WORKSHOP SUMMARY
Childhood cancer is an area of oncology that has seen both remarkable progress as well as substantial continuing challenges. While survival rates for some pediatric cancers present a story of success, for many types of pediatric cancers, little progress has been made. The American Cancer Society’s 2014 Facts and Figures special section featured detailed childhood cancer statistics (affecting children ages 0–14) that laid out a helpful benchmark for quantifying the progress and the problems that remain (ACS, 2014). But setting aside the statistics, when speaking of cancer or any other serious illness affecting children, even one diagnosis or death is one too many. Many cancer treatments are known not only to cause significant acute side effects, but also to lead to numerous long-term health risks and reduced quality of life. Even in cases where the cancer is considered curable, the consequences of treatment present substantial long-term health and psychosocial concerns (i.e., late effects) for children, their families, their communities, and our health system.
To examine specific opportunities and suggestions for driving optimal care delivery supporting survival with high quality of life, the National Cancer Policy Forum of the Institute of Medicine (IOM) and the American
Cancer Society co-hosted a workshop1 on “Comprehensive Cancer Care for Children and Their Families,” which convened experts and members of the public on March 9 and 10, 2015, in Washington, DC. At this workshop, clinicians and researchers in pediatric oncology, palliative, and psychosocial care, along with representatives from the U.S. Food and Drug Administration (FDA), National Cancer Institute (NCI), Children’s Oncology Group, pharmaceutical companies, and patient advocacy organizations, discussed and developed a menu of options for action to improve research, quality of care, and outcomes for pediatric cancer patients and their families. In addition, parents of children with cancer and pediatric cancer survivors shared their experiences with care and provided poignant personal perspectives on specific quality-of-life concerns and support needs for children and families across the life spectrum. Audience participation throughout the workshop also provided important insights, reactions, and ideas.
The presentations and discussions represented a unique dialogue among passionate family members, highly dedicated clinician investigators, and advocates who strive not only for every child with cancer to be cured, but also for the child and family to have every opportunity to maintain quality of life throughout the illness course and beyond. The first day of the workshop included presentations providing an overview of the pediatric cancer landscape, prioritizing quality of life in the research and development pipeline, and optimizing clinical care and care transitions. On the second half-day, the presentations and discussion addressed specific opportunities for patient and family engagement in research and outcomes reporting, as well as opportunities for collecting, documenting, and using these and other needed data. Topics discussed included
- Fostering research and drug/therapeutic and diagnostic development for pediatric cancers that prioritizes increased survival and high quality of life;
- Developing, embedding, and documenting patient- and family-reported outcome measures and findings to support delivery of
___________________
1 The workshop was organized by an independent planning committee whose role was limited to the identification of topics and speakers. The workshop summary has been prepared by the rapporteurs as a factual account of what occurred at the workshop. Statements, recommendations, and opinions expressed are those of individual presenters and participants and are not necessarily endorsed or verified by the IOM. They should not be construed as reflecting any group consensus.
- optimal care that helps minimize pain, symptoms, distress, and other suffering as part of disease-directed treatment and follow-up care;
- Improving and expanding early integration of pediatric palliative care and psychosocial care in all care settings to support emotional and physical functioning, care continuity, and goal-concordant care for the affected child and family members;
- Enhancing access to high-quality end-of-life care in all care settings, as well as bereavement care for families;
- Routinely screening to assess and address patient and family needs for palliative, psychosocial, and rehabilitation support as part of childhood cancer treatment and long-term survivorship follow-up care across multiple transition points and care settings;
- Facilitating clear communication and smooth transitions from acute cancer care to long-term follow-up care across the life spectrum;
- Minimizing, monitoring, and treating side effects and late effects across the care continuum;
- Enhancing and expanding quality-of-life–focused data captured in pediatric oncology registries and other databases to guide improved care integration for pediatric cancer patients; and
- Examining the impact of childhood cancer diagnosis and treatment on family food, energy, and housing security and emerging care models to address health disparities.
The workshop focused on potential actions to address quality-of-life and quality-of-care improvements for children with cancer and their families across all care settings and care transitions. Participants were encouraged to consider connections between what we do in research, how we bring that into the care of patients, and how we continue to engage parents and families as voices that remind us of the importance of moving forward on their behalf and on behalf of their children. The planning process for this workshop assembled experts in different disciplines who would not typically convene to discuss shared challenges, objectives, and steps forward. As such, the very process of preparing this workshop exemplified what clinicians and health systems are striving to achieve for children and families—high-quality cancer care delivery across the care continuum that promotes truly interdisciplinary, person-centered, and family-oriented care.
This workshop’s particular emphasis on children also complements three other recent IOM initiatives, including IOM’s workshop on Identifying and Addressing the Needs of Adolescents and Young Adults with Cancer
(IOM, 2013b), as well as two consensus reports for seriously ill adults, Delivering High-Quality Cancer Care: Charting a Course for a System in Crisis (IOM, 2013a) and Dying in America: Improving Quality and Honoring Individual Preferences Near the End of Life (IOM, 2014). The suggestions put forth at this workshop build on those bodies of work by detailing the specific needs of children and families, including early integration of palliative and psychosocial care along with disease-directed treatment, for any age and for any disease stage, to improve the quality of life—not just at the end of life, but throughout the illness course and well beyond for surviving children and surviving family members. Public policy advocacy promoting these quality-of-life priorities is also escalating with the support of an organized Patient Quality of Life Coalition2 and associated legislative campaign.
This report is a summary of the presentations and discussions at the workshop. A broad range of views and ideas were presented and a summary of suggestions from individual participants is provided in Box 1. The workshop Statement of Task and Agenda can be found in the Appendix. The speakers’ presentations (as PDF and audio files) have been archived.3
OVERVIEW OF THE CURRENT LANDSCAPE IN PEDIATRIC CANCER RESEARCH AND TREATMENT
The workshop began with several presentations that provided an overview of the current landscape in pediatric cancer research and treatment, with some emphasis on the unique challenges and opportunities in pediatric drug development, as well as challenges in addressing treatment toxicities and late effects.
Most of modern oncology is underpinned by early advances in treating pediatric cancer. In introducing the first workshop session, Phillip Pizzo, professor of pediatrics/infectious diseases at Stanford University, explained that pediatric oncology has really been the exemplar of many key elements in quality health care, including interdisciplinary team-based care, the connection of compassionate health care professionals, translational research that brings basic discovery from the laboratory to the clinic and back, continuity over the life journey, and combining compassion with care along the continuum. Gregory Reaman, associate director of the Office of
___________________
2 See www.patientqualityoflife.org (accessed May 29, 2015).
3 See https://www.iom.edu/Activities/Disease/NCPF/2015-MAR-09.aspx (accessed March 15, 2015).
BOX 1
Suggestions Made by Individual Workshop Participants
Improve and Accelerate Pediatric Cancer Drug Development
- Maximize use of regulatory authority provided through existing legislation to address indication-based waivers. (Greg Reaman)
- Create incentives that reduce industry risk much earlier in the process to encourage new pediatric drug development, particularly when no adult indication exists. (Christina Bucci-Rechtweg, Reaman)
- Conduct long-term longitudinal observation studies and combination therapy toxicity studies for newer targeted cancer agents in pediatric populations to evaluate the short- and long-term safety concerns. (Reaman)
- Mandate studies of relevant adult drugs in pediatric populations earlier in the testing process. (Reaman)
- Provide more funding mechanisms, such as small business innovation research grants and targeted use of disease foundation funding, to support drug development for pediatric cancers. (Beth Ann Baber)
- Support more patient-focused drug development that includes quality-of-life assessments. (Reaman)
- Enhance collaboration among all stakeholders (e.g., the Children’s Oncology Group members, academic centers, clinicians, patient advocacy groups, and pharmaceutical companies) to define trial outcome measures and facilitate pediatric drug development. (Bucci-Rechtweg, Reaman, Lillian Sung, Christina Theodore-Oklota)
- Support research on late effects to understand the pathogenesis and dose dependence of late effects, to develop risk prediction models, and to develop targeted interventions to reduce risks. (Smita Bhatia)
Improve Access to Early Pediatric Palliative and Psychosocial Care
- Develop and disseminate evidence-based standards in pediatric palliative care and psychosocial support, and facilitate and incentivize implementation. (Peter Brown, Paul Jacobsen, Mary Jo Kupst, Lori Wiener, Joanne Wolfe)
- Include core competencies in pediatric palliative care in the curricula of schools of medicine, nursing, social work, psychology, and counseling. (Chris Feudtner)
- Provide more information to parents about how pediatric palliative care can help them and their children throughout the care continuum. (Wiener)
- Integrate palliative and psychosocial care services, with a sound business plan and program support, into all pediatric oncology practices. (Feudtner, Wiener)
- Prioritize communication and care supporting family-oriented shared decision making and goal-concordant treatment, including establishing payment mechanisms for coordinated care planning. (Jennifer Mack)
- Train health care providers to initiate advanced care planning discussions. (Mack)
- Include screening for psychosocial needs of children and families in accreditation requirements. (Brown)
- Embed the team approach to palliative and psychosocial care with oncology treatment that integrates expertise of social workers, psychologists, psychiatrists, and child life specialists throughout the care continuum. (Wiener)
- Support more research on pediatric palliative care and psychosocial needs across the care continuum. (Feudtner, Anne Kazak, Kupst, Mack, Wolfe)
- Determine the optimal methods and timing for psychosocial screening and assessment, and support the development of targeted or personalized interventions. (Kazak, Kupst)
Improve and Expand the Use of Pediatric Patient-Reported Outcomes (PROs)
- Define and routinely collect a core group of PROs from patients and parents, and integrate them into the health care delivery system to improve clinical care. (Mary Brigid Bradley-Garelik, Pamela Hinds, Theodore-Oklota, Bryce Reeve, Sung)
- Include PROs as a default in pediatric cancer clinical trials, and ensure that the data are analyzed and published. (Reeve, Sung)
- Educate clinicians and administrators on the value of PROs. (Reeve)
- Educate children and parents about the importance of self-reporting and how their clinical care team will use the information. (Hinds)
- Develop a dynamic, integrated electronic system to routinely screen children for symptoms and other key patient outcomes to provide real-time feedback to clinicians. (Reeve)
- Develop PROs that are appropriate for patients in different age groups. (Sung)
Improve Long-Term Follow-Up Care and Outcomes
- Optimize long-term health of survivors by partnering with parents and patients to capture data on issues related to growth and development, impairment of vital organ function, fertility and reproduction, second cancers, and the impact of all of these sequelae on quality of life. (Bhatia)
- Provide a treatment summary and survivorship care plan to patients upon completion of treatment for pediatric cancers. (Lisa Schwartz)
- Educate clinicians, patients, and parents about the long-term complications of pediatric cancer treatment, follow-up care needs, and health promotion. (Bhatia, Schwartz)
- Continue to monitor and address the long-term health and well-being of childhood cancer survivors, particularly the chronic health conditions and life-threatening or fatal conditions that increase over time. (Bhatia, Kevin Oeffinger)
- Standardize long-term follow-up care and update guidelines regularly. (Schwartz, Bruce Waldholtz)
- Identify best practices and best models of care for transitions from active treatment to short- and long-term follow-up. (Kupst, Schwartz)
- Assess and address transition readiness. (Schwartz)
- Direct high-risk patients to specialty clinics for long-term follow-up care. (Oeffinger)
- Identify the demographics, disease characteristics, and treatment profiles that predict risk or resiliency post-treatment. (Patricia Ganz, Kazak, Schwartz)
- Support a national registry of cancer survivors to enable follow-up care and research on long-term complications of cancer treatments. (Peter Adamson, Richard Aplenc, Bhatia, Ganz)
- Standardize information collection to assess endpoints across different health care systems. (Reeves)
- Develop information and decision support tools (e.g., prompts and drop-down menus in electronic medical records) to inform primary care providers (physicians, nurse practitioners, nurses, social workers, etc.) about unique care needs based on a patient’s cancer history. (Kupst, Oeffinger, Phillip Pizzo, Waldholtz)
- Systematically collect standardized sociodemographic variables in pediatric cancer clinical trials, and direct patients in need to existing support programs. (Kira Bona)
Hematology and Oncology Drug Products in the Center for Drug Evaluation and Research at FDA, agreed, adding that childhood cancer care today involves a “unique integration of clinical practice, patient management, and clinical research [coupled with] a highly effective national clinical trials infrastructure”—all pivotal to the successes achieved.
Today, cancer is diagnosed in an estimated 10,380 children ages 0–14 each year (ACS, 2014), and the number of childhood cancer survivors in the United States was estimated to be 388,501 as of January 1, 2011, of whom 83.5 percent were at least 5 years post diagnosis (Phillips et al., 2015). However, despite notable advances in treatment and resulting improvements in survival for some types of childhood cancer, it remains the leading cause of disease death among children, with approximately 1,250 children losing their lives to cancer each year (ACS, 2014).
Childhood cancers typically are quite different from adult cancers, and the smaller number of pediatric cancer cases overall presents barriers in conducting the large-scale clinical research necessary to develop and deliver new treatment breakthroughs, particularly for the less commonly occurring cancers. As a result, some types or stages of pediatric cancers have not experienced any significant treatment advances or improved survival (Smith et al., 2014). “Any disease is rare until it knocks on your door,” said Jonathan Agin, director of external affairs for the Max Cure Foundation, development liaison and general counsel for the Children’s Cancer Therapy Development Institute, and the father of a daughter he lost to DIPG (diffuse intrinsic pontine glioma), still one of the most lethal childhood cancers. Jennifer Cullen, director of epidemiologic research at the Department of
Defense Center for Prostate Disease Research, and the mother of a daughter who was diagnosed with medulloblastoma and died after 13 months of grueling treatment and suffering many treatment-related complications, stressed that “the public health importance of childhood cancer is obviously not a function of the sheer volume of new cases that occur each year. It is a function of the high malignant potential of each of those cases, the devastating impact of treatment on the survivors, and the many years of life lost for each of those who don’t survive.”
Smita Bhatia, director of the Institute for Cancer Outcomes and Survivorship at the University of Alabama at Birmingham (UAB) School of Medicine, described major landmarks in pediatric oncology, noting that once it became apparent that chemotherapy and radiation therapy could cure children of cancers, “We threw the kitchen sink at our children.” In the 1980s and 1990s, researchers began to document the long-term effects of cancer therapies and see the relationship between dose or type of therapy given and the adverse effects experienced. Recognizing that radiation therapy was responsible for many of those long-term sequelae, physicians began substituting effective drugs for radiation therapy, as well as tailoring therapy based on risk factors for developing late effects from cancer treatment.
But many pediatric patients still receive aggressive treatments with a high probability of significant long-term side effects, such as altered neurological development of the child. Concerns about these late effects can sometimes influence the choice of treatments. For example, Beth Anne Baber, chief executive officer, director, and co-founder of The Nicholas Conor Institute for Pediatric Cancer Research, said she and her husband decided against standard treatment (bone marrow transplant) for their son who had developed a brain cancer at the age of 15 months. She said bio-marker tests suggested that he did not have an aggressive tumor and such transplants have serious short- and long-term side effects. “We decided to downgrade the chemotherapy protocol because we wanted our child to have the highest quality of life. If it wasn’t for a long period of time, he would at least be able to be a child,” she said.
Bhatia also discussed efforts to improve treatment outcomes for children with cancer through a focus on improving adherence to treatment protocols. Bhatia noted that studies showing that children with acute lymphoblastic leukemia who do not adhere to the standard 6-MP4 treatment protocol have a higher risk of relapse (Bhatia et al., 2012). Consequently, a
___________________
4 6-mercaptopurine.
comprehensive approach is now under investigation to determine whether it can improve adherence to the medication schedule among pediatric leukemia patients, with the help of text messaging, directly supervised therapy, and education, she said.
Trends and Challenges in Developing Drugs for Pediatric Cancer
One potential way to improve cancer treatment outcomes and reduce treatment toxicities is through molecularly targeted therapies. Such targeted therapies are already available for treating some pediatric cancers, although most targeted therapies are only FDA approved for treating adults (Adamson, 2015). These therapies target genetic alterations that affect the growth pathways involved in cancer. Many genetic flaws have been identified for childhood cancers, which tend to have fewer gene mutations than adult cancers, Reaman reported. But most of these mutations are relatively rare and often do not occur in adult tumors. Most pediatric cancers have mutations in embryonic genes and lack mutations in genes relevant to the molecularly targeted agents already on the market or being developed for adult cancers, with the exception of a few targeting certain types of leukemias and brain tumors (Northcott et al., 2012). “We need to recognize that there is a biologic distinction in the malignancies diagnosed in children and adolescents compared to adults,” said Christina Bucci-Rechtweg, global head of maternal health and pediatric regulatory policy at Novartis Pharmaceuticals Corporation.
Immunotherapies that enhance the immune system’s ability to destroy cancer cells are also showing promise in treating some pediatric cancers, including brain tumors and leukemia (Grupp et al., 2014; Yu et al., 2010). Both immunotherapies and molecularly targeted therapies still have toxicities and pose short- and long-term safety concerns, Reaman noted (Dy and Adjei, 2013). Side effects linked to the use of these therapies could affect the normal development of children and put them at higher risk of developing certain disorders, he stressed, adding that there are insufficient long-term exposure data in adults and little combination toxicity data to guide treatment in children. These new types of drugs “will really be a new paradigm for pediatric cancer and long-term follow-up,” Reaman said.
He noted that it is challenging to do research and drug development for pediatric cancers because fewer than 15,000 pediatric cancers are diagnosed each year in the United States, which means that the number of children eligible for any particular clinical trial is small, especially because molecular
targeted therapies often only work in small subsets of cancers. Traditionally, the challenges associated with the small and scattered numbers of pediatric cancer patients available for clinical trials have been met with multicenter clinical trials in the United States. To test targeted agents, such trials might have to expand globally to enroll sufficient numbers of pediatric patients, said Malcolm Smith, associate branch chief for pediatric oncology at NCI. “It’s really important to nurture the infrastructures and collaborations that exist and to strengthen our ability to collaborate with partners in Europe, Asia, and other places,” he said.
Even if this challenge can be overcome, pharmaceutical companies are reluctant to develop drugs that will have such a small market, Reaman noted. He pointed out that the genetic changes responsible for some pediatric cancers, such as Ewing’s sarcoma, have been known for some time, but drug companies are not using this information to develop agents that target these genetic defects. Baber noted that drug companies have more incentives to develop drugs for rare chronic diseases in children in which the drug will be taken during the entire lifetime, as opposed to drugs for pediatric cancers, which are taken for a much shorter time period, and thus have a limited potential to achieve a return on the investment to test the drugs.
The higher bar set for cancer drugs for children versus adults also dampens the enthusiasm of companies to develop them, Reaman said. Drugs that target adult cancers can receive FDA approval based on evidence of short-term benefit to patients—the ability to extend life by a few months—whereas more long-term benefits with minimal side effects are usually demanded of drugs for pediatric cancers, according to Reaman. Smith agreed, noting that most regulatory approvals for adult indications are for people who are not expected to live for a long period after their treatment, whereas children could potentially live for many decades after treatment, “We want to know the impact of a new drug on the survivorship for the pediatric patient, including its likelihood of inducing cognitive or cardiac disorders and second cancers,” he explained.
Consequently, most cancer drugs are initially developed for adults, and if they are found to be ineffective for that population, they are abandoned without further testing to see if they might useful for treating pediatric cancers. “Unfortunately, that deprives us of the opportunity to test what might be a promising drug in the pediatric population,” Reaman said. Developing formulations that are appropriate for children can also be challenging, especially because many new cancer medications are given orally. “Giving a capsule that would choke a horse to a 2-year-old child is not possible,” Reaman said.
He suggested several possible avenues for encouraging more drug development for pediatric cancers, including current legal incentives. To repurpose targeted cancer drugs approved for adults so they can be used for pediatric cancers, Reaman suggested maximizing the regulatory authority provided by current legislation to address “indication-based waivers.” For example, the Pediatric Research Equity Act requires drug companies to study their products in children under certain circumstances. When pediatric studies are required, they must be conducted with the same drug and for the same use for which they were approved in adults (Yao, 2013). This may require redesigning early-phase studies to enable more appropriate dosing for children. To encourage more expedient development of pediatric cancer drugs, FDA could mandate that studies of relevant adult drugs be conducted on pediatric populations earlier in the testing process, Reaman noted.
A bigger challenge is fostering the development of new cancer drugs that are not already in use in adults and that would target pediatric cancers. According to Reaman, there is currently no “legislative fix” for this. He suggested providing incentives to industry that may reduce the risk of such early development, instead of just providing extended market exclusivity, patent extensions, and other financial incentives that are only offered once a drug has proven its worth. Bucci-Rechtweg agreed, stressing that current incentives benefit companies late in the life cycle of drugs rather than earlier in drug development. She added that one incentive would be a more expedient review process by FDA because reducing the time lines for reviewing a new drug provides financial benefits to the drug’s sponsor. In 2012, FDA created a program in which it could give priority review vouchers to sponsors of drugs for rare pediatric diseases (Varond and Walsh, 2014). But that benefit has been diluted by other priority review mechanisms at FDA, Reaman said.
Reaman suggested that another option is to develop public–private partnerships to fund the development of new drugs for childhood cancers. NCI is already engaged in such partnerships to support the development of targeted drugs for Ewing’s sarcoma and neuroblastoma. Collaborations among private foundations can also be useful (see Box 2). Lee Greenberger, chief scientific officer at the Leukemia & Lymphoma Society (LLS), noted that when LLS recognized that a study could be expanded to address several different types of leukemia, they provided $100,000 to fund it and asked the St. Baldrick’s Foundation, which funds the most promising research aimed at curing childhood cancers, to match that grant. “The concept of collaboration across foundations is very important,” he said. LLS also collaborates with the NCI-supported Children’s Oncology Group (COG) to
BOX 2
Leukemia & Lymphoma Society—Funding Research
Lee Greenberger of the Leukemia & Lymphoma Society (LLS) described the research funding activities of LLS and how they can serve as a model for fostering drug development for pediatric cancers. The Society’s mission is to cure leukemia, lymphoma, and myeloma and to improve the quality of life of patients with these blood cancers and their families. Since the Society was formed in 1954, it has invested more than a billion dollars in research grants. It currently has 320 active academic grants, including career development training awards, translational bench-to-bedside awards, “new idea” awards, and awards for specialized centers of research.
Greenberger noted that their career development awards for researchers early in their careers have been especially productive because those who receive them tend to continue to do research on blood cancers. Some ended up running major programs in this area 15 to 20 years later, including two researchers at the forefront of discovering or testing innovative immunotherapies that are showing promise in treating a number of cancers.
A more recent LLS initiative is the Society’s Therapy Acceleration Program (TAP) aimed at accelerating innovative therapies that are first in their class. TAP helps bridge the “valley of death” between basic and applied research by enabling promising agents in late preclinical development to enter and progress from this late discovery stage through Phase III clinical trials. “Drugs in the early state of development are at the biggest risk of failure. Couple that with a rare disease and you often see biotech companies and particularly big pharma run in the other direction,” Greenberger said. TAP was started “as a way to get these therapies and drive them into the clinic and find out if they will work,” he said. Once that initial evidence is available, drug companies are more likely to do the additional testing needed to bring a drug into the market. TAP includes “Biotechnology Accelerator Awards,” which are partnerships with biotech companies in which the Society splits the costs of pursuing an interesting idea with therapeutic potential, as well as “Academic Concierge Awards” in which the Society will work with outside companies who they fully fund to make a drug and do the necessary preclinical studies on it so that an academic investigator can test it in a clinical trial. TAP also has a clinical trials program that helps patients gain access to trials in their local communities.
In the 8 years it has been in operation, TAP has had 47 partnerships, 25 of which are still active, and a total of $80 million has been invested in the program. In combination with the Society’s other grant programs, TAP has fostered Food and Drug Administration approvals of three drugs for blood cancers and helped advance more than a dozen other promising agents into the clinical trial pipeline. “The Therapy Acceleration Program has been a good model to advance opportunities, particularly for rare cancers,” Greenberger said. “We are making good progress toward cures for blood cancers, with increases in survival not in terms of months, but in terms of years.”
SOURCE: Greenberger presentation, March 9, 2015.
fund clinical trials of drugs for leukemias or lymphomas, although he noted that such collaborations are sometimes difficult to manage due to different goals and time lines.
Pizzo noted that the Cystic Fibrosis Foundation collaborated with researchers who discovered drugs that target some of the genetic defects that cause cystic fibrosis and fostered the development of those drugs, which are currently on the market. “Could their approach work for developing drugs for rare pediatric cancers?” he asked, noting that even outside of pediatric oncology, pharmaceutical companies are sinking less money into funding research and development, so “partnerships with foundations and academia become ever more important.” He added that parents “can make a huge difference in helping to lead that effort.”
Reaman echoed the plea for parents and advocates to become more involved in drug development and noted that advocates did make a difference in adult drug development. “I think there is enormous opportunity for families of patients,” he said. However, he added that the one-foundation-one-disease approach may fracture the ability to make progress, noting that there are about 3,000 organizations involved in raising money related to cancer, all with their own agendas. Otis Brawley, chief medical officer of the American Cancer Society, also stressed the need to avoid “disease Olympics,” in which advocates press for research on one type of cancer at
the expense of research on another. “We are all arguing for a larger slice of the same size pie,” he said. He pointed out that many genetic discoveries on pediatric cancers have proven beneficial in adult cancers and vice versa. “All these cancers have things in common and we need to realize that if one cancer is bettered, all cancers are really bettered.”
Baber suggested more general public funds, such as small business innovation research grants, could be used to support drug development for pediatric cancers, and that there be more targeted use of disease foundation funds for drug discovery and development. She stressed the gap between government and foundation funding for research that generates data on promising drug targets, and the pharmaceutical and venture capital funding of clinical trials based on those discoveries.
Baber became aware of that gap when her 15-month-old son was diagnosed with a brain tumor. At that time she and her husband both conducted basic research on the DNA damage and repair pathways that can determine cancer susceptibility, and they were advocates for the precision medicine approach that this research suggests is possible. They were shocked to discover a lack of biomarkers available for clinical use that could help them decide the best treatment for their son, even though researchers had published papers on potentially useful biomarkers.
To bridge that gap over what some called the “valley of death” in the development pathway, investigators need access to more tumor samples with linked patient outcome data, as well as venture philanthropy and appropriate industry incentives to validate the biomarkers and potential drugs that had been discovered, Baber suggested. “There’s a huge gap between a promising discovery and a clinical trial, especially for children, and that gap is widening as the economy has suffered earthquakes,” she said. Closing that gap will require collaboration among all stakeholders, including patients and families, clinicians, funding agencies, philanthropic organizations, academic research institutions, the pharmaceutical industry, regulatory agencies, advocates, policy makers, and payers, she stressed.
Reaman also suggested there should be more patient-focused drug development, in which the benefits of treatments are also measured by how they reduce disease-related symptoms and enhance function in daily life, rather than simply focusing on length of survival. Smith stressed this as well, noting that current legislative incentives and industry paradigms for drug development are not patient focused and do not foster the conduct of the clinical trials that may be most needed for specific patient populations. Instead of asking what is the best clinical trial for a given drug, researchers should
be thinking about the most important therapy question that needs to be answered for a given cancer patient population, he said. This is especially true for rare pediatric cancers in which the number of patients available for clinical trials is limited, he pointed out. “We need to think about the key factors associated with treatment for a specific population—the key short-term and long-term issues that diminish quality of life or quality of survivorship—and based on that, determine the most promising clinical research opportunities we could explore for that patient population,” Smith said.
INTEGRATING PEDIATRIC PALLIATIVE CARE: ENSURING CHILD AND FAMILY WELL-BEING ALONG THE CONTINUUM
A key focus of the workshop was the integration of palliative care throughout the pediatric cancer care continuum to improve quality of life as well as survival. For children and their families, treating the pain, symptoms, and stress of cancer is as important as treating the disease (Kaye et al., 2015; Levetown et al., 2001; Schwantes and O’Brien, 2014). Pediatric palliative care lessens physical, psychosocial, emotional, and existential suffering and focuses on improving quality of life for both the child and family. It is appropriate at any age and any disease stage and should be provided along with curative treatment. While palliative care may be delivered by oncology practitioners, they may ask for the help of a specialized team of physicians, nurses, social workers and other professionals who work with them to provide an extra layer of support addressing the child’s and family’s specific quality-of-life needs. Pediatric palliative care specialists also help parents and children have a voice in realizing their treatment goals.
While earlier studies have repeatedly demonstrated the frequency and intensity of children’s suffering from symptoms like pain, breathlessness, fatigue, anxiety, sadness, and other forms of distress resulting from rigorous cancer treatments, unpredictable setbacks, and repeated invasive procedures, parents and health care providers are not always aware of extent of the affected child’s suffering. One study found that about half of cancer patients ages 7 to 12 who completed a questionnaire reported experiencing fatigue and about one-third reported having pain. Nearly half also reported being worried or nervous. When their parents were asked to fill out the same survey on behalf of their children, 43 percent rated their child’s pain or distress differently than the child did (Patel et al., 2011). Another study found that 89 percent of children who died of cancer experienced substantial suffering
in the last month of life and that there was significant discordance between the parent and physician reports of the child suffering (Wolfe et al., 2000a).
Addressing these concerning findings, evidence emerging over the past decade has firmly established the importance of pairing palliative care, including psychosocial support, with oncology treatment for adults and children in all care settings throughout cancer treatment and across the continuum of survivorship. Palliative care is also naturally aligned with other interventions such as cancer rehabilitation that focus on treating specific impairments and improving function as well as alleviating symptoms. Together, these integrated services offer vital support for maintaining patient and family quality of life during and after disease-directed treatment. As such, multiple professional organizations and accrediting entities have now endorsed early integration of palliative care to improve the quality of care for all seriously ill adults and children across the full trajectory of care (AAP, 2000; ACS, 2014; CoC, 2012; IOM, 2003b, 2015; Levetown et al., 2001; Smith et al., 2012; WHO, 2015).
Despite recognition of the importance of palliative care for pediatric patients, health care professionals have been slow to implement recommended pediatric palliative care in their practices and institutions, according to Lori Wiener, co-director of Behavioral Science Core and director of the Psychosocial Support and Research Program in the Pediatric Oncology Branch of the NCI Center for Cancer Research. One study found that the two main barriers to pediatric palliative care integration were ineffective communication (including about palliative care) between health care providers and families, and a lack of resource alignment with patient and family needs (Kassam et al., 2013).
Generalist Plus Specialist Palliative Care
A large majority of health care providers lack formal education, training, or experience in pediatric palliative care or in providing care for children at the end of life, said Wiener. Several participants stressed the importance of supporting initiatives for training and increasing access to both generalist- and specialist-level palliative care in pediatric oncology programs, and making these essential services available in all settings where children receive cancer care—whether inpatient, ambulatory clinic, or at home. Training needs noted for physicians, nurses, social workers, child life specialists, and other professionals specifically included enhancing communication skills (such as discussing prognosis, goals of care, and care
transitions), pain and symptom management, sensitivity to cultural and spiritual beliefs, as well as grief and bereavement care.
Generalist palliative care involves the basic management of pain, symptoms, and communications (e.g., discussions of prognosis) that every oncology clinician should be able to provide, while specialty palliative care is provided by a specially trained team who may be consulted for difficult-to-manage symptoms, complex family dynamics, or challenging care decisions, particularly when the first level of palliative care still leaves the patient or family suffering (Quill and Abernethy, 2013). Both levels of care can and should coexist, support each other, and expand palliative care delivery, said Chris Feudtner, the Steven D. Handler Chair of Medical Ethics and director of the Department of Medical Ethics at The Children’s Hospital of Philadelphia. He added that this is particularly important in caring for children, because not all children’s hospitals have pediatric palliative care teams and the number of specialist-level pediatric palliative care practitioners available is not sufficient to manage the palliative care needs of all seriously ill children in all care settings. “It is something that is added and does not supplant the primary team, but rather complements what they are doing and often can be delivered concurrently with disease-directed therapy,” he said.
End-of-Life Care and Bereavement Care
For children with cancers that are not responsive to treatment, high-quality pediatric end-of-life care (such as hospice care) is needed. These services may be available via a free-standing hospice facility, a hospital, or at home, but many workshop participants stressed that such care must be accessible to all families where they live. More than 3,000 hospices in the United States currently will provide end-of-life care for children, but there is a dearth of free-standing, pediatric in-patient hospice programs, Wiener reported. The first such program opened in California in 2004. Feudtner and Joanne Wolfe, director of pediatric palliative care at Children’s Hospital Boston and division chief of the Pediatric Palliative Care Service in the Department of Psychosocial Oncology and Palliative Care at the Dana-Farber Cancer Institute, also noted that many of these programs are understaffed or underfunded. For example, efforts to build a pediatric hospice targeting unmet end-of-life and respite care needs for children and families in Seattle5 have been stalled for lack of funding.
___________________
Unfortunately, even when hospice care is available in the community, it does not always adequately meet the specific needs of pediatric patients and their families. One father reported that the care his son received while he was at home, in his final days, was nowhere near the quality of care that he received while he was getting treatment. He explained that because the home hospice nurse assigned to his son’s care was trained only in adult care, she was not comfortable administering the pain medications prescribed for her pediatric patient to relieve his suffering. As a result, 7-year-old Evan died in his home experiencing excruciating pain, breathlessness, and associated anxiety—circumstances that were also extremely distressing for his parents (see Box 3). After hearing Gavin Lindberg’s story about Evan’s preventable suffering, as well as Gavin and his wife Wendy’s ongoing grief and distress in its aftermath, Wiener stressed that no family should ever be told they can have home hospice care for their child if they do not have a pediatric provider available to support them.
The death of a child has a profound and lasting impact on the entire family. Bereavement care is provided to help support coping and recovery for the surviving parents and siblings after a child dies. Bereaved parents have been shown to be at increased risk for prolonged grief, isolation, potential economic and health decline, and behavioral health and emotional concerns, Wiener reported (Rosenberg et al., 2012). One study of parents whose children had died of cancer between 6 months and 6 years ago found that 40 percent of the parents expressed the need for bereavement services, but were not receiving any. More than one-third had received such service, but had dropped out because they believed the therapist did not understand them. In another study, nearly half expressed a need for bereavement services 2 to 4 years after the loss of their child (Lichtenthal, 2015). However, screening for bereavement needs, if it is performed at all, is usually only done within the first year after the death of the child, Wiener noted. “After a child dies and a family leaves the center where the child was treated, they are at home without any of us except for an occasional phone call,” she pointed out, adding, “There is a real drop in services that is unconscionable to me.” One parent shared that after his son’s death, the hospice nurse “was more interested in collecting and accounting for the oral pain medications that we had in the house than in comforting us or even sticking around to wait until the funeral home arrived.”
BOX 3
Parent Reflections on Suffering
Three parents who had lost children to cancer spoke about their experiences and provided personal perspectives on the suffering they witnessed and experienced firsthand.

Jennifer Cullen’s daughter Alexandra was diagnosed with medulloblastoma in 2011, just shy of her 4th birthday. She suffered 13 months of debilitating procedures before her death. Her treatment led to extensive and unrelenting mouth sores, severe sepsis when she was neutropenic, and finally, a seizure that led to blindness, muteness, and complete incapacitation. Cullen also described the worst horrors she and her husband endured as parents: “The realization that [Alexandra] would die. Zipping up a white body bag. Purchasing a pink coffin. And then having to carry on with life and raise a son.” At the workshop, just 3 years after her daughter’s death, Cullen courageously expressed a sense of hope, purpose, and determination that the collective experiences of the families of children with cancer will matter. “Because of what we have gone through, there is an enormous determination among all of us that we will make this matter—that the cancer knowledge and delivery of care can and should improve, including palliative care,” she stressed.
Gavin Lindberg’s son Evan was 3 years old when he was diagnosed with neuroblastoma and 7 when he died from his cancer at home. “There was not one day in those 4 years that Evan wasn’t either going through treatment or recovering from treatment. It was just absolutely brutal,” Lindberg said. Although Lindberg felt that Evan received excellent oncology care at Children’s National Medical Center, Memorial Sloan Kettering Cancer Center, and The Children’s Hospital of Philadelphia, and all three physicians who had cared for him at those institutions agreed that the best place for Evan to spend his final days was at home, his home hospice care was abysmal.
“I remember telling his end-of-life physician at Children’s National that our sole priority was to make sure that Evan did not suffer and was not in pain and was as comfortable as possible,” Lindberg said. But unfortunately, care supporting those goals was not provided adequately.
The end-of-life physician Evan had through Children’s National never met him before or after he started to receive hospice care at home. “The person who cared for my son at home when he needed it the most never met him and never spoke to him,” Lindberg stressed. In addition, he added, “The wonderful oncology nurses at Children’s National never had an opportunity to care for our son while he was at home. Instead we had nurses who came to see us from an adult hospital. Their experience and expertise was in caring for adults.”

Because the nurses were not comfortable administering intravenous pain medicines to pediatric patients, Evan was not given effective pain relievers and experienced extreme discomfort, distress, and anxiety. In addition, Evan had respiratory challenges that were not appropriately anticipated or addressed. When his end-of-life physician was called and asked to address these issues, the doctor said to expect Evan would live another week or two, but he died the next morning “after a horrific night that my wife and I will forever have seared in our memory,” Lindberg said. “Unfortunately, there are a lot of kids like Evan and that is just simply unacceptable in this country. Home was the right place for my son to pass, but what was wrong was the type of care he received. We put our trust and faith in the providers and in the system and that was a mistake on our part. There was a lack of communication. There was a lack of transparency about what was happening and why. Children with cancer fight too hard every single day to be left with a fate like that. If we can’t get this right, then shame on us. The hospice system failed our son and as a result, we feel like we failed our son. Those thoughts stay with you. On your worst days, they haunt you.”
Lindberg stressed the need to improve accessibility of high-quality end-of-life care for children at home. “Every terminally ill child in the home care setting should have the right to be cared for by doctors and nurses who have the experience and expertise in all key facets of pediatric hospice care to make these kids comfortable. I don’t want to hear about challenges with reimbursement, licensing challenges that prevent a physician and nurse to go from one jurisdiction to another and I don’t want to hear about funding issues. Because we can fix licensing, fund-
ing, and everything that is wrong with this system that made that little boy have his final days play out the way that they did,” he said. During the panel discussion, a participant noted that his daughter also received inadequate home hospice care until the adult nurse caring for her was replaced with a pediatric nurse.

Victoria Sardi-Brown lost her son Mattie, who had bone cancer, when he was 7 years old. Mattie died in the hospital. Throughout his 14 months of treatment, Mattie also experienced tremendous pain that was not validated and treated adequately by his practitioners. Nor did they ever use a distress thermometer or other assessment tool to gauge his degree of distress and pain. “Mattie’s death was so traumatic and his pain was so enormous that he had to be put into a coma to die,” Sardi-Brown said. The Browns’ experience led them to create the Mattie Miracle Cancer Foundation, whose goal is to create and implement a national standard for psychosocial care for children with cancer and their families from time of diagnosis, throughout treatment, into survivorship or end-of-life and bereavement care. “Integrating psychosocial care and palliative care is vital along the entire cancer care trajectory for positive outcomes. Cancer care is much more than just about the medicine. It must integrate psychosocial distress and pain management needs of the patient to be effective,” Sardi-Brown stressed.
Otis Brawley of the American Cancer Society underscored the importance of these moving personal testimonies that so glaringly reveal the shortcomings of cancer care for children and their families. “These stories will hopefully bring more awareness of the problem, which will require momentum to address. Big momentum comes from parents with personal experiences talking to people on Capitol Hill who have been elected. We can give them the true numbers and a book that summarizes all of the problems, but ultimately it is the parents of the kids and the kids talking that get these people to care,” he said.
Although conversations about a child’s cancer are difficult to have with parents, such communication is considered key in identifying and helping to allay the psychological and physical problems they are experiencing. Jennifer Mack, co-director of the Pediatric Hematology/Oncology Fellowship Program at the Dana-Farber Cancer Institute, stressed that communication allows for the development of shared knowledge between the practitioner and the child and family. It also can relieve distress and uncertainty and provides supportive care by building a therapeutic relationship. “Talking and listening are some of the most important things we do. Communication creates an opportunity for thoughtful decision making based on the personal values of parents and children,” Mack said.
Because of the worry that relaying bad news may cause distress and take away the hope of patients and their families, many clinicians avoid opportunities to communicate and wait for patients or parents to ask for information instead of offering it, or speak in euphemisms or offer overly optimistic information, according to Mack. That could help explain why one study found that more than 60 percent of parents of children with cancer were overly optimistic about their children’s prognosis relative to what the doctor reported (Mack et al., 2007a). In the end-of-life setting, parents of children who ultimately died of cancer tended to recognize that the child had no realistic chance of cure more than 3 months later than the physician did (Wolfe et al., 2000b). Brawley added that physicians may not convey bad news to parents “because sometimes it is hard for the doctor to accept that the patient is dying.” But parents who understand that their children have a poor prognosis are more likely to have do-not-resuscitate orders in place and use less cancer-directed therapy at the end of life, suggesting that this understanding of prognosis impacts the kinds of decisions that patients and their families make about care, Mack said (Wolfe et al., 2000b).
Although it seems counterintuitive, honest communication of bad news can not only be helpful, it can also relieve distress, Mack explained. Her studies show that parents of children with cancer consider communication about prognosis to be very important to them and helpful to decision making, even when they also find it upsetting, and parents who feel they have too little information about prognosis are actually those most likely to feel upset. Strikingly, parents who receive more extensive prognostic information are also those who report feeling the most hopeful, even when the child’s prognosis is poor. Prognostic disclosure is also linked with a greater
peace of mind and with greater trust in the physician (Mack et al., 2006, 2007b, 2009).
Although these findings can be puzzling at first glance, Mack pointed out studies that show uncertainty is distressing and makes people fear the worst. Honest communication can relieve that uncertainty and distress (Mack et al., 2006, 2007b, 2009). “Parents and children are worried about these issues, whether or not we address them. If we address them, we can help manage those fears and also correct misconceptions,” she said. She stressed that “When we communicate about difficult subjects, we also affirm that we will be with the parent and the child through tough times, and parents who know what is ahead feel more prepared to be there for their children. Ultimately, promoting false hope is not a goal of medicine, but being with patients and families through hard times is.”
Victoria Sardi-Brown, the parent of a child who died from cancer, noted that it was a nurse, not a doctor, who pulled her aside to tell her that her child was dying. “If I and my husband had understood what his medical trajectory was, then I would have felt more empowered as a parent to make better decisions for him. Once I found out we were dealing with end-of-life care, I did have hope. Hope changes along the continuum. When hope for a cure went out the window, then we hoped for a more sound, humane, and less painful death,” she said. “Empowerment and communication go hand in hand.”
Such communication and empowerment is important at the end of life, but families should be having conversations with their providers early on about care options and planning for their children, said Wolfe. She added that “sometimes it is really the child that gets empowered, and when the child becomes empowered, that helps empower the parents.”
Wiener also stressed the importance of doing care planning soon after diagnosis to help ensure treatments are aligned with patient and family goals. Both advanced care planning and palliative care are associated with positive outcomes, she noted, including care consistent with patient preferences, better quality of life, less distress, and longer survival. Adolescent patients are often capable of doing such planning and appreciate the opportunity, Wiener found, and she published ways to assess the readiness for such conversations and how to engage the patients’ families in such discussions (Wiener et al., 2013).
Wiener developed an advanced planning guide for adolescents and young adults called Voicing My Choices.6 The guide considers issues critical
___________________
6 See http://www.agingwithdignity.org/voicing-my-choices.php (accessed May 29, 2015).
in their development stage, including identity, autonomy, the importance of family and friends, how they want to be remembered, how they find meaning in their life, and their spiritual thoughts. The guide “allows youth to be able to document decisions that bring them peace and comfort,” Wiener said. Since the guide was first published in October 2012, patients have requested more than 20,000 copies, “which speaks to the need for a tool to open up these conversations,” she said. A recent IOM report also recognizes the importance of such conversations and recommends a life-cycle model, in which advanced care planning occurs at key developmental milestones, including when a life-limiting illness is diagnosed (IOM, 2015). This plan should be revisited periodically by both patient and providers and should become more specific as changing health status warrants.
Listening is another key component of provider communication and the ability of health care practitioners to administer effective palliative care, Mack stressed, as it builds the therapeutic relationship and gives patients and their families the opportunity to explore what is important or what is lacking in their care. “This sets the stage for formulating the goals of care,” she said. Helpful questions include those that ask patients and their families to contemplate the future and indicate what is most important to them and what they are most worried about, as well as what hopes they have; parents are asked what it means to them to be a good parent in the situation they are facing (Feudtner, 2009; Feudtner et al., 2015; Hurwitz et al., 2004).
When a child’s cancer is incurable, many providers rely on goal-oriented decision making as an important way to make decisions about care, but such decision making is needed throughout the child’s illness and can help the transition to end-of-life care, Mack said. “Making goals a part of decision making from the time of diagnosis can help us learn what matters to the parent and the child, and care can be framed in the context of these goals,” she stressed. One study found that clinicians often reach a decision about the preferred direction of care and then present it to parents, but parents often wish for a more active role in care decisions (de Vos et al., 2015). Parents also often seek options beyond what is offered by the oncologist, who needs to listen to those options before making recommendations, Mack said (Bluebond-Langner et al., 2007).
Wiener pointed out that one reason effective communication about palliative care is often not provided is because palliative care suffers from an identity problem—many clinicians mistakenly equate palliative care with end-of-life care and hospice (Parikh et al., 2013)—and the majority of health care practitioners lack formal skills training to be comfortable in
providing pediatric palliative care (Wiener et al., 2015b). Most pediatric oncologists learn to give such care by trial and error (Hilden et al., 2001), with 71 percent of pediatric hematology/oncology fellowships lacking training in palliative care (Roth et al., 2009), Wolfe reported. Nurses also often lack training in pediatric palliative care, she added (Pearson, 2013).
Parents frequently are not knowledgeable about palliative care or misunderstand it as appropriate only at the end of life when cure is not possible. Building on consumer research commissioned in 2011 by the Center to Advance Palliative Care (CAPC) and the American Cancer Society (CAPC, 2011), the American Childhood Cancer Organization (ACCO) adapted some of the poll questions to ask parents of children with cancer about their palliative care knowledge. As with the CAPC poll, few parents in the ACCO survey were knowledgeable about palliative care, but the majority (86 percent; Kirch and Ullrich, 2014) confirmed they would want it for their child when palliative care was defined as care focused on quality of life for the patient and family that manages the pain, symptoms, and stress of serious illness and can be provided along with curative treatment, Wiener noted.
“We need to not think of palliative care as an on–off switch, but as a dimmer switch where palliative care is given alongside potentially curative treatments—a continuum where palliative care plays a role in each particular moment of the patient’s disease or cancer journey,” Wiener said (Tsai and CPS, 2008). Wolfe presented a model of pediatric palliative care delivery across the care continuum (see Figure 1), and added, “Palliative care enhances well-being and strength and resilience, and all of that is needed in order to be able to have the reserve to undergo cancer treatment successfully.”

FIGURE 1 Model of pediatric palliative care delivery across the care continuum.
SOURCE: Wolfe presentation, March 9, 2015.
Emerging evidence supports the notion that pediatric palliative care is beneficial to children and their families, Wolfe reported. Pediatric cancer patients who received this care were more likely to have fun (70 percent versus 45 percent) and to experience events that added meaning to life (89 percent versus 63 percent) (Friedrichsdorf et al., 2015). Children receiving pediatric palliative care also experience shorter hospitalizations and fewer emergency department visits (Ananth et al., manuscript in preparation). In addition, families who received palliative care for their children with cancer report improved communication (Kassam et al., 2015). When children with cancer and their parents are accurately told what palliative care offers, most indicated they would want to meet a palliative care team around the time of diagnosis (Levine et al., 2015).
However, despite the need for pediatric palliative care, few facilities adequately provide it, Wolfe noted. Contrary to American Academy of Pediatrics (AAP) policy, which recommends broad availability of pediatric palliative care services based on child-specific guidelines and standards (AAP, 2000), only 58 percent of COG member institutions have access to a pediatric palliative care service (Johnston et al., 2008). Nearly one-third of children’s hospitals lack a pediatric palliative care program, or if they have one, they are often understaffed (Feudtner et al., 2013). Another study found that only 3 of 15 valued elements of palliative care were accessible to the families of children with cancer (Kassam et al., 2013).
Several studies done by Wolfe and others have documented that there is insufficient relief of the pain and suffering of pediatric cancer patients, even at facilities that offer robust palliative care. Most children in the last month of life suffer from pain, fatigue, and difficulty breathing, according to the reports of parents (Wolfe et al., 2000a). Another study found that most children with advanced cancer reported experiencing pain, fatigue, and other symptoms that were linked to high distress levels.
Feudtner stressed that pain medications can be effective. “At least half the time we are asked to do palliative care consults that improve pain management by using the available medicines more skillfully,” he said. But he added that “the availability of medications that we would like to be able to use is restricted by formulary restrictions.” Wolfe noted, “We are complacent as providers and as families—we expect that because these children are getting cancer treatment, they have to experience all of this distress. We haven’t challenged ourselves to raise the bar and to raise parent expectations that we can do better.”
Policy Opportunities to Improve Pediatric Palliative Care Across the Care Continuum
Several workshop participants suggested various policy measures that could improve access to high-quality pediatric palliative care across the care continuum, including developing standards and incentives for such care, educating health care providers and parents about palliative care, and providing more funding for and research on such care. Wiener pointed out the need for more evidence-based standards in pediatric palliative care, noting that there is only one standard, which is supported by 36 studies: “Youth and their families should be introduced to palliative care concepts to reduce suffering throughout the disease process regardless of disease status” (Weaver et al., 2015, p. 9). “Evidence-based standards are needed, but just as important are the policies and funding to help implement them,” Wiener said, adding “the most beautiful, heroic evidence-based interventions can go from the lab bench to the park bench if we don’t have systematic [implementation]. The same is true for standards.”
Peter Brown, co-founder of the Mattie Miracle Cancer Foundation, also called for the development of standards for palliative care and psychosocial support, similar to medical standards of care. He suggested that the federal government should deem this care as essential and mandate coverage through the Centers for Medicare & Medicaid Services (CMS), saying that eventually, insurance companies would likely follow the CMS lead. He also suggested that accreditation entities for health care institutions should require screening for psychosocial needs.
Wiener suggested that core competencies in pediatric palliative care be implemented in schools of medicine, nursing, social work, psychology, and counseling. Feudtner called on medical schools to train physicians in practicing kindness, and imbuing them with a sense of duty and responsibility around kindness.
Parents also need to be educated about pediatric palliative care and how it can help them and their children throughout the care continuum, Wiener pointed out. Once they are aware of what palliative care offers, few parents would turn it down, she said. “If a child comes in with a cardiac condition, do we say to the parents, would you like to go to the cardiologist? No, we make the referral right away because we want the child to have the very best care possible. Similarly, I don’t know of very many parents, who when told by a physician ‘I want to make sure your child gets the very best care,’ would turn down palliative care,” Wiener said. Wolfe added that there are family
education guides and resources relevant to palliative care that should be offered to parents, including those that prepare them for having a conversation about the child’s potential death, so when the child asks “Am I going to die?” they know what to answer. “These different guides and conversation tools can be taught and handed to families so they don’t live in fear of being asked that question,” she said.
Wiener suggested integrating a palliative care service into pediatric oncology practices, but noted, “One does not need to have a palliative care service to integrate palliative care concepts, such as paying attention to symptoms, personal goals and values, and quality of life throughout care and extending beyond the hospital into the ambulatory and home setting.” She suggested taking a team approach to palliative care and relying on psychosocial oncology professionals, including social workers, psychologists, psychiatrists, and child life specialists, who are trained in providing palliative care throughout the disease trajectory and into survivorship. “We can’t work in our individual silos or we will get absolutely nowhere—we are better together,” she said.
Several participants also called for more research on pediatric palliative care. Feudtner observed that there is a huge gap in what is known and what needs to be known in this area. Wolfe said one study found that the key gaps center around knowing what matters most to parents and their children receiving palliative care, defining the best practices in pain and symptom management, implementing effective strategies in alleviating suffering, and identifying the bereavement needs of families (Steele et al., 2008). Another study provided the basis for consensus on 20 pediatric palliative care research priorities thematically grouped into decision making, care coordination, symptom management, and quality improvement (Baker et al., 2015). “The research needs are vast,” Wolfe said, and stressed that families are often willing to participate in such research. Mack also stressed the need to support research on the most effective communication techniques to use when providing pediatric palliative care.
Several participants pointed out the lack of funding for pediatric palliative care programs. “Many of the pediatric palliative care teams that are operating in different children’s hospitals are doing their work with a promissory note. We need to move beyond that and build vibrant pediatric palliative care programs at all centers with a good business plan underneath,” said Feudtner. Lindberg added, “I don’t want to hear there is no funding because that isn’t helpful to a parent who is facing losing their child tomorrow or the week after. It is too important not to be supported.” Reimbursement
strategies are also needed for the advanced care planning and other conversations health care providers have with their patients and families as part of their palliative care, Mack said. “These are time-intensive conversations that take place over and over again during the span of treatment.” She also noted that support is needed for programs that train practitioners on how to have those conversations.
Wolfe noted public policy and other incentives to increase pediatric hospice options, including a Massachusetts palliative care program that embeds pediatric teams within hospice programs throughout the Commonwealth that has “raised the bar in terms of access to home-based hospice care,” she said. Other states, such as California and Florida, have Medicaid waiver programs that enable enhanced pediatric hospice care in the home setting, she added. “Although the numbers are small, the ripple effect is vast, and we need these home-based programs to be able to take care of our children in the highest possible quality,” she said.
PSYCHOSOCIAL CHALLENGES AND OPPORTUNITIES
From the time of diagnosis onward, cancer poses many psychosocial challenges for a child as well as for his or her family, including anxiety over treatment or the possibility of dying, the stress of dealing with demanding treatments, and school and peer issues. Workshop participants discussed the psychosocial needs of children and their families and provided examples of ways to predict and screen for distress as well as interventions to alleviate distress. Participants also discussed standards of psychosocial care, capacity for providing that care, and research needs.
Mary Jo Kupst, Professor Emeritus at the Medical College of Wisconsin, gave an overview of some of these psychosocial challenges, beginning with diagnosis when there can be stress from information overload, with families expected to make rapid treatment decisions so the child can quickly begin treatment. In addition, both the patients and their families are navigating a new environment with new staff, especially if care occurs away from their communities. Even if families remain in the same town, they are spending an inordinate amount of time away from their homes and in the hospital with their children, who are receiving painful or distressing procedures with disturbing side effects. Despite all these pressures, “Cancer doesn’t occur in a vacuum and life still goes on and you have to deal with family, school, social changes, activities, work, and finances,” Kupst said (Kupst and Bingen, 2006; Kupst and Patenaude, 2015a,b; Rodriguez et al., 2012).
Once treatment becomes more routine, “families have told us this is a time when their emotions start bubbling up so that becomes a very difficult time as well. There are also physical changes, adherence challenges, school and peer issues, and an overall increased strain on the family because the pressures on them do not stop, but just keep going,” Kupst stressed. Ending treatment is a positive time, she noted, “but many families tell us that it is a loss of a safety net and support that you had almost on a daily basis for a long time as you transition to other and new providers” (Wakefield et al., 2011).
Survivorship requires continued monitoring of health status for the late effects of treatment that can disrupt life plans and goals. Surviving cancer can affect academic, vocational, social, and spiritual pursuits, Kupst pointed out (CCSS, 2015). When relapse and recurrence occurs, there is more treatment and uncertainty and a greater threat of death and difficulty maintaining hope. This is especially true once the child progresses to end-of-life care. “Families have told us that there was so much optimism at diagnosis, but once the child is dying you have to anticipate reality—the loss of your life if you are the patient, or the loss of the child if you are the parent. Parents need to talk and support children through this stage when they themselves are trying to deal with it and face the possibility of life after the child’s death, and trying to make sure that the child will be remembered. There is a need for comfort and support during and after this time,” Kupst stressed.
Psychosocial Needs for Pediatric Patients
How pediatric patients react psychologically to a diagnosis of cancer and the subsequent treatment depends on their developmental stage, Kupst noted. “There’s no one-size-fits-all reaction,” she said. Very young children have a number of issues they have to cope with, including fear of separating from their parents or of having painful or frightening treatments. In response to these stresses, their behavior can change and regress. They may attempt to control what they cannot control, have tantrums, cling to their parents, exhibit aggressive behavior, or withdraw from social interactions, Kupst said. Thus, she said the need for psychosocial support varies greatly among pediatric patients, and interventions should be targeted to the needs of individual patients.
Disruption of school is one of the biggest strains on school-aged children. Eric Sandler, Courtesy Associate Professor in the Division of Hematology/Oncology at the University of Florida College of Medicine,
Jacksonville, and a parent of a child with cancer, noted he had to take his daughter out of the public school system and enroll her in a private school because “no matter what we did, we couldn’t get the services that she needed,” despite having an Individual Education Plan developed based on her needs. School-aged children also experience a loss of peer interactions and activities while undergoing treatment, as well as distress over various procedures to which they are subjected. These older children have a greater understanding of the seriousness of their cancer and may seek emotional and social support. One parent of a 7-year-old diagnosed with cancer noted that after undergoing major treatments that put him in a wheelchair, he suffered from depression, anxiety, and posttraumatic stress disorder.
Adolescents have many of the same issues as younger children, Kupst pointed out, with the addition of the strain the cancer is placing on their quest for independence at this stage. “Adolescents are thrown back into a dependent relationship with their parents and with the medical and oncology staff. Adolescent emotions are intense anyway, but this need for independence makes it doubly hard,” she said. Adolescents with cancer have an increased need for and use of social support, and are more focused on identity issues and image. They tend to use humor, self-talk, diversion, and positive reappraisal to cope with the stress of having cancer, but can also exhibit risk-taking behavior, according to Kupst (Kupst and Bingen, 2006).
Cancer in young adults may postpone, interrupt, or alter their romantic relationships and academic or vocational pursuits. They tend to have more concerns about how the long-term effects of cancer will affect their careers, fertility, and finances, Kupst said. Their coping strategies are similar to those of adolescents with cancer, she added, although more research is needed in this regard.
Children at all developmental stages have to face how cancer alters their relationships with their peers at a time when peer relationships are extremely important, said Robert Noll, professor of pediatrics, psychiatry, and psychology at the Children’s Hospital of Pittsburgh. To assess peer relationships among pediatric cancer patients, he relies on two sets of peer measures: what the child is like and whether the child is liked by peers. Such peer reports tend to be reliable and predictable of occupational and social success, according to Noll. Although he expected pediatric cancer survivors would be isolated and victimized, have fewer friends, and be less well liked, he did not find evidence for that, except for those who had brain tumors. The only difference peers reported were that cancer survivors were less aggressive and disruptive (Reiter-Purtill et al., 2003).
However, peer reports on pediatric survivors of brain tumors, provided 3.5 years on average after diagnosis, found they were lacking in leadership; were more sensitive, isolated, and victimized; were not well liked; and did not have many friends (Salley et al., 2015; Vannatta et al., 1998). “In terms of social function in children with cancer, the group of kids who are at very high risk are brain tumor survivors,” Noll stressed. When this becomes a problem for these children is not known exactly, but Noll suspects that when they first return to school after treatment they may socially resemble those of their peers, but over time they start to develop not only neurocognitive problems, but some significant social problems. “We need to think about when this unfolds because if we are going to allocate resources, we need to target them at the right time,” he said.
Psychosocial Needs of Families
Pizzo stressed that pediatric cancer “is truly a family event of extraordinary proportions.” Paul Jacobsen, associate center director in the Division of Population Science at the H. Lee Moffitt Cancer Center and Research Institute, added, “from a psychosocial perspective, the unit of care is the family.” Several parents of pediatric cancer patients shared their perspectives about the impact of their child’s cancer treatment on both the child and the family (see Box 4). Pediatric cancer is devastating to families, but Kupst said that most children and families are ultimately able to cope with the disease, treatment, and its aftermath (Abrams et al., 2007; Kazak et al., 2012; Noll and Kupst, 2007; Vrijmoet-Wiersma et al., 2008; Wechsler and Sanchez-Iglesias, 2013). “But it is life changing. You don’t get over it but you get better at coping with it. A number of findings demonstrate the importance of early and continuing communication and support from the whole cancer team,” she said. Pamela Hinds, The William and Joanne Conway Chair in Nursing Research, director of the Department of Nursing Research and Quality Outcomes, associate director of the Center for Translational Science at the Children’s National Health System, and professor of pediatrics at George Washington University, stressed that a child’s psychological and physical well-being depends on that of his or her parents, so “if we are going to treat the child, we need to also treat the family.” She suggested reaching out to parents while their child is still under treatment. “We have the opportunity to make a difference in family health while they are within our reach, and they may not be within the reach of a health care professional shortly after leaving us.”
BOX 4
Parent Perspectives on Cancer Treatment

Several parents of children with cancer spoke of their personal experiences and the strain their child’s cancer treatment placed on both the child and the family. Eric Sandler noted the fatigue and social and school difficulties his daughter experienced during and after treatment for leukemia. Even though he is a pediatric oncologist, he said he did not fully appreciate the difficulty of these challenges until he had to deal with his own daughter’s cancer.
Other parents spoke of rigorous cancer treatments and the psychological toll it took on their child and themselves.

Jennifer Cullen’s daughter Alexandra was only 4 years old when she underwent extensive treatment for a brain tumor, including having a hole drilled in her skull and a shunt put in to relieve intracranial pressure. Alexandra had such extensive mouth sores from high-dose chemotherapy that she had to be fed intravenously, and the treatment required around-the-clock baths to wash off chemicals that can cause skin burns. “Imagine waking up a 4-year-old at 2 a.m. and putting her in a shower,” Cullen said.
Victoria Sardi-Brown’s son was 7 when he underwent high-dose chemotherapy regimens, three limb-salvaging surgeries, radiation therapy, a sternotomy, and experimental immunotherapies to treat his bone cancer. “He had all sorts of things bombarding his body and in 14 months Mattie was transformed from an active and happy child to one who was unable to walk and function independently. It was very hard for a 7-year-old to be transformed in that way,” she said. As a consequence, Mattie had clinical depression, anxiety, and posttraumatic stress disorder, which required him to take psychotropic medications. “We think of childhood cancer as a physical disease, but it is really much more than that,” Sardi-Brown stressed, adding, “It stuns me that there is a disparity in the psychosocial care and services offered in this country among hospitals. The predominant focus is always on medical care and drug development, yet any of us who has helped a child endure medical treatment knows that there are psychosocial issues just as complex and heartbreaking to manage, such as when your child tells you he feels ugly and that no one wants to be his friend because he is so different, or when he is in such excruciating pain that he is screaming uncontrollably, or worse, when he is telling you he knows he is dying.”
Anne Kazak, co-director of the Nemours Center for Healthcare Delivery Science and professor of pediatrics at the Thomas Jefferson University, agreed, stressing that although the majority of families are resilient, many have psychosocial concerns that could be addressed with evidence-based treatments. She suggested such interventions identify the “hot spots” of the family and deliver treatments specific to those particular problems. Joanne Wolfe added that for any child with a serious or life-threatening illness, throughout the course of that illness, “We need to embrace an individualized blending of care directed at the underlying illness, and at the physical, emotional, social, and spiritual needs of the child and family with continuous reevaluation and adjustment.”
Kupst pointed out the variability in how families cope with cancer, and said that the ability of the child to cope influences how well the parents cope and vice versa. “A number of studies report parents saying, ‘if my child is doing okay then I am doing okay,’” Kupst said, and she noted that if family support is lacking there is more reliance on social support and vice versa. Some families pull together in response to cancer, while others are pulled apart by the disease. Paying attention to these individual differences and how a family’s ability to cope changes over time is important, she said. A cross-sectional study that takes only a snapshot at one time might indicate a family is doing poorly, while a longitudinal study may show them adapting and improving over time (Compas et al., 2012; Folkman and Moskowitz, 2004).
Kupst outlined the early factors that predict how well a family will cope with their child’s cancer, including
- Previous functioning, life events, and experiences with stress (Alderfer et al., 2009; Bruce, 2006; Long and Marsland, 2011; Okado et al., 2014; Vrijmoet-Wiersma et al., 2008);
- Previous ways of coping, and early responses to diagnosis and treatment (Alderfer et al., 2009; Kupst et al., 1995; Vrijmoet-Wiersma et al., 2008);
- Family resources, supports, and concurrent stresses; socially isolated families tend to have poorer outcomes (Bruce, 2006; Patenaude and Kupst, 2005; Vrijmoet-Wiersma et al., 2008);
- Family adaptiveness and cohesion (Alderfer et al., 2009; Long and Marsland, 2011); and
- Developmental level of the child (Alderfer et al., 2010; Long and Marsland, 2011; Okado et al., 2014; Vrijmoet-Wiersma et al., 2008).
Later in the course of the illness, other factors predict the family’s ability to adapt and their psychosocial outcomes, including parent–child coping strategies (Klassen et al., 2011; Kupst et al., 1995; Long and Marsland, 2011; Okado et al., 2014; Pai et al., 2006), social-environmental and cultural variables (Gray et al., 2014; Klassen et al., 2011), and other concurrent stresses (Kupst and Patenaude, 2015). This suggests the need for “personalized” psychosocial interventions, Kupst and others stressed. “We always need to keep the individual child and family in mind and what is going to work best for whom,” she said.
Wolfe added there should be interventions that target “an individual’s experience of suffering.” Her studies found that one-quarter to one-third of children and families need more intensive intervention and help. These children and families experience significant distress, posttraumatic stress, or other problems at some time during and after treatment that indicate need for increased care (Abrams et al., 2007; Aldridge and Roesch, 2007; Kazak et al., 2012; Patenaude and Kupst, 2005). Posttraumatic stress is often high in parents of children with cancer and in pediatric cancer survivors, but can lessen over time. Psychological growth also can occur over time, Kupst noted (Picoraro et al., 2014). “The good news is that we have greater knowledge of the psychosocial aspects of pediatric cancer and have improved assessment and screening measures that are specific to pediatric cancer, as well as good neurocognitive and patient and family-reported measures,” she said. There are also more evidence-based interventions, especially starting early in treatment, Kupst added.
Predicting and Screening for Distress
Identifying children and families in distress is a first step in targeting psychosocial interventions to address their unique needs. Kazak described her Pediatric Psychosocial Preventative Health Model (PPPHM), in which more intensive treatments are reserved for those families that need it the most (see Figure 2). In this model, all families are provided basic psychoeducation and family-centered support. All families are also screened for risk indicators of a compromised ability to psychologically cope with the child’s cancer. Families with more acute or elevated distress or significant risk factors are provided with interventions specific to their symptoms or needs. Families with severe, escalating, or persistent distress are offered more intensive clinical psychosocial services. “These families are the easiest to recognize and the most difficult to treat. They are going to require the expertise of a
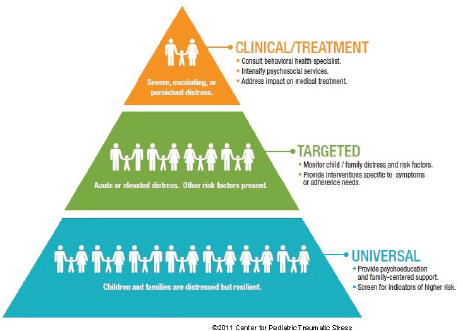
FIGURE 2 Pediatric Psychosocial Preventative Health Model. This model uses a three-tiered approach to address the psychosocial care needs of the child and family. Basic psychoeducation, family-centered support, and screening for psychosocial risk factors are provided to all families. There are three levels of psychosocial risk: universal, targeted, and clinical/treatment. Interventions are tailored to families based on their unique needs and risk level.
SOURCE: Kazak presentation, March 10, 2015; Center for Pediatric Traumatic Stress, 2011. Reproduced with permission from the Center for Pediatric Traumatic Stress (CPTS) at Nemours Children’s Health System © 2014–2015 All rights reserved. The PPPHM image may not be reproduced in any form for any purpose without the express written permission of CPTS. To obtain permission to use or view the most recent version of the PPPHM, please contact CPTS at psychosocialassessmenttool@nemours.org.
behavioral health specialist,” Kazak said. But she noted that the lines distinguishing different levels of this pyramid are not rigid and there is movement among levels, suggesting the need to “screen, watch, and evaluate families.”
Kazak and others have developed a number of screening tools for psychosocial distress, with varying complexity. One of the easiest and briefest screening tools is called a distress thermometer and is akin to the pain thermometer patients use to report their degree of pain. In addition to rating their distress on a scale of 1 to 10, there are additional categories for
difficulties that patients or their families can check off. Structured clinical interviews can generate a richer amount of information, but take more time to conduct. These interviews are not widely used because of the training and time involved in conducting them, according to Kazak. There are also standardized instruments for measuring distress that take less time to use, including one Kazak developed called the Psychosocial Assessment Tool (PAT).
PAT is a brief parent-reported screen of psychosocial risk. It has also been translated into several languages, and there are low-literacy versions in English and Spanish. PAT is composed of a two-page questionnaire and is currently being used in more than 50 sites in the United States and 30 sites internationally, mostly in oncology settings, Kazak reported. Based on research findings and clinical expertise, PAT generates a numerical score for the answers parents give in a number of domains, including family demographics, structure, resources, beliefs, problems, stress responses, and social resources (see Table 1). That score is used to determine the family’s level in the PPPHM. PAT has been clinically validated and is available in a number of formats, including paper or digital versions compatible with iPads and electronic medical record systems. PAT can also be accessed via its website online.
TABLE 1 Psychosocial Assessment Tool
|
|
||
| Domains | Subscales | Scoring/Interpretation |
|
|
||
| Demographic Diagnosis Family structure Family resources Social support Child knowledge of disease School enrollment School placement Child problems Sibling problems Family problems Family beliefs Stress responses Infants/young children Traumatic stress responses Suicidality |
Structure/resources Family problems Social support Stress reactions Child problems Sibling problems Family beliefs |
Items are scored “positive” Total score = sum of scales Clinically relevant but unscored |
|
|
||
NOTE: PPPHM = Pediatric Psychosocial Preventative Health Model.
SOURCE: Kazak presentation, March 10, 2015.
A study of pediatric cancer patients and their families at several institutions using PAT indicated that 50 to 75 percent of families scored in the lowest (universal) tier of the pyramid, with 24 to 36 percent of families in the second (targeted) tier of the pyramid, and 4 to 18 percent of families in the top (clinical) tier (Kazak et al., 2015). For families in the universal tier of the pyramid, Kazak created a guide to resources and tools for health practitioners that can be accessed on the Internet (www.healthcaretoolbox.org). Families can also use the website. A more recent study of PAT found Spanish-speaking families were twice as likely as English-speaking families to fall in the clinical level that requires the most intensive intervention.
Kazak pointed out a number of challenges to screening families for psychosocial distress, including the stigma many families attach to being referred to a psychologist (e.g., that it indicates a weakness or psychological abnormality). This can be countered by making screening universal and by providing education on comprehensive care, Kazak suggested. A lack of time and personnel can also be an impediment to screening. PAT takes 5 to 10 minutes to complete, but a Web-based version makes it easy to integrate into the workflow of a clinic, she said. Alternatively, more time can be scheduled for appointments so families have time to fill out the questionnaire when they arrive. Administrators can also use aggregate data from PAT screening to argue for more psychosocial personnel when resources are available for them, Kazak added, noting that some practices are also linking the screening to pathways of care for which there are billing codes (Kazak et al., 2016).
Parental Interventions to Alleviate Distress
One way to address family distress following a cancer diagnosis is to target psychosocial interventions to the parents. For example, to help parents cope with their child’s diagnosis of cancer and its aftermath, Noll collaborated with several investigators to create a training intervention called Bright IDEAS, which teaches parents problem-solving skills. This intervention has parents identify what their problem is, define and evaluate their options, act, and then see if the action helps to resolve the problem. Studies of mothers of pediatric cancer patients found that the intervention was acceptable and more effective at reducing the mothers’ distress than standard supportive therapy. The training was most effective for minority mothers, single mothers, and mothers with a low socioeconomic status (Sahler et al., 2005).
Only four pediatric cancer centers in the United States offer such problem-solving skills training when families are first diagnosed. The intervention is not widely used because it is labor intensive and requires trained personnel. To increase accessibility, Noll developed an online version that would not only reduce the need for personnel to offer the training, but might make the training more amenable to parents who can do it whenever they have some free time. He is currently evaluating the online program to see if it is as effective as the in-person program conducted by trained staff. He is also, in conjunction with COG, training more than 200 nurses, social workers, and psychologists to increase the number of providers so that every pediatric cancer center in the United States can offer the problem-solving skills training.
Practitioners are also applying other interventions to support and address the psychosocial needs of children with cancer and their families, but one study found that only 9 percent of families at COG institutions were offered empirically supported interventions, and only about half were offered any type of psychosocial care within the first month after treatment began, Kazak reported (Selove et al., 2012). “We know that many families do not receive the care they need and the care that they could benefit from,” she said.
Building Capacity for Psychosocial Care
There was some discussion about who should be responsible for offering psychosocial care for pediatric cancer patients and their families, and who should financially support it. Kupst stressed that integrating psychosocial care in pediatric oncology requires collaborative multidisciplinary care with physicians, nurses, social workers, psychologists, and pastors. She suggested such care be embedded in cancer centers because families are not likely to travel to another site to receive such care.
Even when centers offer psychosocial care, families may not have access to this care when they need it, Kupst and others noted, due to staffing shortages or lack of insurance to pay for such care. One participant pointed out that in Florida, Medicaid does not reimburse for psychosocial care or psychology services. Bhatia pointed out that there are a limited number of social workers trained in oncology (IOM, 2013a), and Brawley stressed that many clinics lack social workers entirely.
Some institutions have funds to support the provision of psychosocial care, but many do not, Kupst noted. She suggested that the way to encour-
age reimbursement by insurance companies is to demonstrate the costs and benefits of such care. Jacobsen added, “You always hear there are no resources, and yet suddenly, when [distress screening] is a mandate from the Commission on Cancer, the valves begin to open up.” He pointed out that insurance companies are increasingly replacing fee-for-service reimbursements with bundled care payments. “Bundled care either represents our greatest promise or our greatest challenge, because if we can get into bundled care packages, then there will be some reimbursement for what we provide. If we do not, then we are sunk. That is why it is critical that we are at the table when these pathways are being developed and when these contracts are being written,” Jacobsen said.
Kazak noted that screening is one of the activities encouraged by the Affordable Care Act. But she added that a big issue for offering services and having them reimbursed is defining the credentials people need to have to provide them. “How low can we go? There are disasters waiting to happen if you don’t have the appropriate level of expertise,” she said, adding, “If we really endorse family-centered care and take it seriously, then we need to make sure we have the types of expertise to do it.”
Anna Muriel, chief of the Division of Pediatric Psychosocial Oncology at the Dana-Farber Cancer Institute, pointed out that pediatric oncology is a smaller venue than adult oncology, so offering psychosocial care at all cancer clinics that treat children may not be feasible. She asked which institutions should be responsible for providing psychosocial care—children’s hospitals or oncology practices? Kazak responded that “in developing standards, we are looking for how we can best fan this out into practice and we are not quite sure how to do that yet.” Jacobsen added, “It may not be important where that care resides, but you need to have some process in place to know that the minimum levels of acceptable care are being delivered and there should be some way of measuring to see that it was done.”
Several participants at the workshop voiced the need for evidence-based standards for psychosocial care in pediatric cancer. “Standards of care are broad statements that will be interpreted differently at different institutions, but the goal is to make sure that the kinds of terrible things that happened to the parents of cancer patients who spoke today do not happen again, because that is unacceptable,” Jacobsen said. He noted that a 2008 IOM report, Cancer Care for the Whole Patient: Meeting Psychosocial Needs, recom-
mended as a standard of cancer care that practitioners should identify each patient’s psychosocial needs and have a plan to link them with the services that can address those needs.
Kupst noted that although standards have been defined, they have not been implemented (Wiener et al., 2015a). To further implementation of such standards, she emphasized the importance of a strong evidence base and strong support from stakeholders, including families, patients, providers, and other influential groups. Jacobsen agreed that publishing guidelines is not sufficient for their implementation, based on the experience of implementing standards for psychosocial care into adult oncology practices. He said there is also an important role for family and patient advocates. “We do the science push, including the systematic reviews, and publish guidelines and assume the world is going to listen to us and do exactly what we say, but it doesn’t work that way. What is key is to also build a demand for the market, the intervention, and for your recommendations,” Jacobsen said.
Standards take a long time, much expertise, and many resources to be implemented, he said, because of all the groundwork that needs to be done to coordinate relevant professional organizations and ensure accreditation of the standards by these organizations (Kerner et al., 2005). For example, despite the 2008 IOM recommendation to identify and address the psychosocial needs of cancer patients (IOM, 2008), it was not until 2012 that the American College of Surgeons Commission on Cancer issued a new set of patient-centered standards of care that included psychosocial distress screening as one of the standards. But this standard was not required as a phase-in for accreditation by the commission until 2015 (CoC, 2014).
The commission’s new patient-centered standards of care should make a substantial impact on adult cancer care because it accredits approximately 1,500 cancer centers, which treat about 75 percent of Americans with cancer in the United States. To further ensure the implementation of these standards and answer questions and concerns about them, the American Psychosocial Oncology Society, the Association of Oncology Social Work, and the Oncology Nursing Society formed a joint task force that offered concrete recommendations for implementing distress screening in cancer programs. These recommendations were published in late 2014 (Pirl et al., 2014). In addition, a broad coalition of providers, patient and family advocacy organizations, foundations, and cancer centers worked together to create a resource guide for meeting the commission’s standards, and also provided examples of best practices and a comprehensive national listing
of psychosocial services. But even that was insufficient for small community cancer practices, Jacobsen noted, so two of his colleagues applied for and received an NCI grant that enables cancer practices to participate in workshops and meet with experts and consultants who can help them build an action plan for how their institutions can implement psychosocial care.
Jacobsen added that the 2008 IOM report also recommended instituting quality oversight mechanisms for measuring and reporting on the quality of cancer care, and he was asked by the American Society of Clinical Oncology (ASCO) to develop indicators of the quality of psychosocial cancer care and ways to code for them in medical records (Jacobsen et al., 2011). ASCO incorporated those indicators in their Quality Oncology Practice Initiative, a voluntary quality assessment and improvement program for oncology clinical practices that enables them to track how well they are following standards of cancer care. The psychosocial indicators provided evidence in the medical chart that the emotional well-being of the patient was assessed within 1 month of the first visit with a medical oncologist, and that if a problem with emotional well-being was identified, action was taken to address the problem or an explanation was provided for no action. But between 2009 and 2013, progress made in adherence to both standards was minimal. “Audit and feedback alone is not sufficient. We need to do more,” Jacobsen said.
He also stressed that “you cannot have a one-size-fits-all approach because you are dealing with major cancer centers that have very good resources as well as small community-based cancer centers that have very few resources. So you have to provide a situation where all boats rise and not just some boats.” He added that providers want and need expert assistance in formulating and implementing changes to improve the psychosocial care for their patients.
The NCI Community Cancer Centers Program7 has the Psychosocial Matrix Assessment Tool that community cancer practices can use as a self-assessment tool to evaluate and improve the psychosocial care they deliver, with criteria derived from IOM recommendations, Jacobsen reported (Forsythe et al., 2013). A study of 16 practices using this self-assessment tool found that at baseline, most were providing only Level 1 care, which means that they identified the need for psychosocial care and suggested resources, but there was no systematic referral pathway for such care. Two years later, the percentage providing only Level 1 care dropped to 25 per-
___________________
7 See http://ncccp.cancer.gov.
cent, with most practices having either a systematic referral pathway or staff trained in psychosocial care to provide such care or a consultation onsite (Forsythe et al., 2013). “It shows remarkable progress in a short period of time by pointing out where there are deficiencies and giving practices with a commitment to provide psychosocial services some guidance for how to do it,” Jacobsen said.
He noted that patients and families already regard psychosocial services as important, so more effort needs to be made to convince clinicians and clinical practices who are wondering why they should invest in these services and devote staff expertise to them. He also stressed that once market demand is built for psychosocial services for children and families, capacity to meet that demand at cancer centers and practices must also be built.
With impetus and ongoing support by the Mattie Miracle Cancer Foundation, more than 40 professionals and advocates over the past 3 years have been attending congressional briefings and think-tank conferences and conducting other intensive work to develop psychosocial standards for care for children with cancer and their families, Kupst reported. This group is trying to publish and disseminate the standards as well as gather support for them from key oncology and advocacy organizations so they can be implemented successfully across all children’s cancer centers. Kazak added that a special upcoming issue of Pediatric Blood and Cancer will be devoted to psychosocial standards. Sardi-Brown suggested that COG support psychosocial standards of care and implement them within COG institutions. Peter Adamson, chair of the Children’s Oncology Group and the Alan R. Cohen Endowed Chair in Pediatrics at The Children’s Hospital of Philadelphia, replied that psychosocial care is a high priority at COG, but “NCI and COG cannot do it alone. Advocacy groups and foundations are critically important.”
Kupst said there was a need for more research devoted to the development of targeted or personalized interventions and more longitudinal research to better understand how psychosocial needs change or stabilize during and after treatment. She and Kazak also said it was important to determine the optimal methods and timing of screening and assessment. “We need to know if screening leads to more effective treatments that match families’ needs. Does it promote adjustment and help us deliver that care earlier or more effectively?” Kazak said. Mack suggested more research to
identify the best communication strategies that providers should employ when talking to the parents of children with cancer.
The workshop also included extensive presentations and discussion about effectively integrating use of patient-reported outcomes (PROs) as well as parent-reported outcomes in pediatric cancer to improve clinical research, care, and overall experiences for children and their families. Also discussed were challenges and strategies for assessing pain and other suffering in younger children, and the limitations of using parents as proxies for reporting children’s symptom burden. In addition, participants explored ways to expand use of pediatric PROs in cancer centers and clinical trials.
In the adult cancer world, there is increasing recognition of the value of PROs that communicate the patient experience, said Lillian Sung, associate professor of hematology/oncology at The Hospital for Sick Children. FDA defines a PRO as “a measurement based on a report that comes directly from the patient (i.e., study subject) about the status of a patient’s health condition without amendment or interpretation of the patient’s response by a clinician or anyone else” (FDA, 2009, p. 32). PROs may capture information about symptoms experienced by patients such as fatigue, nausea, and pain; physical functions such as the ability to walk, breathe, eat, and drink; and psychosocial symptoms, such as sadness, worry, and anger. In addition, PROs may be used to evaluate the level of satisfaction with care and treatment adherence, Sung noted.
Sung said PROs are important because if they are not measured, the full impact of a treatment on the child will not be known. Whenever possible, the child’s voice should be elicited because there is mounting evidence that clinicians and perhaps parents may not accurately assess what the child is actually experiencing, Sung stressed. Bryce Reeve, associate professor of health policy and management at the Lineberger Comprehensive Cancer Center at the University of North Carolina at Chapel Hill, agreed, saying, “We as clinicians and as a health care delivery system are not addressing the needs, especially psychosocial needs, from the child’s perspective. We see a gap between what we think we know the child is experiencing and what the child is actually reporting.” He added that “The evidence shows that there is a disconnect between what we think the children and adolescents are experiencing and what the children and adolescents report they are experiencing.” For example, one study of 7- to 12-year-olds asked them to
report their degree of fatigue and then correlated it to reports of their fatigue made by their parents or nurses. This study found a correlation of 0.35 (on a scale of 0 to 1, with 1 being a perfect match) between what the children reported and what their parents reported. The correlation between the children’s self-reports and the reports of their nurses was even lower at 0.16 (Hockenberry et al., 2003). Other studies in different types of pediatric oncology settings consistently find the same low correlation between what children report and what their clinicians report, according to Reeve (Glaser et al., 1997; Le Gales et al, 1999; Parsons et al., 1999).
Reeve pointed out that 60 to 70 percent of children with cancer participate in clinical trials and such studies mandate collection of adverse event data by clinicians. Consequently, if clinicians are underreporting the number and severity of symptoms relative to that of children, “We underestimate the impact of cancer and treatment on the child’s life.” He suggested establishing a dynamic, integrated electronic system to routinely screen children for symptoms and other key patient outcomes that can be used to provide real-time feedback to clinicians. He also suggested educating clinicians and administrators on the value and effective use of PROs. “Like weight and blood pressure, think of PRO as a vital sign that we always should be collecting at all routine assessments, overall,” Reeve stressed (Feeny, 2013). “Enhancing the child’s voice will make them more involved in [his or her] care, help clinicians to better manage symptoms, and hopefully this will result in better treatment adherence and better overall outcomes. Researchers will also better understand the impact of a cancer and its treatment on children’s lives,” he said.
PROs are important to measure in research studies, Sung added, because the information gathered from them can inform the care of future patients, enabling parents and clinicians to anticipate and prepare for what the child is likely to experience with his or her treatment. Physicians may then be able to lessen or prevent complications by providing prophylactic interventions for those patients that studies show are most likely to be at risk. PROs can also enable families and clinicians to make better decisions when there is more than one treatment option for the patient. As Sung noted, factors that affect such decision making include how treatments affect survival, quality of life, and cost. When survival differences between treatment options are large, PROs are less likely to influence treatment
decisions, but they still might if the PRO is disabling and permanent, she said. The more common situation is when the survival differences among treatment options are small or uncertain. In these situations, PROs can impact the decisions families make about the treatment their child receives.
Christina Theodore-Oklota, senior outcomes research scientist at Genentech, agreed with the importance of PROs in aiding treatment decisions and said Genentech strives to incorporate them into their clinical trials. She stressed that the patient is the expert on what it is like to be in treatment so patient feedback is essential. “Deciding what treatment protocol to go on is one of the most important decisions a family can make. We want to arm you with as much data as possible,” she said. Genentech is currently collaborating with pediatric patients to develop ways to measure PROs across age groups in a more efficient way, asking fewer questions while targeting those effects that matter the most, Theodore-Oklota said. “We are using these patient-centered data to make decisions as we move molecules forward” through the drug development pipeline, she added.
Despite the importance of PROs in pediatric oncology, Sung found that only about 10 percent of pediatric Phase II/III therapeutic studies conducted by COG between 2001 and 2013 had a quality-of-life aim (Whitlow et al., 2014). By contrast, 92 percent of adult trials conducted between 1990 and 2007 by the National Cancer Institute of Canada Clinical Trials Group included a quality-of-life aim (Brundage et al., 2007). Reeve noted that Canadian clinical trials incorporate PROs because their default mode assumes that every clinical trial has a PRO, with exceptions only when investigators can justify why PROs are unnecessary or not relevant to their studies. He suggested COG adopt this same default mechanism for PROs in their clinical trials.
Few current COG therapeutic studies incorporate PROs, according to Sung. She made several suggestions for strategies to effectively capture and incorporate PRO data in pediatric oncology research undertaken by COG. One was to identify trial characteristics that would mandate PRO incorporation into Phase II/III therapeutic clinical trials when seeking approval from NCI and COG early in the clinical trial development process. Sung also stressed the importance of eliciting PROs from patients with a poor prognosis. “It is really critical that we measure PROs in the Phase I study in patients who are unlikely to be cured. That is the setting in which PROs really matter and can impact parents’ decision making.” She suggested that parent and patient support groups should have a role in defining the types of clinical trials that should incorporate PRO endpoints, in addition
to a broader role in all aspects of PRO elicitation, including ensuring that PROs are collected in general and in helping to emphasize PRO elicitation in poor-prognosis settings.
But Noll countered that although he supports using PROs in clinical trials, it sometimes may be more appropriate to do neurocognitive assessments8 of children rather than to collect their PROs. “PROs might not be quite as significant as alternative strategies for evaluating children,” he said, noting that in six ongoing COG clinical trials, such assessments are used to determine neurocognitive outcomes over time.
Adamson added that in order for measures to be incorporated into research protocols, they have to play a role in answering a specific research question. “You don’t just start collecting the data and then later figure out the research question related to those data,” he said. “The primary question in any research study is certainly the most important. The list of what else people want to ask goes from here to down around the block, with the potential to do lots of correlative science and hypothesis generating. Many of those questions are good questions, but many do not garner funding, and unfortunately the funding we now receive truly limits what we can ask.” He pointed out that COG has experienced a 30 percent decrease in its funding over the past decade. “We cannot do all of these important initiatives unless we convince Congress that they are important initiatives. The only way we are going to instill a sense of urgency is if we get the community together to say this is unacceptable,” Adamson stressed.
Sung suggested mandating that PRO endpoints that are included in clinical studies be analyzed and published, noting that often they are not. Among the 16 COG clinical trials she mentioned previously that included quality-of-life aims, only 7 successfully accrued enough patients to complete the data analyses. Only 56 percent presented their findings at a conference, and only 38 percent were published in peer-reviewed literature. “When we do research and don’t disseminate the knowledge we gather, we do a disservice to families and patients who have generously donated their time and effort,” she said.
Complementary to Sung’s findings in the academic realm, TheodoreOklata noted that PROs are lacking in pediatric trials conducted by pharmaceutical companies. “As an industry we have mostly failed in the last several years at both collecting patient-centered outcomes in pediatric
___________________
8 Assessment via tests that measure neuropsychological and behavioral functioning, for example, thinking, learning, and remembering.
clinical trials and disseminating these data post-trial,” she said, adding, “These gaps in understanding patient reports in pediatrics and how behind we are compared to the adult cancer world are kind of scary. We have often not asked patients the questions. If we did, we asked too many items. We asked the wrong person to tell us about treatment effects. We did not ask long enough to really understand the long-term effects, and did not share what we found in accessible ways, so patients and families can make an educated decision about their treatment when comparing potential options. But acknowledging what we have not done is the best way to lay a solid foundation to do things right and even better moving forward and to turn this challenge into a tremendous opportunity.” Bucci-Rechtweg agreed, stressing that “what industry needs to do is work closer with children and families, advocates, investigators, and regulators to understand what domains are relevant to facilitating early drug development that can help us to make regulatory decisions. It is so important to collaborate with all of our external partners.”
Sung also suggested bringing PROs to the patient bedside to improve patient–provider communication and alert providers to key distressing symptoms as well as to improve patient and family satisfaction. She noted that previously reported PROs can save time during clinic encounters and may improve symptom control and supportive care measures (Chen et al., 2013; Kotronoulas et al., 2014).
Wolfe added that parents want PROs so they can be more aware of what their children are experiencing. Wiener agreed, noting that although a child may be grumpy and showing other signs of distress, when parents ask them how they are feeling they often respond that they are “fine.” But PRO questionnaires can elicit more detailed and descriptive information, for which parents are grateful. “Parents are desperate to really find out how the child is doing,” Wiener said. Hinds agreed, noting that parents appreciate when the child’s voice is respected; it helps foster a more trusting relationship with the clinical care team when PROs are used (Coyne and Gallagher, 2011; Mack et al., 2011; Miller and Jawad, 2014; Runeson et al., 2002). She stressed that “the child’s voice is crucial because without it we cannot know the full impact of our protocol-driven therapies and we cannot know the full impact of our nursing, medical, and psychosocial care.” However, Hinds also noted that there are circumstances in which inviting the child
voice might disrupt family boundaries, in which case practitioners should seek permission from those parents first before inviting the child voice and reinforce that the parents will still have their say. “What I don’t want to see happening in care, particularly at end of life, is that we usurp the parent role,” she said.
Hinds suggested explaining the importance of self-reporting to children and parents and how the clinician team will respect and honor their PROs. “We need to be able to measure the child and parent voices consistently at meaningful points. We should not settle for just embedding a child voice measure in a clinical trial. We should purposefully link it to trusted endpoints and to make important clinical interpretations that guide care,” Hinds said. Reeve agreed that PROs should be collected routinely from children and their caregivers and integrated into the health care delivery system, with the information used to both inform clinical research and improve clinical care.
The State of the Science for Pediatric PROs
Hinds noted several milestones in the past 15 to 20 years that are moving the field of pediatric PROs forward, especially in oncology. Studies have demonstrated that it is feasible during cancer treatment to solicit the child’s perspective on symptoms, function, and quality of life. In addition, research shows that children are willing to report from the moment of diagnosis until completion of treatment or death, and that parents are willing to have their child report once they understand the importance of the child voice and how it will be used (Hinds et al., 2013). In the limited number of pediatric clinical trials in which PROs have been embedded, parent and child participation rates are higher, according to Hinds. But only rarely in pediatric oncology is the child’s voice linked to care outcomes and endpoints, and even more rarely is the child’s voice measured at the end of life, she added (Hinds et al., 2007).
Hinds noted that researchers have developed a small number of pediatric PRO measures that can be used during cancer treatment. These measurement tools include those that children and adolescents can use to rate their fatigue or nausea, a more comprehensive PRO tool called the Patient Reported Outcomes Measurement Information System (PROMIS) (see Box 5), which Hinds helped to develop, and the Pediatric Patient-Reported Outcomes Common Terminology Criteria for Adverse Events (PRO-CTCAE) (see Box 6).
BOX 5
Patient Reported Outcomes Measurement Information System (PROMIS)
Pamela Hinds, director of Nursing Research and Quality Outcomes at the Children’s National Health System, described PROMIS, which she developed with others and with support from the National Institutes of Health Common Fund Initiative. Items measured in the system were gathered from a literature review and from focus groups with well children; children with chronic illnesses such as sickle cell anemia, asthma, and juvenile arthritis; and children with acute illnesses such as cancer. Parents were also part of these focus groups. The system is publicly available, not disease specific, and designed for children ages 8 to 18 (Quinn et al., 2014; Varni et al., 2014, 2015). So far, researchers have clinically validated a number of the measures used in PROMIS, including those for emotional distress, pain interference, fatigue, physical functioning, and peer relationships. Others are currently being evaluated, including those that measure stress, pain behavior, intensity, and quality, family belongingness, and subjective well-being, as well as parent measures.
A cross-sectional study of PROMIS found that it was feasible to use and that the measures were acceptable for children during acute care treatment for cancer and into survivorship, Hinds reported (Menard et al., 2014). A second study, which conducted the measures in the system at three points that spanned the course of chemotherapy in the same children, found the sensitivity of the measures did not change as the children grew older and most progressed to survivorship (Hinds et al., 2013). A third study embedded the PROMIS pediatric measures in a Phase I
Sung suggested defining and validating a core group of pediatric PRO symptoms and functions that should be routinely measured. A core set of PRO measures would enable researchers to compare data across studies and disease groups and would provide a rich foundation for meta-analysis and discerning trends over time, she said. Developing such a core group would require the input of patients, parents, COG, NCI, and other funders, she added. “If we are designing randomized trials with PRO endpoints, we need to understand what the smallest changes are in fatigue, pain, and quality of life that matter to patients and their families,” she said. Mary Brigid Bradley-Garelik, director of oncology global clinical research at Bristol-Myers Squibb, added that the
clinical trial and demonstrated the acceptability and feasibility of asking a child whose cancer was incurable to report on symptoms and function at two different time points prior to knowing the status of response to the experimental agent. “The level of participation in this study rivals and exceeds previous reported levels of participation with more traditional pediatric oncology self-report measures, and very few of our measures were not completed,” Hinds said.
Additional analyses of PROMIS data revealed a subset of children who reported a high degree of symptoms prior to beginning treatment and continuing through treatment. “There is a subset of children and adolescents who suffer greatly across all measurement points,” Hinds noted. “We need to know who they are.” She discovered another subset that reported a low degree of symptoms throughout the entire study (Buckner et al., 2014). “We need to know who these children are, too. If we can identify subgroups early in treatment we will match our clinical care, whether it is supportive or other forms of care, to do better by them,” Hinds said. She also pointed out that some patient-reported outcomes used in PROMIS might predict treatment response. She found that those children who indicated they had been very tired prior to starting chemotherapy consistently reported a high degree of symptoms throughout the study, and this might have some prognostic value.
Noll said that in his studies, children’s PROMIS self-reports about their social issues did not correlate with the reports from their peers. “The peer PROMIS dimension is more about social self-concept, but not about peer relationships,” he said.
SOURCE: Hinds presentation, March 10, 2015.
pharmaceutical industry would appreciate having a core set of clinically validated pediatric PROs.
Sung also suggested developing PROs that can be used in adolescent and young adult (AYA) oncology for patients between ages 15 and 40. “We don’t know if AYA patients suffer disproportionately because we don’t have adequate instrumentation to be able to answer this question. Almost all pediatric instruments go up to age 18 and almost all adult instruments start at age 18 and go up,” Sung said. Reeve agreed, noting the importance of having PROs that are tailored to developmental stage. “We need to make sure we have metrics to follow that child from a very young age through adolescence and to a young adult stage,” he said.
BOX 6
Pediatric Patient-Reported Outcomes Common Terminology Criteria for Adverse Events (PRO-CTCAE)
Bryce Reeve of the University of North Carolina at Chapel Hill reported on the multisite study of the pediatric PRO-CTCAE tool he helped to develop, which children and adolescents can use to report their symptoms while undergoing cancer treatment. To develop this tool, pediatricians across all seven participating sites identified which of the more than 790 types of adverse events listed in the Common Terminology Criteria for Adverse Events are prevalent and clinically relevant for children undergoing cancer treatment. This resulted in a list of 16 core terms as well as 47 more terms that were translated into words a child would understand. “Abdominal pain” was translated into “stomach pain,” for example, and “urinary incontinence” was translated into “wet yourself by accident.” The list also included psychosocial measures such as depression and anxiety.
Researchers designed PRO-CTCAE to be used within an electronic system. Children can use a tablet or laptop in the office, in the hospital, or at home to report their symptoms. Information is translated instantly into the traditional grading system used for adverse events and transmitted to the clinician, who can use it to make a more accurate assessment of these adverse effects. “We assume that this will not only improve their toxicity rating accuracy, but also help them respond to the needs of the
Reaman said there are several challenges to developing PROs for children with cancer, including making them comprehensible, relevant, and age appropriate. There is limited success in using PROs as endpoints in oncology in general because of the potential confounding effect of medicines given simultaneously and the requirement for blinded and randomized trials, Reaman added. He stressed that PROs need to be objective and have quantifiable measures. Noll agreed, noting that a response such as “I feel good today” is not a measurable response. Reaman noted that function is probably more quantifiable and is something that FDA has an interest in seeing reported in clinical trials. “I would explore functional assessments, which in the long run are as important, if not more important, with respect to quality of life,” he said. Reeve agreed on the necessity to identify core symptoms and functional impacts.
Reaman and several other speakers also suggested more stakeholder coll-
particular child. We are not replacing the clinician’s job at making that grading, but we are helping to inform their decision about the toxicity grading overall,” Reeve said.
All information gathered is instantly entered into the child’s electronic medical record and integrated with their clinical and biological data. A dashboard summarizes results in a meaningful way and creates automated alerts or triggers when the child is experiencing serious events, such as grade 3 pain that needs immediate attention. The system can also provide treatment recommendations based on guidelines and make other referrals as necessary. All the data collected from the multiple participating hospitals are stored at a central data warehouse. “This gives us a unique opportunity to use collective and aggregated data and to make quality improvement initiatives based on those data. We can also do comparative effectiveness research building on this aggregated dataset,” Reeve said.
He stressed that the system builds on the 2009 National Cancer Policy Forum workshop report that described the benefits of a rapid learning system for cancer care and delivery (IOM, 2010). “Although this report doesn’t refer to pediatrics, I think we can use it as a model to think about how we can integrate this into the pediatric setting to help rapidly use data for care and for comparative effectiveness research,” Reeve said.
SOURCE: Reeve presentation, March 10, 2015.
aboration in developing PROs for children with cancer. Sung agreed, noting that “COG is completely siloed from the research conducted in pediatric oncology practices in the community as well as from the adult oncology community, from which we have a lot to learn, and from industry. There’s a major need to bring together like minds to be able to learn what the best approach is to go forward, and industry is a tremendously important partner in this initiative.” Theodore-Oklota added, “Industry has to work proactively with the cooperative groups, academic centers, clinicians, and patient advocacy groups to make sure we are getting a very robust collection of individuals contributing to what they think is important to be measured on a trial.”
Adamson said other stakeholders should also be included, such as regulators from the European Medicines Agency and members of Congress. Bucci-Rechtweg pointed out that when forging agreement on what core PROs should be gathered, there should be recognition for the different
ways PROs are used in industry trials versus clinical care. A drug company wants to use PROs in its clinical trials to aid its assessment of whether a drug should proceed down the clinical testing pipeline, whereas clinicians and payers may want to see PROs more indicative of the costs and benefits of a treatment. Bucci-Rechtweg also suggested that companies share the tools they are developing to measure pediatric PROs and that there be other opportunities for cross-portfolio collaboration. Companies are generally reluctant to share information or tools with their competitors, but might do so if given the proper incentives, such as expediting review time lines of their products by FDA. “We need to think about opening pathways for early discussion across companies,” she said.
Tina Shih, professor of health economics and chief of the Section of Cancer Economics and Policy in the Department of Health Services Research at the University of Texas MD Anderson Cancer Center, stressed that the PROs collected in clinical studies often cannot be used for cost-effectiveness analyses because of missing cost data and health utility9 information. She added that payers tend to look at short-term costs and want to see budget impact modeling rather than a lifetime cost-effectiveness analysis. “To provide the evidence that payers need, we have to collect the kind of data they are looking for and run the kinds of analyses that would be helpful to make decisions,” Shih said. But Sung responded that it can be difficult to collect the health utility information that payers need in pediatric studies. “It is a cognitively heavy task,” she said.
Sung noted that FDA states in their guidance on PROs that “for patients who cannot respond for themselves, we encourage observer reports that include only those events or behaviors that can be observed” (FDA, 2009). It is not always feasible for children to report their own outcomes, she pointed out, especially young or acutely ill children. Should parents be their proxies in these instances? She said studies show that clinicians’ reports correlate poorly with pediatric patient reports, so they would not make reli-
___________________
9 Utility is a term used by economists to signify the satisfaction accruing to a person from the consumption of a good or service. This concept is applied in health care to mean the individual’s valuation of their state of well-being deriving from the use of health care interventions. See http://www.nlm.nih.gov/nichsr/edu/healthecon/glossary.html (accessed June 15, 2015).
able proxies. She said there may be moderate to good agreement between patient and parent reports when the PedsQL Quality of Life Inventory is administered. However, systematic reviews indicate that differences are also frequently observed between parent and pediatric patient reports (Jardine et al., 2014; Upton et al., 2008). She added that there is debate about whether those differences are clinically meaningful and whether parents tend to overestimate or underestimate their child’s quality of life.
Instead of using parents as proxies for children who are too young or sick to report outcomes, observed events or behaviors such as crying or not eating could be recorded, as FDA suggests. But Sung noted that children with cancer cry for many reasons other than pain, so crying might not be a good surrogate for pain. Children also often do not eat for reasons other than the effects of their cancer or its treatment, as can be seen in normal children who are picky eaters, she added.
Another alternative is to create simple symptom screening tools for young children. But the simpler the rating system, the less sensitive it is for detecting differences that might be important to the child. Accessing these children to apply the screening may also not be possible, Sung noted. She concluded that for children who are too young or sick to self-report, there is a role for parents and guardians as proxy respondents, but also a need to identify feasible means for children self-reports to supplement those proxy reports.
Reeve pointed out that both PROMIS and PRO-CTCAE (see Boxes 5 and 6) have parent proxy reports as well as self-reports from young children and reports from clinicians. “We’re going to look at that triad of reports and see what types of symptoms parents and clinicians are better or worse at reporting and understanding what the child is experiencing,” he said. He added that probably for the more observable type of symptoms, such as nausea and vomiting, reports from parents and clinicians will tend to agree with the child reports, while there will be less agreement for the psychological types of symptoms. Reeve said these studies will indicate whether parent or clinician reports can be relied on when children cannot convey reports themselves.
Reeve is currently studying PRO-CTCAE only in children ages 7 and older, not in younger children. “As we start to move into the younger age ranges, we may change the way we ask the questions and may use more graphic approaches such as happy and sad faces. However, there is a certain age range where the child may not be able to self-report, so we will have to rely on the parents’ reported data,” Reeve said.
Reaman suggested there may be a role for combining PROs with observable outcomes. He stressed that FDA guidance on PROs was developed for adults, but that the agency is interested in developing pediatric PROs not just for children with cancer, but for children with inflammatory bowel disease and other disorders. “There’s an opportunity for us to be creative and merge or integrate proxy-reported outcomes with PROs,” he said.
A portion of the workshop was devoted to presentations on the long-term effects of cancer treatment and how to prevent and screen for them. Bhatia detailed the long-term physical consequences. “When we describe the long-term sequelae in childhood cancer survivors, we are talking about issues related to growth and development, impairment of vital organ function, fertility and reproduction, and secondary neoplasms, and the impact of all this on the quality of life of our cancer survivors,” Bhatia said. Researchers have linked specific cancer treatments to many of these late health complications of pediatric cancer, as indicated in Figure 3. For example, the steroids and
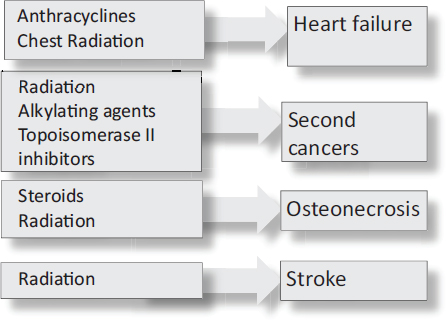
FIGURE 3 Therapeutic exposures and adverse events. Specific cancer treatments have been linked to specific long-term health complications.
SOURCE: Bhatia presentation, March 9, 2015.
anthracyclines used in cancer treatments can cause the death of bone tissue, requiring hip replacements at a young age, or enlarged hearts that cause early heart failure. Radiation therapy in pediatric cancer patients can cause major musculoskeletal deformities or induce breast or brain tumors. Various chemotherapies can cause fatal leukemias, Bhatia reported.
Bhatia and Kevin Oeffinger, director of the Cancer Survivorship Center in the Departments of Medicine and Pediatrics at the Memorial Sloan Kettering Cancer Center, reported on the NCI Childhood Cancer Survivor Study (CCSS) of more than 24,000 patients that has been ongoing since 1994 and involves 26 institutions across the country. The CCSS has demonstrated that nearly three-quarters of pediatric cancer survivors will have at least one chronic condition 30 years after they were first diagnosed with cancer, and the incidence of life-threatening or fatal conditions approaches 40 percent at this time (Armstrong et al., 2014; Oeffinger et al., 2006) (see Figure 4). “The burden of chronic health conditions in our childhood survivors increases as they move away from diagnosis,” Bhatia said. The substantial gap between mortality curves of childhood cancer survivors and that of the general population is also sobering (Oeffinger et al., 2006), Bhatia noted, concluding, “The implications of a cure are not trivial.”
Chronic fatigue was a problematic long-term side effect of cancer treatment described by cancer survivor Melinda Marchiano and by Sandler, the parent of a cancer survivor. Marchiano said, “I don’t think there has been a moment in the 7 years since I finished my treatment for Hodgkin’s lymphoma when I haven’t been tired.” Sandler noted that recent Scandinavian studies have started to link chronic fatigue to survivorship of pediatric cancer. He said that prior to these reports, experts were dismissive that fatigue could be linked to surviving pediatric cancer, perhaps because survivors were not reporting it to their physicians and instead were assuming the fatigue was due to something else.
Patricia Ganz, Distinguished Professor at the University of California, Los Angeles (UCLA), Fielding School of Public Health, and Director, Cancer Prevention & Control Research, Jonsson Comprehensive Cancer Center, UCLA, noted that her studies of women with breast cancer found their fatigue linked to genetic variations in the promoter region for some of the pro-inflammatory cytokines. “We find that people who are fatigued before they ever start their cancer treatment continue to be fatigued afterward,” she said, suggesting there might be a biological cause and that risk may be biological. Ganz pointed out that early social trauma and deprivation might prime the hypothalamic-pituitary axis of some cancer patients
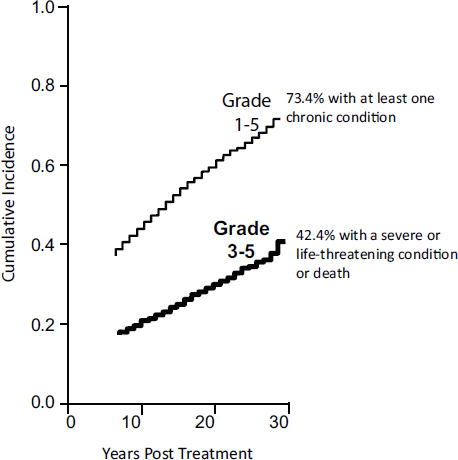
FIGURE 4 Chronic conditions in adult survivors of childhood cancer. The incidence and severity of chronic health conditions increases as childhood cancer survivors move away from diagnosis.
SOURCES: Oeffinger presentation, March 10, 2015; Oeffinger et al., 2006. Reprinted with permission from Massachusetts Medical Society.
for different stress responses to their cancer treatments than others. “This might explain why some patients given the same chemotherapy regime are very troubled by it, while others are extremely resilient and have few symptoms,” she said, adding, “We cannot just think about the symptoms and the self-report, but also need to connect them to the biology and use them to inform our interventions because the kinds of interventions we need to do for patients may vary depending on the mechanism by which they develop the symptoms.”
Others spoke of the long-lasting psychosocial effects linked to cancer treatment, including eating disorders as well as the fear of recurrence that persists long after the cancer treatment ends. For example, Marchiano said she developed a hatred of food due in part to stomach pain that she experienced after treatment, and she needed to undergo counseling to overcome her eating disorder. “That truly saved my life. I got down to 79 pounds at one point, so it was a very life-threatening time,” she said. Sandler noted that his daughter, an adult survivor of pediatric leukemia, still has difficulties relating to her peer group. Lisa Schwartz, psychologist in the Division of Oncology at The Children’s Hospital of Philadelphia and assistant professor of pediatrics, University of Pennsylvania School of Medicine, reported on two studies of pediatric cancer patients that found they often had fears and anxiety about the cancer returning a year after treatment (Hobbie et al., 2010; Wakefield et al., 2012). But she said such fears are also still prevalent in long-term survivors. “It is astonishing how many of the long-term survivors I see at The Children’s Hospital of Philadelphia live with this intense fear that their cancer will come back, even if they are 10 years off treatment,” she said.
Researchers are gathering information to help predict which cancer survivors are likely to develop long-term complications, Bhatia reported. For example, studies show that the likelihood of developing heart failure after taking anthracyclines is related to the dose of the drug, with most people not experiencing the condition unless they received doses greater than 250 milligrams per meter squared (Blanco et al., 2012). But there is variability in the heart damage that occurs after anthracycline treatment, even when doses are kept the same, Bhatia noted. Some people develop heart failure after receiving a dose that is much lower, while others can receive higher doses and not develop the condition.
To identify the cause of that variability, Bhatia used a “molecular epidemiology” approach, in which she and her colleagues compared the RNA or DNA specimens from cancer survivors who developed late effects with those who did not. Her studies revealed genetic variants that protected patients or put them at increased risk of developing heart failure from anthracyclines (Blanco et al., 2012; Wang et al., 2014). When these genetic variants were added into a predictive model based on radiation and anthracycline doses received, the ability to predict which patients would
develop heart failure increased from 69 percent predicted by the clinical parameters alone to 80 percent when the genetic factors were included (Armenian et al., 2013).
Screening for and Preventing Complications
Oeffinger reported on ways to prevent or alter the course of late effects of childhood cancers. He noted two IOM reports on cancer survivorship (IOM, 2003a, 2005) that recommend monitoring survivors early on for recurrence, then continuing to monitor them for second or third cancers or other late effects “with an eye toward early diagnosis and intervention.” COG also established guidelines for screening childhood cancer survivors that are currently being harmonized with similar guidelines developed by other countries, he reported (SIGN, 2013; SKION, 2010; UKCCSG, 2005).
Both the IOM and COG recognize that general cancer prevention strategies are important for cancer survivors, Oeffinger said. Strategies include tobacco cessation or avoidance, physical activity, and appropriate calcium intake. Counseling and education of survivors is also important so they seek the follow-up care they need (Oeffinger, 2002; Oeffinger and Hudson, 2004). But some survivors, such as women who were treated for Hodgkin’s lymphoma with chest radiation, need more targeted screening measures. Studies have found that one-third of these women develop breast cancer by age 50, a much higher incidence compared with the general population (4 percent) and comparable to the risk experienced by women with a BRCA1 mutation. As a result, COG and the international harmonization group recommended they receive screening through annual mammograms and breast magnetic resonance imaging beginning at age 25, or 8 years after radiation therapy, whichever occurs later (Bhatia et al., 1996, 2003; Henderson et al., 2010a; Mulder et al., 2013).
“We are just dipping our toes into this field,” Bhatia noted, with researchers currently conducting studies of interventions aimed at preventing long-term complications in pediatric patients at high risk due to the treatments they received. For example, Saro Armenian at City of Hope is embarking on a Phase II clinical trial using carvedilol (a beta-blocker) to reduce the risk of anthracycline-related heart failure. Another Phase II clinical trial led by Bhatia will test whether low-dose tamoxifen can reduce the risk of breast cancer developing in women who received radiation therapy to the chest as young girls. Because it is known that young girls who receive
bone marrow transplants from unrelated donors have an increased risk of developing cervical cancer, another study led by Wendy Landier at City of Hope is assessing whether vaccination against the human papilloma virus is safe and immunogenic among cancer survivors.
When asked by Pizzo whether the information Bhatia and others are gathering can be used to predict and possibly prevent long-term effects from pediatric cancer treatments, she responded that was not always possible because of the new treatments being used in pediatric oncology that have unknown effects in the long term. “Usually these complications have a latency period that can be as long as decades. So to anticipate these issues can be a problem,” she said, stressing that this makes it imperative that “whenever we are introducing a new drug that we follow our patients long term and don’t let them out of sight. We don’t say that you are done and release them into the world without follow-up.” She said current findings might be used to create a clinical profile or gene signature for patients who are especially vulnerable to certain long-term complications and should be followed aggressively. Ganz also stressed that a national registry is needed to detect the rare long-term complications from cancer treatments.
Several participants noted the value of a national registry of cancer survivors that enables such patient follow-up as well as research on long-term complications of cancer treatments. Bhatia noted that within COG, Adamson initiated Project Every Child, which is enrolling every child with cancer treated in COG trials into its registry along with collecting and storing the child’s biospecimens. The registry documents the therapeutic exposures of these patients, and researchers can use the clinically annotated specimens to study the risks of and susceptibilities for complications linked to certain therapies. But she added that it would be helpful if the United States could follow the Scandinavian model of linking all of its registries.
Richard Aplenc, section chief for hematologic malignancies at The Children’s Hospital of Philadelphia and associate professor of pediatrics at the University of Pennsylvania, suggested working toward the development of a comprehensive, integrated data registry for children with cancer, which will involve engaging patients, families, health care providers, data holders, researchers, regulatory agencies, and funding agencies. “It is difficult to overstate the importance of everybody being willing to contribute data and to work out the regulatory and ethical processes and safeguards that need to exist for this sort of work to happen,” he said, pointing out the significant privacy concerns that are raised when data are shared in a national registry.
Childhood cancer survivors and their families go through many care transitions during the course of the care continuum, including transitioning from acute care to follow-up care, and from follow-up care to survivorship care, with the transition from pediatric to adult care occurring at some point. Several speakers pointed out the frequent shortcomings of these care transitions and ways to improve them.
Transitioning Off Treatment to Follow-Up Care
Schwartz said patients and their families struggle with the transition off treatment and into follow-up care. At this point in the care trajectory, patients can potentially become lost to follow-up care, but there are still opportunities to keep patients engaged. She said that young cancer survivors need interventions that bolster their motivation for follow-up care, and that patients, parents, and providers, including general practitioners, need better education about what that care involves.
Many children and their parents find that when the children’s treatment has ended and they transition into follow-up care, their lives do not return to normal because of lingering psychological or physical problems. A review of studies comparing children transitioning off acute care with their healthy peers found that these children often had lower psychological well-being, reduced physical functioning, sleep and behavioral problems, social difficulties and isolation, and reduced self-esteem. However, some studies found that children recovering from their treatments showed better behavior, higher self-worth, and improved social and emotional roles (Wakefield et al., 2010).
Qualitative data collected in these studies also showed mixed feelings, with some patients reporting feelings of joy and relief coupled with exhaustion, adjustment difficulties, and concerns about cancer recurrence. One patient quoted said, “I know I am supposed to be happy, but I am not. How do I know there isn’t a cancer cell floating around? I still have pain (in back and legs) and have to go to PT [physical therapy] all the time. I can’t keep up with my friends and feel so out of the loop. I am way behind in school and I feel exhausted after a few periods. Seriously, this sucks.”
Parents of children finishing cancer treatment also reported having feelings of relief mixed with fears of relapse as well as anxiety, posttraumatic stress, and physical and emotional exhaustion. But these parents reported
improvements over time, with some of those feelings returning when the child undergoes follow-up cancer screening (Wakefield et al., 2011).
In another study, parents reported concern about interpreting physical symptoms and whether they might indicate cancer recurrence after treatments end. They also reported wanting to stay connected to their oncologists, and needing information about survivorship care and long-term effects, such as infertility (Hobbie et al., 2010).
Based on these findings, Schwartz said, transitions from acute therapy to follow-up care should include education on dealing with the problems of daily management and information on diagnosis and ongoing precautions. She said families should also be offered a plan for the child’s off-therapy care and emotional support, and help in dealing with anxiety. Families indicated they needed easily accessible information, such as information booklets or websites, with support groups the least favored mode of intervention (Wakefield et al., 2012).
“Although we have some lovely, hypothesis-generating data, we really don’t have a good idea from rigorous research about what is happening in this time period off treatment,” Schwartz summarized. Ganz emphasized the need to integrate psychosocial care during transition, noting the two-way connection between the mind and the body and biological reasons to support the notion that one can affect the other. “Getting that mental health care early on is something we have to demand. It is not frivolous or extra care. It is part of taking care of the whole person,” she said.
Transitioning to Adult Follow-Up Care
Several speakers presented evidence that most pediatric cancer survivors do not transition adequately to appropriate long-term follow-up care despite the great need for such care, in which they are monitored and treated for the various ailments they are likely to experience. Schwartz cited a study showing that fewer than 30 percent of adult childhood cancer survivors are receiving cancer-focused follow-up care (Nathan et al., 2009). Bhatia said CCSS indicated that only about 35 percent of childhood cancer survivors are aware of being at risk for serious health problems due to their treatments. “This impairs their ability to seek or receive appropriate long-term follow-up care. Those of us who have children in their 20s know that they will never go to the doctor, even if their mothers have set up the appointments for them,” Bhatia said.
For example, despite the previously mentioned recommendation for
early breast cancer screening for women who received radiation therapy for a childhood cancer, one study of such women in the United States and Canada found that only 10 percent reported at age 30 that they had had two or more screening mammograms in the past 4 years. At any age, these women who had received chest radiation during childhood were not much more likely to undergo breast cancer screening regularly than their sisters, Oeffinger found (Oeffinger et al., 2009). Another study at St. Jude Children’s Research Hospital found that when adult survivors were brought back to St. Jude’s for health assessments, clinicians there often detected serious medical conditions, such as enlarged hearts or heart valve disorders, lung dysfunction, or breast cancer, that had not been diagnosed by their usual care providers (Hudson et al., 2013).
“Patients are often running around with undiagnosed and serious problems that have not yet been detected or are not symptomatic,” Oeffinger stressed. Bhatia added that most cancer survivors are seen by primary care providers, as opposed to oncologists or other providers at cancer centers (Oeffinger et al., 2004). “Primary care providers are primarily responsible, but they are unfamiliar with the problems faced by the childhood cancer survivors,” Bhatia said. “We need to increase the awareness of long-term complications among clinicians.”
Oeffinger reported on studies in which 75 to 80 percent of general internists and family physicians surveyed reported they had not seen a patient in the past 5 years whom they knew had childhood cancer, and 72 percent of internists and 48 percent of family physicians had never seen a cancer treatment summary (Henderson et al., 2010b; Nathan et al., 2013; Suh et al., 2014). “Many of these patients are floating around with their cancer history buried in their past medical history,” Oeffinger said. One of the studies gave these providers a hypothetical case of a young woman who had survived Hodgkin’s lymphoma and found that fewer than one-quarter recommended that this hypothetical patient undergo breast cancer and cardiac screening, although about three-quarters recommended thyroid screening. (All three types of screening should be done on these patients, according to COG recommendations.) Pediatric oncologists scored better on their recommendations, but still only 25 percent recommended that the patient undergo all three forms of screening (Henderson et al., 2010c; Nathan et al., 2013; Suh et al., 2014). “The pediatric oncologists are not
often aware of the specifics of what the risks are or what the recommendations might be,” Oeffinger noted.
Oeffinger stressed that primary care physicians might be more inclined to follow the COG recommendations for adult survivors of childhood cancer if they were more concisely and simply indicated in the survivorship care plan and the cancer treatment summary. “Primary care doctors will tell you that childhood cancer doesn’t even make it to the top 200 list of things they see in their offices, so there is no reason they will get educated about it other than to know what to pay attention to,” he said. He noted that several studies show these providers would like to see a cancer treatment summary and survivorship care plan if it is kept short, is in plain English, and clearly indicates what the physicians’ focus needs to be. These physicians also indicated that when issues come up with patients who are childhood cancer survivors, they would like to be able to communicate with the pediatric oncology team that treated them. Consequently, in his own oncology practice, he said, “my goal is to have everything on one page without using super small fonts and just provide the key information—the treatment given, its potential effects, the screening recommendations, and contact information for getting in touch with our group.”
Marchiano noted that when she went to see a primary care physician and told him she was a cancer survivor, his response was to merely congratulate her without considering the long-term health implications of that fact. When he did not adequately address the continual fatigue she was experiencing, her mother talked her into seeing a provider at a cancer survivorship program. Oeffinger noted that there are long-term follow-up programs at virtually every pediatric cancer institution now, so “If a patient is followed at a cancer center and they are not referred to a long-term follow-up program, that is not the standard of care.”
Bruce Waldholtz of the American Cancer Society suggested that there be prompts and drop-down menus in electronic medical records that indicate to the primary care physician that the patient he or she is seeing is a cancer survivor, and what special screening or care that patient requires because of the cancer history. “We need to standardize survivorship care and demand that it be part of the electronic medical record programs,” he said.
Bhatia also spoke about the need to educate patients and their parents about long-term complications and health promotion. She conducted a
study which showed that after receiving three annual educational sessions delivered at a survivorship clinic, survivors had a significantly improved awareness of their personal risks for nine potential complications of cancer treatment. But one-third of those survivors still did not correctly identify their health risks.
Marchiano also stressed the importance of educating patients before they transition from cancer treatment (see Box 7). “After treatment I was just thrown out into the world and I didn’t know much. There is such a lack of education and knowledge being distributed to survivors. Knowledge is power. It can empower survivors. I don’t have to let the cancer define me, but at the same time I can know and understand what my life is like now and what to look for,” she said. She added that “I am not informed in the sense that I don’t know what things can be helped and what things I am just going to have to live with—what things I should be seeking out medical care for, what I can take steps toward actually getting help with.”
Schwartz emphasized the importance of a treatment summary and survivorship plan for survivors and recommended education for patients, parents, and providers on survivorship care needs. The guidelines for such care also need to be up to date. But it is also critical to measure transition readiness when pediatric patients are transitioning to adult care, she said. That readiness does not just depend on acquisition of disease knowledge and skills, she added, “especially in oncology, where there is such an emotional and traumatic history for these patients and families and such a bond with the pediatric cancer center. There are a lot more complicating factors involved to get them ready to make that transfer to the adult health care system.”
Schwartz developed a comprehensive model of transition readiness for AYAs, and is developing a measure of transition readiness (Schwartz et al., 2011, 2013). Schwartz’s model includes preexisting factors, such as sociodemographics, culture of the family, health care access and insurance, medical status and risk, and neurocognition and IQ. These factors can influence more modifiable variables, such as disease knowledge, beliefs, expectations and goals, relationships, and psychosocial and emotional functioning. Providers and parents can target these modifiable factors to improve the transition process. When Schwartz asked patients, parents, and providers to prioritize which of those factors were most important to transitioning to adult care, parents and providers tended to rate relationships the highest, while patients tended to consider access and insurance the most important factors. “It was hard for parents and providers to let go, whereas for patients
BOX 7
A Pediatric Cancer Survivor’s Perspective

A pediatric cancer survivor’s perspective was provided by Melinda Marchiano, who was diagnosed with Hodgkin’s lymphoma when she was 13. She had chemotherapy and radiation. She finished treatment in May 2008, but found the transition off therapy to be difficult, both physically and emotionally. “I found survivorship to mean lying on the couch, not having the end of treatment anymore as my goal, and still struggling and feeling horrible and not knowing when it was going to end and how long I would have to wait,” Marchiano said.
She noted that during her treatment, an emotional numbness developed that enabled her to survive it, but once treatment ended, emotional issues that had been kept suppressed rose to the surface. Counseling helped her regain her emotional footing, she said, and stressed that this counseling was provided through the support of a private foundation.
She noted that she was not informed of what physical or emotional issues to expect after treatment or what screening measures she should have for late effects that might develop. Marchiano is currently a junior in college and has numerous activities, such as dance and volunteering at a pediatric cancer center. At the same time, she has not recovered from her chronic fatigue, confusion, and mental fog, she said. “In so many ways I am still waiting,” she said. “There have been moments when I have wondered, when I was lying in bed, if I had known what quality of life would be for cancer survivors, would I have fought so hard? Then there are moments when I get to see other childhood cancer patients and tell them ‘I made it through this and you can do it too.’ I want other survivors to be able to experience life after cancer and to be able to pursue their passion with a new perspective and without a cost of the cure,” she said.
it was more logistical, [that is], I will move on, but what is in my insurance and how do I make this transition happen?” she said (Schwartz et al., 2013).
In Schwartz’s study of young adult survivors of cancer with an average age of 27, 55 percent reported seeing an adult practitioner in the past 12 months for their cancer follow-up care. Half of those practitioners were primary care providers, about one-third were providers at a specialized practice such as an adult survivorship clinical or adult oncology practice, and the remainder had a shared model of care, attending both a specialized survivorship clinic and seeing a primary care provider for their cancer-related follow-up care. Nearly all who did not seek cancer-related follow-up care had a visit with a primary care provider.
Several factors predicted whether these young adults received follow-up care, including having private health insurance and low posttraumatic stress scores. A preliminary analysis found that those receiving follow-up care often reported that their parent was the primary decision maker for their health and that they had a high motivation to acquire such care. “Transition readiness is complex and patient motivation is key,” Schwartz said.
Schwartz is currently testing the use of a texting system to see if it can further improve the transition to follow-up cancer care within 1 year of treatment among cancer survivors aged 12 to 25. She and her colleagues are sending the participants daily text messages about general ways to stay healthy as well as more tailored information about what they should or should not be doing based on their cancer treatment history. Texts are also sent regarding goals they have set for themselves, such as reengaging in school or exercising more. The texts are designed to inform, motivate with goals and encouragement, and keep them engaged with their health care. Appointment reminders are also texted.
An analysis of the first 22 patients to complete the 16-week program indicated that most found the information to be understandable and the right amount, and that they would recommend the texting to others. Compared to a control group who merely received a booklet for cancer survivors, the participants who received the texts rated them easier to make time for, understand, and use, and the texting was also rated as having a greater impact on their physical health and quality of life. One participant wrote, “I actually really benefitted from everything I learned. I think more people should be concerned with what happens after cancer, and be told what is good or bad to do. When I finished treatment, I felt like I was just thrown back into the real world and felt really lost. I feel this whole study has been really helpful to me truly.”
In another study reported by Oeffinger, cancer survivors were randomly assigned to either a control group, which received a cancer treatment summary and survivorship care plan, or to an intervention group that received the treatment summary and survivorship care plan as well as a few calls from an advanced-practice nurse that were followed up with some reminders. Twelve months later, about half of those who received the more extensive intervention had an echocardiogram, compared to less than a quarter of those in the control group (Hudson et al., 2014). Similarly, an ongoing study aims to identify what information provided to childhood cancer survivors will better enable them to seek the breast screening they require.
Schwartz and Oeffinger both stressed that education and communication about follow-up care has to be tailored to the developmental stage of the patient. “Our information needs to change over time as their development changes over time,” Schwartz said. She also suggested helping parents teach their children about their treatment history “and not have the parents be the sole keepers of that history.” Oeffinger added, “Remember the patient is a child growing into an adult. They have different needs at different time points, and so do their parents. As they separate out, keep that communication going. Even if they can’t come back to a specialized long-term follow-up program, they should have a basic knowledge of what they need.” Noll added that young adults are in a developmental stage in which they generally are not aware of or wish to pay attention to their health care needs. “The challenges for cancer survivors get amplified by the normal developmental stages they are going through,” he said.
Young adult cancer survivors may also have insurance issues that prevent them from acquiring the follow-up care they need, Oeffinger pointed out. He expects the Affordable Care Act will increase the number of survivors with some level of insurance coverage, and it will prevent health insurers from denying coverage to survivors because of preexisting conditions. However, most pediatric cancer survivors need more intensive screening for breast cancer, secondary cancers, and other conditions for which insurers may not provide sufficient coverage. Although insurers must reimburse for preventive measures recommended by the U.S. Preventive Services Task Force, most survivors of childhood cancer do not fall in the age categories covered by those recommendations, and the Task Force is unlikely to issue recommendations specific to this population, Oeffinger noted. Even when the services they receive are partially covered by health insurance, out-of-pocket expenses can still be prohibitive because pediatric
cancer survivors need to see an assortment of specialists on a regular basis for the rest of their lives. “We will have to think outside of the box and consider what sort of ‘medical home’ might be appropriate for childhood cancer survivors,” he said.
Oeffinger noted the impracticality of pediatric oncologists providing long-term follow-up care for their patients who survive into adulthood. Instead he suggested a risk-stratification approach formulated by colleagues in the United Kingdom. These researchers stratified cancer survivors based on what treatments they received, and follow-up care was tailored to risk. The lowest risk group includes those who only had surgery or low-risk chemotherapy. An intermediate-risk group included those who had chemotherapy or low-dose cranial radiation. The highest risk group included patients who had radiotherapy and what they termed “mega therapy,” which would include bone marrow transplant. Patients in the low-risk group were seen by general practitioners and received reminders by mail or telephone to schedule an appointment every 1 to 2 years. For the intermediate group, follow-up care was led by a primary care physician or nurse, and patients were seen every 1 to 2 years. Those of highest risk were seen annually at a medically supervised late-effects clinic (Eiser et al., 2006; Wallace et al., 2001). “This makes sense because you are using your resources where they are most needed,” Oeffinger pointed out.
Oeffinger briefly described a risk-stratified longitudinal approach, developed in the United States, in which care is shared between the cancer center providers and the primary care physician, with a transition to the community in most cases (McCabe et al., 2013) (see Figure 5). Implicit in this model is the notion that “This is not a one-time hand-off of care because there is communication that should be ongoing, based on the risk of the individual. This model enables us to allocate our resources to those patients who most need them, especially in an economically constrained environment,” Oeffinger said.
Many moderate to large cancer centers now have or are currently developing clinics focused on long-term follow-up and late effects, Kupst said, noting that at these specialized centers, pediatric cancer survivors are seen into their 40s or even longer. “Many survivors want to continue to come back to these centers,” she said, although most eventually transition to seeing an internist. She added that sometimes staff from the clinic where the
patient received cancer treatment will work with providers at the survivorship clinic to help ease the patient’s care transition. More research needs to be done to assess which care models work best, she added. But many cancer survivors do not live near a large institution that offers specialized survivorship care, so they are seen by internists. To aid their care, Kupst suggested working with adult internal medicine or family medicine organizations to ensure education in survivorship care for these providers. Pizzo agreed, but added, “It is not just physicians. We ought to be looking at the role of nurses, nurse practitioners, social workers, and others who can really collaborate in making sure these transitions occur.”
Schwartz summarized the potential pros and cons of receiving survivorship care at a cancer center, in a community setting, or in a shared care model (Friedman et al., 2006; Singer et al., 2013) (see Figure 6). But she asserted, “We really don’t know much about best practices. We can hypothesize that there are pros and cons of these care settings, but we really need more recommendations in this area. There may not be a one size fits all.” According to Schwartz, current recommendations stress (1) that transition must be well organized and coordinated, (2) that there is a treatment summary and survivorship plan for these patients, and (3) that education, including up-to-date guidelines, needs to be provided to patients, parents, and providers on survivorship care and needs. There also should be some assessment of readiness for transition so that patients can become more autonomous in their care and engage in long-term follow-up, she added (Friedman et al., 2006; Singer et al., 2013).
Building the workforce for survivorship care was also discussed at the workshop. Oeffinger said both pediatric and adult oncology workforces are already overwhelmed and insufficient for the number of cancer survivors needing care. In addition, he said primary care physicians have too many preventive health care issues to tend to within short patient visits so they are not likely to fill the survivorship care gap. He suggested making greater use of advanced-practice nurses for this care.
One participant said her daughter, a survivor of childhood cancer, had trouble getting referrals from her pediatric cancer care facility to cardiologists and endocrinologists knowledgeable about the long-term follow-up care needed for patients like her daughter. Consequently, her daughter received the wrong heart medicine, which caused lymphedema. Her daughter also had inadequate medical care during pregnancy, and as a result, she gave birth prematurely. “It is because of inexperienced doctors that don’t know how to treat these kids that more health problems are added to their
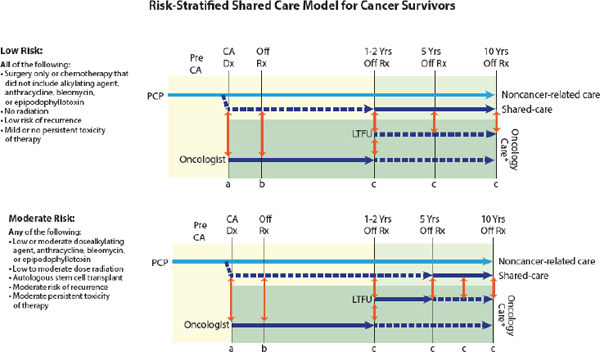
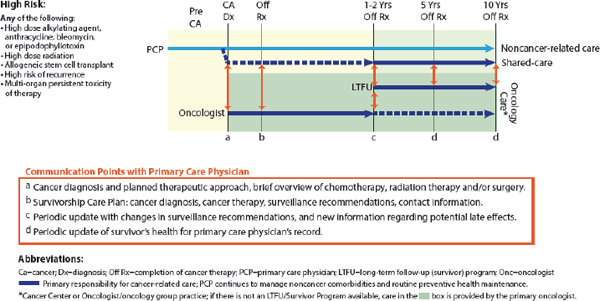
FIGURE 5 Risk-stratified shared care model for cancer survivors. In this model, cancer survivors were stratified based on their cancer treatment and thus their risk of developing late health complications. Follow-up care was tailored to risk level.
SOURCES: Oeffinger presentation, March 10, 2015; McCabe et al., 2013. Reprinted with permission from Elsevier.
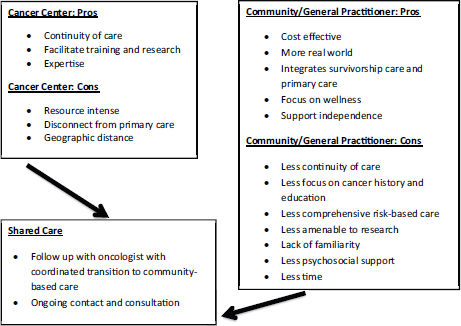
FIGURE 6 The pros and cons of survivorship care models. The potential pros and cons of receiving survivorship care at a cancer center, in a community setting, or in a shared care model are outlined in each box.
SOURCES: Schwartz presentation, March 9, 2015; Friedman et al., 2006; Singer et al., 2013.
plate. It is not a case of her not being empowered because she couldn’t find the right doctors,” this participant said.
Oeffinger agreed that patient empowerment is not sufficient to ensure appropriate care if there is a lack of specialists, such as cardiologists, with expertise in the medical issues that survivors have to confront. He suggested that high-risk patients be seen in specialty clinics for cancer survivors. These clinics employ physicians with expertise in the issues these survivors face and are growing in number, he said.
Ganz responded to this mother by saying, “One of the problems is we trust doctors and a lot of them don’t know anything about this.” She suggested getting second opinions and taking the time to find someone who is an expert in dealing with the rare late effects that pediatric cancer survivors experience. “We have to make sure that these survivors and their families are empowered to go somewhere where there is an expert who has seen [many] people with the condition in question,” she said.
Several gaps in knowledge were noted regarding transitions in the cancer care continuum. Schwartz suggested identifying the demographics, disease characteristics, and treatment profiles that confer risk or resiliency after treatment. “We need to understand who is going to adjust well after treatment, and who is going to continue to need more intensive services,” she said. She also called for research that will provide practitioners with a better understanding of patients’ and parents’ perspectives of care and concerns at the end of treatment. Research is also needed to identify best practices of care for pediatric patients as they transition off treatment and onto adult survivorship care, as well as best models of survivorship care. She added that research could identify factors that predict whether patients will seek appropriate care.
PEDIATRIC ONCOLOGY DATA COLLECTION AND REGISTRIES
Several examples of registries and databases for pediatric cancer were described at the workshop. Participants discussed challenges in pediatric oncology data collection, including how to integrate and store data in a central database that can be used by researchers and practitioners, and ways to measure poverty and incorporate socioeconomic status in clinical trials and link it to outcomes.
Integrating Databases for Research
The rarity of pediatric cancers makes it difficult to conduct research at individual treatment centers. Aplenc said that COG is the largest cooperative pediatric oncology group in the world, and the only organization that can engage enough participants to run a randomized clinical trial on as many as 1,000 children. However, he said COG does not have the resources to monitor all toxicities efficiently, identify costs and perform cost-effectiveness analyses, or conduct comparative effectiveness of treatments in clinical trials. To improve those capabilities, Aplenc was involved in an initiative that integrated and combined COG’s data with that of the Pediatric Health Information Systems (PHIS) database composed of data from 46 free-standing U.S. pediatric hospitals, including 6 hospitals reporting laboratory, radiology, and microbiology results, and the dates and charges for all the care provided to individual patients over time (Aplenc et al., 2012).
He and his colleagues have used the newly merged database to report toxicities more efficiently. Instead of relying on the traditional method for such reports, which involve data managers manually combing through the data to identify adverse events and then having them evaluated and graded by physicians, the researchers used the microbiology data in the system to report life-threatening strep infections that are frequent in leukemia patients. This method was able to detect 92 percent of the infections that occurred, with a false-positive rate of only 3 percent. This compares favorably with the sensitivity and false-positive rates of standard manual methods for abstracting the data, according to Aplenc (Miller et al., 2013). “This is very promising and we are in the process of thinking about how to expand this method to other laboratory values and beyond the six PHIS sites that collect laboratory data,” he said.
Aplenc also used the merged database to estimate treatment costs without needing to manually go through case reports and gather cost data. For example, his analyses have revealed no significant difference in cost in delivering an investigative drug in addition to standard chemotherapy versus standard chemotherapy alone because the room and board charges are twice the cost of pharmacy charges (Getz et al., 2015).
Large integrated databases are useful not only for research, but to improve care in a learning health care system, Reeve stressed. He suggested collaborations between electronic medical record vendors and the Office of the National Coordinator and other stakeholders to standardize what information is collected “so we have the ability to synergize our endpoints across different health care systems.”
Lori Minasian, deputy director of the NCI Division of Cancer Prevention, noted that the more data collected, the more difficult it might be to make sense of the data, “to separate the signal from the noise. How do we prioritize what we ask, how we ask it, and how do we integrate [the data] into other datasets? Often the data are collected for very specific purposes, which makes it complicated when you try to merge datasets,” she said.
Reeve responded that although there are symptoms specific to certain cancers or treatments, there are core symptoms common to all, such as fatigue and pain, and collecting data on at least these symptoms should be a priority. To avoid burdening respondents and collecting data that do not add pertinent information, Reeve also suggested using a dynamic system that does not collect details on a specific symptom unless the child indicates
it is present. For example, PRO-CTCAE will first ask a child if he or she is experiencing pain and will not offer more questions about pain unless the child answers affirmatively that pain is present.
Another participant raised the concern about the mental health variables collected and stored in registries. The validity of those measures and how they will be used by others could be problematic. Using a single self-report scale to measure depression is not sufficient to diagnose depression, he noted. “We need to be very careful in defining and measuring these things because of the unintended consequence of there being a breach of privacy and subsequent impact on the child,” he said. “The registry may indicate the child had depression, even though [he or she] had not been formally diagnosed.” Reeve agreed that caution is warranted in “how we frame and describe when a child self-reports symptoms that are associated with depression.” He noted that any symptoms of depression reported by a child would serve as a red flag in PRO-CTCAEs and could trigger a referral to a mental health provider, who would further evaluate the patient. Only a diagnosis of depression from such a provider would be entered into the child’s medical record. Noll added that one of the advantages to using a system such as PAT is that it is not linked to psychiatric diagnoses that remain in the children’s medical records.
Pediatric Proton Consortium Registry
Torunn Yock, director of pediatric radiation oncology at Massachusetts General Hospital and associate professor at Harvard Medical School, said that brain radiotherapy is known to affect neurocognition, hearing, endocrine function, and psychosocial abilities, and can also trigger secondary malignancies. Outside of the brain, radiation also has functional and cosmetic effects. Proton radiation is a new type of radiation that clinicians are increasingly using to treat pediatric tumors because it can more precisely target the tumor with a dose of radiation. Because proton therapy reduces the dose of radiation to normal tissues by a factor of two to three, it theoretically should reduce the incidence and severity of late effects seen with radiation therapy. But the data to support that claim are still sparse and spread out among various facilities. To better gather those data and use them in research aimed at assessing the long-term effects of proton therapy and how they compare to standard radiation therapy,
Yock and her collaborators developed the Pediatric Proton Consortium Registry (PPCR).10
PPCR is a repository to collect data on the acute and late toxicities of proton therapy. These data should enable comparative effectiveness research comparing proton radiation to other forms of radiation therapy. The registry uses a secure Web-based data collection system and online survey application called REDCap, which is funded and supported by the National Institutes of Health. The database includes information about patients’ baseline demographics, diagnosis, and health inventories, as well as details about radiation therapy, surgery, and other relevant treatment. A third category of information is follow-up data, such as the degree of disease control and late effects of treatment. All radiation plans and pertinent diagnostic imaging, both at baseline and at follow-up, are exported to a quality assurance radiation oncology center where COG houses all of its radiation data on its protocols, with the expectation that the database will facilitate research in the future with COG datasets, Yock said.
As of March 2015, 6 proton center sites are participating in the registry and have enrolled more than 700 patients. Six additional sites are expected to enroll in 2015. About 60 percent of patients enrolled have brain tumors. PROs and parent proxy reports are expected to be incorporated into the database starting in 2015, Yock said. Information related to socioeconomic status might also be added to the database. A long-term goal is to automatically extract data from electronic medical records, Yock said. Bridges could be created to other datasets as well, such as billing data, that could expand the usefulness of the data for comparative effectiveness and cost-effectiveness research as well as for research that links PROs to outcomes. Yock would also like to incorporate a blood and tissue bank component to the registry that researchers can use to make genetic correlations between the radiation received and outcomes and adverse effects. The database was started with funds from Massachusetts General Hospital and NCI, but will need additional funding sources to be sustainable, she said.
Yock stressed that pediatric oncology registries such as PPCR may offer a low-cost way to leverage more information out of existing trial datasets, such as those from COG trials. They might also incorporate PRO measures more easily than traditional treatment trials working with constrained resources.
Bhatia asked Yock how follow-up of patients is ensured with PPCR, given that the patient population is mobile and difficult to track. Yock
___________________
10 See https://clinicaltrials.gov/ct2/show/NCT01696721 (accessed May 18, 2015).
responded that e-mail addresses are gathered from parents and patients. She added that when patients turn 18, the reconsenting process provides another opportunity to acquire their e-mail addresses. “Our hope is to use the REDCap function to send out a basic but important health questionnaire asking about any new surgeries, new health diagnoses, and current medication list, etc.,” Yock said.
Measuring Poverty and Its Impacts
Researchers have noted for some time that black children with leukemia tend to fare worse with treatment in COG trials than white children. Aplenc was able to use the PHIS-COG merged database to discern which of many possible factors are responsible for this health disparity. He said there are many possible drivers, including differences in access to care, concomitant illness, disease characteristics, or treatment responses. Using data from the merged database, he discovered that the factor most responsible for the different outcome in black children compared with white children was the greater likelihood of whites to have private insurance and to receive more care in an intensive care unit (Kavcic et al., 2013; Maude et al., 2014). Such a study could not be done using COG data alone because these data do not include how sick patients are when they first enter the hospital, information that was critical to the analysis the researchers conducted, Aplenc explained.
Kira Bona, instructor in pediatric hematology/oncology and St. Baldrick’s Foundation Fellow at the Dana-Farber/Boston Children’s Cancer and Blood Disorders Center, described measures of poverty prevalence that can be used to screen for at-risk families. “Measurement of social determinants of health in pediatric oncology represents a significant gap in our research base and a potential opportunity for significant improvements in children’s outcomes,” she said. She noted there are limited data regarding social determinants of health and clinical outcomes in pediatric oncology, although poverty has been correlated with negative health outcomes in pediatric primary care and subspecialties, including higher rates of hospitalization, poor health, injury, and infectious disease (Bloom et al., 2011; Cook et al., 2004, 2008; IOM, 2014; Singh, 2010; Yoo et al., 2009).
Poverty can be measured in several ways, Bona said, including household income data that can be derived from U.S. Census measures linked to zip code. Income as a percentage of the federal poverty level is often used as a standard measure. Alternatively, there are measures of material
hardship,11 including a lack of basic needs such as food, housing, and energy, which she said can be assessed by asking fewer than 10 questions within 2 minutes, a task that can be done in most clinical settings. In 2013, about one out of every five children in the United States was living in poverty as defined by household income (Annie E. Casey Foundation, 2015), and the same percentage were found to live in households experiencing food insecurity (USDA, 2015).
Recent publications have demonstrated that childhood cancer treatment is financially costly for families, with at least one in four families reporting losing more than 40 percent of their annual household income due to their child’s cancer treatment (Bona et al., 2011, 2015; Eiser and Upton, 2007; Warner et al., 2014). Other studies show that family poverty is linked to poorer adherence to oral chemotherapy regimens and lower overall survival for children with cancer (Bhatia et al., 2014; Bona et al., manuscript in preparation; Gupta et al., 2014; Lightfoot et al., 2012).
Bona argued that these findings are significant because poverty can be targeted by various measures, including federal and state subsidies for food, housing, and energy. For example, research shows that food subsidies can decrease the risk of children being underweight or in poor health; public housing subsidies can reduce the risk of undernutrition in children; and subsidies for heating bills can decrease the odds of children being hospitalized or having a nutritional deficiency (Black et al., 2004; Frank et al., 2006; Meyers et al., 2005). Simply screening for material hardship in a clinic-based setting increased the number of families receiving these subsidies and subsequently improved child health outcomes (Garg et al., 2015).
Despite the link between poverty and health outcomes in children, there is no systematic collection of measures of poverty in pediatric cancer clinical trials, Bona noted. When data on poverty or other social determinants are collected for studies, they often are of nontargetable factors such as zip codes or degree of parental educational attainment as proxies for household income levels, Bona added. Given that most children with cancer are treated within a clinical trial, she suggested collecting poverty data and linking it to overall survival, event-free survival, relapse rates, acute and late toxicities, and quality of life.
Her studies of poverty among children with leukemia found that those
___________________
11 These measures employ direct indicators of consumption and physical living conditions to examine whether families meet certain basic needs. See http://aspe.hhs.gov/hsp/materialhardship04/execsum.htm (accessed June 16, 2015).
children living in high-poverty areas had significantly lower overall survival rates than children living in low-poverty areas. A subanalysis revealed that children living in high-poverty areas were more likely to experience early relapse, which is often difficult to treat successfully (Bona, 2015). Another study of material hardship in families of children with cancer found that 20 percent of newly diagnosed families reported food, housing, or energy insecurity in the 6 months prior to the child’s diagnosis of cancer. Despite meeting with social workers who could help them address their concrete resource needs when the child first began treatment, families experiencing material hardship increased from 20 percent to 30 percent 6 months later, with 25 percent of the families reporting annual income losses of more than 40 percent in those first 6 months of chemotherapy due to their child’s treatment. Bona also found that 38 percent of families whose children received bone marrow transplants reported in the 6 months post transplant that they had experienced food, housing, or energy insecurity. In this study, children in homes with material hardship had an increased risk of graft-versus-host disease compared to those children living in homes without material hardship (Bona et al., manuscript in preparation).
“These are data suggesting poverty may be mediating some of our pediatric oncology outcomes,” Bona said. She said material hardship is a targetable measure that is feasible to collect, remediable with targeted interventions, and correlated with general pediatric child health outcomes. Material hardship is more prevalent in the patient population than many of the histological or molecular subtypes of cancers currently targeted with chemotherapies, she noted. “Poverty crosses all histologic subtypes of cancer and all risk group stratifications. Unlike molecular targets, this is something widely prevalent in all of our families and is likely driving disparities in outcomes, which we can do something about,” Bona said.
She suggested engaging in systematic and standardized collection of sociodemographic variables in clinical trials that can be linked directly to outcomes of interest. “This is essential if we are going to target and improve this potential driver of outcomes,” she said, adding, “Integrating systematic screening for material hardship in the clinical setting can happen immediately, and it is an opportunity for targeted intervention for quality of life for families because regardless of its relationship with relapse or overall survival, material hardship in families of children being treated for cancer is certainly a source of suffering we can do something about.” Just a few questions need to be added to questionnaires to ascertain the various forms of material hardship, as indicated in Table 2.
TABLE 2 Operational Definitions of the Various Forms of Material Hardship
|
|
|
| Material Hardship | Criteria |
|
|
|
| Housing insecurity |
|
| Food insecurity |
|
| Energy insecurity |
|
|
|
|
SOURCE: Bona presentation, March 10, 2015.
Bona also made some policy suggestions based on her findings, including increasing the eligibility of families experiencing material hardship to access existing governmental support programs and family leave with pay. She also suggested that insurers consider the costs and benefits of addressing the social determinants of health among their clients.
Bona’s talk stimulated discussion on how poverty might influence outcomes in children with cancer and whether targeting it could improve outcomes. Bhatia suggested studying whether poverty could be affecting outcomes by delaying diagnosis, which Bona said she is planning to explore with her research. Minasian suggested also collecting information on communication formats and assessing whether parents experiencing financial hardship are likely to have cell phones so they can receive reminders about appointments or follow-up care from their child’s health practitioners.
Feudtner stressed the data Bona presented showing that poverty is a “huge risk factor for cure and survivorship, so it seems to be a huge opportunity to improve outcomes. If somebody had stood up here yesterday and said I have a drug that will cut mortality risk by one-third, we would all be clamoring for it.” Bona agreed, saying, “I think this represents an enormous opportunity for pediatric oncology to decrease our residual mortality and a huge opportunity for targeting interventions for kids in a way that we do not currently do.”
But Bona stressed that her data are preliminary and based on small studies that have not yet been replicated in larger trials. “I don’t want to overstate the results we have,” she said. “They are suggestive of something that deserves investigation.” Aplenc agreed, noting that the results Bona saw may not hold true for children with other types of cancer for which they receive chemotherapy in the hospital rather than in the home setting (e.g., acute myelogenous leukemia [AML]). Patients with acute lymphoblastic leukemia whom Bona studied received their chemotherapy at home in an unobserved fashion for 2 to 3 years. But he added that if “this is broadly applicable, at least in the AML setting, targeting poverty may have more potential than any targeted therapy we have. The only targeted therapy we have for AML is sorafenib (Nexavar) and this is for 8 percent of kids, not 25 to 30 percent,” Aplenc said.
Feudtner responded that most newer chemotherapies are given orally, so poverty may emerge as a more significant factor in the success of a child’s care. Aplenc added that studies of adults with chronic myelogenous leukemia find that they are more likely to be cured in Sweden as opposed to the United States because the expensive drugs used to treat them are subsidized by the Swedish government, unlike in the United States, where the co-payments for the same drugs can be astronomical.
But Brawley said, “I am not sure that by fixing social determinants of health at the time of diagnosis we can do a great deal to fully address the problem. I actually think the time to address social determinants is from conception of the child rather than at the time of diagnosis of the disease.”
At the end of the workshop, Brawley provided his perspective on the key messages, such as the importance of providing a means for children and families to report symptoms, including psychosocial distress, and to have
those symptoms effectively addressed. “We learned that we need to listen to the child and family about symptoms and toxicities,” he said.
Another key message was the need to better integrate palliative care as a central component of cancer treatment from the point of diagnosis onward. He stressed the value of incorporating palliative care across the care continuum for all patients and families who need it; it is not just for end-of-life care.
He also noted the importance of psychosocial care during and after cancer treatment. Brawley said the posttraumatic stress that children and their parents often have after cancer diagnosis and treatment “is not a disorder, but a normal response,” and needs to be addressed with appropriate psychosocial care.
Another recurrent message was the need to document, monitor, and if possible, prevent the long-term effects of childhood cancer treatments.
Brawley also noted the research on social determinants of health and cancer outcomes reported at the workshop, and the need to consider and ameliorate health disparities. The workshop also made apparent the need to standardize pediatric palliative care and psychosocial care to ensure that every child and caregiver, no matter where they are located and what their ethnicity is, can receive the high-quality care they deserve, he said.
In closing, Wolfe thanked the participants for their willingness to break down silos and share ideas. She said many great suggestions had been discussed that could help make quality of life for children and families a priority of cancer research and clinical care (see Box 1). She also emphasized that “we need less variability across our centers, greater uniformity, and greater access to measures to both continue monitoring and improve our care outcomes.”
The next key steps are to prioritize and disseminate what we know, and to continue refining pediatric cancer care based on novel work, Wolfe said. “These questions are important for us to answer as a community. Our work is not done until all families facing the unthinkable receive optimal care that supports cure and quality of life—so they have the opportunity to both survive and thrive.”
AAP (American Academy of Pediatrics). Committee on Bioethics and Committee on Hospital Care. 2000. Palliative care for children. Pediatrics 106(2):351-357.
Abrams, A. N., E. P. Hazen, and R. T. Penson. 2007. Psychosocial issues in adolescents with cancer. Cancer Treatment Reviews 33(7):622-630.
ACS (American Cancer Society). 2014. Cancer facts & figures 2014. Atlanta, GA: American Cancer Society.
Adamson, P. C. 2015. Improving the outcome for children with cancer: Development of targeted new agents. CA: A Cancer Journal for Clinicians 65:212-220.
Alderfer, M. A., I. Mougianis, L. P. Barakat, D. Beele, S. DiTaranto, W. T. Hwang, A. T. Reilly, and A. E. Kazak. 2009. Family psychosocial risk, distress, and service utilization in pediatric cancer: Predictive validity of the psychosocial assessment tool. Cancer 115(18 Suppl):4339-4349.
Alderfer, M. A., K. A. Long, E. A. Lown, A. L. Marsland, N. L. Ostrowski, J. M. Hock, and L. J. Ewing. 2010. Psychosocial adjustment of siblings of children with cancer: A systematic review. Psycho-oncology 19(8):789-805.
Aldridge, A. A., and S. C. Roesch. 2007. Coping and adjustment in children with cancer: A meta-analytic study. Journal of Behavioral Medicine 30(2):115-129.
Ananth, P., P. Melvin, J. Wolfe, J. G. Berry. 2014. Trends in hospital utilization among children receiving inpatient palliative care. Poster presented at the 2014 American Academy of Hospice and Palliative Medicine Annual Assembly, San Diego, CA.
Annie E. Casey Foundation. 2015. KIDS COUNT Data Center. http://datacenter.kidscount.org (accessed May 29, 2015).
Aplenc, R., B. T. Fisher, Y. S. Huang, Y. Li, T. A. Alonzo, R. B. Gerbing, M. Hall, D. Bertoch, R. Keren, A. E. Seif, L. Sung, P. C. Adamson, and A. Gamis. 2012. Merging of the National Cancer Institute funded Cooperative Oncology Group data with an administrative data source to develop a more effective platform for clinical trial analysis and comparative effectiveness research: A report from the Children’s Oncology Group. Pharmacoepidemiology and Drug Safety 21(Suppl 2):37-43.
Armenian, S. H., Y. Ding, G. Mills, C. Sun, K. Venkataraman, F. L. Wong, S. L. Neuhausen, D. Senitzer, S. Wang, S. J. Forman, and S. Bhatia. 2013. Genetic susceptibility to anthracycline-related congestive heart failure in survivors of haematopoietic cell transplantation. British Journal of Haematology 163(2):205-213.
Armstrong, G. T., T. Kawashima, W. Leisenring, K. Stratton, M. Stovall, M. M. Hudson, C. A. Sklar, L. L. Robison, and K. C. Oeffinger. 2014. Aging and risk of severe, disabling, life-threatening, and fatal events in the Childhood Cancer Survivor Study. Journal of Clinical Oncology 32(12):1218-1227.
Baker, J. N., D. R. Levine, P. S. Hinds, M. S. Weaver, M. J. Cunningham, L. Johnson, D. Anghelescu, B. Mandrell, D. V. Gibson, B. Jones, J. Wolfe, C. Feudtner, S. Friebert, B. Carter, and J. R. Kane. 2015. Research priorities in pediatric palliative care. Journal of Pediatrics. http://www.sciencedirect.com/science/article/pii/S0022347615004709 (accessed July 8, 2015).
Bhatia, S., L. L. Robison, O. Oberlin, M. Greenberg, G. Bunin, F. Fossati-Bellani, and A. T. Meadows. 1996. Breast cancer and other second neoplasms after childhood Hodgkin’s disease. New England Journal of Medicine 334(12):745-751.
Bhatia, S., Y. Yasui, L. L. Robison, J. M. Birch, M. K. Bogue, L. Diller, C. DeLaat, F. Fossati-Bellani, E. Morgan, O. Oberlin, G. Reaman, F. B. Ruymann, J. Tersak, A. T. Meadows, and Late Effects Study Group. 2003. High risk of subsequent neoplasms continues with extended follow-up of childhood Hodgkin’s disease: Report from the Late Effects Study Group. Journal of Clinical Oncology 21(23):4386-4394.
Bhatia, S., W. Landier, M. Shangguan, L. Hageman, A. N. Schaible, A. R. Carter, C. L. Hanby, W. Leisenring, Y. Yasui, N. M. Kornegay, L. Mascarenhas, A. K. Ritchey, J. N. Casillas, D. S. Dickens, J. Meza, W. L. Carroll, M. V. Relling, and F. L. Wong. 2012. Nonadherence to oral mercaptopurine and risk of relapse in Hispanic and non-Hispanic white children with acute lymphoblastic leukemia: A report from the Children’s Oncology Group. Journal of Clinical Oncology 30(17):2094-2101.
Bhatia, S., W. Landier, L. Hageman, H. Kim, Y. Chen, K. R. Crews, W. E. Evans, B. Bostrom, J. Casillas, D. S. Dickens, K. W. Maloney, J. P. Neglia, Y. Ravindranath, A. K. Ritchey, F. L. Wong, and M. V. Relling. 2014. 6-MP adherence in a multiracial cohort of children with acute lymphoblastic leukemia: A Children’s Oncology Group study. Blood 124(15):2345-2353.
Black, M. M., D. B. Cutts, D. A. Frank, J. Geppert, A. Skalicky, S. Levenson, P. H. Casey, C. Berkowitz, N. Zaldivar, J. T. Cook, A. F. Meyers, T. Herren, and Children’s Sentinel Nutritional Assessment Program Study Group. 2004. Special supplemental nutrition program for women, infants, and children participation and infants’ growth and health: A multisite surveillance study. Pediatrics 114(1):169-176.
Blanco, J. G., C. L. Sun, W. Landier, L. Chen, D. Esparza-Duran, W. Leisenring, A. Mays, D. L. Friedman, J. P. Ginsberg, M. M. Hudson, J. P. Neglia, K. C. Oeffinger, A. K. Ritchey, D. Villaluna, M. V. Relling, and S. Bhatia. 2012. Anthracycline-related cardiomyopathy after childhood cancer: Role of polymorphisms in carbonyl reductase genes. A report from the Children’s Oncology Group. Journal of Clinical Oncology 30(13):1415-1421.
Bloom, B., R. A. Cohen, G. Freeman. 2011. Summary health statistics for U.S. children: National Health Interview Survey, 2010. National Center for Health Statistics. Vital Health Statistics 10(250):8-11.
Bluebond-Langner, M., J. B. Belasco, A. Goldman, and C. Belasco. 2007. Understanding parents’ approaches to care and treatment of children with cancer when standard therapy has failed. Journal of Clinical Oncology 25(17):2414-2419.
Bona, K. 2015. Poverty in pediatric oncology: Future directions for outcomes measurement. Presented at the IOM Workshop on Comprehensive Cancer Care for Children and Their Families, Washington, DC. http://www.iom.edu/~/media/Files/Activity%20Files/Disease/NCPF/2015-MAR-09/Bona.pdf (accessed May 29, 2015).
Bona, K., J. Bates, and J. Wolfe. 2011. Massachusetts’ pediatric palliative care network: Successful implementation of a novel state-funded pediatric palliative care program. Journal of Palliative Medicine 14(11):1217-1223.
Bona, K., W. B. London, D. Guo, G. Abel, L. Lehmann, and J. Wolfe. 2015. Prevalence and impact of financial hardship among New England pediatric stem cell transplantation families. Biology of Blood and Marrow Transplantation 21(2):312-318.
Bona, K., W. London, D. Guo, D. Frank, and J. Wolfe. Manuscript in preparation. Impact of socioeconomic status on relapse and survival for children treated on Dana-Farber Cancer Institute ALL Consortium Protocols (2000-2010).
Bruce, M. 2006. A systematic and conceptual review of posttraumatic stress in childhood cancer survivors and their parents. Clinical Psychology Review 26(3):233-256.
Brundage, M., D. Osoba, A. Bezjak, D. Tu, M. Palmer, J. Pater, and National Cancer Institute of Canada Clinical Trials Group. 2007. Lessons learned in the assessment of health-related quality of life: Selected examples from the National Cancer Institute of Canada clinical trials group. Journal Clinical Oncology 25(32):5078-5081.
Buckner, T. W., J. Wang, D. A. DeWalt, S. Jacobs, B. B. Reeve, and P. S. Hinds. 2014. Patterns of symptoms and functional impairments in children with cancer. Pediatric Blood & Cancer 61(7):1282-1288.
CAPC (Center to Advance Palliative Care). 2011. Public opinion research on palliative care: A report based on research by public opinion strategies.
CCSS (Childhood Cancer Survivor Study). 2015. Publications. https://ccss.stjude.org/published-research/publications.html (accessed May 29, 2015).
Chen, J., L. Ou, and S. J. Hollis. 2013. A systematic review of the impact of routine collection of patient reported outcome measures on patients, providers and health organisations in an oncologic setting. BMC Health Services Research 13:211.
CoC (Commission on Cancer). 2012. Cancer program standards 2012: Ensuring patient-centered care. Chicago, IL: American College of Surgeons.
CoC. 2014. Accreditation committee clarifications for standards 3.1 patient navigation process and 3.2 psychosocial distress screening. https://www.facs.org/publications/newsletters/coc-source/special-source/standard3132 (accessed May 9, 2015).
Compas, B. E., S. S. Jaser, M. J. Dunn, and E. M. Rodriguez. 2012. Coping with chronic illness in childhood and adolescence. Annual Review of Clinical Psychology 8:455-480.
Cook, J. T., D. A. Frank, C. Berkowitz, M. M. Black, P. H. Casey, D. B. Cutts, A. F. Meyers, N. Zaldivar, A. Skalicky, S. Levenson, T. Heeren, and M. Nord. 2004. Food insecurity is associated with adverse health outcomes among human infants and toddlers. Journal of Nutrition 134(6):1432-1438.
Cook, J. T., D. A. Frank, P. H. Casey, R. Rose-Jacobs, M. M. Black, M. Chilton, S. Ettinger de Cuba, D. Appugliese, S. Coleman, T. Heeren, C. Berkowitz, and D. B. Cutts. 2008. A brief indicator of household energy security: Associations with food security, child health, and child development in U.S. infants and toddlers. Pediatrics 122(4):e867-e875.
Coyne, I., and P. Gallagher. 2011. Participation in communication and decision-making: Children and young people’s experiences in a hospital setting. Journal of Clinical Nursing 20(15-16):2334-2343.
Dalal, S., S. Palla, D. Hui, L. Nguyen, R. Chacko, Z. Li, N. Fadul, C. Scott, V. Thornton, B. Coldman, Y. Amin, and E. Bruera. 2011. Association between a name change from palliative to supportive care and the timing of patient referrals at a comprehensive cancer center. Oncologist 16(1):105-111.
de Vos, M. A., A. P. Bos, F. B. Plotz, M. van Heerde, B. M. de Graaff, K. Tates, R. D. Truog, and D. L. Willems. 2015. Talking with parents about end-of-life decisions for their children. Pediatrics 135(2):e465-e476.
Dy, G. K., and A. A. Adjei. 2013. Understanding, recognizing, and managing toxicities of targeted anticancer therapies. CA: A Cancer Journal for Clinicians 63(4):249-279.
Eiser, C., and P. Upton. 2007. Costs of caring for a child with cancer: A questionnaire survey. Child: Care, Health and Development 33(4):455-459.
Eiser, C., K. Absolom, D. Greenfield, A. Glaser, B. Horne, H. Waite, T. Urquhart, W. H. Wallace, R. Ross, and H. Davies. 2006. Follow-up after childhood cancer: Evaluation of a three-level model. European Journal of Cancer 42(18):3186-3190.
FDA (U.S. Food and Drug Administration). 2009. Guidance for industry patient-reported outcome measures: Use in medical product development to support labeling claims. Health and Quality of Life Outcomes 4:79.
Feeny, D. 2013. Health-related quality-of-life data should be regarded as a vital sign. Journal of Clinical Epidemiology 66(7):706-709.
Feudtner, C. 2009. The breadth of hopes. New England Journal of Medicine 361(24):2306-2307.
Feudtner, C., J. Womer, R. Augustin, S. Remke, J. Wolfe, S. Friebert, and D. Weissman. 2013. Pediatric palliative care programs in children’s hospitals: A cross-sectional national survey. Pediatrics 132(6):1063-1070.
Feudtner, C., J. K. Walter, J. A. Faerber, D. L. Hill, K. W. Carroll, C. J. Mollen, V. A. Miller, W. E. Morrison, D. Munson, T. I. Kang, and P. S. Hinds. 2015. Good-parent beliefs of parents of seriously ill children. JAMA Pediatrics 169(1):39-47.
Folkman, S., and J. T. Moskowitz. 2004. Coping: Pitfalls and promise. Annual Review of Psychology 55:745-774.
Forsythe, L. P., J. H. Rowland, L. Padgett, K. Blaseg, S. D. Siegel, C. M. Dingman, and T. A. Gillis. 2013. The cancer psychosocial care matrix: A community-derived evaluative tool for designing quality psychosocial cancer care delivery. Psycho-oncology 22(9):1953-1962.
Frank, D. A., N. B. Neault, A. Skalicky, J. T. Cook, J. D. Wilson, S. Levenson, A. F. Meyers, T. Heeren, D. B. Cutts, P. H. Casey, M. M. Black, and C. Berkowitz. 2006. Heat or eat: The low income home energy assistance program and nutritional and health risks among children less than 3 years of age. Pediatrics 118(5):e1293-e1302.
Friedman, D. L., D. R. Freyer, and G. A. Levitt. 2006. Models of care for survivors of childhood cancer. Pediatric Blood & Cancer 46(2):159-168.
Friedrichsdorf, S. J., A. Postier, J. Dreyfus, K. Osenga, S. Sencer, and J. Wolfe. 2015. Improved quality of life at end of life related to home-based palliative care in children with cancer. Journal of Palliative Medicine 18(2):143-150.
Garg, A., S. Toy, Y. Tripodis, M. Silverstein, and E. Freeman. 2015. Addressing social determinants of health at well child care visits: A cluster RCT. Pediatrics 135(2):e296-e304.
Getz, K. D., Y. Li, T. A. Alonzo, M. Hall, R. B. Gerbing, L. Sung, Y. S. Huang, S. Arnold, A. E. Seif, T. P. Miller, R. Bagatell, B. T. Fisher, P. C. Adamson, A. Gamis, R. Keren, and R. Aplenc. 2015. Comparison of in-patient costs for children treated on the AAML0531 clinical trial: A report from the children’s oncology group. Pediatric Blood & Cancer (May 6, 2015 [Epub ahead of print]).
Glaser, A. W., K. Davies, D. Walker, and D. Brazier. 1997. Influence of proxy respondents and mode of administration on health status assessment following central nervous system tumours in childhood. Quality of Life Research 6(1):43-53.
Gray, W. N., L. J. Szulczewski, S. M. Regan, J. A. Williams, and A. L. Pai. 2014. Cultural influences in pediatric cancer: From diagnosis to cure/end of life. Journal of Pediatric Oncology Nursing (July 10, 2014 [Epub ahead of print]).
Grupp, S. A., S. L. Maude, P. Shaw, R. Aplenc, D. M. Barrett, C. Callahan, A. Chew, S. F. Lacey, B. L. Levine, J. J. Melenhorst, L. Motley, S. R. Rehingold, A. Shen, D. T. Teachey, P. A. Wood, D. L. Porter, and C. H. June. 2014. T cells engineered with a chimeric antigen receptor (CAR) targeting CD19 (CTL019) have long term persistence and induce durable remissions in children with relapsed, refractory ALL. Abstract presented at American Society of Hematology Meeting and Exposition, San Francisco, CA. http://www.bloodjournal.org/content/124/21/380?sso-checked=true (accessed July 8, 2015).
Gupta, S., R. Sutradhar, A. Guttmann, L. Sung, and J. D. Pole. 2014. Socioeconomic status and event free survival in pediatric acute lymphoblastic leukemia: A population-based cohort study. Leukemia Research 38(12):1407-1412.
Henderson, T. O., A. Amsterdam, S. Bhatia, M. M. Hudson, A. T. Meadows, J. P. Neglia, L. R. Diller, L. S. Constine, R. A. Smith, M. C. Mahoney, E. A. Morris, L. L. Montgomery, W. Landier, S. M. Smith, L. L. Robison, and K. C. Oeffinger. 2010a. Systematic review: Surveillance for breast cancer in women treated with chest radiation for childhood, adolescent, or young adult cancer. Annals of Internal Medicine 152(7):444-455; W444-W454.
Henderson, T. O., F. J. Hlubocky, K. E. Wroblewski, L. Diller, and C. K. Daugherty. 2010b. Physician preferences and knowledge gaps regarding the care of childhood cancer survivors: A mailed survey of pediatric oncologists. Journal of Clinical Oncology 28(5):878-883.
Hilden, J. M., E. J. Emanuel, D. L. Fairclough, M. P. Link, K. M. Foley, B. C. Clarridge, L. E. Schnipper, and R. J. Mayer. 2001. Attitudes and practices among pediatric oncologists regarding end-of-life care: Results of the 1998 American Society of Clinical Oncology survey. Journal of Clinical Oncology 19(1):205-212.
Hinds, P. S., J. Brandon, C. Allen, N. Hijiya, R. Newsome, and J. R. Kane. 2007. Patient-reported outcomes in end-of-life research in pediatric oncology. Journal of Pediatric Oncology 32(9):1079-1088.
Hinds, P. S., S. L. Nuss, K. S. Ruccione, J. S. Withycombe, S. Jacobs, H. DeLuca, C. Faulkner, Y. Liu, Y. I. Cheng, H. E. Gross, J. Wang, and D. A. DeWalt. 2013. PROMIS pediatric measures in pediatric oncology: Valid and clinically feasible indicators of patient-reported outcomes. Pediatric Blood & Cancer 60(3):402-408.
Hobbie, W. L., S. K. Ogle, M. Reilly, J. P. Ginsberg, M. Rourke, S. Ratcliffe, and J. A. Deatrick. 2010. Identifying the educational needs of parents at the completion of their child’s cancer therapy. Journal of Pediatric Oncology Nursing 27(4):190-195.
Hockenberry, M. J., P. S. Hinds, P. Barrera, R. Bryant, J. Adams-McNeill, C. Hooke, C. Rasco-Baggott, K. Patterson-Kelly, J. S. Gattuso, and B. Manteuffel. 2003. Three instruments to assess fatigue in children with cancer: The child, parent and staff perspectives. Journal of Pain and Symptom Management 25(4):319-328.
Hudson, M. M., K. K. Ness, J. G. Gurney, D. A. Mulrooney, W. Chemaitilly, K. R. Krull, D. M. Green, G. T. Armstrong, K. A. Nottage, K. E. Jones, C. A. Sklar, D. K. Srivastava, and L. L. Robison. 2013. Clinical ascertainment of health outcomes among adults treated for childhood cancer. Journal of the American Medical Association 309(22):2371-2381.
Hudson, M. M., W. Leisenring, K. K. Stratton, N. Tinner, B. D. Steen, S. Ogg, L. Barnes, K. C. Oeffinger, L. L. Robison, and C. L. Cox. 2014. Increasing cardiomyopathy screening in at-risk adult survivors of pediatric malignancies: A randomized controlled trial. Journal of Clinical Oncology 32(35):3974-3981.
Hurwitz, C. A., J. Duncan, and J. Wolfe. 2004. Caring for the child with cancer at the close of life: “There are people who make it, and I’m hoping I’m one of them.” Journal of the American Medical Association 292(17):2141-2149.
IOM (Institute of Medicine). 2003a. Childhood cancer survivorship: Improving care and quality of life. Washington, DC: The National Academies Press.
IOM. 2003b. When children die: Improving palliative and end of life care for children and their families. Washington, DC: The National Academies Press.
IOM. 2005. From cancer patient to cancer survivor: Lost in translation. Washington, DC: The National Academies Press.
IOM. 2008. Cancer care for the whole patient: Meeting psychosocial needs. Washington, DC: The National Academies Press.
IOM. 2010. A foundation for evidence-driven practice: A rapid learning system for cancer care: Workshop summary. Washington, DC: The National Academies Press.
IOM. 2013a. Delivering high-quality cancer care: Charting a new course for a system in crisis. Washington, DC: The National Academies Press.
IOM. 2013b. Identifying and addressing the needs of adolescents and young adults with cancer: Workshop summary. Washington, DC: The National Academies Press.
IOM. 2014a. Capturing social and behavioral domains and measures in electronic health records: Phase 1. Washington, DC: The National Academies Press.
IOM. 2014b. Capturing social and behavioral domains and measures in electronic health records: Phase 2. Washington, DC: The National Academies Press.
IOM. 2015. Dying in America: Improving quality and honoring individual preferences near the end of life. Washington, DC: The National Academies Press.
Jacobsen, P. B., D. Shibata, E. M. Siegel, J. H. Lee, W. J. Fulp, C. Alemany, G. Abesada-Terk, Jr., R. Brown, T. Cartwright, D. Faig, G. Kim, R. Levine, M. J. Markham, F. Schreiber, P. Sharp, and M. Malafa. 2011. Evaluating the quality of psychosocial care in outpatient medical oncology settings using performance indicators. Psycho-oncology 20(11):1221-1227.
Jardine, J., S. V. Glinianaia, H. McConachie, N. D. Embleton, and J. Rankin. 2014. Self-reported quality of life of young children with conditions from early infancy: A systematic review. Pediatrics 134(4):e1129-e1148.
Johnston, D. L., K. Nagel, D. L. Friedman, J. L. Meza, C. A. Hurwitz, and S. Friebert. 2008. Availability and use of palliative care and end-of-life services for pediatric oncology patients. Journal of Clinical Oncology 26(28):4646-4650.
Kassam, A., J. Skiadaresis, S. Habib, S. Alexander, and J. Wolfe. 2013. Moving toward quality palliative cancer care: Parent and clinician perspectives on gaps between what matters and what is accessible. Journal of Clinical Oncology 31(7):910-915.
Kassam, A., J. Skiadaresis, S. Alexander, and J. Wolfe. 2015. Differences in end-of-life communication for children with advanced cancer who were referred to a palliative care team. Pediatric Blood & Cancer (April 16, 2015 [Epub ahead of print]).
Kavcic, M., B. T. Fisher, K. Torp, Y. Li, Y. S. Huang, A. E. Seif, M. Vujkovic, and R. Aplenc. 2013. Assembly of a cohort of children treated for acute myeloid leukemia at freestanding children’s hospitals in the United States using an administrative database. Pediatric Blood & Cancer 60(3):508-511.
Kaye, E. C., J. Rubenstein, D. Levine, J. N. Baker, D. Dabbs, and S. E. Friebert. 2015. Pediatric palliative care in the community. CA: A Cancer Journal for Clinicians (May 7, 2015 [Epub ahead of print]).
Kazak, A. E., M. Brier, M. A. Alderfer, A. Reilly, S. Fooks Parker, S. Rogerwick, S. Ditaranto, and L. P. Barakat. 2012. Screening for psychosocial risk in pediatric cancer. Pediatric Blood & Cancer 59(5):822-827.
Kazak, A. E., S. Schneider, S. Didonato, and A. L. Pai. 2015. Family psychosocial risk screening guided by the Pediatric Psychosocial Preventative Health Model (PPPHM) using the Psychosocial Assessment Tool (PAT). Acta Oncologica 54(5):574-580.
Kazak, A., S. Didonato, S. Schneider, and A. Pai. 2016. Assessing family psychosocial risks in pediatric cancer. In Pediatric psychosocial oncology: Textbook for multidisciplinary care, edited by A. Abrams, A. Muriel, and L. Wiener. New York: Springer.
Kerner, J. F., J. Guirguis-Blake, K. D. Hennessy, P. J. Brounstein, C. Vinson, R. H. Schwartz, B. A. Myers, and P. Briss. 2005. Translating research into improved outcomes in comprehensive cancer control. Cancer Causes & Control 16(Suppl 1):27-40.
Kirch, R. A., and C. K. Ullrich. 2014. Putting pediatric palliative care in prime time. Paper presented at the 2014 Palliative Care in Oncology Symposium. http://meetinglibrary.asco.org/content/137764-153 (accessed June 3, 2015).
Klassen, A. F., S. J. Anthony, A. Khan, L. Sung, and R. Klaassen. 2011. Identifying determinants of quality of life of children with cancer and childhood cancer survivors: A systematic review. Supportive Care in Cancer 19(9):1275-1287.
Kotronoulas, G., N. Kearney, R. Maguire, A. Harrow, D. Di Domenico, S. Croy, and S. MacGillivray. 2014. What is the value of the routine use of patient-reported outcome measures toward improvement of patient outcomes, processes of care, and health service outcomes in cancer care? A systematic review of controlled trials. Journal of Clinical Oncology 32(14):1480-1501.
Kupst, M. J., and K. Bingen. 2006. Stress and coping in the pediatric cancer experience. In Comprehensive handbook of cancer & sickle cell disease. New York: Oxford University Press. Pp. 35-52.
Kupst, M. J., and A. F. Patenaude. 2015a. Coping with pediatric cancer. In Pediatric psycho-oncology: A quick reference on the psychosocial dimensions of cancer symptom management. New York: Oxford University Press. Pp. 241-252.
Kupst, M. J., and A. F. Patenaude. 2015b. Coping, adaptation, and resilience in pediatric cancer: Current perspectives. In Psychosocial oncology: Textbook for multidisciplinary care, edited by A. Abrams, A. Muriel, and L. Wiener. New York: Springer.
Kupst, M. J., M. B. Natta, C. C. Richardson, J. L. Schulman, J. V. Lavigne, and L. Das. 1995. Family coping with pediatric leukemia: Ten years after treatment. Journal of Pediatric Psychology 20(5):601-617.
Le Gales, C., N. Costet, J-C. Gentet, C. Kalifa, D. Frappaz, C. Edan, E. Sariban, D. Plantaz, and F. Doz. 1999. Cross-cultural adaptation of a health status classification system in children with cancer. First results of the French adaptation of the health utilities index marks 2 and 3. International Journal of Cancer 83.
Levetown, M., M. Barnard, L. Byock, B. Carter, S. Connor, and J. Darville. 2001. A call for change: Recommendations to improve the care of children living with life-threatening conditions. Alexandria, VA: National Hospice and Palliative Care Organization.
Levine, D., B. Mandrell, H. Symons, D. Wendler, and J. Baker. 2015. Evaluating supportive care for children with cancer: A multi-institutional survey study of pediatric oncology patients and parents. Jounal of Pain and Symptom Management 49(2):350.
Lichtenthal, W., G. W. Corner, C. R. Sweeney, L. Wiener, K. Roberts, B. Breitbart, D.W. Kissane, and H. G. Prigerson. 2015. Mental health services for parents who lost a child to cancer: If we build them, will they come? Journal of Clinical Oncology 33(20):2246-2253.
Lightfoot, T. J., W. T. Johnston, J. Simpson, A. G. Smith, P. Ansell, S. Crouch, E. Roman, and S. E. Kinsey. 2012. Survival from childhood acute lymphoblastic leukaemia: The impact of social inequality in the United Kingdom. European Journal of Cancer 48(2):263-269.
Long, K. A., and A. L. Marsland. 2011. Family adjustment to childhood cancer: A systematic review. Clinical Child and Family Psychology Review 14(1):57-88.
Maciasz, R. M., R. M. Arnold, E. Chu, S. Y. Park, D. B. White, L. B. Vater, and Y. Schenker. 2013. Does it matter what you call it? A randomized trial of language used to describe palliative care services. Supportive Care in Cancer 21(12):3411-3419.
Mack, J. W., J. Wolfe, H. E. Grier, P. D. Cleary, and J. C. Weeks. 2006. Communication about prognosis between parents and physicians of children with cancer: Parent preferences and the impact of prognostic information. Journal of Clinical Oncology 24(33):5265-5270.
Mack, J. W., E. F. Cook, J. Wolfe, H. E. Grier, P. D. Cleary, and J. C. Weeks. 2007a. Understanding of prognosis among parents of children with cancer: Parental optimism and the parent–physician interaction. Journal of Clinical Oncology 25(11):1357-1362.
Mack, J. W., J. Wolfe, E. F. Cook, H. E. Grier, P. D. Cleary, and J. C. Weeks. 2007b. Hope and prognostic disclosure. Journal of Clinical Oncology 25(35):5636-5642.
Mack, J. W., J. Wolfe, E. F. Cook, H. E. Grier, P. D. Cleary, and J. C. Weeks. 2009. Peace of mind and sense of purpose as core existential issues among parents of children with cancer. Archives of Pediatric Adolescent Medicine 163(6):519-524.
Mack, J. W., J. Wolfe, E. F. Cook, H. E. Grier, P. D. Cleary, and J. C. Weeks. 2011. Parents’ roles in decision making for children with cancer in the first year of cancer treatment. Journal of Clinical Oncology 29(15):2085-2090.
Maude, S. L., J. C. Fitzgerald, B. T. Fisher, Y. Li, Y. S. Huang, K. Torp, A. E. Seif, M. Kavcic, D. M. Walker, K. H. Leckerman, T. J. Kilbaugh, S. R. Rheingold, L. Sung, T. E. Zaoutis, R. A. Berg, V. M. Nadkarni, N. J. Thomas, and R. Aplenc. 2014. Outcome of pediatric acute myeloid leukemia patients receiving intensive care in the United States. Pediatric Critical Care Medicine 15(2):112-120.
McCabe, M. S., A. H. Partridge, E. Grunfeld, and M. M. Hudson. 2013. Risk-based health care, the cancer survivor, the oncologist, and the primary care physician. Seminars in Oncology 40(6):804-812.
Menard, J. C., P. S. Hinds, S. S. Jacobs, K. Cranston, J. Wang, D. A. DeWalt, and H. E. Gross. 2014. Feasibility and acceptability of the patient-reported outcomes measurement information system measures in children and adolescents in active cancer treatment and survivorship. Cancer Nursing 37(1):66-74.
Meyers, A., D. Cutts, D. A. Frank, S. Levenson, A. Skalicky, T. Heeren, J. Cook, C. Berkowitz, M. Black, P. Casey, and N. Zaldivar. 2005. Subsidized housing and children’s nutritional status: Data from a multisite surveillance study. Archives of Pediatric and Adolescent Medicine 159(6):551-556.
Miller, T. P.,M. Kavcic, Y. Li, T. A. Alonzo, M. Hall, Y. Huang, R. B. Gerbin, B. T. Fisher, S. Luger, D. Rubin, A. Troxel, L. Sung, A. S. Gamis, and R. Aplenc. 2013. Accuracy of adverse event reporting compared to patient chart abstraction on a Phase III NCI-funded clinical trial for pediatric acute myeloid leukemia: A report from the Children’s Oncology Group. Blood 122(21):931.
Miller, V. A., and A. F. Jawad. 2014. Relationship of youth involvement in diabetes-related decisions to treatment adherence. Journal of Clinical Psychology in Medical Settings 21:183-189.
Mulder, R. L., L. C. Kremer, M. M. Hudson, S. Bhatia, W. Landier, G. Levitt, L. S. Constine, W. H. Wallace, F. E. van Leeuwen, C. M. Ronckers, T. O. Henderson, M. Dwyer, R. Skinner, K. C. Oeffinger, and International Late Effects of Childhood Cancer Guideline Harmonization Group. 2013. Recommendations for breast cancer surveillance for female survivors of childhood, adolescent, and young adult cancer given chest radiation: A report from the International Late Effects of Childhood Cancer Guideline Harmonization Group. Lancet Oncology 14(13):e621-e629.
Nathan, P. C., J. S. Ford, T. O. Henderson, M. M. Hudson, K. M. Emmons, J. N. Casillas, E. A. Lown, K. K. Ness, and K. C. Oeffinger. 2009. Health behaviors, medical care, and interventions to promote healthy living in the childhood cancer survivor study cohort. Journal of Clinical Oncology 27(14):2363-2373.
Nathan, P. C., C. K. Daugherty, K. E. Wroblewski, M. L. Kigin, T. V. Stewart, F. J. Hlubocky, E. Grunfeld, M. E. Del Giudice, L. A. Ward, J. M. Galliher, K. C. Oeffinger, and T. O. Henderson. 2013. Family physician preferences and knowledge gaps regarding the care of adolescent and young adult survivors of childhood cancer. Journal of Cancer Survivorship 7(3):275-282.
Noll, R. B., and M. J. Kupst. 2007. Commentary: The psychological impact of pediatric cancer hardiness, the exception or the rule? Journal of Pediatric Psychology 32(9):1089-1098.
Northcott, P. A., D. T. Jones, M. Kool, G. W. Robinson, R. J. Gilbertson, Y. J. Cho, S. L. Pomeroy, A. Korshunov, P. Lichter, M. D. Taylor, and S. M. Pfister. 2012. Medulloblastomics: The end of the beginning. National Review of Cancer 12(12):818-834.
Oeffinger, K. C. 2002. Longitudinal cancer-related health care for adult survivors of childhood cancer. https://www.iom.edu/~/media/Files/Activity%20Files/Disease/NCPF/BackgroundPaperLongitudinalCancerRelatedHealthCareforAdultSurvivorsofChildhoodCancer.pdf (accessed June 2, 2015).
Oeffinger, K. C., and M. M. Hudson. 2004. Long-term complications following childhood and adolescent cancer: Foundations for providing risk-based health care for survivors. CA: A Cancer Journal for Clinicians 54(4):208-236.
Oeffinger, K. C., A. C. Mertens, M. M. Hudson, J. G. Gurney, J. Casillas, H. Chen, J. Whitton, M. Yeazel, Y. Yasui, and L. L. Robison. 2004. Health care of young adult survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. The Annals of Family Medicine 2(1):61-70.
Oeffinger, K. C., A. C. Mertens, C. A. Sklar, T. Kawashima, M. M. Hudson, A. T. Meadows, D. L. Friedman, N. Marina, W. Hobbie, N. S. Kadan-Lottick, C. L. Schwartz, W. Leisenring, L. L. Robison, and Childhood Cancer Survivor Study. 2006. Chronic health conditions in adult survivors of childhood cancer. New England Journal of Medicine 355(15):1572-1582.
Oeffinger, K. C., J. S. Ford, C. S. Moskowitz, L. R. Diller, M. M. Hudson, J. F. Chou, S. M. Smith, A. C. Mertens, T. O. Henderson, D. L. Friedman, W. M. Leisenring, and L. L. Robison. 2009. Breast cancer surveillance practices among women previously treated with chest radiation for a childhood cancer. Journal of the American Medical Association 301(4):404-414.
Okado, Y., A. M. Long, and S. Phipps. 2014. Association between parent and child distress and the moderating effects of life events in families with and without a history of pediatric cancer. Journal of Pediatric Psychology 39(9):1049-1060.
Pai, A. L., D. Drotar, K. Zebracki, M. Moore, and E. Youngstrom. 2006. A meta-analysis of the effects of psychological interventions in pediatric oncology on outcomes of psychological distress and adjustment. Journal of Pediatric Psychology 31(9):978-988.
Parikh, R. B., R. A. Kirch, T. J. Smith, and J. S. Temel. 2013. Early specialty palliative care—translating data in oncology into practice. New England Journal of Medicine 369(24):2347-2351.
Parsons, S. K., S. E. Barlow, S. L. Levy, S. E. Supran, and S. H. Kaplan. 1999. Health-related quality of life in pediatric bone marrow transplant survivors: According to whom? International Journal of Cancer Supplement 12:46-51.
Patel, S. K., W. Mullins, A. Turk, N. Dekel, C. Kinjo, and J. K. Sato. 2011. Distress screening, rater agreement, and services in pediatric oncology. Psycho-oncology 20(12):1324-1333.
Patenaude, A. F., and M. J. Kupst. 2005. Psychosocial functioning in pediatric cancer. Journal of Pediatric Psychology 30(1):9-27.
Pearson, H. N. 2013. “You’ve only got one chance to get it right”: Children’s cancer nurses’ experiences of providing palliative care in the acute hospital setting. Issues in Comprehensive Pediatric Nursing 36(3):188-211.
Phillips, S. M., L. S. Padgett, W. M. Leisenring, K. K. Stratton, K. Bishop, K. R. Krull, C. M. Alfano, T. M. Gibson, J. S. de Moor, D. B. Hartigan, G. T. Armstrong, L. L. Robison, J. H. Rowland, K. C. Oeffinger, and A. B. Mariotto. 2015. Survivors of childhood cancer in the United States: Prevalence and burden of morbidity. Cancer Epidemiology, Biomarkers, & Prevention 24(4):653-663.
Picoraro, J. A., J. W. Womer, A. E. Kazak, and C. Feudtner. 2014. Posttraumatic growth in parents and pediatric patients. Journal of Palliative Medicine 17(2):209-218.
Pirl, W. F., J. R. Fann, J. A. Greer, I. Braun, T. Deshields, C. Fulcher, E. Harvey, J. Holland, V. Kennedy, M. Lazenby, L. Wagner, M. Underhill, D. K. Walker, J. Zabora, B. Zebrack, and W. A. Bardwell. 2014. Recommendations for the implementation of distress screening programs in cancer centers: Report from the American Psychosocial Oncology Society (APOS), Association of Oncology Social Work (AOSW), and Oncology Nursing Society (ONS) Joint Task Force. Cancer 120(19):2946-2954.
Quill, T. E., and A. P. Abernethy. 2013. Generalist plus specialist palliative care—creating a more sustainable model. New England Journal of Medicine 368(13):1173-1175.
Quinn, H., D. Thissen, Y. Liu, B. Magnus, J. S. Lai, D. Amtmann, J. W. Varni, H. E. Gross, and D. A. DeWalt. 2014. Using item response theory to enrich and expand the PROMIS® pediatric self report banks. Health and Quality of Life Outcomes 12:160.
Reiter-Purtill, J., K. Vannatta, C. A. Gerhardt, J. Correll, and R. B. Noll. 2003. A controlled longitudinal study of the social functioning of children who completed treatment of cancer. Journal of Pediatric Hematology/Oncology 25(6):467-473.
Rodriguez, E. M., M. J. Dunn, T. Zuckerman, K. Vannatta, C. A. Gerhardt, and B. E. Compas. 2012. Cancer-related sources of stress for children with cancer and their parents. Journal of Pediatric Psychology 37(2):185-197.
Rosenberg, A. R., K. S. Baker, K. Syrjala, and J. Wolfe. 2012. Systematic review of psychosocial morbidities among bereaved parents of children with cancer. Pediatric Blood & Cancer 58(4):503-512.
Roth, M., D. Wang, M. Kim, and K. Moody. 2009. An assessment of the current state of palliative care education in pediatric hematology/oncology fellowship training. Pediatric Blood & Cancer 53(4):647-651.
Runeson, I., I. Hallstrom, G. Elander, and G. Hermeren. 2002. Children’s participation in the decision-making process during hospitalization: An observational study. Nursing Ethics 9(6):583-598.
Sahler, O. J., D. L. Fairclough, S. Phipps, R. K. Mulhern, M. J. Dolgin, R. B. Noll, E. R. Katz, J. W. Varni, D. R. Copeland, and R. W. Butler. 2005. Using problem-solving skills training to reduce negative affectivity in mothers of children with newly diagnosed cancer: Report of a multisite randomized trial. Journal of Consulting and Clinical Psychology 73(2):272-283.
Salley, C. G., L. L. Hewitt, A. F. Patenaude, M. W. Vasey, K. O. Yeates, C. A. Gerhardt, and K. Vannatta. 2015. Temperament and social behavior in pediatric brain tumor survivors and comparison peers. Journal of Pediatric Psychology 40(3):297-308.
Schwantes, S., and H. W. O’Brien. 2014. Pediatric palliative care for children with complex chronic medical conditions. Pediatric Clinics of North America 61(4):797-821.
Schwartz, L. A., L. K. Tuchman, W. L. Hobbie, and J. P. Ginsberg. 2011. A social-ecological model of readiness for transition to adult-oriented care for adolescents and young adults with chronic health conditions. Child: Care, Health and Development 37(6):883-895.
Schwartz, L. A., L. D. Brumley, L. K. Tuchman, L. P. Barakat, W. L. Hobbie, J. P. Ginsberg, L. C. Daniel, A. E. Kazak, K. Bevans, and J. A. Deatrick. 2013. Stakeholder validation of a model of readiness for transition to adult care. JAMA Pediatrics 167(10):939-946.
Selove, R., T. Kroll, M. Coppes, and Y. Cheng. 2012. Psychosocial services in the first 30 days after diagnosis: Results of a web-based survey of Children’s Oncology Group (COG) member institutions. Pediatric Blood & Cancer 58(3):435-440.
SIGN (Scottish Intercollegiate Guidelines Network). 2013. Sign 132 long term follow up of survivors of childhood cancer. Edinburgh, Scotland: Healthcare Improvement Scotland. http://www.sign.ac.uk/guidelines/index.html (accessed May 29, 2015).
Singer, S., M. E. Gianinazzi, A. Hohn, C. E. Kuehni, and G. Michel. 2013. General practitioner involvement in follow-up of childhood cancer survivors: A systematic review. Pediatric Blood & Cancer 60(10):1565-1573.
Singh, G. 2010. Child mortality in the United States, 1935-2007: Large racial and socioeconomic disparities have persisted over time. A 75th anniversary publication. Health Resources and Services Administration, Maternal and Child Health Bureau. Rockville, MD: U.S. Department of Health and Human Services.
SKION (Stichting Kinderoncologie Nederland). 2010. Richtlijn follow-up na kinderkanker meer dan 5 jaar na diagnose. https://www.skion.nl/voor-patienten-en-ouders/late-effecten/533/richtlijn-follow-up-na-kinderkanker (accessed May 29, 2015).
Smith, M. A., S. F. Altekruse, P. C. Adamson, G. H. Reaman, and N. L. Seibel. 2014. Declining childhood and adolescent cancer mortality. Cancer 120(16):2497-2506.
Smith, T. J., S. Temin, E. R. Alesi, A. P. Abernethy, T. A. Balboni, E. M. Basch, B. R. Ferrell, M. Loscalzo, D. E. Meier, J. A. Paice, J. M. Peppercorn, M. Somerfield, E. Stovall, and J. H. Von Roenn. 2012. American Society of Clinical Oncology Provisional Clinical Opinion: The Integration of Palliative Care into Standard Oncology Care. Journal of Clinical Oncology 30:880-887.
Steele, R., H. Bosma, M. F. Johnston, S. Cadell, B. Davies, H. Siden, and L. Straatman. 2008. Research priorities in pediatric palliative care: A delphi study. Journal of Palliative Care 24(4):229-239.
Suh, E., C. K. Daugherty, K. Wroblewski, H. Lee, M. L. Kigin, K. A. Rasinski, J. S. Ford, E. S. Tonorezos, P. C. Nathan, K. C. Oeffinger, and T. O. Henderson. 2014. General internists’ preferences and knowledge about the care of adult survivors of childhood cancer: A cross-sectional survey. Annals of Internal Medicine 160(1):11-17.
Tsai, E., and CPS (Canadian Paediatric Society). 2008. Advance care planning for paediatric patients: Position statement of the Canadian Paediatric Society. Paediatrics & Child Health 13(9):791-796.
UKCCSG (UK Children’s Cancer Study Group). 2005. Therapy-based long-term follow-up. http://www.cclg.org.uk/dynamic_files/LTFU-full.pdf (accessed May 29, 2015).
Upton, P., J. Lawford, and C. Eiser. 2008. Parent–child agreement across child health-related quality of life instruments: A review of the literature. Quality of Life Research 17(6):895-913.
USDA (U.S. Department of Agriculture) Economic Research Service. 2015. Food security in the United States. http://www.ers.usda.gov/data-products/food-security-in-the-unitedstates.aspx (accessed May 29, 2015).
Vannatta, K., M. A. Gartstein, A. Short, and R. B. Noll. 1998. A controlled study of peer relationships of children surviving brain tumors: Teacher, peer, and self ratings. Journal of Pediatric Psychology 23(5):279-287.
Varni, J. W., B. Magnus, B. D. Stucky, Y. Liu, H. Quinn, D. Thissen, H. E. Gross, I. C. Huang, and D. A. DeWalt. 2014. Psychometric properties of the PROMIS® pediatric scales: Precision, stability, and comparison of different scoring and administration options. Quality of Life Research 23(4):1233-1243.
Varni, J. W., D. Thissen, B. D. Stucky, Y. Liu, B. Magnus, J. He, E. M. DeWitt, D. E. Irwin, J. S. Lai, D. Amtmann, and D. A. DeWalt. 2015. Item-level informant discrepancies between children and their parents on the PROMIS pediatric scales. Quality of Life Research (January 6, 2015 [Epub ahead of print]).
Varond, A. J., and A. K. Walsh. 2014. FDA clarifies its rare pediatric disease priority review voucher program. FDA Law Blog, November 20. http://http://www.fdalawblog.net/fda_law_blog_hyman_phelps/2014/11/fda-clarifies-its-rare-pediatric-disease-priorityreview-voucher-program.html (accessed May 8, 2015).
Vrijmoet-Wiersma, C. M., J. M. van Klink, A. M. Kolk, H. M. Koopman, L. M. Ball, and R. Maarten Egeler. 2008. Assessment of parental psychological stress in pediatric cancer: A review. Journal of Pediatric Psychology 33(7):694-706.
Wakefield, C. E., J. McLoone, B. Goodenough, K. Lenthen, D. R. Cairns, and R. J. Cohn. 2010. The psychosocial impact of completing childhood cancer treatment: A systematic review of the literature. Journal of Pediatric Psychology 35(3):262-274.
Wakefield, C. E., J. K. McLoone, P. Butow, K. Lenthen, and R. J. Cohn. 2011. Parental adjustment to the completion of their child’s cancer treatment. Pediatric Blood & Cancer 56(4):524-531.
Wakefield, C. E., P. Butow, C. A. Fleming, G. Daniel, and R. J. Cohn. 2012. Family information needs at childhood cancer treatment completion. Pediatric Blood & Cancer 58(4):621-626.
Wallace, W. H., A. Blacklay, C. Eiser, H. Davies, M. Hawkins, G. A. Levitt, M. E. Jenney, and Late Effects Committee of the United Kingdom Children’s Cancer Study. 2001. Developing strategies for long term follow up of survivors of childhood cancer. BMJ 323(7307):271-274.
Wang, X., W. Liu, C. L. Sun, S. H. Armenian, H. Hakonarson, L. Hageman, Y. Ding, W. Landier, J. G. Blanco, L. Chen, A. Quinones, D. Ferguson, N. Winick, J. P. Ginsberg, F. Keller, J. P. Neglia, S. Desai, C. A. Sklar, S. M. Castellino, I. Cherrick, Z. E. Dreyer, M. M. Hudson, L. L. Robison, Y. Yasui, M. V. Relling, and S. Bhatia. 2014. Hyaluronan synthase 3 variant and anthracycline-related cardiomyopathy: A report from the Children’s Oncology Group. Journal of Clinical Oncology 32(7):647-653.
Warner, E. L., A. C. Kirchhoff, G. E. Nam, and M. Fluchel. 2014. Financial burden of pediatric cancer for patients and their families. Journal of Oncology Practice (October 14, 2014 [Epub ahead of print]).
Weaver, M. S., K. E. Heinze, C. J. Bell, L. Wiener, A. M. Garee, K. P. Kelly, R. L. Casey, A. Watson, P. S. Hinds, and Pediatric Palliative Care Special Interest Group at Children’s National Health System. 2015. Establishing psychosocial palliative care standards for children and adolescents with cancer and their families: An integrative review. Palliative Medicine (April 28, 2015 [Epub ahead of print]).
Wechsler, A. M., and I. Sanchez-Iglesias. 2013. Psychological adjustment of children with cancer as compared with healthy children: A meta-analysis. European Journal of Cancer Care (England) 22(3):314-325.
Whitlow, P. G., M. Caparas, P. Cullen, C. Trask, F. Schulte, L. Embry, R. Nagarajan, D. L. Johnston, and L. Sung. 2014. Strategies to improve success of pediatric cancer cooperative group quality of life studies: A report from the Children’s Oncology Group. Quality of Life Research (November 29, 2014 [Epub ahead of print]).
WHO (World Health Organization). 2015. WHO definition of palliative care. http://www.who.int/cancer/palliative/definition/en (accessed May 15 2015).
Wiener, L., S. Zadeh, L. H. Wexler, and M. Pao. 2013. When silence is not golden: Engaging adolescents and young adults in discussions around end-of-life care choices. Pediatric Blood & Cancer 60(5):715-718.
Wiener, L., A. Viola, J. Koretski, E. D. Perper, and A. F. Patenaude. 2015a. Pediatric psycho-oncology care: Standards, guidelines, and consensus reports. Psycho-oncology 24(2):204-211.
Wiener, L., M. S. Weaver, C. J. Bell, and U. M. Sansom-Daly. 2015b. Threading the cloak: Palliative care education for care providers of adolescents and young adults with cancer. Jouranl of Clinical Oncology for Adolescents and Young Adults 5:1-18.
Wolfe, J., H. E. Grier, N. Klar, S. B. Levin, J. M. Ellenbogen, S. Salem-Schatz, E. J. Emanuel, and J. C. Weeks. 2000a. Symptoms and suffering at the end of life in children with cancer. New England Journal of Medicine 342(5):326-333.
Wolfe, J., N. Klar, H. E. Grier, J. Duncan, S. Salem-Schatz, E. J. Emanuel, and J. C. Weeks. 2000b. Understanding of prognosis among parents of children who died of cancer: Impact on treatment goals and integration of palliative care. JAMA 284(19):2469-2475.
Yao, L. 2013. FDA takes step to encourage pediatric drug studies. FDAVoice (blog), August 26. http://http://blogs.fda.gov/fdavoice/index.php/tag/best-pharmaceuticals-forchildren-act-bpca (accessed May 8, 2015).
Yoo, J. P., K. S. Slack, and J. L. Holl. 2009. Material hardship and the physical health of school-aged children in low-income households. American Journal of Public Health 99(5):829-836.
Yu, A. L., A. L. Gilman, M. F. Ozkaynak, W. B. London, S. G. Kreissman, H. X. Chen, M. Smith, B. Anderson, J. G. Villablanca, K. K. Matthay, H. Shimada, S. A. Grupp, R. Seeger, C. P. Reynolds, A. Buxton, R. A. Reisfeld, S. D. Gillies, S. L. Cohn, J. M. Maris, P. M. Sondel, and Children’s Oncology. 2010. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. New England Journal of Medicine 363(14):1324-1334.




































































































