This page intentionally left blank.
The following four hypothetical scenarios demonstrate how to use the tools described in this Guide to Developing Standard Operating Procedures. The entries in the four forms were completed by the expert committee and are solely for illustrative purposes; real situations will call for procedures that are suited to the specific laboratory surroundings and personnel.
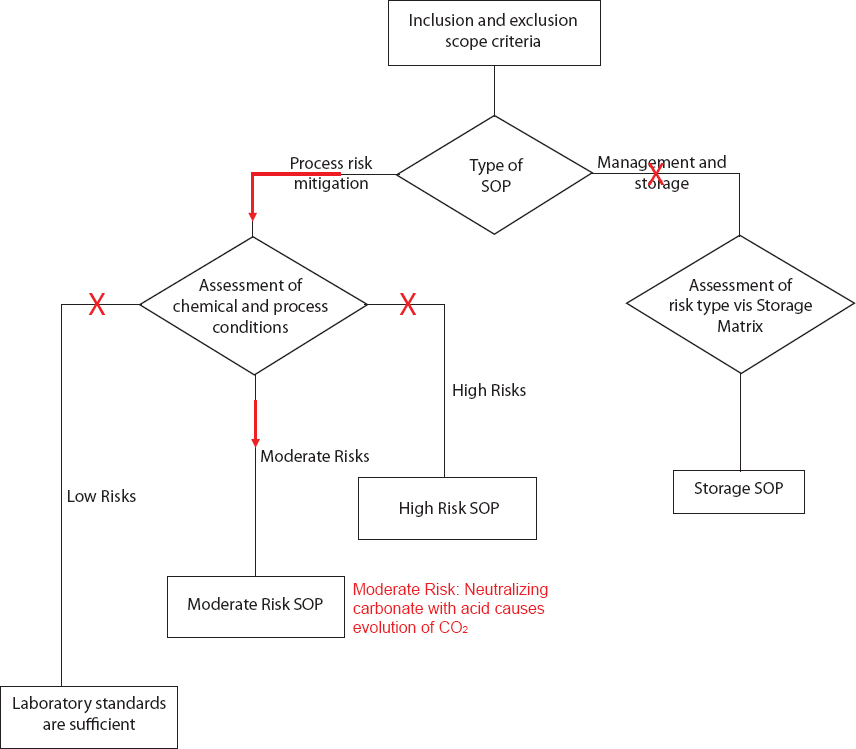
SCENARIO 1: NEUTRALIZATION OF CARBONATE BY ACID
Overview: This scenario describes a situation in which a student with moderate experience is conducting a routine procedure of neutralizing a carbonate species with acid in a chemical laboratory at a university.
SCENARIO:
The student wants to hydrolyze an ester. The chemical reaction is carried out in an aqueous-organic medium with sodium carbonate or bicarbonate as the base. The desired product is soluble in the excess aqueous base. To obtain the product, the medium is neutralized with concentrated hydrochloric acid. The use of concentrated acid helps keep the total reaction fluid volume low, thereby optimizing precipitation or extraction of the product. The vigorous effervescent release of carbon dioxide, however, is a potential concern to the student. How should the student proceed?
Suggested solution:
To conduct the reaction safely, the student uses the Risk Assessment Flowchart to determine whether the laboratory standards are sufficient or whether a standard operating procedure is needed.
Moderate Risk Standard Operating Procedure Form
1. TITLE AND DESCRIPTION OF EXPERIMENT OR PROCESS: Hypothetical workup of a reaction medium requiring neutralization of carbonates using concentrated hydrochloric acid
2. PREPARER(S): [name]
3. LOCATION: Laboratory
4. AUTHORIZED PERSONNEL (WITH CONTACT INFORMATION)
a. PRINCIPAL INVESTIGATOR (PI)/SUPERVISOR: [name] (555) 343-1515
b. STUDENT(S)/TECHNICIAN(S)/OPERATOR(S): [name] (555) 321-5762
c. OTHERS TO BE NOTIFIED (e.g., other workers in the same laboratory, or other members of the research group): [name] (555) 724-8921
5. POTENTIAL HAZARDS: Concentrated hydrochloric acid (HCl, 37%). Hydrochloric acid is corrosive and causes burns on eyes, skin, and digestive and respiratory tracts. It may be fatal if inhaled or swallowed. Repeated exposure may cause erosion of exposed teeth.
6. DESCRIBE ALL SPECIAL REQUIREMENTS FOR SPECIFIC ITEMS THAT REQUIRE A GREATER LEVEL OF SAFETY
| Planned chemicals involved | Concentrated hydrochloric acid (HCl, 37%) |
| Personal protective equipment | Lab coat, long pants, closed-toed shoes, double gloves (vinyl gloves over nitrile gloves), safety goggles |
| Engineering and environmental controls | Any handling of concentrated hydrochloric acid outside of the fume hood is limited and done within closed containers only. |
| Operational ranges and conditions | See special handling section. |
| Special handling and storage requirements | Neutralization should take place in a large-mouth open vessel (a beaker, not an Erlenmeyer flask) in a hood. It is often prudent to chill the carbonate mixture in an ice-salt bath and to stir the mixture vigorously while the acid is added. If a product precipitates at neutrality, then remove it by filtration. If extraction is necessary to obtain the product, then take great care that gas evolution has ceased before the contents are transferred to a separatory funnel. Shaking a separatory funnel with a gas-evolving solution will often blow the stopper and jettison the contents.
Unused chemicals are stored in 2.5 L glass containers in a specially designed and designated acid storage cabinet. In addition, small amounts (40 mL) of hydrochloric acid can be stored in beakers with a glass lid in the fume hood. The aqueous waste is stored in a 4 L High-Density Polyethylene (HDPE) storage bottle in the fume hood or in the designated acid cabinet. No acid is stored outside this cabinet or the fume hood, even temporarily. |
| Spill and accident procedure as needed | An acid-base spill cleanup kit should be used only for very small spills up to 50 mL, following the instructions provided for the kit. Otherwise, secondary containment should be used that contains the entire spill. In the event of a small spill, clean up the spill immediately. If the spill is large, then isolate the area, deny entry, leave the room, and call appropriate emergency responders. If exposed, then remove clothing and use the emergency shower located directly outside of room. If someone is incapacitated, then call 911 and initiate first aid if possible. |
| Waste handling and disposal | For spills: place the used absorbent in 4 L HDPE storage bottle for pick-up. For the aqueous waste left after removal of the product, attach Hazardous Waste Label, accumulate said waste according to laboratory requirements, and contact the waste pick-up operator. |
7. IDENTIFY ANY TRAINING NEEDS No specific training required
| Specific training required |
|
| Users requiring training |
|
| Date completed |
9. VERIFICATION AND REVIEW
By signing below, you acknowledge that you have read, understand, and approve the SOP.
| PI name (please print) | Professor [name] |
| PI signature | Professor |
| Safety staff name (please print) | [name] |
| Safety staff signature | signature |
| Current date | 01/29/2016 |
| Date of SOP expiration (e.g., 1 year) | 01/29/2017 |
9. LIST OF REFERENCES (include Safety Data Sheets, Globally Harmonized System, any outside personnel consulted in the preparation of the document, peer reviewers, etc.)
Moderate Risk Standard Operating Procedure Review and Modifications Log
| Were modifications made to current SOP? | Yes |
| If no, then list date of approval | |
| Last reviewed by (please print) | |
| If yes, then list and describe modifications |
| Were modifications made to current SOP? | Yes |
| If no, then list date of approval | |
| Last reviewed by (please print) | |
| If yes, then list and describe modifications |
| Were modifications made to current SOP? | Yes |
| If no, then list date of approval | |
| Last reviewed by (please print) | |
| If yes, then list and describe modifications |
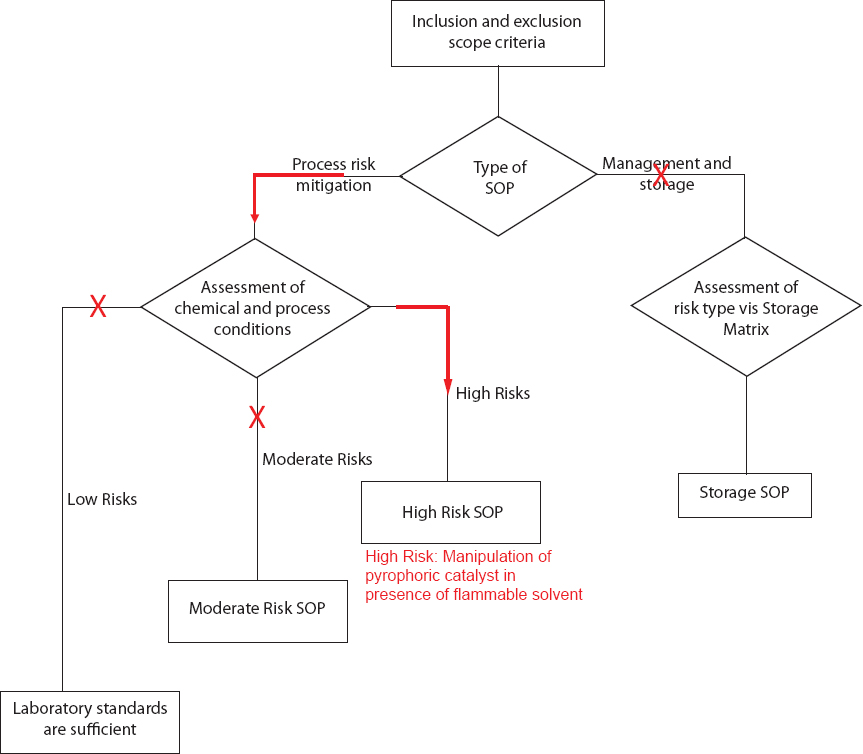
SCENARIO 2: USE AND FILTRATION OF A PYROPHORIC CATALYST
Overview: This scenario describes a situation where an inexperienced researcher wants to complete a reaction using a pyrophoric heterogeneous catalyst, followed by a filtration workup step.
SCENARIO:
A newly hired researcher in an industrial laboratory is using a pyrophoric heterogeneous catalyst in a hydrogenation reaction. After reviewing section 9.7.4 of the National Academies of Sciences, Engineering, and Medicine report, A Guide to Prudent Chemical Management, the researcher discovers special hazards that may be encountered when filtering the hydrogen-saturated catalyst from the flammable organic reactants.
Suggested solution:
To minimize fires and explosions during the filtration step, the researcher goes through a hazard assessment checklist. After completing the assessment checklist, the researcher concludes that he/she will need a High Risk SOP.
High Risk Standard Operating Procedure Form
1. TITLE AND DESCRIPTION OF EXPERIMENT OR PROCESS: Pyrophoric/ignitable catalyst: Procedure for addition to reaction vessel and separation from reaction mixture by filtration
2. PREPARER(S): [name]
3. LOCATION: industrial laboratory
4. AUTHORIZED PERSONNEL (WITH CONTACT INFORMATION)
a. PRINCIPAL INVESTIGATOR (PI)/SUPERVISOR: [name] (555) 123-4567
b. STUDENT(S)/TECHNICIAN(S)/OPERATOR(S): [name] (555) 401-6333
c. OTHERS TO BE NOTIFIED (e.g., other workers in the same laboratory, or other members of the research group): [name] (555) 202-3333
5. IDENTIFY ANY TRAINING NEEDS
| Experimental techniques | Filtration techniques, vacuum trap for solvents |
| Specific training required | See #12 Waste disposal |
| Users requiring training | |
| Date completed | 12/15/2015 |
6. DETAILED PROCESS DESCRIPTION
This SOP covers the use of a wide variety of pyrophoric catalysts. The catalysts may become pyrophoric after exposure to reducing agents (e.g., organic solvents, hydrogen) or are pyrophoric as obtained from vendors.
It is essential that the activated catalyst does not become dry or exposed to air, especially in the presence of solvent vapors, or a fire will result. Raney metal catalysts are pyrophoric as obtained from vendors and are typically provided as water slurries to prevent ignition. These catalysts must be wetted during all manipulations.
The fire potential is mitigated by (1) keeping the pyrophoric catalyst wetted with solvent and/or water during all stages of handling, including final transfer to a waste container, (2) maintaining an inert gas purge or blanket above the pyrophoric catalyst, and (3) eliminating clutter and any additional fuel sources from the immediate work area.
| List ranges for variables | Temperature: ambient |
| Pressure: n/a | |
| Viscosity: n/a | |
| Flammability: reaction solvents are flammable; catalyst may be pyrophoric | |
| Other: | |
| List of operational ranges and conditions |
|
| Materials to be used | Chemicals: pyrophoric catalyst, reaction solvent, Celite |
| Equipment: reaction vessel, filter flask, filter, inert gas source |
DETAILED PROCEDURE:
Column A: Process Steps provide detailed step-by-step normal operating procedures, including the transfer of chemicals between or across laboratories, production of gases, workup of products, and preparation of wastes.
Column B: Safety Notes describe how the risk can be mitigated (i.e., minimize clutter, control flammables, secure gas cylinders, ensure good condition of pump belts).
| Column A: Process Steps | Column B: Safety Notes | |
| 1.0 | Charging pyrophoric catalyst to a vessel. | Engineering controls: The following operations are conducted in a functioning fume hood with sash. PPE requirements: Flame-resistant lab coat, face shield, gloves appropriate for the solvent being used (consult a glove selection guide). |
| 1.1 | Clear the hood where the procedure will take place; move flammables, squirt bottles (acetone, methanol, etc.), and clutter out of the immediate vicinity. | Have Metal-X extinguishing agent and a squirt bottle of water within reach of the work area. Although Metal-X extinguishing is an ideal product for this purpose, alternatives would be a bucket of sand and/or finely divided NaCl. Ensure local fire extinguishers are in their proper locations and are fully charged. |
| 1.2 | Purge the vessel with inert gas for at least 5 minutes. |
| 1.3 | Weigh out the catalyst and add it to the vessel, continuing to purge the vessel with an inert gas. | Most unactivated metal catalysts (e.g., Pd/C, Ru/C, PtO2, Ni/Kieselguhr) are not pyrophoric as obtained from vendors, but they become pyrophoric upon exposure to certain solvents (especially alcohols) and hydrogen.
Raney metal catalysts (e.g., Raney Nickel) are typically provided and weighed/manipulated as water slurries. These catalysts will ignite upon air exposure and will dry if allowed. Do not purge the vessel too vigorously; otherwise, the purge may blow the finely divided catalyst into the hood. |
| 1.4 | Slowly add the desired solvent to the vessel while maintaining the inert gas purge. | |
| 1.5 | Add any other reagents to the vessel while maintaining the inert gas purge. | For Steps 1.3, 1.4, and 1.5 ensure that the catalyst and solvent are in contact only when under inert atmosphere, thus preventing the possibility of ignition and fire. |
| 2.0 | Filtering a pyrophoric heterogeneous catalyst from a reaction solution. | Engineering controls: The following operations are conducted in a fume hood with sash.
PPE requirements: Flame-resistant lab coat, face shield, gloves appropriate for the solvent being used (consult a glove selection guide). |
| 2.1 | Clear the hood of clutter and flammables. | Eliminate any additional fuel sources in the event of a fire. |
| 2.2 | Ensure fire extinguishers are in their proper location and ready for use. | Have Metal-X extinguishing agent and a squirt bottle of water within reach of the work area. Although Metal-X-extinguishing is an ideal product for this purpose, alternatives would be a bucket of sand and/or finely divided NaCl. |
| 2.3 | Choose an appropriate filter and filter flask and secure them in place to the flex frame. Have a means with which to blanket the headspace above the filter with inert gas. | A tall filter is preferable because it allows enough solvent volume above the solid catalyst to blanket the catalyst and protect it from air exposure during filtration. Use a receiving flask large enough to hold the filtrate plus washings. Clamp the apparatus to the flex frame to prevent spills during filtration. |
| 2.4 | Slurry pack the filter with Celite in wash solvent using sufficient Celite to give a uniform 1-2 cm thick bed. | The vacuum system is protected from solvent contamination by means of appropriate traps. |
| 2.5 | Open the vessel containing the reaction mixture and purge with inert gas. |
| 2.6 | With inert gas purging of both the vessel and the filter, carefully pour the reaction mixture onto the Celite bed. Apply slight vacuum to the receiving flask to begin the filtration. Do not filter all of the solvent; leave a small amount of solvent above the catalyst solids. | DO NOT PERMIT THE CATALYST ON TOP OF THE CELITE BED TO BE EXPOSED TO AIR. FIRE WILL OCCUR IF THE CATALYST IS EXPOSED TO AIR.
Adjust the vacuum as needed to give an adequate filtration rate. Maintain a nitrogen blanket above the Celite/catalyst bed. |
| 2.7 | Rinse the vessel with solvent and add to the filter as in Step 2.6. When the vessel has been adequately rinsed, add water using a squirt bottle and set aside. | |
| 2.8 | Wash the catalyst solids with solvent as needed. When filtering the final wash, allow the solvent level to drop to just above the solid level and then stop the filtration. Remove the filter from the receiving flask and then add water to the filter to wet the catalyst. Move the receiving flask to another area of the hood. | DO NOT PERMIT THE CATALYST ON TOP OF THE CELITE BED TO BE EXPOSED TO AIR—A FIRE WILL OCCUR.
Relocate the receiving flask to another area in the hood so that it will not contribute to a fire during catalyst disposal. |
| 2.9 | Add water to a jar or waste container. Purge this container with inert gas. Quickly transfer the filter contents to the waste jar using a spatula and water. Keep the catalyst solids under inert gas as much as possible during this transfer. Rinse the filter with water until all catalyst is transferred to the container. | Do not mix other types of waste in this container. |
| 2.10 | After purging the waste container with inert gas, properly cap and label the container. | Label the waste jar properly, and use appropriate warning signs. |
7. ENGINEERING CONTROLS (check all that apply and provide a detailed description)
| Engineering Controls | Check box if applicable | Description |
| Ex. Glove box | Use inert air (N2 or Ar) | |
Fume hood or glove box |
|
|
| Special ventilation | ||
| HEPA-filtered vacuum lines | ||
| Non-reactive containers | ||
| Pressure relief devices | ||
| Temperature control | ||
| Bench paper, pads, plastic-backed paper | ||
| Special signage | ||
| Safe sharp devices | ||
| Other safety devices used: |
8. PERSONAL PROTECTIVE EQUIPMENT (check all that apply)
| Personal Protective Equipment | Check box if applicable | Description |
| Gloves | Appropriate for solvent being used. (consult a glove selection guide) | |
Lab coats |
|
Flame resistant |
| Suits | ||
| Aprons | ||
| Long pants | ||
| Close-toed shoes | ||
| Long sleeves | ||
| Safety glasses | ||
| Goggles | ||
| Face shields | ||
| Respirators (include cartridge type and cartridge change out schedule) | ||
| Hearing protection (include level of protection needed) | ||
| Special equipment (i.e., blast shields, special enclosures | ||
| Other PPE |
9. WORK PRACTICE CONTROLS
| Controls | Description |
| Designated areas | Do not work alone—have others present |
| Procedures for requesting emergency assistance | Follow lab emergency procedures |
| Emergency phone numbers | 911 EHS number |
| Locations of fire alarms, fire extinguishers, fire blankets, eye washes, showers, etc. | Eye washes and shower are located in Room 101. Fire blanket is located on west wall to entrance door to lab. |
| Emergency responders | |
| Workers on shifts | yes |
| Training on all experimental techniques and experiments | yes |
| Restricting access; locks | yes |
| Housekeeping | Eliminate clutter and any additional fuel sources from the immediate work area. |
| Lockout/tagouta procedure plan | n/a |
| After-hour procedures | No work after office hours. |
| Preventive maintenance | n/a |
a Lockout/tagout refers to specific procedures to safeguard researchers from an unexpected startup of machinery and equipment, or a release of hazardous energy during service or maintenance activities.
10. MONITORING
None required for this procedure
| Monitoring | Description |
| Personnel exposure monitoring | n/a |
| Leak checking | n/a |
| Gas and spill release monitoring | n/a |
| Temperature and pressure | n/a |
| Alarms | n/a |
11. SPILL AND ACCIDENT PROCEDURES
Wearing a flame-resistant lab coat, eye protection, and gloves, immediately cover spilled pyrophoric catalyst with water (squirt bottle) or Metal-X. Then thoroughly soak some paper towels with water. Use the wetted paper towels to mop up the spilled catalyst. Immediately place the used paper towels in the waste container designated for pyrophoric catalysts—do not allow the contaminated towels to dry in the air. Purge the waste container with inert gas and then cap tightly.
| Description and Location | |
| Secondary containment | |
| Spill kits | |
| Emergency shutdown procedures | |
| Process shutdown | |
| Persons to inform |
12. WASTE DISPOSAL PROCEDURES (include segregation of acids, bases, halogenated compounds, PPE, collection and transport procedures, proper documentation, regulations, etc.)
It is suggested that waste containers containing pyrophoric catalysts contain ample water to ensure the solids remain wetted. The container will be flushed with inert gas and then tightly capped. The container is designated for pyrophoric catalysts only, and no other types of waste can be added to it. Spent pyrophoric catalysts are generally designated as reactive waste and are handled accordingly.
13. STORAGE (check all that apply)
Ventilated enclosures ![]()
Refrigeration ![]()
Gas cabinet ![]()
Compliance with regulations ![]()
Expiration date:
Inventory:
Other:
Most vendor-supplied metal catalysts are not supplied in an activated state and are not pyrophoric until activated. Normal storage procedures can be applied. Raney metal catalysts are provided as water slurries to prevent ignition. These samples are stored in tightly capped containers to prevent them from drying out. Inspect routinely and segregate from flammable materials.
14. TRANSPORTATION PROCEDURES:
(e.g., secondary container for transport between and across laboratories; secondary containment for experimental apparatus)
Secondary container for transport between and across laboratories
Secondary containment for experimental apparatus
15. PEER REVIEW
| Name (please print) | [name] |
| Signature | signature |
| Date | 01/31/2016 |
| Notes |
| Name (please print) | [name] |
| Signature | signature |
| Date | 02/03/2016 |
| Notes |
16. VERIFICATION AND REVIEW
By signing below, you acknowledge that you have read, understand, and approve the SOP.
| PI name (please print) | [name] |
| PI signature | signature |
| Safety staff name (please print) | [name] |
| Safety staff signature | signature |
| Current date | 02/10/2016 |
| Date of SOP expiration (e.g., 1 year) | 02/10/2017 |
17. LIST OF REFERENCES (include Safety Data Sheets, Globally Harmonized System, any outside personnel consulted in preparation of document, peer reviewers, etc.)
High Risk Standard Operating Procedure Review and Modifications Log
| Were modifications made to current SOP? | Yes |
| If no, then list date of approval | |
| Last reviewed by (please print) | |
| If yes, then list and describe modifications |
| Were modifications made to current SOP? | Yes |
| If no, then list date of approval | |
| Last reviewed by (please print) | |
| If yes, then list and describe modifications |
| Were modifications made to current SOP? | Yes |
| If no, then list date of approval | |
| Last reviewed by (please print) | |
| If yes, then list and describe modifications |
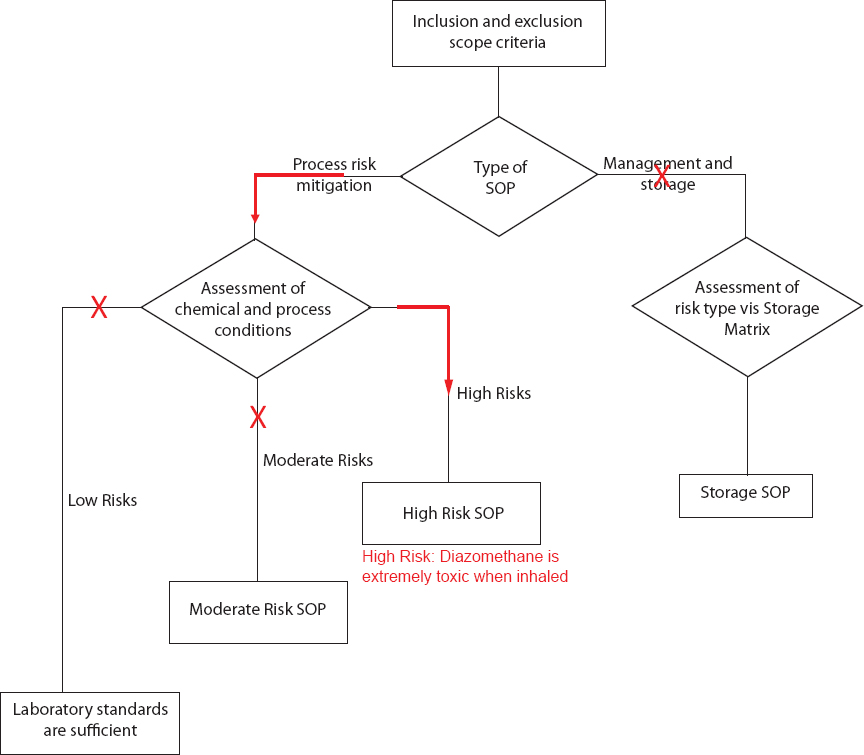
SCENARIO 3: TOXIC AND EXPLOSIVE GASES: HANDLING DIAZOMETHANE AND ALTERNATIVES
Overview: This scenario describes a situation where a graduate student wants to run a reaction that calls for diazomethane. After completing the hazard assessment checklist, she develops a High Risk SOP. Her advisor, who reviews the SOP, does not approve of the use of diazomethane and suggests an alternative compound (trimethylsilyl)diazomethane (TMSD).
SCENARIO:
An experienced student wants to use diazomethane as an alkylating agent in her reaction. Diazomethane has been flagged as highly hazardous because of its flammability, explosive potential, and toxicity as noted in sections 7 and 9 of the National Academies of Sciences, Engineering, and Medicine report, Chemical Laboratory Safety and Security. The student immediately recognizes that she will need to develop a High Risk SOP. She asks her advisor to review her SOP. He does not approve the SOP, even though the student followed and completed the correct steps.
Suggested solution:
The advisor recognized that diazomethane can be substituted with (trimethylsilyl)diazomethane (TMSD) because it is less likely to explode as it generates diazomethane in situ and may be used for the reaction. TMSD is commercially available in anhydrous hexanes or diethyl ether, and the respective Safety Data Sheets are available online. Although explosive accidents have not occurred with TMSD, TMSD is far from being a perfect surrogate. The toxicity of TMSD is still high, with at least two recorded deaths from inhalation.
High Risk Standard Operating Procedure Form
1. TITLE AND DESCRIPTION OF EXPERIMENT OR PROCESS: Toxic and Explosive Gases: Safe Handling of Diazomethane and Alternatives
2. PREPARER(S): [name]
3. LOCATION: Room 101 Chemistry Hall
4. AUTHORIZED PERSONNEL (WITH CONTACT INFORMATION)
a. PRINICIPAL INVESTIGATOR (PI)/SUPERVISOR: [name] (555) 854-9654
b. STUDENT(S)/TECHNICIAN(S)/OPERATOR(S): [name] (555) 202-2020
c. OTHERS TO BE NOTIFIED (e.g., other workers in the same laboratory, or other members of the research group): [name] (555) 333-4444
5. IDENTIFY ANY TRAINING NEEDS: No specific training required
| Experimental techniques | |
| Specific training required | |
| Users requiring training | |
| Date completed |
6. DETAILED PROCESS DESCRIPTION See SDS for CAS Registry Number 18107-18-1
| List ranges for variables | Temperature: |
| Pressure: | |
| Viscosity: | |
| Flammability: | |
| Other: | |
| List of operational ranges and conditions |
|
| Materials to be used | Chemicals: (trimethylsilyl)diazomethane |
| Equipment: Diazomethane will be generated in situ from TMSD. Diazomethane is known to detonate on sharp glass or metal surfaces. Use a special diazomethane flask with fire-polished Clear-Seal™ joints. |
DETAILED PROCEDURE:
Column A: Process Steps provide detailed step-by-step normal operating procedures, including the transfer of chemicals between or across laboratories, production of gases, workup of products, and preparation of wastes.
Column B: Safety Notes describe how the risk can be mitigated (i.e., minimize clutter, control flammables, secure gas cylinders, ensure good condition of pump belts).
| Column A: Process Steps | Column B: Safety Notes | |
| 1.0 | In situ synthesis of diazomethane from TMSD and substrate methylation. | Engineering controls: The following operations are conducted in a functioning fume hood with sash. Diazomethane explosions can be highly energetic, and blast shields are recommended.
PPE Requirements: Wear respiratory protection and full face protection. Avoid breathing vapors, mist, or gas. If contact is likely, then double glove using a second exam-grade nitrile glove under the utility-grade nitrile glove. Wear a flame-resistant lab coat. Remove all sources of ignition. |
| 1.1 | Assemble a three-neck reaction flask containing a pressure-equalizing addition funnel, gas inlet/outlet, thermometer, and magnetic stir bar. | Use cut-resistant gloves when assembling glassware. |
| 1.2 | Charge the reaction flask with solvent and reagents, and then chill to 0°C using an ice bath. | Place the ice bath on top of a magnetic stirrer. |
| 1.3 | Charge the addition funnel with TMSD solution. | |
| 1.4 | Add TMSD in commercially supplied solvent in dropwise fashion to an excess of the substrate for methylation. TMSD is used because it is less likely to explode as it generates diazomethane in situ and is commercially available in anhydrous hexanes or diethyl ether. | Stir the mixture at a moderate rate to ensure methylation. If the yellow color of diazomethane is present, then slow the rate of addition of TMSD and increase the stirring speed.
If large quantities of diazomethane are to be generated, even in a high-flow hood, then some safety authorities recommend wearing a respirator. |
| 2.0 | Isolation and clean-up. | The deactivation of both diazomethane and TMSD is accomplished in the acetic acid quench. Explosive hazard and toxic vapor hazards are mitigated. PPE: exam-grade nitrile gloves are sufficient. |
| 2.1 | Add excess acetic acid until any yellow color dissipates and gas evolution ceases. | Diazomethane is yellow, and reaction completion is usually signaled by the loss of yellow color. Both diazomethane and TMSD are destroyed by acetic acid. |
| 2.2 | Subsequent workup is by published methods. | Ensure any additional hazards are evaluated in published methods. |
7. ENGINEERING CONTROLS (check all that apply and provide a detailed description)
| Engineering Controls | Check box if applicable | Description |
| Ex. Glove box | Use inert air (N2 or Ar) | |
Fume hood or glove box |
|
|
| Special ventilation | ||
| HEPA filtered vacuum lines | ||
| Non-reactive containers | ||
| Pressure relief devices | ||
| Temperature control | ||
| Bench paper, pads, plastic-backed paper | ||
| Special signage | ||
| Safe sharp devices | ||
| Other safety devices used: |
8. PERSONAL PROTECTIVE EQUIPMENT (check all that apply)
| Personal Protective Equipment | Check box if applicable | Description |
| Gloves | Nitrile 8-mil gloves recommended. If contact is likely, then double glove using a second exam-grade nitrile glove first and the utility-grade nitrile glove second. | |
Lab coats |
|
Flame resistant |
| Suits | ||
| Aprons | ||
| Long pants | ||
| Close-toed shoes | ||
| Long sleeves | ||
| Safety glasses | ||
| Goggles | ||
| Face shields | ||
| Respirators (include cartridge type and cartridge change out schedule) | ||
| Hearing protection (include level of protection needed) | ||
| Special equipment (i.e. blast shields, special enclosures | ||
| Other PPE |
9. WORK PRACTICE CONTROLS
| Controls | Description |
| Designated areas | Fume hood with sash |
| Procedures for requesting emergency assistance | Follow lab standards |
| Emergency phone numbers | 911 Fire Department Number |
| Locations of fire alarms, fire extinguishers, fire blankets, eye washes, showers, etc. | One fire alarm in Room 101 Chemistry Hall. Three fire blankets located on the walls of Room 101. Shower located near back wall of Room 101. |
| Emergency responders | 911 |
| Workers on shifts | yes |
| Training on all experimental techniques and experiments | yes |
| Restricting access; locks | yes |
| Housekeeping | yes |
| Lockout/tagouta procedure plan | yes |
| After-hour procedures | no |
| Preventive maintenance | yes |
a Lockout/tagout refers to specific procedures to safeguard researchers from an unexpected startup of machinery and equipment, or a release of hazardous energy during service or maintenance activities.
10. MONITORING
| Monitoring | Description |
| Personnel exposure monitoring | Air samples should be taken in the user’s breathing zone. The collection of vapors by an adsorption tube containing a resin coated with octanoic acid, followed by desorption with carbon disulfide can be measured by gas chromatographic analysis. |
| Leak checking | |
| Gas and spill release monitoring | |
| Temperature and pressure | |
| Alarms |
11. SPILL AND ACCIDENT PROCEDURES
| Description and Location | |
| Secondary containment | n/a |
| Spill kits | |
| Emergency shut down procedures | If the researcher has inhaled diazomethane or TMSD, then seek immediate exposure to fresh air followed by prompt care at an emergency room. |
| Process shutdown | If spill happens inside fume hood, then immediately close sash and let evaporate. Activate emergency exhaust. |
| Persons to inform |
12. WASTE DISPOSAL PROCEDURES (include segregation of acids, bases, halogenated compounds, PPE, collection and transport procedures, proper documentation, regulations, etc.)
After the diazomethane solution is quenched, dispose of it through normal waste streams.
13. STORAGE (check all that apply) Storage of diazomethane solutions is highly discouraged
Ventilated enclosures ![]()
Refrigeration ![]()
Gas cabinet ![]()
Compliance with regulations ![]()
Expiration date:
Inventory:
Other:
14. TRANSPORTATION PROCEDURES:
(e.g., secondary container for transport between and across laboratories; secondary containment for experimental apparatus)
n/a
15. PEER REVIEW
| Name (please print) | [name] |
| Signature | signature |
| Date | 08/13/2016 |
| Notes |
| Name (please print) | |
| Signature | |
| Date | |
| Notes |
16. VERIFICATION AND REVIEW
By signing below, you acknowledge that you have read, understand, and approve the SOP.
| PI name (please print) | [name] |
| PI signature | signature |
| Safety staff name (please print) | [name] |
| Safety staff signature | signature |
| Current date | 08/15/2016 |
| Date of SOP expiration (e.g., 1 year) | 01/15/2017 |
17. LIST OF REFERENCES (include Safety Data Sheets, Globally Harmonized System, any outside personnel consulted in preparation of document, peer reviewers, etc.)
SDS for (trimethylsilyl)diazomethane—CAS Registry Number 18107-18-1
High Risk Standard Operating Procedure Review and Modifications Log
| Were modifications made to current SOP? | Yes |
| If no, then list date of approval | |
| Last reviewed by (please print) | |
| If yes, then list and describe modifications |
| Were modifications made to current SOP? | Yes |
| If no, then list date of approval | |
| Last reviewed by (please print) | |
| If yes, then list and describe modifications |
| Were modifications made to current SOP? | Yes |
| If no, then list date of approval | |
| Last reviewed by (please print) | |
| If yes, then list and describe modifications |
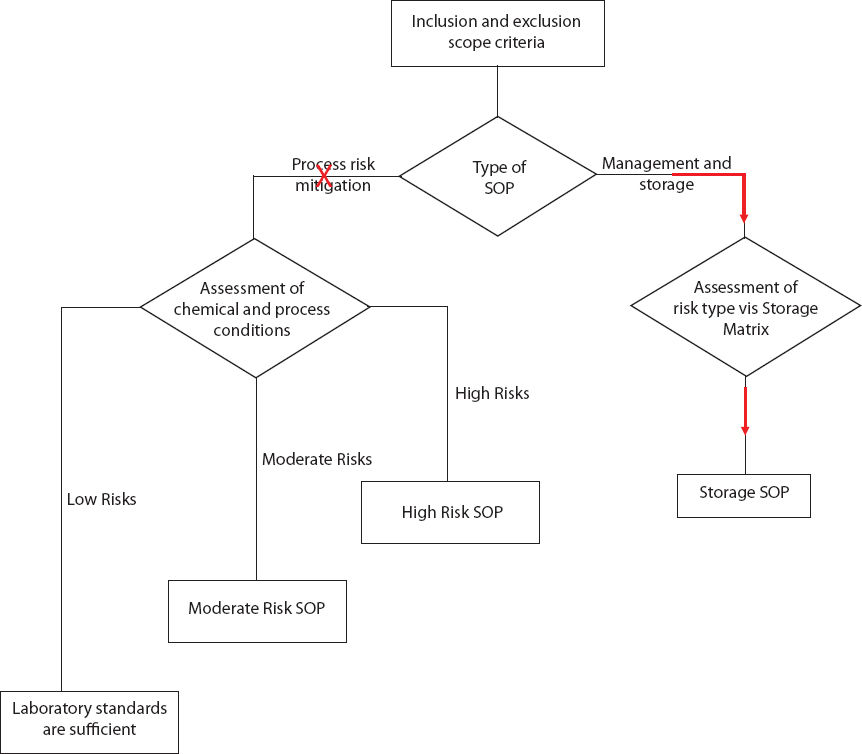
SCENARIO 4: REAGENT STORAGE
Overview: This scenario describes a situation in which a laboratory manager in a large academic laboratory receives three requests from chemistry graduate students for chemical reagents:
- Sodium Azide, 5 g
- Piperonal, 500 g
- Diazald, 25 g
SCENARIO:
The University has a different level of experience and safety awareness with each of these reagents. Exemplary execution of the pre- and post-ordering activities and storage process for these three reagents is shown below:
Suggested Solution:
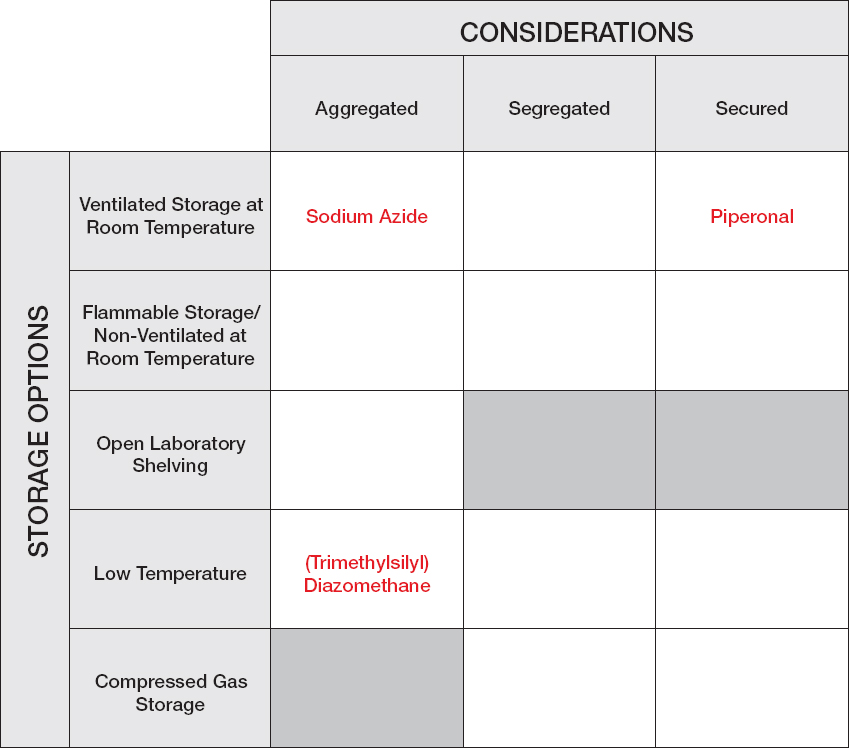
Sodium Azide: When the laboratory manager receives the request for sodium azide, he searches the chemical reagent inventory list and realizes that this reagent has not previously been ordered for use in the department. He accesses the vendor’s website to review the Safety Data Sheet (SDS) and notes warnings of acute toxicity (oral, dermal, inhalation), “Health Hazard” for respiratory sensitization, and “Environment” for hazard to the aquatic environment, both acute and chronic. Azides in combination with acid or acid vapors can form deadly hydrazoic acid, a toxic gas. Azides also present a severe explosion risk when shocked or heated. It is suggested that sodium azide be stored tightly in closed containers in a well-ventilated and dry area away from acid. Because of the explosive potential and safety and security concerns, sodium azide should be avoided in a synthesis if a reasonable alternative exists whenever possible. The student consults the literature and does not find an alternative that will work in the synthetic reaction. After reviewing the SDS and confirming that the reagent is not on the University’s Dual-Use Chemicals List, the laboratory manager places the order for this chemical reagent.
While waiting for the bottle of sodium azide to arrive from the vendor, the laboratory manager uses the Chemical Storage Matrix to identify the chemical’s storage requirements: Are there special temperature requirements? Does the reagent need to be segregated from other chemicals? Are there special security considerations? There are no special requirements for temperature to store sodium azide. Storage in standard ventilated room temperature reagent cabinets will segregate the chemical from acids (because acids are segregated). Therefore, the laboratory manager arrives at the conclusion that “Aggregated—Ventilated Room Temperature” in an area that is temperature and humidity controlled is the proper location for this reagent. Out of an abundance of caution, the laboratory manager stores sodium azide in a secondary container with Drierite (anhydrous sodium sulfate). For future reference, this reagent is added to the University’s list of chemical reagents with the storage designation “Aggregated—Ventilated Room Temperature” with secondary containment. When the student picks up the chemical reagent, the laboratory manager provides a copy of the Safety Data Sheet, notes the hazardous nature of the chemical, and recommends that the student speak with his or her professor before running the first reaction to discuss safe handling for the reaction. In that discussion, the student and professor agree that a Moderate Risk SOP should be drafted for the use of this reagent. While that SOP is being drafted, the student performs the initial probe reactions using sodium azide on a very small scale (< 10 mmol), mitigating the danger of this reaction.
Piperonal: Following the University’s SOP for chemical reagent management, the laboratory manager scans the University’s Dual-Use Chemicals List prior to ordering the chemical and notes that this reagent is on the list
because of its potential to be used for the synthesis of illicit drugs. Although the reagent has been used before at the University, there is presently no material available. The laboratory manager finds that piperonal is a volatile aldehyde that releases a vapor that can induce central nervous system depression. The laboratory manager also reviews the catalog of the chemical supplier and learns that this reagent is available in much smaller quantities than the 500 g bottle requested by the student. As a result, the laboratory manager alerts the student to the fact that this reagent is on the Dual-Use Chemical List and inquires whether 25 g will meet the student’s needs. The student explains that he recommended the 500 g bottle because the unit price at 500 g is one-quarter the unit price at 25 g. After the laboratory manager explains that the best practice guideline is to order the minimum quantity of dual-use chemicals, the student agrees that 25 g is sufficient for his research needs. The laboratory manager then emails the student’s professor as required by the SOP for reagent management to request authorization to order this dual-use reagent. The professor and the student discussed the use of this reagent at the previous day’s group meeting, so the professor immediately approves the request, and the laboratory manager places the order. Upon its arrival, the reagent is placed in a locked storage cabinet, which is ventilated at room temperature. The student is notified of the reagent’s arrival and is reminded that the reagent must be checked out and returned the same day so that it can be secured.
Diazald: Diazald is a common precursor for the generation of diazomethane. The laboratory manager is aware of the danger of diazomethane and of an alternative reagent to diazald. The laboratory manager emails the professor (and copies the student) asking for verification of the need for this chemical reagent. In this case, the professor was unaware that the student was approaching the chemical reaction using the diazald procedure for generation of diazomethane. The professor suggests that the student first try the reaction with (trimethylsilyl) diazomethane, a safer alternative. The laboratory manager searches the existing chemical inventory and finds a recently ordered bottle (2 M dissolved in diethyl ether) in one of the laboratory refrigerators. The student borrows the existing diazald and discusses prudent safeguards with another student in the laboratory with experience with the reagent before performing the reaction.
CHEMICAL STORAGE MATRIX