ALLISON M. OKAMURA AND TANIA K. MORIMOTO
Stanford University
Many medical interventions today are qualitatively and quantitatively limited by human physical and cognitive capabilities. Robot-assisted intervention techniques can extend humans’ ability to perform surgery more accurately and less invasively using novel physical designs and computer control. Hundreds of thousands of surgical procedures are now done annually using robots, typically teleoperated by human surgeons. Commercial surgical robots such as the da Vinci Surgical System (DiMaio et al. 2011) are designed as general tools that can be used for a variety of procedures and patient populations. But because of their limited dexterity, high cost, and large footprint in the operating room, there are many scenarios in which current clinical robots cannot be used to perform minimally invasive medical procedures (Herron and Marohn 2008; Taylor and Stoianovici 2003). The next generation of medical robots will be much more personalized—capable of being rapidly designed, manufactured, and controlled for a specific patient and procedure.
DESIGN OF PERSONALIZED MEDICAL ROBOTS
Each patient presents a design opportunity. A path from a feasible entry point on the surface of the body to the target, such as a cancerous tumor or kidney stone, can be planned based on patient-specific anatomy and mechanical models of tissue acquired via new elastographic imaging techniques. Based on this path, a unique robotic steerable needle or catheter design will achieve the most minimally invasive trajectory possible, thus increasing accuracy, minimizing trauma, and ideally decreasing recovery time and chance of infection. This capacity is particularly useful in addressing the needs of specialized patient groups, includ-
ing children and people with rare diseases, who may otherwise not receive the optimal treatment.
In many procedures, the path of least resistance from a feasible entry point on the surface of the body to a target for treatment has multiple curved segments, so a snakelike device with the ability to change its shape along its length is ideal. To avoid the “curse of dimensionality” (the challenge of modeling and controlling a system with hundreds of individual degrees of freedom), a useful robot design should require only a few input degrees of freedom, yet have the ability to achieve a large variety of physical configurations. Steerable needles (Reed et al. 2011) have this property, but require relatively large reaction forces from tissue and cannot work in free space.
One of the most promising approaches is the concentric tube robot (also known as the active cannula), which consists of nested hollow, precurved, superelastic tubes. As the curved tubes are inserted and rotated with respect to each other, they interact such that their common axis conforms to some combined curvature, causing the overall shape of the robot to change. Because concentric tube robots derive bending actuation from the elastic energy stored in the backbone, they do not require reaction forces to bend and can be used in free space. The concept for the active cannula was simultaneously developed in 2006 (Sears and Dupont 2006; Webster et al. 2006), and recent work has provided a comprehensive analysis of concentric tube robot design and kinematics (Gilbert and Webster 2013; Lock and Dupont 2011; Rucker et al. 2010; Webster et al. 2008).
One example of concentric tube robot design is given in the context of accessing hard-to-reach upper-pole kidney stones in pediatric patients (Morimoto et al. 2013). Because of their smaller body surface area compared to adults, as well as the proximity of the upper kidney to the diaphragm and the pleura, traditional straight needle- and catheter-based approaches can be dangerous. To eliminate these risks, the ideal path would begin below the 12th rib, snake up through the renal pelvis, and curve toward the upper pole of the kidney. The exact dimensions for curvatures and segment lengths of the tubes can be gauged from patient-specific CT scans. Based on kinematic models (Dupont et al. 2010; Sears and Dupont 2007; Webster et al. 2008, 2009), sets of tubes can be identified that follow the desired path through patient tissue (Figure 1).
MANUFACTURING OF PERSONALIZED MEDICAL ROBOTS
A combination of modular robot architecture and novel manufacturing techniques is beginning to enable fast manufacturing and assembly of robotic manipulators that can achieve a variety of design objectives. Primarily these robots will be long, thin, flexible devices whose actuators remain outside the body and whose components that enter the body are sterile and disposable—they are small,
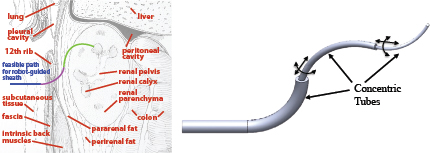
FIGURE 1 Personalized medical robot design uses knowledge of patient anatomy (left) to select the number, shape, and length of robotic elements (right) to reach a target in the safest, most minimally invasive fashion possible. Adapted from Morimoto et al. (2013).
inexpensive, and do not need to be overdesigned for repeated use. The nondisposable base of the robot can consist of modular units.
In the case of a modular concentric tube robot design, a single module includes two motors that allow a tube to be both inserted and rotated with respect to the tubes around it (Figure 2). The outermost tube to be inserted is clamped in the modular unit at the end of the base closest to the patient, while the subsequent tubes (with increasingly smaller diameters) are axially aligned in units further behind. Units can be added or removed based on the number of tubes needed for the specific procedure and patient.
The disposable components of the robot can be either specifically designed for each patient or chosen from a set that has been previously designed and optimized for a particular population of patients (e.g., children). A patient-specific design requires the manufacture of numerous disposable components. In one method for active cannula manufacturing, superelastic (e.g., Nitinol) tubes are heat treated to take on the desired shapes.
Recent work has taken advantage of advances in 3D printing to quickly and cheaply produce patient-specific devices (Figure 2). The use of 3D printing is becoming more widespread in the medical field for anatomy visualization to improve surgical planning (Dankowski et al. 2014; Schwaiger et al. 2012) and for the production of customized implants for patients with special requirements and size constraints (Abdel-Sayed and von Segesser 2011). 3D printing is also increasingly used for manufacturing medical robots, from rehabilitation devices to minimally invasive surgical robots (Roppenecker et al. 2013). The benefits of 3D printing include speed, the use of multiple materials in a single part, and the ability to embed sensors in a mechanical structure.

FIGURE 2 (Left) Active cannula-driving robot module. (Right) Example sets of 3D-printed tubes that can be used to construct an active cannula system.
CONTROL OF PERSONALIZED MEDICAL ROBOTS
Surgical robots that go deep into the body require a combination of low-level autonomous control and high-level human control. Human teleoperation directs the robot tip motions and treatments, while the underlying control system achieves the necessary robot configuration to minimize invasiveness. Seamless integration of preoperative plans and real-time medical imaging provide effective feedback to achieve the desired clinical outcomes. Examples of control systems that involve both low-level autonomous control and high-level human control include teleoperators that combine haptic (force feedback) guidance for steerable needles (Majewicz and Okamura 2013) and operator tip control for active cannulas (Burgner et al. 2011).
CONCLUSION
The next generation of medical robots will be personalized to enable treatment using devices optimized for a particular patient’s body and malady. Advances in medical imaging, path planning, design, manufacturing, control, and human-machine interaction all contribute to this goal.
REFERENCES
Abdel-Sayed P, von Segesser LK. 2011. Rapid prototyping for training purposes in cardiovascular surgery. In Advanced Applications of Rapid Prototyping Technology in Modern Engineering, ed. Hoque ME, Chapter 4. Rijeka, Croatia: InTech.
Burgner J, Swaney PJ, Rucker DC, Gilbert HB, Nill ST, Russell PT, Weaver KD, Webster RJ. 2011. A bimanual teleoperated system for endonasal skull base surgery. In Proceedings of the IEEE/RSJ International Conference on Intelligent Robots and Systems, San Francisco, September 25–30. pp. 2517–2523.
Dankowski R, Baszko A, Sutherland M, Firek L, Kałmucki P, Wróblewska K, Szyszka A, Groothuis A, Siminiak T. 2014. 3D heart model printing for preparation of percutaneous structural interventions: Description of the technology and case report. Kardiologia Polska 72(6):546–551.
DiMaio S, Hanuschik M, Kreaden U. 2011. The da Vinci surgical system. In Surgical Robotics: Systems Applications and Visions, Chapter 9. New York: Springer Science and Business Media.
Dupont PE, Lock J, Itkowitz B, Butler E. 2010. Design and control of concentric-tube robots. IEEE Transaction on Robotics 26(2):209–225.
Gilbert HB, Webster RJ. 2013. Can concentric-tube robots follow the leader? In Proceedings of the 2013 IEEE International Conference on Robotics and Automation, Karlsruhe, Germany, May 6–10. pp. 4866–4872.
Herron DM, Marohn M. 2008. A consensus document on robotic surgery. Surgical Endoscopy 22:313–325.
Lock J, Dupont PE. 2011. Friction modeling in concentric-tube robots. In Proceedings of the 2011 IEEE International Conference on Robotics and Automation, China. pp. 1139–1146.
Majewicz A, Okamura AM. 2013. Cartesian and joint space teleoperation for nonholonomic steerable needles. In Proceedings of the IEEE World Haptics Conference South Korea. pp. 395–400.
Morimoto TK, Hsieh MH, Okamura AM. 2013. Robot-guided sheaths (RoGS) for percutaneous access to the pediatric kidney: Patient-specific design and preliminary results. In Proceedings of the 2013 ASME Dynamic Systems and Control Conference, Palo Alto, October 21–23. doi: 10.1115/DSCC2013-3917.
Reed KB, Majewicz A, Kallem V, Alterovitz A, Goldberg K, Cowan NJ, Okamura AM. 2011. Robot-assisted needle steering. Robotics and Automation Magazine 18:35–46.
Roppenecker DB, Pfaff A, Coy JA, Leuth TC. 2013. Multi arm snake-like robot kinematics. In Proceedings of the 2013 IEEE/RSJ International Conference on Intelligent Robotics and Systems, Japan. pp. 5040–5045.
Rucker DC, Jones BA, Webster RJ. 2010. A geometrically exact model for externally loaded concentric-tube continuum robots. IEEE Transactions on Robotics 26(5):769–780.
Schwaiger J, Kagerer M, Traeger M, Gillen S, Dobritz M, Kleeff J, Feussner H, Lueth TC. 2012. Manufacturing of patient-specific pancreas models for surgical resections. In Proceedings of the 2012 IEEE International Conference on Robotics and Biomimetics, China. pp. 991–998.
Sears P, Dupont PE. 2006. A steerable needle technology using curved concentric tubes. In Proceedings of the 2006 IEEE/RSJ International Conference on Intelligent Robots and Systems, China. pp. 2850–2856.
Sears P, Dupont PE. 2007. Inverse kinematics of concentric-tube steerable needles. In Proceedings of the 2007 IEEE International Conference on Robotics and Automation, Italy. pp. 1887–1892.
Taylor RH, Stoianovici D. 2003. Medical robotics in computer-integrated surgery. IEEE Transactions on Robotics and Automation 19(5):765–781.
Webster RJ, Okamura AM, Cowan NJ. 2006. Toward active cannulas: Miniature snake-like surgical robots. In Proceedings of the 2006 IEEE/RSJ International Conference on Intelligent Robots and Systems, China. pp. 2857–2863.
Webster RJ, Romano JM, Cowan NJ. 2008. Kinematics and calibration of active cannulas. In Proceedings of the 2008 IEEE International Conference on Robotics and Automation, USA. pp. 3888–3895.
Webster R, Romano J, Cowan N. 2009. Mechanics of precurved-tube continuum robots. IEEE Transaction on Robotics 25(1):67–78.
This page intentionally left blank.






