Summary
The Special Supplemental Nutrition Program for Women, Infants, and Children (WIC) was launched in 1974. Its goal was to provide supplemental foods that would supply nutrients lacking in the diets of low-income pregnant, breastfeeding, or postpartum women, infants, and children less than 5 years of age, who had at least one nutritional risk factor. The WIC program also provides nutrition education and referrals to health and social services. The U.S. Department of Agriculture’s Food and Nutrition Service (USDA-FNS) requested that the Institute of Medicine (IOM) undertake a review of the WIC food packages to align the program with dietary guidance in the 2015 Dietary Guidelines for Americans. In response, the IOM convened the Committee to Review WIC Food Packages (the committee) to address this task. This, the phase I interim report, is the second of three reports. The first report, Review of WIC Food Packages: An Evaluation of White Potatoes in the Cash Value Voucher: Letter Report, recommended allowing white potatoes for purchase with the cash value voucher. This second report presents the evidence, analyses, and framework that will be applied to develop the final report (phase II), which will include recommendations.
In the final report, recommendations for revisions to the WIC food packages will build on the revisions recommended in the 2006 IOM WIC report (implemented in 2009) and the evidence presented here, including an update and additional analyses. This interim (phase I) report contains an evidence-based review of relevant scientific literature, analyses of dietary intakes as well as food expenditure data and data on breastfeeding trends. The dietary intake evaluation included comparison of WIC participants
to WIC-eligible nonparticipants. A comparison of intakes before the 2009 food package changes to after these changes will be presented in the final report (phase II). The committee identified possible priority nutrients and food groups that could be used to address nutritional inadequacies (see Chapter 1 for the complete statement of task).
To design the phase I approach, the committee reviewed the key objectives of the WIC program and relevant changes to the WIC population, food packages, and dietary guidance and eating patterns among U.S. populations that occurred since the last IOM review of WIC food packages. Based on its preliminary review of evidence, the committee developed the approach to the task outlined below.
THE COMMITTEE’S APPROACH
The committee’s information-gathering activities included convening two workshops, conducting a comprehensive literature and report review, analyzing data, considering comments from the public and information obtained from committee member visits to WIC clinics and shopping with WIC vouchers. Data analyses were conducted with two national datasets. First, an independent evaluation of National Health and Nutrition Examination Survey (NHANES) data was conducted to examine intakes of nutrients and food groups of WIC participants and WIC-eligible nonparticipants (low-income and pregnant, breastfeeding, or postpartum women; infants; and children ages 1 to less than 5 years). Second, the Food Acquisition and Purchase Survey (FoodAPS) data were evaluated to determine the contribution of WIC foods to household food expenditures. Approaches to a sensitivity analysis and a regulatory impact analysis were developed, to be completed in phase II. The sensitivity analysis will evaluate the effect of major food package changes on nutrient and food group intakes and package cost. The regulatory impact analysis will assess the impact of WIC food package changes on program participation, the value of food packages, and program cost and administration. To serve as the baseline for the sensitivity and regulatory impact analyses evaluations, the committee developed an approach to generating baseline food package nutrient profiles and determining costs of the food packages.
Application of Current Dietary Guidance to the Task
The recommendations of the Scientific Report of the 2015 Dietary Guidelines Advisory Committee (2015 DGAC report), along with the Dietary Reference Intakes (DRIs), served as the basis for evaluation of nutrient and food intake adequacy in this report. The USDA’s Healthy U.S.
Food Pattern served as the basis for comparison of food group intakes by WIC participants and WIC-eligible nonparticipants. Other key recommendations in the 2015 DGAC report included identification of shortfall nutrients and nutrients of public health concern, and limits for sodium, solid fat, and added sugars intakes.
Nine shortfall nutrients were identified in the 2015 DGAC report (vitamin A, vitamin D, vitamin E, vitamin C, folate, calcium, magnesium, fiber, potassium, as well as iron for premenopausal females). Among these shortfall nutrients, calcium, vitamin D, fiber, and potassium were classified as nutrients of public health concern because their under-consumption has been linked to adverse health outcomes. Iron was a shortfall nutrient of public health concern for adolescent females and premenopausal adult females. A specific limit for cholesterol intake was not indicated, and the recommended sodium intake limit for the general population was set at 2,300 mg per day.
The Dietary Guidelines for Americans and 2015 DGAC report apply only to individuals ages 2 years and older. Therefore, the committee compiled current published dietary guidance for individuals younger than 2 years of age issued by the American Academy of Pediatrics and other authoritative groups to evaluate adequacy of the diets of WIC participants of these ages.
Analyses of NHANES
Analyses to determine estimated nutrient and food group intakes used relevant NHANES data. Subgroups of interest include WIC participants as well as low-income, potentially eligible (pregnant, postpartum, or breastfeeding women; infants; and children less than 5 years of age) WIC nonparticipants. At the time of this report, the indicator to identify WIC participants was not available for the most recent NHANES release, 2011–2012. Therefore, a comparison of nutrient or food intakes among WIC participants before the 2009 food package changes to those after the changes could not be conducted. Moreover, although the 2009–2010 NHANES data allowed comparison of WIC participants to WIC-eligible nonparticipants, this period covered the change in food packages and was not considered appropriate for the evaluation of pre- or post-food package change intakes. All low-income WIC-eligible individuals in the NHANES 2011–2012 dataset were analyzed as a proxy for WIC participants. In phase II, the WIC indicator will be applied to the NHANES 2011–2012 dataset if the sample sizes are sufficient. Finally, the committee developed a nutrient-based diet quality index for evaluation of the overall nutrient adequacy and applied the Healthy Eating Index-2010 (HEI-2010) for evaluation of food group intakes.
KEY CONCLUSIONS
Preliminary Nutrient and Food Group Priorities
The committee’s reviews of the scientific literature, analyses described in Chapters 4 and 5, as well as nutrition-related health risks reviewed in Chapter 6, led to the identification of potential target nutrients and food groups for WIC participants of specific ages. These findings are organized in the tables that follow, by age group. Indicated in the tables with a “![]() ” are: (1) nutrients for which inadequacy is apparent in more than 5 percent of the indicated age subgroup, or nutrients that are prioritized based on other information (see Table S-1a), (2) nutrients for which mean usual intakes fall below the adequate intake (AI) value (see Table S-1b), (3) nutrients for which more than 5 percent of the population exceeds the Tolerable Upper Intake Level (UL) (see Table S-2), and (4) food groups for which intakes of at least 50 percent of the population fall below or above recommendations (see Table S-3).
” are: (1) nutrients for which inadequacy is apparent in more than 5 percent of the indicated age subgroup, or nutrients that are prioritized based on other information (see Table S-1a), (2) nutrients for which mean usual intakes fall below the adequate intake (AI) value (see Table S-1b), (3) nutrients for which more than 5 percent of the population exceeds the Tolerable Upper Intake Level (UL) (see Table S-2), and (4) food groups for which intakes of at least 50 percent of the population fall below or above recommendations (see Table S-3).
Conclusions Based on Phase I Findings
In addition to the nutrients and food groups identified above, the committee’s approach to information gathering led to the key findings contained in Chapter 11. Here, the committee presents the overall conclusions, based on the phase I review and resulting findings. The findings, conclusions, and supporting evidence will be used in conjunction with additional planned analyses to develop the committee’s recommendations in phase II.
- Participation in WIC has declined recently. The reasons for this are likely multifaceted and cannot be attributed to the initial rollout of the food package changes. Paper vouchers are being replaced by electronic benefits transfers (EBTs), which may improve program participation as well as redemption of issued benefits.
- There are some racial and ethnic differences in satisfaction with specific items in the food packages, but, aside from the limited availability of Kosher and Halal food options, the packages appear to be broadly culturally suitable.
- Both women and children (ages 2 to less than 5 years) WIC participants had low or inadequate intakes of several nutrients that could potentially be addressed with food package changes (see Tables S-1a and S-1b). These inadequacies may be linked to food intakes that fell below recommendations for specific food groups (see Table S-3).
TABLE S-1a Nutrients with Evidence of Inadequate Intakea in the Diets of WIC Participant Subgroups
| Nutrient | Pregnant, BF, or PP Women, 19 to 50 Years | FF Infants 6 to Less Than 12 Months | Breastfed Infants 6 to Less Than 12 Months | Children 1 to Less Than 2 Years | Children 2 to Less Than 5 Years |
|---|---|---|---|---|---|
| Calcium | |||||
| Copper | |||||
| Iron | |||||
| Magnesium | |||||
| Zinc | |||||
| Vitamin A | |||||
| Vitamin Dc | |||||
| Vitamin E | |||||
| Vitamin C | |||||
| Thiamin | |||||
| Riboflavin | |||||
| Niacin | |||||
| Vitamin B6 | |||||
| Folate | |||||
| Protein | |||||
NOTES: BF = breastfeeding; FF = formula fed; PP = postpartum. Table is based on results for WIC participating individuals in NHANES 2005–2008. The committee found no evidence of inadequate intake in the diets of formula-fed infants 0 to 6 months of age.
a Nutrients listed represent those for which 5 percent or more of each population subgroup had intakes below the Estimated Average Requirement (EAR), unless otherwise noted.
b Based on the committee’s literature review findings of a high risk of low iron intakes in breastfeeding infants.
c Based on serum 25(OH)D below 40 nmol/L. Serum levels were not available for infants.
d More than 5 percent of this subgroup had intakes below the Acceptable Macronutrient Distribution Range (AMDR).
SOURCES: As indicated in Table 11-1a of this report. See Chapter 3 for details on determination of nutrient adequacy.
- Women, infants, and children had excessive intakes of several nutrients (see Table S-2). In some cases, these excessive intakes may be addressed with changes to the food packages; in other cases, they may be addressed with nutrition education.
- Inasmuch as the sample size of low-income women in the 2011–2012 analysis was small, it was not possible to estimate the proportion of the population with food group intakes that were inadequate or excessive compared to recommended intakes. Small sample sizes for some of the population subgroups are likely to limit further disaggregation into WIC participants and WIC-eligible nonparticipating individuals. Therefore, in phase II, mean intakes can be compared among groups and to recommendations, but a population-level comparison to recommended intakes for women before and after the 2009 food package changes is unlikely to be possible.
- The committee notes that the NHANES 2005–2008 nutrient and food intake data do not capture the impact of the 2009 food package changes. Results from these survey years are therefore not suitable to serve as the sole basis for final determination of nutrient and food group priorities in phase I. The nutrient and food group gaps identified in this report will be re-evaluated in phase II as the NHANES 2011–2012 “WIC” identifier is incorporated into the analysis.
- Breastfeeding promotion and support appear to play a role in the improvement of breastfeeding initiation, duration, and exclusivity among WIC participants. The 2009 changes to the food package to improve support for breastfeeding women were associated with
TABLE S-1b Nutrients for Which Mean Usual Intake Falls Below the Adequate Intake (AI) in the Diets of WIC Participant Subgroups*
| Nutrient | P, BF, or PP Women, 19 to 50 Years | FF Infants 0 to 6 Months | Children 1 to Less Than 2 Years | Children 2 to Less Than 5 Years |
|---|---|---|---|---|
| Potassium | ||||
| Choline | ||||
| Fiber | ||||
NOTES: BF = breastfeeding; FF = formula fed; P = pregnant; PP = postpartum. Table is based on results for WIC participating individuals in NHANES 2005–2008. Mean intakes of infants 6 to less than 12 months of age fell above the AI.
* Because breastmilk intakes were not quantified, nutrient intake of breastfeeding infants in NHANES were not analyzed.
SOURCES: As indicated in Table 11-1b of this report. See Chapter 3 for details on determination of nutrient adequacy.
-
only limited positive changes in breastfeeding behavior. There may be additional possibilities for aligning the food packages with support for breastfeeding women.
- The current WIC food packages provide adequate options for participants with most major food allergies, celiac disease, and food intolerances, but inclusion of substitutions for eggs and fish may be warranted.
- Vendors and manufacturers were able to adapt to the 2009 food package changes with some challenges. It is important to consider the feasibility of potential future food package changes from the perspectives of vendors and food manufacturers.
The committee’s phase II activities will include an update to the comprehensive scientific literature review that was conducted for this interim report, an evaluation of nationwide costs and distribution of foods to ensure that the recommended new food packages are efficient for nationwide distribution, and sensitivity and regulatory impact analyses. The committee will conduct a sensitivity analysis that will consider the effect of major recommended alternative food items and changes in quantity relevant to priority nutrient intakes, intakes of food groups and subgroups, and cost. Then the committee will conduct a regulatory impact analysis that
TABLE S-2 Micronutrients with Evidence of Intakes Exceeding the Tolerable Upper Intake Level (UL)* in the Diets of WIC Participant Subgroups
| Nutrient | P, BF, or PP Women, 19 to 50 Years | FF Infants 6 to Less Than 12 Months | Children 1 to Less Than 2 Years | Children 2 to Less Than 5 Years |
|---|---|---|---|---|
| Copper | ||||
| Iron | ||||
| Selenium | ||||
| Sodium | ||||
NOTES: BF = breastfeeding; FF = formula fed; P = pregnant; PP = postpartum. Table is based on results for WIC participating individuals in NHANES 2005–2008. Only nutrients with intakes above recommended levels in more than 5 percent of the population for at least one population subgroup are presented. The committee’s literature review found no evidence of excess nutrient intake for breastfeeding infants or formula-fed infants 0 to 6 months of age.
* Nutrients represent those for which 5 percent or more of the population subgroup exceeded the UL.
SOURCES: As indicated in Table 11-2 of this report. See Chapter 3 for details on determination of excessive intake.
TABLE S-3 Food Groups with Evidence of Intakes Below and Above Amounts Recommended in the DGAC 2015 Report in the Diets of WIC Participant Subgroups
| Food Group | P, BF, or PP Women, 19 to 50 Yearsa | Children 2 to Less Than 5 Yearsb |
|---|---|---|
| Intakes Below Recommended Amounts | ||
| Total fruit | ||
| Total vegetables | ||
|
Dark green |
||
|
Total red and orange |
||
|
Beans and peas |
||
| Total starchy | ||
| Other vegetables | ||
| Total grains | ||
|
Whole grains |
||
| Total protein foods | ||
| Seafood | ||
|
Nuts, seeds, and soy |
||
|
Total dairy |
||
| Oils | ||
| Intakes Above Recommended Amountsd | ||
| Solid fat | ||
| Added sugars | ||
NOTES: BF = breastfeeding; DGAC 2015 = Scientific Report of the 2015 Dietary Guidelines Advisory Committee; P = pregnant; PP = postpartum. Food groups and subgroups listed are those for which 50% or more of the population subgroup had intakes falling below levels recommended in the 2015 DGAC report, or in the case of food groups to limit, above levels recommended in the 20155 DGAC report. The table is based on results for WIC participating women and children in NHANES 2005–2008. The USDA food patterns do not apply to infants and children less than 2 years of age; thus, these age groups were omitted from the table. The committee’s literature review found no evidence to support that specific food group intakes are low among breastfeeding infants, although low intake of iron-containing foods may be of concern.
a Based on the 2015 DGAC report food pattern for a 2,200 kcal diet, which was the EER calculated for women in this report.
b Recommended intakes were generated by weighting the 1,000 and 1,300 (averaged from 1,200 and 1,400 kcal patterns) kcal food patterns in a 1:3 ratio. This results in a food pattern equivalent to approximately 1,225 kcals, slightly under the EER calculated for children 2 to 5 years of age of approximately 1,300 kcals; therefore, intakes for this age group in comparison to recommendations may be slightly overestimated.
c Too few individuals in NHANES 2005–2008 for this age group reported consumption to produce population-level estimates of intake, suggesting that intakes may be low.
d Indicates usual mean intake levels above the upper limit defined by the 2015 DGAC report food pattern comparisons for each age group.
SOURCES: As indicated in Table 11-3 of this report. See Chapter 3 for details on methods applied.
will assess the impact of proposed WIC food package changes on program participation, the value of the food packages, and program cost and administration. Additional details of the approaches to be used for the different activities are discussed in Chapter 3.
GUIDING PRINCIPLES AND FRAMEWORK FOR REVISION OF THE WIC FOOD PACKAGES
The criteria that the committee established to underpin the phase II analyses and evaluation and to guide development of its recommendations are presented in Box S-1 and incorporated into Figure S-1. The final criteria were only slightly modified from those applied by the IOM (2006) Committee to Review WIC Food Packages because, after a thorough review of the evidence, the committee concluded that these criteria were comprehensive and remained relevant. These criteria reflect the committee’s priorities to first, meet the goals of the WIC program; second, respond to the requirement that the WIC food packages be aligned with the 2015 DGA; and third, provide a package that is acceptable to participants and feasible to implement at every level.
The criteria outlined above will be further explored (and possibly revised) in phase II after an update of the phase I review as well as consideration of the results of the analysis of nutrient and food consumption by WIC participants in NHANES 2011–2012 and limitations related to cost. The committee’s proposed process for revising the WIC food packages in phase II is illustrated in Figure S-1. The objective is to ensure that the revisions fall within the criteria outlined in the previous section, with attention to cost constraints. First, the current food packages will be evaluated for
the nutrients and food groups provided and alignment with dietary guidance, as well as the challenges faced during implementation. After reviewing this information, the committee will identify priority changes in the food packages and test possible changes in an iterative fashion to align with the criteria and ensure overall program cost neutrality (the sensitivity analysis). During this process, the criteria or framework may be modified if deemed necessary. The committee anticipates that this process will involve trade-offs, with final recommendations guided by the criteria and cost constraints. Once the iterations result in changes that meet the final criteria, recommendations will be finalized. A regulatory impact analysis will then be conducted to assess the impact of changes in WIC food packages on program participation, the value of the food packages as selected,1 and program costs and administration.
__________________
1 The value that individuals place on the change resulting from a particular regulatory alternative.
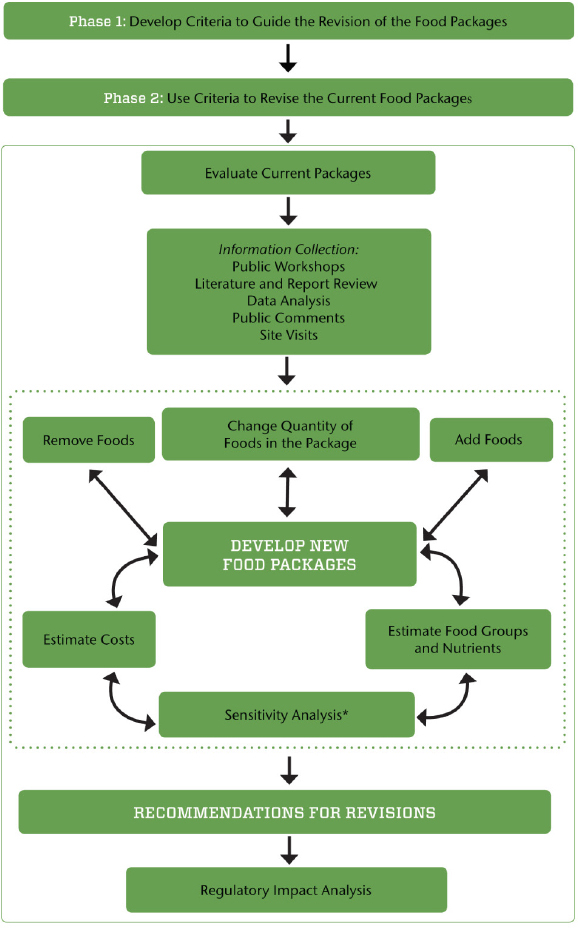
NOTE: The dotted line indicates components of the process that iterate until the criteria for food package revisions are met (see Box S-1).
* The sensitivity analysis includes considerations for maintaining the cost neutrality of the overall WIC food packages.












