Design of Animal Models to Probe the Mechanisms of Multiple Chemical Sensitivity
Meryl H. Karol
INTRODUCTION
Recent years have witnessed the emergence of a syndrome called multiple chemical sensitivity (MCS) characterized by recurrent symptoms related to multiple organ systems (1). Symptoms associated with the disorder include: wheeze, cough. shortness of breath, as well as headache, fatigue, depression, confusion, muscle ache, weakness, and gastrointestinal upset (2). The ailment has been associated with diverse environmental exposures, most frequently to volatile organic solvents (2).
As with many newly recognized medical syndromes, development of an animal model has been suggested as an approach for gaining increased understanding of the pathogenesis of the disorder. Critical design of an animal model is essential to permit both acquisition of meaningful information relative to disease onset and pathogenesis, and to allow scientific acceptance of the model. With MCS, however, the broad range of symptoms, together with their subjective nature, makes it difficult to design an appropriate animal model.
The alternative approach frequently used for development of animal models, simulation of causal exposure conditions, is also fraught with difficulty. Exposures associated with onset of the syndrome are poorly defined and typically involve mixtures of chemicals, glues, solvents, and pesticides (2).
Design of an animal model for pulmonary disease resulting from environmental exposures will be discussed and emphasis placed on features of the model which have application to MCS.
ANIMAL MODELS OF ENVIRONMENTAL LUNG DISEASES
Animal models have been developed for numerous environmental lung diseases including: allergic hypersensitivity (3), acute (4) and chronic byssinosis (5), organic disease toxic syndrome (6), sensory irritation (7), as well as for chronic bronchitis, emphysema and lung cancer (8). Many have proved beneficial in elucidation off disease pathogenesis (8), progression (5) or in identification of biomakers associated with chemical exposures (9). The
characteristics of an ideal animal model have been summarized (Table 1). The use of small animals wherever possible is both economically beneficial and fosters use of the lowest possible phylogenetic species. Exposure of the model should resemble as closely as possible that of the human, to enable distribution of the chemical xenobiotic to appropriate target organs. Lastly, endpoints of toxicity must be carefully selected to resemble, or have relevance to, those observed in humans. Endpoints should occur with low spontaneous incidence.
TABLE 1
|
Features of an Ideal Animal Model for Environmental Disease |
|
|
— |
Use of a small animal species |
|
— |
Exposure through a natural route |
|
— |
Similarity in site of lesion development in humans |
|
— |
Similarity in cell type affected |
|
— |
Low incidence of toxicity end point occurrence in controls |
|
— |
Similar biological handling of toxicant (absorption, metabolism, storage, excretion) to humans |
|
Modified from ref. 8. |
|
Attention to design in development of an animal model for pulmonary sensitivity (10) has allowed its use in study of several environmentally related lung disorders. The basic features allowing for exposures and monitoring of responses are presented in Figure 1 and listed in Table 2. Guinea pigs are used since they are readily accessible, small rodents known to demonstrate strong allergic anaphylactic lung responses. Exposure is by inhalation of airborne atmospheres of chemical xenobiotics. Chemical atmospheres are generated in the mixing chamber. The concentration of xenobiotic, as well as the size of aerosol particles is established from samples taken at the ports in the mixing chamber. The desired chemical concentrations are achieved by adjusting the generator feed and airflow exhaust from this chamber.
Animals are exposed to this atmosphere by connecting individual plethysmographs to the mixing chamber using polytetrafluoroethylene (PTFE) tubing. The system is designed to accommodate 4 plethysmographs simultaneously.
Although exposure is predominantly via the inhalation route, dermal contact with the xenobiotic also occurs. Further, through grooming maneuvers and operation of the respiratory escalator, ingestion of the xenobiotic also occurs. Additionally, deliberate exposure through the ingestion route can be provided in this model by incorporating xenobiotics into the food supply placed within, each plethysmograph.
During exposure, animals are neither sedated nor restrained within plethysmographs. The benefit derived from this design is the maintenance of a normal body position and therefore an appropriate breathing pattern of animals. This results in the deposition of inhaled particles in the appropriate regions of the respiratory tract and thereby allows
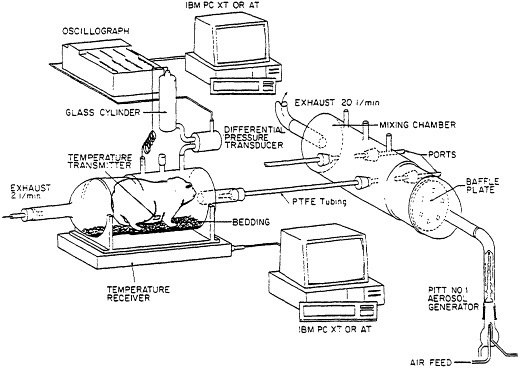
Figure 1 Schematic presentation of the exposure system and equipment for monitoring of pulmonary and febrile responses to airborne xenobiotics. (From ref. 11 with permission).
TABLE 2
|
Features of an Animal Model for Pulmonary Sensitivity |
|
|
— |
Use of guinea pigs (250-400g) |
|
— |
Exposure by inhalation of airborne chemicals |
|
— |
Use of unsedated and unrestrained animals |
|
— |
Avoidance of immunologic adjuvants to achieve sensitization |
|
— |
Passive recording of responses without disturbance of animals |
|
— |
Continuous monitoring of pulmonary and febrile responses for 24 hours |
suitable biologic handling of xenobiotics (see Table 1).
A second feature of the model is the avoidance of adjuvants to induce immunologic sensitization and of physical restraint within the chamber.
Exposure duration may extend from seconds to hours since atmospheres are continuously created using an aerosol or dust (11) generator.
Monitoring of Responses
An essential feature of this model is the provision for passive monitoring of responses. Continuous recording of breathing pattern is achieved through the use of pressure transducers. Core temperature is monitored using radio frequency transmitters and receivers (see Figure 1). Fever has been associated with several pulmonary syndromes including hypersensitivity pneumonitis and organic dust toxic syndrome.
All readings are collected electronically without disturbance of animals (12), and recorded continuously for up to 24 hrs. The latter feature permits detection of reactions, such as breathing difficulty and fever, which have a variable time of onset. This feature was paramount to identification of late-onset airway hypersensitivity responses to ovalbumin (13) and tuberculin protein (14). The model is applicable for study of illness thought to result from environmental contact.
EVALUATION OF ANIMAL MODELS
Once developed, an animal model must be evaluated. Ideally, the syndrome elicited in the model will closely resemble that reported for the human experience. Such situations generate confidence that mechanisms of pathogenesis in the animal system will have relevance for extrapolation to human disease pathogenesis. The guinea pig model for environmental lung disease has been evaluated for three clinical syndromes as described below.
Respiratory Hypersensitivity
Respiratory hypersensitivity to aeroallergens is characterized by occurrence of immediate, or late-onset airway disturbance (15). Evaluation of the model has been performed by comparison of responses in the guinea pig with those reported for clinical sensitization. Characteristic features of clinical sensitivity are occurrence of early and late onset falls in FEV1 and eosinophilic inflammation of the lung (15). The guinea pig model of pulmonary sensitivity demonstrated each of these responses (12). Animals responded to inhalation provocation challenge with an immediate airway constrictive response (see Figure 2). Recovery was complete within one hour. Continuous monitoring of the breathing pattern revealed a second response with maximum intensity occurring about 5 hours following provocation challenge. This late-onset response resolved over several hours.
At the height of response, histopathologic examination of pulmonary tissue revealed eosinophilic infiltration of the submucosal and epithelial regions of both the large and small airways (12, 13). Since eosinophilic inflammation is a primary characteristic of allergic pulmonary sensitivity, the animal system demonstrated the major physiological and histopathological features of the human syndrome it was designed to model.
The animal model of allergic sensitivity has been additionally evaluated by comparison of immunologic findings in the guinea pigs with those from clinical patients. In experimental studies of TDI hypersensitivity, antibodies produced in guinea pigs were shown to have specificity towards 2,4 and 2,6 TDI isomers (16). Further, the antibody titer was found to vary directly with the concentration of TDI to which animals had been exposed
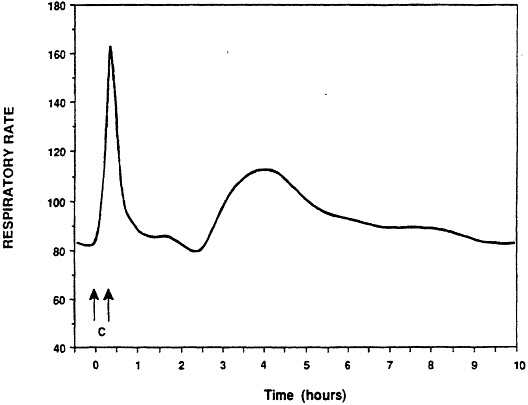
Figure 2 Immediate- and late-onset pulmonary responses of a sensitized guinea pig to ovalbumin. Arrows indicate the period of inhalation challenge. The immediate response occurred within 21 minutes of inhalation challenge. The late-onset response was observed 3.5-4.5 from the start of the challenge exposure. (from ref. 12 with permission).
(17). Both the antibody specificity and its concentration-dependent production were found in sera from individuals occupationally exposed to TDI (16,18).
Guinea Pig Model of Airway Hyperreactivity
One of the cardinal features of asthma is the presence of ahoy hyperreactivity (AHR) (19). This condition is most frequently assessed as a response to a lower than normal amount of inhaled histamine or methacholine. Study of the pathogenesis of asthma requires methodology for detecting occurrence of AHR. The basic guinea pig model for environmental lung disease was applied to detection of AHR. Methodology was developed to quantify airway reactivity in animals based upon their airway response to increasing concentrations of inhaled histamine (20).
To validate the model, it was necessary to assess its sensitivity in detecting transient AHR associated with an immunologic hypersensitivity response. Accordingly, a study was undertaken to determine whether the newly developed methodology could detect AHR which had been established using conventional methodology (21). Data presented in Figure 3 indicate the occurrence of AHR in each of the experimental animals following the hypersensitivity episode (22).
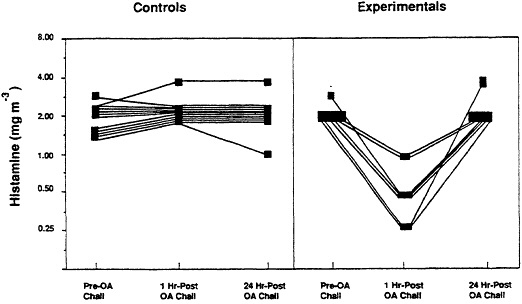
Figure 3 Airway reactivity of control and ovalbumin (OA)- guinea pigs prior to and following inhalation challenge with antigen. Hyperreactivity of experimental animals was apparent 1 hr post OA challenge by the response to lower than normal concentrations of histamine. (from ref. 22 with permission).
The model was further evaluated by comparing its sensitivity for detecting AHR with that of the previously employed methodology. The sensitivity of the two methods appeared comparable. From measurement of dynamic lung compliance and tidal volume (21), reactivity increased from 2.5 to 0.75 mgm-3 histamine required to evoke response. Using the non-invasive methodology (22), reactivity increased from 2.10 mgm-3 histamine to 0.5 mgm-3 required for response. The non-invasive methodology had the additional advantage of accommodating repeated measurements over a prolonged period of time as would be necessary for study of a chronic disease such as asthma.
ANIMAL MODEL FOR ORGANIC DUST TOXIC SYNDROME
A syndrome characterized by fever and neutrophilic alveolitis has been described (23). The disease is associated with environmental exposure to organic dusts and accordingly has
been named Organic Dust Toxic Syndrome (ODTS). In order to investigate both the conditions conducive to development of the syndrome, and mechanisms underlying the neutrophilic inflammation, an animal model was sought.
The basic guinea pig inhalation model had features desirable for application to ODTS (Table 2). These features included: capability to provide prolonged exposures via the inhalation route, and to monitor pulmonary and febrile responses which might develop over several hours. In addition, bronchoalveolar lavage could be readily performed in guinea pigs for the evaluation of neutrophilic pulmonary inflammation.
The model was adapted to study of ODTS by exposing animals for 6 hr to atmospheres of cotton dust obtained from a cotton mill in Memphis, TN (6). Monitoring of animals revealed development of the cardinal features of the syndrome, ie., fever, and neutrophilia (Figures 4,5).
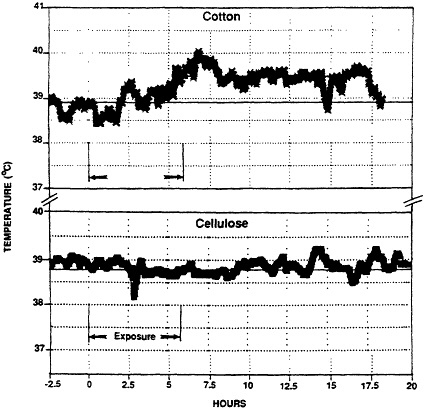
Figure 4 Febrile response of a guinea pig upon inhalation of cotton dust (upper) and lack of response to cellulose dust. Exposure period is indicated by arrows. The febrile response (1.1°C rise in temperature) was sustained for several hours. (from ref. 6 with permission).
Assessment of the model was achieved by further experiments using ''control'' dusts. Frequently, in developing animal models, high dosages (or concentrations) of xenobiotics are employed. The rationale for this procedure is based on the fundamental dose-response
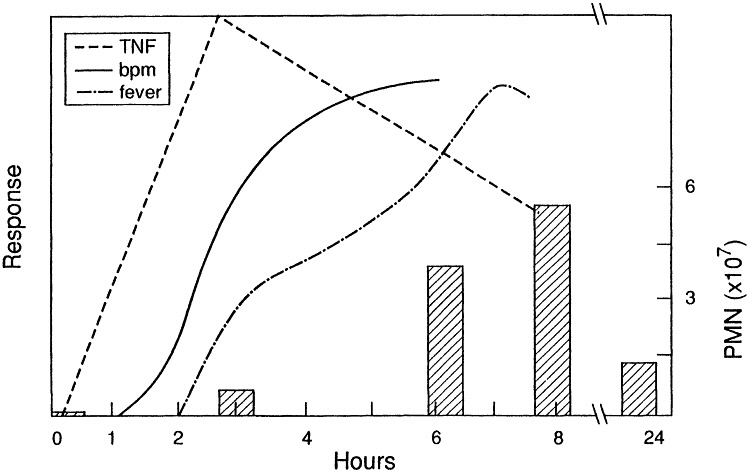
Figure 5 Physiological changes in guinea pigs resulting from inhalation of cotton dust. Hatched bars indicate polymorphonuclear (PMN) leukocyte count (right axis). Left axis: tumor necrosis factor (TNF) activity, breathing frequency, and fever response all shown relative to corresponding pre-exposure values. (from ref. 6 with permission).
principle of toxicology, ie., that if an agent is responsible for an effect, high concentrations of agent will produce sizable responses which can be readily measured. However, high concentrations of agents may create unnatural effects, such as those resulting from overload of biologic systems. For example, inhalation of high concentrations of particulates produced dust overload in rats, resulting in reduced alveolar clearance and altered particle retention (24). The possible occurrence of such effects must be avoided in design of animal models. Experiments must always be performed however, to test for their existence.
Validation of the model for ODTS was achieved by assessing the response of guinea pigs to a high concentration of cellulose dust (22), a dust without reported adverse health effects. Cellulose was selected since it represents the structural matrix of cotton but is devoid of the chemical and biological contaminants usually associated with cotton dust. The absence of both a febrile response, (see Figure 4) and evidence of alveolar inflammation in animals exposed to 41 mgm-3 cellulose, indicated that the response previously detected with cotton dust was not a result of dust overload in the lungs. Rather, it represented pulmonary toxicity resulting from the cotton dust.
AN ANIMAL MODEL OF MCS
Acceptance of an animal model is dependent upon how well the model reproduces the symptom complex associated with the particular disorder. With MCS, the symptoms are highly variable, involving many organ systems. Acceptance of a model for MCS will likely be difficult since there are no objective criteria which are accepted as defining the disorder. Further, the "adaptation" phenomenon, ie., unresponsiveness to acute chemical exposure, implies that negative finding are to be expected and occurrence of symptoms will be unpredictable.
The design of the animal model described above is compatible with study of chronic disease since animals are not instrumented and measurements (such as respiratory patterns and core temperature) are obtained passively and continuously. In addition, blood samples can be obtained readily for immunoglobulin and lymphocyte assessments. PAST (radioallergosorbent) testing can be done to ascertain food intolerance. The model also allows assessment of hypersensitivity, either by skin testing for cutaneous sensitivity, or through provocative inhalation challenge with aeroallergens.
Validation of a model for MCS will be difficult. Which symptoms will be accepted as representing the disorder? Will the absence of immunologic or pulmonary function changes signify a valid consequence of exposure, or an inadequacy of the animal model? Thus, although the animal model described here has many features which make it attractive for use in study of MCS, the absence of responses in animals would be hard to interpret. "Adaptation" could be invoked as a possible reason for unresponsiveness.
A more productive approach for development of an animal model for MCS would appear to be experimental reproduction of exposure conditions associated with development of the disorder. Continuous assessment could be made of physiologic changes (for example, changes in respiratory, neurologic, behavioral or immunologic function). The changes could then be assessed in control animals treated identically to those exposed, but without xenobiotic presence.
It should be noted that reproduction of causative exposure conditions of MCS presents a considerable difficulty since exposures associated with development of the disorder are not well defined. Concentrations of chemicals have not been reported. Moreover, implicated
atmospheres are typically variable and complex. One novel solution to this problem would be to introduce animals into the actual environment associated with MCS and to monitor reactions of the animals. However, the uncharacterized and unpredictable nature of exposures associated with development of MCS makes this a difficult undertaking. Simulation of "contaminated" environments appears a more practical yet improbable alternative.
SUMMARY
MCS is a newly recognized disorder associated with exposure to environmental chemicals. Symptoms associated with it are highly diverse and involve many organ systems. Development of an animal model to study the pathogenesis of the disorder is a laudable goal but standard approaches appear implausible. The difficulty lies both with the absence of a clear definition of the disorder and with lack of diagnostic criteria. Further, phenomena associated with the syndrome, such as adaptation, implies that absence of symptoms may be interpreted as a representative "response".
Approaches to animal model development typically focus on either reproduction of responses or of exposures associated with the particular disorder. For MCS, both approaches present difficulties since there is an absence of agreement on diagnostic criteria and little information on agents, lengths of exposure, or number of repeated exposures necessary for development of the disorder.
Animal models have been developed for many environmentally related disorders and have provided information essential to elucidation of disease pathogenesis, diagnosis and treatment. It is hoped that this approach can be applied to study of MCS since much remains to be learned about this very puzzling but increasingly reported disorder.
ACKNOWLEDGEMENT
Support from NIEHS 01532 is gratefully acknowledged.
References
1. Cullen, M.R. The worker with multiple chemical sensitivity: an overview. In: Workers with Multiple Chemical Sensitivity. State of the Art Reviews, Occupational Medicine (M.R. Cullen, Ed.) Hanley and Belfus, Inc., Philadelphia, PA., pp. 655-662 (1987).
2. Cone, J.E., Harrison, R. and Reiter, R. Patients with multiple chemical sensitivities: Clinical diagnostic subsets among an occupational health clinic population. In: Workers with Multiple Chemical Sensitivity. State of the Art Reviews, Occupational Medicine (M.R. Cullen, Ed.) Hanley and Belfus, Inc., Philadelphia, PA, pp. 721-738 (1987).
3. Thorne, P.S., Hillebrand, J., Magreni, C., Riley, E.J. and Karol, M.H. Experimental sensitization to subtilisin. I. Production of immediate-and late-onset pulmonary responses. Toxicol. Appl. Pharmacol. 86:112-123 (1986).
4. Ellakkani, M.A., Alarie, Y.C., Weyel, D.A., Mazumdar, S. and Karol, M.H. Pulmonary reactions to inhaled cotton dust: An animal model for byssinosis. Toxicol. Appl. Pharmacol. 74: 267-284 (1984).
5. Ellakkani, M.A., Alarie, Y., Weyel, D. and Karol, M.H. Chronic pulmonary effects in guinea pigs from prolonged inhalation of cotton dust. Toxicol. Appl. Pharmacol. 88:354-369 (1987).
6. Griffiths-Johnson, D., Ryan, L. and Karol, M.H. Development of an animal model for organic dust toxic syndrome. Inhal. Toxicol. (1991) (in press).
7. Alarie, Y., Kane, L. and Barrow, C. Sensory irritation: the use of an animal model to establish acceptable exposure to airborne chemical irritants. In: Toxicology: Principles and Practices, Vol. 1. (A.L. Reeves, Ed.) John Wiley and Sons, Inc., New York, NY, 1980, pp. 48-92.
8. Hakkinen, P.J. and Witschi, H.P. Animal Models. In: Toxicology of Inhaled Materials. (H.P. Witschi and J.D. Brain, Eds.) Handbook of Experimental Pharmacology Vol 75, Springer-Verlag, Berlin Heidelberg, 1985, pp. 95-114.
9. Karol, M.H., Thorne, P.S. and Hillebrand, J.A. The immune response as a biological indicator of exposure. In: Occupational and Environmental Chemical Hazards (V. Foa, E.A. Emmett, M. Maroni, A. Colombi, Eds.) Ellis Horwood Ltd., Chichester, 1987, pp. 86-90.
10. Karol, M.H., Dixon, C., Brady, M. and Alarie, Y. Immunologic sensitization and pulmonary hypersensitivity by repeated inhalation of aromatic isocyanates. Toxicol. Appl. Pharmacol. 53: 260-270 (1980).
11. Weyel, D.A., Ellakkani, M., Alarie, Y. and Karol, M. An aerosol generator for the resuspension of cotton dust. Toxicol. Appl. Pharmacol. 76: 544-547 (1984).
12. Karol, M.H., Hillebrand, J.A. and Thorne, P.S. Characteristics of weekly pulmonary hypersensitivity responses elicited in the guinea pig by inhalation of ovalbumin aerosols. Toxicol. Appl. Pharmacol. 100: 234-246 (1989).
13. Thorne, P.S. and Karol, M.H. Association of fever with late-onset pulmonary hypersensitivity responses in the guinea pig. Toxicol. Appl. Pharmacol. 100:247-258 (1989).
14. Stadler, J. and Karol, M.H. Experimental delayed-onset pulmonary hypersensitivity: Identification of retest reactions in the lung. Toxicol. Appl. Pharmacol. 65: 323-328 (1982).
15. Frigas, E. and Gleich, G.J. The eosinophil and the pathophysiology of asthma. J. Allergy Clin. Immunol. 77:527-537 (1986).
16. Jin, R. and Karol, M.H. Specificity of antibodies to toluene diisocyanate identified in workers and induced in an animal model. Am. Rev. Respir. Dis. 143:A439 (1991).
17. Karol, M.H. Concentration-dependent immunologic response to toluene diisocyanate (TDI) following inhalation exposure. Toxicol. Appl. Pharmacol. 68: 229-241 (1983).
18. Karol, M.H. Survey of industrial workers for antibodies to toluene diisocyanate. J. Occup. Med. 23: 741-747 (1981).
19. Armour, C.L., Black, J.L. and Johnson, P.R.A. A role for inflammatory mediators in airway responsiveness. In: Mechanisms In Asthma: Pharmacology, Physiology and Management (C.L. Armour, J.L. Black, Eds.) Alan R. Liss, Inc., New York, NY, 1988, pp. 99-110.
20. Thorne, P.S. and Karol, M.H. Assessment of airway reactivity in guinea pigs: Comparison of methods employing whole body plethysmography. Toxicology 52: 141-163 (1988).
21. Popa, V., Douglas, J.S. and Bouhuys, A. Airway responses to histamine, acetylcholine, and propanolol in anaphylactic hypersensitivity in guinea pigs. J. Allergy Clin. Immunol. 51: 345-356 (1973).
22. Griffiths-Johnson, D. and Karol, M.H. Validation of a non-invasive technique to assess development of airway hyperreactivity in an animal model of immunologic pulmonary hypersensitivity. Toxicology 65: 283-294 (1991).
23. Rask-Andersen, A. Organic dust toxic syndrome among farmers. Brit. J. Ind. Med. 46: 233-238 (1989).
24. Stober, W., Morrow, P.E. and Morawietz, G. Alveolar retention and clearance of insoluble particles in rats simulated by a new physiology-oriented compartmental kinetics model. Fund. Appl. Toxicol. 15:329-349 (1990).












