WORKSHOP SUMMARY
In recent years, the field of oncology has witnessed a number of technological advances, including more precise radiation therapy and minimally invasive surgical techniques. Three-dimensional (3D), stereotactic, and proton beam radiation therapy, as well as laparoscopy and robotic surgery, can enhance clinicians’ ability to treat conditions that were clinically challenging with conventional technologies, and may improve clinical outcomes or reduce treatment-related problems for some patients. Both patients and physicians seek access to these new technologies, which are rapidly being adopted into standard clinical practice. Such demand is often propelled by marketing that portrays the new technologies as the “latest and greatest” treatments available. However, evidence is often lacking to support these claims, and these novel technologies usually come with higher price tags and are often used to treat patients who might have achieved similar benefits from less expensive, conventional treatment.
__________________
1 The planning committee’s role was limited to planning the workshop. The workshop summary has been prepared by the rapporteurs as a factual account of what occurred at the workshop. Statements, recommendations, and opinions expressed are those of individual presenters and participants and are not necessarily endorsed or verified by the Institute of Medicine. They should not be construed as reflecting any group consensus.
The increased cost of novel treatments without adequate assessment of how they affect patient outcomes is a pressing concern given that inappropriate use of expensive technologies is one of the key factors that threaten the affordability of cancer care in the United States (IOM, 2013; Shih et al., 2013). To explore these issues further, the National Cancer Policy Forum (NCPF) of the National Academies of Sciences, Engineering, and Medicine brought together experts and members of the public for the workshop “Appropriate Use of Advanced Technologies for Radiation Therapy and Surgery in Oncology” on July 20 and 21, 2015, in Washington, DC. This is the third NCPF workshop in a series examining the affordability of cancer care. The first entailed a broad overview of the many components of cancer care, while the second focused on the affordability of drug therapies for cancer (IOM, 2013, 2014). At this workshop, clinicians, researchers, and patients along with representatives from industry, the Food and Drug Administration (FDA), the National Cancer Institute (NCI), and the Centers for Medicare & Medicaid Services (CMS) discussed topics related to radiation therapy and surgery for cancer, including
- clinical benefits and comparative effectiveness of emerging advanced technologies for cancer treatment in radiation therapy and surgery, as well as research gaps that are challenging to close;
- factors driving the diffusion of new technologies into oncology practice;
- oversight, training, credentialing, and reimbursement for use of innovative technologies;
- evidence on the overuse, underuse, or misuse of novel technologies; and
- potential strategies to assess the value and promote optimal use of new technologies in cancer treatment.
This report is a summary of the presentations and discussions at the workshop. A broad range of views and ideas were presented and a summary of suggestions for potential solutions from individual participants is provided in Box 1. The workshop Statement of Task can be found in Appendix A and the workshop agenda can be found in Appendix B. The speakers’ presentations (as PDF and audio files) have been archived at https://iom.nationalacademies.org/Activities/Disease/NCPF/2015-JUL-20.aspx.
BOX 1
Suggestions Made by Individual Workshop Participants
Regulatory Oversight
- Assign regulatory authority for oversight of complex medical procedures. (Kessler)
- Require more precision in intended use for medical devices. (Kessler)
- Strengthen the oversight of medical devices with postmarketing studies. (Kessler)
- Eliminate self-referral exemptions for complex in-office ancillary services under the federal Stark Law. (Beyer, Mohler, Williams)
Training and Monitoring Performance
- Establish more rigorous standards for training and credentialing radiation oncologists and surgeons. (Ashley, Hu, Vikram)
- Validate new tools to assess surgical proficiency. (Ashley, Miller) • Require surgical credentialing and regular testing in simulators. (Ashley, Mohler)
- Collect risk-adjusted, physician-specific outcomes data. (Ashley, Miller)
Generating Evidence to Assess Medical Technologies
- Establish a systematic and well-defined framework to generate evidence for assessing new technologies. (Tunis)
- Make data collection and integration a priority. (Whelan)
- Create a data platform to collect, connect, and manage data from multiple systems that are currently siloed. (Smith)
- Work with industry to ensure that critical data needed to assess technologies are collected in electronic medical records. (Beyer)
- Reduce reliance on randomized controlled trials as the gold standard for evidence development to assess new technologies and use novel study methods to assess comparative effectiveness. (Beyer, Dignam, Kessler, Mohler, Steinberg, Tunis)
- Use a structured, well-defined, stepwise process to sequentially conduct more rigorous studies as evidence accumulates. (Kessler)
- Mandate provider participation in registries such as those established by the American College of Surgeons. (Hoey)
- Compare quality-adjusted life years and other clinically meaningful health outcomes that patients identify with and value
-
when conducting comparative effectiveness research. (Dignam, Lawrence, Steinberg, Weichselbaum)
- Include cost-effectiveness assessments as secondary objectives in clinical trials. (Efstathiou)
- Devote more federal funding for technology development. (Yu)
- Use financial incentives (including restrictions on use) to spur trials of devices. (Zietman)
Payment Strategies for Optimal Use of Medical Technologies
- Establish transparent and consistent payment systems that recognize and support the need for evidence development to assess long-term benefits and value. (Steinberg, Yu, Zietman)
- Use coverage with evidence development more frequently to collect long-term data on new medical devices and procedures. (Hahn, Kessler, Steinberg, Tunis, Yu)
- Encourage collaboration between radiation oncologists and payer groups to determine optimal use of new technologies. (Efstathiou)
- Use value-based payment models to incentivize the triple aim of improved patient experience, improved health for populations, and lower per capita cost. (Bekelman, Steinberg)
Utilization and Clinical Guidance
- Establish high-volume specialty centers in which physicians have expertise in a new technology. (Ashley, Hu, Weichselbaum)
- Encourage medical professional societies to review the information available on new technologies and provide a stamp of approval once they think the evidence is sufficient to support clinical uses. (Beyer, Zietman)
- Use treatment pathways to foster value-based care. (Steinberg)
- Evaluate the value of treatment options as evidence accumulates on effectiveness. (Bekelman)
- Use price transparency and engage patients in care decisions to foster appropriate adoption and de-adoption of technologies. (Bekelman, Darien, Farrington, Jagsi, Smith, Steinberg)
The workshop began with overviews of several technologies that have recently become available as cancer treatment options, including radio-therapies designed to more precisely target tumors, and laparoscopic and robotic surgical procedures that are less invasive than standard surgeries.
Technology Advances in Radiation Therapy for Cancer
About half of cancer patients receive radiotherapy directed at tumors or the tumor bed, with the goal of shrinking or eliminating the tumors or preventing local recurrence, said Carol Hahn, associate professor of radiation oncology at the Duke University Medical Center. In conventional radiation therapy, a limited number of X-ray beams are delivered to the tumor region. The planning of the beams is performed in just two dimensions rather than three.
The advent of sophisticated computer software and 3D imaging enabled 3D conformal radiotherapy; radiation beams are shaped and then combined to more precisely fit the profile of the targeted tumor. A more evolved form of 3D conformal radiotherapy is intensity-modulated radiotherapy (IMRT). With IMRT, the radiation delivered is made to more tightly conform with the 3D shape of the tumor by controlling or modulating the radiation beam’s intensity. Radiation dose intensity is greatest for the gross tumor volume, while radiation to neighboring normal tissue can be decreased or, by careful planning, avoided completely. In this manner, for example, physicians can focus radiation on a pancreatic tumor while sparing the nearby kidneys and spinal cord from radiation damage. With head and neck cancer, IMRT reduces the radiation dose to the parotid gland and structures essential for swallowing, while in the brain it reduces the dose to the optic nerve and pituitary structures. In prostate cancer, IMRT reduces the dose of radiation to the rectum, while in gynecological cancers, it reduces the dose to the small bowel and bone marrow, said Steve Chmura, Associate Professor of Radiation and Cellular Oncology at the University of Chicago. With real-time imaging and adjustments, IMRT can even be used to deliver radiation to a moving target, such as a lung tumor that moves with breathing, he said. Companies started offering IMRT-capable machines and software by 2000; by 2002 Medicare “gave it the stamp of approval” by establishing a reimbursement code for it, said James Yu, assistant professor of therapeutic radiology at Yale University. A number of treatment machines with this capability have FDA clearance.
The ability to use IMRT to deliver high doses of radiation while sparing normal tissues has enabled physicians to target tumors that were previously inaccessible with surgery or conventional radiation therapy, Chmura noted. IMRT can also be used in place of multiple surgeries for metastatic disease. “We can use this technology to treat a group of patients who we couldn’t really treat before,” Chmura said.
Another new evolution in radiation therapy is stereotactic body radiation therapy (SBRT). Here a large number of tiny beams coming from multiple directions combine to form a highly conformal treatment for small tumors. Radiation oncologists are putting SBRT to new uses and, in particular, it is finding a role in the ablation of metastases. Chmura noted that metastatic breast cancer sometimes presents as a limited number of tumors in just a few organs in the body (oligometastatic). Instead of surgically removing these tumors or treating them solely with chemotherapy, there has been increasing use of stereotactic radiation therapy to treat them (Lewis et al., 2015). “Before 1995 almost nobody even considered using this technology to treat metastases. Now this is almost universally done in the absence of any good level 1 evidence,” Chmura stressed. A large randomized clinical trial (NRG-BR002) is currently assessing the effectiveness of using radiation therapy combined with drug therapy for metastatic cancer versus drug therapy alone. “If ablative radiation therapy improves overall survival, it should lead to a true paradigm shift in the multidisciplinary treatment of these women who have limited metastatic disease. I would also hope that if it does not show an advantage in terms of overall survival, then off-protocol use of such radiation therapy, which is now so widespread, will stop,” Chmura said.
Another recent development in 3D conformal radiation therapy is the use of proton beams instead of X-rays. Traditional X-rays weaken in intensity as they pass through the body; as a result, more radiation is deposited in the normal tissue above the tumor than in the tumor. Radiation is also deposited beyond the tumor site where the beam exits the body, explained Anthony Zietman, Shipley Professor of Radiation Oncology at Massachusetts General Hospital. To counteract the loss of beam intensity with depth into the body, higher doses of radiation are used in conventional radiation therapy. In contrast, proton beams can be accelerated into the body so that little radiation is deposited until the protons start to slow down, at which point all of the radiation is deposited, with little residual radiation occurring beyond the tumor. The FDA approved proton therapy to treat cancer in the 1980s, Zietman reported, and there currently are more
than 40 proton-beam centers operating worldwide. Fifteen are located in the United States, with an additional 10 more in planning stages.2
Because less radiation is administered with proton beam radiation therapy (PBRT) and there is no exit dose of radiation once the beam leaves the tumor, this technology should enable the delivery of higher radiation doses that in theory should lead to better tumor response, while reducing the late effects of radiation compared to conventional radiation therapy, according to Zietman. He emphasized that children are uniquely sensitive to radiation, which can adversely affect their growth, development, and intellectual capacity, as well as put them at high risk of developing subsequent radiation-induced cancers. For example, children whose bone cancers are successfully treated with radiation therapy have a 20 percent risk of developing a subsequent radiation-induced cancer in their lifetime, and that secondary cancer is likely to be fatal, Zietman stressed.
“Anything we can do to reduce the amount of radiation delivered to normal tissues of children is a good thing,” he said. “There is pretty much unanimity among the radiation oncology community globally that it is appropriate to treat children with proton beam therapy,” he added. Whether PBRT improves outcomes in children has not been assessed with randomized controlled trials (RCTs) because of the unwillingness to enroll children in a control group with standard radiation therapy. However, modeling studies suggest that the number of secondary tumors developing in children treated with PBRT is at least 50 percent less than with conventional radiation therapy, Zietman noted (Miralbell et al., 2002).
In adults, PBRT is used to treat complex tumors of the skull, eye, or spine, not because there is evidence that this treatment has acceptable outcomes for these indications, but because there are no feasible or reasonable surgical or conventional radiation therapy alternatives for such tumors, according to Zietman.
To expand their patient base and make the technology more financially sustainable, many PBRT centers are also experimenting with using the treatment in other cancers and assessing whether it improves outcomes, he added. Due to its precision and lower radiation doses, PBRT is being used to treat cancers that may not be amenable to conventional radiation therapy because of concerns of damaging nearby tissues. For example, PBRT has been used to treat left-sided breast cancer (which is located closer to the heart), and pancreatic, peritoneal, paranasal sinus, lung, and liver tumors.
__________________
2 See http://www.proton-therapy.org (accessed October 20, 2015).
“Protons can be used to very sharply treat in situations where we previously could not treat at all,” Zietman said.
However, most adults treated with PBRT in the United States have prostate cancer. The treatment is thought to be less likely to cause the incontinence, impotence, and other serious side effects seen with conventional therapies for this type of cancer. “The surgical and old radiation options have a bad reputation, so men began to seek what appeared to be a very attractive alternative,” Zietman said. Because of PSA (prostate-specific antigen) testing that can identify low-risk prostate cancers that previously were not detected, “there is this huge uptick in men seeking proton beam radiation for their prostate cancer treatment ahead of the evidence,” he said. Although an RCT he conducted found PBRT very effective for prostate cancer (Zietman et al., 2010), evidence is limited on whether it actually improves outcomes compared to other forms of radiation therapy, which is discussed further in the section on “Evaluation of Comparative Effectiveness.”
Zietman added that PBRT is not a static technology, but rather is continuously evolving. “It is not fixed in time, but instead there are many technical and biological advances that are being progressively brought into proton beam therapy,” Zietman stressed.
Technology Advances in Surgery for Cancer
Cancer surgery techniques have changed dramatically over the past 25 years. For example, prior to the 1990s, the only way to access tumors in the abdomen was to make a sizable incision through the skin, muscles, and other tissue in the abdominal wall, causing significant trauma and wound repair, and disruption of gastrointestinal functioning, said Richard Whelan, chief of surgical oncology at the Mount Sinai Health System. In the early 1990s, surgeons began using video laparoscopy, which enabled access to and removal of an abdominal tumor via a few small incisions in the belly through which a lighted tube, camera, and surgical tools are inserted. By causing less abdominal wall trauma and injury, such laparoscopic surgery reduces pain, the length of hospital stays, and the likelihood of infection. It enables more rapid recovery, quicker resumption of bowel function, and earlier return to work, he said. Minimally invasive surgery also causes less scarring and fewer troublesome adhesions between the abdominal wall and the viscera. This should result in fewer bowel obstructions and incisional hernias, according to Whelan, as well as less expense.
But the potential benefits of laparoscopic surgery were not assessed in studies before the technique was widely adopted in clinical practice after a few published case reports. As Whelan noted, “No decision was made by any entity as to when this would be rolled out. Data and scientific evaluation occurred after it was initially adopted.” Gall bladder removal was the first common operation that surgeons started doing laparoscopically. Results were generally favorable, although initially there were higher rates of common bile duct injuries in the laparoscopic cases compared to open surgery cases. But there continued to be a big push by patients and physicians to conduct the procedure laparoscopically. “Market pressures are huge in terms of driving these new treatments and approaches,” Whelan noted. “The laparoscopic methods became the gold standard overnight and there was no way to get the horse back in the barn once this method had been unveiled.”
Colorectal surgeons were more reluctant to use laparoscopic surgery to remove colon tumors because the procedure is more complicated than for a gall bladder removal. “Technically it is very difficult to operate on large organs in small spaces using the equivalent of chopsticks,” said David Miller, assistant professor of urology at the University of Michigan School of Medicine. There also were concerns that laparoscopic surgery might increase the likelihood of tumor spread because of anecdotal reports of tumors forming at incision sites. This also occurs after open abdominal surgery, but less frequently. Because of those concerns, Whelan and other early adopters conducted RCTs that compared laparoscopic removal of the colon cancers with open incision surgery to remove the cancerous tissue. “This was ultra-unique: Randomized trial data and basic science findings preceded large-scale adoption,” Whelan noted. “This has yet to be repeated in the surgical world because surgeons tend not to ask the scientific questions.”
Whelan reported that the trials did detect short-term differences in immune response, with open surgery suppressing immune function more than the laparoscopic colon surgery. In addition, the rate of wound complications was significantly lower for the laparoscopically treated patients than those who had open surgery. Several studies found no difference in the 5-year disease-free survival and overall survival in patients who had laparoscopic surgery versus open surgery, relieving suspicions that the technique could worsen cancer spread (Fleshman et al., 2007; Nelson et al., 2004).
The latest technical advance in laparoscopic surgery is the addition of a computer console that surgeons can use to manipulate robot arms attached with surgical tools to perform surgery (see Figure 1). The robotic wrists can be moved in multiple ways that the surgeon’s own wrists cannot, enabling
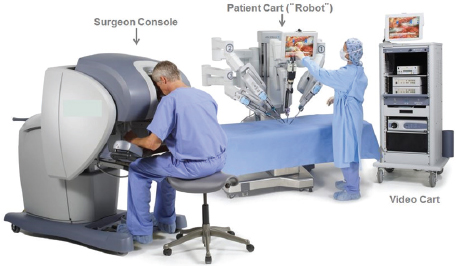
FIGURE 1 Robotic surgery platform.
SOURCE: Wright presentation, July 20, 2015. © Intuitive Surgical, Inc. 2015.
greater mobility and more precision of movement. The 3D display on the console, with the aid of 3D glasses, also gives the surgeon a better view of the surgical field. “It is fundamentally different in terms of the depth of vision you see with the tissue and the clarity of the structures, which I personally believe makes a difference in my ability to do an operation,” Miller said. For surgeons inexperienced in laparoscopic surgery, robotic laparoscopic surgery is easier to learn, according to Whelan. But robotic operations take longer.
The FDA has cleared robotic surgical systems for use in a wide range of procedures. Currently about 85 percent of prostatectomies (removal of the prostate) in the United States are done with robotic laparoscopies, Whelan said. They are also used to surgically treat rectal cancers, with the potential advantage that it may be easier to preserve rectal sphincter functioning. However, studies have not yet demonstrated that the robotic procedure is superior to laparoscopic surgery, according to Whelan, and for gynecology surgeries, the complication rate is higher for robotic operations. “Unless the clinical results show robotic surgery to be superior in a meaningful way, it is going to be hard to justify the outlay of the high purchase price of the robot and the growth of this. However, centers that have these robots feel they have to use them and there is a push on the institutional level for the use of these robots,” Whelan said.
Some evidence indicates that robotic surgery is enabling certain procedures that normally would not be undertaken, Miller reported. He noted that a minimally invasive removal of the portion of a kidney that has a tumor is difficult to do, so instead most patients have the entire kidney removed. But one study found that after hospitals acquired robots, surgeons there did about 35 percent more partial nephrectomies (Sivarajan et al., 2015). Hu stressed that the ability to avoid complete removal of the kidney in these patients should help to avert the chronic renal insufficiency and associated complications that patients are likely to experience in the long term when their entire kidney is removed.
Robotic surgery is also now commonly used in gynecologic surgery, said Jason Wright, division chief of gynecologic oncology at Columbia University College of Physicians and Surgeons. One in nine women will have a hysterectomy during her lifetime, mostly due to precancerous changes, with about 10 percent of hysterectomies performed for malignant indications, he noted (Jacoby et al., 2009). Hysterectomies can be done through vertical or horizontal abdominal incisions, laparoscopically, robotically, or vaginally. Vaginal removal is the most advantageous because it does not require any abdominal incisions, but it is mostly used for pelvic organ prolapse and not for cancer surgeries, according to Wright.
Laparoscopic hysterectomies have been in practice for about 25 years. They are now routinely taught to residents in obstetrics/gynecology, and are widely done by community physicians, Wright reported. More recently, surgeons have begun using robotic surgery for hysterectomies. This requires more and larger incisions than laparoscopic hysterectomies (see Figure 2). In addition to improving the 3D visualization of the surgical field and increased range of motion of the instrumentation, robotic surgery also offers enhanced surgeon ergonomics, which is a significant advantage because laparoscopic hysterectomies often take about 4 hours to perform, Wright said. He added that a primary advantage for robotics in gynecologic surgery is that they enable surgeons to do more technically challenging cases with a minimally invasive approach. Robotics started to diffuse into gynecologic cancer care around 2009, he said, although there are minimal data on robotic surgery outcomes.
EVALUATION OF COMPARATIVE EFFECTIVENESS
New technologies are often much more expensive than those already used in clinical practice. Given the rising costs of cancer care, many speakers
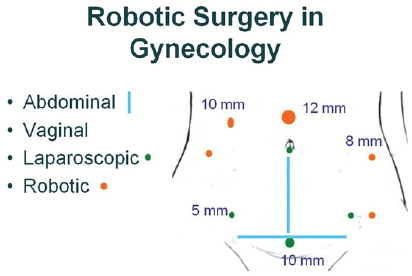
FIGURE 2 Surgical options in gynecology. Robotic surgery requires more and larger incisions (orange dots) compared to laparoscopic surgery (green) dots. Blue lines indicate incisions used in traditional abdominal surgery. No incisions are made in the abdomen for vaginal surgery.
SOURCE: Wright presentation, July 20, 2015.
expressed concern about broad use of new technologies unless they offer more benefits and/or lower risks than standard technologies or procedures, meaning there is evidence of their comparative effectiveness. The bulk of the evidence regarding the comparative effectiveness of new radiation therapies and surgical technologies is derived from observational studies, as opposed to the gold standard of RCTs. Many findings are mixed or insufficient to justify the widespread use these innovative therapies have already had in the clinic, several speakers reported.
Chmura said children are especially vulnerable to the adverse effects of radiation on the brain, which can affect their growth and development and hearing, but studies suggest that the lower doses used in IMRT can reduce hearing loss or loss of growth hormone (Huang et al., 2002; Zhu and Merchant, 2003).
Chmura also reported on several studies that found IMRT caused less radiation damage to normal tissue than conventional two-dimentional (2D) or 3D radiation therapy when used to treat gynecologic cancers, breast
cancers, and head and neck cancers in adults (Brixey et al., 2002; Merchant et al., 2006; Mundt et al., 2002; Nutting et al., 2011; Pignol et al., 2008). Jason Efstathiou, director of the Genitourinary Division in the Department of Radiation Oncology at Massachusetts General Hospital, also cited a British study that found that compared to conventional 2D radiation therapy, 3D conformal radiation therapy significantly reduced the incidence of proctitis in prostate cancer patients (Dearnaley et al., 1999).
However, Bhadrasain Vikram, chief of the Clinical Radiation Oncology Branch at the NCI, noted a British study that found that although IMRT given to patients with head and neck cancer reduced the side effect of dry mouth, patients who had IMRT had worse tumor control. “The question is not whether IMRT or protons can reduce toxicity, but whether they reduce toxicity without compromising tumor control. The second part of the question simply hasn’t been addressed in any meaningful fashion and there is a possibility that this technology that is widely used in the community now and costs more may provide worse tumor control,” he stressed.
Although studies have shown that PBRT reduces the amount of radiation that is deposited in healthy tissues compared to IMRT (Efstathiou et al., 2009; Trofimov et al., 2007) (see Figure 3), studies have yet to show definitively that this is clinically meaningful, according to Efstathiou. He noted that retrospective studies done to date have offered conflicting evidence on whether PBRT causes less or more side effects when used to treat prostate cancer (Gray et al., 2013; James et al., 2012; Kim et al., 2011; Sheets et al., 2012). He suggested more definitive evidence might be provided from a randomized prospective study of IMRT versus PBRT for prostate cancer that is currently accruing patients (NCT01617161).
Because of the difficulties of conducting randomized controlled studies on PBRT, which are explored further in the section on “Potential Research Challenges,” there is also limited evidence on whether PBRT improves outcomes compared to conventional radiation therapy with X-ray beams. One study that compared PBRT outcomes in adults with those who received conventional radiation therapy and whose outcomes were reported in a cancer database (Surveillance, Epidemiology, and End Results Program, or SEER3) found that those patients who received PBRT developed half
__________________
3 See http://seer.cancer.gov (accessed October 15, 2015).
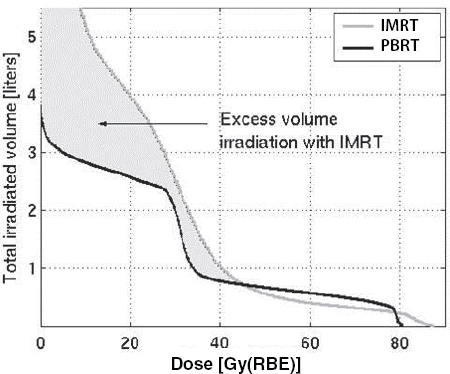
FIGURE 3 Whole-body radiation dose: Marked reduction in integral dose.
NOTES: Gy(RBE) = grays (relative biological effectiveness); IMRT = intensity-modulated radiation therapy; PBRT = proton beam radiation therapy.
SOURCE: Efstathiou presentation, July 21, 2015 (Trofimov et al., 2007). Adapted from International Journal of Radiation Oncology*Biology*Physics. Reprinted with permission from Elsevier.
as many secondary cancers as those who received conventional radiation therapy, Zietman reported (Chung et al., 2013). But he noted these results are contested by others who assert that the study design was flawed (Bekelman et al., 2013b).
A few studies using databases found PBRT was not more effective at treating prostate cancer than conventional therapies, as was expected (Coen et al., 2012; Sheets et al., 2012), but there is mixed evidence on whether PBRT actually reduces the adverse outcomes compared to other therapies, according to Zietman (Colaco et al., 2015; Gray et al., 2013; Sheets et al., 2012; Talcott et al., 2010). “Is it a better treatment? We don’t know,” Zietman said. Smith reported on a study that used Medicare claims data to compare the side effects of PBRT and IMRT for the treatment of
prostate cancer. This study found a slight decrease in the number of genitourinary complications within 6 months of the radiation treatment, but at 12 months following therapy, no differences were detected (Yu et al., 2013).
The standard treatment for early-stage non-small-cell lung cancer is surgical removal of the affected lung lobe. But some elderly patients whose health is compromised by other conditions cannot tolerate such surgery, leading to the suggestion that these patients might have better outcomes when treated with stereotactic radiation. Grace Smith, assistant professor of radiation oncology at the University of Texas MD Anderson Cancer Center, said that one study conducted using the SEER database found that stereotactic radiation therapy was as effective as surgical removal of a lung lobe, in terms of overall survival and lung cancer survival (Shirvani et al., 2014). The same researchers found that stereotactic radiation was the least costly option for up to 5 years after the treatment (Smith et al., 2015). A different cost study also suggested that the radiation option was less costly (Shah et al., 2013). “Advanced technology can sometimes be less costly than prevailing practice when applied to the right patient population,” said Smith.
In a prospective randomized clinical trial to compare two treatment regimens for patients with brain metastases (stereotactic radiosurgery alone or combined with whole-brain radiation), investigators found no difference in survival, even though radiosurgery alone resulted in worse intracranial tumor control, Smith reported. Patients treated solely with stereotactic radiosurgery had improved cognitive and functional status, leading the authors of the study to recommend using radiosurgery alone for such patients (Brown et al., 2015). “In this case, toxicity differences really tipped the balance in terms of what was affecting the decision making for the treatment,” Smith said. But another study done on the same population found that radiosurgery alone may be more costly due to the increased use of follow-up treatments of the tumor (Lal et al., 2012).
Considering the skill of the operator and the type of robotic device used is important when assessing the comparative effectiveness of a robotic surgical procedure, said James Hu, director of the LeFrak Center for
Robotic Surgery at Weill Cornell Medical Center. The performance of surgeons varies tremendously, Hu noted, citing one study of patients who had a prostatectomy, which found that recovery of erectile function varied between 10 and 50 percent at 12 months after controlling for patient characteristics (Vickers et al., 2008). Studies suggest that the threshold of experience needed to decrease complications with robotic surgery is about 150 cases, whereas the threshold for achieving a reduction in the rate of positive tumor margins to less than 10 percent is 1,600 cases. Operative time plateaus at 750 cases and preservation of sexual function thresholds at 1,400 cases (Alemozaffar et al., 2012; Ou et al., 2011; Sooriakumaran et al., 2011). “A great deal of experience needs to be attained in order to achieve some of these landmarks,” Hu said. Another study Hu cited found that the type of surgical system used influenced the types of robotic malfunctions and clinical consequences (Lucas et al., 2012).
According to Miller, the evidence on the comparative effectiveness of robotic laparoscopy compared to standard laparoscopy or open surgery is limited and mainly stems from observational studies at single institutions that may not consider the variability of surgical experience or surgical systems. These studies suggest that compared to open surgery, robotics facilitates smaller incisions, shorter hospital stays, and easier short-term recovery (Nix et al., 2010; Rocco et al., 2009). There are mixed results on whether robotics reduces the complication rate compared to open surgery. With regard to prostatectomies, studies have not consistently shown a benefit of robotic surgery in terms of functional outcomes, such as urinary control and sexual function, nor is there evidence that it affects prostate cancer outcomes, according to Miller.
Hu reported that his study using Medicare claims found that robotic surgery was associated with more diagnoses of erectile dysfunction and incontinence compared to open surgery (Hu et al., 2009). But a secondary analysis of these findings indicated that the higher rate of diagnoses of erectile dysfunction might be due to the higher expectations of patients choosing the robotic procedure, he said. These men may be more likely to seek potency rehabilitation and prescriptions postoperatively. A more recent Swedish study that used highly specific patient-reported outcomes on erectile function found that robotic surgery decreased the incidence of erectile dysfunction compared to open surgery to remove the prostate (Haglind et al., 2015). Another recent study also found that sexual and urinary function were better in men who had prostatectomies with robotic surgery compared to open surgery, Hu noted (O’Neil et al., 2015). “As
surgeons progress beyond the learning curve, there are some benefits now to the robotic approach in terms of functional outcomes,” Hu said.
A recent study he did found that the positive surgical margin rate is lower for robotic versus open surgery for prostatectomy, perhaps due to better visualization as well as lower blood loss (Hu et al., 2009). He said that another study yet to be published found that there is less use of additional therapies, such as androgen deprivation therapy or radiation therapy, within a median follow-up of 7 years, in patients who had robotic prostatectomies compared to those who had the open procedure. In this study, which used Medicare data, patients who received the robotic surgery also had greater overall survival.
Wright reported on studies of robotic hysterectomy surgery. He said results indicate that robotic surgery is associated with improved outcomes compared to open abdominal surgery. However, there is minimal to no benefit compared to laparoscopic hysterectomies, nor is there a difference seen in complications and lymph node yields, which is a surrogate for quality in gynecologic oncology (Gaia et al., 2010; Wright et al., 2012).
Only a few randomized controlled studies have been done, or are currently ongoing, to compare robotics to open surgery or to standard laparoscopy for other types of cancer. One trial done at Memorial Sloan Kettering Cancer Center randomized patients to have their bladders removed robotically or in an open surgery. This study found that patients had lower blood loss, but longer operating room time, with robotic surgery, and there was no difference in the length of hospital stay or rates of complications (Bochner et al., 2014). However, Miller and Hu said this study has been criticized, with a published paper pointing out that the greater experience of the surgeons with open-surgery removal of the bladder was not comparable to the lesser expertise of those surgeons who did the robotic-assisted bladder removal (Desai et al., 2002). Another study (ROLARR trial)4 compared robotic surgery for rectal cancer to laparoscopic surgery and the percentage of cases that had to convert to open surgery. No significant differences were found in the conversion rate, positive margin rate, lymph node yield, or 30-day mortality. But the study surgeons on average had nearly four times more experience with a laparoscopic approach compared with their robotic surgeon colleagues in the study. “Comparisons of surgical devices and techniques must be made beyond the surgeon training curves,” Hu stressed.
__________________
4 See https://clinicaltrials.gov/ct2/show/NCT01736072 (accessed October 26, 2015).
Ralph Weichselbaum, chair of radiation and cellular oncology at the University of Chicago, questioned the notion that robotic prostate surgery might be superior to open surgery, assuming the latter surgery is done competently. “There is a lot of intersurgeon variability so it is going to be impossible to show it is better,” he said. Hu responded that a study of robotic versus open surgery done by Sanda found that robotic surgery decreased the intersurgeon variation in results related to blood loss, length of stay, and operative time. He agreed, however, that with regard to sexual functioning and other quality of life variables, “one could question what does a 10 percent difference really mean in terms of functional status recovery.” Hu pointed out that “there is still a need to define these benefits, although I think it will be difficult to turn back the clock on this no matter what.” But Whelan countered, “The fact that the horse is out of the barn [for using robotic surgery] for prostates does not make it right to recommend it.”
Theodore Lawrence, chair of radiation oncology at the University of Michigan Medical School, suggested that when researchers do comparative effectiveness research, they compare quality-adjusted life years (QALYs). Weichselbaum and Miller agreed that such comparisons should be made for technologies as well as for drugs, and that for prostate cancer treatment, the factors that influence such an analysis would include the number of years men are impotent or incontinent following treatment.
Yu summarized the research on IMRT, PBRT, and robotic surgery by saying, “Evidence is being generated, but it’s just not generated for all the situations where these technologies are being applied.”
The rapid adoption of IMRT, PBRT, laparoscopies, and robotics for the treatment of cancer prior to extensive evidence development is explained, in part, by how devices and procedures are regulated. Michael O’Hara, deputy director of the Division of Radiological Health at the FDA, described how the FDA clears or approves new devices for market entry. To aid these determinations, the agency classifies a new device based on the level of risk it poses to patients. Class I devices pose the lowest risk and can enter the market after manufacturers register and list the device with the FDA.
In contrast, Class II devices, which are viewed as having intermediate risk, require an application for FDA clearance to enter the market
through the FDA’s 510(k)5 program. New devices are cleared for market through this avenue if manufacturers show that the new devices are similar to devices already legally on the market, have the same indication for use, and have the same technological characteristics, or the differences in technology characteristics do not raise new safety or effectiveness issues. “Basically this category says, ‘I’m just like company X’s device that’s already on the market,’” O’Hara said. Proton accelerators used in PBRT are considered Class II devices and are usually cleared with a general indication for use, meaning they can be used for cancer treatment anywhere on the human body, according to O’Hara, rather than limiting use to treat only certain types of cancers.
Class III devices pose the highest risk and require premarket approval by the FDA. To garner that approval, device manufacturers must show results from well-controlled clinical trials or other objective information that demonstrates safety and effectiveness. Device sponsors have to provide information on benefits versus risks, conditions of device use, device safety, performance, and reliability.
To conduct clinical trials of devices that are considered to pose significant risk to patients, such as implantable devices, devices used to support or sustain life, or devices to diagnose or treat disease, manufacturers must apply for an investigational device exemption from the FDA.
Once devices are on the market, the FDA requires mandatory medical device reports from manufacturers, importers, and device user facilities. Facilities have to report any deaths to the FDA and the manufacturer within 10 days, and serious injuries to the manufacturer or to the FDA if the manufacturer is unknown within the same time frame. Based on such reports, the FDA can request or order a recall of a medical device. The manufacturer of the recalled device is responsible for notifying its customers of the recall, and providing instructions to prevent further problems.
Many people claim the FDA review process for devices is less rigorous than for drugs, said Stanley Ashley, professor of surgery at the Brigham and Women’s Hospital (see Box 2). James Dignam, associate professor of biostatistics at the University of Chicago, agreed and said that not only does the FDA require a lower level of evidence for devices versus drugs, but that “formal regulatory control is absent in surgery.” Wright said this lack of regulation fostered the rapid adoption of robotic surgery.
__________________
5 See http://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/DeviceApprovalsandClearances/510kClearances (accessed October 15, 2015).
BOX 2
Development and Regulation: Drugs Versus Devices
Several speakers pointed out major differences in how drugs are developed and regulated compared to that of devices or procedures. Clinical trial results are required for Food and Drug Administration (FDA) approval of a new drug entering the market, whereas clinical trial results often are not needed for new devices to be put into general use, said Tina Shih from the MD Anderson Cancer Center. Drugs also require an indicated use on their labels, whereas devices may not require such a specific indication, and for them, consequently, “The line is very blurred between indicated and off-label use,” she said. In addition, manufacturers usually set drug prices, with patent protections initially enabling them to charge large fees for new drugs before a steep decline in price after they go generic. In contrast, devices not only require a price for the device, which is set by the manufacturer, but a price for the procedure that uses the device, the latter price usually set by payers.
For devices there is a depreciation and eventual replacement over time. Devices also usually require a large capital investment component that can create incentive for the owner to use the devices to recapture their investment. But as Bhadrasain Vikram at the National Cancer Institute pointed out, the fundamental difference between drugs and devices is that “for drugs, the FDA and insurers demand Level 1 evidence, but not for devices. Drug trials are generally funded by industry, and by the time you make a coverage decision, efficacy data and comparative efficacy data are usually available, whereas with device trials it’s the other way around. These device trials are not funded by industry, by and large. Many of them are funded by taxpayers through the [National Institutes of Health] and by the time the trial starts, the device is already in widespread use.”
Justin Bekelman from the University of Pennsylvania added that pharmaceutical companies spend about 30 percent of their budgets on research and development compared to only about 10 percent spent on research by device companies. However, if there was a change in policy requiring more evidence generation for regulation of devices, he noted, the increased cost due to generating that evidence would probably be passed on to payers and patients. Also, unlike for drug clinical trials, in which there are fewer differences in how clinicians deliver the intervention being studied, studies on new surgical techniques are difficult to conduct reliably because of differences in surgical technique and expertise among participating clinicians, Hu asserted.
Ashley also emphasized that neither the FDA nor any other government entity reviews procedures using devices for safety and effectiveness. Larry Kessler, professor and chair of health services at the University of Washington School of Public Health, agreed, noting that procedures “fall in the Bermuda Triangle between NIH [National Institutes of Health], [the] FDA, and CMS and other reimbursement agencies. Nobody legally has the authority to regulate procedures, and it’s a gap that Congress should talk about filling.” In the past, Congress has avoided regulating procedures because they are seen as falling under the practice of medicine, which government agencies cannot regulate, he said.
Kessler also stressed that the general indications given for devices, such as for robotic surgery systems, enable them to enter the market without having to generate evidence to support more specific claims, such as the notion that they can lower the complication rate from prostatectomies, that are later made by those that produce or use the devices. “If the company only makes the general claim, then it becomes time for both professional societies and reimbursement agencies to hold the company to that and not give them excess reimbursement for [additional] claims. There’s a circle here that you can make if companies are held to what they are trying to do, and it’s a pretty big loophole,” Kessler said. “We can make this [regulatory] pathway more accessible and scientifically sound if we make it clear to clinicians and companies that the path forward will require adequate clinical studies in order to get the right indication for which they are actually designing the product. The FDA should insist on more precision in intended use, and it can do so.”
Kessler also called for strengthening the oversight of medical devices with postmarket studies. He noted the potential for using the National Patient-Centered Clinical Research Network (PCORnet) of the Patient-Centered Outcomes Research Institute (PCORI) and the Medical Device Epidemiology Network Initiative (MDEpiNet), whose mission is to bridge evidence gaps by developing datasets and innovative methods for conducting robust studies (see Box 3). David Beyer, medical director of the Cancer Center at Sedona, added that medical professional societies can review the information available for new technologies and put their stamp of approval on them once they think the evidence is sufficient to support clinical uses.
Justin Bekelman, associate professor of radiation oncology at the University of Pennsylvania, stressed that there should be a balance between having high evidence standards and facilitating innovation. “If we relax the
Several workshop participants described databases that may be useful for gathering evidence on the effectiveness or comparative effectiveness of new technologies.
International Robotic Cystectomy Consortium
The International Robotic Cystectomy Consortium (IRCC) is an integrated database for participating institutions that was formed in 2006 to collect outcomes data on patients who received robotic surgery to remove the bladder (Mohler).
Medical Device Epidemiology Network Initiative (MDEpiNet)
Sponsored by the Food and Drug Administration (FDA), MDEpiNeta is a collaborative program through which the FDA and external partners share information and resources to enhance understanding of the safety and effectiveness of medical devices after they are marketed. By bridging gaps in evidence, developing datasets, and creating new methods of conducting robust analytic studies, MDEpiNet aims to develop new ways to study medical devices to improve the understanding of their safety and effectiveness throughout their life cycle (Kessler).
National Radiation Oncology Registry (NROR)
The NRORb pilot in non-metastatic prostate cancer was a collaborative quality improvement initiative of the Radiation Oncology Institute (ROI) and the American Society for Radiation Oncology (ASTRO) aimed at assessing the feasibility of capturing real-world data on the delivery and outcome of care. The pilot was completed in the summer of 2015, revealing attainment of most quality metrics and also illustrating some key lessons learned, including legal and regulatory barriers as well as data entry and financial burdens. This project has been instrumental in informing ASTRO’s future decisions and endeavors focused on real-world, real-time data capture aimed at improving quality of care (Beyer).
standards we’ll innovate faster, but we’ll make more errors as we innovate. But in the context of system change, rather than individual patient change, that’s probably better than what we do now, which is more like pre- and then post-market study,” he said.
PCORnet
Created by the Patient-Centered Outcomes Research Institute (PCORI), the National Patient-Centered Clinical Research Network of PCORI (PCORnet)c provides a real-world coordinated platform for conducting observational studies, as well as large, fast, and inexpensive randomized pragmatic trials. PCORnet integrates health data for studies and catalyzes research partnerships among clinical data research networks based in health care systems, such as hospitals and health centers, and patient-powered research networks run by groups of patients and their partners who are focused on one or more specific conditions, or communities and individuals interested in sharing health information and participating in research (Tunis).
SEER and SEER Medicare Database
The Surveillance, Epidemiology, and End Results (SEER) Programd of the National Cancer Institute is an authoritative source of information on cancer incidence and survival in the United States. SEER collects and publishes cancer incidence and survival data from population-based cancer registries covering approximately 28 percent of the U.S. population. SEER registries routinely collect data on patient demographics, primary tumor site, tumor morphology and stage at diagnosis, first course of treatment, and follow-up for vital status. The SEER Medicare Databasee links SEER data to Medicare claims data, providing information on treatments that Medicare patients received and their costs in addition to the clinical, demographic, and cause of death information (Efstathiou, Smith).
__________________
See a http://www.fda.gov/MedicalDevices/ScienceandResearch/EpidemiologyMedicalDevices/MedicalDeviceEpidemiologyNetworkMDEpiNet/default.htm; b https://www.astro.org/Practice-Management/NROR/Index.aspx; c http://www.pcornet.org; d http://seer.cancer.gov/about/overview.html; e http://healthcaredelivery.cancer.gov/seermedicare/overview (all URLs accessed September 11, 2015).
RAPID WIDESPREAD ADOPTION OF NEW TECHNOLOGIES
Several speakers noted the rapid widespread adoption of new technologies in cancer care, which can be premature given the lack of evidence. Economic and other factors have sometimes fostered the overuse of such expensive technologies, some participants observed.
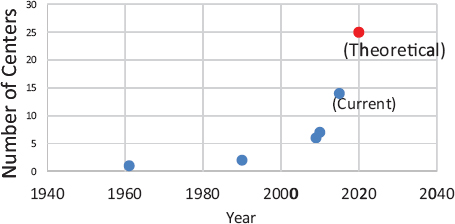
FIGURE 4 Proton beam therapy centers in operation in the United States.
SOURCE: Yu presentation, July 20, 2015.
In just 10 years, beginning in 1999, IMRT replaced 3D conformal radiotherapy as the main radiotherapy treatment for prostate cancer, Yu noted (Raldow et al., 2015). Expansion in the number of facilities that provided IMRT was then followed by rapid growth in the number of facilities that provided PBRT. In 2009 there were 6 PBRT centers; that expanded to 14 centers in operation in 2015, with 11 more under construction in the United States, Yu reported6 (see Figure 4).
Simultaneously, the number of facilities offering robotic surgery has exploded. According to Tina Shih from the MD Anderson Cancer Center, in 1999, there were only 2 robotic surgery machines in the United States, but by 2015 that number had risen to more than 2,200. Robotic surgery is now used for urological, gynecological, colorectal, endocrine, thoracic, and head and neck cancers, Miller reported (see Table 1). The increased use of robotics for prostatectomies is especially striking, with most being done robotically now nationwide (see Figure 5).
Potential Misuse of New Technologies
Miller noted that the international community has not had the rapid growth of robotic technology that the United States has experienced. From
__________________
6 See http://www.proton-therapy.org/map.htm (accessed September 11, 2015).
TABLE 1 Common Robotic Applications for Cancer Surgery
| Category | Types of Cancer |
| Urological | Prostate, bladder, kidney |
| Gynecological | Uterine, cervical, ovarian |
| Gastrointestinal | Colorectal |
| Endocrine | Pancreas, thyroid |
| Thoracic | Lung, esophageal |
| Head and Neck | Tonsil, tongue base |
SOURCE: Miller presentation, July 20, 2015.
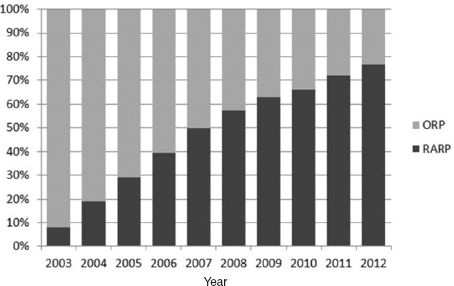
FIGURE 5 Current usage of robot-assisted prostatectomies.
NOTES: ORP = open radical prostatectomy; RARP = robot-assisted radical prostatectomy.
SOURCE: Hu presentation, July 20, 2015. Reprinted from Journal of Urology with permission from Elsevier.
2004 to 2011, the number of robot-assisted laparoscopic procedures in the United States grew from about 10,000 to more than 300,000. International usage increased to only 50,000 procedures in 2011 (Cooper et al., 2013). Miller suggested that some use of robotic surgery as well as other technologies in this country may not be appropriate.
There has been a striking increase in robotic prostatectomies and
IMRT among older men, many of whom have low-risk, slow-growing tumors that are not likely to be lethal. In previous years, these men would have received a recommendation for watchful waiting rather than surgery, Miller stressed (Jacobs et al., 2013; Makarov et al., 2011). “It’s concerning if you do a perfect prostatectomy but the patient isn’t likely to benefit from it. We have to continue to think about overtreatment,” Miller cautioned. (This is discussed further in Box 4.)
There has also been a rapid shift in the technologies used for gynecologic surgery. Wright pointed out that despite little to no evidence that robotic hysterectomies have better outcomes than laparoscopic hysterectomies,
BOX 4
Evolution of Prostate Cancer Treatment
The evolution of prostate cancer treatment over the past two decades reveals the impact of new technologies and how quickly they are adopted and used, even for patients for whom they are not likely to offer a benefit, several speakers showed. Jason Efstathiou of Massachusetts General Hospital noted that in 1995 the only option for radiation therapy for prostate cancer was conventional external beam radiation. By 2015, however, the options had multiplied, as indicated in Figure 6, and now 95 percent of the time, intensity-modulated radiotherapy (IMRT) is the type of external radiation used to treat prostate cancer, he said.
Proton beam radiation therapy (PBRT) is also used to treat prostate cancer. Ron Kline from the Centers for Medicare & Medicaid Services, said that few added benefits of PBRT have been definitively demonstrated for prostate cancer in the 10 years it has been in use, and the treatment is three to five times the cost of conventional radiation therapy, so “it’s hard to argue proton beam therapy for prostate cancer.” Zietman agreed, noting that fewer prostate cancer patients are now being treated with PBRT. “The prostate question is answering itself—the prostate patients are disappearing,” he said.
Several participants expressed the concern that prostate cancer is being overtreated due to the advent of new technologies. As James Mohler of the Roswell Park Cancer Institute pointed out, “We diagnose a lot more men with prostate cancer than those who die of it, but we don’t know what technology to use. Most men should just get active surveillance, but they are not because there are financial pressures and incentives that prevent this practice.” He noted that in urology practices that do treatment self-referrals, substantially fewer men with prostate
and robotic surgery costs more and takes longer, robotic hysterectomies for minimally invasive endometrial cancer increased from 45 percent of hysterectomies in 2008 to 60 percent in 2010 (Wright et al., 2012). Similarly, the number of robotic hysterectomies done for benign conditions increased from nearly zero in 2007 to 10 percent of all cases in 2010. In addition, Wright’s study found that in the first quarter after hospitals adopted robotic surgery, the percentage of robotic hysterectomies for benign conditions doubled (Wright et al., 2013).
Innovative treatments can do substantial harm if they are adopted prematurely, several participants noted. For example, Reshma Jagsi, associate
cancer are followed with active surveillance and more are treated with IMRT compared to those receiving care in a National Comprehensive Cancer Network center (Mitchell, 2013) (see Table 2).
Miller also stressed the concern that men with low-risk prostate cancer might be treated with robotic prostatectomy instead of the more appropriate watchful waiting. He cited a study that found that the number of prostatectomies dramatically increases once facilities acquire robotic systems (Makarov et al., 2011). Another study found that an increasing number of low-risk and elderly patients who are unlikely to die from prostate cancer are being treated with robotic prostatectomy and IMRT (Jacobs et al., 2013).
Miller said that registry data for urologists in the state of Michigan from 2012 to 2015 indicate that 90 percent of laparoscopic prostatectomies in the state are now done robotically. “At least for prostatectomies, robotics is here and highly prevalent. The question is has it achieved the promise that we hoped it would,” he said. He noted some studies have detected unexpected adverse effects of robotic prostatectomies, including a greater risk of genitourinary complications compared to open prostatectomies (Barry et al., 2012; Gandaglia et al., 2014). He added that there are concerns in both the scientific and lay press that there is underreporting of adverse effects of robotic prostatectomies (Cooper et al., 2013). “The robot is not a panacea and there have been some unintended consequences that have been associated with the introduction of robotics that we ought to consider,” Miller said.
He noted the intensive consumer-directed advertising by hospitals linked to the introduction of robotics in urology and in other fields. “These strong forces driving the introduction and adoption for this technology weren’t always harmonized well with preparedness for implementation of the technology. That is a challenge that still exists,” Miller said.
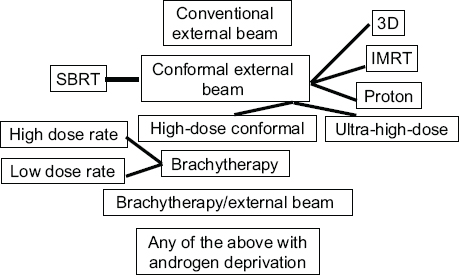
FIGURE 6 Radiation therapy options for prostate cancer treatment in 2015.
NOTES: 3D = three-dimensional; IMRT = intensity-modulated radiation therapy; SBRT = stereotactic body radiation therapy.
SOURCE: Efstathiou presentation, July 21, 2015.
professor of radiation oncology at the University of Michigan, described an innovative treatment for early-stage breast cancer, known as accelerated partial-breast irradiation (APBI), which targets only a portion of the breast with higher doses of radiation so that radiation treatment can be completed within 1 week (compared to 6 weeks for standard whole breast radiation therapy). Radiation can be in the form of external beams, or brachytherapy, which involves implanting radioactive seeds in the area of the cancer. She cited studies which found that once Medicare began reimbursing for APBI, despite a lack of extensive clinical evidence that it was safe and effective, physicians increasingly prescribed it; more than one-quarter of early-stage breast cancer patients in large urban areas of the country now receive APBI therapy (Hattangadi et al., 2012; Presley et al., 2012). However, some studies done after Medicare reimbursements began found that the therapy was linked to poorer cosmetic outcomes and greater rates of skin and wound complications (Jagsi et al., 2010; Presley et al., 2012).
The rapid and widespread adoption of new technologies prematurely before sufficient evidence has been gathered to assess them is also problematic because it is difficult to conduct the studies needed to gather evidence after technologies are in clinical use. As noted by Sean Tunis, founder and
| Versus Urologists Working at National Comprehensive Cancer Network Centers | ||||||||
| Treatment | Self-Referring Urologists in Private Practice | Non–Self-Referring Urologists Employed by the National Comprehensive Cancer Network |
||||||
| Preownership Period (N = 2,620) | Ownership Period (N = 2,449) | Change | P Value | Preownersh Period (N = 1,044 | ip Ownership Period ) (N = 600) | Change | P Value | |
| IMRT delivery by self-referring group (%) | 9.0 | 42.0 | 33.0 | <.001 | — | — | — | — |
| IMRT delivery by other provider (%) | — | 4.5 | — | — | 7.9 | 8.3 | 0.4 | 0.78 |
| Brachytherapy (%) | 17.6 | 2.7 | −14.9 | <.001 | 6.3 | 8.5 | 2.2 | 0.09 |
| Prostatectomy (%) | 16.4 | 12.8 | −3.6 | <.001 | 28.5 | 27.0 | −1.5 | 0.50 |
| Androgen-deprivation therapy (%) | 17.4 | 7.4 | −10.0 | <.001 | 12.0 | 9.7 | −2.3 | 0.14 |
| Active surveillance (%) | 33.9 | 27.6 | −6.3 | <.001 | 44.3 | 45.0 | 0.7 | 0.79 |
| Other procedure (%) | 5.7 | 3.0 | −2.7 | <.001 | 1.0 | 1.5 | 0.5 | 0.30 |
| Time from diagnosis to treatment (days) | 80.0±35.9 | 71.2±31.1 | −8.8 | <.001 | 84.4±38.9 | 82.0±36.7 | −2.4 | 0.39 |
NOTES: ADT = androgen deprivation therapy; AS = active surveillance; Brachy = brachytherapy; IMRT = intensity-modulated radiation therapy; NCCN = National Comprehensive Cancer Network; Rad Px = radical prostatectomy.
SOURCE: Mohler presentation, July 21, 2015. Mitchell, 2013. Reprinted with permission from Massachusetts Medical Society.
CEO of the Center for Medical Technology Policy, “It’s always too early to evaluate medical technologies until suddenly it’s too late.”
The Difficulties of De-Adoption
Several speakers noted that once technologies and medical practices become ingrained, it is difficult to de-adopt them if evidence accumulates showing they do not have the best outcomes, or that their outcomes are no better than less expensive and more convenient treatments. “Adoption happens quickly, perhaps too quickly, and de-adoption often happens too slowly,” Bekelman said.
Examples of slow de-adoption are prevalent for breast cancer treatment, Bekelman said. He said studies show that whole-breast radiation with higher doses but fewer treatments (hypofractionated radiation) has equivalent outcomes to conventional radiation therapy with more treatments at lower doses, yet hypofractionated whole-breast radiation has not been widely adopted by the medical community, despite it being a shorter course of therapy that is more convenient and less costly (Bekelman et al., 2014). Similarly, a single radiation treatment for bone metastases is as effective as multiple treatments, and despite guidelines recommending the former, it is rarely done (Bekelman et al., 2013a). Recent studies also indicate that for elderly women taking hormone therapy for breast cancer with a low risk of recurrence, a lack of radiation therapy does not substantially increase their risk of dying from breast cancer (Hughes et al., 2013). Yet that evidence has not deterred radiation therapy for such patients, with studies finding that the majority of these women still receive radiation therapy (Palta et al., 2015; Soulos et al., 2012).
“It’s very hard to convince patients and physicians alike to omit treatments. Both of these groups tend to be risk averse,” Jagsi said, noting that both physicians and their patients worry that if radiation therapy is not pursued and cancer recurs, they will deeply regret deciding not to have the treatment. She added that physicians also face strong financial disincentives to omit therapy in our current fee-for-service system. Evidence that financial incentives influence which breast cancer treatments are prescribed is suggested by studies that found hypofractionated whole-breast radiation therapy was more quickly adopted in Canada, which has a universal health care system, than in the United States, Jagsi said (Ashworth et al., 2013; Bekelman et al., 2014).
However, Wright observed that de-adoption of a potentially dangerous
technology can happen rapidly if there is enough public pressure to do so. He gave the example of the rapid and widespread adoption of electric power morcellation, which is used to mince the uterus into small pieces that can be easily removed in vaginal or laparoscopic hysterectomies. It involves using a small electromechanical device with a rotating blade that was first developed in 1993. Over the past two decades, the FDA approved a number of these devices, and they have diffused rapidly into gynecological surgery practice, according to Wright.
However, questions have been raised about the safety of electric power morcellation when a hysterectomy is done to remove one or more apparent fibroids. When a fibroid is actually a uterine leiomyosarcoma tumor or contains such a tumor, the possibility exists that morcellation could disseminate the leiomyosarcoma cells within the abdomen and/or pelvis. (Determining whether a fibroid is malignant before surgery usually cannot be done.) This possibility had not been adequately assessed before surgeons began conducting electric power morcellations, Wright noted. However, subsequent studies did raise concerns. For example, one study found that women with uterine leiomyosarcoma have a three-fold increase in the rate of death when undergoing power morcellation compared to those who had a hysterectomy without the morcellation procedure (Park et al., 2011). In 2013, a case was widely publicized in the lay press about a woman who was presumed to have benign fibroid tumors, had power morcellation, and then was found to have disseminated uterine sarcoma. This case, along with the published data, led to a black box warning from the FDA against using laparoscopic power morcellators in the removal of the uterus (hysterectomy) or fibroids (myomectomy) in the vast majority of women (FDA, 2014). Some insurance companies also eliminated reimbursement for hysterectomies performed with an electric power morcellator.
“The pendulum swung pretty rapidly to elimination of morcellation,” Wright noted, although there is evidence that use of the device in younger women having hysterectomies could slightly reduce their risk of dying compared to having abdominal hysterectomies or laparotomies without the device. “This controversy demonstrates some of the difficulties with surgical innovation and some of the non-medical factors, including public opinion, that influence the conversation,” Wright concluded.
Potential Incentives for Rapid Adoption and Overuse of New Technologies
Several speakers noted various incentives for the rapid adoption and inappropriate use of new technologies once they come on the market, including
- training that residents and physicians in practice acquire using the technology that may make them more comfortable with it than conventional technology (Ashley);
- high costs of the technology, which can prompt practitioners and hospitals who own it to use it frequently in order to make it financially sustainable (Zietman);
- overenthusiasm and marketing hype for new technology and the assumption, based on minimal evidence, that it will improve care (Hu); and
- willingness of payers to reimburse the use of the new technology (Yu).
Yu noted it is commonly assumed that new technologies will provide an extra benefit for patients and a competitive advantage for practices. When deciding whether to adopt the new technology, practitioners also consider the skills and knowledge required to use it, the evidence supporting it, the stability in the patient need for it, and the return on investment for it (Dirksen et al., 1996; Geroski, 1999; Hall and Khan, 2003). IMRT was rapidly adopted because many of those factors were favorable for the technology, Yu said. The cost of adopting IMRT was not excessive compared to the revenue that could be generated from it, and competition among radiation oncology providers is so great that many sought out the competitive advantage IMRT initially gave them. In addition, the skill set and team knowledge needed for IMRT are relatively accessible for most radiation oncology teams, and many viewed the technology as providing better clinical outcomes based on the evidence available at the time. Consequently IMRT rapidly diffused into practice (Mell et al., 2005).
In contrast, the capital costs of PBRT are much greater and are the largest barrier for investors, although the advent of superconducting synchrocyclotrons in 2011 has lowered their cost somewhat, Yu said. PBRT also requires a significant amount of team knowledge, and payers tend to perceive the costs of PBRT as being greater than the benefits based on the
evidence collected to date (Beck, 2015). This mixed bag of positive and negative factors has led to slower diffusion of PBRT into practice compared to IMRT, Yu said, but it is currently poised to be rapidly adopted as more residents are being trained to use it and as competition increases among cancer treatment facilities.
Training also influences the adoption of robotic techniques. Yu suggested, and Wright agreed, that due to a lack of experience with laparoscopic surgery, some surgeons opt to do robotic surgery as a means to do a minimally invasive surgery instead of open surgery. Many residents are not trained in laparoscopic surgery, Wright noted, so “Until there is change in reimbursement policy, you’ll continue to see uptake of robotic surgery.”
Yu and others also noted that overenthusiasm for new technologies can prompt widespread adoption that is premature. “How do we distinguish between when we’re blinded by earnest enthusiasm or when we’re advocating for a transformative technology?” he asked. Efstathiou noted, “We’re living in a state of gizmo idolatry. Technology is great but it can be seductive and expensive and that needs to be addressed.” Jagsi also noted, “Lower tech approaches for breast radiotherapy have been less quickly adopted than higher tech approaches in the United States, even when the former have been more firmly grounded in evidence.”
Several participants pointed out that provider ownership of a new technology strongly influences how much it is used in a practice or facility. Stephen Williams, a urologist at the University of Texas MD Anderson Cancer Center, reported that such a conflict of interest is regulated to some degree by the Stark Law, which is a federal law enacted in 1993 that makes it generally illegal for a physician to refer Medicare or Medicaid patients for designated health services in which the physician has a financial interest. This law prohibits many physician self-referral arrangements. But physician group practices are exempt for in-office ancillary services if the group practice meets specific criteria. Physicians in these practices may self-refer if the services are personally performed or supervised by another physician in the same group practice. Because radiation therapies like IMRT are generally provided onsite by urologist-owned integrated centers, there is no violation of the Stark Law (Falit et al, 2010). The Stark Law also does not apply to specific types of facilities, such as ambulatory surgical centers or whole hospitals (Mitchell, 2005).
But one study using California data found that more than 60 percent of urologists who have self-referral arrangements for magnetic resonance imaging (MRI) and computed tomography (CT) scanners may be in violation of the Stark Law (Mitchell, 2005). Another study found that urologists’ new ownership of IMRT technology led to an 18 percent increase in self-referrals to treat prostate cancer and a reduction in the use of less expensive brachytherapy (Mitchell, 2013). The researchers concluded that referral by urologists to an IMRT service in which they have a financial interest is associated with increased use of IMRT. Similar conclusions were reached in a study by Williams in which he found a significantly increased use of IMRT to treat prostate cancer patients in integrated self-referring practices compared to nonintegrated practices, not only for high-risk patients, but also for favorable-risk patients, for whom practice guidelines recommend active surveillance rather than radiation therapy. Williams concluded, “There is a need for health policy reform to guide appropriate utilization so that we can optimize the treatment and care of our patients.”
Patricia Ganz, director of cancer prevention and control research at the University of California, Los Angeles (UCLA), asked if the radiation oncologists in integrated practices have different expertise than those who choose to practice elsewhere. Yu and Williams responded that these radiation oncologists and the urologists they work with are not any less qualified than other radiation oncologists, but rather they have chosen to specialize in prostate cancer and be an employee of a urologist who will send them patients. But Weichselbaum countered, “It’s clear from the data that both the urologist and the radiation oncologist are putting financial gain ahead of patient decisions.” Williams added, “They engage in financial arrangements which in turn increase the self-referral patterns.”
Marc Hartstein, director of the Hospital and Ambulatory Policy Group at CMS, said this should not be surprising because “human beings respond to economic incentives. Research on fee for service suggests that when you pay somebody for doing one thing, then they are going to do more of that thing. So a lot of health services delivery system reforms are designed to create bundles or packages and episodes of care that lack incentives to do more, but to instead provide the right care and reward good quality care. It’s unfortunate but true that decisions about patient care are not always driven by what’s in the best interest of the patient, but are driven by the economics of the way human beings operate.” Jagsi added, “Reimbursement mechanisms can clearly create perverse financial incentives and gizmo idolatry.”
Beyer noted, “We have real problems with how incentives are driving care and influencing adoption of advanced technologies. Where the incentive exists, people are going to react to it. So we have to do something about this—ask Congress to shut down this self-referral loophole because it has absolutely perverted a lot of what we are discussing in advanced technologies.” James Mohler, chair of urology at the Roswell Park Cancer Institute, agreed, adding, “The in-office exception to the Stark Law is an error that can be corrected.” He noted that the in-office exception was meant to allow a laboratory test or simple radiographic study to be performed on physician-owned equipment in the physician’s office. Without such an exception, for example, a physician would not be allowed to do a urinalysis on a patient with symptoms of a bladder infection. But such an exception never should have been extended to expensive technologies such as MRIs or IMRT, he said.
Efstathiou suggested that incentives might be better aligned with quality patient care in multidisciplinary clinics, noting that low-risk prostate cancer patients seen in such clinics have double the adoption of active surveillance compared with those who see single providers (Aizer et al., 2013). Mohler responded that he operates in a multidisciplinary clinic and he finds these clinics to be very expensive and time-inefficient, and that a less expensive alternative would be to properly close the self-referral loophole in the Stark Law. Beyer added that although there is ample evidence that multidisciplinary clinics improve care, “They are underfunded, difficult to coordinate, and mainly exist in large academic institutions. They don’t exist out in that real world that I live in. It’s a great idea where it can happen, but it’s hard to throw that out into the world at large.”
Financial incentives can also foster the use of an expensive new technology, such as PBRT, for patients who may not benefit from it (e.g., patients with low-risk prostate cancers) to help pay for the cost of making the technology available for the rare pediatric cancer patients who are likely to benefit, said Peter Johnstone, radiation oncology clinical director at the Moffitt Cancer Center (see Box 5). “When people spend that money on expensive PBRT systems, then they are going to want it back,” he stressed.
Another factor influencing the spread of new technologies is media and institutional marketing hype about how these novel technologies are better than existing options, several speakers noted. “The advertising message from institutions that have robotic surgery capabilities is ‘Come here
Peter Johnstone from the Moffitt Cancer Center explained the economics of proton beam radiation therapy (PBRT) and how difficult it is to make a PBRT facility financially sustainable. The profitability of PBRT depends on patient throughput, which in turn depends on how complex and time consuming patient cases are. He said most people agree that the best use of PBRT is in treating complex pediatric cancers, but these are relatively rare cases that take up multiple treatment slots so it is difficult to achieve a return on investment based on that use alone. Consequently, most PBRT facilities are built with the additional aim of treating simpler cases, most notably prostate cancer cases. “Kids are the people that need this the most and for whom it’s best prescribed, but you lose money on kids,” Johnstone said.
Johnstone argued that the current expansion of PBRT facilities may not be sustainable given the limited number of patients for whom the treatment is beneficial above and beyond other less expensive therapies. For example, his modeling of the number of PBRT rooms needed to treat the population in the Atlanta area indicated that four treatment rooms are needed. Emory University in Atlanta is currently building a facility that has four PBRT treatment rooms, but that new facility will only be economically sustainable if all the patients in the Atlanta area are treated at Emory when they require PBRT. However, Emory has competition from other hospitals in the Atlanta area that may also build such facilities so they can be on equal footing with Emory. “They have a bunch of cancer centers that are not affiliated with Emory and have no reason to refer their patients to those four rooms at Emory, so the mere fact that you have four rooms built in a city that needs four rooms doesn’t necessarily mean the patients will go there,” Johnstone stressed.
He compared having a PBRT facility in a city to having a franchise for a sports team in a city. For sports teams, franchises limit the number that are based in the same city, so only very large cities have more than one team. However, there are no franchises for proton centers to ensure that sufficient numbers of patients use them to make them economically sustainable, Johnstone pointed out.
Many states have Certificate of Need (CON) laws that can limit the creation of new medical facilities or the acquisition of new medical tech-
nologies, such as a PBRT system. CON laws are designed to contain costs for health services by matching availability of expensive facilities to the needs of the patient populations. But studies show CON laws have had little influence on the dissemination of robotic surgery for prostate cancer or the use of intensity-modulated radiotherapy in elderly patients with early cancers, Johnstone reported (Falchook and Chen, 2015; Jacobs et al., 2012). He said CON laws are even less effective in limiting the spread of highly expensive technologies that cost $100 million or more because they are governed by market forces, especially venture capital money. “That’s part of the problem of how we got into protons. People with venture capital money thought protons were a good thing to do,” Johnston said. He added that there has been a lack of valid pro formas delineating expected use and profit for many medical technology ventures.
Anthony Zietman from Massachusetts General Hospital noted, “It may be that the number of proton centers that we have in the United States will be unsustainable when the evidence finally comes in.” He reported that the British did a needs assessment and determined that one PBRT facility is needed per 30 million people, which suggests the United States should have 11 proton centers, but it has 15, with 10 more in the planning stages. “We are market based so we have a boom, then a bust, and then we settle out appropriate use,” he said. “Whether all of these centers will open will depend on national and payer policy.”
Johnstone noted future factors that might make PBRT facilities more economically viable, including consolidation of health care facilities and sharing of assets that boost the number of patients per proton center, as well as expansion in the use of PBRT to treat other cancers such as breast or lung cancer. But if facilities continue to compete for available patients, as they currently are doing in New Jersey and New York, an expanding patient base may not be adequate. In addition, Johnstone expects reimbursement for PBRT to decrease over time because reimbursement for all aspects of health care is decreasing. Zietman said the cost of PBRT is also decreasing, although it is still about three times the cost of standard radiation. However, he expects the efforts being made to miniaturize proton accelerators will drive the cost down.
for the latest in cancer treatment.’ It is unfair and unreasonable marketing and we can make it worse by overplaying the claims based on the fact that it has bells and whistles and is exciting and very modern,” Whelan said. “There’s a lot of hype around these therapies, a lot of enthusiasm from patients for what they see as a silver bullet that is not going to affect their functional outcomes.”
Wright noted that in New York, he has seen patients request robotic surgery after trying out the robot at the local shopping center. “There’s certainly this role of marketing to hospitals and patients as well as to physicians,” he said. One study assessed information about robotic gynecologic surgery on hospital websites and found most made claims that this type of surgery was less painful, enabled shorter recoveries, and caused less scarring, blood loss, and infection, despite there being little data to back up those claims, Wright stressed. Often the treatment was described as “cutting-edge technology” and the marketing implied that “you owe it to yourself ” (Schiavone et al., 2012). “There are lots of non-medical reasons that are driving technologies like this,” he said.
Beyer described a study which found that 45 percent of online promotions for robotic surgery were on hospital websites. There is a lot of such direct-to-consumer advertising as well as direct-to-provider marketing, Efstathiou noted. Michael Steinberg, chair of radiation oncology at UCLA, said this is compounded by a cultural attitude that cancer must be treated, no matter what the cost. “Cancer was a sacred cow until recently, with studies showing cancer care costs rising at twice the rate of health care costs in general and cancer care having the highest out-of-pocket expense of all disease entities,” he said (Elkin and Bach, 2010; Zafar et al., 2013).
Ganz stressed, “If you have a hammer, everything is a nail, and you are going to use it for everything. We have robots that are being used for surgical procedures where they are not necessary. IMRT is used for all sorts of treatments for which it may not necessarily be superior to standard radiation therapy and we’re going to have the same problem with protons. We are using these technologies in situations where it is not appropriate and someone is not going to survive a long time to get a benefit from it, and we are also using them where there is no evidence [of benefit].”
Other than having insurance companies deny payment for inappropriate use, “How do we prevent the creep of ‘we have the machine so we’re going to use it?’” Ganz asked. Zietman suggested that Choosing Wisely7
__________________
7 See http://www.choosingwisely.org (accessed October 15, 2015).
and other medical specialty society guidances can indicate when it is appropriate to use new technologies. He noted that among its five Choosing Wisely recommendations, the American Society for Radiation Oncology (ASTRO) recommends that physicians carefully consider other options before recommending PBRT to patients with prostate cancer. Although those recommendations “are toothless because they are not enforced, specialty societies can at least push the physicians in an ethical direction,” he said. But Ann Geiger, acting associate director of the Healthcare Delivery Research Program at the NCI, noted that Choosing Wisely is not structured to prevent new and often premature technologies from rapidly taking root in medical practice. “Choosing Wisely is wonderful, but it doesn’t address how we keep people from getting something they don’t need so that in 10 years we are not trying to take something away. Behavioral economics shows that taking things away, even if people don’t benefit from them, is very difficult for people to accept because it feels like a loss,” Geiger said.
Although there are exceptions, the costs of most new technologies used in cancer care exceed the costs of previously used technologies. For example, the cost of treating prostate cancer with 3D conformational radiotherapy in 2005 was about $20,000 per patient, whereas the cost of IMRT was about $10,000 more than that, and the cost of PBRT was an additional $14,000 more, Yu reported (James et al., 2012; Nguyen et al., 2011). Steinberg added that charges and reimbursements for technical procedures do not reflect the actual costs, and that charges for the same procedure can vary widely between different providers and settings. Shih pointed out that between 2003 and 2009, radiation oncology ranked highest as the group generating the most increased Medicare expenditures compared to 2002 (Alhassani et al., 2012). Yu added that IMRT was responsible for the increasing costs of radiation therapy from 2002 to 2008, and for prostate cancer therapy alone, PBRT has the potential to cost Medicare hundreds of millions of dollars beyond the cost of IMRT (Konski, 2011; Shen et al., 2014). He noted, however, that the costs of IMRT technology are decreasing. Thomas Farrington, president and founder of the Prostate Health Education Network, suggested that SBRT has a lower cost than these other innovative radiotherapy technologies. But Yu noted he did a study that compared SBRT to IMRT and found that SBRT was linked to a greater
rate of toxicity, with other studies of patient-reported outcomes showing results similar to what he found.
A similar cost trend is observed for newer surgical techniques. Compared to open surgery, laparoscopic surgery is more expensive upfront due to high equipment costs and higher procedure costs, mostly due to longer surgical time. But because laparoscopic surgery can reduce complications for certain surgeries and enable some patients to leave the hospital sooner, it is still a more economical alternative than abdominal surgery in some cases, Whelan reported. For example, laparoscopic ventral hernia repair surgeries are more than $3,000 less costly than open surgeries, and open surgical removal of all or part of the colon is 1.26 times more expensive than laparoscopic removal. Laparoscopic liver and pancreatic resections and gastric bypass surgery are also less expensive than open surgery (Ecker et al., 2015; Limongelli et al., 2014; Livingston, 2005).
However, robotic surgery increases the cost of the surgery, on average, by about 13 percent, according to a study cited by Shih (Barbash and Glied, 2010). Wright reported that the median cost of a robotic hysterectomy is about $1,600 greater than a standard laparoscopic procedure (Wright et al., 2012). The cost of removing the prostate, colon, or bladder with robotic assistance is about $2,000 to $4,000 more than with standard open surgery, according to Hu (Marino et al., 2015).
Whelan said that the added costs of robotic surgeries, with limited evidence of their greater effectiveness, “makes it hard to invest in these robots and train people to use them when there are cheaper alternatives. It is very hard to justify robotic surgery in an economy that is so strapped to pay for health care.” But Miller suggested looking at the benefits of robotic surgery over a longer time frame and noted that due to reduced complications, analyses using price standardization and risk-adjusted episode costs for 90 days after surgery find the costs of robotic and open prostatectomies equivalent from a payers’ perspective, although the hospital still has larger costs with robotics given its initial large investment in the technology (manuscript in development).
Several speakers noted the tremendous upfront costs involved in purchasing the equipment to deliver new technologies. Robotic systems cost about $2 million, Whelan reported, plus there are yearly maintenance costs of about $150,000 per robot, and additional costs for the specialized tools needed for the robot. Miller noted that the disposable equipment alone for robotic surgery can cost $1,500 to $2,000 per patient. Costs for robots continue to be high in the United States because there is only one vendor
for these devices in this country, unlike the multiple vendors of robots internationally. Mohler noted that the lack of competition in robot vendors has not only made robots more expensive but has also stifled innovation.
The costs of a proton accelerator needed to deliver PBRT is especially expensive, with installation costs that exceed those of nearly any other medical device in use, according to Zietman. The initial $30 million to $180 million investment in this technology impedes research on its safety and effectiveness, Zietman noted. “If an institution invests $150 million in a proton facility, it is tough to then ask it to do research with no reimbursement. The reimbursement system and the way protons have been rolled out in this country has been an economic trap from which we need to extricate ourselves,” he said. He added that technical advances have the possibility of miniaturizing proton facilities and cutting their costs and are avidly being pursued by researchers.
Hu noted that device manufacturers are increasingly becoming more conscious of the costs of their new technologies and are providing spreadsheets with assumptions about the pricing and profit structure of hospitals when using new technologies, such as robotics. These analyses suggest certain operations, such as robotic hernia repair or kidney removal, are not financially sustainable. “There are attempts to help us better select which procedures are appropriate, and which are non-starters to begin with from a profit standpoint,” he said.
One participant stressed that when considering costs, there should also be consideration of how various technological procedures affect the quality of life of patients and patient costs that are not just monetary. Many patient quality-of-life costs are unknown because they are often not measured. These costs include long functional recovery times, including delays in returning to work, and long-term complications of therapy or late disease recurrence, as can be seen in the second and third tiers of the diagram of costs of treatments for head and neck cancers in Figure 7 (de Souza and Seiwert, 2013). Researchers also frequently neglect to measure more subjective patient-reported outcomes, such as treatment-related anxiety, stress on the caregiver, convenience, and ability to maintain employment, Steinberg said.
Given the rapidly rising costs of health care in the United States, there is increasing concern that expensive new technologies may not provide sufficient value compared to standard interventions. Steinberg began a
FIGURE 7 Dimensions of value in head and neck cancer treatment.
SOURCE: Steinberg presentation, July 21, 2015. Adapted from Porter, 2010. Reprinted with permission from Massachusetts Medical Society.
discussion of the value of new technologies by pointing out that the U.S. per capita health care expenditure has been rising since 1950 and is twice that of other developed countries, with no demonstrable difference in health outcomes (OECD, 2014). Over a 10-year period, the extra health care spending in the United States bought 10 percent more office visits, the same number of overnight stays in the hospital, about 80 percent more MRI scans and twice the number of CT scans (with the associated radiation dose), a doubling of the cost of specialty drugs, and more proton facilities, he said. During the same 10-year period, the adult life expectancy in the United States increased by only 1 year, roughly half of the average gain in life expectancy achieved by other Organisation for Economic Co-operation and Development countries (2.2 years). In addition, between 1998 and
2008, out-of-pocket health care expenses more than doubled, a trend that some have termed “financial toxicity” linked to treatment (Kaiser Family Foundation, 2008; Zafar et al., 2013).
These statistics suggest that volume of care does not equate with quality or value of care in our fee-for-service system that encourages overuse, Steinberg noted. This is compounded by patients incorrectly assuming that providers prescribe with the patients’ best interests in mind when they are also motivated by financial considerations, he stressed. Consequently, some applications of new technologies and other medical innovations may not be offering good value in health care, which Steinberg defined as health outcomes divided by dollars spent through the entire cycle of care (Porter, 2010).
Steinberg pointed out that value is assessed differently by payers and manufacturers. The conceptual framework of value used by manufacturers is dominated by comparative clinical effectiveness, additional benefits, and the intrinsic value of having multiple treatment options, whereas the conceptual framework of value used by payers in the United States is dominated by comparative clinical effectiveness and budget impact, he said. Medicare in particular determines the value and reimbursement amounts for new technologies based on whether they provide “substantial clinical improvement” (see Box 6). “The best policy in the near future might be that payers become more transparent and consistent while focusing more on the balance of long-term benefits of cost in their conception of value, and that manufacturers begin to view affordability as a mutual and immediate imperative,” Steinberg said. He added that payment systems should recognize and support the need for evidence development. Yu also noted, “The question is not simply whether a technology is efficacious, but could the money spent on it be used for other purposes as well.”
Steinberg also described the California Technology Assessment Forum’s procedure for determining the value of new technologies, which have influenced payer coverage decisions (see Box 7).
Many speakers discussed challenges in generating the evidence needed to support the use of new technologies in the clinic. Topics discussed included the time and cost of research, data collection and integration, evolving technologies and expertise, and quality control.
Several speakers emphasized that one of the most formidable barriers to
BOX 6
Medicare Reimbursement Decisions
Marc Hartstein, director of the Centers for Medicare & Medicaid Services (CMS) Hospital and Ambulatory Policy Group, and Tamara Syrek Jensen, director of the CMS Coverage and Analysis Group, described the process for determining whether Medicare will provide reimbursement for a new technology and if so, what the reimbursement rate should be. Jensen noted that national coverage decisions generally take 9 months and involve 6 months of in-house discussions and reviews, after which a proposed decision is posted followed by a 30-day public comment period. At the end of that comment period, CMS posts the final decision on their website.
Legally, Medicare is expected to cover medical treatments that are “reasonable and necessary.” The operational definition CMS uses to define reasonable and necessary is that there is adequate evidence to conclude that the item or service improves clinically meaningful health outcomes for the Medicare population. In addition to reviewing the evidence that a treatment offers clinical improvement, CMS also considers whether to narrow the coverage decision for specific patient populations, practitioner specialties, provider volumes, or other factors to ensure positive outcomes. Precedents in other coverage decisions are also factored in, but as Jensen emphasized, “Any decision we make is always based on the evidence and that’s how we defend it.”
For treatments that seem promising but for which there is not yet sufficient evidence for a definitive coverage decision, CMS can use “coverage with evidence development” (CED). Technologies provisionally covered under CED would be reimbursed only if patient outcomes are documented in a registry or a clinical study. Once enough data are collected to answer outstanding questions CMS has on the technology, a final coverage decision is made.
Jensen noted that new devices being studied for Food and Drug Administration (FDA) review under an investigational device exemption may also be covered by Medicare if they meet certain criteria. In addition, Medicare patients being treated in a clinical trial sponsored by the National Institutes of Health (NIH), the FDA, or the Agency for Healthcare Research and Quality (AHRQ) are eligible to have their routine costs in the clinical trial reimbursed by Medicare, and in some cases, the investigative item or service is also covered. She also described a new parallel review process in which manufacturers provide data to both the FDA and CMS so that regulatory and reimbursement decisions can be made simultaneously.
CMS also collaborates with NIH to gather the evidence needed to make coverage decisions, and with AHRQ, which undertakes technology assessments and helps determine the questions that need to be answered with CED.
Hartstein reported that new technologies and the medical procedures in which they are used must have an established benefit category in the Medicare legal statute in order for Medicare to reimburse them. These benefit categories include physician services, services and supplies, hospital services, and X-ray, radium, and radioactive isotope therapy, including materials and services of technicians. There is a subcategory under hospital services for new technologies not yet incorporated into the hospital charges data reported to Medicare. According to Hartstein, it can take as long as 4 years for Medicare to acquire enough cost data on new technologies for the agency to determine appropriate reimbursement. Because of that long lag time, special provisions in Medicare regulations enable a pass-through payment for new technologies if they are recognized as offering substantial clinical improvement over treatments currently covered by Medicare.
As Hartstein and others pointed out, when new technologies first come into the market, there is not a great deal of evidence on which to base determinations of substantial clinical improvement. Consequently, the agency has been highly criticized by vendors for not approving enough technologies and for having opaque criteria. But he noted that if precise criteria were used, there would probably be fewer positive coverage determinations because of the need to meet some statistical test “or hard and fast criteria rather than us using our judgment,” he said. “Some things are easy to see as offering substantial clinical improvement and others are not.”
Tina Shih of the MD Anderson Cancer Center asked Hartstein why Medicare reimburses intensity-modulated radiotherapy (IMRT) at three times the rate of standard external beam radiation therapy. He responded that when reimbursing hospitals for IMRT, Medicare relies on price data submitted by hospitals. When reimbursing physicians for IMRT, more complex methods are used to determine payment rates, but essentially those rates are based on the resource inputs associated with doing the service, he said. But he pointed out that payment rates are continually being updated by CMS. “We’re always trying to refine and improve the information that we’re using to determine payment rates. We’re always striving to achieve an ideal, but we’re never actually getting there.”
Bhadrasain Vikram from the National Cancer Institute noted that NIH-funded studies found that higher doses of radiation used with IMRT benefit some patients, but harm others. For example, one study found that increasing the radiation dose for prostate cancer treatment benefited a small proportion of patients, but also caused more toxicity. He asked if Medicare coverage determinations factor in such new evidence that would limit the patient population for whom new technologies should be provided. Jensen responded that Medicare does consider such new evidence and will alter their coverage determinations accordingly, although she added, “It certainly is much harder once you’ve said ‘yes’ to then move to a ‘no.’ But we have walked down that path where if there is a clear indication that the harm outweighs the benefit, we revise our coverage determination to narrow it or pull it into coverage with evidence development.” When Vikram asked how that revision process gets started, Jensen responded that CMS can initiate it internally or initiate it in response to a request made by others. “Anyone can ask for a national coverage determination, and I’ve had several [cases in which we were] asked to do a national coverage determination to actually issue a ‘no,’” although the agency tries to take a neutral stance when responding to such requests, she said.
research on new technologies is that they can enter the market with insufficient evidence. Tunis said that because patients and their care providers can access these new technologies in clinical practice without having to participate in a clinical trial, they lack incentives to participate in studies to assess the interventions. Dignam said that the common notion that new technologies are better than old ones, compounded by easy access to these technologies, results in frequent failures of clinical trials due to lack of accrual. He gave the example of a trial of SBRT versus surgery for lung cancer, in which not enough patients were willing to be randomized to receive one or the other treatment, perceiving SBRT as a much better alternative. In addition, institutions are reluctant to conduct clinical trials of a technology, Weichselbaum observed. “Once you invest $250 million in PBRT, you are not going to do a trial that shows it doesn’t work. That’s not going to happen,” he said. Providers are also not willing to conduct
BOX 7
California Technology Assessment Forum
Michael Steinberg of the University of California, Los Angeles, described the California Technology Assessment Forum (CTAF), a panel of physicians and lay members that reviews evidence on new medical procedures, processes, and therapies to assess their clinical effectiveness and value. CTAF is funded by a foundation established by Blue Cross/Blue Shield, but operates separately and independently from them.
After CTAF conducts its systematic evidence reviews, it uses decision analysis and cost-effectiveness analysis tools to determine its findings. CTAF then rates technologies based on a combination of the net comparative health benefit (total benefits minus total harms) and the level of certainty on the evidence for that net benefit. Using that rating system, CTAF then determines a “care value” for the treatment based on cost effectiveness, including the incremental cost per outcomes achieved; other benefits and disadvantages that wouldn’t normally be tabulated in clinical studies, such as convenience of the treatment or public health benefits; and contextual considerations not related to cost, such as the burden of the illness and whether there are current treatment alternatives or whether the illness affects a high-priority population. Such care values inform Medicare coverage decisions and clinical practice recommendations, according to Steinberg.
CTAF also assesses care values within the broader health care system. A provisional “Health System Value” is determined based on the care value and the potential impact of the changes the introduced treatment would have on the health care budget. After reviewing whether mechanisms are in place to manage the affordability of the new intervention, CTAF gives the proposed intervention a “Health System Value.”
studies because of their own biases or financial incentives to keep using a technology in which they are already invested, Vikram noted.
On the other hand, it can be challenging to conduct nonbiased research on technologies have not spread sufficiently. For example, Dignam wanted to compare the outcomes for PBRT versus standard radiation therapy for the brain cancer glioblastoma. But not all cancer centers have PBRT, and
those that do may serve a different patient population than those that do not, which can bias the study.
Lack of Integrated and Accessible Data
Given the difficulties of conducting randomized trials of technologies already in use, researchers have used health care data to compare outcomes of patients receiving the new technology versus those who received standard treatment in what are known as “observational studies.” James Hoey, executive chair of Elektra Holdings, Inc., noted that nearly every U.S. hospital that treats cancer patients has a registry, originally established by the American College of Surgeons, that is codified and uses dedicated trained registrars. These registries have demographic and tumor data as well as surgical data, he said, although they lack other treatment data and outcomes data other than mortality. “The infrastructure is already here in the United States to collect good observational data and we just need more backing, prodding, or demanding by ASCO [American Society of Clinical Oncology] and ASTRO to make mandatory these datasets now considered optional,” he said. Miller added that there is also a growing effort to publicly publish surgical patient outcomes data garnered from Medicare claims.
Smith of the MD Anderson Cancer Center noted that although vast quantities of health care data are generated daily that have measures of toxicity, treatment outcomes, and comparative costs, retrospective data mining of that information can be challenging because of difficulties accessing or integrating the multiple sources of data needed to do such research. “The barrier is not so much a lack of data as a lack in design to optimally connect the data. In our current systems, data from various sources are siloed without a platform to connect, collect, and manage the entirety of data from multiple systems,” she said. She suggested creating a platform for these data that is interconnected, while carefully ensuring privacy of the individual patients contributing to these data.
Smith noted recent open-access data efforts in which patients participating in clinical trials volunteer to have their data stored in a centralized database and then disseminated to other researchers wishing to conduct additional studies beyond the clinical trial in which the patients originally participated. She added that efforts have been made to gather and integrate a number of sources of data in the National Radiation Oncology Registry. This was a pilot project involving multiple centers with an interconnected
platform that seeks to prospectively collect data on benchmark measures, practices, and effectiveness of different treatments (see Box 3).
Whelan agreed with the need to make data more integrated and accessible and suggested this be done on a national scale. “The only people who have databases are those who pay for them themselves—they raise the money to hire employees to get the data, to call the patients, and the data are siloed. We as a country need to find a way to make data collection and integration a priority,” he said.
To fully assess new technologies, researchers need cost data, but Smith noted that true cost data are difficult to obtain and there is currently no consensus on how to reconcile divergent cost data from different sources. There is also a lack of consensus on how to translate data into a quantitative metric that can be practical for actual treatment decision making, she added.
Comparative effectiveness studies also require long-term outcomes, which are often lacking, Whelan added. Beyer and Kessler also emphasized the lack of long-term outcomes data and the difficulties of acquiring such data from clinical trials. “There’s no way to get prostate cancer mortality data quickly from randomized trials,” Kessler said. “We need to gather that long-term data by pushing coverage with evidence development at the national and state level,” as well as having private insurers cover new technologies while ensuring data are gathered on long-term outcomes akin to the Massachusetts Blue Cross/Blue Shield effort, which is described further in Box 8. “Data registries should be the default, not the exception,” Kessler stressed. “When fancy technologies get on the market, coverage with evidence development should be the expectation, with only a few cases not being required to do that. This is a matter for the reimbursement agencies as well as NIH and the FDA to be more insistent about,” Kessler said. But Beyer pointed out that data registries are expensive.
Smith noted that electronic medical records are not sufficient to capture the data needed for comparative effectiveness research on new technologies for cancer treatment. Additional data are needed on long-term treatment toxicity, quality, and cost. Beyer added that electronic health records “don’t talk well to each other and we have data in a hodge-podge of different ways because every one of us uses our records a little differently.” He recommended working with industry to ensure that critical data needed to assess technologies are collected in all electronic medical records.
BOX 8
Physician–Payer Collaboration to Optimize Use of New Technologies
Jason Efstathiou of Massachusetts General Hospital described a collaboration between radiation oncologists and a payer—Massachusetts Blue Cross/Blue Shield—to determine the optimal use of intensity-modulated radiotherapy (IMRT). In this collaboration, the insurer convened an advisory council composed of radiation oncologists from 11 academic and private practices throughout the state. This council examined the evidence and noted types of cancers for which there was strong consensus that IMRT was beneficial and recommended these be covered by the insurer. For other types of cancers, the council considered normal tissue constraints on use of radiation therapy garnered from the National Cancer Institute Cooperative Group trials as well as Quantitative Analysis of Normal Tissue Effects in the Clinic (QUANTEC) guidelines. If the established constraints could not be met with conventional radiation therapy, the council recommended allowing IMRT as a reimbursable treatment (Steingisser et al., 2014).
In the two years prior to implementation of the council’s recommendations for IMRT reimbursement, IMRT use had increased by 20 percent, with conventional radiation declining by 3 percent. Within 1 year of implementing the council’s reimbursement recommendations in 2011, there was a 17 percent decrease in the use of IMRT and a 6 percent increase in the use of three-dimensional conformal radiation (Steingisser et al., 2014).
Summing up, Efstathiou stressed that the collaboration between Blue Cross/Blue Shield and radiation oncologists resulted in consensus
“Otherwise we’re going to be spending millions of dollars on registries that won’t answer questions,” Beyer stressed.
Data quality is also critical for drawing accurate conclusions from studies. “We need quality assurance for some kind of centralized system. It is not as simple as making sure the databases can talk to each other,” Smith said. Dignam noted that high-quality data registries have features that trial databases typically have, such as active data ascertainment, clearly defined inclusion criteria, and data auditing. He added that registries may be more comprehensive because they often include data that are not collected in clinical trials. He suggested that if high-quality statistical analyses are
development of IMRT criteria despite a lack of high-level evidence (Level 1 evidence based on randomized controlled trials). Such criteria reduced the use of IMRT and radiation therapy expenses. Blue Cross/Blue Shield in Massachusetts estimated that in the first year they saved $4.7 million, while administrative expenses remained the same, and their review process for IMRT claims became simpler. “This established a community standard of care in collaboration with providers that may be a useful model for other new technologies for which the science is not mature and the clinical outcomes data are evolving,” Efstathiou said.
He added that the council of radiation oncologists convened by Blue Cross/Blue Shield in Massachusetts continues to meet to update their IMRT guidelines and to discuss creating guidelines for other advanced technologies, such as stereotactic radiation techniques and proton beam radiation therapy (PBRT). “We should encourage similar collaborative proactive models of payer involvement,” Efstathiou said. He noted that Blue Cross/Blue Shield has been similarly proactive in Michigan, where it is helping to support a registry on IMRT use in breast and lung cancer in which care providers at the University of Michigan and elsewhere in the state are actively participating.a This registry will include measures of toxicity and quality of life. In response to a question, Efstathiou commented that the Massachusetts model for determining IMRT reimbursement standards is scalable and could be applied nationally.
__________________
aSee https://mroqc.org (accessed November 16, 2015).
applied to such data, they may be more useful than clinical trials. However, Dignam also cautioned that there have been many data-mining disasters that led to erroneous conclusions. He said it is critical for investigators to understand the limitations of the data and the inherent risks of retrospective data analyses.
Long Time Frames and Large Number of Patients Needed
Prospective studies with a prespecified plan for data collection and analysis at the study outset have their own challenges, however. These stud-
ies typically take long periods of time to complete. In addition, prospective clinical trials need to enroll large numbers of patients if the differences among treatments are small, Zietman noted. Dignam also emphasized the need to enroll many patients to ensure the reliability of conclusions in RCTs, and that there is a trade-off between confidence in one’s results and the size of the trial, with smaller trials often unable to reliably demonstrate that one treatment is superior to another. He stressed that showing a treatment is not inferior to another in what is known as a “non-inferiority trial” is not the same as showing it is a superior treatment. “We need to be clear when we’re designing and interpreting studies, what exactly we’re aiming to achieve,” he said. Patients not complying with the treatment they were randomized to receive can also make clinical trials difficult to interpret, with such noncompliance rendering the two arms of the study similar, Dignam added.
To detect late complications, data collection in a trial must continue over a long period of time, said Beyer, emphasizing that “we need follow-up to look at end results that are meaningful.” For example, detecting a reduction in the rare secondary cancers that develop due to radiation treatment requires long-term trials with thousands of patients, Dignam said. But during the 10 years that may be needed to complete a trial, methods of treatment may have changed, or the technology may have become outdated, making the results of the trial potentially invalid or irrelevant, Smith and Zietman said.
Evolving Technology and Expertise
Evolving expertise regarding new technologies as well as evolution in the technologies themselves that make them a moving target also hamper retrospective data-mining studies of comparative effectiveness, several participants said. These changes over time can also affect the reliability of clinical trial results. This is especially true for new surgical procedures and devices. “How can we design trials that would help us prove the next technology when that technology has a steep learning curve and when the people who are popularizing it are just figuring it out as they go?” Beyer asked. Miller responded that the United Kingdom does postimplementation surveillance for new surgical procedures and has a process for carrying out pragmatic trials, but he said a similar framework would be difficult to adopt in the U.S. health care system.
When conducting clinical trials, researchers should have a process to ensure the quality of care delivery, especially for innovative technologies such as radiation therapies or laparoscopic or robotic surgeries, Chmura and Zietman noted, but such quality assurance is increasingly inadequate because of research budget cutbacks, Chmura said. Vikram pointed out that the NCI still demands quality assurance for clinicians participating in the IMRT studies they fund. “You have to at least radiate a piece of plastic accurately in an NCI-funded trial,” he said, but in community practices in most states, there are no performance requirements for treating with IMRT. Lawrence noted that in a recent Dutch trial of a stomach surgery technique, participating surgeons were trained in the technique before the start of the trial. Ashley added that some U.S. trials of sentinel node biopsies, total mesorectal excision for rectal cancer, and other operations have had credentialing requirements for participation, including a minimum number of cases and submission of a video or a photograph of the specimen.
Others noted the difficulty in conducting double-blind studies of surgical procedures. “In surgery it’s very hard to have a double-blind study because you know what is being applied,” said John Gardenier, who is retired from the Centers for Disease Control and Prevention, adding “you cannot have a placebo effect by not treating patients.” In addition, surgeons may be reluctant to test the effectiveness of new techniques that many view as adaptations of current procedures. Dignam noted, “You’re tweaking or modifying a procedure, so the expectation is it will be good or better perhaps, and that the effects are incremental or a logical extension of existing treatment. Similarly, when we vary radiation doses or delivery schedules, we’re not looking for profound differences so perhaps we don’t have the same expectation of testing.”
A lack of funding for trials of new devices is also problematic, Steinberg noted. “Device companies are a fraction of the size of drug companies. How do we get the funding to do the needed trials?” he asked. Jay Roy, Elekta (a device manufacturer), added that “the margin a device company makes on a device versus what a drug company makes on a drug is not even comparable.” Zietman responded that financial incentives are needed to spur trials of devices. “There has to be some combination of restriction of who
can use the new technology and a tie-in between new technology use and evidence development and payment. That is the only way around it I can see,” he said.
Yu referred to a 1979 Institute of Medicine (IOM) report that recommended federal funding of large-scale technology development projects and pointed out that insufficient or “on-again-off-again” funding for technology development has had disastrous effects (IOM, 1979). More federal funding for technology development is still needed today, according to Yu. “Since about half of all cancer patients require radiation therapy and only 1.6 percent of NIH cancer funding went to radiation-related research in 2013, we need to greatly increase NIH funding for radiation oncology-specific research and fund new technology assessment,” Yu said (Brown and Adler, 2015; Steinberg et al., 2013).
A portion of the workshop was devoted to discussion of the advantages and limitations of data-mining and observational studies versus RCTs, as well as ways to design studies that can more easily and reliably assess the effectiveness or comparative effectiveness of new technologies.
Observational Studies Versus Randomized Controlled Trials
Retrospective observational studies that analyze data already collected are affected by biases in the data that can persist even when statistical methods are used to account for such bias, Smith stressed. For example, the patients, physicians, or facilities who opt to adopt and use new technologies may have different characteristics compared to those that do not. There can also be differences based on when patients were treated that makes comparisons biased, especially given that technologies and the skills of physicians who use them tend to improve over time, she added. Efstathiou noted that because of private insurers’ frequent unwillingness to cover treatment with certain new technologies for patients under the age of 65, studies of such technologies tend to be more populated with elderly patients on Medicare, which can bias their results.
Several speakers pointed out that RCTs often do not confirm the findings of studies done on data from SEER and Medicare databases. They expressed concern that the populations compared in the latter studies are not balanced and that researchers fail to correct for biases. Miller said, “We
don’t have the resources to do an RCT in every situation,” but that even when observational studies are done well, with adjustments for potential biases, there is often still disagreement in the results of these studies compared to those from RCTs. “This is a tough nut to crack,” he said.
Yu noted that previous IOM reports recommended that clinical investigators use other methods in addition to RCTs for evidence generation because of the high costs of clinical trials and the rapidly changing nature of technology, though appropriate judgment is needed to assess the loss of information against the gains in technical and economic feasibility (IOM, 1979, 2013). “We need to enable and use a learning health care system to provide insights and evidence. We need to let the data talk to each other and set the data free,” Yu said.
But Lawrence noted that complications are often not as well documented in observational data as they might be in an RCT in which people are asked specific questions at certain times during the course of treatment. He asked when an observational study is sufficient and when an RCT is necessary. Jagsi responded by noting that the observational data often cannot be used reliably to determine whether the intervention has caused the outcomes measured. In addition, observational data may not accurately capture an outcome of interest, such as toxicity. “Observational data have greater generalizability and RCT data have better causal mechanisms, so when you put the two together you can really be like the blind man and the elephant and feel out different parts. They are complementary sources of information we need to pursue,” she said. Wright agreed from a surgical perspective, noting that whether observational studies can be done depends on the quality and extensiveness of the data source, with some medical records not sufficiently capturing complications, whereas the National Surgical Quality Improvement Program abstracts patient charts and has a very defined data collection process. “Probably we need to rely on multiple sources of data,” he said.
Tunis noted that “if we limit our evidence generation to the 3 to 5 percent of patients who enroll in trials, we’re not going to make a dent in the number of questions we really need to answer.” In addition, RCTs are slow and expensive, and although “they will get the right answer, by the time the answer comes out, it’s sort of like the game is over because we’re on to the next generation of technology,” he said. Consequently, Tunis suggested exploring options for equipping the health care delivery system to more efficiently generate evidence. “Is the RCT ready for retirement and do we need to come up with new models? It’s really important to think about methods
that have a way of generating evidence that’s in some way consistent with the kinds of decisions we need to make and the evolution of health care,” he said. At the same time, he noted the shortcomings of observational studies. “The unfortunate truth is sometimes with anything short of a randomized controlled trial, you get the answer horribly wrong, in which case you have harmed a lot of people before you figure that out.”
Mohler noted that randomized trials are not done to determine if other new devices, such as iPhones, are better. Instead, manufacturers do statistical process control in which they serially capture data to assess if a series of events worsen or improve. They use this information to determine if the new device is better, and can make such determinations at a much quicker pace than researchers conducting clinical trials on new medical devices. “We should be bold and get rid of randomized controlled trials. We need to just say they’re impractical. It takes thousands of events and patients to figure out which treatment is better, so every time somebody says, ‘We need a randomized clinical trial,’ I just say, ‘You all are dreaming.’ We can’t afford the cost or the time for it.”
Robert Carlson, CEO of the National Comprehensive Cancer Network, responded, “No matter how granular registry data are, there are biases that occur, and sometimes strong biases that occur before the data are ever generated.” He gave the example of high-dose chemotherapy for high-risk metastatic breast cancer. Data from nonrandomized observational studies indicated that this treatment offered a major survival advantage. But the RCT showed that the favorable results were due to a selection bias, and that the high-dose therapy was not more advantageous—and actually may have been deleterious due to the severe toxicities associated with the treatment regimen. He noted that medical oncologists were very skilled at selecting patients who could tolerate high-dose therapy. “These women were healthier and more fit, so they did better on the high-dose therapy. The results seen had nothing to do with the actual therapy that was delivered,” Carlson stressed. “There are important clues and information we can get from nonrandomized, high-quality registry data, but we have to be very cautious that we don’t get fundamentally misled and follow a pathway that has nothing to do with the effectiveness of treatment, but everything to do with the biases of physicians.”
Mohler responded by pointing out that “we are overly obsessed with randomized clinical trials.” He noted that often such trials for advanced prostate cancer show that new agents extend survival between 2.4 and 5 months. “As a surgeon, I think, ‘So what.’ There are places where
randomized controlled trials need to be done, but we can’t get obsessed and say we can’t adopt any new technology without one,” he said. Mohler also suggested exploring more innovative statistical designs for observational studies that can offer alternatives to RCTs.
Steinberg referred to a journal article in which the author suggested the appropriate balance between RCTs and observational studies (Rawlins, 2008). He also pointed out that in the current era of precision medicine, an infinite number of RCTs will need to be done on all the subtypes of cancers that are being identified or their results will not be generalizable, “so we need to accept all types of evidence to move the field forward, particularly as it relates to technology.” Beyer added that if RCTs are taken to the extreme, an RCT would be required before a new technology, such as IMRT, can be used on each organ in the body, on each disease, and on each stage of the disease. “There are times when a randomized trial can answer an important question, and there are times when the randomized trial just isn’t going to be the way to go, but we need to have other methods that we can look at, other ways that we can answer questions in ways that the larger world will look at and believe,” he said.
Kessler agreed and stressed the need for a framework to determine what kinds of studies need to be done and when. He added that observational studies “are good for generating signals and should be part of the learning health care system that gives us feedback that we can then use to generate the right next kind of studies.” He suggested a structured, well-defined, stepwise process to sequentially conduct more rigorous studies, to replace the ad hoc way in which evidence is currently gathered. “We should progressively have more rigorous, more expensive, and higher validity studies, and at some point in that continuum, the payers will agree there’s enough evidence to pay for it, but before the randomized trials are done,” he said. Dignam suggested taking an observational-randomized controlled clinical trial approach in which a trial is embedded within a registry that captures all the data needed. This would be more expensive to do than typical clinical trials, but worth doing, he said.
Dignam reported on several new study methods that can be used to assess the comparative effectiveness of new technologies, including cluster RCTs, causal inference analyses, and propensity score adjustments. With cluster RCTs, instead of randomly assigning patients to receive a new
technology or not and then comparing outcomes, institutions or centers are randomized to use the new technology or procedure and are compared to those not using the new intervention. Cluster RCTs are easier to implement than standard RCTs, Dignam noted, but there can be confounding of the center and treatment. For example, patients treated in centers in urban areas that are large enough to support a proton beam facility may differ from those patients treated in centers that lack the technology, and those differences may bias results.
However, Kessler pointed out that a special cluster RCT known as a “stepped-wedge trial” may be feasible for generating unbiased results if the diffusion of new technologies can be systematically controlled via gradual introduction of reimbursement at different centers. With such a trial, researchers may be able to collect valuable outcomes data that are similar to those collected in a standard RCT, he said. Kessler added that investigators are increasingly using stepped-wedge studies in comparative effectiveness research. He also suggested conducting international trials because some countries adopt new medical technologies more readily than others, and researchers can use this variability to assess the benefits and costs of a new technology or procedure. “The international market is not so dissimilar from us, but they are slower to adopt,” he said. Stepped-wedge study designs and international studies “would give a lot of bang for the buck and there are opportunities for companies, regulatory agencies, and NIH to leverage this,” Kessler said.
Some experts claim that large databases can overcome some of the inherent bias in data mining, but Dignam stressed, “Big Data is not really the answer because it just puts more precision on biased estimates. You really need bias control, not just bigger numbers.” One way to control bias so researchers can determine whether an association between a new technology and certain outcomes seen in observational studies is causal is to model the data and make adjustments for possible confounders and biases. Propensity score adjustment is a more systematic approach to such modeling that determines the probability of a given treatment choice, based on the factors that influence that choice, and then adjusts treatment groups accordingly using stratification, matching, or weighting (Austin, 2011). Propensity score matching, for example, attempts to mimic randomization by creating a treatment patient group that is comparable on all observed factors to those not receiving the treatment. Instrumental variable analysis is another systematic approach for modeling observational data and involves identifying variables strongly related to treatment choice, but not outcomes.
Standardization of these factors can control for known confounders similar to randomization, according to Dignam (Hadley et al., 2010).
Dignam ended his presentation by suggesting that researchers “use the best methods available and work hard to get good end points that are feasible in this changing technology landscape. We need to think about more creative trial designs and how far we can go with them and improve trial accrual.” He added, “Technology raises evidence-generation challenges, but they are not insurmountable and patients deserve the same high-level evidence for technologies as for other [treatment] modalities, and certainly in the current environment, the [health care] systems and payers expect the same.”
Vikram raised the possibility of keeping a registry of patients who decline to be randomized in clinical trials so that those results could be compiled and used in studies for comparison. Dignam responded that he did not know of any cases where this had been done, but he said that in theory, such an approach should not undermine the validity of the randomized trial. Efstathiou noted that in his study of prostate cancer treatment, he is collecting information on patients who do not accept randomization for treatment and those who volunteer to enroll in the study, but then withdraw for lack of insurance coverage of the treatment under investigation.
There was some discussion about how studies should be designed to ensure that the results are not only reliable but also applicable to the clinical populations who will ultimately be treated with the intervention studied. Participants also discussed the need to generate data on quality of life and cost effectiveness to inform treatment decisions by patients and their physicians.
Ganz asked if there is a way to base device approval on studies done in appropriate populations, noting that older populations often are not included in trials of new treatments, even though such treatments are later commonly prescribed for elderly patients. Jensen agreed that if results from a trial cannot be generalized to certain populations, it will be hard to make treatment decisions for that population. However, she cautioned that having adequate representation of various populations should not be a rate-limiting step in clinical research. Yu responded that clinical trials frequently do not have sufficient representation of all relevant patient groups and reiterated the importance of incorporating novel forms of comparative effectiveness research as a complement to RCTs.
To aid clinical decisions, Steinberg suggested that studies should include quality-of-life measurements and other meaningful health outcomes that patients identify with and value. In addition, Efstathiou stressed that once appropriate end points are determined and measured, researchers have to decide which findings are clinically meaningful versus just statistically meaningful. Dignam agreed, noting, “It no longer flies to talk solely about the change as a percentage of the mean or something. We need to translate it back into what does that mean on the ground, in terms of the patient’s experience, and that can be elusive,” he said. Weichselbaum added that it is important not only to determine what a clinically meaningful difference is, but how much that difference is worth. “If there’s a 2 percent difference that is statistically significant, is it worth $10 billion to have the new technology adopted all over?” he asked. Efstathiou agreed that is a valid question that underlines the importance of including cost-effectiveness assessments as secondary objectives in clinical trials. In a clinical trial in which he is involved, researchers are collecting both direct and indirect cost data, using health care utilization forms over time, and developing relationships with payers who provide much of the cost data.
TRAINING AND MONITORING CLINICAL PERFORMANCE
Even if a new surgical procedure is shown to have clinical value, several participants noted that surgeons may not have enough experience with the procedure to perform it properly due to insufficient training and a lack of rigorous credentialing and clinical privileging. Ron Kline, Medical Officer for the Patient Care Models Group at the CMS Center for Medicare & Medicaid Innovation, said that a five-fold variation in the complication rate of prostatectomies has been noted even at a large and well-respected, high-volume institution, so “If you just simply monitor the surgeons and improve their quality, you would have a much greater effect on health care in prostatectomy in the United States than anything a robot has been able to achieve.” Miller agreed, saying, “Ultimately it is the surgeons who are doing the operations and not the robots.” Hu also noted that injuries and death that occur during robotic surgery are largely due to the inexperience of the surgeons that use the robots. Concerns have also been raised about whether surgeons who have been trained primarily using robotic technology have the skills to convert to open surgical procedures should the need arise due to complications encountered during surgery.
Training and Credentialing of Surgeons
Ashley reported that the Accreditation Council for Graduate Medical Education (ACGME)8 oversees surgical training in medical residency programs and the American Board of Medical Specialties oversees surgeons’ Board Certification in subspecialties, as well as their Maintenance of Certification (MOC), a system of ongoing professional development and practice assessment and improvement. Ashley noted that the MOC practice assessment for general surgery is minimal and requires surgeons to participate in a national database “that is not really providing surgeon-specific data or really anything on [long-term] outcomes,” he said.
In addition, hospitals have their own credentialing for their surgeons that confirms their qualifications, as well as privileging that authorizes them to perform specific patient care activities. State licensure is required, and the Joint Commission9 provides some general guidelines for credentialing and privileging. Surgeons must also have the proper credentialing to bill CMS. Initial credentialing is based on confirmation that the surgeon has attained a medical degree, fulfilled the requirements of residency, is board certified, and has no history of malpractice or criminal offenses.
Privileging is based on a review of the surgeon’s training, expertise, and scope of practice by the chief or chair of surgery. Most specialties have a set of core privileges usually acquired during residency as well as advanced privileges, the latter being surgical procedures done so infrequently that training in residency is insufficient and candidates need additional oversight before they can perform these procedures on their own. The Joint Commission recommends a focused practice performance evaluation that is applied to new staff members during their first 6 months and ideally requires another physician closely overseeing their care.
Advanced privileges may require precepting or proctoring by senior partners in a practice. A preceptor, usually a more experienced surgeon in a practice, helps the learning surgeon acquire new skills by overseeing their surgeries and providing feedback about their performance. He or she oversees the learner’s surgeries, assists in some complex cases, and takes over the surgery when necessary. But as Ashley noted, “People have gotten too busy to do that. In an academic setting that’s easier, but [precepting] still doesn’t happen with any regularity.” A proctor’s role is to assess skills and report
__________________
8 See http://www.abms.org/board-certification (accessed September 11, 2015).
9 See http://www.jointcommission.org (accessed October 15, 2015).
back for privileging decisions. Proctors are generally observers who did not participate in training or precepting. But even proctoring, which requires a regular presence in the operating room to assess the skills of the surgeon, is unusual, Ashley noted. “How much precepting or proctoring happens varies widely depending on the institution,” he said.
Ashley pointed out several challenges to training, credentialing, and privileging surgeons, including significant variation in the volume of procedures, the independence of the trainees, and the assessment of surgical skills. “The program director signs something at the end that says the trainee is competent to practice, but there’s very little assessment of individual procedures and everybody has a different learning curve,” he said. He noted that the ACGME is moving toward having competency-based training, but this is also challenging because of the lack of validated direct assessment tools. Miller mentioned that a group of surgical experts are currently using a systematic approach, known as the Delphi method,10 to identify key steps of surgical procedures as well as a grading metric for those steps. “Eventually that should lead to better outcomes so you don’t have to do a thousand surgeries to be where you need to be,” he said.
Another challenge to certifying a surgeon’s competency to perform certain surgeries is the debate about what skills learned performing one type of surgery are transferrable to another. For example, does the ability to perform one operation on a specific organ certify a surgeon to perform a much different operation on the same organ? “There are limits to what skills are transferable and where those lie, which I don’t think anybody has defined,” Ashley said.
He also stressed that there is a difference in being able to confidently perform an operation versus being proficient and mastering the technique. He said many surgical procedures have long learning curves, and a growing body of data show that skills for surgical procedures continue to improve until surgeons reach their 50s, after which they start to decline. He noted that in many countries in Europe, surgeons continue to work under a senior supervising professor until mid-career. “That’s very different than finishing an ACGME residency and believing you are competent to go out into practice,” Ashley said.
Ganz and Whelan concurred, noting that low volumes for certain surgeries preclude the development of expertise at some institutions. “We have no way to corral the appropriate volume in a [care] setting in the U.S., and
__________________
10 See http://www.rand.org/topics/delphi-method.html (accessed October 15, 2015).
in our own institution, we can’t get the few surgeons that do only two low anterior resections a year with horrible results to stop doing it. The chair tries to stop them, but they claim they have trained,” Whelan said. Ganz asked, “It may take 10 to 15 years to learn how to use a robot effectively, so does it make sense for us to allow any surgeon who says she or he is qualified to do this kind of procedure to do it? The young people coming out of surgical residencies today may have the newest technologies, but they don’t have the years of experience that the folks out in the field have. How do we manage this tension of volume experience that the mature surgeon has with technical innovations, which may or may not be valuable?” Miller noted that three academic institutions announced recently that they would implement strict volume standards for a number of surgical procedures, including some that were cancer related.
Several participants suggested establishing high-volume specialty centers in which physicians have expertise in a new technology. “There is abundant evidence from a lot of specialties that the more you do, the better you get at it. If there were better organization of where we access high-technology, we would have more experienced physicians doing it,” said Weichselbaum.
Hu noted that due to limitations in funds in its national health care system, Canada has only 10 robots, which only a few surgeons use to operate in Centers of Excellence. “There is some inequity in terms of how technology rolls out there, but it was done more thoughtfully than in our free-market economy medical care fashion. Clearly there is need for regulation when you think about the learning curves and the sacrifices of patient outcomes made along those learning curves,” he said.
Ashley concurred and noted that there is an increasing number of institutions developing skill centers and making large investments in training programs. He noted that Methodist Hospital is trying to position itself as “the place surgeons come from all over Texas to get credentialed, recredentialed, work in a skills lab, or get proctored to do robotic surgery,” and the American College of Surgeons is currently accrediting such education institutes.
Monitoring of Surgical Performance
The Joint Commission recommends ongoing professional performance evaluations for physicians more than once per year, Ashley reported, and recredentialing is usually done every 2 years. Ideally, recredentialing should
be based on outcome measures, such as complication and fatality rates, length of hospital stays and readmissions, and appropriateness of care, he said. But he added that in general surgery, none of the commonly available measures are surgeon specific and “nobody does enough of any single procedure to get good data that are risk adjusted so you can use them to compare outcomes.” Consequently, surgeons are recredentialed based on process measures, such as medical record completions, emergency room availability when on call, etc., as well as patient complaints and malpractice cases.
Ashley noted several challenges in monitoring a surgeon’s performance, including a wide variation among different institutions in their performance evaluation criteria; a lack of risk-adjusted, physician-specific data; and physicians working at multiple facilities that do not share data between them. But Miller stressed that collecting and sharing such performance data are critical. He said his facility participates in a surgical registry and works with Blue Cross/Blue Shield to provide to each surgeon on a quarterly basis their comparative complication rates as well as comparative patient-reported quality-of-life outcomes. “The degree to which that has accelerated interest in and commitment to improvement is remarkable, and without such feedback the ability to improve is difficult. There has to be a feedback loop on quality improvement,” Miller said.
Training and Oversight for New Procedures
Ashley noted that although training in new medical procedures previously often followed the maxim “see one, do one, teach one,” medical residents today do not have the opportunity to function independently when doing surgery. “They don’t come out of training with the confidence or the ability to safely do a new procedure themselves and we haven’t compensated for that,” Ashley said. He noted that laparoscopy created a big technical change in surgery and doctors were performing the procedure after taking only a 2-day class on it, in which they practiced on animals. This led to an increased complication rate initially for certain laparoscopic surgeries.
Currently there are no standards for what is considered “new” in surgery and there is no national review process for new procedures, Ashley reported. No organization oversees surgeons’ adoption of new procedures beyond hospitals or surgery departments, although payers can limit adoption to some extent. In addition, there is no oversight for training for new procedures. Such training often falls by default to device manufacturers who provide it in the operating room. “There’s a gray line between whether the
manufacturer is teaching or helping the surgeon do a new procedure and whether they are actually doing the procedure,” Ashley said. He added that new procedure standards for privileging take time to ascertain. “We didn’t know what was needed for robotics initially,” he said. He also stressed that “the need to stay competitive may trump patient safety,” with financial incentives sometimes fostering premature use of a new technique.
Ashley summed up his presentation by saying, “We have a pretty significant credentialing gap, not just for new procedures, but for the initial privileging process and recredentialing.” He also pointed out the need to not make training, credentialing, and privileging so onerous that beneficial new techniques do not spread quickly, giving the example of the slow adoption of sentinel lymph node biopsies at small rural institutions many years after national organizations stipulated the procedure as the standard of care. Fifteen years after the technique debuted, only 50 percent of rural surgeons were doing these biopsies compared with 80 percent of urban surgeons, Ashley said. Striking the right balance in credentialing “is something we need to think about as a profession,” he added.
Ashley made a few suggestions for how to improve the training and monitoring of surgical performance, such as credentialing and regular testing in simulators akin to how the Federal Aviation Administration tests the skills of pilots. Vikram stressed, however, that unless there is enforcement of the use of these virtual tools, people may not use them. Mohler noted that Roswell has a program that simulates robotic operations, a lab for surgeons to practice their operations on pigs, as well as an observation program in the operating room in which more than 300 surgeons from all over the world have participated. “We realize that no one is wise enough to do robotic surgery alone. It’s a team enterprise and we always test drive in the porcine lab anything that’s new, whether that’s a procedure, surgeon, or assistant.” He mentioned that he had not done a robotic surgery in 3 weeks, and plans to use the simulation lab “to warm up and make sure that I can do justice to the person sitting in front of me,” he said. He cited a study that found robotic simulators useful, as well as one that found simulation-based training to be economically feasible (Chowriappa et al., 2015; Rehman et al., 2013).
Ashley noted the need for other validated tools to assess surgical proficiency and described one attempt to do this, in which 20 bariatric surgeons had their surgeries videotaped and their procedures assessed by 10 reviewers using objective standards to assess their technical skills. This study found a correlation between the skills ratings of the surgeons and their complication
rates and use of health care resources due to readmissions, emergency room visits, and having to repeat the surgery (Birkmeyer et al., 2013). “This is the kind of data we need and where we’re headed,” Ashley said.
Miller noted a study by Hu which found that video review of surgical residents’ robotic prostatectomy procedures combined with performance feedback from peers on a social media site was linked to residents feeling more comfortable with robotic surgery and more satisfied with the learning experience (Carter et al., 2015). Similarly, in another study, videos of robotic prostatectomies performed by experienced surgeons were reviewed and ranked by their peers as well as anonymous “crowd workers” from Amazon’s Mechanical Turk Program (Ghani et al., 2016). “We’ve been able to show that if you assess technical skill using validated instruments and fully trained surgeons, there is a gradation of skill as assessed by your peers. There’s tremendous promise for the ability of video review to identify surgeons that may benefit from coaching or additional proctoring,” Miller said. “If we are going to be doing robotic surgery in a majority of patients, we need to not only focus on selecting the right patients, but also think about how to improve our technical skills in this area,” Miller concluded.
Gardenier noted use of the American Board of Surgery In-Training Examination (ABSITE) scoring11 in the initial credentialing for new general surgeons and asked if something similar could be applied to new technologies. Ashley responded that ABSITE and other similar tools are designed as learning tools to identify gaps in knowledge, but not to assess the proficiency of practitioners.
Ashley said there is increasing interest in providing coaching for new surgeons, or for surgeons learning new techniques or procedures. Such coaching could be done by retired surgeons, he said. He also described the role of Advanced Procedures and Technology Committees (APTCs), which review new technologies not currently offered in hospitals. Such reviews determine whether the new procedure is an extension of procedures already privileged, meaning it entails transferrable skills, or an advanced procedure or technology that will require additional training and privileging. A physician or institution submits the review request with information regarding the procedure, indications, potential benefits and risks, and necessary equipment, training, and privileging criteria. The APTC multidisciplinary committee of experts then assesses whether the facility has a need for the treatment or device and recommends a training and privileging plan. Ashley also pointed
__________________
11 See http://www.absurgery.org/default.jsp?certabsite (accessed September 11, 2015).
out that the American Association of Gynecologic Laparoscopists developed a set of guidelines for privileging that includes privileging criteria for both basic and more advanced procedures (AAGL, 2014).
Ashley noted that at Brigham and Women’s Hospital, privileges for gynecologic robotic surgery require the surgeon to take a validated skills course, show proof of robotic proficiency, and then undergo a preceptorship with an expert robotic surgery proctor for a minimum of three cases. Proof of proficiency can be a formal standardized postresidency training that includes dry and animal laboratory practicums with industry certification, at least three case observations, and evidence of full proficiency at digital simulation. Alternatively, proof of proficiency can be based on formal postgraduate-level training that includes a minimum of 15 cases with more than 20 percent console time within 12 months, and evidence of full proficiency at digital simulation. Surgeons from other hospitals applying for robotic privileges require a letter from their previous department chair certifying that they have conducted a minimum of 10 cases (Gargiulo, 2014).
Renewal of robotic surgery privileging requirements at Brigham and Women’s Hospital depends on how many cases a surgeon has done per year, with renewals denied to those who have done no cases, and with no requirement for demonstration of skills or proctoring for surgeons who have done 12 or more cases. Roswell requires surgeons to do at least 10 robotic cases per year for their credentialing, whereas UCLA requires 10 robotic cases over a 2-year span, according to Mohler, who noted that he personally “would not let anyone operate on me who does 10 robotic cases a year—that’s not even one a month.”
Vikram also noted that facilities often fail the credentialing test required for participation in clinical trials of advanced radiation technologies. “Depending on the anatomic site, between one-third and two-thirds of the facilities fail that test on the first attempt. And these are the crème de la crème as many of them are academic institutions that participate in NIH-funded clinical trials,” he said. Nonetheless, there is no requirement in most states for physicians using the radiation technologies to show their proficiency, as is done for participation in a clinical trial. He asked, “What is the responsibility of the profession versus the government in implementing or enforcing something like this?”
Bekelman agreed that “clinical trial credentialing can sometimes be more stringent than the quality assurance that happens in the routine real world.” He added, “It’s an important question to consider as the technologies become much more advanced, especially with proton therapy, where
we know that the beam at one institution is not like the beam at another.” Hu agreed that more rigorous standards need to be applied to training and credentialing of both radiation oncologists and surgeons. “We all recognize that there’s a need for this and all it will take is a highly publicized case for regulation to come in, in the absence of surgeons or others being more proactive about the quality improvement that needs to take place,” he said.
Several speakers described payment models and related approaches that could encourage evidence gathering on new treatments, and help to ensure they are being applied appropriately. Topics discussed included
- coverage with evidence development;
- accountable care organizations;
- new pricing schemes and price transparency; and
- delivery system innovation.
Yu, Hahn, and Tunis all suggested greater use of coverage with evidence development (CED) to enable continuing collection of data on use and outcomes after a technology enters the market. Yu suggested applying CED to all new radiation technologies so that all patients undergoing treatment with an innovative radiation technology are enrolled in a study that assesses outcomes. When Chmura raised the question of who will pay for the development of the large registries needed for CED, Jensen responded that if CMS makes a national coverage determination for a new technology that entails CED, then it will pay for the new technology and related costs of care, but it does not cover the costs of a registry, which are usually supported by specialty societies, academic centers, or manufacturers. “It’s a shared responsibility,” she said. Tunis added that “there needs to be a systematic and well-defined framework that we make sure everybody follows,” noting that payers are often reluctant to partner in the evidence generation process due to a lack of consensus on what needs to be done when and how.
Tunis also stressed that CED recognizes that “the accumulation of certainty about the risks and benefits of new technologies is a continuous function. There’s an artificial notion that at some magical moment a new intervention goes from being investigational to being medically necessary, but that’s clearly a fiction. Yet that’s the sort of fiction payers live by.” He added, “Having policy mechanisms that are more flexible and adaptable
and that allow for coverage of emerging technologies in the context of clinical investigation is incredibly important.” But Tunis also noted that CED is difficult to implement, with the Medicare statutory authority for it “extremely weak,” he said, and “private payers, with a few exceptions, are not generally enthusiastic. These projects are incredibly labor intensive.”
Tunis said the move toward accountable care organizations12 (ACOs) and a greater emphasis on population health might appropriately constrain rapid diffusion of new technologies while promoting more coverage with evidence development. Hu pointed out that ACOs are increasingly offering an alternative to fee-for-service payments, and suggested the move to ACOs will be accompanied by self-policing within surgical departments and by hospital administrators. He said this could result in patients having to pay more out of pocket for robotic prostatectomies and other procedures of uncertain value, as occurs in countries where insurers do not cover these procedures.
Bekelman suggested several other potential alternatives to fee-for-service payments that might offer better incentives for appropriate use of new medical technologies. One option, a risk-sharing model, would be to increase the professional fees of oncologists while giving them responsibility for all other costs, with the expectation that they will lower costs of care. This is an approach used by CMS in its recently developed Oncology Care Model.13 Alternatively, they could be paid a lump sum for each patient. Such bundled care is another form of risk sharing.
The risk-sharing model provides incentive to oncologists to minimize costs by minimizing hospitalizations and unnecessary treatment. Risk sharing could nudge innovation toward lower-risk and lower-cost technologies and accelerate de-adoption of inappropriate technologies, Bekelman said (Bekelman et al., 2014). “We might not be treating with extended fractionation schedules as much if we knew we only had a lump sum of money to work with. But at the same time, this risk sharing could have unintended consequences because radiation oncologists could cherry-pick [patients], induce demand [among] other patients . . . or stint on care,” he added. Bekelman also noted the numerous challenges in determining how to price treatments in a bundled care payment system for radiation therapies, given the multimodality field of radiation oncology and the fact that radiation oncologists are not responsible for much of the care that cancer patients
__________________
12 See https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/ACO/index.html (accessed October 15, 2015).
13 See https://innovation.cms.gov/initiatives/oncology-care (accessed November 3, 2015).
receive, including whether they are admitted into hospitals or whether they have surgery or chemotherapy.
Price transparency for patients is another way to potentially encourage appropriate adoption and de-adoption of technologies, Bekelman and others noted. Steinberg stressed, “The provider and the patient don’t possess the same information about cost and quality, which precludes patients from picking the best possible health care.” One study found that when patients were given the opportunity to compare the costs of treatments they could receive, they chose less expensive laboratory tests and imaging, compared to those not aware of costs (Whaley et al., 2014). “Price transparency may impact oncology because . . . patients have a real sense of different treatments and their costs,” Bekelman said.
Patient advocate Gwen Darien, executive vice president of programs and services at the Cancer Support Community, noted that although some cancer patients may opt not to pursue a very expensive treatment if they thought the costs were so much greater than the potential benefits, others will want to do anything possible to treat their cancers. But another cancer survivor, Thomas Farrington, stressed that if patients knew not just the costs but the values of new technologies, they could make better decisions and foster the proper adoption and de-adoption of new treatments. If patients knew, for example, that the outcomes for two treatments are likely to be the same but one is more expensive, then they would be more likely to choose the less expensive but equally effective alternative and that could lead to more appropriate use of new technologies, he said.
For cost transparency to be effective, there needs to be shared decision making between patient and provider, Bekelman stressed. “It’s important to describe not just the outcomes that are important to our patients, but also the associated costs with those outcomes, and to work with patients to make that decision together as part of participatory decision making,” he said. Smith added that it is important when conveying the cost and value of a treatment to patients to discuss not only costs to payers, but costs for patients themselves, including out-of-pocket costs and productivity costs. But the degree to which patients want to engage in shared decision making about their treatments varies from patient to patient. Jagsi stressed the importance of determining with patients how much they wish to be involved in the decision making and what is important to them. “We need to bring cost into these discussions in a sensitive way that empowers patients and allows them to emphasize the things that they value,” she said. Darien added, “If patients feel like they’re prepared to make decisions and there is
true shared decision making, they are less likely to have decisional regret” about not pursuing more aggressive treatments.
Another payment model described by Bekelman would link reimbursement to evidence of comparative effectiveness. If there is evidence of superior comparative effectiveness when CMS decides to cover a new intervention, the payment for new technology would be based on existing formulas (Pearson and Bach, 2010). But if there is evidence that the new intervention is merely comparable to another standard treatment, payment would be equal to that of the standard treatment, an approach referred to as reference-based pricing, Bekelman explained. If there is insufficient evidence to judge comparative effectiveness, then CMS would use an approach referred to as dynamic pricing, in which the treatment would be priced initially according to existing formulas, with a subsequent review 3 years later, at which point price adjustments could be made based on the evidence accrued during that time (Bekelman and Hahn, 2014; Elkin and Bach, 2010). Bekelman said this payment model is appealing because of how it could limit adoption of treatments whose costs far exceed their benefits, and that dynamic pricing also would provide an incentive to de-adopt treatments if they are later shown not to be superior. But he added that the model may not be feasible given the long time frames needed to conduct the cancer clinical trials to acquire the comparative effectiveness evidence.
Steinberg also advocated for a value-based payment model in which higher prices are paid for treatments showing better treatment outcomes because he said it incentivizes the triple aim of improved patient experience, improved health for populations, and lower per capita cost. He agreed that the reference pricing described by Bekelman would be a useful way to ensure value-based care. Steinberg also suggested that treatment pathways, as opposed to guidelines, can foster value-based care. Guidelines are usually written by specialty societies and tend to be inclusive and provide many treatment options, he said. In contrast, pathways are specifically defined processes of care and usually are developed by payers or by clinicians in a particular region or community through a consensus-based process looking at efficacy and cost, patient acceptance and efficiency, and local care factors. He noted that US Oncology found that an on-pathway approach to treating non-small cell lung cancer resulted in similar survival, but a 30 percent decrease in costs due to careful selection of drugs compared to a nonpathway approach (Neubauer et al., 2010).
Bekelman also suggested that delivery system reforms could help optimize adoption of valuable new technologies and de-adoption of low-value
technologies already in use by evaluating the value of treatment options as evidence accumulates on effectiveness, as depicted in the intervention ladder in Figure 8 (Nuffield Council on Bioethics, 2007). In this model, choice of treatments could be guided by incentives, disincentives, and default mechanisms. Bekelman said that “each rung on the ladder can be experimented on to determine whether it will work, and we already know in some cases it does work.” But he added that there could also be unintended consequences with this approach. Utilization management can slow clinical care, and patients with real indications may not be able to access certain treatments with limited evidence, such as PBRT, he said.
Bekelman concluded that adoption and de-adoption of new technologies should be based on the evidence of their value. “We shouldn’t expect the pace of adoption to be speedy or not speedy, but we should expect it to respond reasonably to evidence generated, and we may get a more potent response if we link evidence to payment reform. Delivery system innova-
FIGURE 8 The intervention ladder.
SOURCE: Bekelman presentation, July 20, 2015; Adapted from “Public Health: Ethical Issues” Nuffield Council on Bioethics. See http://nuffieldbioethics.org/report/public-health-2/policy-process-practice (accessed November 18, 2015).
tion holds great potential not only in radiation oncology, but throughout medicine,” Bekelman said.
But Tunis noted the challenge of trying to simultaneously optimize good-quality evidence about effectiveness, innovation, and rapid access to new technologies, and value and affordability. “It’s really difficult to optimize across all three of these—you can have innovation and evidence, but it won’t be cheap, so what is going to get us the best balance?” he asked.
In closing remarks, Tina Shih from the MD Anderson Cancer Center noted that the main goal for many of the new radiation technologies being used to treat cancer is to spare normal tissue from harm. Although there is well-accepted evidence to support the use of advanced technologies in some cases (e.g., PBRT for pediatric cancers of the head and neck), the clinical benefits of using these technologies to treat many common cancer sites in adults is not clear. The value of using robotic surgery for most cancer is also uncertain, she said. Robotic surgery enhances 3D visualization and instrument rotation, and may limit the incision size and shorten the length of a hospital stay, but there is mixed evidence regarding how it affects functional outcome and cancer control, she noted.
Shih said there are relatively fewer resources to conduct clinical trials of medical technologies compared to drugs, and noted that reimbursement practices can facilitate or hinder accrual to clinical trials that evaluate new technologies. She summarized the numerous challenges in gathering evidence to assess the comparative value of new medical technologies in the absence of clinical trials, including a lack of cost data, reliance on observational data that may not be of high quality, the inability to distinguish sources of variability (the device versus the provider versus the procedure), and difficulty to establish causality effects. High-quality national registries, better integration of existing databases, international studies, and advanced statistical methods might alleviate some of those challenges, Shih stressed. But she added that they will not address “cancer exceptionalism”—the notion that every treatment is well justified for cancer—which in the past has made it difficult to assess value in oncology care. She said this attitude has slowly been changing because of the high cost of cancer care, but there is still pressure to provide new treatments.
Shih summarized the diffusion of advanced technologies into clinical practice, noting that IMRT is already broadly diffused into oncology prac-
tice, but PBRT is still at an earlier stage of clinical adoption. Robotic surgery has saturated the market for prostatectomies, and is expanding rapidly into gynecologic oncology. She reiterated that adoption of new technologies tends to happen much faster than de-adoption if they are shown to be of low value, with technologies abandoned only in rare cases, often due to loss of reimbursement. She added that it is important to remember that treatment itself can be harmful.
Shih noted financial incentives that have fostered the spread of new technologies in the clinic, including the capital investment providers have in these highly expensive technologies. “It is important to differentiate between technology substitution, which is replacing old technology with new technology, and market expansion, which is applying new technology to a population that may or may not need it. That might lead to overtreatment,” Shih said. She asked whether the speed of diffusion of new technologies could be determined by their value, so that higher-value technologies diffuse faster into practice.
Shih also summarized some of the innovative ways to incorporate value into payment schemes, including the framework for value-based payments established by the California Technology Assessment Forum, the guidelines-based approach used by Massachusetts Blue Cross/Blue Shield to reimburse for IMRT, and various ways to institute CED so that reimbursement is linked to generation of evidence to assess value.
In closing, Shih also reminded participants about the importance of engaging patients in clinical decisions and listening to patient voices in assessing the value of advanced medical technologies.
AAGL (American Association of Gynecologic Laparoscopists). 2014. Guidelines for privileging for robotic-assisted gynecologic laparoscopy. Journal of Minimally Invasive Gynecology 21(2):157-167.
Aizer, A. A., J. J. Paly, and J. A. Efstathiou. 2013. Multidisciplinary care and management selection in prostate cancer. Seminars in Radiation Oncology 23(3):157-164.
Alemozaffar, M., A. Duclos, N. D. Hevelone, S. R. Lipsitz, T. Borza, H.-Y. Yu, K. J. Kowalczyk, and J. C. Hu. 2012. Technical refinement and learning curve for attenuating neurapraxia during robotic-assisted radical prostatectomy to improve sexual function. European Urology 61(6):1222-1228.
Alhassani, A., A. Chandra, and M. E. Chernew. 2012. The sources of the SGR “hole.” New England Journal of Medicine 366(4):289-291.
Ashworth, A., W. Kong, T. Whelan, and W. J. Mackillop. 2013. A population-based study of the fractionation of postlumpectomy breast radiation therapy. International Journal of Radiation Oncology*Biology*Physics 86(1):51-57.
Austin, P. C. 2011. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behavioral Research 46(3):399-424.
Barbash, G. I., and S. A. Glied. 2010. New technology and health care costs—the case of robot-assisted surgery. New England Journal of Medicine 363(8):701-704.
Barry, M. J., P. M. Gallagher, J. S. Skinner, and F. J. Fowler. 2012. Adverse effects of robotic-assisted laparoscopic versus open retropubic radical prostatectomy among a nationwide random sample of Medicare-age men. Journal of Clinical Oncology 30(5):513-518.
Beck, M. 2015. Big bets on proton therapy face uncertain future. Wall Street Journal, May 26.
Bekelman, J. E., and S. M. Hahn. 2014. Reference pricing with evidence development: A way forward for proton therapy. Journal of Clinical Oncology 32(15):1540-1542.
Bekelman, J. E., A. J. Epstein, and E. J. Emanuel. 2013a. Single- vs multiple-fraction radiotherapy for bone metastases from prostate cancer. JAMA 310(14):1501-1502.
Bekelman, J. E., T. Schultheiss, and A. B. De Gonzalez. 2013b. Subsequent malignancies after photon versus proton radiation therapy. International Journal of Radiation Oncology*Biology*Physics 1(87):10-12.
Bekelman, J. E., G. Sylwestrzak, J. Barron, J. Liu, A. J. Epstein, G. Freedman, J. Malin, and E. J. Emanuel. 2014. Uptake and costs of hypofractionated vs conventional whole breast irradiation after breast conserving surgery in the United States, 2008-2013. JAMA 312(23):2542-2550.
Birkmeyer, J. D., J. F. Finks, A. O’Reilly, M. Oerline, A. M. Carlin, A. R. Nunn, J. Dimick, M. Banerjee, and N. J. Birkmeyer. 2013. Surgical skill and complication rates after bariatric surgery. New England Journal of Medicine 369(15):1434-1442.
Bochner, B. H., D. D. Sjoberg, and V. P. Laudone. 2014. A randomized trial of robot-assisted laparoscopic radical cystectomy. New England Journal of Medicine 371(4):389-390.
Brixey, C. J., J. C. Roeske, A. E. Lujan, S. D. Yamada, J. Rotmensch, and A. J. Mundt. 2002. Impact of intensity-modulated radiotherapy on acute hematologic toxicity in women with gynecologic malignancies. International Journal of Radiation Oncology*Biology*Physics 54(5):1388-1396.
Brown, J. M., and J. R. Adler. 2015. Is equipment development stifling innovation in radiation oncology? International Journal of Radiation Oncology*Biology*Physics 92(4):713-714.
Brown, P. D., A. L. Asher, K. V. Ballman, E. Farace, J. H. Cerhan, S. K. Anderson, X. W. Carrero, F. G. Barker, R. L. Deming, and S. Burri. 2015. NCCTG N0574 (Alliance): A Phase III randomized trial of whole brain radiation therapy (WBRT) in addition to radiosurgery (SRS) in patients with 1 to 3 brain metastases. ASCO Annual Meeting Proceedings.
Carter, S. C., A. Chiang, G. Shah, L. Kwan, J. S. Montgomery, A. Karam, C. Tarnay, K. A. Guru, and J. C. Hu. 2015. Video-based peer feedback through social networking for robotic surgery simulation: A multicenter randomized controlled trial. Annals of Surgery 261(5):870-875.
Chowriappa, A., S. J. Raza, A. Fazili, E. Field, C. Malito, D. Samarasekera, Y. Shi, K. Ahmed, G. Wilding, and J. Kaouk. 2015. Augmented reality-based skills training for robot-assisted urethrovesical anastomosis: A multi-institutional randomised controlled trial. BJU International 115(2):336-345.
Chung, C. S., T. I. Yock, K. Nelson, Y. Xu, N. L. Keating, and N. J. Tarbell. 2013. Incidence of second malignancies among patients treated with proton versus photon radiation. International Journal of Radiation Oncology*Biology*Physics 87(1):46-52.
Coen, J. J., A. L. Zietman, C. J. Rossi, J. A. Grocela, J. A. Efstathiou, Y. Yan, and W. U. Shipley. 2012. Comparison of high-dose proton radiotherapy and brachytherapy in localized prostate cancer: A case-matched analysis. International Journal of Radiation Oncology*Biology*Physics 82(1):e25-e31.
Colaco, R. J., B. S. Hoppe, S. Flampouri, B. T. McKibben, R. H. Henderson, C. Bryant, R. C. Nichols, W. M. Mendenhall, Z. Li, and Z. Su. 2015. Rectal toxicity after proton therapy for prostate cancer: An analysis of outcomes of prospective studies conducted at the University of Florida Proton Therapy Institute. International Journal of Radiation Oncology*Biology*Physics 91(1):172-181.
Cooper, M. A., A. Ibrahim, H. Lyu, and M. A. Makary. 2013. Underreporting of robotic surgery complications. Journal for Healthcare Quality 37(2):133-138.
de Souza, J., and T. Seiwert. 2013. A value framework in head and neck cancer care. American Society of Clinical Oncology Educational Book e304-e309.
Dearnaley, D. P., V. S. Khoo, A. R. Norman, L. Meyer, A. Nahum, D. Tait, J. Yarnold, and A. Horwich. 1999. Comparison of radiation side-effects of conformal and conventional radiotherapy in prostate cancer: A randomised trial. Lancet 353(9149):267-272.
Desai, M. M., I. S. Gill, J. H. Kaouk, S. F. Matin, G. T. Sung, and E. L. Bravo. 2002. Robotic-assisted laparoscopic adrenalectomy. Urology 60(6):1104-1107.
Dirksen, C. D., A. H. Ament, and P. M. Go. 1996. Diffusion of six surgical endoscopic procedures in the Netherlands. Stimulating and restraining factors. Health Policy 37(2):91-104.
Ecker, B. L., G. E. Savulionyte, J. Datta, K. R. Dumon, J. Kucharczuk, N. N. Williams, and D. T. Dempsey. 2015. Laparoscopic transhiatal esophagectomy improves hospital outcomes and reduces cost: A single-institution analysis of laparoscopic-assisted and open techniques. Surgical Endoscopy 29(9):1-8.
Efstathiou, J. A., A. V. Trofimov, and A. L. Zietman. 2009. Life, liberty, and the pursuit of protons: An evidence-based review of the role of particle therapy in the treatment of prostate cancer. The Cancer Journal 15(4):312-318.
Elkin, E. B., and P. B. Bach. 2010. Cancer’s next frontier: Addressing high and increasing costs. JAMA 303(11):1086-1087.
Falchook, A. D., and R. C. Chen. 2015. Association between certificate of need legislation and radiation therapy use among elderly patients with early cancers. International Journal of Radiation Oncology*Biology*Physics 2(91):448-450.
Falit, B. P., C. P. Gross, and K. B. Roberts. 2010. Integrated prostate cancer centers and over-utilization of IMRT: A close look at fee-for-service medicine in radiation oncology. Internaitonal Journal of Radiation Oncology*Biology*Physics 76(5):1285-1288.
FDA (Food and Drug Administration). 2014. FDA warns against using laparoscopic power morcellators to treat uterine fibroids. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm424435.htm (accessed November 4, 2015).
Fleshman, J., D. J. Sargent, E. Green, M. Anvari, S. J. Stryker, R. W. Beart, Jr., M. Hellinger, R. Flanagan, Jr., W. Peters, and H. Nelson. 2007. Laparoscopic colectomy for cancer is not inferior to open surgery based on 5-year data from the cost study group trial. Annals of Surgery 246(4):655-664.
Gaia, G., R. W. Holloway, L. Santoro, S. Ahmad, E. Di Silverio, and A. Spinillo. 2010. Robotic-assisted hysterectomy for endometrial cancer compared with traditional laparoscopic and laparotomy approaches: A systematic review. Obstetrics & Gynecology 116(6):1422-1431.
Gandaglia, G., J. D. Sammon, S. L. Chang, T. K. Choueiri, J. C. Hu, P. I. Karakiewicz, A. S. Kibel, S. P. Kim, R. Konijeti, and F. Montorsi. 2014. Comparative effectiveness of robot-assisted and open radical prostatectomy in the postdissemination era. Journal of Clinical Oncology 32(14):1419-1426.
Gargiulo, A. R. 2014. Computer-assisted reproductive surgery: Why it matters to reproductive endocrinology and infertility subspecialists. Fertility and Sterility 102(4):911-921.
Geroski, P. A. 1999. DP2146 models of technology diffusion. London, UK: Centre for Economic Policy Research.
Ghani, K. R., D. C. Miller, S. Linsell, A. Brachulis, B. Lane, R. Sarle, D. Dalela, M. Menon, B. Comstock, T. S. Lendvay, J. Montie, and J. O. Peabody. Measuring to improve: Peer and crowd-sourced assessments of technical skill with robot-assisted radical prostatectomy. European Urology 69(1):302.
Gray, P. J., J. J. Paly, B. Y. Yeap, M. G. Sanda, H. Sandler, J. M. Michalski, J. A. Talcott, J. J. Coen, D. A. Hamstra, and W. U. Shipley. 2013. Patient-reported outcomes after 3-dimensional conformal, intensity-modulated, or proton beam radiotherapy for localized prostate cancer. Cancer 119(9):1729-1735.
Hadley, J., K. R. Yabroff, M. J. Barrett, D. F. Penson, C. S. Saigal, and A. L. Potosky. 2010. Comparative effectiveness of prostate cancer treatments: Evaluating statistical adjustments for confounding in observational data. Journal of the National Cancer Institute 102(23):1780-1793.
Haglind, E., S. Carlsson, J. Stranne, A. Wallerstedt, U. Wilderäng, T. Thorsteinsdottir, M. Lagerkvist, J.-E. Damber, A. Bjartell, and J. Hugosson. 2015. Urinary incontinence and erectile dysfunction after robotic versus open radical prostatectomy: A prospective, controlled, nonrandomised trial. European Urology 68(2):216-225.
Hall, B. H., and B. Khan. 2003. Adoption of new technology. Cambridge, MA: National Bureau of Economic Research.
Hattangadi, J. A., N. Taback, B. A. Neville, J. R. Harris, and R. S. Punglia. 2012. Accelerated partial breast irradiation using brachytherapy for breast cancer: Patterns in utilization and guideline concordance. Journal of the National Cancer Institute 104(1):29-41.
Hu, J. C., X. Gu, S. R. Lipsitz, M. J. Barry, A. V. D’Amico, A. C. Weinberg, and N. L. Keating. 2009. Comparative effectiveness of minimally invasive vs open radical prostatectomy. JAMA 302(14):1557-1564.
Huang, E., B. S. Teh, D. R. Strother, Q. G. Davis, J. K. Chiu, H. H. Lu, L. S. Carpenter, W.-Y. Mai, M. M. Chintagumpala, and M. South. 2002. Intensity-modulated radiation therapy for pediatric medulloblastoma: Early report on the reduction of ototoxicity. International Journal of Radiation Oncology*Biology*Physics 52(3):599-605.
Hughes, K. S., L. A. Schnaper, J. R. Bellon, C. T. Cirrincione, D. A. Berry, B. McCormick, H. B. Muss, B. L. Smith, C. A. Hudis, and E. P. Winer. 2013. Lumpectomy plus tamoxifen with or without irradiation in women age 70 years or older with early breast cancer: Long-term follow-up of CALGB 9343. Journal of Clinical Oncology 31(19):2382-2387.
IOM (Institute of Medicine). 1979. Medical technology and the health care system: A study of the diffusion of equipment-embodied technology. Washington, DC: National Academy Press.
IOM. 2013. Delivering high-quality cancer care: Charting a new course for a system in crisis. Washington, DC: The National Academies Press.
IOM. 2014. Ensuring patient access to affordable cancer drugs: Workshop summary. Washington, DC: The National Academies Press.
Jacobs, B. L., Y. Zhang, T. A. Skolarus, J. T. Wei, J. E. Montie, F. R. Schroeck, and B. K. Hollenbeck. 2012. Managed care and the diffusion of intensity-modulated radiotherapy for prostate cancer. Urology 80(6):1236-1242.
Jacobs, B. L., Y. Zhang, F. R. Schroeck, T. A. Skolarus, J. T. Wei, J. E. Montie, S. M. Gilbert, S. A. Strope, R. L. Dunn, and D. C. Miller. 2013. Use of advanced treatment technologies among men at low risk of dying from prostate cancer. JAMA 309(24):2587-2595.
Jacoby, V. L., A. Autry, G. Jacobson, R. Domush, S. Nakagawa, and A. Jacoby. 2009. Nationwide use of laparoscopic hysterectomy compared with abdominal and vaginal approaches. Obstetrics & Gynecology 114(5):1041-1048.
Jagsi, R., M. A. Ben-David, J. M. Moran, R. B. Marsh, K. A. Griffith, J. A. Hayman, and L. J. Pierce. 2010. Unacceptable cosmesis in a protocol investigating intensity-modulated radiotherapy with active breathing control for accelerated partial-breast irradiation. International Journal of Radiation Oncology*Biology*Physics 76(1):71-78.
James, B. Y., P. R. Soulos, J. Herrin, L. D. Cramer, A. L. Potosky, K. B. Roberts, and C. P. Gross. 2012. Proton versus intensity-modulated radiotherapy for prostate cancer: Patterns of care and early toxicity. Journal of the National Cancer Institute 105(1):25-32.
Kaiser Family Foundation. 2008. HRET survey of employer-sponsored health benefits, 1999-2008: Median family income from U.S. Census Bureau. Menlo Park, CA: Henry J. Kaiser Family Foundation.
Kim, S. C., C. Song, W. Kim, T. Kang, J. Park, I. G. Jeong, S. Lee, Y. M. Cho, and H. Ahn. 2011. Factors determining functional outcomes after radical prostatectomy: Robot-assisted versus retropubic. European Urology 60(3):413-419.
Konski, A. 2011. The war on cancer: Progress at what price? Journal of Clinical Oncology 29(12):1503-1504.
Lal, L. S., S. D. Byfield, E. L. Chang, L. Franzini, L.-A. Miller, R. Arbuckle, L. Reasonda, C. Feng, A. Adamus, and J. M. Swint. 2012. Cost-effectiveness analysis of a randomized study comparing radiosurgery with radiosurgery and whole brain radiation therapy in patients with 1 to 3 brain metastases. American Journal of Clinical Oncology 35(1):45-50.
Lewis, S. L., S. Porceddu, N. Nakamura, D. A. Palma, S. S. Lo, P. Hoskin, D. Moghanaki, S. J. Chmura, and J. K. Salama. 2015. Definitive stereotactic body radiotherapy (SBRT) for extracranial oligometastases. American Journal of Clinical Oncology. [Epub ahead of print.] www.ncbi.nlm.nih.gov/pubmed/25647831 (accessed January 5, 2016).
Limongelli, P., C. Vitiello, A. Belli, M. Pai, S. Tolone, G. del Genio, L. Brusciano, G. Docimo, N. Habib, and G. Belli. 2014. Costs of laparoscopic and open liver and pancreatic resection: A systematic review. World Journal of Gastroenterology 20(46):17595.
Livingston, E. H. 2005. Hospital costs associated with bariatric procedures in the United States. The American Journal of Surgery 190(5):816-820.
Lucas, S. M., E. A. Pattison, and C. P. Sundaram. 2012. Global robotic experience and the type of surgical system impact the types of robotic malfunctions and their clinical consequences: An FDA MAUDE review. BJU International 109(8):1222-1227.
Makarov, D. V., B. Y. James, R. A. Desai, D. F. Penson, and C. P. Gross. 2011. The association between diffusion of the surgical robot and radical prostatectomy rates. Medical Care 49(4):333-339.
Marino, P., G. Houvenaeghel, F. Narducci, A. Boyer-Chammard, G. Ferron, C. Uzan, A.-S. Bats, P. Mathevet, P. Dessogne, and F. Guyon. 2015. Cost-effectiveness of conventional vs robotic-assisted laparoscopy in gynecologic oncologic indications. International Journal of Gynecological Cancer 25(6):1102-1108.
Mell, L. K., A. K. Mehrotra, and A. J. Mundt. 2005. Intensity-modulated radiation therapy use in the U.S., 2004. Cancer 104(6):1296-1303.
Merchant, T. E., E. N. Kiehna, C. Li, H. Shukla, S. Sengupta, X. Xiong, A. Gajjar, and R. K. Mulhern. 2006. Modeling radiation dosimetry to predict cognitive outcomes in pediatric patients with CNS embryonal tumors including medulloblastoma. International Journal of Radiation Oncology*Biology*Physics 65(1):210-221.
Miralbell, R., A. Lomax, L. Cella, and U. Schneider. 2002. Potential reduction of the incidence of radiation-induced second cancers by using proton beams in the treatment of pediatric tumors. International Journal of Radiation Oncology*Biology*Physics 54(3):824-829.
Mitchell, J. M. 2005. Effects of physician-owned limited-service hospitals: Evidence from Arizona. Health Affairs 24:W5.
Mitchell, J. M. 2013. Urologists’ use of intensity-modulated radiation therapy for prostate cancer. New England Journal of Medicine 369(17):1629-1637.
Mundt, A. J., A. E. Lujan, J. Rotmensch, S. E. Waggoner, S. D. Yamada, G. Fleming, and J. C. Roeske. 2002. Intensity-modulated whole pelvic radiotherapy in women with gynecologic malignancies. International Journal of Radiation Oncology*Biology*Physics 52(5):1330-1337.
Nelson, H., D. J. sarget, S. Wieand, J. Fleshman, M. Anvari, S. Stryker, R. Beart, M. Hellinger, R. Flanagan, W. Peters, and D. Ota. 2004. A comparison of laparoscopically assisted and open colectomy for colon cancer. New England Journal of Medicine 350(20):2050.
Neubauer, M. A., J. R. Hoverman, M. Kolodziej, L. Reisman, S. K. Gruschkus, S. Hoang, A. A. Alva, M. McArthur, M. Forsyth, and T. Rothermel. 2010. Cost effectiveness of evidence-based treatment guidelines for the treatment of non-small-cell lung cancer in the community setting. Journal of Oncology Practice 6(1):12-18.
Nguyen, P. L., X. Gu, S. R. Lipsitz, T. K. Choueiri, W. W. Choi, Y. Lei, K. E. Hoffman, and J. C. Hu. 2011. Cost implications of the rapid adoption of newer technologies for treating prostate cancer. Journal of Clinical Oncology 29(12):1517-1524.
Nix, J., A. Smith, R. Kurpad, M. E. Nielsen, E. M. Wallen, and R. S. Pruthi. 2010. Prospective randomized controlled trial of robotic versus open radical cystectomy for bladder cancer: Perioperative and pathologic results. European Urology 57(2):196-201.
Nuffield Council on Bioethics. Public health: Ethical issues. 2007. London, UK. http://nuffieldbioethics.org/wp-content/uploads/2014/07/Public-health-ethical-issues.pdf (accessed November 4, 2015).
Nutting, C. M., J. P. Morden, K. J. Harrington, T. G. Urbano, S. A. Bhide, C. Clark, E. A. Miles, A. B. Miah, K. Newbold, and M. Tanay. 2011. Parotid-sparing intensity modulated versus conventional radiotherapy in head and neck cancer (PARSPORT): A Phase 3 multicentre randomised controlled trial. Lancet Oncology 12(2):127-136.
OECD (Organisation for Economic Co-operation and Development). 2014. Country statistical profile: United States 2014/1. Paris: OECD Publishing.
O’Neil, B., T. Koyama, J. Rudd, A. Peter, C. Matthew, M. Goodman, S. Greenfield, A. Hamilton, K. Hoffman, and R. Hoffman. 2015. PII-LBA5 evidence of superior quality of life after robotic prostatectomy: Results from a population-based analysis. Journal of Urology 193(4):e411-e412.
Ou, Y. C., C. R. Yang, J. Wang, C. K. Yang, C. L. Cheng, V. R. Patel, and A. K. Tewari. 2011. The learning curve for reducing complications of robotic-assisted laparoscopic radical prostatectomy by a single surgeon. BJU International 108(3):420-425.
Palta, M., P. Palta, N. A. Bhavsar, J. K. Horton, and R. C. Blitzblau. 2015. The use of adjuvant radiotherapy in elderly patients with early-stage breast cancer: Changes in practice patterns after publication of Cancer and Leukemia Group B 9343. Cancer 121(2):188-193.
Park, J.-Y., S.-K. Park, D.-Y. Kim, J.-H. Kim, Y.-M. Kim, Y.-T. Kim, and J.-H. Nam. 2011. The impact of tumor morcellation during surgery on the prognosis of patients with apparently early uterine leiomyosarcoma. Gynecologic Oncology 122(2):255-259.
Pearson, S. D. and P. B. Bach. 2010. How Medicare could use comparative effectiveness research in deciding on new coverage and reimbursement. Health Affairs 29(10):1796-1804.
Pignol, J.-P., I. Olivotto, E. Rakovitch, S. Gardner, K. Sixel, W. Beckham, T. T. Vu, P. Truong, I. Ackerman, and L. Paszat. 2008. A multicenter randomized trial of breast intensity-modulated radiation therapy to reduce acute radiation dermatitis. Journal of Clinical Oncology 26(13):2085-2092.
Porter, M. E. 2010. What is value in health care? New England Journal of Medicine 363(26):2477-2481.
Presley, C. J., P. R. Soulos, J. Herrin, K. B. Roberts, B. Y. James, B. Killelea, B.-A. Lesnikoski, J. B. Long, and C. P. Gross. 2012. Patterns of use and short-term complications of breast brachytherapy in the national Medicare population from 2008-2009. Journal of Clinical Oncology 30(35):4302-4307.
Raldow, A. C., C. J. Presley, J. B. Yu, L. D. Cramer, P. R. Soulos, J. B. Long, D. V. Makarov, and C. P. Gross. 2015. Dissemination of new technologies: Cost and temporal trends in curative therapy for prostate cancer, edited by Y. C. C. Center. New Haven, CT: Cancer Outcomes, Public Policy and Effectiveness Research Center.
Rawlins, M. 2008. De testimonio: On the evidence for decisions about the use of therapeutic interventions. Clinical Medicine 8(6):579-588.
Rehman, S., S. J. Raza, A. P. Stegemann, K. Zeeck, R. Din, A. Llewellyn, L. Dio, M. Trznadel, Y. W. Seo, and A. J. Chowriappa. 2013. Simulation-based robot-assisted surgical training: A health economic evaluation. International Journal of Surgery 11(9):841-846.
Rocco, B., D. V. Matei, S. Melegari, J. C. Ospina, F. Mazzoleni, G. Errico, M. Mastropasqua, L. Santoro, S. Detti, and O. De Cobelli. 2009. Robotic vs open prostatectomy in a laparoscopically naive centre: A matched-pair analysis. BJU International 104(7):991-995.
Schiavone, M. B., E. C. Kuo, R. W. Naumann, W. M. Burke, S. N. Lewin, A. I. Neugut, D. L. Hershman, T. J. Herzog, and J. D. Wright. 2012. The commercialization of robotic surgery: Unsubstantiated marketing of gynecologic surgery by hospitals. American Journal of Obstetrics and Gynecology 207(3):174.
Shah, A., S. M. Hahn, R. L. Stetson, J. S. Friedberg, T. T. Pechet, and D. J. Sher. 2013. Cost-effectiveness of stereotactic body radiation therapy versus surgical resection for Stage I non-small cell lung cancer. Cancer 119(17):3123-3132.
Sheets, N. C., G. H. Goldin, A.-M. Meyer, Y. Wu, Y. Chang, T. Stürmer, J. A. Holmes, B. B. Reeve, P. A. Godley, and W. R. Carpenter. 2012. Intensity-modulated radiation therapy, proton therapy, or conformal radiation therapy and morbidity and disease control in localized prostate cancer. JAMA 307(15):1611-1620.
Shen, X., T. N. Showalter, M. V. Mishra, S. Barth, V. Rao, D. Levin, and L. Parker. 2014. Radiation oncology services in the modern era: Evolving patterns of usage and payments in the office setting for Medicare patients from 2000 to 2010. Journal of Oncology Practice 10(4):e201-e207.
Shih, Y.-C. T., P. A. Ganz, D. Aberle, A. Abernethy, J. Bekelman, O. Brawley, J. S. Goodwin, J. C. Hu, D. Schrag, and J. S. Temel. 2013. Delivering high-quality and affordable care throughout the cancer care continuum. Journal of Clinical Oncology 31(32):4151-4157.
Shirvani, S. M., J. Jiang, J. Y. Chang, J. Welsh, A. Likhacheva, T. A. Buchholz, S. G. Swisher, and B. D. Smith. 2014. Lobectomy, sublobar resection, and stereotactic ablative radiotherapy for early-stage non-small cell lung cancers in the elderly. JAMA Surgery 149(12):1244-1253.
Sivarajan, G., G. B. Taksler, D. Walter, C. P. Gross, R. E. Sosa, and D. V. Makarov. 2015. The effect of the diffusion of the surgical robot on the hospital-level utilization of partial nephrectomy. Medical Care 53(1):71-78.
Smith, B. D., J. Jiang, J. Y. Chang, J. Welsh, A. Likhacheva, T. A. Buchholz, S. G. Swisher, and S. M. Shirvani. 2015. Cost-effectiveness of stereotactic radiation, sublobar resection, and lobectomy for early non-small cell lung cancers in older adults. Journal of Geriatric Oncology 6(4):324-331.
Sooriakumaran, P., M. John, P. Wiklund, D. Lee, A. Nilsson, and A. Tewari. 2011. Learning curve for robotic assisted laparoscopic prostatectomy: A multi-institutional study of 3794 patients. Minerva Urologica e Nefrologica—The Italian Journal of Urology and Nephrology 63(3):191-198.
Soulos, P. R., B. Y. James, K. B. Roberts, A. C. Raldow, J. Herrin, J. B. Long, and C. P. Gross. 2012. Assessing the impact of a cooperative group trial on breast cancer care in the Medicare population. Journal of Clinical Oncology 30(14):1601-1607.
Steinberg, M., W. H. McBride, E. Vlashi, and F. Pajonk. 2013. National Institutes of Health funding in radiation oncology: A snapshot. International Journal of Radiation Oncology*Biology*Physics 86(2):234-240.
Steingisser, L., B. Acker, S. Berman, M. J. Brenner, B. A. Bornstein, P. Busse, T. J. FitzGerald, J. O. Jacobson, E. Jekowsky, and L. A. Kachnic. 2014. Bending the cost curve: A unique collaboration between radiation oncologists and Blue Cross Blue Shield of Massachusetts to optimize the use of advanced technology. Journal of Oncology Practice 10(5):e321-e327.
Talcott, J. A., C. Rossi, W. U. Shipley, J. A. Clark, J. D. Slater, A. Niemierko, and A. L. Zietman. 2010. Patient-reported long-term outcomes after conventional and high-dose combined proton and photon radiation for early prostate cancer. JAMA 303(11):1046-1053.
Trofimov, A., P. L. Nguyen, J. J. Coen, K. P. Doppke, R. J. Schneider, J. A. Adams, T. R. Bortfeld, A. L. Zietman, T. F. DeLaney, and W. U. Shipley. 2007. Radiotherapy treatment of early-stage prostate cancer with IMRT and protons: A treatment planning comparison. International Journal of Radiation Oncology*Biology* Physics 69(2):444-453.
Vickers, A. J., F. J. Bianco, M. Gonen, A. M. Cronin, J. A. Eastham, D. Schrag, E. A. Klein, A. M. Reuther, M. W. Kattan, and J. E. Pontes. 2008. Effects of pathologic stage on the learning curve for radical prostatectomy: Evidence that recurrence in organ-confined cancer is largely related to inadequate surgical technique. European Urology 53(5):960-966.
Whaley, C., J. S. Chafen, S. Pinkard, G. Kellerman, D. Bravata, R. Kocher, and N. Sood. 2014. Association between availability of health service prices and payments for these services. JAMA 312(16):1670-1676.
Wright, J. D., W. M. Burke, E. T. Wilde, S. N. Lewin, A. S. Charles, J. H. Kim, N. Goldman, A. I. Neugut, T. J. Herzog, and D. L. Hershman. 2012. Comparative effectiveness of robotic versus laparoscopic hysterectomy for endometrial cancer. Journal of Clinical Oncology 30(8):783-791.
Wright, J. D., C. V. Ananth, S. N. Lewin, W. M. Burke, Y.-S. Lu, A. I. Neugut, T. J. Herzog, and D. L. Hershman. 2013. Robotically assisted vs laparoscopic hysterectomy among women with benign gynecologic disease. JAMA 309(7):689-698.
Yu, J. B., P. R. Soulos, J. Herrin, L. D. Cramer, A. L. Potosky, K. B. Roberts, and C. P. Gross. 2013. Proton versus intensity-modulated radiotherapy for prostate cancer: patterns of care and early toxicity. Journal of the National Cancer Institute 105(1):25-32.
Zafar, S. Y., J. M. Peppercorn, D. Schrag, D. H. Taylor, A. M. Goetzinger, X. Zhong, and A. P. Abernethy. 2013. The financial toxicity of cancer treatment: A pilot study assessing out-of-pocket expenses and the insured cancer patient’s experience. Oncologist 18(4):381-390.
Zhu, Y., and T. E. Merchant. 2003. Effects of hypothalamic equivalent uniform dose on growth hormone secretion. International Journal of Radiation Oncology*Biology*Physics 57(2):S374.
Zietman, A. L., K. Bae, J. D. Slater, W. U. Shipley, J. A. Efstathiou, J. J. Coen, D. A. Bush, M. Lunt, D. Y. Spiegel, and R. Skowronski. 2010. Randomized trial comparing conventional-dose with high-dose conformal radiation therapy in early-stage adenocarcinoma of the prostate: Long-term results from Proton Radiation Oncology Group/American College of Radiology 95-09. Journal of Clinical Oncology 28(7):1106-1111.


















































































