The Air Force Health Study (AFHS) was a longitudinal, prospective epidemiologic study initiated by the U.S. Air Force in 1979. Its original purpose was to determine whether U.S. Air Force personnel (all men) who had participated in Operation Ranch Hand—the program responsible for herbicide spraying in Vietnam—had experienced adverse health outcomes as a result of their service. The study protocol consisted of three components: a retrospective mortality study, a retrospective morbidity study, and a 20-year prospective follow-up study. The most resource intensive of the components, as well as the basis for the current research initiative, was the prospective follow-up study, which consisted of six comprehensive exams. A comprehensive set of clinical measurements and observations were made and biological specimens (serum, whole blood, urine, semen, and adipose tissue) were obtained and preserved at each of the six exams, or cycles. Extensive health questionnaires addressing health, lifestyle, socioeconomic status, employment, and other potential sources of environmental exposures were administered during each exam cycle. Other information was obtained from personal medical records and from the participants’ families. Data collection and analysis for the formal study was completed in 2006. However, due to a congressional directive, the extensive health data and accompanying biospecimens have to date remained available as a resource for additional research through the Institute of Medicine (IOM).
This chapter summarizes information on the AFHS relevant to the consideration of its further disposition. It begins with an overview of the design of the study. A detailed description of the data and biospecimens collected during the course of the AFHS that remain useful for conducting new research studies
is then provided. The chapter ends with a summary of the findings, conclusions, and recommendations made in the 2006 Disposition of the Air Force Health Study report, the effort that laid the foundation for the current research program.
The protocol for the AHFS was developed by a team of principal investigators and other researchers from the Air Force School of Aerospace Medicine at Brooks Air Force Base in San Antonio, Texas. It had three major components: a retrospective mortality study, a retrospective morbidity study, and a 20-year prospective morbidity follow-up study.1 The protocol was extensively and independently reviewed, revised, and rereviewed by the Air Force and academic and scientific institutions, including the National Research Council of the National Academy of Sciences, the University of Texas School of Public Health in Houston, the Air Force Scientific Advisory Board, and the Armed Forces Epidemiological Board until the 11th version was implemented in 1982 (AFHS, 1982, 1984a). Beginning in 1980, the Advisory Committee on Special Studies Relating to the Possible Long-Term Health Effects of Phenoxy Herbicides and Contaminants—also known as the Ranch Hand Advisory Committee—was convened as the entity responsible for overseeing and monitoring the conduct of the study.
The prospective morbidity study collected data through comprehensive physical examinations (including laboratory work and biospecimen draws), participant questionnaires, and reviews of personal medical records. These in-depth information collection efforts took place at six time points in years 1, 3, 5, 10, 15, and 20 of the study. Figure 2-1 depicts the study timeline including the time of exposure to herbicides, duration of the AFHS from protocol development to termination, and IOM custodianship. In all, 2,758 individuals participated in at least one exam cycle (IOM, 2006).
Ranch Hand personnel—1,2422—were identified and individually matched on age, race, and military occupation (differentiated into five categories: officer/pilot, officer/navigator, officer/other, enlisted/flight engineer, and enlisted/other) to comparison participants for the first exam cycle (1982) (AFHS, 1984a). The comparison participants served in the U.S. Air Force between 1962 and 1971, were stationed in and flew cargo operations elsewhere in Southeast Asia during the Vietnam conflict, had not been exposed to tactical herbicides, and were assumed to be similar to the Ranch Hands regarding lifestyle, training profiles, and socioeconomic factors. Each Ranch Hand was matched to a pool of 8–10
_______________
1 The IOM report Disposition of the Air Force Health Study provides a more detailed explanation of the design and results from the mortality and retrospective morbidity study components (IOM, 2006).
2 The exact number of Ranch Hands varies between published reports, but 1,242 reflects the number who had served in Vietnam and were not killed in action; however, not all of them ultimately participated in the Air Force Health Study (deceased before 1982, unlocatable, or refused) (IOM, 2006).
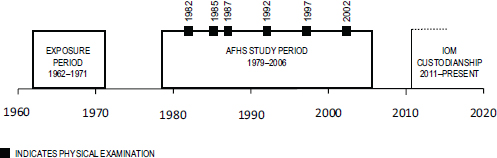
FIGURE 2-1 AFHS timeline.
comparisons,3 who were selected based on the first living and compliant person randomly selected from the individual-level pool. Individual comparison participants remained associated with their matched Ranch Hand for the duration of the study, but those who died, dropped out, or were lost to follow-up were replaced with the next best comparable control who was living and agreed to participate (AFHS, 1982). Although not representative of the U.S. population, the sample was diverse in terms of socioeconomic status and educational background.
Questionnaires were administered and physical examinations were conducted by contract personnel who were blinded to the herbicide exposure status of participants (AFHS, 1982, 1984a, 2005). The questionnaire was developed by the National Opinion Research Center in cooperation with the principal investigators and included questions on a broad range of health effects as identified by studies of humans and animals exposed to phenoxy herbicides and dioxin, hypothetical health effects based on biochemical and biological systems, and veterans’ complaints and public perception of health effects to the herbicides used (AFHS, 1984a). Portions of previously field-tested questionnaires were also incorporated in the questionnaire to maximize their validity (AFHS, 1984a). Baseline questionnaires were administered by trained professionals of Louis Harris and Associates, Inc., in participants’ homes; spouses were also interviewed. In subsequent cycles, under subcontract to Science Applications International Corporation (SAIC),4 the National Opinion Research Center administered all questionnaires, including the baseline questionnaire for new participants. In-home interviews to complete the baseline questionnaire for new participants continued through part
_______________
3 The committee notes that the comparison cohort was selected to be comparable to the Ranch Hands, but was not truly matched in an epidemiologic sense because the follow-up time was not identical for Ranch Hands and their comparison subjects if the comparison was replaced over the duration of the study.
4 SAIC was a contract service provider to the AFHS for all exam cycles. In cycles 2–6 SAIC administered the questionnaires and conducted the physical exams at the Scripps Clinic and Research Foundation. SAIC created and documented the protocols for collection, shipment, assay, and storage for the biological specimens for cycles 2–6 and created data dictionaries for later cycles.
of cycle 3 (1987), after which all questionnaires were administered at the physical exam site (AFHS, 2005). New participants—primarily replacement comparison participants—received the same questionnaire that was administered at the cycle 1 evaluation as well as the current cycle questionnaire (AFHS, 2005). An interval questionnaire was developed and updated for each cycle to capture new information and update existing information for all returning participants. Questions in the follow-up cycles were formulated to cover the time interval since the last questionnaire (Robinson, 2007). When a question was added to the questionnaire for a particular follow-up cycle, it was included in subsequent questionnaires to establish a longitudinal record for the item (AFHS, 2005).
All study participants who had completed the questionnaire were invited to complete a 2.5-day physical examination. Persons who refused to complete a questionnaire were excluded from the physical exam. If the person who refused were in the comparison group, a compliant replacement participant was selected from among the other comparisons in the matched set. Examiners were required to strictly adhere to the handbook included in the protocol to ensure high-quality data collection and to minimize variability. All laboratory tests were also subject to strict protocols and quality control (AFHS, 1984a). Participants were asked to bring a copy of their medical and dental records to the physical exam. These records were reviewed and information from them extracted and added to the AFHS repository. As with the questionnaire, the physical examination included health outcome measures and endpoints that were known or suspected to be affected by phenoxy herbicides and dioxin exposure based on results from scientific literature reviews (AFHS, 1984a). The final version included those outcome measures and endpoints that were feasible, practical, and of limited invasiveness (IOM, 2006). The baseline physical examinations were conducted by Kelsey-Seybold Clinic, P.A., in Houston, Texas. All physical examinations for cycles 2–6 were administered by Scripps Clinic and Research Foundation in La Jolla, California. It took approximately 10 months to conduct physical exams for all participants (Cecil, 1986).
The AFHS assets consist of electronic data from questionnaires, physical exams, and other sources as well as frozen laboratory biospecimens. The following section details the voluminous amounts of health and other types of data collected.
Information was gathered through comprehensive physical examinations, questionnaires, and reviews of medical records. Box 2-1 summarizes the major types of information collected. At the end of the AFHS in 2006, more than 123,000
BOX 2-1
Summary of Clinical, Laboratory, and Questionnaire Information Collected by the Air Force Health Study
Biospecimens: serum, whole blood, adipose tissue, semen, urine
Clinical measures: spirometry, chest radiographs, electrocardiograms, psychological testing, dermatology exams, peripheral vascular exams, neurological assessments
Demographics: education, employment, income, military experience, age, race
Endpoints by organ system: neurologic, endocrine, pulmonary, immunologic, renal, gastrointestinal, hepatic, hematologic, dermatologic, psychiatric, neoplasia, cardiovascular, reproductive
Familial factors: marital and fertility history, child and family health
General health: health habits, physical activities, leisure activities, toxic substances exposures, vital status, cause of death
electronic files were stored on AFHS servers and later transferred to the IOM. These files were stored as text files, images of X-rays and electrocardiograms, analysis files (Statistical Analysis System databases and programs, Fortran programs, Excel spreadsheets), PDFs of questionnaires and physical exam forms, and codes, including translation values to those used in data files. Content of the files includes questionnaires and physical exams from all six cycles, some of the analysis files used to create the AFHS reports for each cycle, diagnostic codes, dioxin analyses for cycle 3, dioxin data for cycles 3–6, last known addresses of all cohort members updated in 2007, journal articles and AFHS technical reports, master files to link personally identifiable information to case numbers, medication codes, the questionnaire on exposure to herbicides administered to Ranch Hand ground crew, mortality information updated in 2007, tour of duty histories, and freezer location of specimens (Robinson, 2007). About 25,000 of transferred files were considered high priority by the advisory committee and the IOM data staff and required analysis and processing for the creation of the master data files. These master data files were needed so data and samples could be provided to researchers. Each exam cycle includes its own master data files, which are used as the basis for all analyses, and within a cycle, there are separate files for different components of the cycle’s data gathering effort. Data in these files are stored by the participant’s unique identification number. Preparation of data files continued
throughout the entire duration of the research program, with more than 50,000 files processed as of November 1, 2014.
Questionnaire data included information relating to demographics; employment; child and family health; health habits; recreation, leisure, and physical activities; toxic exposures; military experience; and wartime herbicide exposure. Data collected during physical examinations comprise indices of health status that encompassed general health and endpoints by major organ system. Appendix B of the Disposition of the Air Force Health Study report provides an extensive summary of the health outcomes and endpoints collected in the course of the AFHS by cycle and organ system, including the more than 200 laboratory tests performed on the biospecimens. Table 2-1 provides an overview of the number of consented participants that completed the questionnaire and physical exam in each examination cycle; those data are available for use in research.
Additional sources of data collected in the course of the AFHS included medical records from the participants’ physicians, dentists, and other health providers; vital status records, such as birth and death certificates; and military administrative records that contained duty station orders, flight records, performance reports, awards and decorations, and discharge documents (AFHS, 1984a). These data and other exam materials such as electrocardiogram strips, chest radiographs, and high-resolution dental videos were scanned and stored as image files in irregular formats. All of these files were transferred to the IOM when it became the custodian of the AFHS assets and have the potential to be used in research; however, the IOM has been unable to extract and incorporate those data into a usable form (see Chapter 4).
Another component of the AFHS focused on reproductive outcomes because the possibility of increased risk of birth defects in children of Vietnam veterans was of concern. Reproductive outcome information was collected in all six cycles. In addition to the participant questionnaires that included questions on marital and reproductive history, data were gathered and compiled from interviews with current and former spouses and partners conducted in cycles 1–4. Permission
TABLE 2-1 Number of Consented Participants Who Completed Questionnaires and Physical Exams by Year Collected
| Component | 1982 Cycle 1 |
1985 Cycle 2 |
1987 Cycle 3 |
1992 Cycle 4 |
1997 Cycle 5 |
2002 Cycle 6 |
Completed all six cycles | ||
| Questionnaire | 1,983 | 1,884 | 1,869 | 1,816 | 1,711 | 1,569 | 1,269 | ||
| Ranch Hand | 908 | 851 | 839 | 800 | 726 | 645 | 577 | ||
| Comparison | 1,075 | 1,033 | 1,030 | 1,016 | 985 | 924 | 692 | ||
| Physical Exam | 1,846 | 1,884 | 1,869 | 1,817 | 1,711 | 1,571 | 1,214 | ||
| Ranch Hand | 866 | 851 | 839 | 800 | 726 | 645 | 561 | ||
| Comparison | 980 | 1,033 | 1,030 | 1,017 | 985 | 926 | 653 | ||
for the release of medical data and records related to the participant, spouse, and children was also obtained. In total, data were collected on 9,921 conceptions and 8,100 live births. Other information collected included fertility, miscarriages, stillbirths, induced abortions, intrauterine growth retardation, birth defects, and general health and well-being of children through age 18 (IOM, 2006; Michalek et al., 1998). These analyses were limited to those participants for whom serum dioxin was measured in the 1987 or 1992 exam cycles (IOM, 2006).
Although some participants (233 Ranch Hands5) were considered exposed to high levels of military herbicides, most were not. Some of the men were exposed to elevated levels of dioxin, but the comparison participants and even most of the group that was potentially exposed to herbicides had serum dioxin levels well within population background levels.6 Herbicide exposure was approximated through a series of serum dioxin assays performed in cycles 3–6, but AFHS reports warned that this qualitative exposure index was “not a good measure of actual dioxin exposure” (AFHS, 1991; IOM, 2006, p. 42). Comparison participants were considered to have background levels of dioxin (defined as ≤ 10 ppt), and Ranch Hands with levels unknown or ≤ 10 ppt were excluded from analyses. Pavuk and colleagues (2014) measured serum concentrations of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) and other dioxin-like compounds in samples from 777 Ranch Hands and 1,173 comparisons who participated in the 2002 exam cycle. The total dioxin equivalents in Ranch Hands (with the exception of dibenzo-p-dioxin congeners) were not statistically different from those of the comparisons or those reported for similarly aged males in the 2001–2002 National Health and Nutrition Examination Survey results. These findings provide empirical evidence that apart from TCDD, Ranch Hands were not more highly exposed to dioxin-like compounds than were the comparisons or the general U.S. population. Should potential exposure to dioxin be considered a confounder for certain research questions, the cohort could be restricted to comparisons participants only or all participants with dioxin levels equal to or below background levels.
The AFHS data represents an older cohort of participants, significant numbers of whom have been diagnosed with several types of conditions or diseases, and some who have died of various causes. Table 2-2 shows the numbers of unique Ranch Hand and comparison participants that have International Classification of Diseases, Ninth Revision (ICD-9) diagnoses by system category. For example, a person with ischemic heart disease and diabetes will be counted in both the
_______________
5 Determined by a compilation of different dioxin measures used by the original AFHS custodians where dioxin level was > 90 ppt.
6 Dioxins and dioxin-like compounds are a broad class of chemical compounds that persist in the environment and are found to be present in human tissues at low concentrations primarily through ingestion of meat and other animal products (CDC, 2009). Other compounds enter the body through inhalation exposures, some of which are toxic, such as TCDD, a contaminant of the Agent Orange herbicide used in Vietnam. Toxic equivalency has been shown to be affected by age, gender, race, smoking status, and percent body fat (Ferriby et al., 2007).
TABLE 2-2 Diagnoses by System for Ranch Hand and Comparison Participants Who Consented to Having Their Data and Biospecimens Used in New Research Studies
| Diagnosis Category (ICD-9 code) | Ranch Hands N = 922 | Comparisons N = 1,288 | Total N = 2,210 | |
| Circulatory disease (390–459) | 837 | 1,155 | 1,992 | |
| Respiratory disease (460–519) | 686 | 935 | 1,621 | |
| Digestive disease (520–579) | 455 | 582 | 1,037 | |
| Cancer (140–239) | 591 | 814 | 1,405 | |
| Endocrine disease (240–279) | 725 | 1,012 | 1,737 | |
| Nervous system disease (320–359) | 432 | 565 | 997 | |
| Genitourinary disease (580–629) | 677 | 910 | 1,587 | |
| Infectious or parasitic disease (001–139) | 661 | 879 | 1,540 | |
| Mental disorders (290–319) | 692 | 915 | 1,607 | |
circulatory disease and the endocrine disease categories. However, if a person has been diagnosed with more than one condition in a category, they are only counted once in that category. The counts include both deceased and living individuals.
Cause-specific and all-cause mortality comparison studies of Ranch Hands and their comparisons have been made throughout the original AFHS and updated at a few points since its termination (AFHS, 1984b; Pavuk et al., 2006). Mortality data was provided to the IOM for 460 consented Ranch Hand and comparison participants. Since the previous update in 2003, an additional 155 Ranch Hands and 188 comparisons have died. In all, information and biospecimens are available on 2,210 Ranch Hand and comparison participants, including 757 deceased participants, that can be used in new research studies. Updated mortality information was requested through 2012 (the last complete year available).
Using mortality information updated through December 31, 2003, Pavuk and colleagues reported on a cohort of 20,343 veterans (1,263 Ranch Hands and the pool of 19,080 comparable participants) who were followed after their service in Vietnam. After adjusting for year of birth and military occupation, the authors found the all-cause relative risk (RR) of death was significantly increased (RR = 1.25, 95% CI: 1.1, 1.4) in Ranch Hands relative to comparison participants (Pavuk et al., 2006). The increased death rate was mostly attributable to increases from diseases of the circulatory system (RR = 1.4, 95% CI: 1.1, 1.8). The RR of death from cancer was not significantly increased. The number of deaths from other categories of diseases, including respiratory, endocrine, and digestive diseases, was too small for relative risks to reach statistical significance but risk appeared to be somewhat elevated among AFHS participants. Stratifying participants by dioxin exposure level did not result in any significant increases in the risk of death overall for all causes or by cancer in the cohort. However, deaths from circulatory diseases showed a significant increasing trend for level of dioxin exposure and risk of death caused by circulatory diseases.
The AFHS repository contains more than 91,000 unaliquoted biospecimens.7 The number of samples varies by type of specimen, participant, and cycle. These were collected from study participants at each exam cycle and preserved to be used for future analyses. In the course of the AFHS, more than 200 clinical laboratory tests and measures were conducted and evaluated, and more than 60 of these were measured at all six cycles, such as red and white blood cell counts, hemoglobin, platelet count, total testosterone, bilirubin, cholesterol, and glucose. The number and type of laboratory tests performed at each physical examination changed over time reflecting changes in science and technology. Tests included blood draws, urine and semen collections, skin and fat biopsies, and stool smears; however, skin biopsies and stool smears were not retained for future research. The AFHS biospecimens are unique in that some types—such as serum and urine—were collected longitudinally across multiple cycles, while semen and whole blood were collected at a single exam cycle and adipose tissue was collected only from a subset of individuals in one exam cycle. A separate blood draw was performed to obtain samples for a TCDD (dioxin) assay carried out by the Centers for Disease Control and Prevention that was performed on 777 Ranch Hand and 1,174 matched control participants during cycles 3–6. Multiple blood samples were drawn for a subset of the population for use in dioxin biological half-life studies.
Biospecimen collection activities were conducted by contractors for all cycles. A detailed protocol is not available for cycle 1, but the protocols for collection, shipment, and storage of various biospecimens for cycles 2–6 are documented. Serum was isolated from blood samples within 2 hours of blood collection (Pavuk et al., 2007). Biospecimen aliquots were packaged on dry ice and shipped overnight to the AFHS facilities in San Antonio, Texas, where they were maintained in –70°C freezers (IOM, 2006). No samples were lost for any of the six cycles during shipment from the exam site to Brooks Air Force Base in San Antonio, Texas, or when the freezers were transferred from Brooks Air Force Base to Wright-Patterson Air Force Base in Dayton, Ohio. Because the IOM does not have an in-house capability to maintain biospecimens, the AFHS biospecimens are currently securely stored and maintained at the Air Force Research Laboratory of Wright-Patterson Air Force Base under the aegis of the IOM.
USEFULNESS OF THE BIOSPECIMEN REPOSITORY
The stored biospecimens can be linked to a wealth of information collected from the physical exams, clinical measurements, and questionnaires over time. The AFHS assets are exceptional in the sheer multitude and range of types of
_______________
7 Samples collected and stored in large volumes for laboratory or chemical analysis that have not been divided into smaller volumes.
information available for each participant. In addition to the large amount of clinical information associated with this collection, there are two additional valuable features of the biospecimen repository, as identified in the 2006 report Disposition of the Air Force Health Study. First, the repository contains matched sample types—serum, whole blood, adipose tissue, semen, and urine—for certain cycles. Specimens collected at each examination were collected on the same day. Matched samples are valuable for research because they can be used to investigate the correlation of findings in one sample type, such as adipose tissue, with another sample type that is less invasive or more routinely obtained, such as serum.8 This type of biological sample collection is seldom conducted despite its recognized value because of the difficulty and expense. Moreover, it is rare to find adipose and semen samples in biological sample repositories, and even rarer to find them matched with serum as in the AFHS.
Second, the repository contains serum and urine specimens collected longitudinally (in other words, repeat samples taken from participants at several different cycles) between 1982 and 2002 from a relatively large population of men. The repository contains information from 2,210 participants who participated in at least one cycle and gave consent for their materials to be used in research. Table 2-1 provides the number of individuals that completed the questionnaire and physical exam by cycle. As noted earlier, many of the same questions were asked and information on health endpoints collected at each cycle to establish a longitudinal record. Table 2-3 provides the number of participants with biospecimens available for each of the six collection time points.
Blood serum (multiple aliquots ranging between 2–10 mL per aliquot) was collected at each exam, and urine was collected at the first three exams. There are 1,204 participants (556 Ranch Hands and 648 comparisons) with blood serum samples available for all six cycles. These matched and longitudinally collected samples have been stored and maintained under conditions that permit their continued viability and use in research. Semen and serum samples collected in 1982 are 30 years old, but Pavuk (2006) and current investigators have shown that time of storage does not appear to affect their viability or properties. The specimens have been found to be suitable for many current assays and potentially for assays not yet identified. Chapter 3 has further discussion on time of storage and effect on viability.
The importance of collecting, documenting, and storing biological samples for research was not as appreciated at the time the AFHS was conducted as it is now. As noted by the National Cancer Institute,
The lack of standardized, high-quality biospecimens is widely recognized as a significant roadblock to cancer research…. One of the most difficult problems that will drive 21st century cancer research [is] … the limited availability of carefully collected and controlled,
_______________
8 For example, one could examine levels of various lipids in serum and markers of lipid breakdown products in matched adipose tissue collected on the same day.
high-quality human biospecimens annotated with essential clinical data and properly consented for broad investigational use. (NCI, 2014)
In a survey of 456 U.S. biological sample repositories, only 7% were established in 1980 or earlier while 59% were established after 2001 (Henderson et al., 2013). It would be extremely difficult, if not impossible, to independently replicate the samples (let alone the voluminous amounts of accompanying detailed data) collected from a similar population over a similar time period from biological repositories in operation at the time. Given the rapid development of new analysis techniques and instrument technologies; assay methods with improved analytical sensitivity and specificity; and an abundance of existing proteomic, metabolomic, and genetic methods that can be applied to specimens and data from the AFHS,
TABLE 2-3 Number of Consented Participants with Biospecimens Available by Type and Year Collected*
| Specimen Type | 1982 Cycle 1 |
1985 Cycle 2 |
1987 Cycle 3 |
1992 Cycle 4 |
1997 Cycle 5 |
2002 Cycle 6 |
|||
| Blood Serum | |||||||||
| Ranch Hand | 858 | 849 | 831 | 799 | 723 | 644 | |||
| Comparison | 975 | 1,027 | 1,023 | 1,011 | 977 | 924 | |||
| Total | 1,833 | 1,876 | 1,854 | 1,810 | 1,700 | 1,568 | |||
| Whole Blood | |||||||||
| Ranch Hand | 644 | ||||||||
| Comparison | 923 | ||||||||
| Total | 1,567 | ||||||||
| Urine | |||||||||
| Ranch Hand | 666 | 840 | 827 | ||||||
| Comparison | 580 | 1,020 | 1,018 | ||||||
| Total | 1,246 | 1,860 | 1,845 | ||||||
| Semen | |||||||||
| Ranch Hand | 668 | ||||||||
| Comparison | 780 | ||||||||
| Total | 1,448 | ||||||||
| Adipose Tissue | |||||||||
| Ranch Hand | 98 | ||||||||
| Comparison | 144 | ||||||||
| Total | 242 | ||||||||
* Available amounts will change as investigators use the specimens. Data are current as of November 1, 2014.
the potential value and utility of the AFHS specimens is far greater than what was originally imagined at the beginning of the study.
Not only does the AFHS repository contain matched and longitudinally collected samples, but the biospecimens are linked to data collected at each exam cycle including routine information (e.g., height, weight, blood pressure) as well as medical histories and risk factors (such as history of smoking and alcohol use or other avenues of exposure to specific chemicals), which is extremely valuable for epidemiologic research. The linked information allows researchers to stratify participants on the basis of important factors related to an outcome or disease that might not otherwise be available in other cohorts that do not have these large amounts of clinical information linked to the biospecimens. For example, researchers could investigate levels of blood glucose or other biomarkers of diabetes and risk of stroke, myocardial infarction, or other diseases in the AFHS cohort over the 20-year duration of data and specimen collection. Testing of longitudinally collected samples for the same biomarker offers an exceptional opportunity to determine whether changes in a particular biomarker predispose an individual to develop a specific disease or outcome.
The AFHS repository contains several types of biospecimens—some of which are held by relatively few biological sample repositories—that are valuable because they can be linked to detailed information about the individuals who participated in the study and because they were collected on the same date of the examination cycle, making it possible to determine associations between various biomarkers of exposure and outcomes of interest. The sections below address the various sample types and their research value.
Adipose Tissue
Adipose tissue is seldom found in biorepositories. Indeed, in a 2014 survey of 261 U.S. biological sample repositories, none reported adipose tissue (Edwards et al., 2014). Adipose tissue is useful for many types of epidemiologic investigations. For example, it is useful in nutritional studies because there is evidence that measurement of adipose tissue biomarkers is a more accurate measure of long-term dietary intake than dietary questionnaires because they rely on objective and measurable outcomes rather than memory, self-reported information, or interviewer-collected information that might be biased (Baylin et al., 2002). Adipose tissue has been used to quantify various biomarkers including fatty acid intakes (Baylin et al., 2002), long-term intake of carotenoids (El-Sohemy et al., 2002), and other dietary components.
Measurement of various biomarkers in adipose tissue has also been used as an indicator of cachexia (a wasting of lean and adipose mass that is often associated
with cancer) (Batista et al., 2013). Adipose tissue is used to quantify exposure to lipophilic pesticides and chemicals including dioxin, and it has been proposed as a useful tissue for measuring biomarkers of cancer (particularly colorectal and breast cancer) (Campbell et al., 2009) as well as adipose tissue dysfunction that may lead to obesity (Kruijsdijk et al., 2009). Adipose tissue can be used to confirm the presence of disease, such as light chain amyloidosis (Lavatelli et al., 2008) and to examine DNA methylation marks to make comparisons of chemical exposure-related effects within the tissue.
Semen
Like adipose tissue, semen is seldom available in biological repositories; in a survey of 261 U.S. facilities, only one held semen (Edwards et al., 2014). Because it contains citric acid, amino acids, fructose, various enzymes, potassium, zinc, and various other components, it is useful for many types of epidemiological studies. The semen from a single ejaculation varies from 2–5 mL and may contain up to 600 million sperm. The viscous fluid in semen provides a nutrient-rich medium through which sperm can move and can also act as the carrier for sexually transmitted diseases.
Semen is most often used in studies of fertility, and there are many biomarkers to quantify in vivo fertility. The classic biomarker tests of fertility in semen are measures of follicle stimulating hormone, luteinizing hormone, and testosterone levels; however, new fertility biomarkers are being developed continuously and may provide equivalent results (Sabetian et al., 2010). Some seminal plasma proteins are negatively related to fertility (seminal plasma proteins 1 and 2) while others are positively related to fertility (cysteine-rich secretory protein 3) (Novak et al., 2010).
Semen biomarkers may be used in studies of prostate cancer. For example, Selth and colleagues (2014) showed that levels of microRNAs in seminal fluid were increased in prostate tumors and may help to detect prostate cancer at early stages. Semen has also been used to identify prostatitis (an inflammation of the prostate gland that affects about 10% of U.S. men). For example, one study identified 59 putative biomarkers in seminal fluid that may represent early markers of prostatitis (Kagedan et al., 2012).
Semen has been used to quantify exposure to pesticides and other chemicals with some studies suggesting that semen quality may be reduced in normally fertile men who have been exposed to pesticides and other agricultural chemical including the herbicides alachlor, atrazine, and diazinon (Swan et al., 2003). In addition, semen has been used to examine occupational exposure to polycyclic aromatic hydrocarbons. Workers highly exposed to these chemicals exhibit higher levels of sperm DNA damage and higher levels of polycyclic aromatic hydrocarbons biomarkers than nonexposed workers (Hsu et al., 2006). In addition, various biomarkers, including those for nutritional factors, oxidative damage, and antioxi-
dants, may be measured in seminal fluid to determine nutritional status and levels of intensive exercise (Tartibian and Maleki, 2012).
Serum
Serum samples were collected at every exam cycle, and are used in a variety of standard clinical tests of physiologic conditions such as blood sugar levels and liver function, owing to the minimally invasive nature of the sample collection and the stability of many analytes during transport and processing. Serum can also be used to identify biomarkers that serve as surrogate markers of disease where direct sampling of the diseased organ is not possible. For example, serum samples can be used to test for circulatory protein biomarkers of cardiovascular disease (Battistonia et al., 2012), and a panel of multiple serum protein biomarkers was found to enable assessment of disease activity in rheumatoid arthritis (Centola et al., 2013). Other serum protein biomarkers have been characterized for early detection of colorectal cancer (Chen et al., 2014).
Serum samples can be used to analyze nonprotein biomarkers, such as metabolites (Psychogios et al., 2011), microRNAs (Condorelli et al., 2014), and circulating tumor DNA (Nguyen and Sim, 2014). MicroRNAs are known to be relatively stable in frozen serum, and microRNA profiles have been associated with different tumor types as well as a range of diseases such as cardiovascular disease, stroke, and multiple sclerosis (Reid et al., 2010). Circulating tumor DNA—which is fragmented tumor DNA that can be extracted from either serum or whole blood—can be analyzed for a variety of research purposes, including early detection and assessment of molecular heterogeneity of overall disease (Diaz and Bardelli, 2014) and tumor-specific genetic and epigenetic markers in gliomas of various grades (Lavon et al., 2010).
Urine
Unlike adipose tissue and semen, urine is a relatively common type of sample available in biological repositories because it is easily collected and useful for many types of epidemiologic studies. In their review of U.S. biological sample repositories, Edwards and colleagues (2014) found about one-third of them held urine samples. Urine is principally water and contains various organic compounds, nitrogen, salts, proteins, hormones, and other products. Typical basic urine tests include color, clarity, odor, specific gravity, pH, protein, glucose, nitrites, leukocyte esterases, ketones, blood cells, crystals, and bacterial or yeast cells and/or parasites. The type of urine test (one time or 24-hour) may also influence the tests that can be performed. Many studies collect a convenience sample of urine (taken at one time point); however, the protocol of the AFHS specified a 24-hour urine collection, of which 100 cc were aliquoted and frozen for future research (AFHS, 1984a). The 24-hour urine collection is more valuable than a convenience sample
because it permits a more comprehensive examination of urine components over the course of 1 day and also permits a one-time estimation of normal liquid intake for the individual. Outcomes measured by the AFHS included volume, delta-aminolevulinic acid, coproporphyrins, uroporphyrins, porphobilinogen, and creatinine.
Urinary biomarkers have been developed to measure an array of factors related to various diseases, nutritional status, early- and late-stage cancer, and exposure to pesticides and chemicals. Such biomarkers include those that can assist in detecting early stages of diabetic kidney disease (Wang et al., 2013) and detection of multiple sclerosis (Dobson, 2012). Although urine has long been used to quantify drug use, more recent applications allow quantification of alcohol use. Ethyl glucuronide is a highly sensitive marker of alcohol consumption—it is so sensitive that individuals who recently used household products containing alcohol, but did not consume alcohol, can test positive (Zolas and Sher, 2013). In addition to drugs and alcohol, urine can often be used to quantify exposure to various pesticides and other chemicals, but measured exposure may yield different results depending whether adjustments are made by volume or by creatinine (Fortin et al., 2008). Urine is also commonly used to measure recent dietary intakes of various foods. For example, the presence of urinary 1- and 3-methylhistidine is an indicator of recent meat consumption (Cross et al., 2011). Research using urine to attempt to discover biomarkers for early detection of disease, especially cancers, continues to be an active area of study. Discovery of such noninvasive methods and measures that could accurately be used for early detection of these diseases, especially in clinical settings, would be an important advancement.
Whole Blood
Whole blood samples, which are stable during prolonged storage, were collected only in the final AFHS exam cycle (2002), and are common in biological sample repositories. Whole blood samples can be used to extract DNA that can then be analyzed to provide information on genetic markers of disease predisposition or prognosis. For example, DNA analysis of single nucleotide polymorphisms (single nucleotide polymorphisms genotyping) has been used to identify genetic markers conferring susceptibility to Crohn’s disease (Yamazaki et al., 2005), age-related macular degeneration (Klein et al., 2005), and associations with environmental contaminants, such as dioxin-like compounds (Urban et al., 2011).
Continuing advances in technologies for sequencing of DNA now enables data from an entire genome to be obtained from a single sample aliquot in a short period of time. In the past 10 years, new types of sequencing instruments have been introduced that have accelerated data collection rates for DNA sequencing from years to weeks. For example, the data to decipher an entire human genome can be available within 2 weeks. Advances in technology are likely to further
reduce the time needed for generating data from whole-genome sequencing, and further transform how such research is conducted (Mardis, 2013).
Extracted DNA can be used to analyze epigenetic modifications of DNA such as methylation. Epigenetic processes alter the accessibility and expression of genes without changing the DNA sequence. Methylation has important implications for normal development and disease, and thus profiling DNA methylation across the genome is important for understanding the influence of epigenetics. Technology for analyzing DNA methylation has progressed to the point where “analyses that previously were restricted to specific loci can now be performed on a genome-scale and entire methylomes can be characterized at single-base-pair resolution” (Laird, 2010, p. 191). Altered DNA methylation marks can be linked with environmental exposures and to various cell types (Hou et al., 2012; Perera and Herbstman, 2011).
Ideally, whole genome data generated by genetic and epigenetic analyses by a single investigator will also be made available to other investigators (see Chapter 4), so the samples do not need to be used to generate the whole genome data more than once. The high-throughput nature of next-generation sequencing methods will allow investigators to use the subset of the data that are relevant to their study.
FINDINGS, CONCLUSIONS, AND RECOMMENDATIONS FROM DISPOSITION OF THE AIR FORCE HEALTH STUDY
Before the AFHS was scheduled to end in 2006, Congress asked the IOM to convene an expert committee and conduct an assessment to address several questions regarding the disposition of the AFHS, and specifically whether there was scientific merit in retaining and maintaining the data and biospecimens. The IOM appointed a committee to address the questions, and the key findings, conclusions, and recommendations from Disposition of the Air Force Health Study are summarized in Box 2-2. That committee concluded that there was scientific merit in retaining and maintaining the assets after the study’s scheduled termination date and recommended that further study using the AFHS data and biospecimens was advisable (IOM, 2006). Both the IOM committee and the Ranch Hand Advisory Committee, the interagency workgroup established in 1979 to oversee the AFHS, concluded that the AFHS assets have value beyond studying the effects of dioxin and herbicide exposure on health (RHAC, 2000).
The IOM committee found that the medical records, other study data, and biospecimens collected over the study’s duration had been properly maintained; however, it noted that the assets were not organized and documented in a manner that allowed them to be easily understood, evaluated, managed, or analyzed by persons outside of the AFHS researchers, program staff, and personnel. Proper organization and documentation of both the data and biospecimens is necessary to maximize their effective use, especially future use by persons outside of the
BOX 2-2
Summary of Key Findings, Conclusions, and Recommendations from the Disposition of the Air Force Health Study Report
- There is scientific merit in retaining and maintaining the medical records, other study data, and laboratory specimens collected in the course of the Air Force Health Study (AFHS) after the study’s currently scheduled termination date: no other epidemiologic dataset on Vietnam veterans contains as detailed information over as long a time period, the data appear to be of high quality and the specimens well preserved, and analysis of the assets has contributed to the literature addressing the health of Vietnam veterans.
- Obstacles exist to retaining and maintaining the AFHS data assets. These relate to factors intrinsic to the study’s design, resulting from implementation decisions made by the investigators, relating to documentation and organization of the data assets, and addressing the preservation of the privacy of the study subjects and the confidentiality of their personal information. The committee believes that the identified obstacles are surmountable.
- Further study of the AFHS medical records, other study data, and laboratory specimens is advisable. This should be accomplished by making these materials available for research via a custodian that takes an active role in fostering use of the assets. Five years after the chosen custodian assumes responsibility, a committee should be convened to evaluate whether any further support should be extended to the maintenance of access to the data or the biospecimens.
- The potential value and relevance of further study of the AFHS data assets rest in the application of the results of future health research on the data assets. This research could encompass using novel analysis approaches, employing new technology and techniques, and examining data and outcomes not evaluated to date. The cost of such work will vary greatly, depending on the research question that is addressed.
- The committee cannot offer a specific recommendation on the federal or nonfederal entity best suited to continue the study of the AFHS data assets but has identified a number of options that could be pursued successfully.
- Independent oversight of future research using the AFHS data assets is advisable, and should be provided through the review of proposals for scientific merit and adherence to ethical, legal, and related considerations by an Institutional Review Board and, separately, an advisory and oversight board. Additionally, research should be carried out in a manner transparent to study subjects, through systematic communication of research plans and results.
SOURCE: IOM, 2006.
AFHS. At the time of writing that report, much of the knowledge and nuances of the data files was stored “in the collective minds and personal notes of the AFHS staff charged with maintaining it” (IOM, 2006, p. 85). As part of its recommendation to organize and document the assets prior to the scheduled termination date, the committee specified such actions as creating detailed and comprehensive data dictionaries for master data files for each examination cycle; a comprehensive dictionary of the variables contained in the master data files, organized by examination cycle and by questionnaire, physical examination report, or other data intake instrument; a master codebook that would constitute a comprehensive distillation of database contents, such as from which examination cycles a particular piece of information was gathered and the variable name(s) associated with that information over the course of the study; and a document or database describing the contents, format, and location of the AFHS collection of assets that have been scanned into PDF image files. Other items that the committee believed should be included in the comprehensive data dictionary were a synopsis of the variable names and their descriptions, the summary variables created and codes used, the number of study participants examined for each test or outcome, changes in the database structure from the previous examination, and relevant notes on data comparability between cycles. The committee recommended that these documents be in a form and format that facilitates easy access to their contents. For those variables specific to the laboratory results, it suggested that the dictionary contain descriptions of assays, units of measurement and normal ranges, and data codes. Notation should include whether any attributes of a variable have changed over the course of the study; for example, noting increased sensitivity of a biomarker where in earlier exams a participant might have tested negative and with increased sensitivity may have then tested positive in a later exam, although the value may be the same.
As is the case with the electronic data, the Disposition of the Air Force Health Study committee noted that obstacles, including privacy concerns, exist in maintaining the laboratory biospecimens. Privacy concerns relate to safeguarding the personally identifiable information linked to the biospecimens and any information, such as a particular diagnosis, that can be determined from the biospecimens, rather than the fact that DNA can be extracted from some of the biospecimens. (DNA, although unique for each individual, is not itself identifiable unless matched to another identified sample.)
The committee was told of a planned reassay on a selected set of biospecimens using endpoints with recorded historical values to evaluate the stability and condition of the biospecimens, but results were not available at the time the report was published. The committee recommended that the Air Force fully document and inventory the biospecimens prior to the study’s scheduled termination date because such an activity would be vital to facilitating any possible future use. Recommended actions that should be part of completing an inventory of the biospecimens included verifying each biospecimen’s location and ascertaining the
number and volume of aliquots and type of samples; visually inspecting each specimen during the verification process to identify any problematic samples; creating a single biospecimen database that includes case number, exam cycle, specimen type, and freezer location and compiling all information regarding biospecimen history (receipt, realiquoting, freeze–thaw cycles, dispersal, etc.) into a single reference database; and compiling all protocols regarding receipt, maintenance, dispersal, and return of biospecimens for all cycles into a single reference document.
The IOM committee further concluded that concerns regarding privacy and other “ethical, legal, and social issues are not an intrinsic obstacle to retaining and maintaining the AFHS medical records, other study data, and laboratory specimens” (IOM, 2006, p. 116) and are, in fact, surmountable. Examples of these ethical, legal, and social issues include maintaining confidentiality and security of participants’ identifiable health information and obtaining informed consent from the cohort participants to use the assets in broad research. However, the committee also noted that attention to such concerns and issues must be a major consideration in decisions regarding the future disposition and use of these assets. Given this, and that the committee concluded that there is scientific merit in retaining and maintaining the AFHS data and biospecimens, the committee further concluded, “Further study of the AFHS medical records, other study data, and laboratory specimens is advisable. The potential value and relevance of extending the study of the AFHS data assets rests in the application of the results of future research on the assets” (IOM, 2006, p. 116). The committee then identified several possible applications that are further explored in Chapter 3 along with the many nuances and steps between the recommendations made by the 2006 IOM committee and implementing them into a successful management program.
AFHS (Air Force Health Study). 1982. An epidemiologic investigation of health effects in Air Force personnel following exposure to herbicides: Study protocol, initial report. SAM-TR-82-44. Brooks AFB, TX: USAF School of Aerospace Medicine.
AFHS. 1984a. An epidemiologic investigation of health effects in Air Force personnel following exposure to herbicides: Baseline morbidity study results. NTIS AD-A-138-340. Brooks AFB, TX: USAF School of Aerospace Medicine.
AFHS. 1984b. An epidemiologic investigation of health effects in Air Force personnel following exposure to herbicides: Mortality update: 1984. NTIS AD-A-162-687. Brooks AFB, TX: USAF School of Aerospace Medicine.
AFHS. 1991. An epidemiologic investigation of health effects in Air Force personnel following exposure to herbicides. Mortality update: 1992. AL-TR-1991-0132. Brooks AFB, TX: Armstrong Laboratory.
AFHS. 2005. An epidemiologic investigation of health effects in Air Force personnel following exposure to herbicides. 2002 follow-up examination results. AFRL-HE-BR-SR-2005-0003. Brooks AFB, TX: Epidemiologic Research Division, Armstrong Laboratory.
Batista, M. L., M. Olivan, P. S. M. Alcantara, R. Sandoval, S. B. Peres, R. X. Neves, R. Silverio, L. F. Maximiano, J. P. Otoch, and M. Seelaender. 2013. Adipose tissue-derived factors as potential biomarkers in cachectic cancer patients. Cytokine 61:532-539.
Battistonia, A., S. Rubattua, and M. Volpea. 2012. Circulating biomarkers with preventive, diagnostic and prognostic implications in cardiovascular diseases. International Journal of Cardiology 157(2):160-168.
Baylin, A., E. Kabagambe, X. Siles, and H. Campos. 2002. Adipose tissue biomarkers of fatty acid intake. American Journal of Clinical Nutrition 76:750-757.
Campbell, K. L., K. W. Makar, M. Kratz, K. E. Foster-Schubert, A. McTiernan, and C. Ulrich. 2009. A pilot study of sampling subcutaneous adipose tissue to examine biomarkers of cancer risk. Cancer Prevention Research 2:37-42.
CDC (Centers for Disease Control and Prevention). 2009. Fourth national report on human exposure to environmental chemicals. Atlanta, GA: CDC.
Cecil, P. F. 1986. Herbicidal warfare: The Ranch Hand Project in Vietnam. New York: Praeger.
Centola, M., G. Cavet, Y. Shen, S. Ramanujan, N. Knowlton, K. A. Swan, M. Turner, C. Sutton, D. R. Smith, D. J. Haney, D. Chernoff, L. K. Hesterberg, J. P. Carulli, and J. R. Curtis. 2013. Development of a multi-biomarker disease activity test for rheumatoid arthritis. PLoS ONE 8(4):e60635.
Chen, H., S. Werner, S. Tao, I. Zörnig, and H. Brenner. 2014. Blood autoantibodies against tumor-associated antigens as biomarkers in early detection of colorectal cancer. Cancer Letters 346(2):178-187.
Condorelli, G., M. V. Latronico, and E. Cavarretta. 2014. MicroRNAs in cardiovascular diseases: Current knowledge and the road ahead. Journal of the American College of Cardiology 63(21):2177-2187.
Cross, A. J., J. M. Major, and R. Sinha. 2011. Urinary biomarkers of meat consumption. Cancer Epidemiology, Biomarkers and Prevention 20:1107-1111.
Diaz, L. A., Jr., and A. Bardelli. 2014. Liquid biopsies: Genotyping circulating tumor DNA. Journal of Clinical Oncology 32(6):579-586.
Dobson, R. 2012. Urine: An under-studied source of biomarkers in multiple sclerosis? Multiple Sclerosis and Related Disorders 1:76-80.
Edwards, T., R. J. Cadigan, J. P. Evans, and G. E. Henderson. 2014. Biobanks containing clinical specimens: Defining characteristics, policies, and practices. Clinical Biochemistry 47:245-251.
El-Sohemy, A., A. Baylin, E. Kabagambe, A. Ascherio, D. Spiegelman, and H. Campos. 2002. Individual carotenoid concentrations in adipose tissue and plasma as biomarkers of dietary intake. American Journal of Clinical Nutrition 76:172-179.
Ferriby, L. L., J. S. Knutsen, M. Harris, K. M. Unice, P. Scott, P. Nony, L. C. Haws, and D. Paustenbach. 2007. Evaluation of PCDD/F and dioxin-like PCB serum concentration data from the 2001-2002 National Health and Nutrition Examination Survey of the United States population. Journal of Exposure Science Environmental Epidemiology 17(4):358-371.
Fortin, M. C., G. Carrier, and M. Bouchard. 2008. Concentrations versus amounts of biomarkers in urine: A comparison of approaches to assess pyrethroid exposure. Environmental Health 7(55):1-13.
Henderson, G. E., R. J. Cadigan, T. P. Edwards, I. Conlon, A. G. Nelson, J. P. Evans, A. M. Davis, C. Zimmer, and B. J. Weiner. 2013. Characterizing biobank organizations in the U.S.: Results from a national survey. Genome Medicine 5(3):1-12.
Hou, L., X. Zhang, D. Wang, and A. Baccarelli. 2012. Environmental chemical exposures and human epigenetics. International Journal Epidemiology 41(1):79-105.
Hsu, P. C., I. Y. Chem, C. H. Pan, K. Y. Wu, M. H. Pan, J. R. Chen, C. J. Chen, G. P. Chang-Chien, C. H. Hsu, C. S. Liu, and M. T. Wu. 2006. Sperm DNA damage correlates with polycyclic aromatic hydrocarbons biomarker in coke-oven workers. International Archives of Occupational & Environmental Health 79:349-356.
IOM (Institute of Medicine). 2006. Disposition of the Air Force Health Study. Washington, DC: The National Academies Press.
Kagedan, D., I. Lecker, C. Batruch, C. Smith, I. Kaploun, K. Lo, I. Grober, E. Diamandis, and K. Jarvi. 2012. Characterization of the seminal plasma proteome in men with prostatitis by mass spectrometry. Clinical Proteomics 9:2-20.
Klein, R. J., C. Zeiss, E. Y. Chew, J. Y. Tsai, R. S. Sackler, C. Haynes, A. K. Henning, J. P. San-Giovanni, S. M. Mane, S. T. Mayne, M. B. Bracken, F. L. Ferris, J. Ott, C. Barnstable, and J. Hoh. 2005. Complement factor H polymorphism in age-related macular degeneration. Science 308(5720):385-389.
Kruijsdijk, R. C. M., E. Van Der Wall, and F. L. J. Visseren. 2009. Obesity and cancer: The role of dysfunctional adipose tissue. Cancer Epidemiology, Biomarkers and Prevention 18(10):2569-2578.
Laird, P. W. 2010. Principles and challenges of genome-wide DNA methylation analysis. Nature Reviews Genetics 11(3):191-203.
Lavatelli, F., D. H. Perlman, B. Spencer, T. Prokaeva, M. E. McComb, R. Théberge, L. H. Connors, V. Bellotti, D. C. Seldin, G. Merlini, M. Skinner, and C. E. Costello. 2008. Amyloidogenic and associated proteins in systemic amyloidosis proteome of adipose tissue. Molecular and Cellular Proteomics 7(8):1570-1583.
Lavon, I., M. Refael, B. Zelikovitch, E. Shalom, and T. Siegal. 2010. Serum DNA can define tumor-specific genetic and epigenetic markers in gliomas of various grades. Neuro-Oncology Journal 12(2):173-180.
Mardis, E. R. 2013. Next-generation sequencing platforms. Annual Review of Analytical Chemistry 6:287-303.
Michalek, J. E., A. J. Rahe, and C. A. Boyle. 1998. Paternal dioxin, preterm birth, intrauterine growth retardation, and infant death. Epidemiology 9(2):161-167.
NCI (National Cancer Institute). 2014. Mission and goals of the biorepositories and biospecimen research branch. http://biospecimens.cancer.gov/about/overview.asp (accessed November 7, 2014).
Nguyen, A. H., and S. J. Sim. 2014. Nanoplasmonic biosensor: Detection and amplification of dual bio-signatures of circulating tumor DNA. Biosensors and Bioelectronics 67:448-449.
Novak, S., T. A. Smith, F. Paradis, L. Burwash, M. K. Dyck, G. R. Foxcroft, and W. T. Dixon. 2010. Biomarkers of in vivo fertility in sperm and seminal plasma of fertile stallions. Theriogenology 74:956-967.
Pavuk, M. 2006. Testing the viability of stored frozen serum samples from the Air Force Health Study using human multi-analyte profiles (MAP). AFRL-HE-BR-TE-2007-0015. Brooks-City-Base, TX: Air Force Research Laboratory Human Effectiveness Directorate, Information Operations and Special Program Division.
Pavuk, M., N. S. Ketchum, and K. A. Fox. 2006. Mortality in US Air Force veterans of Operation Ranch Hand. Organohalogen Compounds 68:2136-2139.
Pavuk, M., D. G. Patterson, Jr., W. E. Turner, L. L. Needham, and N. S. Ketchum. 2007. Polychlorinated dibenzo-p-dioxins (PCDDs), polychlorinated dibenzofurans (PCDFs), and dioxin-like polychlorinated biphenyls (PCBs) in the serum of US Air Force veterans in 2002. Chemosphere 68(1):62-68.
Pavuk, M., D. G. Patterson, and W. E. Turner. 2014. Serum concentrations of TCDD and other dioxin-like compounds in US Air Force veterans of Operation Ranch Hand. Chemosphere 102:18-23.
Perera, F., and J. Herbstman. 2011. Prenatal environmental exposures, epigenetics, and disease. Reproductive Toxicology 31(3):363-373.
Psychogios, N., D. D. Hau, J. Peng, A. C. Guo, R. Mandal, S. Bouatra, I. Sinelnikov, R. Krishnamurthy, R. Eisner, B. Gautam, N. Young, J. Xia, C. Knox, and D. S. Wishart. 2011. The human serum metabolome. PLoS ONE 6(2):e16957.
Reid, G., M. B. Kirschner, and N. Van Zandwijk. 2010. Circulating microRNAs: Association with disease and potential use as biomarkers. Critical Reviews in Oncology/Hematology 80(2):193-208.
RHAC (Ranch Hand Advisory Committee). 2000 (October 19–20). Meeting minutes. http://www.fda.gov/ohrms/dockets/ac/00/transcripts/3654t1.pdf (accessed November 7, 2014).
Robinson, J. 2007. Overview of resources provided by AFHS to MFUA. Document given to MFUA upon transfer of AFHS assets.
Sabetian, S., A. Ardekani, M. Hodjat, M. M. Akhondi, and H. Soltanghoraee. 2010. Comparing seminal plasma biomarkers between normospermic and azoospermic men. Journal of Reproduction and Infertility 11(1):39-46.
Selth, L. A., M. J. Roberts, C. W. K. Chow, V. R. Marshall, S. Doi, L. M. Vincent, L. M. Butler, M. F. Lavin, W. D. Tilley, and F. Gardner. 2014. Human seminal fluid as a source of prostate cancer specific microRNA biomarkers. Endocrine Related Cancer 21(4):L17-L21.
Swan, S. H., R. L. Kruse, F. Liu, D. B. Barr, E. Z. Drobnis, J. B. Redmon, C. Wang, C. Brazil, J. W. Overstreet. 2003. Study for Future Families Research Group. Semen quality in relation to biomarkers of pesticide exposure. Environmental Health Perspectives 111(12):1478-1484.
Tartibian, B., and B. H. Maleki. 2012. The effects of honey supplementation on seminal plasma cytokines, oxidative stress biomarkers, and antioxidants during 8 weeks of intensive cycle training. Journal of Andrology 33:449-461.
Urban, J. D., R. A. Budinsky, and J. C. Rowlands. 2011. Single nucleotide polymorphisms in the human aryl hydrocarbon receptor nuclear translocator (ARNT) gene. Drug Metabolism and Pharmacokinetics 26(6):637-645.
Wang, C., C. C. Li, W. Y. Gong, and T. Lou. 2013. New urinary biomarkers for diabetic kidney disease. Biomarker Research 1:9-12.
Yamazaki, K., D. McGovern, J. Ragoussis, M. Paolucci, H. Butler, D. Jewell, L. Cardon, M. Takazoe, T. Tanaka, T. Ichimori, S. Saito, A. Sekine, A. Iida, A. Takahashi, T. Tsunoda, M. Lathrop, and Y. Nakamura. 2005. Single nucleotide polymorphisms in TNFSF15 confer susceptibility to Crohn’s disease. Human Molecular Genetics 14(22):3499-3506.
Zolas, P. T., and A. Sher. 2013. Ethyl glucuronide: A biomarker for acute alcohol ingestion. Journal of Pharmacy and Alternative Medicine 2(2):1-4. http://iiste.org/Journals/index.php/JPAM/article/download/6884/7023 (accessed December 30, 2014).






















