Subra, R. 1983. The regulation of preimaginal populations of Aedes aegypti L. (Diptera:Culicidae) on the Kenya coast. I. Preimaginal population dynamics and the role of human behavior. Annals of Tropical Medicine and Parasitology 77:195-201.
Trenberth, K. E., and J. T. Fasullo. 2013. Climate extremes and climate change: The Russian heat wave and other climate extremes of 2010. Journal of Geophysical Research: Atmospheres 117:D17103.
Turell, M. J. 1993. Effects of environmental temperature on the vector competence of Aedes taeniorhynchus for Rift Valley fever and Venezuelan equine encephalitis viruses. American Journal of Tropical Medicine and Hygiene 49:672-670.
Turell, M. J., M. O’Guinn, D. J. Dohm, and J. W. Jones. 2001. Vector competence of North American mosquitoes (Diptera: Culicidae) for West Nile virus. Journal of Medical Entomology 38:130-134.
Van Den Hurk, A. F., S. B. Craig, S. M. Tulsiani, and C. C. Jansen. 2010. Emerging tropical diseases in Australia. Part 4. Mosquito-borne diseases. Annals of Tropical Medicine and Parasitology 8:623-640.
A8
CHANGING PARADIGMS FOR TICK-BORNE DISEASES IN THE AMERICAS1
Christopher D. Paddock,2Robert S. Lane,3J. Erin Staples,4and Marcelo B. Labruna5
Introduction
Ticks transmit a greater diversity of viral, bacterial, and protozoan diseases than any other arthropod vector on earth (Jongejan and Uilenberg, 2004; IOM, 2011). Through 2014, at least 27 ecologically and epidemiologically distinct tickborne diseases were identified in the Western Hemisphere; remarkably, nearly half of these were discovered during the last 20 years (see Table A8-1). Against this background of expanding pathogen recognition are also unprecedented surges in the incidence of several tick-borne infections throughout the Americas.
During 2013, 48,821 cases of autochthonous, nationally notifiable, vector-borne disease were reported to the United States Centers for Disease Control and Prevention (CDC) (CDC, 2014). Overall, approximately 95 percent of reported
___________________
1 The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
2 Rickettsial Zoonoses Branch, Centers for Disease Control and Prevention, Atlanta, GA.
3 Department of Environmental Science, Policy and Management, University of California, Berkeley, CA.
4 Arboviral Diseases Branch, Centers for Disease Control and Prevention, Ft. Collins, CO.
5 Faculdade de Medicina Veterinária e Zootecnia, Universidade de São Paulo, São Paulo, Brazil.
TABLE A8-1 Tick-Borne Pathogens Affecting Humans in the Western Hemisphere
| Pathogen | Year Identified as a Cause of Tick-Borne Disease | Principal Tick Vector(s) | Country or Countries with Cases of Disease |
|---|---|---|---|
| Rickettsia rickettsii | 1909 | Dermacentor andersoni Dermacentor variabilis Rhipicephalus sanguineus Amblyomma sculptum Amblyomma aureolatum | Canada, United States, Mexico, Costa Rica, Panama, Colombia, Brazil, Argentina |
| Borrelia mazzottii | 1921 | Carios talaje | Mexico, Panama |
| Francisella tularensis | 1924 | D. variabilis D. andersoni Amblyomma americanum | Canada, United States, Mexico |
| Borrelia venezuelensis | 1927 | Carios rudis | Colombia, Venezuela |
| Borrelia turicatae | 1930 | Ornithodoros turicata | United States, Mexico |
| Borrelia hermsii | 1935 | Ornithodoros hermsi | Canada, United States |
| Borrelia parkeri | 1941 | Ornithodoros parkeri | United States |
| Colorado tick fever virus | 1946 | D. andersoni | Canada, United States |
| Lineage I Powassan virus | 1963 | Ixodes marxi Ixodes cookei Ixodes spinipalpis | Canada, United States |
| Babesia microti | 1970 | Ixodes scapularis | United States |
| Borrelia burgdorferi | 1982 | I. scapularis Ixodes pacificus | Canada, United States |
| Ehrlichia chaffeensis | 1987 | A. americanum | United States |
| Babesia duncani | 1993 | Unknown | United States |
| Anaplasma phagocytophilum | 1994 | I. scapularis I. pacificus | Canada, United States |
| Babesia divergens-like organism | 1996 | Unknown | United States |
| Rickettsia africae | 1998 | Amblyomma variegatum | Guadeloupe |
| Ehrlichia ewingii | 1999 | A. americanum | United States |
| Lineage II Powassan virusa | 2001 | I. scapularis D. andersoni | Canada, United States |
| Rickettsia parkeri | 2004 | Amblyomma maculatum Amblyomma triste Amblyomma tigrinum | United States, Uruguay, Argentina |
| Rickettsia sp. 364D | 2010 | Dermacentor occidentalis | United States |
| Pathogen | Year Identified as a Cause of Tick-Borne Disease | Principal Tick Vector(s) | Country or Countries with Cases of Disease |
|---|---|---|---|
| Rickettsia sp. Atlantic rainforest | 2010 | Amblyomma ovale A. aureolatum | Brazil |
| Ehrlichia muris-like agent | 2011 | I. scapularis | United States |
| Heartland virus | 2012 | A. americanum | United States |
| Borrelia americana | 2013 | Unknown | United States |
| Borrelia andersonii | 2013 | Unknown | United States |
| Borrelia miyamotoi | 2013 | I. scapularis I. pacificus | United States |
| Borrelia mayonii | 2014 | I. scapularis | United States |
NOTES: In some circumstances the distribution of the pathogen in ticks extends to other countries from which no documented cases of human disease have been reported. Dates approximate the recognition of a specific agent and its direct association with ticks and disease in humans. In some instances the named disease preceded discovery of the causative agent by many years. In other situations, the discovery of the agent in ticks preceded its association with human disease, or the agent was discovered simultaneously with the disease but remained without a formal name or was misidentified as another species before its correct designation.
a Also known as deer tick virus, this agent was first detected in Dermacentor andersoni ticks in Colorado in 1952 (Thomas et al., 1960; Kuno et al., 2001).
SOURCES: Ricketts, 1909; Bates et al., 1921; Parker et al., 1924; Dunn, 1927; Weller and Graham, 1930; Wheeler et al., 1935; Davis et al., 1941; Florio et al., 1946; McLean et al., 1963; Western et al., 1970; Steere et al., 1983; Maeda et al., 1987; Quick et al., 1993; Bakken et al., 1994; Herwaldt et al., 1996; Parola et al., 1998; Buller et al., 1999; Kuno et al., 2001; Paddock et al., 2004; Shapiro et al., 2010; Spolidorio et al., 2010; Pritt et al., 2011; McMullan et al., 2012; Gugliotta et al., 2013; Clark et al., 2013, Clark et al., 2014; Pritt et al., 2014.
cases of vector-borne disease were associated with ticks, making these the most medically important group of arthropods in the United States. Lyme disease alone accounted for almost 75 percent of all reported cases of indigenously acquired vector-borne disease. This compilation does not include many other regionally important and occasionally life-threatening tick-borne infections such as Colorado tick fever (CTF), tick-borne relapsing fever, and Heartland virus infection that are not nationally notifiable (Forrester et al.; 2015, Yendell et al., 2015; Pastula et al., 2014). In comparison, indigenously acquired mosquito- and flea-borne diseases comprised only approximately 5 percent of the nationally reported cases of vector-borne disease for 2013.
Since 2000, the numbers of reported cases of notifiable tick-borne diseases in the United States have followed consistent upward trends (see Figures A8-1, A8-2, and A8-3). During 2000–2008, the annual reported incidence of Rocky Mountain spotted fever (RMSF) in the United States increased from 1.7 to 9.4 cases per million persons, representing the steepest rise to the highest rate ever recorded (Openshaw et al., 2010). Likewise, from 2000 to 2007, the incidence of infections caused by Anaplasma phagocytophilum and Ehrlichia chaffeensis increased linearly, from 0.80 to 3.0 and 1.4 to 3.0 cases per million population, respectively (Dahlgren et a1., 2011). Nonetheless, these figures underestimate the true burden of tick-borne infections (IOM, 2011). Recent analyses of Lyme disease statistics provide a salient example. Using data acquired from a survey of 7 large commercial laboratories in the United States that performed tests for Lyme disease during 2008, investigators identified an estimated 240,000 to 440,000 source patients for that year (Hinckley et al., 2014). Although Lyme disease is the most commonly reported arthropod-borne infection in the United States, fewer than 30,000 cases were reported to the CDC in 2008, suggesting that national surveillance underestimates the annual magnitude of Lyme disease by about a factor of 10.
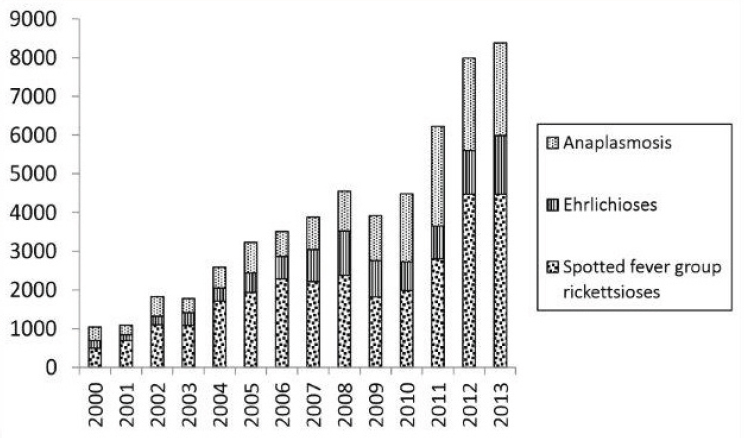
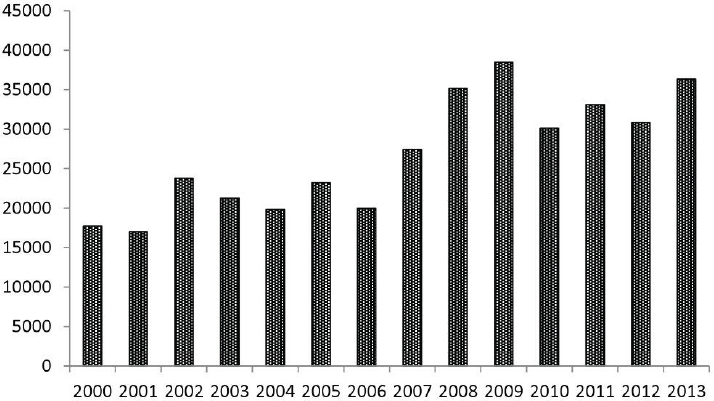
SOURCE: Adams et al., 2014 (CDC) (http://www.cdc.gov/lyme/stats/chartstables/reportedcases_statelocality.html).
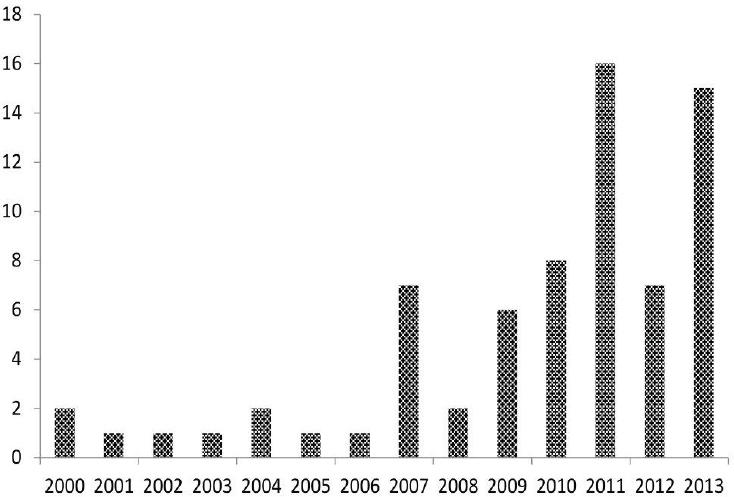
SOURCE: ArboNET, Arboviral Diseases Branch, Centers for Disease Control and Prevention (http://www.cdc.gov/powassan/statistics.html).
Similar trends in rising case counts of tick-borne diseases have been identified in other countries of the Western Hemisphere. In several states of Brazil, the number of reported cases of Brazilian spotted fever (BSF) has risen steadily during the last decade (see Figure A8-4; Amâncio et al., 2011; Barros e Silva et al., 2014). Since 2004, RMSF has reemerged in many regions of Mexico, particularly in the states of Baja California, Sonora, and Yucatan (Zavala-Castro et al., 2008; Bustamente Moreno and Pon Méndeza, 2010a; Álvarez Hernández and Contreras Soto, 2013). Similar trends have been recognized in Colombia (Hidalgo et al., 2007, 2011) and Panama (Estripeaut et al., 2007; Tribaldos et al., 2011), where RMSF reemerged more than 50 years after the sentinel outbreaks were identified in these countries during the first half of the 20th century (Patino et al., 1937; Rodaniche and Rodaniche, 1950).
Collectively, these observations highlight several recurring themes: (1) the scope and magnitude of tick-borne diseases are continuously evolving and expanding; (2) changes in the distribution and determinants of these diseases may occur over relatively brief intervals of time and space; and (3) the epidemiology of historically recognized tick-borne infections may evolve alongside the discovery of newly characterized pathogens. The following discussion examines the growing list of tick-borne pathogens, explores new perspectives on the pathogenesis of these infections in humans, and briefly considers certain aspects of the dynamic and multifaceted natural histories of these diseases in the Western Hemisphere.
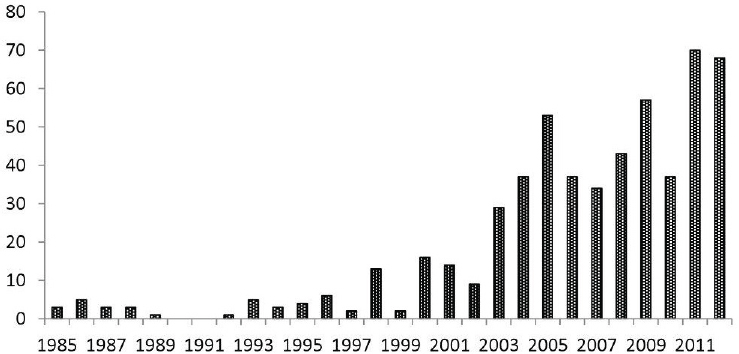
SOURCE: Data from São Paulo State government’s Centro de Vigilância Epidemiológica “Alexandre Vranjac” (CVE).
The Expanding Diversity of Tick-Borne Pathogens
Thirteen newly recognized tick-borne pathogens have been identified and characterized in the Western Hemisphere during the last 20 years (see Table A8-1). Perhaps more remarkable is the diversity of organisms represented by these newly identified pathogens, including several arboviruses and various Borrelia, Ehrlichia, and Rickettsia species, as well as the recognition that certain tick species can transmit multiple pathogens, in some cases as many as seven. It is highly probable that many tick-borne viruses and bacteria will be discovered throughout the enormously broad and ecologically diverse expanse of the Western Hemisphere. By example, a molecular survey of host-seeking western black-legged ticks (Ixodes pacificus) and small mammals from a single county bordering San Francisco Bay in California revealed an unprecedented seven Borrelia species, including two emerging human pathogens and two species novel for North America (Fedorova et al., 2014). Some recently identified tick-borne agents, such as Rickettsia parkeri, Rickettsia 364D (provisionally named Rickettsia philipii), and lineage II Powassan virus (also known as deer tick virus), represent bacteria or viruses that were identified in ticks many decades before they were formally recognized as human pathogens, including some that are evincing increased disease burdens (Kuno et al., 2001; Paddock et al., 2004; Shapiro et al., 2010; Nofchissey et al., 2013; El Khoury et al., 2013). Nonetheless, many of these agents are new to science and medicine, and even virulent pathogens, such as Heartland virus, continue to be identified, often by coupling classical diagnostic methods like cell culture isolation and electron microscopy with evolving molecular technologies (McMullan et al., 2012; Goldsmith et al., 2013).
The initial recognition of a tick-borne disease agent typically lags behind its detection in nature. Indeed, these agents characteristically afflict human populations as cryptic or misidentified infectious processes for decades or even centuries before these are correctly characterized. RMSF was identified in a “typhus fever” patient who died in Maryland in 1901 by testing autopsy tissues 90 years later. This retrospective diagnosis preceded the first official description of RMSF in the eastern United States by 30 years (Dumler, 1991). Historical accounts of a life-threatening, typhus-like illness, afflicting 44 persons in a small settlement in North Carolina during the summer of 1759, also document an illness clinically and epidemiologically suggestive of RMSF, 140 years before the disease was “discovered” in the western United States in 1900 (Tigertt, 1987). A febrile, highly lethal disease locally called febre pintada (spotted fever) has been recorded in the Brazilian state of Minas Gerais, an area where BSF is endemic (Amâncio et al., 2011), since the beginning of the 17th century (Magalhães, 1952).
The Lyme disease spirochete, Borrelia burgdorferi sensu stricto, hereinafter B. burgdorferi, is another case in point. DNA of B. burgdorferi was amplified from archival specimens of white-footed mice (Peromyscus leucopus) collected in Massachusetts during the 1890s (Marshall et al., 1994) and from black-legged ticks (Ixodes scapularis) collected on Long Island, New York during the 1940s
(Persing et al., 1990), which establishes the presence of the pathogen in vector and reservoir hosts in the northeastern United States decades before the formal recognition of Lyme disease. Willy Burgdorfer’s epochal discovery of this spirochete in I. scapularis ticks collected from vegetation on Shelter Island, New York, represents one of the major biomedical breakthroughs of the 20th century (Burgdorfer et al., 1982; Burgdorfer, 1984). Lyme disease was initially attributed to a single bacterial species, but subsequently was found to be caused by several closely related species forming the ever-expanding B. burgdorferi sensu lato (s. l.) complex. Nineteen additional species have been confirmed or proposed since B. burgdorferi was characterized and named in 1984 (Margos et al., 2011; Rudenko et al., 2011; Ivanova et al., 2013), and more undescribed species await characterization (Fedorova et al., 2014). Borrelia burgdorferi was the sole member of the complex thought to infect humans in North America for 3 decades until B. bissettii-like spirochetes were detected in three residents of a rural community in north-coastal California (Girard et al., 2011), and B. americana and B. andersonii were incriminated as human pathogens in the southeastern United States (Clark et al., 2013). More recently, B. americana-like strains were recovered from patients residing in the northeastern, southeastern, northwestern, and southwestern United States (Clark et al., 2014).
Discovery of new agents has important ramifications that may change clinical and epidemiological perceptions of previously identified tick-borne infections. In Missouri, investigators used molecular methods to discriminate infections caused by Ehrlichia ewingii from those caused by E. chaffeensis (Buller et al., 1999). That finding revealed a clinically and ecologically similar illness previously obscured because of overlapping disease manifestations and a shared tick vector. Indeed, surveys examining the relative prevalence of Ehrlichia spp. in reservoir hosts and Amblyomma americanum ticks in the United States suggest that E. ewingii occurs in these species at frequencies similar to, or in some cases greater than, infection with E. chaffeensis (Paddock and Yabsley, 2007). However, E. ewingii appears to cause a milder illness, particularly in immunosuppressed patients. Without molecular methods, these infections would have remained submerged among those caused by E. chaffeensis, contributing to a falsely heterogeneous description of E. chaffeensis ehrlichiosis. Likewise, an ehrlichial species very closely related to Ehrlichia muris (designated the E. muris-like agent) identified by molecular methods from patients in Minnesota and Wisconsin, appears to cause most and perhaps all of the serologically diagnosed cases of ehrlichiosis in the upper Midwestern United States, where neither E. chaffeensis nor E. ewingii are endemic (Pritt et al., 2011; Hoang Johnson et al., 2015). Because the E. muris-like agent appears to cause milder disease in humans than E. chaffeensis, and is transmitted by I. scapularis ticks rather than by A. americanum ticks (Stromdahl et al., 2014), the clinical, epidemiological, and ecological features of these diseases are distinct (Hoang Johnson et al., 2015).
Equally telling is a recent example involving arboviruses. A retrospective evaluation of 14 patients diagnosed with Powassan encephalitis in New York State from 2004 to 2012 yielded laboratory and epidemiological evidence indicating that many of these cases were caused by lineage II (i.e., deer tick virus) rather than lineage I (i.e., classical Powassan) virus (El Khoury et al., 2013). The ecologies of the two lineages of Powassan virus are markedly different: lineage I is maintained principally between Ixodes cookei ticks and groundhogs (Marmota momax) or Ixodes marxi and striped skunks (Mephitis mephitis), whereas lineage II is maintained predominantly between I. scapularis and deer mice (Peromyscus maniculatus). Because I. cookei and I. marxi ticks rarely attach to humans, the number of Powassan cases caused by lineage I is almost certainly lower than those caused by lineage II Powassan virus which is more readily transmitted to humans by I. scapularis ticks. Furthermore, I. scapularis ticks may be coinfected with lineage II Powassan virus, B. burgdorferi, A. phagocytophilum, or other zoonotic agents that can confound the clinical and epidemiological features of Powassan encephalitis caused by lineage II virus. Close examination of three other newly recognized tick-borne diseases further illustrates the foregoing general trends.
Rickettsia parkeri
Disease caused by R. parkeri was first described in 2004 (Paddock et al., 2004). Unrecognized infections undoubtedly have occurred in humans for many years before the index case, as suggested by descriptions of non-fatal cases of RMSF associated with attachment-site ulcers from coastal areas of Virginia and Maryland during the 1920s and 1930s. Although R. parkeri rickettsiosis and RMSF are clinically, epidemiologically, and ecologically distinct diseases (Paddock and Goddard, 2015; Romer et al., 2011), cases of R. parkeri rickettsiosis have been embedded among national surveillance data for RMSF for decades. Accordingly, the reporting category for RMSF in the United States was modified in 2010 to include diseases caused by R. parkeri and other spotted fever group rickettsioses (Openshaw et al., 2010). Through 2014, cases of R. parkeri rickettsiosis have been identified from Alabama, Florida, Georgia, Kentucky, Maryland, Mississippi, North Carolina, Texas, and Virginia. Moreover, the magnitude of R. parkeri rickettsiosis is likely greater than currently appreciated because 8–56 percent of Amblyomma maculatum ticks, the principal vector species, are infected with R. parkeri (Paddock and Goddard, 2015). By comparison, Rickettsia rickettsii, the etiologic agent of RMSF, was detected in only 1 (0.02 percent) of 5,286 Dermacentor variabilis ticks removed from humans during one U.S. study from 1997–2009 (Stromdahl et al., 2011).
During the last decade, R. parkeri was detected in at least three human-biting Amblyomma tick species in Argentina, Brazil, Peru, and Uruguay, and more than 15 cases of R. parkeri rickettsiosis were identified in South America though 2014
(Romer et al., 2011, 2014). In Brazil, the discovery in 2010 of a second pathogen, closely related to R. parkeri and designated Rickettsia sp. Atlantic rainforest (Spolidorio et al., 2010), helped solve an epidemiological conundrum created by vastly different clinical features described for the same tick-borne disease. During 2007–2012, 734 laboratory confirmed cases of BSF were reported to the National Disease Surveillance System in Brazil, including 180 (24.5 percent) and 324 (44 percent) from the states of Santa Catarina and São Paulo, respectively. Surprisingly, no BSF-associated deaths were reported from Santa Catarina during this period, whereas the case-fatality rate of BSF in São Paulo was approximately 41 percent (Barros e Silva et al., 2014). A careful comparison of clinical characteristics of BSF patients in Santa Catarina versus São Paulo revealed marked differences in severity, which suggests that cases designated as “BSF” in these two states were in fact two distinct diseases (Angerami et al., 2009). Although no significant differences were identified between the frequency of fever, rash, or malaise, the rates of hemorrhage, severe neurological manifestations, and death differed notably (see Table A8-2). Acarological surveys from different regions of Santa Catarina subsequently identified Rickettsia sp. Atlantic rainforest infecting approximately 3–9 percent of human-biting Amblyomma spp. ticks in these areas with no evidence of R. rickettsii (Medeiros et al., 2011; Barbieri et al., 2014).
| Sign or Symptom | São Paulo (n = 126) | Santa Catarina (n = 61) | P value |
|---|---|---|---|
| Fever | 112 (89 percent) | 58 (95 percent) | 0.16 |
| Rash | 44 (35 percent) | 30 (49 percent) | 0.06 |
| Malaise | 73 (58 percent) | 35 (57 percent) | 0.9 |
| Adenopathy | 5 (4 percent) | 30 (49 percent) | < 0.01 |
| Petechiae | 46 (36 percent) | 5 (8 percent) | < 0.01 |
| Hemorrhage | 33 (26 percent) | 1 (2 percent) | |
| Hypotension | 30 (24 percent) | 2 (3 percent) | < 0.01 |
| Coma | 24 (19 percent) | 0 | < 0.01 |
| Convulsion | 18 (14 percent) | 0 | < 0.01 |
| Death | 46 (37 percent) | 0 | < 0.01 |
SOURCE: Adapted from Angerami et al., 2009.
These findings strongly suggest that reported cases of tick-borne spotted fever in Santa Catarina are caused by a Rickettsia species different than the pathogen associated with classical BSF in São Paulo.
Borrelia miyamotoi
Until PCR and sequencing techniques came into routine use during the early 1990s, B. miyamotoi had been categorized unknowingly with B. burgdorferi s. l. for more than a decade. First described in Japan in 1995 and named in honor of tick researcher Kenji Miyamoto, this relapsing-fever group spirochete was isolated initially from Ixodes persulcatus ticks and the blood of a rodent (Apodemus argenteus) (Fukunaga et al., 1995). Borrelia miyamotoi was subsequently detected in North America in I. scapularis ticks, and 1.9–2.5 percent of host-seeking nymphs collected in Connecticut, Maryland, New Jersey, New York, or Rhode Island were found to contain this Borrelia species (Scoles et al., 2001). In Canada, B. miyamotoi was detected in 23 (0.5 percent) of 4,938 I. scapularis ticks collected by passive surveillance in eight provinces during 2012 (Dibernardo et al., 2014). Borrelia miyamotoi is passed transstadially and transovarially within I. scapularis ticks, and the white-footed mouse has been incriminated as a reservoir host. Approximately 12 percent of all Borrelia-positive ticks detected in the areas surveyed by Scoles et al., (2001) were infected with B. miyamotoi versus the Lyme disease spirochete B. burgdorferi (88 percent), which indicates that a sizable proportion of spirochete-positive ticks previously thought to contain B. burgdorferi by microscopy were instead infected with this novel Borrelia. The authors also posited, correctly as it turned out, that at least some of the B. burgdorferi infections reported earlier in wild-caught I. scapularis larvae were probably B. miyamotoi, not B. burgdorferi. The foregoing assumptions are supported convincingly by experimental and field and laboratory evidence for I. scapularis and other members of the medically relevant I. persulcatus group of ticks, such as I. pacificus (Lane and Burgdorfer, 1987; Rollend et al., 2013; Padgett et al., 2014). In the study by Lane and Burgdorfer (1987), spirochetes visualized in tissue smears of I. pacificus F2 larval progeny, but not those present in all three parasitic stages of the F1 generation, were reactive with a monoclonal antibody (H5332) once deemed specific for B. burgdorferi, but now recognized to be more broadly reactive with other borreliae.
A 13-year survey carried out in 24 of California’s 58 counties revealed that about half of spirochete-infected I. pacificus adults assayed for borreliae were infected with B. miyamotoi and the other half with B. burgdorferi s. l. (Padgett et al., 2014). These results have important epidemiological implications, namely, that a considerable percentage of adult ticks thought to be infected with B. burgdorferi when assayed 20–30 years earlier using less specific serological methods are likely to have been infected with B. miyamotoi, and that the risk of human exposure to B. burgdorferi and B. miyamotoi following the bite of
an adult I. pacificus is similar (Padgett et al., 2014). Nevertheless, the risk in California of acquiring infection with either spirochete from an adult I. pacificus tick is very low, as less than 1 percent of 6,036 tested adult ticks were infected with either B. burgdorferi or B. miyamotoi. By comparison, 3.2 percent of 2,188 nymphal I. pacificus were infected with B. burgdorferi versus 1.4 percent with B. miyamotoi.
On a global scale, multiple species of Ixodes ticks and several small mammals and birds are known to host B. miyamotoi in Asia, Europe, and North America. In the United States, B. miyamotoi infects white-footed mice in the northeastern and north-central regions (Barbour et al., 2009), and wild turkeys (Meleagris gallopavo) in the south-central region (Scott et al., 2010); whereas, nothing is known about its vertebrate hosts in the western United States. As is true for so many emerging tick-borne illnesses, the full clinical spectrum produced by different genogroups of B. miyamotoi requires clarification. What is clear, however, is that infection with B. miyamotoi may cause much more than a mild relapsing fever-like illness (Krause et al., 2013, 2014; Chowdri et al., 2013), as reported recently for an elderly patient who developed meningoencephalitis following infection with B. miyamotoi in the northeastern United States (Gugliotta et al., 2013).
Heartland Virus and Other Potentially Tick-Borne Arboviruses
The first human cases of Heartland virus disease were discovered when the virus was cultured serendipitously from blood specimens of two Missouri patients who were suspected initially to have E. chaffeensis ehrlichiosis (McMullan et al., 2012). Although the cell cultures showed cytopathic effects, no ehrlichial morulae were identified; subsequently, electron microscopy identified a Bunyavirus, and next-generation sequencing further characterized this pathogen as a newly recognized Phlebovirus (see Figure A8-5A). Because Heartland virus grows slowly in Vero cells that traditionally are used to isolate arboviruses, this virus may not have been readily discovered if the clinical samples had been received by an arbovirology laboratory. Prior to this discovery, no human pathogenic Phlebovirus was known to occur in the Western Hemisphere (Matsuno et al., 2014). The most closely related virus, severe fever with thrombocytopenia syndrome virus described from China in 2011, demonstrates only 70 percent homology on nucleic acid sequencing (Yu et al., 2011; McMullan et al., 2012). Despite its recent identification in 2009, and given the similar clinical features of Heartland virus with other tick-borne bacterial or rickettsial diseases, it is likely that cases of Heartland virus disease may have been misdiagnosed clinically as one of these diseases in the United States for many years (Figure A8-5B). The percent nucleotide divergence of Heartland virus strains from various locations in the United States suggest that these foci had been evolving separately for quite some time (Muehlenbachs et al., 2014). Through 2014, cases of Heartland virus disease,
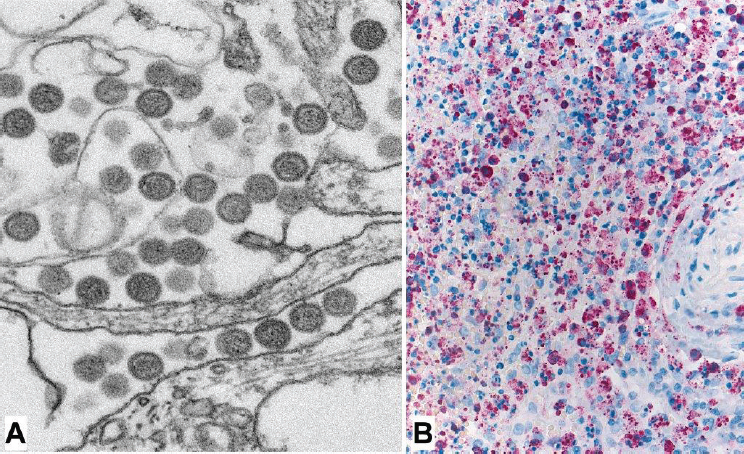
SOURCES: Images courtesy of Cynthia Goldsmith, CDC; Sherif Zaki, CDC.
including several deaths, have been identified in Georgia, Kentucky, Missouri, Tennessee, and Oklahoma (Pastula et al., 2014; CDC unpublished data), and it is likely that the distribution of Heartland virus in the United States will resemble closely that of its vector, A. americanum (Savage et al., 2013).
The recent discovery of Bourbon virus, a newly recognized Thogotovirus isolated from an ill patient in Kansas, resulted from a careful laboratory assessment of a patient suspected initially to be infected with Heartland virus (Kosoy et al., 2015). Although it is currently unknown how Bourbon virus is transmitted to humans, the initial case-patient reported tick exposure and removed an imbedded tick several days prior to the onset of illness characterized by fever, fatigue, thrombocytopenia, and leukopenia. Despite treatment with doxycycline and other antimicrobial agents, the patient failed to improve, developed multiorgan failure and died 11 days after illness onset from cardiopulmonary arrest. Testing of the patient’s specimen for Heartland virus antibodies using plaque reduction neutralization revealed a unique virus that was subsequently identified by next-generation sequencing and phylogenetic analysis as a member of the genus Thogotovirus. Thogotoviruses primarily associated with hard or soft ticks (McCauley et al., 2012), and field studies are in progress to determine if Bourbon virus is yet another example of the expanding diversity of tick-borne pathogens.
The Expanding Clinical Spectrum of Tick-Borne Diseases
Our understanding of the pathogenesis of tick-borne diseases has become more nuanced during the last 50 years, augmented by the identification of host factors that place persons at risk for more severe illness, and by the recognition that some strains of a specific pathogen may dictate specific manifestations or severity. Increasing awareness of the astonishing complexity among the pathogen, its vector, and the human host has been leveraged by transformational advances in diagnostic techniques that accurately provide species and strain identity of the infectious agent.
Underrecognized Manifestations of Previously Recognized Pathogens
During 2012–2013, three cases of Lyme carditis associated with sudden cardiac deaths were identified by postmortem examinations of patients ranging in age from 26 to 38 years (see Figure A8-6; Ray et al., 2013). Prior to this report, only four deaths attributed to Lyme carditis had been described since the initial characterization of the disease in the early 1980s. Indeed, fatal carditis is considered an extremely rare manifestation of Lyme disease. A retrospective evaluation
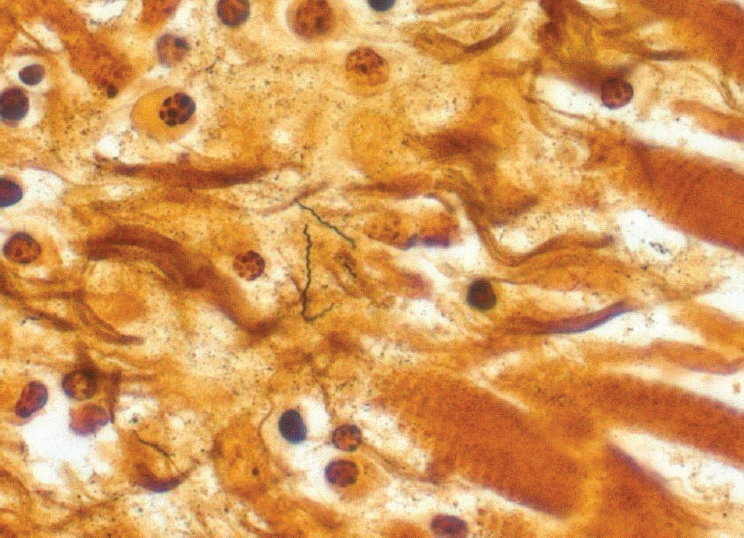
SOURCE: Image courtesy of Atis Muehlenbachs, CDC.
of 121,894 cases of Lyme disease from seven selected high-incidence states that occurred between 1995 and 2013 identified two suspected cases of fatal Lyme disease carditis, representing only 0.002 percent of the total patients and 0.1 percent of the 1,696 cases for whom carditis was documented (Forrester et al., 2014). Nonetheless, the frequency of sudden death attributable to cardiac infection with B. burgdorferi, albeit rare, may be greater than previously believed.
Before 2003, nearly all reported cases of Powassan virus disease experienced severe neurologic illness, usually meningoencephalitis (Artsob, 1988; Gholam et al., 1999). Since then, several cases without neurologic features have been documented (Hoang Johnson et al., 2010). In addition, an increasing number of aseptic meningitis cases due to Powassan virus infections have been reported (Minnesota Department of Health, 2014). These less severe clinical presentations were associated mainly with lineage II Powassan virus infections originating from the Midwest (Neitzel et al., 2013; Hoang Johnson et al., 2010; Ebel et al., 1999; Brackney et al., 2008). Some of these perceived differences in severity between the two lineages might be due to increased recognition and testing in certain locations of potentially less severe disease cases (Neitzel et al., 2013; Hoang Johnson et al., 2010; Hinten et al., 2008); whereas, ecological data suggest that there has been a true increase in the circulation of lineage II Powassan virus over the last 30 years (Nofchissey et al., 2013).
Strain-Specific Variations Associated with Virulence and Tissue Tropism
Advanced molecular techniques have allowed strain separation of R. rickettsii isolates obtained from patients in North, Central, and South America. In the United States and Mexico, two predominant genogroups have been associated with disease in humans, while cases of RMSF in Central and South America have been associated exclusively with a third distinct genogroup (Paddock et al., 2014; Labruna et al., 2014). Case-fatality rates of RMSF in Costa Rica, Panama, Colombia, Brazil, and Argentina characteristically are three to four times greater than those observed in the United States, and an unusually virulent genogroup of R. rickettsii may be responsible for the higher case-fatality rates in many Latin American countries (Parola et al., 2009; Labruna et al., 2014).
In north coastal California, 13 outer-surface-protein (ospC) allelles belonging to 12 genotypes of B. burgdorferi were identified among several thousand host-seeking I. pacificus nymphs (Girard et al., 2009). Approximately 20 ospC genotypes had been described from North America through 2008, including at least 4 and possibly as many as 9 that are invasive for humans. The most prevalent genotype in northern California is a novel strain, designated H3, previously not detected in B. burgdorferi-infected I. scapularis ticks in the northeastern United States, or in humans anywhere. Surprisingly, strain H3 was found in 25 percent of 222 B. burgdorferi-infected nymphs collected from dense woodlands in Mendocino County (Girard et al., 2009). The presence of this strain, and the
absence of a few other strains causing disseminated disease in people in the northeastern United States, especially the highly invasive ospC genotypes I and K, may represent one of several factors contributing to the low incidence of Lyme disease in California. Intriguingly, serum specimens from 24 percent of the subjects tested from a community at high risk for Lyme disease in the same county were PCR positive for B. burgdorferi infection (Girard et al., 2011). Among 20 B. burgdorferi-infected study subjects whose spirochete DNA could be typed, 95 percent contained the highly invasive ospC genotype A even though only 11 percent of infected nymphs collected countywide harbored this strain (Girard et al., 2009).
Co-Infections with Multiple Tick-Borne Agents
Increasing awareness that a single tick species may contain multiple and varied pathogenic agents has leveraged medical awareness that human hosts may be infected simultaneously by two or more pathogens following a single tick bite or from concurrent single-pathogen tick attachments. The most widely described examples represent co-infections transmitted by I. scapularis and include instances of Lyme disease and babesiosis, Lyme disease and human anaplasmosis, and Lyme disease, human anaplasmosis, and babesiosis (Krause et al., 1996; Swanson et al., 2006; Horowitz et al., 2013). The risk for co-infection with multiple pathogens differs by geographic location and depends to a large degree on the prevalence of the pathogens in reservoir hosts and tick species. Nonetheless, the risk of exposure to multiple pathogens following a single tick bite are not well understood, and a recent study suggests that certain co-infections may occur at frequencies other than predicted by independent assortment of the various pathogens. Specifically, evaluation of questing nymphal I. scapularis ticks collected in Dutchess County, New York, during 2011–2012 revealed 83 percent more co-infections with B. microti and B. burgdorferi than predicted by chance alone; whereas, fewer confections with B. microti and A. phagocytophilum were identified than predicted by chance (Hersh et al., 2014).
In the United States, the highest frequencies of co-infections in humans have been reported from New England (Swanson et al., 2006). From one study, 75 (39 percent) of 192 patients from Massachusetts and Connecticut diagnosed with Lyme disease, human anaplasmosis, or babesiosis during 1997–2000 were co-infected with two or more pathogens (Krause et al., 2002). Co-infected patients are significantly more likely to present with a greater diversity of signs and symptoms, as well as longer durations of illness, caused in part by a delay in diagnosis of the secondary or tertiary co-infection (Krause et al., 2002; Horowitz et al., 2013). Co-infections also occur with Powassan virus and A. phagocytophilum (Hoang Johnson et al., 2010), and with Heartland virus and Ehrlichia chaffeensis (CDC, unpublished data).
Host Factors and Clinical Expression of Disease
African-American male patients in the United States with glucose-6-phosphate dehydrogenase (G6PD) deficiency have a greater likelihood of experiencing severe or fatal RMSF than patients with normal G6PD activity (Walker et al., 1983a,b). Specifically, the genotype represented by G6PD A- was identified four times more often than the expected frequency in that cohort of RMSF patients. The overall frequency of G6PD A- also is generally higher among the at-risk population of several Latin America countries versus the United States and may contribute to the much greater case-fatality rates associated with R. rickettsii infections in South America. For example, the overall case-fatality rate of BSF in Minas Gerais State during 2000 to 2008 was 40 percent (Amâncio et al., 2011), and in São Paulo State during 2007–2012 it was 41 percent (Barros e Silva et al., 2014). By contrast, contemporary RMSF case-fatality rates in the United States have been less than 5 percent (Openshaw et al., 2010).
Advanced age is a risk factor for disease severity for many tick-borne infections. By example, the age-specific incidence for ehrlichiosis and anaplasmosis show a striking age-related increase in frequency among older persons (Demma et al., 2005b). Cholesterol dependence by the pathogenic bacteria E. chaffeensis and A. phagocytophilum may correlate with greater disease severity in older patients, because cholesterol levels typically rise with increasing age, and these bacteria lack the genes necessary for the biosynthesis of lipid A (Lin and Rikihisa, 2003). Furthermore, symptoms of babesiosis are more diverse, longer lasting, and more frequently require hospitalization in elderly patients than in younger individuals (Krause et al., 2003). Despite children being tested for Heartland virus, none has tested positive, and all known cases of Heartland virus disease have occurred in adults (Pastula et al., 2014), and the few deaths attributed to Heartland virus occurred in persons older than 60 years of age, many of whom had co-morbid conditions (Muehlenbachs et al., 2014; CDC, unpublished data). Although it is unclear why older adults are more likely to have more clinically apparent or severe disease when infected with Heartland virus, this finding mirrors what occurs with many other viral infections, such as West Nile virus and influenza virus (Lindsey et al., 2010; Quandelacy et al., 2014).
Recent Epidemiological and Ecological Shifts in Ticks and Tick-Borne Diseases
Tick-borne zoonoses are highly sensitive to manifold factors, often anthropogenic, that include microclimate, climate, host availability, habitat fragmentation, invasive forest pathogens and land use (Levia et al., 2012; Swei et al., 2012; Pfäffle et al., 2013; Léger et al., 2013). Changes in one or more of these variables often create ecological ripples across landscapes that culminate in modified environments favorable for the propagation and perpetuation of certain tick vectors. These dynamic and cumulative processes, associated intimately with concurrent
movements of pathogens, reservoir hosts, and host species, result in the emergence of tick-borne infections in human populations that reside or intrude into regions newly colonized by the particular tick species (Ogden et al., 2013). On a microscopic level, the perception of ticks as “crawling pins” has evolved into a far more complex host–pathogen association, as microbiome analyses reveal a remarkable diversity of bacteria and viruses that coexist within these arthropods and likely affect pathogen transmission.
Epidemiological Changes Over Time and Space
During the 1970s, most cases of CTF occurred among males aged 20-39 years (Goodpasture et al., 1978; Spruance and Bailey, 1973). More recently CTF cases in Montana, Utah, and Wyoming from 1995–2003 occurred in a higher proportion of females and people older than 50 years (Brackney et al., 2010). Changes in care-seeking behavior, testing, or surveillance practices, or true differences in exposures during recreational activities among persons of all ages and both sexes, may underlie these demographic changes. Overall, the number of CTF cases has decreased dramatically from more than 200 cases diagnosed per year in the United States from 1970–1984 to a median of 55 cases per year from 1987–2001 and only 5 cases per year from 2002—2012 (Bowen, 1988; Marfin and Campbell, 2005; Yendell et al., 2015). This decline may be an artifact of changes in testing and reporting practices. For example, Colorado historically reported the largest number of CTF cases (Bowen, 1988; Tsai, 1991; Marfin and Campbell, 2005), and when CTF was removed from the list of notifiable conditions in Colorado in 1997, the number of nationally reported cases plummeted (Yendell et al., 2015).
The ecology and epidemiology of Lyme disease on the West Coast differs markedly from that in the northeastern United States. Californians are exposed to infected I. pacificus ticks predominantly in rural or semirural settings year-round in less populated northern counties while they recreate or work outdoors versus mainly peridomestic exposure in suburban areas in the Northeast (Lane et al., 1992; Salkeld et al., 2014). Risk factors for exposure to I. pacificus nymphs in California include spending time in forested areas having an annual growing degree-day range of 2,600 to 3,000 (Eisen et al., 2006), and having contact with wood by sitting atop logs, gathering firewood, or woodcutting (Lane et al., 1992, 2004). Moreover, Lyme disease is a highly focal disease in California, with more than four-fifths of cases reported from northern counties, especially the sparsely populated northwestern coastal region (Eisen et al., 2006). Acarological, demographic, and climatic factors contribute to the low statewide incidence (about 0.2 cases per 100,000 population) of Lyme disease. Most residents live in suburban or urban areas in more arid southern counties where both the projected (Eisen et al., 2006) and known acarologic risks are low (Lane et al., 2013). Although more than 37 million residents reside in the state’s 58 counties, half of the entire population is concentrated in Los Angeles, San Diego, Orange, Riverside, and
San Bernardino counties, where B. burgdorferi-infected ticks are rarely encountered and diurnal questing by I. pacificus nymphs is minimal (Lane et al., 2013).
Range Expansions of Medically Important Ticks
Many shifts in the distribution and abundance of tick species in North America occurred during the last 50 years. Some of these observations reflect the ebb and flow of species movement within an ancestral range that is modulated by constant human intervention (Spielman et al., 1993; Paddock and Yabsley, 2007; Paddock and Goddard, 2015). The rise of I. scapularis populations throughout much of eastern North America reflects a series of anthropogenically driven events during the mid-19th century to the present, whereby reversal of post-Columbian deforestation, increased deer abundance, and increased development and use of forested sites by humans resulted in a proliferation of black-legged ticks and recognition of at least 7 I. scapularis-borne pathogens (Spielman et al., 1985; Spielman et al., 1993; IOM, 2011; Pritt et al., 2011; Hoang Johnson et al., 2015). Current data indicate that the expansion of geographic range of blacklegged ticks has proceeded largely through progressive and local migration events from southern populations to proximate northern locations (Khatchikian et al., 2015). In the mid-1980s, Ipswich, Massachusetts, represented the northernmost distribution of I. scapularis in the northeastern United States. Within a decade, however, this tick had spread northward to the Bar Harbor region in Maine.
Similar changes in the distribution and abundance of black-legged tick populations took place across the central and upper Midwestern United States during the past 30 years. A significant increase in the prevalence of I. scapularis on white-tailed deer occurred along the Wisconsin River Valley during 1981–1994 (Riehle and Paskewitz, 1996). No specimens of I. scapularis were found during an acarological survey in the early 1990s around Chicago; whereas, established populations were detected within 20 years throughout several northeastern Illinois counties located adjacent to and inclusive of this large metropolitan area (Rydzewski et al., 2012). Recent incursions of black-legged ticks also were identified across the lower peninsula of Michigan (Hamer et al., 2010). Finally, B. burgdorferi-infected I. scapularis ticks have spread throughout southern Canada, with recent invasion events in southwestern Quebec and southern Ontario being ascribed to long-distance dispersal by migratory birds (Ogden et al., 2013).
Acarological surveys conducted at the end of the 20th century identified established populations of A. americanum throughout many regions of New York State where none were noted approximately 50 years earlier (Means and White, 1997; Paddock and Yabsley, 2007). Similarly, retrospective assessment of various state and national tick collections revealed only isolated and sporadic records of A. americanum in Nebraska during 1944–1973. Collection records of this species increased markedly during 1987–2011, and it now represents the second most frequently reported tick in the state (Cortinas and Spomer, 2013). Indeed, various
data sources suggest a general northward shift in the distribution of A. americanum throughout much of the Midwest and northeastern United States during the last 50 years (Springer et al., 2014).
The distribution of A. maculatum was described 70 years ago as occupying a narrow band extending 100–150 miles inland from the Gulf Coast of Texas, across the southern states, to the Atlantic Coast of South Carolina. Since then, collection data suggest qualitative and quantitative changes in the historically accepted range of A. maculatum, including established populations more than 250 miles inland in several states bordering the Gulf of Mexico as well as northern expansions in many mid-Atlantic states (Nadolny et al., 2015). Established populations of A. maculatum now occur in several states where few or no records of this species existed during the first half of the 20th century, including Arkansas, Delaware, Kansas, Kentucky, North Carolina, Oklahoma, and Virginia, and confirmed cases of R. parkeri rickettsiosis have been documented in several of these states (Paddock and Goddard, 2015).
Amblyomma variegatum, the tropical bont tick, was introduced into the Caribbean on cattle imported from Senegal, Gambia, and Guinea to Guadeloupe during the early 1800s, and is now established on more than 15 islands in this region (Parola et al., 2009; Léger et al., 2013). A. variegatum is a primary vector of Rickettsia africae, the etiologic agent of African tick-bite fever and R. africae-infected populations of A. variegatum have been identified throughout much of the Caribbean, including Guadeloupe, Martinique, St. Lucia, Nevis, St. Kitts, Antigua, Dominica, Montserrat, and the U.S. Virgin Islands (Kelly et al., 2010). In 1998, the first case of African tick-bite fever acquired in the Western Hemisphere was documented in a traveler from Guadeloupe, and it is likely that many other undocumented cases occur annually in the Caribbean (Parola et al., 1998).
Capybara and the Reemergence of Brazilian Spotted Fever
The state of São Paulo in southeastern Brazil has accounted for nearly half of all laboratory-confirmed cases of BSF during the past 30 years (Barros e Silva et al., 2014). Notably, the number of BSF cases has gradually increased from 3 in 1985 to 68 in 2013, with annual fatality rates always around 40 percent. Most of these cases occurred in rural areas where capybaras (Hydrochoerus hydrochaeris) sustain large populations of the tick vector Amblyomma sculptum, a member of the Amblyomma cajennense species complex (Nava et al., 2014). Besides its role as a major host for A. sculptum, capybaras also serve as reservoir hosts of R. rickettsii (Labruna, 2013). Capybaras infected with R. rickettsii can maintain rickettsiae in their bloodstream for several days to weeks at levels sufficient to infect noninfected ticks, thereby amplifying rickettsial infection among the tick population (Souza et al., 2009). Because R. rickettsii is only partially maintained through vertical transmission in A. sculptum (Soares et al., 2012), capybaras play a major role in the ecology of R. rickettsii in BSF-endemic areas in São Paulo.
Indeed, the increasing number of BSF cases has been directly attributed to the increasing expansion of capybara populations in the state of São Paulo during the same period (Labruna, 2013).
During the last 5 decades, the state of São Paulo has gone through substantial landscape transformation, in which three factors have played a major role in the expansion of capybaras: (1) the tremendous agricultural expansion of sugar cane, a preferred food source of capybara, that has developed over recent years throughout Brazil as ethanol has emerged as a biofuel; (2) the creation of strict laws prohibiting the hunting of wildlife, which protect capybaras even in urban and semiurban areas; and (3) the elimination of natural predators of capybara such as jaguars from these same areas (Ferraz et al., 2007; Moreira et al., 2013). Capybara are remarkably prolific breeders, and females can birth 6 pups each year; indeed, the density of capybara in some BSF-endemic areas of the state of São Paulo are estimated to be 40 to 60-fold higher than the densities observed in natural environments, such as Pantanal and Amazon (Ferraz et al., 2010). Collectively these changes have modified capybara behavior such that large peridomestic populations exist that enhance the likelihood of human exposure to tick vectors of R. rickettsii.
Rhipicephalus sanguineus and the Ecology of RMSF in Western North America
For almost a century, D. variabilis and Dermacentor andersoni were considered to be the most important vectors of RMSF in the United States. During 2002–2004, 16 cases of Rocky Mountain spotted fever were identified in a 6700-km2 region of rural eastern Arizona (Demma et al., 2005a). From an epidemiological perspective, this was a highly unusual event, as only 8 cases of RMSF had been reported from the entire state during the preceding 15 years. An ecological assessment revealed large numbers of free-roaming dogs and Rhipicephalus sanguineus ticks in all life stages distributed abundantly at the case-patient households and surrounding environment (Nicholson et al., 2006). R. rickettsii was detected in approximately 5 percent of the nonengorged ticks and 10 percent of the engorged ticks (Eremeeva, 2012). Neither D. variabilis nor D. andersoni were found at any of the case-acquisition sites despite repeated acarological evaluations. Although investigators in the 1930s determined that Rh. sanguineus was a competent experimental vector of R. rickettsii (Parker et al., 1933), a role for this tick in the natural history of RMSF in the United States had not been demonstrated before this outbreak. It is now recognized that the specific ecological circumstances that perpetuated epidemic RMSF in these small communities also exist in other areas of Arizona. During 2003–2012, more than 250 authochthonous cases of RMSF, including 19 deaths, were reported from this state alone. During 2009–2012, the average annual incidence of RMSF in Arizona was approximately 136 cases per 100,000 persons, more than 150 times the U.S. national average (Drexler et al., 2014).
In-depth studies in Mexico during the 1940s identified Rh. sanguineus as a vector for R. rickettsii during outbreaks of RMSF in several northern states including Sonora and Sinaloa (Mariotte et al., 1944; Bustamente and Varela, 1947). In 2003, investigators in Mexicali, Mexico, determined that 60 percent of stray and privately owned dogs in the city were infested with Rh. sanguineus (Tinoco-Gracia et al., 2009). These findings heralded an epidemic of RMSF that occurred in Mexicali and other areas of Baja California during 2009, resulting in more than 1,000 confirmed and probable infections (Bustamente Moreno and Pon Méndez, 2010a). Surveys for Rh. sanguineus identified these ticks in all 14 districts of Mexicali, where 96 percent of the cases occurred (Sanchez et al., 2009; Bustamente Moreno and Pon Méndez, 2010b). These outbreaks are not necessarily generalizable to other regions of the United States or to other countries in the Americas. However, it appears that Rh. sanguineus is a far more important vector of RMSF than previously believed. While R. rickettsii has been detected or isolated from Rh. sanguineus ticks at different BSF-endemic areas of southeastern Brazil (Cunha et al., 2009; Gehrke et al., 2009; Moraes-Filho et al., 2009; Pacheco et al., 2011), cases have not been associated with this tick species in Brazil. Thus far, R. rickettsii-infected Rh. sanguineus ticks have been collected only from areas where the classical vectors of R. rickettsii—A. sculptum and Amblyomma aureolatum—were also present (Labruna, 2009).
Conclusions and Future Perspectives
The contemporary pace of tick-borne pathogen discovery has produced a litany of newly recognized agents and may well escalate with the advent of metagenomics. Many candidate tick-borne pathogens already have been suggested based on data from animal experimentation, serologic reactivity to particular antigens, or anecdotal reports of non-characterized illnesses following tick bites (see Table A8-3). Focused endeavors to determine viral etiologies of tick-borne disease in the New World will undoubtedly reveal novel pathogens. In North America, there are currently four tick-associated viral agents of disease—Colorado tick fever virus, Heartland virus, and Powassan lineage I and II viruses. This likely represents only a fraction of the pathogenic tick-borne arboviruses in the Western Hemisphere. Several viruses in the Bunyaviridae and Arenaviridae families have been detected with increasing frequency in human-biting ticks, and it would be surprising if some of these occasionally did not infect people (Briese et al., 2014; Pinto da Silva et al., 2005; McElroy Horne and Vanlandingham, 2014; Sayler et al., 2014). Recently, virome analyses of I. scapularis and D. variabilis have uncovered multiple previously undescribed viruses, including novel nairoviruses and phleboviruses of potential relevance for public health (Tokarz et al., 2014b; Matsuno et al., 2014). Similar studies examining the viromes of I. pacificus, D. occidentalis, Rh. sanguineus, A. maculatum, O. hermsi, and other human-biting ticks in the United States will likely yield other tick-borne viruses.
TABLE A8-3 Candidate Tick-Borne Pathogens in the Western Hemisphere
| Agent | Tick associate | Data to suggest pathogenicity | Reference(s) |
|---|---|---|---|
| Rickettsia canadensis | Haemaphysalis leporispalustris | Tick transmission of agent to guinea pigs; fever in infected guinea pigs; seroconversion to R. canadensis antigens in febrile patients. | McKiel et al., 1967 Burgdorfer, 1968 Bozeman et al., 1970 Wenzel et al., 1986 |
| Rickettsia sp. Tillamook | Ixodes pacificus | Death of infected mice. | Hughes et al., 1976 |
| Rickettsia sp. parumapertus | Dermacentor parumapertus | Fever in infected guinea pigs. | Philip and Hughes, 1953 |
| Rickettsia rhipicephali | Rhipicephalus sanguineus | Fever and death in infected meadow voles. | Burgdorfer et al., 1975 |
| Punta Salinas virus | Ornithodoros amblus | Undifferentiated febrile illness in persons bitten by O. amblus | Clifford et al., 1980 |
| Sixgun City virus | Argas cooleyi | Illness in suckling mice; related viruses cause disease in humans. | Yunker et al., 1972 |
| Tacaribe virus | Amblyomma americanum | Associated with a non-fatal infection in a laboratory worker. | Sayler et al., 2014 |
| Borrelia bissettii | Ixodes pacificus | DNA detected in human serum; cause of illness in Europe; induces pathology in mice. | Girard et al., 2011 Schneider et al., 2008 |
Virus isolations and serological surveys from multiple species of wildlife collected in California suggest that CTF virus, or a very similar virus, is widespread across several west-central counties, far beyond the historically recognized and relatively limited distribution of CTF in the far northeastern corner of that state (Lane et al., 1982). Remarkably, no pathogenic viruses of humans have been catalogued from among the 28 species of human-biting ixodid ticks in South America (Guglielmone et al., 2006; Nava et al., 2014), where the potential for similar discoveries is enormous.
Prospecting archival specimen banks containing blood, serum, or tissue samples from patients for whom a recognized tick-borne disease was suspected but unconfirmed with existing assays is expected to yield novel agents as well. A retrospective survey of 29 patients for whom RMSF was suspected clinically and epidemiologically identified 3 individuals who seroconverted to Ehrlichia antigens and subsequent prospective evaluation at a hospital in eastern Georgia where one of these patients had been treated identified 3 more individuals with acute ehrlichiosis (Fishbein et al., 1987). In a similar manner, fatal cases of Heartland virus infection have been identified retrospectively from autopsy specimens from at least 4 case-patients initially suspected to have died from ehrlichiosis (Muehlenbachs et al., 2014; CDC, unpublished data).
Investigators throughout the 20th century commented on the diversity of prokaryotic species that co-infected medically important ticks (Cowdry, 1925; Steinhaus, 1942; Martin and Schmidtmann, 1998), but contemporary molecular techniques now reveal a far greater assemblage of bacterial residents than ever imagined. The growing recognition that complex and interacting microbial communities exist within medically important ticks, and that these interactions may influence pathogen prevalence, heralds an important and evolving area of research in tick-borne diseases (Clay et al., 2006). Amblyomma americanum, a vector of E. chaffeensis and E. ewingii, contains many other bacterial taxa, including Acidobacteriales, Bacilliales, Burkholderiales, Caulobacterales, Enterobacteriales, Flavobacteriales, Legionellales, Pseudomonadales, Rhizobiales, Rickettsiales, and Sphingomonadales (Clay et al., 2008; Ponnusamy et al., 2014; Williams-Newkirk et al., 2014). Additional data indicate that the composition of these bacterial communities are remarkably dynamic and change in response to environmental stimuli, during acquisition of blood meals, and between various life stages (Menchaca et al., 2013; Williams-Newkirk et al., 2014). The complexity of tick-associated microbial communities extends beyond bacteria: A. americanum is also a vector of Heartland virus, as well as host to another bunyavirus (Lone Star virus) and a newly identified rhabdovirus (Long Island tick rhabdovirus) (Swei et al., 2013; Tokarz et al., 2014a).
In certain situations, microbial communities appear to modulate the frequency of pathogen transmission. By using an antibiotic to perturb the composition of the indigenous gut microbiota of larval I. scapularis ticks, investigators detected qualitative alterations in the tick peritrophic membrane that significantly reduced colonization by B. burgdorferi spirochetes (Narasimhan et al., 2014). During a multistate survey of field-collected I. scapularis ticks, male ticks harboring an uncharacterized and presumably nonpathogenic Rickettsia species had significantly lower rates of infection with B. burgdorferi than Rickettsia-free males (Steiner et al., 2008). Also, ticks cannot simultaneously maintain more than one Rickettsia species by vertical transmission, as demonstrated by the exclusion of transovarial transmission of R. rickettsii by Rickettsia peacockii in D. andersoni (Burgdorfer et al., 1981), Rickettsia rhipicephali by Rickettsia montanensis
in D. variabilis (Macaluso et al., 2002), and R. rickettsii by Rickettsia bellii in Amblyomma dubitatum (Sakai et al., 2014). This rickettsial interference phenomenon presumably explains the uneven distribution of RMSF cases in the Bitterroot Valley of western Montana. Almost all patients acquire the infection from exposures to D. andersoni ticks on the western side of the valley. It is believed that R. peacockii, a nonpathogenic Rickettsia species infecting up to 80 percent of D. andersoni ticks collected from the eastern side of the valley, prevents transovarial transmission of R. rickettsii. In contrast, almost all tick isolates of R. rickettsii from the Bitterroot Valley have originated from its western slopes, where only 8–16 percent of D. andersoni are infected with R. peacockii (Burgdorfer et al., 1981). These findings could stimulate new areas of research and exploration regarding control and prevention of certain tick-borne diseases by manipulating the microbiome of medically important ticks using strategies similar to those proposed for control of certain insect pests (Douglas, 2007).
Investigators who consider the complete ecological framework in which a particular tick-borne pathogen resides are poised to make remarkable discoveries. Unprecedented advances have been made in genetics, biochemistry, and molecular biology, but in many cases, these advances are applied to pursuits that are independent of or entirely unconnected to the natural history of the disease. In this context, many endeavors in this discipline result in highly compartmentalized studies that rely on elegant and sophisticated techniques, but are divorced from the ecology of the pathogen, its vectors, and its hosts (IOM, 2008). Future explorations in tick-borne disease research hold tremendous promise, but erosion of expertise in many core disciplines could seriously undermine the foundation upon which many past discoveries were based. During the last 50 years, transformational advances in molecular technology have fueled the discovery and characterization of multiple tick-borne pathogens. Ironically, the number of scientists who pursue fundamental studies in tick taxonomy, vector–pathogen–host interactions, and basic transmission dynamics, has diminished considerably during this same period. The identification of ticks should be founded predominantly on morphology, and it is axiomatic that any subsequent ecologic or epidemiologic conclusions based on an incorrect identification of the vector species are erroneous and misleading. Unfortunately, diminishing numbers of contemporary investigators have a solid foundation in tick taxonomy (Estrada-Peña et al., 2013). Identification methods based on molecular data or proteome analysis such as mass spectrometry are under development, but are not considered reference standards for tick identification, and cannot be developed or validated without convincing morphological correlation. Emphasis in these areas of expertise clearly needs to be maintained and fortified. Despite an increasingly diverse catalogue of tick-borne diseases in the United States and other countries of the Western Hemisphere, many of the resources that are necessary to properly explore the transmission dynamics, reservoir hosts, and human epidemiologic
and clinical features for tick-borne pathogens are declining, particularly in state health departments (Hadler et al., 2014).
A more complete understanding of the ecological and biological factors responsible for expanding distributions of tick vectors and reservoir hosts, as well as the microbiological dynamics within ticks that modulate pathogen emergence, is needed to develop effective strategies to mitigate the rising incidence of tickborne diseases in the Americas. To achieve this goal, more vector biology training centers and programs that offer balanced curricula fostering ecological as well as molecular and quantitative approaches are essential, as are more academic and governmental-funded field-related job opportunities (Glaser, 2010). Also, a more concerted effort must be made by national funding agencies to promote and support field studies because these form the bedrock upon which successful epidemiological interventions are based. Lastly, vector-borne disease scientists need to become better advocates for their work, and more clearly articulate the benefits of this research to public health and welfare (Porter, 2014).
Dedication
We are honored to dedicate this article to the memory of Dr. Willy Burgdorfer (1925–2014), a world-class medical entomologist who not only discovered the Lyme disease spirochete, but made many other significant discoveries about various tick-borne agents that have a bearing on public health. He also was a very generous and kind individual, and a highly effective mentor who willingly shared his broad expertise about ticks and tick-borne diseases while training numerous neophytes in the field. Several decades ago, Willy informed one of us presciently: “There is no such thing as a clean tick.” How accurately he foretold the future with his characteristic acumen.
References
Adams, D. A., R. A. Jajosky, U. Ajani, J. Kriesman, P. Sharp, D. H. Onwen, A. W. Schley, W. J. Anderson, A. Grigoryan, A. E. Aranas, M. S. Wodajo, J. P. Abellera, and Centers for Disease Control and Prevention. 2014. Summary of notifiable diseases – United States, 2012. Morbidity and Mortality Weekly Report 61:1-121.
Álvarez Herández, G., and J. J. Contreras Soto. 2013. Letalidad por fiebre manchada po Rickettsia rickettsii en pacientes de un hospital pediátrico del estado de Sonora, 2004-2012. Salud Pública de México 55:151-152.
Amâncio, F. F., V. D. Amorim, T. L. Chamone, M. G. de Brito, S. B. Calic, A. C. Leite, G. L. Fraga, and M. L. Ferraz. 2011. Epidemiological characteristics of Brazilian spotted fever in Minas Gerais State, Brazil, 2000-2008. Cad Saúde Pública, Rio de Janeiro 27:1969-1976.
Angerami, R. N., A. M. R. da Silva, E. M. M. Nascimento, et al. 2009. Brazilain spotted fever: Two faces of the same disease? A comparative study of clinical aspects between an old and a new endemic area in Brazil. Clinical Microbiology and Infectious Disease 15:207-208.
Artsob, H. 1988. Powassan encephalitis. In The arboviruses: Epidemiology and ecology, edited by T. P. Monath. Vol. 4. Boca Raton, FL: CRC Press. Pp. 29-49.
Bakken, J. S., J. S. Dumler, S. M. Chen, M. R. Eckman, L. L. Van Etta, and D. H. Walker. 1994. Human granulocytic ehrlichiosis in the upper Midwest United States. A new species emerging? Journal of the American Medical Association 272:212-218.
Barbieri, A. R. M., J. M. Filho, F. A. Nieri-Bastos, J. C. Souza Jr., M. P. Szabo, and M. B. Labruna. 2014. Epidemiology of Rickettsia ssp. strain Atlantic rainforest in a spotted fever-endemic area of southern Brazil. Ticks and Tick-Borne Diseases 5:848-853.
Barbour, A. G., J. Bunikis, B. Travinsky, A. G. Hoen, M. A. Diuk-Wasser, D. Fish, and J. I. Tsao. 2009. Niche partitioning of Borrelia burgdorferi and Borrelia miyamotoi in the same tick vector and mammalian reservoir species. American Journal of Tropical Medicine and Hygiene 81:1120-1131.
Barros e Silva, P. M. R., S. C. Pereira, L. X. Fonseca, F. V. P. Maniglia, S. V. Oliveira, and E. P. de Caldas. 2014. Febre maculosa: Uma análise epidemiológica dos registros do sistema de vigilância do Brasil. Scientia Plena 10:1-9.
Bates, L. B., L. H. Dunn, and J. H. St. John. 1921. Relapsing fever in Panama. The human tick, Ornithodoros talaje, demonstrated to be the transmitting agent of relapsing fever in Panama by human experimentation. American Journal of Tropical Medicine 1:183-210.
Bowen, G. S. 1980. Colorado tick fever. In: The arboviruses: Epidemiology and ecology, edited by T. P. Monath. Vol. 4. Boca Raton, FL: CRC Press. Pp. 159-176.
Bozeman, F. M., B. L. Elisberg, J. W. Humphries, K. Runcik, and D. R. Palmer, Jr. 1970. Serologic evidence of Rickettsia canada infection in man. Journal of Infectious Diseases 121:367-371.
Brackney, D. E., R. A. Nofchissey, K. A. Fitzpatrick, et al., 2008. Stable prevalence of Powassan virus in Ixodes scapularis in a northern Wisconsin focus. American Journal of Tropical Medicine and Hygiene 79:971-973.
Brackney, M. M., A. A. Marfin, J. E. Staples, L. Stallones, T. Keefe, W. C. Black, and G. L. Campbell. 2010. Epidemiology of Colorado tick fever in Montana, Utah, and Wyoming, 1995-2003. Vector Borne and Zoonotic Diseases 10:381-385.
Breise, T., R. Chowdry, A. Travassos de Rosa, S. K. Hutchinson, V. Popov, C. Street, R. B. Test, and W. I. Lipkin. 2014. Upolu virus and Aransas Bay viruses, two presumptive bunyaviruses, are novel members of the family Orthomyxoviridae. Journal of Virology 88:5298-5309.
Buller, R. S., M. Arens, S. P. Himmel, C. D. Paddock, J.W. Sumner, Y. Rikihisa, A. Unver, M. Gaudreault-Keener, F. A. Manian, A. M. Liddell, N. Schmulewitz, and G. A. Storch. 1999. Ehrlichia ewingii, a newly recognized agent of human ehrlichiosis. New England Journal of Medicine 341:148-155.
Burgdorfer, W. 1968. Observations of Rickettsia canada, a recently described member of the typhus group rickettsiae. Journal of Hygiene, Epidemiology, and Microbiology 12:26-31.
Burgdorfer, W. 1984. Discovery of the Lyme disease spirochete and its relation to tick vectors. Yale Journal of Biology and Medicine 57:515-520.
Burgdorfer, W., S. F. Hayes, and A. J. Mavros. 1981. Nonpathogenic rickettsiae in Dermacentor andersoni: A limiting factor for the distribution of Rickettsia rickettsii. In: Rickettsiae and rickettsial diseases, edited by W. Burgdorfer and R. L. Anacker. New York: Academic Press. Pp. 585-594.
Burgdorfer, W., A. G. Barbour, S. F. Hayes, J. L. Benach, E. Grunwaldt, and J. P. Davis. 1982. Lyme disease–a tick-borne spirochetosis? Science 216:1317-1319.
Bustamente, M. E., and G. Varela. 1947. IV. Estudios de fiebre manchada en Mexico: Papel del Rhipicephalus sanguineus en ala transmission de la fiebre manchada en la Republica Mexicana. Revista del Instituto de Salubridad Y Enfermedades Tropicales 8:139-141.
Bustamente Moreno, J. G., and A. Pon Méndez. 2010a. Actualización en la vigilancia epidemiológica de “rickettsiosis”. Part I. Epidemiológia Boletin 6:1-4.
Bustamente Moreno, J. G., and A. Pon Méndez. 2010b. Actualización en la vigilancia epidemiológica de “rickettsiosis”. Part II. Epidemiológia Boletin 7:1-3.
CDC (Centers for Disease Control and Prevention). 2014. Notice to readers: Final 2013 reports of nationally notifiable infectious diseases. Morbidity and Mortality Weekly Report 63:702-715.
Chowdri, H. R., J. L. Gugliotta, H. K. Goethert, P. J. Molloy, S. L. Sterling, and S. R. Telford III. 2013. Borrelia miyamotoi infection presenting as human granulocytic anaplasmosis: A case report. Annals of Internal Medicine 159:21-27.
Clark, K. L., B. Leydet, and S. Hartman. 2013. Lyme borreliosis in human patients in Florida and Georgia, USA. International Journal of Medical Sciences 10:915-931.
Clark, K. L., B. F. Leydet, and C. Threlkeld. 2014. Geographical and genospecies distribution of Borrelia burgdorferi sensu lato DNA detected in humans in the USA. Journal of Medical Microbiology 63:674-684.
Clay, K., O. Klyachko, N. Grindle, et al. 2008. Microbial communities and interactions in the Lone Star tick, Amblyomma americanum. Molecular Ecology 17:4371-4381.
Clifford, C. M., H. Hoogstraal, F. J. Radovsky, D. Stiller, and J. E. Keirans. 1980. Ornithodoros (Alectorobius) amblus (Acarina: Ixodoidea: Argasidae): Identity, marine bird and human hosts, virus infections, and distribution in Peru. Journal of Parasitology 66:312-323.
Cortinas, R., and S. Spomer. 2013. Lone Star tick (Acari: Ixodidae) occurrence in Nebraska: Historical and current perspectives. Journal of Medical Entomology 50:244-251.
Cowdry, E. V. 1925. A group of microorganisms transmitted hereditarily in ticks and apparently unassociated with disease. Journal of Experimental Medicine 41:817-830.
Cunha, N. C., A. H. Fonseca, J. Rezende, T. Rozental, A. R. M. Favacho, J. D. Barreira, C. L. Massard, and E. R. S. Lemos. 2009. First identification of natural infection of Rickettsia rickettsii in the Rhipicephalus sanguineus tick, in the State of Rio de Janeiro. Pesquisa Veterinaria Brasileira 29:105-108.
Dahlgren, F., E. Mandel, J. Krebs, R. Massung, and J. H. McQuiston. 2011. Increasing incidence of Ehrlichia chaffeensis and Anaplasma phagocyophilum in the United States, 2000-2007. American Journal of Tropical Medicine and Hygiene 85:124-131.
Davis, G. E., H. L. Wynns, and D. L. Beck. 1941. Relapsing fever: Ornithodoros parkeri, a vector in California. Public Health Reports 56:2426-2428.
Demma, L. J., M. S. Traeger, W. L. Nicholson, C. D. Paddock, D. M. Blau, M. E. Eremeeva, G. A. Dasch, M .L. Levin, J. Singleton Jr., S. R. Zaki, J. E. Cheek, D. L. Swerdlow, and J. H. McQuiston. 2005a. Rocky Mountain spotted fever from an unexpected vector in Arizona. New England Journal of Medicine 353:587-594.
Demma, L. J., R. C. Holman, J. H. McQuiston, J. W. Krebs, and D. L. Swerdlow. 2005b. Epidemiology of human ehrlichiosis and anaplasmosis in the United States, 2001-2002. American Journal of Tropical Medicine and Hygiene 73:400-409.
Dibernardo, A., T. Cote, N. H. Ogden, and L. R. Lindsay. 2014. The prevalence of Borrelia miyamotoi infection, and co-infections with other Borrelia spp. in Ixodes scapularis ticks collected in Canada. Parasites and Vectors 7:183.
Douglas, A. E. 2007. Symbiotic microorganisms: Untapped resources for insect pest control. Trends in Biotechnology 25:338-342.
Drexler, N., M. Miller, J. Gerding, S. Todd, L. Adams, F. S. Dahlgren, N. Bryant, E. Weis, K. Herrick, J. Francies, K. Komatsu, S. Piontkowski, J. Velascosoltero, T. Shelhamer, B. Hamilton, C. Eribes, A. Brock, P. Sneezy, C. Goseyun, H. Bendle, R. Hovet, V. Williams, R. Massung, and J. H. McQuiston. 2014. Community-based control of the brown dog tick in a region with high rates of Rocky Mountain spotted fever, 2012-2013. PLoS ONE 9: e112368.
Dumler, J. S. 1991. Fatal Rocky Mountain spotted fever in Maryland—1901. JAMA 265:718.
Dunn, L. H. 1927. Studies on the South American tick, Ornithodoros venezuelensis Brumpt, in Colombia. Its prevalence, distribution, and importance as an intermediate host of relapsing fever. Journal of Parasitology 13:249-255.
Ebel, G. D., I. Foppa, A. Spielman, and S. R. Telford, III. 1999. A focus of deer tick virus transmission in the north central United States. Emerging Infectious Diseases 5:570-574.
Eisen, R. J., R. S. Lane, C. L. Fritz, and L. Eisen. 2006. Spatial patterns of Lyme disease risk in California based on disease incidence data and modeling of vector-tick exposure. American Journal of Tropical Medicine and Hygiene 75:669-676.
El Khoury, M. Y., J. F. Camargo, J. L. White, et al., 2013. Potential role of deer tick virus in Powassan encephalitis cases in Lyme disease-endemic areas of New York, USA. Emerging Infectious Diseases 19:1926-1933.
Eremeeva, M. E. 2012. Molecular epidemiology of rickettsial diseases in North America. Ticks and Tick-Borne Diseases 3:331-336.
Estrada-Peña, A., J. S. Gray, O. Kahl, R. S. Lane, and A. M. Nijhof. 2013. Research on the ecology of ticks and tick-borne pathogens—methodological principles and caveats. Frontiers in Cellular and Infection Microbiology 3:1-12.
Estripeaut, D., M. G. Aramburú, X. Sáez-Llorens, H. A. Thompson, G. A. Dasch, C. D. Paddock, S. Zaki, and M. E. Eremeeva. 2007. Rocky Mountain spotted fever, Panama. Emerging Infectious Diseases 13:1763-1765.
Fedorova, N., J. E. Kleinjan, D. James, L. T. Hui, H. Peeters, and R. S. Lane. 2014. Remarkable diversity of tick or mammalian-associated borreliae in the metropolitan San Francisco Bay Area, California. Ticks and Tick-Borne Diseases 5:951-961.
Ferraz, K. M. P. M. B., S. F. B. Ferraz, J. R. Moreira, H. T. Z. Couto, and L. M. Verdade. 2007. Capybara (Hydrochoerus hydrochaeris) distribution in agroecosystems: A cross-scale habitat analysis. Journal of Biogeogeography 34:223-230.
Ferraz, K. M. P. M. B., B. Manly, and L. M. Verdade. 2010. The influence of environmental variables on capybara (Hydrochoerus hydrochaeris: Rodentia, Hydrochoeridae) detectability in anthropogenic environments of southeastern Brazil. Population Ecology 52:263-270.
Fishbein, D. B., L. A. Sawyer, C. J. Holland, E.B. Hayes, W. Okoroanyanwu, D. Williams, K. Sikes, M. Ristic, and J. E. McDade. 1987. Unexplained febrile illnesses after exposure to ticks: infection with an Ehrlichia? Journal of the American Medical Association 257:3100-3104.
Florio, L., M. D. Stewart, and E. R. Mugrage. 1946. The etiology of Colorado tick fever. Journal of Experimental Medicine 83:1-10.
Forrester, J. D., J. Meiman, J. Mullins, R. Nelson, S.H. Ertel, M. Cartter, C.M. Brown, V. Lijewski, E. Schiffman, D. Neitzel, E. R. Daly, A. A. Matthewson, W. Howe, L. A. Lowe, N. R. Kratz, S. Semple, P. B. Backenson, J. L. White, P. M. Kurpiel, R. Rockwell, K. Walker, D. H. Johnson, C. Steward, B. Batten, D. Blau, M. DeLeon-Carnes, C. Drew, A. Muehlenbachs, J. Ritter, J. Sanders, S. R. Zaki, C. Molins, M. Schriefer, A. Perea, K. Kugeler, C. Nelson, A. Hinckley, P. Mead, and Centers for Disease Control and Prevention (CDC). 2014. Update on Lyme carditis, groups at high risk, and frequency of associated sudden cardiac death—United States. Morbidity and Mortality Weekly Report 63:982-983.
Forrester, J. D., A. M. Kjemtrup, C. L. Fritz, N. Marsden-Haug, J. B. Nichols, L. A. Tengelsen, R. Sowadsky, E. DeBess, P. R. Cieslak, J. Weiss, N. Evert, P. Ettestad, C. Smelser, J. Iralu, R. J. Nett, E. Mosher, J. S. Baker, C. van Houten, E. Thorp, A. L. Geissler, K. Kugeler, and P. Mead. 2015. Tickborne relapsing fever—United States, 1990-2011. Morbidity and Mortality Weekly Report 64:58-60.
Fukunaga, M., Y. Takahashi, Y. Tsuruta, O. Matsushita, D. Ralph, M. McClelland, and M. Nakao. 1995. Genetic and phenotypic analysis of Borrelia miyamotoi sp. nov., isolated from the ixodid tick Ixodes persulcatus, the vector for Lyme disease in Japan. International Journal of Systematic Bacteriology 45:804-810.
Gehrke, F. S., G. S. Gazeta, E. R. Souza, A. Ribeiro, M. T. Marrelli, and T. T. S. Schumaker. 2009. Rickettsia rickettsii, Rickettsia felis and Rickettsia sp. TwKM03 infecting Rhipicephalus sanguineus and Ctenocephalides felis collected from dogs in a Brazilian spotted fever focus in the State of Rio de Janeiro/Brazil. Clinical Microbiology and Infection 15:267-268.
Gholam, B. I., S. Puksa, and J. P. Provias. 1999. Powassan encephalitis: A case report with neuropathology and literature review. Canadian Medical Association Journal 161:1419-1422.
Girard, Y. A., B. Travinsky, A. Schotthoefer, N. Fedorova, R. J. Eisen, L. Eisen, A. G. Barbour, and R. S. Lane. 2009. Population structure of the Lyme borreliosis spirochete Borrelia burgdorferi in the western black-legged tick (Ixodes pacificus) in northern California. Applied and Environmental Microbiology 75:7243-7252.
Girard, Y. A., N. Fedorova, and R. S. Lane. 2011. Genetic diversity of Borrelia burgdorferi and detection of B. bissettii-like DNA in serum of north-coastal California residents. Journal of Clinical Microbiology 49:945-954.
Glaser, V. 2010. An interview with Robert Lane, Ph.D. Vector Borne and Zoonotic Diseases 10:211-215.
Goldsmith, C. S., T. G. Ksiazek, P. E. Rollin, J. A. Comer, W. L. Nicholson, T. C. T. Peret, D. D. Erdman, W. J. Bellini, B. H. Harcourt, P. A. Rota, J. Bhatnagar, M. D. Bowen, B. R. Erickson, L. K. McMullan, S. T. Nichol, W. J. Shieh, C. D. Paddock, and S. R. Zaki. 2013. Cell culture and electron microscopy for identifying viruses in diseases of unknown cause. Emerging Infectious Diseases 19:886-891.
Goodpasture, H. C., J. D. Poland, D. B. Francy, G. S. Bowen, and K. A. Horn. 1978. Colorado tick fever: Clinical, epidemiological, and laboratory aspects of 228 cases in Colorado in 1973-1974. Annals of Internal Medicine 88:303-310.
Guglielmone, A. A., L. Beati, D. M. Barros-Battesti, M. B. Labruna, S. Nava, J. M. Venzal, A. J. Mangold, M. P. Szabo, J. R. Martins, D. Gonzalez-Acuna, and A. Estrada-Pena. 2006. Ticks (Ixodidae) on humans in South America. Experimental and Applied Acarology 40:83-100.
Gugliotta, J. L., H. K. Goethert, V. P. Berardi, and T. S. Telford III. 2013. Meningoencephalitis from Borrelia miyamotoi in an immunocompromised patient. New England Journal of Medicine 368:240-245.
Hadler, J. L., D. Patel, K. Bradley, J.M. Hughes, C. Blackmore, P. Etkind, L. Kan, J. Getchell, J. Blumenstock, J. Engel, and Centers for Disease Control and Prevention (CDC). 2014. National capacity for surveillance, prevention, and control of West Nile virus and other arbovirus infections—United States, 2004 and 2012. Morbidity and Mortality Weekly Report 63:281-284.
Hamer, S. A., J. I. Tsao, E. D. Walker, and G. J. Hickling. 2010. Invasion of the Lyme disease vector Ixodes scapularis: Implications for Borrelia burgdorferi endemicty. EcoHealth 7:47-63.
Hersh, M. H., R. S. Ostfeld, D. J. McHenry, M. Tibbetts, J. L. Brunner, M. E. Killilea, K. LoGiudice, K. A. Schmidt, and F. Keesing. 2014. Co-infection of blacklegged ticks with Babesia microti and Borrelia burgdorferi is higher than expected and acquired from small mammal hosts. PLoS ONE 9:e99348.
Herwaldt, B., D. H. Persing, E. A. Précigout, W. L. Goff, D. A. Mathiesen, P. W. Taylor, M. L. Eberhard, and A. F. Gorenflot. 1996. A fatal case of babesiosis in Missouri: Identification of another piroplasm that infects humans. Annals of Internal Medicine 124:643-650.
Hidalgo, M., L. Orejuela, P. Fuya, P. Carrillo, J. Hernandez, E. Parra, C. Keng, M. Small, J. P. Olano, D. Bouyer, E. Castaneda, D. Walker, and G. Valbuena. 2007. Rocky Mountain spotted fever, Colombia. Emerging Infectious Diseases 13:1058-1060.
Hidalgo, M., J. Miranda, D. Heredia, P. Zambrano, J. F. Vesga, D. Lizarazo, S. Mattar, and G. Valbuena. 2011. Outbreak of Rocky Mountain spotted fever in Córdoba, Colombia. Memórias do Instituto Oswaldo Cruz 106:117-118.
Hinckley, A. F., N. P. Connally, J. I. Meek, B. J. Johnson, M. M. Kemperman, K. A. Feldman, J. L. White, and P. S. Mead. 2014. Lyme disease testing by large commercial laboratories in the United States. Clinical Infectious Diseases 59:676-681.
Hinten, S. R., G. A. Beckett, K. F. Gensheimer, E. Pritchard, T. M. Courtney, S. D. Sears, J. M. Woytowicz, D. G. Preston, R. P. Smith Jr., P. W. Rand, E. H. Lacombe, M. S. Holman, C. B. Lubelczyk, P. T. Kelso, A. P. Beelen, M. G. Stobierski, M. J. Sotir, S. Wong, G. Ebel, O. Kosoy, J. Piesman, G. L. Campbell, and A. A. Marfin. 2008. Increased recognition of Powassan encephalitis in the United States, 1999-2005. Vector-Borne and Zoonotic Diseases 8:733-740.
Hoang Johnson, D. K., J. E. Staples, M. J. Sotir, D. M. Warshauer, and J. P. Davis. 2010. Tickborne Powassan virus infections among Wisconsin residents. Wisconsin Medical Journal 109:91-97.
Hoang Johnson, D. K., E. K. Schiffman, J. P. Davis, D. F. Neitzel, L. M. Sloan, W. L. Nicholson, T. R. Fritsche, C. R. Steward, J. A. Ray, T. K. Miller, M. A. Feist, T. S. Uphoff, J. J. Franson, A. L. Livermore, A. K. Deedon, E. S. Theel, and B. S. Pritt. 2015. Human infection with Ehrlichia muris-like pathogen, United States, 2007-2013. Emerging Infectious Diseases 21:1785-1790.
Horowitz, H. W., M. E. Aguero-Rosenfeld, D. Holmgren, D. McKenna, I. Schwartz, M.E. Cox, and G. P. Wormser. 2013. Lyme disease and human granulocytic anaplasmosis coinfection: Impact of case definition on coinfection rates and illness severity. Clinical Infectious Diseases 56:93-99.
Hughes, L. E., C. M. Clifford, R. Gresrink, et al. 1976. Isolation of a spotted fever group rickettsia from the Pacific coast tick, Ixodes pacificus, in Oregon. American Journal of Tropical Medicine and Hygiene 25:513-516.
IOM (Institute of Medicine). 2008. Vector-borne diseases: Understanding the environmental, human health and ecological connections. Washington, DC: The National Academies Press. Pp. 65-69.
IOM. 2011. Critical needs and gaps in understanding prevention, amelioration, and resolution of Lyme and other tick-borne diseases: The short-term and long-term outcomes. Washington, DC: The National Academies Press. Pp. 221-266.
Ivanova, L. B., A. Tomova, D. González-Acuña, R. Murúa, C. X. Moreno, C. Hernández, J. Cabello, C. Cabello, T. .J. Daniels, H. P Godfrey, and F. C. Cabello. 2014. Borrelia chilensis, a new member of the Borrelia burgdorferi sensu lato complex that extends the range of this genospecies in the Southern Hemisphere. Environmental Microbiology 16:1069-1080.
Jongejan, F., and G. Uilenberg. 2004. The global importance of ticks. Parasitology 129(Suppl):S3-S14.
Kelly, P., H. Lucas, L. Beati, C. Yowell, S. Mahan, and J. Dame. 2010. Rickettsia africae in Amblyomma variegatum and domestic ruminants on eight Caribbean islands. Journal of Parasitology 96:1086-1088.
Khatchikian, C. E., M. A. Prusinski, M. Stone, P. B. Backenson, I. N. Wang, E. Foley, S. N. Seifert, M. Z. Levy, and D. Brisson. 2015. Recent and rapid population growth and range expansion of the Lyme disease tick vector, Ixodes scapularis, in North America. Evolution 69:1678-1689.
Kosoy, O. I., A. J. Lambert, D. J. Hawkinson, et al. 2015. Novel Thogotovirus associated with a febrile illness and death, United States 2014. Emerging Infectious Diseases. 21:760-764.
Krause, P. J., S. R. Telford III, A. Spielman, V. Sikand, and R. Ryan. 1996. Concurrent Lyme disease and babesiosis. Evidence for increased severity and duration of illness. Journal of the American Medical Association 275:1657-1660.
Krause, P. J., K. McKay, C. A. Thompson, V. K. Sikand, R. Lentz, T. Lepore, L. Closter, D. Christianson, S. R. Telford, D. Persing, J. D. Radolf, A. Spielman, and Deer-Associated Infection Study Group. 2002. Disease-specific diagnosis of coinfecting tickborne zoonoses: Babesiosis, human granulocytic ehrlichiosis, and Lyme disease. Clinical Infectious Diseases 34:1184-1191.
Krause, P. J., K. McKay, J. Gadbaw, D. Christianson, L. Closter, T. Lepore, S. R. Telford 3rd, V. Sikand, R. Ryan, D. Persing, J. D. Radolf, A. Spielman, and Tick-Borne Infection Study Group. 2003. Increasing health burden of human babesiosis at endemic sites. American Journal of Tropical Medicine and Hygiene 68:431-436.
Krause, P. J., S. Narasimhan, G. P. Wormser, L. Rollend, E. Fikrig, T. Lepore, A. Barbour, and D. Fish. 2013. Human Borrelia miyamotoi infection in the United States. New England Journal of Medicine 368:291-293.
Krause, P. J., S. Narasimhan, G. P. Wormser, A. G. Barbour, A. E. Platonov, J. Brancato, T. Lepore, K. Dardick, M. Mamula, L. Rollend, T. K. Steeves, M. Diuk-Wasser, S. Usmani-Brown, P. Williamson, D. S. Sarksyan, E. Fikrig, D. Fish, and Tick-Borne Diseases Group. 2014. Borrelia miyamotoi sensu lato seroreactivity and seroprevalence in the northeastern United States. Emerging Infectious Diseases 20:1183-1190.
Kuno, G., H. Artsob, N. Karabatsos, K. R. Tsuchiya, and G. J. J. Chang. 2001. Genomic sequencing of deer tick virus and phylogeny of Powassan-related viruses in North America. American Journal of Tropical Medicine and Hygiene 65:671-676.
Labruna, M. B. 2009. Ecology of Rickettsia in South America. Annals of the New York Academy of Sciences 1166, 156-166.
Labruna, M. B. 2013. Brazilian spotted fever: The role of capybaras. In: J. R. Moreira et al. (eds.), Capybara: Biology, use and conservation of an exceptional neotropical species. New York: Springer Science+Business Media. Pp. 371-383.
Labruna, M. B., F. C. P. Santos, M. Ogrzewalska, E. M. M. Nascimento, S. Colombo, A. Marcili, and R. Angerami. 2014. Genetic identification of rickettsial isolates from fatal cases of Brazilian spotted fever and comparison with Rickettsia rickettsii isolates from the American continents. Journal of Clinical Microbiology 52:3788-3791.
Lane, R. S., R. W. Emmons, V. Devlin, D. V. Dondero, and B. C. Nelson. 1982. Survey for evidence of Colorado tick fever virus outside of the known endemic area in California. American Journal of Tropical Medicine and Hygiene 31:837-843.
Lane, R. S., and W. Burgdorfer. 1987. Transovarial and transstadial passage of Borrelia burgdorferi in the western black-legged tick, Ixodes pacificus (Acari: Ixodidae). American Journal of Tropical Medicine and Hygiene 37:188-192.
Lane, R. S., S. A. Manweiler, H. A. Stubbs, E. T. Lennette, J. E. Madigan, and P. E. Lavoie. 1992. Risk factors for Lyme disease in a small rural community in northern California. American Journal of Epidemiology 136:1358-1368.
Lane, R. S., D. B. Steinlein, and J. Mun. 2004. Human behaviors elevating exposure to Ixodes pacificus (Acari: Ixodidae) nymphs and their associated bacterial zoonotic agents in a hardwood forest. Journal of Medical Entomology 41:239-248.
Lane, R. S., N. Fedorova, J. E. Kleinjan, and M. Maxwell. 2013. Eco-epidemiological factors contributing to the low risk of human exposure to ixodid tick-borne borreliae in southern California, USA. Ticks and Tick-Borne Diseases 4:377-385.
Léger, E., G. Vourc’h, L. Vial, C. Chevillon, and K. D. McCoy. 2013. Changing distributions of ticks: Causes and consequences. Experimental and Applied Acarology 59:219-244.
Levia, T., M. Kirkpatrick, M. Mangel, and C. Wilmers. 2012. Deer, predators, and the emergence of Lyme disease. Proceedings of the National Academy of Sciences 109:10942-10947.
Lin, M., and Y. Rikihisa. 2003. Ehrlichia chaffeensis and Anaplasma phagocytophilum lack genes for lipid A biosynthesis and incorporate cholesterol for their survival. Infection and Immunity 71:5324-5331.
Lindsey, N. P., J. E. Staples, J. A. Lehman, and M. Fischer. 2010. Surveillance for human West Nile virus disease–United States, 1999—2008. Morbidity and Mortality Weekly Report 59:SS-2.
Macaluso, K. R., D. E. Sonenshine, S. M. Ceraul, and A. F. Azad. 2002. Rickettsial infection in Dermacentor variabilis (Acari: Ixodidae) inhibits transovarial transmission of a second Rickettsia. Journal of Medical Entomology 39:809-813.
Maeda, K., N. Markowitz, R. C. Hawley, M. Ristic, D. Cox, and J. E. McDade. 1987. Human infection with Ehrlichia canis, a leukocytic rickettsia. New England Journal of Medicine 316: 853-856.
Magalhães, O. 1952. Contribuição ao conhecimento das doenças do grupo tifo exantematico. Instituto Oswaldo Cruz, Rio de Janeiro. 968pp.
Marfin, A., and G. Campbell. 2005. Colorado tick fever and related Coltivirus infections. In Tickborne diseases of humans, edited by J. Goodman. Washington, DC: ASM Press. Pp. 143-149.
Margos, G., S. A. Vollmer, N. H. Ogden, and D. Fish. 2011. Population genetics, taxonomy, phylogeny and evolution of Borrelia burgdorferi sensu lato. Infection, Genetics and Evolution 11:1545-1563.
Mariotte, C. O., M. E. Bustamente, and G. Varela. 1944. Hallazgo del Rhipicephalus sanguineus Latreille infectado naturalmente con fiebre manchada de las Montañas Rocosas, en Sonora (Mexico). Revista del Instituto de Salubridad Y Enfermedades Tropicales 5:297-300.
Marshall, W. F., III, S. R. Telford III, P. N. Rys, B. J. Rutledge, D. Mathiesen, S. E. Malawista, A. Spielman, and D. H. Persing. 1994. Detection of Borrelia burgdorferi DNA in museum specimens of Peromyscus leucopus. Journal of Infectious Diseases 170:1027-1032.
Martin, P. A., and E. T. Schmidtmann. 1998. Isolation of aerobic microbes from Ixodes scapularis (Acari: Ixodidae), the vector of Lyme disease in the eastern United States. Journal of Economic Entomology 91:864-868.
Matsuno, K., C. Weisend, M. Kajihara, C. Matysiak, B. N. Williamson, M. Simuunza, A. S. Mweene, A. Takada, R. B. Tesh, and H. Ebihara. 2015. Comprehensive molecular detection of tick-borne phleboviruses leads to the retrospective identification of taxonomically unassigned bunyaviruses and the discovery of a novel member of the genus Phlebovirus. Journal of Virology 89:594-604.
McCauley, J. W., S. Hongo, N. V. Kaverin, G. Kochs, R. A. Lamb, M. N. Matrosovich, D. R. Perez, P. Palese, R. M. Presti, and E. Rimstad. 2012. Family Orthomyxoviridae. In Virus taxonomy: Classification and nomenclature of viruses, edited by A. M. Q. King, M. J. Adams, E. B. Carstens, and E. J. Lefkowitz. Ninth Report of the International Committee of Taxonomy of Viruses. New York: Elsevier Inc. Pp. 749-761.
McElroy Horne, K., and D. L. Vanlandingham. 2014. Bunyavirus-vector interactions. Viruses 6:4373-4397.
McKiel, J. A., E. J. Bell, and D. B. Lackman. 1967. Rickettsia canada: A new member of the typhus group of rickettsiae isolated from Haemaphysalis leporispalustris ticks in Canada. Canadian Journal of Microbiology 13:503-510.
McLean, D. M., and R. P. B. Larke. 1963. Powassan and Silverwater viruses: Ecology of two Ontario arboviruses. Canadian Medical Association Journal 88:182-185.
McMullan, L. K., S. M. Folk, A. J. Kelly, A. MacNeil, C. S. Goldsmith, M. G. Metcalfe, B. C. Batten, C. G. Albarino, S. R. Zaki, P. E. Rollin, W. L. Nicholson, and S. T. Nichol. 2012. A new phlebovirus associated with severe febrile illness in Missouri. New England Journal of Medicine 367:834-841.
Means, R. G., and D. J. White. 1997. New distribution records of Amblyomma americanum (L.) (Acari: Ixodidae) in New York State. Journal of Vector Ecology 22:133-145.
Medeiros, A. P., A. P. de Souza, A. B. de Moura, M. S. Lavina, V. Belatto, A. Sartor, F. A. Nieri-Bastos, L. J. Richtzenhain, and M. B. Labruna. 2011. Spotted fever group Rickettsia infecting ticks (Acari: Ixodidae) in the state of Santa Catarina, Brazil. Memorias do Instituto Oswaldo Cruz, Rio de Janeiro 106:926-930.
Menchaca, A. C., D. K. Visi, O. F. Strey, P. D. Teel, K. Kalinowski, M. S. Allen, and P. C. Williamson. 2013. Preliminary assessment of microbiome changes following blood-feeding and survivorship in the Amblyomma americanum nymph-to-adult transition using semiconductor sequencing. PLoS ONE 6:e67129.
Moreira, J. R., K. M. P. M. B. Ferraz, E. A. Herrera, and D. W. Macdonald. 2013. Capybara - biology, use and conservation of an exceptional Neotropical species. New York: Springer, 419 pp.
Minnesota Department of Health. 2014. Powassan (POW) Virus Information for Health Professionals. Available at: http://www.health.state.mn.us/divs/idepc/diseases/powassan/hcp.html. Accessed on 16 December 2014.
Moraes-Filho, J., A. Pinter, R. C. Pacheco, T. B. Gutmann, S. O. Barbosa, M. A. Gonzales, M. A. Muraro, S. R. Cecilio, and M. B. Labruna. 2009. New epidemiological data on Brazilian spotted fever in an endemic area of the state of São Paulo, Brazil. Vector-Borne and Zoonotic Diseases 9:73-78.
Muehlenbachs, A., C. R. Fata, A. J. Lambert, C. D. Paddock, J. O. Velez, D. M. Blau, J. E. Staples, M. B. Karlekar, J. Bhatnagar, R. S. Nasci, and S. R. Zaki. 2014. Heartland virus-associated death in Tennessee. Clinical Infectious Diseases 59:845-850.
Nadolny, R., H. Gaff, J. Carlsson, and D. Gauthier. 2015. Comparative population genetics of two invading ticks: Evidence of the ecological mechanisms underlying tick range expansions. Infection, Genetics and Evolution 35:153-162.
Narasimhan, S., N. Rajeevan, L. Liu, Y. O. Zhao, J. Heisig, J. Pan, R. Eppler-Epstein, K. DePonte, D. Fish, and E. Fikrig. 2014. Gut microbiota of the tick vector Ixodes scapularis modulate colonization of the Lyme disease spirochete. Cell Host and Microbe 15:58-71.
Nava, S., L. Beati, M. B. Labruna, A. G. Cáceres, A. J. Mangold, and A. A. Guglielmone. 2014. Reassessment of the taxonomic status of Amblyomma cajennense (F.) with the description of three new species, Amblyomma tonelliae n. sp., Amblyomma interandinum n. sp. and Amblyomma patinoi n. sp., and reinstatement of Amblyomma mixtum, and Amblyomma sculptum (Ixodida: Ixodidae). Ticks Tick-borne Diseases 5:252-276.
Neitzel, D. F., R. Lynfield, and K. Smith. 2013. Powassan virus encephalitis, Minnesota, USA. Emerging Infectious Diseases 19:686.
Nicholson, W. L., C. D. Paddock, L. Demma, M. Traeger, B. Johnson, J. Dickson, J. McQuiston, and J. Swerdlow. 2006. Rocky Mountain spotted fever in Arizona: Documentation of heavy environmental infestations of Rhipicephalus sanguineus at an endemic site. Annals of the New York Academy of Sciences 1078:338-341.
Nofchissey, R. A., E. R. Deardorff, T. M. Blevins, M. Anishcenko, A. Bosco-Lauth, E. Berl, C. Lubelczyk, J. P. Mutebi, A. C. Brault, G. D. Ebel, and L. A. Magnarelli. 2013. Seroprevalence of Powassan virus in New England deer, 1979—2010. American Journal of Tropical Medicine and Hygiene 88:1159-1162.
Ogden, N. H., S. Mechai, and G. Margos. 2013. Changing geographic ranges of ticks and tick-borne pathogens: Drivers, mechanisms and consequences for pathogen diversity. Frontiers in Cellular and Infection Microbiology 3:1-11.
Openshaw, J. J., D. L. Swerdlow, J. W. Krebs, R.C. Holman, E. Mandel, A. Harvey, D. Haberling, R. F. Massung, and J. H. McQuiston. 2010. Rocky Mountain spotted fever in the United States: Interpreting contemporary increases in incidence. American Journal of Tropical Medicine and Hygiene 83:174-182.
Pacheco, R. C., J. Moraes-Filho, E. Guedes, I. Silveira, L. J. Richtzenhain, and R. C. Leiti. 2011. Rickettsial infections of dogs, horses and ticks in Juiz de Fora, southeastern Brazil, and isolation of Rickettsia rickettsii from Rhipicephalus sanguineus ticks. Medical and Veterinary Entomology 25:148-155.
Paddock, C. D., and M. J. Yabsley. 2007. Ecological havoc, the rise of white-tailed deer, and the emergence of Amblyomma americanum-associated zoonoses in the United States. Current Topics in Microbiology and Immunology 315:289-324.
Paddock, C. D., and J. Goddard. 2015. The evolving medical and veterinary importance of the Gulf Coast tick (Acari: Ixodidae). Journal of Medical Entomology 52:230-252.
Paddock, C. D., J. W. Sumner, J. A. Comer, S. R. Zaki, C. S. Goldsmith, J. Goddard, S. L. McLellan, C. L. Tamminga, and C. A. Ohl. 2004. Rickettsia parkeri: a newly recognized cause of spotted fever rickettsiosis in the United States. Clinical Infectious Diseases 38:805-811.
Paddock, C. D., A. M. Denison, R. R. Lash, L. Liu, B. C. Bollweg, F. S. Dahlgren, C. T. Kanamura, R. N. Angerami, F. C. Pereira dos Santos, R. Brasil Martines, and S. E. Karpathy. 2014. Phylo-geography of Rickettsia rickettsii genotypes associated with fatal Rocky Mountain spotted fever. American Journal of Tropical Medicine and Hygiene 91:589-597.
Padgett, K., D. Bonilla, A. Kjemtrup, I. M. Vilcins, M. Hardstone Yoshimizu, L. Hui, M. Sola, M. Quintana, and V. Kramer. 2014. Large scale spatial risk and comparative prevalence of Borrelia miyamotoi and Borrelia burgdorferi sensu lato in Ixodes pacificus. PLoS ONE 9:e110853.
Parker, R. R., R. R. Spencer, and E. Francis. 1924. Tularemia XI. Tularemia infection in ticks of the species Dermacentor andersoni Stiles in the Bitterroot Valley, Montana. Public Health Reports 39:1057-1073.
Parker, R. R., C. B. Philip, and W. L. Jellison. 1933. Potentialities of tick transmission in relation to geographical occurrence in the United States. American Journal of Tropical Medicine and Hygiene 13:341-379.
Parola, P., J. Jourdan, and D. Raoult. 1998. Tick-borne infection caused by Rickettsia africae in the French West Indies. New England Journal of Medicine 338:1391.
Parola, P., M. B. Labruna, and D. Raoult. 2009. Tick-borne rickettsioses in America: Uunanswered questions and emerging diseases. Current Infectious Disease Reports 11:40-50.
Pastula, D. M., G. Turabelidze, K. F. Yates, T. F. Jones, A. J. Lambert, A. J. Panella, O. I. Kosoy, J. O. Velez, M. Fischer, and E. Staples. 2014. Heartland virus disease–United States, 2012-2013. Morbidity and Mortality Weekly Report 63:270-271.
Patino, L., A. Afanador, and J. H. Paul. 1937. A spotted fever in Tobia, Colombia. American Journal of Tropical Medicine and Hygiene 17:639-53.
Persing, D. H., S. R. Telford III, P. N. Rys, D. E. Dodge, T. J. White, S. E. Malawitsa, and A. Spielman. 1990. Detection of Borrelia burgdorferi DNA in museum specimens of Ixodes dammini ticks. Science 249:1420-1423.
Pfäffle, M., N. Littwin, S. V. Muders, and T. N. Petney. 2013. The ecology of tick-borne diseases. International Journal for Parasitology 43:1059-1077.
Philip, C. B., and L. E. Hughes. 1953. Disease agents found in the rabbit tick, Dermcentor parumapertus, in the southwestern United States. Sixth International Congress of Microbiology, Rome 5:541-548.
Pinto da Silva, E. V., A. P. A. Travassos da Rosa, M. R. T. Nunes, J. P. Diniz, R. B. Tesh, A. C. R. Cruz, C. M. A. Vieira, and P. F. C. Vasconcelos. 2005. Araguari virus, a new member of the family Orthomyxoviridae: Serologic, ultrastructural, and molecular characterization. American Journal of Tropical Medicine and Hygiene 73:1050-1058.
Ponnusamy, L., A. Gonzalez, W. Van Treuren, S. Weiss, C. Parobek, J. J. Juliano, R. Knight, R. M. Roe, C. S. Apperson, and S. R. Meshnick. 2014. Diversity of Rickettsiales in the microbiome of the lone star tick, Amblyomma americanum. Applied and Experimental Microbiology 80:354-359.
Porter, J. E. 2014. Time to speak up for research. Science 344:1207.
Pritt, B. S., L. M. Sloan, D. K. Johnson, et al. 2011. Emergence of a new pathogenic Ehrlichia species, Wisconsin and Minnesota, 2009. New England Journal of Medicine 365:422-429.
Pritt, B. S., L. M. Sloan, D. K. Hoang Johnson, et al. 2014. Emergence of a novel Borrelia sp. agent pathogenic for humans in the upper Midwestern United States, 2012-2014. 63rd Annual Meeting of American Society for Tropical Medicine and Hygiene, New Orleans, LA. Late-breaker abstract 3238.
Quandelacy, T. M., C. Viboud, V. Charu, M. Lipsitch, and E. Goldstein. 2014. Age- and sex-related risk factors for influenza-associated mortality in the United States between 1997-2007. American Journal of Epidemiology 179:156-167.
Quick, R. E., B. L. Herwaldt, J. W. Thomford, M. E. Garnett, M. L. Eberhard, M. Wilson, D. H. Spach, J. W. Dickerson, S. R. Teleford 3rd, K. R. Steingart, R. Pollock, D. H. Persing, J. M. Kobayashi, D. D. Juranek, and P. A. Conrad. 1993. Babesiosis in Washington State: A new species of Babesia? Annals of Internal Medicine 119:284-290.
Ray, G., T. Schulz, W. Daniels, E. R. Daly, T. A. Andrew, C. M. Brown, P. Cummings, R. Nelson, M. L. Cartter, B. Backenson, J. L. White, P. M. Kurpiel, R. Rockwell, A. S. Rotans, C. Hertzog, L. S. Squires, J. V. Linden, M. Prial, J. House, P. Pontones, B. Batten, D. Blau, M. DeLeon-Carnes, A. Muehlenbachs, J. Ritter, J. Sanders, S. R. Zaki, P. Mead, A. Hinckley, C. Nelson, A. Perea, M. Schriefer, C. Molins, and J. Forrester. 2013. Three sudden cardiac deaths associated with Lyme carditis—United States, November 2012-July 2013. Morbidity and Mortality Weekly Report 62:993-996.
Ricketts, H. R. 1909. A micro-organism which apparently has a specific relationship to Rocky Mountain spotted fever. A preliminary report. Journal of the American Medical Association 52:379-384.
Riehle, M., and S. M. Paskewitz. 1996. Ixodes scapularis (Acari: Ixodidae): Status and change in prevalence and distribution in Wisconsin between 1981 and 1994 measured by deer surveillance. Journal of Medical Entomology 33:933-938.
Rodaniche, E. C., and A. Rodaniche. 1950. Spotted fever in Panama. Isolation of the etiologic agent from a fatal case. American Journal of Tropical Medicine and Hygiene 30:511-517.
Rollend, L., D. Fish, and J. E. Childs. 2013. Transovarial transmission of Borrelia spirochetes by Ixodes scapularis: A summary of the literature and recent observations. Ticks and Tick-Borne Diseases 4:46-51.
Romer, Y., A. C. Seijo, F. Crudo, W. L. Nicholson, A. Varela-Stokes, R. R. Lash, and C. D. Paddock. 2011. Rickettsia parkeri rickettsiosis in Argentina. Emerging Infectious Diseases17:1169-1173.
Romer, Y., S. Nava, F. Govedic, G. Cicuttin, A. M. Denison, J. Singleton, A. J. Kelly, C. Y. Kato, and C. D. Paddock. 2014. Rickettsia parkeri in different ecological regions of Argentina and its association with Amblyomma tigrinum as a potential vector. American Journal of Tropical Medicine and Hygiene. 91:1156-1160.
Rudenko, N., M. Golovchenko, L. Grubhoffer, and J. H. Oliver, Jr. 2011. Updates on Borrelia burgdorferi sensu lato complex with respect to public health. Ticks and Tick-Borne Diseases 2:123-128.
Rydzewski, J., N. Mateus-Pinilla, R. E. Warner, J. A. Nelson, and T. C. Velat. 2012. Ixodes scapularis (Acari: Ixodidae) distribution surveys in the Chicago metropolitan region. Journal of Medical Entomology 49:955-959.
Sakai, R. K., F. B. Costa, T. E. H. Ueno, D. G. Ramirez, J. F. Soares, A. H. Fonseca, M. B. Labruna, and D. M. Barros-Battesti. 2014. Experimental infection with Rickettsia rickettsii in an Amblyomma dubitatum tick colony, naturally infected by Rickettsia bellii. Ticks and Tick-Borne Diseases 5:917-923.
Salkeld, D. J., M. B. Castro, D. Bonilla, A. Kjemtrup, V. L. Kramer, R. S. Lane, and K. A. Padgett. 2014. Seasonal activity patterns of the western black-legged tick, Ixodes pacificus, in relation to onset of human Lyme disease in northwestern California. Ticks and Tick-Borne Diseases 5:790-796.
Sanchez, R., C. Alpuche, H. Lopez-Gatell, C. Soria, J. Estrada, H. Olguin, M.A. Lezana, W. Nicholson, M. Eremeeva, CDC Infectious Disease Pathology Branch, G. Dasch, M. Fonseca, S. Montiel, S. Waterman, and J. McQuiston. 2009. Rhipicephalus sanguineus–associated Rocky Mountain spotted fever in Mexicali, Mexico: Observations from an outbreak in 2008-2009. Paper presented at the 23rd Annual meeting of the American Society for Rickettsiology, Hilton Head, South Carolina (abstract 75).
Savage, H. M., M. S. Godsey Jr., A. Lambert, N. A. Panella, K. L. Burkhalter, J. R. Harmon, R. R. Lash, D. C. Ashley, and W. L. Nicholson. 2013. First detection of Heartland virus (Bunyaviridae: Phlebovirus) from field-collected arthropods. American Journal of Tropical Medicine and Hygiene 89:445-452.
Sayler, K. A., A. F. Barbet, C. Chamberlain, W. L. Clapp, R. Alleman, J. C. Loeb, and J. A. Lednicky. 2014. Isolation of Tacaribe virus, a Caribbean arenavirus, from host-seeking Amblyomma americanum ticks in Florida. PLoS ONE 9:e115769.
Schneider, B. S., M. E. Schriefer, G. Dietrich, M. C. Dolan, M. G. Morshed, and N. S. Zeidner. 2008. Borrelia bissettii isolates induce pathology in a murine model of disease. Vector-Borne and Zoonotic Diseases 8:623-633.
Scoles, G. A., M. Papero, L. Beati, and D. Fish. 2001. A relapsing fever group spirochete transmitted by Ixodes scapularis ticks. Vector-Borne and Zoonotic Diseases 1:21-34.
Scott, M. C., M. E. Rosen, S. A. Hamer, E. Baker, H. Edwards, C. Crowder, J. I. Tsao, and G. J. Hickling. 2010. High-prevalence Borrelia miyamotoi infection among wild turkeys (Meleagris gallopavo) in Tennessee. Journal of Medical Entomology 47:1238-1242.
Shapiro, M. R., C. L. Fritz, K. Tait, C. D. Paddock, W. L. Nicholson, K. F. Abramowicz, S. E. Karpathy, G. A. Dasch, J. W. Sumner, P.V . Adem, J. J. Scott, K. A. Padgett, S. R. Zaki, and M. E. Eremeeva. 2010. Rickettsia 364D: A newly recognized cause of eschar-associated illness in California. Clinical Infectious Diseases 50:541-548.
Soares, J. F., H. S. Soares, A. M. Barbieri, and M. B. Labruna. 2012. Experimental infection of the tick Amblyomma cajennense, Cayenne tick, with Rickettsia rickettsii, the agent of Rocky Mountain spotted fever. Medical and Veterinary Entomology 26:139-151.
Souza, C. E., J. Moraes-Filho, M. Ogrzewalska, F. C. Uchoa, M. C. Horta, S. S. Souza, R. C. Borba, and M. B. Labruna. 2009. Experimental infection of capybaras Hydrochoerus hydrochaeris by Rickettsia rickettsii and evaluation of the transmission of the infection to ticks Amblyomma cajennense. Veterinary Parasitology 161:116-121.
Spielman, A., M. L. Wilson, J. F. Levine, and J. Piesman. 1985. Ecology of Ixodes dammini-borne human babesiosis and Lyme disease. Annual Review of Entomology 30:439-460.
Spielman, A., S. R. Telford III, and R. J. Pollak. 1993. The origins and course of the present outbreak of Lyme disease. In Ecology and environmental management of Lyme disease, edited by H. S. Ginsberg. New Brunswick, NJ: Rutgers University Press. Pp. 83-96.
Spolidorio, M. G., M. B. Labruna, E. Mantovani, P. E. Brandao, L. J. Richtzenhain, and N. H. Yoshinari. 2010. Novel spotted fever group rickettsiosis, Brazil. Emerging Infectious Diseases 16:521-523.
Springer, Y. P., L. Eisen, L. Beati, A. M. James, and R. J. Eisen. 2014. Spatial distribution of counties in the continental United States with records of occurrence of Amblyomma americanum (Ixodida:Ixodidae). Journal of Medical Entomology 51:342-351.
Spruance, S. L., and A. Bailey. 1973. Colorado tick fever: A review of 115 laboratory confirmed cases. Archives of Internal Medicine 131:288-293.
Steere, A. C., R. L. Grodzicki, A. N. Kornblatt, J. E. Craft, A. G. Barbour, W. Burgdorfer, G. P. Schmid, E. Johnson, and S. E. Malawista. 1983. The spirochetal etiology of Lyme disease. New England Journal of Medicine 308:733-740.
Steiner, F. E., R. R. Pinger, C. N. Vann, N. Grindle, D. Vivitello, K. Clay, and C. Fuqua. 2008. Infection and co-infection rates of Anaplasma phagocytophilum variants, Babesia spp. Borrelia burgdorferi, and the rickettsial endosymbiont in Ixodes scapularis (Acari: Ixodidae) from sites in Indiana, Maine, Pennsylvania, and Wisconsin. Journal of Medical Entomology 45:289-297.
Steinhaus, E. A. 1942. The microbial flora of the Rocky Mountain wood tick, Dermacentor andersoni Stiles. Journal of Bacteriology 44:397-404.
Stromdahl, E. Y., J. Jiang, M. Vince, and A. L. Richards. 2011. Infrequency of Rickettsia rickettsii in Dermacentor variabilis removed from humans, with comments on the role of other human-biting ticks associated with spotted fever group rickettsiae in the United States. Vector-Borne and Zoonotic Diseases 11:969-976.
Stromdahl, E., S. Haner, S. Jenkins, L. Sloan, P. Williamson, E. Foster, R. Nadolny, C. Elkins, M. Vince, and B. Pritt. 2014. Comparison of phenology and pathogen prevalence, including infection with the Ehrlichia muris-like (EML) agent, of Ixodes scapularis removed from soldiers in the Midwestern and the northeastern United States over a 15 year period (1997-2012). Parasites and Vectors 7:553.
Swanson, S. J., D. Neitzel, K. D. Reed, and E. A. Belongia. 2006. Coinfections acquired from Ixodes ticks. Clinical Microbiology Reviews 19:708-727.
Swei, A., C. J. Briggs, R. S. Lane, and R. S. Ostfeld. 2012. Impacts of an introduced forest pathogen on the risk of Lyme disease in California. Vector-Borne and Zoonotic Diseases 12:623-631.
Swei, A., B. J. Russell, S. N. Naccache, B. Kabre, N. Veeraraghavan, M. A. Pilgard, B. J. B. Johnson, and C. Y. Chiu. 2013. The genome sequence of lone star virus, a highly divergent bunyavirus found in the Amblyomma americanum tick. PLoS ONE 8:e62083.
Thomas, L. A., R. C. Kennedy, and C. L. Eklund. 1960. Isolation of a virus closely related to Powassan virus from Dermacentor andersoni collected along North Cache la Poudre River, Colo. Proceedings of the Society of Experimental Biology and Medicine 104:355-359.
Tigertt, W. D. 1987. A 1759 spotted fever epidemic in North Carolina. Journal of the History of Medicine and Allied Sciences 42:296-304.
Tinoco-Gracia, L., H. Quiroz-Romero, M. T. Quintero-Martínez, et al., 2009. Prevalence of Rhipicephalus sanguineus ticks on dogs in a region on the Mexico-USA border. Veterinary Record 164:59-61.
Tokarz, R., S. Sameroff, M. S. Leon, K. Jain, W. I. Lipkin. 2014a. Genome characterization of Long Island tick rhabdovirus, a new virus identified in Amblyomma americanum ticks. Virology Journal 11:26.
Tokarz, R., S. H. Williams, S. Sameroff, M. S. Leon, K. Jain, W. I. Lipkin. 2014b. Virome analysis of Amblyomma americanum, Dermacentor variabilis, and Ixodes scapularis ticks reveals novel highly divergent vertebrate and invertebrate viruses. Journal of Virology 88:11480-11492.





































