaBDE is through the ingestion of dust or, for infants, ingestion of breast milk, followed by food and water ingestion and dermal absorption (Lorber 2008; Petito Boyce et al. 2009; EPA 2010a). Inhalation may also be a relevant route of exposure (EPA 2010b). Children have higher levels of exposure to decaBDE than do adults (Petito Boyce et al. 2009), likely due to higher hand- to- mouth behavior.” Information about exposure of decaBDE and alternatives is shown on Table 12-8 on toxicokinetics.
- Environmental exposures from EPA 2014i: “Environmental releases of decaBDE can occur during each stage of a product’s life cycle, including chemical manufacturing, product manufacturing, product storage and use, and end-of-life handling (EPA 2009)”. This is expected to be true for alternatives, as well. Tables 12-9, 12-10, and 12-11 list persistence, transport, and bioaccumulation levels for decaBDE and alternatives.
TABLE 12-6 Human Health Effects Data from Dfe’s DecBDE Alternatives Assessment
| Human Health Effects | ||||||||||||
| Chemical | CASRN | Acute Toxicity | Carcinogenicity | Genotoxicity | Reproductive | Developmental | Neurological | Repeated Dose | Skin Sensitization | Respiratory Sensitization | Eye Irritation | Dermal Irritation |
| Decabromodiphenyl Ether | 1163-19-5 | L | M | L | L | H | L | M | L | L | L | |
| Decabromodiphenyl Ethane | 84852-53-9 | L | M§ | L | L | H§ | L | L | L | VL | VL | |
| Antimony Trioxidea | 1309-64-4 | L | M* | M | M | L | L | H | L | L | M | |
| Resorcinol Bis-Diphenylphosphate; RDP | 125997-21-9 | L | M§ | L | L | M | M | M | L | L | VL | |
| Triphenyl Phosphate | 115-86-6 | L | M | L | L | L | L | H | L | L | VL | |
NOTE: VL = Very Low hazard L = Low hazard M = Moderate hazard H = High hazard VH = Very High hazard Endpoints (VL, L, M, H, and VH) were assigned based on empirical data. Endpoints in italics (VL, L, M, H, and VH) were assigned using values from predictive models and/or professional judgment.
§Based on analogy to experimental data for a structurally similar compound.
*Ongoing studies may result in a change in this endpoint.
a This compound is included in the ongoing EPA Work Plan evaluation for Antimony Trioxide.
SOURCE: Adapted from EPA 2014i.
TABLE 12-7 Ecotoxicity Data from DfE’s Alternatives Assessment
| Aquatic Toxicity** | Environmental Fate | ||||
| Chemical | CASRN | Acute | Chronic | Persistence | Bioaccumulation |
| Decabromodiphenyl Ether | 1163-19-5 | L | L | VH | H |
| Decabromodiphenyl Ethane | 84852-53-9 | L | L | VH | H |
| Antimony Trioxidea | 1309-64-4 | H | M | HR | L |
| Resorcinol Bis-Diphenylphosphate; RDP | 125997-21-9 | VH | VH | M | H‡ |
| Triphenyl Phosphate | 115-86-6 | VH | VH | L | M |
NOTE: VL = Very Low hazard L = Low hazard M = Moderate hazard H = High hazard VH = Very High hazard Endpoints (VL, L, M, H, and VH) were assigned based on empirical data. Endpoints in italics (VL, L, M, H, and VH) were assigned using values from predictive models and/or professional judgment.
‡ The highest hazard designation of any of the oligomers with MW <1,000.
R Recalcitrant: Substance is comprised of metallic species that will not degrade, but may change oxidation state or undergo complexation processes under environmental conditions.
**Aquatic toxicity: EPA/DfE criteria are based in large part upon water column exposures which may not be adequate for poorly soluble substances such as many flame retardants that may partition to sediment and particulates.
a This compound is included in the ongoing EPA Work Plan evaluation for Antimony Trioxide. SOURCE: Adapted from EPA 2014i.
| Toxicokinetics | |
| DecaBDE | Although experimental findings in human and animal studies suggest that decaBDE is poorly absorbed following oral and dermal administration, even low levels of decaBDE are physiologically relevant due to its chemical properties. 82.5-91.3% of decaBDE is eliminated from the body in the feces with ≤0.05% excreted in urine. DecaBDE is mainly excreted as unchanged parent compound but may also be excreted in the form of metabolites. Some conversion of parent compound may be mediated by intestinal epithelium or microflora. Monitoring studies in humans, with unknown levels of exposure, demonstrate that decaBDE can be absorbed, distributed to mammary tissue, and secreted in human breast milk during lactation. |
| Alternative | Expected Toxicokinetics |
| DBDPE | Decabromodiphenyl ethane, as a neat material, is estimated to not be absorbed through the skin and to have poor skin absorption when in solution. Decabromodiphenyl ethane is expected to have poor absorption via the lungs and gastrointestinal (GI) tract. Decabromodiphenyl ethane is poorly absorbed in the GI tract following oral exposure and is mainly excreted in the feces. If absorption does occur, decabromodiphenyl ethane is distributed to the serum, liver, kidney, and adipose tissues and undergoes biotransformation to form metabolites. |
| ATO | Antimony trioxide is expected to have no absorption through skin and has poor absorption through the lungs and gastrointestinal (GI) tract, according to experimental data. Following oral exposure, the majority of antimony trioxide is excreted in the feces. The compound accumulates in lungs with inhalation exposure due to slow absorption and clearance. |
| RDP | Resorcinol bis-diphenylphosphate was readily absorbed via the oral route and was absorbed to a lesser extent following dermal exposure. Metabolism was extensive with metabolites excreted in feces, urine, and in expired air as CO2. |
| TPP | Triphenyl phosphate is hydrolyzed in the liver to produce diphenyl phosphate as the primary metabolite. TPP can be detected in human breast milk. |
| Note: Italicized text taken from EPA 2014i. | |
Step 7: Identify Safer Alternatives
The combined hazard table for decaBDE, DBDPE, ATO, RDP, and TPP from the DfE’s DecaBDE AA report is shown in Table 12-12.
“Confidence in the categorization of endpoint hazard levels,” in Section 4.2: Data Sources and Assessment Methodology of the DfE DecaBDe AA, deals with how data were collected, prioritized and reviewed for use in the development of hazard profiles. According to the report, “High-quality experimental studies lead to a thorough understanding of behavior and effects of the chemical in the environment and in living organisms. Analog approaches and SAR-based estimation methods [were] also useful tools and are discussed throughout this section” (EPA 2014i).
KayDisplay recognizes that there are varying levels of confidence (per Chapter 6) in the different end point categorizations (vH, H, M, L, vL), and the company understands that measured data are not necessarily higher confidence than models. However the company has insufficient expertise to differentiate the confidence levels, and therefore will assume approximately equal confidence levels for the categorizations of end points for the purpose of this assessment.
- Relative hazards: In reviewing the hazard summary table for the alternatives, KayDisplay finds that DBDPE/ATO shows improvements over decaBDE in repeated dose toxicity and irritation, but not in the original areas of concern (persistence, bioaccumulation, and neurodevelopmental toxicity), nor in transformation products. RDP/ATO shows improvements over decaBDE/ATO in the original areas of concern, but does not offer clear improvements in every impact area, and appears to have higher aquatic toxicity.
- Trade-off resolution: In order to help resolve this trade-off and make a decision, KayDisplay had originally considered applying a scoring scheme. However, the company found that constructing a robust scoring scheme, or chemical ranking and scoring (CRS) system, is difficult and can lead to incorrect conclusions (Davis et al. 1994; Swanson and Socha 1997). For example, if a scoring system assigned each chemical very high (vH) four points, each high (H) three points, each medium (M) two points, each low (L) one point, and each very low (vL) zero points, the results would indicate that a substance with all Ms (score 28) would appear worse than a PBT like decaBDE (score 23) if each end point were
TABLE 12-9 Persistence for DecaBDE and Alternatives
| Persistence | |
| DecaBDE | VERY HIGH: Empirical and predicted data indicate that all PBDEs (including decaBDE) are highly persistent in the environment (Environment Canada 2006), and decaBDE has been found in high and increasing concentrations in the sediment of lakes, rivers, streams and estuaries (Song, Li et al. 2005; Environment Canada 2006; Illinois Environmental Protection Agency 2006). The persistence potential for decaBDE is Very High; it is not expected to degrade rapidly under aerobic conditions. Slow degradation through debromination may occur under anaerobic conditions. The anaerobic experimental results are indicative of limited removal, but at very low rates that are possibly background level degradation under the test conditions. Experimental studies indicate no degradation after 2 weeks in a ready biodegradation test, but no data were located for soil or water. Results from biodegradation estimation models also suggest decaBDE is recalcitrant under aerobic conditions. Non-guideline experimental studies indicate decaBDE may be capable of undergoing limited anaerobic biodegradation; however the removal rate also suggests Very High persistence. The initially formed degradation products are also expected to be persistent. DecaBDE is not expected to hydrolyze in the environment based on experimental data. Experimental data indicate that decaBDE may undergo photolysis to debrominated transformation products. Data concerning the kinetics of these photolysis reactions were not located. |
| Alternative | Expected Persistence |
| DBDPE | VERY HIGH: Very high persistence of decabromodiphenyl ethane is expected based on experimental biodegradation data. Decabromodiphenyl ethane was determined to not be readily biodegradable in a 28-day MITI test, nor was it inherently degradable in a 90-day aerobic sewage/soil test using pre-exposed inoculum. Decabromodiphenyl ethane is not expected to undergo hydrolysis since it does not contain hydrolysable functional groups. The atmospheric half-life of decabromodiphenyl ethane is estimated to be 4.5 days, although it is expected to exist primarily in the particulate phase in air. Laboratory studies have demonstrated photolysis of decabromodiphenyl ethane, although the rate of this process under environmental conditions has not been established. |
| ATO | HIGH: Antimony trioxide is an inorganic substance containing metallic atoms that are likely to be found in the environment for more than 180 days after release, resulting in a very high persistence/recalcitrant hazard designation. Based on water solubility studies under a range of pH values, antimony trioxide is expected to slowly dissolve, resulting in the release of antimony ions and, depending on pH, be oxidized or reduced to other oxidation states. Additionally, results from a pure culture study using autotrophic bacterium indicate that antimony may be oxidized by bacteria. Antimony trioxide is not anticipated to undergo hydrolysis under environmental conditions. Antimony trioxide does not contain functional groups expected to absorb light at environmentally significant wavelengths, and therefore is not expected to photolyze. No degradation processes for antimony trioxide under typical environmental conditions were identified. |
| RDP | MODERATE: Moderate persistence is expected for resorcinol bis- diphenylphosphate based on experimental biodegradation studies that indicate the potential for biodegradation of the commercial polymeric mixture. The commercial mixture was determined to be inherently biodegradable using the guidelines of Directive 84/449/EEC, C.6 “Biotic degradation - the Closed Bottle test” test. After 28 days, 37% biodegradation occurred, and after 56 days, 66% biodegradation occurred. Resorcinol bis-diphenylphosphate oligomers (n=1 and n=2) do not contain chromophores that absorb at wavelengths >290 nm, and therefore, are not expected to be susceptible to direct photolysis by sunlight. The atmospheric half-life of resorcinol bis-diphenylphosphate oligomers are estimated to be 6.1 (n=1) and 4.1 (n=2) hours, although they are expected to exist primarily in the particulate phase in air. Enzymatic or basic hydrolysis leading to the production of phenol (CASRN 108-95-2), diphenyl phosphate (CASRN 838-85-7), and resorcinol (CASRN 108-46-3) through sequential dephosphorylation is theoretically possible but has not been demonstrated. |
| TPP | LOW: The persistence of triphenyl phosphate is based on experimental data. Under aerobic conditions in a Japanese MITI ready biodegradability test (OECD Test Guidelines (TG) 301C), 90% biodegradation of triphenyl phosphate occurred after 28 days, and 93.8% triphenyl phosphate removal as dissolved organic carbon (DOC) occurred over 20 days in an OECD 303A guideline study. TPP does not meet the criteria for very low persistence because the percent removal in the criteria does not occur within a 10-day window. In loamy sand, a half-life of 37 days was observed under aerobic conditions. Triphenyl phosphate was determined to be inherently biodegradable in a river die-away test, after degrading 100% over 3 days in river water. Triphenyl phosphate may degrade under anaerobic conditions, with primary degradation of 31.1% after 3 days (89.7% after 40 days) in river sediment. However, removal under anaerobic conditions is not anticipated to be an important fate process. Triphenyl phosphate will undergo hydrolysis under alkaline conditions, with half-lives of 3 days at pH 9; it is relatively stable to hydrolysis under neutral and acidic conditions, with half-lives of 28 days at pH 5 and 19 days at pH 7. Triphenyl phosphate is not expected to be susceptible to direct photolysis by sunlight, since it does not absorb light at wavelengths >290 nm. The atmospheric half-live of vapor-phase triphenyl phosphate is estimated to be 12 hours. |
| Note: Italicized text taken from EPA 2014i. | |
TABLE 12-10 Transport for DecaBDE and Alternatives
| Transport | |
| DecaBDE | DecaBDE has also been measured in ambient atmospheric particulates (Illinois Environmental Protection Agency 2006) and in the Arctic environment, providing evidence that it is subject to long-range transport (Environment Canada 2006). |
| The transport evaluation for decaBDE is based on both estimated and experimental physical and chemical properties. Based on the Level III fugacity models incorporating the located experimental property data, decaBDE is expected to partition primarily to soil. It is not expected to dissociate at environmentally-relevant pHs. DecaBDE is expected to have low mobility in soil based on its estimated Koc. Therefore, leaching of decaBDE through soil to groundwater is not expected to be an important transport mechanism. Estimated volatilization half-lives for a model river indicate that it will have moderate potential to volatilize from surface water. Volatilization potential from a model lake is expected to be low. In the atmosphere, decaBDE is expected to exist primarily in the particulate phase. Particulate phase decaBDE will be removed from air by wet or dry deposition. | |
| Alternative | Expected Transport |
| DBDPE | Based on the Level III fugacity models incorporating the located experimental property data, decabromodiphenyl ethane is expected to partition primarily to soil. Decabromodiphenyl ethane is expected to be immobile in soil based on its estimated Koc. Leaching of decabromodiphenyl ethane through soil to groundwater is not expected to be an important transport mechanism. Estimated volatilization half-lives indicate that it will be non-volatile from surface water. Volatilization from dry surface is also not expected based on its vapor pressure. In the atmosphere, decabromodiphenyl ethane is expected to exist solely in the particulate phase, based on its estimated vapor pressure. Particulates may be removed from air by wet or dry deposition. |
| ATO | The limited mobility observed under experimental conditions and the low vapor pressure indicates that antimony trioxide is anticipated to partition predominantly to soil and sediment. It will not volatilize from water. Soil mobility and sediment adsorption tests indicate that antimony trioxide will be immobile in soil, and therefore will not be expected to migrate into groundwater. |
| RDP | The environmental fate is described for the oligomer where n=1, which is the primary component of the commercial product. Based on the Level III fugacity models incorporating the located experimental property data, resorcinol bis-diphenylphosphate is expected to partition primarily to soil and sediment. Resorcinol bis-diphenylphosphate is expected to be immobile in soil based on its estimated Koc. Leaching of resorcinol bis-diphenylphosphate through soil to groundwater is not expected to be an important transport mechanism. Estimated volatilization half-lives indicate that it will be non-volatile from surface water. Volatilization from dry surface is also not expected based on its vapor pressure. In the atmosphere, resorcinol bis-diphenylphosphate is expected to exist solely in the particulate phase, based on its estimated vapor pressure. Particulates may be removed from air by wet or dry deposition. The higher MW components of the commercial product are anticipated to behave similarly to that described above. |
| TPP | Level III fugacity models incorporating available physical and chemical property data indicate that at steady state, TPP is expected to be found primarily in soil and, to a lesser extent, water. Triphenyl phosphate is expected to have moderate mobility in soil, based on measured Koc values in silty clay, loamy sand, and silt loam. Leaching through soil to groundwater may occur, though it is not expected to be an important transport mechanism. Triphenyl phosphate may volatilize from moist soil and water surfaces based on its Henry’s Law constant. Volatilization from dry surface is not expected based on its vapor pressure. In the atmosphere, triphenyl phosphate is expected to exist in both the vapor phase and particulate phase. Particulates may be removed from air by wet or dry deposition. |
| Note: Italicized text taken from EPA 2014i. | |
equally weighted. A weighted scoring scheme could be an improvement, but as noted above, constructing a robust weighted scoring scheme is difficult and would be beyond the capabilities of KayDisplay.
Instead of creating its own system, KayDisplay referred to the “Hazard Assessment Module” of the IC2, which recommends using GreenScreen® for Safer Chemicals as a way of integrating information across human health and environmental topics (Clean Production Action 2014).
The GreenScreen® benchmark scoring system uses structured decision logic to assign a single integer score to each chemical being assessed. This scheme incorporates national and international precedents to weigh and prioritize combinations of hazard end points.
The GreenScreen® defines four hazard levels for substances:
- Benchmark 1 — “Avoid - Chemical of High Concern”
TABLE 12-11 Bioaccumulation for DecaBDE and Alternatives
| Bioaccumulation | |
| DecaBDE | HIGH: Laboratory studies demonstrate decaBDE’s bioavailability and metabolism in fish (Illinois Environmental Protection Agency 2006). DecaBDE has been detected in some but not all species of fish studied (Dodder et al. 2002; European Chemicals Bureau 2002; Johnson-Restrepo et al. 2005; Environment Canada 2009; Roberts et al. 2011). Also, decaBDE has been measured in birds and their eggs (Lindberg et al. 2004; Vorkamp et al. 2005) and in mammals, including polar bears, seals, marmots, and foxes (Christensen et al. 2005; Illinois Environmental Protection Agency 2006; Voorspoels et al. 2006; Environment Canada 2009). Further, terrestrial species tend to have higher levels of decaBDE than aquatic species for both birds (Jaspers et al. 2006) and mammals (Christensen et al. 2005). These observations indicate bioavailability of decaBDE to wildlife and human food sources, with potential for bioaccumulation and biomagnification of decaBDE and/or its degradation products. Based on estimated BAF values suggesting that the potential for bioaccumulation is high and located monitoring data indicating that decaBDE has been detected in higher trophic level organisms. DecaBDE degradation, transformation, and metabolism products also contribute to the high bioaccumulation hazard designation. These compounds are lower brominated congeners and also have been detected in monitoring studies (ATSDR 2004). |
| Alternative | Expected Bioaccumulation |
| DBDPE | HIGH: The bioaccumulation hazard designation is estimated based on decabromodiphenyl ethane monitoring data reporting detections in many different species, including those higher on the food chain. Although the estimated bioaccumulation factor is low, the persistence of decabromodiphenyl ethane and its detection in many species from different habitats and trophic levels indicates high potential for bioaccumulation hazard in aquatic or terrestrial species. |
| ATO | LOW: Antimony trioxide is an inorganic compound and is not expected to bioaccumulate. |
| RDP | HIGH: The estimated BCF value for the n=1 component has high potential for bioaccumulation. The higher MW oligomers that may be found in this mixture (n=2, 3, 4…) are expected to have moderate or low potential for bioaccumulation based on their large size and low solubility according to the polymer assessment literature (Boethling and Nabholz1997). |
| TPP | MODERATE: There is moderate potential for bioaccumulation based on experimental BCF values. |
| Note: Italicized text taken from EPA 2014i. | |
- Benchmark 2 — “Use but Search for Safer Substitutes”
- Benchmark 3 — “Use but Still Opportunity for Improvement”
- Benchmark 4 — “Prefer - Safer Chemical”
“Each benchmark includes a set of criteria that a chemical, along with its known and predicted transformation products, must pass” (Rossi and Heine 2007). For example, if a chemical met any of the following criteria, it would be classified as “Benchmark 1:
- a. PBT = High P + High B + [very High T (Ecotoxicity or Group II Human) or High T (Group I or II* Human)]
- b. vPvB = very High P + very High B
- c. vPT = very High P + [very High T (Ecotoxicity or Group II Human) or High T (Group I or II* Human)]
- d. vBT = very High B + [very High T (Ecotoxicity or Group II Human) or High T (Group I or II* Human)]
- e. High T (Group I Human)” (Clean Production Action 2011)
The criteria for each benchmark become progressively more demanding, with Benchmark 4 representing the most preferred (least hazardous) chemicals.
GreenScreen® attempts to use all available data, including analogs, models, and expert judgment, to assess end points. It has a hierarchy of data adequacy to establish whether the hazard data were of sufficient quality to meet the requirements of the assessment process. End points with insufficient information to assess the hazard are assigned a data gap (DG). There are also minimum datasets which, if not met, will either lower the score or result in the chemical receiving a rating of “U,” denoting that there is insufficient data to enable evaluation. This is consistent with KayDisplay’s choice in Step 2 to be labeled, “missing data neutral.”
As noted above, KayDisplay does not have chemists or toxicologists on staff, and therefore cannot complete GreenScreen® in-house. However, GreenScreen® is aligned with the DfE hazard criteria, and the Clean Production Action has published draft benchmark scores for many of the substances in the DfE DecaBDE AA (see Table 12-11).
Based on the GreenScreen® scores, RDP (Benchmark 2) with TPP (Benchmark 2) appears safer than DecaBDE (Benchmark 1) or DBDPE (Benchmark 1) with ATO (Benchmark 1). However, KayDisplay headquarters are located in Washington State, where water issues are of the highest priority, so the company will further investigate the potential aquatic toxicity of RDP/TPP.
KayDisplay was able to contact the chemical supplier of RDP, and the team learned that commercial formulations of RDP, which contain TPP contamination (<5%), have been subjected to acute ecotoxicity testing, and that the commercial mixture shows no toxicity at the maximum water solubility level, using what is called the Water Accommodated Fraction (WAF) methodology in accordance with OECD guidance. Although RDP/TPP will most likely sequester in sediments, tests using aquatic organisms as surrogates indicate that concerns with water issues are minimal and, for this application it appears to be acceptable.
Conclusion
Based on these analyses, KayDisplay concludes that alternatives based on RDP/TPP meet the requirement of being safer than those based on the original DecaBDE/ATO, so RDP/TPP alternatives will be evaluated further. Alternatives based on DBDPE/ATO (Benchmark 1) will not be evaluated further because DBDPE/ATO is only minimally safer than the original DecaBDE/ATO and does not meet the goal of being Benchmark 2 or better.
Steps 8-13
Once alternatives based on DBDPE/ATO have been eliminated, the remaining alternatives are:
- PPE/HIPS with RDP/TPP
- PC/ABS with RDP/TPP
Both alternatives meet the ecolabel requirement. However, the PPE/HIPS option with RDP/TPP offers a lower cost, but may not meet flammability and performance targets. In contrast, the PC/ABS option with RDP/TPP costs more, but is likely to meet flammability requirements and offer performance and aesthetic benefits. It is clear that additional assessments must be completed to select and implement a single alternative.
As noted earlier, Steps 8- 13 will not be completed as part of this case study.
TABLE 12-12 Combined Hazard Table from DfE Alternatives Analysis
| Human Health Effects | Aquatic Toxicity** | Environmental Fate | ||||||||||||||
| Chemical | CASRN | Acute Toxicity | Carcinogenicity | Genotoxicity | Reproductive | Developmental | Neurological | Repeated Dose | Skin Sensitization | Respiratory Sensitization | Eye Irritation | Dermal Irritation | Acute | Chronic | Persistence | Bioaccumulation |
| Decabromodiphenyl Ether | 1163-19-5 | L | M | L | L | H | L | M | L | L | L | L | L | VH | H | |
| Decabromodiphenyl Ethane | 84852-53-9 | L | M§ | L | L | H § | L | L | L | V L | V L | L | L | VH | H | |
| Antimony Trioxide1 | 1309-64-4 | L | M* | M | M | L | L | H | L | L | M | H | M | HR | L | |
| Resorcinol Bis-Diphenylphosphate; RDP | 125997-21-9 | L | M§ | L | L | M | M | M | L | L | V L | VH | VH | M | H‡ | |
| Triphenyl Phosphate | 115-86-6 | L | M | L | L | L | L | H | L | L | V L | VH | VH | L | M | |
NOTE: VL = Very Low hazard L = Low hazard M = Moderate hazard H = High hazard VH = Very High hazard Endpoints (VL, L, M, H, and VH) were assigned based on empirical data. Endpoints in italics (VL, L, M, H, and VH) were assigned using values from predictive models and/or professional judgment.
§ Based on analogy to experimental data for a structurally similar compound. * This alternative may contain impurities. These impurities have hazard designations that differ from the flame retardant alternative, Brominated poly(phenylether), as follows, based on experimental data: HIGH for human health, HIGH for aquatic toxicity, VERY HIGH for bioaccumulation, and VERY HIGH for persistence. This chemical is subject to testing in an EPA consent order for this endpoint.
* Ongoing studies may result in a change in this endpoint.
‡ The highest hazard designation of any of the oligomers with MW <1,000.
R Recalcitrant: Substance is comprised of metallic species that will not degrade, but may change oxidation state or undergo complexation processes under environmental conditions.
**Aquatic toxicity: EPA/DfE criteria are based in large part upon water column exposures which may not be adequate for poorly soluble substances such as many flame retardants that may partition to sediment and particulates.
1 This compound is included in the ongoing EPA Work Plan evaluation for Antimony Trioxide.
SOURCE: EPA 2014i.
TABLE 12-13 Clean Production Action Draft Benchmark Scores
| Substance | Draft Benchmark score | Basis of Benchmark Score |
| DecaBDE | Benchmark 1 | Very high persistence; high bioaccumulation; high developmental toxicity (1a, 1c, 1e). |
| DBDPE | Benchmark 1 | Very high persistence; high bioaccumulation; high developmental toxicity (1a, 1c, 1e). |
| ATO | Benchmark 1 | High systemic repeat dose toxicity and very high persistence (1c). |
| RDP | Benchmark 2 | Very high ecotoxicity (2f); moderate Group I human toxicity end points (carcinogenicity) (2e); and high bioaccumulation and moderate toxicity (2d). |
| TPP | Benchmark 2 | Moderate Group I human toxicity end points (carcinogenicity and endocrine activity) (2e); high Group II human toxicity end points (repeat dose systemic) and very high ecotoxicity end points (acute and chronic aquatic toxicity) (2f). |
This page intentionally left blank.
CASE STUDY 2: CHEMICAL SUBSTITUTION OF A HAZARDOUS BIOLOGICALLY ACTIVE COMPOUND (GLITAZONE)
In this case study, an alternatives assessment will be performed on three chemicals that were originally developed as pharmaceutical agents. The rationale for choosing this example was driven in part by the committee’s statement of task requiring examples demonstrating “how high throughput and high content data streams could inform assessment of potentially safer substitutes early in the chemical development process” (see Chapter 1). This case study was specifically intended to illustrate how in silico and in vitro high throughput screening (HTS) data, animal toxicity data, and human health outcome data can be used to assess potential hazards associated with a chemical substitution.
When considering this case study, it is important to note the following:
- This case study represents a hypothetical situation where there is a need to find a substitution for a biologically active ingredient that has been identified to cause severe liver injury. This was the result of accidental ingestion by humans during or after the use of the product containing this active ingredient.
- Although based on a real-life historical problem, the presentation of data has been adapted to illustrate the use of the committee’s framework. The approach shown is for illustration purposes only and is not intended as a commentary on any drug development or regulatory process.
- Many of the comparisons made here are based on data and knowledge that were not available at the time of regulatory approval for these drugs. The human health observations associated withthese chemicals drove much of the scientific investigation that led to the development of some of the key in vitro assays and their implications for safety that are discussed in this case study.
- This case study is not intended to imply that all chemical alternatives should be held to the same level of stringency (e.g., as commonly used in the development of pharmaceuticals).
- Publicly available data have been used throughout this case study. For example, the mammalian safety assessments for all three chemicals are taken from the original Summary Basis of Approval documents that are publicly available from the FDA through the Freedom of Information Act. These studies were conducted according to Good Laboratory Practice (GLP) guidelines and formed the basis for regulatory approval.
Steps 1- 4 of the Framework
Step 1: Identify Chemical of Concern
Concerns for human health have been identified with the primary biologically active ingredient, (RS)-5-(4-[(6-hydroxy-2,5,7,8-tetramethylchroman-2-yl)methoxy]benzyl)thiazolidine-2,4-dione, in a product that is widely used across the world. This ingredient (Figure 12-2) is commonly referred to by its abbreviated trade name, Glitazone-T, and is the chemical of concern in this scenario. Numerous reports of severe liver injury, sometimes fatal, in people exposed to products containing Glitazone-T have come to light, so there is a desire to reduce human exposure, eliminate Glitazone-T from the product, or find an alternative chemical substitute for this active ingredient.
Step 2: Scoping and Problem Formulation
Glitazone-T is the primary biologically-active ingredient in the products in which it is used. The exact mechanism of action of Glitazone-T has not been clearly established, although its stimulatory effect on the peroxisomal proliferator activated receptor gamma (PPARγ) is well known and thought to play a key role in its biological effectiveness. In vitro experiments with Glitazone-T showed that the activity of PPARγ increased by 50% at a concentration of 0.72 µM when tested in transfected HepG2 cells. In 3T3-L1 adipocytes, it was shown to reduce the uptake of 2-deoxyglucose by 50% at a concentration of 2 µM.51
Regulatory authorities have identified Glitazone-T as having potential adverse effects on human health. Products containing this active ingredient have been linked to numerous cases of severe liver injury, and in some cases, these effects result in fatalities (Watkins and Whitcomb 1998). The bioavailability of Glitazone-T is approximately 58%. Product effectiveness requires relatively high concentrations in the final formulation. As a
_____________
51 Data available from FDA Summary Basis of Approval by FOIA request.
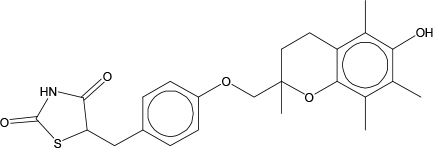
FIGURE 12-2 Chemical structure of Glitazone-T, CAS # 97322-87-7.
consequence, it is estimated that the maximum adult human daily exposure to the active ingredient is approximately 400 mg through the normal use of products containing Glitazone-T. Any proposed alternative must satisfy government bodies and product consumers that it has a substantially improved safety profile for human health.
Other considerations in Step 2 include identification of the following:
- Stakeholders: Relevant internal stakeholder groups include safety experts, chemists, and pharmacologists. External stakeholders include relevant advocacy groups and regulatory agencies. These groups may have differing views on the relative importance of the various aspects of an alternatives assessment, such as the relative weight given to functional performance vs. environmental or human health concerns for any proposed alternative.
- Guiding assumptions and values implicit in the assessment: Avoid persistent, bioaccumulative, and toxic (PBT) chemicals. Whenever possible, the GreenScreen® for Safer Chemicals® classification system will be used to assign health and ecological hazard ratings.
- Function and performance requirements for the substance of concern and alternatives: Complete removal of Glitazone-T would eliminate any functional use of those products where this active ingredient is included, rendering the product nonviable from an economic perspective. Any alternative must be able to replace the biological activity of Glitazone-T, including activation of PPARγ, which is thought to be critical to the beneficial effects observed from using this class of product.
- Hazards of concern and potential exposure tradeoffs that should be evaluated in the assessment: Alternatives to Glitazone-T must have a lower potential for causing human hepatotoxicity. Ecotoxicity must also be considered, since release of Glitazone-T and its alternatives to wastewater can occur. Because of the beneficial aspects of the product, human health considerations are considered a primary motivation.
- Assessment Steps to be completed: Steps 1-8 and 10 should be completed. Because product use is anticipated to be similar, a comparative Exposure Assessment (Step 6.3) and Life Cycle Thinking (Step 8) should be adequate and the optional Step 9 not needed.
- Identify safer alternatives: In Step 7, assessments of in vivo data will be completed using the GreenScreen® tool. GreenScreen® assessments may be supplemented with additional data sources, such in vitro and in silico investigations, if needed. Remaining data gaps will be handled in accordance with the GreenScreen® guidelines. End points with insufficient information to assess the hazard are assigned a data gap (DG). For illustration purposes, the uncertainty of each in vivo finding will also be considered.52 Factors used to evaluate parameter uncertainty will include robustness of the data (e.g., multiple studies, multiple species, adequacy of the reporting of the results), and model uncertainty (e.g., relevance of an assay end point to a human health end point of concern). A neutral approach to uncertainty and missing data will be used in this example (see Chapter 9 for more details).
- Life Cycle Thinking (Step 8) will qualitatively determine if there are differences in material or energy flow or synthetic history exist between
_____________
52 Strategies for handling uncertainty in other endpoints could also be developed.
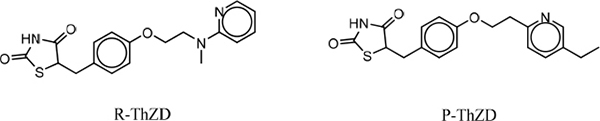
FIGURE 12-3 Chemical structures of R-ThZD and P-ThZD.
the original chemical (Glitazone-T) and the potential alternatives.
Step 3: Identify Potential Alternatives
Numerous structural analogs to Glitazone-T are available, but for the most part these were deemed to have either lower potency against the PPARγ receptor or had physicochemical properties, such as solubility or bioavailability, that would reduce their effectiveness as a replacement for Glitazone-T. However, two viable alternatives have been identified: 5-(4-{2-[Methyl(2-pyridinyl)amino]ethoxy}benzyl)-1,3-thiazolidine-2,4-dione, commonly referred to as R-ThZD and 5-{4-[2-(5-Ethyl-2-pyridinyl)ethoxy]benzyl}-1,3-thiazolidine-2,4-dione, also known as P-ThZD. Structures for these alternatives are shown in Figure 12-3.
In in vitro experiments, R-ThZD and P-ThZD were shown to increase the activity of PPARγ by 50% at concentrations of 0.082 µM and 0.81 µM, respectively, when tested in transfected HepG2 cells. In in vitro 3T3-L1 adipocytes R-ThZD and P-ThZD were shown to reduce the uptake of 2-deoxyglucose by 50% at concentrations of 50 nM and 3 µM, respectively.
Step 5: Assess Physicochemical Properties
General assessment of physicochemical properties indicates that both alternatives have similar physical characteristics in terms of their melting point, boiling point, and vapor pressure (see Table 12-14). However, computational assessments of the aqueous solubility of both R-ThZD and P-ThZD suggest that these chemicals are significantly more water soluble than Glitazone-T.
Assessment of Ecological Impact Based on Physicochemical Properties
Comparison of the physicochemical properties of Glitazone-T with the other two Glitazone alternatives show the same thiazolidinone ring structure, but Glitazone-T has a phenolic functional group as well as a prospectively liable, if masked, carbonyl group (Weltman et al 2011). The pKa (base) value is also orders of magnitude different between these chemicals. Hence the environmental fate and impact of Glitazone-T, its metabolites, or degradation products are uncertain.
Assessment of the ecological impact of a chemical and its degradation or metabolic products is best based on direct data. For P-ThZD, it has been experimentally determined that it and its major metabolites do not significantly bioaccumulate, persist in the aquatic environment, show toxicity to aquatic organisms, or become absorbed by sewage solids (Drug Bank, 2013a). An evaluation of R-ThZD can be carried out by comparison of physicochemical properties of P-ThZD and R-ThZD. Both P-ThZD and R-ThZD have similar chemical structures, functional groups, molecular weights, and logPs, as well as calculated pKas and polar surface areas (psa). It is reasonable to assume that environmental binding, persistence, degradation, and transformation of R-ThZD is well modeled by P-ThZD (Drug Bank, 2013b). In terms of chemical structure, the only difference is in the substitution of pyridine rings, which would have a minor effect on the reactivity.
Assessment of Human Health Impacts Based on Physicochemical Properties
In comparing the physicochemical properties of R-ThZD and P-ThZD to Glitazone-T, it can be hypothesized that the lower LogP values for R-ThZD and P-ThZD and higher predicted aqueous solubility (see Table 12-12) will increase their relative bioavailability when compared to Glitazone-T. Given that the in vitro potency of R-ThzD is superior to
TABLE 12-14: Physicochemical Properties for Glitazone-T, P-ThZD, and R-ThZD
| Property | Glitazone-T (EC50 = 0.72µM) | P-ThZD (EC50 = 0.81µM) | R-ThZD (EC50 = 0.082µM) |
| MW | 441.5 | 356.4 | 357.4 |
| cLogPa | 5.585 | 3.533 | 3.02 |
| Polar surface area | 110.16 | 93.59 | 96.83 |
| LogD (shake flask pH 7.4) | 3.65 | 2.45 | 1.93 |
| Aqueous solubility (pred.) | 0.04 mg/ml | 46.8 mg/ml | 1033 mg/ml |
| Rule of 5 violations | 1 | 0 | 0 |
| Acid pKa | 6.27 | 6.27 | 6.27 |
| Melting point | 184oC (exp.) | 271oC (pred.) | 153oC (exp.) |
| Boiling point @ 760 mmHg (pred.) | 657 ± 55 °C | 575 ± 45°C | 585 ± 35°C |
| Vapor pressure at 25°C (pred.) | 0.0 ± 2.1 mmHg | 0.0 ± 1.6 mmHg | 0.0 ± 1.6 mmHg |
aValues for cLogP in this table were determined using the Biobyte software package.
SOURCE: ChemSpider 2014a, b,c.
that for Glitazone-T against the PPARγ receptor, and the in vitro potency of P-ThzD is comparable to that for Glitazone-T, then higher bioavailability of these alternatives will lead to a decrease in their relative concentrations in the end products. A direct result will be a reduction in the level of human exposure to these biological active ingredients, assuming that similar product usage patterns are equivalent.
Step 6.1: Assess Chemical Hazards for Human Health
This section examines the various data streams available for hazard assessment by looking at in silico, in vitro, and in vivo data.
Computational Assessment of Safety
In silico predictions for a variety of different properties were obtained for Glitazone-T, P-ThZD, and R-ThZD using some available quantitative structure activity relationship (QSAR) models. Model outputs include predictions of cytotoxicity to cells; inhibition of the human Ether-a-go-go Related Gene (hERG) ion channel that is associated with prolonged cardiac QT interval; volume of distribution; free fraction in human plasma; and other end points (Table 12-15). The rationale for choosing these predicted properties is explained in more detail in Chapter 8.
- Cytotoxicity: Compounds that cause cytotoxicity at lower in vitro concentrations will generally have a higher probability of causing toxicity in vivo at lower plasma concentrations (Greene et al. 2010a). The in silico predictions suggest that P-ThZD and R-ThZD will have a higher LC50 values for cytotoxicity in cells compared to Glitazone-T. Thus, cytotoxicity associated with these chemicals likely occurs at higher in vivo (plasma) concentrations.
- hERG inhibition: hERG channel inhibition has been shown to cause QT interval prolongation in humans. This alteration of the cardiac electrical cycle has been implicated in the onset of ventricular tachyarrhythmias like torsades de pointes, which can result in sudden death. In silico predictions suggest that there is no increased risk of hERG Inhibition with either R-ThZD or P-ThZD when compared to GitazoneT.
- Volume of distribution, free fraction, and passive permeability: Volume of distribution (Vdss) has
TABLE 12-15 Various Predicted Properties for Glitazone-T, P-ThZD, and R-ThZD
| Predicted Property | Glitazone-T | P-ThZD | R-ThZD |
| Cytotoxicity LC50 | 79.7 µM | 254 µM | 259 µM |
| hERG (human Ether-a-go-go Related Gene) IC50 | 19.2 µM | 13.5 µM | 19.4 µM |
| VDss (L/kg) Volume of distribution | 0.615 | 0.538 | 0.3214 |
| Fu (%) (Free fraction in human plasma) | 0.00154 | 0.00976 | 0.00369 |
| RRCK (Russ Ralph Canine Kidney) (x 10-6 cm/sec) Passive permeability | 5.16 (Moderate) | 28.5 (High) | 26.2 (High) |
| MDR (Multidrug Resistance) efflux Pgp (P-glycoprotein) active efflux | 2.04 (Low) | 0.967 (Low) | 0.914 (Low) |
| Structural alerts | Yes Thiazolidinedione | Yes Thiazolidinedione | Yes Thiazolidinedione |
| Mitochondrial dysfunction | High | Medium | Medium |
| BSEP (Bile Salt Export Pump) Inhibition @100 μM | 75% | 83% | 80% |
Note: Data for this table were generated by a committee member using unpublished Pfizer data.
-
been shown to correlate with the lowest observable adverse effect level (LOAEL) in preclinical studies, where higher Vdss values lead to lower LOAEL concentrations (Sutherland et al. 2012). The Vdss for all three compounds is predicted to be low, suggesting that there would be no substantial increased safety concern from either R-ThZD or P-ThZD when compared with Glitazone-T. Passive permeability is linked to bioavailability, where highly permeable compounds have high bioavailability. Moderate passive permeability was predicted for Glitazone-T, whereas R-ThZD and P-ThZD are expected to be higher, indicating that R-ThZD and P-ThZD would have better bioavailability. In addition, since the pharmacological action of a compound is generally driven by the unbound fraction in vivo, then a higher free fraction indicates that lower total drug doses would be needed to elicit the desired effect of the compound. The free fraction for both R-ThZD and P-ThZD is predicted to be ~9-fold and ~3-fold higher than Glitazone-T, suggesting that the overall exposure required to achieve the intended effect would be lower.53
- Thiazolidinedione structural alert: The thiazolidinedione substructure has been identified as a structural alert associated with hepatotoxicity resulting in liver failure and/or cholestatic hepatitis (Greene et al. 2010b). Cyp3A4 enzyme induction has also been observed with compounds containing this structural group. The mechanism of toxicity is thought to be via CYP mediated oxidation of the activated methylene to give a reactive quinoid intermediate (see Figure 12-4), which can be trapped with glutathione (GSH) in a reactive metabolite assay. All three compounds contain this structural alert, so it cannot be determined if the two alternatives, R-ThZD and P-ThZD, would have an improved safety profile when compared to the hepatotoxic Glitazone-T compound.
- Mitochondrial dysfunction and BSEP inhibition: Many eukaryotic cells derive the majority of their energy needs from the mitochondrial
_____________
53 This observation could also affect wastewater concentrations of these compounds.
FIGURE 12-4 Formation of a reactive quinoid intermediate.
production of adenosine triphosphate (ATP). Interfering with mitochondrial production of ATP will deplete cellular energy stores, and may result in cellular stress and cell death. Glitazone-T is predicted to have a high likelihood of having an adverse impact on mitochondrial function, whereas R-ThZD and P-ThZD are predicted to have only a moderate likelihood of having an effect on mitochondrial function. Therefore, it might be expected that both R-ThZD and P-ThZD would have a lower likelihood of having adverse safety effects. As a co-factor to mitochondrial dysfunction, inhibition of the Bile Salt Extraction Pump (BSEP), an energy-dependent transporter, has been linked to causing cholestasis and hepatic injury. All three compounds are predicted to have similar inhibitory effects on BSEP. This information doesn’t allow for differentiating between these chemicals on the basis of this potential mechanism of liver injury.
Based on the in silico analysis, R-ThZD and P-ThZD offer a slightly more favorable hazard profile than Glitazone-T due to a lower predicted potential for causing cytotoxicity, better predicted bioavailability, and lower plasma protein binding.
Using In Vitro Data to Assess Safety Hazards
- In vitro absorption, distribution, metabolism, and excretion (ADME) assessments: When comparing in vitro ADME data for all three compounds, R-ThZD was shown to have moderate metabolic stability in human liver microsomes and hepatocytes, whereas Glitazone-T had poor detection sensitivity in the experimental conditions. R-ThZD also had good passive permeability compared to Glitazone-T. No data were available for P-ThZD, but based on its close structural similarity and similar physicochemical properties to R-ThZD, along with similar in silico predictions for passive permeability, Vdss and protein binding, it might be expected that these two compounds would show similar profiles in the in vitro systems. Despite some observed differences in their interactions with specific biological pathways or proteins, metabolic stability and permeability have been shown to be strongly correlated with physicochemical characteristics, such as lipophilicity and pKa.
-
In vitro safety assays: Glitazone-T was shown to cause cytotoxicity at lower concentrations (LC50=78μM) compared to R-ThZD (259µM) and P-ThZD (263µM) in immortalized human liver epithelial (THLE) cells, but no significant difference was noted in a human liver carcinoma (HepG2) cell line. This might be due to the increased sensitivity of THLE to compounds that affect mitochondrial function when compared with HepG2 cells. This relative difference in mitochondrial dysfunction was confirmed in vitro, where Glitazone-T shows both uncoupling and inhibitory effects on isolated mitochondria at significantly lower concentrations than that observed for either R-ThZD or P-ThZD (see Table 12-16). However, it is worth noting that P-ThZD had a significantly greater inhibitory effect on the BSEP transporter than either Glitazone-T or R-ThZD.
When comparing these compounds for their effects on endoplasmic reticulum stress, which has been linked to a number diseases, R-ThZD showed an measurable increase in the nuclear translocation of XBP1, part of the endoplasmic reticulum stress pathway, at much lower concentrations than Glitazone-T and P-ThZD, which may indicate a slightly higher concern for adverse effects with R-ThZD.
TABLE 12-16 In Vitro Safety Data.
| In Vitro Safety Assay | Glitazone-T (EC50 = 0.72 µM) | P-ThZD (EC50 = 0.81 µM) | R-ThZD (EC50 = 0.082 µM) |
| Cytotoxicity in THLE cells Cytotoxicity in HepG2 cells | 78 µM 242 µM | 263 µM >300 µM | 259 µM >276 µM |
| XBP1 Reporter Assay (ER Stress) | 144 µM | 279 µM | 46 µM |
| BSEP Inhibition (Bile Salt Extraction Pump) | 9.1 µM | 0.15 µM | 5.2 µM |
| Mitochondrial Uncoupling (Isolated mitochondria) | 22.9 nmol/mg | >100 nmol/mg | 88.7 nmol/mg |
| Mitochondrial Inhibition (Isolated mitochondria) | 55 nmol/mg | >100 nmol/mg | >100 nmol/mg |
| Off-target pharmacology (%inhib@10μM >50%) | Dopamine Transporter, Norepinephrine Transporter, Thyroid Hormone Receptor, GABA A, CYP3A4, H3 | None | None |
NOTE: Data for this table were generated by a committee member using unpublished Pfizer data.
-
Finally, R-ThZD and P-ThZD had fewer off-target effects when compared to Glitazone-T in a panel of biochemical binding assays. Greater target promiscuity has been linked to a higher likelihood of observing toxicity at lower exposures (see Chapter 8 for more details).
- ToxCast data: Glitazone-T and P-ThZD have been profiled in numerous in vitro assays as part of the ToxCast initiative. Figure 12-5 shows the in vitro profile in the Apredica high content assays, where data for P-ThZD Glitazone-T are presented. This figure illustrates that Glitazone-T has increased effects on p53 and mitochondrial membrane potential when compared to P-ThZD, which suggests that P-ThZD may have a better safety profile.
Similarly, when comparing the profiles for these two compounds in the Attagene nuclear hormone receptor panels in Figure 12-6, it can be seen that aside from the intended biological activity of these molecules, Glitazone-T is having an effect on more of these receptors than P-ThZD. Based on these observations, it may be expected that P-ThZD would have a better safety profile than Glitazone-T.
This trend is also observed when comparing the in vitro profiles of the two chemicals in the BioSeek platform (Figure 12-7), where it can be observed that Glitazone-T has a much stronger response across almost all of the measured end points when compared to the profile for P-ThZD. Similar data were not available for R-ThZD, but based on the close structural similarity and similar physicochemical properties to R-ThZD, along with similar in silico predictions for passive permeability, Vdss and protein binding, it might be expected that these two compounds would show similar profiles in the in vitro systems, although differences could be present based on the observation that these two chemicals have different activities for XBP1 and BSEP.
Glitazone-T is more cytotoxic in THLE cells when compared to P-ThZD and R-ThZD, which probably reflects its greater impact on mitochondrial function. Similarly, there is a general lack of off-target activity for P-ThZD and R-ThZD when compared to Glitazone-T. Although P-ThZD is a more potent inhibitor of the BSEP transporter than either R-ThZD or Glitazone-T, this finding by itself may not translate into a direct biological effect in vivo. R-ThZD has a greater impact on inducing ER stress based on the XBP1 reporter assay, and so may be expected to show in vivo toxicity at lower plasma concentrations than P-ThZD. From the ToxCast profiles, P-ThZD has a “cleaner” profile across the three assay platforms when compared to Glitazone-T. Therefore, it might be expected to have a better in vivo safety profile aside from those effects related to the primary mechanism of action of these compounds. ToxCast data were not available for R-ThZD, and so comparisons between these two alternatives cannot be made. Based on the in vitro assessments of Glitazone-T, P-ThZD, and R-ThZD, it can be inferred that both P-ThZD and R-ThZD
would have fewer effects on a biological system compared to Glitazone-T, making them potentially viable safer alternatives.
Mammalian Toxicity Assessment
Comparisons between Glitazone-T, R-ThZD, and P-ThZD were made based on the available data using the GreenScreen® classification system.54
Acute mammalian toxicity: Glitazone-T has an acute oral LD50 of greater than 2000 mg/kg in multiple species, and so it receives a hazard designation of Low. P-ThZD, however, has an acute oral LD50 = 181mg/kg in mice, which is considered to be Very High. Similarly, R-ThZD has a mouse LD50 = 300 mg/kg, so its acute mammalian toxicity is categorized as High.
Carcinogenicity: In mice, Glitazone-T showed an increased hemangiosarcoma incidence in females at 400 mg/kg and in males and females at 800 mg/kg. In mice, Glitazone-T showed an increased hepatocellular carcinoma incidence in females at 800 mg/kg (Herman et al. 2002). P-ThZD showed benign and/or malignant transitional cell neoplasms in rats at 4 mg/kg/day and an increased incidence of urinary bladder tumors at 63 mg/kg. R-ThZD showed a significant increase in benign adipose tissue tumors (lipomas) in rats at doses greater than or equal to 0.3 mg per kg (mg/kg/day) for 104 weeks. On the basis of this evidence, all three chemicals are categorized as Moderate.
Mutagenicity/genotoxicity: Glitazone-T was not mutagenic in bacteria at concentrations up to 10,000 µg/plate, with or without metabolic activation. In a Chinese hamster fibroblast assay, both aneuploid cells and giant cell forms were noted after exposure to 2.9 µg/ml without metabolic activation for 48 hours. With activation, the number of cells with endoreduplicated chromosome was increased with Glitazone-T at 58 and 64 µg/ml. Pronounced cytotoxicity and increased structural chromosome aberrations frequency were observed following 6 hours of exposure to Glitazone-T at 178 µg/ml without activation and at 163 µg/ml with activation. Results of the in vitro mouse lymphoma mutation assay at cytotoxicity-limited concentrations up to 30 µg/ml were mixed because minimal, but significant increases in mutation frequency were noted in two out of five trials without metabolic action and in two out of six trials with activation. The unscheduled DNA synthesis was observed in hepatocytes isolated 2 or 24 hours post-dose from rats given single oral doses of Glitazone-T at 1,000, 1,500, or 2,000 mg/kg. Thus, it is concluded that the Glitazone-T genotoxic potential should be categorized as Moderate.
P-ThZD showed no mutagenic or genotoxic potential in bacterial mutagenicity studies, in vitro mammalian tests, and in vivo micronucleus studies. Thus, it can be concluded that P-ThZD genotoxic potential should be categorized as Low.
The overall genotoxicity potential of R-ThZD appears to be equivocal since the tests of chromosomal aberration, unscheduled DNA, and in vivo mouse micronucleus were all negative, while the incidence of forward mutations at the thymidine kinase locus of mouse lymphoma LS 178Y cells was increased by R-ThZD in triplicate assays in the presence of S-9 mix. Thus, it can be concluded that R-ThZD genotoxic potential should be categorized as Moderate.
Reproductive & developmental toxicity: Pregnancy duration was slightly shorter in rats given Glitazone-T at 1000 mg/kg when compared with untreated controls. Growth rate of rat pups was reduced in both sexes following high dose (2000 mg/kg/day) Glitazone-T. This effect was particularly pronounced between postnatal days 29 to 57. Aside from these findings, Glitazone-T had little or no effect on fertility, teratology, and peri- and post-natal development in rodents and rabbits. Based on this information, the reproductive hazard categorization is Low and the developmental hazard categorization is Very Low.
In studies with P-ThZD (Takeda Canada 2012), rats exhibited delayed parturition, embryotoxicity, delayed development, and reduced fetal weights at oral doses > 40 mg/kg/day. In rabbits, embryotoxicity was observed at an oral dose of 160 mg/kg. Based on this information, both the reproductive and the developmental hazard categorizations are Moderate.
R-ThZD treatment of rabbits and rats was studied by GSK (GSK 2012). Treatment of rats during early pregnancy did not result in notable implantation or embryo impacts. However, treatment of both rats and rabbits during mid-late pregnancy was associated with growth retardation and fetal death. Teratogenicity was not observed. Placental pathology was observed with R-ThZD treatment of rats (>3 mg/kg/day) but not in rabbits (100 mg/kg/day). When rats were treated during pregnancy and lactation with R-ThZD, reductions in
_____________
54 Alternative (e.g., GHS) classification schemes could be used.
FIGURE 12-5 Apredica assay profiles for Glitazone-T (Trogliatazone) and P-ThZD (Pioglitazone). NOTE: Data in figure are from EPA ToxCast Initiative; figure generated using Spotfire.
FIGURE 12-6 Attagene Nuclear Hormone Receptor panel assay profiles for Glitazone-T (Trogliatazone) and P-ThZD (Pioglitazone). NOTE: Data in figure are from EPA ToxCast Initiative; figure generated using Spotfire.
FIGURE 12-7 BioSeek panel assay profiles for Glitazone-T (Trogliatazone) and P-ThZD (Pioglitazone). NOTE: Data in figure are from EPA ToxCast Initiative; figure generated using Spotfire. Conclusions from the In Vitro Safety Data.
litter size and neonatal viability were observed. Postnatal growth retardation that was reversible after puberty was also seen. The no-effect dose for effects on the placenta, embryo, and offspring was 0.2 mg/kg/day in rats and 15 mg/kg/day in rabbits. Fertility was decreased at a dose of 40 mg/kg per day, and estrous cyclicity was altered at 2 mg/kg per day, but these effects were not noted at doses less than 0.2 mg/kg per day. These effects were attributed to altered plasma levels of progesterone and estradiol. Based on this information, the reproductive and developmental hazard categorizations are High.
Neurotoxicity: In rats given amorphous Glitazone-T at 6, 25, 100, or 400 mg/kg by gavage for 13 weeks, there were no deaths or drug-related clinical signs. Based on this information, the neurotoxicity hazard categorization is considered Low. No functional or behavioral toxicity was observed in offspring of rats given oral doses up to 80 mg/kg of P-ThZD. Based on this information the neurotoxicity hazard categorization is considered Moderate.
In a 13-week dietary range-finding study, mice were given R-ThZD at doses of 0, 0.4, 2, 10, or 20 mg/kg/day by dietary admixture. There was no mortality. No remarkable clinical signs were noted except firm, but palpable, swellings in the scapular areas noted in 14/16 animals in the high-dose group and 6/l6 animals dosed at 10 mg/kg/day. Based on this information, the neurotoxicity hazard categorization is considered Moderate.
Repeated dose toxicity: In 13-week studies with Glitazone-T, dose-related increases in absolute and relative liver weight of 21%-75% in male rats at 400 mg/kg and 14%-48% in female rats at 50 mg/kg were observed. Heart weight and its body weight ratios in female rats increased 28%-53% at 200 and 400 mg/kg at week 13, respectively. No effects in dogs or monkeys given up to 400 mg/kg/day for 28 days. Based on this information, the repeat dose hazard categorization is Moderate.
Anemia with reduced erythrocytes, hematocrit and hemoglobin concentration, and splenic extramedullary hematopoiesis were present in rats after 13 weeks of oral administration of P-ThZD at doses of 100 or 300 mg/kg. The toxicological no effect dose might be near 30 mg/kg. Based on this information, the repeat dose hazard categorization is Moderate.
In a 13-week study, there was a dose-related increase in scapular adipose tissue weight in female
mice at 2 mg/kg/day. Because this brown adipose tissue is not found in people, this response is not considered relevant. In males, there was a slight increase of 10% in kidney weight at 2 mg/kg/day and above. An increase of up to 16% in heart weight at 10 and 20 mg/kg/day was noted. This end point was chosen as the point of departure because heart effects were noted in longer-term studies in multiple species. Based on this information, the repeat dose hazard categorization is Moderate.
Respiratory and skin sensitization: No information is available to assess the respiratory and skin sensitization hazards associated with Glitazone-T, P-ThZD, or R-ThZD. Therefore, the respiratory and skin sensitization hazard categorization is Unknown.
Eye and Skin irritation/corrosivity: No information is available to assess the eye and skin irritation and the corrosive hazards associated with Glitazone-T, P-ThZD, or R-ThZD. Therefore the eye and skin irritation and corrosivity hazard categorization is Unknown.
Mammalian toxicity summary: Table 12-17 summarizes the mammalian toxicity assessment based on the GreenScreen® classification system.
Step 6.2: Assess Ecotoxicity (Chemical Hazards)
This section compares the environmental toxicity of three compounds: P-ThZD, R-ThZD, and Glitazone-T. There is sufficient experimental data for P-ThZD to characterize the aquatic toxicity by comparing the measured toxic end points to the thresholds described in several chemical alternatives assessments. There is, however, a lack of directly measured empirical data to characterize the aquatic toxicity of R-ThZD or Glitazone-T. The toxicity of the latter two chemicals, compared to P-ThZD, was estimated based on the chemical properties and reactivity of these chemicals. There is, however, uncertainty in any conclusions when comparing a chemical with an experimentally well-defined toxicity (P-ThZD) relative to the other two alternatives, which have no direct measurements of aquatic toxicity. The latter is a significant data gap in making any comparison.
There is no terrestrial toxicity data for any of these three compounds. However, the mammalian toxicity data generated to estimate human toxicity (see Mammalian Toxicity Summary) can be used to compare the toxicity of these three compounds to small mammals.
TABLE 12-17 Summary of Mammalian Toxicity Assessment
| Alternative | Acute mammalian | Carcinogenicity | Mutagenicity/Genotoxicity | Reproductive Toxicity | Developmental Toxicity | Neurotoxicity | Repeated Dose | Respiratory and Skin Sensitization | |||||||||||||||||||
| V H |
H | M | L | V H |
H | M | L | V H |
H | M | L | H | M | L | V L |
H | M | L | V L |
H | M | L | H | M | L | ||
| Glita zone -T | DG | ||||||||||||||||||||||||||
| P-ThZ D | DG | ||||||||||||||||||||||||||
| R-ThZ D | DG | ||||||||||||||||||||||||||
Note: Toxicity data has been benchmarked using the GreenScreen® system. The uncertainty associated with each toxicological finding is depicted by colors (green = minimal uncertainty, yellow= moderate uncertainty, orange = highly uncertain, gray = data gap)
Aquatic Toxicity
Weltman et al. (2011) provide an assessment of the environmental fate and effects of P-ThZD conducted as a higher-tier assessment triggered by exceeding screening criteria under a preliminary evaluation based on “Guideline on the Environmental Risk Assessment of Medicinal Products from Human Use”(EMA 2006). The data generated included various physical-chemical parameters (e.g., biodegradation, Kow, aerobic transformation in sediments, Koc) and toxicity to sewage microorganisms. The aquatic toxicity was characterized based on toxicological testing with a freshwater algae (species not provided), a freshwater invertebrate (Daphnia magna), and an early life stage fish (species not provided).
The algal test was a 72-hour exposure that measured the algal response as average specific growth rate and yield (as cell number) over a range of concentrations. The testing provided a no observed effect concentration (NOEC) and percentage effect (relative to controls) of EC10, EC20, and EC50. The EC10 was the lowest effect level measured. The invertebrate test was a 21-day test that measured the parental mortality and reproduction (as neonates per female) over a range of concentrations. The testing provided a NOEC for reproduction, an overall NOEC (reproduction and mortality), and a percentage mortality (relative to controls and measured as immobile adults) of EC20, EC40, and EC50. The EC20 was the lowest effect measured.
The fish early life stage test derived a NOEC in a 21-day test (range of concentrations) based on larval survival (post-hatch) and growth of larvae over the course of the test.
Several of the existing chemical assessment alternatives reviewed in this report (Chapter 7) use the type of ecological toxicity test data measured in this study of P-ThZD to characterize the acute and chronic toxicity of a chemical based on a range of thresholds. Table 12-18 summarizes the thresholds and categories provided by the four chemical alternatives assessments that provide quantitative characterizations of toxicity.
Characterization of Aquatic Toxicity
The various categories in Table 12-18 were applied to the toxicity data from Weltman et al. (2011) to characterize the aquatic toxicity of P-ThZD. The toxicity data for algae included the following:
- Algal toxicity (growth rate) had a measured EC10 at 0.702 mg/L, but there was no further response at higher concentrations. The authors report the EC50 at some concentration above 0.851 mg/L. Therefore the characterization of the EC50 for growth rate as very high toxicity is a conservative (i.e., environmentally protective) characterization. The actual EC50 may be higher.
- Algal toxicity (yield) had a measured EC10 at 0.189 mg/L and a measured EC20 at 340 mg/L, but there was no further response at higher concentrations. The authors report the EC50 at some concentration above 0.851 mg/L. Therefore, the characterization of the EC50 for yield as very high toxicity is a conservative (i.e., environmentally protective) characterization. The actual EC50 may be higher.
- Weltman et al. (2011) estimate the overall NOEC for algae at 0.189 mg/L, which was the EC10 for the yield end point. They did not estimate a LOEC. However, we used the EC20 for yield (the first measured response above the NOEC), 0.340 mg/L as the LOEC.
The toxicity data for invertebrates were chronic end points (21- day test) and included the following:
- Invertebrate mortality (measured as invertebrate mobility) had a chronic LOEC of 0.0387 mg/l based on a LC20 for adult mobility.
- A NOEC of 0.296 mg/l and a LOEC of 0.530 for reproduction measured as the number of offspring produced per adult D. magna.
- An estimated overall NOEC of 0.7530 (Weltman et al. 2011).
The fish early life stage toxicity tests indicated no response in survival of fry over the course of the test (32 days). The estimated NOEC and LOEC for body weight were 0.0584 mg/L and 0.1296 mg/L, respectively.
Table 12-19 provides this comparison. The aquatic toxicity for P-ThZD is generally characterized as high toxicity, with the exception of the characterization of NOEL under the P20ASys.
In terms of structure, the difference in the compounds is only in the substitution of pyridine rings, which would have a minor effect on the reactivity. This analysis indicates that the toxicity of the two compounds is likely to be similar.
| Acute Toxicity | Chronic Toxicity | |||||
| End point | End point Thresholds (mg/L) | Assigned Category | Endpoint | End point Thresholds (mg/L) | Assigned Category | |
| DfE | EC50 or LC50 | <1 | Very High | LOEC | <0.1 | Very High |
| 1 - 10 | High | 0.1 - 1 | High | |||
| 10 - 100 | Moderate | >1 - 10 | Moderate | |||
| >100 | Low | >10 | Low | |||
| IC2 | 96 hr LC50 (fish); 48 hr EC50 (crustacean); 72 hr or 96 hr ER50 (algae or aquatic plants) | <1 | Very High | |||
| 1 - 10 | High | |||||
| 10 - 100 | Moderate | |||||
| >100 | Low | |||||
| TURI P2OASys | LC50 (aquatic) | <0.1 | 10 | NOAEC (fish) | <0.00002 | 10 |
| 0.1 - 1 | 8 | 0.0002 | 8 | |||
| 1 - 50 | 6 | 0.002 | 6 | |||
| 50 - 1000 | 4 | 0.02 | 4 | |||
| > 1000 | 2 | <0.2 | 2 | |||
| LC50 (plant) | <0.1 | 10 | ||||
| 0.1 - 1 | 8 | |||||
| 1 - 10 | 6 | |||||
| 10 - 100 | 4 | |||||
| > 100 | 2 | |||||
| Guide on Sustainable Chemicals | NOEC | <0.01 | Not Toxic | |||
Step 6.3: Conduct Comparative Exposure Assessment
Measurement of plasma protein binding in human serum showed that Glitazone-T was greater than 99.9% bound to protein, whereas P-ThZD and R-ThZD were 99.2% and 99.7% bound, respectively. Therefore, the free concentration available for the intended pharmacological action will be approximately seven times greater in the case of P-ThZD and two times greater for R-ThZD. These differences in free concentrations and absorption result in lower concentrations being required of both P-ThZD and R-ThZD to achieve the same biological effect compared to Glitazone-T, assuming equivalent potency against the PPARγ receptor across all three chemicals.
In vitro experiments have shown a 50% increase in PPARγ activity following exposure of transfected HepG2 cells with 0.72 µM Glitazone-T. In 3T3-L1, adipocytes Glitazone-T was shown to reduce the uptake of 2-deoxyglucose by 50% at a concentration of 2 µM. The bioavailability (the amount entering the bloodstream) of Glitazone-T is approximately 58%; for product effectiveness, it is necessary to have relatively high concentrations. As a result, it is estimated that the maximum adult human daily exposure to the active ingredient is in the region of 400 mg through the normal use of products containing Glitazone-T.
During in vitro experiments, R-ThZD and P-ThZD were shown to increase the activity of PPARγ by 50% at concentrations of 0.082 µM and 0.81 µM, respectively, when tested in transfected HepG2 cells. In 3T3-L1, adipocytes R-ThZD and P-ThZD were shown to reduce the uptake of 2-deoxyglucose by 50% at concentrations of 50 nM and 3 µM, respectively. The bioavailability of P-ThZD and R-ThZD is 81% and 60%, respectively, and the free concentrations in plasma are seven times greater for P-ThZD and two times greater for R-ThZD when
TABLE 12-19 Summary of Toxicity Data for P-ThZD
| Toxic Category By Chemical Alternatives Assessment Method | |||||
| End Point | Measured Value | DfE | IC2 | P2OAsys | Guide on Sustainable Chemicals |
| Algae End Points | |||||
| Algae 72- hour EC50 (growth rate) | >0.851 mg/L | Very High | Very High | 8 | NA |
| Algae 72- hour EC50 (yield) | >0.851 mg/L | Very High | Very High | 8 | NA |
| Algae 72- hour NOEC | 0.189 mg/L | NA | NA | NA | Toxic |
| Algae 72- hour LOEC | 0.340 mg/L | High | NA | NA | NA |
|
Invertebrate End Points |
|||||
| D. magna LOEC (LC20 for adult mobility) | 0.0387 mg/L | Very High | NA | NA | NA |
| D. magna NOEC (for reproduction) | 0.296 mg/L | NA | NA | NA | Toxic |
| D. magna LOEC (for reproduction) | 0.530 mg/L | Very High | NA | NA | NA |
| D. magna overall NOEC | 0.0753 mg/l | NA | NA | NA | Toxic |
|
Fish End Points |
|||||
| Fish 32- day NOEC (early life stage body weight) | 0.0584 mg/L | 2 | Toxic | ||
| Fish 32- day LOEC (early life stage body weight) | 0.1296 mg/L | Very High | Toxic | ||
compared to Glitazone-T. Based on these data, it is anticipated that concentrations of the biological ingredient in products will be substantially reduced; the anticipated maximum daily exposure to the active ingredient will be in the region of 45 mg in the case of P-ThZD and 4 mg in the case of R-ThZD.
Step 7: Are Alternatives Considered Safer?
Based on the available data, there are numerous ways to visualize and compare the profiles of the chemicals. No one way is considered as the preferred method. In all cases, one effect has not been deliberately ranked over another. Table 12-18 shows one approach incorporating the data into a single rank ordering of alternatives.
Another way to visualize and rank order these compounds would be to use the ToxPi, software, as explained in Appendix C and Reif et al. 2013. This software allows the categories of data to be grouped and weighted, if desired, to give a graphic comparison of chemicals. In addition to the graphic comparison, ToxPi software can be used to calculate an overall score for each chemical, using all the domains of data. In addition, the impact of giving more weight to some evidence categories on the overall ranking of compounds can easily be explored.
For the purpose of illustrating the effect that relative weightings can have on an overall assessment and ranking, data were grouped into seven logical categories or slices as outlined in Table 12-21. For the purpose of the illustration, the individual data points were rescaled to fall between 0 and 1, where “1” represents the most favorable value of the three for the data point in question and the rest are converted to a fraction of this data point. It should
be noted that for some properties, lower numbers are considered more favorable than higher ones. For this reason, the calculations were adjusted to compensate for this directionality. Finally, no absolute thresholds were defined for an assay or property values because this was beyond the scope of the committee.
In Figure 12-8, the different slices of the pie charts represent the different components of the physicochemical properties, in vitro data, and in vivo and in silico predictions. In this example of data integration, the in vivo safety and exposure assessments carry the highest weighting, as illustrated by the lengths of the arcs for each slice. Preclinical ADME and in vitro data were the next highest weightings, with off-target activity, in silico predictions and physicochemical properties given the lowest weightings.
The relative ranking of each chemical can be seen in the three data points that the arrows point to. The higher ToxPi score represents a more favorable compound. In this example, P-ThZD had the best score, with R-ThZD in second place, and Glitazone-T the least favorable. As shown by the relative size of each slice, Glitazone-T was ranked last because of lower (unfavorable) scores in exposure, in vitro safety, off-target activities, and physicochemical properties.
In Figure 12-9, greater emphasis was placed on the in vivo (e.g., animal) safety assessments, increasing this to contribute 50% of the overall score for each compound. This was done to illustrate the effect of putting greater weight on the safety of a product over the functional use of the alternatives. In this case, Glitazone-T was the most favorable option, with P-ThZD second and R-ThZD the least favored.
In these analyses, the committee recognizes that there are varying levels of confidence in the different end point categorizations. In the illustration with mammalian toxicity data, uncertainty was considered and handled using a Missing-Data-Neutral approach (see Chapter 9 for more details). In this approach, the presence of uncertainty and missing data are noted, but would not exclude, or otherwise demote, the alternative at this point in the selection process.
Step 8: Life Cycle Thinking
In Step 8 (Life Cycle Thinking), it is first important to map the product system. For an agent like Glitazone-T, the key elements of the product system include: (a) transportation and storage of raw materials; (b) initial production of the active ingredient; (c) secondary processing resulting in the production of the product formulation; (d) product storage and distribution; (e) auxiliary operations, including disposal of production waste products; (f) therapeutic usage; and (g) post-consumer disposal and environmental fate of the drug and its metabolites (Mata et al. 2012). Life Cycle Thinking did not identify a significant difference in these areas, when the life cycle of the original chemical was compared to that of the alternative. Thus, additional screening life cycle analyses or more quantitative analyses were not required.
Step 10: Identify Acceptable Alternatives
In Figure 12-10, ToxPi was used to integrate different types of information (as discussed in Chapter 9). Specifically, ToxPi is used to combine the data from the human exposure assessments with the functional efficacy of each compound at the PPAR receptor, to incorporate a measure of functional performance into the weighting and ranking process. In addition, the relative contribution from the exposure and performance slices were increased to give exposure and performance the greatest emphasis, followed by in vivo safety, in vitro safety, preclinical ADME with in silico predictions, and physicochemical properties, off-target activities having the lowest weight. This illustrates the impact that weighting of functional performance as the highest criteria for selection can have at the integration step and how it may influence the outcome of an alternatives assessment. In this case, R-ThZD was the most favorable option, with P-ThZD in second place, and Glitazone-T least favored.
From these examples, it becomes clear that each of the three chemicals can be ranked as the most favorable, depending on the relative emphasis placed on the data points available. Depending on the entity performing the alternatives assessment, subtle differences in a chemical’s attributes and rankings may lead to selection (or deselection) of an alternative. In this case, each of the alternatives has one or more human health or ecological hazards that may be desirable to avoid. Therefore, some framework users may initiate additional research and development efforts (Step 13).
The real-life outcome was that Glitazone-T
(Troglitazone) was withdrawn from the market in 2000, less than three years after regulatory approval, as a result of cases of severe liver injury in patients taking the drug. R-ThZD (Rosiglitazone) was approved in 1999 and reached peak sales of $2.5 billion in 2007, but was finally withdrawn in 2012, after reports linked the drug to cardiac toxicity. P-ThZD (pioglitazone) was approved by the FDA in 1999 and achieved sales worth $2.4 billion in 2008. It is still prescribed today, but has been withdrawn in some markets because of concerns with its association to bladder cancer after extended periods of treatment. Additional research and development efforts have led to the development of novel pharmaceutical treatment options for type 2 diabetes mellitus.
TABLE 12-20 Incorporation of Data into a Single Rank Ordering of Alternatives.
Note: A relative level of preference is assigned where “1” is the most preferable and “3” is the least preferable.
| Category of Data | End Point | Glitazone-T | P-ThZD | R-ThZD |
| Exposure assessment | Estimated daily exposure | 3 | 2 | 1 |
| Physicochemical data | LogP/LogD | 3 | 2 | 1 |
| Polar surface area | 1 | 1 | 1 | |
| Aqueous solubility | 3 | 2 | 1 | |
| Predicted properties | Cytotoxicity LC50 | 3 | 1 | 1 |
| hERG IC50 | 1 | 1 | 1 | |
| Volume of distribution | 1 | 1 | 1 | |
| Free fraction in human plasma | 3 | 1 | 2 | |
| Passive permeability | 2 | 1 | 1 | |
| MDR efflux | 1 | 1 | 1 | |
| Structural alerts | 1 | 1 | 1 | |
| Mitochondrial dysfunction | 3 | 2 | 2 | |
| BSEP inhibition @ 100μM | 3 | 3 | 3 | |
| In vitro safety assays | Cytotoxicity in THLE & HepG2 cells | 3 | 1 | 1 |
| XBP1 reporter assay (ER Stress) | 2 | 1 | 3 | |
| BSEP inhibition | 1 | 3 | 2 | |
| Mitochondrial uncoupling | 3 | 1 | 2 | |
| Mitochondrial inhibition | 3 | 1 | 1 | |
| Off-target pharmacology | 3 | 1 | 1 | |
| Mammalian exposure | Bioavailability | 3 | 1 | 2 |
| Protein binding | 3 | 1 | 2 | |
| Mammalian toxicity | Acute toxicity | 1 | 2 | 2 |
| Carcinogenicity | 2 | 2 | 2 | |
| Mutagenicity/Genotoxicity | 2 | 1 | 2 | |
| Reproductive toxicity | 1 | 2 | 3 | |
| Developmental toxicity | 1 | 2 | 3 | |
| Neurotoxicity | 1 | 2 | 2 | |
| Repeated dose toxicity | 2 | 2 | 2 | |
| Ecological toxicity | Aquatic Toxicity | 3 | 3 | 3 |
| TOTAL SCORES | 62 | 45 | 50 | |
TABLE 12-21 Components of ToxPi Slices in Case Study Illustration
| Category or Slice | Properties, assays or data |
| Functional efficacy | Human: T1/2, Tmax, AUC, PPB, Cmax, projected human exposure or dose |
| Preclinical ADME | Bioavailability (rat, monkey and dog), VDss, Rat Cmax, Rat Tmax, Rat AUC |
| In silico predictions | BSEP inhib, hERG inhib, MDR and RRCK, calculated rat PPB, THLE cytotoxicity, calculated human PPB |
| Off-target activity | % inhib @ 10μM values for the following Cerep targets: COX2, Dopamine Transporter, 5-HT transporter, PPAR gamma, PDE3, Na channel, Ca Channel, CB1, M1, Glucocorticoid, GABAA, Mu, Beta2, D1, H1, Alpha1, NE Transporter, 5HT2b. |
| In vitro safety | Cytotoxicity LC50 in HepG2 cells at 24 hrs in glucose and galactose, XBP1 activation assay, Caspase 3/7 activation, Mitochondrial inhibition and uncoupling, BSEP inhibition, cytotoxicity LC50 in HepG2 and THLE cells at 72 hrs in glucose containing media |
| In vivo safety | Assessments of mutagenicity, carcinogenicity, reproductive toxicity, neurotoxicity, repeat dose toxicity, acute toxicity, developmental toxicity |
| Physicochemical properties | LogP, LogD, PSA, PSA/MW, cSolubility, Acidic pKa, Basic pKa |
NOTE: AUC = area under receiver operating characteristic curve; PPB = parts per billion; Cmax = maximum concentration; VDss = volume of distribution at steady-state; Tmax = time of maximum plasma concentration; BSEP = bile salt export pump; hERG = human Ether-à-go-go-Related Gene; MDR = multi-drug resistant; THLE = T-antigen-immortalized human liver epithelial; COX2 = cyclooxygenase-2; 5-HT = serotonin transporter; PPAR = peroxisome proliferator-activated receptor; PDE3 = phosphodiesterase 3; Na = sodium; Ca = calcium; CB1 = cannabinoid receptor 1; GABAA = γ-Aminobutyric acid a; NE = norepinephrine; 5HT2b = 5-Hydroxytryptamine receptor 2B; LC50 = lethal concentration 50; XBP1 = X-box binding protein 1; LogP = partition coefficient; LogD = distribution coefficient; PSA = prostate-specific antigen
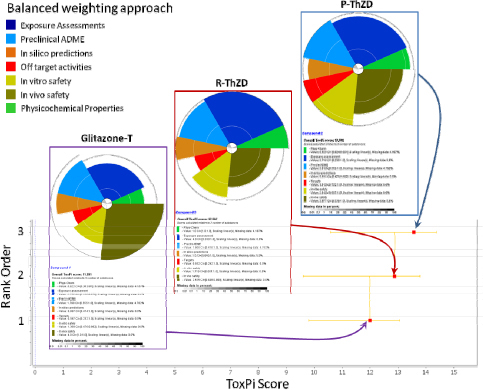
FIGURE 12-8 ToxPi visualization of data by data type and resultant rank ordering of chemicals.