Tribaldos, M., Y. Zaldivar, S. Bermudez, F. Samudio, Y. Mendoza, A. A. Martinez, R. Villalobos, M. E. Eremeeva, C. D. Paddock, K. Page, R. E. Smith, and J. M. Pascale. 2011. Rocky Mountain spotted fever in Panama: A cluster description. Journal of Infectious in Developing Countries 5:737-741.
Tsai, T. F. 1991. Arboviral infections in the United States. Infectious Diseases Clinics of North America 5:73-102.
Walker, D. H., H. K. Hawkins, and P. Hudson. 1983a. Fulminant Rocky Mountain spotted fever. Its pathologic characteristics associated with glucose-6-phosphatate dehydrogenasease deficiency. Archives of Pathology and Laboratory Medicine 107:121-125.
Walker, D. H., D. L. Radisch, and H. N. Kirkman. 1983b. Haemolysis with rickettsiosis and glucose-6-phosphate dehydrogenase deficiency. Lancet 2:217.
Weller, B., and G. M. Graham. 1930. Relapsing fever in central Texas. Journal of the American Medical Association 95:1834-1835.
Wenzel, R. P., F. G. Hayden, D. H. M. Gröschel, et al. 1986. Acute febrile cerebrovasculitis: A syndrome of unknown, perhaps rickettsial cause. Annals of Internal Medicine 104:606-615.
Western, K. A., G. D. Benson, N. N. Gleason, G. R. Healy, and M. G. Schultz. 1970. Babesiosis in a Massachusetts resident. New England Journal of Medicine 283:854-856.
Wheeler, C. M., W. B. Herms, and K. F. Meyer. 1935. A new tick vector of relapsing fever in California. Proceeding of the Society of Experimental and Biological Medicine 32:1290-1292.
Williams-Newkirk, A. J., L. A. Rowe, T. R. Mixson-Hayden, and G. A. Dasch. 2014. Characterization of the bacterial communities of life stages of free living lone star ticks (Amblyomma americanum). PLoS ONE 9:e102130.
Yendell, S. J., M. Fischer, and J. E. Staples. 2015. Colorado tick fever in the United States, 2002-2012. Vector Borne and Zoonotic Diseases 15:311-316.
Yu, X.-J., M.-F. Liang, S.-Y. Zhang, Y. Liu, J.-D. Li, Y.-L. Sun, L. Zhang, Q. F. Zhang, V. L. Popov, C. L, J. Qu, Q. Li, Y.-P. Zhang, R. Hai, W. Wu, Q. Wang, F.-X. Zhan, X.-J. Wang, B. Kan, S.-W. Wang, K.-L. Wan, H.-Q. Jing, J.-X. Lu, W.-W. Yin, H. Zhou, X.-H. Guan, J.-F. Liu, Z.-Q. Bi, G.-H. Liu, J. Ren, H. Wang, Z. Zhao, J.-D. Song, J.-R. He, T. Wan, J.-S. Zhang, X.-P. Fu, L.-N. Sun, X.-P. Dong, Z.-J. Feng, W.-Z. Yang, Y. Hong, Y. Zhang, D. H. Walker, Y. Wang, and D.-X. Li. 2011. Fever with thrombocytopenia associated with a novel bunyavirus in China. New England Journal of Medicine 364:1523-1532.
Yunker, C. E., C. M. Clifford, L. A. Thomas, et al. 1972. Isolation of viruses from swallowticks, Argas cooleyi, in the southwestern United States. Acta Virologica 16:415-421.
Zavala-Castro, J. E., K. R. Dzul-Rosado, J. J. Arias León, D. H. Walker, and J. E. Zavala-Velázquez. 2008. An increase in human cases of spotted fever rickettsiosis in Yucatan, Mexico, involving children. American Journal of Tropical Medicine and Hygiene. 79:907-910.
A9
EMERGING VECTOR-BORNE DISEASES IN THE UNITED STATES: WHAT IS NEXT, AND ARE WE PREPARED?
Lyle R. Petersen, Roger S. Nasci, Charles B. Beard, and Robert F. Massung1
The emergence of West Nile virus in the United States in 1999 dramatically illustrated the vulnerability of the United States to exotic vector-borne diseases.
___________________
1 Division of Vector-Borne Diseases, Centers for Disease Control and Prevention.
The sociologic, environmental, and technologic drivers of vector-borne disease emergence globally and in the United States, such as expanded travel and trade, changing land use, human population growth, urbanization, and climate change, are well known and many are accelerating (Kilpatrick and Randolph, 2012; Sutherst, 2004). As such, a bewildering array of vector-borne problems has confronted the United States in recent years. New pathogens, such as the chikungunya virus, have come from abroad (Leparc-Goffart et al., 2014). Other endemic pathogens, such as Lyme disease, have markedly increased in incidence and geographic distribution (Bacon et al., 2008). Still others, such as the Heartland and Bourbon viruses, have been newly discovered, in part, by combining traditional microbiological methods with technological advances in genetic sequencing (Kosoy et al., 2015; McMullan et al., 2012). It is evident that emerging vector-borne diseases will continue to tax our public health and medical care systems for years to come. The question remains whether we will be prepared.
Current Situation in the United States
Arthropod-borne viruses (arboviruses), bacteria, and to a much lesser extent, parasites, are medically important vector-borne pathogens in the United States. Ticks and mosquitoes principally vector the arboviruses; whereas, ticks vector most vector-borne bacteria and parasites. As such, this report will focus on mosquito- and tick-borne diseases.
Arbovirus Transmission—General Aspects
The arboviruses circulate in complex transmission cycles that most often involve a vertebrate host and arthropod vector. The short mosquito generation time and time between blood meals permit rapid pathogen amplification in mosquito-borne transmission cycles and, hence, development of large human outbreaks of sudden onset that garner public attention. The mosquito-borne arbovirus amplification cycle is stochastic, and as such, may be subject to substantial random variability. Furthermore, it is influenced by factors not easily measured, such as immunity in birds, or predicted far in advance, such as weather. As a result, prediction of mosquito-borne arboviral disease outbreaks has proven notoriously difficult.
While many variations exist, arboviral transmission cycles can be simplified into two scenarios that influence many aspects of pathogen ecology, epidemiology, and strategies for control (see Figure A9-1). In the first scenario (the zoonoses), humans do not efficiently infect arthropod vectors; thus, humans do not contribute to the maintenance of the pathogen and are considered incidental hosts. In the United States, rodents and birds serve as the most important vertebrate hosts, while ticks and mosquitoes are the most important arthropod vectors.
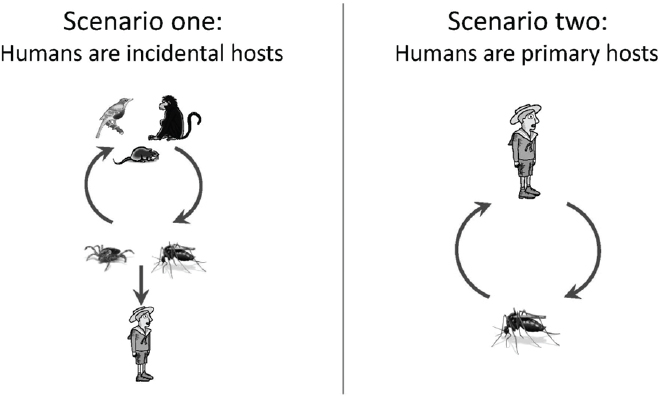
High turnover rates in many animal reservoirs (e.g., small rodents, birds) limit herd immunity, facilitating the long-term maintenance of the pathogen in nature.
In the second scenario (the anthroponoses), humans develop a sufficient titer of the pathogen to efficiently infect mosquito vectors and can maintain the pathogen without other vertebrate hosts. In the United States, the only important arthropod vectors for this transmission pattern are Aedes aegypti and Aedes albopictus mosquitoes. Among the two, Aedes aegypti is a superior vector as it lives around human habitation and preferentially feeds on humans, often biting many persons in a single blood meal. These outbreaks occur mainly in tropical areas of the United States where Aedes aegypti mosquitoes are abundant (Ramos et al., 2008). Outbreaks can be explosive and may continue until sufficient human herd immunity develops.
Mosquito-Borne Arboviruses—Humans as Incidental Hosts
Mosquito-borne arboviruses have attained substantial public health importance in recent years in the United States. Among the arboviruses using humans as incidental hosts, West Nile virus produces by far the highest human infection incidence, greatest morbidity, and highest number of deaths (Petersen and Fischer, 2012). It was first recognized in the Western Hemisphere during an epizootic in birds and an outbreak of encephalitis in humans in 1999 in the New York City area (Nash et al., 2001). The presence of competent Culex mosquito vectors and ubiquitous avian vertebrate hosts throughout the United States permitted the virus’ rapid spread to the West Coast by 2004 (Petersen and Hayes,
2008). West Nile virus is now widely endemic; human cases have occurred in all of the contiguous states, with Midwestern states in particular having recurring high incidence (see Figure A9-2) (Petersen et al., 2013). Hundreds of neuroinvasive disease cases now occur each year; regional outbreaks in 2002, 2003, and 2012 each resulted in nearly 2,000 neuroinvasive disease cases (see Figure A9-3). Outbreaks tend to occur during heat waves, likely because increased temperatures shorten the extrinsic incubation period (time from infection to infectiousness) and increase viral levels in mosquitoes, both factors conducive to amplifying transmission (Kilpatrick et al., 2008).
Although West Nile virus outbreaks are largely unpredictable, intensive surveillance in urban settings can indicate impending outbreaks with sufficient lead-time to implement safe and highly effective emergency adult mosquito control measures (Carney et al., 2008, 2011; Healy et al., 2015; Ruktanonchai et al., 2014). Unfortunately, many communities have failed to implement adequate mosquito-based surveillance, and even when data are available, concerns about cost and pesticide use often delay or prohibit application of control measures (Chung et al., 2013).
West Nile virus was shown to be a transfusion-transmitted infection in 2002 (Pealer et al., 2003). In contrast to most viral transfusion-transmitted infections
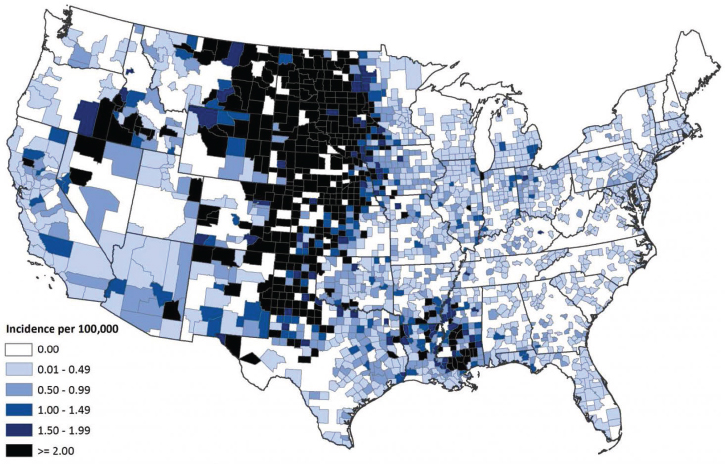
SOURCE: Centers for Disease Control and Prevention (http://www.cdc.gov/westnile/resources/pdfs/data/7-wnv-neuro-incidence-by-county-map_1999-2014_06042015.pdf).
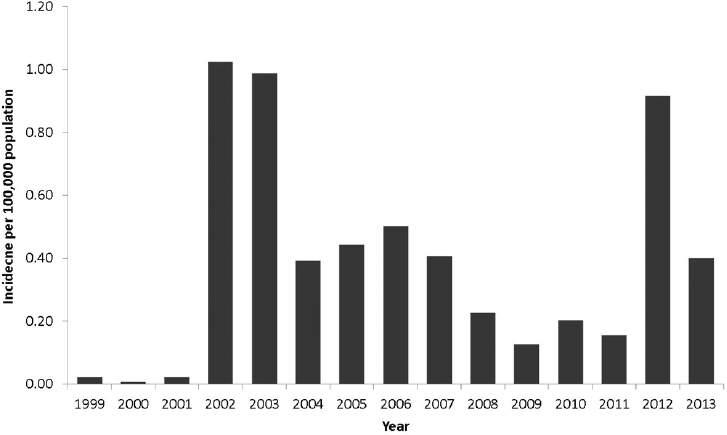
SOURCE: Centers for Disease Control and Prevention (http://www.cdc.gov/westnile/resources/pdfs/cummulative/99_2013_neuroinvasivebyyear.pdf).
that cause risk by virtue of the chronicity of asymptomatic viremia in potential donor populations, the extremely high population incidence of West Nile virus infection during outbreaks produces risk despite the short duration of viremia in humans (Petersen and Busch, 2010). Since 2003, universal blood screening by nucleic acid amplification testing has nearly eliminated transfusion transmission from West Nile virus (Busch et al., 2005; Stramer et al., 2005).
Other important arboviruses involving humans as incidental hosts include the eastern equine encephalitis and La Crosse encephalitis viruses. Although their incidence has been relatively stable, their geographic distributions have changed in recent years. Evidence of increased eastern equine encephalitis virus transmission has been detected in the upper Northeast, possibly due to changes in habitat structure or climate that have influenced transmission ecology (Gibney et al., 2011). The distribution of La Crosse encephalitis virus has expanded from upper Midwestern states to those in the southeastern and mid-Atlantic regions for unclear reasons. The incidence of St. Louis encephalitis virus, which caused thousands of neuroinvasive disease cases in the mid-1970s (Creech, 1977), has been quite low in recent years, possibly because West Nile virus may have displaced St. Louis encephalitis virus in its similar ecological niche (Reisen et al., 2008).
Mosquito-Borne Arboviruses—Humans as Primary Hosts
Globally, the dengue and chikungunya viruses are now by far the most important arboviruses that use humans as primary vertebrate hosts. Thousands of dengue-infected travelers return to the contiguous United States each year from dengue endemic tropical areas (Mohammed et al., 2010), and more than 2,400 travelers returning to the contiguous United States with chikungunya virus infection were reported during the first year of its circulation in the Americas (CDC, unpublished data). Dengue and chikungunya viruses are now significant health concerns in many areas of the United States where competent mosquito vectors reside and autochthonous transmission may occur.
Dengue incidence has increased several fold in the past 15 years in endemic areas of the Western Hemisphere, which includes Puerto Rico and the U.S. Virgin Islands. Nevertheless, the spread and impact of dengue in the contiguous United States has been limited by the sporadic and limited distribution of Aedes aegypti and likely by other sociologic factors such as the widespread use of air conditioning (Ramos et al., 2008). While Aedes albopictus is a competent mosquito vector whose distribution extends throughout much of the eastern United States, recent dengue outbreaks in the contiguous states have only occurred in the southern states in areas with significant Aedes aegypti populations, suggesting a limited potential for Aedes albopictus to cause outbreaks (Bouri et al., 2012). The four dengue viruses have no known important animal reservoir.
The first autochthonous transmission of chikungunya virus in the Western Hemisphere was noted on the Caribbean island of St. Martin in late 2013 (Leparc-Goffart et al., 2014). Chikungunya virus uses the same transmission ecology as dengue (Vega-Rua et al., 2014), and the widespread distribution of the Aedes aegypti mosquito in the region permitted the virus’ spread throughout the Caribbean, Central America, and parts of Mexico and South America within a year, causing more than one million recorded cases, including more than 30,000 suspect cases in Puerto Rico (Pan American Health Organization, 2014; Sharp et al., 2014). However, despite more than 2,400 imported cases reported in the contiguous United States in 2014, only 11 autochthonous cases were recorded, all in south Florida (Kendrick et al., 2014). These findings suggest that chikungunya will follow a pattern similar to that of dengue in the contiguous United States.
Tick-Borne Arboviruses
Colorado tick fever virus has historically been the most important tickborne arbovirus in the United States, although reported incidence has decreased in recent years, possibly due to decreased surveillance (Yendell et al., 2015). However, the incidence of Powassan virus has increased, with 6 to 12 cases now reported each year across an expanding geographic range (Centers for Disease Control and Prevention, 2015). Two types of Powassan virus in the United States are linked to human disease. The first type, often called lineage 1 Powassan virus,
is associated with Ixodes cookei or Ixodes marxi ticks, which infrequently bite humans. Lineage 2 Powassan virus, sometimes called deer tick virus, is associated with Ixodes scapularis ticks (El Khoury et al., 2013). While it is not clear if a true increase or enhanced recognition account for the increasing reported Powassan virus disease incidence, other diseases associated with Ixodes scapularis ticks, such as Lyme disease, human anaplasmosis, and babesiosis, have greatly increased in incidence in recent years (see below).
The combination of traditional microbiologic methods, next generation sequencing, and focused surveillance efforts has resulted in identification of two novel pathogenic tick-borne arboviruses in the last 3 years in the United States. Heartland virus, the first pathogenic phlebovirus identified in North America, causes a febrile illness that can be fatal (McMullan et al., 2012; Muehlenbachs et al., 2014; Pastula et al., 2014). It is transmitted by Amblyomma americanum ticks, which are widely distributed in much of the Midwestern, southern, and eastern United States (Savage et al., 2013). Consequently, human cases have been identified over a wide geographic distribution, but disease and infection incidence remain unknown. Heartland virus is most closely related to the newly discovered severe fever with thrombocytopenia syndrome virus (SFTS) found in China, Korea, and Japan (Lei et al., 2015; Park et al., 2014; Saito et al., 2015).
More recently, Bourbon virus was discovered from a Kansas fatality with a history of tick bite (Kosoy et al., 2015). Bourbon virus is a type of thogotovirus, which belongs to the orthomyxovirus virus family. This is the first thogotovirus identified in the Western Hemisphere. Bourbon virus is genetically similar to tick-borne viruses found in Eastern Europe, Africa, and Asia. Human incidence and distribution as well as the arthropod vector and vertebrate hosts of Bourbon virus are unknown. The recent discoveries of Heartland and Bourbon viruses suggest that further efforts may yield additional novel tick-borne arboviruses, some possibly of public health significance.
Tick-Borne Bacterial Infections
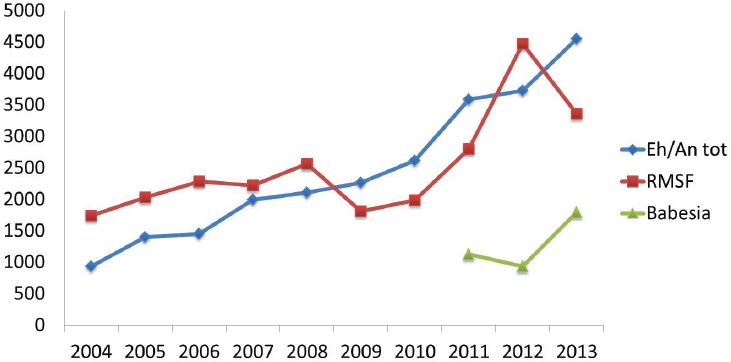
The reported incidence of nearly all tick-borne bacterial infections has markedly increased in recent years (see Figures A9-4 and A9-5). The distributions of the major tick-borne diseases are geographically circumscribed by the distributions of their respective tick vectors (see Figure A9-6). Of particular concern is the expansion and increasing frequency of Amblyomma americanum (Lone Star tick), the vector of Ehrlichia chaffeensis and Heartland virus; and Ixodes scapularis (blacklegged tick), the vector of a wide array of bacterial (Borrelia burgdorferi, Anaplasma phagocytophilum, Borrelia miyamotoi), viral (Powassan virus), and parasitic (Babesia microti) pathogens.
Ixodes scapularis has a 2-year life cycle. Larvae and nymphs feed mostly on mice and other rodents, which serve as vertebrate hosts for Borrelia burgdorferi, the cause of Lyme disease in the United States, and Anaplasma phagocytophylum,
SOURCE: Centers for Disease Control and Prevention.
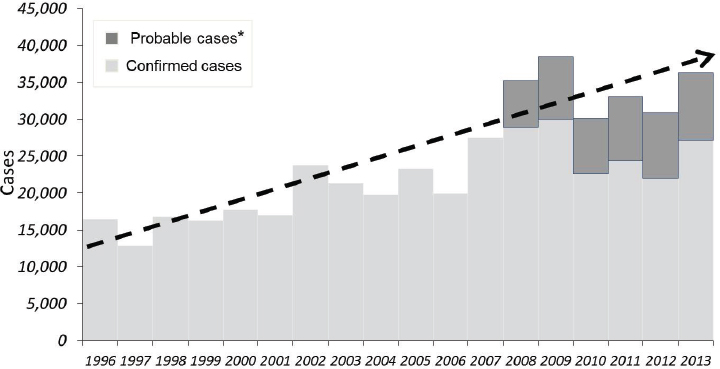
SOURCE: Centers for Disease Control and Prevention, 2008 (http://www.cdc.gov/lyme/stats/graphs.html).
*National Surveillance case definition revised in 2008 to include probable cases.
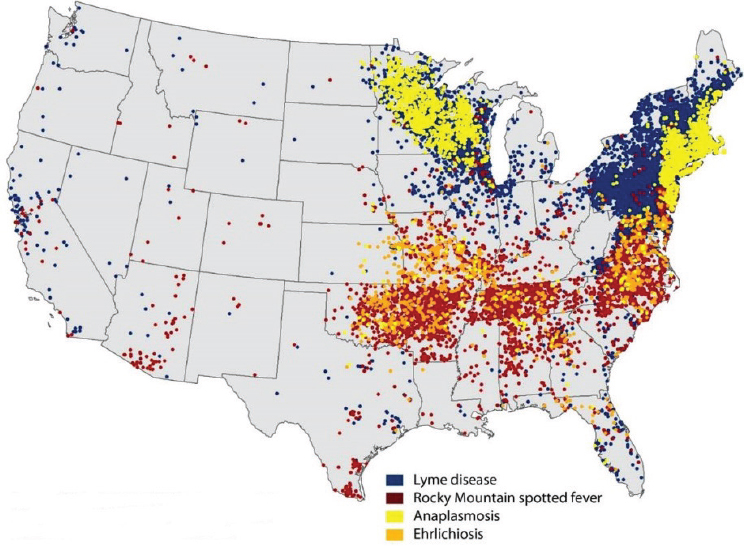
NOTE: Each dot represents county of residence and does not necessarily indicate location of exposure.
SOURCE: Centers for Disease Control and Prevention, 2014.
the etiologic agent of anaplasmosis. Adult Ixodes scapularis ticks feed and mate on white tailed deer, whose greatly increased numbers in recent decades have likely contributed to expanding Ixodes scapularis tick populations (Spielman et al., 1985). At the same time, suburbanization of woodlands and other habitats has put people in close proximity to deer and ticks. Lyme disease by far has the highest incidence of the tick-borne diseases in the United States. Approximately 35,000 Lyme disease are reported annually (see Figure A9-5) over a widening geographic area (see Figure A9-7). Data indicate that Lyme disease is significantly underreported; the true incidence may be 10 times greater than the number reported (Hinckley et al., 2014).
Unfortunately the relentless increase in diseases spread by Ixodes scapularis ticks remains unchecked. Reducing deer populations has been effective in reducing tick populations and human disease in some settings, but large-scale controlled studies have not been done to demonstrate efficacy (Kilpatrick et al., 2014). Four-post deer feeding stations that reduce tick populations on deer have also been used with mixed results (Grear et al., 2014; Hoen et al., 2009). Other host-targeted approaches include using bait boxes to apply acaricides to mice, feed antibiotics to mice, or vaccinate mice (Gomes-Solecki et al., 2006; Piesman,
2006). While some of these approaches have yielded promising results in the laboratory and small-scale field studies, efficacy on reducing human disease has not yet been studied in controlled trials. Acaricides are commonly applied around the perimeter of homes in an attempt to reduce tick abundance and human disease. Unfortunately, a large recent placebo-controlled study showed that this approach substantially reduced tick populations in treated areas but failed to reduce tick exposure or tick-borne disease incidence (CDC, unpublished data). Since Borrelia burgdorferi is not transmitted to humans unless Ixodes scapularis has been attached for at least 24 hours, tick checks and removing attached ticks can be an effective preventive measure. However, the nymphal ticks are very small and easily missed. A human vaccine for Lyme disease was introduced and subsequently taken off the market, with the manufacturer citing poor sales (Poland, 2011).
Rocky Mountain spotted fever (Rickettsia rickettsii) is a significant pathogen in the United States, particularly because of its severe and fatal course if left untreated. Its incidence has increased in recent years (see Figure A9-5), but it is not
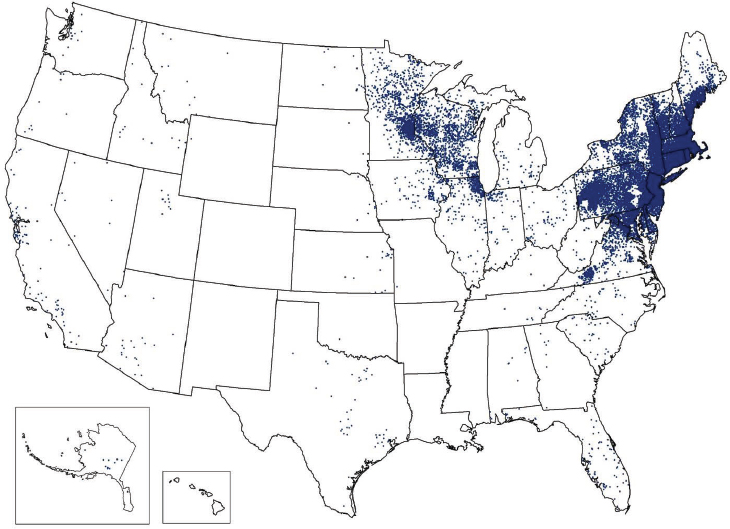
NOTE: Each dot is placed in the county of residence and does not necessarily indicate the location of exposure.
SOURCE: Centers for Disease Control and Prevention (http://www.cdc.gov/lyme/stats/index.html).
clear to what extent this increase is due to a true increase, increased recognition, or confusion with other Rickettsial diseases. Diagnosis largely rests on serologic tests for which cross-reactivity among the rickettsia can create diagnostic uncertainty. Several tick species vector Rocky Mountain spotted fever, which account for its wide geographic distribution. Tick control is impractical in most areas as cases are widely dispersed geographically and temporally. One exception has been the emergence of Rocky Mountain spotted fever on Native American reservations in Arizona, where RMSF incidence exceeds that of the rest of the United States by at least 10 times (Holman et al., 2009). Transmission in this instance is related to brown dog ticks (Rhipicephalus sanguineous) and uncontrolled dog populations (Nicholson et al., 2006). The lack of other tick vectors and vertebrate hosts facilitates disease control, or even elimination, in these settings. Interventions to reduce stray dogs, apply tick-control dog collars to all dogs, and apply acaricides around homes have resulted in greater than 95 percent reductions in tick populations (Drexler et al., 2014). The sustainability of this program and its effect on human disease incidence has not yet been determined.
Several other tick-borne bacterial pathogens are emerging or have been newly discovered in the United States. Ehrlichiosisis is caused by at least three different ehrlichial species in the United States: Ehrlichia chaffeensis, Ehrlichia ewingii, and a third Ehrlichia species provisionally called Ehrlichia muris-like (EML) (Paddock and Yabsley, 2007; Pritt et al., 2011). The Amblyomma americanum tick is the primary vector of both Ehrlichia chaffeensis and Ehrlichia ewingii in the United States, and the geographic range of this tick is expanding northward along the Atlantic coast, and in mid-Atlantic and Midwestern states (Cortinas and Spomer, 2013; Springer et al., 2014). The incidence of ehrlichiosis is increasing and is a growing public health concern (see Figure A9-4). The causes of this increase are not understood. Reported fatality rates range from 1-4 percent. Anaplasma phagocytophylum, the cause of human anaplasmosis, has a lower fatality rate and like other pathogens spread by Ixodes scapularis, is increasing and expanding in distribution (see Figure A9-4). Anaplasma phagocytophylum has also been identified rarely in Ixodes pacificus ticks (Western black-legged tick).
Borrelia miyamotoi infection has been described in several patients in the United States and has been found both in Ixodes scapularis and Ixodes pacificus ticks (Gugliotta et al., 2013; Krause et al., 2013; Padgett et al., 2014). The incidence and clinical importance of this infection are unknown. Several new Rickettsial infections have been discovered in recent years. Rickettsia parkerii, vectored by Amblyomma maculatum (Gulf Coast tick), causes a systemic illness with an eschar at the site of the tick bite in coastal areas of the eastern and southern United States (Paddock et al., 2004). Rickettsia 364D, vectored by Dermacentor occidentalis (Pacific Coast tick), also causes a febrile illness with an eschar at the site of the tick bite in coastal regions of Northern California (Johnston et al., 2013).
Tick-Borne Parasitic Infections
While parasitic vector-borne diseases are major scourges worldwide, only Babesia microti, the cause of babesiosis, carries significant public health impact in the United States (Vannier and Krause, 2012). Babesiosis has only been reportable since 2011, but as with other pathogens spread by Ixodes scapularis, data suggest increasing incidence (see Figure A9-5). Babesiosia microti can be transmitted by blood transfusion and has become a transfusion transmission concern in endemic areas (Herwaldt et al., 2011). Babesiosis can be fatal if untreated.
What Is Next?
Three major factors alone or in combination are likely to drive future vector-borne disease trends in the United States: (1) importation of exotic pathogens and vectors, (2) evolving epidemiology and ecology of recognized pathogens currently endemic to the United States, and (3) discovery of new pathogens already endemic to the United States.
Importation of Novel Pathogens and Vectors
Increasing travel and trade undoubtedly will introduce new vectors and pathogens to the United States. The introduction of West Nile virus to the New York City area in 1999 was not predicted. Similarly, accurate prediction of the arrival of new pathogens will be a formidable challenge. However, monitoring global trends in vector-borne disease distribution will be of benefit, as demonstrated by the observation that the global expansion of chikungunya would likely result in the virus’ introduction into the Western Hemisphere. This allowed health agencies in the region to establish laboratory and other response capacity beforehand. Nevertheless, nobody could have predicted that it would first take hold on the tiny island of Saint Martin, particularly by an Asian genotype virus likely originating from Southeast Asia or the Western Pacific. Based on India having been the primary source of traveler-related chikungunya cases before 2014 (Lindsey et al., 2015), most had expected that the East-Central-South African genotype virus circulating in India would be introduced to the Americas.
The epidemiology and public health significance of newly imported exotic pathogens may be very difficult to predict. For example, the epidemiology of West Nile virus could not have been ascertained at the time of its introduction and only became apparent after more than a decade of observation. Similarly, while it was expected that chikungunya would produce large outbreaks in the Americas, it remains unknown whether the virus will become permanently established in the Western Hemisphere and how long outbreaks will persist and to what extent they will affect the United States.
In addition to exotic viruses, mosquito species not endemic to the United States have been introduced and established populations across the country,
adding new potential vectors to the ecosystems. This trend was first noted over 200 years ago with the introduction of Aedes aegypti from Africa, and its subsequent widespread establishment and transmission of yellow fever and dengue viruses throughout the Western Hemisphere. Aedes albopictus, the Asian tiger mosquito, has received the most attention because of its peridomestic habitats, human biting tendencies, and competence to transmit several arboviruses found in the United States (Benedict et al., 2007). However, there are other recently introduced mosquito species expanding in different areas of the United States, including two species introduced from Asia (Aedes japonicus, Aedes togoi), one from the Caribbean (Aedes bahamensis), and one from Australia (Aedes notoscriptus) (Belton and Belton, 1990; Kaufman and Fonseca, 2014; O’Meara et al., 1989).
Evolving Epidemiology of Pathogens Endemic to the United States
Of the arboviruses endemic to the contiguous United States, West Nile virus will likely remain of paramount importance as continued unpredictable focal and regional outbreaks. The St. Louis encephalitis virus caused large outbreaks in the Midwest in the mid-1970s for reasons that still defy explanation and thus is of potential concern despite its decreasing incidence in recent years. It is unknown whether the shifting geographic distributions of the La Crosse encephalitis and eastern equine encephalitis viruses will continue, or if the epidemiology of Powassan virus will change as a result of its apparently new association with Ixodes scapularis.
For tropical areas of the United States, such as Puerto Rico and the U.S. Virgin Islands, the trend toward larger dengue outbreaks will likely continue as it has in the Caribbean and Latin America in recent years. Accordingly, more traveler-associated cases can be expected, with the potential to cause outbreaks in areas of the contiguous United States with Aedes aegypti populations. The distribution of Aedes aegypti is expanding in certain areas, such as in California, placing new areas at risk (Gloria-Soria et al., 2014).
As discussed earlier, the expanding geographic distribution and increasing prevalence of Ixodes scapularis ticks will likely continue unabated. Thus, increasing incidence of the diseases they carry—Lyme disease, anaplasmosis, Powassan virus, babesiosis, and Borrelia miyamotoi infection—is expected. The eventual extent of this geographic expansion and disease incidence are unknown but could dwarf the current reality. One important consideration is that unlike the Midwestern, Mid-Atlantic, and Northeastern states, the reported incidences of Lyme disease and anaplasmosis in the Western coastal states are not increasing, presumably because of differences in the ecology of its western tick vector, Ixodes pacificus, compared to Ixodes scapularis ticks found in other disease endemic areas.
For Rocky Mountain spotted fever, it is difficult to determine eventual trends as the root causes of recent increases in incidence remain unknown. The one exception is that successful control efforts could abate the increasing incidence of Rocky Mountain spotted fever on Native American reservations in Arizona if they are maintained and expanded.
Newly Discovered Endemic Pathogens
The discovery of an unprecedented number of tick-borne pathogens in recent years has resulted from dedicated efforts of astute clinicians, microbiologists, and entomologists. These efforts have been supplemented by advances in nucleic acid sequencing that allow for the rapid and efficient identification and characterization of new agents. Nevertheless, decades of inattention have led to identification of few novel arboviruses compared to nonvector-borne viruses (Rosenberg, 2015; Rosenberg et al., 2013), suggesting that renewed efforts at identification of previously unidentified vector-borne pathogens could be quite fruitful.
Some novel or previously unrecognized pathogens may have considerable public health significance. The high prevalence of antibodies to Heartland virus in multiple animal species over a broad geographic area in endemic regions for Amblyomma americanum, an aggressive human biting tick, suggests substantial potential for human exposure (Bosco-Lauth et al., 2015). The vectors, animal hosts, human incidence, and disease spectrum of the newly recognized Bourbon virus are unknown.
In addition, genetic mutations in domestic or exotic vector-borne pathogens may alter vector competence, host range, or pathogenicity. For example, genetic changes in the West Nile virus have affected avian mortality and augmented temperature-dependent changes in viral replication in vector mosquitoes (Brault et al., 2007; Kilpatrick et al., 2008), while genetic changes in the chikungunya virus increased the fitness of Aedes albopictus as a vector (Tsetsarkin et al., 2007).
Are We Prepared?
Considerable investments by the U.S. government for vector-borne disease surveillance and research following the introduction of West Nile virus in 1999 led to substantial short-term improvements in surveillance; our understanding of the epidemiology, ecology, microbiology, and pathogenesis of vector-borne disease; and diagnosis and recognition of endemic and novel agents. However, if we are to detect and respond to new and exotic pathogens and to reverse the increasing incidence trends of endemic vector-borne diseases, existing capacities must be strengthened and new capacities must be developed (see Table A9-1).
| Capacities | Current needs |
|---|---|
| Surveillance |
|
| Diagnosis and pathogen recognition |
|
| Research and research capacity |
|
| Prevention: Vector control |
|
| Capacities | Current needs |
|---|---|
|
|
| Prevention: Vaccine development and licensure |
|
| Prevention: Other modalities |
|
| Therapeutics |
|
Surveillance
Surveillance is a foundational capacity required to determine trends in currently endemic, newly emerging, and exotic pathogens. As surveillance may require human, animal, and vector components, considerable technical expertise is required at national, state, and local levels, a capacity that may take years to develop. Two national surveillance systems monitor vector-borne diseases in the contiguous United States.
The ArboNET surveillance system, developed in 2000 to track the spread of West Nile virus across the United States, is the only surveillance system in the world that tracks human arboviral disease cases as well as environmental indicators of arbovirus transmission activity, such as arbovirus infection in mosquito vectors, avian amplifier hosts, veterinary cases, and vectors in real time. It has expanded in scope, now tracking 14 arboviral diseases. However, capacities related to the conduct of entomologic surveillance required for early detection of impending West Nile virus outbreaks, and for comprehensive arbovirus diagnostic testing at state health department laboratories have diminished (Hadler et al., 2014). Retaining capacities at state and local levels is important, particularly in high population centers where control efforts could substantially reduce human morbidity and mortality from West Nile virus.
The TickNET surveillance and prevention effectiveness program is small in scope relative to the large and growing burden of tick-borne diseases. The sheer number of Lyme disease cases and the difficulties in verifying them present a formidable challenge to health departments in highly endemic areas, producing considerable undercounting and surveillance artifact. New surveillance paradigms based on a sampling approach rather than attempting to capture and verify every case need to be considered. In addition, expansion of TickNET surveillance activities to allow added emphasis on other emerging and newly discovered tickborne diseases will permit improved understanding of their epidemiology and their public health impact.
Diagnosis and Pathogen Recognition
For the arboviral diseases, widespread development of nucleic acid detection testing capacities has greatly improved diagnostic sensitivity and specificity for dengue and chikungunya, particularly during early disease when diagnosis is most clinically relevant. Nucleic acid detection tests have also been adapted to the identification of West Nile virus in mosquitoes and birds, making monitoring of these indicators of impending human risk more readily available. Nevertheless, serologic tests remain an important component of arboviral diagnosis because human diagnostic samples are often obtained after detectable viremia has subsided. The plaque reduction neutralization test (PRNT) is the most specific serologic test and thus remains the gold-standard serologic confirmatory method. However, the PRNT is performed mostly in reference laboratories as it is technically
demanding, involves culture of live virus, and is slow and time consuming. An alternative to the PRNT would be of great benefit, although none has yet been identified.
The diagnosis of bacterial vector-borne diseases, particularly during early disease, remains problematic. Lyme disease diagnostics are complicated by the low sensitivity of serology-based diagnostic tests during early Lyme disease and diagnosis often relies on recognition of the erythema migrans rash, which may not occur, can be atypical, and can mimic the rashes of other diseases, such as the southern tick associated rash illness (STARI). Established serologic diagnostic algorithms currently use the Western blot test as the second tier in a 2-tier algorithm. Western blot results are interpreted according to the presence of a certain number of specific bands; however, difficulties in identifying the bands have led to considerable confusion, uncertainty, and misinterpretation of results. Other promising serologic diagnostic algorithms not involving the Western blot need to be fully evaluated and adopted as a standard. Ultimately, creation of sensitive and specific diagnostic tests for all stages of disease that don’t rely on serology would be of considerable benefit.
Early recognition and treatment dramatically reduces disease morbidity and mortality from Rocky Mountain spotted fever; however, initial symptoms are nonspecific and serologic tests of acute- and convalescent-phase sera are often required for definitive diagnosis. Thus, laboratory confirmation of infection is too late to be clinically useful, and serologic cross-reactivity with other Rickettsia may prohibit definitive diagnosis. Development of sensitive nucleic acid or other early detection tests are urgently needed. While ehrlichiosis and anaplasmosis have lower mortality than Rocky Mountain spotted fever, diagnosis of early disease often relies on serologic methods that have limited use in the acute care setting.
Next generation nucleic acid sequencing undoubtedly will continue to be developed as a tool to identify new vector-borne pathogens, particularly when combined with a concerted effort to establish surveillance and research protocols to identify patients with illnesses of unknown etiology following potential vector exposure. Reference laboratories need to be equipped with these new technologies and associated data management and analytic capabilities. When new human pathogens are discovered, epidemiologic investigation and fieldwork are required to identify potential vectors, enzootic transmission cycles, clinical spectrum, and incidence and geographic distribution of disease.
Research and Research Capacity
Improved surveillance combined with field- and laboratory-based research has improved our understanding of environmental influences on vector-borne disease transmission, pathogen-host interactions, and microbiologic basis of transmission and virulence. While a detailed discussion of research needs is
beyond the scope of this report, this research base provides the underpinnings for the development of all prevention measures. However, research in these areas has decreased, particularly for ecological- and field-based investigations, and as a result, academic programs have diminished, particularly those specializing in medical entomology, the vector component of vector-borne disease. A new pipeline of investigators capable of bridging the gap between laboratory and field research will ensure continued development and evaluation of new intervention methods. This will require partnerships with academic research institutions to address staffing and other critical research areas.
The difficulties in predicting vector-borne disease have resulted in new modeling efforts. Additional expertise and collaborations of modelers with epidemiologists, ecologists, and other subject matter experts have resulted in more realistic and robust models of vector-borne disease transmission and improved estimates of disease burden. The eventual usefulness of vector-borne disease modeling and need for further development in this arena requires an improved understanding of transmission ecology and epidemiology, and will be predicated on the demonstrated benefit of these models on public health practice and policy.
Prevention: Vector Control
Unfortunately, our capacities to control vector-borne diseases through vector control measures remain quite limited, and when effective prevention methods do exist, they are often inadequately employed. For example, as previously mentioned, surveillance indicators reflecting infection rates in mosquito vectors can predict West Nile outbreaks with sufficient lead time to mobilize safe and effective control measures in urban areas, yet inadequate surveillance effort, public concerns about pesticides, lack of local control capacity, or inability to mobilize funds quickly often delay or prohibit implementation of control measures when and where they would be most effective. Greater understanding of these barriers may promote development of measures to mitigate them, particularly in large metropolitan areas where West Nile virus prevention and control efforts would have the biggest impact.
Much of the mosquito control capacity in the United States is developed and funded at local levels for reducing the impact of mosquitoes on quality of life, with vector control capacity benefitting the community as a result of this support. Rather than trying to increase vector control capacity across the board, which seems unrealistic in communities without the need or desire to support nuisance mosquito control, a robust surveillance program, coupled with a rapidly deploy-able national or regional emergency response capacity should be developed to address the often focal and sporadic West Nile virus outbreaks. This would not only benefit West Nile virus control, but could be used to address other new or emerging mosquito-borne diseases, or situations that develop following natural disasters such as hurricanes and floods.
Highly effective, scalable, and cost-effective tick or mosquito control methods proven to reduce human illness for most vector-borne pathogens in contemporary settings do not exist. Given the substantial public health impact of the diseases they vector, surprisingly few resources are devoted to developing and field-testing new pesticide- or nonpesticide-based control measures. Nevertheless, several novel alternatives to pesticide-based approaches or novel pesticide delivery systems for mosquito and tick control have been developed. For Ixodes scapularis, several products that do not contain synthetic pesticides, such as entomopathogenic fungi, nootkatone, and reservoir-targeted vaccines have been developed and are being evaluated and novel pesticide delivery systems have been developed and some are in use, such as bait boxes and 4-posters designed to apply pesticides to the vertebrate hosts of ticks. For Aedes aegypti, lethal ovitraps, insect growth regulator auto-dissemination devices, and release of Wolbachia-infected or genetically-modified mosquitoes that produce non-viable offspring are among approaches currently under development. Extended release tick control collars are a promising approach for Rocky Mountain spotted fever in locations where dogs are the primary reservoir.
Nevertheless, it is yet to be determined if any of these approaches will be sufficiently scalable and effective in reducing human disease to impact the upward trend in vector-borne disease over the long run. Entomologic and ecologic field research and randomized trials with human disease outcomes are needed; however, this research takes considerable time to complete since vector activity follows annual cycles. At the current pace, decades may pass before effective entomologic control measures are developed and proven effective in reducing human illness on a large scale. As explained above, the human resources required to conduct this research are diminishing.
An Institute of Medicine Report from 2003 warned of the diminishing supply of public health pesticides for vector control resulting from the considerable costs of registration and reregistration relative to the limited size of the public health market (IOM, 2003). The situation has not improved and the cost of developing and registering novel active ingredients or formulations, or for repurposing products developed for agriculture to public health uses remains extraordinarily high. Introduction of new classes of pesticides with unique modes of action and new formulations to improve delivery characteristics is essential to overcome resistance to extant pesticides and to provide the options required for successful integrated vector management programs. New programs to support research and development and streamlined pathways to registration would greatly enhance options for vector management.
Prevention: Vaccines
Given the difficulties with developing, implementing, and sustaining entomologic control measures, creation of human vaccines for the most common
vector-borne diseases is an attractive avenue of pursuit. While yellow fever and Japanese encephalitis vaccines have long been effective and cost-efficient prevention modalities for residents of or travelers to endemic areas, no human vaccines are available for vector-borne diseases endemic to the United States. Dengue vaccines are the furthest along in development, with one having completed phase-3 trials (Capeding et al., 2014; Villar et al., 2015). This vaccine would likely be of considerable public health benefit despite its incomplete protection to all four dengue serotypes. Other dengue vaccines in late-stage development might confer better protection, but are years away from becoming commercially available. Because dengue is only endemic in tropical areas of the United States, such as Puerto Rico and the U.S. Virgin Islands, manufacturers may not put priority on vaccine pursuing licensure in the United States. Nevertheless, given the difficulties with Aedes aegypti control and substantial dengue public health impact in Puerto Rico, preparations for the introduction of a vaccine in Puerto Rico should continue, including the development of appropriate surveillance tools so that vaccine effectiveness and impact can be assessed.
Licensed West Nile virus equine vaccines have dramatically reduced equine neuroinvasive disease incidence in the United States (Gardner et al., 2007). While human vaccines have been developed and have undergone successful phase-2 clinical trials (De Filette et al., 2012), phase-3 trials have not been attempted because of uncertain market potential for a West Nile virus vaccine and the considerable logistical difficulties in conducting a phase-3 efficacy trial for a sporadic and geographically dispersed disease that largely occurs in rural and suburban settings. Defining the public health cost-benefit for a West Nile vaccine will help determine future market potential and a clear and cost-efficient pathway to licensure must be identified.
The difficulties with Ixodes scapularis control and extremely high Lyme disease incidence warrant accelerated development and licensure of a safe and effective next-generation human Lyme disease vaccine that requires fewer inoculations and with long-lasting efficacy (Shen et al., 2011). Unfortunately, controversies surrounding the previous Lyme disease vaccine have undoubtedly reduced manufacturer interest in further vaccine development (Poland, 2011).
Prevention: Other Modalities
Insect repellents, though demonstrated to effectively reduce human–vector contact, are often infrequently used, even during well-publicized outbreaks. Development of new repellent active ingredients as well as improvements in repellent formulation, such as repellent-containing soaps and spatial repellents that are effective both indoors and outdoors, may improve repellent use and might provide additional protection. Also, investigations to determine to what degree repellents must be used to provide public health benefit should be conducted. Expanded programs to increase public awareness of vector-borne disease, with
the goal of increasing use of personal protective measures, could increase the appropriate use of personal protective measures.
Universal viral nucleic acid screening of blood doors has nearly eliminated the threat of transfusion-associated West Nile virus infection. Nevertheless, this screening is costly and does not cover other vector-borne agents of proven or theoretical transfusion-transmission risk, such as babesiosis. Development of effective and practical pathogen reduction techniques for all blood components would obviate the need to screen for multiple pathogens and would help prevent transfusion transmission of newly emerging vector-borne disease pathogens (Petersen and Busch, 2010). For example, pathogen reduction technology was implemented for platelet screening in French overseas territories experiencing chikungunya outbreaks (Petersen and Epstein, 2014).
Therapeutics
Several potential therapeutics for West Nile virus have been developed; however, as with vaccine development, its sporadic and dispersed epidemiology has precluded evaluation of clinical efficacy (Jester et al., 2006). Thus, no clinical trials for treatment of West Nile virus are currently underway. A clear and cost-efficient pathway to licensure is required before further late-stage clinical development will commence. This is a universal problem for all emerging infectious diseases of this kind; solving it would be broadly useful.
Doxycycline is the preferred treatment for Rocky Mountain spotted fever, other rickettsiosis, and Lyme disease; however, concerns about dental staining stemming from the experience with early tetracycline formulations still lead to warnings against its use in children. Evidence suggests that modern doxycycline formulations do not cause dental staining. It is important to educate the public and health care providers about the lack of evidence between doxycycline use and dental staining in both children and adults.
Recognition of impending dengue hemorrhagic fever and close monitoring of fluid and electrolytes markedly reduce morbidity and mortality. Continued efforts to promote effective practice guidelines are needed. In addition, inexpensive and rapid tests that indicate impending dengue hemorrhagic fever would be of considerable clinical benefit.
Chikungunya virus carries considerable morbidity, and therapeutic options for pain management and for reduction of several arthritic sequelae have not been fully evaluated in controlled clinical trials. Given that chikungunya is likely to be epidemic for years to come, controlled clinical trials are needed.
Concluding Remarks
The United States is faced with an unprecedented array of imported vector-borne disease pathogens, substantial increases in endemic vector-borne diseases
of major public health importance, and newly discovered endemic vector-borne diseases of yet to be determined public health significance. The etiologies underlying these trends are likely accelerating. Following the importation of West Nile virus into the United States, capacities to address emerging vector-borne disease threats, particularly the development of arboviral disease surveillance systems, were greatly augmented. Nevertheless, capacities on nearly all fronts—surveillance, basic and applied research, and prevention—have eroded in recent years, at a time when the need has never been greater. Ramping up effective control programs, such as those for West Nile virus, and developing and identifying new scalable methodologies proven effective on reducing human illness from diseases spread by Aedes aegypti mosquitoes and Ixodes scapularis ticks are needed. The development of innovative, cost-effective paradigms for bringing new public health pesticides, vaccines, and therapeutics to market is a prerequisite for spurring their development and bringing them to market. Without significant advances on all these fronts, the gap between the increasing impact of vector-borne diseases in the United States and our capacity to effectively respond to them will become ever larger.
References
Bacon, R. M., K. J. Kugeler, P. S. Mead, Centers for Disease Control and Prevention. 2008. Surveillance for Lyme disease—United States, 1992-2006. MMWR Surveillance Summary 57(10): 1-9.
Belton, P., and O. C. Belton. 1990. Aedes togoi comes aboard. Journal of the American Mosquito Control Association 6(2):328-329.
Benedict, M. Q., R. S. Levine, W. A. Hawley, and L. P. Lounibos. 2007. Spread of the tiger: Global risk of invasion by the mosquito Aedes albopictus. Vector Borne Zoonotic Diseases 7(1):76-85.
Bosco-Lauth, A. M., N. A. Panella, J. J. Root, T. Gidlewski, R. R. Lash, J. R. Harmon, K. L. Burkhalter, M. S. Godsey, H. M. Savage, W. L. Nicholson, N. Komar, and A. C. Brault. 2015. Serological investigation of Heartland virus (Bunyaviridae: Phlebovirus) exposure in wild and domestic animals adjacent to human case sites in Missouri 2012-2013. American Journal of Tropical Medicine and Hygiene 92:1163-1167.
Bouri, N., T. K. Sell, C. Franco, A. A. Adalja, D. A. Henderson, and N. A. Hynes. 2012. Return of epidemic dengue in the United States: Implications for the public health practitioner. Public Health Report 127(3):259-266.
Brault, A. C., C. Y. Huang, S. A. Langevin, R. M. Kinney, R. A. Bowen, W. N. Ramey, N. A. Panella, E. C. Holmes, A. M. Powers, and B. R. Miller. 2007. A single positively selected West Nile viral mutation confers increased virogenesis in American crows. Nature and Genetics 39(9):1162-1166.
Busch, M. P., S. Caglioti, E. F. Robertson, J. D. McAuley, L. H. Tobler, H. Kamel, J. M. Linnen, V. Shyamala, P. Tomasulo, and S. H. Kleinman. 2005. Screening the blood supply for West Nile virus RNA by nucleic acid amplification testing. New England Journal of Medicine 353(5):460-467.
Capeding, M. R., N. H. Tran, S. R. Hadinegoro, H. I. Ismail, T. Chotpitayasunondh, M. N. Chua, C. Q. Luong, K. Rusmil, D. N. Wirawan, R. Nallusamy, P. Pitisuttithum, U. Thisyakorn, I. K. Yoon, D. van der Vliet, E. Langevin, T. Laot, Y. Hutagalung, C. Frago, M. Boaz, T. A. Wartel, N. G. Tornieporth, M. Saville, A. Bouckenooghe, and C. Y. D. Study Group. 2014. Clinical efficacy and safety of a novel tetravalent dengue vaccine in healthy children in Asia: A phase 3, randomised, observer-masked, placebo-controlled trial. Lancet 384(9951):1358-1365.
Carney, R. M., S. Husted, C. Jean, C. Glaser, and V. Kramer. 2008. Efficacy of aerial spraying of mosquito adulticide in reducing incidence of West Nile Virus, California, 2005. Emerging Infectious Diseases 14(5):747-54.
Carney, R. M., S. C. Ahearn, A. McConchie, C. Glasner, C. Jean, C. Barker, B. Park, K. Padgett, E. Parker, E. Aquino, and V. Kramer. 2011. Early warning system for West Nile virus risk areas, California, USA. Emerging Infectious Diseases 17(8):1445-1454.
Centers for Disease Control and Prevention. 2015. Powassan Virus: Statistics and Maps. http://www.cdc.gov/powassan/statistics.html.
Chung, W. M., C. M. Buseman, S. N. Joyner, S. M. Hughes, T. B. Fomby, J. P. Luby, and R. W. Haley. 2013. The 2012 West Nile encephalitis epidemic in Dallas, Texas. Journal of the American Medical Association 310(3):297-307.
Cortinas, R., and S. Spomer. 2013. Lone star tick (Acari: Ixodidae) occurrence in Nebraska: Historical and current perspectives. Journal of Medical Entomology 50(2):244-251.
Creech, W. B. 1977. St. Louis encephalitis in the United States, 1975. Journal of Infectious Diseases 135(6):1014-1016.
De Filette, M., S. Ulbert, M. Diamond, and N. N. Sanders. 2012. Recent progress in West Nile virus diagnosis and vaccination. Veterinary Research 43(1):16.
Drexler, N., M. Miller, J. Gerding, S. Todd, L. Adams, F. S. Dahlgren, N. Bryant, E. Weis, K. Herrick, J. Francies, K. Komatsu, S. Piontkowski, J. Velascosoltero, T. Shelhamer, B. Hamilton, C. Eribes, A. Brock, P. Sneezy, C. Goseyun, H. Bendle, R. Hovet, V. Williams, R. Massung, and J. H. McQuiston. 2014. Community-based control of the brown dog tick in a region with high rates of Rocky Mountain spotted fever, 2012-2013. PLoS One 9(12):e112368.
El Khoury, M. Y., J. F. Camargo, J. L. White, B. P. Backenson, A. P. Dupuis 2nd, K. L. Escuyer, L. Kramer, K. St George, D. Chatterjee, M. Prusinski, G. P. Wormser, and S. J. Wong. 2013. Potential role of deer tick virus in Powassan encephalitis cases in Lyme disease-endemic areas of New York, U.S.A. Emerging Infectious Diseases 19(12):1926-1933.
Gardner, I. A., S. J. Wong, G. L. Ferraro, U. B. Balasuriya, P. J. Hullinger, W. D. Wilson, P. Y. Shi, and N. J. MacLachlan. 2007. Incidence and effects of West Nile virus infection in vaccinated and unvaccinated horses in California. Veterinary Research 38(1):109-116.
Gibney, K. B., S. Robinson, J. P. Mutebi, D. E. Hoenig, B. J. Bernier, L. Webber, C. Lubelczyk, R. J. Nett, and M. Fischer. 2011. Eastern equine encephalitis: An emerging arboviral disease threat, Maine, 2009. Vector Borne Zoonotic Diseases 11(6):637-639.
Gloria-Soria, A., J. E. Brown, V. Kramer, M. Hardstone Yoshimizu, and J. R. Powell. 2014. Origin of the dengue fever mosquito, Aedes aegypti, in California. PLoS Neglected Tropical Diseases 8(7):e3029.
Gomes-Solecki, M. J., D. R. Brisson, and R. J. Dattwyler. 2006. Oral vaccine that breaks the transmission cycle of the Lyme disease spirochete can be delivered via bait. Vaccine 24(20):4440-4449.
Grear, J. S., R. Koethe, B. Hoskins, R. Hillger, L. Dapsis, and M. Pongsiri. 2014. The effectiveness of permethrin-treated deer stations for control of the Lyme disease vector Ixodes scapularis on Cape Cod and the islands: A five-year experiment. Parasitic Vectors 7:292.
Gugliotta, J. L., H. K. Goethert, V. P. Berardi, and S. R. Telford, 3rd. 2013. Meningoencephalitis from Borrelia miyamotoi in an immunocompromised patient. New England Journal of Medicine 368(3):240-5.
Hadler, J. L., D. Patel, K. Bradley, J. M. Hughes, C. Blackmore, P. Etkind, L. Kan, J. Getchell, J. Blumenstock, J. Engel, Control Centers for Disease and Prevention. 2014. National capacity for surveillance, prevention, and control of West Nile virus and other arbovirus infections—United States, 2004 and 2012. Morbidity and Mortality Weekly Report 63(13):281-284.
Healy, J. M., W. K. Reisen, V. L. Kramer, M. Fischer, N. P. Lindsey, R. S. Nasci, P. A. Macedo, G. White, R. Takahashi, L. Khang, and C. M. Barker. 2015. Comparison of the efficiency and cost of West Nile virus surveillance methods in California. Vector Borne Zoonotic Diseases 15(2):147-155.
Herwaldt, B. L., J. V. Linden, E. Bosserman, C. Young, D. Olkowska, and M. Wilson. 2011. Transfusion-associated babesiosis in the United States: A description of cases. Annals of Internal Medicine 155(8):509-519.
Hinckley, A. F., N. P. Connally, J. I. Meek, B. J. Johnson, M. M. Kemperman, K. A. Feldman, J. L. White, and P. S. Mead. 2014. Lyme disease testing by large commercial laboratories in the United States. Clinical Infectious Diseases 59(5):676-681.
Hoen, A. G., L. G. Rollend, M. A. Papero, J. F. Carroll, T. J. Daniels, T. N. Mather, T. L. Schulze, K. C. Stafford 3rd, and D. Fish. 2009. Effects of tick control by acaricide self-treatment of white-tailed deer on host-seeking tick infection prevalence and entomologic risk for Ixodes scapularis-borne pathogens. Vector Borne Zoonotic Diseases 9(4):431-438.
Holman, R. C., J. H. McQuiston, D. L. Haberling, and J. E. Cheek. 2009. Increasing incidence of Rocky Mountain spotted fever among the American Indian population in the United States. American Journal of Tropical Medicine and Hygiene 80(4):601-605.
IOM (Institute of Medicine). 2003. Microbial threats to health: Emergence, detection, and response. Washington, DC: The National Academies Press.
Jester, P. M., S. J. Tilden, Y. Li, R. J. Whitley, and W. M. Sullender. 2006. Regulatory challenges: Lessons from recent West Nile virus trials in the United States. Contemporary Clinical Trials 27(3):254-259.
Johnston, S. H., C. A. Glaser, K. Padgett, D. A. Wadford, A. Espinosa, N. Espinosa, M. E. Eremeeva, K. Tait, B. Hobson, S. Shtivelman, C. Hsieh, and S. L. Messenger. 2013. Rickettsia spp. 364D causing a cluster of eschar-associated illness, California. Pediatric Infectious Disease Journal 32(9):1036-1039.
Kaufman, M. G., and D. M. Fonseca. 2014. Invasion biology of Aedes japonicus japonicus (Diptera: Culicidae). Annual Review of Entomology 59:31-49.
Kendrick, K., D. Stanek, C. Blackmore, Centers for Disease Control and Prevention. 2014. Notes from the field: Transmission of chikungunya virus in the continental United States—Florida, 2014. Morbidity and Mortality Weekly Report 63(48):1137.
Kilpatrick, A. M., and S. E. Randolph. 2012. Drivers, dynamics, and control of emerging vector-borne zoonotic diseases. Lancet 380(9857):1946-1955.
Kilpatrick, A. M., M. A. Meola, R. M. Moudy, and L. D. Kramer. 2008. Temperature, viral genetics, and the transmission of West Nile virus by Culex pipiens mosquitoes. PLoS Pathogens 4(6):e1000092.
Kilpatrick, H. J., A. M. LaBonte, and K. C. Stafford. 2014. The relationship between deer density, tick abundance, and human cases of Lyme disease in a residential community. Journal of Medical Entomology 51(4):777-784.
Kosoy, O., A. J. Lambert, D. J. Hawkinson, D. M. Pastula, C. S. Goldsmith, D. C. Hunt, and J. E. Staples. 2015. Novel Thogotovirus species associated with febrile illness and death, United States, 2014. Emerging Infectious Diseases 21:5.
Krause, P. J., S. Narasimhan, G. P. Wormser, L. Rollend, E. Fikrig, T. Lepore, A. Barbour, and D. Fish. 2013. Human Borrelia miyamotoi infection in the United States. New England Journal of Medicine 368(3):291-293.
Lei, X. Y., M. M. Liu, and X. J. Yu. 2015. Severe fever with thrombocytopenia syndrome and its pathogen SFTSV. Microbes and Infection 17(2):149-154.
Leparc-Goffart, I., A. Nougairede, S. Cassadou, C. Prat, and X. de Lamballerie. 2014. Chikungunya in the Americas. Lancet 383(9916):514.
Lindsey, N. P., H. E. Prince, O. Kosoy, J. Laven, S. Messenger, J. E. Staples, and M. Fischer. 2015. Chikungunya virus infections among travelers—United States, 2010-2013. American Journal of Tropical Medicine and Hygiene 92(1):82-87.
McMullan, L. K., S. M. Folk, A. J. Kelly, A. MacNeil, C. S. Goldsmith, M. G. Metcalfe, B. C. Batten, C. G. Albarino, S. R. Zaki, P. E. Rollin, W. L. Nicholson, and S. T. Nichol. 2012. A new phlebovirus associated with severe febrile illness in Missouri. New England Journal of Medicine 367(9):834-841.
Mohammed, H. P., M. M. Ramos, A. Rivera, M. Johansson, J. L. Munoz-Jordan, W. Sun, and K. M. Tomashek. 2010. Travel-associated dengue infections in the United States, 1996 to 2005. Journal of Travel Medicine 17(1):8-14.
Muehlenbachs, A., C. R. Fata, A. J. Lambert, C. D. Paddock, J. O. Velez, D. M. Blau, J. E. Staples, M. B. Karlekar, J. Bhatnagar, R. S. Nasci, and S. R. Zaki. 2014. Heartland virus-associated death in Tennessee. Clinical Infectious Diseases 59(6):845-850.
Nash, D., F. Mostashari, A. Fine, J. Miller, D. O’Leary, K. Murray, A. Huang, A. Rosenberg, A. Greenberg, M. Sherman, S. Wong, and M. Layton. 2001. The outbreak of West Nile virus infection in the New York City area in 1999. New England Journal of Medicine 344(24):1807-1814.
Nicholson, W. L., C. D. Paddock, L. Demma, M. Traeger, B. Johnson, J. Dickson, J. McQuiston, and D. Swerdlow. 2006. Rocky Mountain spotted fever in Arizona: Documentation of heavy environmental infestations of Rhipicephalus sanguineus at an endemic site. Annals of the New York Academy of Science 1078:338-341.
O’Meara, G. F., V. L. Larson, D. H. Mook, and M. D. Latham. 1989. Aedes bahamensis: Its invasion of south Florida and association with Aedes aegypti. Journal of the American Mosquito Control Association 5(1):1-5.
Paddock, C. D., and M. J. Yabsley. 2007. Ecological havoc, the rise of white-tailed deer, and the emergence of Amblyomma americanum-associated zoonoses in the United States. Current Topics in Microbiology and Immunology 315):289-324.
Paddock, C. D., J. W. Sumner, J. A. Comer, S. R. Zaki, C. S. Goldsmith, J. Goddard, S. L. McLellan, C. L. Tamminga, and C. A. Ohl. 2004. Rickettsia parkeri: A newly recognized cause of spotted fever rickettsiosis in the United States. Clinical Infectious Diseases 38(6):805-811.
Padgett, K., D. Bonilla, A. Kjemtrup, I. M. Vilcins, M. H. Yoshimizu, L. Hui, M. Sola, M. Quintana, and V. Kramer. 2014. Large scale spatial risk and comparative prevalence of Borrelia miyamotoi and Borrelia burgdorferi sensu lato in Ixodes pacificus. PLoS One 9(10):e110853.
Pan American Health Organization (PAHO). 2014. Chikungunya: PAHO/WHO Data, Maps and Statistics. http://www.paho.org/hq/index.php?option=com_topics&view=rdmore&cid=7928&Itemid=40931&lang=en.
Park, S. W., M. G. Han, S. M. Yun, C. Park, W. J. Lee, and J. Ryou. 2014. Severe fever with thrombocytopenia syndrome virus, South Korea, 2013. Emerging Infectious Diseases 20(11):1880-1882.
Pastula, D. M., G. Turabelidze, K. F. Yates, T. F. Jones, A. J. Lambert, A. J. Panella, O. I. Kosoy, J. O. Velez, M. Fisher, E. Staples, Centers for Disease Control and Prevention. 2014. Notes from the field: Heartland virus disease - United States, 2012-2013. Morbidity and Mortality Weekly Report 63(12):270-271.
Pealer, L. N., A. A. Marfin, L. R. Petersen, R. S. Lanciotti, P. L. Page, S. L. Stramer, M. G. Stobierski, K. Signs, B. Newman, H. Kapoor, J. L. Goodman, and M. E. Chamberland. 2003. Transmission of West Nile virus through blood transfusion in the United States in 2002. New England Journal of Medicine 349(13):1236-1245.
Petersen, L. R., and M. P. Busch. 2010. Transfusion-transmitted arboviruses. Vox Sanguinis 98(4): 495-503.
Petersen, L. R., and J. S. Epstein. 2014. Chikungunya virus: New risk to transfusion safety in the Americas. Transfusion 54(8):1911-1915.
Petersen, L. R., and M. Fischer. 2012. Unpredictable and difficult to control—the adolescence of West Nile virus. New England Journal of Medicine 367(14):1281-1284.
Petersen, L. R., and E. B. Hayes. 2008. West Nile virus in the Americas. Medical Clinics of North America 92(6):1307-1322, ix.
Petersen, L. R., A. C. Brault, and R. S. Nasci. 2013. West Nile virus: Review of the literature. Journal of the American Medical Association 310(3):308-315.
Piesman, J. 2006. Strategies for reducing the risk of Lyme borreliosis in North America. International Journal of Medical Microbiologist 296(Suppl 40):17-22.
Poland, G. A. 2011. Vaccines against Lyme disease: What happened and what lessons can we learn? Clinical Infectious Diseases 52(Suppl 3):s253-s258.
Pritt, B. S., L. M. Sloan, D. K. Johnson, U. G. Munderloh, S. M. Paskewitz, K. M. McElroy, J. D. McFadden, M. J. Binnicker, D. F. Neitzel, G. Liu, W. L. Nicholson, C. M. Nelson, J. J. Franson, S. A. Martin, S. A. Cunningham, C. R. Steward, K. Bogumill, M. E. Bjorgaard, J. P. Davis, J. H. McQuiston, D. M. Warshauer, M. P. Wilhelm, R. Patel, V. A. Trivedi, and M. E. Eremeeva. 2011. Emergence of a new pathogenic Ehrlichia species, Wisconsin and Minnesota, 2009. New England Journal of Medicine 365(5):422-429.
Ramos, M. M., H. Mohammed, E. Zielinski-Gutierrez, M. H. Hayden, J. L. Lopez, M. Fournier, A. R. Trujillo, R. Burton, J. M. Brunkard, L. Anaya-Lopez, A. A. Banicki, P. K. Morales, B. Smith, J. L. Munoz, S. H. Waterman, and Dengue Serosurvey Working Group. 2008. Epidemic dengue and dengue hemorrhagic fever at the Texas-Mexico border: Results of a household-based seroepidemiologic survey, December 2005. American Journal of Tropical Medicine and Hygiene 78(3):364-369.
Reisen, W. K., H. D. Lothrop, S. S. Wheeler, M. Kennsington, A. Gutierrez, Y. Fang, S. Garcia, and B. Lothrop. 2008. Persistent West Nile virus transmission and the apparent displacement St. Louis encephalitis virus in southeastern California, 2003-2006. Journal of Medical Entomology 45(3):494-508.
Rosenberg, R. 2015. Detecting the emergence of novel, zoonotic viruses pathogenic to humans. Cellular and Molecular Life Sciences 72(6):1115-1125.
Rosenberg, R., M. A. Johansson, A. M. Powers, and B. R. Miller. 2013. Search strategy has influenced the discovery rate of human viruses. Proceedings of the National Academy of Science of the United States of America 110(34):13961-13964.
Ruktanonchai, D. J., S. Stonecipher, N. Lindsey, J. McAllister, S. K. Pillai, K. Horiuchi, M. Delorey, B. J. Biggerstaff, T. Sidwa, J. Zoretic, R. Nasci, M. Fischer, and S. L. Hills. 2014. Effect of aerial insecticide spraying on West Nile virus disease—north-central Texas, 2012. American Journal of Tropical Medicine and Hygiene 91(2):240-245.
Saito, T., K. Fukushima, K. Umeki, and K. Nakajima. 2015. Severe fever with thrombocytopenia syndrome in Japan and public health communication. Emerging Infectious Diseases 21(3):487-489.
Savage, H. M., M. S. Godsey Jr., A. Lambert, N. A. Panella, K. L. Burkhalter, J. R. Harmon, R. R. Lash, D. C. Ashley, and W. L. Nicholson. 2013. First detection of heartland virus (Bunyaviridae: Phlebovirus) from field collected arthropods. American Journal of Tropical Medicine and Hygeine 89(3):445-452.
Sharp, T. M., N. M. Roth, J. Torres, K. R. Ryff, N. M. Perez Rodriguez, C. Mercado, M. D. Pilar Diaz Padro, M. Ramos, R. Phillips, M. Lozier, C. S. Arriola, M. Johansson, E. Hunsperger, J. L. Munoz-Jordan, H. S. Margolis, B. R. Garcia, Centers for Disease Control and Prevention. 2014. Chikungunya cases identified through passive surveillance and household investigations—Puerto Rico, May 5-August 12, 2014. Morbidity and Mortality Weekly Report 63(48):1121-1128.
Shen, A. K., P. S. Mead, and C. B. Beard. 2011. The Lyme disease vaccine—a public health perspective. Clinical Infectious Diseases 52(Suppl 3):s247-s252.
Spielman, A., M. L. Wilson, J. F. Levine, and J. Piesman. 1985. Ecology of Ixodes dammini-borne human babesiosis and Lyme disease. Annual Review of Entomology 30:439-460.
Springer, Y. P., L. Eisen, L. Beati, A. M. James, and R. J. Eisen. 2014. Spatial distribution of counties in the continental United States with records of occurrence of Amblyomma americanum (Ixodida: Ixodidae). Journal of Medical Entomology 51(2):342-351.
Stramer, S. L., C. T. Fang, G. A. Foster, A. G. Wagner, J. P. Brodsky, and R. Y. Dodd. 2005. West Nile virus among blood donors in the United States, 2003 and 2004. New England Journal of Medicine 353(5):451-459.
Sutherst, R. W. 2004. Global change and human vulnerability to vector-borne diseases. Clinical Microbiology Reviews 17(1):136-173.
Tsetsarkin, K. A., D. L. Vanlandingham, C. E. McGee, and S. Higgs. 2007. A single mutation in chikungunya virus affects vector specificity and epidemic potential. PLoS Pathogens 3(12):e201.
Vannier, E., and P. J. Krause. 2012. Human babesiosis. New England Journal of Medicine 366(25):2397-2407.



























