Physicochemical Properties and Environmental Fate
Knowledge of the physicochemical properties of potential chemical alternatives is a requirement of the alternatives assessment process for two reasons. First, the inherent hazard of a chemical, such as its capacity to interfere with normal biological processes, and its physical hazards and environmental fate (degradation, persistence) are determined by its intrinsic physicochemical properties and the system with which it is interacting. For organic and inorganic chemicals, these intrinsic properties are determined by molecular structure, while for materials, they are determined by composition, size, structure, and morphology. Second, physicochemical properties can be used to eliminate from consideration chemicals that are likely to exhibit particular physical or toxicological hazards. As important as these data are, obtaining them is relatively fast and inexpensive, and can be readily done at the initial stages of the alternatives assessment.
This chapter provides a general background on physicochemical properties and briefly reviews experimental and computational methods that could be used to determine physicochemical properties. Current approaches for assessing physicochemical properties in several alternatives assessment frameworks are then discussed, followed by the details behind assessment of physicochemical properties and their relevance in predicting environmental fate and transport and human health hazards and ecotoxicity. Finally, the committee provides additional instructions on the implementation of Step 5 in its framework.
Box 5-1 provides a brief description of the elements of the committee’s suggested approach.
PHYSICOCHEMICAL PROPERTIES OF INTEREST
For the purpose of this report, we broadly define physicochemical properties as physical properties, solvation properties related to interactions with different media, and properties or molecular attributes that define intrinsic chemical reactivity. The physicochemical properties of interest to chemical alternatives assessment can be used to identify physical hazards and to understand or predict a chemical’s environmental fate, human toxicity, or ecotoxicity (see Figure 5-2). The committee cautions that given the active research in the field and the potential for special case concerns to arise for a given compound (e.g., atmospheric reactivity), the properties highlighted in this chapter
BOX 5-1
Elements of Step 5 (Assessing Physicochemical Properties)
The assessment of physicochemical properties is an early step (Figure 5-1) in alternatives assessment because physical hazards, environmental fate, and intrinsic human health hazards and ecotoxicity are directly related to a chemical’s intrinsic physicochemical properties (Figure 5-1). Physicochemical properties such as those indicative of physical hazards could be used to eliminate particular chemicals from consideration and prioritize chemicals for further screening for human and ecotoxicological effects. A number of properties can be informative to alternatives assessment, as described in detail in this chapter, and a high-priority data set is also defined. Property data can be obtained from experimental or in silico (estimation) methods. In fact, state-of-the-art methodologies are making in silico methods increasingly reliable, low-cost approaches.
The suggested uses of physicochemical property data are:
- To identify the potential for direct physical hazards posed by the chemical or material.
- To determine the environmental compartment(s) into which the chemicals will partition.
- To estimate the potential for bioconcentration and bioavailability.
- To estimate the likely route(s) of mammalian exposure and bioavailability, and the likelihood for high aquatic toxicity.
- To estimate the potential for inducing human toxicity.
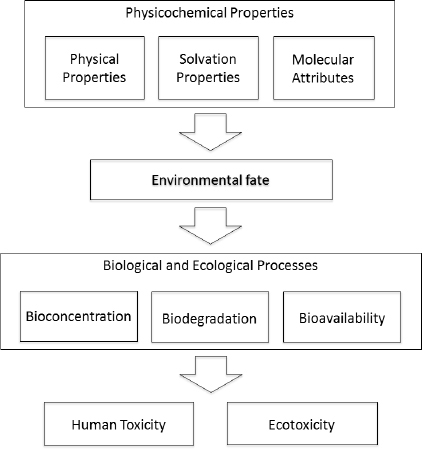
FIGURE 5-2 Scheme illustrating the relationships between the three primary types of physicochemical properties assessed in the committee’s framework and their relationship to environmental fate, biological and ecological processes, and toxicity.
should be seen as general guidance, and care should be taken by the assessor to ensure that all appropriate physicochemical properties are identified for a given compound and system.
Physical Properties
Physical properties include freezing point, boiling point, melting point, infrared spectrum, electronic parameters, viscosity, and density. Some of these physical properties (e.g., electronic parameters, molecular weight, boiling/freezing point) are directly associated with environmental fate and health effects.
Solvation Properties
Solvation properties14 describe a chemical’s interactions with different phases and its partitioning between phases. Solvation properties of interest in alternatives assessment can be divided into three main types: (a) phase partitioning, (b) solubility, and (c) colligative properties:
- Phase partitioning. A partition-coefficient or distribution-coefficient is defined mathematically as the ratio of concentrations of a given compound across two mixed, immiscible phases at equilibrium. In the context of a chemical alternatives assessment, important partition coefficients are often measured in the liquid phase. Though partitioning can be measured across a range of solvents and phases, the phase partition coefficient most often encountered when assessing physicochemical properties is from a system where one solvent is water or an aqueous phase and the second is organic and hydrophobic, such as 1-octanol (i.e.,
_____________
14 The terms solvation properties and solution properties are often used interchangeably. Solvation is the term used in this report.
octanol/water partition coefficient [Kow] represented by P).15
![]()
- Solubility. This chemical property refers to the ability of a given substance (the solute) to dissolve in a solvent. The primary measurement of interest in chemical alternatives assessment is solubility in water.
- Colligative properties. Colligative properties are properties of solutions that are not dependent on the chemical species but instead on the ratio of the number of solute particles to the number of solvent molecules in a solution. Examples of colligative properties include lowering of vapor pressure, elevation of boiling point, and depression of freezing point. Colligative properties generally do not play a significant role in alternatives assessments and are not discussed further in this report.
Molecular Attributes
The term molecular attribute is used to describe properties related to molecular shape and size. For the purposes of this report, the committee considers electronic parameters of molecules (e.g., frontier orbital energies and polarizability) that affect chemical reactivity as a type of molecular attribute.
Environmental Partitioning
In addition to the partition coefficient P, there are other media-specific partition coefficients that can provide valuable information about environmental fate, such as a chemical’s phase partition coefficient in soil and water (Kd) and in water and air (Kw/g, Henry’s law). As will be discussed in a later section, these coefficients provide insight into environmental partitioning of the molecule and the potential for bioaccumulation. As with other physicochemical properties, some of these values must be directly measured and some may be estimated.
MEASURED PHYSICOCHEMICAL PROPERTY VALUES
An extensive review of the experimental measurement of a chemical’s physicochemical properties is beyond the scope of this report. Measured values of these properties often can be obtained from the scientific literature (Leo 1995). Some useful databases include: the National Institute of Standards and Technology Search for Species Data (NIST 2011) and the Syracuse Research Corporation’s CHEMFATE Chemical Search database (SRC 2014). Since there is a wide range of environmental conditions of interest (especially temperature and pH), there are often no suitable literature values available. In those cases, direct measurement or estimation through computational approaches is required.
The OECD Guidelines for the Testing of Chemicals is a review of approximately 100 testing methods used by various governmental and non-governmental entities to identify and characterize potential hazards of new and existing chemicals (OECD 2014a). OECD test guidelines exist for the measurement of a variety of physicochemical properties, including Kow and determination of pH, vapor pressure, density, water solubility, and melting and boiling points, among others. A number of comprehensive review texts have been authored on the measurement and estimation of physicochemical properties (Boethling and Mackay 2000). In cases where measurement is not feasible or is prohibited by cost, estimated parameters can be determined through a variety of methods, as discussed in the next section.
METHODS FOR ESTIMATING SELECT PHYSICOCHEMICAL PROPERTIES
This section briefly discusses the increasing number of computational, or in silico, tools available for estimation of the key physicochemical properties included in the committee’s framework. These tools provide a rapid means for obtaining physicochemical data, often at a lower cost when compared with experimental measurement. A number of different software packages and algorithms are available for predicting physicochemical properties, and predictions are often in excellent agreement with experimentally-derived values. The user of such tools, however, must have a basic understanding of the inherent advantages and limitations of the various algorithms as they relate to the accuracy of physicochemical property prediction. Here we will briefly explore two broad categories of properties discussed in the chapter that are most amenable to accurate estimation—solvation properties and electronic parameters.
_____________
15 In this chapter, P will be used interchangeably with Kow, reflecting preferences in terminology across relevant fields.
Solvation Properties
Phase Partitioning
Molecular hydrophobicity (or lipophilicity) is expressed as P or D and is one of the most studied physicochemical properties in organic and medicinal chemistry. LogD is defined as the ratio of the concentration of compound in the lipid phase to the concentration of all species (ionized and un-ionized) in an aqueous phase at a given pH. This ratio is directly affected by the pH of the system; thus, this information is often included as a subscript, logDpH. LogP is defined as the logarithm of the ratio of unionized compound in each phase.
LogD for acids/bases can be readily calculated from logP when pKa values are known. Thus, only methods for determining logP will be discussed here. Two types of in silico methods for estimating logP exist: those based on chemical structure, which are well established, and those based on spectroscopic data, which are fairly novel. There are five classifications of structure-based computational methods: whole molecule methods (which use only molecular parameters, such as size, polarizability and H-bond acceptor strength), atom-based, fragment-based, constructionist, and reductionist (Leo 2000). While some of these approaches use atomic- or fragment-based prediction algorithms, where a molecule is dissected into fragments (and its logP value is obtained by summing the hydrophobic contributions of each fragment), others use whole molecule attributes that take into account conformations (Meylan and Howard 1995; Muehlbacher et al. 2011). The most commonly used group contribution tools, such as ALOGP, CLOGP, ACD, and KOWWIN, have a coefficient of determination (r2) in the range of 0.90-0.95 (An et al. 2014). Although these tools are very fast and accurate, these methods often show lower accuracy when externally validated (r2 = 0.51-0.91). An and colleagues determined that this could be “due to limitations in the applicability domains to structures containing predefined fragments” (An et al. 2014). In particular, the authors identified concerns about the performance of compounds containing phosphorus, halogens, and other heteroatoms. They noted that there were clear disagreements between measured values of logP and those calculated by predictive programs. A detailed discussion of these nuances is available (Voutchkova et al. 2012). The algorithms based on spectroscopic data do not require knowledge of exact chemical structure (Voutchkova-Kostal et al. 2013). Although fairly new, they report performance on par with those of structure-based approaches, but their full applicability has not been determined (An et al. 2014).
Aqueous Solubility
Aqueous solubility is a direct measure of the hydrophobicity of a substance. The solubility equation developed by Yalkowsky can be used to estimate intrinsic water solubility at 25oC (logS) for structurally diverse organic substances (Ran and Yalkowsky 2001). This equation uses regression-derived correlation with logP and melting point (MP) for solids:
logS = 0.8 – logP – 0.01(MP – 25)
Other factors that influence water solubility include temperature and pressure, neither of which is accounted for in this equation (Jorgensen and Duffy 2002). Another effect that should be considered arises from salinity (“salting-out”), which indicates that this equation is not appropriate for use with high-melting, non-ionic solids (Voutchkova et al. 2012).
pKa
pKa values provide insights into the lipophilicity and solubility of ionizable compounds. This, in turn, can be used to better anticipate and predict the compound’s toxicokinetic behavior for processes such as gastrointestinal absorption, membrane permeability, protein binding, and metabolic transformations. Therefore, research has led to the development of computational tools for pKa determination. As noted in the 2012 Handbook of Green Chemistry (Voutchkova et al. 2012):
In silico pKa methods are fast, cost-effective, and mostly reliable (some reporting correlation with experiment as high as 0.90) … [T]hey can also provide structural assignment and identify which ionization center in the molecule corresponds to each pKa value, and also predict the pKa values of tautomers. Most of these methods use linear free energy relationships with Hammett σ and Taft σ* constants for the calculation of microscopic and macroscopic ionization constants (Shields and Seybold, 2013). Some more fundamental approaches use semiempirical and higher level quantum calculations; however, these are problematic for larger systems, since
they require calculating very small differences in the energy of relatively large molecules (Shields and Seybold, 2013)…. Importantly, as with all methods that require parameterization, the choice of in silico pKa prediction tool should be guided by the type of compounds being analyzed, as every parameterization yields outliers (usually containing specific functional groups), and its range of applicability is limited by the training set used.
Molecular Attributes: Electronic Properties
Knowing the calculated electronic properties of molecules can be a useful part of a first-tier estimation of a chemical’s reactivity with biological targets. For some end points, electronic properties have been shown to be helpful in identifying chemicals of high toxicity. These properties can be readily estimated with quantum mechanic calculations when the chemical structure is known. A multitude of electronic properties and molecular attributes have been used to describe biological activity of chemicals. Some of these relate to molecular size, shape, and volume, others relate to the distribution of electrons in the molecule, and yet another set is based on frontier orbital energies.
Properties that describe molecular size and shape include solvent accessible surface area, molecular volume, globularity, and ovality, and they can be related to bioavailability and reactivity. Accurate estimation of these attributes based on chemical structure necessitates prior optimization of the geometry via a conformational analysis and energy optimization.
Properties related to electron distribution are often related to chemical reactivity and biological activity (Voutchkova 2012). For example, molecular electronic dipole moments, μ, and dipole polarizabilities, α, are important in determining the energy, geometry, and intermolecular forces of molecules. Electric dipole moment μc is classically expressed as a sum of discrete charges, qi, multiplied by the position vector, ri, from the origin to the ith charge. Polarizability is the relative tendency of a charge distribution (ρ(r), an atom or molecule’s electron cloud) to be distorted by an external electric field. Thus, the quantum method and the basis set16 used impact the dipole moment and polarizability calculations.
Electronic properties based on frontier orbital energies are closely related to chemical and biological reactivity. Frontier molecular orbital (FMO) theory, pioneered by Fukui and coworkers (Fukui, et al. 1952), mathematically defined the role that frontier orbitals play on chemical reactivity. This theory is now well accepted in the field. In addition to the energies of the frontier orbitals (Highest Occupied Molecular Orbital [HOMO], Lowest Unoccupied Molecular Orbital [LUMO)], and the energy gap [∆E] between the HOMO and LUMO orbitals), electronic properties can include chemical softness/hardness, chemical potential, and electrophilic index, to name a few.
Rather than attempting to provide a detailed description of these properties and their relation to biological activity, instead we illustrate the potential utility of one such property to alternatives assessment. This property is the HOMO–LUMO gap, which is a known measure of kinetic stability, such that a molecule with a small HOMO–LUMO gap (see Figure 5-3) is considered chemically reactive for covalent bonding (Kostal et al. in press). In the section of this chapter entitled “Use of Physicochemical Properties to Predict Aquatic Bioavailability and Toxicity,” there is an example of the applicability of HOMO-LUMO gap for identifying chemicals most likely to exhibit high acute aquatic toxicity.
When calculating any electronic properties, the choice of quantum mechanical approach (i.e., semiempirical, ab initio, and density functional methods) should be made judiciously. Recent advances have made density functional theory (DFT) methods comparable in accuracy to post-Hartree-Fock ab intio methods and often represent the optimal method of choice, especially for larger molecules. Semi-empirical methods can be accurate and are notably faster than ab initio or DFT methods; however, their performance is tied to the training set used in their development. Thus, these semi-empirical methods should always be benchmarked against experimental data or higher-level calculations for any given application.
_____________
16A basis set is a collection of vectors that defines a space in which a problem is solved. In quantum chemistry, the “basis set” usually refers to the set of (nonorthogonal) one-particle functions used to build molecular orbitals.
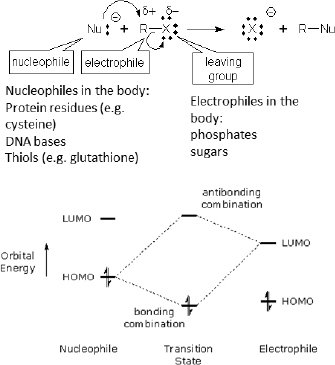
FIGURE 5-3 Relation of frontier molecular orbital energies to covalent interactions of nucleophiles and electrophiles, as illustrated with a generic nucleophilic substitution (Sn2) reaction. The reactivity of nucleophiles with HOMO energies close to the LUMO energies of the electrophiles will be higher than ones with larger differences, assuming steric effects are held constant.
PHYSICOCHEMICAL PROPERTIES IN OTHER FRAMEWORKS
Requirements for the collection and interpretation of physicochemical data are present in other frameworks, but specific guidance for the evaluation of these properties is not always included. For example, the disclosure of some physicochemical properties is required for registration under the European Union’s REACH process (ECHA 2014c), but interpretation of those data is not.
Physical Hazards
The IC2 framework evaluates reactivity and flammability and uses the GreenScreen® methodology to categorize chemicals, which requires data on flammability and explosibility (Rossi and Heine 2007). The German Guide notes that physicochemical hazards may make certain chemicals difficult for workers to handle and pose safety hazards due to flammability and explosibility. It provides a chart with some guidance on categorization of different physical hazards based on common labeling standards.
EPA’s DfE evaluates physical hazards using the United Nation’s GHS, which is an internationally recognized structure for communication of a range of hazards (UNECE 2013a). In total, the GHS identifies 16 types of physical hazards. GHS also provides a structure for classifying these hazards, facilitating direct comparisons to be made across materials. Annex 8 of the 2013 edition provides an example of how to carry out a GHS classification (UNECE 2013b). The DfE framework lists several GHS categories, including those in the 2011 Alternatives Assessment Criteria for Hazard Evaluation. These criteria include explosibility, self-reactive substances, substances that on contact with water emit flammable gases, oxidizing gases, oxidizing liquids and solids, organic peroxides, self-heating substances, and corrosivity to metals as physical hazards of concern (EPA 2011a). These categories are explained in more detail in Table 5-1.
TABLE 5-1 GHS Criteria used by DfE for the Classification of Physical Hazards
| Physical Hazards | Very High | High | Moderate | Low |
| Explosives | GHS Unstable Explosive | GHS Explosive Division 1.1 (Mass explosion hazard), 1.2 (Severe projection hazard), or 1.3 (Fire, Blast hazard or projection hazard) | GHS Explosive Division 1.4 (Fire or projection hazard), or 1.5 (may mass explode in fire) | GHS Explosive Division 1.6 (Extremely insensitive articles with no mass explosion hazard) or not classifiable as an explosive by GHS |
| Self-reactive Substances | GHS Type A (Detonates/Deflagrates rapidly) or B (Liable to undergo thermal explosion) | GHS Type C (Possesses explosive properties) or D (Detonates partially when heated in confinement) | GHS Type E (Does not detonate when heated in confinement) or F (No effect when heated in confinement, not explosive) | GHS Type G (Thermally stable) or GHS not classified |
| Substances that on contact with water emit flammable gases | GHS Category 1 (In contact with water releases flammable gases which may ignite spontaneously) | GHS Category 2 (In contact with water releases flammable gases | GHS Category 3 (In contact with water releases flammable gases) | GHS not classified |
| Oxidizing Gases | GHS Category 1 (May cause or intensify fire; oxidizer) | GHS not classified | ||
| Oxidizing Liquids and Solids | GHS Category 1 (May cause fire or explosion; strong oxidizer) | GHS Category 2 (May intensify fire; oxidizer) | GHS Category 3 (May intensify fire; oxidizer) | GHS not classified |
| Organic Peroxides | GHS Type A (Heating may cause an explosion) or B (Heating may cause a fire or explosion) | GHS Type C (Heating may cause a fire) or D (Heating may cause a fire) | GHS Type E (Heating may cause a fire) or F (Heating may cause a fire) | GHS Type G (No hazard label) or not classified |
| Self-heating Substances | GHS Category 1 (Self-heating; may catch fire) | GHS Category 2 (Self-heating in large quantities; may catch fire) | GHS not classified | |
| Substances corrosive to metal | GHS Category 1 (May be corrosive to metals) | GHS not classified | ||
SOURCE: EPA 2011a and UNECE 2011.
TABLE 5-2 End Points, Thresholds, and Categories used to Evaluate Bioaccumulation Potential in Chemical Alternatives Assessment Frameworks Reviewed by the Committee. TURI’s P2OASys worksheet returns numerical values based on a scale of 1 to 10 to represent relative hazard from low to high.
| End Point | Framework | Threshold | Category |
| Log Kow | DfE | < 2 | Low |
| IC2 | ≥ 5 | Very high | |
| < 4.5 | High | ||
| 4-4.5 | Moderate | ||
| TURIa | ≥ 6 | 10 | |
| < 6 | 8 | ||
| < 4 | 6 | ||
| < 2 | 4 | ||
| BAF/BCF (Bioaccumulation Factor/Bioconcentration Factor) (mg/L) | DfE | > 5000 | Very high |
| 1000-5000 | High | ||
| 100 ≤ 1000 | Moderate | ||
| < 100 | Low | ||
| IC2 | > 5000 | Very high | |
| 1000-5000 | High | ||
| 500 ≤ 1000 | Moderate | ||
| 100 ≤ 500 | Low | ||
| <100 | Very low | ||
| TURIa | ≥ 1000 | 10 | |
| < 1000 | 8 | ||
| < 200 | 6 | ||
| < 100 | 4 | ||
| < 10 | 2 | ||
aCategory values calculated from the Pollution Prevention Options Assessment System (P2OASys) worksheet, September 2014. The P2OASys worksheet returns numerical values based on a scale of 1 to 10 to represent relative hazard from low to high.
SOURCE: EPA 2012; IC2 2011; TURI 2010
Solvation Properties and Molecular Attributes
Several reviewed frameworks provide an analytical system for assessing exposures on the basis of physicochemical properties or bioaccumulation.17 For example, the IC2 framework lists a variety of physicochemical properties that should be considered when assessing exposure pathways, including: volatility/vapor pressure, molecular weight and size, solubility, logP (as Kow), boiling point, melting point, density/specific gravity, pH, corrosivity, and dissociation constant. All but one of the alternatives assessment hazard classification schemes include a metric for bioaccumulation (see Appendix B for more information). Across a number of frameworks, the octanol–water partition coefficient logP or Kow is used as an indicator of hydrophobicity. Table 5-2 shows the characterization and ranges that define classification scores for logP for three frameworks.
Among the frameworks, the potential for bioaccumulation generally is considered very high when logP exceeds 5 to 6 and generally considered low when the logP < 2. It should be noted, however, that a compound with a high logP value may be rapidly metabolized or degraded, and in these cases, would not bioaccumulate.
The DfE evaluates the ability of a chemical to bioaccumulate. When measured data are unavailable,
_____________
17 “Bioaccumulation is defined as the accumulation of chemicals in the tissue of organisms through any route, including respiration, ingestion, or direct contact with contaminated water, sediment, and pore water in the sediment” (EPA 2000).
the DfE will consider the octanol-water (Kow) and octanol-air (Koa) partition coefficients. If the Kow and Koa have not been experimentally determined, then the DfE indicates that these values can be estimated using models, including KOWWIN and KOAWIN, available through EPI Suite18 or SPARC.19 Another appropriate method for determining these end points can also be used.
PHYSICOCHEMICAL PROPERTIES IN THE COMMITTEE’S FRAMEWORK
After reviewing the research literature and existing frameworks described in Chapter 2, the committee identified a high-priority data set of physicochemical property data. These properties are listed in Table 5-3, together with a brief description of the committee’s rationale for their inclusion. In general, the committee selected those physicochemical properties that could support the following uses in an alternatives assessment:
- To identify the chemical or material’s potential for posing a direct physical hazard.
- To determine the environmental compartment(s) into which the chemical or material will partition.
- To estimate the potential for the chemical to bioconcentrate20 and/or be bioavailable.21
- To estimate likely route(s) of mammalian exposure and bioavailability, as well the likelihood for high aquatic toxicity.
This section describes in further detail how physicochemical properties can be used to inform an alternatives assessment with respect to evaluation of physical hazards, environmental fate, and human/ecotoxicological toxicity end points.
Physical Hazards
The first step in using physicochemical properties is to determine the likely physical hazards. For much of this report, the primary consideration of the hazards and impacts of a potential chemical substitution is directed toward the post-manufacturing, consumer, use, and end-of-life phases. When considering physical hazards in particular, this focus may shift, as many substances that are considered non-physical hazards at the consumer phase may pose greater risks at the manufacturing and transport stages.22 The committee believes that for most cases, undesired physical hazard concerns that carry over to the consumer realm are most likely limited to flammability, gases under pressure, oxidizing liquids, and corrosivity to metals. There are, of course, rare cases where these properties might be desired in the final product (fireworks, lighter fluid, etc.).
Identification and classification of the physical hazards posed by potential alternatives allows for direct comparison and consideration of the risk and process safety management strategies that a company would need to develop when a given material or chemical alternative is chosen. As mentioned earlier, the GHS is a useful aid in evaluating and classifying physical hazards (see Table 5-1 for an example of its application to the DfE framework).
_____________
18 Estimation Program Interface (EPI) SuiteTM. The EPI Suite™ is a Windows®-based screening-level tool developed by the EPA’s Office of Pollution Prevention Toxics and Syracuse Research Corporation (SRC). This tool provides a suite of physical/chemical property and environmental fate estimation programs.
19 SPARC (SPARC Performs Automated Reasoning in Chemistry). This EPA predictive modeling system is used to estimate chemical reactivity parameters and physical properties for a wide range of organic molecules. “SPARC is being designed to provide physical properties and chemical reactivity parameters describing factors for air, water and other environmental media needed to develop and apply models such as the Environmental Fate Simulator and Reaction Pathway Simulator” (EPA 2013b).
20 Bioconcentration is a process leading to a higher concentration of a substance in an organism than in environmental media to which it is exposed (IUPAC 1993).
21 In toxicology, bioavailability is that fraction of the total amount of material in contact with a portal of entry (lung, gastrointestinal tract, skin) that then enters the blood. In contrast, an ecotoxicologist may define bioavailability as that fraction of material solubilized in the water column under certain conditions of hardness and pH. An aquatic toxicologist might consider contaminants, which are soluble under specific stream conditions, to be bioavailable to fish or benthic organisms (EPA 1994).
22 One example is household baking flour. On a shelf in a pantry, there is little physical hazard posed to the average home. However, the manufacturing process must be carefully managed to avoid the serious explosive hazard posed by the flammable particles suspended in air.
TABLE 5-3 High-priority Data Set of Physicochemical Processes and Rationale for their Inclusion
| Type | Property | Rationale for Inclusion |
| Physical properties | Flammability | Associated with flammability hazard |
| Corrosivity | Associated with ability to gradually destroy materials by chemical reactions | |
| Oxidizing ability | Associated with ability to give off oxidizing substances or oxidize combustible materials, increasing fire or explosion hazards | |
| Melting and boiling point | Impacts environmental fate and transport, as well as potential bioavailability | |
| Vapor pressure | Impacts environmental fate and transport, as well as potential bioavailability | |
| Solvation properties | Acidity (pKa) | Determines ionization state in the environment as well as in biological compartments; ionization state in turn impacts other properties, such as water solubility and partition coefficients, which directly impact toxicokinetics |
| Aqueous solubility | Reflects ability to partition into aquatic environment | |
| Octanol-water partition coefficient (logP) | Important determinant of human/mammalian oral and skin bioavailability; relevance to acute & chronic aquatic toxicity (narcosis) and directly related to bioconcentration | |
| Henry’s law constant (logPw/g) | Relevance to environmental partitioning and transport as well as human/mammalian alveolar absorption | |
| Electronic parameters | Frontier orbital energies (HOMO, LUMO) | Reflects chemical reactivity with nucleophiles and electrophiles, which translates to reactivity with biomolecules in vivo |
| Molecular electronic dipole moments, μ, and dipole polarizabilities, α | Important in determining the energy, geometry, and intermolecular forces of molecules, and are often related to biological activity | |
| Inherent measures of environmental fate | Biodegradation | Indicator of persistence, and persistence is tied to ecotoxicity |
| Bioconcentration factor (BCF) | Bioconcentration enhances the hazard potential of lipophilic chemicals; BCFs provide a comparative basis for assessing the potential for a chemical to have effects that resonate through the food chain. | |
Physicochemical Properties and Environmental Fate: Compartments of Concern
The second step in using physicochemical property data is to determine the environmental compartment(s) into which the chemicals will partition (environmental partitioning). The chemical’s physical state, which can be predicted on the basis of melting point, boiling point, and vapor pressure, can indicate which environmental compartments—air, water, sediment, biota, soil— into which the chemical will partition. Highly volatile chemicals, for example, will escape from soil or water and primarily be present in the air. Conversely, chemicals with a high propensity to sorb onto organic carbon or move into lipid phases are likely to remain in soils or sediments or move into biota, respectively.
Aqueous solubility will provide information about whether a chemical will dissolve in water, a starting point for understanding its fate and transport into the water column or sediment. This understanding is further enhanced by knowledge of phase partition coefficient, logP. In general, chemicals with higher logP values will be more likely to cross into and be retained by biota, although there are significant exceptions to this rule (e.g., large molecules that cannot cross biological membranes). Chemicals with the propensity to environmentally partition into sediments will be more likely to sorb onto soils, so the soil-water phase partition coefficient (Kd) will be informative for both systems. For some chemicals, transformation processes need to be considered because transformed or metabolized products often have different physicochemical properties; thus, they may reside in different environmental compartments.
To obtain a sense of the escape potential for a given chemical, unit world models developed by Mackay and Paterson (1991) for organic chemicals and by Diamond et al. (1990) for metals provide a structural framework for determining potential chemical distribution based on intrinsic properties of fugacity (f), which is an inherent chemical property that governs the relative concentrations of chemicals in different environmental and biotic compartments. These models have been applied to ecological systems by Harvey et al. (2007) and Farley et al. (2011), who also provide examples of how to apply these model concepts to hazard assessments. Their application will be discussed in more detail in the next section.
Physicochemical Properties and Persistence, Bioaccumulation, and Biotransformation
Organisms take up and eliminate chemicals at different rates; when excretion or metabolic detoxification is slower than uptake, the chemical (or chemicals) accumulates in the organism, resulting in prolonged tissue delivery (Luoma and Rainbow 2005). Some chemicals increase in concentration at each level of the food chain; these chemicals are said to biomagnify. Because of the potential of some chemicals to biomagnify and persist in the food web, bioaccumulative substances require special consideration because they may pose a greater hazard than chemicals that are rapidly eliminated and do not accumulate.
Large chemical structural databases combined with recent developments in quantitative structure property relationships have greatly expanded the potential for rapid assessment of chemicals (Howard and Muir 2011). Thus far, efforts using these models have proven successful in identifying potentially persistent, bioaccumulative, and toxic (PBT) chemicals. (For more information about the application of physicochemical data to ecotoxicology, see Chapter 7).
Use of Physicochemical Properties to Predict the Persistence of Organic Chemicals
As defined by Pavan and Worth, “the persistence of a substance is the length of time it remains in a particular environment before it is physically transported to another compartment and/or is chemically or biologically transformed” (Pavan and Worth 2008). Most alternatives assessment frameworks consider persistence because molecules that persist will have increased concentrations, and possibly higher impacts, in environmental compartments and are more likely to bioaccumulate. For some classes of materials, it is possible to obtain useful, predictive information about potential persistence from physicochemical data, such as structural markers on the molecule and partition coefficients. For example, Howard and Muir (2010) screened more than 2,2000 commercial chemicals with in silico and expert judgment approaches and identified physicochemical properties that could be used to classify chemicals as persistent in the atmosphere (atmospheric oxidation half-life > 2 days), or potentially susceptible to long-range transport (logP > -5 and < -1).
The persistence of a chemical in the environment is often measured, or estimated, in
terms of its biodegradation.23 There are numerous modes of degradation that depend on the environmental conditions, types of microbes present, and the structure of the chemical. Degradation is typically quantified based on the extent of removal of dissolved organic compounds within an aqueous medium of a chemical and is expressed as a percentage of degradation in a given time.24
Degradation is usually a complex, multistep process that often produces chemical intermediates. These intermediates may present additional environmental hazards or persist if they are not readily degraded. Some transformations can increase the toxicity of the parent compound (e.g., methylation of mercury; photoinduction of polycyclic aromatic hydrocarbons [PAHs]), whereas other reactions may decrease the toxicity of a chemical. Biodegradation can also change the distribution of components within environmental compartments (e.g., due to ion formation from inorganic chemicals).
Chemical degradation, a subset of biodegradation, may involve a number of chemical reaction steps depending on the environmental conditions and the chemical structure (Khetan and Collins 2007). Chemical degradation processes include hydrolysis, photochemical transformations, and the action of microbial species (Khetan and Collins2007). The modes of degradation depend on the environmental compartment and conditions (pH, UV irradiation, microorganism population, etc). The diversity of conditions and chemical reactivities means that the results of degradation testing are sensitive to the conditions of the test. To complement those tests, researchers have developed some “rules of thumb” (see Box 5-2) to estimate or predict chemical degradation based on chemical functional groups and structure.
In addition, some models and databases have been developed to predict degradation rates (Arnot et al 2005). Examples of these models include:
BOX 5-2
Structural Attributes that Enhance Biodegradation
- Minimal number of halogens (especially F and Cl).
- Minimal chemical branching (especially quaternary C).
- Minimal number of tertiary amine, nitro, nitroso, azo, and arylamino groups.
- Minimal number of polycyclic residues (especially more than three fused rings).
- Presence of esters (including phosphonates).
- Presence of oxygen atoms.
- Presence of short linear alkyl chains (< 4 C) or phenyl rings that can act as sites for oxygenase enzyme activity.
SOURCE: Meylan et al. 2007; Howard and Muir 2013.
- Group contribution models that estimate and predict thermodynamic and other properties from molecular structures; for example, BIOWIN (Boethling et al 2004).
- Expert judgment criteria for biodegradability based upon “rule of thumb” models (Meylan et al. 2007).
- Degradation pathways model, including probabilistic models that calculate the probabilities of the individual transformations; for example, CATABOL (Dimitrov et al. 2007).
Several research challenges remain with respect to obtaining biodegradation data. These challenges include:
- Predicting degradation fragments.
- The need to develop more predictive structure/degradation relationships (SDRs) for parent chemicals and degradates.
- Predicting the rates of degradation for a new or previously unstudied chemical.
There are also a number of other factors that need to be considered when evaluating measured or predicted degradation data of organic chemicals. These include:
- Potential trade-offs between aquatic toxicity and degradation. Improving biodegradation often increases aquatic toxicity and may reduce durability.
- The initial stages of polymer degradation may make components more bioavailable until they are later degraded (Platt 2006). This can be a
_____________
23 Biodegradation is the process by which microbial organisms transform or alter (through metabolic or enzymatic action) the structure of chemicals introduced into the environment (EPA 2012f).
24 In the design of chemicals and components for formulating products, there is a tension between stability and degradation. During use, the chemical is expected to be stable. Upon release into the environment, the chemical should rapidly degrade. Ideally, it should form degradates that do not persist and are less toxic than the parent chemical. This type of strategy is one of the guiding principles of green chemistry (EPA 2014b).
major difference from the degradation of smaller molecules.
Use of Physicochemical Properties to Predict the Bioavailability of Inorganic Chemicals
Characterizing the lifetime of metals in the environment is difficult because the interactions are highly dependent on the characteristics of the environmental system where they are released. Leaching25 and aging26 are related to conditions within the soils and sediments, so predicting environmental hazard based solely on standard aquatic toxicity tests using dissolvable salts is not adequate. Therefore, the field of ecotoxicology is becoming increasingly reliant on sediment and soil toxicity test protocols that include leaching and aging steps or the application of bioavailability models to adjust data acquired under laboratory conditions to realistic conditions in soils, sediments, and water (Santore et al. 2002; Smolders et al. 2009).
Metals newly introduced into soils or sediments are more bioavailable than those that have aged for months to years. Metals are initially leached from soils or sediments, a process that happens relatively quickly (i.e., weeks to months), followed by a slow aging process (i.e., years), which results in decreased toxicity to sediment or soil organisms over time. Therefore, toxicity studies conducted with soil or sediment freshly amended with metal salts will result in effects at much lower concentrations than will be observed in real-world situations (Besser et al. 2011).
Aging occurs due to several different processes, including sorption to aluminum, manganese, or iron oxides and eventual incorporation of the metal ion into the crystalline structure of the mineral soil or sediment particles (Adriano 2001). The rate of chemical sorption to oxides, clays, other minerals, or organic matter is determined by the strength and number of negatively charged binding sites in the soil or sediment particle which, in turn, are influenced by the amount of aluminum, iron, or silicon present. Sorption reactions are reversible and highly dependent on pH, with higher rates of sorption occurring at higher pH, increasing bioavailability as pH decreases (i.e., becomes more acidic). Redox potential influences the bioavailability of cationic metals; highly insoluble sulfides of metals form under reducing conditions, such as those found in saturated soils or anoxic sediments. Therefore, the type of environment to which the metal is introduced also influences the degree of toxicity that might be expected, although this differs by metal. The strength of attraction between metal ions and charged sites is a function of the affinity of the metal to the charged site relative to its affinity for water molecules. Copper generally has the highest rate of sorption, followed in descending order by nickel, cobalt, lead, cadmium, and zinc. This order differs slightly for electrostatic binding to clays and other negatively charged particles, with nickel having the highest binding affinity and lead the lowest.
Binding affinity also influences the toxicity of cationic metals. For example, the gill of aquatic organisms is negatively charged and acts as another binding site for some metal ions (Playle 2004). Toxicity depends on the relative binding strength of the biotic ligand and other negatively charged particles in the water (e.g., organic matter, iron sulfides) and competition for the binding sites by other metals. The biotic ligand model can be used to predict toxicity for a given metal if the concentrations of other major cations are known (DiToro et al. 2001). This model adjusts values from standard toxicity tests to different types of aquatic environments and may affect the relative hazard of the different metals.
Anionic metals and metalloids such as molybdenum, arsenic, mercury, and selenium also bind to iron oxides, but binding decreases with increasing pH, which is opposite to what occurs with cationic metals. Therefore, toxicity of these metals differs substantially from that of the cationic metals in the same environment. Furthermore, methylation of metalloids plays a very important role in increasing their mobility and uptake as well as their ability to biomagnify in the food chain. Well-known examples of this phenomenon is the observation that methylated arsenic is less toxic than its inorganic form, while methylated mercury and organoselenium species are more toxic. Methylation is a biological process that occurs in bacteria, with the initial step occurring in sediments under reducing conditions (low oxygen) and the presence of high organic matter (Jonnalagadda and Rao 1993).
Because plants, invertebrates, and soil microorganisms interact with the soil or sediment pore water, the amount of free metal ions in solution is the most important determinant of toxicity. Plant roots may exude phytochelatins that
_____________
25 Leaching is the process by which soluble materials in the soil, such as salts, are washed into a lower layer of soil or dissolved and carried away by water (USGS 2014).
26 Aging refers to reduced bioavailability over time (Kelsey and Alexander 1997).
bind metals to either facilitate or exclude their uptake, while at the same time reducing pH of the soil or sediment to make nutrients more bioavailable (Pal and Rai 2010).
Cationic metals can occur as various ionic species, some of which are more soluble and therefore more bioavailable than others. For example, chromium is present in solution as Cr+6, which is a highly bioavailable and toxic ion, while it is bound to soils and most sediments as Cr+3, which is less toxic. Similarly, antimony trioxide (SbO3) is highly insoluble, whereas antimony trichloride (SbCl3) is not, which makes the latter less bioavailable. Toxicity studies with the soluble species of a metal, where the free ion is readily available, are useful for predicting effects to aquatic organisms, but generally are of little predictive value for soil or sediment organisms, largely due to the length of time needed for dissolution into the pore water and the confounding factors of pH and salinity from the added chloride (Smolders et al. 2009). In light of this complexity, no one physicochemical property or set of properties is currently adequate to define all toxicity concerns if metals are present in the structure of the compound and have the potential to become freely available during the degradation process. Toxicity testing and evaluation as described in the following chapters should be carried out to identify concerns related to the presence of metals in a compound.
Use of Physicochemical Properties to Predict Bioaccumulation
Bioaccumulation potential (B) is represented in most alternatives assessment schemes by the bioconcentration factor (BCF). The BCF is the ratio of the amount of chemical in an aquatic organism (usually fish) to the amount of chemical in the water under conditions of equilibrium. An alternative approach is to measure the bioaccumulation factor (BAF), which is the ratio of the amount of chemical in the fish to the amount in both food and water, expressed on a molar basis and frequently normalized to lipid content. Standard test protocols for these factors are available, but may be difficult to conduct and interpret due to several factors described in the literature (Fraunhofer Institute 2007).
LogP is a good surrogate for determining the extent to which a chemical would thermodynamically distribute between the lipids of biological organisms and water. In general, very lipophilic substances (ones with logP > 5) have the greatest potential to bioaccumulate. However, lipophilicity also affects whether a chemical will be taken up by the organism (i.e., its bioavailability). For example, chemicals with logP > 5 are primarily taken up from the diet, and the BAF is higher than the BCF. Chemicals with logP < 5 are primarily absorbed from the water, and the BCF and BAF are equal (Mackay et al. 2013). Aquatic organisms may need to be exposed to chemically treated water for 60 days or more before reaching chemical equilibrium. This is true for chemicals with slow excretion or metabolism rates, during which time dilution by growth generally occurs. While the BAF provides a more realistic measure of exposure to hydrophobic chemicals, additional uncertainty is introduced because the BAF includes partitioning of the chemical between water and food and simplifying assumptions about dietary preferences (Mackay et al. 2013). However, given that standard protocols have been developed to provide guidance for conducting BAF tests to enable comparability among chemicals, these data should be given preference over BCF values for estimating bioaccumulation potential in hazard classification and ranking. Note that for regulatory schemes where hazard classification is required (such as REACH or the GHS for transportation labeling), binning chemicals by whether they are non-accumulative (BCF < 2,000), somewhat accumulative (2,000 < BCF < 5,000) or very bioaccumulative (BCF > 5,000) is sufficient.
In the absence of measured BAF, it is theoretically possible to calculate the BAF from a measured BCF. Bioaccumulation for fish (BAFF) is the bioconcentration factor based on freely dissolved chemical concentration (BCFD) for its food items (phytoplankton such as algae) times the ratio of the uptake rate from the diet (KD) and the uptake rate from the water via respiration (KR). This is expressed as BAFF = (1+ KD/ KR) X BCFD. Furthermore, the tendency for a chemical to biomagnify can be quantified by the ratio of two trophic levels (BAF2/BAF1), with BAF2 being a higher trophic level than BAF1. However, because the diet for higher trophic-level species includes species that have a BAFF, calculating the BCFD can become quite complex.
A recent article addresses the question of whether BCF or BAF should be used to predict bioaccumulation potential. It concludes that for BCF and BAF values predicted by the EPA’s EPI SUITE software, both BCF and BAF values provide comparable information (Costanza et al. 2012). The threshold values proposed by Costanza and coworkers (2012) are as follows:
- Not significantly bioaccumulative: BCF or BAF < 1,000.
- Bioaccumulative: BCF or BAF 1,000 and < 5,000.
- Highly bioaccumulative: BCF or BAF > 5,000.
Another approach for comparing bioaccumulation potential between chemicals is the use of chemical fugacity. Fugacity (f), expressed as Pascals (Pa), is an inherent chemical property that governs the relative concentrations of chemicals in different environmental and biotic compartments. Each type of media (air, water, lipid, biota) has an inherent fugacity capacity (Z) that defines the amount of a chemical fugacity that can be retained within that material, where Z is expressed as (mol/m3 x Pa). Therefore, the ability of a chemical to bioaccumulate in any organism is a function of its chemical fugacity and the fugacity capacity of that organism for that chemical. Fugacity ratios between biota and their environment can be compared among chemicals to determine which chemical is most likely to bioaccumulate, or comparisons can be made between trophic levels to determine biomagnification potential (Burkhard et al. 2012; Mackay et al. 2013). Because fugacity capacity is a function of the Henry’s Law constant and the logP for each chemical, these properties can be used to rank chemicals on their potential to bioaccumulate. The fugacity capacity for water, Zwater, is equal to 1/H, where H is the Henry’s Law constant for the target chemical. Therefore, in a closed system, a chemical with a smaller Henry’s Law constant will partition to a greater extent in water than one with a larger Henry’s Law constant. For an organism, fugacity capacity is equivalent to Zwater times the P and percent lipid. Therefore, a chemical with a small Henry’s Law constant and high logP will be most likely to bioaccumulate, and a comparative ranking scheme can be developed based on the ratio of these two parameters.
Current hazard classification and ranking schemes use BCF and BAF for aquatic organisms. Questions remain about whether aquatic BCF and BAF values are predictive for terrestrial organisms, where uptake into the food chain begins with movement of chemicals from soils into plants. Plant uptake of chemicals is highly related to soil sorptive properties of the chemical, solubility into soil pore water, and active uptake by plants. Terrestrial animals have different amounts of lipids than fish, making it questionable to directly extrapolate fish BAF values to birds and mammals. The relative fugacity approach described above, however, is equally applicable to terrestrial and aquatic systems, so it may form the basis of an approach for determining bioaccumulation (and biomagnification) potential in terrestrial systems.
Use of Physicochemical Properties to Predict Aquatic Bioavailability and Toxicity
Bioavailability is a measure of the amount of a chemical and the rate at which it crosses a barrier of the external environment and enters an organism’s circulation. From there, the chemical can reach tissues in living systems and interact with cellular macromolecules. Adapted from the study of metals in the environment, chemical bioaccessibility, or environmental availability, can be defined as the amount of a chemical “in soil, sediment, water, or air that is available for physical, chemical, and biological modifying influences (e.g., fate, transport, and bioaccumulation)” (McGeer et al. 2004). For a chemical to exert a toxic effect, it typically must be bioavailable at a level that allows the chemical (or its metabolite) to reach a biochemical target, where it can exert its toxicologic effect. Blocking or reducing bioavailability is one potential means for reducing the intrinsic toxicity of a chemical (Voutchkova et al. 2010). While the lack of bioavailability is an indicator that the compound is likely to have low toxicity, high bioavailability does not suggest the compound is necessarily highly toxic.
Aquatic bioavailability: The scientific literature characterizes trends that allow comparative assessment of bioavailability in different species and through different routes of exposure. For example, in aquatic species, it is known that bioavailability is positively correlated with logP of the chemical, although the linearity of this relationship is not clearly defined (Pärt 1989). It is also known that aqueous solubility, molecular size, and ionization state also influence bioavailability. The logP at environmental or biological pH (i.e., logD) has been proposed as a measure that correlates with partitioning and ionization. The Biotic Ligand Model (Janssen et al. 2003) is useful when considering metal bioavailability to aquatic species as it relates competitive metal binding to ecotoxicological effects (Tessier and Turner 1996).
Aquatic toxicity. An example of how physicochemical properties can be directly used to estimate an ecotoxicological end point is acute aquatic toxicity. The physicochemical property limits listed in Table 5-4 are known to favor reduced acute and/or chronic aquatic toxicity. Meeting two or three property limits has been shown to substantially increase the probability that a chemical will have low
TABLE 5-4 Changes in Physicochemical Properties to Favor Reduced Aquatic Toxicity
| Physicochemical Property | Changes |
| Molecular size and weight | Generally, as molecular weight increases, aquatic bioavailability and toxicity decrease. At MW > 1000 amu, bioavailability is negligible. Caution must be taken, however, to consider possible breakdown products that may have MW < 1000 amu and exert toxicity. |
| Octanol-water partition Coefficient (logP) and octanol-water distribution coefficient at biological pH (logD7.4) | logP usually correlates exponentially with acute aquatic toxicity by narcosis for non-ionic organic chemicals up to a value of about 5-7. Chemicals with logP < 2 have a higher probability of having low acute and chronic aquatic toxicity (Voutchkova et al. 2011). For ionizable organic chemicals, logD7.4 is a more appropriate measure: ionizable compounds with logD7.4 < 1.7 have been shown to have increased probability of being safe to freshwater fish than those with logD7.4 > 1.7 (Kostal et al. in press). |
| Water solubility | Generally, compounds with higher logP have lower water solubility. Very poorly water-soluble chemicals (<1 ppb) generally have low bioavailability and are less toxic. |
| ∆E energy [HOMO-LUMO] | The ∆E reflects broad chemical reactivity. It was recently reported that chemicals with ∆E > 9 eV (as calculated by semi-empirical methods) or > 6.5 eV (as calculated by DFT) are much less likely to be acutely or chronically toxic to aquatic species (Voutchkova-Kostal et al. 2012; Kostal et al. in press). |
or no aquatic toxicity. This is one example of the use of global reactivity parameters to assess fundamental chemical reactivity that relates to biological activity, but other approaches may exist.
Use of Physicochemical Properties to Estimate Mammalian/Human Toxicokinetics
In addition to the use of physicochemical data to predict aquatic toxicity, these properties can also be used to estimate the toxicity of a given chemical in humans and other animals as they influence toxicokinetic and toxicodynamic parameters.27 While the toxicodynamic interactions of chemicals are very challenging to relate to specific physicochemical properties, the influence of such properties on toxicokinetic behavior of chemicals can be more readily defined and used to prioritize the human health assessment of chemical alternatives.
The key toxicokinetic processes are absorption, distribution, metabolism, and excretion. The focus here is on the influence of physicochemical properties on the rate of absorption of a chemical into the bloodstream, its distribution to the organs and tissues, and its rate of elimination (clearance) of a compound.
The most prominent properties that have been shown to impact chemical toxicokinetics include:
- molecular size and shape,
- lipophilicity and hydrophobicity,
- ionization potential or pKa, and
- hydrogen bonding.
Physicochemical Properties That Influence Bioavailability in Humans
Chemicals that are highly bioavailable to mammals through particular exposure routes have also been defined by a set of property limits. These property limits were originally defined to assess the probability of drug candidates entering the human body, and are therefore highly dependent on the expected route of exposure. The property limits
_____________
27 Note that “pharmacokinetic” and “pharmacodynamic” are often used interchangeably with the terms “toxicokinetic” and “toxicodynamic.”
| Exposure Route | Physicochemical Property | Property Limit |
| Ocular | Water solubility | Variable |
| Molecular size | < 500 Da (corneal epithelium) | |
| < 10000 Da (conjunctival epithelium) | ||
| Vapor pressure | < 0.0001 mm Hg | |
| Oral | Molecular size | < 500 Da |
| LogP | 0-5 | |
| Non-ionized at GI tract pH | ----- | |
| Respiratory (Lungs) | Particle size | < 5 µm |
| Molecular size | < 400 Da | |
| Vapor pressure | < 0.0001 mm Hg | |
| Dermal | Molecular size | < 400 Da |
| LogP | 0-6 | |
| Presence of solvents | ----- | |
| Ionization (polar, ionized) | ----- | |
associated with increased bioavailability through the four main routes of exposure have been discussed in detail in the medicinal chemistry literature, and review articles are available (DeVito, and Garrett 1996; Voutchkova et al. 2010). These property limits are provided in Table 5-5 and are further discussed in Chapter 8. The inverse of these property limits is likely to increase the probability of minimal human bioavailability, but concrete studies to support this assertion are still lacking.
Ocular bioavailability: The topical delivery of pharmaceuticals for the treatment of the anterior segment of the eye (i.e., cornea, conjunctiva, sclera, anterior uvea), where the bulk of the research in this area has been done, has proven challenging, largely due to the complex structure and variety of clearance pathways and barriers that can reduce absorption and remove xenobiotics from the eye. For example, the flow of lacrimal fluid quickly removes most instilled compounds from the surface of the eye.
Mechanism of delivery and exposure most relevant for the consideration of chemical alternatives is that of direct absorption through the cornea or through systemic exposure; this is reflected in the values presented in Table 5-5. The vapor pressure of the material reflects the potential for gas-phase exposure to the compound. The importance of molecular size reflects the paracellular pore sizes in the corneal and conjunctival epitheliums, and lipophilicity appears to affect the route of entry into the body, whether through the cornea (reduced absorption of compounds with high lipophilicity) or the conjunctiva (where lipophilicity appears to play no role in absorption).
Oral bioavailability: As defined by Varma et al., “Oral bioavailability (F) is a product of fraction absorbed (Fa), fraction escaping gut-wall elimination (Fg), and the fraction escaping hepatic elimination (Fh)” (Varma et al. 2010). The property limits for oral bioavailability are well characterized. Lipinski identified four physicochemical properties that govern optimal oral absorption: molecular weight (MW) < 500 amu; octanol/water partition coefficient (logP) < 5; number of hydrogen bond donor atoms (HBD) < 5; and the number of hydrogen bond
acceptor atoms (HBA) < 10 (Lipinski et al. 1997). Although there are numerous exceptions (Ganesan 2008), chemicals are generally less likely to have good oral absorption if they violate two or more of these physicochemical “rules.”
Varma and coworkers (2010) also evaluated the physicochemical space for optimum human oral bioavailability. They showed that molecular weight, ionization state, lipophilicity, polar descriptors, and free rotatable bonds (RB) influenced oral bioavailability, stating that:
These trends were due to a combination of effects of the properties on Fa and first-pass elimination (Fg and Fh). Higher [molecular weight] significantly impacted Fa, while Fg and Fh decreased with increasing lipophilicity. Parabolic trends were observed for bioavailability with polar descriptors. Interestingly, RB has a negative effect on all three parameters, leading to its pronounced effect on bioavailability (Varma et al., 2010).
Dermal bioavailability: Dermal or topical absorption predictive models have been in existence since the early 1990s, when Potts and Guy (1992) published a simple model that showed a relationship between the molecular volume or molecular weight and the lipophilicity of a chemical and its ability to permeate the skin. Although many other models have been proposed and published, most rely on related properties to determine the skin permeation rate. A framework incorporating the impact of exposure scenarios and application conditions on risk assessment of chemicals applied to skin is described in a number of key references (Ibrahim et al. 2012).
Respiratory bioavailability: Nasal uptake and regional deposition are influenced by the physical and chemical properties of the inhaled material, including water solubility, reactivity, and airborne concentration (Morgan and Monticello 1990). The pharmacokinetics of inhaled particles is also dependent upon physicochemical properties of the particles, including aerodynamic diameter (size) and solubility (Kreyling et al. 2013). The size of the particle will influence where it deposits within the respiratory tract; for example, particles under 1µm penetrate to the alveoli and over 30 µm rarely progress farther than the upper respiratory tract.28 Knowledge of the particle size distribution of any powder, mist, aerosol, or other similar material is important for identifying hazards that should be eliminated or managed through the use of appropriate engineering, procedural, and personal protective equipment control at the sites of manufacture and use.
In addition to size, other physical and chemical properties can also influence transpulmonary transport (Holder 2012; Ibrahim and Garcia-Contreras 2013). These include molecular weight, melting point, boiling point, vapor point, molecular polarity, Henry’s phase distribution, and the extrinsic properties of pressure (P) and moles (n). Localized tissue responses and respiratory tract absorption of deposited metals are also highly dependent upon chemical solubility, particle size, and surface area, which contribute to metal release from the inhaled particle (Kang et al. 2011; Oberdorster 1996).
Physicochemical Properties that Influence Distribution in Living Organisms
Volume of distribution (Vd): One important estimate of a compound’s distribution that has been demonstrated to have a link to toxicity in animals is the volume of distribution, Vd. If a quantity of compound is introduced into the body, some amount will enter into the bloodstream and some will undergo different processes that remove it from the bloodstream, such as uptake by tissues and elimination from the body. Vd is defined as the theoretical volume of blood plasma required to achieve that concentration if no removal processes were occurring. If Vd is roughly equivalent to the total blood volume of the organism or individual, then no uptake is occurring. If Vd is higher than the total blood volume of the organism, then it indicates that some amount of compound has been lost from the bloodstream by those processes. The higher the Vd, the greater the distribution of the compound thoughout the body is likely to be. Those drugs that are lipophilic at pH 7.4 are likely to have higher values of Vd than those that are ionized or those that have a high affinity for plasma binding protein. The Vd directly influences the half-life of a compound, whereby large Vd leads to a longer half-life; that is, it prolongs the duration of exposure. The Vd has also been shown to influence the lowest observable adverse effect level (LOAEL). In rodent studies, a larger value for Vd generally results in a lower LOAEL (Sutherland et al. 2012).
Plasma protein binding (PPB): In general, xenobiotics within in vivo systems are either (i)
_____________
28 Note that larger particles may not be inhaled, but upon deposition in the nose, mouth, and throat may still enter the body by mucociliary clearance and ingestion.
bound to proteins and lipids in plasma (more commonly referred to as plasma protein binding [PPB]), (ii) bound to proteins and lipids in tissues, or (iii) unbound and free to diffuse among the aqueous environment of the blood and tissues (Smith et al. 2010). PPB strongly influences Vd and the half-life of chemicals in the body (Hollósy et al. 2006) because it is typically the unbound fraction of xenobiotics that interacts with protein receptors, forms DNA adducts, or interferes with a biological system in other ways to produce either a pharmacologic or toxicologic effect. Studies have shown that chemicals that interact with a protein receptor (e.g., the estrogen receptor) and are also highly bound to plasma proteins, will generally require higher doses to achieve the required free concentrations to elicit an equivalent response to a chemical that has a lower PPB level, provided the rate and fraction absorbed for both are equivalent. Physicochemical properties that influence PPB include lipophilicity, as measured by logP, and pKa. In general, chemicals with high lipophilicity and/or ones with acidic character will have a smaller unbound fraction, and thus a greater degree of PPB, than more hydrophilic or basic compounds (Vallianatou et al. 2013).
Physicochemical Properties that Influence Elimination/Clearance in Living Organisms
Clearance (CL) describes the rate of elimination of a given chemical to its concentration in plasma and is expressed as volume of distribution cleared per unit time. Total clearance describes the elimination of a chemical from the body without identifying the mechanisms involved (e.g., metabolism, urinary or biliary excretion, etc), but most chemicals are eliminated primarily via the liver and/or kidney.
Clearance is one of the most important pharmacokinetic parameters. It is affected significantly by the PPB of the chemical, because only the free fraction can be cleared. The clearance of the unbound chemical, CLu, is independent of the PPB. Thus, CLu only depends on chemical structure and physicochemical properties. For example, the rate of clearance is heavily dependent on the distribution coefficient of the chemical at biological pH (7.4), expressed as log D7.4 (Zhivkova and Doytchinova 2013).
In sum, examining physicochemical properties can be used to help screen chemicals for their potential to induce human toxicity. For example, the lack of bioavailability and high clearance often indicate that the compound is likely to have low mammalian toxicity. However, high bioavailability and low clearance do not necessarily indicate that the compound is highly toxic. More retrospective and prospective analyses are needed to inform decisions about the use of materials that pose environmental risks. In some cases, development of specialized analytic methodology will be required. Continued assessment of known hazardous compounds will be important. For the present and for the immediate future, decisions will have to be made on the basis of limited data and information.
IMPLEMENTATION OF STEP 5 IN THE COMMITTEE’S FRAMEWORK
The implementation of Step 5 requires a comparative approach to the evaluation of the chemical of concern and its alternatives. In essence, information concerning the chemical of interest serves as a “baseline” for all subsequent comparisons. Completion of this step requires several broad activities, including:
- a. Identification of the chemical of interest, chemical alternatives, and their most likely degradates or metabolites. Whenever possible, primary data about the identity and structure of the degradates and metabolites should be used. A variety of software tools can also be used to predict degradate and metabolite structures. Chemical identity includes the chemical name, chemical formula and structure, and whenever possible, the Chemical Abstracts Service (CAS) registry number.
- b. Compilation of the minimal data set described in Table 5-3. Data should be compiled for the chemical of interest (serves as the baseline for subsequent comparisons), chemical alternatives, and their most likely degradates or metabolites. Physicochemical data to be collected and analyzed can be either measured or estimated values. Missing data should be clearly identified as such. All data sources, including software programs used to estimate physicochemical parameters, should be documented, and judicious awareness of the applicability domain of the estimation tool(s) should be used. The completed data set should be represented in a tabular or graphical display.
- c. The compiled data should be categorized in such a way to determine the relative difference (such as high, medium, or low) between the physicochemical property of a chemical alternative and the chemical of interest. Widely accepted categorization tools like GHS available
- for some physicochemical properties (e.g., flammability, corrosivity, oxidizing ability) should be used. The committee also provided categorization systems used by other frameworks for logP, vapor pressure, and several other physicochemical properties of interest. In some cases (e.g., aquatic solubility), the comparison of a physicochemical property is intended to identify potential differences in the environmental compartment(s) into which the chemical or material will partition. As a minimum, the identity of the environmental compartments of concern should be documented. In other cases, secondary end points (e.g., bioconcentration factor, or BCF) could be estimated from the physicochemical property data. Categorization schemes for BCF are also available in other frameworks and tools, such as GreenScreen®, and could be used with the committee’s framework.
- d. Some physicochemical data can be used to estimate likely route(s) of mammalian exposure and bioavailability, as well the likelihood for high aquatic toxicity. Information gleaned for physicochemical properties should be made available to members of the assessment team performing Step 6 (comparative exposure assessment, ecotoxicity hazard assessment, and human health hazard assessment).
- e. Compilation of physicochemical property data may require an iterative approach. For example, the evaluation of degradates and metabolites may occur at later stages of the alternatives assessment process. Staging of effort may increase efficiency when a large number of alternatives are initially identified. In this case, some alternatives may be removed from consideration because of other factors (e.g., inherent toxicity).
- f. It is not typically anticipated that a compound will be eliminated from consideration based on physicochemical properties alone. The exception to this would likely occur in the case where property data for a chemical reveal a high risk of physical hazards, such as flammability and explosibility, especially when these are not desirable properties of the alternative. Elimination of chemicals with undesirable physical hazards may be particularly critical if the consumer will be directly exposed to the chemical in question (as opposed to an intermediate in a production/synthesis process, which is only handled under controlled conditions).