Microbial Ecology of Hydraulic Fracturing
KELVIN B. GREGORY
Carnegie Mellon University
Advances in drilling and stimulation technologies have greatly improved the economics of oil and gas production from deep, tight, hydrocarbon-rich shale formations. But the unconventional drilling required for this production is associated with challenges in the management of both the wastewater that is coproduced at the surface and the microbial communities in this water.
The focus of this article is on the microbial ecology and biogeochemical processes that impact the production of oil and gas, management of wastewater (both flowback and produced water), and product quality from hydraulically fractured wells. Hydraulic fracturing is discussed from the perspective of water management, including the volume and makeup of fracturing fluids that give rise to produced water microbiology. Recent studies of the chemistry and microbiology of produced water from the Marcellus and Barnett shale regions are discussed. Microbial ecology present at wellheads is described as well as that of stratified impoundments for produced water. The concluding section considers the implications of microbial control for unconventional production and identifies research needed to address them.
HYDRAULIC FRACTURING OVERVIEW
Horizontal drilling, a technology that has been broadly applied since the 1980s, allows access to a far greater portion of a formation than vertical wells by following the horizontal contour of the formation for thousands of meters. In this manner, horizontal wells also greatly reduce surface impacts by minimizing the number of wells required to develop a particular area. Hydraulic fracturing (often called fracking) is used in conjunction with horizontal wells to increase the
permeability of a formation and extend the radius of influence of the wellbore for an overall increase in the productive area of the reservoir.
Hydraulic fracturing entails the pumping of fluid into the wellbore at a rate that exceeds the capacity of the formation to accept without fracturing. A number of fracturing fluids exist, the most common of which is a mix of water (as a solvent for chemical modifiers), sand (for proppant), and chemicals. This fluid is pumped into the wellbore at pressures of 500–900 atm, depending on the needs of the target formation. The proppant flows into preexisting and newly initiated fractures and holds them open after pumping has stopped and pressure decreases. In the Marcellus shale region, hydraulic fracturing may require 6–15 million liters of water, depending on the depth to the target formation and length of the horizontal leg of the well (Gregory et al. 2011).
FLOWBACK AND PRODUCED WATER
Generation of Fluid
After the pumping for hydraulic fracturing but before the production of hydrocarbons, the pressure in the well is allowed to dissipate; fluid returns to the surface through the wellhead and is collected and stored. This initial fluid, which is produced to the surface for about 14 days before product recovery, is called flowback. Thereafter, water produced to the surface (along with hydrocarbon products) over the lifetime of the well is called produced water. The difference between flowback and produced water is largely operational; the rate at which flowback returns to the surface is greater and the strength lower than that of produced water.
In terms of content, both flowback and produced water are a mixture of hydraulic fracturing fluid and water present in the formation. The quantity and quality of flowback vary among unconventional oil and gas plays and according to the hydraulic fracturing procedure used. In the Marcellus shale, for example, flowback returns 9–53 percent (average 10 percent) of the injected volume (Vidic et al. 2013). In terms of the quality of the fluid, the concentration of total dissolved solids (TDS, including salts and metals) in the water that returns to the surface varies over time; the earliest flowback (e.g., the first 2 days) resembles the hydraulic fracturing fluid itself and has the lowest concentration of TDS, while the later flowback (e.g., after day 10) and the produced water have much higher TDS, more like the chemistry of the formation water.
Generally speaking, the composition of produced water from a formation is similarly variable (Barbot et al. 2013). The study by Barbot and colleagues reveals that the TDS in produced water from the Marcellus varied from ~1 to 345 g/L, with an average of 106 g/L. The authors also found that the TDS in the produced water was predominantly from the ions (Cl−, Na+, and Ca2+) and further characterized by high concentrations of magnesium, barium, and strontium. Organic compounds in produced water include those introduced with the fracturing fluid
as well as polycyclic aromatic hydrocarbons (PAHs), heterocyclic compounds, alkyl phenols, aromatic amines, alkyl aromatics (alkyl benzenes, alkyl biphenyls), long-chain fatty acids, and aliphatic hydrocarbons (Orem et al. 2014), all of which may be used as carbon sources and electron donors for bacterial growth.
Disposition of Fluid
Most produced water in the United States is disposed through deep-well injection (Clark and Veil 2009). However, because there are few deep-well injection options in Pennsylvania, reuse of flowback as the makeup water for subsequent hydraulic fracturing has become the preferred management option in the Marcellus region. This reuse also reduces both the need for freshwater withdrawals and the costs incurred for transportation and treatment or disposal. Produced water brines are used for subsequent hydraulic fracturing, diluted with a freshwater source, and treated to remove solids and divalent cations before reuse. Although the reuse of flowback (and produced) water for hydraulic fracturing is a novel technology and management solution for oil and gas wastewater brines, a recent analysis of data from the Pennsylvania Department of Environmental Protection revealed a ~90 percent reuse rate of oil and gas brines from the Marcellus (Maloney and Yoxtheimer 2012).
Flowback is typically impounded at the surface before disposal, treatment, or reuse. While treatment or disposal may take place immediately, reuse for subsequent fracturing requires storage of a variable volume of wastewater (from 10,000 to 60,000 m3, depending on the intended use and the number of wells served) for variable periods of time (weeks or months); for this it is either transported by truck or pumped to centralized impoundments that serve multiple wells or multiple pads with multiple wells.
In storage, microbes in the flowback and produced fluids evolve and give rise to water management challenges such as malodorous compounds, biofouling of the formation and production equipment and infrastructure, biocorrosion, and alteration of the solubility of metals including radionuclides. Issues associated with the proliferation of bacteria during oil and gas production are ubiquitous, manifest themselves throughout the production infrastructure, and are costly to manage (Ollivier and Magot 2005).
MICROBIAL COMMUNITIES IN PRODUCED FLUIDS
Microbial processes that impact oil production from conventionally developed reservoirs are well documented (Van Hamme et al. 2003). In these reservoirs, the stimulation of bacteria may result in reservoir fouling, biocorrosion, and product souring (sulfidization), or, conversely, provide benefit by enhancing product recovery and the removal of soured product and paraffins. The microbial ecology of these processes in conventional reservoirs is well understood, but there are
few studies of the detrimental or beneficial impacts of bacteria or the microbial ecology of unconventional oil and gas development, despite similar concerns and knowledge that the microbiology is costly to control.
From Aerobic to Anaerobic Bacteria
A recent study of wellhead samples of Marcellus flowback and produced water reveals that the microbial community changes over time together with the geochemistry (Murali Mohan et al. 2013b). On day 1 of the flowback the community closely resembles that of the fracturing fluid and the source water (Figure 1). The microbes are most similar to species associated with nonhalophilic, aerobic, and phototrophic metabolisms, all of which would be expected in water from a freshwater surface source. However, later in the flowback period, the TDS increase as do the number of bacterial species that are most closely related to halophilic, thermophilic anaerobes. The rise in the abundance of halophilic anaerobes occurs at the expense of the nonhalophilic aerobes and phototrophs and at the expense of microbial diversity (Murali Mohan et al. 2013b).
Figure 1 shows a gradual decrease in Rhodobacterales (freshwater phototrophs) and increase in Halanaerobiales (anaerobic halophiles) during hydraulic fracturing of a well in the Marcellus shale. The emergence of the halophiles was concomitant with the emergence of anaerobic geochemistry (e.g., Fe2+ and HS−) in the water and led to the loss of virtually all species present in the initial flowback except the Halanaerobiales, which eventually represented more than 99 percent of the community (Cluff et al. 2014). Bacteria identified in a sample of produced water were closely associated anaerobic, fermentative, and sulfur-reducing bacteria in the Halanaerobiales. A study of flowback from the Barnett shale revealed a community that was similarly changing with time, adapting to anoxic and saline conditions (Struchtemeyer and Elshahed 2011).
Possible Sources of Bacteria
It is important to note that next generation sequencing revealed that the anaerobic and halophilic species present in great abundance in the produced water were also present in very low abundance in hydraulic fracturing fluid. This suggests that the organisms in the produced water may originate at the surface and be introduced by hydraulic fracturing rather than native to the connate water in the deep subsurface reservoir. But there is a chicken and egg problem: trucks and equipment used for handling the water likely were exposed to brines from hydraulic fracturing, so the source of the organisms could be any equipment used for hauling the water. It is therefore difficult to pinpoint the source of the organisms in flowback and produced water.
Although bacteria are well known to inhabit deep subsurface environments (Fredrickson and Balkwill 2006), their presence in connate brine from the
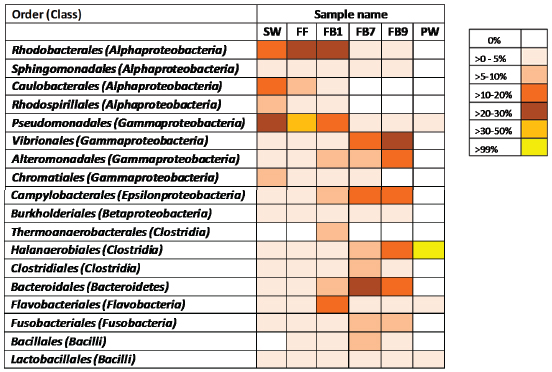
FIGURE 1 Heat map revealing the relative abundance of bacterial taxa in water samples associated with hydraulic fracturing of the Marcellus Shale as revealed by 454 pyrosequencing. FF=fracturing fluid; FB1, 7, 9=flowbackon days 1, 7, 9; PW=produced water on day 187; SW=source water.
Marcellus and Barnett formations has not been documented. Obtaining representative samples that are assured to be free of bacterial contamination from drilling or sampling is difficult and costly as the source formations are in extremely low permeability rock. Any bacteria that were present when the formation sealed were at temperatures and pressures sufficient to produce natural gas from biosolids—more than 120°C for millions of years. With those temperatures, durations, and limited permeability for nutrient delivery, the rapidly evolving robust microbial communities in wellhead samples are not likely. However, shallow and lower-temperature hydrocarbon-rich formations and those that contain natural fractures (which may serve as pathways for the exchange of fluids, as in the Antrim basin in Michigan) are more likely to have a native community (Martini et al. 1998).
Regardless of the source of organisms at the wellhead, they come in contact with equipment for handling and transporting fluids and eventually are in an impoundment where new geochemistry, impacted by management strategy and the surface environment, drives further changes in these adaptive bacterial communities.
MICROBIAL COMMUNITIES IN IMPOUNDMENTS
Impoundments for storage of flowback and produced water have robust and dynamic microbial communities that correspond to the geochemistry of the impoundment water. Impoundments receive water and bacteria from a variety of sources, including wells, source water, drilling fluids (Struchtemeyer et al. 2011), equipment, and the environment (e.g., rain, dust, animals, runoff). Which species are capable of surviving depends on their ability to adapt to brine concentrations above 100 g/L and to the spatially and temporally dynamic geochemistry that results from environmental processes and human intervention during impoundment management.
The only study of the microbiology in flowback water impoundments finds that such microbial communities stratify with the impoundment chemistry and are impacted by management strategy (Murali Mohan et al. 2013a). The onset of anaerobic conditions in an impoundment may be detected by malodorous compounds from bacterial activity associated with volatile fermentation products and sulfide gas; this aesthetic issue is commonly controlled by the addition of biocides or aeration of the impoundment.
Samples collected at depths ranging from the surface to the bottom of aerated and unaerated impoundments revealed that the geochemistry and microbiology in the aerated impoundment were homogeneous throughout (Figure 2). Moreover, findings show that the microbial community at all depths contained species that were most similar to known aerobes and phototrophs. This follows from the expected vertical mixing of the community between the bottom and surface depths from vigorous aeration. In contrast, the untreated impoundment was geochemically and microbially stratified: bacteria that were most similar to aerobes and
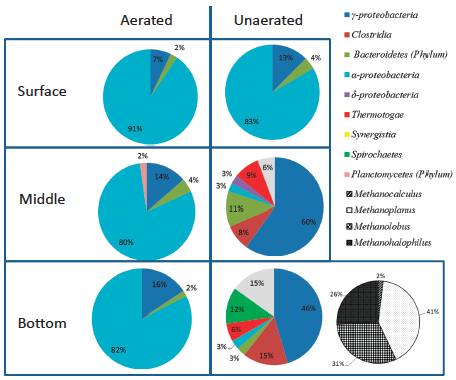
FIGURE 2 Relative abundance of bacterial taxa in flowback water impoundments at various depths. Data generated by clone libraries and subsequent sequencing of 16s rRNA genes recovered from samples. Sequences with similarity to Archaea were recovered only from the bottom of the unaerated impoundment and are shown in black and white. Data adapted from Murali Mohan et al. (2013a).
phototrophs were confined to the surface layer and anaerobes (such as sulfidogenic and methanogenic bacteria) were confined to the anoxic middle and bottom layers. In all samples, regardless of treatment or depth, the species present were most similar to taxa that are known halophiles, showing that the brine conditions of the water are an overarching driver of the ecology.
The study does not reveal the activity of the organisms, but it does suggest that the bacteria in impoundments adapt to the conditions imposed, despite the addition of biocides to the initial fracturing water (Murali Mohan et al. 2013a), as reported from studies of flowback from the Barnett shale as well (Struchtemeyer and Elshahed 2011).
IMPLICATIONS FOR UNCONVENTIONAL OIL AND GAS PRODUCTION
Research into the microbiology of unconventional oil and gas development is a nascent focus area for engineers and scientists. There are some similarities with conventional petroleum microbiology, but many research questions remain about water management associated with unconventional development. Their answers will affect the practice as well as the economic and environmental sustainability of hydraulic fracturing for oil and gas production.
Most importantly, studies to date indicate that the microbial communities present in wellheads and impoundments appear to be dynamic in time and space, indicating that biocides may not be working or used as intended. Moreover, microbial diversity drops sharply during flowback as it becomes enriched with survivor species. The implication is that recycling of flowback and produced water for subsequent hydraulic fracturing may introduce to new wells deleterious bacteria that are adapted to the harsh environment of the well (and biocides). These bacteria grow quickly, advance the onset of sulfide production, and are more resistant to treatment options.
Studies have also shown the presence and abundance of species that are similar to Halanaerobium congolense, a sulfidogen. Sulfide production in wells is associated with human and environmental health risks, corrosion, and costly degradation of product quality. This organism has been identified in produced waters from conventional development, but is of significance here because it cannot reduce sulfate and instead uses thiosulfate and sulfur as electron acceptors for sulfide production. Because standardized tests for assessing sulfidogenic potential in produced water rely on the numbers of sulfate-reducing bacteria, they will yield false negative reports for sulfide production potential, a risk for the industry. New tests that enable assessment of sulfide production from sulfur-reducing bacteria are needed for unconventional wells.
REFERENCES
Barbot E, Vidic NS, Gregory KB, Vidic RD. 2013. Spatial and temporal correlation of water quality parameters of produced waters from Devonian-Age shale following hydraulic fracturing. Environmental Science and Technology 47:2562–2569.
Clark CE, Veil JA. 2009. Produced Water Volumes and Management Practices in the United States. ANL/EVS/R–09/1. Environmental Science Division, Argonne National Laboratory.
Cluff MA, Harsock A, MacRae JD, Carter K, Mouser PJ. 2014. Temporal changes in microbial ecology and geochemistry in produced water from hydraulically fractured Marcellus shale gas wells. Environmental Science and Technology 48:6508–6517.
Fredrickson JK, Balkwill DL. 2006. Geomicrobial processes and biodiversity in the deep terrestrial subsurface. Geomicrobiology Journal 23:345–356.
Gregory KB, Vidic RD, Dzombak DA. 2011. Water management challenges in development of shale gas with hydraulic fracturing. Elements 7:181–186.
Maloney KO, Yoxtheimer DA. 2012. Production and disposal of waste materials from gas and oil extraction from the Marcellus shale play in Pennsylvania. Environmental Practices 14:278–287.
Martini AM, Walter LM, Budai JM, Ku TCW, Kaiser CJ, Schoell M. 1998. Genetic and temporal relations between formation waters and biogenic methane: Upper Devonian Antrim Shale, Michigan Basin, USA. Geochimica et Cosmochimica Acta 62:1699–1720.
Murali Mohan A, Hartsock A, Hammack RW, Vidic RW, Gregory KB. 2013a. Microbial communities in flowback water impoundments from hydraulic fracturing for recovery of shale gas. FEMS Microbial Ecology 86:567–580.
Murali Mohan A, Hartsock A, Bibby KJ, Hammack RW, Vidic RW, Gregory KB. 2013b. Microbial community changes in hydraulic fracturing fluids and produced water from shale gas extraction. Environmental Science and Technology 47:13141−13150.
Ollivier B, Magot M. 2005. Petroleum Microbiology. Washington: ASM Press.
Orem W, Tatu C, Varonka M, Lerch H, Bates A, Engle M, Crosby L, McIntosh J. 2014. Organic substances in produced and formation water from unconventional natural gas extraction in coal and shale. International Journal of Coal Geology 126:20–31.
Struchtemeyer CG, Elshahed MS. 2011. Bacterial communities associated with hydraulic fracturing fluids in thermogenic natural gas wells in North Central Texas, USA. FEMS Microbial Ecology 81:13–25.
Struchtemeyer CG, Davis JP, Elshahed MS. 2011. Influence of the drilling mud formulation process on the bacterial communities in thermogenic natural gas wells of the Barnett Shale. Applied and Environmental Microbiology 77:4744–4753.
Van Hamme JD, Sing A, Ward OP. 2003. Recent advances in petroleum microbiology. Molecular and Microbiology Reviews 67:503–549.
Vidic RD, Brantley SL, Vandenbossche JM, Yoxtheimer D, Abad JD. 2013. Impact of shale gas development on regional water quality. Science 340:1235009.
This page intentionally left blank.










