4
Membrane Behavior and Microchemical Systems
Key Points
- While a great deal is known about membrane function and overall structure, little is known about the organization of lipids in the membrane that form mesoscale structures and how they modulate the function of membrane-bound proteins.
- Given the ability to create asymmetric vesicles—those with different lipid mixtures in the inner and outer layer—opportunities and challenges exist for research on these systems to gain a better understanding of biomembrane structure and function.
- Digital microfluidics is a member of a family of techniques that have interesting mesoscale characteristics and challenges associated with them but that can also serve as tools to understand questions related to molecular transport.
- Microchemical systems present several opportunities for mesoscale chemical engineering by manipulating local mass and heat transport and chemical phenomena at micrometer to millimeter scales.
- There are many challenges confronting those who are attempting to turn the promise of mesoscale chemical engineering into practical devices.
The workshop’s second panel featured three speakers. Erwin London, Professor of Biochemistry and Cell Biology and Professor of Chemistry at Stony Brook University, spoke about the collective mesoscale behavior of the lipid bilayer in biological membranes as studied using a new experimental model that more closely represents the asymmetric composition of the cell membrane. Aaron Wheeler, the Canada Research Chair of Bioanalytical Chemistry at the University of Toronto, described his group’s work developing digital microfluidic systems that can manipulate fluid droplets in, around, and through solid materials. Benjamin Wilhite, Associate Professor of Chemical Engineering at Texas A&M University, reviewed recent advances in both microreactor development and membrane research, with an emphasis on energy-related applications. A panel discussion moderated by session chair Vernon Anderson, Program Director in the Division of Pharmacology, Physiology, and Biological Chemistry at the National Institute of General Medical Sciences and a member of the workshop organizing committee, followed the three presentations.
ASYMMETRIC LIPID VESICLES FORMATION AND PROPERTIES
The lipid bilayer that forms the membrane that surrounds all cells is a mesoscale structure comprising individual lipid molecules, each with a polar and nonpolar region, that self-assemble and self-organize with the polar portions in contact with water, explained Erwin London. Proteins, he added, are embedded within the lipid bilayer. While a great deal is known about membrane function and overall structure, little is known about the organization of lipids in the membrane that form mesoscale structures and how they modulate the function of membrane-bound proteins. It is also still a mystery
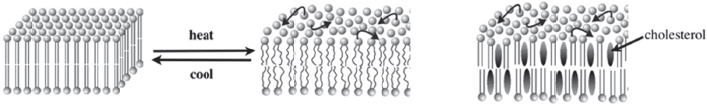
Figure 4-1 Lipid bilayers can exist in different physical states. SOURCE: London (2014). Reproduced with permission from Erwin London.
as to why natural lipids have so many different chemical structures, he added.
Lipid bilayers can exist in different physical states, London explained (Figure 4-1). In the solid-like “gel” state, the acyl chains are packed tightly in a highly ordered, almost crystalline, arrangement. Lateral diffusion within the membrane is slow in this state. At higher temperatures, a transition occurs, characterized by a melting temperature that depends on the type of lipids in the bilayer. This transition leads to a liquid-like disordered state, called the Ld state, where lateral motions are fast and in which the acyl chains have kinks in them. A third physical state, which occurs only when cholesterol is present in the membrane and is known as the Lo state, is both liquid and ordered. London characterized this as an interesting state of matter that shares properties with both the solid-like gel state and the liquid-like disordered state. In this third physical state lateral motions are fast but the acyl chains are extended, straight, and tightly packed against themselves and the cholesterol molecules.
Saturated lipids—those without double bonds—can pack together more easily and bilayers formed from saturated lipids have high melting temperatures and tend to form the solid, gel-like state at physiological temperatures. Unsaturated lipids have cis double bonds that act as permanent kinks that tend to inhibit lipid–lipid interactions. As a result, they pack together more loosely and have low melting temperatures. London said that in the 1960s and 1970s, when researchers came to appreciate the ways different lipids pack together, the question arose as to how the mixtures of lipids in a real cell membrane would behave, if phase separation occurred within membranes, and if the interesting biology that occurs in a membrane would take place in regions where coexisting domains occur.
It turns out that in model membrane systems coexisting domains are observed and studied (Figure 4-2), but in real cell membranes these domains are vanishingly small. These different domains in model and real systems are important because each membrane protein has a particular affinity for these different domains, with the result that mesoscale lipid structure has a direct effect on protein–protein interactions. For example, proteins with similar affinities may interact in the same domain or be segregated from each other if their affinities are different. A protein’s conformation, and therefore its activity, can also be affected by the domain state. London noted that as these domains form or break up, or when smaller domains coalesce into larger domains, it can turn on or off biological processes driven by membrane-bound proteins. Ordered lipid domains, also known as lipid rafts, have been proposed to play a role in signal transduction, sorting of proteins to different cell membranes, infection by bacteria and viruses, and other membrane processes. “So the question we’re faced with is how can we better understand the formation of these domains, what are the principles that are behind their formation, and what are the principles that control their size,” said London. He added that this last question is a contentious subject among researchers who are trying to understand why they are small in many systems and larger in many of the model systems.
One of the issues that the field faces is that many of the model membranes, which are made by mixing lipids in water, are symmetric, while real cell membranes are asymmetric—they have a different lipid composition in the inner and outer layers (Figure 4-3). In mammalian membranes, the outer layer, or leaflet, is rich in sphingolipids and the inner leaflet is rich in aminolipids such as phosphatidylethanolamine and phosphatidylserine. Being able to reproduce this type of distribution is important because the sphingolipids and cholesterol
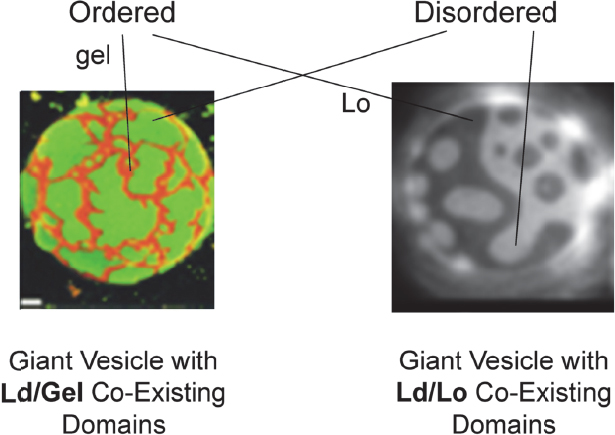
Figure 4-2 Membranes with mixtures of lipids can have coexisting domains with different compositions and different states. SOURCE: Feigenson and Buboltz (2001, right) and Veatch and Keller (2003, left). Reprinted with permission from Biophysical Journal.
create ordered domains and the sphingolipids are restricted largely to the outer leaflet. At the same time, membrane reorganization in the outer leaflet is somehow transmitted to the inner leaflet and the proteins in the inner leaflet respond to clustering in the outer leaflet, London explained.
To study the effects of asymmetry, London and his collaborators create model asymmetric membranes using a family of molecules known as cyclodextrins, which are cyclic glucose polymers containing six to eight glucose units. Cyclodextrins are hydrophilic but with a hydrophobic cavity that can be used to bind lipids from a donor vesicle and deposit them in the outer leaflet of an acceptor vesicle of different lipid composition. The result is an asymmetric vesicle that can be separated by centrifugation. London’s group recently improved this method so that they can create asymmetric vesicles with controlled amounts of cholesterol in the inner and outer leaflets that come close to mimicking the plasma membrane from cells.
Using this method, London’s group has been studying coupling between the outer and inner leaflets. They have created vesicles with one of two different sphingolipids in the outer leaflet: milk sphingomyelin, which has a long hydrocarbon chain that extends into the inner leaflet, and egg sphingomyelin, which has shorter hydrocarbon chains and does not extend into the inner leaflet. The outer leaflet of these vesicles also contains cholesterol and an unsaturated lipid—1,2-dioleoylsn-glycero-3-phosphocholine (DOPC)—that forms disordered domains, while the inner leaflet only contains DOPC. The outer leaflet has a mixture of ordered and disordered domains, while the inner leaflet by itself would only form the disordered state.
To probe the two leaflets, London’s group used fluorescent dyes that locate either in Ld or Lo domains. Images of the asymmetric vesicles show that the inner leaflet, which has no sphingolipid, has responded to the outer leaflet and formed ordered domains that correspond to where the outer leaflet has formed ordered domains, demonstrating that there is strong interleaflet coupling. In vesicles with
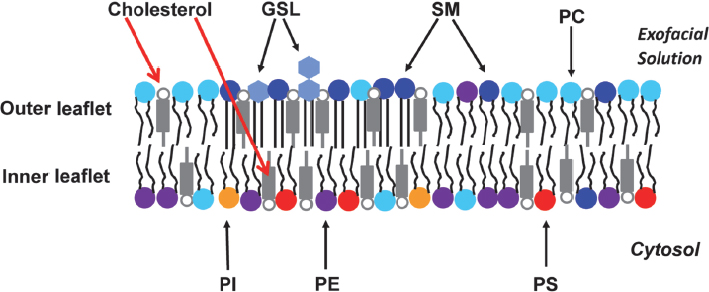
Figure 4-3 Lipid bilayers in cells are asymmetric. In mammalian cells, the major lipids in the outer leaflet are sphingomyelin (SM), glycosphingolipid (GSL), phosphatidylcholine (PC), cholesterol, and some phosphatidylethanolamine (PE). The inner leaflet’s major lipids are PE, phosphatidylserine (PS), phosphatidylinositol (PI), cholesterol, and some PC. SOURCE: London (2014). Reproduced with permission from Erwin London.
milk sphingomyelin, the inner leaflet properties match those of the outer leaflet, which London said suggests that the ordered domains forming in the inner leaflet are more similar to those in the outer leaflet. In the vesicles with egg sphingomyelin, the properties of ordered domains in the inner leaflet are different than those in the outer leaflet, suggesting that with longer hydrocarbon chains that can interdigitate with the inner leaflet, there is much stronger coupling. London noted that the results of these experiments also suggest that the ordered domains of the inner leaflet are enriched in cholesterol.
London concluded his presentation by listing some of the opportunities and challenges that exist for research in this area given the availability of asymmetric vesicles that can be used to gain a better understanding of biomembrane structure and function:
- What lipids reduce or resist this interlipid coupling in vivo?
- What controls where membrane proteins go and how do they partition between these domains in realistic asymmetric membranes?
- What controls the affinity of membrane proteins for different membrane domains?
- How does domain structure impact membrane protein interactions, conformation, and function?
- Can the principles identified in studying asymmetric membranes be extended to enable the preparation of vesicles or materials other than lipids in an asymmetric layer-by-layer fashion?
- What are the principles that control domain size, which can vary from large domains to nanoscale domains?
- Can we create domain patterned materials with controlled mesoscale heterogeneity in terms of domain size and chemical composition?
- Can we use asymmetric vesicles for improved encapsulation applications such as drug delivery?
DIGITAL MICROFLUIDICS: NOT JUST FOR LIQUIDS ANYMORE
As introduced by Aaron Wheeler, digital microfluidics is a member of a family of techniques that have interesting mesoscale characteristics and challenges associated with them but that can also serve as tools to understand some of the questions that this workshop is addressing. This is particularly true for questions related to molecular transport. He
noted that though the focus is on one set of techniques in this talk, there are quite a few different tools to study transport phenomena that use enclosed microchannels of a wide variety of interesting geometries.
As an example of a digital microfluidic device, Wheeler showed a schematic of a system consisting of a droplet trapped between an insulator sitting above an array of electrodes and a counter electrode. The insulator is a critical design element that enables fine control over the electrodes. With this system Wheeler’s group can charge a given electrode and “drag” the droplet over to the charged region of the device. He noted that his group has developed computational models to describe this type of system, and they understand that the forces being applied to the droplet are on the order of tens of micronewtons. Devices such as these can be used to carry out chemical reactions in a precise and controlled manner thanks to the ability to deliver droplets to specific places on the microfluidic device. In the example he showed, the droplets are on the order of tens of nanoliters in volume and the reaction space was a few hundred microns. Wheeler explained that this type of device enables programmed chemistry to take place with controlled, small quantities of reagent delivered with exquisite precision in terms of sequence and location.
Wheeler said that while much of the work in digital microfluidics has been done using liquids or even cells, he began asking himself a number of years ago what would happen to this type of system if he used it with solids and for making three-dimensional heterogeneous systems. To do that, his group had to answer a number of questions, such as what happens to the driving and resistive forces in a three-dimensional heterogeneous system and how can they be used and under what conditions to move solids through such a device. In working to answer these questions, his group has been using digital microfluidics to move hydrogels, polymer plugs, and magnetic particles. “Each of these systems has its own unique challenges and questions associated with it,” Wheeler explained as he described the device that his group created to work with hydrogels.
The first question his group explored was what would happen to the hydrogel in a digital microfluidic device as reagents were moved through the device. In one set of experiments, members of his research group showed that they could move water droplets through a hydrogel without disturbing the hydrogel. “We weren’t sure what to expect, and in fact we thought that perhaps the presence of the hydrogel would present some significant impediments to moving the droplet, but that turns out not to be the case at all,” he explained. Given that result, his group then followed the diffusion of a tracer either into or out of the gel in a passive state, where the droplet is sitting in place, to calculate a diffusion coefficient, and it matched what they expected from the literature. However, moving the droplet repeatedly through the hydrogel triggers an active transport process that is three times faster than passive diffusion. Moreover, this active transport rate changes depending on the velocity of droplet movement, the size of the gel, and the size of the droplet. Wheeler noted that this type of device could prove useful for studying transport under varying conditions.
One application of digital microfluidics with solids that his group has been exploring involves the notion of confining catalytic systems to take advantage of different rates or types of chemistry. One such project involves confining proteolytic enzymes to hydrogels to create a device for use in proteomic analysis, and it started with developing an epoxide-based chemical reaction to tether these enzymes throughout the hydrogel. Confocal microscopy showed that this immobilization process did distribute enzyme throughout the three-dimensional structure of the hydrogel. With this chemistry in hand, Wheeler’s group then created microreactors with different enzymes connected to microfluidic channels through which they could deliver samples for proteomic analysis and then collect the enzymatic digest for analysis. The eventual goal of this work is to create a device containing a large number of different proteases to produce multiple protein digests that will provide a high level of amino acid sequence coverage to enable high-throughput proteomics. Currently, this project is at the stage where Wheeler’s group has started collaborating with a mass spectroscopy company that wants to use these devices in conjunction with automated mass spectroscopic analysis of the protein digests.
Another project from his laboratory aims to develop hydrogel-containing microfluidic devices for growing mammalian cells. Before describing this device, he reminded the workshop that mammalian cells grown in a three-dimensional conventional gel
develop with a different phenotype compared to when they are grown in two dimensions. Though the three-dimensional growth results in a phenotype that is closer to in vivo phenotypes, many researchers choose not to grow three-dimensional structures because the fragility of the conventional gels makes the process arduous. Building on the knowledge within the group, they first refined the process for creating gels for this device, for example, group developing gel crosslinking methods and ways of controlling surface energies that enable them to form a wide variety of gel shapes. With these techniques in hand, his team has built microfluidic devices with arrays of gels that are each addressable individually.
When cells are placed in and evenly distributed throughout these devices, they grow in a three-dimensional arrangement with the same phenotype expected from the literature for cells grown using conventional gel systems. However, when comparing the robustness of the conventionally grown three-dimensional cellular spheroids with those grown in the hydrogels, a significant and surprising difference was identified. The resulting cell spheroids grown within the microgels are far more robust than those grown in conventional gels. This makes it possible to deliver reagents to these cells in ways that were not possible with those grown in conventional gels. One potential application for these devices that his group is exploring with collaborators is to produce an array-based three-dimensional tissue culture system for medical screening applications. He noted that “this is an interesting system where there may be some opportunity for mesoscale science . . . and again I’ll point out that the surface has just been scratched.” He concluded his talk by noting that “these types of tools may end up being useful for studying some of the things that this workshop is interested in exploring.” He also noted in answering a question from a workshop participant that these microfluidic devices work in air, oil, and other environments and that once the device is set up, operations are carried out electronically.
ENGINEERING AT THE MESOSCALE: MICROREACTORS AND MEMBRANES
Benjamin Wilhite began the last presentation in this session with the comment that, from his perspective as a chemical engineer, he has a different concept of mesoscale. “To the chemical engineer, microchemical systems present several opportunities for mesoscale chemical engineering by manipulating local mass and heat transport and chemical phenomena at micrometer to millimeter scales,” he said. From the perspective of a materials scientist, he added, membrane materials present several opportunities for mesoscale chemistry by manipulating the chemical structure of membranes at the mesoscale in a way that enables new kinds of separations to occur. He also noted that chemical engineers usually work at such large scales of reactors, reactants, and catalysts that the local manipulation of chemistry at the mesoscale is not something that has been considered very often. That awareness did start to change about 20 years ago, he added, when chemical microreactors were first developed. The ability to build miniaturized chemical reactors created a situation where chemical engineers had to start paying attention to various physical processes such as fluid dynamics, laminar flow, and conductive heat transfer that become dominant in these smaller systems. “What that allowed us to do to solve those problems was to manipulate, by way of the structure of the device, the local transport rates in such ways as to manipulate indirectly the chemistry of the reaction,” Wilhite explained. “Finally, we can live up to what all engineers aspire to be, which are master manipulators,” he added.
The first decade of microreactors saw a wealth of firsts, and all could be summed up with catchphrase, “on a chip”: the first gas–solid catalytic reactor on a chip (Srinivasan et al. 1997), the first gas–liquid mixer on a chip, the first gas–liquid disengaging unit on a chip, the first gas separation unit on a chip, the first fully integrated trickle-bed microreactor on a chip (Wilhite et al. 2008), and so on. This decade established the basic chemical engineering operations that were possible on a chip and how to perform them, as well as what did and did not work. Since the 2000s, the emphasis has shifted from the novel to the practical, and research has proceeded down one of two paths: either creating lab-on-a-chip devices for chemical analysis, such as the work that Wheeler described, or building devices that perform chemical processing and replace the large-scale reactors and processing equipment that are traditionally in the realm of chemical engineering.
Researchers going down this second path, including Wilhite, soon realized that chemical
engineering on individual chips was never going to replace chemical refineries because of the scale of industrial chemical production per month. Research then began to look at ways of coupling different devices, much like Lego bricks, into modular reforming processes that operate at the mesoscale rather than the microscale and that can produce enough of a chemical to be useful on an industrial scale. Wilhite described a few of these modular chemical plants. One such device can perform laboratory-scale organic systems with modules that can be assembled and disassembled quickly to make different products as needed. “This has great potential in the chemical industry where nowadays the market is volatile in terms of what chemicals need to be made for society,” said Wilhite. “The idea of having a chemical plant that you design and build and make money off of for 20 years without the feedstock or the desired product changing, those days are gone. Now we need modular chemical plants that can be quickly field stripped, reassembled, and retrofitted.”
Another promising area of research and development activity aims at creating a personal “turnkey” chemical plant capable of converting natural gas to hydrogen as needed at home or at a local fueling station, which could enable an immediate transition to hydrogen fuels for automobile use by leveraging the existing natural gas infrastructure. Similarly, researchers are working on a modular system that would convert the natural gas now being flared at the wellhead of what are known as stranded oil wells—those too far from a natural gas pipeline for it to be economical to run additional line to capture the gas associated with shale oil—into synthesis gas using Fischer-Tropsch chemistry. The resulting liquid fuel could then be more readily and economically captured and transported. Wilhite noted that Qatar is already doing this on a large scale to turn natural gas into diesel fuel.
There are many challenges confronting those who are attempting to turn the promise of mesoscale chemical engineering into practical devices. One of the first challenges researchers addressed was the difficulty in getting liquids to mix in these mesoscale devices. Mixing at the bulk scale is dominated by turbulent motion of free liquid when agitated, and mixing is controlled either by stirring or not stirring a mixture of liquids. At the mesoscale, mixing is dominated by the diffusion of momentum to such an extent that when two fluid streams are brought together in microchannels they do not mix at all on any kind of useful time frame. However, forming channels with submillimeter ridges and fins on the walls disrupts laminar flow and exquisite control of mixing (Liu et al. 2013, Stroock and Whitesides 2003).
Another challenge that Wilhite and his group addressed was to develop the means of manipulating gas-phase chemistry at membrane surfaces. One of the first examples of accomplishing this was when Wilhite’s group created a device that used a membrane to remove contaminants from a natural gas stream that would rapidly corrode a second, more expensive catalytic palladium-containing membrane (Figure 4-4) (Kim et al. 2010, Moreno, Damodharan, and Wilhite 2010). Not only did this system eliminate corrosion of the catalytic membrane, but it also increased the rate of permeation through that second membrane by about 30 percent, Wilhite explained.
Heat transfer in microreactors has also proven to be a challenge, particularly with regard to preventing heat loss in a device with hundreds of tiny channels passing through a block of material. The key to addressing this problem was to develop manufacturing techniques that would isolate each microchannel and remove the common solid substrate (Besser 2011), Wilhite said. “What that has allowed us to do is to focus the patterning of a catalyst within a heat-exchanging microreactor so that we can control the local mesoscale rates of heat transfer, rates of heat production, and rates of heat utilization by a chemical reaction, in order to get significant improvement in the amount of reaction we can push through one small volume,” said Wilhite.
In the final portion of his presentation, Wilhite discussed some of the challenges of using membranes in gas separations. Membrane-based gas separation has become the standard industrial process since the first gas-separation membranes were commercialized in the 1980s as a less-expensive and lower-energy-use alternative to distillation. The main challenge is overcoming the inherent limit to the efficiency of these membranes, a property revealed by what is known as a Robeson map that plots purity of the separation versus the permeability of the membrane to that gas. To overcome the tradeoff between purity and rate of separation, researchers have been adding structured
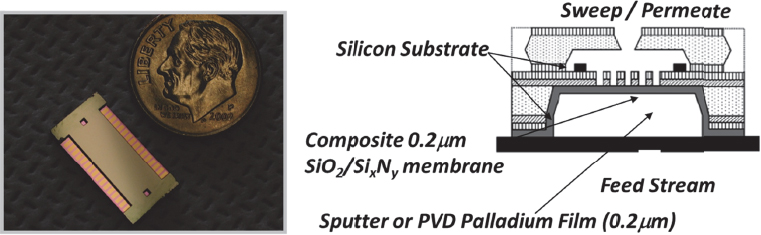
Figure 4-4 Manipulation of gas-phase chemistry at membrane surfaces enabled the first demonstration of portable high-purity hydrogen generation from methanol. SOURCE: Wilhite, Schmidt, and Jensen (2004). Reprinted with permission from Industrial & Engineering Chemistry.
inorganic materials such as zeolites and metal oxide frameworks to the polymer membranes (Kim, D., et al. 2014).
Wilhite and his collaborators have developed a layer-by-layer deposition technique that allows them to control the chemistry of deposition by building a film one layer at a time, alternating positively charged and negatively charged polymers. He noted that this process is not as precise as atomic layer deposition, but that it works well for creating membranes. The example he described used polyethyleneimine and polyacrylic acid and the membrane grows by allowing the polymers to adhere electrostatically to the surface. By adjusting the pH of the dip solutions, it is possible to tune the level of charge in the resulting membrane film as well as the crosslinking between the polymer layers. The result is a thick, robust membrane with well-defined pores that will separate hydrogen gas from carbon monoxide, carbon dioxide, oxygen, and nitrogen with performance characteristics that exceed the upper bounds predicted by the Robeson map by an order of magnitude. “We are able to have a higher separation than anything else in the literature because of using mesoscale chemistry to manipulate the deposition conditions used to make the membrane,” Wilhite said in closing.
In response to a question from Yi Lu about how to incorporate different enzymes into a membrane with known spatial definition, London replied that this is a difficult task in an asymmetric lipid bilayer. “Controlling protein orientation is still a mystery,” London explained, adding that there is no solution to this problem at present. One possibility would be to manipulate the shape of the proteins given that orientation in the lipid bilayer is controlled to some degree by protein shape. Wheeler added that Petra Dittrich and her colleagues at ETH Zurich are exploring some innovative approaches to achieve protein orientation.
Vernon Anderson from the National Institutes of Health asked about the lateral stability of proteins in the artificial bilayers that London creates. London replied that proteins move laterally quickly, on the order of many times per second, but that the rate of flipping from one side of the membrane to the other is very slow and reaches hours or days.
Jim De Yoreo asked Wilhite about work that might have been done to make synthetic analogs of sequence-defined polymers that can operate in drug environments. Wilhite replied that he did not know of any work in this area but that the membranes he and his collaborators make will separate gases under drug conditions and that the membranes have good stability when used in dry conditions. Even in the absence of water, these membranes maintain their structure and their separation properties.
London then fielded a question from Wendy Shaw, who asked if he had any details on what was driving the phase separation seen with different membranes and if microfluidics could help gain
insights into that process. He replied that the driving force of the phase separation is simply the tendency of one lipid to prefer a solid phase and the other to prefer a liquid phase at some intermediate temperature at which these two states will exist. Van der Waals forces drive the lipids to pack tightly in the solid state, and this is amplified by the presence of cholesterol, which also likes to pack tightly in the presence of water.
Andrew Borovik then asked Wheeler if his devices could be used to deliver electrons as well as reagents in a spatially and temporally defined manner. Wheeler’s answer was yes, and that his group has in fact done that kind of chemistry for electroanalysis and that it should be possible for electrocatalysis, too. Borovik commented that this capability could be useful not just for doing chemistry but also to better understand the mechanisms of electrocatalytic reactions.
Bruce Garrett asked Wilhite if the problem of heat transfer was now solved given the results he obtained, and Wilhite said that it was not. Heat, he said, is still lost to such an extent that it cannot yet be recaptured to drive other reactions on the chip. Researchers are working on this problem but it has yet to be solved. Wilhite also responded to a question from Yong Wang of the Pacific Northwest National Laboratory about whether industry is working to scale these devices to reach the necessary capacity for its purposes by noting that industry is still concerned about how to monitor and control these devices and about the robustness of the systems. Because the channels are packed tightly into these devices, it is difficult presently to also fit in the amount of instrumentation needed to monitor chemical processes to the extent that they are monitored now in large petrochemical and fine chemical manufacturing facilities.
Lu then asked Wilhite if the devices can operate under pressure, and Wilhite said that this, too, is a challenge because the devices as they are currently made are welded laminates that can fail under pressure. Going to a tube-within-a-tube design for creating channels reduces the number of welds needed to hold the device together. London asked if three-dimensional printing can overcome this problem by eliminating the need to weld layers together, and Wilhite said this was an interesting possibility, one that he wants to explore. Cristian Contescu from Oak Ridge National Laboratory invited Wilhite to use the three-dimensional printers in the manufacturing demonstration facility there and then asked Wilhite about the cost of these devices and whether it would ever be practical to use these devices on a large scale. Wilhite acknowledged that cost was a big issue and that there are groups working to reduce the cost of manufacturing the devices by using stamping and rolling processes instead of micromachining in order to scale and automate production of the devices. He noted, too, that tube-in-tube systems should also be less expensive to make.










