4
Challenges and Potential Solutions to Enable Development of Successful Treatments
Highlights
- Early detection and early effective treatment may prevent depression from becoming chronic, debilitating, and relapsing (Sahakian).
- Experimental medicine models that enable modeling the disease process in humans may provide efficacy signals early in the development of new treatments (Harmer).
- Stratification of patients into narrow groups for clinical trials of cognition in depression may improve the efficiency of the trial and help address pseudo-specificity, but may also limit generalizability (Fava, Keefe, Laughren).
- Cognitive assessments are widely used to assess the effects of treatment on cognition, yet functional measures may provide more clinically meaningful measures (Harvey).
- Subjective and objective measures of cognition both have value in assessing treatment effects, yet both also have disadvantages and often do not correlate with one another (Areán, Fava, Harvey).
- Brain changes across the lifespan must be taken into account when selecting assessment tools for clinical studies (Areán).
- Innovative technologies, such as those using smartphones, have the potential to provide continuous assessments of mood and cognition and could also be useful in treatment decisions (Areán).
NOTE: These points were made by the individual speakers identified above; they are not intended to reflect a consensus among workshop participants.
Several workshop participants outlined challenges and potential solutions that would enhance studies of cognitive improvement in depression. Progress will require clarification of definitions and consideration of a variety of design and assessment strategies, all of which impact the ability of a trial to demonstrate effectiveness in a reasonable time frame and with a manageable number of participants, said several participants. As mentioned by Barbara Sahakian and others, depression studies are further constrained by the fact that, because most patients with depression are treated by primary care physicians, tools for the trials need to be simple enough to be used in the primary care setting.
EARLY DETECTION AND TREATMENT AND THE IMPORTANCE OF BIOMARKERS
Cognitive dysfunction is present even in the first episode of MDD, and persists even after other symptoms of depression have abated (Bora et al., 2013; Trivedi and Greer, 2014). In addition, untreated depression leads to poorer response to treatment with antidepressant medications, a lower rate of remission, a higher risk of chronicity, and a higher number of recurrences, according to a systematic review and meta-analysis of studies by Lucio Ghio and colleagues that looked at the relationship between the duration of untreated depression and clinical outcomes (Ghio et al., 2014). What this means, said Sahakian, is that early detection and early effective treatment may prevent depression from becoming debilitating, chronic, and relapsing.
Tools needed to achieve early detection and predict response to treatment include a range of biomarkers, including genetic, neuroimaging, cognitive, and other physiologic measures (Insel et al., 2013). For example, studies from Ian Goodyer’s and Sahakian’s group have shown that elevated morning cortisol levels in adolescent boys signal an elevated risk of MDD, and that a genetic marker associated with serotonin (5-HTTLPR) as well as early exposure to childhood adversity predict deficits in cognitive and emotional processing in adolescents (Owens et al., 2012, 2014). These markers can be measured objectively and easily, said Sahakian, offering the means to detect individuals at risk of developing depression 1 year later.
Neuropsychological tests may also be useful to detect early signs of depression or predict response to treatment. Amit Etkin described the International Study to Predict Optimized Treatment in Depression (iSPOT-D), in
which 1,008 unmedicated patients were randomized to treatment with one of three common antidepressants over an 8-week period and followed with a broad cognitive battery. The aim of the study was to determine whether performance on a standardized test battery of cognitive and emotional function was predictive of remission or response to treatment. The battery included tests of psychomotor function, decision speed, verbal memory, working memory, cognitive flexibility, attention, response inhibition, information processing speed, executive function, emotion identification reaction time, and emotion bias reaction time. The study showed that a subgroup of depressed patients that could be discriminated based on cognitive test performance had poorer treatment outcomes, suggesting that a composite biomarker based on the antidepressant outcome and test performance could be used to predict treatment outcome (Etkin et al., 2014). Another study by Walsh and colleagues suggested that tests of working memory, such as the n-back verbal memory task, may predict clinical outcome (Walsh et al., 2007).
Lack of efficacy is the main reason for drug development failures (Hay et al., 2014) yet the current phased approach to drug development means that efficacy is typically not assessed until a relatively late stage of development, after substantial resources have been invested (Kola and Landis, 2004). Novel candidate treatments are often screened for efficacy using preclinical animal models, but these models have low predictive validity, according to Catherine Harmer. She proposed using an experimental medicine approach, which models the disease process in humans by testing how various parameters (e.g., different experimental compounds, dosing, etc.) impact how individuals suffering from MDD perform on neurocognitive tests. This approach aims to provide answers to key questions early in development.
Drug development for depression has largely been built on the success of selective serotonin reuptake inhibitors (SSRIs) and thus has led to a generation of similar drugs that focus on serotonin. More recently, therapeutic delay, or the delayed onset of antidepressant drug action, prompted the search for neurobiological correlates that are expressed in a delayed manner, such as changes in plasticity. A third approach, advocated by Harmer, is to assess the effects of antidepressants on neural and psychological processes that are important in the early stages of depression; for
example, the effects of drugs on negative biases in emotional processing, or hot cognition. Indeed, studies show that early antidepressant drug treatment can affect hot cognitive bias even before patients notice improvements in mood, suggesting that antidepressants may work not directly as mood enhancers, but indirectly by changing the way information is processed (Harmer et al., 2009; Roiser and Sahakian, 2013).
Harmer showed data from her studies in patients with depression on their ability to pick up on happy facial cues (Harmer et al., 2009). In comparison to healthy controls, depressed patients find it much more difficult to perform this task; however, after just one dose of the noradrenergic reuptake inhibitor reboxetine, patients show an improved ability to recognize happy facial expressions. They do not feel any better and are no less depressed, said Harmer, but these early changes in emotional processing suggest they are already processing emotional cues in a more positive way, which would be expected to have therapeutic benefit over time. Moreover, these findings suggest this type of model might be used to screen new treatments early in the development process. Further investigation showed that the model met a number of key criteria that allow it to be used for this purpose:
- The model is sensitive to a range of established antidepressants with different neurochemical actions.
- Early effects predict treatment response.
- The model can discriminate between ineffective and effective agents.
- It is sensitive to novel mechanisms of action.
- It can be used to generate hypotheses, calculate dosing information, or identify subgroups useful for randomized clinical trials.
- It can be used in healthy people as well as in depressed patients (Harmer et al., 2009; Pringle et al., 2013; Roiser and Sahakian, 2013; Shiroma et al., 2014).
This marker is now being used by five pharmaceutical companies to explore new candidate treatments for depression and anxiety at an early stage of development, said Harmer. For example, in a collaboration with Eli Lilly and Company on the development of a new drug with a novel mechanism of action that showed good results in preclinical animal models, Harmer and colleagues showed that the emotional processing response at 1 week not only provided an early marker of likely efficacy, but also predicted a subgroup of responders.
Harmer said it also may be possible to use these same kinds of models to understand and predict mechanisms of action on cold cognitive targets such as memory, executive function, and depression. The CANTAB, for example, has been used extensively to understand the impact of drug treatment on cognition and the mechanisms behind drug efficacy, and to identify which patient groups are most likely to benefit from specific drugs (Turner et al., 2003, 2004a,b).
The n-back task is one specific test used to assess visual working memory in both healthy and clinical populations, said Harmer. As mentioned earlier, depressed patients find it hard to switch off the default-mode network, but have increased activation in the task-positive network. Indeed, using the n-back test, investigators showed that depressed patients exhibited the characteristic hyperactivity of task-positive circuits, and that treatment with fluoxetine failed to normalize this overactivity (Walsh et al., 2007). This suggested that the n-back test might be useful as an experimental model to separate out mood and cognitive effects of novel antidepressants on cognition. To test this hypothesis, Harmer said that she and her colleagues used the n-back test in combination with fMRI in patients randomized to receive vortioxetine or placebo for 10 days. Vortioxetine is a novel, multimodal antidepressant that has been shown to have positive effects on cognition in depression (Katona et al., 2012; McIntyre et al., 2014). In both healthy controls and depressed patients in remission, vortioxetine treatment improved both subjective and objective measures of cognition and resulted in decreased neural activity across the brain regions that are affected in depression, that is, a reduced activation of the DLPFC and increased deactivation of parts of the DMN. The fact that these effects were seen in healthy people demonstrates the usefulness of this approach in early-stage drug development, said Harmer. However, she noted that more sensitive tasks may be needed to demonstrate cognitive improvements in individuals who are cognitively healthy.
A variety of study designs may be used in evaluating treatments aimed at improving cognition in patients with depression. Three specific types—adjunctive, acute-phase, and switching—were mentioned frequently during the workshop. The choice of design affects the duration and size of the study as well as inclusion and exclusion criteria. Other
important trial design decisions involve the choice of control, that is, active comparator, placebo, or both, and the study population.
Design Type
Adjunctive approaches, where a second treatment is added to an existing treatment regimen, may be particularly useful to address cognitive impairment in depression because cognitive symptoms frequently remain even after mood has improved in response to treatment with an antidepressant, said Tiffany Farchione, deputy director, Division of Psychiatry Products at FDA. A concern from the regulators’ perspective is whether the second drug improves depression overall or specifically targets cognition. Thomas Laughren referenced the lisdexamfetamine trial as an example in which patients improved on both the cognitive measure and the MADRS when lisdex (in comparison to placebo) was added. However, a more careful look at scores on MADRS items suggested that the drug was specifically targeting cognition. Laughren suggested that this design represents one way of addressing concerns regarding pseudo-specificity, which is discussed in more detail in Chapters 5 and 6.
Acute-phase designs target the acute phase of the illness. Such designs might incorporate three arms with two different treatments (active control and investigational agent) as well as a placebo arm, and could enable targeting of both depression and cognitive impairment, while also addressing the concern of pseudo-specificity. Although both treatments might show antidepressant effects, if the investigational agent but not the active control also improves cognition, this could be taken as evidence that the agent specifically targets cognition. The vortioxetine study (CONNECT) is an example of this approach. The CONNECT study compared vortioxetine and duloxetine, both active agents, against a placebo with results demonstrating that both treatments significantly improved depressive symptoms based on the MADRS scale, but only vortioxetine was found to be efficacious in improving cognitive function in depressive patients (Mahableshwarkar et al., 2015).
Switching designs, in which patients in a residual phase of depression or remission are randomized to continue on one antidepressant or switch to a second drug that is thought to improve cognition might also be useful, said Laughren; however, he said he is not aware of anyone using this design in depression studies.
Controls
The use of an active comparator versus placebo as the control represents another important design consideration. According to Farchione, the agency has a strong preference for an active comparator, typically another antidepressant.
Study Population
Depression is a heterogeneous condition, affecting people across the lifespan and varying in terms of symptomatology and response to treatment. Because not all patients with depression have cognitive impairment, enrichment for those who do makes sense for trials aimed at improving cognition in depression, Fava said. Beyond selecting individuals with cognitive impairment, other population decisions include whether to include untreated patients, those who have responded to treatment and remitted, or those who have responded but have residual cognitive symptoms. Each choice has advantages and disadvantages, depending on the trial being conducted. For example, for a trial of an augmentation therapy, enrichment with patients who have residual cognitive symptoms may make the most sense.
Stratifying patients into narrow groups for clinical trials may, in addition to improving the efficiency of the trial, help deal with pseudo-specificity, said Richard Keefe. For example, a recent study of lisdexamfetamine enriched the study population by selecting patients with remitted depression and no attention deficit hyperactivity disorder (ADHD) in order to remove the confounding effects of ADHD on measures of attention (Madhoo et al., 2014). Fava, however, said a simpler design would be to start with remitted MDD patients. While enrichment may be deemed necessary to ensure that a trial is able to demonstrate a treatment effect, selecting more narrow groups for clinical trials also has a downside in terms of the generalizability of the results. To illustrate this point, Laughren described a meta-analysis of placebo-controlled antidepressant trials conducted by FDA, in which out of nearly 100,000 patients in the analysis, there were only 8 suicides. This suggests that suicidal patients were excluded from most controlled trials, despite the fact that suicidality is a significant issue in depression (Stone et al., 2009).
Enrichment may be achieved using either objective or subjective measures, each of which has advantages and disadvantages. This is discussed in more detail in the section on assessment.
Future Directions in Study Design and Content
Keefe suggested a number of changes to study design that could increase the field’s likelihood of identifying an efficacious drug. These changes include using study designs and statistical methods that maximize test validity and minimize confounding factors, assessing treatment effects on specific domains of cognitive function. Other changes in design that were mentioned by several participants included assessing changes over time rather than only at baseline and end of study, and identifying signals that can be assessed in 1 week or 48 hours to enable faster trials, particularly in Phases I and II. Adaptive approaches such as those used in the I-SPY2 TRIAL for breast cancer therapies might also be useful for testing treatments for cognitive impairment in depression, said Thomas Insel (Barker et al., 2009). This approach uses a clustered randomized design to match experimental therapies with the appropriate patients, using interim outcomes to adapt the trial as it progresses.
Keefe also called for an increased number of larger-scale, longer-term, placebo-controlled studies, as well as further research to overcome the substantial methodological limitations of prior investigations and a more systematic examination of the cognitive effects of pharmacotherapy in MDD, similar to what is under way in schizophrenia. More studies are also needed to optimize the use of neurostimulation and psychotherapy as treatments for cognition in depression, said Diego Pizzagalli. One fundamental question that needs answering, he said, is whether improvements in cognition are mediated directly or indirectly by improvements in symptoms of depression. Pizzagalli also called for studies that demonstrate target engagement with specific interventions.
A number of other suggestions emerged related to the concept of stratifying participants to deal with heterogeneity. Rather than using depression as the target, Insel suggested that stratification could enable development of a “purer culture” of participants with a specific problem than can be addressed with drugs, devices, or psychotherapy. Pizzagalli suggested using neural navigation to identify people who might disproportionately benefit from various interventions based on neural function and dysfunction. Patricia Areán suggested not looking at cognitive impairment as an outcome, but as a way of identifying “flavors” of depression. For example, there is some evidence that certain interventions are particularly effective on those who have a depressive disorder and executive dysfunction, However, Etkin noted that while many participants expressed support for the idea of subtyping, tools are needed to show whether a treatment
effect is a subgroup effect or a general effect. Matching mechanism to intervention would be helpful in clarifying the reasons for a treatment effect or lack of effect.
Assessment of cognition and function can be accomplished using both objective measures that are performance based, as well as subjective observational, self-reported, and informant-reported measures. Whatever the approach, the requirements are essentially the same: validity, adequate psychometric properties, practicality, and tolerability, said Philip Harvey, professor of psychiatry and behavioral sciences at the University of Miami Miller School of Medicine. The measure must also be sensitive to treatment effects and clinically meaningful, a point that was raised by several participants, including regulators. Yet while these general requirements are the same across studies and across disease conditions, the components of individual tests may vary.
Cognition Versus Function
Naturally, cognitive measures are widely used in studies to assess the effects of a treatment on cognition, but the need to find measures that are clinically meaningful to patients has prompted many investigators to consider functional measures as an alternative or adjunct. Harvey and colleagues conducted a study of clinically stable patients with schizophrenia, schizoaffective disorder, bipolar disorder, and depression, using a battery of neuropsychological tests assessing eight cold cognitive domains. The study showed that cognitive performance profiles were similar across all four groups, although there were quantitative differences, with schizophrenia patients exhibiting more severe cognitive impairment (Harvey et al., 2015; Reichenberg et al., 2009). However, functional impairments vary substantially among these different groups. Many patients with schizophrenia have never held down a job, for example, while patients with MDD typically have experienced greater lifetime achievement not only in employment, but in social activities and education as well. Harvey suggested that in patients with depression who were previously functional but became unable to resume productive activities, one possible functional outcome measure for MDD trials might be returning to work or resuming other activities.
Objective Versus Subjective
Objective measures have the advantage of norms that are relatively devoid of bias; however, norms are population based and do not reflect premorbid performance levels. In contrast, subjective, self-reported measures are able to capture perception of change from premorbid levels; however, a patient’s perception may be affected by cognitive appraisal, self-esteem, depression, and anxiety. Linking either objective or subjective measures to functional change may be one way to ensure that the results are clinically meaningful, said Fava (see Figure 4-1).
A recent meta-analysis of studies using objective measures of impaired cognition in depression showed that several tests—including the Stroop task, Trail Making Test B (TMT-B), and n-back—show robust impairments in depression (Snyder, 2013). Fava and colleagues have taken a different approach to assessing components of cognition such as attention and memory, developing a subjective self-rated scale called the Cognitive and Physical Functioning Questionnaire (CPFQ) (Fava et al., 2009). The CPFQ asks simple questions such as “How has your ability to find words been over the past month?” to which patients respond using a 6-point Likert scale ranging from “greater than normal” to “totally absent.” The CPFQ has demonstrated internal consistency, high test-retest reliability, and sensitivity to change with treatment, and has been used in a number of studies from Fava’s group. In addition, it has shown that even people who respond to treatment continue to report impairments in attention, memory, word finding, and mental acuity (Fava et al., 2006, 2009).
Fava and colleagues have also used the CPFQ to explore how heterogeneity of depression affects cognition. Correlating CPFQ results with data from the Harvard National Depression Screening Day Scale (HANDS), they found that the residual symptoms of cognitive impairment by self-report hold no association with the core symptoms of MDD (Pedrelli et al., 2010).
Fava’s team has also looked at the overlap between objective (DSST, TMT-B, Cognitive Reflection Test [CRT], one-back) and subjective (CPFQ) measures of cognitive impairment using data from a clinical trial of vortioxetine. These studies have not yet been published, but suggest there is only partial overlap between subjective and objective measures. However, subjective impairment correlated with greater severity of depression
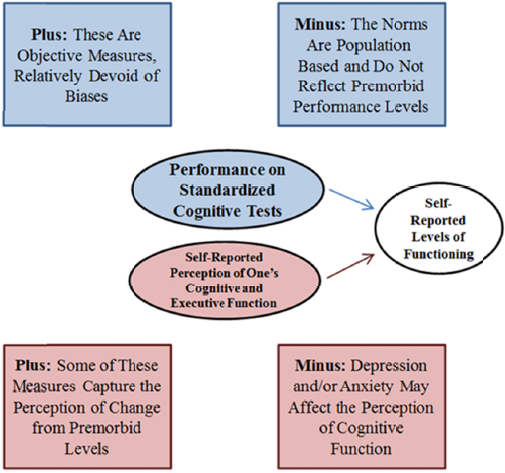
FIGURE 4-1 Objective versus subjective measures of cognition.
SOURCE: Presented by Maurizio Fava at the IOM Workshop on Enabling Discovery, Development, and Translation of Treatments for Cognitive Dysfunction in Depression, February 24, 2015.
and greater functional impairment, whereas objective impairment correlated only with functional impairment, but not severity. In the lisdexamfetamine study mentioned earlier, both self- and informant-reported measures showed significant improvement, but a computerized cognitive test battery did not (Madhoo et al., 2014). Fava and colleagues concluded that both subjective and objective measures have value, and that regardless of the measure, the functional impairment drives the outcome.
Harvey’s group has been experimenting with interview-based strategies aimed at assessing cognition and function in both patients and informants. When using such measures, the difference between patient and informant reports appears to be especially important. For example, in a recent study of patients with schizophrenia, the single best predictor of everyday disability was the size of the discrepancy between the patient’s assessment of his or her cognition and the clinician’s impression, with patients tending to significantly overestimate their own cognitive function (Gould et al., 2015). Harvey concluded that patients’ impressions of the severity of their disturbance was affected by their mood, and not correlated with objective measures.
Several participants expressed concern about the use of self-report measures because of concerns about the effects of mood or the patient’s lack of insight or awareness about their condition. In a study of 30 patients with bipolar depression, patients’ self-report of their functioning or the severity of their disturbance was uncorrelated with any objective performance-based measures, suggesting that mood or other factors may markedly influence patients’ self-assessment (Harvey et al., 2015). However, Areán noted that while subjective measures may not be useful as standalone assessments, they can be useful as initial indicators that something is wrong. Moreover, she and Harvey added that in depression, even so-called objective measures are affected by amotivation and anhedonia, and that this can confound the results.
Co-Primary Measures
Harvey noted that in both Alzheimer’s disease and schizophrenia trials, regulators have asked for co-primary measures in addition to cognitive performance, and both performance-based and interview-based measures have been used to determine functional capacity. Co-primary measures are thought to increase the face validity of the trial, resulting in better acceptance by consumers and clinicians, according to Michael Green, professor-in-residence in the Department of Psychiatry and Biobehavioral Sciences at the University of California, Los Angeles, Geffen School of Medicine and Co-Principal Investigator of the MATRICS project. The MATRICS-CT (Co-Primary Translation) initiative conducted a Validation of Intermediate Measures (VIM) study to assess various potential co-primary measures (Green et al., 2011). This study determined that the UPSA correlated well with the MATRICS
Consensus Cognitive Battery (MCCB) as the primary outcome measure, while self-reported measures did not.
In another study of patients with schizophrenia, a blinded clinician interview-based assessment (the Schizophrenia Cognition Rating Scale, or SCoRS) correlated with neuropsychological test performance as well as real-world functioning, but self-reported cognitive performance did not (Keefe et al., 2006). The SCoRS also demonstrated sensitivity to treatment effects, said Harvey. However, interviews with the patient but not the informant did not correlate, indicating that sensitivity to treatment effects is not necessarily linked to the questions that are asked, but rather who provides the answers. These data suggest that both clinician interview-based and performance-based assessments of cognition and function correlate with neuropsychological tests, and thus that they are essentially interchangeable.
Harvey described another study, again in schizophrenia, that assessed the independence of benefit from cognitive remediation and skills training with the UPSA, MCCB, and a clinician-based assessment of function, the Specific Level of Functioning Scale (SLOF). Cognitive remediation alone improved only neuropsychological test (MCCB) scores, while skills training alone improved only everyday outcome (UPSA) scores; only the combined therapy improved scores on all three tests. What this suggests, said Harvey, is that if a drug or treatment has a meaningful effect size, changes in everyday outcome could be used as a measure. Indeed, the UPSA has been widely used for conditions other than schizophrenia and bipolar disorder. In support of this view, in a study of patients with both schizophrenia and bipolar disorder, the UPSA scores were shown to correlate with independence in residential functioning (Mausbach et al., 2010).
Harvey concluded that validated performance-based measures of functional capacity that are related to everyday outcomes are optimal for assessing treatment outcomes.
Assessment Across the Lifespan
Given the brain changes that occur across the lifespan, Areán emphasized the need to take into account lifespan issues when doing assessments. In particular, children and older adults exhibit somewhat different symptoms and may respond differently to medication and behavioral interventions than do those in the middle years, yet these populations are difficult to recruit for studies and, as a result, existing longitudinal stud-
ies and clinical trials may not fully represent the full spectrum of people with depression and other mood disorders.
Areán also explored issues important to consumers. Interestingly, the treatment outcomes important to children and older adults with depression align, although they are applied in different contexts. For instance, both groups express concern about sleep, social contact, and the ability to concentrate and focus. Researchers have been historically interested in testing cognitive domains such as executive function, attentional bias and reward, motivation, and valuation, all of which affect everyday function and social contact. However, assessment in both children and older adults brings with it certain challenges. Both groups are notoriously poor reporters of their own mood, said Areán, and children may have a hard time naming what they are experiencing. In addition, assessment can be particularly burdensome in these groups: in children because of distraction and in elders because of fatigue. Another important consideration for children is the context in which an assessment is done; for example, teachers may report different behavior from parents. Some objective functional measures may be particularly useful in these populations, said Areán. For example, in older adults, driving performance is directly related to the degree of cognitive impairment, and in children school performance may be directly related to the degree of cognitive and emotional impairment. So combined assessments of behavior, cognition, and mood may be the most valid assessments of treatment effectiveness.
Innovative Tools for Assessment
Many participants spoke of the need to embrace innovation for the assessment and treatment of cognitive impairment in depression. Assessment can benefit from increased use of technology, ranging from the development of touchscreen computerized tests of hot cognition with domains including emotional processing, social cognition, motivation, and reward to the use of ubiquitous computing tools. These new tools have the potential not only to increase the accuracy of assessment, but may improve engagement and provide more meaningful data, said Areán. She also mentioned the need to look at outcomes other than mood and cognition that may be more salient to people’s concerns.
Areán has been working with a number of innovative technologies designed to assess mood across the lifespan. One of these, Ginger.io, runs passively in the background on a smartphone, assessing activity, sleep patterns, and social connectedness in real time across many days or
weeks. The tool can also push surveys to participants about various issues such as mood and medication compliance. Tools such as this one have been used in treatment studies to collect data unobtrusively about changes in daily mobility and function that reflect changes in mood and that may be predictive of outcome.
Cognitive Health Corporation is using a variety of computer platforms and technologies to assess changes in mood and cognition by looking at eye movement, eye tracking, facial expressions, activity, coordination, manual dexterity, fine-motor dexterity, and voice data. In designing these tools, developers have been cognizant of the need not only to accurately assess cognition, but also to engage patients in the activity through games and provide outcome measures that are meaningful to patients. Many of these tools offer an additional advantage in that they can be adapted to individual performance at baseline or as the trial progresses, analyzing data in real time and optimizing information from previous trials. This improves accuracy and lessens the amount of time required for testing.
Areán described a study she is currently running to test whether mobile apps can improve mood, concentration, and motivation in people with depression. The BRIGHTEN study (brightenstudy.com), funded by an R34 grant from NIMH, recruited nearly 1,700 volunteers in only 6 months, with a broad age and geographical representation. They are now collecting cognitive, mood, and activity data on these participants.
Sahakian suggested that in combination with deep “in-clinic” profiling of cognition, frequent assessments using in-home or mobile computing technologies (such as CANTAB mobile1) could provide combined cognitive, behavioral, and functional assessments that are more individualized and clinically meaningful to patients (see Figure 4-2). Sahakian thought that some nonverbal tests would have the advantage of being culture-free, not dependent on language, and less affected by language level. Sahakian also pointed out that computerized tests have the advantage of being objective, less affected by tester bias, and could more accurately measure speed of response. These assessments could also be combined with data from biomarker, neuroimaging, genetic, and other physiologic studies for a much richer understanding of cognitive impairment in depression. Sahakian also suggested that games on iPads or
_____________
1See http://www.cambridgecognition.com/healthcare/cantabmobile (accessed June 17, 2015).
smartphones may be useful in reducing attentional bias or anhedonia and increasing motivation (Sahakian et al., in press).
As an example of a potential model for this type of approach, Sahakian discussed the National Institute for Health Research (NIHR) and the Medical Research Council jointly-funded feasibility study for intensive phenotyping of 24 preclinical Alzheimer’s disease patients. The study, which involves industry collaboration, aims to identify biomarkers that change over periods of months, rather than years. It will use an extensive range of magnetic resonance imaging and cognitive testing, along with additional clinical testing and biomarkers, at multiple frequent intervals over the course of three months. The goal is that these biomarkers could be used in a range of follow-on trials.2
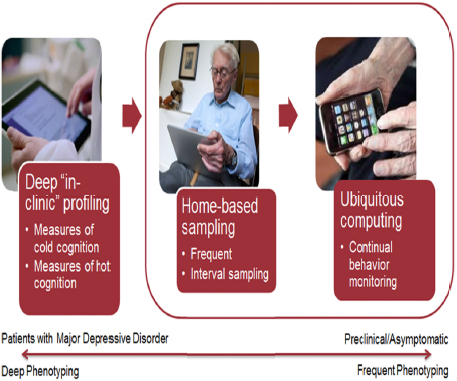
FIGURE 4-2 In-depth assessment of cognition in depression and frequent monitoring of changes in severity.
SOURCE: Barbara Sahakian presentation, February 24, 2015.
_____________
2See http://www.mrc.ac.uk/research/facilities/dementias-platform-uk (accessed June 17, 2015).
Innovation may also be useful in terms of recruiting and retaining patients for clinical trials. For example, the social media website company PatientsLikeMe (www.patientslikeme.com) has enrolled tens of thousands of people with mood disorders and is now interested in adding cognitive assessment to their tool box, according to Insel. Many of the patients who enroll provide consent to be contacted again for upcoming studies.
Future Directions in Assessment
In addition to the need to embrace innovation, a major issue that arose during workshop discussions was the need for alignment on appropriate assessments to use in clinical trials. Two approaches to assessment that seemed diametrically opposed emerged: (1) homing in on what is important to patients, or (2) focusing on mechanisms. Etkin suggested that the challenge is to think about mechanisms from an explanatory perspective that translates work across the field into a set of common data elements, and determine how to measure these elements and why we should care about them.
A question frequently asked throughout the day was whether a MATRICS-like process is needed to gain consensus on an assessment battery. Pizzagalli noted that the lack of a common battery such as MATRICS makes the integration of findings challenging. This view was supported by William Potter, senior advisor in the Office of the Director of NIMH. Potter suggested that pooling data from different sources, which would require agreeing on a common core of measures, might expedite the process of reaching a better understanding. Madhukar Trivedi, Betty Jo Hay Distinguished Chair in Mental Health at University of Texas Southwestern Medical School, suggested aligning on four to seven measures that would capture the important aspects of the illness, then developing metrics that would represent a meaningful change in these measures.
However, several participants shied away from the MATRICS-like approach. For example, Fava favored deemphasizing a specific set of measures in favor of looking at specific measures based on the mechanism of action of a particular drug, and combining that with a functional measure. Several other speakers and audience participants also expressed concern that a MATRICS-like approach could stifle innovation.
In the absence of a MATRICS-like approach, Laughren asked how the field would be able to gain more clarity about a pathway forward,
which he said is needed to give drug companies confidence to enter this space. Insel replied that although we may not need a MATRICS battery, we might want to develop a set of standard measures that can be integrated across multiple studies to allow larger datasets to be compared.
Laughren asked if we need a cognitive assessment at all, or whether we should go straight to a functional measure. While some participants favored this approach, Trivedi raised the concern that such an approach would not assess what a molecule is actually doing in the brain. He argued that something more proximal to brain changes is important because so many factors (work, social life, etc.) influence functional measures. Areán added that regardless of intervening variables, what matters to patients is whether something makes them feel better and leads to better functioning. However, she and others agreed that it remains important to know the mechanism of how a treatment works. For example, said Fava, if statins were discovered simply because they improved morbidity and mortality, we might not have known about their effects on cholesterol and inflammation.


















