A7
CLIMATE TELECONNECTIONS, WEATHER EXTREMES, AND VECTOR-BORNE DISEASE OUTBREAKS1
Kenneth J. Linthicum,2,3Assaf Anyamba,4,5Seth C. Britch,1,2Jennifer L. Small,3,4and Compton J. Tucker3,4
Introduction: Vectors, Ecology, and Climate
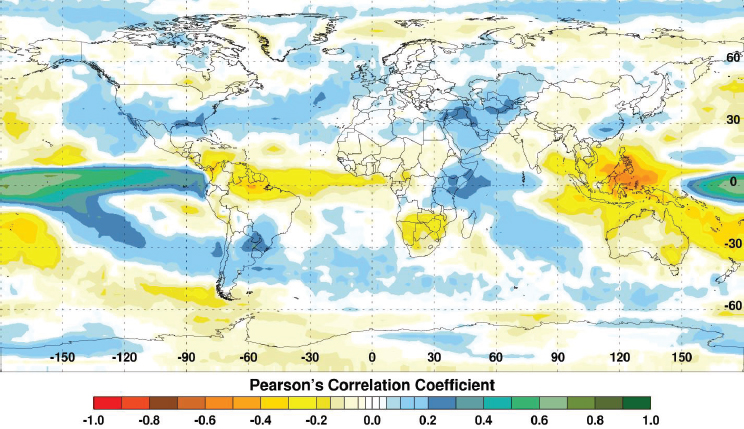
Fluctuations in climate lead to extremes in temperature, rainfall, flooding, and droughts. These climate extremes create ideal ecological conditions that promote mosquito-borne disease transmission that impacts global human and animal health (see Figure A7-1). For example, abnormally high temperatures can affect mosquito populations by reducing mosquito survival, altering susceptibility of mosquitoes to pathogens, increasing mosquito development rates, changing their seasonal activity, increasing pathogen replication and shortening the extrinsic incubation period in the mosquito, and changing disease transmission patterns and seasonality (Gubler et al., 2001; Epstein, 2005; Linthicum et al., 2014). Elevated rainfall may increase immature habitats for mosquitoes, and elevated humidity can increase mosquito survival (Turell et al., 2001; Glass et al., 2000; Reisen et al., 1993). Drought conditions can change immature mosquito habitats and enhance container breeding mosquito habitats (Chretien et al., 2007). One well-known driver of such global-scale climate fluctuations is the El Niño/Southern Oscillation (ENSO) phenomenon that is exemplified by periodic extreme warming and cooling of the eastern equatorial Pacific Ocean with attendant consequences on precipitation and temperature worldwide especially across the global tropics. Such extremes include flooding as a result of persistent and above-normal rainfall and drought resulting from extended periods of below-normal rainfall and above-normal temperatures (see Figure A7-2). Such extremes in regional climate can create ecological conditions that influence the emergence of mosquito vectors, their distribution and abundance, population dynamics, and transmission of mosquito-borne disease (Anyamba et al., 2012). In this paper we show that outbreaks of Rift Valley fever and chikungunya, two important emerging mosquito-borne diseases, are coupled to specific climate anomaly patterns.
___________________
1 Modified by authors from PLOS Neglected Tropical Diseases doi:10.1371/journal.pntd.0001465 and PLoS ONE doi:10.1371/journal.pone.0092538 for inclusion in this workshop summary.
2 USDA-ARS Center for Medical, Agricultural, and Veterinary Entomology, 1600 S.W. 23rd Drive, Gainesville, FL 32608.
3 Mosquito and Fly Research Unit.
4 Biospheric Sciences Laboratory.
5 NASA Goddard Space Flight Center.
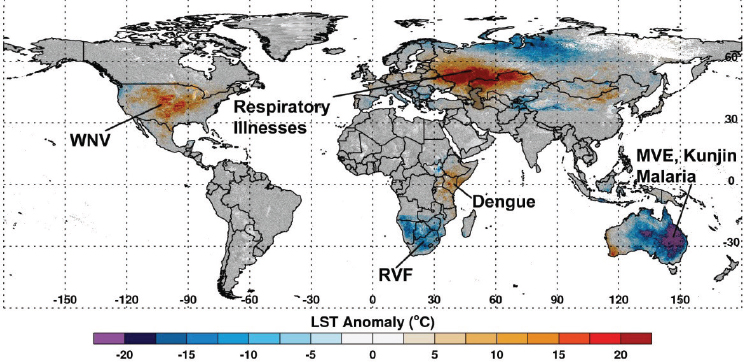
SOURCE: Assaf Anyamba (USRA at NASA Goddard Space Flight Center) and Kenneth Linthicum.
Data: LST data from the MODIS instrument on-board NASA’s Earth Observing System Terra satellite.
Next we describe significant worldwide weather anomalies that impacted vector-borne disease outbreaks during the 2010–2012 period. Using 2000–2012 normalized difference vegetation index (NDVI) and land surface temperature (LST) data from NASA’s satellite-based Moderate Resolution Imaging Spectroradiometer (MODIS) we map the magnitude and extent of these weather anomalies for diverse regions including the continental United States, Russia, East Africa, Southern Africa, and Australia, and we demonstrate that shifts in temperature and/or precipitation have significant impacts on ecology patterns with attendant consequences for public health. Weather extremes resulted in excessive rainfall and flooding as well as severe drought which created exceptional conditions for extensive mosquito-borne disease outbreaks of Rift Valley fever, Murray Valley encephalitis, dengue, West Nile virus disease, and air pollution associated with extensive fires and high temperatures. Finally we describe climate teleconnections between several vector-borne, rodent-borne, and environmentally linked diseases, and describe how risks may develop if El Niño conditions develop in the winter of 2014 and spring of 2015 (Chretien et al., 2015).
SOURCE: Anyamba et al., 2012. Available from PLoS Neglected Tropical Diseases under Creative Commons license.
Climate Teleconnections and Vector-Borne Disease Patterns
ENSO is a climate phenomenon that is associated with extremes in global climate on interannual time scales through its dislocation of major centers of and redistribution of precipitation across the global tropics and extra-tropics. It has been shown to be associated with the occurrence of several human and animal diseases including Rift Valley fever, Murray Valley encephalitis, chikungunya, malaria, hantavirus pulmonary syndrome, and cholera precipitated by extremes in rainfall and temperature among other factors (Anyamba et al., 2009; Linthicum et al., 1999; Nicholls, 1986; Bouma and Dye, 1997; Kovats et al., 2003; Engelthaler et al., 1999; Pascual et al., 2000). The impact of ENSO on earth’s climate system, especially over the tropics, through interannual variations in temperature, atmospheric circulation, and rainfall at various distant locations, is termed teleconnection. These teleconnections produce differential anomaly patterns in major climate variables with near-cyclical transitions through time from the warm El Niño phase to the cold La Niña phase with a periodicity of 5–7 years (Diaz and Markgraf, 2000). There is likelihood for outbreaks of mosquito-borne diseases to occur at or near the same time during an ENSO cycle, demonstrating how extremes in rainfall resulting in either persistent flood or severe drought can
influence the regional dynamics of mosquito vector populations at distant locations around the world, especially in the tropics (Nicholls, 1986; Glantz, 1991; Engelthaler et al., 1999; Linthicum et al., 1999).
Globally the El Niño phase of ENSO causes predictable patterns of flooding and drought. Figure A7-2 depicts the close correlation between sea surface temperatures (SST) and rainfall anomalies. There is a strong tendency for above (below) normal rainfall during El Niño (La Niña) events over East Africa (Southern Africa, Southeast Asia). Elevated rainfall conditions and floods generally occur over Eastern Africa, the southern half of the United States, Southern Brazil/Northern Argentina, eastern and central Pacific Islands, Ecuador, and Peru. Reduced rainfall conditions leading to drought occur over a large area of Southeast Asia, Australia, northern and north-eastern Brazil, and Southern Africa. The opposite conditions may prevail during the La Niña phase of ENSO (Glantz, 1991).
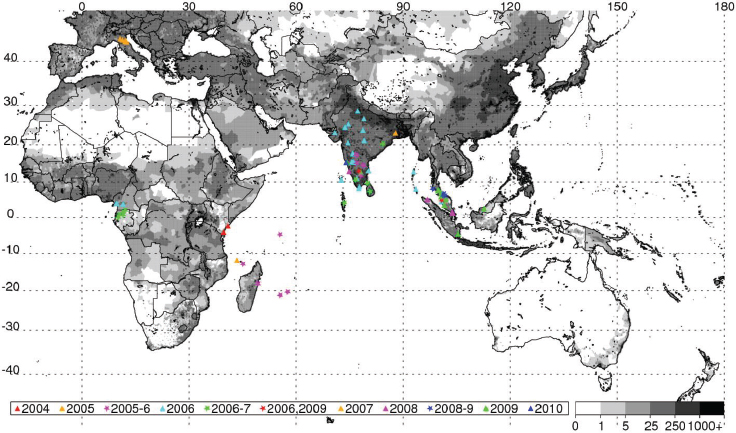
ENSO-produced extremes in regional climate can create ecological conditions that influence the transmission of mosquito-borne diseases of global public health relevance (Gubler et al., 2001; Gage et al., 2008). Teleconnection studies examining the link between climate and disease have been limited by poor reporting and a lack of georeferenced disease data. We analyzed and illustrated how recent outbreaks of two mosquito-borne diseases, chikungunya and Rift Valley fever (Chretien et al., 2007; Gage et al., 2008; Panning et al., 2008; Anyamba et al., 2009, 2010, 2012), over Africa, the western Indian Ocean basin islands, and Asia were linked to specific climate anomaly patterns. We georeferenced disease occurrence records, and examined the spatial and temporal associations of these disease outbreaks and how they were impacted by variable climate and ecological patterns.
The relationship between rainfall and Rift Valley fever was determined through a logistic regression of presence or absence of disease reports on cumulative rainfall anomalies for the 4 months immediately preceding each Rift Valley fever outbreak (Anyamba, 2009; data not shown). There was a significant relationship found between cumulative rainfall anomalies and Rift Valley fever presence with at least 99.9 percent confidence. This relationship for East Africa, Sudan, and South Africa was strongly positive. Figure A7-3 shows that for each of the selected outbreak locations in each region, persistent above-normal rainfall for 3–4 months preceded the first case of Rift Valley fever. Rainfall anomalies for each of these regions at the times of disease activity are depicted in Figure A7-4 and listed in Table A7-1. The magnitude of such rainfall anomalies creates ideal ecological conditions for an increase in Rift Valley fever mosquito vector emergence and survival. These findings confirmed experimental (Linthicum et al., 1984) and field (Linthicum et al., 1985) observations that persistent, widespread, and above-normal rainfall is required to flood mosquito habitats to produce ecological conditions supporting the emergence of virus-infected ground pool Aedes mosquito populations and ultimately lead to large populations of vector competent mosquitoes on a large geographic scale that would result in a Rift
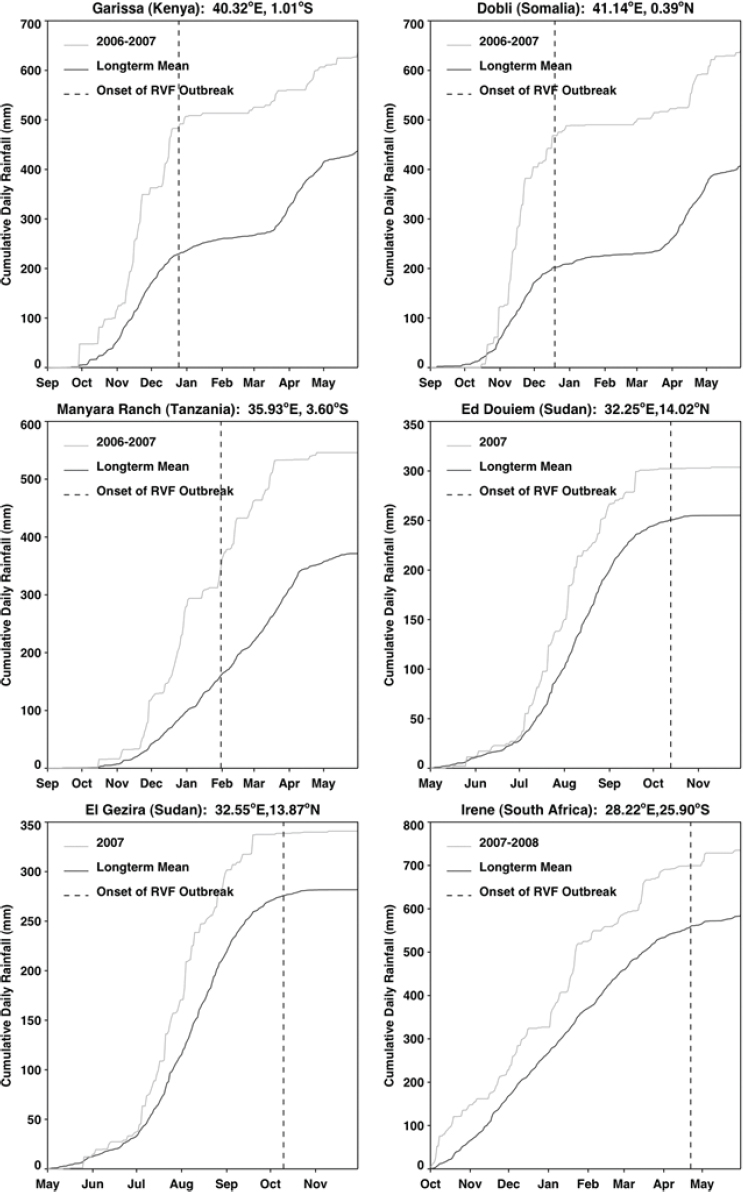
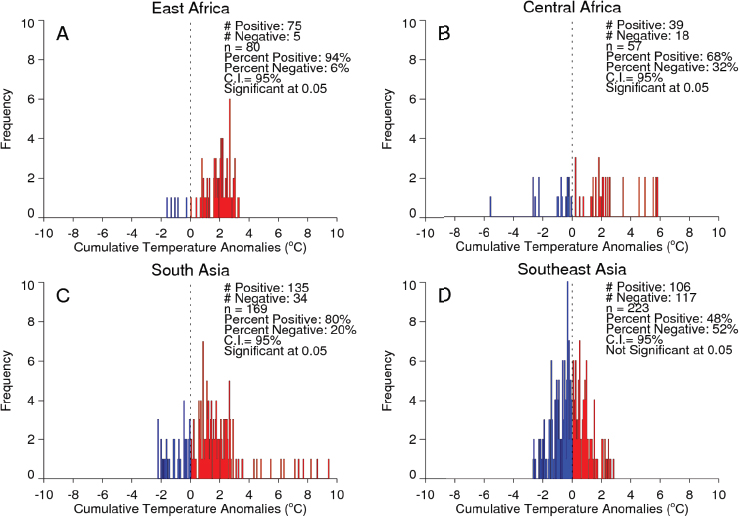
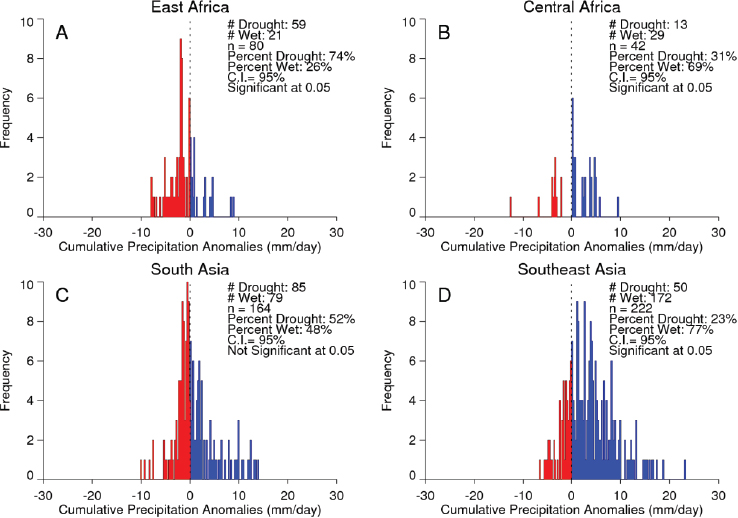
Valley fever epizootic. However, in Madagascar a negative relationship was found, with the model predicting higher odds of Rift Valley fever outbreaks when rainfall was less than normal (see Figure A7-4). Although the 2008 Madagascar Rift Valley fever outbreak was initially triggered by elevated rainfall (Anyamba et al., 2010), the subsequent spread of the outbreak may have been caused by the introduction of infected livestock from the southern part of the country to areas in the north where competent Culex mosquito vectors are associated with human habitations. Large outbreaks of chikungunya have historically been transmitted by Aedes aegypti mosquitoes in large highly populated urban areas of Asia and highly populated areas of Africa with smaller outbreaks in rural areas. The location of chikungunya outbreaks between 2004 and 2010 in relation to human population density is illustrated in Figure A7-5. Outbreaks occurred in coastal urban centers with large population densities (Chretien et al., 2007; Anyamba et al., 2010). Chikungunya cases between January 1979 and February 2010 were analyzed by Anyamba et al. (2012) and examined for relationships with surface air temperature anomalies and precipitation anomalies as shown in Figures A7-6 and A7-7, respectively. These figures show the frequency distribution of the number of reported chikungunya outbreak events against rainfall and temperature anomalies. Rainfall and temperature anomalies were calculated by subtracting the long-term 1979–2010 monthly rainfall or temperature means from the rainfall or temperature values in each month in each year of the study period, such that the rainfall or temperature anomaly for each month could be plotted as greater than or less than the zero long-term baseline (shown as a vertical dotted line in Figures A7-5 and A7-6). Temperature anomalies which persisted over a 4-month period were classified as hot if anomalies were > 0 or cool if < 0. Precipitation anomalies which persisted over a 4-month period were classified as drought if < 0 and wet if > 0. In East Africa, Central Africa, and South Asia, 94 percent, 68 percent, and 80 percent of the outbreaks, respectively, occurred during warmer-than-normal temperatures, and these differences were significant at P < 0.05 (see Figures A7-6A–C). In Southeast Asia, however, 52 percent of the outbreaks occurred during cooler than normal temperatures (see Figure A7-6D). In East Africa chikungunya reports were significantly positively correlated with drought conditions at P < 0.05 (see Figure A7-7A), and were not significantly correlated in South Asia (see Figure A7-7C).
SOURCE: Anyamba et al., 2012b, reproduced with permission from IEEE.
SOURCES: (A-Left) Anyamba et al., 2012. Available from PLoS Neglected Tropical Diseases under Creative Commons license; (B-Right) Anyamba et al., 2012b, reproduced with permission from IEEE.
In Central Africa (see Figure A7-7B) and in Southeast Asia (see Figure A7-7D) outbreaks were significantly negatively correlated with drought at P < 0.05. The positive correlation between chikungunya outbreaks and warmer than normal temperatures in Africa and South Asia was consistent with nonsylvatic transmission by Ae. aegypti and Ae. albopictus in highly populated domestic settings where domestic and peridomestic stored water supplies were the likely source of the mosquitoes (Chretien et al., 2007; Anyamba et al., 2012). Our analyses suggest that in a changing and variable climate, mosquito-transmitted viruses and their mosquito vectors are going to adapt to the existing climatic and ecological conditions in new regions, and disease transmission will vary accordingly and may not be the same manifestation as observed in the original endemic regions. Combining satellite-derived measurements and analyses of climate and ecology with an understanding of mosquito vector biology and human and animal population immunity status can contribute substantially towards reducing the global burden of vector-borne diseases. Better understanding of climate teleconnection
| Region | Season | Total (mm) | Mean (mm) | Anomaly (%) |
|---|---|---|---|---|
| East Africa (Somalia/Kenya)a | September -December 2006 | 495.51 | 247.36 | 102.54 |
| Sudan | June-September 2007 | 563.95 | 354.23 | 63.77 |
| US (Texas) | June-August 2011 | 59.40 | 174.11 | –65.88 |
| East Africa (Somalia/Kenya)b | December 2010-February 2011 | 40.65 | 80.94 | –52.26 |
| South Africa (Free State/North West) | December 2010-February 2011 | 363.92 | 253.69 | 43.45 |
| SE Australia (New South Wales) | September-November 2010 | 255.28 | 95.59 | 174.01 |
a Refers to the specific location of Rift Valley outbreak in 2006–2007.
b Location of cluster of dengue outbreaks in East Africa in 2010–2011.
SOURCE: Data from NASA Goddard Space Flight Center (http://precip.gsfc.nasa.gov/), as described by Adler et al., 2003.
events and their link to mosquito-borne diseases will likely allow parts of Africa, the Indian Ocean basin islands, and elsewhere within the greater tropics to have from several months to more than a year warning prior to Rift Valley fever outbreaks, permitting more efficacious targeting of vaccine, virus surveillance, mosquito control, animal quarantine, and public education strategies.
Extreme Weather and Disease Outbreaks
The anomalous conditions observed during 2010–2012 were the most extreme weather events in the 12-year record of Terra MODIS data, and they present a good opportunity to quantify these weather impacts on mosquito-transmitted diseases using various satellite-based parameters of surface conditions. The timing and unique intensity of these events is corroborated by analyses using longer-term climate data sets (Trenberth and Fasullo, 2012; Blunden and Arndt, 2013; Hoerling et al., 2013). Anyamba et al. (2012, 2014) postulated that because both severe drought and flooding may create ecological conditions for enhancing vector-borne disease emergence, then under such extreme weather events as have been documented for 2010–2012 significant increases
SOURCE: Anyamba et al., 2012. Available from PLoS Neglected Tropical Diseases under Creative Commons license.
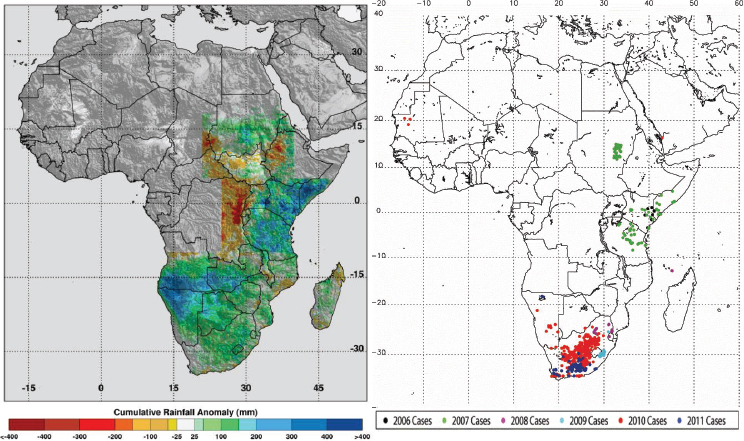
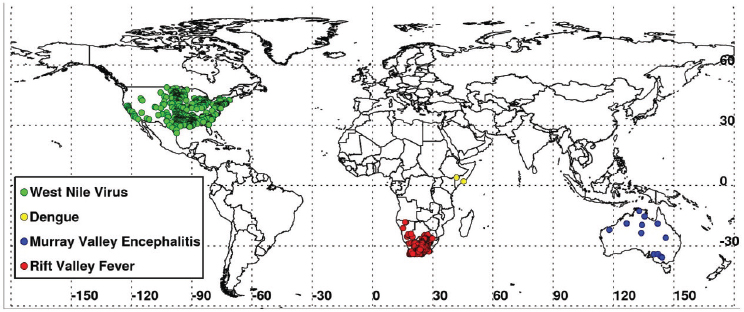
in outbreaks of vector-borne diseases under conditions of both extreme drought and above-normal rainfall should be observed. They mapped locations of documented 2010–2012 extremes at selected locations around the globe using various satellite datasets, and described and illustrated the impacts of these extremes on epidemics/epizootics of West Nile virus disease (USA 2012), dengue (East Africa 2010–2011), Murray Valley encephalitis (SE Australia 2010), and Rift Valley fever (East Africa 2006, Sudan 2007, South Africa 2010–2011) for the selected regions shown in Figures A7-4 and A7-8. Rainfall statistics for regions where and times when these outbreaks occurred are shown in Table A7-1. Rainfall deficits were associated with elevated West Nile virus and dengue transmission, and surpluses associated with Murray Valley encephalitis and Rift Valley fever outbreaks.
Record high temperatures and persistent drought (rainfall shortfalls of 65 percent) (Table A7-1) were linked to the highest period of West Nile virus activity on record in Texas and the rest of the continental United States (see Figure A7-9A, B). The 2012 epidemic of West Nile virus disease across the continental United States (see Figure A7-8) was the largest such outbreak since the introduction of West Nile virus into the United States in 1999, and the spike in human
SOURCE: Anyamba et al., 2012. Available from PLoS Neglected Tropical Diseases under Creative Commons license.
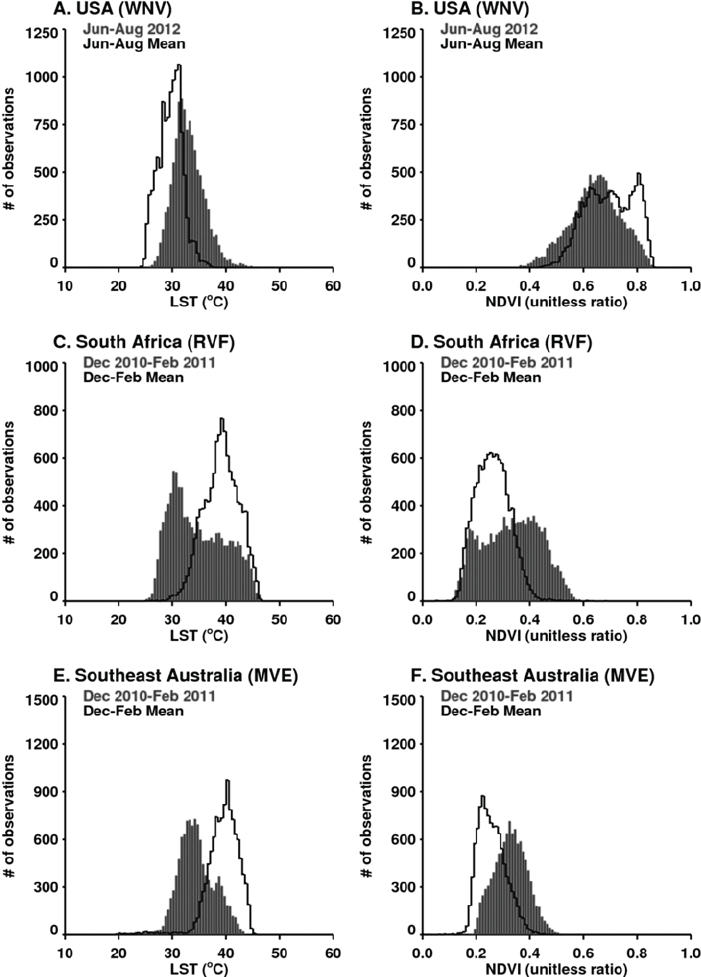
West Nile virus disease cases in 2012 can in part be associated with extreme drought (see Figure A7-9B; Epstein and Defilippo, 2001). Mean summer temperatures in June to August 2012 ranged from 30°C to 33°C, exceeding long-term means (see Figure A7-9A). Elevated temperatures may have increased the efficiency of transmission of West Nile virus by both Culex pipiens and Cx. tarsalis mosquitoes, the likely vectors, by elevating population development and survival, biting rates, and viral replication within these mosquito species (Kilpatrick et al., 2008; Johnson and Sukhdeo, 2013; Moudy et al., 2007).
Across Eurasia, the summer drought of June–August 2010 was centered in western Russia (see Figure A7-1) with the drought area extending to Belarus, Poland, Germany, Ukraine, and Kazakhstan (Munich, 2010; Trenberth and Fasullo, 2012). Cumulative seasonal land surface temperatures reached as high
SOURCE: Anyamba et al., 2012. Available from PLoS Neglected Tropical Diseases under Creative Commons license.
as 20°C above normal with declines in NDVI of up to 40 percent below normal (see Figure A7-1). As in the southern United States, the drought caused extreme fire conditions. The fires led to smoke pollution and an increase in the number of upper respiratory illnesses, and resulted in more than 15,000 deaths in Russia during the 2010 summer.
In 2011 an unprecedented extensive dengue outbreak occurred in East Africa and was associated with anomalously elevated land surface temperatures and drought (52 percent below-normal rainfall) (see Table A7-1, Figure A7-8). Dengue virus, transmitted by Ae. aegypti mosquitoes, has been linked to increased storage of water around households during hot, dry climatic conditions in densely populated areas (Epstein, 2005; Chretien et al., 2007; Padmanabha et al., 2010). These conditions are thought to increase populations of that mosquito species
SOURCE: Anyamba et al., 2014. Available from PLoS ONE under Creative Commons license.
(Subra, 1983). Extensive drought and elevated higher temperatures likely increased the abundance of container-breeding dengue virus vector mosquitoes in urban settings leading to the dengue outbreaks focused in Mogadishu, Somalia, and Mandera, Kenya (IRIN, 2011) (see Table A7-1, Figure A7-8).
During the extended La Niña event of 2010–2011 more than 40 percent higher than normal rainfall fell in much of South Africa. These extremely wet conditions led to the flooding of low-lying areas, or dambos/pans, and created ideal ecological conditions to hatch ground pool Aedes species mosquito eggs infected with Rift Valley fever virus. Additionally, there was a downward shift in mean seasonal (December–February) temperatures from about 40°C to 30°C in South Africa as shown in Figures A7-9C and A7-9D. These cool and wet conditions, which persisted through December 2010 to February 2011, permitted increased mosquito vector populations and increased virus infection rates in mosquitoes (Turell, 1993; Grobbelaar et al., 2011), and subsequent Rift Valley fever virus transmission. The outbreak was the most extensive and widespread epizootic/epidemic of Rift Valley fever observed in the region since the 1970s (see Figure A7-8), severely impacting domestic animal production and human health in southern Africa (Grobbelaar et al., 2011; Metras et al., 2012). MODIS time series NDVI and LST anomalies during the 2010/11 La Niña were the most persistent and extreme anomalies ever observed for the southern Africa region during the 12-year history record of consistent measurements.
Accordingly, the peak of the La Niña event from November 2010 to January 2011 produced persistent and heavy rainfall, cooler temperatures, vegetation
SOURCE: Anyamba et al., 2014. Available from PLoS ONE under Creative Commons license.
growth, and created the ideal conditions for increasing Murray Valley encephalitis virus mosquito vector populations. These conditions (174 percent above-normal rainfall) led to outbreaks of the virus over northern and eastern Australia (Knox et al., 2012) (see Table A7-1, Figure A7-8). Culex annulirostris, the primary Murray Valley encephalitis mosquito vectors, propagate well during cooler temperatures associated with heavy rainfall periods in the tropics and subtropics (Van Den Hurk et al., 2010). During this epidemic period there was a reduction in mean seasonal temperatures from 40°C to 30°C in the period from December 2010 to January 2011 compared to the long-term mean distribution for eastern Australia (see Figures A7-9E and A7-9F).
These regional examples described above illustrate how extreme weather events can impact mosquito-borne disease outbreaks. These environmentally enhanced outbreaks can vary globally depending on the virus and its transmission ecology and the geographic location. The status of a particular disease, its seasonality, and other factors may enhance the potential for globalization of such pathogens (Anyamba et al., 2012). The analysis of temperature and vegetation conditions presented here and in Anyamba et al. (2014) provides a method for consistently quantifying weather extremes from region to region, and demonstrates the unique capability of satellite data in monitoring and mapping the magnitude and extent of such events. It is likely that as extreme weather events become more common under a changing and more variable climate (Cai et al., 2015), countries will face challenging and costly adaptation strategies.
Summary: Extremes and the Near Future
The National Oceanic and Atmospheric Administration’s (NOAA’s) Climate Prediction Center (CPC) issued, on December 4, 2014, their most recent El Niño conditions advisory (http://www.cpc.ncep.noaa.gov/products/analysis_monitoring/enso_advisory/index.shtml). The advisory indicated that there is a 65 percent chance that El Niño conditions will be present during the Northern Hemisphere winter and last into the Northern Hemisphere spring of 2015. Additionally, the RVF Monitor website (http://www.ars.usda.gov/Business/docs.htm?docid=23464) has the current suite of satellite global climate surveillance products for monitoring El Niño and vegetation conditions and implications for Rift Valley fever activity in Africa and the Arabian Peninsula region. The global products including NDVI, SST, and outgoing longwave radiation (OLR—a proxy indicator for rainfall), as well as data from terrestrial rainfall monitoring stations, are useful in illustrating the current situation of global climate anomalies with implications for public health.
The U.S. Department of Defense Armed Forces Health Surveillance Center’s Global Emerging Infections Surveillance and Response System (DoD-AFHSC/GEIS); United States Department of Agriculture-Agricultural Research Service Center for Medical, Agricultural, and Veterinary Entomology (USDA-ARS/
CMAVE); and the Global Inventory Modeling and Mapping Studies (GIMMS) Group at NASA Goddard Space Flight Center monitor global climate and ecological conditions of relevance to various disease outbreaks. The current NOAA El Niño watch forecasts a 65 percent chance of the development of El Niño conditions this winter 2014 and into the coming spring 2015, which may result in climate perturbations and anomalies that will affect various vector-borne and rodent-borne pathogen ecologies globally and likely result in disease outbreaks. Given current observations and forecast information, the following regions are at increased risk for disease outbreaks (see Figure A7-10):
- Indonesia, Malaysia, Thailand, and most of the Southeast Asia Islands: High likelihood of increased dengue fever and possibly chikungunya transmission caused by drought conditions which (1) increase water storage around houses leading to elevated Ae. aegypti populations and (2) elevate ambient air temperatures which will reduce the extrinsic incubation period for the virus in Ae. aegypti and Ae. albopictus mosquito vectors increasing vectorial capacity; and likelihood of increased respiratory illnesses attributable to uncontrolled burning of tropical forests during extreme drought conditions.
- Coastal Peru, Venezuela, and Colombia: Elevated risk of malaria epidemics due to elevated anopheline mosquito populations that will develop when various types of potential habitats are flooded after heavy rainfall.
- Bangladesh, coastal India, and Sri Lanka: Elevated risk for cholera and malaria outbreaks due to increased rainfall and flooding.
- East Africa (Kenya, Tanzania, Uganda, Somalia, and Ethiopia): Elevated risk for Rift Valley fever and malaria illnesses resulting from elevated mosquito vector populations, as well as increased risk for cholera due to heavy rainfall in dry land areas and human contamination of water supply. Note: In spite of significant protective Rift Valley fever virus antibodies in livestock older than 3 years, infected mosquitoes may be produced in the region and significant transmission may occur particularly in areas not affected during the recent 2006–2007 epizootic/epidemic. In addition, areas previously affected have now restocked after the recent severe drought from 2010–2011. There also is a risk for co-infection with other mosquito-borne diseases such as dengue, chikungunya, and o’nyong’nyong.
- Southwest United States (New Mexico, Arizona, Colorado, Utah, Texas, and California): Hantavirus pulmonary syndrome and plague due to elevated rodent populations caused by heavy rainfall. In addition, elevated potential for transmission of arboviruses, such as West Nile virus, caused by heavy rainfall and elevated culicine mosquito populations.
- Southern and Southeast United States, particularly along the Gulf Coast: Elevated rainfall conditions may increase Ae. albopictus and Ae. aegypti populations, potentially increasing the likelihood of local transmission of
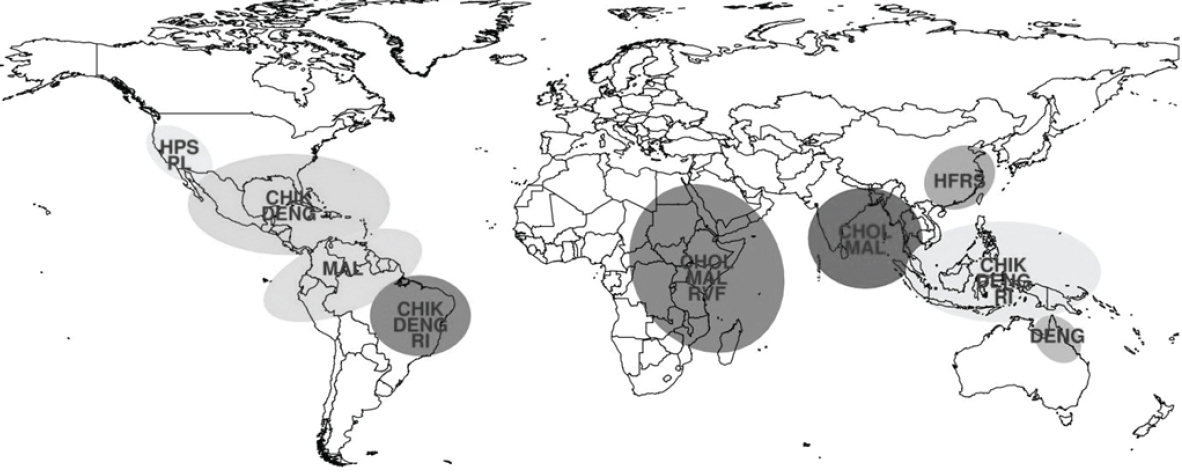
NOTES: CHIK = Chikungunya; CHOL = Cholera; DENG = Dengue Fever; HFRS = Hemorrhagic Fever with Renal Sundrome; HPS = Hantavirus Pulmonary Syndrome; MAL = Malaria; PL = Plague; RI = Respiratory Illness; RVF = Rift Valley Fever.
SOURCE: Chretien et al., 2015. Available from PLoS Currents Outbreaks under Creative Commons license.
-
dengue and chikungunya virus within existing or potential endemic areas or following continued pathogen introduction from current outbreaks in the Caribbean Islands and/or Central/South America.
- Northeast Brazil: Elevated risk for dengue and respiratory illnesses due to drought conditions and potential large-scale forest fires. Additionally, increased risk for chikungunya introduction from the ongoing outbreak in the Caribbean and/or Central/South America into Brazil.
As described by Anyamba et al. (2012, 2014) outbreaks of mosquito-borne diseases on epidemic scales, such as those experienced during 2005–2011 in Africa, the western Indian Ocean islands, and in Asia, place a huge burden on public health care systems and the economy. Outbreaks such as those of chikungunya are also an impediment to tourism, a major contributor to the gross national product of countries and small island nation states everywhere. Given the potential implications from the developing El Niño in the winter of 2014 and spring of 2015 it is imperative that countries in potentially impacted regions anticipate the changing, variable, and extreme nature of the climate to prevent or minimize the emergence and reemergence of such diseases. There is an urgent need for public health authorities to take advantage of climate observations and analyses in times of extreme climate variability to enhance response and mitigation planning including: vector surveillance and control, virus surveillance, vaccination, and public education in areas that may be impacted by disease outbreaks. In addition, climate-based predictions offer opportunities for virologists, epidemiologists, entomologists, physicians, and veterinarians to understand the biological and cyclic nature of these diseases and how their episodic occurrence relates to livestock and human immunity in recently infected areas, and the potential for reemergence of the diseases in livestock and human populations.
References
Adler, R. F., G. J. Huffman, A. Chang, R. Ferraro, P.-P. Xie, J. Janowiak, B. Rudolf, U. Schneider, S. Curtis, D. Bolvin, A. Gruber, J. Susskind, P. Arkin, and E. Nelkin. 2003. The version-2 Global Precipitation Climatology Project (GPCP) monthly precipitation analysis (1979-present). Journal of Hydrometeorology 4:1147-1167.
Anyamba, A., J.-P. Chretien, J. Small, C. J. Tucker, P. B. Formenty, J. H. Richardson, S. C. Britch, D. C. Schnabel, R. L. Erickson, and K. J. Linthicum. 2009. Prediction of a Rift Valley fever outbreak. Proceedings of the National Academy of Sciences of the United States of America 106:955-959.
Anyamba, A., K. J. Linthicum, J. Small, S. C. Britch, E. Pak, S. de La Rocque, P. Formenty, A. W. Hightower, R. F. Breiman. J.-P. Chretien, C. J. Tucker, D. Schnabel, R. Sang, K. Haagsma, M. Latham, H. B. Lewandowski, S. Osman Magdi, M. Ally Mohamed, P. M. Nguku, J.-M. Reynes, and R. Swanepoel. 2010. Prediction, assessment of the Rift Valley fever activity in East and Southern Africa 2006–2008 and possible vector control strategies. American Journal of Tropical Medicine and Hygiene 83(S2):43-51.
Anyamba, A., K. J. Linthicum, J. L. Small, K. M. Collins, C. J. Tucker, E. W. Pak, S. C. Britch, J. R. Eastman, J. E. Pinzon, and K. L. Russell. 2012. Climate teleconnections and recent patterns of human and animal disease outbreaks. Public Library of Science Neglected Tropical Diseases 6(1):e1465. doi:10.1371/journal.pntd.0001465.
Anyamba, A., J. L. Small, S. C. Britch, C. J. Tucker, E. W. Pak, C. A. Reynolds, J. Crutchfield, and K. J. Linthicum. 2014. Recent weather extremes and impacts on agricultural production and vector-borne disease outbreak patterns. Public Library of Science ONE 9(3): e92538. doi:10.1371/journal.pone.0092538.
Blunden, J., and D. S. Arndt. 2013. State of the climate in 2012. Bulletin of the American Meteorological Society 94:S1-S258.
Bouma, M. J., and C. Dye. 1997. Cycles of malaria associated with El Niño in Venezuela. Journal of the American Medical Association 278:1772-1774.
Cai, W., G. Wang, A. Santoso, M. J. McPhaden, L. Wu, F.-F. Jin, A. Timmermann, M. Collins, G. Vecchi, M. Lengaigne, M. H. England, D. Dommenget, K. Takahashi, and E. Guilyardi. 2015. Increased frequency of extreme La Niña events under greenhouse warming. Nature Climate Change 5:132-137.
Chretien, J.-P., A. Anyamba, S. A. Bedno, R. F. Breiman, R. Sang, K. Sergon, A.M. Powers, C. O. Onyango, J. Small, C. J. Tucker, and K. J. Linthicum. 2007. Drought-associated chikungunya emergence along coastal East Africa. American Journal of Tropical Medicine and Hygiene 76:405-407.
Chretien, J.-P., A. Anyamba, J. L. Small, S. C. Britch, J. L. Sanchez, A. C. Halbach, C. J. Tucker, and K. J. Linthicum. 2015. Global climate anomalies and potential infectious disease risks: 2014-2015. PLoS Currents Outbreaks Jan 26. Edition 1. doi: 10.1371/currents.outbreaks.95fbc4a8f b4695e049baabfc2fc8289f.
Diaz, H. F., and V. Markgraf. 2000. El Niño: Historical and paleoclimatic aspects of the Southern Oscillation. New York: Cambridge University Press. Pp. 323-348.
Engelthaler, D. M., D. G. Mosley, J. E. Cheek, C. E. Levy, K. K. Komatsu, P. Ettestad, T. Davies, D. T. Tanda, L. Miller, J. W. Frampton, R. Porter, and R. T. Bryan. 1999. Climatic and environmental patterns associated with hantavirus pulmonary syndrome, Four Corners region, United States. Emerging Infectious Diseases 5:87-94.
Epstein, P. R. 2005. Climate change and human health. New England Journal of Medicine 353:1433-1436.
Epstein, P. R., and C. Defilippo. 2001. West Nile virus and drought. Global Change and Human Health 2(2):105-107. doi:10.1023/A:1015089901425
Gage, K. L., T. R. Burkot, R. J. Eisen, and E. B. Hayes. 2008. Climate and vector-borne diseases. American Journal of Preventive Medicine 35:436-450.
Glantz, M. H. 1991 Introduction. In Teleconnections linking worldwide climate anomalies, edited by M. H. Glantz, R. W. Katz, and N. Nicholls. New York: Cambridge University Press. Pp. 1-12.
Glass, G. E., J. E. Cheek, J. A. Patz, T. M. Shields, T. J. Doyle, D. A. Thoroughman, D. K. Hunt, R. E. Enscore, K. L. Gage, C. Irland, C. J. Peters, and R. Bryan. 2000. Using remotely sensed data to identify areas of risk for hantavirus pulmonary syndrome. Emerging Infectious Diseases 63:238-247.
Grobbelaar, A. A., J. Weyer, P. A. Leman, A. Kemp, J. T. Paweska, and R. Swanepoel. 2011. Molecular epidemiology of Rift Valley fever virus. Emerging Infectious Diseases 17:2270-2276.
Gubler, D. J., P. Reiter, K. L. Ebi, W. Yap, R. Nasci, and J. A. Patz. 2001. Climate variability and change in the United States: Potential impacts on vector- and rodent-borne diseases. Environmental Health Perspectives 109(S2):223-233.
Hoerling, M., A. Kumar, R. Dole, J. W. Nielse-Gammon, J. Eischeid, A. Kumar, J. W. Nielsen-Gammon, P. Pegion, J. Perlwitz, X.-W. Quan, T. Zhang, P. Pegion, and M. Chen. 2013. Anatomy of an extreme event. Journal of Climate 26: 2811-2832. doi: http://dx.doi.org/10.1175/JCLI-D-12-00270.1
IRIN (Integrated Regional Information Networks). 2011. Kenya: Medics overwhelmed as dengue fever spreads. IRIN Humanitarian News and Analysis. http://www.irinnews.org/Report/93848/KENYA-Medics-overwhelmed-as-dengue-fever-spreads (accessed April 10, 2013).
Johnson, B. J., and M. V. K. Sukhdeo. 2013. Drought-induced amplification of local and regional West Nile virus infection rates in New Jersey. Journal of Medical Entomology 50:195-204.
Kilpatrick, A. M., M. A. Meola, R. M. Moudy, and L. D. Kramer. 2008. Temperature, viral genetics, and the transmission of West Nile virus by Culex pipiens mosquitoes. Public Library of Science Pathogens 4: e1000092. doi: 10.1371/journal.ppat.1000092.
Knox, J., R. U. Cowan, J. S. Doyle, M. K. Ligtermoet, J. S. Archer, J. N. C. Burrow, S. Y. C. Tong, B. J. Currie, J. S. Mackenzie, D. W. Smith, M. Catton, R. J. Moran, C. A. Aboltins, and J. S. Richards. 2012. Murray Valley encephalitis: A review of clinical features, diagnosis and treatment. Medical Journal of Australia 196:322-326.
Kovats, R., M. J. Bouma, S. Hajat, E. Worrall, and A. Haines. 2003. El Niño and health. Lancet 362:1481-1489.
Linthicum, K. J., F. G. Davies, C. L. Bailey, and A. Kairo. 1984. Mosquito species encountered in a flooded grassland dambo in Kenya. Mosquito News 44:228-232.
Linthicum, K. J., F. G. Davies, A. Kairo, and C. L. Bailey. 1985. Rift Valley fever virus (family Bunyaviridae, genus Phlebovirus). Isolations from Diptera collected during an interepizootic period in Kenya. Journal of Hygiene-Cambridge 95:197-209.
Linthicum, K. J., A. Anyamba, C. J. Tucker, P. W. Kelley, M. F. Myers, and C. J. Peters. 1999. Climate and satellite indicators to forecast Rift Valley fever epidemics in Kenya. Science 285:397-400.
Linthicum, K. J., A. Anyamba, B. Killenbeck, W.-J. Lee, H. C. S. Lee, T. A. Klein, J.-C. Kim, J. A. Pavlin, S. C. Britch, S. Small, C. J. Tucker, and J. C. Gaydos. 2014. Association of temperature and historical dynamics of malaria in the Republic of Korea, including reemergence in 1993. Military Medicine 179:806-814.
Métras, R., T. Porphyre, D. U. Pfeiffer, A. Kemp, P. N. Thompson, L. M. Collins, and R. G. White. 2012. Exploratory space-time analyses of Rift Valley fever in South Africa in 2008–2011. Public Library of Science Neglected Tropical Diseases 6(8):e1808. doi:10.1371/journal.pntd.0001808.
Moudy, R. M., M. A. Meola, L. L. Morin, G. D. Ebel, and L. D. Kramer. 2007. A newly emergent genotype of West Nile virus is transmitted earlier and more efficiently by Culex mosquitoes. American Journal of Tropical Medicine and Hygiene 77:365-370.
Munich, R. E. 2010. Geo risks research: Heat wave, drought, wildfires in Russia (Summer 2010). https://www.munichre.com/site/touchnaturalhazards/get/documents_E439647210/mr/assetpool.shared/Documents/5_Touch/_NatCatService/Catastrophe_portraits/event_report_hw_dr_wf_russia_touch_en.pdf (accessed January 29, 2015).
Nicholls, N. A. 1986. A method for predicting Murray Valley encephalitis in southeast Australia using the Southern Oscillation. Australian Journal of Experimental Biology & Medical Science 64:587-594.
Padmanabha, H., E. Soto, M. Mosquera, C. C. Lord, and L. P. Lounibos. 2010. Ecological links between water storage behaviors and Aedes aegypti production: Implications for dengue vector control in variable climates. Ecohealth 7:78-90.
Panning, M., K. Grywna, M. van Esbroeck, P. Emmerich, and C. Drosten. 2008. Chikungunya fever in travellers returning to Europe from the Indian Ocean region, 2006. Emerging Infectious Diseases 14:416-422.
Pascual, M., X. Rodó, S. P. Ellner, R. Colwell, and M. J. Bouma. 2000. Cholera dynamics and El Niño-Southern Oscillation. Science 289:1766-1769.
Reisen, W. K., R. P. Meyer, S. B. Presser, and J. L. Hardy. 1993. Effect of temperature on the transmission of Western equine encephalomyelitis and St. Louis encephalitis viruses by Culex tarsalis (Diptera, Culicidae). Journal of Medical Entomology 30:151-160.



















