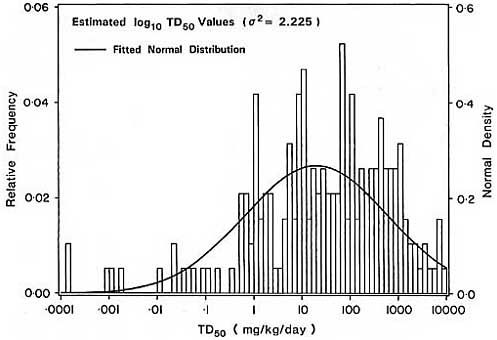
nonzero dose level, which precluded the use of the Weibull model to estimate the TD50 (see annex A for a detailed discussion of this issue). The median TD50 based on the fitted lognormal distribution was approximately 29 mg/kg/day, with 10th and 90th percentiles of 0.5 and 896 mg/kg/day, respectively. Although the inter-decile range is limited to a range of potencies of about 2,000-fold, the observed potencies again vary by more than eight orders of magnitude due in large part to the very low TD50 value for TCDD.
Rulis (1986) suggested that this distribution of carcinogenic potency could be used to establish a level of exposure below which regulatory attention would not be required. Such a threshold of regulation would be established on the basis of a lower quantile of the distribution of TD50s, on the assumption that a new untested chemical would be unlikely to be more potent than most known animal carcinogens (Munro, 1990). This concept has also been considered by Zeise et al. (1984).
The distribution of TD50s in Figure 1a is subject to overdispersion, since each individual TD50 is subject to experimental error. The distribution of true TD50s may be determined using the shrinkage estimators described in annex B. This technique adjusts for overdispersion by ''shrinking" each estimated TD50 towards the mean TD50 on a logarithmic scale, using a shrinkage factor determined by the relative magnitude of the variation within and between experiments (see annex B for details). Due to the large variation in TD50s noted in different studies, and the comparatively small standard error for individual TD50s, application of the shrinkage estimator in this case reduces the variance of the logarithm (base 10) of the TD50 from 2 = 2.2 to 2 = 1.8 (Figure 1b).
2.4 Classification of Carcinogens
Based on an evaluation of 237 chemical carcinogens tested in the U.S. National Toxicology Program, Rosenkranz & Ennever (1990) showed that carcinogens that are active at multiple sites in more than one species tend to be more potent than carcinogens that affect a single species or a single tissue. McGregor (1992) recently examined the characteristics of chemical carcinogens in different categories used by the International Agency for Research on Cancer to classify the strength of evidence for carcinogenicity. Carcinogens in Group 1 (known human carcinogens) tended to be more potent in rodents than carcinogens in Group 2A (probable