This chapter provides background information about the process planned for use at the Blue Grass Chemical Agent Destruction Pilot Plant (BGCAPP) to dispose of the nerve agents and energetic materials contained in the nerve agent munitions stored at the Blue Grass Army Depot (BGAD). Subsections provide an overview of the entire process, including a discussion of the production and characterization of the hydrolysates, a description of the supercritical water oxidation (SCWO) system, and a description of the water recovery system (WRS). Two earlier National Research Council (NRC) reports also provide background information about the SCWO system (NRC, 2013) and the WRS (NRC, 2012) and may be of interest to readers of this report.
BRIEF DESCRIPTION OF THE BGCAPP PROCESS
The energetic materials and the nerve agents stored in munitions at BGAD will be removed from the projectiles and rockets that contain them in a munitions pretreatment area. The energetic materials in the munitions include RDX (C4H8N8O8), HMX (C3H6N6O6), TNT (C7H5N3O6), nitroglycerin (C3H5N3O9), nitrocellulose, and tetryl (C7H5N5O8). The chemical agents include GB (C4H10FO2P) and VX (C11H26NO2PS). Munitions containing H (C4H8Cl2S) are also stored at BGAD. These will be destroyed by a separate explosive destruction technology.
These materials will then be neutralized separately by high-temperature caustic hydrolysis. This process breaks up large complex molecules into smaller ones, destroying the nerve agents and eliminating their acute toxicity. Thus, the hydrolysate should no longer contain GB or VX. The drained projectile bodies, along with any material adhering to them, will be thermally treated in the metal parts treater at 1,000°F for 15 minutes. The offgas from the metal parts treater (MPT) goes to a thermal oxidizer unit, which operates at approximately 2,200°F and has a 3-sec residence time, to destroy any trace organic materials. The rocket warheads and bursters will be hydrolyzed in the energetics batch hydrolyzers (EBHs). Residual metal parts will also be sent to the MPT. The metal from the MPT will go offsite for disposal or recycling.
After laboratory analysis to ensure the hydrolysates meet release requirements, including that agent has been destroyed to regulatory requirements, the VX, GB, and energetics hydrolysates will be stored separately outdoors in closed tanks. The energetics hydrolysate will be diluted and acidified to facilitate the subsequent removal of aluminum in the aluminum filtration system, as discussed later in this chapter. The GB and VX hydrolysates will then be individually blended with energetics hydrolysate, combined with additives (e.g., HCl, H2SO4, NaCl, S) to improve SCWO process-ability, and then, along with fuel and compressed air, be fed to a SCWO reactor for the purpose of converting the organic carbon to CO2. The air feed provides the oxygen for oxidation, and the fuel (isopropyl alcohol) is used to maintain the reactor temperature. Additional SCWO feed streams include spent decontamination solution, condensate from the metal parts treater offgas treatment system, and other condensates. Sufficient mixing of the various components in the SCWO feed stream should help to ensure that a homogeneous solution enters the SCWO reactor. Quench water is added at the bottom of the SCWO reactors to cool the SCWO effluent and to keep salts in solution downstream of the SCWO reactors. The SCWO facility consists of three identical SCWO trains. Each SCWO train consists of a feed module and a reactor module. The processing of the hydrolysates with SCWO is required to comply with regulatory permits as well as to meet requirements of the Chemical Weapons Convention (CWC). The treatment process is shown in the block flow diagram of Figure 2-1.
After being cooled, the SCWO reactor effluent will be separated into gas and liquid phases. The gas phase passes through carbon filters before being released to the atmosphere. If the composition of the liquid phase meets treatment specifications, it proceeds to the SCWO effluent tank and is then treated in the WRS to produce a brine stream for
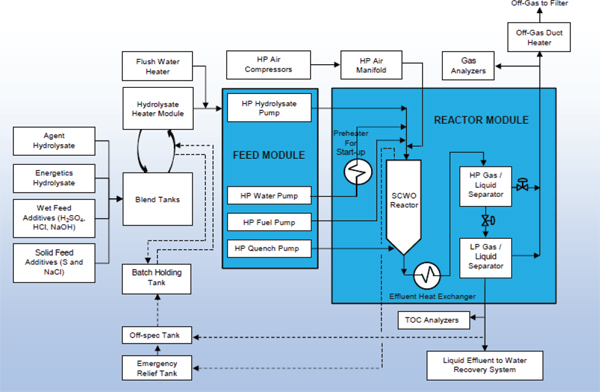
FIGURE 2-1 Schematic diagram of SCWO reactors. SOURCE: George Lucier, deputy chief scientist, BGCAPP; Dave Linkenheld, SCWO start-up supervisor; and Larry Austin, waste manager, BGCAPP, “SCWO Process: Cradle to Grave,” presentation to the committee on January 28, 2015.
ultimate offsite disposal and a product water stream that will be recycled as quench water for the SCWO reactors. Off-spec SCWO effluent will go to the off-spec tank, be blended with hydrolysate, and go through the reactor again until the treatment specifications are met.
CHARACTERIZATION OF AGENT AND ENERGETICS HYDROLYSATES
This section provides information about the likely compositions of the three different hydrolysates. The information comes from an understanding of the chemistry that occurs during hydrolysis and from process simulations performed by BGCAPP. The total volume of hydrolysate anticipated to be generated at BGCAPP is approximately 2.5 million gallons, with about 7 percent of this volume coming from VX neutralization, 37 percent from GB, and the remaining 56 percent from energetics.
The actual compositions of the hydrolysates that will be produced at BGCAPP are not yet known with certainty, because no detailed, quantitative, analytical characterization of actual hydrolysate will be available until the plant begins to generate hydrolysate. Moreover, the variability in the hydrolysates’ compositions due to variability in the compositions of the agent and energetics themselves is not known. Nevertheless, enough was known about the anticipated compositions of these streams to permit formulation of the simulated hydrolysates used in the SCWO first-of-a-kind (FOAK) testing.
Finding 2-1. The compositions of the hydrolysates and their batch-to-batch variability have only been estimated at this point. No detailed, quantitative analysis is available.
Nerve agent GB will be neutralized in caustic at 160°F for 4 hr. The chemistry of GB neutralization has been well documented and is well known. The main products are sodium isopropyl methylphosphonate (IMP) and sodium fluoride (NaF). Tables 2-1, 2-2, and 2-3 provide the compositions of the GB hydrolysate simulant, the energetics hydrolysate simulant, and the blended simulant used in FOAK testing of SCWO. Note that the simulated hydrolysates were not intended to contain all of the chemical species that could be present in actual agent hydrolysate. Rather, they were designed to mimic the solids-forming elements, heating value, and content of key species—tributylamine for GB and organic nitrogen for VX (see next subsection)—expected in the actual agent hydrolysate (BPBG, 2013).
TABLE 2-1 Simulated GB Agent Hydrolysate Used in FOAK Testing
| Constituent (7.5 wt% reacted GB) (without additives) | Wt% | |||
| Deionized water | 87.03 | |||
| Sodium fluoride (NaF) | 1.84 | |||
| Sodium hydroxide (NaOH) | 3.84 | |||
| Dimethylmethyl phosphonate (C3H9O3P) (DMMP) | 5.91 | |||
| 100% Isopropanol (C3H8O) (IPA) | 1.18 | |||
| Tri-n-butylamine (C12H27N) | 0.20 | |||
| Total | 100.00 | |||
SOURCE: BPBG (2013).
TABLE 2-2 Simulated Energetics Hydrolysate Used in GB FOAK Testing
| Constituent (with additives) | Wt% | |||
| Deionized water | 78.14 | |||
| Sodium chloride (NaCl) | 5.30 | |||
| Sodium sulfate (Na2SO4) | 12.71 | |||
| Sodium nitrite (NaNO2) | 0.39 | |||
| Sodium formate (NaCHO2) | 1.18 | |||
| 35% Hydrochloric acid (HCl) | 1.92 | |||
| Sulfur (S) | 0.36 | |||
| Total | 100.00 | |||
SOURCE: BPBG (2013).
TABLE 2-3 Simulated Blended Hydrolysate Used in FOAK Testing for GBa
| Constituent (with additives) | Wt%b | |||
| Deionized water | 80.64 | |||
| Sodium sulfate (Na2SO4) | 9.14 | |||
| Sodium chloride (NaCl) | 3.81 | |||
| Sodium hydroxide (NaOH) | 1.08 | |||
| Dimethyl methylphosphonate C3H9O3P (DMMP) | 1.66 | |||
| 35% Hydrochloric acid (HCl) | 1.38 | |||
| Sodium formate (NaCHO2) | 0.85 | |||
| Sodium fluoride (NaF) | 0.52 | |||
| 100% Isopropanol (C3H8O) (IPA) | 0.33 | |||
| Sodium nitrite (NaNO2) | 0.28 | |||
| Sulfur (S) | 0.26 | |||
| Tri-n-butylamine (C12H27N) (TBA) | 0.06 | |||
| Total | 100.00 | |||
a2.5 parts simulated energetics hydrolysate to 1 part simulated agent hydrolysate.
bAgent hydrolysate simulant constituents are diluted further by the additives used in the energetics hydrolysate recipe; hence the dilution factor is 3.558, not 3.5. For DMMP, for example, we have [(102.33 × 2.5) + 100]/100 = 3.558; 5.91/3.558 = 1.66 wt%.
SOURCE: BPBG (2013).
In addition to the components listed in the tables, actual GB hydrolysate will also contain trace components such as dithiane, diisopropyl methylphosphonate (DIMP), triisopropyl phosphate, N,N′-diisopropyl urea (DIPU), N-hexyl butanamide, dibutylacetamide, and dibutylbutanamide (Malloy et al., 2007).
Nerve agent VX will be neutralized in caustic at 194°F for 9 hr. The chemistry of VX neutralization has also been well documented and is well known. The main products from caustic hydrolysis of VX are sodium ethyl methylphosphonate and 2-(diisopropylamino)ethane thiol (sodium salt). A secondary pathway (about 10 percent of the VX) leads to ethanol and EA 2192,1 which then hydrolyze to sodium methylphosphonate and 2-(diisopropylamino)ethane thiol (sodium salt). Tables 2-4, 2-5, and 2-6 provide the compositions of the VX hydrolysate simulant, energetics hydrolysate simulant, and blended simulant, respectively, used in FOAK testing of SCWO. Note that the simulated hydrolysates were not intended to contain all of the chemical species that could be present in actual agent hydrolysate. Rather, they were designed to mimic the solids-forming elements, heating value, and content of key species (i.e., tributylamine for GB and organic nitrogen for VX) expected in the actual agent hydrolysate (BPBG, 2013).
In addition to the components in the simulants listed in the tables above, actual VX hydrolysate also contains traces of many other compounds, the presence of which has been detected but the precise amounts of which are not known. These trace components include the following (Dejarme and Lecakes, 2008):
- [2-(Diisopropylamino)ethyl]-(2-mercaptoethyl) sulfide
- 1,2-Bis-(2-diisopropylaminoethyl) ethane
- Cyclohexanamine, N-cyclohexyl-
- Morpholine, 4-phenyl-
- N-Isopropylethylenediamine
- (Diisopropylamino)ethanol
- 1,2-Bis-(2-diisopropylaminoethyl) ethane
- 1,3-Dicyclohexylcarbodiimide
- 2-(Diisopropylamino)ethanethiol
- 2-Diisopropylaminoethyl ethyl ether
- 2-Diisopropylaminoethyl ethyl sulfide
- Acetamide, N-(3-methyl-2-buten-1-yl)-
- Acetamide, N-cyclohexyl-
- Arsine, triphenyl-
- Bis(diisopropylaminoethyl) disulfide
- Cyclohexanamine
- Ethane, 1,2-bis(methylthio)-
______________
1 EA-2192 is a decomposition product of VX that is nearly as toxic as VX. It is a Schedule 1A chemical under the CWC.
TABLE 2-4 Simulated VX Agent Hydrolysate Used in FOAK Testing
| Constituent (16.6 wt% reacted VX) (without additives) | Wt% | |||
| Deionized water | 58.18 | |||
| Sodium hydroxide (NaOH) | 6.37 | |||
| Dimethyl methylphosphonate (C3H9O3P) (DMMP) | 7.41 | |||
| 85% Diethanolamine (C4H11NO2) (DEA) | 7.76 | |||
| 56% Sodium isethionate (C2H5NaO4S) (SI) | 15.63 | |||
| Ethanol (C2H6O) (denatured) | 4.64 | |||
| Total | 100.00 | |||
SOURCE: BPBG (2013).
TABLE 2-5 Simulated Energetics Hydrolysate Used in VX FOAK Testing
| Constituent (with additives) | Wt% | |||
| Deionized water | 80.32 | |||
| Sodium chloride (NaCl) | 4.26 | |||
| Sodium sulfate (Na2SO4) | 10.24 | |||
| Sodium nitrite (NaNO2) | 0.32 | |||
| Sodium formate (NaCHO2) | 0.94 | |||
| 35% Hydrochloric acid (HCl) | 1.74 | |||
| 93% Sulfuric acid (H2SO4) | 0.91 | |||
| Sodium chloride (NaCl) | 1.28 | |||
| Total | 100.00 | |||
SOURCE: BPBG (2013).
TABLE 2-6 Simulated Blended Hydrolysate Used in VX FOAK Testinga
| Constituent (with additives) | Wt%b | |||
| Deionized water | 74.18 | |||
| Sodium sulfate (Na2SO4) | 7.40 | |||
| 56% Sodium isethionate (C2H5NaO4S) (SI) | 4.34 | |||
| Sodium hydroxide (NaOH) | 1.77 | |||
| Sodium chloride (NaCl) | 4.00 | |||
| 85% Diethanolamine (C4H11NO2) (DEA) | 2.15 | |||
| Dimethyl methylphosphonate C3H9O3P (DMMP) | 2.06 | |||
| Ethanol (C2H6O) (denatured) | 1.29 | |||
| 35% Hydrochloric acid (HCl) | 1.25 | |||
| Sodium formate NaCHO2 | 0.68 | |||
| 93% Sulfuric acid (H2SO4) | 0.65 | |||
| Sodium nitrite (NaNO2) | 0.23 | |||
| Total | 100.00 | |||
a2.5 parts simulated energetics hydrolysate to 1 part simulated agent hydrolysate.
bAgent hydrolysate simulant constituents are diluted further by the additives used in the energetics hydrolysate recipe; hence the dilution factor is 3.558, not 3.5. For DMMP, for example, we have [(102.33 × 2.5) + 100]/100 = 3.558; 5.91/3.558 = 1.66 wt%.
SOURCE: BPBG (2013).
- Ethane, 1-[(2-diisopropylamino)ethylthio]-2-[(2-diisopropylamino)ethyldithio]-
- N,N-Diisopropylformamide
- Urea, 1-(3-chloropropyl)-3-cyclohexyl-
- Urea, N,N′-bis(1-methylethyl)-
Chemical Weapons Convention Treaty Chemicals in Agent Hydrolysate
In addition to the chemicals in the agent hydrolysate simulant recipes and the trace components listed above for VX hydrolysate, the actual GB and VX hydrolysates may contain chemicals regulated by the Chemical Weapons Convention (CWC). This depends on the chemical reaction pathway that occurs during hydrolysis. The CWC identifies three classes of chemicals on the basis of the scale of their use in legitimate, nonweapons applications. Schedule 1 chemicals have no (or few) uses aside from chemical weapons. Schedule 2 chemicals have small-scale applications, and Schedule 3 chemicals have large-scale applications apart from chemical weapons. Chemicals in each schedule are classified as Part A (can be used directly as weapons) or Part B (can be used to manufacture chemical weapons). The agent hydrolysates may contain chemicals that are listed as Schedule 1A (i.e., EA2192). Schedule 2 compounds that might be present include ethyl methyl phosphonic acid (EMPA), methyl phosphonic acid (MPA), O-ethyl methyl phosphonothioic acid (EMPSH), and isopropyl methyl phosphonic acid (IMPA).
Energetics Hydrolysate Composition
In addition to the nerve agents GB and VX, the M55 rockets in the BGAD stockpile also contain energetic materials, i.e., bursters and propellant from rocket motors. This material will be physically separated during the dismantling of the rockets and will be neutralized at 240-300°F in the EBHs.2 The chemistry of the hydrolysis step and the resultant composition of the hydrolysate have been well documented. Representative compositions of energetics hydrolysate were given above in Tables 2-2 and 2-5. The energetic materials are expected to form C1 compounds such as formate and formaldehyde, along with N2O, NH3, and N2. One of the other by-products of energetics hydrolysis is cyanide. BGCAPP has established specific hydrolysis reactor conditions that are designed to significantly reduce the presence of cyanide in the hydrolysate before it is transferred to the SCWO facility.3 Other components likely to be present in the energetics hydrolysate include acetate, aluminum, ammonia,
______________
2 Only propellant from rocket motors contaminated with agent will be processed through the EBHs.
3 J. Barton, chief scientist, BPBG, “Cyanide Mitigation and Worker Protection,” presentation to the Kentucky Chemical Demilitarization Citizens’ Advisory Commission and Chemical Destruction Community Advisory Board on May 7, 2014, http://www.slideshare.net/acwanews/cyanide-mitigation-and-worker-protection-may-7-2014.
beryllium, calcium, chloride, chromium, cobalt, copper, fluoride, formate, HMX, iron, lead, magnesium, manganese, mercury, molybdenum, nickel, nitrate-N, nitrite-N, phosphorus, potassium, silver, sodium, sulfate, TNT, and zinc (Bonnett and Elmasri, 2002).
The rocket shipping and firing tubes (SFTs) contain polychlorinated biphenyls (PCBs). The PCBs were used as a lubricant to make it easier to slide the rockets into the SFTs.4 PCB concentrations in the SFTs range from under 50 parts per million (ppm) to more than 2,000 ppm. The SFTs may exit the BGCAPP processes with the rocket motors when separated from the nonleaking agent-filled warheads to be treated or disposed of at a permitted, offsite Toxic Substances Control Act facility. For approximately 200 “leaker” rockets, the rocket motors will be processed through the EBHs, and the hydrolysates from these EBH batches can be expected to contain PCBs leached from the SFTs. BGCAPP calculated that a conservative estimate of the PCB concentrations in each batch of energetics hydrolysate would be 44 ppm.5
Dissolved aluminum is present in the energetics hydrolysate because both the M56 warhead and the fuze in an M55 rocket are made of mostly aluminum. During energetics hydrolysis some of this aluminum will react with water to form soluble aluminum species. This aluminum is particularly problematic for the SCWO process, as past studies have shown that aluminum forms solid precipitates in the SCWO reactor that interfere with the flow of materials through the reactor. As a result, most of the aluminum must be removed from the alkaline energetics hydrolysate before it can be blended with agent hydrolysate and treated by SCWO. The energetics hydrolysate will therefore be treated by adding acid to precipitate the aluminum as aluminum hydroxide, after which the slurry will be dewatered using a filter press. The filter cake will then be collected and sent for offsite disposal. The liquid filtrate from the belt press will be collected in storage tanks where it will be kept for blending with agent hydrolysate prior to being fed to the SCWO.
GENERAL DESCRIPTION OF SUPERCRITICAL WATER OXIDATION
SCWO has been studied for nearly 40 years. Several companies have developed technologies to use SCWO for different applications, including the treatment of diverse waste materials, water purification on long-term manned space missions, recovering precious metals from catalytic materials, and producing heat and power (e.g., SCWO of coal–water slurries). This extensive experience with SCWO has revealed that it can be very effective for a wide range of feed materials and that the main long-term operating challenges relate to corrosion and the management of salts in the reactor.
The SCWO process can mineralize organic materials by reacting them with an oxidant (often O2 in air) in water above its critical temperature and pressure [Tc = 705°F (374°C), Pc = 218 atm (3,200 psi)]. Organic carbon is converted to CO2, hydrogen is converted to water, and N, S, and P heteroatoms are converted to N2, sulfate, and phosphate, respectively. The elementary chemical reactions that occur during SCWO are analogous to those that occur during combustion. However, the lower temperature, higher pressure, and abundance of water in SCWO alter some of the reaction pathways from those expected in combustion.
When a mixture is above its thermodynamic critical temperature, it can exist only as a single fluid phase. Coexisting liquid and gas phases cannot form. Thus, SCWO allows the organic material, the oxidant, and water to exist in a single fluid phase under reaction conditions. This absence of fluid phase boundaries prevents interphase transport processes from limiting reaction rates. Consequently, SCWO reactions are rapid, and complete mineralization can often be achieved in seconds.
Since the oxidation reaction is exothermic, the temperature of the reactor effluent [e.g., 1,112°F (600°C)] typically exceeds that of the reactor feed. This thermal energy from the reaction can be captured in a well-engineered process through heat integration and then used again in the process. In short, SCWO provides an option for the rapid and nearly complete oxidative destruction of organic material. The process allows for analysis of the effluent before release so that one can be assured that desired destruction efficiencies are being achieved.
The SCWO environment can be corrosive, especially if a region exists in the process where the aqueous feed stream is in the dense, near-critical state [around 572-662°F (300-350°C)]. The presence of halogens also increases corrosion rates. There have been different approaches to handling corrosion in SCWO systems, including preventing corrosive species from reaching reactor surfaces, altering the feed stream composition or processing conditions to reduce corrosion, or managing corrosion by using sacrificial reactor liners made from materials such as titanium.
Though liquid water is a good medium for dissolving salts, supercritical water is not. It has a much lower density and far fewer hydrogen bonds, so it is not a favorable medium for ion formation. Indeed, ions in supercritical water typically exist as ion pairs. Thus, any salts present in the feed stream and any salts that form during SCWO may precipitate under SCWO operating conditions. This can lead to salts accumulating in, and plugging, the SCWO reactor. Different methods have been examined for dealing with salt precipitation or accumulation in SCWO reactors. These methods include avoiding precipitation by operating at high densities (very high supercritical pressures), allowing precipitation but avoiding accumulation (e.g., by keeping the salts mov-
______________
4 Annual Status Report on the Disposal of Chemical Weapons and Materiel for Fiscal Year 2007, www.peoacwa.army.mil/wp-content/uploads/annual_status_report_disposal_of_chemical_weapons_materiel_fy07.pdf.
5 Battelle Calculation Continuation Sheet, Concentration of PCBs in one EBH batch, no date.
ing through the reactor), and allowing both precipitation and accumulation but periodically removing the salt deposits from the reactor walls (e.g., by brushing or scraping).
Supercritical Water Oxidation at BGCAPP
BGCAPP has three identical vertically-oriented SCWO tubular reactors that will operate in continuous flow mode at about 1,150°F (621°C) for GB hydrolysate and 1,175°F (635°C) for VX hydrolysate, and 235 atm (3,454 psi). Each reactor is 10 ft high and 7-5/8 in. in diameter. The reactor residence time is about 10 sec. Hydrolysate, fuel (isopropanol), and air enter the top of the reactor and effluent exits from the bottom, where quench water is added (see Figure 2-1). Each reactor can process 1,000 lb/hr of blended hydrolysate feed.
Both corrosion and salt management are relevant concerns for the SCWO process at BGCAPP. Phosphate and fluoride ions, which are known corrosive agents, will be present in the feed to the SCWO reactor. BGCAPP will deal with corrosion by employing a grade 2 titanium reactor liner that will be periodically replaced as it corrodes. Replacement every 300-400 hours is anticipated, depending on the agent hydrolysate being treated. Salts will be managed by adding sulfur and chloride to the feed such that a mixture of NaCl and Na2SO4 will coprecipitate from solution in the SCWO reactor. This salt mixture is a liquid under a known range of conditions, so it will flow down the vertical reactor to the quench zone at the bottom and should not cause deposits or plugging in the upper reactive reaches of the SCWO reactor.
As discussed above, BGCAPP will use SCWO to treat blended agent (GB or VX) and energetics hydrolysates. Previous pilot-scale work verified that SCWO can achieve high destruction efficiencies for actual hydrolysate produced from both nerve agents and from energetic materials as well as for blended hydrolysate (BPBGT, 2014). Additionally, FOAK testing showed that the reactors to be used at BGCAPP can operate successfully for extended periods of time when the feed stream is simulated hydrolysate. FOAK testing provided 756 hr (not continuous) of reactor operation with simulated hydrolysates (BPBG, 2013). FOAK testing showed that the total organic carbon content in the effluent was <5 ppm and that the salts management strategy was effective. Moreover, the FOAK testing showed that replacement of the thermowells would need to be more frequent (about every 100 hr) than replacement of the SCWO reactor liner (about every 300-400 hours of operation) (BPBGT, 2014).
Finding 2-2. Only hydrolysate simulants have been processed through the full-scale SCWO unit during FOAK testing. Actual hydrolysate, however, has been tested at the pilot scale.
Finding 2-3. SCWO has been demonstrated to be a robust method for treating a wide range of organic wastes, including both actual and simulated hydrolysates. Lack of knowledge of the precise composition of the hydrolysates and their variability does not diminish confidence in the ability of SCWO to destroy the organic carbon present therein.
DESCRIPTION OF THE BGCAPP WATER RECOVERY SYSTEM
A reverse osmosis (RO) system is to be used to desalinate a blend of SCWO effluent, cooling tower blowdown water, and steam boiler blowdown water.6 Water softeners will be used to remove calcium from the cooling tower and steam blowdown water, to avoid fouling of the RO units. According to BPBG, 2007, ion exchange beds will be used to soften this stream prior to blending it with the SCWO effluent. The RO system is intended to produce a permeate stream and a reject stream. The permeate will be recycled as quench water for the SCWO reactors. The spent softener regenerant7 will be combined with RO reject water for final disposal.
The WRS system was designed to do the following:
- Operate with an efficiency of 70 percent water recovery with a maximum of 500 mg/L total dissolved solids in the permeate and
- Ensure one and a half day’s storage of RO permeate to permit SCWO operation in case the WRS is temporarily not operating.8
To accomplish these operations, the WRS will have the following equipment:
- Three SCWO effluent storage tanks, where the effluent will be analyzed to ensure that the total organic carbon (TOC) concentration is less than 10 ppm;
- A conventional pretreatment system consisting of coagulation, media filtration, and antiscalant addition;
- Three spiral wound RO units (two operational, one spare); and
- Storage tanks to hold RO permeate to periodically clean the RO membranes and to provide SCWO reactor quench water.
The pretreatment portion of the WRS will remove suspended solids, while the RO system will reduce the total dissolved solids. Figure 2-2 shows the flow of material from hydrolysis, through the SCWO process (shown in
______________
6 Blowdown water is water that is drained from cooling equipment or boilers to remove minerals that accumulate over time. By definition, they tend to concentrate calcium and other dissolved impurities.
7 The regenerant is the waste solution resulting when a high concentration solution of NaCl is used to renew the hardness removal capacity of the ion exchange resin. It typically contains high concentrations of NaCl, calcium salts, and magnesium salts.
8 NRC Request Update, NRC#08, received via e-mail on February 16, 2015.
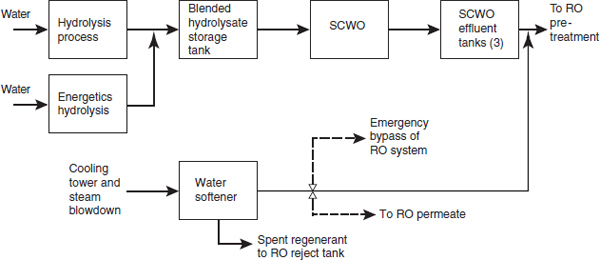
FIGURE 2-2 Flow of material from hydrolysis, through SCWO, and until the pretreatment step in the WRS. SOURCE: NRC, 2012.
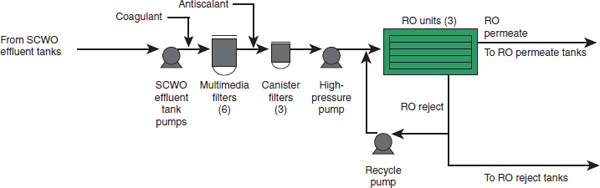
FIGURE 2-3 Process flow diagram for the WRS, including the pretreatment and RO system. SOURCE: NRC, 2012.
Figure 2-1), up to the pretreatment step in the WRS. It also indicates where the cooling tower and steam blowdown is blended with the SCWO effluent. Figure 2-3 shows the flow of material through the WRS.
Bonnett, P.C., and B. Elmasri. 2002. Base Hydrolysis Process for the Destruction of Energetics Material. AD-E402 946. Picatinny Arsenal, N.J.: U.S. Army Armament Research, Development and Engineering Center.
BPBG (Becthel Parsons Blue Grass). 2007. SCWO Building Water Recovery—R.O. Unit Process Flow Diagram, Rev. 6, Sheet 1/2, October 2. Richmond, Ky.: Blue Grass Chemical Agent Destruction Pilot Plant Project.
BPBG. 2013. Test Report for Supercritical Water Oxidation (SCWO) First-of-a-Kind (FOAK) Test (Preliminary Draft). SDN-24915-00-GQYGGEN-00094. Richmond, Ky.
BPBGT (Becthel Parsons Blue Grass Team). 2014. Characterization of Supercritical Water Oxidation (SCWO) Operating Requirements, Rev. 0. 24915-00-30R-SCWO-00001. Richmond, Ky.
Dejarme, L., and G.D. Lecakes. 2008. Bench-Scale Evaluation of VX Hydrolysis, TRRP #11, Test Report, Rev. 2. Aberdeen, Md.: Battelle Eastern Science and Technology Center.
Malloy IV, T.A., L. Dejarme, C. Fricker, J. Guinan, G.D. Lecakes, and A. Shaffer. 2007. Bench-Scale Evaluation of GB Hydrolysis, TRRP #02a Phase II, Test Report, Rev. 0. Aberdeen Proving Ground, Md.: Program Manager for Assembled Chemical Weapons Alternatives.
NRC (National Research Council). 2012. Letter Report: The Blue Grass Chemical Agent Destruction Pilot Plant’s Water Recovery System. Washington, D.C.: The National Academies Press.
NRC. 2013. Assessment of Supercritical Water Oxidation System Testing for the Blue Grass Chemical Agent Destruction Pilot Plant. Washington, D.C.: The National Academies Press.







