The Shale Gas Revolution: A Methane-to-Organic Chemicals Renaissance?
ERIC E. STANGLAND
Dow Chemical Company
The increasing availability of domestic shale oil and gas has resulted in a return to profitability for the US chemical industry, spurring 148 projects and $100 billion dollars in new capital investment over the next 10 years (ACC 2014).
A significant feature of major US shale gas plays (e.g., Bakken and Eagle Ford) is that they have large relative quantities of condensate or wet natural gas that contain ethane and propane fractions from which ethylene and propylene, the primary olefin feedstocks of the modern organic chemical industry, are derived. Ethylene, increasingly derived in the United States from the steam cracking of ethane (SCE), is the dominant organic chemical in the world, with a world production capacity of 123,000 kilotonnes per year (kta). The United States produces 24,000 kta of ethylene with a 10,000 kta increase in capacity expected over the next 10 years—a nearly 50 percent increase (Devanney 2011).
The US-produced ethane increase since 2007 has lowered the relative price such that ethane is now trading at fuel (methane) value (Figure 1). The net effect is an increase in relative profits for chemical producers after conversion of ethane to ethylene and then to polymers and derivatives. Moreover, the relative pricing of US ethane to global naphtha, which trades at the price of oil, is driving both the US competitive advantage for chemical production investment and the continuing US trend toward cracking a lighter feedstock to produce US ethylene supply. Additional US investment is garnered from announced capital and processes to directly address the decreasing amounts of heavier industry feedstocks such as propylene and C4 that result from decreased naphtha cracking. The continued viability of these US chemical industry trends is based on the future pricing of ethane relative to other potential fungible feedstocks (such as naphtha) and is a complex function of production and global import/export dynamics.
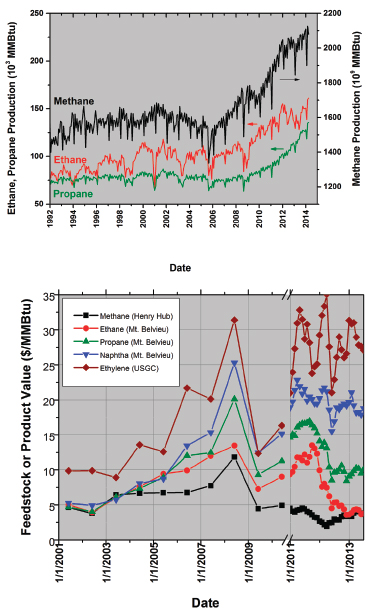
FIGURE 1 (a) Production levels of dry methane to ethane and propane from US gas wells. (b) Value of various potential fuels relative to ethylene on the US Gulf Coast (USGC). Data before the plot break (symbol and line) are plotted as the June average value for that year, whereas data after the plot break are plotted weekly. Data sources: EIA 2014a-d, ICIS Pricing Report 2014a,b.
The chemical industry upgrades potential fuels such as ethane to ethylene and derivatives. Although wet shale sources rich in ethane are providing this US boom, Figure 1a also shows that the greatest source of natural gas is dry gas, or methane. Most methane is used as a fuel for heating value or for electricity generation (Figure 2, inset). The chemical industry derives some chemical value from methane, in the form of both ammonia and methanol, but mostly it uses methane as a fuel.
Methane has long held unrealized feedstock potential for organic chemical producers because it has typically traded below the cost of many potential feedstocks. The increasing availability of domestic methane will inevitably again raise questions about the viability of producing higher-value ethylene and propylene derivatives from this abundant natural gas resource. But the direct use of methane as a feedstock for derivatives remains an economically tantalizing and elusive challenge. To date, and not for lack of effort, no process that directly uses methane to produce olefins operates economically in the United States. After decades of research, a burning question remains for the chemical industry: Is methane a fuel or feedstock?
ETHYLENE PRODUCTION
The desired use of methane as an organic chemical feedstock unfortunately converges with certain technological and economic realities in how the world produces ethylene. The incumbent technology for ethylene production is the hydrocarbon steam cracker. In the United States this is increasingly becoming the ethane steam cracker, a mature and successful technology that fundamentally consists of two parts: a reaction plant and a separation plant (Cesar 2003; Sundaram et al. 2000; van Goethem 2006; Zimmermann and Walzl 2000).
The reaction plant is a natural gas (methane)–fired furnace in which steam and ethane react inside high-alloy metal tubes at residence times of less than 1 second to produce a mixture of unreacted ethane, ethylene, propane, propylene, hydrogen, methane, and a small amount of heavier hydrocarbons. This cracked gas mixture is water-quenched and treated to remove impurities such as CO2 and H2S as well as alkynes (e.g., acetylene), which are hydrogenated before downstream separations. The separation plant uses high-pressure steam generated during energy cross exchange from the cracker furnace effluent to drive compression turbines, liquefying the cracking products for separation by a series of cryogenic distillations of component pairs (olefin-paraffin) that have very similar relative volatilities.
The steam cracker is thus akin to a small power generation plant where fuel (methane) is used to generate electricity (ethylene). This technology is practiced at tremendous scales in a single plant, with single train capacities approaching 1,500 kta of ethylene, or around 175,000 kilograms of olefins processed every hour. The size of these complexes is growing as ethylene producers seek to capitalize on SCE production scaling laws that are less than unity, extracting maximum profitability for capital invested.
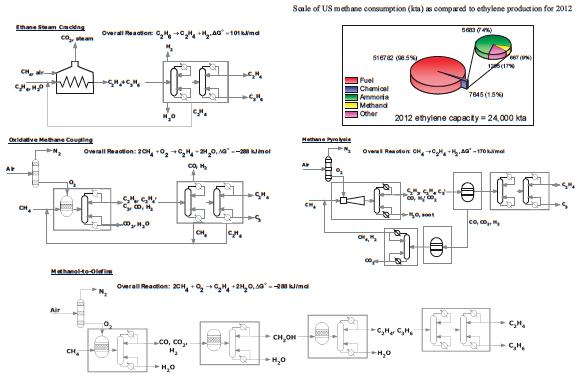
FIGURE 2 Simplified flow sheets showing an ethane steam cracker and some known processes that could produce ethylene from methane resources. Inset pie charts give additional information on the size of methane-to-chemical streams as compared to the fuels market. Labeled major reactor and separation process sections, when considering capital expenditure, are grouped into boxes, without implied meaning as to the actual complexity or real number of units in each section. Source inset data: Devanney 2013.
The use of methane as a feedstock for ethylene, or the displacement of any part of conventional technology, will require market risk that will be justifiable only if the capital and variable cost intensity of any conceived methane process is significantly lower than conventional technology. If there is no feedstock variable cost advantage, as is now the case with methane and ethane trading at parity, then any US-built methane-to-ethylene process, with or without required oxidants, will likely require a huge capital reduction relative to the SCE process for legitimate attention (Lange 2005).
METHANE-TO-ETHYLENE
The chemical industry makes a significant amount of non-polymeric chemicals from methane, including refinery hydrogen, ammonia, methanol, and liquids (fuels) via Fischer-Tropsch synthesis. These chemicals share a common derivation from synthesis gas (CO, H2) that is readily made from the partial oxidation of methane. Methane-to-ethylene routes have been envisioned and investigated at varied scale, but they have been historically disadvantaged by fixed capital and/or variable costs in geographies with direct access to sufficient ethane.
The utilization of a methane derivative, methanol, to produce olefins via the methanol-to-olefins (MTO) process is taking root in ethane-poor China, where regionally advantaged cheap and abundant coal resources outweigh the increased process complexity of MTO relative to SCE. MTO also has an advantage in potential methane-to-ethylene processes because its methanol feedstock is a world-fungible commodity that can be decoupled from olefin synthesis, reducing risk to producers interested only in olefins and derivatives. The technology, in effect, cheats the direct methane-to-ethylene challenge by first forming in succession the metastable products synthesis gas and methanol. The need for increased capital for MTO relative to SCE is one penalty for the cheating.
In contrast to MTO, direct methane conversion technologies, such as oxidative coupling of methane (OCM) or methane pyrolysis (MP), suffer from product selectivity losses as the conversion increases. Process flow sheets for these processes are shown in Figure 2. In OCM, the product ethane has C-H bonds more reactive to oxygen than those of methane, decreasing useful selectivity as conversion is increased to economic levels. The resulting CO2 must be rejected as lost carbon. In MP, the high temperatures necessary to overcome the free-energy hurdle in reaction are favorable for carbon-carbon bond scission, resulting in the formation of soot instead of the desired acetylene, ethylene, and H2. Selectivity loss can be partially overcome with unique burner design, using substoichiometric amounts of oxygen to provide enthalpy while preserving more carbon product, but significant CO2 rejection is unavoidable. Despite these challenges, both OCM and MP are possible at large scale.
With generic polyethylene trading at an equivalent of $42/MMBtu (ICIS Pricing Report 2014c)—more than 8 times the value of methane—why is SCE still
the preferred method for the manufacture of ethylene in most geographies? The most important answer to this question is evident from Figure 3 when considering relative capital intensity (shown in the size of the circles). Even after considering that methane is required to fuel the furnaces responsible for the endothermic ethane-to-ethylene chemistry, the steam cracker and its separation train have been remarkably energy integrated over the technology lifetime, resulting in an ethane use efficiency of greater than 85 percent and a total carbon efficiency (methane + ethane) from the plant of nearly 60 percent. Favorable SCE capacity-scaling laws per unit product give the SCE complex a total fixed capital and variable cost advantages relative to MTO, OCM, and MP in world geographies with access to cheap methane and ethane. Processes with higher capital costs for equivalently sized methane-to-ethylene plants cannot economically compete at the current valuation of ethylene derivatives in the marketplace.
Notwithstanding the success of SCE, Figure 3 also suggests that the most significant potential liability of SCE in a carbon-tax world is its overall thermodynamic efficiency of less than 20 percent—significantly less than the 50–60 percent efficiency at which combined-cycle power plants can generate electricity from natural gas. The equivalent retail value of electricity ($29/MMBtu) (EIA 2014b) relative to generic polyethylene ($42/MMBtu) shows the value society places on ethylene derivatives. Chemical producers are willing, and able, to trade lower energy efficiency, and higher greenhouse gas emissions (primarily CO2), to deliver this product to market.
For chemical industry engineers, efforts to increase the sustainability of chemicals and chemical processes are at the forefront of many modern challenges. In the chemical industry one could define sustainability as the selection of chemical feedstocks to ensure that derived chemical processes are the most efficient from both 1st and 2nd thermodynamic law perspectives as well as from other socioeconomic factors such as the ultimate cost of those products (Banholzer and Jones 2013; IEA 2013). For most historic and US Gulf Coast chemical production, what SCE lacks in energy efficiency is compensated for by the lower risk of return on borrowed capital for plant construction and depreciation.
Figure 3, however, does provide an answer to the question: Is methane fuel or feedstock? There is obvious value in methane as a sustainable feedstock, not just a fuel. Both MTO and OCM have the potential to be more thermodynamically and carbon efficient than SCE, whereas envisioned methane pyrolysis processes fall short. The overall reactions for SCE and MP are strongly endothermic, and methane must be burned to provide energy for these plants, whereas the overall exothermic reactions for MTO, and particularly OCM, take advantage of the naturally higher energy density of methane itself to drive the relevant reactions in one vessel, albeit with the help of an oxidant. At the current valuation of ethylene relative to methane fuel, the choice of oxidants appears limited to oxygen (Lange 2005).
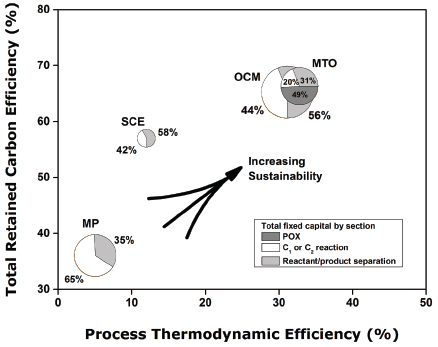
FIGURE 3 Comparison of methane-to-ethylene processes relative to ethane steam cracking (SCE) in terms of total carbon efficiency (including methane fuel usage) and 2nd law thermodynamic process efficiency. Process thermodynamic efficiency was calculated by using the ratio of the estimated process Gibbs free energy change relative to the change for primary methane reaction and separation of pure reactants and products at 298 K. The size of each pie is proportional to the total fixed capital for each process. The pie is divided by the percentage of fixed capital in the partial oxidation (POX), C1 or C2 reaction, and separation sections. Economics and thermodynamics have been derived from the relevant SRI or Nexant process economic reports, scaled to 1,000 kta (kilotonnes per year) olefin capacity. For the methanol-to-olefins (MTO) case, the scaling basis was 66–33% ethylene and propylene mix at 1,000 kta and for oxidative coupling of methane (OCM) it was 86–14% ethylene and propylene mix at 1,000 kta. In all processes, steam was rejected at 413 K for thermodynamic analysis. MP=methane pyrolysis; SCE=steam cracking of ethane. Data sources: Cesar (2003); IHS (1994); Nexant (2009); Wan (2007).
ENGINEERING CHALLENGES FOR SUSTAINABLE METHANE-TO-ETHYLENE
What challenges, if solved, would allow for increased monetization of US natural methane resources to higher derivatives? First, overall methane-to-ethylene capital must be reduced. The reasons for the higher cost are evident in the tradeoffs for each chemistry: the multiple world-scale unit complexity of MTO, the low per-pass methane conversion with large CO2-scrubbing units of OCM, and the multiple reactor capital units in MP of pyrolysis, acetylene hydrogenation, and COx hydrogenation needed to boost carbon selectivity. However, all of these processes share a common thread with SCE. The capital distributions shown in Figure 3 suggest that nearly 50 percent of the total fixed capital resources are tied to the separation and purification of ethylene, not the reaction step. While reaction section capital improvements in the form of catalysts or novel reactor design may reduce reaction capital, one cannot significantly reduce overall methane-to-ethylene capital without holistically addressing both the reaction and separation section.
The primary method of ethylene purification in all cases is cryogenic distillation. The low relative volatility difference between olefin and paraffin, and inherent low boiling point of C1 and C2 hydrocarbons, make compression and distillation a significant cost contributor to any methane-ethane conversion process that does not have 100 percent olefin selectivity. The ability of distillation to scale relatively economically, despite its high energy requirement for separation, makes it the industry separation method of choice (Neelis et al. 2008).
Replacement of distillation by lower capital and energy use options is the second challenge. The incumbency of distillation for ethylene production demonstrates the technical and economic deficiencies of potentially less energy-intensive technologies employing mass-separating agents or membranes to perform the needed separations at scale. Figure 4 makes clear that heat transfer loss and separations require the majority of energy for these processes. It is also well known that capital intensity scales with increasing needs of heat transfer duties (Lange 2001). In addition, Figure 4 shows that, on a relative basis, the operators and innovators of SCE processes have made energy integration a priority, and the energy use of SCE is low relative to alternatives despite the fact that 80 percent of the heat generated in the SCE process must be used for heat transfer and separation even after discounting for reaction enthalpy. This SCE efficiency complicates the replacement challenge.
CONCLUSION
Significant reinvestment in the US chemical industry has resulted from the influx of shale gas ethane into the marketplace. Industry is currently employing mature steam cracking technology to increase production of polyethylene to
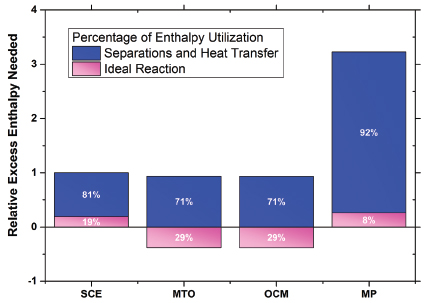
FIGURE 4 Relative energy use for the separations and reaction sections of methane-to-ethylene processes as compared to steam cracking of ethane (SCE), which is set at a value of 1 for this plot. Typical SCE energy use for this analysis was 14,440 Btu/lb ethylene. A positive excess enthalpy value represents specific heat needed in addition to any that can be recovered from any primary exothermic chemistry (a negative relative value) in the cases of oxidative coupling of methane (OCM) and methanol-to-olefins (MTO). MP=methane pyrolysis. Data sources: Cesar (2003); HIS (1994); Nexant (2009); Wan (2007).
monetize these resources. The unavoidable larger fraction of concomitant methane that accompanies shale gas liquids once again raises old questions regarding the economic viability of methane-to-ethylene transformation processes relative to methane’s use solely as a fuel. Despite potential thermodynamic advantages for use of methane as a polyolefin source, all known methane-to-ethylene processes suffer from significantly higher capital intensities relative to incumbent steam cracking technology, discouraging domestic adoption. Solutions to the elusive challenge of a methane-to-ethylene process will require innovations from practitioners in multiple disciplines: chemists and chemical engineers for design of new catalysts and integrated chemical processes, materials engineers for development of new separation materials, computer and information engineers for new ways to control complex chemical processes, and executive entrepreneurs who are willing to be the first to take on the risk.
REFERENCES
ACC [American Chemistry Council]. 2014. US Chemical Investment Linked to Shale Gas Reaches $100 Billion. Washington. Available at www.americanchemistry.com/Media/PressReleasesTranscripts/ACC-news-releases/US-Chemical-Investment-Linked-to-Shale-Gas-Reaches-100-Billion.html.
Banholzer WF, Jones ME. 2013. Chemical engineers must focus on practical solutions. AIChE Journal 59(8):2708–2720.
Cesar MA. 2003. Process Economics Program, Steam Cracking for Olefins Production. Englewood, CO: SRI Consulting.
Devanney MT. 2011. CEH Marketing Research Report: Ethylene. Chemical Engineering Handbook. Englewood, CO: SRI Consulting.
Devanney MT. 2013. CEH Marketing Research Report: Natural Gas. Chemical Engineering Handbook. Englewood, CO: SRI Consulting.
EIA [US Energy Information Administration]. 2014a. US Natural Gas Monthly Supply and Disposition Balance. Washington. Available at www.eia.gov/naturalgas/.
EIA. 2014b. Average Retail Price of Electricity to Ultimate Customers. Washington. Available at http://www.eia.gov/electricity.
EIA. 2014c. Natural Gas Plant Field Production. Washington. Available at www.eia.gov/naturalgas/.
EIA. 2014d. Natural Gas Pricing. Washington. Available at www.eia.gov/naturalgas/.
ICIS Pricing Report. 2014a. Ethylene (US Gulf). Houston: Reed Business Information Limited. Available at www.icispricing.com.
ICIS Pricing Report. 2014b. Feedstocks Report. Houston: Reed Business Information Limited. Available at www.icispricing.com.
ICIS Pricing Report. 2014c. Polyethylene (USA). Houston: Reed Business Information Limited. Available at www.icispricing.com.
IEA [International Energy Agency]. 2013. Technology Roadmap: Energy and GHG Reductions in the Chemical Industry via Catalytic Processes. Paris. Available at www.iea.org/publications/freepublications/publication/technology-roadmap-chemical-industry-via-catalytic-processes.html.
IHS. 1994. Ethylene from Methane. Process Economics Program. Englewood, CO: SRI Consulting.
Lange J-P. 2001. Economics of Alkane Conversion. In: Sustainable Strategies for the Upgrading of Natural Gas: Fundamentals, Challenges, and Opportunities. Derouane EG, Parmon V, Lemos, F, Ramôa Ribeiro F, eds. Dordrecht: Springer.
Lange J-P. 2005. Fuels and chemical manufacturing: Guidelines for minimizing the production costs. CATTECH 5(2):82–95.
Neelis M, Worrell E, Masanet E. 2008. Energy Efficiency Improvment and Cost Savings Opportunities for the Petrochemical Industry, LBNL-964E. Berkeley: E.O. Lawrence Berkeley National Laboratory.
Nexant. 2009. Gas to Ethylene, PERP 08-09S10. Chemsystems Process Evaluation/Research Planing (PERP) Program Report. San Francisco.
Sundaram KM, Shreehan MM, Olszewski EF. 2000. Ethylene. Kirk-Othmer Encyclopedia of Chemical Technology. Hoboken, NJ: John Wiley & Sons, Inc.
Van Goethem MWM, Barendregt S, Grievink J, Moulijn JA, Verheijen PJT. 2006. Ideal chemical conversion concept for the industrial production of ethene from hydrocarbons. Industrial & Engineering Chemistry Research 46(12):4045–4062.
Wan V. 2007. Methanol to Olefins. Process Economics Program Report 261. Englewood, CO: SRI Consulting.
Zimmermann H, Walzl R. 2000. Ethylene. In Ullmann’s Encyclopedia of Industrial Chemistry. Winheim: Wiley-VCH Verlag GmbH & Co. KGaA.










