This page intentionally left blank.
INTRODUCTION
The Risk Assessment Flowchart is designed to aid in the assessment of the risks associated with specific process and chemicals. The flowchart is divided into two tracks: (1) Process Risk Mitigation and (2) Management and Storage of Chemicals. Development of a Chemical Storage standard operating procedure (SOP; see Chapter 3 for more details), which is the endpoint for the Management and Storage of Chemicals track, would be appropriate for all hazardous chemicals.
MANAGEMENT AND STORAGE
The laboratory worker will use scope criteria to determine whether the risk is inherent in the processing of the chemical or in the management and storage of the chemical to determine whether an SOP is needed for Process Risk Mitigation or Management and Storage of Chemicals (Figure 2-1). If the proper procedures for handling and storing the chemical are unclear, then the worker will select the Management and Storage track as the default. Proper storage is a concern for all hazardous chemicals, even those that will be used in an experiment scheduled for the future. The Chemical Storage Matrix, which will assist in development of the Management and Storage SOP, is presented in Chapter 3.
PROCESS RISK MITIGATION
The worker will select the Process Risk Mitigation track for all processes. Once on this path, the worker will assess the characteristics of each chemical and the processes to be used.
Assessment of Chemicals
The worker will assess the safety-related characteristics of each chemical used in the experiment or operation to determine whether it presents high, moderate, or low risk. Safety-related characteristics include toxicity and health hazards (acute and chronic), flammability, reactivity, and whether the chemical is an oxidizer or peroxide-former. This information can be obtained from Globally Harmonized System (GHS) labeling, Safety Data Sheets, and other literature sources listed in Appendix B.
The worker will also consider the scale of the experiment or operation. For example, the laboratory standard may call for a Moderate Risk SOP if only a small amount (milliliters) of a highly flammable and moderately toxic solvent will be used but a High Risk SOP if the same solvent will be used at a substantially larger scale. Certain chemicals, regardless of quantity, can pose a high risk under harsh weather conditions, such as high level of humidity and/or temperature, which would dictate use of a High Risk SOP.
Assessment of Process Conditions
After assessing the chemical’s characteristics, the worker will assess all of the process conditions, which include temperature, pressure, scale, the number of times the process will be repeated, and the training of laboratory personnel. Process conditions also include any hazards inherent in the equipment being used and the location where the process will be performed, for example, in a chemical hood or lab benchtop. Together, the assessment of chemical characterstics and process conditions will indicate a low, moderate, or high level of risk (see Table 2-1).
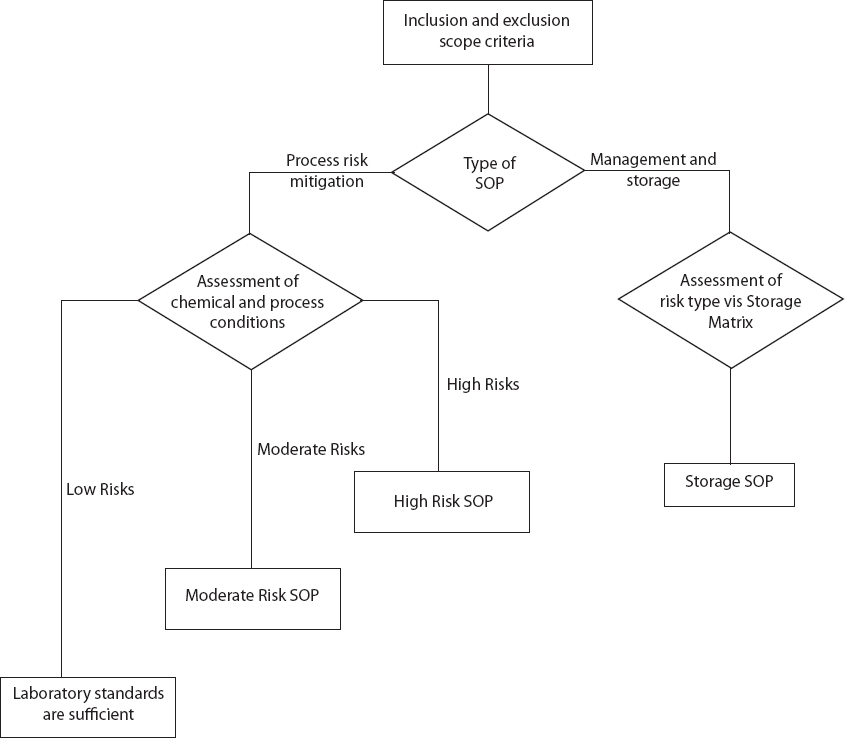
TABLE 2-1 Types of Risk
| Type of Risk | Description |
| Low Risk | No additional controls are required. |
| Moderate Risk | Consider whether the risks can be lowered, where applicable, to a tolerable, preferably acceptable, level, while accounting for the costs of such risk reduction measures. Processes of moderate risk may include neutralization of a carbonate. |
| High Risk | Substantial efforts should be made to minmize the risk. Risk reduction measures should be implemented urgently. Processes of high risk may involve the use of pyrophorics, explosives, or high volumes of flammable solvents, infrequently performed activities with a high potential for failure, and/or first-time activities with less experience. |
If the process conditions pose a low level of risk, then the existing laboratory standards1 will be sufficient to minimize concerns about the health and safety of the laboratory personnel and others in the surrounding area. At a minimum, sufficient laboratory standards will address personal apparel and personal protective equipment, laboratory housekeeping, emergency action plan, safety equipment such as eye washes, showers, and fire extinguishers, and handling of chemical waste.
If the process conditions pose a moderate level of risk, then the worker will develop a Moderate Risk SOP. An example of a moderate-risk process is neutralization of carbonate with acid in a workup step in a chemical synthesis (see Chapter 5, Scenario 1).
Finally, if the process conditions pose a high level of risk, then the personnel will develop a High Risk SOP. Examples of high-risk processes are the use and filtration of a pyrophoric palladium on carbon catalyst from a hydrogenation reaction in methanol solvent (see Chapter 5, Scenario 2) or the handling of diazomethane (see Chapter 5, Scenario 3). When deciding between a Moderate Risk and a High Risk SOP, the worker must consider the level of experience of the laboratory personnel performing the process.
__________________
1 Each laboratory develops and implements a written program that sets forth procedures, equipment, personal protective equipment, and work practices that are capable of protecting employees from the health hazards presented by hazardous chemicals used in that particular workplace.
This page intentionally left blank.







