Summary1
Every patient is unique, and the evolving field of precision medicine aims to ensure the delivery of the right treatment to the right patient at the right time. In an era of rapid advances in biomedicine and enhanced understanding of the genetic basis of disease, health care providers increasingly have access to advanced technologies that may identify molecular variations specific to an individual patient, which subsequently can be targeted for treatment. Known as biomarker tests for molecularly targeted therapies, these complex tests have the potential to enable the selection of the most beneficial treatment (and also to identify treatments that may be harmful or ineffective) for the molecular underpinnings of an individual patient’s disease. Such tests are key to unlocking the promise of precision medicine.
Biomarker tests for molecularly targeted therapies represent a crucial area of focus for developing methods that could later be applicable to other areas of precision medicine. The appropriate regulatory oversight of these tests is required to ensure that they are accurate, reliable, properly validated, and appropriately implemented in clinical practice. Moreover, common evidentiary standards for assessing the beneficial impact of biomarker-guided therapy selection on patient outcomes, as well as the effective collection and sharing of information related to those outcomes, are urgently needed to better inform clinical decision making. Getting
___________________
1 This summary does not include references. Citations for the findings presented in the summary appear in subsequent chapters of the report.
biomarker tests right is imperative because a bad biomarker test is as problematic and potentially harmful as a bad drug.
Therein is the complicated issue addressed by this study: How do we ensure patients have timely access to appropriate tests that may accurately direct targeted therapies, while at the same time protect them from potential harm due to the adoption of poorly validated tests or inappropriately used tests? Patients with life-threatening diseases, in particular, cannot afford to wait for the answer to this question. The Institute of Medicine appointed an independent committee of experts to examine regulatory, reimbursement, and clinical practice policy issues that currently influence the adoption of biomarker tests for molecularly targeted therapies into routine clinical practice.
Biomarker test development and use are accelerating at a rapid rate, propelled by new research discoveries enabling even deeper understanding of the genetic basis of disease. However, the appropriate adoption and broader implementation into routine clinical use of such tests is held back by several interrelated factors: (1) lack of consensus over common evidentiary standards; (2) inefficient and inconsistent regulatory and reimbursement approaches; (3) the need for an effective framework for collecting patient data on tests, treatments, and outcomes; and (4) the need to translate such data into new knowledge to improve patient care and outcomes. Addressing these challenges will enable biomarker tests for molecularly targeted therapies to realize their potential to advance the clinical practice of precision medicine.
CONCLUSIONS
The first conclusion from the committee’s deliberations is that the full potential of precision medicine will not be realized without accurate, reliable, clinically useful, and appropriately implemented biomarker tests for molecularly targeted therapies. Second, an integrated approach is necessary to effectively address the diverse challenges in clinical practice, regulation, reimbursement, and other interrelated issues associated with this highly complex area of health care. The committee proposes a rapid learning system as a framework for its recommendations because the evolving field of precision medicine requires an approach for accelerated learning that integrates research and clinical practice to enhance patient care and improve clinical outcomes.
Third, substantial variation in the evidence used to inform regulatory, reimbursement, and treatment decisions ultimately limits the adoption of potentially useful biomarker tests and targeted therapies into clinical practice. Innovative, customized policy approaches that reflect a clear
understanding of the unique evidentiary issues related to these complex and rapidly evolving tests are needed.
Fourth, the ability to implement a rapid learning system for biomarker tests for molecularly targeted therapies is currently limited, in part, because complete results of complex biomarker tests (including tests performed using next-generation sequencing, or NGS, technologies) are not widely available in the electronic health record (EHR), nor are these data structured for integration into clinical practice and research. The ability to track long-term outcomes for patients treated with molecularly targeted therapies is essential to understanding the potential benefits as well as risks of these complex tests and associated therapies. The development of one or more large, integrated, interoperable, and accessible clinical database(s) on patient outcomes related to use of biomarker tests for molecularly targeted therapies is critical to accelerate progress in precision medicine and to improve patient care.
Finally, the committee concludes that precision medicine may have the unintended consequence of intensifying disparities in access to advanced health care services such as biomarker testing for molecularly targeted therapies. Improved patient and provider education about precision medicine as well as improved collaboration across health care settings may help to reduce disparities.
RECOMMENDATIONS
The committee’s recommendations focus on achieving 10 goals to further advance the development and appropriate use of biomarker tests for molecularly targeted therapies (see Box S-1). The recommended approaches to achieving these goals are designed to address a range of policy challenges; some of the committee’s recommendations are intentionally broad, while others are more focused. Though the recommendations focus on diverse areas for improvement, they are linked together by a common understanding: properly validated, appropriately implemented biomarker tests hold the potential to enhance patient care and improve outcomes, and therefore addressing the challenges facing such tests is critical.
The committee’s 10 recommendations are presented as interrelated components of a rapid learning system for biomarker tests for molecularly targeted therapies (see Figure S-1). Rapid learning systems serve as useful approaches to facilitate knowledge generation, and continuous learning, and accelerate the translation of lessons learned into better patient care and improved clinical outcomes. Thus, the committee’s vision identifies opportunities to improve the policy environment, data infrastructure, and
patient care processes that influence biomarker tests, while maintaining patients as the focal point.
Integrated Approach to Implementing the Committee’s Recommendations
The committee recognizes that the creation of its proposed rapid learning system will require a significant amount of time, planning, resources, and collaboration among a range of stakeholders. Though ideally implemented as a unified set, some of the committee’s recommendations, such as those related to EHRs and processes to improve patient care, can be implemented as stand-alone measures. By contrast, the policy recommendations are clearly interrelated and would be most effective if implemented together.
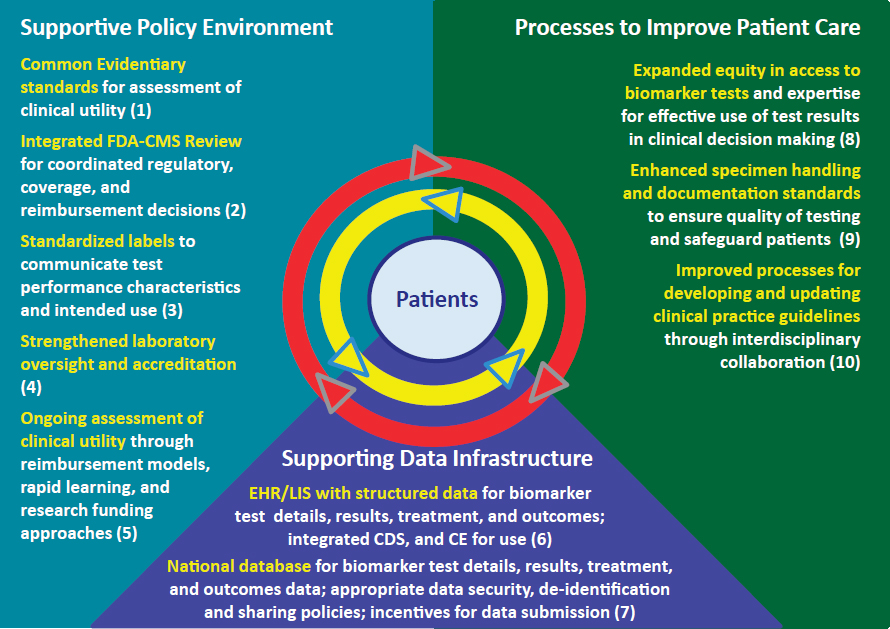
NOTES: Numbers in parentheses refer to committee’s recommendations. CDS = clinical decision support; CE = continuing education; CMS = Centers for Medicare & Medicaid Services; EHR/LIS = electronic health record/laboratory information system; FDA = Food and Drug Administration.
The committee recommends that initial implementation efforts focus on the foundational recommendations, as shown in Figure S-2. This approach takes into account the need for and feasibility of achieving each component of the proposed rapid learning system. For instance, the committee recommends that the Department of Health and Human Services (HHS) should immediately begin to facilitate a process for development of common evidentiary standards of clinical utility for biomarker tests for molecularly targeted therapies. One mechanism for the development of such standards could be convening one or more independent, public–private multistakeholder bodies.
Other initial steps include HHS convening a task force to plan and execute the development of a national data repository to ensure that database development efforts proceed apace. At the same time, developers of EHRs and laboratory information systems (LISs) should make products available that can properly manage the data requirements of a rapid learning system for biomarker tests for molecularly targeted therapies.
The process for developing evidentiary standards would inform other aspects of the committee’s integrated framework. For example, common evidentiary standards would facilitate the ongoing assessment of biomarker tests’ clinical utility (whether use of the test leads to improved patient outcomes), which also would involve the flow of patient biomarker test data and information into a national data repository. The committee’s recommended integrated review process for coordinated regulatory, coverage, and reimbursement decisions for biomarker tests for molecularly targeted therapies also would be aligned with common evidentiary standards.
Other interrelated actions recommended by the committee include the development of standardized test labels to communicate test performance characteristics and intended use(s) and rating of the evidence of a test’s clinical validity (accuracy of a test for a specific purpose) and clinical utility; such standards would also evolve out of the standards development process. The committee’s recommendations regarding updating and strengthening laboratory oversight and accreditation would improve the quality of biomarker testing and would enhance the implementation of the recommendations described above. Ultimately, the combined impact of the committee’s recommended changes would translate into more efficient and effective processes to improve patient care, which would be further enhanced through implementation of the committee’s specific recommendations relating to equity in access to biomarker tests, improved specimen standards, and coordinated development of clinical practice guidelines.
Health care providers, patients and patient advocates, researchers, test and drug developers and manufacturers, and policy makers all have
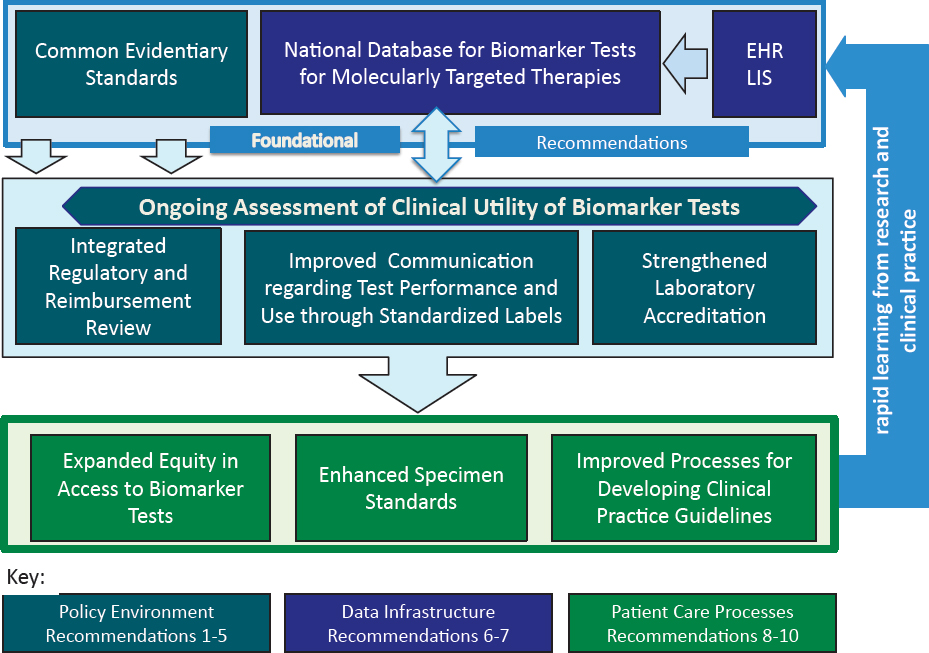
NOTE: EHR = electronic health record; LIS = laboratory information system.
important insights to offer on the most effective way to implement the committee’s recommendations. Leveraging the expertise and influence of this diverse stakeholder community will be critical to enhance the appropriate adoption of biomarker tests for molecularly targeted therapies into routine clinical practice. Although some recommendations provide specific direction to individual stakeholders, the full realization of the committee’s vision of a rapid learning system requires collaboration among multiple stakeholders (see Figure S-3).
Common Evidentiary Standards
Uncertainty resulting from a lack of common evidentiary standards for clinical utility is a significant limiting factor for patients, health care providers, payers, and test developers. The committee’s recommendation that HHS should facilitate the development of such standards recognizes the need for national leadership to bring all relevant stakeholders together. Doing so could provide a forum for sharing stakeholders’ diverse perspectives and support the collaboration needed to forge agreement on the critical issue of establishing common evidentiary standards for biomarker tests for molecularly targeted therapies. Such standards will be integral to consistent regulatory, coverage, and reimbursement decisions.
The committee emphasizes that evidentiary standards evolve over time in this rapidly changing and highly complex field. Thus, HHS should ensure ongoing support to continually refine common evidentiary standards. As these standards are developed and modified, they will inform the development of clinical guidelines and be reflected in clinical standards of care.
Goal 1: Establish common evidentiary standards of clinical utility—using evidence generated both within and outside the context of clinical trials—across all stakeholders.
Recommendation 1: The Secretary of the Department of Health and Human Services (HHS) should facilitate the development of common clinical utility evidentiary standards that are applied for initial and ongoing coordinated regulatory, coverage, and reimbursement decisions for biomarker tests for molecularly targeted therapies. One mechanism for development of these evidentiary standards could be convening one or more independent, public–private, multistakeholder bodies.
- Consistent and coordinated evidentiary standards and study design approaches, including rapid learning systems, should
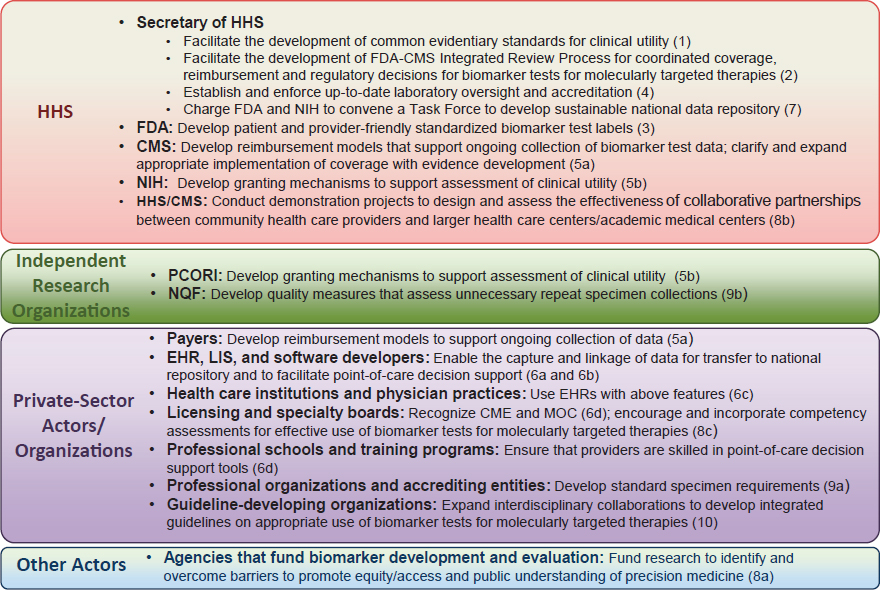
NOTES: Recommendation number in parentheses. CME = continuing medical education; CMS = Centers for Medicare & Medicaid Services; EHR = electronic health record; FDA = Food and Drug Administration; HHS = Department of Health and Human Services; LIS = laboratory information system; MOC = maintenance of certification; NIH = National Institutes of Health; NQF = National Quality Forum; PCORI = Patient-Centered Outcomes Research Institute.
-
be developed that simultaneously accommodate the various types of decisions (including clinical, regulatory, coverage/reimbursement, and guideline recommendations), and facilitate the ongoing development of evidence of clinical utility.
- Involvement of a variety of stakeholders will be critical to ensure that clinical utility studies are designed to reflect a range of decision-making needs and to strike an acceptable balance between ideal utility assessment and study feasibility. Stakeholders participating in these initiatives should include patients, health care providers, clinical practice guideline developers, public and private payers (including the Centers for Medicare & Medicaid Services), the Food and Drug Administration, test developers, pharmaceutical companies, molecular pathologists, clinical laboratory geneticists, and research funders (e.g., the Patient-Centered Outcomes Research Institute, the National Institutes of Health, and the Agency for Healthcare Research and Quality).
- Recognizing that evidentiary standards for clinical utility may vary across diseases, HHS could determine that more than one advisory body may be necessary to develop such disease-specific standards.
- Standards for ongoing development of clinical utility evidence will be used to guide the creation of new labels for biomarker tests and corresponding therapies (see Recommendation 3), and for guideline development (see Recommendation 10).
- Analytic and clinical validity of biomarker tests should be assured prior to assessing clinical utility.
- HHS should continue to support ongoing refinement of common evidentiary standards as they evolve.
Integrated Regulatory and Reimbursement Review
The inefficiencies created by the misalignment of the regulatory and reimbursement decision processes represent a significant challenge to the effective and timely implementation of appropriate biomarker tests for molecularly targeted therapies into clinical practice. The committee emphasizes the need for a coordinated Food and Drug Administration (FDA) and Centers for Medicare & Medicaid Services (CMS) process that enables an integrated concurrent review for regulatory, coverage, and reimbursement decisions for biomarker tests, including in vitro diagnostics (IVDs), laboratory-developed tests (LDTs), multianalyte tests such as NGS, and any associated molecular therapies (see Figure S-4). The committee recognizes the different statutory authority of the two agencies and
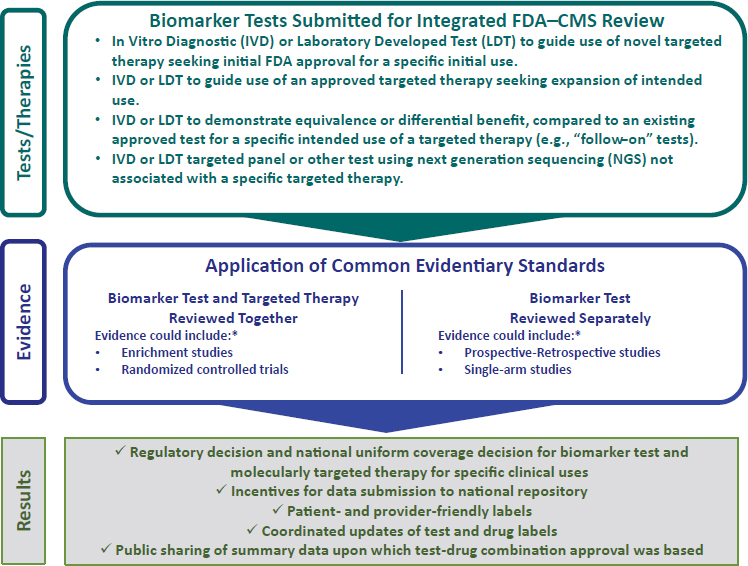
NOTE: CMS = Centers for Medicare & Medicaid Services; FDA = Food and Drug Administration. * See Table 3-1 for descriptions of various approaches to evidence generation.
their distinct evidentiary requirements. The committee is not calling for statutory reconciliation of the two agencies; rather it emphasizes the need for the two agencies to work closely together to coordinate more effectively the decision-making process for regulatory, coverage, and reimbursement decisions related to a small subset of clinical tests: biomarker tests for molecularly targeted therapies.
Goal 2: Establish a more coordinated and transparent federal process for regulatory and reimbursement decisions for biomarker tests for molecularly targeted therapies.
Recommendation 2: The Secretary of the Department of Health and Human Services should facilitate the development of a new integrated federal review process involving the Food and Drug Administration and the Centers for Medicare & Medicaid Services, as a pathway for coordinated regulatory, coverage, and reimburse-
ment decisions for biomarker tests for molecularly targeted therapies (including in vitro diagnostics, laboratory developed tests, and multianalyte tests performed using current or new technologies, and any corresponding molecularly targeted therapies).2 This coordinated pathway should accomplish all of the following through application of common evidentiary standards (as described in Recommendation 1):
- Primary (and follow-on) biomarker test review and approval with detailed test labeling requirements (as described in Recommendation 3).
- Drug review and approval with detailed labeling that includes standardized biomarker test information (as described in Recommendation 3), when occurring concurrently with biomarker test review.
- A national uniform coverage decision for a biomarker test and molecularly targeted therapy in specific clinical uses, including financial incentives for data submission on use and outcomes (see Recommendation 7).
- A defined process for coordinated updates of biomarker test and drug labels.
- Public sharing of the summary data upon which the review process based the approval and coverage decisions for a biomarker test and drug combination.
Test Performance and Intended Use Information
New patient- and health care provider-friendly labeling information, including a rating system that ranks the evidence to support the clinical validity and clinical utility of any biomarker test for molecularly targeted therapy, would increase the transparency of test performance and intended use. This would enable health care providers to clearly identify which test to order, while supporting patient engagement. Labeling information would be revised as further evidence develops through clinical use of the test.
Goal 3: Enhance communication to patients and providers about the performance characteristics and evidence for use of specific biomarker tests for molecularly targeted therapies.
___________________
2 This coordinated pathway is designed to reflect the current predominant fee-for-service reimbursement system for clinical tests.
Recommendation 3: The Food and Drug Administration (FDA) should develop a patient- and provider-friendly standardized label for biomarker tests (including in vitro diagnostics and laboratory developed tests) to facilitate transparency of test performance characteristics and the level of evidence for the intended use(s) of the test. FDA or laboratory accrediting bodies should approve the label for each biomarker test, including tests not reviewed through the integrated process specified in Recommendation 2.
- Labels should prominently feature an easily understood ranking system (e.g., 4-star scales) separately for the evidence to support the clinical validity and clinical utility for each intended clinical use of a test. The evidence ranking standards could be developed by the process described in Recommendation 1.
- Labeling should be subject to expedited revision as further evidence develops, providing an incentive for developers to establish the clinical utility of their products.
- Labels should use standardized terminology and should be clear enough for patients to understand as well as sufficiently useful to inform clinical decision making and to provide a basis for reimbursement.
Enhanced Laboratory Oversight
CMS regulates all clinical laboratories through the Clinical Laboratory Improvement Amendments (CLIA). Regulatory oversight under CLIA is widely viewed as insufficient for increasingly complex biomarker tests for molecularly targeted therapies.
Goal 4: Update and strengthen the oversight and accreditation of laboratories providing biomarker tests for molecularly targeted therapies.
Recommendation 4: The Secretary of the Department of Health and Human Services should establish and enforce up-to-date laboratory accreditation standards for biomarker tests for molecularly targeted therapies, either through the Centers for Medicare & Medicaid Services’ Clinical Laboratory Improvement Amendments (CLIA) or in collaboration with an existing up-to-date accreditation organization. Reimbursement for such biomarker testing should be dependent on meeting these standards.
- Current CLIA standards are inadequate for current advanced biomarker tests performed using next-generation sequencing and other emerging technologies.
- These standards should comply with test labeling requirements (see Recommendation 3).
Ongoing Assessment of Clinical Utility
It is important to view the generation of evidence of the clinical utility of any biomarker test for molecularly targeted therapies as a continuous process; for a biomarker test that is ultimately demonstrated to have clinical utility, the quality of evidence improves over time, progressing along a continuum from investigational/experimental to adequate for initial clinical use, eventually attaining stronger evidence of clinical utility for various intended uses (see Figure S-5).
In many cases, new, promising biomarker tests may be implemented in clinical practice without sufficient data to support definitive reimbursement decisions using current coverage decision approaches. It is important that CMS and other payers develop payment models to support ongoing data collection required to establish sufficiently robust evidence to confirm the clinical utility of promising biomarker tests. These data would be instrumental for evolving payment determinations, includ-
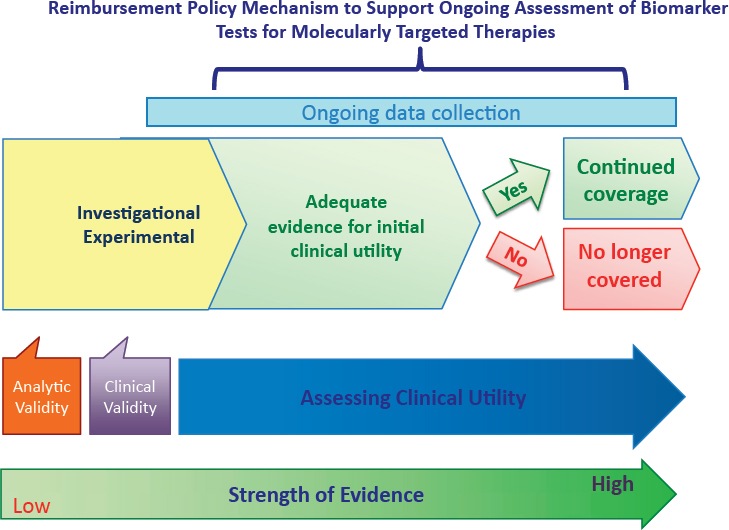
ing whether to discontinue payment for a specific biomarker test for which the clinical utility is not confirmed through additional evidence development.
Consistent with the committee’s vision of a rapid learning system, and the central role of data in learning and knowledge generation, the committee recommends that CMS should seek to clarify and expand appropriate implementation of coverage with evidence development (CED), which has potential to be an effective policy lever to generate evidence to support reimbursement decisions for promising technologies such as biomarker tests for molecularly targeted therapies.
Goal 5: Ensure ongoing assessment of the clinical utility of biomarker tests for molecularly targeted therapies.
Recommendation 5a: When existing evidence of clinical utility is sufficient for initial use of a biomarker test for a molecularly targeted therapy, the Centers for Medicare & Medicaid Services (CMS) and other payers should develop reimbursement models that support the ongoing collection of data within a rapid learning system. Such data will be used further to assess the evidence of clinical utility.
Potential approaches that payers could use to support this data collection include the following:
-
Reimbursement for biomarker tests that meet predefined clinical and evidentiary criteria (see Recommendation 1), with the requirement for ongoing postmarket data collection and assessment (through the national database as proposed in Recommendation 7).
- – These data could support decisions for continued reimbursement or provide the rationale for discontinued reimbursement for a specific biomarker test and its molecularly targeted therapy for specific patient groups.
- Reimbursement for biomarker tests with data collection for patient populations for which the evidence is less substantial, such as rare diseases or underrepresented populations and less studied groups.
- Consider innovative incentives to promote the submission of data to the national repository for biomarker tests and molecularly targeted therapies that have initial evidence of clinical utility.
- CMS should seek to clarify and expand appropriate implementation of coverage with evidence development, which has potential to be an effective policy lever to generate evidence to support
reimbursement decisions for promising technologies such as biomarker tests for molecularly targeted therapies.
Recommendation 5b: The Patient-Centered Outcomes Research Institute and the National Institutes of Health, as well as other funding groups, should develop granting mechanisms that support the assessment of the clinical utility of biomarker tests for molecularly targeted therapies using rapid learning approaches.
Development and Use of Effective EHRs
The committee highlights the critical role of EHRs and LISs in data collection and clinical decision support and underscores the importance of ensuring that EHRs are appropriately developed to facilitate the collection of real-time patient test, treatment, and outcomes data in a structured format. Moreover, EHR patient portals should be designed to provide relevant educational information for patients as well as links to detailed test label information. It is critical not only that vendors and software developers generate effective tools, but that health care providers take advantage of those tools to facilitate point-of-care decision support for biomarker test ordering, reporting, and clinical decision making. Appropriately structured EHR and LIS data will facilitate data transfer to a national data repository recommended by the committee.
Goal 6: Ensure development and use of EHRs and related biomedical informatics tools and assessments that support the effective clinical use of biomarker tests for molecularly targeted therapies.
Recommendation 6a: Electronic health record (EHR) and laboratory information system (LIS) vendors and relevant software developers should enable the capture and linkage of biomarker tests, molecularly targeted therapies, and longitudinal clinical patient data in the EHR to facilitate data transfer into one or more national databases (as described in Recommendation 7).
The information to be structured in the EHR should include, at a minimum:
- Biomarker test specimen requirements (type, amount, handling).
- Specific biomarker test results and interpretation (including actionable panel or next-generation sequencing test results).
- Treatments prescribed and diagnostic tests ordered (whether based on the biomarker test result or not).
- Longitudinal clinical patient data.
The information to be structured in the LIS should include, at a minimum:
- Biomarker test descriptions (assay method, analytes assessed, test performance characteristics, quality metrics, and bioinformatics tools).
Recommendation 6b: Electronic health record (EHR) vendors and relevant software developers should enable EHRs to facilitate point-of-care decision support for biomarker test ordering, reporting, and shared clinical decision making.
- EHR decision support should be layered: highly focused for within the office visit and more detailed for before or after the visit.
- EHRs should allow for incorporation of practice guidelines and pathways as decision support, and also allow tracking compliance.
- Patient portals linked to EHRs should provide biomarker test result information in a patient-friendly manner.
- To enhance patient understanding, relevant educational materials should be accessible from within the portal.
- Portals should include linkages to test labels (see Recommendation 3).
Recommendation 6c: Health care institutions and physician practices should use electronic health records (EHRs) that facilitate point-of-care decision support for biomarker test ordering, reporting, and clinical decision making. This point-of-care decision support should align with available evidence-based clinical practice guidelines.
Recommendation 6d: Licensing and specialty boards should recognize Continuing Medical Education, Continuing Education Units, and Maintenance of Certification achieved through interaction with point-of-care decision support educational materials.
- Professional schools, post-graduate training programs, specialty boards, and continuing education programs should ensure that providers are skilled in the use of point-of-care decision support tools.
National Data Repository
The committee recognizes that much biomarker test data are not available publicly; rather, they are maintained in separate siloes at individual
institutions—a seemingly incongruous situation of a tremendous volume of genomic and genetic data combined with inability to access the data for broader learning purposes. In an effort to promote the public sharing of critical biomarker test data, the committee calls on HHS to facilitate collaboration between FDA and the National Institutes of Health (NIH) to convene a task force to develop a national repository of data related to biomarker tests and corresponding molecularly targeted therapies. HHS should provide incentive payments to encourage all health systems and providers to submit their data to the national repository, which will be built and made accessible with appropriate de-identification and data security measures.
Goal 7: Develop and maintain a sustainable national database for biomarker tests for molecularly targeted therapies through biomedical informatics technology to promote rapid learning for the improvement of patient care.
Recommendation 7: The Secretary of the Department of Health and Human Services (HHS) should charge the Food and Drug Administration (FDA) and National Institutes of Health (NIH) to convene a task force (comprising FDA, the Centers for Medicare & Medicaid Services, the Department of Veterans Affairs, NIH, the Department of Defense, the Patient-Centered Outcomes Research Institute, and other public and private partners) to develop a sustainable national repository of biomarker tests, molecularly targeted therapies, and longitudinal clinical patient data to facilitate rapid learning approaches.
- This prospective, integrated, and structured database should include biomarker test description, test results and interpretation, treatment decisions and outcomes, other relevant electronic health record data generated during clinical practice, clinical trial data, billing/reimbursement data, patient-reported outcomes, and longitudinal clinical patient data.
- The national repository should be built and made accessible with appropriate de-identification, data security, and patient consent measures.
- HHS should provide incentives to encourage data submission by all health care providers/health systems.
Equitable Access
Patients of particular economic, ethnic, and cultural backgrounds and geographic locations may face challenges in obtaining access to precision
medicine’s complex tools such as biomarker tests for molecularly targeted therapies. Dedicated research resources should support a comprehensive investigation to identify existing barriers to equitable access, and subsequently develop approaches to address them. Moreover, collaboration between community health care providers and larger health care centers or academic medical centers should be examined to determine potential impact on access for patients in remote and/or underserved areas.
Goal 8: Promote equity in access to biomarker tests for molecularly targeted therapies and the expertise for effective use of the results in clinical decision making.
Recommendation 8a: Agencies that fund the development or evaluation of biomarkers should include funding to identify and overcome barriers to promote equity, access, and public understanding of precision medicine.
- Potential challenges include but are not limited to: economic factors, cultural/ethnic heterogeneity, geographic diversity, and the complexity of precision medicine.
Recommendation 8b: The Secretary of the Department of Health and Human Services and the Centers for Medicare & Medicaid Services (CMS) should conduct demonstration projects to enable and assess the effectiveness of collaboration between community health care providers and larger health care centers and/or academic medical centers to be part of a rapid learning system.
The demonstration projects should examine:
- Use of reimbursement incentives by CMS for the multidisciplinary collection and review of patient data with clinical recommendations, using distance technology or telemedicine.
- Reimbursement by CMS for genetic counseling services.
Recommendation 8c: Licensing and specialty boards should ensure that health care professionals have and maintain competencies needed for effective use of biomarker tests for molecularly targeted therapies.
- Providers should demonstrate competency in communicating with patients about biomarker tests for molecularly targeted therapies.
Enhanced Specimen Standards
The reliability of biomarker test results depends on the quality of the patient specimens. If the specimen is inadequate for tests that need to be conducted, repeat biopsy procedures may be required to obtain samples sufficient for testing, exposing the patient to unnecessary risk. Professional organizations and health care institutions should develop and implement standards for specimen adequacy and handling, as well as relevant documentation in the EHR and/or LIS.
Goal 9: Enhance specimen handling and documentation to ensure patient safety and the accuracy of biomarker test results.
Recommendation 9a: Professional organizations and accrediting entities should develop, and health care institutions and providers should implement, standards for specimen requirements, handling, and documentation (see Recommendation 6a) through an interdisciplinary effort, including pathologists, interventionalists, surgeons, and other relevant experts.
- Health care professionals who collect, process, and handle (label and ship) patient biomaterials for biomarker testing should ensure that adequate tissue is acquired to perform all necessary testing; that patients are protected from unnecessary/repeated procedures; and that samples are properly handled, with documentation in the electronic health record and/or the laboratory information system.
Recommendation 9b: The National Quality Forum should develop quality measures that assess unnecessary repeat specimen collections.
Improved Clinical Practice Guideline (CPG) Development Processes
Increasingly, a broader base of interdisciplinary expertise is needed to generate trustworthy CPGs related to complex biomarker tests. Consistent with the committee’s vision of a rapid learning system, CPGs serve an important educational purpose—both for clinical decision making as well as for test and drug labeling—and should consider the evolving nature of evidence of clinical utility for biomarker tests for molecularly targeted therapies.
Goal 10: Improve the processes for developing and updating clinical practice guidelines for the effective use of biomarker tests for molecularly targeted therapies.
Recommendation 10: Guideline-developing organizations (e.g., the College of American Pathologists, Association for Molecular Pathology, American College of Medical Genetics and Genomics, American College of Cardiology, National Comprehensive Cancer Network, American Heart Association, American Society of Clinical Oncology, American College of Physicians, and others) should expand interdisciplinary collaborations to develop integrated guidelines on the appropriate use of biomarker tests for molecularly targeted therapies.
- Guidelines should be updated regularly and at intervals appropriate to advances in the field, widely disseminated, user-friendly, and developed with patient participation. They should conform to standards articulated by authoritative groups, including the Institute of Medicine and Guidelines International Network.
- Guideline developers should consider the evolving clinical utility evidence, relative to the standards discussed in Recommendation 1, and from the proposed rapid learning system for biomarker tests.
- The National Guideline Clearinghouse should expand its work in reviewing and rating guidelines.
- Electronic health records (EHR) vendors/EHR purchasers should ensure that recommendations from high-quality guidelines are available within the EHR at the point of care (see Recommendation 6).
- Frequently updated guidelines should serve as input to the iterative updating of test and drug labeling by the integrated federal review process (see Recommendation 2).
The committee’s proposed rapid learning system is expressly designed to promote the proper development, effective and ongoing assessment, and appropriate use of biomarker tests for molecularly targeted therapies. Such a supportive framework would enable precision medicine’s promising treatment-tailoring technologies to realize their full potential to improve patient outcomes.
This page intentionally left blank.






















