Thangamani, S., S. Higgs, S. Ziegler, D. Vanlandingham, R. Tesh, and S. Wikel. 2010. Host immune response to mosquito-transmitted chikungunya virus differs from that elicited by needle inoculated virus. PLoS One 5(8):e12137.
Thiberville, S. D., N. Moyen, L. Dupuis-Maguiraga, A. Nougairede, E. A. Gould, P. Roques, and X. de Lamballerie. 2013. Chikungunya fever: Epidemiology, clinical syndrome, pathogenesis and therapy. Antiviral Research 99(3):345-370.
Tsetsarkin, K. A., D. L. Vanlandingham, C. E. McGee, and S. Higgs. 2007. A single mutation in chikungunya virus affects vector specificity and epidemic potential. PLoS Pathogens 3(12):e201.
Tsetsarkin, K. A., C. E. McGee, S. M. Volk, D. L. Vanlandingham, S. C. Weaver, and S. Higgs. 2009. Epistatic roles of E2 glycoprotein mutations in adaption of chikungunya virus to Aedes albopictus and Ae. aegypti mosquitoes. PLoS One 4(8):e6835
Vazeille, M., L. Mousson, E. Martin, and A. B. Failloux. 2010. Orally co-infected Aedes albopictus from La Reunion Island, Indian Ocean, can deliver both dengue and chikungunya infectious viral particles in their saliva. PLoS Neglected Tropical Diseases 4(6):e706.
Vega-Rua, A., K. Zouache, R. Girod, A. B. Failloux, and R. Lourenco-de-Oliveira. 2014. High level of vector competence of Aedes aegypti and Aedes albopictus from ten American countries as a crucial factor in the spread of Chikungunya virus. Journal of Virology 88(11):6294-6306.
Voss, J. E., M. C. Vaney, S. Duquerroy, C. Vonrhein, C. Girard-Blanc, E. Crublet, A. Thompson, G. Bricogne, and F. A. Rey. 2010. Glycoprotein organization of chikungunya virus particles revealed by X-ray crystallography. Nature 468(7324):709-712.
Wu, S. J., G. Grouard-Vogel, W. Sun, J. R. Mascola, E. Brachtel, R. Putvatana, M. K. Louder, et al. 2000. Human skin Langerhans cells are targets of dengue virus infection. Nature Medicine 6(7):816-820.
A11
VECTOR-BORNE DISEASE EMERGENCE AND SPREAD IN THE EUROPEAN UNION1
Jan C. Semenza2
The emergence and spread of vector-borne diseases (VBD) in Europe is a function of biotic (living organisms in an ecosystem), abiotic (nonliving elements in an ecosystem) and socioeconomic drivers of disease. Permissive circumstances that coincide in time and space can trigger an outbreak of VBD. Anticipating and elucidating such an outbreak requires a systems perspective. In a foresight study, the European Centre for Disease Prevention and Control mapped the interrelated and interdependent nature of disease drivers in order to predict the abrupt emergence of infectious disease threats by 2020 in Europe (Suk and Semenza, 2011). The most significant infectious disease drivers for Europe were grouped into three broad categories: globalization and environmental change, social and
___________________
1 Modified by author from Int. J. Environ. Res. Public Health 2015, 12(6), 6333−6351, doi:10.3390/ijerph120606333 for inclusion in this workshop summary.
2 European Centre for Disease Prevention and Control.
demographic change, and the public health system. Their relation to VBD is briefly described below.
Globalization and Environmental Change
These two factors are recognized as significant disease drivers. They include the steadily expanding reach of travel and trade and population movements. Global disease dispersal is aided by a dense network of air traffic and shipping routes (Semenza et al., 2014; Thomas et al., 2014). They have facilitated the arrival, establishment, and spread of invasive pathogens to novel geographic destinations, including dengue, malaria, chikungunya, and West Nile (Randolph and Rogers, 2010). Approximately 60 percent of human pathogens are estimated to be of zoonotic origin, in that they can be transmitted from animals to humans (Karesh et al., 2012). Thus, land use can indirectly determine the spread of zoonotic diseases through different exposure pathways in urban, suburban, and rural settings with a wider range of animal habitats such as pastures, arable fields, and managed forests (Karesh et al., 2012; Patz et al., 2004). Urbanization, urban sprawl, and high-population densities have also been associated with VBD emergence (Jones et al., 2008). Habitat encroachment and habitat destruction can result in displacement of wild animals into novel environments that can have a bearing on exposure patterns to infectious pathogens. Climatic conditions are also significant drivers of VBD as some of the vectors are cold-blooded; thus, climate change can shift the geographical ranges of VBD transmission (McMichael, 2013b; Lindgren et al., 2012; Confalonieri et al., 2007; Altizer et al., 2013; Semenza and Menne, 2009; Semenza et al., 2012).
Social and Demographic Change
The human world is currently experiencing shifts in demographic profiles, social inequality, and lifestyles. Socially and economically disadvantaged groups suffer disproportionally from infectious diseases in Europe (Semenza and Giesecke, 2008; Suhrcke et al., 2011). In the 1990s, during times of economic hardship, individuals in Central and Eastern Europe resorted to mushroom harvesting in wooded areas, and thereby increased their risk of tick-borne encephalitis (Stefanoff et al., 2012). The economic crisis in Kosovo in 1999–2000 resulted in the abandonment of food stores with the subsequent rise in rodent populations which led to the emergence of tularemia (Reintjes et al., 2002). The 2007 mortgage foreclosures in the Californian housing market resulted in many abandoned homes with swimming pools, increasing breeding habitats for mosquitoes, which was linked to an unexpectedly early seasonal increase in West Nile virus cases (Reisen et al., 2008).
The Public Health System
This includes surveillance and reporting, research and development, animal and food safety and health care. However, current surveillance systems might not be adequately equipped to cope with the arrival and dispersal of tropical pathogens commonly associated with warmer temperatures (Lindgren et al., 2012; Semenza and Domanović, 2013). Contamination of blood products from donors infected with known, unexpected, or unknown pathogens represents a significant threat to the blood supply and thus to public health. Current microbial blood-safety practices might not be adequate to cope with global environmental change (Semenza and Domanović, 2013). Research and development of novel surveillance systems and of pathogen-reduction technologies for the blood supply might reduce the risk from these emerging threats (Semenza et al., 2013; Lindgren et al., 2012; Semenza and Domanović, 2013). Access to health care is an important determinant for early treatment for a VBD and can help interrupt the outbreak by removing a host from the transmission cycle.
Eight plausible threat scenarios facing the European Union by 2020 were formulated, based on the line-listing of different drivers for infectious diseases from the ECDC foresight study described above (Suk and Semenza, 2011). These threat scenarios were selected based on the plausibility of the event, potential severity of the scenario in terms of burden of disease, and relevance of the scenario to multiple EU member states. They were primarily intended for prioritization of public health interventions and health policy decision making. These plausible scenarios were used to develop tangible steps to mitigate the potential public health fallout from such infectious disease threats (Suk and Semenza, 2011). One plausible scenario included a VBD outbreak triggered by environmental/climate change, travel and tourism, global trade, and social inequality. The VBD scenario considered a threat from the introduction of new disease vectors, which creates new opportunities for disease transmission; expanded ability of vectors to transmit pathogens (e.g., by mutation); and a shift in the transmission range of diseases, hosts, and vectors due to socioeconomic factors and climate change.
The drivers and plausible scenarios for 2020 were compared to the threat events that actually occurred between 2008 and 2013 and detected through ECDC’s epidemic intelligence activities (Semenza et al., 2016). ECDC conducts epidemic intelligence by monitoring all events (e.g., from media reports) that could potentially be a threat to public health in Europe. Event-based data collected at ECDC are verified and archived on a daily basis and used to generate the Communicable Disease Threats Reports (CDTR); these are weekly reports summarizing information gathered through epidemic intelligence regarding communicable disease threats of concern to the EU. Data and epidemiological information for each event were extracted from the CDTR, epidemiological reports and communications, ECDC rapid risk assessments, threat assessments, mission reports, and scientific publications. The threat events were sorted into 10 threat categories:
- Vector and rodent-borne diseases,
- Food and water-borne diseases,
- Other zoonosis,
- Vaccine-preventable diseases,
- Multidrug-resistant diseases,
- Health care-associated infections,
- Injecting drug-use associated diseases,
- Sexually transmitted infections,
- Pandemic influenza,
- Air-borne infections.
A total of 116 threats, 10 threat categories, and 17 drivers in 3 driver categories, were recorded (see Figure A11-1). Vector- and rodent-borne disease events were the second most common threat events in the database with 27 individual events. The event-based database did not comprehensively capture mortality and morbidity (ECDC has a dedicated repository for notifiable infectious diseases), but 64 deaths and 4,748 illnesses were attributed to those 27 individual VBD
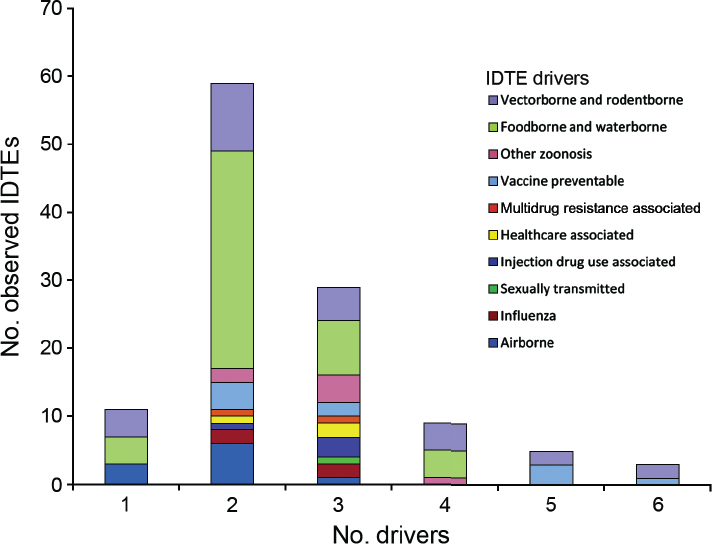
NOTE: This figure is a new visualization of the same data set that was presented at the workshop.
SOURCE: Semenza et al., in press with Emerging Infectious Diseases. Data from ECDC.
events. These events included the large dengue outbreak in Madeira (Lourenco and Recker, 2014) (over 2,000 cases) and the West Nile fever outbreak in Southeast Europe with over 260 cases in Greece alone (Paz et al., 2013).
Based on a logistic regression analysis of all the drivers for the vector and rodent-borne diseases category, the natural environment, climate, and lifestyle scored as the top three drivers. “Natural environment” was the sole driver in four events, a codriver in two events with climate, and one of four drivers in four events. The majority of these events were West Nile fever outbreaks where environmental and climatic determinates obviously play an important role (Paz and Semenza, 2013). The lifestyle driver pertained to a large outbreak of hantavirus in Germany in 2010 where the bank vole populations had increased substantially due to excessive seed production the previous year (Faber et al., 2010); human behavior favouring exposure and potentially increased dust production following dry and warm weather were attributed to the outbreak.
Comparing the scenarios of the 2020 foresight study (Suk and Semenza, 2011) with the threat events that actually occurred between 2008 and 2013 reveals some similarities. The large dengue outbreak in Madeira was sparked by the importation of viremic air traffic passengers in an environment where the vector had recently been introduced. Environmental and climatic conditions contributed to the upsurge of WNF in Southeast Europe (Paz and Semenza, 2013). Social inequality was a factor in the emergence of malaria in Greece in 2009 where migrant workers from endemic countries were part of the malaria transmission cycle (Sudre et al., 2013). However, regardless of the accuracy of such foresight studies VBD continue to emerge and spread in the European Union. Traditional public health strategies might not suffice to cope with the public health challenges associated with global environmental change. ECDC has developed a pragmatic approach to tackle these emerging threats which are described below.
The European Environment and Epidemiology (E3) Network
Many environmental drivers can be considered epidemic precursors of disease (see Figure A11-2). Monitoring changes in environmental conditions can help anticipate, or even forecast, an upsurge of disease (Lindgren et al., 2012; Semenza and Menne, 2009; Semenza et al., 2013). However, traditional environmental and infectious disease epidemiology is hampered by a number of shortcomings when it comes to the public health challenges from global environmental change (McMichael, 2013a). Environmental, climatic, or epidemiologic data often lack historic baseline measurements which make comparison and extrapolation difficult. The effects of global environmental change do not adhere to typical effect-response relationships which traditional epidemiologic methods have been refined (and perfected) to measure. The pathways tend to be more complex and sometimes protracted; they can be direct but more often than not indirect, diffuse or delayed (Butler and Harley, 2010). Estimating future health risks requires
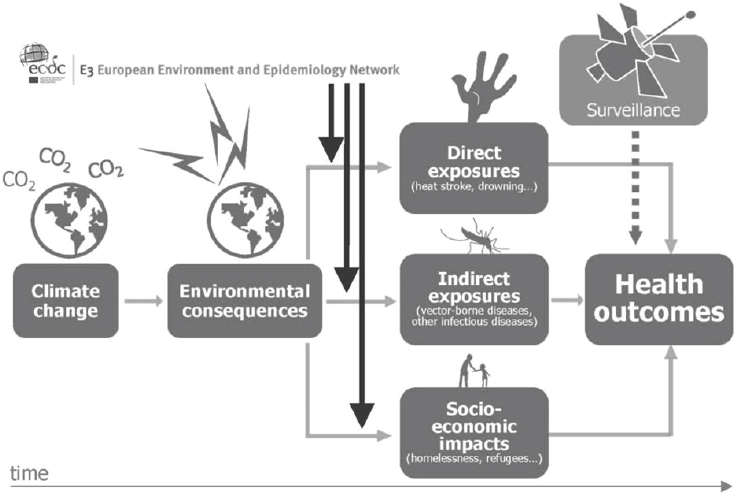
SOURCE: © European Centre for Disease Prevention and Control (ECDC) (www.ecdc.europa.eu).
interdisciplinary collaborations to develop scenario-based models. Moreover, detection of health endpoints with traditional surveillance methods suffers from significant time lags because of delays in case identification, diagnosis, and reporting, which can result in exposure misclassification and confounding. A geographic shift in infectious diseases might also lead to an expansion of the disease burden into new areas and might therefore be missed. Thus, these changes in the risk profile for human populations call for novel approaches to assess interconnected and interdependent risks (Altizer et al., 2013; Woolhouse, 2011).
Rapid developments in geographic information systems over the last decades have facilitated the management and use of spatial data for analytic epidemiology. A number of tools are now available over the web to explore and map spatial data that adhere to the standards of geographic data (e.g., INSPIRE directive). Risk models can then be used for the quantitative estimation of dynamic risks by taking into account changes in disease drivers. With this approach, future risk under different scenarios can be estimated.
The European Centre for Disease Prevention and Control (ECDC) has recognised the need for a proactive approach to deal with global environmental change (Lindgren et al., 2012; Semenza and Menne, 2009; Semenza et al., 2013). ECDC has developed an infrastructure coined the European Environment and
Epidemiology (E3) Network to help monitor environmental and climatic conditions related to infectious disease threats (see Figure A11-2) (Semenza et al., 2013; Semenza and Menne, 2009). The hub is composed of a data repository, a geoportal for data visualization and dissemination, and online tools that support the analysis of environmental, climatic and social drivers of infectious diseases (see Figure A11-3) (ECDC, 2014). The E3 Network provides technical support for the reporting, monitoring, analysis, and mapping of data and enhances the analytical capacity of existing resources in Europe. Results have been disseminated to policy makers, public health practitioners, European Union and international agencies, other governmental sectors, and nongovernmental organisations.
With the E3 Network, climatic, weather, and environmental data can be merged and integrated with health data in order to provide support tools for decision makers (see Figure A11-4) (Lindgren et al., 2012; Semenza and Menne, 2009; Semenza et al., 2013). Easy and rapid linkage of data for novel analyses provides novel opportunities to deal with the complex nature of interconnected and interdependent risks. Such an approach can rapidly identify geographic areas of increased risk at a certain point in time. It can also define high-risk populations that are particularly vulnerable to exposure and guide public health interventions.
SOURCE: © ECDC.
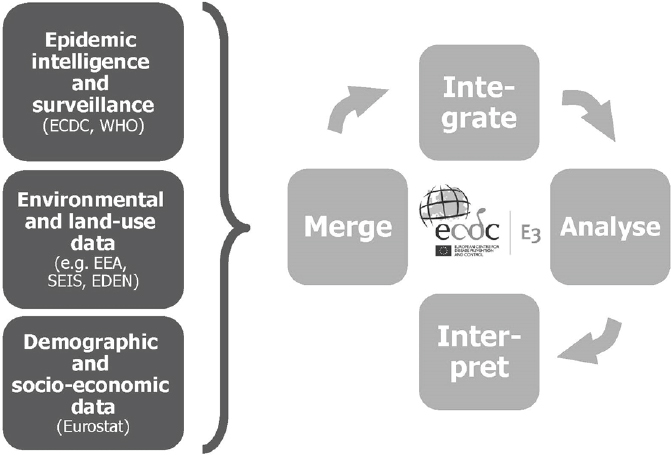
SOURCE: © ECDC.
Information from these analyses can provide lead time for outreach to the public and engagement of health care providers. It can also be used to set public health policies and inform civil society about potential consequences of global environmental change on public health.
The initial building-block of the E3 Network was a set of data that was assembled through a research project of the Directorate-General for Research and Innovation of the European Commission entitled Emerging Diseases in a Changing European Environment project (EDEN). The processing of these data sets, and those continuously assembled from other sources, with regular outputs from advanced scientific analysis, serve as a reference point (ECDC, 2014). It also supports data exchanges and scientific collaborations between member states, researchers, ECDC experts, and other authorised users across geographical and political boundaries in the European Community, with particular interest in the area of climatic change adaptation, landscape epidemiology, and emerging disease threats. The data of the E3 Network have been used in a number of case studies, three of which are briefly described below.
Intercepting Vector-Borne Disease Emergence and Spread: Case Studies
Environmental Suitability of Malaria Transmission in Greece
E3 data were used to predict the environmental suitability of malaria transmission in Greece (Sudre et al., 2013). In the past, malaria was endemic in Greece, but due to a successful malaria control and elimination programmes the country was declared malaria free in 1974 (Sabatinelli et al., 2001; Danis et al., 2011). Yet, importation of malaria has continued to occur accompanied by sporadic autochthonous transmission (Vakali et al., 2012; Kampen et al., 2002, 2003). A cluster of six locally acquired Plasmodium vivax cases without travel history to an endemic area was discovered in 2009; a total of 267 malaria cases were noted by Greek health authorities between 2009 and 2012, although some reported a travel history (Danis et al., 2011).
Nevertheless, the continuing transmission of P. vivax by indigenous vectors in areas with permissive environmental and climatic conditions could potentially signal the reemergence of malaria in Greece. Delineating specific areas environmentally suitable for transmission could direct and focus malaria control efforts. To assess the location of exposure of locally acquired malaria for such a suitability map, a standardized questionnaire was administered to each malaria case in Greece by a health officer. Cases with a travel history were excluded from this analysis as the goal was to delineate the areas environmentally suitable for autochthonous malaria transmission in Greece. By defining the environmental and climatic profile of areas with active transmission cycles between 2009 and 2012 other areas at risk for malaria re-emergence in Greece could then be defined.
Georeferenced environmental and climatic information for Greece and several other data sources were retrieved from the E3 Network data repository and other sources and processed for spatial modeling (Scharlemann et al., 2008; Earth Resources Observation and Science Center USGS EROS, 2005; European Environment Agency, 2011; The Joint Research Centre, 2009). They included demographic indicators, land cover categories, altitude, seasonal variations of vegetation, temperature, and so on. Using nonlinear discriminant analysis (NLDA) available in eRiskMapper version 1.1.4, a disease risk map was developed of areas suitable for persistent malaria transmission (Sudre et al., 2013) (see Figure A11-5).
Areas of environmental suitability for malaria transmission were characterized by warmer temperatures; low elevation; intensive, year round irrigated agriculture with complex cultivation patterns. Elevated temperatures (both nighttime and daytime temperature parameters were predictive in this model) can accelerate mosquito and parasite development which are likely contributing factors to mosquitoes presence and potentially to malaria transmission. This suitability map matched the historical distribution of malaria in Greece, particularly in the Peloponnese, the west coast of Central Greece and Epirus, and the east part of central Greece.
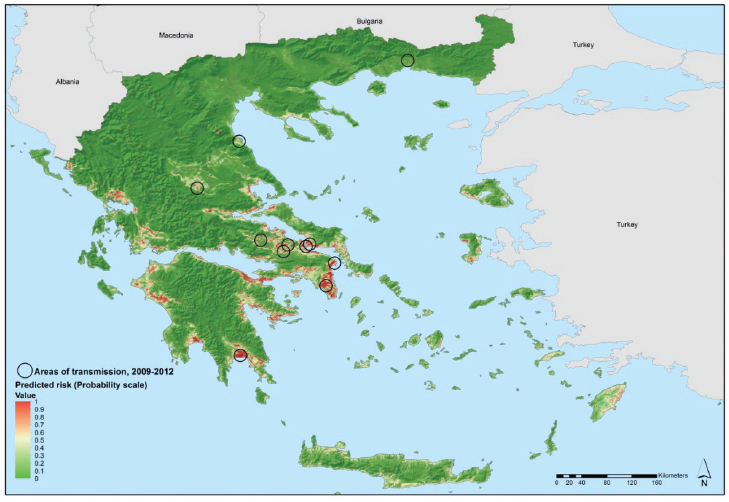
NOTE: Values from 0 to 0.5 (dark to light green) indicate conditions not favorable for malaria transmission (based on locally acquired cases); yellow to dark red areas delineate conditions increasingly favorable for transmission (values from 0.5 to 1).
SOURCE: Sudre et al., 2013.
This map was shared with public health practitioners in Greece responsible for integrated preparedness and response activities. Using EU structural funds, transmission was eventually interrupted in 2013 through targeted epidemiological and entomological surveillance, vector control activities, and awareness rising among the general population and health workers, in the areas environmentally suitable for transmission.
Environmental Determinants of West Nile Fever
Transmission of WNF is determined by environmental/climatic and biological drivers (Randolph and Rogers, 2010; Paz and Semenza, 2013). For sustained transmission to take hold at a given place and time, susceptible birds have to come in contact with infected birds in the presence of competent vectors. The avian transmission cycle is then amplified by local birds at which point the transmission can spill over to dead-end hosts such as humans or horses through
bridge vectors that feed on both birds and humans/horses (Paz and Semenza, 2013). A crucial aspect of WNV amplification among competent insect vectors and vertebrate hosts is also their population densities which determine the intensity of transmission. Vector population densities depend on temperature which accelerates the growth rates (Reisen et al., 2006). Moreover, elevated temperature decreases the timing between blood meals but accelerates viral replication rates and thus the transmission of WNV (Andrade et al., 2011). Thus, permissive weather and environmental conditions are responsible for sustained local transmission whereas migratory birds and short distance vector transportation affect the geographic dispersion.
In Europe, several WNF outbreaks have been linked with elevated ambient temperature (Paz and Albersheim, 2008; Savage et al., 1999; Paz et al., 2013). For example, Southeastern Europe was afflicted by a heat wave at the end of July to mid-August of 2010 which was followed by an outbreak of WNF cases (Paz et al., 2013). Temperature deviations above the 30 year mean struck Russia (deviations > 9°C), Romania (> 5°C), Turkey (> 5°C), and Greece (> 3°C) where 419, 57, 47, and 262 cases of WNF were reported, respectively (Figure A11-6). A number of meteorological variables were examined but temperature was the most significant one: in ‘colder’ countries of more northern latitudes a statistically significant correlation between number of WNF cases and temperature was observed, with time lags of up to 4 weeks from the onset of the temperature raise; in contrast, warmer and more southern countries presented correlations without these time lags (Paz et al., 2013). It has been noted that eruptions of WNF in previously unaffected areas tend to occur in years when summer temperatures deviate from the norm and that continued transmission can occur the following years even at normal summer temperatures (Reisen et al., 2006).
The notion that the initial outbreak is associated with a heat wave but not the subsequent ones has been observed in a number of settings (Epstein, 2001; Paz et al., 2013; Reisen et al., 2006; Pecoraro et al., 2007; Soverow et al., 2009).
To examine other environmental variables as predictors of WNF risk (Ozdenerol et al., 2013) we tested the contribution of remotely sensed temperature, the state of vegetation and water bodies, and bird migratory routes. The analysis was performed at the district level where each district was considered “infected” if WNF human cases were reported there that year, and as “noninfected” otherwise.
The number of WNF cases from 2002 to 2011 was assembled from ECDC surveillance data, peer-reviewed papers and the grey literature to fit the models. ECDC surveillance data for 2012 and 2013 were used for external validation. We used univariate and multivariate logistic regression models to assess the probability of a district to be categorized as WNV positive. The status of infection was set as the response variable, and anomalies of temperature, wetlands, and bird migratory routes were set as explanatory variables. In the final multivariate logistic regression model, parameters of WNV risk at district level for 2002–2011 were:
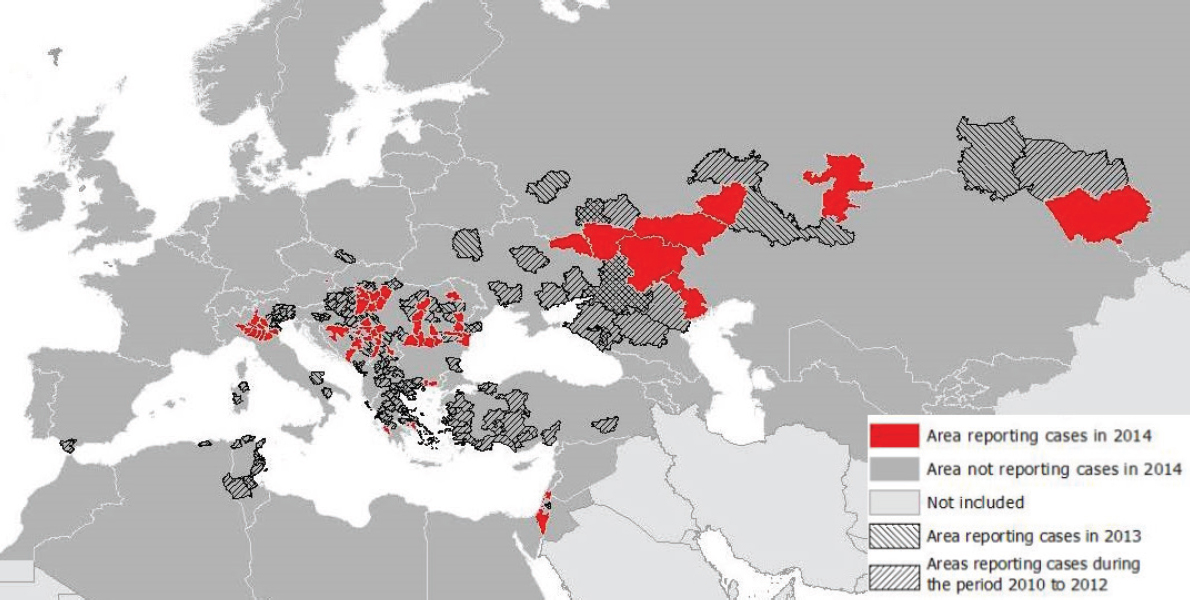
NOTE: Transmission season 2014 and previous transmission seasons; accessed November 20, 2014.
SOURCE: ECDC.
July temperature anomalies, the anomaly of the Modified Normalized Difference Water Index (MNDWI) (Xu, 2006) in early June, an outbreak the previous year, the size of the human population, wetlands and the type of avian flyways. Model validation with 2012 and 2013 data showed a good level of prediction; thus, July temperature anomalies and MNDWI can be considered determinants for WNV transmission in Europe. These models indicate that risk maps for WNV transmission can be assembled with up-to-date anomalies of July temperatures for a given year along with the MNDWI (Semenza et al., 2016).
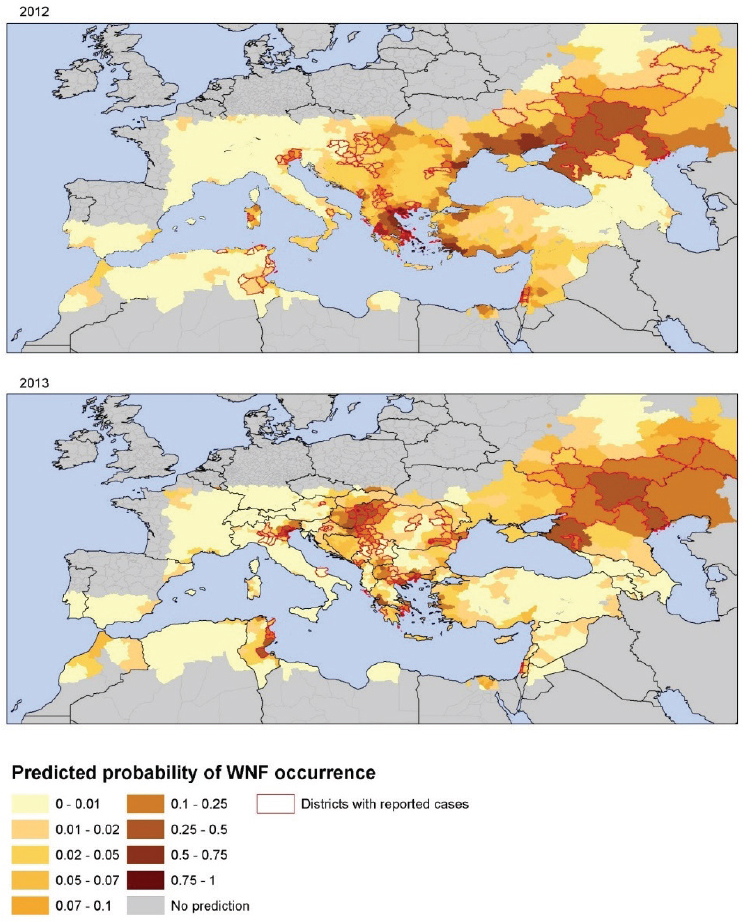
These two environmental determinants lend themselves for an integration of environmental monitoring in public health surveillance systems of human cases, serological surveillance of domestic and wild avifauna, and entomological surveillance (Ozdenerol et al., 2013; Kwan et al., 2012; Semenza and Zeller, 2014). Figure A11-7 displays the spatial heterogeneity of the probability of WNV infection per district in 2012 and 2013 as predicted by this model (Tran et al., 2014). Central and Eastern Europe, Turkey, Israel, and Tunisia were predicted to have higher risk values for 2012. In comparison with Figure A11-6, WND cases were notified in all of the predicted high-risk areas, except in Ukraine, and Turkey. Tunisia, Northern Italy, Northern Greece, Central Europe, and South Russia scored the highest predicted values in agreement with main areas of transmission in 2013 (see Figure A11-7). These findings indicate that the variables in this model can in part describe the variability in WNV transmission in Europe at the district level. Applying temperature anomalies for July can produce short-term and even long-term predictive maps of the probability of WNV infections.
Needless to say, besides these environmental/climatic drivers, biological drivers such as the presence and abundance of avian hosts and mosquito vectors of WNV need to be better described in order to more accurately predict the transmission of WNF in Europe (Paz and Semenza, 2013).
Dengue Dispersal Through Air Traffic
Dengue is by far the most significant mosquito-borne viral disease affecting humans globally, but there is currently no efficacious vaccine available for dengue (Simmons et al., 2012; Capeding et al., 2014). Tens of millions of cases occur annually resulting in approximately 20,000–25,000 deaths predominantly in children (Gubler, 2002; Mackenzie et al., 2004; Simmons et al., 2012; WHO, 2013; Bhatt et al., 2013). Transmission occurs largely in tropical and subtropical regions of the world, threatening almost half of the world’s population (WHO, 2013). In continental Europe limited outbreaks have occurred in areas infested by two of the mosquito vectors, Aedes albopictus and Aedes aegypti. Aedes aegypti is the predominant mosquito vector that transmits the dengue virus to humans, whereas Aedes albopictus is a less effective vector (Lambrechts et al., 2010).
Ae. aegypti is not present on continental Europe but was first reported in 2005 on the Portuguese island of Madeira and has subsequently invaded the
SOURCE: Tran et al., 2013. Reproduced with permission from BioMed Central.
southern part of the island (ECDC, 2012). In Europe, Ae. albopictus has been reported in at least 15 countries (either established or recently recorded) and continues to broaden its reach. The development period for Ae. albopictus begins in April and dwindles off in October/November based on entomological monitoring activities in the Mediterranean (Giatropoulos et al., 2012; Tran et al., 2013; Zitko and Merdic, 2014); however, the time of peak activity for Ae. albopictus are the summer months.
Travellers from the tropics or subtropics, can be considered at risk for dengue virus (DENV) infection (Gardner et al., 2012). Through international air travel, infected travellers can arrive in Europe during their viremic period, and be bitten by local Aedes mosquitoes (Vaughn et al., 2000). These infected mosquitos can subsequently transmit DENV locally and trigger an outbreak. In Europe, transmission has in fact occurred in areas where Aedes mosquitoes are present (La Ruche et al., 2010; Gjenero-Margan et al., 2011). In 2010, two dengue cases without recent travel history or blood transfusion were recognized in Southern France (La Ruche et al., 2010) and two other dengue cases in Croatia (Gjenero-Margan et al., 2011). Thus, for the first time in decades, local transmission had occurred in Europe. In 2012, an epidemic of over 2,000 dengue cases erupted in Madeira, Portugal, in areas where Ae. aegypti is known to be present (ECDC, 2012).
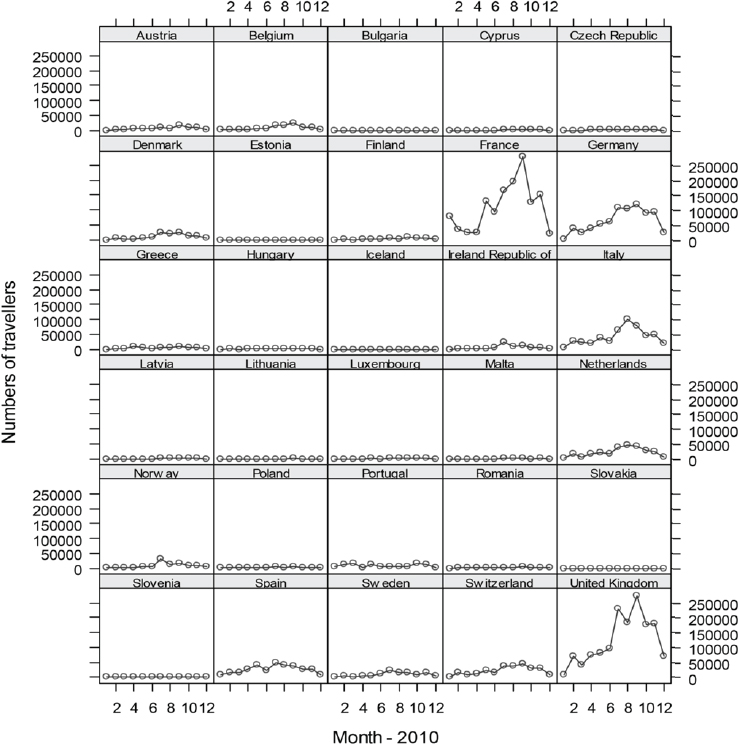
With the goal to quantify the risk of dengue importation in areas where local transmission could occur (due to the presence of the vector) we took into account the global disease burden and seasonality of dengue, the volume and seasonal fluctuations of travellers originating from dengue-affected areas, and the seasonality and distribution of competent mosquito populations within Europe (Semenza et al., 2014). We developed a model based on 2010 data that relates air travellers from dengue affected areas to dengue importation to Europe. Over 5.8 million air passengers entered Europe from dengue-affected areas in 2010; country-level arrival by month is illustrated in Figure A11-8 (Semenza et al., 2014). The final European destinations were mapped as a function of the volumes of global air travellers arriving from areas with dengue activity during 2010; the spatial extent of the Ae. albopictus distribution (from the E3 data repository) was overlaid (see Figure A11-9). Milan and Rome received over half, and Barcelona 14 percent of these travellers that enter Europe from dengue-active/affected areas (Semenza et al., 2014).
Imported dengue cases were significantly related to the monthly number of travellers arriving from dengue-affected locations. We developed a hierarchical multivariate model for imported dengue cases in 2010: the adjusted incidence rate ratio was 1.09 with a 95 percent confidence interval of [1.01–1.17] for every 10,000 traveller increase (Semenza et al., 2014). This corresponds to a 9 percent increase in the incidence of imported cases for every additional 10,000 travellers arriving from dengue-affected areas, all other predictors in the model being constant. In August, September, and October the rate ratio was 1.70 (95% CI:
SOURCE: Semenza et al., 2014. Available from PLoS Neglected Tropical Diseases under Creative Commons license.
1.23–2.35), 1.46 (95% CI: 1.02–2.1), and 1.35 (95% CI: 1.01–1.81), respectively (Semenza et al. 2014).
This empirical model for 2010 aims to quantify the association between the number of monthly in-coming travellers with the number of monthly dengue importations at the country level. The main driver of dengue importation and its pattern into EU countries can be described with high spatial and temporal resolution international air traffic data (see Figure A11-9) (Semenza et al., 2014). Moreover, the model accounts for dengue seasonality in the origin countries since
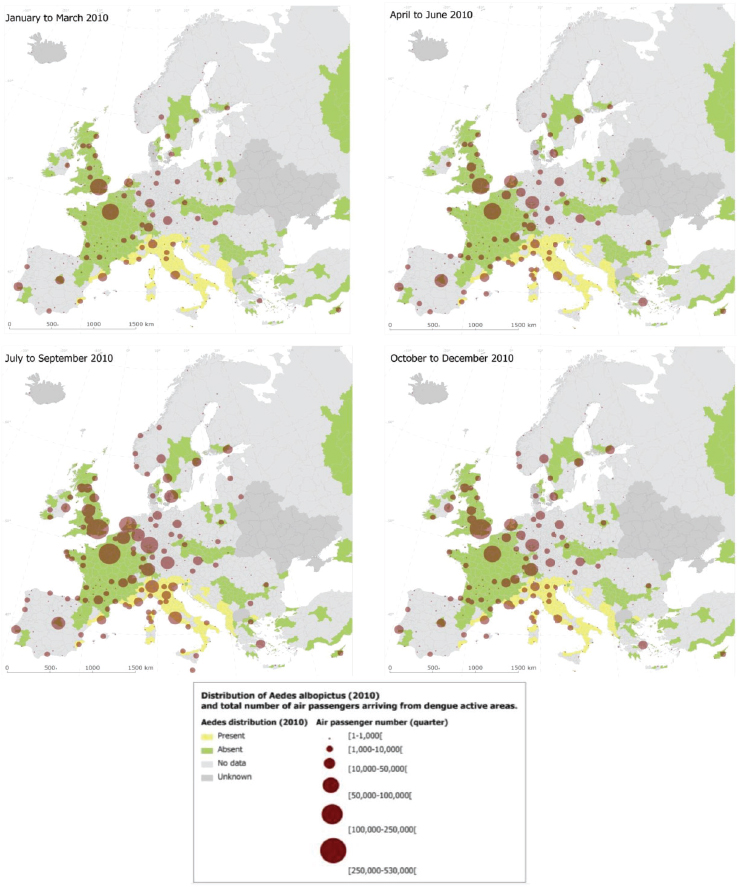
SOURCE: Semenza et al., 2014. Available from PLoS Neglected Tropical Diseases under Creative Commons license.
dengue presence was recorded by month and documents that the importation risk for 2010 was the highest between August and October.
Disease dispersal through international air traffic is the inevitable consequence of globalization. Pathogen introduction is difficult to intercept, and public
health has to resort to early detection, rapid response, and effective control measures to contain potential dengue establishment and spread (Hufnagel et al., 2004; Semenza and Zeller, 2014). The approach presented here could be translated to other settings in support of integrated surveillance of human cases and vectors (Lindgren et al., 2012; Semenza et al., 2014; Suk and Semenza, 2014). Such empirical models lend themselves to guide public health responses and can be developed into early warning systems of emerging risks (Nichols et al., 2014; Semenza et al., 2013).
Conclusion
Vector-borne diseases are a threat to global public health, including Europe. Mounting an effective public health response to these threats obviously includes awareness rising among the general public, public health practitioners, and policy makers about disease vectors and their relationship with infectious diseases. Exposure prevention through personal protection and vector control are essential activities of effective public health practice. However, intercepting the emergence and spread of vector-borne diseases can contain escalating human and financial costs of a potential epidemic. Monitoring environmental/climatic precursors of these outbreaks through early warning systems can help predict the emergence and spread of vector-borne diseases (Nichols et al., 2014; Semenza et al., 2013). Forecasts and predictions can be developed by linking the monitoring of environmental/climatic precursors to dedicated disease surveillance systems with integrated vector surveillance of invasive and endemic vector species as described in this chapter.
In recognition of the above, the European Commission emphasises the need to strengthen public health preparedness, including surveillance and monitoring. Specifically, DG SANCO acknowledges the importance of the E3 Network:
By connecting these sources of information, the E3 network should bolster European early warning for climate-related disease events. It should also enable forecasting and risk mapping of infectious disease incidence in relation to environmental changes. (Commision of the European Communities, 2009)
Monitoring the upstream environmental, climatic, and socioeconomic drivers of disease can provide the lead time for a swift public health response in order to contain human and financial costs associated with VBD emergence and spread in the European Union.
Acknowledgments
I am grateful for the contribution of my collaborators to these E3 projects; in particular Drs. B. Sudre, J.E. Suk, M. Rossi, T. Oni, E. Lindgren, S. Paz, A. Tran, J. Sears, W. Van Bortel, H. Zeller, V. Estevez and K. Kahn.
References
Altizer, S., R. S. Ostfeld, P. T. J. Johnson, S. Kutz, and C. D. Harvell. 2013. Climate change and infectious diseases: From evidence to a predictive framework. Science 341(6145):514-519.
Andrade, C. C., P. D. Maharaj, W. K. Reisen, and A. C. Brault. 2011. North American West Nile virus genotype isolates demonstrate differential replicative capacities in response to temperature. Journal of General Virology 92(Pt 11):2523-2533.
Bhatt, S., P. W. Gething, O. J. Brady, J. P. Messina, A. W. Farlow, C. L. Moyes, J. M. Drake, J. S. Brownstein, A. G. Hoen, O. Sankoh, M. F. Myers, D. B. George, T. Jaenisch, G. R. Wint, C. P. Simmons, T. W. Scott, J. J. Farrar, and S. I. Hay. 2013. The global distribution and burden of dengue. Nature 496(7446):504-507.
Butler, C. D., and D. Harley. 2010. Primary, secondary and tertiary effects of eco-climatic change: The medical response. Postgrad Medical Journal 86(1014):230-234.
Capeding, M. R., N. H. Tran, S. R. S. Hadinegoro, H. I. H. J. M. Ismail, T. Chotpitayasunondh, M. N. Chua, C. Q. Luong, K. Rusmi, D. N. Wirawan, R. Nallusamy, P. Pitisuttithum, U. Thisyakorn, I. Yoon, D. Vliet, E. Langevin, T. Laot, Y. Hutagalung, C. Frago, M. Boaz, A. Wartel, N. G. Tornieporth, M. Saville, and A. Bouckenooghe. 2014. Clinical efficacy and safety of a novel tetravalent dengue vaccine in healthy children in Asia: A phase 3, randomised, observer-masked, placebo-controlled trial. Lancet 384(9951):1358-1365.
Commision of the European Communities. 2009. Human, animal and plant health impacts of climate change. http://ec.europa.eu/health/ph_threats/climate/docs/com_2009-147_en.pdf (accessed December 1, 2014).
Confalonieri, U., B. Menne, R. Akhtar, K. L. Ebi, M. Hauengue, R. S. Kovats, B. Revich, and A. Woodward. 2007. Human health, In Climate change 2007: Impacts, adaptation and vulnerability. Contribution of Working Group II to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change, edited by M. L. Parry, O. F. Canziani, J. P. Palutikof, P. J. van der Linden, and C. E. Hanson. Cambridge, UK: Cambridge University Press.
Danis, K., A. Baka, A. Lenglet, W. Van Bortel, I. Terzaki, M. Tseroni, M. Detsis, E. Papanikolaou, A. Balaska, S. Gewehr, G. Dougas, T. Sideroglou, A. Economopoulou, N. Vakalis, S. Tsiodras, S. Bonovas, and J. Kremastinou. 2011. Autochthonous Plasmodium vivax malaria in Greece, 2011. European Surveillance 16(42)pii:19993.
Earth Resources Observation and Science Center USGS EROS. 2005. Global 30 Arc-Second Elevation Dataset (GTOPO30).
ECDC (European Centre for Disease Prevention and Control). 2012. Dengue outbreak in Madeira, Portugal 2012. http://www.ecdc.europa.eu/en/press/news/Lists/News/ECDC_DispForm.aspx?List=32e43ee8%2De230%2D4424%2Da783%2D85742124029a&ID=866&RootFolder=%2Fen%2Fpress%2Fnews%2FLists%2FNews (accessed August 1, 2014).
ECDC. 2014. Tender specifications for analysis of environmental drivers of infectious diseases framework service contract. http://e3devint.ecdcnet.europa.eu/SitePages/Vibrio%20Risk%20Map.aspx (accessed December 1, 2014).
Epstein, P. R. 2001. West Nile virus and the climate. Journal of Urban Health 78(2):367-371.
European Environment Agency. 2011. Corine Land Cover 2000 seamless vector data—version 15 (08/2011). Copenhagen, Denmark: European Environment Agency.
Faber, M. S., R. G. Ulrich, C. Frank, S. O. Brockmann, G. M. Pfaff, J. Jacob, D. H. Kruger, and K. Stark. 2010. Steep rise in notified hantavirus infections in Germany, April 2010. Euro Surveillance 15(20) pii=19574.
Gardner, L. M., D. Fajardo, S. T. Waller, O. Wang, and S. Sarkar. 2012. A predictive spatial model to quantify the risk of air-travel-associated dengue importation into the United States and Europe. Journal of Tropical Medicine 2012:103679.
Giatropoulos, A., N. Emmanouel, G. Koliopoulos, and A. Michaelakis. 2012. A study on distribution and seasonal abundance of Aedes albopictus (Diptera: Culicidae) population in Athens, Greece. Journal of Medical Entomology 49(2):262-269.
Gjenero-Margan, I., B. Aleraj, D. Krajcar, V. Lesnikar, A. Klobucar, I. Pem-Novosel, S. Kurecic-Filipovic, S. Komparak, R. Martic, S. Duricic, L. Betica-Radic, J. Okmadzic, T. Vilibic-Cavlek, A. Babic-Erceg, B. Turkovic, T. Avsic-Zupanc, I. Radic, M. Ljubic, K. Sarac, N. Benic, and G. Mlinaric-Galinovic. 2011. Autochthonous dengue fever in Croatia, August-September 2010. European Surveillance 16(9).
Gubler, D. J. 2002. The global emergence/resurgence of arboviral diseases as public health problems. Archives of Medical Research 33(4):330-342.
Hufnagel, L., D. Brockmann, and T. Geisel. 2004. Forecast and control of epidemics in a globalized world. Proceedings of the National Academy of Sciences of the United States of America 101(42):15124-15129.
The Joint Research Centre. 2009. Raster data on population density using Corine Land Cover 2000 inventory. Copenhagen, Denmark: European Environment Agency.
Jones, K. E., N. G. Patel, M. A. Levy, A. Storeygard, D. Balk, J. L. Gittleman, and P. Daszak. 2008. Global trends in emerging infectious diseases. Nature 451(7181):990-993.
Kampen, H., E. Maltezos, M. Pagonaki, K. P. Hunfeld, W. A. Maier, and H. M. Seitz. 2002. Individual cases of autochthonous malaria in Evros Province, northern Greece: Serological aspects. Parasitology Research 88(3):261-266.
Kampen, H., J. Proft, S. Etti, E. Maltezos, M. Pagonaki, W. A. Maier, and H. M. Seitz. 2003. Individual cases of autochthonous malaria in Evros Province, northern Greece: Entomological aspects. Parasitology Research 89(4):252-258.
Karesh, W. B., A. Dobson, J. O. Lloyd-Smith, J. Lubroth, M. A. Dixon, M. Bennett, S. Aldrich, T. Harrington, P. Formenty, E. H. Loh, C. C. MacHalaba, M. J. Thomas, and D. L. Heymann. 2012. Ecology of zoonoses: Natural and unnatural histories. Lancet 380(9857):1936-1945.
Kwan, J. L., B. K. Park, T. E. Carpenter, V. Ngo, R. Civen, and W. K. Reisen. 2012. Comparison of enzootic risk measures for predicting West Nile disease, Los Angeles, California, USA, 2004-2010. Emerging Infectious Diseases 18(8):1298-1306.
La Ruche, G., Y. Souares, A. Armengaud, F. Peloux-Petiot, P. Delaunay, P. Despres, A. Lenglet, F. Jourdain, I. Leparc-Goffart, F. Charlet, L. Ollier, K. Mantey, T. Mollet, J. P. Fournier, R. Torrents, K. Leitmeyer, P. Hilairet, H. Zeller, W. Van Bortel, D. Dejour-Salamanca, M. Grandadam, and M. Gastellu-Etchegorry. 2010. First two autochthonous dengue virus infections in metropolitan France, September 2010. European Surveillance 15(39):19676.
Lambrechts, L., T. W. Scott, and D. J. Gubler. 2010. Consequences of the expanding global distribution of Aedes albopictus for dengue virus transmission. PLoS Neglected Tropical Diseases 4(5):e646.
Lindgren, E., Y. Andersson, J. E. Suk, B. Sudre, and J. C. Semenza. 2012. Public health. Monitoring EU emerging infectious disease risk due to climate change. Science 336(6080):418-419.
Lourenco, J., and M. Recker. 2014. The 2012 Madeira dengue outbreak: Epidemiological determinants and future epidemic potential. PLoS Neglected Tropical Diseases 8(8):e3083.
Mackenzie, J. S., D. J. Gubler, and L. R. Petersen. 2004. Emerging flaviviruses: The spread and resurgence of Japanese encephalitis, West Nile and dengue viruses. Nature Medicine 10(12 Suppl):S98-S109.
McMichael, A. J. 2013a. Impediments to comprehensive research on climate change and health. International Journal of Environmental Research and Public Health 10(11):6096-6105.
McMichael, A. J. 2013b. Globalization, climate change, and human health. New England Journal of Medicine 368(14):1335-1343.
Nichols, G. L., Y. Andersson, E. Lindgren, I. Devaux, and J. C. Semenza. 2014. European monitoring systems and data for assessing environmental and climate impacts on human infectious diseases. International Journal of Environmental Research and Public Health 11(4):3894-3936.
Ozdenerol, E., G. N. Taff, and C. Akkus. 2013. Exploring the spatio-temporal dynamics of reservoir hosts, vectors, and human hosts of West Nile virus: A review of the recent literature. International Journal of Environmental Research and Public Health 10(11):5399-5432.
Patz, J. A., P. Daszak, G. M. Tabor, A. A. Aguirre, M. Pearl, J. Epstein, N. D. Wolfe, A. M. Kilpatrick, J. Foufopoulos, D. Molyneux, and D. J. Bradley. 2004. Unhealthy landscapes: Policy recommendations on land use change and infectious disease emergence. Environmental Health Perspectives 112(10):1092-1098.
Paz, S., and I. Albersheim. 2008. Influence of warming tendency on Culex pipiens population abundance and on the probability of West Nile fever outbreaks (Israeli Case Study: 2001-2005). Ecohealth 5(1):40-48.
Paz, S., and J. C. Semenza. 2013. Environmental drivers of West Nile fever epidemiology in Europe and Western Asia--a review. International Journal of Environmental Research and Public Health 10(8):3543-3562.
Paz, S., D. Malkinson, M. S. Green, G. Tsioni, A. Papa, K. Danis, A. Sirbu, C. Ceianu, K. Katalin, E. Ferenczi, H. Zeller, and J. C. Semenza. 2013. Permissive summer temperatures of the 2010 European West Nile fever upsurge. PLoS One 8(2):e56398.
Pecoraro, H. L., H. L. Day, R. Reineke, N. Stevens, J. C. Withey, J. M. Marzluff, and J. S. Meschke. 2007. Climatic and landscape correlates for potential West Nile virus mosquito vectors in the Seattle region. Journal of Vector Ecology 32(1):22-28.
Randolph, S. E., and D. J. Rogers. 2010. The arrival, establishment and spread of exotic diseases: Patterns and predictions. Nature Review Microbiology 8(5):361-371.
Reintjes, R., I. Dedushaj, A. Gjini, T. R. Jorgensen, B. Cotter, A. Lieftucht, F. D’Ancona, D. T. Dennis, M. A. Kosoy, G. Mulliqi-Osmani, R. Grunow, A. Kalaveshi, L. Gashi, and I. Humolli. 2002. Tularemia outbreak investigation in Kosovo: Case control and environmental studies. Emerging Infectious Diseases 8(1):69-73.
Reisen, W. K., Y. Fang, and V. M. Martinez. 2006. Effects of temperature on the transmission of West Nile virus by Culex tarsalis (Diptera: Culicidae). Journal of Medical Entomology 43(2):309-317.
Reisen, W. K., R. M. Takahashi, B. D. Carroll, and R. Quiring. 2008. Delinquent mortgages, neglected swimming pools, and West Nile virus, California. Emering Infectious Diseases 14(11):1747-1749.
Sabatinelli, G., M. Ejov, and P. Joergensen. 2001. Malaria in the WHO European Region (1971-1999). European Surveillance 6(4):61-65.
Savage, H. M., C. Ceianu, G. Nicolescu, N. Karabatsos, R. Lanciotti, A. Vladimirescu, L. Laiv, A. Ungureanu, C. Romanca, and T. F. Tsai. 1999. Entomologic and avian investigations of an epidemic of West Nile fever in Romania in 1996, with serologic and molecular characterization of a virus isolate from mosquitoes. American Journal of Tropical Medicine and Hygiene 61(4):600-611.
Scharlemann, J. P., D. Benz, S. I. Hay, B. V. Purse, A. J. Tatem, G. R. Wint, and D. J. Rogers. 2008. Global data for ecology and epidemiology: A novel algorithm for temporal Fourier processing MODIS data. PLoS One 3(1):e1408.
Semenza, J. C. 2015. Prototype early warning systems for vector-borne diseases in Europe. International Journal of Environmental Research and Public Health 12(6):6333-6351; doi:10.3390/ijerph120606333.
Semenza, J. C., and D. Domanović. 2013. Blood supply under threat. Nature Climate Change 3:432-435.
Semenza, J. C., and J. Giesecke. 2008. Intervening to reduce inequalities in infections in Europe. American Journal of Public Health 98(5):787-792.
Semenza, J. C., and B. Menne. 2009. Climate change and infectious diseases in Europe. Lancet Infectious Diseases 9(6):365-375.
Semenza, J. C., and H. Zeller. 2014. Integrated surveillance for prevention and control of emerging vector-borne diseases in Europe. European Surveillance 19(13).
Semenza, J. C., J. E. Suk, V. Estevez, K. L. Ebi, and E. Lindgren. 2012. Mapping climate change vulnerabilities to infectious diseases in Europe. Environmental Health Perspectives 120(3):385-392.
Semenza, J. C., B. Sudre, T. Oni, J. E. Suk, and J. Giesecke. 2013. Linking environmental drivers to infectious diseases: The European environment and epidemiology network. PLoS Neglected Tropical Diseases 7(7):e2323.
Semenza, J. C., B. Sudre, J. Miniota, M. Rossi, W. Hu, D. Kossowsky, J. E. Suk, W. Van Bortel, and K. Khan. 2014. International dispersal of dengue through air travel: Importation risk for Europe. PLoS Neglected Tropical Diseases 8(12):e3278.
Semenza, J. C., E. Lindgren, L. Balkanyi, L. Espinosa, M. S. Almqvist, P. Penttinen, J. Rocklöv. Determinants and Drivers of Infectious Disease Threat Events in Europe. 2016. Emerging Infectious Diseases 22(4): 581-589.
Semenza, J. C., A. Tran, L. Espinosa, B. Sudre, D. Domanovic, S. Paz. Climate change projections of West Nile virus infections in Europe: Implications for blood safety practices. Environmental Health (in press).
Simmons, C. P., J. J. Farrar, V. Nguyen v, and B. Wills. 2012. Dengue. New England Journal of Medicine 366(15):1423-1432.
Soverow, J. E., G. A. Wellenius, D. N. Fisman, and M. A. Mittleman. 2009. Infectious disease in a warming world: How weather influenced West Nile virus in the United States (2001-2005). Environmental Health Perspectives 117(7):1049-1052.
Stefanoff, P., M. Rosinska, S. Samuels, D. J. White, D. L. Morse, and S. E. Randolph. 2012. A national case-control study identifies human socio-economic status and activities as risk factors for tick-borne encephalitis in Poland. PLoS One 7(9):e45511. doi: 10.1371/journal.pone.0045511.
Sudre, B., M. Rossi, W. Van Bortel, K. Danis, A. Baka, N. Vakalis, and J. C. Semenza. 2013. Mapping environmental suitability for malaria transmission, Greece. Emerging Infectious Diseases 19(5):784-786. doi: 10.3201/eid1905.120811.
Suhrcke, M., D. Stuckler, J. E. Suk, M. Desai, M. Senek, M. McKee, S. Tsolova, S. Basu, I. Abubakar, P. Hunter, B. Rechel, and J. C. Semenza. 2011. The impact of economic crises on communicable disease transmission and control: A systematic review of the evidence. PLoS One 6(6):e20724.
Suk, J. E., and J. C. Semenza. 2011. Future infectious disease threats to Europe. American Journal of Public Health 101(11):2068-2079.
Suk, J. E., and J. C. Semenza. 2014. From global to local: Vector-borne disease in an interconnected world. European Journal of Public Health 24(4):531-532.
Thomas, S. M., N. B. Tjaden, S. van den Bos, and C. Beierkuhnlein. 2014. Implementing cargo movement into climate based risk assessment of vector-borne diseases. International Journal of Environmental Research and Public Health 11(3):3360-3374.
Tran, A., G. L’Ambert, G. Lacour, R. Benoit, M. Demarchi, M. Cros, P. Cailly, M. Aubry-Kientz, T. Balenghien, and P. Ezanno. 2013. A rainfall- and temperature-driven abundance model for Aedes albopictus populations. International Journal of Environmental Research and Public Health 10(5):1698-1719.
Tran, A., B. Sudre, S. Paz, M. Rossi, A. Desbrosse, V. Chevalier, and J. C. Semenza. 2014. Environmental predictors of West Nile fever risk in Europe. International Journal of Health Geographics 13(1):1-11.
Vakali, A., E. Patsoula, G. Spanakos, K. Danis, E. Vassalou, N. Tegos, A. Economopoulou, A. Baka, A. Pavli, C. Koutis, C. Hadjichristodoulou, and T. Kremastinou. 2012. Malaria in Greece, 1975 to 2010. Eurosurveillance 17(47):pii=20322.
Vaughn, D. W., S. Green, S. Kalayanarooj, B. L. Innis, S. Nimmannitya, S. Suntayakorn, T. P. Endy, B. Raengsakulrach, A. L. Rothman, F. A. Ennis, and A. Nisalak. 2000. Dengue viremia titer, antibody response pattern, and virus serotype correlate with disease severity. Journal of Infectious Diseases 181(1):2-9.
WHO (World Health Organization). 2013. Dengue 2014. http://www.who.int/denguecontrol/en/ (accessed September 21, 2014).
Woolhouse, M. 2011. How to make predictions about future infectious disease risks. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences 366(1573):2045-2054.
Xu, H. Q. 2006. Modification of normalised difference water index (NDWI) to enhance open water features in remotely sensed imagery. International Journal of Remote Sensing 27(14):3025-3033.
Zitko, T., and E. Merdic. 2014. Seasonal and spatial oviposition activity of Aedes albopictus (Diptera: Culicidae) in Adriatic Croatia. Journal of Medical Entomology 51(4):760-768.






















