A6
DRIVERS, DYNAMICS, AND CONTROL OF EMERGING VECTOR-BORNE ZOONOTIC DISEASES1
A. Marm Kilpatrick2and Sarah E. Randolph3
Summary
Emerging vector-borne diseases are an important issue in global health. Many vector-borne pathogens have appeared in new regions in the past two decades, while many endemic diseases have increased in incidence. Although introductions and emergence of endemic pathogens are often considered to be distinct processes, many endemic pathogens are actually spreading at a local scale coincident with habitat change. We draw attention to key differences between dynamics and disease burden that result from increased pathogen transmission after habitat change and after introduction into new regions. Local emergence is commonly driven by changes in human factors as much as by enhanced enzootic cycles, whereas pathogen invasion results from anthropogenic trade and travel where and when conditions (eg, hosts, vectors, and climate) are suitable for a pathogen. Once a pathogen is established, ecological factors related to vector characteristics can shape the evolutionary selective pressure and result in increased use of people as transmission hosts. We describe challenges inherent in the control of vector-borne zoonotic diseases and some emerging non-traditional strategies that could be effective in the long term.
Introduction
In the past three decades, many vector-borne pathogens (VBPs) have emerged, creating new challenges for public health (Weaver and Reisen, 2010). Some are exotic pathogens that have been introduced into new regions, and others are endemic species that have greatly increased in incidence or have started to infect local human populations for the first time. Here, we review the drivers of these processes. Of particular interest are zoonoses that are maintained by transmission in wildlife but also affect people who have been bitten by infected vectors. Additionally, we draw from lessons learned from diseases that now use only people as transmission hosts, such as malaria and dengue.
___________________
1 Reprinted from Lancet, Vol. 380, Pages 1946−1955. Copyright 2012, with permission from Elsevier.
2 Department of Ecology and Evolutionary Biology, University of California, Santa Cruz, Santa Cruz, CA, USA.
3 Oxford Tick Research Group, Department of Zoology, University of Oxford, Oxford, UK.
Clinicians have an important role alongside disease ecologists and epidemiologists in the study of the causes of an outbreak and minimisation of the burden of disease, because the effectiveness of control is improved by rapid identification (Lloyd-Smith et al., 2003; Ferguson et al., 2006). In many cases, clinicians are on the first line of detection of these epidemics because clusters of patients present with novel sets of symptoms; evidence of new outbreaks then has to be passed to public health agencies for appropriate management. New high-throughput technologies for detection and identification of novel genetic material in samples taken from patients can greatly aid this process (Gaynor et al., 2007; Lipkin, 2010). Additionally, data obtained via mobile phones and online social networks checked against expert assessment of plausibility offer the potential to detect changes in spatial and temporal patterns of illness in real time so that new epidemics can be detected early (Brownstein et al., 2009).
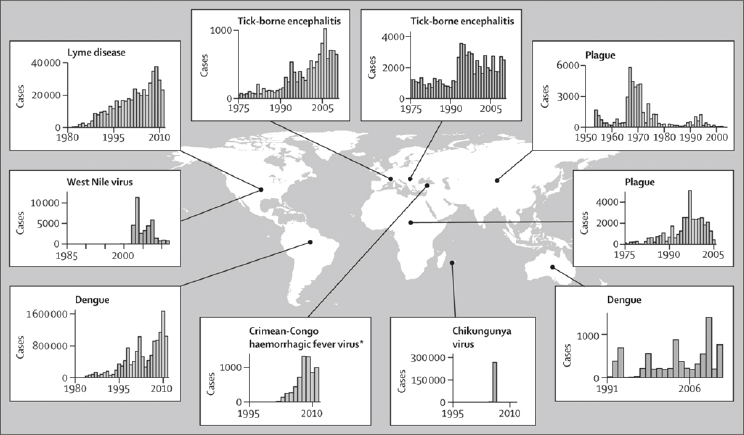
West Nile virus and chikungunya virus are among the best understood zoonotic VBPs to have emerged in the past two decades and show just how explosive epidemics can be in new regions (see Figure A6-1). In 1999, the New York City Department of Health (NY, USA) reported a cluster of patients with meningoencephalitis associated with muscle weakness; epidemiological evidence suggested that an arbovirus (ie, a virus transmitted by arthropod vectors) was a probable
cause (Nash et al., 2001). Clinicians and veterinarians collaborated to identify the aetiological agent as West Nile virus, but unfortunately identification and initial control efforts did not prevent the virus spreading from the east to the West Coast of North America within 4 years (CDC, 2012a; Kilpatrick, 2011), causing national epidemics in 2002 and 2003.
Similarly, on the Indian Ocean island of Réunion in 2005, hundreds of patients had painful and disabling polyarthralgia, and a subset presented with neurological signs or fulminant hepatitis (Pialoux et al., 2007). A second wave of such symptoms in 2006 exceeded all expectations, eventually affecting more than a third of the entire population of 770,000 people (Pialoux et al., 2007). The causative agent was identified as chikungunya virus, which is also causing continuing epidemics in India, with several million cases so far (Pialoux et al., 2007; Yergolkar et al., 2006; Schuffenecker et al., 2006). Other introductions of VBPs have caused smaller outbreaks but have been important in the expansion of the range of human populations at risk. For example, dengue virus has spread to Hawaii (Effler et al., 2005), Zika virus to the Micronesian island of Yap (Duffy et al., 2009), and chikungunya virus to Europe (Rezza et al., 2007). Whether the outbreak of chikungunya in Europe died out naturally because of the arrival of the temperate autumn or was interrupted by mosquito control efforts is unclear.
These past experiences—together with increases in the known drivers of pathogen introduction—suggest that future introductions are likely (see Table A6-1). A key challenge arises from the non-specificity and similarity of symptoms caused by many of these viruses, especially Zika virus, dengue, and chikungunya virus that all present with acute fever similar to many diseases endemic in the tropics, such as malaria (San Martin et al., 2010; Duffy et al., 2009). This difficulty makes rapid identification methods (Gerstl et al., 2010) and high-quality laboratory-based diagnoses necessary for accurate surveillance and appropriate treatment. Recent advances in identification of unknown pathogens with deep sequencing and microarrays should enable rapid identification of novel or introduced pathogens (Yozwiak et al., 2012). A key need is to develop diagnostics for point-of-care use for infection and exposure to allow for proper assessments of case fatality ratios and disease burden.
The emergence of endemic VBPs is usually thought to be a qualitatively different process from the arrival of exotic ones, but in some cases increases in incidence of endemic VBPs result more from spread into new areas than increases in local transmission. A combination of local spread and an increase in transmission potential in situ is also possible. Lyme disease is perhaps the best understood example of a mixed emergence. Reported cases (and estimated illnesses) have roughly tripled since 1990 in the USA (see Figure A6-1), appeared increasingly in Canada (Ogden et al., 2009), and apparently increased by between two and ten times in various parts of Europe where diagnosis and reporting are more variable. Evidence for the importance of local invasion in the USA comes from counties in the states of Wisconsin and Virginia, where Lyme cases have
| Regions at Risk | Endemic Region | Pathways for Introduction* | |
|---|---|---|---|
| Japanese encephalitis virus | Americas | Asia | Infected livestock |
| Rift Valley fever virus | Americas, southern Europe | Africa, Asia | Infected livestock |
| Venezuelan equine encephalitis virus | Europe, Asia, Africa | Americas | Infected livestock |
| Chikungunya virus | Europe, Americas, Australia | Africa, Asia | Infected people |
| Mayaro virus | Africa, Asia, Europe | South America | Infected people |
| Zika virus | Europe, Americas | Africa, Asia | Infected people |
| Crimean-Congo haemorrhagic fever | North Africa, east Asia, central and western Europe | Africa, Asia, Europe | Infected livestock |
| Dengue virus | Southern Europe | Southern hemisphere | Infected people |
| West Nile virus | Central Europe, Turkey | Africa, Asia, Europe, Australia | Migratory or dispersing birds |
| Sindbis virus | Northern Europe | Africa, Asia, Australia | Migratory or dispersing birds |
* Infected mosquitoes transported via aeroplanes are a potential pathway for all these pathogens (except Crimean-Congo haemorrhagic fever which is tick borne) in addition to pathways listed.
SOURCE: Adapted from Kilpatrick et al., 2006a.
only been reported in the past decade and few if any cases occurred previously (CDC, 2012). By contrast, in the state of Connecticut—where the first cases of Lyme were detected 30 years ago—incidence of the disease has hardly risen in the past decade (CDC, 2012b).
In Europe and Eurasia, the substantial rise in cases of Lyme disease and other tick-borne diseases, including babesiosis, ehrlichiosis, and rickettsiosis, and tick-borne encephalitis, is due as much or more to upsurges within pre-existing ranges of the vector ticks (principally Ixodes ricinus and Ixodes persulcatus) as to the establishment of enzootic cycles in new places. Zoonotic VBPs with other types of vectors also represent an important and growing threat in some places, such as those that cause Chagas disease, plague, and leishmaniasis (Hotez et al., 2008). Strong evidence suggests that ecological and human factors have had important roles in establishment of the differential patterns of increased incidence of all these diseases, while increasing awareness and testing by clinicians has contributed to improved reporting of cases.
Differences in the drivers of emergence of exotic and endemic VBPs have important implications for their subsequent dynamics, where they will emerge,
* Crimean-Congo haemorrhagic fever virus is shown as endemic to Turkey because there is evidence of its presence there many years before its appearance in people.
and the efforts that can be made to control or eliminate them. We consider each of these aspects in turn, illustrated by some of the more notable examples across the globe. We argue that viewing emerging endemic pathogens as invading at a local scale can be used to take a prospective approach to prevention and control.
Emergence of Exotic Versus Endemic Pathogens
Arrival of Exotic Pathogens
The main driver of pathogen introductions in the past five decades—the accelerating increase in trade and travel—is well known. What is less discussed
is that four centuries of trade and travel set the stage for many present pathogen introductions. In the 17th to 19th centuries, shipping traffic resulted in the transport of larvae of several important mosquito species, such as Aedes aegypti (a vector of dengue, yellow fever, chikungunya virus, and others), Culex pipiens (a vector of West Nile virus) and Culex quinquefasciatus (a vector of West Nile virus and filariasis) (Fonseca et al., 2006; Bryant et al., 2007; Farajollahi et al., 2011).
Some pathogens (eg, Plasmodium vivax) were introduced to new continents and became established coincident with or shortly after these early vector introductions because they cause chronic infections in people that are still infectious after weeks or months of travel (Mendis et al., 2001). Other pathogens that have only short periods of infectiousness in people, including yellow fever virus and dengue virus, could also reach distant regions centuries ago because pathogen transmission cycles could occur aboard ships in which vectors were present and could reproduce (Farajollahi et al., 2011).
The growth in air travel enabling global transit in a single day (see Figure A6-2) has accelerated introductions because it has allowed many pathogens that cause acute infectiousness (eg, chikungunya and West Nile viruses) to reach other continents within the few days that hosts are infectious, and even during the latent period for some diseases (Kilpatrick et al., 2006a). Several of
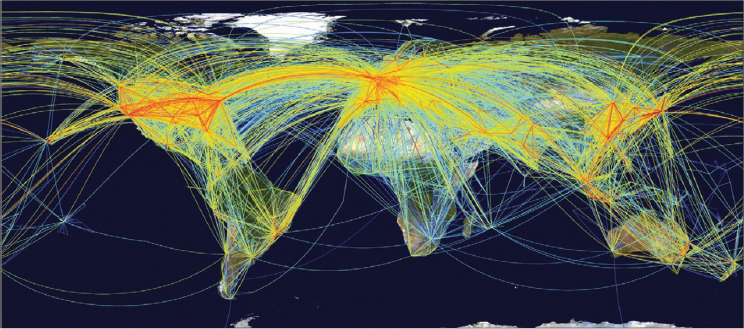
SOURCE: Adapted from Hufnagal et al., 2004.
these pathogens were also aided by the 20th century introductions of another key vector, Aedes albopictus (Reiter, 2010; Tatem et al., 2006). Thus, the most recent wave of pathogen introductions, and those likely to occur in the near future, take place against the backdrop of centuries of vector introductions that enable establishment.
A key result of an already well established vector population and a highly suitable environment (including hosts and climate) is that many introduced pathogens cause explosive epidemics in which a large fraction of the population at risk is infected in the first few years after introduction (see Figure A6-1). High vector populations (relative to host abundance) result in a high basic reproduction number (R0) of the pathogen, and if the host population is immunologically naive to the introduced pathogen, as is usually the case, then the effective pathogen reproduction number (Reff) is close to the maximum R0. This high Reff leads to another common pattern, which is that the intense and rapid initial spread of a novel pathogen is frequently followed by a substantial decrease in case burden shortly after introduction, especially on a local scale, as the fraction of the population that is immune to infection rises (Kilpatrick, 2011). This pattern both contrasts with, and has similarities to, the emergence of endemic diseases.
Emergence of Endemic Pathogens
Emergence of endemic VBPs is frequently associated with changes in land use (Lambin et al., 2010) or socioeconomic conditions (Randolph, 2010), and these transitions control the dynamics of disease emergence. For pathogens affected by land-use change, the rise in case numbers is often gradual (see Figure A6-1), paralleling changes in the pathogen’s abiotic and biotic environment. By contrast, the increased incidence of endemic disease driven by changes in socioeconomic conditions can be abrupt if the shift is rapid, such as that caused by political upheavals, military conflicts, or natural disasters (see Figure A6-1).
Changes in land use affect VBPs by altering the interactions and abundance of wildlife and domestic hosts, vectors, and people, with some diseases better understood than are others (Lambin et al., 2010). In the Amazon and east Africa, deforestation increases standing water and sunlight and enhances the breeding success of some mosquito species, which can increase risk of malaria. Further increases in urbanisation frequently eliminate anopheline mosquito habitat and have reduced malaria elsewhere (Yasuoka and Levins, 2007). In northeastern North America, reforestation during the 20th century is thought to have allowed recolonisation by deer and the consequent expansion of the range of ticks (Ixodes scapularis), underpinning the emergence of Lyme disease in the mid-20th century (Barbour and Fish, 1993). Deer (Odocoileus virginianus in the USA and Capreolus in Europe) have a key role in feeding adult Ixodes ticks, although they are actually incompetent hosts for the Lyme disease bacterial spirochaetes. Additionally, in the past three decades, fragmentation of forests in eastern regions
of Canada and the USA and changes in predator communities (Levi et al., 2012) have altered the host community for ticks and the Lyme bacterium Borrelia burgdorferi, and might have increased relative abundance of small mammals (white-footed mice [Peromyscus leucopus], eastern chipmunks [Tamias striatus], and shrews [Sorex spp and Blarina brevicauda]) that are the principal transmission hosts for Lyme disease spirochaetes. These changes in the host community can result in increased spirochaete infection prevalence in nymphal ticks (Logiudice et al., 2008). A key remaining question is how fragmentation and hunting-induced changes in the host community affect the abundance of infected nymphal ticks, which is the key metric for disease risk.
Changes in land use might also be responsible for recent emergent foci of Crimean-Congo haemorrhagic fever virus within its large range through parts of Africa, Asia, southeastern Europe, and the Middle East. By contrast with typical sporadic outbreaks of only a few cases, an exceptional epidemic occurred in Turkey, starting with about 20 cases in 2002, and rising to nearly 1,400 cases by 2008 (see Figure A6-1). Most infections occurred in agricultural and animal husbandry workers via tick bites and direct contamination from infected animals. Changes in land cover associated with political unrest and reduced agricultural activities might have allowed colonisation by wildlife and subsequent tick population growth, as is thought to have precipitated the first recorded epidemic of Crimean-Congo haemorrhagic fever in Crimea in 1944–45 (Hoogstraal, 1979). The case fatality rate (5 percent) in Turkey has been much lower than is usually observed (20–30 percent) (Hoogstraal, 1979; ErgÖnül, 2006), creating some uncertainty about the cause of this epidemic. This uncertainty emphasises the need for accurate and systematic diagnosis through effective point-of-care methods.
Increases in incidence can also result from changes in socioeconomic and human activities, such as expansion into risky new habitats for exploitation or dwelling, or land-cover change, such as reforestation of previously agricultural areas (Barbour and Fish, 1993; Chaves et al., 2008; Barrett and Higgs, 2007; Hay et al., 2005). Human infection with VBPs increases with the product of entomological risk (the abundance of infected vectors) and exposure of people to vectors, which can change independently and sometimes synergistically. For example, the incidence of dengue is higher on the Mexican side of the Mexico–Texas border than on the other (Reiter et al., 2003), where open windows compensate for the absence of air-conditioning but expose people to mosquito biting.
Exposure to ticks, paradoxically, might be higher in people of high and low income than in those with intermediate income (see Figure A6-3). Incidence of Lyme disease in parts of Europe has been shown to be higher in people with high income living in new homes in broad-leaf woodlands where wildlife co-occur, including rodents and birds that serve as reservoirs for spirochaetes and ticks (Linard et al., 2007). Generally, outdoor recreational opportunities associated with wealth can result in increased exposure to vectors. Conversely, hardship precipitated by population displacements due to civil conflict, loss of protective
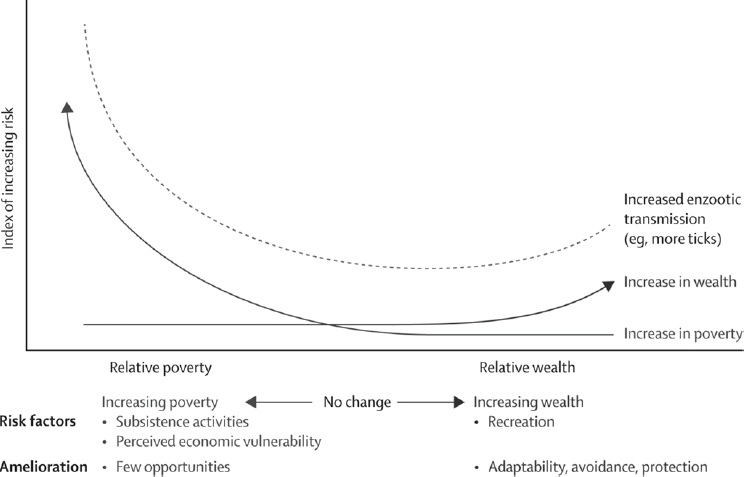
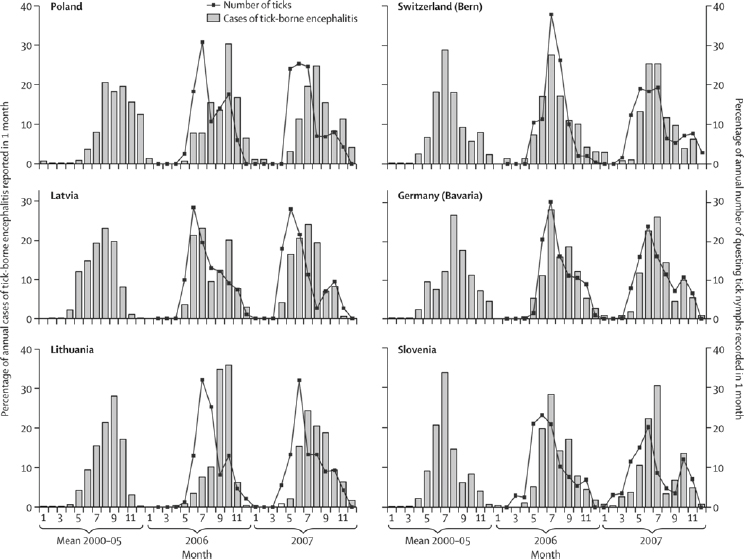
housing through natural disasters, or use of natural environmental resources driven by economic transitions can lead to increased contact between people and vectors (Randolph, 2010; Beyrer et al., 2007). A clear example comes from a large upsurge of tick-borne encephalitis in 2009, immediately after the economic downturn in three eastern European countries that already had high background poverty and where foods are harvested from forests for subsistence and commerce (Godfrey and Randolph, 2011). Human activities resulting in exposure to VBPs is sometimes reflected in different seasonal patterns, such as cases of tick-borne encephalitis in different parts of Europe (see Figure A6-4). In eastern Europe, the timing of cases matches the season of forest food harvest more closely than the seasonal pattern of tick abundance, while in western Europe the earlier peak of cases coincides with summer recreational activity.
Poverty and wealth, however, probably affect final disease outcomes asymmetrically, because economic duress restricts the potential for ameliorative actions (eg, limiting of outdoor activities, protection from vector bites, or costly vaccination in the case of tick-borne encephalitis). This hypothesis could partly explain the difference between a large upsurge (two to 30 times) in reported tick-borne encephalitis cases in the early 1990s in central and eastern Europe
after the fall of Soviet rule and a gradual and steady increase in western Europe (Figure A6-1) (Randolph, 2010). Political and civil unrest that commonly occur with armed conflict could also account for the sudden re-emergence of plague in Vietnam in the late 1960s and in Madagascar and Mozambique at the end of the 1980s (Duplantier et al., 2005). Failure of public health services and overcrowded, unsanitary living conditions increased human contact with flea-bearing rodents and decreased routine surveillance, allowing rapid emergence with no warning. These examples of social strife enabling new epidemics of vector-borne diseases will probably recur, and awareness and investment in public health infrastructure can help to reduce their effect.
Understanding of the mechanistic processes linking land use and socioeconomic conditions with disease enables prediction of future trends and control or mitigation. Economic and public health assistance could be targeted towards populations at high disease risk because of social strife caused by conflict or natural disasters, and urban planning could be used to minimise the use of risky habitat by people for living and recreation. Unfortunately, although correlations exist between land use and disease incidence or measures of risk, rigorous and
mechanistic analyses that identify causal factors that are needed for intelligent urban planning are absent in most cases. For example, in the USA, specific types of land use (agriculture and urbanisation) are associated with a higher incidence of West Nile virus in people at the county scale, but the mechanism underlying this pattern is unknown (Kilpatrick, 2011; Bowden et al., 2011). This gap in our knowledge makes it difficult to anticipate and avoid future epidemics associated with rapid urbanisation and land-use change.
Climate Change and Vector-Borne Diseases
Although several components of vector-borne disease systems (principally the vector and the pathogen) are highly sensitive to climate, evidence shows that climate change has been less important in the recent emergence of vector-borne
diseases than have changes in land use, animal host communities, human living conditions, and societal factors, probably because of countering influences of climate (see Box A6-2). An exception seems to be the increased transmission of VBPs with warming along the cold latitudinal and altitudinal edges of their present distribution. The differential effect of climate at the edge and core of a pathogen’s distribution stems partly from the non-linear relation between the fraction of the population exposed in an epidemic and transmission potential (which can be quantified as R0). Specifically, initial increases in R0 to more than one (ie, allowing pathogen spread to create an epidemic) lead to large rises in case burden, but further increases in R0 have diminishing effects, especially for pathogens with sterilising immunity. Expansions in the distribution of a disease might have disproportionate effects on public health if the newly exposed populations have little immunity. Examples of VBP range expansions along cold edges are dengue virus in
Texas, USA (Brunkard et al., 2007), Lyme disease in Canada (Ogden et al., 2009), and tick-borne encephalitis at increasing altitude in Slovakia (Lukan et al., 2010).
In core transmission areas, not only are the effects of climate change less important than other factors, but warming might even decrease transmission if decreases in vector survival overwhelm other factors (Randolph and Rogers, 2000) (see Box A6-2). An analysis of several decades of severe malaria incidence (the best studied disease with respect to climate change) at five locations spanning a range of elevations in western Kenya identified initial rises in incidence followed by two decades of decreases at two locations and increases with high variability in three others (Chaves et al., 2012). These mixed patterns challenge expectations that continuing climate change will lead to increased malaria and suggest that changes in transmission potential of malaria and other VBPs are primarily driven instead by a mix of factors such as demographic shifts, land-use change, interventions (eg, bed nets), drug resistance, and climate. The relative contributions of each factor can be rigorously assessed only by careful comparisons of the same pathogen over time and with valid accurate baseline data, which were lacking in a previous study (Gething et al., 2010).
Evolution of Vector-Borne Pathogens
One underappreciated aspect of growing human populations, global land-use change, and the introduction of human commensal vectors is the selective pressure exerted on pathogens, causing them to evolve to take advantage of new environments, including hosts and vectors. Both West Nile virus and chikungunya evolved rapidly (a feature typical of viruses and especially RNA viruses) (Holmes, 2003) after being introduced to new locations and encountering new anthropophilic vectors. The original genotype of West Nile virus (NY99) was replaced by another (WN02) (Davis et al., 2005) that differs by three consensus nucleotide changes and exhibits increased transmission efficiency in C pipiens and Culex tarsalis mosquitoes (Moudy et al., 2007; Kilpatrick et al., 2008). Similarly, on Réunion between 2005 and 2006, one nucleotide change occurred in chikungunya virus that increased infection in the recently introduced mosquito species Aedes albopictus (Tsetsarkin and Weaver, 2011). The same genetic change appeared independently in viruses isolated from Réunion, west Africa, and Italy, but was not identified in mosquitoes from India at the start of the continuing epidemics there in 2006 (de Lambellerie et al., 2008). When A albopictus rather than A aegypti became the main vector in India from 2007, however, the same genetic substitution spread rapidly and subsequent substitutions seem to be enabling even more efficient virus circulation and persistence, which could presage further expansion of the chikungunya virus (de Lambellerie et al., 2008).
More generally, the transmission of many VBPs is less efficient when the vector feeds on several hosts, only some of which can be infected by the pathogen (Kilpatrick et al., 2007). It is no coincidence that the dominant human VBPs
malaria and dengue are transmitted most intensely where they are vectored by mosquitoes that feed almost entirely on people. What has been less appreciated is the selective pressure imposed on zoonotic pathogens (especially those for which people are still a dead-end host) to adapt to be efficiently transmitted by human specialist vectors like Anopheles gambiae, A aegypti, and, to a slightly lesser extent, A albopictus (which sometimes feeds on non-human mammals and birds) where people are highly abundant. As the abundance of human commensal vectors increases with urbanisation and deforestation, so do the opportunities for strictly human transmission of pathogens.
Control of VBPs
Novel introductions and increases in incidence of endemic VBPs draw attention to the need for effective control and treatment of individuals with associated diseases. A key challenge in the attempt to control many VBPs is that they are zoonotic and transmission intensity in vectors is driven primarily by wildlife reservoirs. As a result, the dominant method used to control directly transmitted pathogens—vaccines—protects only individuals with financial and logistical access and has no effect on underlying transmission intensity. Thus, natural or vaccine-acquired herd immunity has no protective effect in people, and exposure is governed primarily by contact with vectors.
Control of zoonoses in wildlife is difficult at best, and eradication is often impossible (Barrett and Higgs, 2007). Vaccines for wildlife hosts—in development for West Nile virus (Kilpatrick et al., 2010) and field tested at a small scale for Lyme borreliosis (Tsao et al., 2004)—offer some reasons for optimism, but substantial work remains before they can be deployed as effective instruments on a large scale. Additionally, for vector-borne pathogens, transmission is thought to be frequency dependent, such that culling of livestock or wildlife that decreases host abundance (short of eradication) might increase transmission. Vectors are likely to seek out, feed on, and infect the hosts that remain after culling efforts, and the remaining hosts will subsequently be fed on by a greater number of susceptible vectors per host than they were before culling (Wonham et al., 2004). Control of frequency-dependent pathogens by culling would thus be expected to result in short but intensified epizootics that could lead to additional human infections, with the exact public burden depending in part on patterns of vector feeding on people and other hosts (Kilpatrick et al., 2006c, 2007).
Another control strategy used for VBPs, active or passive use of animals to divert vector feeding away from people to protect them against infection (so-called zooprophylaxis) (Hess and Hayes, 1970), has had mixed effects. Feeding on additional alternative hosts sometimes results in increased vector densities, which could result in higher transmission even if a smaller proportion feed on people (Yamamoto et al., 2009; Cohen and Gurtler, 2001). A more recent incarnation of this basic idea—termed the dilution effect—postulates that naturally
occurring biodiversity could, in some instances, also divert vectors from infectious hosts (Ostfeld and Keesing, 2000). As with empirical attempts of zooprophylaxis, the effects of biodiversity, or, more accurately, variable host community assemblages, are not uniform with respect to risk of infection, because of the complexity of interactions between hosts, vectors, and pathogens (Randolph and Dobson, 2012; Kilpatrick et al., 2006b). The more direct strategy of vector control targeted at larval mosquitoes (including elimination of larval habitat) has been more effective than has zooprophylaxis and has even resulted in local eradication of a disease (Killeen, 2003). Additionally, new techniques to develop vectors resistant to pathogens by infecting them with naturally occurring intracellular insect parasites (eg, Wolbachia) offer some promise (Hoffman et al., 2011).
In many cases, the most effective long-term public health strategies will combine efforts by clinicians and public health officials to treat and alter the behaviour of patients to avoid infection with actions by others to reverse the ecological drivers of transmission. Behavioural change is especially important at the leading edge of invading endemic or exotic pathogens where personal protective behaviours are often absent. Reversal of ecological drivers of disease emergence necessitates identification of the causes of increases in incidence and subsequent targeting with appropriate control measures, which needs integration between researchers, public health agencies, the government, and the public. For example, risk related to specific types of land use could be ameliorated by urban planning and management of host and vector communities through landscaping, hunting, or restoration of ecological communities.
Similarly, increases in incidence related to socioeconomic changes could be reduced with prudent development and assistance after disasters and social upheaval (Bogich et al., 2012). The vaccination campaign against tick-borne encephalitis, for example, targeted children in Latvia in response to the massive upsurge in incidence in the early 1990s. This campaign, together with a reduction in high-risk activities in tick-infested forests (presumably as a result of enhanced awareness), effectively reduced the mean national incidence by 74 percent by 1999, with the greatest reductions in counties where incidence was previously highest (Šumilo et al., 2008). Even modest changes in societal structure and socioeconomic development can increase exposure to zoonoses; an awareness of changing risk would allow communication of appropriate warnings to alert unsuspecting members of the public. Prevention of the introduction of foreign pathogens is far more difficult than is control of endemic VBPs because it is an inevitable result of the globalisation of trade and travel. History suggests that successful control needs prompt identification, swift action, and occasionally draconian social measures.
Conclusions
VBPs impose an important global burden on public health, including widespread human diseases that were formerly zoonotic, such as malaria and dengue, as well as zoonotic diseases for which people are dead-end hosts, such as Lyme disease, West Nile virus, and Crimean-Congo haemorrhagic fever. Widespread land-use change, globalisation of trade and travel, and social upheaval are driving the emergence of zoonotic VBPs, including along local invasion fronts. Recognition that a large fraction of the public health burden of both endemic and exotic VBPs comes from infection at the invading front would enable prospective action to address the ecological and sociological drivers of transmission. Financial and technological hurdles persist in developing countries, making diagnosis and control difficult where the diseases are stubbornly most prevalent. Inadequate knowledge prevents populations in developed countries from taking actions that would minimise the diseases’ effects. Development projects that address disease can help to overcome these challenges, and clinicians and public health professionals can play important parts in the reduction of the burden of vector-borne disease.
Search Strategy and Selection Criteria
We searched PubMed and ISI Web of Knowledge with the terms “emerging infection,*” “vector-borne diseas.*” “zoonos*” or names of specific vector-borne infections, in combination with “control,” “exotic,” “climate change,” “socioeconom,*” “land use,” or “evolution” for reports published in any language before July, 2012. Searches were done at all stages, from the initial drafting of the paper to submission of the revised and final version. We also relied on our own familiarity with the scientific literature. We largely selected reports from the past 6 years, but did not exclude older publications that were informative and useful. We also searched the reference lists of reports identified by our searches and selected those that we judged to be relevant. Reviews and book chapters are cited to provide readers with comprehensive sources of references, but primary research is also included where possible within the space allowed. Our reference list was modified on the basis of comments from peer reviewers.
Contributors
AMK and SER conceived the ideas and wrote the report.
Conflicts of Interest
We declare that we have no conflicts of interest.
Acknowledgements
AMK acknowledges funding from the National Science Foundation and the National Institutes of Health.
References
Barbour, A.G., and D. Fish. 1993. The biological and social phenomenon of Lyme disease. Science 260: 1610–16.
Barrett, A.D.T., and S. Higgs. 2007. Yellow fever: a disease that has yet to be conquered. Annual Review of Entomology 52: 209–29.
Beyrer, C., J.C. Villar, V. Suwanvanichkij, S. Singh, S.D. Baral, and E.J. Mills. 2007. Neglected diseases, civil conflicts, and the right to health. Lancet 370: 619–27.
Bogich, T.L., R. Chunara, D. Scales, et al. 2012. Preventing pandemics via international development: a systems approach. PLoS Medicine 9: e1001354.
Bowden, S.E., K. Magori, and J.M. Drake. 2011. Regional differences in the association between land cover and West Nile virus disease incidence in humans in the United States. Am J Trop Med Hyg 84: 234–38.
Brownstein, J.S., C.C. Freifeld, and L.C. Madoff. 2009. Digital disease detection: harnessing the web for public health surveillance. New England Journal of Medicine 360: 2153–57.
Brunkard, J.M., J.L.R. Lopez, J. Ramirez, et al. 2007. Dengue fever seroprevalence and risk factors, Texas–Mexico border, 2004. Emerg Infect Dis 13: 1477–83.
Bryant, J.E., E.C. Holmes, and A.D.T. Barrett. 2007. Out of Africa: a molecular perspective on the introduction of yellow fever virus into the Americas. PLoS Pathogens 3: 668–73.
Centers for Disease Control and Prevention. 2012a. West Nile virus. http://www.cdc.gov/ncidod/dvbid/westnile/index.htm (accessed Oct 2, 2012).
Centers for Disease Control and Prevention. 2012b. Reported Lyme disease cases by state, 2002–2011. http://www.cdc.gov/lyme/stats/chartstables/reportedcases_statelocality.html (accessed Sept 10, 2012).
Chaves, L.F., J.M. Cohen, M. Pascual, and M.L. Wilson. 2008. Social exclusion modifies climate and deforestation impacts on a vector-borne disease. PLoS Neglected Tropical Diseases 2: e176.
Chaves, L.F., M. Hashizume, A. Satake, and N. Minakawa. 2012. Regime shifts and heterogeneous trends in malaria time series from Western Kenya Highlands. Parasitology 139: 14–25.
Cohen, J.E. and R.E. Gurtler. 2001. Modeling household transmission of American trypanosomiasis. Science 293: 694–98.
D’Ortenzio, E., M. Grandadam, E. Balleydier, et al. 2011. A226V Strains of chikungunya virus, Reunion Island, 2010. Emerging Infectious Diseases 17: 309–11.
Davis, C.T., G.D. Ebel, R.S. Lanciotti, et al. 2005. Phylogenetic analysis of North American West Nile virus isolates, 2001–2004: evidence for the emergence of a dominant genotype. Virology 342: 252–65.
de Lambellerie, X., E. Leroy, R.N. Charrel, K.A. Tsetsarkin, S. Higgs, and E.A. Gould. 2008. Chikungunya virus adapts to tiger mosquito via evolutionary convergence: a sign of things to come? Virol J 3:33.
Duffy, M.R., T.H. Chen, W.T. Hancock, et al. 2009. Zika virus outbreak on Yap Island, Federated States of Micronesia. N Engl J Med 360: 2536–43.
Duplantier, J.M., J.B. Duchemin, S. Chanteau, and E. Carniel. 2005. From the recent lessons of the Malagasy foci towards a global understanding of the factors involved in plague reemergence. Veterinary Research 36: 437–53.
Effler, P.V., L. Pang, P. Kitsutani, et al. 2005. Dengue fever, Hawaii, 2001–2002. Emerging Infectious Diseases; 11: 742–49.
Ergönül, Ö. 2006. Crimean-Congo haemorrhagic fever. Lancet Infect Dis 6: 203–14.
Farajollahi, A., D.M. Fonseca, L.D. Kramer, and A.M. Kilpatrick. 2011. Bird biting mosquitoes and human disease: a review of the role of Culex pipiens complex mosquitoes in epidemiology. Infection, Genetics and Evolution 11: 1577–85.
Ferguson, N.M., D.A.T. Cummings, C. Fraser, J.C. Cajka, P.C. Cooley, and D.S. Burke. 2006. Strategies for mitigating an influenza pandemic. Nature 442: 448–52.
Fonseca, D.M., J.L. Smith, R.C. Wilkerson, and R.C. Fleischer. 2006. Pathways of expansion and multiple introductions illustrated by large genetic differentiation among worldwide populations of the southern house mosquito. American Journal of Tropical Medicine and Hygiene 74: 284–89.
Gaynor, A.M., M.D. Nissen, D.M. Whiley, et al. 2007. Identification of a novel polyomavirus from patients with acute respiratory tract infections. PLoS Pathogens 3: e64.
Gerstl, S., S. Dunkley, A. Mukhtar, M. De Smet, S. Baker, and J. Maikere. 2010. Assessment of two malaria rapid diagnostic tests in children under five years of age, with follow-up of false-positive pLDH test results, in a hyperendemic falciparum malaria area, Sierra Leone. Malaria Journal 9: 28.
Gething, P.W., D.L. Smith, A.P. Patil, A.J. Tatem, R.W. Snow, and S.I. Hay. 2010. Climate change and the global malaria recession. Nature 465: 342–45.
Godfrey, E.R., and S.E. Randolph. 2011. Economic downturn results in tick-borne disease upsurge. Parasites and Vectors 4: e35.
Gould, E.A., and S. Higgs. 2009. Impact of climate change and other factors on emerging arbovirus diseases. Transactions of the Royal Society of Tropical Medicine and Hygiene 103: 109–21.
Gray, J.S., H. Dautel, A. Estrada-Pena, O. Kahl, and E. Lindgren. 2009. Effects of climate change on ticks and tick-borne diseases in Europe. Interdisciplinary Perspectives in Infectious Diseases 2009: 593232, 12 pages.
Hay, S.I., C.A. Guerra, A.J. Tatem, P.M. Atkinson, and R.W. Snow. 2005. Urbanization, malaria transmission and disease burden in Africa. Nat Rev Mic 3: 81–90.
Hess, A.D., and R.O. Hayes. 1970. Relative potentials of domestic animals for zooprophylaxis against mosquito vectors of encephalitis. American Journal of Tropical Medicine and Hygiene 19: 327–34.
Hoffmann, A.A., B.L. Montgomery, J. Popovici, et al. 2011. Successful establishment of Wolbachia in Aedes populations to suppress dengue transmission. Nature 476: 454–57.
Holmes, E.C. 2003. Error thresholds and the constraints to RNA virus evolution. Trends in Microbiology 11: 543–46.
Hoogstraal, H. 1979. Epidemiology of tick-borne Crimean Congo hemorrhagic fever in Asia, Europe, and Africa. J Med Entomol 15: 307–417.
Hotez, P.J., M.E. Bottazzi, C. Franco-Paredes, S.K. Ault, and M.R. Periago. 2008. The neglected tropical diseases of Latin America and the Caribbean: a review of disease burden and distribution and a roadmap for control and elimination. PLoS Neglected Tropical Diseases 2: e300.
Hufnagel, L., D. Brockmann, and T. Geisel. 2004. Forecast and control of epidemics in a globalized world. Proc Natl Acad Sci USA 101: 15124–29.
Killeen, G.F. 2003. Following in Soper’s footsteps: northeast Brazil 63 years after eradication of Anopheles gambiae. Lancet Infectious Diseases 3: 663–66.
Kilpatrick, A.M. 2011. Globalization, land use, and the invasion of West Nile virus. Science 334: 323–27.
Kilpatrick, A.M., P. Daszak, S.J. Goodman, H. Rogg, L.D. Kramer, C. Cedeño, and A.A. Cunningham. 2006a. Predicting pathogen introduction: West Nile virus spread to Galapagos. Conservation Biology 20: 1224–31.
Kilpatrick, A.M., P. Daszak, M.J. Jones, P.P. Marra, and L.D. Kramer. 2006b. Host heterogeneity dominates West Nile virus transmission. Proceedings of the Royal Society B: Biological Sciences 273: 2327–33.
Kilpatrick, A.M., L.D. Kramer, M.J. Jones, P.P. Marra, and P. Daszak. 2006c. West Nile virus epidemics in North America are driven by shifts in mosquito feeding behavior. PLoS Biology 4: 606–10.
Kilpatrick, A.M., L.D. Kramer, M.J. Jones, P.P. Marra, P. Daszak, and D.M. Fonseca. 2007. Genetic influences on mosquito feeding behavior and the emergence of zoonotic pathogens. American Journal of Tropical Medicine and Hygiene 77: 667–71.
Kilpatrick, A.M., M.A. Meola, R.M. Moudy, L.D. Kramer. 2008. Temperature, viral genetics, and the transmission of West Nile virus by Culex pipiens mosquitoes. PLoS Pathogens 4: e1000092.
Kilpatrick, A.M., A.P. Dupuis, G.J.J. Chang, and L.D. Kramer. 2010. DNA vaccination of American robins (Turdus migratorius) against West Nile virus. Vector Borne Zoonotic Disease 10: 377–80.
Lafferty, K.D. 2009. The ecology of climate change and infectious diseases. Ecology 90: 888–900.
Lambin, E.F., A. Tran, S.O. Vanwambeke, C. Linard, and V. Soti. 2010. Pathogenic landscapes: interactions between land, people, disease vectors, and their animal hosts. International Journal of Health Geographics 9: 54.
Levi, T., A.M. Kilpatrick, M. Mangel, and C.C. Wilmers. 2012. Deer, predators, and the emergence of Lyme disease. Proceedings of the National Academy of Sciences 109: 10942–47.
Linard, C., P. Lamarque, P. Heyman, et al. 2007. Determinants of the geographic distribution of Puumala virus and Lyme borreliosis infections in Belgium. International Journal of Health Geographics 6: 15.
Lipkin, W.I. 2010. Microbe hunting. Microbiology and Molecular Biology Reviews 74: 363–77.
Lloyd-Smith, J.O., A.P. Galvani, and W.M. Getz. 2003. Curtailing transmission of severe acute respiratory syndrome within a community and its hospital. Proceedings of the Royal Society of London B: Biological Sciences 270: 1979–89.
Logiudice, K., S.T.K. Duerr, M.J. Newhouse, K.A. Schmidt, M.E. Killilea, and R.S. Ostfeld. 2008. Impact of host community composition on Lyme disease risk. Ecology 89: 2841–49.
Lukan, M., E. Bullova, and B. Petko. 2010. Climate warming and tick-borne encephalitis, Slovakia. Emerging Infectious Diseases 16: 524–26.
Martens, W.J.M., L.W. Niessen, J. Rotmans, T.H. Jetten, and A.J. McMichael. 1995. Potential impact of global climate change on malaria risk. Environmental Health Perspectives 103: 458–64.
Mendis, K., B.J. Sina, P. Marchesini, and R. Carter. 2001. The neglected burden of Plasmodium vivax malaria. Am J Trop Med Hyg 64: 97–106.
Moudy, R.M., M.A. Meola, L.L. Morin, G.D. Ebel, and L.D. Kramer. 2007. A newly emergent genotype of West Nile virus is transmitted earlier and more efficiently by Culex mosquitoes. American Journal of Tropical Medicine and Hygiene 77: 365–70.
Nash, D., F. Mostashari, A. Fine, et al. 2001. The outbreak of West Nile virus infection in the New York City area in 1999. New England Journal of Medicine 344: 1807–14.
Ogden, N.H., L.R. Lindsay, M. Morshed, P.N. Sockett, and H. Artsob. 2009. The emergence of Lyme disease in Canada. Canadian Medical Association Journal 180: 1221–24.
Ostfeld, R., and F. Keesing. 2000. The function of biodiversity in the ecology of vector-borne zoonotic diseases. Canadian Journal of Zoology 78: 2061–78.
Pialoux, G., B.A. Gaüzière, S. Jaureguiverry, and M. Strobel. 2007. Chikungunya, an epidemic arbovirosis. Lancet Infect Dis 7: 319–27.
Randolph, S.E., and A.D.M. Dobson. 2012. Pangloss revisited: a critique of the dilution effect and the biodiversity-buffers-infection paradigm. Parasitology 139: 847–63.
Randolph, S.E., on behalf of the EDEN-TBD team. 2010. Human activities predominate in determining changing incidence of tick-borne zoonoses in Europe. European Surveillance 15: 24–31.
Randolph, S.E., and D.J. Rogers. 2000. Fragile transmission cycles of tick-borne encephalitis virus may be disrupted by predicted climate change. Proceedings of the Royal Society B: Biological Sciences 267: 1741–44.
Reiter, P., S. Lathrop, M. Bunning, et al. 2003. Texas lifestyle limits transmission of dengue virus. Emerging Infectious Disease 9: 86–89.
Reiter, P. 2008. Climate change and mosquito-borne disease: knowing the horse before hitching the cart. Scientific and Technical Review OIE 27: 383–98.
Reiter, P. 2010. The standardised freight container: vector of vectors and vector-borne diseases. Scientific and Technical Review OIE 29: 57–64.
Rezza, G., L. Nicoletti, R. Angelini, et al. 2007. Infection with chikungunya virus in Italy: an outbreak in a temperate region. Lancet 370: 1840–46.
Rogers, D.J., and S.E. Randolph. 2006. Climate change and vector-borne diseases. Advances in Parasitology 62: 345–81.
Rohr, J.R., A.P. Dobson, P.T.J. Johnson, et al. 2011. Frontiers in climate change-disease research. Trends in Ecological Evolution 26: 270–77.
Russell, R.C., B.J. Currie, M.D. Lindsay, J.S. Mackenzie, S.A. Ritchie, and P.I. Whelan. 2009. Dengue and climate change in Australia: predictions for the future should incorporate knowledge from the past. Medical Journal of Australia 190: 265–68.
San Martin, J.L., O. Brathwaite, B. Zambrano, et al. 2010. The epidemiology of dengue in the Americas over the last three decades: a worrisome reality. American Journal of Tropical Medicine and Hygiene 82: 128–35.
Schuffenecker, I., I. Iteman, A. Michault, et al. 2006. Genome microevolution of Chikungunya viruses causing the Indian Ocean outbreak. PLoS Medicine 3: 1058–70.
Stenseth, N.C., B.B. Atshabar, M. Begon, et al. 2008. Plague: past, present, and future. PLoS Medicine 5: 9–13.
Šumilo, D., L. Asokliene, T. Avsic-Zupanc, et al. 2008. Behavioural responses to perceived risk of tick-borne encephalitis: vaccination and avoidance in the Baltics and Slovenia. Vaccine 26: 2580–88.
Šumilo, D., L. Asokliene, A. Bormane, V. Vasilenko, I. Golovljova, and S.E. Randolph. 2007. Climate change cannot explain the upsurge of tick-borne encephalitis in the Baltics. PLoS One 2: e500.
Tatem, A.J., S.I. Hay, and D.J. Rogers. 2006. Global traffic and disease vector dispersal. Proceedings of the National Academy of Sciences 103: 6242–47.
Tsao, J.I., J.T. Wootton, J. Bunikis, M.G. Luna, D. Fish, and A.G. Barbour. 2004. An ecological approach to preventing human infection: vaccinating wild mouse reservoirs intervenes in the Lyme disease cycle. Proceedings of the National Academy of Sciences 101: 18159–64.
Tsetsarkin, K.A., and S.C. Weaver. 2011. Sequential adaptive mutations enhance efficient vector switching by Chikungunya virus and its epidemic emergence. PLoS Pathogens 7: e1002412.
Weaver, S.C., and W.K. Reisen. 2010. Present and future arboviral threats. Antiviral Res 85: 328–45.
Wonham, M.J., T. de-Camino-Beck, and M.A. Lewis. 2004. An epidemiological model for West Nile virus: invasion analysis and control applications. Proceedings of the Royal Society B: Biological Sciences 271: 501 07.
Yamamoto, S.S., V.R. Louis, A. Sie, and R. Sauerborn. 2009. The effects of zooprophylaxis and other mosquito control measures against malaria in Nouna, Burkina Faso. Malaria Journal 8: 5.
Yasuoka, J., and R. Levins. 2007. Impact of deforestation and agricultural development on anopheline ecology and malaria epidemiology. American Journal of Tropical Medicine and Hygiene 76: 450–60.
Yergolkar, P.N., B.V. Tandale, V.A. Arankalle et al. 2006. Chikungunya outbreaks caused by African genotype, India. Emerging Infectectious Diseases 12: 1580–83.
Yozwiak, N.L., P. Skewes-Cox, M.D. Stenglein, A. Balmaseda, E. Harris, J.L. DeRisi. 2012. Virus identification in unknown tropical febrile illness cases using deep sequencing. PLoS Neglected Tropical Diseases 6: e1485.




















