On October 17, 2014, spurred by incidents at U.S. government laboratories that raised serious biosafety concerns, the U.S. government launched a 1-year deliberative process to address the continuing controversy surrounding so-called “gain-of-function” (GoF) research on respiratory pathogens with pandemic potential (White House, 2014a).1 GoF research is the latest example of U.S. efforts to develop oversight mechanisms for dual use research in the life sciences that can “reliably identify, and where necessary, mitigate risks while protecting scientific autonomy, discovery and innovation, public health, national security, and other critical interests” (Hebbeler, 2014).2
_______________
1 As discussed in Chapter 3, in virology “gain of function” refers to a type of mutation that results in an altered gene product that possesses a new molecular function or a new pattern of gene expression. A loss-of-function mutation is a type of mutation in which the altered gene product lacks the molecular function of the wild-type gene (Mouse Genome Informatics, http://www.informatics.jax.org/). The specific definition applied in the U.S. government policy is discussed in the text.
2 A 2004 report from the National Research Council, Biotechnology Research in an Age of Terrorism, argued that biotechnology posed a “dual use dilemma” because “the same technologies can be used legitimately for human betterment and misused for bioterrorism” (NRC, 2004:1). Most policy discussions have focused on efforts to address a subset of “dual use research of concern” (DURC), which was defined by the National Science Advisory Board for Biosecurity in 2007 as research that, “based on current understanding, can be reasonably anticipated to provide knowledge, products, or technologies that could be directly misapplied by others to pose a threat to public health and safety, agricultural crops and other plants, animals, the environment, or materiel” (NSABB, 2007:17). Examples of U.S. policy initiatives stimulated by the controversy over GoF research that began in late
The GoF controversy began in late 2011 with the question of whether to publish the results of two experiments involving H5N1 avian influenza and continued to focus on certain research with highly pathogenic avian influenza over the next 3 years.3 The new U.S. policy expanded the scope to include experiments with the coronaviruses that cause Severe Acute Respiratory Syndrome (SARS) and Middle East Respiratory Syndrome (MERS). The heart of the U.S. process is an evaluation of the potential risks and benefits of certain types of GoF experiments with influenza, SARS, and MERS viruses that would “inform the development and adoption of a new U.S. Government policy governing the funding and conduct of gain-of-function research” (White House, 2014a:3). As part of the process, the government also instituted a pause in both new and current funding for some GoF research projects while the evaluation was carried out.
New USG funding will not be released for gain-of-function research projects that may be reasonably anticipated to confer attributes to influenza, MERS, or SARS viruses such that the virus would have enhanced pathogenicity and/or transmissibility in mammals via the respiratory route. The research funding pause would not apply to characterization or testing of naturally occurring influenza, MERS, and SARS viruses, unless the tests are reasonably anticipated to increase transmissibility and/or pathogenicity. In parallel, we will encourage the currently-funded USG and non-USG funded research community to join in adopting a voluntary pause on research that meets the stated definition. (White House, 2014a:2)
Initially, 18 research projects funded by the National Institutes of Health (NIH) were halted, although several involving the effort to develop an animal model for studying MERS were later exempted (Kaiser, 2014).
Two entities were given special responsibilities for supporting the deliberative process. The National Science Advisory Board for Biosecurity (NSABB), a federal advisory committee established in 2004, is to “(1) advise on the design, development, and conduct of risk and benefit assessment studies” and “(2) provide recommendations to the USG [sic]
_______________
2011 include the USG Policy for Oversight of Life Sciences Dual Use Research of Concern (March 29, 2012), the HHS Framework for Highly Pathogenic Avian Influenza Research (2012), the NIH Guidelines for Research Involving Recombinant or Synthetic Nucleic Acid Molecules (November 2013), and the USG Policy for Institutional Oversight of Life Sciences Dual Use Research of Concern (September, 2014). In addition, in response to the laboratory incidents revealed in July 2014, White House Science Advisor John Holdren and Homeland Security Advisor Lisa Monaco issued a memorandum in August 2014 on Enhancing Biosafety and Biosecurity in the United States that “urged departments and agencies to take specific steps to strengthen safety and security” in federal laboratories (Hebbeler, 2014).
3 Information about the initial controversy may be found in NRC (2013).
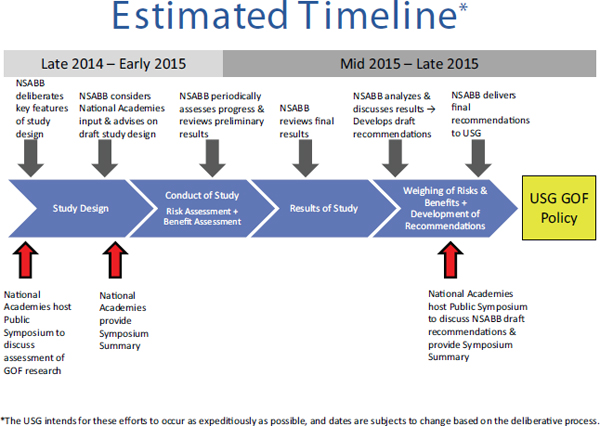
on a conceptual approach to the evaluation of proposed GOF studies” (Stanley, 2014). The formal assessment of the potential risks and benefits would be commissioned by the NIH and carried out by private contractors.4 The National Research Council (NRC) and Institute of Medicine (IOM) of the National Academies5 were asked to “provide a forum for broad public debate, which will inform the NSABB’s deliberations and the development of USG [sic] policy on GOF research” by carrying out two public conferences (Groesch, 2014). The first conference would offer the opportunity for input from a wide range of stakeholders about both general principles that should guide the assessments of benefits and risks and what specific issues should be considered, while the second would provide an opportunity for comments on the NSABB’s draft policy recommendations once the assessments were completed. In each case, a summary report of the meeting would be provided to the NIH within 1 month. The formal statement of task from the NIH for the first meeting may be found in Box 1-1. This document was prepared as a factual summary of that first meeting. The timeline for the entire deliberative process may be found in Figure 1-1.
The first public symposium was organized by a planning committee under the chairmanship of Dr. Harvey Fineberg, Gordon and Betty Moore Foundation. Biographies of the planning committee members may be found in Appendix B. The agenda for the meeting, which was held December 15-16 at the National Academies, may be found in Appendix C. Biographies of the speakers may be found in Appendix D. The symposium was attended by 140 individuals; a list of attendees may be found in Appendix E. In addition, the event was webcast and attracted approximately 300 viewers; an archived version may be found at https://www.youtube.com/playlist?list=PLuTGMA3A_-16HWJ6smsx4w1Bh_2TKf40V.
In his welcoming remarks IOM President Dr. Victor Dzau commented that the symposium was an example of one of the major roles played by the National Academies: providing a neutral forum for the discussion of complex and controversial issues in which science is an essential component. Both he and National Academy of Science (NAS) President Dr. Ralph Cicerone acknowledged the support for the symposium from the NIH as well as several foundations and underscored the importance
_______________
4 The “Sources Sought Notice” from the NIH for the project may be found at https://www.fbo.gov/?s=opportunity&mode=form&id=c134018fd1d008c582b7755be1fc1c06&tab=core&_cview=0.
5 The National Academies is the collective name of the National Academy of Sciences, the National Academy of Engineering, the Institute of Medicine, and the National Research Council.
BOX 1-1
Statement of Task
An ad hoc committee established by the National Research Council and the Institute of Medicine will organize two public symposia on Gain of function (GOF) research. The first symposium, which will be held in the middle of December 2015, will examine the underlying scientific and technical questions that are the source of current discussion and debate over GOF research involving pathogens with pandemic potential. The topics to be addressed include: Principles important for, and key considerations in, the design of risk and benefit assessments of GOF research. This will be informed by discussion of the following topics: Potential benefits of the research, including whether, in addition to generating new scientific knowledge about biological organisms, it will:
- 1. Inform public health responses to a potential pandemic, in particular by supporting surveillance efforts to identify possible pandemic strains and provide more time for preparedness; and
- 2. Facilitate the development of vaccines and antiviral therapeutics.
- Potential risks associated with the research, in particular those related to biosafety and biosecurity.
- Alternative methods that may be employed to yield similar scientific insights and/or potential benefits, while reducing potential risks.
The two-day symposium will invite participants with a wide range of perspectives and expertise, including public health, biosafety, public health surveillance, research, security, drug and vaccine development, and experts from regions of the world where pathogens with pandemic potential are endemic. The symposium will be webcast and the presentations and background materials will be archived online.
SOURCE: NIH, 2014.
of finding a way to allow science to move forward while addressing the concerns that were raised about GoF research.6
In his introduction to the symposium, Fineberg stressed that the event was intended to provide an opportunity for fundamental reflection and reconsideration, with no attempt to reach consensus or force agreement. There are widely divergent views from many areas of expertise about the potential risks and benefits of GoF research, and the debate, like the research, is international. This had been reinforced for him the previous
_______________
6 In addition to the NIH, support for the symposium came from the John D. and Catherine T. MacArthur Foundation, the Alfred P. Sloan Foundation, the Wellcome Trust, and internal National Academies’ funds.
week when he took part in a major meeting in Hannover, Germany, organized by the Volkswagen Foundation and the Max Planck Society that reflected the extensive European engagement with GoF issues.7 Although U.S. policy was the focus of this symposium, the issues are not restricted to the interests, programs, or funding of any one nation. Science is universal, and the possibilities of both the benefits and risks from GoF research are not limited to any country. So the question of how the world can proceed to develop a consensus about a way forward was an essential part of the backdrop.
The symposium was designed to offer an opportunity to explore what issues should be included in the risk and benefit assessments, “to systematically expose, explain, and identify those elements of consideration that should be brought to bear on the question of proceeding or not proceeding with what types of gain-of-function research…. We will have done our job in this next 36 hours if by the end of our conversation we have been able to enumerate in a more systematic way the questions, the points of potential agreement, the points of difference, and what differences would matter the most to the ultimate decisions that would need to be made” (Fineberg, 2014).
This report has been prepared by the workshop rapporteurs as a factual summary of what occurred at the workshop. The planning committee’s role was limited to planning and convening the workshop. The views contained in the report are those of individual workshop participants and do not necessarily represent the views of all workshop participants, the planning committee, or the National Research Council. It offers a summary of the key issues and ideas identified during the symposium, but offers no consensus conclusions or recommendations and is intended to reflect the discussions during the meeting. In order to be as responsive to the charge as possible, it is organized thematically rather than chronologically, so that ideas raised at various points in the symposium are grouped together. Chapter 2 begins with an overview of risk and benefit analysis. It is followed by an overview in Chapter 3 of the science associated with GoF research, the particular characteristics of the experiments that are the subject of the U.S. funding pause and the earlier controversy over influenza, and some of the ideas that were suggested as alternative experimental approaches. Chapter 4 describes the discussions of the major potential benefits claimed for GoF research, while Chapter 5 describes the discussions about potential biosafety and biosecurity risks. Chapter 6 offers a summary of the presentation and discussions of policy issues.
_______________
7 Further information about the Hannover meeting, “Dual Use Research: Biosafety, Biosecurity, Responsibility,” may be found at http://www.volkswagenstiftung.de/dualuseresearch.