Chemical Design: An Opportunity for Innovation
Alternatives assessment as described in this report typically begins with the recognition that a particular chemical is problematic from a health, safety, or environmental standpoint (Step 1 in the committee’s framework), followed by a comparative assessment of potential alternatives. In many cases, alternatives assessment only considers chemical substitutes that have been commercialized, can be readily obtained and, typically, have known physicochemical properties or information about their effects that can be compared. De novo design of new chemicals is a less common, but important approach to finding safer alternatives to existing chemicals.
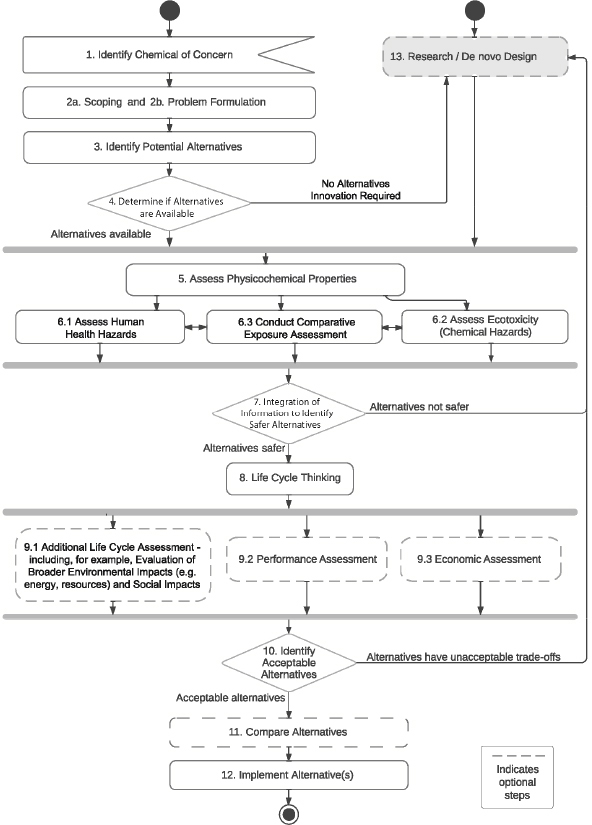
This chapter illustrates how the scientific concepts applied to alternatives assessment and described in earlier chapters can also be applied to the process of designing new alternatives de novo—Step 13, see Figure 13-1 and Box 13-1. Multidisciplinary teams are commonly tasked with this effort. While the term de novo design is used here, the concept of designing chemicals to be inherently safer is often referred to as “green chemistry.” Green chemistry is a proactive approach to reducing the potential for unwanted health and environmental impacts early in chemical design or discovery.
De novo chemical designs begin as drawings of chemical structures on paper or on the computer. At this point, chemical designs are only conceptual; therefore, the properties or effects of the different chemicals cannot be compared through empirical measurement and testing. Actually synthesizing the designed chemical can take many resources and an extended period of time (months to years). Thus, compared to evaluation of existing chemicals that can actually be tested, a different assessment strategy is needed for these conceptual chemicals. The goal is to get rapid, if imperfect, feedback that guides innovators away from candidates that are likely to have undesirable properties or impacts. Such feedback enables innovators to focus on alternatives that are more likely to be successful
BOX 13-1
DESIGN AND INNOVATION AT A GLANCE
- Chemicals of concern can be addressed by developing a new chemical to meet the functional needs or by developing an innovative concept that addresses the problem in a different way.
- The design of new chemicals is an opportunity to address the lack of satisfactory alternatives.
- During the design process, it is important to consider the environmental and health impacts in parallel with performance criteria.
- During the consideration of novel alternative structures, before they have been synthesized, rules of thumb, or general principles; computational methods; and expert systems can be used to predict both physicochemical properties and biological impacts so that the structures selected for further development are the least likely to fail later on because of poor environmental or toxicity performance.
- For newly synthesized candidates, physicochemical properties should be determined to identify which candidates are predicted by these properties to have poor environmental or health performance. Avoid these candidates and use this information as feedback to design.
- In the future, newly synthesized candidates could be screened through a battery of in vitro tests, like those in ToxCast or Tox21, to provide a baseline of information about initial compounds’ potential hazards and effective concentration at a relatively low cost. This would allow triaging and focus on the most promising candidates.
- Potential impacts, health or environmental, should continue to be considered as chemical designs are changed to address performance weaknesses identified later in product development.
and to reallocate resources and effort away from those associated with negative environmental or safety concerns. This chapter describes the design of new chemicals as an opportunity to develop safer chemicals and outlines considerations for scientists who design new chemicals.
INNOVATION WITHIN THE COMMITTEE’S ALTERNATIVES ASSESSMENT FRAMEWORK
In the search for alternatives, there will be cases where alternatives assessment is not, by itself, sufficient to identify a viable option. Considered alternatives may fail on performance, economic, safety, or other grounds. Or, entrepreneurs (and innovators inside a company) may see the alternatives assessment process as an opportunity to create a new compound or an entirely new product concept to satisfy the desired needs of the customer base. In either of these cases, the framework should include information that aids such innovators in their quest to find compounds that offer both better performance and improved environmental and human health attributes compared to the initial chemical of concern. The committee acknowledges that scientists within select companies may practice some, or all, of the suggested approaches described; however, teams tasked with alternatives assessment often have not incorporated these approaches.
Within the Committee’s framework, there are several steps where consideration of de novo designs (Step 13) is important:
- At the decision point in Step 4, if no alternatives are available, or if there is a business opportunity to consider novel alternatives, de novo design should be considered.
- Innovators may also enter Step 13 based on a business opportunity to develop a safer alternative that is not necessarily driven by the identification of a chemical of concern. (This is indicated by the direct point of entry into Step 13 of the committee’s framework diagram.)
- Finally, de novo design may be required (or motivated) by the results of testing at decision points that occur in Steps 7 or 10. Two types of outcomes are likely:
- a. The determination that alternatives have undesirable properties or impacts, leading to additional efforts toward de novo design.
- b. Information from testing provides feedback to inform further optimization of innovative alternatives.
BOX 13-2
LESSONS FROM THE PHARMACEUTICAL INDUSTRY
Consideration of environmental and health consequences of chemical structures and physicochemical properties of new chemicals does not usually take place until the very late in the process, if at all. One example of this reality can be found in the pharmaceutical industry, where in the early 1990s, when the primary focus of development work was developing a potent inhibitor or activator of an intended protein target. Little regard was given to the physicochemical properties that would allow the new drug to be readily absorbed into the bloodstream. In the late 1990s (highlighted by the publication of Lipinski’s “Rule of 5” in 1997 (Lipinski et al. 1997)), awareness of the properties that differentiated compounds with good oral bioavailability from those that were poorly absorbed became a central part of medicinal chemistry thinking. (See Chapter 5 for more details on physicochemical properties and their relationship to bioavailability.)
After solving the problem of bioavailability, the pharmaceutical industry began to realize that safety-related issues were now a significant cause of failure for new drug candidates. As result, much effort has been put into trying to understand the relationships between chemical structure and the toxicity observed for a given compound. By considering what is known about chemical structures and physicochemical properties early in the design process, these problems can be avoided. However, mechanisms of toxicity are often complex and poorly understood, so success in avoiding these problems altogether has been limited. Progress has been made, however, through the use of in silico models and in vitro assays, which can help identify the best compounds to put forward for further development. The thinking is that using these methods can at least improve the odds of success if not guarantee it (see Chapter 8 for more detail).
Despite this increased understanding of the importance of the safety profile that constitutes a successful drug candidate; medicinal chemists will often focus first on optimizing the potency and bioavailability of the molecule, rapidly narrowing down the search to within a single chemical series. Only then will they search for the one with the fewest safety liabilities within a narrow range of available substrates. Perhaps if safety were considered when there were still choices about which option was the optimal chemical series, then it might be possible to select molecules that had the ideal balance of target impact, bioavailability, and toxicity avoidance, leading to higher success rates and increased productivity.
Figure 13-2 shows a typical “front end” of the innovation process that has been modified (shaded boxes) to incorporate early inclusion of safer chemical design principles. The system includes three main activities: opportunity identification and analysis, concept creation, and design. Although innovators typically do not include aspects of safer chemical design in these early stages, the committee believes that this approach can help reduce problems during later stages. A key aspect to finding opportunity (in the business sense) is to truly understand customers’ desired outcomes; as shown in Figure 13-2, such understanding also helps to identify concepts that result in safer products.
As noted, one of the crucial early steps in the innovation process is to develop a deep understanding of “who your customers are” and “what their desired outcomes are” in the context of one’s product or service. For example, when regulatory bodies in Europe raised concerns over the use of phthalate plasticizers in polyvinylchloride-containing toys, one approach to the problem was to create more benign plasticizers (such as the cyclohexyl analog to a phthalate synthesized by BASF). However, customers are not interested in plasticizer design per se, but rather, in a safe, flexible material for use in children’s toys. Focus on this desired outcome can lead one to many possible solutions, such as Dow’s Insite® polymers (thermoplastic elastomers made from ethylene and propylene that are inherently soft and pliable without any need for plasticizer). Successful product design firms typically use a combination of ethnography and voice of the customer analyses to uncover desired customer outcomes, which prove critical to prototype fabrication.
Once a business understands its customer base, structured brainstorming can be used to generate novel solutions. In the case of safer chemical product design, one of Goldberg’s rules of thumb (Goldberg et al., 2003) can be borrowed: innovation by elimination to help create safer products. For example, in the plasticized polyvinylchloride case or the case of brominated flame retardants, removing the need for the problematic chemical while satisfying desired outcomes (an inherently soft material vs. softness through plasticizer or an inherently flame-retardant material vs. addition of flame retardant compounds) can lead to safer products.
Finally, once a promising concept has been generated, it is useful to examine the expected life cycle of a chemical as a way to check for red flags that might appear in the early stages of a product’s lifetime (see Life Cycle Thinking, Chapter 10).
In each of these instances, a consideration of the human health hazards and ecotoxicity is needed, alongside consideration of other environmental impacts and product performance attributes, as early as possible in the design process (ideally, when concepts are being penned to paper). In the traditional approach to innovation, health and environmental concerns are considered, if at all, near the end of the innovation process—only after significant time and resources has been committed to product development and the satisfaction of customer-centric performance criteria. If the goal is to reduce undesirable health and environmental impacts, these issues must be considered early in the design process. Ramani et al. (2010) and others have proposed that many health and safety impacts are “locked in” at the concept stage (before any significant bench work has begun). Consequently, considering these impacts early in the process is necessary to create true eco-innovations, products and services that promise enhanced performance with a reduced footprint.
Although the strategies and tools for safer chemical design provide primarily qualitative guidance, these approaches, when used early and often, can steer innovators away from products unlikely to meet safety criteria. A recent example of this comes from the use of heavy metal-containing nanoparticles (Bystrzejewska-Pitrowska et al. 2009). Despite the exceptional fluorescence properties of CdSe and PbS nanoparticles, each contains heavy metal cations. The presence of those cations might not pose environmental or health and safety concerns for macroscopic thin films embedded in electronic devices, but it is a different story if they are used to cover extremely high surface areas. Then cations from these nanoparticles are more readily released, potentially posing a hazard in many applications. It is now clear that these types of nanoparticles have limited potential due to the toxicity of their constituent elements (Schrand et al. 2010). By considering the safety concerns earlier in the innovation process, development time and resources might have been applied to solutions with environmental and health safety performance on par with their other performance attributes.
Figure 13-2 shows a typical flow diagram for the early stages of the innovation process and how innovators can eliminate potentially problematic
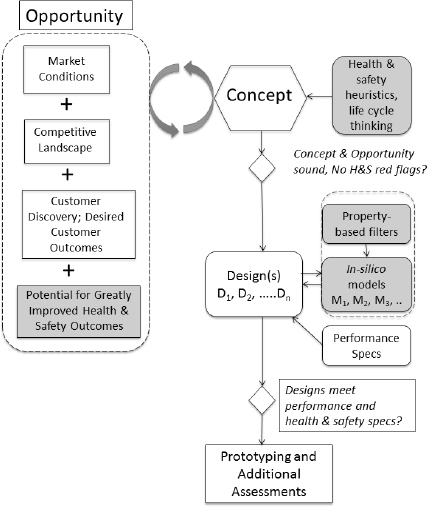
FIGURE 13-2. Flow diagram for the innovation process with the addition of alternatives assessment at the early stages. The traditional front end of the process is indicated by the white boxes, with proposed addition of tools (in shaded boxes) that can aid in design of safer products. Strong opportunity includes identification of a group of customers whose desired outcomes are not being met, a significant market, and typically failings among the competition. The potential for improvements to product safety can be included as a contributor to opportunity as well. Concepts are created to service opportunities. This is known as creating good product-market fit. Designs are then the physical manifestation of a concept (see Box 13-3 for detail). Further, as shown in this chapter, both creation of entirely new concepts or the de novo design of chemicals can benefit from inclusion of safer chemical principles.
design choices. From the perspective of the entrepreneur, the front end of the innovation process usually begins either with the realization that an unfilled, yet lucrative, opportunity exists and/or the identification of a novel concept or solution. Often, there is a gap between the desired outcomes of a significant customer segment (or segments) and current offerings.
Another type of “gap” that could lead to new opportunities could thus be the failings of current offerings due to environmental, health, and human health deficits. Indeed, identification of such opportunities for “green chemistry” or “ecoinnovation” is a potential outcome of Steps 3 and 4 of the committee’s framework. A manufacturer with a product containing a compound of concern may see a lack of satisfactory chemical offerings as a problem that needs to be dealt with, but an innovator will view this same “problem” as the rationale for new concept or business creation. It all depends on how a chemical of concern is perceived. One example of an opportunity created by a substance of concern is California’s effort to phase out perchloroethylene in dry cleaning because of toxicity issues, resulting in the development of a spate of new dry cleaning technologies in the 1990s (Sabanadesan and Vanderlinden 2007). Likewise,
emerging problems related to Bisphenol A use in polycarbonates created the opportunity for a non-Bisphenol A transparent thermoplastic with high-use temperature. In response to this opportunity, Eastman Chemical created Tritan copolyesters, while other companies invested in new, transparent, high-use temperature polyolefins (Nelson and Long 2012).
New Concepts and Chemical Designs
There are two primary approaches to developing innovative solutions that go beyond the consideration of known chemicals. The first involves design and synthesis of a new chemical to directly replace a known chemical of concern, or starting with the “design” step shown in Figure 13-2. This approach typically involves evaluating the function and structure of that chemical and modifying its structure to meet the functional need while reducing the impacts of concern (as illustrated in Case Study 1 on DecaDBE, Chapter 12). The second approach starts in either the “opportunity” or “concept” box in Figure 13-2. It involves identifying or developing novel approaches that seek to duplicate the function of the chemical of concern, not just the chemical itself. One might expect established companies that currently manufacture chemicals or chemical formulations to focus on the first approach (de novo design of a replacement chemical), given the constraints imposed by a mature business model that itself depends upon certain feedstocks or plant configurations. Similarly, one might expect start-up companies or downstream users of chemicals to instead focus on new concepts—providing the desired function without necessarily duplicating the original chemical. For an illustration of the difference between concept and design, see Box 13-3.
In either of these approaches—new design or new concept—innovators should proactively check to see whether there are any environmental, health, or other red flags related to chemical hazard in the design. They should use rules of thumb, structure/function relationships, computational tools, safer chemical lists and guides, and other early indicators to guide design at each stage of innovation. By identifying the functional use clearly early in the process, it may be possible to identify particular areas of concern (e.g., inhalational toxicity for a chemical that will be used as a fragrance or flammability for a product often used near open flames or heat sources) that can be considered during the design process. As noted in Figure 13-2, these early checks can be conducted at each stage of the innovation process, regardless of which approach is used.
A concept is a top-level response that fulfills the desired outcomes of customers, while a design is a more specific manifestation of the concept. It is possible to use health and safety screening tools at both the concept and the design stage. Below are two examples.
Example 1: If the desired customer outcome is “a surface free of bacteria,” one might have:
Concept 1: An antibacterial spray
Design 1A: A spray of triclosan and ethanol
Design 1B: A spray of lactic acid in water
Concept 2: A surface that prevents bacterial colonization.
Design 2A: A silver-functional acrylic coating that kills bacteria on contact.
Design 2B: A shark-scale biomimetic coating that prevents bacteria from sticking.
Example 2: If the desired customer outcome is a “fabric with bright color,” one might have:
Concept 1: Use a dye to color the fabric.
Design 1A: Use a metal-based dye.
Design 1B: Use a dye extracted from a plant or animal
Concept 2: Use reflection from surfaces to create the illusion of color.
Design 2A: Layers of polymer to mimic the Morpho Butterfly (Teijin Fibers MorphoTex)
Design 2B: Rolled layers to mimic the plant Margaritaria Nobilis (Kolle et al. 2013)
Guidance for New Concept Creation
Generally, in its early stages the innovation process is strongly influenced by the needs of the market, and concept creation is guided by an understanding of these market needs (and the competitive landscape). Whereas early inclusion of health, ecotoxicity, and physicochemical principles, as well as Life Cycle Thinking, would be valuable in this process, this is not common. The committee recommends that such inclusions occur early in the process. For example, at the concept stage, use of Life Cycle Thinking can be useful in avoiding undesirable building blocks and stimulating thinking about a novel way to reduce the environmental
footprint (for example, creating inherently flame-retardant materials vs. the use of chemical flame retardants).
Guidance for De Novo Design of Alternative Compounds
In the early design stage, there are a number of approaches (including the descriptions in Chapter 5 about physicochemical properties), which innovators should consider to guide chemical designers and help them select from a number of potential chemical structures. When de novo chemical design is required, consideration of both 1) physicochemical properties and 2) potential biological activities will reduce the likelihood of new chemicals encountering issues as development and further testing proceeds.
The following stages can be used to guide the design of new chemicals. They are tiered and based on the speed with which they can be applied and increasing sensitivity.
Stage 1: Apply qualitative structure-based55 design filters. At this stage, it is useful to screen for chemical functional groups or other structural features that are highly likely to be associated with particular hazards. This can be done before a chemical is synthesized, while it is still in the conceptual phase. A common example of an undesirable feature is the presence of an unhindered aromatic amine, which is strongly associated with carcinogenicity (Benigni and Passerini 2002). Box 13-4 lists various overlapping approaches for qualitative structure-based screening.
Stage 2: Apply qualitative property-based design filters (see Box 13-5) to eliminate chemicals highly likely to exhibit hazards associated with particular undesirable physicochemical properties. As soon as samples of chemicals are synthesized, these physicochemical properties can be measured, or these properties can be predicted based on computational models when chemicals are still in the concept phase.
Stage 3: Apply a more refined set of in silico tools and quantitative models to further assess toxicity hazards. Such models can be either based on structure (Quantitative Structure - Activity Relationships, QSARs) or spectra (Quantitative Spectroscopic Data Activity Relationships, QSDARs). These models will allow screening for additional human and ecotoxicity end points, such as carcinogenicity, mutagenicity, endocrine disruption, etc. For a more information, see QSAR discussions in Chapters 7 and 8. Discretion must be applied to use these models in a way that provides meaningful results. If a candidate chemical is predicted to have high toxicity for one or more end points, it should either be screened out, given a low priority, or redesigned and fed back through the workflow.
Stage 4: Apply mechanistic prediction tools for end points that are available. For the remaining candidates, use of more complex novel high throughput testing and computational models, such as those described in Chapter 8, may further decrease the probability that the candidates proposed will cause unintended consequences. While such models are routinely used in the pharmaceutical industry in drug design to avoid unintended consequences (see Box 13-2), they are underutilized in the rational design of commercial chemicals. The mechanistic underpinning of these models allows a more refined prediction for some end points, such as skin sensitization and carcinogenicity.
Stages 1-4 provide guidance for improving environmental and health attributes, by using available tools before the chemical synthesis stage. In addition to being used to screen out less desirable chemicals in the design stage, the information gathered can inform future designs of analogous alternatives. Although these steps are described in a linear fashion for the sake of simplicity, a strong and continuous flow of information, from the analysis of chemical structure to a description of physicochemical properties, is needed as feedback to guide design of safer alternatives. This type of feedback is key to developing more robust structure/activity relationships for chemical classes.
_____________
55 Note: While structure-based filters and physicochemical property-based filters are described here separately, physicochemical properties obviously stem from structure.
Qualitative screening for chemical functional groups or other features that are highly likely to be associated with particular hazards can be done before a chemical is synthesized, while it is still in the conceptual phase. Design filters are listed here with common names. In reality, the approaches listed here overlap in the concepts they cover.
“Rules of Thumb”: Principles developed from experience that have broad application but are not intended to be strictly accurate or reliable for every situation. They should be used to qualitatively screen for structural features associated with high probability of hazard. Two examples widely used in pharmaceutical chemistry, but not widely applied by those engaged in alternatives assessment, include:
- Avoid unhindered aromatic amines, which are strongly associated with carcinogenicity (Benigni and Passerini 2002).
- Lipinski’s rule of five for drug design56 (Lipinski et al. 1997):
- Number of hydrogen bond donors (nitrogen or oxygen atoms with one or more hydrogen atoms < 5).
- Number of hydrogen bond acceptors (all nitrogen or oxygen atoms < 10).
- Molecular mass < 500 daltons.
- Lipophilicity (logP < 5).
Computational predictive approaches: This refers to computational approaches that strive to predict activity from structural information. These approaches would typically involve the use of various computational methods to calculate structures, properties, or impacts.
Expert rules: Structure- or mechanism-based decision-making approaches that are typically computerized and aim to mimic the integrative analysis that an “expert” would provide. Expert rules may incorporate both rules of thumb and computational learning about toxicity prediction. Expert rules should be used to qualitatively screen for structural features associated with high probability of hazard. One example is DEREK:
- “DEREK is a knowledge-based expert system comprising a number of structural rules that aim to encode structure-toxicity information with an emphasis on mechanisms. The toxicity predictions made by DEREK are the result of two processes. The program checks whether any alerts in the knowledge base match toxicophores in the query structure. The reasoning engine then assesses the likelihood of a structure being toxic. There are 9 levels of confidence: certain, probable, plausible, equivocal, doubted, improbable, impossible, open, contradicted. The reasoning model considers the following information:
- The toxicological end point.
- The alerts that match toxicophores in the query structure.
- The physicochemical property values calculated for the query structure.
- The presence of an exact match between the query structure and a supporting example within the knowledge base” (Saliner et al. 2005).
Structure activity relationships: These are relationships that intend to link specific structural features with biological activity.
_____________
56 These rules of thumb are associated with increased likelihood of oral activity in humans. Avoiding them in chemical design would reduce the likelihood of unwanted oral activity.
BOX 13-5
QUALITATIVE PROPERTY-BASED DESIGN FILTERS: DESIRABLE/UNDESIRABLE PROPERTIES
Structure-property relationships. These are relationships that intend to link specific structural features with particular chemical properties (physicochemical properties).
Physicochemical property-based design guidelines (see also Chapter 5)
Examples of established property-based design guidelines are listed below, but it is clear that there is a need to develop additional guidelines that address materials safety and additional biological end points.
- Rules of thumb for increasing biodegradation according to Williams and Williams (Williams and Williams 2012) are to avoid:
- “Halogens, especially chlorine and fluorine and especially if there are more than three in a small molecule (iodine and (probably) bromine contribute to a lesser extent);
- Chain branching if extensive (quaternary C is especially problematic);
- Tertiary amine, nitro, nitroso, azo, and arylamino groups;
- Polycyclic residues (such as in polycyclic aromatic hydrocarbons), especially with more than three fused rings; heterocyclic residues, for example, imidazole); and
- Aliphatic ether bonds (except in ethoxylates).”
- Criteria for human bioavailability by different exposure routes: If a chemical meets all of the property limits associated with skin, oral, respiratory, or ocular bioavailability, it is likely to pose higher risk of exhibiting human toxicity. While this may not be detrimental, it is reasonable that chemicals with low bioavailability are given higher preference.
- Criteria for aquatic toxicity: If an organic chemical meets the criteria for high risk of acute and/or chronic aquatic toxicity, it should be redesigned, screened out, or given low priority.
- Criteria for physical hazard: These include flammability flash point corrosivity etc.
Redesign of an Existing Chemical
The considerations required for redesigning an existing chemical to minimize hazard while retaining function overlap partly with those outlined in the previous section. Structural optimization to tune biological activity is not uncommon in the pharmaceutical industry, but it is not typically utilized in the rational design of commercial chemicals. This process starts with the identification of the structural core of a chemical that is associated with function. In cases where this is not obvious, the functional core can be identified by understanding how the chemical exerts the desired function. Identifying this motif will allow for the identification of the non-essential structural features of the molecule that could be modified. The possible analogs can then be generated to obtain a set of candidates. These candidates are fed through the above process starting at Stage 2, and proceeding to the end. The result of these workflows will be a number of candidate chemicals that can be carried through the alternatives assessment workflow described earlier in this report.
Looking Forward: New Tools for Early Insights into Toxicity
The stages describe how to use what is known about chemical structures and physicochemical properties to design chemicals that avoid unfavorable characteristics. The structure-based prediction can be conducted before a chemical is even synthesized. Physicochemical properties may be predicted and/or measured. The advent of high throughput testing of chemicals through a large battery of tests designed to identify a number of common toxicity end points is likely to yield yet another opportunity for early insight into toxicity. As described in Chapter 8, computational toxicologists, who evaluate the results of such high throughput robotic testing through hundreds of assays for various end points, are working to discern what type of information they can glean from these approaches, such as the Tox21 or ToxCast batteries of assays developed by EPA, NIH, and FDA collaborations. While the assays have shortcomings, there are indications that batteries of assays may be useful for predicting particular end points.
Furthermore, there are hints that the assays may be even more valuable in predicting the chemical concentration at which biological activity occurs. As the toxicology community moves toward a common understanding about the value that can be gleaned from these assays, it is likely that chemical designers who can synthesize their compounds in a pure enough form to avoid artifacts from the assays could benefit from the ability to quickly screen compounds they are developing.
Where no alternatives exist and a new chemical must be rationally designed, a series of qualitative structure-based or physicochemical property-based design filters can be used to assess chemical designs while they are still conceptual or have only small amounts synthesized, to minimize health and ecotoxicity issues. Then, more refined tools, such as in silico modeling of mechanisms and QSAR and QSDAR, should be used to guide designs that meet environmental and health requirements as well as functional performance. The most important aspect is to consider attributes that increase ecological or health risks, in tandem with other performance attributes, as early as possible in the design process.
The staged evaluation of these novel alternatives is tiered and based on the speed with which they can be applied and increasing sensitivity. The advantage of this approach is that fatally flawed alternatives may be eliminated from consideration earlier in the process. Innovation time and resources can then be focused on viable alternatives, and when more of the actual compound is available for testing, additional information can be obtained.