9
Regulation of Current and Future Genetically Engineered Crops
Given the controversies and broad spectrum of interests surrounding genetic engineering in agriculture, it is not surprising that different countries have developed and adopted diverse regulatory approaches to genetically engineered (GE) plants, crops, and food. The elements of scientific risk assessment are broadly similar among regulatory systems, but policy decisions—which inherently reflect different political and cultural perspectives on risks and benefits—vary considerably. Different cultural traditions, environmental and other societal conditions, and risk tolerances influence decision-makers, and they face political pressures from diverse groups—environmental and food-safety groups, organic-crop producers, large-scale farmers, animal producers, consumers, multinational agricultural companies, and other entities involved in the complex global food production and distribution chain.
As noted in Chapter 3, some regulatory systems reflect policies that are more permissive toward GE crops and foods1 and others reflect policies that are more precautionary. A number of countries have adopted a “process-based” approach to regulation in which foods and crops that have been modified through a specified set of genetic-engineering techniques are
__________________
1 The term GE foods is used here as a shorthand way to refer to various food and feed products produced from GE crops, but few foods were directly “genetically engineered” when the committee was writing its report. Instead, most GE foods contain ingredients derived from GE plants (predominantly maize and soybean). The term is also used to refer to feed, the grains and other products from GE crops fed to animals. However, the term does not include the use of food-processing agents, such as chymosin produced from GE bacteria because these are not “crops” and are therefore beyond the scope of this report.
subject to premarket regulatory safety review for food safety and environmental protection, whereas new foods and crops that have similar traits and were developed through other breeding technologies are not. In addition, as noted in Chapter 6, some regulatory systems for GE crops and foods go beyond food safety and environmental protection to address economic and social issues, such as protecting non-GE agricultural production systems, providing information to consumers through product labels, and taking account of other social and economic concerns.
This chapter reviews illustrative examples of regulatory systems and compares regulation of GE crops with regulation of crops developed through conventional plant breeding. It also analyzes the implications of the emerging genetic-engineering technologies discussed in Chapter 7 for risk, risk assessment, and the scope of GE crop regulatory systems in 2015. Finally, the chapter reviews several critical issues regarding the regulation of current and future GE crops and offers several general and specific recommendations regarding the U.S. regulatory system. The regulatory issues reviewed include the role of product-approval systems in addressing social and economic issues, such as labeling and coexistence; the relationship between expert decision-making and democratic processes, including transparency and public participation; post-approval regulatory authority; and the appropriate scope of premarket regulatory review for plants that have novel traits, including GE crops.
REGULATORY SYSTEMS FOR GENETICALLY ENGINEERED CROPS
In this section, the committee first reviews international agreements that have relevance to the regulation of GE crops and then provides examples of the regulatory systems in three countries and the European Union (EU) to demonstrate different approaches that national or regional governments may take in the oversight of GE crop commercialization.
International Frameworks
To a considerable extent, international trade and other agreements constrain the domestic-product regulation policies of countries that are parties to the agreements. The World Trade Organization (WTO) agreements and the Cartagena Protocol on Biosafety are particularly relevant to the regulation of GE foods and crops.
Safety Assessment of Genetically Engineered Foods
National food-safety regulatory systems of countries that are party to the WTO must be consistent with principles established in the WTO
Agreement on the Application of Sanitary and Phytosanitary Measures (SPS Agreement).2 The SPS Agreement governs measures to protect human, animal, or plant life or health, including food safety. While acknowledging the right of governments to enact such measures, the SPS Agreement also recognizes that such measures can operate as a de facto trade barrier and therefore sets out requirements to minimize trade barriers. Among other things, the SPS Agreement requires that measures be based on scientific principles and not maintained without scientific evidence except measures under Article 5 on which scientific information is insufficient. In such a case, a country may proceed to regulate but must also seek to resolve the scientific uncertainty. To promote harmonization of measures, the SPS Agreement recognizes international standards and guidelines developed by the Codex Alimentarius Commission and several other international organizations. Countries may adopt measures that are stricter than international standards if they are based on appropriate risk assessment. Countries may not adopt measures that are more trade-restrictive than needed to achieve the appropriate level of protection.
To increase the likelihood that countries regulate food safety on the basis of scientific principles, in 2003 the Codex Alimentarius Commission issued guidelines for assessing the safety of foods derived from plants that have recombinant DNA (CAC, 2003a) and principles for risk analysis of foods derived by modern biotechnology3 (CAC, 2003b). The principles refer to risk analysis as including three components: risk assessment, risk management, and risk communication (CAC, 2003b). Risk assessment—an evidence-based process for characterizing the risks posed by a product—is a critical component of the SPS framework (Box 9-1). Countries that follow the Codex risk-assessment process in their domestic GE food-safety regulatory systems are in compliance with the SPS Agreement. As noted in Chapter 5 (see section “Substantial Equivalence of Genetically Engineered and Non–Genetically Engineered Crops”), the EU and many national GE food-safety regulatory systems have incorporated the Codex guidelines.
In general, the Codex guidelines and principles direct developers of GE foods to provide information that enables regulators to assess a variety of food-safety risks:
__________________
2 This discussion focuses on WTO agreements. Many regional and bilateral trade agreements contain similar provisions.
3 The Codex definition of modern biotechnology comes from the Cartagena Biosafety Protocol under the Convention on Biological Diversity. It is defined as the application of in vitro nucleic acid techniques, including recombinant DNA and direct injection of nucleic acid into cells or organelles or the fusion of cells beyond the taxonomic family that overcome natural physiological reproductive or recombinant barriers and that are not techniques used in traditional breeding and selection (CAC, 2003b).
- Description of the GE plant (the crop involved and the nature of the genetic modification event or events).
- Description of the host plant and its use as a food, including the host plant’s cultivation and breeding development and any known toxicity or allergenicity issues.
- Description of donor organisms, including any toxicity or allergenicity issues associated with them.
- Description of the genetic modifications, including details of the method of transformation, the DNA used, the vectors used, and any intermediate hosts that might have been used in the process.
- Characterization of the genetic modifications, including the number and nature of DNA insertions and border regions, the expression of the inserted DNA sequences, and a determination as to whether the expression of any other genes in the host plant has been affected.
- Safety assessment, including
- — Expressed substances (non–nucleic-acid substances): An examination of the toxicity of any expressed products resulting from the genetic event and an evaluation to ensure that toxic components from a donor organism have not been inadvertently transferred. In the case of proteins, it is expected that amino acid sequences will be characterized and the potential for allergenicity determined.
- — Compositional analysis of key components: An examination of key components of the host plant in comparison with the transformed plant. Plants are generally field-trialed under conditions that closely resemble commercial production, and natural variations in key components are considered in any evaluation.
- — Evaluation of metabolites: An evaluation of metabolites that might be produced in the GE plant but not in the original host. The metabolites, if present, need to be assessed for their potential effect on human health.
- — Food processing: Studies that explore the effects of food-processing treatments on components or metabolites of GE foods. The focus is to determine whether an altered protein or metabolite might become toxic after processing in contrast with components of the non-GE counterpart.
- — Nutritional analysis: Same as the compositional analysis except when the genetic insertion is intended to change a key nutritional component, in which case additional testing may be needed to determine the level of the nutrient in question and its effects on human health, taking into account normal consumption patterns and the stability of the trait in multiple production environments.
Environmental Risk Assessment of Genetically Engineered Crops
The WTO Agreement on Technical Barriers to Trade (TBT Agreement) governs a broader set of measures and standards than the SPS Agreement and is intended to address such standards as those designed to protect the environment, promote national security, prevent deceptive marketplace practices, and protect human health and safety (apart from food-safety issues) and animal or plant life or health. The TBT Agreement recognizes the right of governments to adopt such measures but encourages the use of
relevant international standards and nondiscriminatory practices to reduce barriers to trade. Recognizing the broader scope of such measures and different risk preferences in every country, the TBT Agreement does not require such measures to be based on scientific principles but instead emphasizes the nondiscriminatory nature and trade effects of such measures. In other words, the TBT Agreement provides countries with broader latitude than does the SPS Agreement in determining what levels of protection are appropriate. However, if new scientific information shows that the circumstances that gave rise to a measure are no longer valid—so that a perceived risk is found not to exist—the measure would have to be reviewed.
There is no recognized international expert scientific body equivalent to the Codex Alimentarius Commission in environmental protection. Some early international work on the topic of environmental risk assessment of GE crops was carried out by the Organisation for Economic Co-operation and Development (OECD) (OECD, 1986, 1993). The Ad Hoc Technical Expert group of the Cartagena Protocol on Biosafety also develops risk-assessment roadmaps (UNEP, 2014). The approaches to environmental risk assessment of GE crops (or “living modified organisms,” LMOs) adopted by various countries share many elements but differ in level of detail and in specific considerations (EFSA, 2010; Flint et al., 2012).
The 2000 Cartagena Protocol on Biosafety (Biosafety Protocol), developed under the 1992 Convention on Biological Diversity, addresses potential environmental concerns that might be posed by introducing LMOs—such as GE seeds or plants that could propagate—into countries through international trade.4 (It does not apply to pharmaceuticals or goods produced from GE crops, such as cotton or soybean oil, but some provisions apply to GE foods, including GE feed and processing ingredients.) The Biosafety Protocol calls for “Advance Informed Agreements” (AIAs) between exporting and importing countries regarding an initial shipment of an LMO and requires labeling of later shipments of that LMO.5 The purpose of an AIA is to enable an importing country to assess potential environmental risks posed by the LMO before its introduction (through trade) into the country. The Biosafety Protocol expressly adopts the “precautionary principle” that allows countries to deny the importation of a GE product if they consider that there is not enough scientific evidence that
__________________
4 The Cartagena Protocol on Biosafety defines a living modified organism as “any living organism that possesses a novel combination of genetic material obtained through the use of modern biotechnology.” The Biosafety Protocol uses the same definition of modern biotechnology as the Codex Alimentarius Commission (see footnote 3).
5 Many major agricultural exporting nations—including the United States, Argentina, Australia, Canada, and Russia—have not ratified the Biosafety Protocol. Nevertheless, U.S. companies involved in international grain trading comply with the requirements of importing countries.
the product is safe (Box 9-2). The Biosafety Protocol has been the main impetus for food-importing developing countries to develop biosafety approval and regulatory systems under its guidelines. The Biosafety Protocol’s Supplementary Protocol on Liability and Redress establishes a liability mechanism for preventing and redressing environmental harm, but it was not in force when the committee was writing its report. Progress had been made in implementing functional biosafety policies in developing countries, but “translating policy into practice has been slow and laborious,” especially in African countries (Chambers et al., 2014). According to Chambers et al. (2014), commercial GE crops were cultivated in only four African countries,6 and there were confined field trials in six more.7 Other countries were in various stages of developing policy or enacting biosafety legislation when the committee was writing its report.
Socioeconomic Considerations
Both the SPS Agreement and the TBT Agreement represent efforts to reduce impediments to trade by limiting what member countries may do through regulations or practices to create de facto trade barriers. As discussed above, in the case of food safety, restrictions must be based on scientific evidence regarding risk assessment, but other kinds of regulation have more leeway to incorporate nonsafety or socioeconomic issues that represent the diverse values of different countries. The reasons for the differences among countries regarding governance of socioeconomic issues related to GE crops are multifaceted and, as mentioned earlier, include different cultural traditions, values, risk tolerances, and political pressures exerted by diverse groups. Despite those differences, the WTO gives greater weight to scientific evidence related to safety (as opposed to values or fairness) in settling trade disputes, so consideration of socioeconomic issues receives little support in resolving trade disputes between countries. For example, in 2003, the United States, Canada, and Argentina brought a trade-dispute case under the WTO, alleging that the EU had violated the SPS Agreement through its de facto moratorium on approvals of genetically engineered food and feed (WTO, 2006). In its decision, the WTO Dispute Resolution Panel noted that the products had each been reviewed and approved on the basis of a scientific risk assessment and that the EU had not challenged those previous decisions. In its decision, the panel declined to apply the precautionary principle as an established principle of international law and also declined to apply provisions of the Biosafety Protocol, noting that the Biosafety Protocol was not binding on all WTO members (Henckels, 2006).
__________________
6 Burkina Faso, Egypt (until 2012), South Africa, and Sudan.
7 Ghana, Kenya, Malawi, Nigeria, Uganda, and Zimbabwe.
One example of regulation regarding a socioeconomic issue that is not science-based is mandatory labeling of GE foods. As discussed in Chapter 6, a number of countries have adopted mandatory labeling of GE foods on the grounds that labels provide information that enables consumer autonomy and choice. That rationale avoids the need to provide scientific substantiation for the claim that GE foods need to be labeled because they are less safe than non-GE foods. When the committee was writing its report, mandatory labeling of GE foods had not been challenged in the WTO. In 2011, the Codex Alimentarius Commission, which had a standard for GE-food labeling under consideration for a number of years, abandoned the effort in the face of disagreement (CAC, 2011; Miller and Kershen, 2011).
In contrast with the WTO agreements, the Biosafety Protocol, an international environmental agreement rather than a trade agreement, explicitly permits countries to include socioeconomic issues in their LMO biosafety risk assessment in Article 26.1.8 The article has been subject to conflicting interpretations (Horna et al., 2013). In addition to protecting biological
__________________
8 Article 26.1 of the Cartagena Protocol on Biosafety states that: “The Parties, in reaching a decision on import under this Protocol or under its domestic measures implementing the Protocol, may take into account, consistent with their international obligations, socio-economic considerations arising from the impact of living modified organisms on the conservation and sustainable use of biological diversity, especially with regard to the value of biological diversity to indigenous and local communities.”
diversity and human health under the Biosafety Protocol, countries could potentially consider economic effects on farmers or even ethical or religious issues.
Although some international agreements allow the consideration of socioeconomic issues, none require it; trade agreements generally discourage it. As a result, most of the consideration of socioeconomic issues related to GE crops has been at the national level.
National Approaches
Within the overall framework of the various international agreements, national governments have crafted formal regulatory approaches for GE foods and crops that differ in several important ways. First, definitions of the kinds of crops and foods that are subject to regulation vary from country to country. In some cases, a product’s regulation depends on the use of a defined genetic-engineering process; in other cases, products are regulated on the basis of the risk posed by a product’s intended use or characteristics. Second, one way to characterize national regulatory systems is by their approach to genetic engineering, ranging from promotional to preventive (Table 9-1; Paarlberg, 2000; see also Chapter 3 section “Different Policy Approaches to Genetically Engineered Crops and Food”). Third, some national regulatory systems address only biosafety concerns (food safety and environmental protection), whereas others go beyond biosafety considerations to address socioeconomic concerns, such as consumer right-to-know and protection of farmers of non-GE crops from unintended gene flow from GE crops. Fourth, regulatory schemes differ in how they allocate decisions between scientific experts and political bodies that reflect broader societal views (see Munch, 1995; Klinke and Renn, 2002; Renn and Benighaus, 2013).
However, there are also similarities in various national regulatory approaches. Following the standards of such international bodies as the Codex Alimentarius Commission, the elements of the scientific risk-assessment
TABLE 9-1 The Paarlberg Model of Policy Options and Regimes Towards Genetically Engineered (GE) Crops
| Promotional | Permissive | Precautionary | Preventive | |
|---|---|---|---|---|
| Intellectual-property rights | Full patent protection, plus PBRa under UPOVb 1991 | PBR under UPOV 1991 | PBR under UPOV 1978, which preserves farmers’ privilege | No IPRc for plants or animals or IPR on paper that are not enforced |
| Promotional | Permissive | Precautionary | Preventive | |
|---|---|---|---|---|
| Biosafety | No careful screening, only token screening, or approval based on approvals in other countries | Case-by-case screening primarily for demonstrated risk, depending on intended use of product | Case-by-case screening also for scientific uncertainties owing to novelty of genetic-engineering process | No careful case-by-case screening; risk assumed because of genetic-engineering process |
| Trade | GE crops promoted to lower commodity production costs and boost exports; no restrictions on imports of GE seeds or plant materials | GE crops neither promoted nor prevented; imports of GE commodities limited in same way as non-GE commodities in accordance with science-based World Trade Organization standards | Imports of GE seeds and materials screened or restrained separately and more tightly than non-GE seeds and materials; labeling requirements imposed on import of GE foods or commodities | GE seed and plant imports blocked; GE-free status maintained in hopes of capturing export market premiums |
| Food and human health safety and consumer choice | No regulatory distinction drawn between GE and non-GE products in either testing or labeling for product safety | Distinction made between GE and non-GE products on some existing product labels but not so as to require segregation of market channels | Comprehensive labeling of all GE products required and enforced with segregated market channels | GE product sales banned or warning labels that stigmatize GE products as unsafe to consumers required |
| Public research investment | Treasury resources spent on both development and local adaptations of GE crop technologies | Treasury resources spent on local adaptations of GE crop technologies but not on development of new transgenes | No substantial treasury resources spent on either GE crop research or adaptation; donors allowed to finance local adaptations of GE crops | Neither treasury nor donor funds spent on any adaptation or development of GE crop technology |
a Plant breeders’ rights.
b International Union for the Protection of New Varieties of Plants Convention.
c Intellectual-property rights.
SOURCE: Migone and Howlett (2009).
process for food safety and for environmental protection are similar among national regulatory systems.
The section reviews three national approaches and one regional approach for assessing and managing the risks associated with GE crops and foods for food safety and health, environmental effects, and socioeconomic concerns.
United States
U.S. regulatory policy for GE products, including crops and foods, was set out in the 1986 Coordinated Framework for the Regulation of Biotechnology (hereafter referred to as the Coordinated Framework). The Coordinated Framework directed U.S. regulatory agencies to use their existing legal authorities to review the safety of products created with genetic
engineering in the same manner as similar products produced by using conventional breeding (Box 9-3). As a result, how a particular product is regulated depends on its intended use (that is, as a food, drug, or pesticide) or characteristics (that is, as a plant pest). Depending on the characteristics and intended use of a GE product, more than one agency can be involved in a review of a GE crop or a food derived from a GE crop. A maize plant (Zea mays) engineered to express pesticidal proteins is reviewed by all three regulatory agencies: the U.S. Food and Drug Administration (FDA) for food safety, the U.S. Department of Agriculture (USDA) for plant-pest characteristics and other adverse environment effects, and the U.S. Environmental Protection Agency (EPA) to ensure that the plant-expressed pesticide does not pose unreasonable risks to human health or the environment (Figure 9-1).
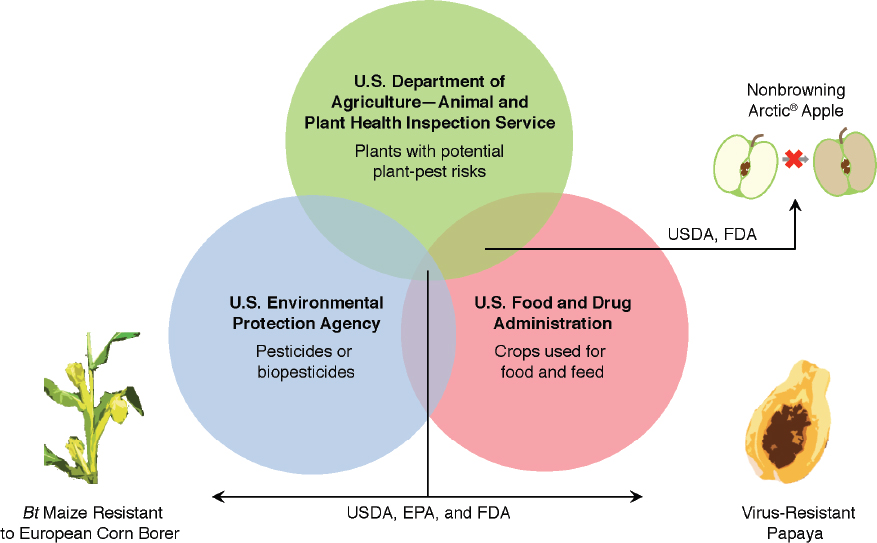
SOURCE: Based on Turner (2014).
Food-Safety Policy for Genetically Engineered Foods. FDA uses its foodsafety authority under the Federal Food, Drug, and Cosmetic Act (FFDCA, 21 U.S.C. §301 et seq) to oversee the safety of foods, including foods derived from GE crops. Unlike drugs, new whole foods are not required to be approved as safe by FDA before they are introduced into the U.S. market. The responsibility for ensuring that a food is safe falls on its manufacturer. If a serious food-safety threat arises after a product is on the market, FDA has authority to recall or seize the product. Historically, novel whole-food varieties developed from conventional breeding have gone directly to market without prior government oversight. FDA notes that the practices used by plant breeders in selecting and developing new varieties of plants have historically “been proven to be reliable for ensuring food safety,” and FDA has therefore not found it necessary to routinely conduct premarket safety reviews of whole foods derived from new plants based on the long record of safe development of such plants (FDA, 1992).
In 1992, FDA issued a policy statement for foods derived from GE crops stating that a whole food derived from a GE crop that was substantially equivalent to its conventionally bred counterpart would be presumed to be as safe as the conventionally bred variety (FDA, 1992). FDA stated that most GE proteins or other GE substances added to a food were likely to be similar to substances already in the food supply and therefore would presumptively be “generally recognized as safe” (GRAS).
In its policy statement, FDA left the door open for the possibility that future food products derived by genetic engineering could differ substantially from their non-GE counterparts or contain new substances that would not be GRAS. In such cases, FDA has the option to consider novel substances in a food to be “food additives,” which are regulated differently from whole foods. Food additives are substances intentionally added to foods (for example, chemical preservatives) and must be approved by FDA as safe before marketing unless they are GRAS. In 1994, the FLAVR SAVR™ tomato was the first whole food from a genetically engineered plant to be reviewed by FDA under its voluntary consultation process. At the same time, at the developer’s request, FDA approved an enzyme (aminoglycoside 3′ phosphotransferase II) encoded by the kanamycin resistance gene in the FLAVR SAVR tomato as a food additive (FDA, 1994).
Like novel whole foods developed through conventional breeding, most foods derived from novel GE crop varieties are not required to be reviewed or approved for safety before going to market. However, FDA has encouraged GE crop developers to consult with FDA voluntarily before going to market and to share with the agency information that the company believes demonstrates that the GE food is substantially equivalent and that any
added substances are safe. The consultation process also gives FDA the opportunity to determine whether an intentionally added substance would be a food additive that would require premarket approval. FDA does not make any safety findings, but it does close the consultation process with a letter stating that FDA has no further questions and reminding the developer of its responsibility to ensure product safety. Through March 2016, FDA has completed 171 consultations (FDA, 2015a). As a practical matter, for marketing purposes, developers have stated to the committee that they view the consultation process as a de facto requirement. FDA has stated that no GE foods that have been evaluated under its voluntary consultation process have gone to market until all FDA safety questions have been resolved.9 In 2001, FDA proposed making the consultation process mandatory, but this proposal was never made final (FDA, 2001).
Under Section 408(c) of the FFDCA, EPA has the responsibility of setting safe tolerances for pesticide residues in food. EPA must set the tolerance at a point that there is a “reasonable certainty of no harm.”
Environmental Policy for Genetically Engineered Crops. Under the Coordinated Framework, both EPA and USDA’s Animal and Plant Health Inspection Service (APHIS) have responsibility for assessing and managing the potential environmental risks posed by some GE crops. Given its general authority to regulate pesticides under the Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA, 7 U.S.C. §135 et seq), EPA has responsibility for approving pesticidal proteins expressed in GE crops (EPA, 2001b). Developers of such pest-resistant plants may not field-test them on more than 10 acres without prior EPA approval and may not release them commercially until EPA has approved them as posing no “unreasonable adverse effects on the environment.”10
APHIS regulates some GE plants under the Plant Protection Act (7 U.S.C. §7758(c)), which generally authorizes the agency to control and prevent the spread of plant pests and noxious weeds. Under its plant pest legal authority, APHIS requires developers of plants that have been genetically engineered by using plant-pest sequences to notify APHIS or to
__________________
9 Questions & Answers on Food from Genetically Engineered Plants. Available at http://www.fda.gov/food/foodscienceresearch/geplants/ucm346030.htm. Accessed November 30, 2015.
10 FIFRA defines unreasonable adverse effects on the environment as “(1) any unreasonable risk to man or the environment, taking into account the economic, social and environmental costs and benefits of the use of any pesticide, or (2) a human dietary risk from residues that result from the use of a pesticide in or on any food inconsistent with the standard under section 408 of the Federal Food, Drug, and Cosmetic Act (21 U.S.C. 346a)” (7 U.S.C. §136(bb)).
obtain a permit before any field testing or environmental release.11 Before commercialization of a GE crop, developers typically seek a “nonregulated status” determination from APHIS, which allows them to grow the crop on a commercial scale without further regulation.12
In some cases, both EPA and APHIS are involved in reviewing a GE crop. For example, both EPA and APHIS review pest-resistant varieties of plants or crops for the risks addressed by their specific legal authority. Although APHIS reviews herbicide-resistant GE crops, EPA’s role is limited to regulating a herbicide that will be applied to a crop (see Chapter 5 section “Regulatory Testing of Crops Resistant to Glyphosate and 2,4-D and of the New Uses of the Herbicides Themselves” for a detailed example).
EPA and APHIS both impose requirements intended to prevent the movement of transgenes from experimental field trials for the GE crops under their jurisdiction. These controls are particularly important because neither the food-safety risks nor the environmental risks associated with GE crops undergoing field trials have been assessed by a regulatory agency. Despite the restrictions on field trials, there have been numerous discoveries of low levels of unapproved GE events in seed, food, and crops (see section “Coexistence” in Chapter 6).
Once a transgenic event in a particular crop species is deregulated by APHIS, there is no further oversight from the agency because in effect the action is a determination that the plant is not within APHIS’s legal authority to regulate. Consequently, USDA has not required post-approval herbicide-resistance management plans. In addition, a deregulated transgenic event may be stacked with other deregulated events if they have been previously approved for a specific crop species without further regulatory oversight by APHIS. For example, once deregulated, GE glyphosate or glufosinate resistance may be stacked in maize with other events without the need for further approval by the agency.
In contrast with APHIS, EPA requires pesticide registrants to report
__________________
11 APHIS’s regulations were initially issued in 1987 (USDA–APHIS, 1987) and have since been amended. APHIS’s rule applies to a regulated article, which is defined as (7 CFR §340.1):
Any organism which has been altered or produced through genetic engineering, if the donor organism, recipient organism, or vector or vector agent belongs to any genera or taxa designated in § 340.2 of this part and meets the definition of plant pest, or is an unclassified organism and/or an organism whose classification is unknown, or any product which contains such an organism, or any other organism or product altered or produced through genetic engineering which the Deputy Administrator determines is a plant pest or has reason to believe is a plant pest.
12 Under APHIS’s regulations, a party may petition USDA for a determination that its plant does not pose a plant-pest risk and therefore should be deregulated. This is also referred to as a Petition for Determination of Nonregulated Status (7 CFR §340.6).
adverse events13 (that is, unexpected potentially harmful effects) and may also require specific post-market monitoring requirements to ensure that the use of products remains consistent with FIFRA’s legal standards. For example, the planting of Bt insect-resistant crops often requires planting of non-GE refuges near GE crops as part of an insect resistance management (IRM) strategy (EPA, 1988). The planting requirements depend on the specific protein responsible for the Bt trait, the crop, and the area of the country where the crop is being grown (EPA, 2001c, 2015; Smith and Smith, 2013). The strategy was introduced to reduce the selection pressure for the evolution of insects resistant to Bt (see Chapter 4). EPA also requires the reregistration of Bt crops and has adjusted the IRM strategy (EPA, 2001a, 2015; Glaser and Matten, 2003). The agency requires annual compliance reporting from the companies that sell the Bt crops. EPA has also restricted planting of Bt cotton in areas where wild cotton grows to prevent the flow of the transgene to wild cotton strains. In 2014, for the first time, EPA required a herbicide-resistance plan as part of a registration for a herbicide to be used with a GE herbicide-resistant crop.14
Socioeconomic Issues. U.S. laws differ markedly in the extent to which they permit or require a regulatory agency to consider economic or other nonsafety issues in making a regulatory decision. For example, under the food-additive provisions of the FFDCA, FDA can approve a food additive only when it finds it to be safe (defined in the law as “reasonable certainty of no harm”). Food must be safe; FDA cannot consider any other factors, including costs. (The same legal standard applies to EPA’s tolerances for pesticide residues in food.)
In contrast, EPA is required by some laws to consider factors other than environmental harm, including economic benefits and costs. For example, FIFRA requires EPA to take into account “the economic, social, and environmental costs and benefits of the use of any pesticide” in making a decision as to whether a pesticide would have an “unreasonable adverse effect on the environment” (7 U.S.C. 136(bb)). The standard of “unreasonableness” recognizes that some magnitude of risk is acceptable as long as it is outweighed by countervailing benefits. More generally, proposed regula-
__________________
13 FIFRA §6(a)2. In 2001, for example, EPA conducted a reassessment of registered Bt maize products in light of concerns about potential adverse effects on monarch butterflies and required additional data from the registrants (EPA, 2001a).
14 As part of the registration for Enlist Duo® herbicide—a combination of 2,4-D and glyphosate for use on herbicide-resistant maize and soybean—EPA required the developer, Dow Agrosciences, to monitor drift issues related to the use of the herbicides and to implement a herbicide resistance management (HRM) plan (EPA, 2014a). When the committee was writing its report, glyphosate was undergoing reregistration, and EPA was reportedly considering requiring an HRM as part of any approval (Gillam, 2015; Housenger, 2015).
tions are reviewed by the Office of Management and Budget to ensure that the economic and other benefits of a proposed rule outweigh its costs (Executive Office of the President, 2011).
The National Environmental Policy Act (NEPA) requires agencies to undertake a broad assessment of the effects of significant agency actions, including the consideration of the “ecological, aesthetic, cultural, economic, social, or health” effects (40 CFR §1508.8).15 However, although agencies must go through this assessment process, NEPA does not give agencies any additional legal authority to make decisions on the basis of those factors. When APHIS deregulates a GE crop, for example, it must conduct an environmental assessment or provide an environmental impact statement to comply with NEPA, but it legally is required to deregulate a GE crop if it is not a plant pest, regardless of the outcome of the NEPA analysis. If the NEPA assessment showed an adverse ecological effect of a GE plant (for example, on air or water quality) that was not a plant-pest risk in the view of APHIS, the plant would still have to be deregulated.
U.S. regulatory agency product approvals are usually represented solely as technical decisions that a product meets the appropriate statutory requirements of safety or efficacy. Agencies generally do not consider, for example, the moral implications of a new product or the fairness of the economic effects on various stakeholders of those decisions. At least in theory, the basic approach of U.S. regulatory policy is to leave such contentious issues to public opinion, various actors, and the marketplace to sort out.
Given this general policy orientation, it is not surprising that U.S. product regulatory agencies have had limited responses to socioeconomic issues, such as consumer right-to-know and effects from GE crop gene flow on non-GE farmers. With regard to mandatory labeling of GE foods, FDA’s position is that it has no legal basis under its general authority to mandate GE labeling. Section 201(n) of FFDCA prohibits food labels from being “false or misleading,” which is defined as a failure “to reveal facts that are material in light of representations made or suggested in the labeling, or material with respect to consequences that may result from the use of the food to which the labeling relates under the conditions of use prescribed in the labeling, or under such conditions of use as are customary or usual.” Under this authority, FDA has required labeling of a number of food processes that change the character of the food (including taste, smell, and texture) that consumers might otherwise be unaware of at the time of sale, such as whether a juice drink has been made from concentrate (21 CFR 102.33(g)). FDA has concluded, however, that as a class there is no “meaningful” difference between a food produced from a GE crop and
__________________
15 EPA is exempt from the procedural requirements of NEPA because its actions are presumed to be consistent with the goals of NEPA.
a conventionally bred crop and that therefore there is no basis to require the disclosure of the use of genetic engineering (FDA 2001, 2015b).16 The fact that consumers may be interested in that information is not sufficient legal grounds to mandate labeling under the FFDCA. FDA’s GE labeling policy was upheld by the court in Alliance for Bio-Integrity v. Shalala, 116 F. Supp. 2d 166 (D.D.C. 2000).
Similarly, neither EPA nor APHIS addresses the economic conflicts that arise from the coexistence of commercial GE and non-GE crops as part of the regulatory-approval process. Neither agency requires post-approval monitoring nor management plans to prevent the low-level presence of GE traits in non-GE crops or foods.17
At the same time, U.S. policy-makers clearly have the authority and ability to respond to social, ethical, and economic concerns through means other than product regulation. The U.S. Congress could address such issues through legislation. Executive branch agencies also have authority outside the product-regulation framework to address some of the concerns. Within USDA, for example, the Agricultural Marketing Service has a long history of working to establish marketing standards, and the secretary of agriculture has made efforts to address coexistence issues through crop insurance and other programs (USDA Advisory Committee, 2012). The Federal Trade Commission and the U.S. Department of Justice have the authority under anti-trust laws to investigate market-distortion issues that might arise from a concentrated seed industry.
Food-Safety and Environmental Risk Assessments. This section looks in more detail at how the United States uses risk assessment to characterize the food-safety and environmental risks of GE crops and foods as part of the product-approval process. The risk assessment determines the kind and quality of data that a developer must supply to the regulatory agencies.
The FDA voluntary consultation process with developers focuses on two major issues as part of the food-safety assessment: the compositional similarity of a whole food to the comparable conventionally bred variety and the safety of any substances intentionally or unintentionally added to the food through the genetic-engineering process. Analysis follows closely the Codex risk-assessment principles and guidelines, discussed above. The
__________________
16 The U.S. Congress has passed laws that have required specific food labels that go beyond FDA’s generic legal authority; the most well-known example is nutrition labeling, which was required by Congress in the 1990 Nutrition Labeling and Education Act (P.L. 101-535).
17 In APHIS’s draft environmental assessment of Dow AgroScience’s Enlist™ maize, it rejected an option to require isolation distances between GE and non-GE varieties as being “inconsistent” with its statutory authority because it had found that the GE maize was not a plant pest (USDA–APHIS, 2011:48).
FDA and EPA food-safety risk assessment processes are discussed in detail in Chapter 5.
With regard to environmental risk assessment, APHIS’s regulations (7 CFR §340.6) outline the types of studies that are necessary to support a determination of nonregulated status; in effect, data are required that would enable APHIS to determine that a plant is not a “plant pest” within its legal authority. APHIS considers, among other things, whether the GE crop is more likely than its non-GE comparator to become invasive or weedy, to be more susceptible to pests or diseases, or to have greater effects on nontarget organisms. APHIS also considers the potential effects of gene flow to wild relatives and other organisms. In effect, APHIS uses the risk-assessment process to determine whether a GE crop is likely to pose a greater “plant pest” risk than a comparable conventionally bred crop variety.
To accompany its permits and deregulation decisions, APHIS is also required to prepare an environmental assessment (EA) or an environmental impact statement (EIS) in compliance with NEPA. The NEPA analysis requires APHIS to consider broader potential environmental effects than whether a plant is a plant pest, as is described above. Although APHIS does not use the non-pest plant aspects of the NEPA analysis as a basis for its decisions, it requires developers to submit data to assess environmental effects.
In its review of a pesticide registration for human health and environmental effects, EPA has not formally published data requirements for plant-incorporated protectants, but the types of studies typically required by EPA for pesticide registration have been set out in regulations (40 CFR 158) and include characterization of introduced genetic material and its expression, a suite of nontarget-organism acute-toxicity studies (mammals, aquatic species, avian species, and beneficial insects), and various environmental-fate studies. Unlike APHIS, EPA does not have to prepare an EA or EIS for its regulatory decisions under NEPA, but its broader environmental risk assessment would cover the same issues as would be required by an EA.
As technology has improved, testing capabilities have expanded, and safety questions around GE varieties have arisen, the number and types of tests that are included in a preapproval package have increased. For example, at the time the committee was writing its report, EPA was in the process of developing possible new data requirements for RNA-interference technology (RNAi) (EPA, 2014b). The list in Table 9-2 provides an example of increasing testing demands required by EPA between 1995 and 2008 for a safety assessment of a new GE variety that incorporates a pesticide.
TABLE 9-2 Safety Assessments Required for Registration of Crops Containing Plant-Incorporated Protectants by the U.S. Environmental Protection Agency (EPA), 1995 and 2008a
| Data Category | Bt bPotato 1995 | Bt Maize 2008 |
|---|---|---|
| Product Characterization | ||
| Identification of the transformation event | X | X |
| Identification of PIPc components | X | X |
| Spectrum of pesticidal activity | X | |
| Mode of action | X | X |
| Certification of limits | X | |
| Characterization of inserted DNA | X | X |
| Characterization of protein(s) – Efficacy | X | |
| Characterization of protein(s) – Expression levels | X | X |
| Characterization of protein(s) – Physiochemical | X | X |
| Demonstration of protein equivalency | X | X |
| Human Health | ||
| Mouse acute oral toxicity | X | X |
| Toxins – Protein database analysis | X | |
| Allergenicity – Stability to heat, SGF,d SIFe | X | |
| Allergenicity – Bioinformatics database analysis | X | |
| Environmental – Nontarget Organisms | ||
| Avian acute oral toxicity (quail/duck) | X | X |
| Avian dietary toxicity (broiler/duck) | ||
| Freshwater fish toxicity | X | |
| Freshwater invertebrate toxicity | X | |
| Estuarine and marine animal toxicity | X | |
| Honeybee toxicity – Larva and adult | X | X |
| Beneficial insect toxicity – Predators | X | X |
| Beneficial insect toxicity – Parasitic wasp | X | X |
| Non-arthropod invertebrate toxicity – Earthworm | X | |
| Synergistic effects from multiple PIPs | X | |
| Environmental – Environmental Fate | ||
| Soil degradation rate | X | X |
| Data Category | Bt bPotato 1995 | Bt Maize 2008 |
|---|---|---|
| Resistance Management Data Requirements | ||
| Target organism susceptibility | X | |
| Simulation models | X | |
| Potential for cross resistance | X | |
| Resistance monitoring plan | X | |
| Remedial action plan | X | |
| Compliance assurance/grower education | X | |
| Conditions of Registration | ||
| Annual report on compliance assurance program | X | |
| Annual report on grower education | X | |
| Annual report on IRMf monitoring | X | |
| Annual sales report | X | |
| Other | ||
| Analytical detection method | X | |
| Public interest document | X | |
a This table includes the information that EPA typically requests from an applicant before the agency will grant commercial approval for a crop containing a plant-incorporated protectant (PIP). Every new registration application does not necessarily contain information for all of the categories listed. For example, Bt maize products that contain both previously registered Bt seeds and non-Bt seeds, known as Refuge in a Bag (RIB), require a new registration. However, EPA does not require a new data submission on effects on nontarget organisms for RIB applicants because that information was provided in the previous application. Applications to register stacks of previously registered events may also refer to elements in data packages submitted earlier. Finally, the table refers to information that accompanies a registration application. Developers of PIP-containing crops must also submit applications, with accompanying data and other forms of information to conduct field trials larger than 10 acres and for EPA either to grant a tolerance exemption or to set a food tolerance for the Bt protein if it is produced by a food or feed crop. That information is publicly available in EPA’s decision documents, the Biopesticide Registration Action Documents. A table of current and previous registrations for PIPS is available at http://www2.epa.gov/ingredients-used-pesticide-products/current-previously-registered-section-3-plant-incorporated. Accessed December 15, 2015.
b Contains a gene or genes from the bacterium Bacillus thuringiensis (Bt).
c PIP = plant-incorporated protectant
d SGF = simulated gastric fluid
e SIF = simulated intestinal fluid
f IRM = integrated resistance management
SOURCE: Compiled by the Biotechnology Industry Organization Science and Regulatory Working Group in March 2012.
European Union
As a regional government, the EU’s approach to regulation obviously differs from that of the United States because it is not based on existing national laws. It has taken a more precautionary approach to approving the commercialization of GE crops.
Safety of Foods Derived from Genetically Engineered Crops and Cultivation of Genetically Engineered Crops. The EU, which consisted of 28 Member States when the committee was writing its report, established a regulatory process for the assessment and approval of GE foods that intentionally incorporates a precautionary approach (see Table 9-1 for a description of the precautionary approach). Assessment and approval are triggered by the presence of a process used to introduce a trait into an organism. Under EU rules, organisms in which the genetic material has been modified in a way that “does not occur naturally by mating and/or natural recombination” are subject to mandatory premarket assessment. Included in that definition are organisms modified through the use of recombinant-DNA technology, micro-injection, and cell fusion whose result is a combination of genetic materials that do not occur naturally. The definition does not include in vitro fertilization, polyploidy induction, selective breeding, crossing, or mutagenesis (Directive 2001/18/EC Annex I A). As a result, new varieties of crops developed through conventional breeding may be introduced into the market without premarket regulatory review and approval. Once a new food from a conventionally bred crop is on the market, the EU and the Member States have the authority to recall it if health or safety issues arise under the General Food Law Regulation.
The procedures for evaluation and approval of a market application for what the EU defines as a genetically modified organism (GMO) are set out in Regulation (EC) No. 1829/2003 on genetically modified food and feed and in Directive 2001/18/EC on the release of GMOs into the environment.18 Under those regulations, the European Food Safety Authority (EFSA), in cooperation with the scientific agencies of Member States, is responsible for making a food-safety and environmental assessment for all applications for GMOs to be used for cultivation, importation, or processing. The centralization of the risk-assessment process provides a “single-door” approach that applies a uniform-risk evaluation process throughout the EU.
EFSA’s role is limited to providing scientific advice. Once EFSA has delivered its opinion on the food-safety and environmental-safety risks associated with an application, the decision to authorize the application,
__________________
18 Because genetically modified organism is a defined term under EU law, this section of the report uses it and such related terms as genetically modified (GM) rather than genetically engineered organism and GE.
whether for cultivation or for the marketing of food or feed without cultivation, is decided by the European Commission and the Member States. The decision process is complex and inherently has a political component, given the need for broad agreement by all the Member States (Figure 9-2). Within 3 months after receiving an EFSA opinion finding that a product does not pose a risk to health or the environment under proposed conditions of use, the Commission makes an initial draft decision. If the Commission proposes to approve the application, its draft decision is then submitted to the Member States, represented in the Standing Committee, for a vote under qualified majority rules.19 If the Standing Committee approves, the Commission adopts the draft approval decision. If the Standing Committee votes no or fails to reach a decision within 90 days, the Commission may resubmit its draft decision to the Appeal Committee. The Member States then vote again on the Commission’s draft decision in the Appeal Committee. Again, if the Member States vote yes with a qualified majority, the Commission adopts the decision. If they vote no, the Commission cannot adopt the proposal. However, as of December 2015, in all cases with respect to Commission draft approvals for GM cultivation or for food and feed, the Member States had failed to reach any decision by a qualified majority vote; there were insufficient votes either to approve or to reject. EU procedures dictate that under such circumstances the Commission is required to adopt and implement its own decision (EC, 2015c). As the Commission has explained, “the reasons invoked by Member States to justify their abstentions or negative votes are sometimes scientific in nature, but in the majority of cases are based on other considerations, reflecting the societal debate in their country” (EC, 2015c).
Given the strong public opposition to GM foods in some Member States, it has been difficult for the EU decision-making process to reach agreements to approve GM food and crop applications even when EFSA’s risk assessments have concluded that a GM food or crop is as safe as non-GM counterparts. Despite the 2006 WTO dispute-settlement body’s decision that the EU’s failure to approve GE food or crop applications was a de facto moratorium in violation of the TBT Agreement (see above section “International Frameworks”), the EU has continued to find it difficult to approve GMO applications. As of June 2015, according to the European Commission, only one crop—Monsanto’s MON810, a maize variety with a Bt gene to protect against the European corn borer (Ostrinia nubilalis)—is
__________________
19 The “qualified majority” decision-making process for GMO approvals is the same general process used in all EU legislative decision-making under Regulation (EC) No 182/2011 (comitology procedure). Under EU voting rules, a qualified majority consists of 55 percent of the Member States (in the case of a Commission proposal) and representing 65 percent of the population. A minority of four Member States can block a proposal (EC, 2015c).
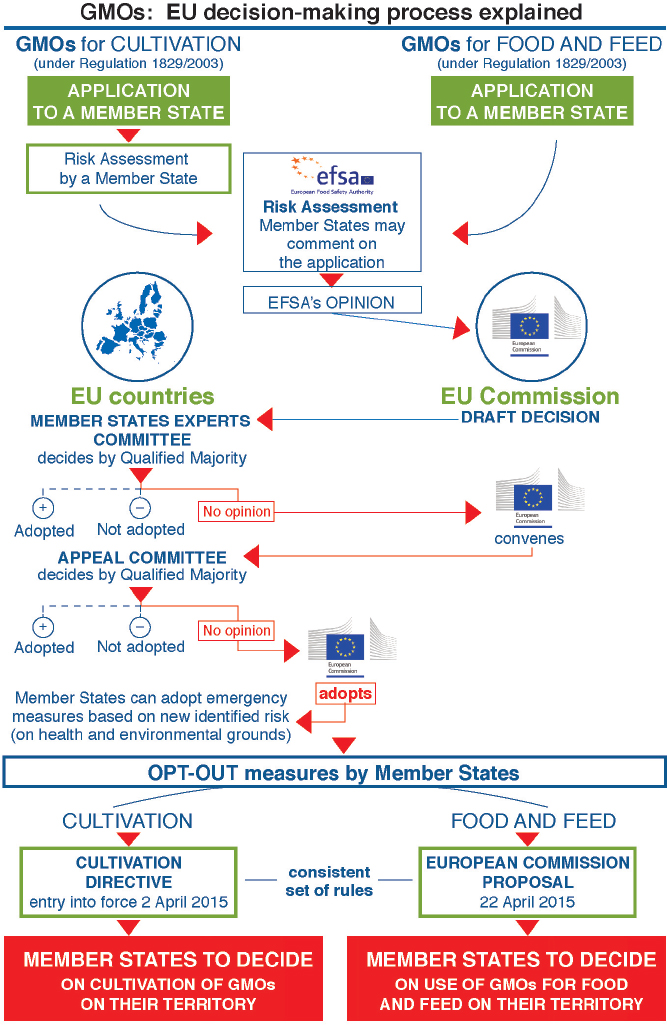
SOURCE: GMOs: EU decision-making process explained. Available at http://ec.europa.eu/food/plant/docs/decision_making_process.pdf. Accessed December 15, 2015.
authorized to be cultivated in the EU, and it was awaiting reauthorization.20 At that time, MON810 was grown in five Member States; Spain accounted for most of the planted hectares, and the variety represented less than 2 percent of the total EU hectares planted to maize. Eight applications for GMO cultivation are pending, four of which have been assessed by EFSA as safe and four of which are awaiting an EFSA opinion (EC, 2015b).
The EU has approved a greater number of applications for the importation of GE food and feed than for cultivation. As of April 2015, the EU had approved 10 new GE crops, to bring the total to 68 GMOs that are authorized in the EU for food and feed purposes, including maize, cotton (Gossypium spp.), soybean (Glycine max), canola (Brassica napus), and sugar beet (Beta vulgaris) (EC, 2015a,b). The great bulk of those GMO imports are in the form of soybean feed for the EU’s livestock sector, which depends heavily on imports. Few, if any, GM food products are available for sale. Most food manufacturers have reformulated their products in Europe to avoid having to label their food as containing GMOs (Wesseler, 2014).
To break the political gridlock surrounding decisions to approve the cultivation of GM crops, the EU in late 2014 adopted new rules to allow Member States to prohibit or restrict the cultivation of an approved GM crop on the basis of nonrisk policy considerations such as environmental or agricultural policy objectives, land-use planning, socioeconomic effects, or coexistence management (Directive EU 2015/412). Although the new rule clearly undercuts the desire to have a consistent and uniform policy in all EU Member States, it will allow Member States that want to grow new GM crops to proceed.
Socioeconomic Issues. The EU has adopted rules that require a GM food, feed, or grain to be labeled. The EU justifies labeling as a right-to-know issue, a right conferred in the European constitution and by international human-rights laws. When it was adopted, EU officials also stated that labeling was required partly to rebuild public confidence in its food-safety system (EC, 2001).
As discussed in Chapter 6 (Box 6-5), the EU has also developed general guidance for managing coexistence between GM and non-GM producers, although the management of coexistence has primarily been left to the Member State level. A number of Member States have adopted requirements that have largely had the effect of protecting non-GM producers.
Food-Safety and Environmental Risk Assessments. EFSA has published its risk-assessment guidelines for both food safety and the environment (EFSA, 2010, 2011b). EFSA’s food-safety risk assessment, like those of
__________________
20 MON810 was initially authorized for use within the EU in 1998.
the Codex guidelines and FDA, starts with the comparison of the GM crop with its conventionally bred counterpart. Information provided by the applicant must include a molecular characterization, which provides information on the structure and expression of the inserted material and on the stability of the intended trait, a toxicological assessment that addresses effects of biologically relevant changes in the GM crop or food on human and animal health, an assessment of potential allergenicity of any novel protein and the whole food, and a nutritional assessment to ensure that food or feed derived from the GM crop is not nutritionally disadvantageous to humans or animals. EFSA guidelines set out the requirements for testing the toxicity of new expressed proteins. Until 2013, the EFSA guidelines did not require animal-feeding studies to test the safety of a whole food unless its composition was substantially different from its non-GM counterpart or there were other indications of unintended effects from a comparative analysis.21 The final risk characterization should demonstrate that the consumption of a food or feed derived from a GM plant is at least as safe as its conventionally bred counterpart and that it is at least as nutritious for humans and animals as a non-GM food or feed.
Before a GM crop can be grown in the EU, an applicant has to submit a data package to enable a Member State to conduct a comprehensive environmental risk assessment (ERA). EFSA has issued guidance on the types of information that applicants must submit and the process that must be followed for an ERA (EFSA, 2010). EFSA also conducts an ERA for the whole EU territory, taking into account the Member State’s ERA and any additional information EFSA may request. The ERA guidelines include seven specific concerns:
- Persistence and invasiveness of the GM crop.
- Plant-to-microorganism gene transfer.
- Interaction of the plant with target organisms.
- Interaction of the plant with nontarget organisms.
- Effects of cultivation, management, and harvesting techniques.
- Effects on biogeochemical processes.
- Effects on human and animal health.
The ERA consists of a full risk assessment, moving through the steps of problem formulation, hazard characterization, exposure characterization, and risk characterization. If risks need to be mitigated, assessment requires the applicant to propose measures for reducing them to a level of “no con-
__________________
21 As noted in Chapter 5, EU regulations adopted in 2013 require EFSA to conduct wholefood rodent feeding studies as part of its risk assessment (Implementing Regulation (EU) 503/203). EFSA has issued guidance on animal-feeding studies (EFSA, 2014).
cern.” EFSA, like its USDA and EPA counterparts, often asks for additional information from the applicant to supplement the original application.
Each application for cultivation also requires a post-market environmental monitoring (PMEM) plan under which the applicant will continue to monitor for potential adverse environmental effects (EFSA, 2011a). PMEM plans are also required for any live GE material (grain or seeds) imported into the EU market.
In preparing risk assessments, EFSA works with scientific bodies in the Member States, including a network of over 100 organizations and authorities in Europe. Member States are given an opportunity to provide input to the EFSA GMO assessments. With its final opinion, EFSA also publishes a summary of the comments and input from Member States.
Canada
Canada takes yet a different approach to regulation. Its system uses the concept of “novelty” in assessing whether there is a need to regulate new crops, regardless of the breeding method used.
Genetically Engineered Crops and Foods. Like the United States, Canada has divided the regulatory responsibilities for GE foods and crops. Health Canada is the agency responsible for food safety in the Canadian regulatory system, and the Canadian Food Inspection Agency (CFIA) is responsible for assessing the environmental effects of new crops.
Unlike the United States, Canada passed new laws to revise its regulatory system to address concerns being raised about GE crops and foods. However, the new laws reflected a policy of focusing on novel foods and novel plant traits rather than on a specific breeding process (genetic engineering) or product category (such as plant pests).22 Thus, the Canadian regulatory system appears to follow a process-neutral approach in determining which foods and plants should be subject to mandatory premarket government review. Instead of focusing on the intended uses or characteristics of a plant or the use of a specific process, the Canadian approach centers on risk: the potential for novel food or environmental exposures.
Consequently, Division 28 of the Food and Drugs Regulation, also referred to as the Novel Foods Regulation, establishes a premarket notification process for all “novel foods,” whether GE or not. “Novel foods”
__________________
22 Canada’s regulatory framework followed 7 years of discussions with stakeholders (Smyth and McHughen, 2012). Early field trials in the 1980s and early 1990s were held under the authority of existing laws, primarily the Seeds Act (1985), the Feeds Act (1983), and the Food and Drugs Act (1985) (Smyth and McHughen, 2012). Regulations implementing the “novel foods” and plants with “novel traits” approaches were first issued by CFIA in 1994.
can be summarized as products that do not have a history of safe use as food, foods that have been subjected to a process that has not previously been used for them and that causes them to undergo a major change, and foods derived from plants or animals that have been genetically modified to introduce or delete traits or to change the anticipated array of characteristics (B.28.001 C.R.C., c. 870 (2014)). The term genetically modify is defined as to change “the heritable traits of a plant, animal, or microorganism” (B.28.001). In an on-line posting of frequently asked questions, Health Canada has indicated that “genetic modification” is not limited to recombinant-DNA technologies but could also include conventional breeding, mutagenesis, and emerging genetic-engineering technologies, such as genome editing (Health Canada, 2015).23 That definition includes only a subset of “new” foods; in particular, developers or importers of foods that have been safely used in other countries or that have only minor processing changes are not required to submit prior notification (Smyth and McHughen, 2012).
Developers and importers of a “novel food” must notify Health Canada at least 45 days before its marketing and submit information sufficient to demonstrate its safety. Health Canada may request additional information; once satisfied that the food is safe, Health Canada notifies the submitter in writing that the information is sufficient and that the agency has “no objection” to its marketing in Canada. No “novel food” may be marketed before receiving authorization from Health Canada. The agency publishes a summary of the notification and its decision on-line.
According to information on the Health Canada website, over 81 GE foods and many more non-GE foods were assessed and approved as novel foods for sale in Canada as of 2015. Non-GE foods include an artificial sweetener (Sucromalt), foods treated with a novel high-pressure process for sanitation, foods with added ingredients (such as phytosterols), and novel non-GE food varieties, including herbicide-resistant sunflower (Helianthus annuus) and mid-oleic sunflower oil. Once a food is approved, there is no requirement for routine post-approval food-safety monitoring, although developers and food manufacturers must report any new adverse safety information.
Environmental risks posed by crops are the responsibility of CFIA, which assesses the environmental safety of plants and the safety of animal feed under the Seeds Act and the Feeds Act. Developers of a plant with “novel traits” (PNT) must obtain authorization from CFIA before conducting confined field trials or unconfined release (including commercialization). A “novel trait” is one that is new to stable, cultivated populations
__________________
23 On its website, Health Canada uses the terms genetically modified and genetically engineered interchangeably. In the discussion in this section, genetically engineered (or GE) is used.
of the plant species in Canada and that has a potential to have a substantial adverse environmental effect (CFIA, 2009). When the committee was writing its report, all GE plants reviewed by CFIA had been considered to contain novel traits. However, as mentioned above, novel traits can also be introduced through non-GE techniques. For example, in 2005, CFIA reviewed and approved BASF Canada’s CLEARFIELD® sunflower, which has a novel trait for resistance to the herbicide imidazolinone (CFIA, 2005). The trait originated in a natural mutation in the wild sunflower population in Kansas and was introduced into domestic germplasm by conventional breeding. CFIA has also reviewed and approved BASF Canada’s CLEARFIELD imidazolinone-resistant trait in canola and wheat (Triticum aestivum) (CFIA, 2007, 2008); in these cases, the traits were introduced through chemically induced seed mutagenesis and interspecific crossing.
So far, all GE crops have been submitted by their developers for regulatory review, but not all future GE crops are expected to have novel traits (Thomas and Yarrow, 2012). Once a PNT has been introduced into the environment, its trait may no longer be considered novel in the Canadian approach. As a result, a later plant of the same species transformed with the same DNA construct and expressing the same traits as an approved variety should not be subject to the full regulatory-approval process (Smyth and McHughen, 2012). In addition, in some cases the developer of a crop with stacked traits, each of which has already been approved, would not have to submit the full regulatory-approval package (CFIA, 1994). In practice, however, developers of varieties stacked with previously approved traits have continued to submit them for full regulatory approval (Thomas and Yarrow, 2012). Furthermore, new crop varieties that confer insect resistance or herbicide resistance will still need to have stewardship plans for managing resistance development even if the traits were already approved. If a plant is no longer a PNT, it may still be a novel food that will require approval by Health Canada.
In the Canadian system, it is the responsibility of the plant breeder to make the initial determination of whether a plant has novel traits. CFIA has issued guidelines to help plant breeders to determine both whether a plant is “new” to the environment and whether it has the potential for environmental harm (CFIA, 2009). A trait will not be considered “new” if it has been observed in a population of the same species cultivated in Canada. Simply increasing the frequency of the trait would not be sufficient for a trait to be considered new, but a trait could be considered new if it is expressed at levels substantially outside observed ranges. In most conventional plant breeding, new varieties display relatively small changes in trait expression that are unlikely to require regulatory review. CFIA has acknowledged that in most cases products of conventional plant breeding are unlikely to pose a risk to the environment. However, the concept of
novelty provides regulatory flexibility and adaptability to cover new crop varieties that pose greater risk, regardless of the method by which they were produced. At the same time, the novelty trigger is somewhat less predictable than a clearly determinable process-based trigger. For that reason, CFIA encourages plant breeders to come in for early consultation during the development process.
Socioeconomic Issues. Canada’s regulatory approach is more similar to the market-oriented approach of the United States than to the social-welfare approach of the EU (Marcoux and Létourneau, 2013). Like the United States, Canada does not require labeling of GE foods. The Canadian government participated in a multistakeholder process with the Canadian Council of Grocery Distributors and the Canadian General Standards Board to develop a standard to guide the use of voluntary labeling to ensure that it is truthful and not misleading. The Standard for Voluntary Labeling and Advertising of Foods that Are and Are Not Products of Genetic Engineering was published as a national standard of Canada in 2004 (Canadian General Standards Board, 2004).
Also like the United States, Canada does not regulate coexistence between GE and non-GE producers (Dessureault and Lupescu, 2014) and, as in the United States, the consequence is that the economic burden of avoiding gene drift and commingling is on the producers of non-GE crops. According to USDA’s Foreign Agriculture Service, however, there is not enough information to determine the extent of unwanted admixture of GE crops and organic crops and the damage entailed (Dessureault and Lupescu, 2014).
The Canadian system includes one aspect that serves socioeconomic ends. Under the Seeds Act, any new variety of a major agricultural crop—whether GE or not—has to receive prior approval from CFIA’s Variety Registration Office after review by an advisory committee with representatives from public and private institutions that examines the new crop variety to ensure that it is at least equal in quality to existing varieties. That approval process is intended to protect Canadian farmers from inferior new crop varieties and to ensure that the new varieties will deliver the benefits as described. However, the office’s focus is on the quality of the new variety, not on the possible economic consequences of its introduction (Smyth and McHughen, 2012).
Food-Safety and Environmental Risk Assessments. Health Canada has published guidelines for the safety assessment of novel foods, detailing the information to be submitted by food manufacturers or importers. The guidelines were derived from the food-safety assessments developed by the OECD, the Food and Agriculture Organization, the World Health Orga-
nization, and the Codex Alimentarius Commission (Health Canada, 1994, amended 2006). A food-safety assessment examines how a food crop was developed, including molecular biological data, the composition and nutritional profile of the novel food compared with non-GE counterpart foods, the potential to introduce new toxins or to cause allergic reactions, and dietary exposure by the average consumer and by sensitive populations, such as children. Health Canada estimates that it typically takes 7–10 years of product development for a company to compile enough data to submit a premarket notification for a novel food (Health Canada, 2015).
Environmental risk assessments are conducted by CFIA. In considering whether a plant meets the environmental-risk part of the novel-trait definition, CFIA focuses on whether a new variety is likely to have a more adverse environmental effect than its non-GE counterpart. Adverse effects to be considered include weediness potential, harmful gene flow, plant-pest potential, effects on nontarget organisms, and other potential adverse effects on biodiversity (CFIA, 2009).
Before any PNT is grown in a confined field trial, the applicant must apply for approval from CFIA and submit a data package with information on the crop variety and the description of the field trial. An authorization for a confined research field trial is subject to general and crop-specific terms and conditions, which are intended to minimize persistence and spread of the plant in the environment and prevent contamination of feed and food with unapproved plant material. Enforcement of those terms and conditions by CFIA involves site inspections during the growing season and post-harvest monitoring (CFIA, 2000). After approval, nonconfidential information about confined research field trials is posted on CFIA’s website.
When field trials have been completed and developers want to commercialize the PNT, developers must apply to CFIA for approval for an unconfined environmental release. Applicants must submit a data package that will permit CFIA to complete a thorough environmental-safety assessment (CFIA, 1994). CFIA compares the environmental effects of the new variety with those of a non-GE counterpart to ensure that it poses no greater environmental risk than the counterpart. CFIA may impose restrictions to manage or mitigate adverse environmental effects. In addition, CFIA requires stewardship plans for herbicide-resistant or insect-resistant crops to prevent the development of resistance and prolong the lifespan and usefulness of the technology. Developers are also expected to implement a post-release monitoring plan for unintended or unexpected environmental effects. Applicants are required to report any adverse information on environmental effects. As with approvals for confined field trials, decisions to approve unconfined environmental releases are posted on CFIA’s website, as are documents explaining the decision reached by CFIA.
Brazil
Unlike the other governments reviewed here, Brazil’s government adopted a regulatory policy when GE crops were already being grown in the country. Economic and environmental concerns played a role in when and what kind of system was put into place.
Food-Safety and Environmental Policy for Genetically Engineered Crops. Brazil’s regulatory scheme for GE foods and crops became law in 2005. Brazil passed its first law for GE foods and crops in 1995, but the law generated protest and controversy after the National Technical Commission of Biosafety (CTNBio) approved a request for the commercial release of a glyphosate-resistant soybean without requiring the completion of an environmental impact report. CTNBio’s authority was challenged in court by the Institute of Consumer Defense and Greenpeace as violating environmental laws.
A lower court issued an injunction against CTNBio’s approval, and the case was taken to a three-judge appeals court, which delayed a decision for several years while there were extensive and often contentious discussions among Brazilian civil-society groups, farmers, biotechnology companies, and government officials (Soares, 2014) about how GE-food and GE-crop approval decisions should be made and who should make them (Schnepf, 2003; Cardoso et al., 2005). Issues related to the roles of democratic decision-making, scientific expertise, the equitable distribution of risks and benefits, and potential effects on the environment and biodiversity were all elements of the debate. In 2003, the controversy was exacerbated by news that substantial amounts of GE soybean seeds had been smuggled from Argentina and illegally planted in parts of southern Brazil and had become commingled with non-GE soybean (Schnepf, 2003). At the time, USDA estimated that 10–20 percent of Brazil’s total soybean crop might have consisted of illegally planted GE soybean varieties (Schnepf, 2003).
In 2003, after two temporary authorizations of the GE soybeans that were already planted and harvested in 2003 and after extended negotiations, new legislation was proposed. The new biosafety framework law was passed in 2005 after long debate as Law No. 11,105.24
The 2005 Brazilian biosafety law established several organizations with different decision-making responsibilities for biotechnology decisions (Figure 9-3). Like the EU, Brazil has a “technical” organization that conducts risk assessments of GE foods and crops (CTNBio) and a separate political decision-making body with final decision-making authority that can weigh
__________________
24 Law No. 11,105 was modified in 2007 by Law No. 11,460 and in 2006 by Decree No. 5591.
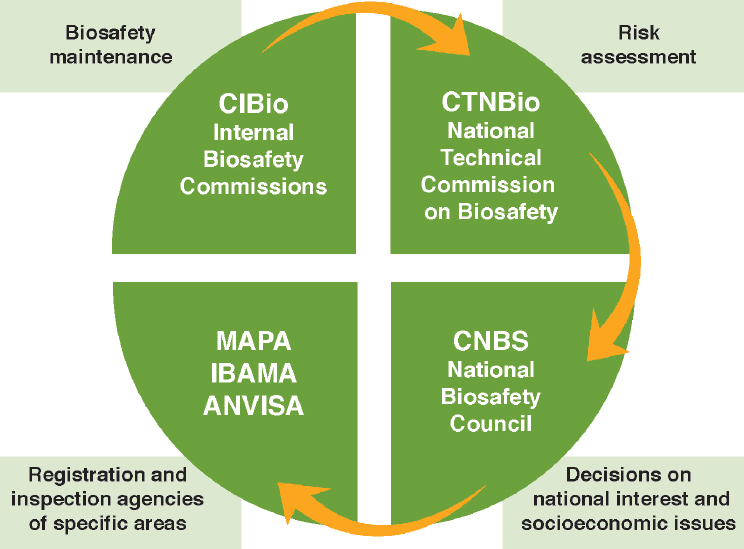
SOURCE: Finardi Filho (2014).
NOTE: MAPA = Ministry of Agriculture, Livestock, and Food Supply; IBAMA = Brazilian Institute of Environment and Renewable Natural Resources; ANVISA = Ministry of Health through the National Surveillance Agency.
nonbiosafety issues, including socioeconomic effects: the National Biosafety Council (CNBS). Unlike the EU, however, Brazil has approved numerous GE crops for cultivation: as of 2014, more than 35 GE varieties (mostly of maize, soybean, and cotton) had been approved for commercialization under the system, and Brazil had become the world’s second largest grower of GE crops.
Under Brazil’s regulatory system, CTNBio is responsible for all technical issues related to biotechnology. It conducts the assessment of food-safety and environmental risks for GE foods and crops, including imports.25
__________________
25 Under Brazil’s law, a GMO is defined as an organism whose genetic material (DNA or RNA) has been modified by “any genetic engineering” technique; genetic engineering is defined as the activity of manipulating DNA or RNA recombinant molecules. A GM byproduct is defined as a product obtained from a GMO that has no autonomous replication capacity or that does not contain a viable GM form. See Article 3 (V and VI).
CTNBio consists of 27 members, including officials in nine federal ministries, 12 technical specialists, and six other specialists in such fields as consumer rights and family farming (Silva, 2014). CTNBio was established under the Ministry of Science and Technology; its members are appointed for 2 years. A majority vote is required to recommend approvals of new biotechnology products. CTNBio’s meetings are open to the public. CTNBio also authorizes all field trials in Brazil; no environmental releases may be conducted before a Certificate of Quality in Biosafety is obtained from CTNBio.
CNBS, also established by law, is in the Office of the President and is responsible for developing and implementing overall national biosafety policies. It considers broader national and socioeconomic implications of agricultural biotechnology. CNBS is a purely political body, consisting of 11 cabinet ministers. Although the 2005 Brazilian biosafety law gives CNBS the authority to make the final decision on commercialization of GE products, as the practice has evolved CNBS views the technical safety determinations by CTNBio as conclusive on biosafety issues and reconsiders CTNBio determinations only when there are issues of national interest or social or economic issues (Silva, 2014). If a socioeconomic issue is raised during the CTNBio risk-assessment process, CNBS can commission a third party to study it. In that respect, Brazil separates the technical assessment from nonbiosafety issues (Ludlow et al., 2013).
Regulation and inspection of GE crop field trials is primarily the responsibility of the Ministry of Agriculture, Livestock, and Food Supply. The Ministry of Health through the National Surveillance Agency inspects the events for toxicity, and the Ministry of the Environment through the Brazilian Institute of Environment and Renewable Natural Resources monitors and inspects the events and their effects on the environment.
After approval, CTNBio retains the authority to suspend or revoke an authorization for an environmental release of a GE crop and its byproducts if there is evidence of adverse effects on the environment or on human or animal health (Soares, 2014). In addition, CTNBio requires post-market environmental monitoring and has required specific post-market studies in several instances to address potential environmental concerns (Mendonça-Hagler et al., 2008).
Socioeconomic Issues. Brazil has adopted a number of policy measures intended to promote coexistence of farmers growing GE crops and other farmers (Soares, 2014). In 2007, CTNBio issued Normative Instruction No. 4, establishing minimum isolation distances between GE and non-GE maize crops. CTNBio has also issued coexistence rules related to the planned release of GE citrus plants (Normative Instruction No. 10) and sorghum (Normative Instruction No. 13). Exclusion zones have been re-
quired to prevent gene flow from GE cotton areas where naturally growing populations of wild cotton occur. Furthermore, no GE crops are permitted in Indian reservations or in officially recognized preservation areas (Mendonça-Hagler et al., 2008).
The Brazilian biotechnology law also establishes a general liability regime, in which persons responsible for damage to the environment or to third parties from GE crops are liable for damages regardless of any negligence on their part (Soares, 2014). The law also provides for civil and criminal penalties for violations of biotechnology regulations and rules.
In 2003, Brazil’s president mandated labeling of GE foods and food ingredients for products exceeding 1.0-percent GE content. The Ministry of Justice issued implementing regulations requiring foods containing more than 1.0 percent to carry a specified transgenic logo, an uppercase “T” in a yellow triangle. In 2008, it was reported that that requirement was not being enforced (Mendonça-Hagler et al., 2008). In 2012, however, Nestlé was fined by a Brazilian court for failing to label GE soybean ingredients found in several of its consumer products (Jornal DCI, 2012).
Food-Safety and Environmental Risk Assessments. CTNBio’s food-safety assessment follows the concept of substantial equivalence and the guidelines of the Codex Alimentarius Commission (Mendonça-Hagler et al., 2008).
A planned release of GE crops into the environment must initially be approved by a company’s Internal Biosafety Committee. After that approval, the applicant submits a dossier for approval to CTNBio. CTNBio has issued regulations specifying the information to be included in the dossier, including information on the GE plant (a description of the modification and the process used, the exogenous DNA or RNA sequence, and genetic characteristics that may affect fitness) and information on the planned release, including protocols for safety and monitoring (Annexes I–IV of Normative Resolution No. 6). CTNBio’s risk assessment evaluates the potential adverse effects of the crop and its byproducts on human and animal health, on the environment, and on plants while “maintaining transparency, the scientific method, and the precautionary principle” (Normative Resolution No. 5, Article 6(I)).
Conclusions on Comparison of Regulatory Approaches
The four regulatory regimes for agricultural products of genetic engineering reviewed above scarcely provide a comprehensive review. Rather, they are intended to present some illustrative examples of the array of policy approaches adopted in different regions. In all cases, the development of rules involved political controversy and took a substantial period to put into place. In the EU, initial regulatory approaches met opposition and were replaced with more stringent regulations and labeling requirements,
and even these have been difficult to implement. Canada’s rules were developed over an extensive period of consultation (Thomas and Yarrow, 2012). Brazil’s initial attempt to regulate GE crops and foods in 1995 broke down in contentious disagreements. Even in the United States, where the basic policy framework was adopted in 1986, there was controversy over some aspects of agency rule-making, including EPA’s proposal to regulate plant-incorporated protectants. (EPA initially proposed the rule in 1994; the final rule was published in 2001.) Regulations continue to evolve. The EU recently adopted changes to allow Member States to opt out of growing EU-approved GE crops (EC, 2015b). In 2015, the U.S. Office of Science and Technology Policy announced a comprehensive review of biotechnology regulations (OSTP, 2015), and in 2016, APHIS published proposals for revising its GE plant regulations (USDA–APHIS, 2016).
The four examples illustrate different approaches to the decision of what kinds of new foods and crops require premarket regulatory review. Both the EU and Brazil have chosen to regulate genetic engineering specifically, excluding conventional and other breeding methods. Canada has taken a different approach, choosing to regulate foods and plants on the basis of novelty and potential for harm, regardless of the breeding technique used. Unlike other countries, the United States has relied on existing product-regulation laws as the basis for regulation of GE crops and products derived from them. Although in theory the United States has adopted a product-based policy, in effect APHIS and EPA both take the breeding process into account in determining which plants to regulate.
The processes used in all four regulatory approaches to assess environmental and food-safety risks are similar and are based on guidelines and recommendations issued by the Codex Alimentarius Commission (in the case of food safety) and other international bodies, such as the OECD (in the case of environmental safety). For both food and environmental safety, the risk-assessment process used by all countries starts with the fundamental idea of comparison of a GE variety with a known, conventionally bred counterpart. Risk assessment focuses on the intended and unintended differences and considers the effects of the differences on relevant endpoints. For food, the primary issues to be considered include the potential effects of compositional changes on nutritional elements, toxicity, and allergenicity. Environmental issues include effects on nontarget organisms, changes in invasiveness or weediness, and potential for unwanted gene transfer to related species. In every case, developers are required to submit a package of data from field trials and other sources to show that the GE variety poses risks no greater than its non-GE counterpart.
Once a risk assessment has been completed, it needs to be decided whether the risk (and its uncertainty) posed by the GE variety is “acceptable” within the country’s legal and cultural framework. The regulatory
regimes handle that risk-management decision differently. In the United States and Canada, the decision is left to regulatory agencies that are expected to consider primarily the narrow biosafety question of whether the GE variety poses a risk substantially greater than its non-GE counterpart. Issues of socioeconomic effects are generally not addressed in the approval decision. The EU and Brazil separate risk assessment and risk management, which is handled by a government body with direct political accountability. In some cases, broader socioeconomic issues, including consumer “right-to-know” and effects on other producers, are brought into the approval decision-making process.
Those differences and conflicts are not unique. The diverse regulatory processes for products of genetic engineering mirror the broader social, legal, and cultural differences among nations. Conflicts also arise in the context of the development of international trade standards and the individual autonomy of nation-states to protect the cultural and social values specific to the countries. The United States has been a world leader in the effort to develop liberalized trade rules. Countries with stronger traditions of social welfare are not likely to be equally enthusiastic about regulatory processes that emphasize benefits of trade. As a result, the conflicts over trade discussed in Chapter 6 that arise from asynchronous product approvals are likely to continue.
FINDING: The diverse regulatory processes for products of genetic engineering mirror the broader social, political, legal, and cultural differences among countries.
FINDING: Conflicts about trade and disagreements about regulatory models are likely to continue to be a part of the international landscape.
REGULATORY IMPLICATIONS OF EMERGING GENETIC-ENGINEERING TECHNOLOGIES
As outlined in Chapter 7, the toolset of genetic engineering is changing rapidly, and new, more specific, and potentially more powerful genetic-engineering technologies are coming into use. As others have noted (Lusser et al., 2012; Lusser and Davies, 2013; Hartung and Schiemann, 2014; Voytas and Gao, 2014), the emerging technologies are likely to challenge regulatory schemes in divergent ways.
An initial issue is whether crops made with the technologies will fall within the definition of GE crops used by various regulatory agencies as a regulatory trigger and therefore be subject to premarket safety reviews. This issue is particularly relevant for regulatory systems that use a process-based definition, although the answer would need to be determined with reference
to the specific language of the law.26 Some GE plants already fall outside existing regulatory definitions (Table 9-3).
Whether that regulatory development is of concern depends on a second critical question: whether crops made with emerging genetic-engineering technologies will have risk characteristics different from plants made with other breeding techniques and, if so, what this means for regulation. Emerging genetic-engineering technologies may also pose challenges for risk assessment. Many of the current risk-assessment guidelines for GE crops are based on the assumption that the plants have been modified with transgenic recombinant-DNA technology that introduces a gene sequence from one organism into the genome of another organism through in vitro manipulation. Knowledge of the biological function and structure of the inserted gene and the donor organism is important in understanding the function of the gene in the new organism and thus a critical component of the risk assessment. Some of the emerging technologies might result in crops that at a genetic level are generally similar to crops engineered with recombinant-DNA technology, in which known DNA from one biological species is added to the genome of another species. Such cases are less likely to present novel challenges for risk assessors unless they involve gene-drive technology (see discussion in Chapter 7). However, future crop varieties could have added DNA sequences that have been computationally designed with no known biological source or could be transformed without the use of recombinant DNA. It is still unknown how, or even whether, such approaches should be regulated.
Some of the emerging genetic-engineering technologies, such as precisely targeted gene knockouts, also have the potential to create novel plant varieties that are hard to distinguish genetically from plants produced through processes that occur in nature and through conventional breeding (Voytas and Gao, 2014). The size and extent of the genetic transformation itself has relatively little relevance to its biological effect and consequently its environmental or food-safety risk. As explained in Chapter 7, small genetic changes can lead to important changes in phenotype, and large genetic changes can lead to relatively trivial changes in phenotype.
Chapter 7 describes several of the technologies for improving plant genetics that are emerging. Genome editing with meganucleases, zinc finger nucleases (ZFN), transcription activator-like effector nucleases (TALENs), and the clustered regularly interspaced palindromic repeats (CRISPR)/Cas9
__________________
26 As often occurs, there may be room for disagreement about the applicability of laws and regulations. Compare, for example, the conclusion of the New Breeding Technology Platform that the EU regulations do not apply to most of the emerging technologies (NBT Platform, 2013) and an analysis by the German Federal Agency for Nature Conservation that comes to the opposite conclusion (Rehder, 2015).
nuclease system will increasingly be used in crop genetic improvement. Synthetic biology, or computationally designed genetics, has been practiced on microorganisms for the last decade but is relatively new for plants (Liu et al., 2013; Liu and Stewart, 2015). Computational design of novel genes and even genomes (Liu and Stewart, 2015) could challenge existing regulations that are process-based.
Genome Editing
Genome editing uses novel altered nucleases and complementary components to edit the sequence and function of genes in situ (Chapter 7). Plant genomes can currently be edited in three ways: a gene can be disabled (knocked out), the sequence of a functional endogenous gene can be changed, and a chromosomal locus can be targeted for the insertion of DNA precisely in that location.
That third outcome of precise transgenic insertion is probably the least problematic for existing process-based regulatory approaches. Precise gene targeting has long been a goal of plant biotechnology; genome-editing methods make precise transgene placement possible (Liu et al., 2013). The addition to a plant of genes or elements that control gene expression would probably be covered as genetic engineering under existing process-based regulatory definitions.
The cases of gene knockouts and small sequence changes are less clear (Jones, 2015). Genome-editing methods—including ZFN, TALEN, or CRISPR—can make small, precise changes or deletions in genetic sequences that can have substantial effects on a plant’s phenotype. (Indeed, the CRISPR/Cas9 system is likely to be a “game-changer” in every application of biology, including in crops (Belhaj et al., 2015).)
Many process-based regulatory systems would not cover such plants despite the important alteration of the plant phenotype. For example, APHIS regulations cover only plants that retain some genetic sequences derived from a known “plant pest” in the final plant. The genome-editing methods are already creating GE plants that have no plant-pest components and are therefore not regulated by APHIS.27Table 9-3 shows that since 2011 APHIS has determined that many new GE events do not fall under its purview because a plant pest is not used to introduce the GE crop. It is interesting to note that when the White House Office of Science and
__________________
27 Cibus, a company in San Diego, California, has commercially introduced in the United States a nontransgenic herbicide-resistant canola developed by using a genome-editing technique to introduce a point mutation to endow the resistant trait. The variety appears not be regulated in the United States. Cibus plans to use similar technology to commercialize other herbicide-resistant crops, including flax and rice (www.cibus.com, accessed April 13, 2016).
TABLE 9-3 Regulated Status of Genetically Engineered Products Submitted in Letters of Inquiry to the U.S. Department of Agriculture’s Animal and Plant Health Inspection Service (APHIS) for Determination of Regulation
| Category | Inquiry Date | Applicant | Host Organism |
|---|---|---|---|
| I Null Segregants |
1/18/2011 | USDA Agricultural Research Service | Plum |
| 1/22/2011 | North Carolina State University | Tobacco | |
| 1/27/2011 | New Zealand Institute for Plant and Food Research | N/A | |
| 12/10/2011 | University of Nebraska | Sorghum | |
| 7/29/2013 | Cellectis | Potato | |
| 3/17/2015 | Agravida | Maize | |
| 4/28/2015 | Arnold & Porter LLP | Tobacco | |
| 8/25/2015 | Calyxt | Wheat | |
| II Gene Delivery Systems |
3/8/1995 | (none listed) | Carnation |
| 12/11/2007 | New Zealand Crop and Food Limited | Petunia | |
| 9/1/2009 | Noble Foundation | Barrel medic (Medicago truncatula) | |
| 9/13/2010 | Scotts Company | Kentucky bluegrass | |
| 1/20/2012 | Ceres, Inc. | Switchgrass | |
| 1/31/2012 | Scotts Company | Kentucky bluegrass | |
| 2/1/2012 | Scotts Company | St. Augustinegrass | |
| 7/23/2012 | Ceres, Inc. | Switchgrass | |
| 7/23/2012 | Ceres, Inc. | Switchgrass | |
| 7/23/2012 | Ceres, Inc. | Switchgrass | |
| 7/23/2012 | Ceres, Inc. | Switchgrass | |
| 7/30/2012 | Del Monte Fresh Produce Company | Pineapple | |
| 9/14/2012 | ArborGen | Pine | |
| 2/22/2013 | Ceres | Sorghum | |
| 3/25/2013 | (CBI redacted) | Kalanchoe blossfeldiana and hybrids | |
| 4/5/2013 | Scotts | Tall fescue | |
| 6/1/2013 | University of Georgia | Soybean | |
| 8/30/2013 | Ceres | Maize | |
| 10/1/2014 | Glowing Plants Inc. | Arabidopsis | |
| 1/13/2015 | B.H. Biosystems | Maize | |
| 12/14/2015 | Bayer CropScience | Tobacco | |
| III Cisgenesis and Intragenesis |
2/8/2012 | University of Florida | Grape |
| 2/23/2012 | Wageningen University | Apple | |
| IV Site-Directed Nucleases |
3/1/2010 | Dow | Maize |
| 3/2/2010 | Dow | Maize | |
| 9/9/2011 | Cellectis | N/A | |
| 9/10/2011 | Cellectis | N/A | |
| 2/7/2014 | Iowa State University | Rice | |
| 11/17/2014 | Cellectis | Soy | |
| 3/12/2015 | Cellectis | Soy | |
| V Other |
3/7/1994 | Washington State University | Rhizobium leguminosarum |
| 2/16/2005 | V.P. Technology Development | Chlamydomonas reinhardtii HSV8 | |
| 4/6/2008 | Coastal Biomarine | Algae strains | |
| 2/21/2011 | Danziger | Baby’s Breath | |
| 6/15/2012 | BioGlow LLC | (CBI redacted) | |
| 10/23/2012 | BioGlow LLC | (CBI redacted) | |
| 1/10/2013 | Rutgers IR4 Project | N/A |
*Transgenic crops modified by targeted deletions during which no plant-pest genetic information is incorporated into the host genome were determined to fall.
SOURCE: Table 1 from Camacho et al. (2014), updated by the committee with letters of inquiry to APHIS dated until April 8, 2016.
| Modification / Phenotype / Product Descrption | Transformation Method | Status |
|---|---|---|
| Accelerated breeding | (none listed) | – |
| Accelerated breeding | (none listed) | – |
| Centromere-mediated chromosome elimination/production of doubled haploids | (none listed) | – |
| Decreased MSH1 Expression | Agrobacterium tumefaciens | – |
| Improved consumer & processing quality | Transient expression of TALENs | – |
| High starch in leaves and stalks | Meganuclease deletions | – |
| Accelerated breeding; ‘reduced harm traits’ | (none listed) | – |
| Improved disease resistance | Transient expression of TALENs | – |
| (none listed) | Agrobacterium tumefaciens | – |
| Altered vegetative pigmentation | Biolistics | – |
| Tnt1 retrotransposon expression (knockout library) | Agrobacterium tumefaciens | Regulated |
| Glyphosate resistant | Biolistics | – |
| Improved biofuel yield potential | Biolistics | – |
| Glyphosate resistant, enhanced turfgrass quality | Biolistics | – |
| Glyphosate resistant, enhanced turfgrass quality | Biolistics | – |
| Enhanced water-use efficiency | Biolistics | – |
| Biomass more easily converted to fermentable sugars | Biolistics | – |
| Biomass more easily converted to fermentable sugars | Biolistics | – |
| Biomass more easily converted to fermentable sugars | Biolistics | – |
| Altered fruit tissue color/anthocyanin content | Agrobacterium tumefaciens | – |
| Improved wood density | Biolistics | – |
| Improved biomass, juice volume & total sugars | Biolistics | – |
| Insertion of rol gene from natural isolate; compact stature | Agrobacterium rhizogenes | – |
| Glyphosate resistant & improved turfgrass quality | Biolistics | – |
| Altered flavonoid profiles | Biolistics | – |
| Improved digestibility, insecticidal properties, improved palatability, drought tolerance, increased seed yield and/or dwarfing | Biolistics | – |
| Bioluminescence | Biolistics | – |
| Improved yield (photosynthetic capacity) | Biolistics | – |
| improved photosynthetic capacity and biomass production | Biolistics | – |
| Increased anthocyanin production (intragenic) | Biolistics | – |
| Scab disease resistance (cisgenic) | Agrobacterium tumefaciens | Regulated |
| Suppressed phytate biosynthesis | Zinc-finger nuclease (EXZACT™) | – |
| Suppressed phytate biosynthesis | Zinc-finger nuclease (EXZACT™) | Regulated* |
| Genome editing (targeted Indels) | Meganuclease (I-Cre1) deletions | – |
| Genome editing (targeted Indels) | Meganuclease (I-Cre1) substitutions or additions | Regulated* |
| Improved disease resistance | Transient expression of TALENs | – |
| FAD2 knockout; improved consumer quality | Transient expression of TALENs | – |
| FAD3 knockout; improved consumer quality | Transient expression of TALENs | – |
| Insect tolerance | (none listed) | – |
| Expression of antibodies for human therapeutics | (none listed) | – |
| Expression of a glucose transporter from Chlorella | (none listed) | – |
| Altered flower color | (none listed) | – |
| (CBI redacted) | (CBI redacted) | – |
| (CBI redacted) | (CBI redacted) | – |
| Plasmid; conferring fus crown rot (tomato) resistance | N/A | – |
NOTE: CBI = confidential business information. In the status column, “–” indicates that APHIS considers that the item in the query would not be regulated and therefore should not enter its regulatory system.
Technology Policy (OSTP) developed the Coordinated Framework in 1986, it was just a few months before the invention of GE plant production by particle bombardment became publicly known (Klein et al., 1987). Most of the applications in Table 9-3 use particle bombardment (also known as the gene gun), a nonbiological method to make GE plants (see Chapter 3). In all likelihood, at the time of adoption in 1986, OSTP and APHIS could not have foreseen GE plants not regulated by APHIS because, until that time, all GE plants had been produced using the plant pathogen Agrobacterium tumefaciens.
It is now possible to use molecular techniques to suppress expression of a protein or disable a protein’s function without having any new DNA added to a plant. This situation mimics what could readily occur in nature or in conventional breeding (Voytas and Gao, 2014). Indeed, wheat has been genome-edited with TALENs and CRISPRs to edit the six copies of a gene in the crop simultaneously to confer resistance to powdery mildew (see Box 7-2). If similar resistance had been achieved through the insertion of genetic material from an unrelated organism, the transformed plant would fall under EPA’s current regulations as a “plant-incorporated protectant.” In the case of knockouts, however, the pest-resistant or virus-resistant plant contains no new genetic material from a nonsexually compatible source and is therefore likely to be exempt from EPA’s registration requirements under its current rules (EPA, 2001b, §174.25).
When the committee was writing its report, most applications of genome editing had been to accomplish gene knockouts. Nevertheless, it was possible to insert DNA and change DNA bases with the new methods (reviewed in Mahfouz et al., 2014; Belhaj et al., 2015). A few changes in an endogenous plant gene can confer an agronomic trait, such as herbicide resistance.28 Thus, small changes in gene sequence in an endogenous gene can result in large phenotype and fitness changes. These small genome edits can sometimes mimic a mutation that can occur in nature. As with knockouts, whether such edited plants would be subject to a premarket regulatory-approval process would depend on the specific wording of the regulations; they would almost certainly not be covered by APHIS despite the addition of a herbicide-resistant trait.
__________________
28 For example, by changing the glyphosate binding site in the 5-enolpyruvylshikimate-3-phosphate synthase (EPSPS) gene by just two nucleotides, which results in changes in two amino acids, the “TIPS” (T102I + P106S) mutation will confer glyphosate resistance. Indeed, that mutation has occurred naturally in the weed Indian goosegrass (Eleusine indica) and endowed resistance higher by a factor of over 2,500 than the wild-type gene and higher by a factor of 600 than the single mutation (P106S) in the EPSPS gene (Yu et al., 2015). The same TIPS mutation has been rendered in the EPSPS gene in tobacco by using TALENs and would be feasible in almost any crop with any of the genome-editing approaches.
Genome editing will force a compelling dilemma for some regulatory approaches: it is now possible to change plant genetics without leaving any trace of genome-editing reagents. In several cases in which the nuclease gene has been segregated away from the site-directed mutation, genome-edited plants have no exogenous DNA (reviewed in Voytas and Gao, 2014). Genetic engineering of the epigenome (see Chapter 7) raises the same issues, inasmuch as there would be no change in the target organism’s DNA.
Synthetic Genes and Genomes
The increased development of synthesized genetic components raises several regulatory issues. Whether the insertion of synthetic promoters and transcription factors into a plant would trigger regulatory review under process-based definitions will depend on the specific wording of the various regulations. Such additions would probably be covered by many process-based approaches, although the APHIS approach of covering plants that have been engineered with the use of plant-pest organisms would not seem to apply to computationally derived sequences. Synthetic DNA sequences with no direct biological species analogue would not fall within the current APHIS regulations, in which the biological source of recombinant DNA plays an important role. In the case of new genome-editing reagents, U.S. regulatory agencies are not structured to regulate the DNA-free delivery of a reagent that produces a targeted mutation but leaves no exogenous DNA footprint with the host genome (see Chapter 7). Indeed, in late 2015, the Swedish Board of Agriculture deemed transgenic Arabidopsis that was genome-edited via CRISPR not subject to regulations and started allowing field tests. The nontransgenic and DNA-free delivery of CRISPR would be even more likely to be exempt from regulation under the Swedish approach because it would not have a transgene.
For risk assessment, synthetic components also raise issues. On the one hand, because the sequences will be not derived from a biological source, they may have no direct analogue or comparator in nature, which could result in additional regulatory uncertainty. On the other hand, a synthetic promoter would probably be streamlined and have more precise function than endogenous promoters (Liu and Stewart, 2016). For example, a synthetic promoter, such as a soybean cyst nematode-inducible promoter designed for expression in soybean roots, is about one-tenth the length of the typical plant promoter and has been computationally designed not to have any cryptic transcriptional start sites or any other issues that could lead to off-target regulation of gene expression (Liu et al., 2014). Therefore, risk potential might actually be decreased by use of some synthetic components.
With the ability to design genes and genetic control elements computationally and with the relatively inexpensive DNA synthesis and assembly
methods that are routinely available (Kosuri and Church, 2014), entire synthetic organelle genomes can be built. Yeast (Saccharomyces cerevisiae) synthetic chromosomes have been built and installed into the genome to replace their endogenous counterparts (Annaluru et al., 2014). Although yeast has a more streamlined nuclear genome than plants, such design features will probably find their way into plants. Indeed, a 150,000–base-pair synthetic chloroplast genome (plastome) is already feasible to design, manufacture, and install into plants (Liu and Stewart, 2015).
A plant with a synthetic plastome would pose a challenge for risk assessments because it might lack a known natural biological comparator, the basis of present substantial-equivalence risk-assessment paradigms. Furthermore, the gene-by-gene regulatory paradigm of incremental improvement could be severely challenged by synthetic genomes or subgenomes in which many genes and traits are changed simultaneously.
RELATED REGULATORY ISSUES
The regulatory process in most countries for current and future GE crops addresses primarily the biosafety of the products. However, additional issues are related to the products, such as coexistence, labeling, post-approval environmental monitoring, and public participation. Here, the committee looks at how regulation may interact with commercialized products and with GE products that may be developed in the future with emerging technologies.
The Role of Product Regulation Beyond Biosafety
As noted above, some countries use their product regulatory systems to address socioeconomic and other policy issues that go beyond the mission of ensuring the safety of food and other products. In the case of GE foods and crops, the two primary issues that emerge are managing coexistence of GE, non-GE, and organic-farm production systems and mandatory labeling of GE foods.
Those issues clearly involve social and economic choices that go beyond scientific assessments of health or environmental safety; ultimately, they inherently involve value choices that science alone cannot answer. It is likely that different societies will balance the competing interests in different ways.
As noted above, product regulation in the United States is primarily viewed as a technical process that does not incorporate broader ethical concerns or issues about the fairness to stakeholders into product-approval decisions. That regulatory approach reflects fundamental cultural values,
including respect for the marketplace and a limited role for government, that may differ in other countries.
That observation does not mean, however, that the issues cannot be addressed by U.S. policy-makers and the private sector as a broader part of technology governance. Outside the product regulation process, the U.S. Congress has addressed a number of economic, ethical, and social concerns, such as animal welfare, protections for research subjects, crop insurance, marketing standards, and voluntary labeling programs. Such issues can also be addressed by nongovernmental actions, including voluntary standard-setting organizations.
On the issue of coexistence, Chapter 6 notes that nonregulatory parts of USDA (such as the Agricultural Marketing Service and the Federal Grain Inspection Service) have a long history of working with the private sector to ensure orderly markets and trade and could address coexistence issues. The secretary of agriculture has also made efforts to address coexistence through the Advisory Committee on 21st Century Agriculture and various workshops. The private sector is playing a major role in developing markets that put together producers and consumers by managing supply chains and contractual obligations. When the committee’s report was being written, the various governance efforts had not been sufficient to address the concerns raised by organic growers and growers of non-GE crops or to meet the need to protect identity-preserved channels for various GE crops that have not received full export approvals. As noted in Chapter 6, the risk of adventitious presence currently affects producers of non-GE crops in the United States.
Mandatory labeling is a similarly complex issue that involves competing values. There clearly are strong nonsafety arguments and considerable public support for mandatory labeling of products containing GE material. On the basis of its review of the evidence on health effects (Chapter 5), the committee does not believe that mandatory labeling of foods with GE content is justified to protect public health. As discussed in more detail later in the present chapter, previous reports from the National Research Council have consistently upheld the view that the process by which a food is made or a crop is bred is a poor indicator of risk. All technologies for improving plant genetics have the potential to change foods in ways that raise safety issues.
As discussed in Chapter 6, however, product labeling serves purposes that go beyond food safety. As with coexistence, U.S. policy-makers and the private sector have the ability to address the broader social and economic issues and to balance the competing interests involved. The marketplace is also responding to consumer interest in avoiding GE foods: the number of
products voluntarily labeled as “non-GMO” has increased dramatically in the last 10 years.29
FINDING: Policy regarding GE crops has scientific, legal, and social dimensions, and not all issues can be answered by science alone. Indeed, conclusions about GE crops often depend on how stakeholders and decision-makers set priorities among and weigh different considerations and values.
RECOMMENDATION: In addition to issues of product safety, socioeconomic issues that go beyond product safety are technology-governance issues that should be addressed by policy-makers, the private sector, and the public in a way that considers competing interests of various stakeholders and inherent tradeoffs.
The Role of Expertise, Public Participation, and Transparency in Product Regulation
Different countries allocate the roles of risk assessment and risk-management decisions in different ways. In the examples included in this report, every country has a technical expert body to conduct a risk assessment of a product seeking regulatory approval. The risk assessment provides a scientifically based evaluation of a product’s overall food-safety and environmental risks. The decision of whether to approve a product for commercialization or to approve it with conditions needed to prevent or mitigate potential harm is the risk-management decision. Depending on the particular law involved, the approval process may take into account such issues as costs, benefits, and socioeconomic effects. For that reason, some countries have chosen to give the risk-management decision to bodies that are more politically accountable and that can reflect public opinion. In the EU, for example, approvals of GE crops and foods involve the representatives of Member States; in Brazil, final approvals are the responsibility of a group of cabinet ministers. In the United States and Canada, the same agency that conducts the risk assessment is also responsible for making
__________________
29 There is no national standard for “non-GE” claims, and FDA has provided guidance for voluntary labeling to ensure that such labels are not misleading (FDA, 2015b). One large voluntary certification and labeling program is operated by the Non-GMO Project, under which foods that are certified to follow the Project’s standards may include the “Non-GMO Project Verified” label on the package (www.nongmoproject.org). The Project states that it has participation from 1,500 brands accounting for more than $11 billion in annual sales. Recently, USDA approved the use of a USDA “Non-GMO/GE Process Verified” label for one food company (NGFA, 2015). Other “non-GE” labels are evolving in the U.S. marketplace (Strom, 2015).
the product-approval decision. Because the approval decision is seen to be more narrowly based on the question of safety as determined by the risk assessment, the U.S. and Canadian approaches give final approval authority to agencies that are more insulated from political and public influences.
The approaches discussed above all attempt to address the tension between expertise and democratic accountability experienced in different contexts (Liberatore and Funtowicz, 2003) in a climate in which some members of the public are growing more and more distrustful of elite experts (Fisher, 2009). Inclusionary approaches are not always successful. For example, Hatanaka and Konefal (2013) described a process in which a participatory approach was attempted to establish the legitimacy and integrity of a sustainability standard. Legitimacy has three interrelated elements: input, procedural, and output. It is assumed generally that there is a positive relationship between the three, that is, legitimacy of any one contributes to the legitimacy of the others. However, it is possible that input legitimacy can contribute to weakened procedural and output legitimacy (Tamm Hallström and Boström, 2010; Hatanaka and Konefal, 2013). Hatanaka and Konefal found that the sustainability standard lacked output legitimacy because too many actors with differing opinions on input watered down the standard during its creation and key actors opted out of the process during contentious negotiations. In another example, Endres (2005) reported a similar outcome related to an effort to create a coexistence working group. The group reached consensus (and near unanimity) on relevant “best management practices” to foster coexistence among organic, non-GE, and GE crop production. However, after initial voting on the proposed best management practices, five members of the group withdrew their support and discontinued participation in the project (Endres, 2005). That withdrawal, despite input legitimacy, led to a failure of output legitimacy.
Despite such failures, institutions (including the National Academy of Sciences and the National Research Council) have responded to the concerns about trust and democratic legitimacy primarily through changes in process to expand transparency and public participation. Many efforts have been made to find innovative ways to include the public in decision-making on issues involving technical or scientific matters (Rowe and Frewer, 2005).
As noted in Chapter 2, international human-rights law protects rights to access to information and public participation and requires that exceptions to these rights be drawn as narrowly as possible. National Research Council committees have long recognized the need for transparency and robust public participation, both generally regarding risk analysis of scientific issues and specifically regarding GE crops. The 1996 National Research Council report discussed in Chapter 2, Understanding Risk: Informing Decisions in a Democratic Society, noted the importance of
including stakeholder participation throughout the risk-assessment process and particularly during the final phase of risk characterization (NRC, 1996:11):
Risk characterization involves complex, value-laden judgments and a need for effective dialogue between technical experts and interested and affected citizens who may lack technical expertise, yet have essential information and often hold strong views and substantial power in our democratic society.
The report noted that risk characterization benefits as much from deliberation with stakeholders as from expert analysis. The process should involve “sufficiently diverse participation from across the spectrum of interested and affected parties to ensure that the important, decision-relevant knowledge enters the process, that the important perspectives are considered, and that the parties’ legitimate concerns about the inclusiveness and openness of the process are addressed” (NRC, 1996:4).
Another National Research Council report, Science and Decisions: Advancing Risk Assessment, offered similar recommendations, urging greater public inclusion in the risk-assessment process, particularly in the early stages of problem formulation, not only to improve public acceptance of the analysis but to improve the analysis for the purposes of risk management (NRC, 2009). Public communication and inclusion are particularly important with respect to emerging genetic-engineering technologies, including areas such as synthetic biology, and prospective regulatory methodologies, such as the use of -omics technologies. Institutions involved in regulating GE crops thus should pay special attention to communicating with the public about and seeking public input regarding how those institutions might regulate emerging technologies and their products and how they might use -omics technologies.
The issues involved in policies regarding genetic engineering are complex and require the input of many stakeholders, particularly as new technologies and new applications are considered (Oye et al., 2014). The importance of transparency and public participation in the risk assessment of GE crops in particular was emphasized in the 2002 National Research Council report Environmental Effects of Transgenic Plants. In assessing environmental risks, including stakeholders and the public is important because there is less consensus about what constitutes an environmental risk—what is worth protecting (NRC, 2002). The committee found that “public confidence in biotechnology will require that socioeconomic impacts are evaluated along with environmental risks and that people representing diverse values have an opportunity to participate in judgments about the impact of the technology” (NRC, 2002:245).
The present committee did not have adequate information on the regulatory-approval processes of other countries to make an informed judgment about the adequacy of transparency and the opportunity for public participation during risk assessment and risk management. However, it was aware of a number of efforts in the EU and elsewhere to engage stakeholders and publics on the issue of GE crops and foods outside the formal product-approval process (Medlock et al., 2007).
In the United States, transparency and opportunities for stakeholder and public participation in regulatory-agency product-approval proceedings are constrained by laws that protect confidential business information and define how and when agencies may communicate with the public, particularly the Freedom of Information Act, which provides the overarching framework for transparency regarding government actions, and the Administrative Procedures Act, which provides rules for public participation in rule-making. Agencies have made commendable efforts to post more of their proposed actions and decisions on-line to make it easier for the public to be aware of or to comment on specific actions. Furthermore, agencies have attempted to create opportunities for discussion with stakeholders and the public beyond the “notice-and-comment” procedure required for agency rule-making. In 2015, APHIS suspended a rule-making proceeding to provide an opportunity for a more flexible engagement with stakeholders and the public on its biotechnology regulations (USDA–APHIS, 2015).
Nevertheless, opportunities for public engagement in an agency decision-making process are limited, and much information submitted to an agency in support of a product approval remains protected as confidential business information. In particular, the committee was aware that the lack of public access to the health and safety data submitted by developers creates distrust in some stakeholders.30 Although agencies publish a summary of their decisions based on the data, the public cannot judge for itself the quality, objectivity, and comprehensiveness of the materials submitted. Given a developer’s self-interest in getting a product approved and its control over the material considered by the agency, the lack of access creates skepticism about the quality of the data. To address that concern, EFSA was planning to make industry data submissions publicly available over the next few years (Rabesandratana, 2015). Some stakeholders have commented on the need for increased GE crop safety research funding for academic scientists not funded by the biotechnology industry to provide peer-reviewed
__________________
30 With respect to FDA’s consultation process, interested parties are able to obtain the developer submissions and related data that are not trade secrets or confidential commercial information from the agency by submitting a Freedom of Information Act request. Health and safety data submitted to FDA as part of final consultations are typically available to the public upon request.
and publicly accessible information. In 2002, the U.S. General Accounting Office (now Government Accountability Office) recommended that FDA randomly verify raw test data that provide the basis of a developer’s submission to enhance its evaluation process and improve credibility (GAO, 2002). When the committee was writing its report, FDA had not indicated whether it had adopted that suggestion.
The committee recognizes the legitimacy of the confidential nature of business information as a rationale for withholding some data from public access and understands that U.S. agencies are constrained by various laws in what they can publicly disclose. Within that framework, however, the committee concludes, on the basis of research findings, that transparency and public participation are critical and urges agencies to ensure that exemptions from disclosure are as narrow as possible. The committee also urges developers to disclose voluntarily as much of the health and safety information submitted to agencies as possible.
FINDING: Transparency and public participation have been shown by research to be critically important for appropriate, sound, and credible governance of all aspects of the development, deployment, and use of GE crops.
RECOMMENDATION: Regulating authorities should be particularly proactive in communicating information to the public about how emerging genetic-engineering technologies (including genome editing and synthetic biology) or their products might be regulated and about how new regulatory methodologies (such as the use of -omics technologies) might be used. They should also be proactive in seeking input from the public on these issues.
RECOMMENDATION: In deciding what information to exclude from public disclosure as confidential business information or on other legal grounds, regulating authorities should bear in mind the importance of transparency, access to information, and public participation and ensure that exemptions are as narrow as possible.
Post-Approval Environmental Monitoring
Premarket regulatory safety reviews are intended to prevent harmful foods or plants from going to market. In many cases, however, regulators know that identified risks exist or are faced with uncertainty about risks. One way to manage those situations is to impose conditions on commercial use that are intended to mitigate potential harm and to require post-approval monitoring to ensure that there are no unexpected adverse
events. Post-market controls and monitoring are critical risk-management tools.
Most of the national GE crop regulatory systems considered in this report routinely impose continuing requirements such as monitoring after crops have been approved. In particular, crops with herbicide-resistant or insect-resistant traits are required by most regulators to have stewardship plans in place to reduce the evolution of insect and weed resistance, including requirements to monitor for resistance and unanticipated adverse effects (see, for example, EFSA, 2010, Part 4; and EFSA, 2011a).
APHIS has taken the position that it lacks the legal authority to require post-market conditions or monitoring. Under APHIS, the final step for a typical crop is deregulation, which is in effect a decision by the agency that the crop is not a plant pest and that it therefore no longer has any legal authority to continue to regulate it. One of the consequences is that APHIS did not require developers to institute any post-approval management practices to reduce the potential for weed resistance to glyphosate, nor did it require developers to monitor for resistance or other unexpected effects. A requirement for monitoring might have prevented the rapid spread of glyphosate-resistant weeds discussed in Chapter 4. An authority to establish and enforce post-approval requirements to reduce resistance or mitigate other environmental effects is a critical tool for risk-management agencies.31 In contrast with APHIS, as noted previously, EPA has exercised its authority under FIFRA to require post-approval monitoring and pest-resistance–management programs for Bt crops and more recently proposed resistance-management programs for some herbicides used with herbicide-resistant crops. Post-approval regulatory authority also enables risk managers to impose conditions on use, such as restrictions intended to reduce the potential for unwanted gene flow, when a risk assessment raises uncertainties and caution is therefore required. Similarly, post-approval monitoring could have alerted APHIS to the increasing spread of glyphosate-resistant weeds at an early stage and enabled it to make mid-course corrections.
RECOMMENDATION: Regulatory agencies responsible for environmental risk should have the authority to impose continuing requirements and require environmental monitoring for unexpected effects after a GE crop has been approved for commercial release.
__________________
31 Post-market authority also enables regulatory agencies to work with affected stakeholders to develop and promote voluntary, community-based pest-resistance–management programs (Iowa State University, 2015).
SCOPE OF PRODUCTS SUBJECT TO PREMARKET REGULATORY SAFETY ASSESSMENT
As noted above, one continuing regulatory issue has been the question of what, if any, new crops and foods should be subject to regulatory scrutiny for safety before going to market. For regulatory efficiency, the goal of any product-regulation system should be to assess premarket safety of those products most likely to pose unacceptable risk. The practical difficulty, of course, is for the regulatory agencies to identify such products in advance while allowing safe and useful products to proceed to market.
Many countries have adopted process-based regulations that require premarket food-safety and environmental protection approvals for crops or foods that have been genetically engineered in specified ways, in part on the assumption that the engineering process or the novel traits that can be introduced by genetic engineering makes such plants more likely to be risky than new crops developed through other breeding techniques.
Previous National Research Council reports have consistently said that the breeding process used to introduce a new trait into a crop is not a particularly useful indicator of new or increased hazards. A 1989 National Research Council report noted that crops “modified by molecular and cellular methods should pose risks no different from those modified by classical genetic methods for similar traits” (NRC, 1989:67). As a 2000 National Research Council report stated, “both methods have the potential to produce organisms of high or low risk” (NRC, 2000:43). In addition, National Academy of Sciences and National Research Council reports have concluded that transgenic techniques create no “unique” categories of hazards (NAS, 1987; NRC, 2000, 2002). As the 2000 report noted, “toxicity, allergenicity, effects of gene flow, development of resistant plants, and effects on non-target species are concerns for both conventional and transgenic pest-protected plants” (NRC, 2000:6). Indeed, the committee found it difficult to conceive of a totally different category of hazard that could be posed by any plant-breeding process.32
By focusing only on particular forms of genetic engineering, such process-based regulatory approaches may be underregulating plants developed with other breeding processes that can pose equal or greater hazards, increase exposure, or create greater uncertainty about risk. The 2004 National Research Council report Safety of Genetically Engineered Foods: Approaches to Assessing Unintended Health Effects found that some breeding processes, including mutagenesis, are more likely to intro-
__________________
32 A possible exception is gene drives in plants. When the present committee was writing its report, a different National Academies committee was investigating gene-drive research. Its report, Gene Drives on the Horizon: Advancing Science, Navigating Uncertainty, and Aligning Research with Public Values, was published in 2016.
duce unintended effects (NRC, 2004) than some other breeding processes. Whether such unintended changes pose environmental or human health risks depends on the specific changes made in the plant (NRC, 2004); many unintended changes are likely to be benign.33
The array of emerging genetic-engineering technologies, including genome editing and synthetic biology, makes it clear that any attempt by regulators to define the scope of a regulatory system through the definition of specified technologies will be rapidly outmoded by new approaches. Many of the emerging technologies will not be covered under existing rules. Some emerging technologies could result in new plant varieties that genetically look very much like the products of conventional cross-breeding, whereas others could result in the introduction of synthetic gene sequences without a natural counterpart, creating uncertainty about potential hazard. Differentiating what is genetic engineering and what is conventional breeding is becoming more difficult.
Although the U.S. regulatory system avoids some of those issues, its emphasis on product categories creates similar issues of inconsistency for environmental risks. APHIS has authority to regulate only narrowly defined plant pests. Therefore, some plants with novel traits (such as herbicide resistance) are reviewed for plant-pest risks before being approved because they contain DNA sequences from plant pests, and other plants with similar traits that have been introduced with techniques that do not require the use of plant-pest genetic sequences may be commercialized without any APHIS regulatory review. Similarly, EPA, as a policy matter, has exempted plants with pest-resistant traits that have been introduced through conventional breeding; as a result, genome editing would most likely not be covered by EPA’s current rules, although EPA is considering possible data requirements for RNAi technology (EPA, 2014b) and other genetic-engineering technologies not currently covered.
In addition, both EPA and APHIS review plants with traits that have been previously reviewed for other crops and varieties and are already in wide use. Earlier National Research Council reports have stressed that risk needs to be determined on the basis of the properties of the modified plant and the specific environment into which it is intended to be introduced. To be consistent with that approach, a more effective regulatory approach would give premarket scrutiny to plants that express traits that are new to established, cultivated crop species and that pose a potential for envi-
__________________
33 In addition to the unintended changes in the plant itself, risk assessors need to consider unexpected or unintended effects of the trait that has been intentionally introduced into the plant. In the environmental assessment, for example, regulators would need to consider whether organisms other than the intended target organism of a plant-incorporated protectant would be unintentionally harmed, whether through direct action (for example, toxicity) or indirect action (for example, loss of habitat).
ronmental harm, regardless of the process used. In concept, that is the approach adopted by Canada for plants with novel traits. The policy focuses appropriately on the two critical elements of risk assessment: hazard and exposure.
The introduction of a novel trait that has not previously been present in an established, cultivated crop species represents a novel exposure and therefore has an increased uncertainty of risk with respect to environmental effect. (Conversely, familiarity with a plant, trait, and the intended environment reduces the uncertainty of a risk assessment.) In contrast, a plant with a relatively small change in a trait that already exists in that environment is less likely to create environmental disruption because organisms in the environment have already been exposed to the trait and environmental responses are already established. The novelty of a trait in a crop species and the power of its expression are relevant to the exposure portion of the risk-assessment analysis.
In addition to exposure, there has to be a hazard—an agent or mechanism that causes some undesirable environmental outcome or increases a food-safety risk. For example, a new GE trait could affect the reproduction of beneficial insects when they are exposed to it in the field, or a plant might contain a protein with known potential for allergenicity.
In many cases, there may be substantial uncertainty about whether there is a hazard at all or how severe the hazard is. As technology provides plant breeders with more powerful tools, it creates the potential to introduce novel traits with which breeders and regulators have no clear comparators or experience. Such cases may be rare, but given the potential for novel exposure, it is a reasonable policy response to review such plants before their release into the environment. Risk managers can obtain additional information under field trial conditions requiring containment and other risk-mitigation measures intended to prevent uncontrolled releases.
A Tiered Approach to Premarket Regulatory Testing
An immediate concern that arises regarding regulation based on the novelty of a trait in a cultivated plant species is that there would be a broad expansion of the varieties that would undergo the full array of premarket testing because it would not be possible to exclude the possibility that an unintended change during any genetic-engineering or conventional-breeding process would lead to novel biological properties. As pointed out above, even a small genetic change could lead to biologically important alterations of a crop, so it would not be possible to exempt plants with small genetic changes.
Over the last 20 years, however, not only genetic-engineering techniques have advanced rapidly but so have other genomic methods, and some of
these, called -omics technologies, enable much more accurate assessment of whether unintended biological changes have occurred in a plant that has been manipulated by conventional-breeding or genetic-engineering processes. As discussed in detail in Chapters 5 and 7, a number of -omics screening methods that can scan almost the entire DNA sequence of a plant and the quantitative profile of its messenger RNAs (mRNA) have been developed. Not quite as advanced are -omics methods for understanding and quantifying a plant’s proteins, epigenome, and other molecules (metabolites), but these methods are advancing rapidly. None of these -omics methods are required by regulatory agencies, but, as reviewed in Chapter 5, they are being used by researchers to compare available GE crops with their non-GE counterparts. When those studies are conducted carefully (that is, with near-isogenic lines grown side by side with identical farming practices, appropriate replication, and good laboratory practices), the only differences in mRNA, protein, and metabolite profiles should be the ones that are intended. The studies reviewed in Chapter 5 bear that out. Other studies that compare the profiles of current GE crops with those of an array of varieties of the same crop also typically find no unexpected alterations.
In Chapter 7, the committee reviews the scientific basis of the -omics technologies and their current limitations. Although the committee emphasizes that finding a difference does not mean that there is a safety risk, not finding any unexpected differences is strong evidence that there is unlikely to be an unintended alteration that could pose a safety risk. The committee outlines research investments that could improve precision while decreasing cost of risk analyses. Most important, the committee develops a flow diagram (Figure 7-6) to explain how the -omics technologies could be used in a tiered approach to risk analysis to streamline testing of many new varieties.
The potential for adopting -omics technologies for regulatory screening purposes has been discussed in disciplines beyond food safety and environmental safety. For example, Marx-Stoelting et al. (2015) discussed the outcome of a workshop evaluating the potential future uses of -omics technologies for regulatory toxicology. Some of the limitations of -omics for regulatory toxicology are related to interpreting differences that are found because most of the compounds to be tested are expected to cause some differences. Liebsch et al. (2011) examined the potential of -omics methods for replacing some animal testing. They also saw the issue of interpreting differences as a challenge. The limitations in interpreting differences do not constitute as great a barrier for testing crops and foods because the finding of no differences is much more likely and useful in the case of crops and foods. Nevertheless, as indicated in Chapter 7, there is a need for investment in publicly accessible databases and improved methods if -omics technologies are to be used in a tiered approach with future GE and conventionally bred crops within current risk-assessment paradigms.
Alternative Policy That Eliminates Premarket Regulatory Review
The committee also considered an alternative regulatory policy that would let all new plant varieties, regardless of the methods by which they are made, go to market without premarket regulatory review and approval and allow regulators to respond if food-safety or environmental issues appear later. (Such products as drugs and pesticides would still, of course, be subject to applicable laws.) That would make plant breeders and food manufacturers primarily responsible for the safety of their products, as is the case for conventionally bred plants and foods. One could argue that the food-safety record of GE crops and foods over the last 20 years suggests that they are just as safe as conventionally bred crops and should not be subject to expensive government regulation on food-safety grounds. As noted in Chapter 6, the costs of the regulatory system can operate as a barrier to entry, particularly to public researchers, small seed companies, and specialty-crop developers that either lack financial resources or do not see the ability to recoup those costs in the marketplace. As a result, critics argue that biotechnology regulation has had the effect of keeping valuable and beneficial new crops and plants off the market and perversely benefiting large seed developers by restricting competition.
That policy option, however, has drawbacks. Although most novel crop varieties are likely to be as safe as those already on the market, some may raise legitimate concerns. As discussed above, it should be possible to distinguish among plants on the basis of their probable risk, taking into account the potential for exposure and harm. Furthermore, the new suite of emerging genetic-engineering technologies discussed in Chapter 7 is dramatically enhancing the ability of scientists to develop potentially effective new plant traits. Future GE crops discussed in Chapter 8 could greatly expand the use of agricultural biotechnology in the development of biofuels, forestry restoration, and industrial bioprocessing and thus potentially lead to new risk-assessment and risk-management issues (NRC, 2015). This policy option thus would have the effect of shifting risk to the public; mitigation measures could be expensive and ineffective, depending on the nature of the post-market problem.
This option has practical drawbacks as well. One of the major economic concerns that has been raised is the issue of coexistence and the need to keep unapproved or undesired genetic traits out of various food- and feed-supply channels. Currently, regulators impose conditions on experimental field trials in an effort to mitigate gene flow of unapproved events from experimental field trials, although adventitious events still occur (see Chapter 6 and Box 3-2 for an example of the consequences of failing to follow those conditions). Without some similar system in place, the market could experience a substantial increase in expensive adventitious events.
Similarly, a regulatory-approval system is essential for global trade to work. Few, if any, importing countries are likely to approve GE food or feed for import or GE seed for cultivation that has not been approved as safe by the relevant regulatory authorities in the exporting country.
Finally, an important effect of a regulatory system is to enable markets by creating a credible and independent process to verify that products are safe. As noted in Chapter 2, publics in many countries, including the United States, are wary about the safety of GE crops and foods. There should be concern about the effect on public opinion if GE crops and foods are brought to market without government review for safety. Without the assurance that there has been some third-party review for safety, consumers’ perceptions about the safety of GE foods and crops might erode completely. Although consumer confidence should not be the only rationale for a product-approval system, it is important to recognize that it is an important social and economic factor (OSTP, 2015).
FINDING: Not having government regulation of GE crops would be problematic for safety, trade, and other reasons and would erode public trust.
RECOMMENDATION: In determining whether a new plant variety should be subject to a premarket government approval for health and environmental safety, regulators should focus on the extent to which the characteristics of the plant variety (both intended and unintended) are likely to pose a risk to health or the environment on the basis of the novelty of traits, the extent of uncertainty regarding the severity of potential harm, and the potential for exposure regardless of the process by which the novel plant variety was bred.
CONCLUSIONS
Current international agreements and national regulatory systems reflect a variety of political and regulatory approaches to GE crops and foods. All the regulatory systems examined in this report use similar risk-assessment methods to analyze the food-safety and environmental risks posed by GE crops and foods on the basis of a comparison with similar existing foods and crops. However, regulatory systems differ in approaches and policy decisions related to risk management and the level of “acceptable” risk. Thus, some countries have adopted more precautionary approaches and included socioeconomic considerations in product approvals, such as the coexistence of GE and non-GE cropping systems and consumer right-to-know.
Although such nonsafety issues are not typically considered by U.S. regulatory agencies, they are nevertheless important technology-governance
issues that can be addressed by policy-makers, the private sector, and the public through a variety of governmental and nongovernmental means that take into account competing interests of stakeholders and inherent tradeoffs involved in any decision.
Accuracy and trust are critical for technology governance. The committee renews the advice from prior National Research Council reports to regulatory agencies to expand efforts to include the public in their deliberations and to make their decisions and the information on which they base their decisions as transparent as possible, recognizing the constraints of various laws that protect confidential business information and other sensitive data. Similarly, the committee emphasizes that governance authorities should actively seek public input on decisions, including decisions regarding how to approach emerging genetic-engineering technologies (such as genome editing and synthetic biology) and their regulation.
The power to require continued monitoring or controls after a crop has been approved is a critical tool for regulators, particularly when there are known risks or there is some residual uncertainty at the time of approval. The development of herbicide resistance in weeds might have been mitigated if APHIS had had the authority to make mid-course corrections after there was experience on a commercial scale.
Prior National Research Council reports have argued that there is no strict dichotomy between genetic engineering and other forms of plant breeding with respect to risk. Recent developments in genome editing and other emerging genetic-engineering technologies make it even more apparent that regulatory approaches that focus on some form of breeding “process” as an indicator of risk are less and less technically defensible. Some emerging genetic-engineering technologies are likely to create new crop varieties that are indistinguishable from those developed with conventional plant breeding, whereas other technologies, such as mutagenesis, that are not covered by existing laws could create new crop varieties with substantial changes to plant phenotypes. The size and extent of the genetic transformation has relatively little relevance to the extent of the change in the plant and consequently to the risk that it poses to the environment or to food safety. The committee recommends the development of a tiered approach to regulation that is based not on the breeding process but on considerations of novelty, potential hazard, and exposure as criteria. The application of -omics technologies can help to provide greater assurance that no unintended differences have been introduced by whatever breeding technique is used.
REFERENCES
Annaluru, N., H. Muller, L.A. Mitchell, S. Ramalingam, G. Stracquadanio, S.M. Richardson, J.S. Dymond, Z. Kuang, L.Z. Scheifele, E.M. Cooper, Y. Cai, K. Zeller, N. Agmon, J.S. Han, M. Hadjithomas, J. Tullman, K. Caravelli, K. Cirelli, Z. Guo, V. London, A. Yeluru, S. Murugan, K. Kandevlou, N. Agier, G. Fischer, K. Yang, J.A. Martin, M. Bilgel, P. Bohutskyi, K.M. Boulier, B.J. Capaldo, J. Chang, K. Charoen, W.J. Choi, P. Deng, J.E. DiCarlo, J. Doong, J. Dunn, J.I. Feinberg, C. Fernandez, C.E. Floria, D. Gladowski, P. Hadidi, I. Ishizuka, J. Jabbari, C.Y.L. Lau, P.A. Lee, S. Li, D. Lin, M.E. Linder, J. Ling, J. Liu, M. London, H. Ma, J. Mao, J.E. McDade, A. McMillan, A.M. Moore, W.C. Oh, Y. Ouyang, R. Patel, M. Paul, L.C. Paulsen, J. Qiu, A. Rhee, M.G. Rubashkin, I.Y. Soh, N.E. Sotuyo, A. Srinivas, A. Suarez, A. Wong, R. Wong, W.R. Xie, Y. Xu, A.T. Yu, R. Koszul, J.S. Bader, J.D. Boeke, and S. Chandrasegaran. 2014. Total synthesis of a functional designer eukaryotic chromosome. Science 344:55–58.
Belhaj, K., A. Chaparro-Garcia, S. Kamoun, N.J. Patron, and V. Nekrasov. 2015. Editing plant genomes with CRISPR/Cas9. Current Opinion in Biotechnology 32:76–84.
Bergkamp, L., and L. Kogan. 2013. Trade, the precautionary principle, and post-modern regulatory process. European Journal of Risk Regulation 4:493–507.
CAC (Codex Alimentarius Commission). 2003a. Guideline for the Conduct of Food Safety Assessment of Foods Using Recombinant DNA Plants. Doc CAC/GL 45-2003. Rome: World Health Organization and Food and Agriculture Organization.
CAC (Codex Alimentarius Commission). 2003b. Principles for the Risk Analysis of Foods Derived from Modern Biotechnology. Doc CAC/GL 44-2003. Rome: World Health Organization and Food and Agriculture Organization.
CAC (Codex Alimentarius Commission). 2011. Compilation of Codex Texts Relevant to Labelling of Foods Derived from Modern Biotechnology. Doc CAC/GL 76-2011. Rome: World Health Organization and Food and Agriculture Organization.
Camacho, A., A. Van Deynze, C. Chi-Ham, and A.B. Bennett. 2014. Genetically engineered crops that fly under the US regulatory radar. Nature Biotechnology 32:1087–1091.
Canadian General Standards Board. 2004. Voluntary Labeling and Advertising of Foods That Are and Are Not Products of Genetic Engineering. CAN/CGSB-32.315-2004. Available at http://www.tpsgc-pwgsc.gc.ca/ongc-cgsb/programme-program/normes-standards/internet/032-0315/documents/commite-commmittee-eng.pdf. Accessed April 9, 2016.
Cardoso, T.A.O., M.B.M. Albuquerque Navarro, B.E.C. Soares, F.H. Lima e Silva, S.S. Rocha, and L.M. Oda. 2005. Memories of biosafety in Brazil: Lessons to be learned. Applied Biosafety 10:160–168.
CFIA (Canadian Food Inspection Agency). 1994. Directive 94–08. Assessment Criteria for Determining Environmental Safety Plants with Novel Traits. Available at http://www.inspection.gc.ca/plants/plants-with-novel-traits/applicants/directive-94-08/eng/1304475469806/1304475550733. Accessed December 1, 2015.
CFIA (Canadian Food Inspection Agency). 2000. Directive Dir2000–07: Conducting Confined Research Field Trials of Plant with Novel Traits in Canada. Available at http://www.inspection.gc.ca/plants/plants-with-novel-traits/applicants/directive-dir2000-07/eng/ 1304474667559/1304474738697. Accessed December 1, 2015.
CFIA (Canadian Food Inspection Agency). 2005. DD2005–50: Determination of the Safety of the BASF Canada Imidazolinone-Tolearnt CLEARFIELD™ Sunflower (Helianthus annuus L.) Hybrid X81359. Available at http://www.inspection.gc.ca/plants/plants-withnovel-traits/approved-under-review/decision-documents/dd2005-50/eng/1311804702962/1311804831279. Accessed December 1, 2015.
CFIA (Canadian Food Inspection Agency). 2007. DD2007-66: Determination of the Safety of BASF’s Imidazolinone-Tolerant CLEARFIELD® Wheat Event BW7. Available at http://www.inspection.gc.ca/plants/plants-with-novel-traits/approved-under-review/decisiondocuments/dd2007-66/eng/1310920107214/1310920203993. Accessed December 1, 2015.
CFIA (Canadian Food Inspection Agency.) 2008. DD2008-73: Determination of the Safety of BASF Canada Inc’s Imidazolinone-Tolerant CLEARFIELD® Canola Quality Indian Mustard Event S006. Available at http://www.inspection.gc.ca/plants/plants-with-noveltraits/approved-under-review/decision-documents/dd2008-73/eng/1310605979718/1310606047946. Accessed December 1, 2015.
CFIA (Canadian Food Inspection Agency). 2009. Directive 2009–09: Plants with Novel Traits Regulated under Part V of the Seeds Regulations: Guidelines for Determining when to Notify the CFIA. Available at http://www.inspection.gc.ca/plants/plants-with-noveltraits/applicants/directive-2009-09/eng/1304466419931/1304466812439. Accessed December 1, 2015.
Chambers, J.A., P. Zambrano, J.B. Falck-Zepeda, G.P. Gruère, D. Sengupta, and K. Hokanson. 2014. GM agricultural technologies for Africa: A state of affairs. Washington, DC: International Food Policy Research Institute.
Dessureault, D., and M. Lupescu. 2014. Canada: Agricultural Biotechnology Annual – 2014. U.S. Department of Agriculture–Foreign Agricultural Service. Available at http://gain.fas.usda.gov/Recent%20GAIN%20Publications/Agricultural%20Biotechnology%20Annual_Ottawa_Canada_7-14-2014.pdf. Accessed December 1, 2015.
Driesen, D. 2013. Cost-benefit analysis and the precautionary principle: Can they be reconciled? Michigan State Law Review 771.
EC (European Commission). 2000. Communication from the Commission on the precautionary principle. COM(2000) 1 final. Available at http://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:52000DC0001&from=EN. Accessed April 9, 2016.
EC (European Commission). 2001. Commission improves rules on labeling and tracing of GMOs in Europe to enable freedom of choice and ensure environmental safety. Brussels.
EC (European Commission). 2015a. Commission authorises 17 GMOs for food/feed uses and 2 GM carnations. Available at http://europa.eu/rapid/press-release_IP-15-4843_en.htm. Accessed November 30, 2015.
EC (European Commission). 2015b. Fact sheet: Questions and answers on EU’s policies on GMOs. Available at http://europa.eu/rapid/press-release_MEMO-15-4778_en.htm. Accessed November 30, 2015.
EC (European Commission). 2015c. Review of the decision-making process on GMOs in the EU: Questions and Answers. Available at http://europa.eu/rapid/press-release_MEMO-15-4779_en.htm. Accessed November 30, 2015.
EFSA (European Food Safety Authority). 2010. Guidance on the environmental risk assessment of genetically modified plants. EFSA Journal 8:1879–1989.
EFSA (European Food Safety Authority). 2011a. Scientific opinion on guidance on the post-market environmental monitoring (PMEM) of genetically modified plants. EFSA Journal 9:2316.
EFSA (European Food Safety Authority). 2011b. Scientific opinion on guidance for risk assessment of food and feed from genetically modified plants. EFSA Journal 9:2150.
EFSA (European Food Safety Authority). 2014. Explanatory statement for the applicability of the guidance of the EFSA scientific committee on conducting repeated-dose 90-day oral toxicity study in rodents on whole food/feed for GMO risk assessment. EFSA Journal 12:3871.
Endres, A.B. 2005. Revising seed purity laws to account for the adventitious presence of genetically modified varieties: A first step towards coexistence. Journal of Food Law and Policy 1:131–163.
EPA (U.S. Environmental Protection Agency). 1988. Guidance for the Reregistration of Pesticide Products Containing Bacillus thuringiensis as the Active Ingredient. Washington, DC: EPA.
EPA (U.S. Environmental Protection Agency). 1998. Guidelines for Ecological Risk Assessment. Federal Register 63:26846–26294.
EPA (U.S. Environmental Protection Agency). 2001a. Opportunity to Comment on Implications of Revised Bt Crops Reassessment for Regulatory Decisions Affecting These Products, and on Potential Elements of Regulatory Options; Announcement of Public Meeting. Federal Register 66:37227–37229.
EPA (U.S. Environmental Protection Agency). 2001b. Regulations Under the Federal Insecticide, Fungicide, and Rodenticide Act for Plant-Incorporated Protectants (Formerly Plant-Pesticides); Final Rule. Federal Register 66:37772–37817.
EPA (U.S. Environmental Protection Agency). 2001c. Biopesticides Registration Action Document—Bacillus thuringiensis Plant-Incorporated Protectants. Available at http://www3.epa.gov/pesticides/chem_search/reg_actions/pip/bt_brad.htm. Accessed November 22, 2015.
EPA (U.S. Environmental Protection Agency). 2014a. Final Registration of Enlist Duo™ Herbicide. Available at http://www2.epa.gov/sites/production/files/2014-10/documents/final_registration_-_enlist_duo.pdf. Accessed November 24, 2015.
EPA (U.S. Environmental Protection Agency). 2014b. Minutes of the FIFRA SAP Meeting Held January 28, 2014 on “RNAi Technology as a Pesticide: Problem Formulation for Human Health and Ecological Risk Assessment. Available at http://www.regulations.gov/#!documentDetail;D=EPA-HQ-OPP-2013-0485-0049. Accessed February 8, 2016.
EPA (U.S. Environmental Protection Agency). 2015. EPA Proposal to Improve Corn Rootworm Resistance Management; Notice of Availability. Federal Register 80:4564–4565.
Executive Office of the President. 2011. Executive Order 13563 of January 18, 2011: Improving Regulation and Regulatory Review. Federal Register 76:3821–3823.
FDA (U.S. Department of Health and Human Services–Food and Drug Administration). 1992. Statement of Policy: Foods Derived From New Plant Varieties. Federal Register 57:22984–23005.
FDA (U.S. Department of Health and Human Services–Food and Drug Administration). 1994. Calgene, Inc.; Availability of Letter Concluding Consultation. Federal Register 59:May 23.
FDA (U.S. Department of Health and Human Services–Food and Drug Administration). 2001. Premarket Notice Concerning Bioengineered Foods; Proposed Rule. Federal Register 66:4706–4738.
FDA (U.S. Department of Health and Human Services–Food and Drug Administration). 2015a. Biotechnology Consultations on Food from GE Plant Varieties. Available at http://www.accessdata.fda.gov/scripts/fdcc/?set=Biocon. Accessed April 11, 2016.
FDA (U.S. Department of Health and Human Services-Food and Drug Administration). 2015b. Guidance for Industry: Voluntary Labeling Indicating Whether Foods Have or Have Not Been Derived from Genetically Engineered Plants. Available at http://www.fda.gov/food/guidanceregulation/guidancedocumentsregulatoryinformation/ucm059098.htm#B. Accessed February 16, 2016.
Finardi Filho, F. 2014. Situación de los cultivos MG en Brasil. Available at_http://www.foroagrario.com/140623_BBr/20140623_Finardi.pdf. Accessed December 15, 2015.
Fisher, F. 2009. Democracy and Expertise: Reorienting Policy Inquiry. Oxford: Oxford University Press.
Flint, S., T. Heidel, S. Loss, J. Osborne, K. Prescott, and D. Smith. 2012. Summary and Comparative Analysis of Nine National Approaches to Ecological Risk Assessment of Living Modified Organisms in the Context of the Cartagena Protocol on Biosafety, Annex III. Montreal: Secretariat of the Convention of Biological Diversity.
GAO (General Accounting Office). 2002. Genetically Modified Foods: Experts View Regimen of Safety Tests as Adequate, but FDA’s Evaluation Process Could be Enhanced. Washington, DC: GAO.
Gillam, C. March 31, 2015. EPA will require weed-resistance restrictions on glyphosate herbicide. Online. Reuters. Available at http://www.reuters.com/article/2015/03/31/usmonsanto-herbicide-weeds-idUSKBN0MR2JT20150331#qsv4p0WODueFQIF8.97. Accessed November 30, 2015.
Glaser, J.A., and S.R. Matten. 2003. Sustainability of insect resistance management strategies for transgenic Bt corn. Biotechnology Advances 22:45–69.
Hammit, J., M. Rogers, P. Sand, and J.B. Wiener, eds. 2013. The Reality of Precaution: Comparing Risk Regulation in the United States and Europe. New York: RFF Press.
Hartung, F., and J. Schiemann. 2014. Precise plant breeding using new genome editing techniques: Opportunities, safety and regulation in the EU. The Plant Journal 78:742–752.
Hatanaka, M. and J. Konefal. 2013. Legitimacy and standard development in multi-stakeholder initiatives: A case study of the Leonardo Academy’s sustainable agriculture standard initiative. International Journal of Sociology of Agriculture and Food 20:155–173.
Health Canada. 1994, amended 2006. Guidelines for the Safety Assessment of Novel Foods. Available at http://www.hc-sc.gc.ca/fn-an/legislation/guide-ld/nf-an/guidelineslignesdirectrices-eng.php. Accessed December 1, 2015.
Health Canada. 2015. GM Foods and Their Regulation. Available at http://www.hc-sc.gc.ca/fn-an/gmf-agm/fs-if/gm-foods-aliments-gm-eng.php. Accessed April 29, 2015.
Henckels, C. 2006. GMOs in the WTO: A critique of the panel’s legal reasoning in EC—Biotech. Melbourne Journal of International Law 7:278–305.
Horna, D., P. Zambrano, and J.B. Falck-Zepeda, eds. 2013. Socioeconomic Considerations in Biosafety Decisionmaking: Methods and Implementation. Washington, DC: International Food Policy Research Institute.
Housenger, J.E. 2015. EPA’s Perspective on Herbicide Resistance in Weeds. Presentation at USDA Stakeholder Workshop on Coexistence, March 12, Raleigh, NC.
Iowa State University. 2015. Resistance Management: Whose Problem and Whose Job? Summary Report. Available at http://www.ipm.iastate.edu/files/page/files/Resistance%20Management%20Meeting%20Summary%20Report%202015_0.pdf. Accessed February 9, 2016.
Jones, H. 2015. Regulatory uncertainty of genome editing. Nature Plants 1:1–3.
Jornal DCI (Diário Comércio Indústria & Serviços/Sao Paulo). August 17, 2012. Justiça manda Nestlé avisar sobre transgênicos. Online. Available at http://www.dci.com.br/capa/justica-manda-nestle-avisar-sobre-transgenicos-id307870.html. Accessed December 3, 2015.
Klein, T.M., E.D. Wolf, R. Wu, and J.C. Sanford. 1987. High-velocity microprojectiles for delivering nucleic acids into living cells. Nature 327:70–73.
Klinke, A., and O. Renn. 2002. A new approach to risk evaluation and management: Risk-based, precaution-based, and discourse-based strategies. Risk Analysis 22:1071–1094.
Kosuri, S., and G.M. Church. 2014. Large-scale de novo DNA synthesis: Technologies and applications. Nature Methods 11:499–507.
Liberatore, A., and S. Funtowicz. 2003. Democratising expertise, expertising democracy: What does it mean, and why bother? Science and Public Policy 30:146–150.
Liebsch, M., B. Grune, A. Seiler, D. Butzke, M. Oelgeschläger, R. Pirow, S. Adler, C. Riebeling, and A. Luch. 2011. Alternatives to animal testing: Current status and future perspectives. Archives of Toxicology 85:841–858.
Liu, W., J.S. Yuan, and C.N. Stewart, Jr. 2013. Advanced genetic tools for plant biotechnology. Nature Reviews Genetics 14:781–793.
Liu, W., and C.N. Stewart, Jr. 2015. Plant synthetic biology. Trends in Plant Science 20:309–317.
Liu, W., and C.N. Stewart, Jr. 2016. Plant synthetic promoters and transcription factors. Current Opinion in Biotechnology 37:36–44.
Liu, W., M. Mazarei, Y. Peng, M.H. Fethe, M.R. Rudis, J. Lin, R.J. Millwood, P.R. Arelli, and C.N. Stewart, Jr. 2014. Computational discovery of soybean promoter cis-regulatory elements for the construction of soybean cyst nematode-inducible synthetic promoters. Plant Biotechnology Journal 12: 1015–1026.
Ludlow, K., S.J. Smyth, and J. Falck-Zepeda. 2013. Socio-Economic Considerations in Biotechnology Regulation. New York: Springer-Verlag.
Lusser, M., C. Parisi, D. Plan, D., and E. Rodríguez-Cerezo. 2012. Deployment of new biotechnologies in plant breeding. Nature Biotechnology 30:231–239.
Lusser, M., and H.V. Davies. 2013. Comparative regulatory approaches for groups of new plant breeding techniques. New Biotechnology 30:437–446.
Mahfouz, M.M., A. Piatek, and C.N. Stewart, Jr. 2014. Genome engineering via TALENs and CRISPR/Cas9 systems: Challenges and perspectives. Plant Biotechnology Journal 12:1006–1014.
Marchant, G.E., and K.L. Mossman. 2004. Arbitrary and Capricious: The Precautionary Principle in the European Union Courts. Washington, DC: AEI Press.
Marchant, G., L. Abbott, A. Felsot, and R. Griffin. 2013. Impact of the precautionary principle on feeding current and future generations. CAST Issue Paper 52-QC. Available at http://www.cast-science.org/download.cfm?PublicationID=276208&File=1030df6c4bf9e6d2086d211a3c242a317a7cTR. Accessed February 9, 2016.
Marcoux, J.M., and L. Létourneau. 2013. A distorted regulatory landscape: Genetically modified wheat and the influence of non-safety issues in Canada. Science and Public Policy 40:514–528.
Marx-Stoelting, P., A. Braeuning, T. Buhrke, A. Lampen, L. Niemann, M. Oelgeschlaeger, S. Rieke, F. Schmidt, T. Heise, R. Pfeil, and R. Solecki. 2015. Application of omics data in regulatory toxicology: Report of an international BfR expert workshop. Archives in Toxicology 89:2177–2184.
Medlock, J., R. Downey, and E. Einsiedel. 2007. Governing controversial technologies: Consensus conferences as a communications tool. Pp. 308–326 in The Public, The Media and Agricultural Biotechnology, D. Brossard, J. Shanahan, and T.C. Nesbitt, eds. Cambridge, MA: CAB International.
Mendonça-Hagler, L., L. Souza, L. Aleixo, and L. Oda. 2008. Trends in biotechnology and biosafety in Brazil. Environmental Biosafety Research 7:115–121.
Migone, A., and M. Howlett. 2009. Classifying biotechnology-related policy, regulatory and innovation regimes: A framework for the comparative analysis of genomics policy-making. Policy and Society 28:267–278.
Miller, H.I., and D.L. Kershen. 2011. A label we don’t need. Nature Biotechnology 29:971–972.
Millstone, E., A. Stirling, and D. Glover. 2015. Regulating genetic engineering: The limits and politics of knowledge. Issues in Science and Technology 31:23–26.
Munch, R. 1995. The political regulation of technological risks: A theoretical and comparative analysis. International Journal of Comparative Sociology 36:109–130.
NAS (National Academy of Sciences). 1987. Introduction of Recombinant DNA-Engineered Organisms into the Environment: Key Issues. Washington, DC: National Academy Press.
NBT Platform. 2013. Legal briefing paper: The regulatory status of plants resulting from New Breeding Technologies. Available at http://www.nbtplatform.org/background-documents/legal-briefing-paper---the-regulatory-status-of-plants-resulting-from-nbts-final-.pdf. Accessed November 10, 2015.
NGFA (National Grain and Feed Association). 2015. USDA Grants First Process Verified Claim for Non-GMO Corn, Soybeans. May 29. http://www.ngfa.org/2015/05/29/usdagrants-first-process-verified-claim-for-non-gmo-corn-soybeans/. Accessed June 17, 2015.
NRC (National Research Council). 1983. Risk Assessment in the Federal Government: Managing the Process. Washington, DC: National Academy Press.
NRC (National Research Council). 1989. Field Testing Genetically Modified Organisms: Framework for Decisions. Washington, DC: National Academy Press.
NRC (National Research Council). 1994. Science and Judgment in Risk Assessment. Washington, DC: National Academy Press.
NRC (National Research Council). 1996. Understanding Risk: Informing Decisions in a Democratic Society. Washington, DC: National Academy Press.
NRC (National Research Council). 2000. Genetically Modified Pest-Protected Plants: Science and Regulation. Washington, DC: National Academy Press.
NRC (National Research Council). 2002. Environmental Effects of Transgenic Plants: The Scope and Adequacy of Regulation. Washington, DC: National Academy Press.
NRC (National Research Council). 2004. Safety of Genetically Engineered Foods: Approaches to Assessing Unintended Health Effects. Washington, DC: National Academies Press.
NRC (National Research Council). 2009. Science and Decisions: Advancing Risk Assessment. Washington, DC: National Academies Press.
NRC (National Research Council). 2015. Industrialization of Biology: A Roadmap to Accelerate the Advanced Manufacturing of Chemicals. Washington, DC: National Academies Press.
OECD (Organisation for Economic Co-operation and Development). 1986. Recombinant DNA Safety Considerations. Paris: OECD.
OECD (Organisation for Economic Co-operation and Development). 1993. Safety Considerations for Biotechnology: Scale-up of Crop Plants. Paris: OECD.
OSTP (Executive Office of the President, Office of Science and Technology Policy). 1986. Coordinated Framework for Regulation of Biotechnology. Federal Register 51:23302. Available at https://www.aphis.usda.gov/brs/fedregister/coordinated_framework.pdf. Accessed December 18, 2015.
OSTP (Executive Office of the President, Office of Science and Technology Policy). 1992. Exercise of Federal Oversight Within Scope of Statutory Authority: Planned Introductions of Biotechnology Products into the Environment. Federal Register 57:6753–6762.
OSTP (Executive Office of the President, Office of Science and Technology Policy). 2015. Memorandum for Heads of Food and Drug Administration, Environmental Protection Agency, and Department of Agriculture. Available at https://www.whitehouse.gov/sites/default/files/microsites/ostp/modernizing_the_reg_system_for_biotech_products_memo_final.pdf. Accessed September 25, 2015.
Oye, K.A., K. Esvelt, E. Appleton, F. Catteruccia, G. Church, T. Kuiken, S. B.-Y. Lightfood, J. McNamara, A. Smidler, and J.P. Collins. 2014. Regulating gene drives. Science 345:626–628.
Paarlberg, R.L. 2000. Governing the GM Crop Revolution: Policy Choices for Developing Countries. Washington, DC: International Food Policy Research Institute.
Rabesandratana, T. 2015. Europe’s food watchdog embraces transparency. Science 350:368.
Rehder, L.E. 2015. Germany: CRISPR and other NBT’s classified as GMOs. Report GM15034, October 30, 2015. U.S. Department of Agriculture–Foreign Agricultural Service. Available at http://gain.fas.usda.gov/Recent%20GAIN%20Publications/CRISPR%20and%20other%20NBT%E2%80%99s%20classified%20as%20GMO%E2%80%98s_Berlin_Germany_10-30-2015.pdf. Accessed December 4, 2015.
Renn, O., and C. Benighaus. 2013. Perception of technological risk: Insights from research and lessons for risk communication and management. Journal of Risk Research 16:293–313.
Rowe, G., and L.J. Frewer. 2005. A typology of public engagement mechanisms. Science, Technology & Human Values 30:251–290.
Schnepf, R. 2003. Genetically Engineered Soybeans: Acceptance and Intellectual Property Rights Issues in South America. Washington, DC: Congressional Research Service.
Silva, J.F. 2014. Brazil: Agricultural Biotechnology Annual – 2014. U.S. Department of Agriculture–Foreign Agricultural Service. Available at http://gain.fas.usda.gov/Recent%20GAIN%20Publications/Agricultural%20Biotechnology%20Annual_Brasilia_Brazil_7-8-2014.pdf. Accessed December 4, 2015.
Smith, M.J., and A.J. Smith. 2013. 2012 Insect Resistance Management (IRM) Compliance Assurance Program Report for Corn Borer-Protected Bt Corn, Corn Rootworm-Protected Bt Corn, Corn Borer/Corn Rootworm-Protected Stacked and Pyramided Bt Corn. Available at http://www.regulations.gov/#!documentDetail;D=EPA-HQ-OPP-2011-0922-0040. Accessed December 15, 2015.
Smyth, S.J., and A. McHughen. 2012. Regulation of genetically modified crops in USA and Canada: Canadian Overview. Pp. 15–34 in Regulation of Agricultural Biotechnology: The United States and Canada, C. Wozniak and A. McHughen, eds. Heidelberg, Germany: Springer.
Soares, E. 2014. Restrictions on Genetically Modified Organisms: Brazil. Washington, DC: Law Library of Congress.
Stirling, A. 2008. Science, precaution, and the politics of technological risk: Converging implications in evolutionary and social scientific perspectives. Annals of the New York Academy of Sciences 1128:95–110.
Strom, S. April 26, 2015. Chipotle to stop using genetically altered ingredients. Online. New York Times. Available at http://www.nytimes.com/2015/04/27/business/chipotle-to-stopserving-genetically-altered-food.html. Accessed November 5, 2015.
Tamm Hallström, K., and M. Boström. 2010. Transnational Multi-stakeholder Standardization: Organizing Fragile Non-state Authority. Cheltenham, UK: Edward Elgar.
Thomas, K., and S. Yarrow. 2012. Regulating the environmental release of plans with novel traits in Canada. Pp. 147–162 in Regulation of Agricultural Biotechnology: The United States and Canada, C. Wozniak and A. McHughen, eds. Heidelberg, Germany: Springer.
Turner, J. 2014. Regulation of Genetically Engineered Organisms at USDA–APHIS. Presentation to the National Academy of Sciences’ Committee on Genetically Engineered Crops: Past Experience and Future Prospects, December 10, Washington, DC.
UNEP (United Nations Environment Programme). 2014. Convention on Biological Diversity: Ad Hoc Technical Expert Group on Risk Assessment and Risk Management Under the Cartagena Protocol on Biosafety. Bonn, 2-6 June 2014. Available at https://www.cbd.int/doc/meetings/bs/bsrarm-05/official/bsrarm-05-01-en.pdf. Accessed February 16, 2016.
USDA Advisory Committee. 2012. Enhancing Coexistence: A Report of the AC21 to the Secretary of Agriculture. Available at http://www.usda.gov/documents/ac21_report-enhancingcoexistence.pdf. Accessed June 16, 2015.
USDA–APHIS (U.S. Department of Agriculture–Animal and Plant Health Inspection Service). 1987. Plant Pests; Introduction of Genetically Engineered Organisms or Products; Final Rule, Federal Register 52:22892–22815.
USDA–APHIS (U.S. Department of Agriculture–Animal and Plant Health Inspection Service). 2011. Dow AgroSciences Petition (09-233-01p) for Determination of Nonregulated Status of Herbicide-Tolerant DAS-40278-9 Corn, Zea mays, Event DAS-40278-9: Draft Environment Assessment. Available at https://www.aphis.usda.gov/brs/aphisdocs/09_23301p_dea.pdf. Accessed November 30, 2015.
USDA–APHIS (U.S. Department of Agriculture–Animal and Plant Health Inspection Service). 2015. APHIS announces withdrawal of 2008 Proposed Rule for Biotechnology Regulations. Available at https://www.aphis.usda.gov/stakeholders/downloads/2015/sa_withdrawal.pdf. Accessed December 4, 2015.
USDA–APHIS (U.S. Department of Agriculture–Animal and Plant Health Inspection Service). 2016. Environmental Impact Statement; Introduction of the Products of Biotechnology. Federal Register 81:6225–6229.
Vogel, D. 2012. The Politics of Precaution: Regulating Health, Safety, and Environmental Risks in Europe and the United States. Princeton, NJ: Princeton University Press.
Von Schomberg, R. 2012. The precautionary principle: Its use within hard and soft law. European Journal of Risk Regulation 2:147–156.
Voytas, D.F., and C. Gao. 2014. Precision genome engineering and agriculture: Opportunities and regulatory challenges. PLoS Biology 12:e1001877.
Wesseler, J. 2014. Biotechnologies and agrifood strategies: Opportunities, threats, and economic implications. Bio-based and Applied Economics 3:187–204.
WTO (World Trade Organization). 2006. Dispute Settlement. Dispute DS291. European Communities-Measures Affecting Approval and Marketing of Biotech Products. Panel Report.
Yu, Q., A. Jalaludin, H. Han, M. Chen, R.D. Sammons, and S.B. Powles. 2015. Evolution of a double amino acid substitution in the 5-enolpyruvylshikimate-3-phosphate synthase in Eleusine indica conferring high-level glyphosate resistance. Plant Physiology 167:1440–1447.




































































