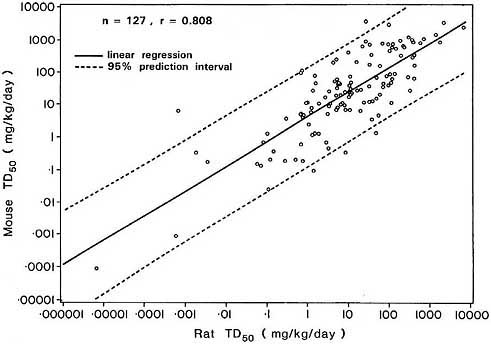
which include a real component. The latter models lead to slightly higher correlations, suggesting that although empirically observed correlations such as that in Figure 5 are largely due to the correlation between the corresponding MTDs, at least part of this association is non-artifactual.
6.2 Extrapolation from Rodents to Humans
Allen et al. (1988ab) and Crump et al. (1989) compared the carcinogenic potency, as measured by the TD25, for 23 chemicals for which suitable dose-response data were available from both human epidemiological studies and animal carcinogenesis bioassays. Several alternative methods of analyzing the animal bioassays were investigated, including the choice of the interspecies dose scaling factor, benign and malignant versus malignant tumors only, and separate versus average results across studies. Most of the methods yielded animal TD25s, that were significantly correlated with human TD25s, with rank correlation coefficients ranging up to 0.9. Although the correlation between potency rankings in animals and humans is high, the error associated with predictions of carcinogenic potency in humans based on animal data is substantial.
Chemotherapeutic agents represent another data base which may be useful in establishing animal-human correlation in carcinogenic potency. Kaldor et al. (1988) obtained estimates of the carcinogenic potency of 15 antineoplastic drugs that increase the risk of secondary tumors (acute non-lymphocytic leukemia). Two sets of TD50 values were obtained from the CPDB for 5 of these agents; the first set involved tumors of any type whereas the second set was restricted to tumors of the hematopoietic system. The potency rankings for 3 nitrogen mustard compounds (cyclophosphamide, chlorambucil, and melphalan) were similar in animals and humans. The most potent of the rat carcinogens, actinomycin D, did not cause leukemia in humans at the doses used, which are limited by its toxicity. The authors found these results encouraging in terms of using animal data to predict potency rankings, but cautioned against quantitative prediction of human carcinogenic potency on the basis of these data.
Goodman & Wilson (1991b) evaluated predictions of cancer risks based on potency values in the CPDB against epidemiological observations