6
Resuscitation Research and Continuous Quality Improvement
Research, along with the implementation of best practices that are based on that research, provides the foundation necessary to improve cardiac arrest outcomes over the next decade. Despite being a leading cause of death in the United States (Taniguchi et al., 2012), cardiac arrest lacks prioritization in terms of national support and collaboration to enhance research efforts. Fundamental gaps in knowledge about the epidemiology, etiology, pathophysiology, and treatment of cardiac arrest contribute to difficulties in establishing national evidence-based practice standards to guide local decision making about practice protocols that can lead to optimal patient care and outcomes. State-level regulation of emergency medical services (EMS) and health care systems, as well as variation in available resources and capacities, further complicates the process. A broader focus on, and investment in, cardiac arrest research is needed to overcome these challenges.
Paradoxically, as knowledge about cardiac arrest expands, it can be increasingly difficult to analyze this knowledge and to uniformly and rapidly translate it into enhanced patient care. Within the current systems of response to cardiac arrest, there are many occasions to generate waste that can affect patient care. In general, patient outcomes are dependent on the following components: (1) a culture that supports scientific pursuit; (2) science that expands the existing knowledge base; (3) translation of this knowledge into evidence-based practice on a wide scale; and (4) care that is provided by competent, well-trained individuals and is subject to formal and continuous quality assessment. Specific barriers and limitations between each component contribute to missed opportunities, waste, and harm, as illustrated in Table 6-1.
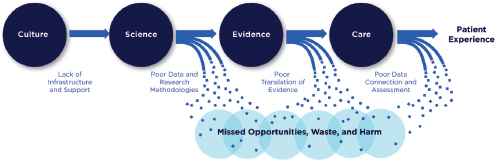
FIGURE 6-1 Schematic of today’s system of response to cardiac arrest.
SOURCE: Adapted from IOM, 2012, p. 15.
Reducing the missed opportunities, waste, and harm at a national level requires known treatments and strategies to improve the system of response, as well as a renewed commitment to research—not only the execution of sound basic, clinical, and translational research, but also a steadfast commitment to the principle of continuous measurement and assessment between and within EMS and health care systems. To achieve this goal, the field of resuscitation science must not only evaluate the current approaches to the treatment of cardiac arrest, but also fundamentally reassess its approach to research and existing mechanisms for generating new knowledge. First, there is a need to improve the current methods for quantifying and understanding the public health problem of cardiac arrest, including both care process and outcomes. Second, it is essential to consider a paradigm shift in how scientific investigation is conducted in order to appreciate the likely complexity of patient populations and treatment effects. Third, it is critical to identify and implement new strategies and devices for improved care delivery. Finally, it is important to reconsider the processes by which experts evaluate scientific evidence and develop and implement guidelines for recommended treatments. In other words, the resuscitation field needs to reappraise how knowledge about cardiac arrest is generated and how best to translate that knowledge into improved patient outcomes (Neumar, 2010). This will require both internal and external support for the resuscitation field.
Previous chapters have examined the evidence on treatments and interventions for out-of-hospital cardiac arrest (OHCA) and in-hospital cardiac arrest (IHCA) and have explored strategies to optimize postarrest care. In this chapter, the committee evaluates the current state of resuscitation research and identifies potential areas for improvement. The first two sections describe the state of current infrastructure, highlighting
promising new areas of research to improve cardiac arrest survival, and the overarching status of cardiac arrest research infrastructure and support. The next section discusses the need for a learning system of response to cardiac arrest based on principles of a learning health care system and formal adoption of continuous quality improvement principles to improve accountability for system performance and cardiac arrest outcomes. The final section talks about the need for increased advocacy to generate sustained and sufficient support for resuscitation research and dissemination of research findings to positively affect clinical practice and patient outcomes.
THE STATE OF RESUSCITATION RESEARCH: ENHANCING THE SCIENCE
To better understand the physiological, social, and environmental risk factors for cardiac arrest and unfavorable health outcomes associated with cardiac arrest, research and specialized expertise are needed across an array of disciplines. High-quality, innovative investigations across the spectrum of research are needed to support further progress in valuable research areas, such as cellular and molecular medicine. From basic science to the translation of evidence into practice in communities across the country, there are a multitude of opportunities along the translation research continuum where the resuscitation field could benefit from targeted improvements. Figure 6-2 depicts the phases of this continuum.
Applying the phases of translational research to cardiac arrest, the figure starts with basic science (T0), where diminishing funding has led to a paucity of cardiac arrest research that focuses on the etiology and physiological mechanisms that underlie many of the complexities noted throughout this report. Without an understanding of physiological mechanisms of action, the translation of new therapies to human studies (T1) decreases. When innovative new therapies are not tested in humans, the translation to patients (T2) is not possible. Translation to practice (T3) and the broader community (T4) is then stifled, resulting in missed opportunities to affect the survival and outcomes of individuals and populations within communities. This section assesses the potential of research, new research models, and emerging technologies and devices to improve treatment protocols for cardiac arrest that will improve patient outcomes.
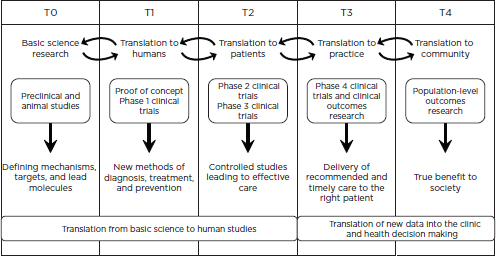
FIGURE 6-2 Operational phases of translational research (T0-T4).
SOURCE: Adapted with permission from Macmillan Publishers Ltd.: Nature Medicine (Blumberg et al., 2012).
Research to Improve Treatments
In recent years, a new paradigm for cardiac arrest response proposed a three-phase model (i.e., electrical, circulatory, and metabolic phases) “to reflect the time-sensitive progression of resuscitation physiology, which in turn requires time-critical interventions” (Weisfeldt and Becker, 2002, p. 3035). Research has demonstrated that early access to existing therapies during the electrical phase (from time of cardiac arrest to approximately 4 minutes following arrest) and the circulatory phase (between 4 and 10 minutes after arrest) can be highly effective (Weisfeldt and Becker, 2002; see also Ewy et al., 2006). After approximately 10 minutes without treatment, patients enter the metabolic phase of cardiac arrest, which involves a number of cascading biochemical pathways that may extend beyond localized organ damage and can result in full-body systemic injury (Bainey and Armstrong, 2014; Frohlich et al., 2013; Kalogeris et al., 2014). The metabolic phase of cardiac arrest is associated with poor survival rates and neurologic and functional outcomes. In this late phase, the standard guideline-recommended therapies, such as CPR and defibrillation, are usually unsuccessful (Weisfeldt and Becker, 2002). Additional research is needed to define the role of alternate techniques for improving blood flow during a cardiac arrest to
maximize the period for successful intervention. Box 6-1 provides other examples of possible research areas by treatment phase.
Metabolic therapies that target specific injury pathways have demonstrated increasing potential to improve survival following prolonged, untreated cardiac arrest (Bartos et al., 2014, 2015; Riess et al., 2014). Furthermore, some studies suggest that the commonly accepted 4-minute time limit beyond which ischemic injuries begin to irreversibly damage critical organs may be extended (Allen and Buckberg, 2012; Allen et al., 2012a,b; Athanasuleas et al., 2006; Trummer et al., 2010, 2014).
Recent investigations have reported that a combination of protective drugs and other treatments may be more effective in delaying the severe biological consequences of prolonged cardiac arrest (Bartos et al., 2015; Boller et al., 2011). Using a combination of therapies targeting the circulatory and metabolic phases of cardiac arrest, animal model research suggests the potential to restore life after sustained periods of clinical death. For example, in a recent laboratory experiment using porcine models, Bartos and colleagues (2015) demonstrated improved rates of survival with minimal or no neurological deficits after 17 minutes of cardiac arrest after administering medications and treatments to mitigate the tissue damage associated with reperfusion injury. Another swine experiment achieved survival following 30 minutes of isolated brain ischemia by infusing a warm blood reperfusate that consisted of free radical scavengers, a calcium chelating compound, inflammatory controls, osmotic controls, and energy substrates for 20 minutes instead of oxygenated blood during reperfusion (Allen et al., 2012a).
In a recent human trial, Australian investigators treated cardiac arrest patients who had an initial cardiac rhythm of ventricular fibrillation (VF), had received traditional advanced cardiac life support (ACLS), but had not achieved return of spontaneous circulation after 30 minutes (among other inclusion criteria) (Stub et al., 2015a). Researchers employed a combination of treatments that included mechanical cardiopulmonary resuscitation (CPR) (due to long periods of resuscitation), intra-arrest cooling via intravenous ice cold saline, rapid initiation of extracorporeal membrane oxygenation, and rapid percutaneous coronary intervention for patients with coronary artery occlusion (Stub et al., 2015a). Of the 26 patients who participated in this study, 14 (54 percent) were discharged from the hospital with favorable neurologic status (Stub et al., 2015a). Although this was a small feasibility study, it demonstrates new possibilities in cardiac arrest recovery and the importance
of funding research to explore new treatment models and therapies in efforts to improve patients’ lives.
BOX 6-1
Examples of Possible Research Areas by Treatment Phase
Electrical Phase
Research to reduce time to defibrillation, including the use of automated external defibrillators (AEDs), implantable medical devices (e.g., implantable cardioverter defibrillators), and wearable defibrillators.
Circulatory Phase
Research to improve efficiency of CPR, which currently only provides 10 to 30 percent of regular blood flow to the heart and approximately 30 to 40 percent to the brain, as demonstrated in animal studies (Meaney et al., 2013).
Research into the effects of alternate manual CPR techniques, such as those that employ active compression and decompression and impedance threshold devices (Pirrallo et al., 2005; Wolcke et al., 2003).
Research involving advanced circulatory systems, such as emergency cardiopulmonary bypass resuscitation, which involves the rapid placement of the arrested patient on a heart-lung machine to provide full blood circulation to the heart and brain (Johnson et al., 2014; Nagao et al., 2000, 2010; Nichol et al., 2006; Wallmuller et al., 2013).
Metabolic Phase
Investigation of interventions designed to control and minimize reperfusion injury, the inflammatory response responsible for cellular death and diminished organ function (Anderson et al., 2006; Bainey and Armstrong, 2014; Becker, 2004; Frohlich et al., 2013; Lavani et al., 2007).
Research on the effects of hypothermia on the cellular, molecular, and physiological effects of ischemia and reperfusion (Bro-Jeppesen et al., 2015; Cronberg et al., 2015; Nolan et al., 2010).
Research on the effects of other interventions targeting post-anoxic and ischemic brain injury.
Research Methodology and Trial Design
Over the past 30 years, many large randomized clinical trials for specific cardiac arrest treatments have found no demonstrated benefit (Aufderheide et al., 2011; Stiell et al., 2011). For instance, a recent study found that targeted temperature management at 33oC did not improve survival or neurological outcomes over targeted temperature management at 36oC (Nielsen et al., 2013). Similarly, research findings related to the impact of mechanical CPR devices are mixed, with trials demonstrating increases in CPR quality, but only nonsignificant impacts on survival to discharge (Hallstrom et al., 2006; Ong et al., 2012b; Wik et al., 2014).
The lack of measured impact in these trials may be attributable to a variety of systemic factors and methodological limitations. One possible explanation is that specific treatments are simply ineffective. As a result, researchers may focus on therapies with a limited likelihood of measurable benefit, including therapies that are based on insufficient preclinical work, that have unknown or unclear mechanisms of action, or have documented ineffectiveness. Decisions made during the design of clinical trials may also reduce the likelihood that the study will result in a demonstrated benefit or identify optimal use. This is especially true if the trials are based on sparse prior information related to the optimal treatment population, treatment efficacy, and the most appropriate biomarker or patient-centered outcome measure. The urgency of identifying new therapies for cardiac arrest can create incentives for researchers to perform confirmatory or even pragmatic trials before clearly establishing efficacy in more controlled and limited settings.
Resuscitation research investigating the efficacy of new treatments should utilize clinical designs and research strategies that have the highest probability of demonstrating benefit after accounting for the heterogeneous nature of cardiac arrest and cardiac arrest patients. Traditional drug and device development processes, which largely uniformly apply and evaluate one therapy at a time regardless of important variations in patient populations, may fail to detect clinically important benefits when therapies are combined or used in select subsets of patients. The complex nature of cardiac arrest will likely require simultaneous testing of multiple treatment and treatment modalities in order to identify new effective treatments. The biggest successes may materialize when multiple treatment approaches are combined and carefully tailored to individual patient characteristics and responses to treatment (i.e., individualized resuscitation).
There is now broader recognition in the scientific community that the traditional sequential series of individual clinical trials—each testing a single treatment in a relatively homogeneous population—is time consuming, resource intensive, and increasingly leads to failed trials late in the testing phase (Berry et al., 2015). An analysis of research into neuroprotectants for ischemic strokes—an area that involves local effects of ischemia and reperfusion—identified more than 40 failed Phase III trials (Minnerup et al., 2014). Recent analyses of the increasing overall failure rates in Phase III pharmaceutical clinical trials have concluded that inadequate Phase II trials (or “learn phase” trials) contribute to, as a primary factor, the low success rates in Phase III trials (Lavine and Mann, 2012; McAuley et al., 2010; Retzios, 2009).
Examples of research strategies and clinical trial designs that could increase the efficiency and success rate of resuscitation research include the increased use of small, hypothesis-generating studies; a more robust exploration of dose effects and modifiers of efficacy (i.e., interactions between treatments and patient characteristics) in learn phase trials; the use of randomized, withdrawal trials; and the implementation of adaptive clinical trials and platform trials (see Table 6-1). For example, a randomized, withdrawal trial could be used to study the use of therapeutic hypothermia. This type of trial would treat all patients with therapeutic hypothermia and then randomize the time of rewarming (the withdrawal of the treatment) only in the subset of patients who demonstrated some benefit as determined by a defined biomarker. Other novel approaches include adaptive and platform trials, which often use response-adaptive randomization so that patients who are enrolled later in the trial are preferentially randomized to treatment arms that are most likely to be beneficial (Berry et al., 2015; HHS, 2010). This approach may improve the risk-to-benefit balance for research subjects, increase the chance of a definitive trial outcome, and increase the number of subjects ultimately treated with the best-performing treatment arm (Meurer et al., 2012). Criteria for defining a positive adaptive or platform trial result should be selected to ensure protection from false positive results (FDA, 2015a).
A strategic approach to implementation of resuscitation science discoveries and new therapies is required to foster evidence adoption, use, and sustainability. Once a new treatment has demonstrated benefit in controlled clinical trials, it must be tested in increasingly realistic settings to confirm effectiveness and to identify the optimal clinical practice
TABLE 6-1 Traditional and Alternative Clinical Trial Designs
| Design | Definition |
| Traditional clinical trial | A prospective biomedical or behavioral research study that is designed to test the safety and effectiveness of a therapeutic agent or intervention (e.g., drug, vaccine, device) using consenting human subjects. These trials generally evaluate the use of a single treatment relative to the standard of care in a relatively homogeneous patient population. |
| Randomized withdrawal trial | Experiments in which subjects who respond positively to an intervention are randomized to continue receiving that intervention or to receive a placebo. This trial design minimizes the time subjects spend receiving a placebo (IOM, 2001, p. 40) and focuses the comparison on the subset of subjects who demonstrate a response to treatment, potentially increasing the ability of the trial to demonstrate benefit. |
| Adaptive clinical trial | A clinical trial design that includes a prospectively planned opportunity for modification of one or more specified aspects of the study design (e.g., randomization ratios, sample size) based on analysis of interim data from study subjects (adapted from FDA, 2015a). |
| Platform trial | A clinical trial designed to simultaneously evaluate multiple treatments or combinations of treatment. This design offers the possibility that some treatments may be removed from the trial and others may be added over time (Berry et al., 2015). |
| Pragmatic trial | This type of trial measures treatment effectiveness or the benefit the intervention produces in routine clinical practice, and it accurately reflects variation in patient populations and care delivery (Patsopoulos, 2011). |
| Randomized registry trial | A large-scale, randomized experiment based on data collected from registries and patient records. These trials are designed to minimize the burden of data collection, increase external validity, and reduce the time to dissemination when compared with traditional clinical trials (Lauer and D’Agostino, 2013). |
protocols. Translational research focuses on the translation of new findings in basic science into new treatments and the adoption of those treatments in practice (Rubio et al., 2010). Table 6-2 offers a summary of key areas of focus for possible future resuscitation research across the spectrum of translational research. In the resuscitation field, guidelines are a predominant mechanism for translating research into practice. (The strengths and weaknesses of national guidelines related to cardiac arrest treatment are discussed later in this chapter.) The rapid translation of basic research findings into new treatments and the adoption of new treatments into practice is more likely if clinical trials and related studies are designed to produce results that can facilitate evidence-based practice.
TABLE 6-2 Key Areas for Resuscitation Science Research
| Major Research Area | Research Subjects |
| Basic science | Mitochondria, energetics, and metabolism; cellular death pathways; loss of ionic control and membrane integrity; reperfusion injury biology; pharmacology, receptors, and channels; endothelial barrier and injury; inflammatory pathways; genetics and epigenetics; neurological injury and cardiovascular injury mechanisms |
| Bioengineering and biosensor science | Sensors of micro- and macro-hemodynamic function; EKG and defibrillator developments; metabolic sensors, redox sensors, and mitochondrial function sensors; optimization of CPR and external compressors; ventilator devices; modulation of thoracic impedance; cardiopulmonary bypass technologies; artificial oxygenation; oxymetry, capnography; early EMS notification technology; prediction of cardiac arrest technologies; vascular access technologies |
| Preclinical science | Oxygenation during resuscitation; pharmacological ACLS protocol testing; combinatorial therapies (multidrug cocktail development); one-size-fits-all versus patient-centered physiologically guided CPR; translational studies on favorable basic science and bioengineering technologies; optimization of hypothermia studies and new hypothermia technology; impact of differing age on resuscitation; ventricular fibrillation mechanisms; defibrillation waveforms; emergency cardiopulmonary bypass mediated resuscitation |
| Clinical studies | Optimal airway and oxygenation studies; ACLS pharmacological studies of epinephrine, vasopressor drugs, and antiarrhythmic drugs; hypothermia studies; optimized CPR technique and protocol studies; clinical studies focused on special populations: studies of neonates, children, and geriatric populations; studies of combinatorial strategies |
| Systems of care | Simulation training, team training, and high-performance CPR; dispatcher-assisted CPR, telemedicine, and remote resuscitation care; social media, crowdsourcing to improve notification, bystander action, and community survival rates; willingness-to-act-to-rescue studies, early recognition of symptoms; optimization of training studies; implementation strategy studies; studies on the impact of public policy, informed consent, and ethics of resuscitation |
| Population studies | Risk factor and genetic markers of cardiac arrest; diversity studies focusing on communities with high- and low-survival rates; racial, ethnic, and socioeconomic factors in incidence and survival; effects of age on incidence and survival; special populations: focused on underserved communities, resource-scarce communities, and diverse populations |
NOTES: This list was generated by review of two prior consensus statements on resuscitation research priorities and was then amended with additional consideration by the IOM Committee on the Treatment of Cardiac Arrest: Current Status and Future Directions. In 2002, the NIH-sponsored PULSE Conference identified five domains for high-priority research that included (1) mechanisms, (2) pharmacology, (3) translational studies, (4) bioengineering, and (5) clinical evaluative research (Becker et al., 2002). In 2005, the American Heart Association sponsored the 2005 Guidelines Conference and identified categories that included medical emergency teams; recognition of cardiac arrest and its causes; body position; electrical defibrillation; blood flow generation; airway management; ventilation; oxygenation; pharmacological interventions; metabolic, temperature, and post-resuscitation management; physiological monitoring and feedback; ethical issues; education and training; and outcomes (Gazmuri et al., 2007). ACLS = advanced cardiac life support; CPR = cardiopulmonary resuscitation; EKG = electrocardiogram.
Emerging Technologies and Devices
Recent progress in science, engineering, health informatics, and mobile technologies has created the potential to revolutionize treatments and care delivery in the field of cardiac arrest and resuscitation. The committee was charged with evaluating the “research, technology transfer and innovation, and implementation gaps,” as well as promising new
strategies that have the potential to improve cardiac arrest outcomes (see Box 1-1). Previous chapters have described innovative new therapies (e.g., hemodynamic support therapies such as extracorporeal membrane oxygenation) and care strategies (e.g., prediction models for early detection of deterioration in IHCA patients) for OHCA and IHCA resuscitation and post-arrest treatment, as well as devices to improve quality of resuscitation treatment (e.g., monitoring and impedance threshold devices). This section examines emerging technologies and devices that are currently in prototype or early preclinical phase testing and, therefore, have not been widely incorporated into clinical practice. Although many of these devices and mobile applications have not yet met the U.S. Food and Drug Administration (FDA) criteria for broad distribution because of the lack of evidence from large-scale clinical trials, the committee recognizes that these state-of-the-art technologies have the potential to significantly improve worldwide survival of cardiac arrest.
Innovations in smartphone and mobile applications, social media, home monitoring devices, and wearable technologies could significantly reduce the time interval between collapse and treatment and substantially improve patient survival rates (Scholten et al., 2011). Collection of data from many of these devices could be used to enhance research and surveillance activities, with appropriate privacy protections, and drive improvements in prevention of cardiac arrest as well as quality of care delivery (Bosley et al., 2013; Chang et al., 2013). Similarly, analytics of patient data from hospital and other health care facilities have the potential to predict cardiac arrest, thereby improving medical treatment and response (Churpek et al., 2012; Ong et al., 2012a). Emerging technologies may support better CPR training and performance by monitoring the rhythm and compression depth with real-time feedback and could be an effective aid in continuing education for first responders (Chang et al., 2015; Fischer et al., 2011; Martin et al., 2013). Finally, outpatient monitoring of patients who are already at greater risk for cardiac arrest (such as a recovering cardiac arrest patient or a patient with a predisposing cardiac condition) could potentially reduce the cost of care across a greater number of patients, especially in rural or underserved areas (Przybylski et al., 2009; Reynolds et al., 2006). Technological innovations for assessing patients undergoing resuscitation have the potential to personalize our approach to patients in cardiac arrest.
Adoption of these mechanical devices and wearable technologies has been limited because of a lack of robust evidence collected from clinical
trials, as well as challenges related to data management and patient privacy issues, reliability and interoperability (Honeyman et al., 2014). Moreover, there are few consensus statements on best practices or implementation of these technologies. FDA recently issued guidelines regarding mobile medical applications, but it has not yet evaluated these in the context of cardiac arrest treatment and care strategies (FDA, 2015c). Box 6-2 provides additional examples of new technologies and devices that could enhance the cardiac arrest chain of survival.
Although the private sector plays an integral role in developing technologies to improve cardiac arrest treatment, the public sector can also support progress through a variety of mechanisms. Research to determine the effectiveness of mobile applications, social media, and innovative devices such as wearable alert systems in improving cardiac arrest survival is paramount. The National Institutes of Health’s (NIH’s) National Institute of Biomedical Imaging and Bioengineering supports research that integrates physical, engineering, and life sciences to accelerate the development and application of biomedical technologies (NIBIB, 2015). Research into new areas, such as advances in imaging or thermometry, may lead to new therapies to restore spontaneous circulation and diagnose or prevent neurological damage after cardiac arrest. Additional research is necessary to develop more complex devices targeting early recognition and response to cardiac arrest, as well as the quality of resuscitation and post-arrest care.
Regulatory controls, such as the premarket approval process, must promote safety without stifling innovation; this constitutes a major challenge within health policy and translational science, both in general and in the field of cardiac arrest in particular (FDA, 2011). As of February 2015, FDA now requires that AEDs and AED accessories (e.g., batteries, electrodes, and adapters) undergo premarket approval before being released for sale to the general public (FDA, 2015b). This decision was made in light of device recalls and reported problems with the design and manufacture of AEDs. The premarket approval process is meant to provide reasonable assurance of the safety and effectiveness of the device, and it includes review of the methods, facilities, and controls involved in the manufacturing, processing, packing, and installation of devices (FDA, 2014). To improve efficiencies in regulatory approval of technology for cardiac arrest, fast-track review, and expedited premarket approval within FDA may be appropriate with post-market surveillance.
BOX 6-2
Examples of Emerging Technologies and Devices
Early Detection and Emergency Alert System
A majority of cardiac arrests occur in the home and are often unwitnessed. Mobile applications, technologies, and devices that can track outpatient physiological data, monitor electrocardiograms and pulse and cardiac arrhythmias, and activate the “chain of survival” could have a profound effect on saving lives. A recent study tested a wrist watch that is designed to send a radio signal to alert emergency medical systems when it detects a loss of pulse (Rickard et al., 2011). It includes a motion sensor that can cancel the alert if the patient is conscious. Although an initial clinical trial demonstrated a 10 percent false-positive rate, such outpatient monitoring can significantly reduce collapse-to-treatment times in cardiac arrest and shows great promise.
Bystander Response Assistance
There are more than 50 smartphone applications currently on the market that can perform a variety of tasks including providing a platform that connects cardiac arrest patients with the nearest bystander or trained first responder, and providing CPR instructions and locating the nearest AEDs (Sakai et al., 2011, 2015; Scholten et al., 2011). Multiple EMS agencies have these mobile applications, which are simultaneously activated by the local public safety agencies along with EMS dispatch for patients who arrest in public locations (PulsePoint, 2015). One study in Sweden found that bystanders arrived before the ambulance over half of the time and performed CPR 30 percent of the time (Ringh et al., 2011). Innovative prototypes for “ambulance drones” have been developed and proposed as a model strategy for reducing collapse-to-defibrillation time (TU Delft, 2014). The EMS provider can air deliver the “ambulance” which contains a functional AED, camera, and speaker, using a built-in global positioning system and can locate the cardiac arrest patient and bystander.
Internal, External, and Wearable Technologies for Defibrillation
Patients at increased risk of cardiac arrest can use wearable external defibrillators as a promising and noninvasive alternative to implantable cardioverter-defibrillators. Wearable defibrillators are FDA approved, covered by many health plans in the United States, and can effectively detect and reverse ventricular tachyarrhythmia (Feldman et al., 2004; Lee and Olgin, 2009). They consist of a lightweight garment that sounds an alarm before delivering a shock. Patients who are conscious can manually turn this off to avoid receiving defibrillation. A number of nonwearable and portable AEDs also are available on the market.
Research and Surveillance Capacity
Data stored by AEDs and implantable cardioverter defibrillators can be transmitted via smartphones and cellular networks to providers for remote monitoring (Crossley et al., 2011). Traditional surveillance systems often cannot accurately capture the time interval between patient collapse and treatment. Data from AEDs as well as wearable devices that allow wireless data export of global-positioning-system or patient-monitored data, can provide information regarding time of collapse, location, and other important factors, and can fill an important gap in current knowledge regarding unwitnessed cardiac arrests. Some investigators have harnessed the power of social media outlets such as Twitter to analyze trends in cardiac arrest and better understand factors that determine public action and response (Bosley et al., 2013).
Devices to Guide and Individualize Treatment
New technologies have the potential to guide and individualize resuscitation efforts. Mechanical CPR devices that allow for individualization (such as discrete input [e.g., gender or body size] or continuous input [e.g., carbon dioxide levels]) have demonstrated efficacy in limited settings. However, a meta-analysis found mixed evidence from clinical trials and did not find a substantial difference between mechanical and manual CPR in increasing survival to discharge (Ong et al., 2012b). Currently, end-tidal carbondioxide monitoring is recommended but is limited to intubated patients (Neumar et al., 2010). Noninvasive tools such as ultrasound and devices that are capable of measuring blood flow during CPR have the potential to change the approach to the treatment of patients in cardiac arrest (Volpicelli, 2011).
INFRASTUCTURE AND SUPPORT FOR RESUSCITATION RESEARCH
Many barriers within the resuscitation research field limit the impact of much-needed cardiac arrest research. Fragmented administrative oversight and coordination across federal research agencies and in academia complicates efforts to better understand the state of resuscitation research and engage in coordinated, collaborative research and funding efforts. Funding that is available for resuscitation research is disjointedly allocated and lacks transparency. Based in part on these persistent challenges, advocacy efforts have not been able to successfully expand available research resources across all levels—from basic research to population-level studies. Ideally, a robust cardiac arrest research agenda would ensure that research findings at the T0 level are transformed into practices
that benefit the community (T4) and improve health. To successfully implement such a broad research agenda, the resuscitation community must first overcome the infrastructure barriers described below in order to ensure a sustainable and active research pipeline that includes sufficient numbers of researchers.
Administrative Oversight and Coordination
Federal agencies charged with activities related to the public’s health and high-quality health care play an important role in the success and failure of local, state, and national efforts to improve outcomes from disease, health conditions, and health-related events—such as cardiac arrest. For example, NIH credits its research for major health advances related to declines in mortality from cancer, heart disease, and stroke, as well as improvements in antiviral therapies for people infected with the human immunodeficiency virus (NIH, 2012b). However, fractured federal oversight, a lack of interagency coordination, and overlapping authorities within federal agencies pose substantial barriers to advancing basic, clinical, and translational research in cardiac arrest treatment. At a national level, federal oversight of responding to and treating cardiac arrest has been largely prescribed by the site of cardiac arrest, with OHCA largely the province of EMS and IHCA the responsibility of hospital-based systems of care. For example, agencies or departments involved with EMS, including the U.S. National Highway Transportation and Safety Administration and the U.S. Department of Defense, may view cardiac arrest treatment as one indicator of the quality of the overarching EMS system (NHTSA, 2009).
NIH, the Centers for Disease Control and Prevention (CDC), and the U.S. Health Resources and Services Administration (HRSA) provide traditional support for general resuscitation research through various grant mechanisms that fund individual research studies, large-scale data collection, and infrastructure. However, within agencies, resuscitation research grants may be spread across a wide range of institutes or offices and various topics (e.g., basic science, training, and treatment protocols) related to, but not necessarily dedicated to, the study of cardiac arrest. For example, although the National Heart, Lung, and Blood Institute (NHLBI) is the official lead institute for resuscitation research within the NIH, funding is spread across multiple NIH institutes, including the National Institute of Neurological Disorders and Stroke (NINDS) and the National Institute of Child Health and Human Development (NICHHD)
(see Figure 6-3). A pediatric cardiac arrest study that targets the circulatory phase of resuscitation could theoretically be funded as a cardiovascular, neurologic, or pediatric study within different NIH institutes. Because the missions and priorities of individual institutes may overlap, the appropriate funding institute is not always apparent to investigators or the public, leading to confusion and suboptimal coordination of activities within the research community. Although this challenge is not unique to cardiac arrest research, it has an ongoing impact on efforts to streamline cardiac arrest research.
NIH also houses the Office of Emergency Care Research, which “coordinates, catalyzes and communicates about NIH funding opportunities in emergency care research and fosters the training of future researchers in this field” (NIH, 2013b). It is a trans-NIH office but, unlike other trans-NIH offices, has no budget for funding research (NIH, 2012a). Although it does not focus exclusively on resuscitation research, the office has worked with NINDS and the NICHHD on research and training activities related to post-resuscitation hypothermia (NIH, 2015c). As mentioned in Chapter 2, the office will also play a role in NIH’s new cross-collaborative clinical trials network for emergency care, Strategies to Innovate EmeRgeNcy Care Clinical Coordinating Center (SIREN), which could be used for resuscitation research.
In addition to support provided by NIH, CDC, and HRSA, a number of other federal agencies and private organizations provide support for cardiac arrest and resuscitation research or whose missions aligns with improving cardiac arrest treatment and care. The U.S. Department of Veterans Affairs (VA) funds multiple projects with various academic institutions to better understand the incidence and response to cardiac arrest. The VA also requires its care facilities to establish an interdisciplinary committee to “review each episode of care where resuscitation was attempted for the purpose of identifying problems, analyzing trends, and improving processes and outcomes,” but the enforcement of this rule between facilities varies (VA, 2015). Although the Patient-Centered Outcomes Research Institute has not focused on cardiac arrest specifically, its mission “to improve patient care and outcomes through patient-centered comparative clinical effectiveness research” is generally applicable to resuscitation research (PCORI, 2014). The Centers for Medicare & Medicaid Services (CMS) reimburses health care providers for particular services and treatments related to cardiac arrest, but the committee
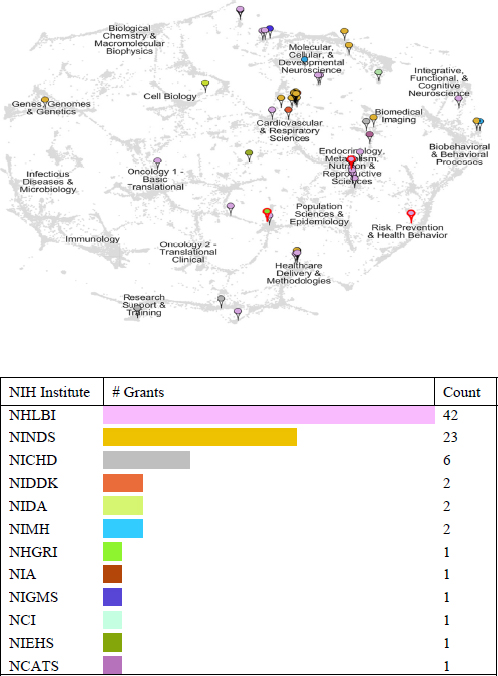
FIGURE 6-3 Location and number of grants related to cardiac arrest within the National Institutes of Health in 2013.
SOURCE: Lathrop, 2014.
did not find any recent initiative that focuses specifically on cardiac arrest survival.1
The Need for Sustained and Coordinated Research Funding
The ability of resuscitation research to improve cardiac arrest outcomes relies on sustained funding and widespread implementation of practices based on newly generated evidence (described in greater detail in the next section). In 1962, Senator Hubert H. Humphrey urged “the creation of NIH centers or institutes that focus on the physiology of death, on resuscitation and on related topics,” underscoring the desire to prolong life, postpone death, and possibly reverse death (Humphrey, 1962, emphasis added). This spurred interest and research related to cardiac arrest treatment. Unfortunately, evolving and shifting priorities within federal agencies and among congressional leadership shifted focus away from cardiac arrest treatment as early as the 1990s and continues to affect current research activities (Thompson et al., 1996).
Many federal agencies and private-sector organizations provide research support related to the study and treatment of cardiac arrest. However, even within a single Institute or office, the exact amount of annual research funding is often difficult to calculate. The majority of NIH funding for cardiac arrest, as for other biomedical research funding through NIH, supports investigator-initiated research applications. As a whole, cardiac arrest funding is not reported as a separate line item in NIH’s annual research portfolio, as other research and disease areas are (e.g., Alzheimer’s disease, brain cancer, traumatic brain injury, and stroke) (NIH, 2015a). Estimates of total annual funding related to cardiac arrest are available, calculated by a search and review of all funded grant awards by search term. The search terms “cardiac arrest” and “resuscitation science” generally identify grants related to the treatment of cardiac arrest, whereas the term “sudden cardiac death” is categorized as an arrhythmia, which also includes prevention-related research on “arrhythmogenesis, genetic and environmental bases of normal cardiac electrical activity and arrhythmias, and etiology of rare and common arrhythmias” (NIH, 2014).
Based on these searches, NIH’s total support related to cardiac arrest in fiscal year 2013 was approximately $107.7 million ($40.3 million for cardiac arrest and $67.4 million for sudden cardiac death and resuscita-
________________
1CMS and CDC are joint leaders of the Million Hearts® Initiative, which focuses on the prevention of heart attacks and stroke (CDC, n.d.).
tion sciences), of which NHLBI provided $85.4 million (Lathrop, 2014). However, these estimates are likely inflated because of overlap in the awards identified using search terms (i.e., more than one search term may be included in the same grant award). Comparatively, stroke, which kills approximately 130,000 people annually received approximately $282 million of NIH’s research budget in 2013 (CDC, 2015; NIH, 2015a). In fiscal year 2013, NIH invested an estimated $5.27 billion on cancer research and about $1.96 billion on cardiovascular disease research (NIH, 2015a). Recent studies have identified potential, untapped sources of funding, including private philanthropy and foundations, the biomedical industry, the insurance industry, and more traditional private-sector organizations, which can be used to complement federal agency investment in resuscitation research (Myerburg and Ullmann, 2015).
Cardiac arrest support falls short when compared to other critical diseases and conditions using additional metrics. A recent study found that, between 1996 and 2006, “advancement in the treatment of acute ST Segment Elevation Myocardial Infarction (STEMI) has led to gradual reduction in the incidence of STEMI related cardiogenic shock irrespective of ethnicities or gender suggesting improving outcome of patients presenting with STEMI” (Movahed et al., 2014). Similarly, acute cardiovascular disease and stroke hospitalizations, mortality, and readmissions rates declined more rapidly between 1999 and 2011 than did other conditions (Krumholz et al., 2014). Figure 6-4 provides a comparison of the number of published randomized control trials, NIH-funded studies, and deaths by STEMI (a specific type of heart attack), stroke, heart failure, and cardiac arrest. Although this is only one study limited by its date of publication, when combined with documented declines in deaths from other, more, well-funded diseases and conditions, this figure further supports assertions of the important relationship between research levels and patient outcomes from cardiac arrest.
Similarly, the grants supporting resuscitation research are distributed across multiple academic institutions throughout the United States, and the current administrative structure of these grants provides very little incentive for cross-institutional collaboration. Some academic institutions have attempted to create translational “centers of excellence” that conduct research from the bench to the bedside. For example, in 2010, four academic medical centers formed the National Post-Arrest Research Consortium through NIH’s Clinical and Translational Research Award program, seeking to “advance science and foster partnerships to speed innovation and accelerate laboratory discoveries into treatments for [cardiac
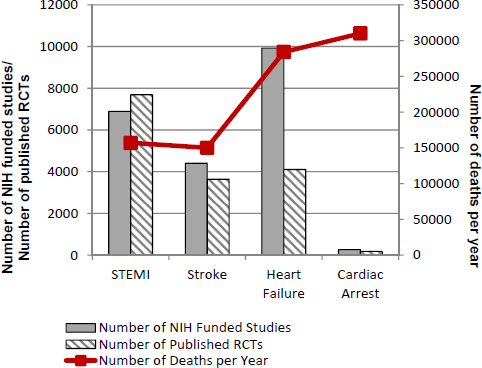
FIGURE 6-4 Comparison of four diseases by annual deaths and specific research support.
NOTE: NIH = National Institutes of Health; RCT = randomized controlled trial; STEMI = ST segment elevation myocardial infarction.
SOURCE: Ornato et al., 2010.
arrest] patients” (Virginia Commonwealth University, 2011). Despite the consortium’s potential, its impact was limited by the number of partnerships within the consortium and available funding.
The ability to successfully treat cardiac arrest depends on having a workforce that knows how to conduct research and translate that research into practice. In addition to the lack of population health data for cardiac arrest (see Chapter 2), there is also a striking lack of a viable preclinical research laboratory enterprises. A viable preclinical enterprise must exist that includes sustainable animal laboratories that use robust animal models, but the current number of active basic science and preclinical laboratories working in the area of cardiac arrest in the United States is in the single digits (Yannopoulos, 2014). Some experts suggest that fewer young scientists are choosing to pursue basic, preclinical, and clinical research in resuscitation because of a lack of sustainable funding
opportunities (Daniels, 2015; Yannopoulos, 2014). This deficit in the academic pipeline has led some organizations to include programs to develop a new generation of researchers in resuscitation and trauma as part of more traditional resuscitation research grants. For example, the Resuscitation Outcomes Consortium funded five training sites as “core leaders” in resuscitation research training and has trainees attend meetings to learn from mentors as they conduct research (Ornato, 2014). Current funding priorities are not likely to entice individual researchers to pursue careers in the treatment of cardiac arrest, which will affect the potential to develop new, effective treatments and therapies.
TRANSLATING SCIENCE INTO PRACTICE: THE ROLE OF EVIDENCE-BASED GUIDELINES
The Institute of Medicine (IOM) defines a “clinical practice guidelines” as a “systematically developed statement to assist practitioner and patient decisions about appropriate health care for specific clinical circumstances” (IOM, 1990, p. 38). “Evidence-based” implies sufficient clinical trial evidence to document the impact and need for each element of a specific guideline. To fully capitalize on the potential of novel research, therapies, and treatment protocols to reduce cardiac arrest death and disability, widespread implementation of evidence-based practice is required. Unfortunately, evidence related to cardiac arrest is lacking.
In the resuscitation field, a number of organizations—including the International Liaison Committee on Resuscitation (ILCOR), the American Heart Association (AHA), American Red Cross, and American College of Cardiology—offer consensus-based guidelines on various cardiac arrest topics (e.g., defibrillation, CPR, IHCA, ACLS, basic life support [BLS], and pediatric treatments). Guidelines, formal recommendations, and advisory statements are updated every 5 years after expert panels analyze all available international science and evidence (see Meaney et al., 2013; Morrison et al., 2013; Nolan et al., 2003; Peberdy et al., 2010). While some guideline-setting organizations have released joint consensus statements on several occasions, there remain slight variations in various guidelines, such as those for pediatric populations or for special circumstances, such as drowning. The availability of different guidelines through different organizations may contribute to general confusion about which guidelines best reflect the current literature and are most appropriate for different audiences.
Because comprehensive data and research studies are largely absent in the resuscitation field, guidelines may reflect extrapolations from existing data and research, as well as practical considerations, rather than a strict interpretation of solid evidence (Neumar, 2010). For example, the AHA and ILCOR guidelines recommend at least 50 millimeters for chest-compression depth during CPR. Yet, there are no high-level studies, such as randomized clinical trials, that compare compression depth. A recent study by Stiell and colleagues (2012) assessed chest compression depth in patients undergoing CPR and found that although a compression depth of 38-51 millimeters was associated with better survival, compression depth greater than 51 millimeters did not further improve rates of return of spontaneous circulation, 1-day survival, or survival to discharge. There was also an inverse relationship between compression depth and compression rate (Stiell et al., 2012). Similar questions about specific guidelines related to optimal chest compression rates or the sequence of treatments in the algorithms for cardiac arrest exist (Wallace et al., 2013). Moreover, the 5-year process for evaluating new evidence and updating recommendations raises questions about whether guidelines can be a timely reflection of a developing evidence base, especially in the time leading up to a new review cycle.
The effect of guidelines, standards, and advisory statements on patient outcomes from cardiac arrest is unclear. Some studies have failed to identify a direct association between implementation of cardiac arrest guidelines and improved patient outcomes (Deasy et al., 2011). For example, a study in which Resuscitation Outcomes Consortium (ROC) investigators assessed the effect of the 2005 Emergency Cardiovascular Care guidelines on survival to hospital discharge using before and after data from 85 EMS agencies found no significant difference in overall survival, which was 5.8 percent prior compared to 6.5 percent after implementation of the new guidelines (Bigham et al., 2011). However, other studies have found that adherence to specific guideline protocols is associated with improved patient outcomes (Kirves et al., 2007; Stub et al., 2015b).
Many factors contribute to difficulties in assessing the impact of guidelines. Numerous organizations, entities, and members of the public rely on guidelines to respond to cardiac arrest, but do not typically engage in systematic analyses of how adherence to guidelines affects cardiac arrest outcomes (Meaney et al., 2013). In addition to the complex nexus of cofounding factors that affect patient outcomes, compliance rates with guideline-recommended practice is low and can be difficult to
measure (Kampmeier et al., 2014; Kirves et al., 2007), and compliance with Utstein guidelines for reporting and research design can be incomplete (Donoghue et al., 2005). Many health care organizations rely on BLS and ACLS certificates to ensure that professional staff is sufficiently trained in various emergency skills, but it can be difficult to assess whether professional certification affects patient outcomes (IOM, 2014). More research is needed to demonstrate the impact of guideline adherence on patient outcomes following cardiac arrest.
Despite the limitations described above, guidelines and associated dissemination efforts do contribute to patient care in cardiac arrest. Guidelines and their associated checklists can reduce unnecessary variations in practice and are a reasonable place to begin process and health outcome assessment. However, recommending specific elements that are based on insufficient evidence as a standard of care has the potential to impede beneficial innovation. Systems of care should be encouraged to adopt guidelines and monitor outcomes to determine optimal, local protocols to enhance survival and good neurologic function following cardiac arrest. This approach will allow for local-level innovations that account for local needs and resources while contributing to a broader and more authoritative evidence base from which to update guidelines.
CONTINUOUS QUALITY IMPROVEMENT
Continuous quality improvement (CQI) is a “process-based, data-driven approach to improving the quality of a product or service, [which] operates under the belief that there is always room for improving operations, processes, and activities to increase quality” (RWJF, 2014). The basic premise of CQI involves capturing, analyzing, and regularly reporting data; translating the data and resulting information into actionable opportunities to improve performance at the local level; and developing plans for process changes that will further support effective, efficient, and value-added interventions (IOM, 2013a). An important component of any CQI effort is evaluating interventions and making adjustments, as needed. Even unsuccessful interventions can ultimately be used to improve survival rates, because they may provide evidence about ineffective treatments and protocols.
In the resuscitation field, adoption of CQI initiatives across EMS systems, health care systems, and hospitals has several distinct advantages. First, the chain of survival identifies groups of care providers
that already or can naturally engage in CQI activities. Using a CQI process to drive clinical excellence based on familiar guidelines reduces disruption and improves adoption of new practices. Second, CQI processes focus on modifiable structures and processes within existing systems with the goal to improve patient, provider, and system outcomes (National Learning Consortium, 2013). Cardiac arrest research and monitoring implementation of best practices can be a natural extension of established CQI activities in different care settings. Third, standardized data collection is central to CQI. Incorporation of standardized cardiac arrest data elements that are harmonized across multiple settings can be collected for purposes of local and national benchmarking to improve cardiac arrest outcomes.
Numerous experts and studies have found that many systems with documented improvements in cardiac arrest survival rates and neurologic and functional outcomes in patients use CQI strategies (Bobrow et al., 2008b; Cobb et al., 1999; Meaney et al., 2013). In a 2013 consensus statement, the American Heart Association noted that, although measuring, reporting, and reacting to resuscitation performance data can positively influence cardiac arrest survival, such activities are not universally employed by health care systems; consequently, outcomes from cardiac arrest remain at unacceptably low and disparate levels (Meaney et al., 2013). The report goes on to recommend that all health care providers should implement a “CPR CQI program that provides feedback to the director, managers and providers” (Meaney et al., 2013, p. 9). Implementation of novel interventions should be monitored and adjusted based on locally identified needs and resources, areas for improvement, and innovations in order to promote the adoption of evidence-based practice. CQI programs should not be used as a disciplinary tool but may be combined with periodic retraining (Resuscitation Academy, 2013). Specific CQI strategies in cardiac arrest include:
- Measuring processes and outcomes associated with resuscitation activities across all settings,
- Benchmarking against best practices and data from comparable systems,
- Providing feedback to teams of providers, and
- Developing strategies to continually improve resuscitation practices (Travers et al., 2010).
Communities and health care systems that have made the greatest strides in improving outcomes have typically established a culture of data collection, evaluation, and reporting that results in necessary adjustments that best fit local needs and capacities. Most communities that have demonstrated the greatest aptitude for reducing deaths from cardiac arrest share one common characteristic—CQI. The examples included in Box 6-4 highlight select communities within the United States that have adopted CQI approaches and local implementation approaches to available guidelines. Proactive leaders within these communities have encouraged and supported the adoption of innovative and adaptive strategies informed by active CQI programs, which use locally available data to improve cardiac arrest survival rates and health outcomes.
There are a number of commonalities shared across the examples provided in Box 6-3. Each of the examples started with recognizing a gap or an area for improvement that required strong leadership to operationalize needed changes. Success was predicated on the implementation of a reliable database that employed standard outcome parameters that could be used to measure and compare outcomes. The systems continually monitored outcomes data and developed non-punitive strategies and interventions to improve survival. These new interventions relied on a culture of innovation and continuous learning with the understanding that not all new approaches were going to lead to success. These elements, combined with appropriate responsibility and authority, need to serve as the foundation for a new paradigm in the treatment of cardiac arrest.
To promote adoption of CQI activities on a widespread scale, organization and agencies have a variety of options, including
- Disseminating guidelines and training materials that incorporate CQI training and examples of innovation;
- Providing forums for exchanging information and sharing experiences in adopting CQI principles;
- Offering and seeking competitive grants for CQI adoption and innovations;
- Hosting regional meetings for EMS agencies to exchange information and best practices for implementing CQI programs on a local level; and
- Creating prizes and other incentives for best innovations, improvement, regional survival rate, and so on.
Effective implementation of CQI programs, whether in the context of OHCA or IHCA, requires ongoing data collection, formal accountability, and flexibility to allow for innovation. As described in Chapter 2, NIH and CDC have supported cardiac arrest data collection and research through various mechanisms, including the ROC Epistry and CARES, respectively. However, CDC shifted funding to the private sector in 2012 for CARES, which has since had to adopt a sustainable, subscription model to support the data set infrastructure and personnel (CDC, 2013a; Slattery and McNally, 2015). Similarly, NIH is not renewing its grant support for ROC, shifting its focus to the newly established SIREN network, which will combine ROC and Neurological Emergencies Treatment Trials resources to better enable research on emergency medicine.2 The creation of a national database of cardiac arrest information may capitalize on natural efficiencies and enable CQI efforts to enhance resuscitation science, therapies, and treatment protocols. Benchmarking associated with CQI and the adoption of guidelines by consensus organizations will also contribute to more reliable systems of data collection.
Implementation science is an important concept in cardiac arrest research and has specific relevance to CQI programs. Implementation science—“the scientific study of methods to promote the integration of research findings and evidence-based interventions into healthcare policy and practice”—is a key element of translational research (Schackman, 2010, p. S27). Implementation science seeks “to understand the behavior of [health care] professionals and other stakeholders as a variable in the sustainable uptake, adoption, and implementation of evidence-based interventions” (NIH, 2013a). The principles of implementation science can complement and inform efforts to adopt a meaningful, system-wide CQI program to improve cardiac arrest outcomes.
As part of performance assessment, organizations need to identify and analyze implementation gaps affecting cardiac arrest research, technology transfer and innovation, and adoption of new discoveries and therapies. Analysis of factors affecting implementation can identify barriers to the adoption of new practices and develop strategies to overcome those barriers. Implementation science may also provide information about perceived acceptability, appropriateness, costs, feasibility, sustainability, safety, effectiveness, and patient-centeredness, in addition to patient outcomes (Proctor et al., 2011). Different implementation models
________________
2Personal communication with J. Brown, NIH, May 22, 2015.
BOX 6-3
Examples of Local Continuous Quality Programs
Arizona implemented a statewide database to measure OHCA incidence and care processes, and EMS systems throughout Arizona initiated a range of interventions and CQI efforts. Based on evidence from laboratory investigations and observational data, some EMS systems implemented and monitored the impact of efforts to increase bystander compression-only CPR training and a new CPR protocol—termed cardiocerebral resuscitation—which called for uninterrupted chest compressions and delayed intubation for OHCA. Between 2005 and 2009, the bystander CPR rate increased from 28.2 to 39.9 percent (Bobrow et al., 2010). Adherence to the cardiocerebral resuscitation protocol was associated with an increase in survival-to-discharge rates from 1.8 to 5.4 percent, in general, and from 4.7 to 17.6 percent for patients with witnessed VF arrest (Bobrow et al., 2008a). Similarly, a 5-year prospective observational study involving 90 EMS agencies found survival-to-discharge rates were 5.2 percent for patients with no bystander CPR, 7.8 percent for patients who received conventional CPR, and 13.3 percent for patients who received compression-only CPR (Bobrow et al., 2010).
The Cardiac Arrest Registry to Enhance Survival (CARES) was established in collaboration between Emory University and CDC with the goal of helping communities determine standard outcome measures for OHCA. CARES promoted the use of quality improvement efforts and benchmarking capability at the local level to improve care and survival (CDC, 2013b). CARES allows participating communities to compare performance data with statistics at the local, state, and national levels in order to improve EMS system practices and improve care (CDC, 2013b). A recent analysis of temporal trends between 2005 and 2012 found that communities enrolled in CARES demonstrated improved risk-adjusted survival rates (from 5.7 to 7.2 percent) for all first-observed cardiac arrest rhythms (i.e., shockable and nonshockable) and decreased rates of neurological disability (Chan et al., 2014). Geographical differences were observed, with the Northeast showing greatest improvements while the Midwest’s survival rates remained stagnant (Chan et al., 2014).
Denver, Colorado, participated in a pilot implementation trial of the Denver High Arrest Neighborhoods to Decrease Disparities in Survival project, applying the principles of CQI systems to improve rates of bystander CPR in “high-risk” neighborhoods. The project involved three phases in which areas with high cardiac arrest rates, and barriers to performing CPR in those areas, were identified. Culturally sensitive CPR programs were implemented, and in the final phase, the program
was evaluated (Sasson et al., 2014). Because of the initial success of this pilot implementation and community engagement, a larger initiative is now being created.
The Get With The Guidelines–Resuscitation Registry is a quality improvement program designed to promote adherence to treatment guidelines for IHCA, by collecting IHCA patient data and providing hospitals with feedback (AHA, 2014). An analysis of IHCA survival and neurologic outcomes between 2000 and 2009 found significant improvements in both outcome measures (Chan et al., 2012). These improvements were consistently observed across all cardiac arrest rhythms, regardless of response to defibrillation (Chan el al., 2012).
King County, Washington, which includes Seattle, has been recognized as a model of innovation and progressive improvement in OHCA treatment, improving survival rates from 35 to 62 percent over a 10-year period (Chatalas and Plorde, 2014; Plorde et al., 2005). The EMS leaders in Seattle and King County have been monitoring outcomes for more than three decades and consistently review data and consider systemwide changes that could further improve the index outcome measure: survival to hospital discharge of patients with witnessed VF arrest. Local CQI efforts have included developing a reliable OHCA database, which is used to conduct formal studies of ways to improve resuscitation, provide feedback on performance, and inform training methods. King County also evaluates the effectiveness of interventions after implementation. For example, a review of pauses in CPR revealed long periods without chest compressions. Based on these findings, the CPR protocol was changed to deliver single shocks with immediate CPR after each shock, increasing survival rates from 33 percent to 46 percent and influencing national CPR guidelines (Rea et al., 2006).
Wake County, North Carolina, which has a population of approximately 840,000 people, implemented a community-wide, three-phase program. Researchers designed interventions that included new CPR training that taught an approach to CPR that minimized interruptions and avoided hyperventilation, the application of the impedance threshold device, and prompt institution of therapeutic hypothermia once the patient arrived at the hospital. An EMS database was also designed to monitor each phase of intervention. After the complete implementation of the program, researchers reported that overall rate of survival to hospital discharge improved by 7.3 percent and that survival for witnessed ventricular tachycardia/VF increased by 27 percent (Hinchey et al., 2010).
with varying degrees of flexibility, emphasis, and applicability exist (Tabak et al., 2012) and can be molded to the specific context and goals of an EMS or health care system (Straus et al., 2013). By integrating CQI programs into existing systems of care, with an understanding of likely barriers affecting both the adoption of CQI principles and implementation of new evidence-based protocols, care providers will learn from their experience and solve problems using a formal, transparent, and systematic approach within the system to achieve improved patient outcomes following cardiac arrest.
STRENGTHENING COORDINATION AND ADVOCACY
Advocacy groups play an important role in providing support for cardiac arrest survivors and for families who have lost loved ones to cardiac arrest. They can also be influential in raising awareness; promoting education and training efforts in communities; lobbying for regulatory changes; and driving policy at the local, state, and national levels. A number of cardiac arrest–related advocacy groups exist, including the Sudden Cardiac Arrest Foundation, Parent Heart Watch, and the Sudden Cardiac Arrest Association. Despite remarkable work in expanding public education and awareness of cardiac arrest, these groups have been less successful at expanding resources for research and education campaigns at a national level when compared with advocacy groups focused on cancer, AIDS, and Alzheimer’s disease research.
Lack of progress may be due, in part, to the poor outcomes associated with cardiac arrest. Because of low survival rates, a large cohort of cardiac arrest survivors is not available to serve as living champions for better treatment and health outcomes. Conversely, those living with conditions such as cancer or AIDS have a more visible and vocal presence in the media and are better able to advocate on behalf of others living with the same condition. The field of resuscitation also suffers from fragmentation and the absence of a single, unified voice to generate the necessary political, private, and public support to advance resuscitation research.
Forming partnerships or collaborations is a common strategy used to promote integrated service delivery across health care and public health service systems. Defined as a “process by which groups come together, establishing a formal commitment to work together to achieve common goals and objectives” (NACCO, 2014), formal collaborations can help align programs and activities across different stakeholders with unique
knowledge, resources, and skills to achieve greater efficiencies, impact, and political and public support. Meaningful partnerships among researchers, health care providers, advocacy organizations, and the community can build an environment of mutual trust and understanding, information exchange and education, and active support and participation (NACCO, 2014).
Collaboratives can have various structures and purposes. Some collaboratives have focused on expanding research capacity and reach. For example, in 2006, NIH established the Clinical and Translational Science Award (CTSA) program to accelerate the translation of research from the most basic levels through the widespread application of research findings at the population level (IOM, 2013b). In addition to providing research resources and support tools, sites within participating academic health centers are required to actively engage community organizations, health care providers, and public health professionals (NIH, 2015b). As noted in the IOM’s 2013 evaluation of the CTSA program, “partnerships with community representatives can identify community health needs and priorities, provide critical input and data on clinically relevant questions, develop culturally appropriate clinical research protocols, promote successful enrollment and retention of research participants, and, ultimately, disseminate and implement research results more effectively” (IOM, 2013b, p. 117). Many of the CTSAs are creating long-term partnerships that are in turn leading to successful community programs and interventions that focus on areas such as diabetes, reducing health disparities, substance abuse, increasing clinical trial participation, and training community leader (IOM, 2013b).
Other collaboratives are formed to promote evidence-based practice. For example, the Brain Attack Coalition was formed to strengthen and promote relationships between its member organizations (including professional, voluntary, and governmental entities) in order to “reduc[e] the occurrence, disabilities and death associated with stroke” (Brain Attack Coalition, n.d.). The Coalition website is maintained by NINDS. The Coalition has engaged in activities related to messaging for National Stroke Awareness Month, a list of stroke systems for public education initiatives, and authored papers outlining guidelines for national stroke centers. The Coalition’s success is due, in part, to adoption of an inclusive multidisciplinary approach to advance large projects and overarching goals, adopting a deliberative process, limiting research to high-impact publications, and focusing on big projects and concepts (Alberts, 2015).
Still other collaboratives have a much broader focus, engaging wide ranges of stakeholders at every level of society to advance short- and long-term goals and change a culture. The Partnership for a Healthier America (PHA) has more than 150 private-sector partners and supporters, “who are increasing access to healthier, affordable meals; creating safer places for children to play; offering more opportunities for kids to get up and move before, during and after school; and helping parents understand how to provide healthier meals” (PHA, 2015b). Each year, PHA holds a national summit, which gathers health experts, policy makers, and business and industry leaders to talk with nonprofit, academic, and government counterparts to find actionable solutions to improve health and well-being in the United States (PHA, 2015a). Although its focus is not research necessarily, PHA provides an example of how a public–private partnership can have a rapid impact on the consciousness of individuals within the United States to catalyze large-scale changes in behaviors, interventions, and education.
Similarly, the epilepsy advocacy community provides an exemplar for inclusive collaboration that could be easily adapted and quite beneficial to the cardiac arrest field. Like cardiac arrest, the epilepsy field has struggled with limited research funding despite a large public health burden. The Epilepsy Leadership Council includes advocacy organizations, health professional organizations, government agencies, NIH institutes, health care providers, and researchers, and was created to explore opportunities for collaboration, common areas of interest, and funding possibilities within the epilepsy research community (American Epilepsy Society, 2015). The members of the Epilepsy Leadership Council meet on a semiannual basis and have established working groups that focus on surveillance and prevention; health care providers; patients, families, and education; and clinical trials (Jacobs, 2015). Following the release of an IOM report on the public health dimensions of epilepsy in 2012, sudden unexpected death in epilepsy became a condition monitored by the Sudden Death in the Young Registry—a step forward in epilepsy surveillance that holds promised for future epidemiological research (Epilepsy Foundation, 2015; Sudden Cardiac Arrest Foundation, 2015). The Epilepsy Leadership Council also maintains websites that focus on clinical trials and opportunities for participation (HERO, 2015) and education for patients and their families (Jacobs, 2015).
Federal support for research and infrastructure and engagement in public health campaigns is more likely when a field has a united front of advocates to communicate the urgency of a problem, why that problem
should be addressed now, and how best to solve the problem. The resuscitation field has the leaders and tools necessary to develop an advocacy network that can help advance the field as a whole in an effort to save lives. Formal collaboration could shape long-term strategies for the field of resuscitation in general, creating an effective advocacy base, unified platform, and evidence-based policies to improve research support and health outcomes from cardiac arrest over the next decade.
CONCLUSION
In conclusion, the committee urges the reprioritization of resuscitation research within the national research agenda. Research is a key component in any national framework to reduce death and disability from cardiac arrest within the next decade. Basic and clinical research offers insights related to the cardiac arrest etiology, pathophysiology, and treatment, and new approaches to the design and conduct of randomized controlled trials may better address the heterogeneity of affected patient populations, cardiac arrest events, and treatment protocols. New technologies and devices are on the near horizon, and regulatory policies and procedures should facilitate rapid evaluation and approval of successful devices and drugs without sacrificing patient safety. Translational research can also inform decisions about how to implement evidence-based practice and spur adoption of emerging therapies and evolving treatment protocols.
Continuous quality improvement is a relatively simple but essential concept to optimize system performance and contribute evidence related to existing treatments and protocols. As new knowledge emerges, local EMS and health care systems have an important responsibility to translate consensus guidelines into local practice protocols that reflect the resources and needs of a specific community. Numerous communities have demonstrated the ability to improve cardiac arrest outcomes through adoption of CQI programs, and these communities should serve as examples to other lower-performing systems. By implementing CQI programs to capture data and generate timely feedback, EMS and health care systems can produce an environment that promotes innovation and inform guideline-setting processes. As more communities begin to engage in data collection and assessment, these data can be used to benchmark additional CQI initiatives, leading to better-informed cardiac arrest research, practice, and policy at national, state, and local levels.
In order to make substantial gains in cardiac arrest treatment, a renewed commitment to resuscitation research support and infrastructure is needed. This commitment requires enhanced transparency and accountability within research-sponsoring organizations and from policy makers for the level and coordination of support dedicated to the treatment of cardiac arrest. This commitment is not likely to occur in a vacuum, however. Examples of successful stakeholder collaborations in various fields demonstrate the potential to effectuate change through coordinated action. With united leadership from key stakeholders, the resuscitation field can elevate its presence and effectively advocate for needed support to engage local, state, and federal stakeholders in a vision to improve cardiac arrest treatment and outcomes through research, evidence-based practice, innovation, and an unwavering commitment to saving lives in communities across the United States.
REFERENCES
AHA (American Heart Association). 2014. Resuscitation fact sheet. http://www.heart.org/idc/groups/heart-public/@private/@wcm/@hcm/agwtg/documents/downloadable/ucm_434082.pdf (accessed June 15, 2015).
Alberts, M. J. 2015. Can we apply lessons learned in stroke centers to change aspects of cardiac arrest care? Presentation before the Committee on the Treatment of Cardiac Arrest: Current Status and Future Directions, Washington, DC.
Allen, B. S., and G. D. Buckberg. 2012. Studies of isolated global brain ischaemia: I. Overview of irreversible brain injury and evolution of a new concept—redefining the time of brain death. European Journal Cardio-Thoracic Surgery 41(5):1132-1137.
Allen, B. S., Y. Ko, G. D. Buckberg, and Z. Tan. 2012a. Studies of isolated global brain ischaemia: II. Controlled reperfusion provides complete neurologic recovery following 30 min of warm ischaemia—the importance of perfusion pressure. European Journal Cardio-Thoracic Surgery 41(5): 1147-1154.
Allen, B. S., Y. Ko, G. D. Buckberg, and Z. Tan. 2012b. Studies of isolated global brain ischaemia: III. Influence of pulsatile flow during cerebral perfusion and its link to consistent full neurological recovery with controlled reperfusion following 30 min of global brain ischaemia. European Journal Cardio-Thoracic Surgery 41(5):1155-1163.
American Epilepsy Society. 2015. Introducing Epilepsy Leadership Council. https://www.aesnet.org/sites/default/files/file_attach/AboutAES/AESConnections/Introducing%20Epilepsy%20Leadership%20Council.pdf (accessed June 15, 2015).
Anderson, T. C., C. Q. Li, Z. H. Shao, T. Hoang, K. C. Chan, K. J. Hamann, L. B. Becker, and T. L. Vanden Hoek. 2006. Transient and partial mitochondrial inhibition for the treatment of postresuscitation injury: Getting it just right. Critical Care Medicine 34(12 Suppl):S474-S482.
Athanasuleas, C. L., G. D. Buckberg, B. S. Allen, F. Beyersdorf, and M. M. Kirsh. 2006. Sudden cardiac death: Directing the scope of resuscitation towards the heart and brain. Resuscitation 70(1):44-51.
Aufderheide, T. P., G. Nichol, T. D. Rea, S. P. Brown, B. G. Leroux, P. E. Pepe, P. J. Kudenchuk, J. Christenson, M. R. Daya, P. Dorian, C. W. Callaway, A. H. Idris, D. Andrusiek, S. W. Stephens, D. Hostler, D. P. Davis, J. V. Dunford, R. G. Pirrallo, I. G. Stiell, C. M. Clement, A. Craig, L. Van Ottingham, T. A. Schmidt, H. E. Wang, M. L. Weisfeldt, J. P. Ornato, and G. Sopko. 2011. A trial of an impedance threshold device in out-of-hospital cardiac arrest. New England Journal of Medicine 365(9):798-806.
Bainey, K. R., and P. W. Armstrong. 2014. Clinical perspectives on reperfusion injury in acute myocardial infarction. American Heart Journal 167(5):637-645.
Bartos, J. A., G. Debaty, T. Matsuura, and D. Yannopoulos. 2014. Post-conditioning to improve cardiopulmonary resuscitation. Current Opinion in Critical Care 20(3):242-249.
Bartos, J. A., T. R. Matsuura, M. Sarraf, S. T. Youngquist, S. H. McKnite, J. N. Rees, D. T. Sloper, F. S. Bates, N. Segal, G. Debaty, K. G. Lurie, R. W. Neumar, J. M. Metzger, M. L. Riess, and D. Yannopoulos. 2015. Bundled postconditioning therapies improve hemodynamics and neurologic recovery after 17 min of untreated cardiac arrest. Resuscitation 87:7-13.
Becker, L. B. 2004. New concepts in reactive oxygen species and cardiovascular reperfusion physiology. Cardiovascular Research 61(3):461-470.
Becker, L. B., M. L. Weisfeldt, M. H. Weil, T. Budinger, J. Carrico, K. Kern, G. Nichol, I. Shechter, R. Traystman, C. Webb, H. Wiedemann, R. Wise, and G. Sopko. 2002. The pulse initiative: Scientific priorities and strategic planning for resuscitation research and life saving therapies. Circulation 105(21):2562-2570.
Berry, S. M., J. T. Connor, and R. J. Lewis. 2015. The platform trial: an efficient strategy for evaluating multiple treatments. Journal of American Medical Association 313(16):1619-1620.
Bigham, B. L., K. Koprowicz, T. Rea, P. Dorian, T. P. Aufderheide, D. P. Davis, J. Powell, L. J. Morrison, and the ROC Investigators. 2011. Cardiac arrest survival did not increase in the Resuscitation Outcomes Consortium after implementation of the 2005 AHA CPR and ECC guidelines. Resuscitation 82(8):979-983.
Blumberg R. S., B. Dittel, D. Hafler, M. von Herrath, and F. O. Nestle. 2012. Unraveling the autoimmune translational research process layer by layer. Nature Medicine 18(1):35-41.
Bobrow, B. J., L. L. Clark, G. A. Ewy, V. Chikani, A. B. Sanders, R. A. Berg, P. B. Richman, and K. B. Kern. 2008a. Minimally interrupted cardiac resuscitation by emergency medical services for out-of-hospital cardiac arrest. Journal of American Medical Association 299(10):1158-1165.
Bobrow, B. J., T. F. Vadeboncoeur, L. Clark, and V. Chikani. 2008b. Establishing Arizona’s statewide cardiac arrest reporting and educational network. Prehospital Emergency Care 12(3):381-387.
Bobrow, B. J., D. W. Spaite, R. A. Berg, U. Stolz, A. B. Sanders, K. B. Kern, T. F. Vadeboncoeur, L. L. Clark, J. V. Gallagher, J. S. Stapczynski, F. LoVecchio, T. J. Mullins, W. O. Humble, and G. A. Ewy. 2010. Chest compression-only CPR by lay rescuers and survival from out-of-hospital cardiac arrest. Journal of American Medical Association 304(13):1447-1454.
Boller, M., S. K. Jung, S. Odegaard, A. Muehlmatt, J. M. Katz, and L. B. Becker. 2011. A combination of metabolic strategies plus cardiopulmonary bypass improves short-term resuscitation from prolonged lethal cardiac arrest. Resuscitation 82(Suppl 2):S27-S34.
Bosley, J. C., N. W. Zhao, S. Hill, F. S. Shofer, D. A. Asch, L. B. Becker, and R. M. Merchant. 2013. Decoding Twitter: Surveillance and trends for cardiac arrest and resuscitation communication. Resuscitation 84(2):206-212.
Brain Attack Coalition. n.d. About us. http://www.brainattackcoalition.org/aboutus.html (accessed June 8, 2015).
Bro-Jeppesen, J., J. Kjaergaard, M. Wanscher, N. Nielsen, H. Friberg, M. Bjerre, and C. Hassager. 2015. Systemic inflammatory response and potential prognostic implications after out-of-hospital cardiac arrest: A substudy of the target temperature management trial. Critical Care Medicine 43(6):1223-1232.
CDC (Centers for Disease Control and Prevention). 2013a. Latest news: CARES funding. https://mycares.net/sitepages/latestnews.jsp (accessed June 15, 2015).
CDC. 2013b. Overview: The Cardiac Arrest Registry to Enhance Survival (CARES). https://mycares.net/sitepages/overview.jsp (accessed June 15, 2015).
CDC. 2015. Stroke facts. http://www.cdc.gov/stroke/facts.htm (accessed June 15, 2015).
CDC. n.d. Million Hearts: The initiative. http://millionhearts.hhs.gov/aboutmh/overview.html (available June 15, 2015).
Chan, P. S., J. A. Spertus, H. M. Krumholz, R. A. Berg, Y. Li, C. Sasson, and B. K. Nallamothu. 2012. A validated prediction tool for initial survivors of in-hospital cardiac arrest. Archives of Internal Medicine 172(12):947-953.
Chan, P. S., B. McNally, F. Tang, and A. Kellermann. 2014. Recent trends in survival from out-of-hospital cardiac arrest in the united states. Circulation 130(21):1876-1882.
Chang, A. M., A. C. Leung, O. Saynisch, H. Griffis, S. Hill, J. C. Hershey, L. B. Becker, D. A. Asch, A. Seidman, and R. M. Merchant. 2013. Using a
mobile app and mobile workforce to validate data about emergency public health resources. Emergency Medicine Journal 31(7):545-548.
Chang, A., L. L. Brown, J. P. Duff, J. Davidson, F. Overly, N. M. Tofil, D. T. Peterson, M. L. White, F. Bhanji, I. Bank, R. Gottesman, M. Adler, J. Zhong, V. Grant, D. J. Grant, S. N. Sudikoff, K. Marohn, A. Charnovich, E. A. Hunt, D. O. Kessler, H. Wong, N. Robertson, Y. Lin, Q. Doan, J. M. Duval-Arnould, and V. M. Nadkarni. 2015. Improving cardiopulmonary resuscitation with a CPR feedback device and refresher simulations (CPR CARES study): A randomized clinical trial. JAMA Pediatrics 169(2):137-144.
Chatalas, H., and M. Plorde, eds. 2014. Division of Emergency Medical Services 2014 Annual Report to the King County Council. Seattle, WA: Emergency Medical Services Division of the Public Health Department—Seattle and King County. http://www.kingcounty.gov/healthservices/health/%7e/media/health/publichealth/documents/ems/2014AnnualReport.ashx (accessed June 8, 2015).
Churpek, M. M., T. C. Yuen, S. Y. Park, D. O. Meltzer, J. B. Hall, and D. P. Edelson. 2012. Derivation of a cardiac arrest prediction model using ward vital signs. Critical Care Medicine 40(7):2102-2108.
Cobb, L. A., C. E. Fahrenbruch, T. R. Walsh, M. K. Copass, M. Olsufka, M. Breskin, and A. P. Hallstrom. 1999. Influence of cardiopulmonary resuscitation prior to defibrillation in patients with out-of-hospital ventricular fibrillation. Journal of American Medical Association 281(13):1182-1188.
Cronberg, T., G. Lilja, J. Horn, J. Kjaergaard, M. P. Wise, T. Pellis, J. Hovdenes, Y. Gasche, A. Aneman, P. Stammet, D. Erlinge, H. Friberg, C. Hassager, M. Kuiper, M. Wanscher, F. Bosch, J. Cranshaw, G. R. Kleger, S. Persson, J. Unden, A. Walden, P. Winkel, J. Wetterslev, and N. Nielsen. 2015. Neurologic function and health-related quality of life in patients following targeted temperature management at 33 degrees C vs 36 degrees C after out-of-hospital cardiac arrest: a randomized clinical trial. JAMA Neurology 72(6):634-641.
Crossley, G. H., A. Boyle, H. Vitense, Y. Chang, and R. H. Mead. 2011. The connect (clinical evaluation of remote notification to reduce time to clinical decision) trial: The value of wireless remote monitoring with automatic clinician alerts. Journal of the American College of Cardiology 57(10):1181-1189.
Daniels, R. J. 2015. A generation at risk: Young investigators and the future of the biomedical workforce. Proceedings of the National Academy of Sciences of the United States of America 112(2):313-318.
Deasy, C., J. E. Bray, K. Smith, R. Wolfe, L. R. Harriss, S. A. Bernard, and P. Cameron. 2011. Cardiac arrest outcomes before and after the 2005 resuscitation guidelines implementation: Evidence of improvement? Resuscitation 82(8):984-988.
Donoghue, A. J., V. Nadkarni, R. A. Berg, M. H. Osmond, G. Wells, L. Nesbitt, and I. G. Stiell. 2005. Out-of-hospital pediatric cardiac arrest: An epidemiologic review and assessment of current knowledge. Annals of Emergency Medicine 46(6):512-522.
Epilepsy Foundation. 2015. SUDEP surveillance efforts. http://www.epilepsy.com/get-help/sudep-institute/sudep-surveillance-efforts (accessed June 15, 2015).
Ewy, G. A., K. B. Kern, A. B. Sanders, D. Newburn, T. D. Valenzuela, L. Clark, R. W. Hilwig, C. W. Otto, M. M. Hayes, P. Martinez, and R. A. Berg. 2006. Cardiocerebral resuscitation for cardiac arrest. The American Journal of Medicine 119(1):6-9.
FDA (U.S. Food and Drug Administration). 2011. Medical devices: Protecting patients and promoting innovation. http://www.fda.gov/NewsEvents/Testimony/ucm278960.htm (accessed June 15, 2015).
FDA. 2014. PMA clinical studies. http://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/HowtoMarketYourDevice/PremarketSubmissions/PremarketApprovalPMA/ucm050419.htm (accessed June 15, 2015).
FDA. 2015a. Draft guidance for industry and Food and Drug Administration staff: adaptive designs for medical device clinical studies. http://www.fda.gov/ucm/groups/fdagov-public/@fdagov-meddev-gen/documents/document/ucm446729.pdf (accessed June 15, 2015).
FDA. 2015b. Effective date of requirement for premarket approval for automated external defibrillator systems (republication). Federal Register 80:5674-5683.
FDA. 2015c. Mobile medical applications: Guidance for industry and Food and Drug Administration staff. Code of Federal Regulation 21(870):5674-5683.
Feldman, A. M., H. Klein, P. Tchou, S. Murali, W. J. Hall, D. Mancini, J. Boehmer, M. Harvey, M. S. Heilman, S. J. Szymkiewicz, and A. J. Moss. 2004. Use of a wearable defibrillator in terminating tachyarrhythmias in patients at high risk for sudden death: Results of the WEARIT/BIROAD. Pacing and Clinical Electrophysiology 27(1):4-9.
Fischer, H., J. Gruber, S. Neuhold, S. Frantal, E. Hochbrugger, H. Herkner, H. Schochl, B. Steinlechner, and R. Greif. 2011. Effects and limitations of an AED with audiovisual feedback for cardiopulmonary resuscitation: A randomized manikin study. Resuscitation 82(7):902-907.
Frohlich, G. M., P. Meier, S. K. White, D. M. Yellon, and D. J. Hausenloy. 2013. Myocardial reperfusion injury: Looking beyond primary PCI. European Heart Journal 34(23):1714-1722.
Gazmuri, R. J., V. M. Nadkarni, J. P. Nolan, H.-R. Arntz, J. E. Billi, L. Bossaert, C. D. Deakin, J. Finn, W. W. Hammill, A. J. Handley, M. F. Hazinski, R. W. Hickey, I. Jacobs, E. C. Jauch, W. G. J. Kloeck, M. H. Mattes, W. H. Montgomery, P. Morley, L. J. Morrison, G. Nichol, R. E. O’Connor, J. Perlman, S. Richmond, M. Sayre, M. Shuster, S. Timerman, M. H. Weil, M. L. Weisfeldt, A. Zaritsky, and D. A. Zideman. 2007. Scientific
knowledge gaps and clinical research priorities for cardiopulmonary resuscitation and emergency cardiovascular care identified during the 2005 international consensus conference on ECC and CPR science with treatment recommendations: A consensus statement from the International Liaison Committee on Resuscitation (American Heart Association, Australian Resuscitation Council, European Resuscitaiton Countil, Heart and Stroke Foundation of Canada, Interamerican Heart Foundation, Resuscitation Council of Southern Africa, and the New Zealand Resuscitation Council); the American Heart Association Emergency Cardiovascular Care Committee; the Stroke Council; and the Cardiovascular Nursing Council. Circulation 116(21):2501-2512.
Hallstrom, A., T. D. Rea, M. R. Sayre, J. Christenson, A. R. Anton, V. N. Mosesso, Jr., L. Van Ottingham, M. Olsufka, S. Pennington, L. J. White, S. Yahn, J. Husar, M. F. Morris, and L. A. Cobb. 2006. Manual chest compression vs use of an automated chest compression device during resuscitation following out-of-hospital cardiac arrest: A randomized trial. Journal of American Medical Association 295(22):2620-2628.
HERO (Human Epilepsy Research Opportunities). 2015. Heroes and clinical research. http://www.epilepsyhero.org (accessed June 12, 2015).
HHS (U.S. Department of Health and Human Services). 2010. Guidance for industry: Adaptive design clinical trials for drugs and biologics. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM201790.pdf (accessed June 15, 2015).
Hinchey, P. R., J. B. Myers, R. Lewis, V. J. De Maio, E. Reyer, D. Licatese, J. Zalkin, and G. Snyder. 2010. Improved out-of-hospital cardiac arrest survival after the sequential implementation of 2005 AHA guidelines for compressions, ventilations, and induced hypothermia: The wake county experience. Annals of Emergency Medicine 56(4):348-357.
Honeyman, E., H. Ding, M. Varnfield, and M. Karunanithi. 2014. Mobile health applications in cardiac care. Interventional Cardiology 6(2):227-240.
Humphrey, H. H. 1962 (October 13). Congressional Record A7837-A7839.
IOM (Institute of Medicine). 1990. Clinical practice guidelines: Directions for a new program. Washington, DC: National Academy Press.
IOM. 2001. Small clinical trials issues and challenges. Washington, DC: National Academy Press.
IOM. 2013a. Best care at lower cost: The path to continuously learning health care in America. Washington, DC: The National Academies Press.
IOM. 2013b. The CTSA program at NIH: Opportunities for advancing clinical research and translational research. Washington, DC: The National Academies Press.
IOM. 2014. Future directions of credentialing research in nursing: Workshop summary. Washington, DC: The National Academies Press.
Jacobs, M. P. 2015. Epilepsy: A model for collaborating—post IOM report. http://www.hhs.gov/advcomcfs/meetings/presentations/iom-epilepsy-experiencemargaret-jacobs-june-2014.pdf (accessed June 15, 2015).
Johnson, N. J., M. Acker, C. H. Hsu, N. Desai, P. Vallabhajosyula, S. Lazar, J. Horak, J. Wald, F. McCarthy, E. Rame, K. Gray, S. M. Perman, L. B. Becker, D. Cowie, A. Grossestreuer, T. Smith, and D. F. Gaieski. 2014. Extracorporeal life support as rescue strategy for out-of-hospital and emergency department cardiac arrest. Resuscitation 85(11):1527-1532.
Kalogeris, T., Y. Bao, and R. J. Korthuis. 2014. Mitochondrial reactive oxygen species: A double edged sword in ischemia/reperfusion vs preconditioning. Redox Biology 2:702-714.
Kampmeier, T. G., R. P. Lukas, C. Steffler, C. Sauerland, T. P. Weber, H. Van Aken, and A. Bohn. 2014. Chest compression depth after change in CPR guidelines—improved but not sufficient. Resuscitation 85(4):503-508.
Kirves, H., M. B. Skrifvars, M. Vahakuopus, K. Ekstrom, M. Martikainen, and M. Castren. 2007. Adherence to resuscitation guidelines during prehospital care of cardiac arrest patients. European Journal of Emergency Medicine 14(2):75-81.
Krumholz, H. M., S. L. Normand, and Y. Wang. 2014. Trends in hospitalizations and outcomes for acute cardiovascular disease and stroke, 1999-2011. Circulation 130(12):966-975.
Lathrop, D. 2014. Charge to the consensus study committee treatment of cardiac arrest: Current status and future directions. Presentation at the first meeting of the Committee on Treatment of Cardiac Arrest: Current Status and Future Directions, Washington, DC. https://www.iom.edu/~/media/Files/Activity%20Files/PublicHealth/TreatmentofCardiacArrest/2014-MAR-12/IOM%20-%20Lathrop.pdf (accessed June 8, 2015).
Lauer, M. S., and R. B. D’Agostino. 2013. The randomized registry trial—the next disruptive technology in clinical research? New England Journal of Medicine 369(17):1579-1581.
Lavani, R., W. T. Chang, T. Anderson, Z. H. Shao, K. R. Wojcik, C. Q. Li, R. Pietrowski, D. G. Beiser, A. H. Idris, K. J. Hamann, L. B. Becker, and T. L. Vanden Hoek. 2007. Altering CO2 during reperfusion of ischemic cardiomyocytes modifies mitochondrial oxidant injury. Critical Care Medicine 35(7):1709-1716.
Lavine, K. J., and D. L. Mann. 2012. Rethinking phase II clinical trial design in heart failure. Clinical Investigation 3(1):57-68.
Lee, B. K., and J. E. Olgin. 2009. Role of wearable and automatic external defibrillators in improving survival in patients at risk for sudden cardiac death. Current Treatment Options in Cardiovascular Mediicne 11(5):360-365.
Martin, P., P. Theobald, A. Kemp, S. Maguire, I. Maconochie, and M. Jones. 2013. Real-time feedback can improve infant manikin cardiopulmonary
resuscitation by up to 79%—a randomised controlled trial. Resuscitation 84(8):1125-1130.
Mazor, K. M., J. E. Sabin, D. Boudreau, M. J. Goodman, J. H. Gurwitz, L. J. Herrinton, M. A. Raebel, D. Roblin, D. H. Smith, V. Meterko, and R. Platt. 2007. Cluster randomized trials: Opportunities and barriers identified by leaders of eight health plans. Medical Care 45(10 Supl 2):S29-S37.
McAuley, D. F., C. O’Kane, and M. J. Griffiths. 2010. A stepwise approach to justify phase III randomized clinical trials and enhance the likelihood of a positive result. Critical Care Medicine 38(10 Suppl):S523-S527.
Meaney, P. A., B. J. Bobrow, M. E. Mancini, J. Christenson, A. R. de Caen, F. Bhanji, B. S. Abella, M. E. Kleinman, D. P. Edelson, R. A. Berg, T. P. Aufderheide, V. Menon, and M. Leary. 2013. Cardiopulmonary resuscitation quality: [corrected] improving cardiac resuscitation outcomes both inside and outside the hospital: A consensus statement from the American Heart Association. Circulation 128(4):417-435.
Meurer, W. J., R. J. Lewis, and D. A. Berry. 2012. Adaptive clinical trials: A partial remedy for the therapeutic misconception? Journal of American Medical Association 307(22):2377-2378.
Minnerup, J., H. Wersching, M. Schilling, and W. R. Schabitz. 2014. Analysis of early phase and subsequent phase III stroke studies of neuroprotectants: Outcomes and predictors for success. Experimental and Translational Stroke Medicine 6(1):2.
Morrison, L. J., R. W. Neumar, J. L. Zimmerman, M. S. Link, L. K. Newby, P. W. McMullan, Jr., T. V. Hoek, C. C. Halverson, L. Doering, M. A. Peberdy, and D. P. Edelson. 2013. Strategies for improving survival after in-hospital cardiac arrest in the United States: 2013 consensus recommendations: A consensus statement from the American Heart Association. Circulation 127(14):1538-1563.
Movahed, M. R., M. F. Khan, M. Hashemzadeh, and M. Hashemzadeh. 2014. Gradual decline in the age-adjusted in-hospital mortality rate from STEMI-related cardiogenic shock irrespective of cause, race or gender with persistent higher mortality rates in women despite multivariate adjustment. Journal of Invasive Cardiology 26(1):7-12.
Myerburg, R. J., and S. G. Ullmann. 2015. Alternative research funding to improve clinical outcomes: The model of prediction and prevention of sudden cardiac death. Circulation: Arrhythmia and Electrophysiology. 8(2):492-498.
NACCHO (National Association of County and City Health Organizations). 2014. Pulling together 5: Section two: Building collaboration. http://www.naccho.org/topics/environmental/pullingtogether/sectiontwo.cfm (accessed June 8, 2015).
Nagao, K., N. Hayashi, K. Kanmatsuse, K. Arima, J. Ohtsuki, K. Kikushima, and I. Watanabe. 2000. Cardiopulmonary cerebral resuscitation using emergency cardiopulmonary bypass, coronary reperfusion therapy and mild
hypothermia in patients with cardiac arrest outside the hospital. Journal of the American College of Cardiology 36(3):776-783.
Nagao, K., K. Kikushima, K. Watanabe, E. Tachibana, Y. Tominaga, K. Tada, M. Ishii, N. Chiba, A. Kasai, T. Soga, M. Matsuzaki, K. Nishikawa, Y. Tateda, H. Ikeda, and T. Yagi. 2010. Early induction of hypothermia during cardiac arrest improves neurological outcomes in patients with out-of-hospital cardiac arrest who undergo emergency cardiopulmonary bypass and percutaneous coronary intervention. Circulation Journal 74(1):77-85.
National Learning Consortium. 2013. Continuous quality improvement (CQI) strategies to optimize your practice. https://www.healthit.gov/sites/default/files/tools/nlc_continuousqualityimprovementprimer.pdf (accessed June 15, 2015).
Neumar, R. W. 2010. From science to guidelines: The future for resuscitation. Signa Vitae 5(Suppl 1):10-12.
Neumar, R. W., C. W. Otto, M. S. Link, S. L. Kronick, M. Shuster, C. W. Callaway, P. J. Kudenchuk, J. P. Ornato, B. McNally, S. M. Silvers, R. S. Passman, R. D. White, E. P. Hess, W. Tang, D. Davis, E. Sinz, and L. J. Morrison. 2010. Part 8: Adult advanced cardiovascular life support: 2010 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation 122(18 Suppl 3):S729-S767.
NHTSA (National Highway Traffic Safety Administration). 2009. Emergency medical services performance measures: Recommended attributes and indicators for system and service performance. Washington, DC: NHTSA http://www.ems.gov/pdf/811211.pdf (accessed June 15, 2015).
NIBIB (National Institute of Biomedical Imaging and Bioengineering). 2015. About NIBIB: Mission. http://www.nibib.nih.gov/about-nibib (accessed June 15, 2015).
Nichol, G., R. Karmy-Jones, C. Salerno, L. Cantore, and L. Becker. 2006. Systematic review of percutaneous cardiopulmonary bypass for cardiac arrest or cardiogenic shock states. Resuscitation 70(3):381-394.
Nielsen, N., J. Wetterslev, T. Cronberg, D. Erlinge, Y. Gasche, C. Hassager, J. Horn, J. Hovdenes, J. Kjaergaard, M. Kuiper, T. Pellis, P. Stammet, M. Wanscher, M. P. Wise, A. Åneman, N. Al-Subaie, S. Boesgaard, J. Bro-Jeppesen, I. Brunetti, J. F. Bugge, C. D. Hingston, N. P. Juffermans, M. Koopmans, L. Køber, J. Langørgen, G. Lilja, J. E. Møller, M. Rundgren, C. Rylander, O. Smid, C. Werer, P. Winkel, and H. Friberg. 2013. Targeted temperature management at 33°C versus 36°C after cardiac arrest. New England Journal of Medicine 369(23):2197-2206.
NIH (National Institutes of Health). 2012a. News and events: NIH creates Office of Emergency Care Research. http://www.nih.gov/news/health/jul2012/nih31.htm (accessed June 15, 2015).
NIH. 2012b. NIH factsheet—NIH: Turning discovery into health. Washington, DC: U.S. Department of Health and Human Services. http://www.oar.
nih.gov/factsheets/pdfs/NIH_FS_NIHandNIHAIDSResearch.pdf (accessed June 15, 2015).
NIH. 2013a. Frequently asked questions about implementation science. http://www.fic.nih.gov/News/Events/implementation-science/Pages/faqs.aspx (accessed June 15, 2015).
NIH. 2013b. News and events: Dr. Jeremy Brown to direct NIH Office of Emergency Care Research. http://www.nih.gov/news/health/jun2013/nigms-25.htm (accessed June 15, 2015).
NIH. 2014. Division of Cardiovascular Sciences (DCVS): Heart Failure & Arrhythmias Branch. http://www.nhlbi.nih.gov/about/org/dcvs (accessed June 15, 2015).
NIH. 2015a. Estimates of funding for various research, condition, and disease categories (RCDC). http://report.nih.gov/categorical_spending.aspx (accessed June 15, 2015).
NIH. 2015b. National Center for Advancing Translational Sciences: Communities and research. http://www.ncats.nih.gov/ctsa/community (accessed June 8, 2015).
NIH. 2015c. NIH emergency care research and training activities. http://www.nigms.nih.gov/about/overview/OECR/pages/programs.aspx (accessed June 16, 2015).
Nolan, J. P., R. W. Neumar, C. Adrie, M. Aibiki, R. A. Berg, B. W. Bbttiger, C. Callaway, R. S. Clark, R. G. Geocadin, E. C. Jauch, K. B. Kern, I. Laurent, W. T. Longstreth, R. M. Merchant, P. Morley, L. J. Morrison, V. Nadkarni, M. A. Peberdy, E. P. Rivers, A. Rodriguez-Nunez, F. W. Sellke, C. Spaulding, K. Sunde, and T. Vanden Hoek. 2010. Post-cardiac arrest syndrome: Epidemiology, pathophysiology, treatment, and prognostication: A scientific statement from the International Liaison Committee on Resuscitation; the American Heart Association Emergency Cardiovascular Care Committee; the Council on Cardiovascular Surgery and Anesthesia; the Council on Cardiopulmonary, Perioperative, and Critical Care; the Council on Clinical Cardiology; and the Council on Stroke (part II) International Emergency Nursing 18(1):8-28.
Ong, M. E., C. H. Lee Ng, K. Goh, N. Liu, Z. X. Koh, N. Shahidah, T. T. Zhang, S. Fook-Chong, and Z. Lin. 2012a. Prediction of cardiac arrest in critically ill patients presenting to the emergency department using a machine learning score incorporating heart rate variability compared with the modified early warning score. Critical Care 16(3):R108.
Ong, M. E., K. E. Mackey, Z. C. Zhang, H. Tanaka, M. H. Ma, R. Swor, and S. D. Shin. 2012b. Mechanical CPR devices compared to manual CPR during out-of-hospital cardiac arrest and ambulance transport: A systematic review. Scandinavian Journal of Trauma, Resuscitation & Emergency Medicine 20:39.
Ornato, J. P. 2015. Resuscitation Outcomes Consortium. Presentation before the Committee on the Treatment of Cardiac Arrest: Current Status and Future Directions, Washington, DC.
Ornato, J. P., L. B. Becker, M. L. Weisfeldt, and B. A. Wright. 2010. Cardiac arrest and resuscitation: An opportunity to align research prioritization and public health need. Circulation 122(18):1876-1879.
Patsopoulos, N. A. 2011. A pragmatic view on pragmatic trials. Dialogues in Clinical NeuroSciences 13(2):217-224.
PCORI (Patient-Centered Outcomes Research Institute). 2014. What we do. http://www.pcori.org/about-us/what-we-do (accessed June 15, 2015).
Peberdy, M. A., C. W. Callaway, R. W. Neumar, R. G. Geocadin, J. L. Zimmerman, M. Donnino, A. Gabrielli, S. M. Silvers, A. L. Zaritsky, R. Merchant, T. L. Vanden Hoek, and S. L. Kronick; for the American Heart Association. 2010. Part 9: Post-cardiac arrest care: 2010 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation 122(18 Suppl 3):S768-S786.
PHA (Partnership for a Healthier America). 2015a. Building a healthier future summit. http://ahealthieramerica.org/summit/about-the-summit (accessed June 12, 2015.
PHA. 2015b. The foundation rises: Constructing a legacy of good health. http://progressreports.ahealthieramerica.org/2014/making-strides (accessed June 8, 2015.
PHA. 2015c. Let’s move! http://ahealthieramerica.org/about/lets-move (accessed June 15, 2015).
Pirrallo, R. G., T. P. Aufderheide, T. A. Provo, and K. G. Lurie. 2005. Effect of an inspiratory impedance threshold device on hemodynamics during conventional manual cardiopulmonary resuscitation. Resuscitation 66(1):13-20.
Plorde, M., T. Hearne, and C. Bradshaw. eds. 2005. Division of Emergency Medical Services 2005Annual Report to the King County Council. Seattle, WA: Emergency Medical Services Division of the Public Health Department—Seattle and King County. http://www.kingcounty.gov/healthservices/health/%7e/media/health/publichealth/documents/ems/2014AnnualReport.ashx (accessed June 8, 2015).
Proctor, E., H. Silmere, R. Raghavan, P. Hovmand, G. Aarons, A. Bunger, R. Griffey, and M. Hensley. 2011. Outcomes for implementation research: Conceptual distinctions, measurement challenges, and research agenda. Administration and Policy in Mental Health 38(2):65-76.
Przybylski, A., J. Zakrzewska-Koperska, A. Maciag, P. Derejko, M. Orczykowski, L. Szumowski, and F. Walczak. 2009. Technical and practical aspects of remote monitoring of implantable cardioverter-defibrillator patients in poland—preliminary results. Kardiologia Polska 67(5):505-511.
PulsePoint. 2015. PulsePoint. http://www.pulsepoint.org (accessed June 15, 2015).
Rea, T. D., M. Helbock, S. Perry, M. Garcia, D. Cloyd, L. Becker, and M. Eisenberg. 2006. Increasing use of cardiopulmonary resuscitation during out-of-hospital ventricular fibrillation arrest: Survival implications of guideline changes. Circulation 114(25):2760-2765.
Resuscitation Academy. 2013. 10 steps for improving survival from sudden cardiac arrest. http://www.resuscitationacademy.com/downloads/ebook/TenStepsforImprovingSurvivalFromSuddenCardiacArrest-RA-eBook-PDFFinal-v1_2.pdf (accessed June 15, 2015).
Resuscitation Academy. 2014. Strategies to improve survival from sudden cardiac arrest: An evidence based analysis. http://www.resuscitationacademy.com/downloads/RA-35-Strategies-to-Improve-CA-SUrvival.pdf (accessed June 15, 2015).
Retzios, A. D. 2009. Why do so many phase 3 clinical trials fail? Issues in Clinical Research: Bay Clinical R&D Services 1-46.
Reynolds, D. W., N. Jayaprasad, and J. Francis. 2006. Remote monitoring of implantable cardioverter defibrillator. Indian Pacing and Electrophysiology Journal 6(4):186-188.
Rickard, J., S. Ahmed, M. Baruch, B. Klocman, D. O. Martin, and V. Menon. 2011. Utility of a novel watch-based pulse detection system to detect pulselessness in human subjects. Heart Rhythm 8(12):1895-1899.
Riess, M. L., T. R. Matsuura, J. A. Bartos, M. Bienengraeber, M. Aldakkak, S. H. McKnite, J. N. Rees, T. P. Aufderheide, M. Sarraf, R. W. Neumar, and D. Yannopoulos. 2014. Anaesthetic postconditioning at the initiation of CPR improves myocardial and mitochondrial function in a pig model of prolonged untreated ventricular fibrillation. Resuscitation 85(12):1745-1751.
Ringh, M., D. Fredman, P. Nordberg, T. Stark, and J. Hollenberg. 2011. Mobile phone technology identifies and recruits trained citizens to perform CPR on out-of-hospital cardiac arrest victims prior to ambulance arrival. Resuscitation 82(12):1514-1518.
Rubio, D. M., E. E. Schoenbaum, L. S. Lee, D. E. Schteingart, P. R. Marantz, K. E. Anderson, L. D. Platt, A. Baez, and K. Esposito. 2010. Defining translational research: Implications for training. Academic Medicine 85(3):470-475.
RWJF (Robert Wood Johnson Foundation). 2014. The science of continuous quality improvement. http://www.rwjf.org/en/how-we-work/rel/researchfeatures/evaluating-CQI.html (accessed June 8, 2015).
Sakai, T., T. Iwami, T. Kitamura, C. Nishiyama, T. Kawamura, K. Kajino, H. Tanaka, S. Marukawa, O. Tasaki, T. Shiozaki, H. Ogura, Y. Kuwagata, and T. Shimazu. 2011. Effectiveness of the new “mobile AED map” to find and retrieve an AED: A randomised controlled trial. Resuscitation 82(1):69-73.
Sakai, T., T. Kitamura, C. Nishiyama, Y. Murakami, M. Ando, T. Kawamura, O. Tasaki, Y. Kuwagata, T. Shimazu, and T. Iwami. 2015. Cardiopulmonary
resuscitation support application on a smartphone-randomized controlled trial. Circulation Journal 79(5):1052-1057.
Sasson, C., M. A. M. Rogers, J. Dahl, and A. L. Kellermann. 2010. Predictors of survival from out-of-hospital cardiac arrest: A systematic review and metaanalysis. Circulation: Cardiovascular Quality and Outcomes 3(1):63-81.
Schackman, B. R. 2010. Implementation science for the prevention and treatment of HIV/AIDS. Journal of Acquired Immune Deficiency Syndrome 55(Suppl 1):S27-S31.
Scholten, A. C., J. G. van Manen, W. E. van der Worp, M. J. Ijzerman, and C. J. Doggen. 2011. Early cardiopulmonary resuscitation and use of automated external defibrillators by laypersons in out-of-hospital cardiac arrest using an SMS alert service. Resuscitation 82(10):1273-1278.
Slattery, D., and B. McNally. 2015 CARES sustainability discussion: Subscription model. Presentation during CARES Webinar, University of Nevada. https://mycares.net/sitepages/latestnews.jsp (accessed June 15, 2015).
Stiell, I. G., G. Nichol, B. G. Leroux, T. D. Rea, J. P. Ornato, J. Powell, J. Christenson, C. W. Callaway, P. J. Kudenchuk, T. P. Aufderheide, A. H. Idris, M. R. Daya, H. E. Wang, L. J. Morrison, D. Davis, D. Andrusiek, S. Stephens, S. Cheskes, R. H. Schmicker, R. Fowler, C. Vaillancourt, D. Hostler, D. Zive, R. G. Pirrallo, G. M. Vilke, G. Sopko, and M. Weisfeldt. 2011. Early versus later rhythm analysis in patients with out-of-hospital cardiac arrest. New England Journal of Medicine 365(9):787-797.
Stiell, I. G., S. P. Brown, J. Christenson, S. Cheskes, G. Nochol, J. Powell, B. Bigham, L. J. Morrison, J. Larsen, E. Hess, C. Vaillancourt, D. P. Davis, C. W. Callaway, Resuscitation Outcomes Consortium (ROC) Investigators. 2012. What is the role of chest compression depth during out-of-hospital cardic arrest resuscitation. Critical Care Medicine 40(4):1192-1198.
Straus, S. E., J. E. Moore, S. Uka, C. Marquez, and A. M. Gulmezoglu. 2013. Determinants of implementation of maternal health guidelines in Kosovo: Mixed methods study. Implementation Science 8:108.
Stub, D., S. Bernard, V. Pellegrino, K. Smith, T. Walker, J. Sheldrake, L. Hockings, J. Shaw, S. J. Duffy, A. Burrell, P. Cameron, V. Smit de, and D. M. Kaye. 2015a. Refractory cardiac arrest treated with mechanical CPR, hypothermia, ECMO and early reperfusion (the CHEER trial). Resuscitation 86:88-94.
Stub, D., R. H. Schmicker, M. L. Anderson, C. W. Callaway, M. R. Daya, M. R. Sayre, J. Elmer, B. E. Grunau, T. P. Aufderheide, S. Lin, J. E. Buick, D. Zive, E. D. Peterson, and G. Nichol. 2015b. Association between hospital post-resuscitative performance and clinical outcomes after out-of-hospital cardiac arrest. Resuscitation 92:45-52.
Sudden Cardiac Arrest Foundation. 2015. NIH and CDC announce grantees for the sudden death in the young registry. http://www.sca-aware.org/scanews/nih-and-cdc-announce-grantees-for-the-sudden-death-in-the-young-registry (accessed June 12, 2015).
Tabak, R. G., E. C. Khoong, D. A. Chambers, and R. C. Brownson. 2012. Bridging research and practice: Models for dissemination and implementation research. American Journal of Preventive Medicine 43(3):337-350.
Taniguchi, D., A. Baernstein, and G. Nichol. 2012. Cardiac arrest: A public health perspective. Emergency Medicine Clinics of North America 30(1):1-12.
Thompson, W. L., R. Bellamy, R. O. Cummins, H. H. Delooz, W. Dick, P. M. Kochanek, J. P. Ornato, E. M. Ricci, M. H. Weil, and P. M. Winter. 1996. Funding resuscitation research. Critical Care Medicine 24(2 Suppl):S90-S94.
Travers, A. H., T. D. Rea, B. J. Bobrow, D. P. Edelson, R. A. Berg, M. R. Sayre, M. D. Berg, L. Chameides, R. E. O’Connor, and R. A. Swor. 2010. Part 4: CPR overview: 2010 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation 122(18 Suppl 3):S676-S684.
Trummer, G., K. Foerster, G. D. Buckberg, C. Benk, C. Heilmann, I. Mader, F. Feuerhake, O. Liakopoulos, K. Brehm, and F. Beyersdorf. 2010. Successful resuscitation after prolonged periods of cardiac arrest: A new field in cardiac surgery. Journal of Thoracic and Cardiovascular Surgery 139(5):1325-1332.
Trummer, G., K. Foerster, G. D. Buckberg, C. Benk, I. Mader, C. Heilmann, O. Liakopoulos, and F. Beyersdorf. 2014. Superior neurologic recovery after 15 minutes of normothermic cardiac arrest using an extracorporeal life support system for optimized blood pressure and flow. Perfusion 29(2):130-138.
TU Delft (Delft University of Technology). 2014. TU Delft’s ambulance drone drastically increases chances of survival of cardiac arrest patients. http://www.tudelft.nl/en/current/latest-news/article/detail/ambulance-dronetu-delft-vergroot-overlevingskans-bij-hartstilstand-drastisch (accessed June 15, 2015).
VA (U.S. Department of Veterans Affairs). 2015. Combined assessment program summary report: Evaluation of quality management in Veterans Health Administration facilities fiscal year 2014 (Report no. 14-00378-208). Washington, DC: U.S. Department of Veterans Affairs. http://www.va.gov/oig/pubs/VAOIG-14-00378-208.pdf (accessed June 15, 2015).
Virginia Commonwealth University. 2011. Researchers form consortium to study post-cardiac arrest patients. http://www.cctr.vcu.edu/news/feature/consortium.html (accessed June 15, 2015).
Volpicelli, G. 2011. Usefulness of emergency ultrasound in nontraumatic cardiac arrest. American Journal of Emergency Medicine 29(2):216-223.
Wallace, S. K., B. S. Abella, and L. B. Becker. 2013. Quantifying the effect of cardiopulmonary resuscitation quality on cardiac arrest outcome: A systematic review and meta-analysis. Circulation: Cardiovascular Quality and Outcomes 6(2):148-156.
Wallmuller, C., F. Sterz, C. Testori, A. Schober, P. Stratil, D. Horburger, M. Stockl, C. Weiser, D. Kricanac, D. Zimpfer, Z. Deckert, and M. Holzer. 2013. Emergency cardio-pulmonary bypass in cardiac arrest: Seventeen years of experience. Resuscitation 84(3):326-330.
Weisfeldt, M. L., and L. B. Becker. 2002. Resuscitation after cardiac arrest: A 3-phase time-sensitive model. Journal of American Medical Association 288(23):3035-3038.
Wik, L., J. A. Olsen, D. Persse, F. Sterz, M. Lozano, Jr., M. A. Brouwer, M. Westfall, C. M. Souders, R. Malzer, P. M. van Grunsven, D. T. Travis, A. Whitehead, U. R. Herken, and E. B. Lerner. 2014. Manual vs. Integrated automatic load-distributing band CPR with equal survival after out of hospital cardiac arrest. The randomized CIRC trial. Resuscitation 85(6):741-748.
Wolcke, B. B., D. K. Mauer, M. F. Schoefmann, H. Teichmann, T. A. Provo, K. H. Lindner, W. F. Dick, D. Aeppli, and K. G. Lurie. 2003. Comparison of standard cardiopulmonary resuscitation versus the combination of active compression-decompression cardiopulmonary resuscitation and an inspiratory impedance threshold device for out-of-hospital cardiac arrest. Circulation 108(18):2201-2205.
Yannopoulos, D. 2014. Guiding medical therapy for cardiac arrest with physiological based observations and solutions. Presentation at the third meeting of the IOM’s Committee on Treatment of Cardiac Arrest: Current Status and Future Directions. https://www.iom.edu/~/media/Files/Activity%20Files/PublicHealth/TreatmentofCardiacArrest/August%202014/Yannopoulos%20IOM%20presentation%208-25-14.pdf (accessed June 15, 2015).
















































