5
Laboratory Regulatory Oversight: Finding the Balance
INDIAN REGULATIONS AND ANIMAL AND HUMAN HEALTH SAFETY
Nitin Jain opened the session with a presentation on Indian regulatory mechanisms. In 1993, considering the increasing risk associated with the use of new technology in laboratories, the National Biotechnology Board issued guidelines for ensuring the safety of laboratory workers. While drafting and preparing these guidelines, the review committee considered local factors, such as resistance to infection, the host-parasite burden in the community laboratory environment, and chances of survival and growth of altered organisms under tropical conditions. Prior to that, in 1986, the Indian government enacted environmental protection rules and regulations defining procedures for handling genetically modified organisms (GMOs) and hazardous microorganisms. These rules, finalized in 1989, are known as the rules for the manufacture, use, import, export, and storage of hazardous microorganisms, genetically engineered (GE) organisms, and cells which were not included in the 1986 Environment Protection Act (EPA).
The 1986 rules outlined six regulatory committees by topical area of research, each with its own set of guidelines.1
- Recombinant DNA Advisory Committee (RDAC)
- Institutional Biosafety Committee (IBSC)
- Review Committee on Genetic Manipulation (RCGM)
_____________________
1 For more information about India’s regulatory committees, see: http://www.moef.nic.in/division/genetic-engineering-approval-committee-geac; accessed April 10, 2016.
- Genetic Engineering Appraisal Committee (GEAC)
- State Biotechnology Coordination Committee (SBCC)
- District Level Committee (DLC)
IBSC and RCGM are involved in approving cases involving the use of genetically modified organisms (GMOs) or living modified organisms (LMOs) in research, and they also conduct biosafety assessments. The 1989 rules describe the approval required for the use of GMOs in plants and medical biotech products. For environmental release of GMOs or LMOs, or for large-scale production of them in the country, approval must to be obtained from the Genetic Engineering Appraisal Committee. DLCs basically function as regional monitoring groups to ensure compliance with the act and associated rules.
Jain provided an overview of the IBSC, including how it functions and its composition, and then briefly described the RCGM and SBCC.
The IBC is a statutory committee constituted by the Provisions of Rules (1989) of the EPA (1986). Organizations undertaking recombinant DNA activities with GMOs, LMOs, or rDNA materials, must have an IBSC, which must be registered with the Department of Biotechnology (DBT) under the Ministry of Science and Technology. An IBSC is initially constituted for three years and thereafter is renewed every two years. Its role is to examine the experimental protocols submitted with applications for research permission. It evaluates the ability of the investigator and his or her staff to conduct the proposed work, and it evaluates the facilities available within the organization to conduct research involving the recombinant DNA technology. The IBSC evaluates any potential danger associated with the work. It also evaluates the biological containment plan and facilities as per the recombinant DNA safety guidelines, and determines whether additional expertise should be considered. If there is need of any additional expertise, the IBSC may solicit expert comments.
An IBSC consists of one chairperson, three internal members, one member secretary who is also an in-house scientist, and one outside expert in the relevant discipline, typically in molecular biology. An IBSC should also have one biosafety officer with medical qualifications adequately trained to offer advice on specialized containment requirements. Finally, one member is nominated by DBT. IBSC members are appointed by the head of the organization, and can be reappointed at the end of a three year term. Membership is usually reviewed annually and appropriately modified based on participation and the requirements of the proposed recombinant DNA research and
developments involving recombinant DNA technology. DBT is to be notified within 2 weeks of any change in IBSC membership or chairmanship.
An IBSC is responsible for reviewing all research and development activity involving recombinant DNA technology of that particular organization. Depending on the category of the experimentation, the IBSC can simply note the information, grant permission for initiating the experiments, or refer the proposed research to RCGM for further review.
Next, Jain described the DBT RCGM, which functions in the Department of Biotechnology, and is responsible for reviewing the reports of all approved/ongoing projects involving the high risk category and control field experiment research in four areas: human and animal healthcare, agriculture, industry, and environmental management. This committee is empowered to visit the experimental facilities where projects with biohazard potential are being pursued prior to the commencement of research to ensure that adequate safety measures are taken as per the recombinant DNA safety guidelines. The committee is also empowered to issue clearance for the import and export of etiologic agents and vectors, germplasm, and so forth, necessary for recombinant DNA experimental work, training, and research.
Lastly, the GEAP functions under the Ministry of Environment and Forests, and is responsible for examining research proposals from the perspective of environmental safety on a case-by-case basis. It is also responsible for examining the environmental aspects of activities involving large scale use of hazardous microorganisms, recombinants in research, and industrial production. Proposals relating to the release of GE organisms and products into the environment, including experimental field trials, are considered by the GEAC. It also examines large scale use of recombinant DNA in products or the elements of GMOs.2
Jain then provided a brief history of regulatory efforts by the Department of Biotechnology from 1990 to 2014. The Department considered the RDAC’s proposed guidelines, which were issued in 1990 (Recombinant DNA Safety Guidelines), and in 1994, the Revised Guidelines on Safety and Biotechnology were issued. To ensure biosafety in India, DBT also developed the following guidelines:
_____________________
2 There are two websites that provide information on activities of RCGM and GEAC: www.dbtbiosafety.nic.in, and www.igmoris.nic.in. Accessed April 10, 2016.
- Revised Guidelines for Research in Transgenic Plants
- Guidelines for Generating Pre-clinical and Clinical Data for Recombinant DNA-Based Vaccines, Diagnostics and Other Biologicals
- Guidelines and Standard Operating Procedures for Confined Field Trials of Regulated GE Plants
- Guidelines for Safety Assessment of Food Derived from GE Plants
- Guidelines and Handbook for Institutional Biosafety Committee (revised in 2011)
Then, in 2012, DBT issued Guidelines on Synthetic Similar Biology and Regulatory Requirements for Marketing Authorization in India.
Issues related to genetic engineering of human embryos and the use of embryos or fetuses in research and human germline gene therapy are excluded from the scope of the 1990 Recombinant DNA Safety Guidelines. Those guidelines cover areas of research involving GE organisms, genetic transformation of green plants and animals, recombinant DNA technology applicable in vaccine development and large scale production, deliberate/accidental release of organisms, plants, animals, and products derived from recombinant DNA technology. Under these guidelines, four levels of risk have been assigned. Classification of organisms within these levels is based on the pathogenicity of the agents, the modes of transmission, the host range of the agent, the availability of effective preventive treatments or curative medicines, the capability to cause disease in humans, animals or plants, and an epidemic caused by microbial strains in India. The guidelines are based on those issued by the World Health Organization.
Jain provided an overview of all the recombinant DNA guidelines:
- Chapter I: defines the scope of the guidelines, including research activity, large-scale operation, and the involvement of risk associated with the accidental or deliberate release of recombinant DNA organisms.
- Chapter II: defines the recombinant DNA classified pathogens, and describes elements of biological and physical containment, such as laboratory safety, safety equipment, facility design, and so forth; the procedure for obtaining approval for large scale experimentation or manufacturing for release of GMOs in the
-
environment is also covered in this chapter. Chapter III: discusses the scope of their various committees, and the functions and implementation structure.
- Chapter IV: describes the containment facility and biosafety practices to be followed while conducting research involving GMOs.
- Chapter V: describes the recombinant DNA safety considerations, and classifies microorganisms on the basis of risk. It also provides the general scientific consideration for the risk assessment while working with microorganisms, hazardous microorganisms or recombinant DNA microorganisms.
Based on the level of the associated risk and the requirement for approval from competent authorities, research activities have been classified into three categories. Category I research activities are exempted from the approval process of the competent authorities, which are RCGM and GEAC. Experiments under Category I involve self-cloning using strains and inter-species cloning of organisms in the same exchanger group, such as organelle DNA including those from chloroplast and mitochondria. This type of activity does not require that the researcher notify RCGM or GEAC.
Category II research activity requires prior notification to the competent authority or RCGM. Experiments falling under the Containment Levels II, III, and IV are considered under Category II, as are experiments involving non-pathogen DNA vector systems and regeneration from single cells. Category II experiments also include those wherein DNA or RNA molecules are derived from any source except viral genomes and transferred to any non-human vertebrate or any invertebrate organism and propagated in containment. Large-scale use of the systems exempted in Category I, such as the large-scale use of recombinants made by self-cloning, are also considered under Category II. Proposals in this category are examined by IBSC, and the researcher must also notify RCGM for record purposes.
Category III research requires prior review and approval by the competent authority. Examples of Category III research include all toxin gene cloning experiments producing LD50 less than 50 micrograms per kg of body weight of vertebrates or large scale growing, research including cultured human cells of recombinant DNA molecules containing complete genes of potentially oncogenic viruses or transformed cellular genes, experiments involving the use of infectious animal and plant viruses in a tissue culture system, experiments
involving gene transfer to whole plants and animals, experiments requiring field testing and the release of recombinant DNA microorganisms or plants, and experiments involving engineered microbes with deletion and certain rearrangements. Category III experiments must be reviewed by the IBC and the RCGM.
Prior to 2003, large-scale research was defined as experimentation using fermentation beyond 20 liters, and it fell into Categories I, II, and III. In such large-scale research, safety criteria are to be compiled first. These criteria include a description of the host organism and the vector, and adherence to good laboratory standard operating procedures (GLSP) when working with genetically-modified organisms. Guidelines also specify the principles of professional safety and hygiene for GLSP as well as the level of containment. The import or receipt of etiological agents and or vectors for human and animal diseases or their carriers is subject to quarantine regulations. Further, if an import is for research purposes, RCGM is the competent authority granting approval, but if the import is for industrial purposes or large-scale manufacturing and further commercializing purposes, the approval-granting authority is GEAC. The 20-litres threshold was relaxed in 2003. The RCGM, using its discretion, may, on a case-by-case basis, permit the applicant to conduct experiments using fermenter capacity of greater than 20 liters exclusively for research purposes, and only to produce sufficient GMOs required to generate pre-clinical and other relevant data to create the product for commercial use. The threshold was also relaxed to ensure that sufficient material is being generated to conduct pre-clinical trials.
Discussion
A participant asked about the Biotechnology Regulatory Authority of India (NBRA) bill being considered by the India parliament at the time of the workshop. Jain replied that the bill was introduced in the 15th Lok Sabha, but with the dissolution of the parliament, that bill was also dissolved, however, it was to be reintroduced at the parliamentary session following the 2014 elections, and he said that it will be included in this Lok Sabha in session at the time of the workshop.
A participant asked if Jain could clarify the qualifications of a biosafety officer on the IBSC. Jain replied that this person is to have medical qualifications, specifically the person is to be a practicing doctor (MBBS) who also understands containment issues.
Another participant asked about the process for the IBSC to identify suitable expertise in complex cases? Is it a regular process across the country, or does each local institution have its own process for identifying the right people? Jain replied that an IBSC functions independently and the chair is empowered to call upon any person he or she may consider suitable.
T. S. Rao added that when the biosafety guidelines were initially created, DBT nominated the biosafety committee members. Since then, however, the number of laboratories and industries conducting this type of research has increased.
A participant noted that sometimes only one or two people physically attend IBSC meetings in the United States. Is there a system in India of defining minimum attendance for a valid meeting? Jain replied that DBT has received inquiries about whether members can join via Skype, so a policy on this will soon be released.
REGULATORY OVERSIGHT OF BSL-3 LABORATORIES
John Kenneth opened his remarks by saying that biosafety per se, as the word suggests, is based primarily on the risk posed by pathogens known to cause primary disease and are classified according to risk level into biosafety levels 1, 2, 3, and 4, in an ascending order of risk. Biosafety containment provides a barrier between disease-causing pathogens and healthy persons and the environment.
There is a tendency for people to focus on one area of specific concern, based on professional interest, be it the environment, a laboratory, etc., but biosafety measures block pathogens from being transmitted between lab workers, hospital staff, general staff, and the public at large. The basic tools to do this are: engineering controls, or the way a facility is designed and constructed; personal protective equipment, and safety equipment; and safe work practices.
Next, Kenneth provided details about BSL-3 laboratory regulations. Indigenous or exotic agents that may cause potentially lethal disease are studied in BSL-3 laboratories because these diseases can be contracted through inhalation. BSL-3 facilities are also required for clinical work, teaching junior scientists, and training that accompanies research. Diseases causing near-certain death are studied in BSL-4 laboratories. Due to the biosafety concerns, everything that goes into and everything that comes out of a BSL-3 laboratory must be regulated, including the
people, the air, the articles, the samples, containers, and waste. Access is controlled and there is a physical separation from other corridors. The air flow is negative pressure, which means that the pressure sucks air into the area, and it does not let air out of that particular area without proper filtration, and it is not recirculated out into the environment. The air quality inside the lab is also controlled.
Physical entry only occurs through an air-lock or an anteroom so that air does not pass unfiltered outside the facility. There is self-closing double door access, and personal protective equipment must be worn inside that air-lock or anteroom. Biosafety cabinets are used inside the facility, and waste and clothing are decontaminated at the exit and removed through a second door. See Figure 5-1.
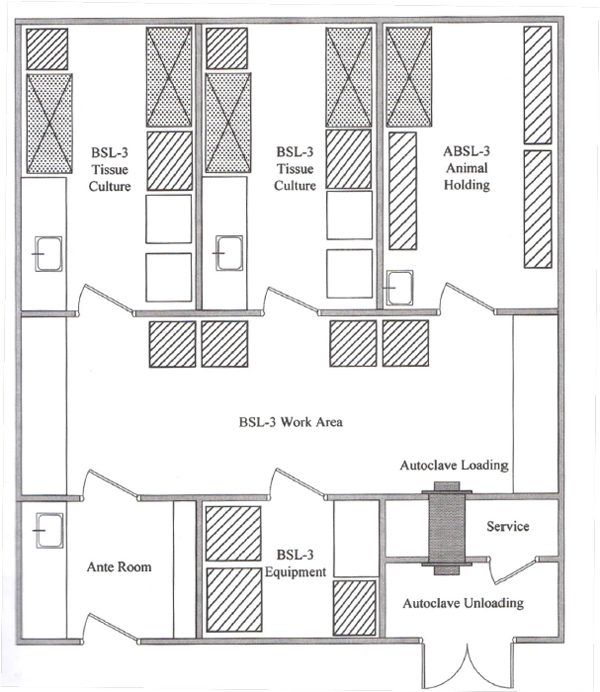
SOURCE: Fleming, Diane O. Hunt, Debra L. 2006. Biological Safety - Principles and Practices, 4th Edition. American Society for Microbiology. Reprinted with permission of the American Society for Microbiology.
The basic principle is to have an inlet, which is an anteroom, and an outlet. Everything in between is totally controlled. Airflow must be unidirectional, and the air change rate must be between 15 and 20 air changes per hour, filtered at the entry. A HEPA filter is required so that the number of pathogens and the number of organisms, entering is limited. The air has to be at least Class D, fewer than 1,000 particles of less than 0.3 nanometers per cubic meter, and have a negative pressure of minus five to 15 centimeters of water. The anteroom is also pressurized, and there is no leak to the external environment. The entire area is engineered so that there is no deposition of organisms. There is a totally smooth and cleanable area that does not have any crevices or niches where organisms can multiply.
The essential issue for personnel safety is “training, training, and more training.” All personnel must have protective equipment, and basic laboratory training. They need to be recertified periodically and all of them must be vaccinated and educated. Consent must also be obtained, because laboratory work can be potentially harmful. Workers are strictly monitored to avoid errors or lapses in concentration, just like airline pilots who cannot work longer than a specified period of time. The importance of training cannot be overstated. Internal and external audits are also necessary.
An ideal BSL-3 team, Kenneth said, would include an experienced team leader, HVAC engineers, engineers for facility and instrumentation, infection control, doctors and nurses, and those who can conduct periodic reassessments and certification.
There are several existing standards, such as the ISO standards, GLP standards by the U.S. Code of Federal Regulations, the Australian National Association of Testing Authorities standards, and checklists for BSL-3 labs developed based on the Biosafety in Microbiological and Biomedical Laboratories (BMBL 5th edition) published by the U.S. Centers for Disease Control and Prevention (CDC).3 The ISO 15189:2012 guideline for medical laboratories is a mature standard.
_____________________
3 For examples of two BSL-3 checklists, see: http://www.selectagents.gov/resources/Checklist-BSL3.pdf; and http://orf.od.nih.gov/PoliciesAndGuidelines/Bioenvironmental/Documents/BSL3CertificationGuidelinesFINAL_508.pdf; accessed April 10, 2016.
In addition to the requisite standards and assessors, India also has a National Accreditation Board for Hospitals. The best experts are chosen to conduct examinations for third party certification. A module to assess BSL-3s is needed, and laboratories need to be overseen with regard to specific areas like air flow, training, readiness, waste disposal, and so forth.
It would also be helpful to extend assessments to include isolation and high security care. Currently, there is no comprehensive oversight of laboratories on an international basis. Every country has its own oversight mechanisms, but it would be ideal to have international standards. However, they most likely would be impossible to implement.
Discussion
The discussion following Kenneth’s presentation focused on accreditation and certification of laboratories.
Kenneth noted that since most research conducted in BSL-3 labs in India involves microbiology, certification of clinical laboratories belongs to the National Accreditation Board for Laboratories. T.S. Rao noted that having third party certification is very important, and welcomed any input on how to organize this.
Another participant followed up on the ISO 15189. The National Accreditation Board trains assessors in India, however, none of them has access to BSL-3 facilities, therefore, in the participant’s view, a third party should be involved in the assessment and accreditation of these labs. Similarly, the third party could seek to have specific assessors who have relevant knowledge and who have passed specific exams, including an additional component on the ISO 15189. T.S. Rao then asked about the guidelines for a BSL-3 model. He believed that this needed to be defined.
Another participant asked if India had a national certification system. Kenneth replied that India has a link to the International Asia Pacific Lab Accreditation Committee as well as to the International Laboratory Accreditation Committee. They audit one another. The participant followed up, asking if this system was specifically for BSL-3 labs. Kenneth replied that no, this system existed for all labs.
A participant noted that there do not appear to be any procedures for other laboratories in India, such as animal science laboratories, other than procedures for medical labs. The existing certification is missing a biosafety component in its overall approach. That should be introduced
in the ISO 17025 accreditation process so that it covers all types of laboratories, including medical and animal science laboratories, the participant said. There is also a lack of expert groups in India who can provide assessments of biosafety laboratories; this aspect is also needed.
Kenneth made a final point. When molecular biology was new, National Accreditation Board for Testing and Calibration Laboratories (NABL) did not have experience in these fields. There were very few who had such experience, so when a specific organization applied for molecular biology certification, he was called to examine it. He proposed that there could be four or five people who are well-trained to assess labs and they could be called upon to assist with lab certification.
One of the workshop participants recounted the initial consideration among scientists of building a BSL-4 lab in India. The government of India was opposed. Together, India and international technical experts visited a number of countries to understand the international experience and an Australian lab was selected as the model for the Indian lab. However, after further analysis, they concluded that BSL-4 labs are very expensive. Another group was later formed, and they visited additional laboratories around the world and selected the Holland lab as a model. The biosafety officer atthe Holland lab assisted in preparing the architectural drawings for the Indian lab along with an architect from Delhi. It took nearly five years to complete the requirements on paper. It was a rigorous exercise. Following this, structural equipment was imported, sold to fabricators, tested, re-tested, and then manufactured. Another participant continued by noting that this experience provided Indian experts with valuable knowledge about designing and operating laboratories to the highest standards, however challenges remain.
An alternative perspective was offered by another participant who cited a great deal of education among laboratory managers and staff in India, sufficient to address problems that may arise independently and with the assistance of international experts. DBT has a full-fledged engineering group, and once the decision has been made to build a lab, the group does so according to the specifications of that particular laboratory. The specific requirements are determined on the basis of a preliminary outline or draft. Rigorous discussions among engineers from all relevant fields follow and a final design is then endorsed. The previous speaker agreed, emphasizing the need to translate that expertise into reality. T.S. Rao requested draft recommendations from workshop participants regarding how to create such a facility.
The previous participant expressed the view that Indian experts are good at fabrication as well as engineering, including pumps for negative pressure, HEPA filters, etc. NABL accreditation is not mandatory for all laboratories. There are many private labs around the country that do not seek NABL accreditation. A regulatory body to certify and recertify labs would be helpful. T.S. Rao noted that the government is still creating the new regulatory, accreditation structure, but NABL could be empowered to do this rather than create a new entity. Another participant reiterated that DBT has the expertise required to build biocontainment labs in India.
Rao stated that the government will assist whenever additional expertise is needed, or wherever there are uniquely governmental functions required because, “we need to make things happen,” and “we need to drive reality.” In response, the need to empower DBT with a greater role was noted because the only BSL-4 lab in the south Asia region is in India, therefore, India has a responsibility, not only to the country, but also to the region. The Indian government is also interested in strengthening the region.
The United States is always concerned about the development of BSL-3s and BSL-4s, noted another workshop participant. In particular, it is important to consider how these labs fit into the global community of well-operating, -managed, -maintained, -certified laboratories. One of the outcomes of this meeting, if it is useful to the Indian government, may be for Indian National Science Academy and the National Academy of Sciences to produce background papers that could provide information on existing animal health and human health laboratories, and on which regulatory structures exist and where gaps still remain. Perhaps at another stage the academies together could provide DBT with a set of recommendations on the types of regulatory needs associated with these labs. These needs could include types of structures and guidelines for oversight and/or advisory bodies as the rapidly expanding BSL capacity comes online in the next decade. Rao agreed that such recommendations would be excellent, and that India would appreciate this assistance.
Another participant noted that there are many BSL-3 labs in India, and in Delhi itself there will be at least four or five; and they are all functioning well. However, they are extremely expensive to maintain. In addition, the reliability of BSL-3 labs needs to be certified over a period of time, not only at the point of initial operation. There have been efforts to try to identify people to certify and recertify these labs. Recertification may require some sophisticated equipment, which will in turn require the
generation of an entirely new type of business. A certification company could certify BSL-3s or BSL-4s in the country. Only with such a model would it be sustainable to invest in this equipment at a national level. Regular funding for maintenance is similarly a critical aspect of sustaining BSL-3 labs and the government lab. Since these labs are extremely expensive, universities or institutional centers may not be able to support them from their own budgets. Rao agreed that the long-term sustainability is an important aspect to continuously consider. This prompted the comment that perhaps it is better to consider BSL-2+ labs for some research instead of BSL-3 labs, because there are probably not more than 13 or 14 labs that actually adhere to the requirements of a BSL-3 lab.
REGULATORY OVERSIGHT OF BSL-4 LABORATORIES
Tom Ksiazek began his discussion of the regulatory framework for BSL-4 labs in the United States with the advent of genetic engineering. In the early 1970s, the book and film The Andromeda Strain triggered concern in the general public about that scientists might inadvertently create and release a superpathogen in the laboratory by manipulating E. coli. Scientists agreed that there ought to be some consideration given to the safety standards under which these experiments were conducted and convened the Asilomar Conference in 1975. The Asilomar Conference led to the National Institutes of Health (NIH) becoming the regulatory agency that established the RAC, issued the NIH Guidelines for Research Involving Recombinant DNA Molecules, and provided assistance to local committees in the application of those guidelines Asilomar also led to the Biosafety in Microbiological and Biomedical Laboratories (BMBL) regulations and guidelines.
The RAC guidelines were published in 1981, and the first edition of the BMBL was released in 1984. They serve as the regulations and guidelines under which all biosafety levels are regulated in the United States. With some caveats, prior to the advent of these guidelines, research conducted in laboratories was regulated through permits. Permits largely regulated exotic organisms and their importation or redistribution in the United States. If Ksiazek wanted to bring Japanese encephalitis into the United States, for example, or obtain Japanese encephalitis from Rockefeller Lab, he would have to obtain a permit to move an exotic agent from one place to another. However, there are no inspections associated with obtaining the permit, so he would have to
describe the facilities, the training, and the type of personnel that would be handling the agent. The same is true for exotic animal pathogens, especially livestock pathogens. These pathogens were and still are regulated by the U.S. Department of Agriculture Animal and Plant Health Inspection Service.
The BMBL and another document called “Chapter 9” became the standard for the operation of these laboratories. The BMBL standards are performance-based rather than prescriptive. They describe the qualities that facilities are to meet to be in compliance with the standards required under other laws. “Chapter 9” actually prescribes the physical constitution of the laboratory in much more detail, which does not leave the architects and engineers with the ability to meet performance standards in the same way. As technology has advanced, laboratory operations have evolved a great deal. With performance-based standards, operators have the obligation to meet them, but the manner in which they do so is not specified because technology continues to develop.
It has been approximately 30 years since the first edition of the BMBL was released; about every 5 to 6 years these documents are modified by the regulating agencies. In the case of human health research, NIH and CDC jointly modify the documents. Outside experts are involved for specific groups of organisms and/or for specific levels of labs. The BMBL is now used as a regulation, although it was clearly developed as a guideline. Under the Select Agent Act, laboratories must be inspected every three years. BSL-3 laboratories receive certification for three years, and there is often one surprise inspection within that three year period. In practice, BSL-4 labs are inspected annually. There are two elements involved in establishing the level of laboratory appropriate to specific types of research. The first is a risk assessment conducted on the organisms themselves. Each organism is assigned to a specific risk group. The qualities that are assessed when making that determination are in Table 5-1.
Determination of the risk is made at a local level, where organisms are assigned to a laboratory that meets the standards for performance. The general principle underlying the risk assessment is personal protection rather than environmental containment. For example, if a researcher is handling a common human pathogen that is found in the community, it is much less important to try to protect the environment more rigorously. However, if the agent is not found in that location and is likely to cause a public health emergency in that community, it is more important to try to rigorously protect the environment.
TABLE 5-1 The Factors Considered in Assessing Organism Risk for Laboratory Biological Safety Levels
| Personal Risk | Environmental Risk |
| Human Pathogen? | Contagious? |
| Laboratory Infections? | Indigenous? |
| Vaccines Available? | Aerosol Infectious? |
| Treatment Available and Effective? | Agricultural Risk? |
| Aerosol Infectious? | |
BSL-4 labs require the building of a box inside of another box, followed by the development of a very strict regimen of procedures. Specific equipment is also designed to keep organisms inside the specified boxes. When Ksiazek began his career at Fort Detrick in the 1980s, regulations were just emerging, and the primary concern of security was the safety of all people who worked in the laboratory facility, so the goal was to keep organisms inside the appropriate location.
Much of the biosafety technology used today was developed at Fort Detrick and transferred to the many other U.S. BSL-3 and BSL-4 facilities: The air supply and exhaust systems are filtered and double-filtered; all of the sewage is incinerated; and, all of the waste exits the facility through autoclaves that are validated with each run. A great deal of effort is expended to certify that this material remains inside the laboratory where the individuals themselves are protected by very rigorous use of personal protective equipment.
There are also secondary safety barriers in facility design. Engineering controls are an important part of these laboratories. Often it is difficult to determine the exact cost of equipping and running them because BSL-3 and BSL-4 labs have traditionally been part of a large physical structure such as Fort Detrick or CDC. Generally, if there was a centralized steam plant or a water chill plant, one could not separate the cost of that particular part of the facility incurred by the lab. Both BSL-3 and BSL-4 facilities generally have what is called single pass air, therefore, whether in summer or winter the cost of air conditioning the lab, for example, remained hidden in the overall physical plant operation of the large enterprise. To provide a sense of scale, the cost of electricity for the high-containment laboratory building at the Galveston National Laboratory in Texas is approximately $2.2 to $2.5 million per year.
Emerging infections continue to create surprises and public health emergencies of considerable size, not only by threatening human health, but also by creating economic consequences to the countries affected. SARS, HIV, and zoonotic pathogens exist in human populations, and can politically destabilize a number of countries. Ksiazek described naturally-occurring emerging infections as the principle biosecurity issue currently facing the United States, although terrorists, as individuals or groups, may use organisms against people in a way that could create considerable problems. He argued that there is less thought given to pathogen risk analysis than perhaps there ought to be. Under the framework of the Select Agent Act that went into effect in 1997 in the United States, training must be documented in ways not previously required.
In December 2013, the select agent regulations went into effect, causing a significant response among the microbiology community due to the number of agents on the official U.S. Select Agent List. There was some agreement within the scientific community that not all agents on the list posed equal risk. As a result of these concerns, there was an effort on the part of the American Society for Microbiology and other large organizations to elevate a small number of agents, perhaps smallpox and 1918 influenza, to the status of Tier 1 agents with the remaining agents being listed at a lower level of risk. What happened in the end was that a fairly extensive list of pathogens became Tier 1 agents, and a smaller number of agents were removed. As a result, if a lab has a Tier 1 agent, the lab must put in place a greater number of biosecurity measures with regard to personnel reliability, the security of data maintained about the agents themselves, and the security of information and data related to the physical plant. Physical security requirements were also increased at facilities that held or were working with Tier 1 agents. There are real costs associated with being able to handle select agents, and those costs have increased with the advent of the Tier 1 category. In Ksiazek’s view, there are Tier I agents that do not have some of the attributes ascribed to them. Some of the individuals involved in the classification of these agents have agreed with Ksiazek.
Ksiazek returned to personnel reliability measures. The Select Agent Act requires that researchers working with Tier 1 agents obtain a security clearance prior to obtaining permission to conduct their experiments. The clearance is granted by the Department of Justice, implemented by the Federal Bureau Investigation, and confirms that the person does not have a criminal background and that there are no other issues that would, in
their view, disqualify that person from handling these agents. When the Act was initially enforced, there were specific instances when clearance investigations discovered that individuals who had worked in these labs for many years had incidents in their past, and they were removed from their positions. Ksiazek stated that there is no ability to appeal these decisions.
Discussion
In response to a question regarding which of the U.S. regulations should perhaps not be replicated in other countries, Ksiazek replied that, as a microbiologist and a public health researcher, he would modulate some of the security regulations. Safety is very important, and the code of practiceBMBL, does a good job of addressing these issues.
Another issue, Ksiazek added, is that prior to the adoption of the Select Agent Act, NIH held the researcher and the safety committee accountable for adherence to the BMBL standards. In other words, if funding came from the federal government, researchers had to meet these requirements. With the advent of the Select Agent Act, the BMBL shifted from being standards to being a regulatory mechanism.
An Indian participant noted that when Indian experts studied the classification of biocontainment labs by biosafety level, there were initially just a few parameters taken into account: an agent’s capability to infect the person working with the organism, the risk posed to the community by the agent, and the availability of preventive and therapeutic measures. There are now four BSL levels. However, there are many other internationally recognized levels, such as BSL-2+, BSL-3+, BSL-3 NRs. What are the distinguishing features between BSL-2+ and BSL-3 labs? Some say that a BSL-2+ lab has different physical structures, whereas a BSL-3 lab has different practices as well.
Ksiazek noted that officially the category of BSL-2+ labs does not exist in the United States. There are instances where, for all practical purposes, these labs do exist. For instance, during the 2009 emergence of H1N1, a special category of lab was created that allowed BSL-2 labs to operate with BSL-3 lab practices to initially handle specimens. There is a laboratory category called “BSL-3 enhanced for individual organisms. The BMBL does have lists and recommended risk levels for these instances. In the United States, BSL-3 labs are not required to have HEPA filtration, although no one would build a facility without it.
ETHICAL CODES RELEVANT TO MEDICAL AND HUMAN HEALTH RESEARCH LABORATORIES
Vasantha Muthuswamy began by defining ethics as a modern code of conduct that determines right and wrong. There are many codes of conduct in the form of guidelines with which individuals voluntarily comply. The first code for biomedical research in India dates to 1980, when it was released as a policy statement on ethical considerations in modern research on human subjects. When these various guidelines were developed, the Belmont Report4 and international guidelines were consulted, and equity, accessibility, and affordability were also taken into consideration. In 1996, a committee that was established by the government of India developed new ethical guidelines, which were listed in the Indian Council of Medical Research (ICMR)-NIH forum in 2000. At that time, Justice Venkatachaliah agreed to chair the committee on one condition: that these guidelines one day be passed as a bill in the Indian Parliament, or made mandatory, so that they would be followed by all. Nearly 20 years later, the bill has not been passed as far as medical research is concerned.
In the meantime, a number of other guidelines have been developed. In 2006, the ethical guidelines for biomedical research of 2000 were revised and newly titled, Ethical Guidelines for Research in Human Participants. Guidelines on stem cell research and therapy have been brought to DBT, and the draft of biobanking guidelines have also been brought forward. There are also guidelines on GMO food safety, and, in the summer of 2008, good clinical laboratory practice (GCLP) guidelines were introduced. ICMR-DBT jointly released guidelines for probiotic research and ICMR recently posted the Code of Conduct for people conducting life-science research online. Another recent development is the ICMR ethics bill, soon to be released. The basic tenets of these guidelines include: autonomy, justice, beneficence, and non-maleficence, as in the Hippocratic Oath.
The 2000 guidelines, updated in 2006, are followed across India. The most recent version of the bill introduced in Parliament will incorporate mandatory adherence and will also establish a biomedical research authority that will ensure the accreditation and registration of all ethics
_____________________
4 The Belmont Report can be found at: http://www/hhs/gov/ohrp/humansubjects/guidance/belmonth.html; accessed April 10, 2016.
committees and Institutional Review Board (IRBs) in India. Likewise, all clinical trial site investigators must be accredited. If the ICMR ethics bill is enacted by Parliament as scheduled, it is to come into effect at the end of 2015.
Muthuswamy then turned to biosafety, biosecurity, and human health safety issues for workers and research participants. The ICMR/GCLP guidelines issued in 2008 are clinical laboratory practice guidelines pertaining to specimen collections and pre-analytical collections.5 Good clinical laboratory practice guidelines are not for reporting quality test results or day-to-day research results, but they are to be followed by medical researchers to generate quality data.
Now that these guidelines are in place, the primary concern is compliance. The laboratory GCLP guidelines pertain to all labs involved in biomedical research: microbiology, serology, hematology, blood banking, molecular biology, molecular pathology, clinical pathology, clinical biochemistry, immunology, immunohematology and immunobiochemistry, histopathology/pathology, and cytology. An issue raised in the United States as well as in India by modern biomedical research is biobanking: The 2006 guidelines addressed DNA and cell line banking, repository collections, research samples, primary use and secondary use, and general principles to be followed. They also enumerated responsibilities given to the IRBs and the IECs to oversee and guide researchers on practices to follow and not to follow. This has become a very important issue for more recent guidelines due to large numbers of existing samples. Efforts are being made to educate people about the significance of addressing stored samples. They are attempting to answer critical questions, such as what types of samples currently exist: coded samples, unknown samples, and samples that must be anonymized. Guidelines should provide details about the benefits of laboratory research with stored biological material, while also addressing concerns such as informed consent. These challenges are not unique to India, although they may be more acute in some countries due to a lack of awareness, even among scientists. Specificities exist in India regarding informing research participants and seeking consent after explaining potential risks and benefits due to the number of languages spoken and varying literacy levels. Several ethical questions remain,
_____________________
5 In international collaborations where the transfer of biological materials is involved, the Indian Ministry of Health’s Screening Committee provides oversight, though the ICMR is also involved.
including the type of consent required for the safe use of biological materials while maintaining privacy. When should waivers of consent be allowed and what are the necessary precautions to be taken?
There are many ways that ethics committees can function. It is common in India to assign compliance grades to institutions from zero to 100 percent, and many institutes receive outstanding assessments. Yet there are other institutions that still lack ethics committees despite many years of efforts. In still other institutes, committees exist on paper only. The primary focus currently is building the capacity of IRB members themselves. There is a tendency to perhaps villainize the IRB. Some members do not realize they are there to guide researchers in doing their work properly; they do not have a policing role. Given that they have considerable responsibility to oversee research across the country, it is essential that members understand their roles clearly.
Modern biology and biotechnology have novel ways of manipulating basic life, Muthuswamy noted; therefore, codes of conduct are needed, particularly with regard to dual-use research. Scientists engaged in such research activities should be aware of the potential associated risks of a broader range of applications (including hostile applications). They should not only be aware of, but also comply with, the requirements of international conventions and treaties relevant to their research work. The aim of codes of conduct for scientists is to ensure that all research activities involving microbial or other biological agents or toxins, whatever their origin or method of production, are only of the types and quantities justified by preventative research or other peaceful purposes. In order to prevent the use of scientific research for purposes of bioterrorism or biowarfare, all persons and institutions engaged in any aspect of scientific research should abide by their codes of conduct.
Responsibility rests with the institution to make the appropriate precautionary arrangements when allowing their laboratories to conduct certain research. To provide all necessary biosafety precautions, risk must be minimized and due care and caution must be taken. The institutions are responsible for having the appropriate committees to oversee the research. They are also involved in decisions pertaining to the publication of dual-use information and knowledge where there are reasonable grounds to believe that there are significant risks that the information and knowledge could be readily misused or inflict serious harm. Once established, the ethical principles upon which the guidelines should be based are transmitted to all who are, or may become, engaged in the conduct of biomedical research.
In 2011, the Association of Microbiologists was formed in India. On that occasion, a new paper on guidelines for microbiologists was published in the Indian Journal of Microbiology. This widely distributed paper was intended to ensure that all microbiologists follow the guidelines. However, even if all the members of the association are aware of the guidelines, we still do not know a great deal about the overall implementation of the guidelines.
Further, capacity building is needed for all those who are involved in this research. At this point, it appears that only those who are involved in GMO research are aware of biosafety and bioethics. Biosafety now applies not only to recombinant DNA research, but also to research on many infectious agents. Researchers working in a BSL-3 or BSL-4 institution may not be aware of the differences in these labs or what steps are to be followed when handling infectious agents. In 1997, the Medical Council of India issued a notification that all medical schools should make bioethics education part of their curriculum, but for various reasons this is still not universally mandatory except at a few institutions. Capacity building, bioethics education, and biosafety training must be part of the curriculum. International collaboration on bioethics and biosafety issues warrant focused attention.
Muthuswamy concluded by underscoring that guidelines need constant updating, new ones must be formulated, and legislation is needed to regulate these guidelines. Regulation is not the final answer, but it is one step forward in informing people about the challenges that these issues present. Unfortunately, even with all of these elements in place, unethical activities will occur. The majority of law abiding people, however, want to follow the rules, and it will be helpful to have laws that guide them as they conduct particular kinds of research.
Discussion
The discussion opened with a question about international collaboration and sample exchange. In many respects, sample exchange is difficult in India due to domestic regulations and the regulations of other countries. Muthuswamy commented that biological samples can be sent to and from India, following the appropriate guidelines, and consent must be obtained from both countries. The purpose of the exchange must be stated, and the appropriate ethics committee must also provide clearance. The approval is handled through the Ministry of Health’s Screening Committee which consists of all relevant government
departments. A subcommittee reviews all applications and provides recommendations for approval or denial of permission for the samples exchange.
T.S. Rao added that he has been involved with the Hatfield Marine Science Center (HMSC) and the development of the sample exchange guidelines of 1997. He has found that it is difficult to ensure that proper credit is given to the researchers in India, and that the rights of the individuals whose samples are transferred and used are protected. Another participant added that at times, if a researcher wants to do a particular test not available in India, it can be done in the other countries and then those samples can be sent back to India, and permissions are given for this to occur. However, all authors must be given proper credit. The Health Minister’s Screening Committee was also concerned that a lot of material would be leaving India without due credit being given. Despite this concern, sample sharing continues. There are some institutes, such as the Institution of Science, where directors have the authority to send some material without the Steering Committee’s approval. Sample exchanges by DST and DBT do not require approval. However, the institutions that actually send requests and submit applications for clearance are far fewer than the actual number of exchanges between Indian organizations and international organizations and institutions. The extent of such research becomes clear when papers are published, and it is unclear as to how samples were sent from India. Who gave permission for so many samples to be garnered for such studies? The majority of institutions do not seek approval from HMSC; there are only a few that are aware of the regulations and apply for approval. Those who do apply go through bureaucratic procedures that delay the process and they often become frustrated. There is so much being done in India, but it is small in comparison with the size of the potential.
Another participant raised the connection between the restrictions on sample exchange and biosecurity concerns. Under export controls, there is a SCOMET list that contains special chemicals, organisms, materials, equipment, and so forth. While DBT did clear some of these samples for exchange, industries send samples to their own facilities after gaining approval. It was under that system that there were some checks on exports.
Rao closed the discussion by reiterating the need for independent regulatory oversight of BSL-3 and BSL-4 labs. He also underscored his request for Indian-U.S. cooperation to develop regulatory guidelines
























