3
Genetically Engineered Crops Through 2015
Having laid the groundwork for its approach to risk and benefit assessment, introduced the major actors operating in the sphere of governance of genetically engineered (GE) crops, and defined the terms commonly used in its report, the committee turns in this chapter to its first charge in the statement of task: an examination of the history of the development and introduction of GE crops, both in and outside the United States. The examination includes not only GE crops that were available in 2015 but GE crops that were developed but not commercialized, GE crops that entered the market but were withdrawn or discontinued, and crops with GE traits that were developed and near market release as of 2015. It also gives an introduction of the government processes that have emerged to regulate GE crops.
THE DEVELOPMENT OF GENETIC ENGINEERING IN AGRICULTURE
People have been domesticating plants for at least 10,000 years. Early plant domestication involved selecting individual plants, fruits, seeds, inflorescences, or other propagules for characteristics of interest. Selected characteristics (traits) included higher yields, reduced toxicity, improved flavor or morphology of seeds or fruits, and seed heads (in grains) or pods (in legumes) that did not shatter and were therefore easier to harvest. Selection permitted people to domesticate numerous wild plants into crops, such as wheat (Triticum aestivum), rice (Oryza sativa), maize (Zea mays), potato (Solanum tuberosum), and tomato (Solanum lycopersicum).
One of the most vivid examples of domestication is maize (corn). Beginning some 6,000–10,000 years ago, ancient Meso-American farmers drastically changed teosinte (Zea mays subsp. parviglumis) through selection (Figure 3-1). Teosinte is a grass species that has numerous lateral branches and cobs with 5–12 individually encapsulated kernels that drop to the ground when ripe. Through human selections based on very rare, desirable attributes caused by naturally occurring mutations, a plant was derived with no lateral branching (that is, a single stalk) and a cob with dozens or even hundreds of large seeds (kernels) that were encased in husk leaves; this resulted in the maize that is grown today (Doebley, 2004; Flint-Garcia, 2013; Wang et al., 2015).
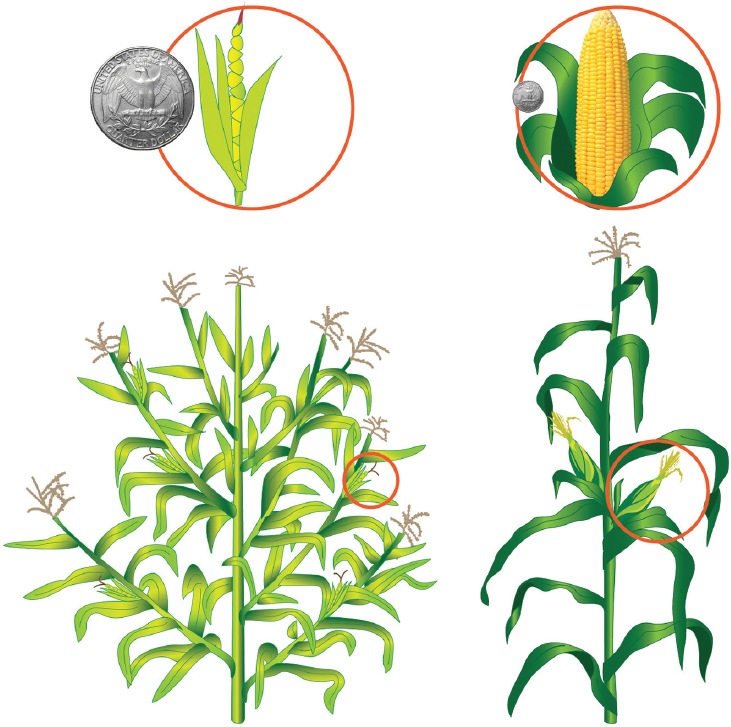
SOURCE: Based on Fuller (2005).
NOTE: The U.S. quarter coin is included for scale (about 2 centimeters in diameter).
Domestication of wild Solanum species native to the American continents through the selection of altered fruit size, fruit shape, seed size, and taste led to the tomato (Bai and Lindhout, 2007); wild tomatoes are generally neither large nor tasty. The progenitors of carrot (Daucus carota subsp. sativus) were woody, gnarly, and white, rather than tasty, uniformly shaped, and orange. Developed first in France and later in the United States, today’s strawberries (Fragaria × ananassa) descend from hybrids of two species, one of which was prized for its flavor (originally found in what is now the U.S. state of Virginia) and the other for its size (grown off the coast of Chile).
The modern era of genetics and plant breeding can be traced to Darwin’s theory of evolution and natural selection and to Mendel’s elucidation of the basic principles of heredity in the mid-1800s. The application of the basic principles of heredity to use the genetic variation (biodiversity) available in a species is a cornerstone of plant breeding. Genetic variation arises naturally in a crop from mutation (changes in the DNA sequence of an individual), recombination of the alleles (variants of a gene) in an individual through sexual reproduction, and introgression of new genes or alleles from a donor species.
Research in the late 19th and early 20th centuries led to a better understanding of genetics, and plant breeders applied this knowledge with increasing precision. They deliberately changed the expression of traits in plants by crossing specific parent plants to produce offspring that had the desired traits. They also discovered methods to accelerate the generation and detection of genetic variation, and this led to targeted and more efficient breeding of improved varieties (for review, see Mba, 2013). DNA mutation is relatively rare in nature (Ossowski et al., 2010), but scientists found that they could use chemicals or radiation to induce mutations in DNA at a much greater frequency (Roychowdhury and Tah, 2013) and thereby increase the genetic variation in the species.1 Natural and human-made mutations are random (in that they can affect any gene), so breeders must evaluate the progeny so that they can discard individuals that have undesirable or even harmful traits and select individuals that have improved characteristics to develop further.
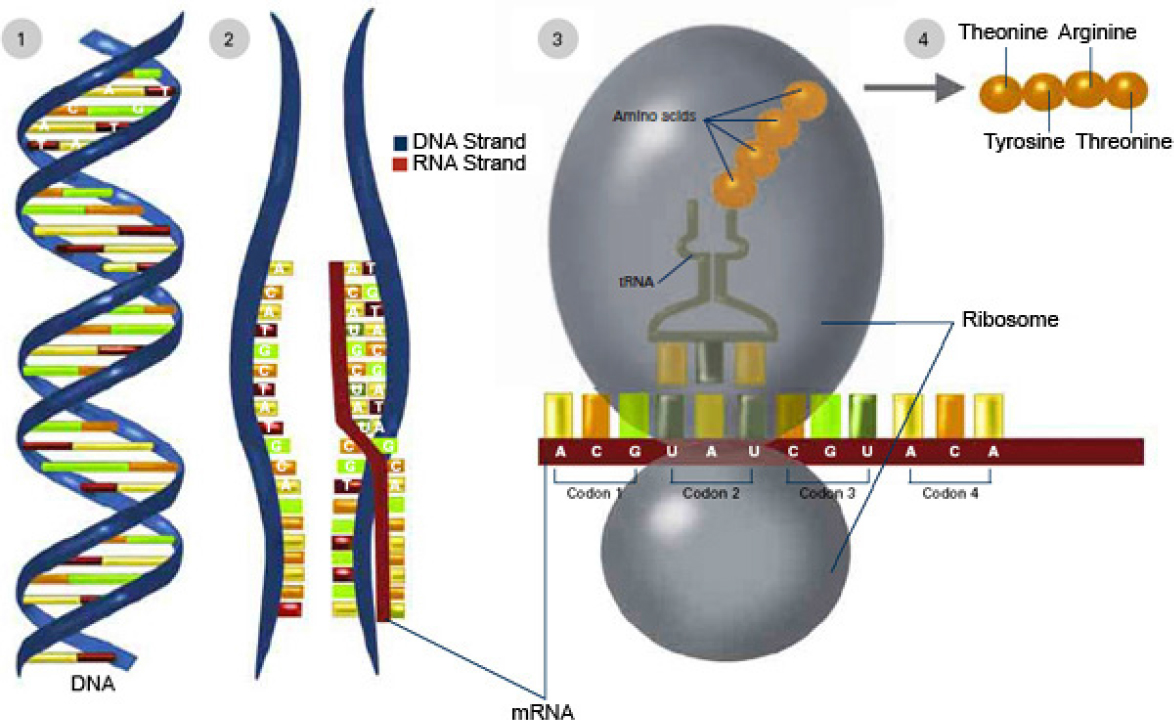
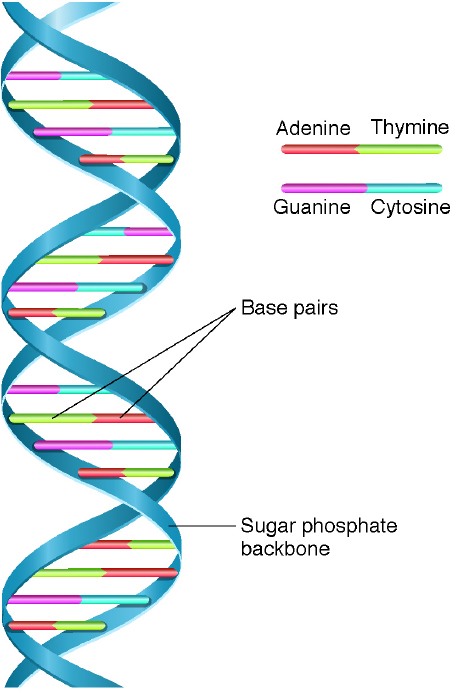
Nearly a century after the discoveries by Darwin and Mendel, Watson and Crick were awarded the 1962 Nobel prize in medicine for discovering the double-helix structure of DNA (Figure 3-2). Holley, Khorana, and Nirenberg received the 1968 Nobel prize in physiology or medicine for deciphering the genetic code related to protein synthesis. Three-base sequences in DNA specify amino acids. These sequences, or “words,” form templates
__________________
1 Ionizing radiation was used to produce several varieties of rice, wheat, barley (Hordeum vulgare), and maize (Roychowdhury and Tah, 2013) and the red-fruited Ruby Sweet and Rio Star grapefruits (Citrus paradisi) (see http://www.texasweet.com/texas-grapefruits-andoranges/texas-grapefruit-history/. Accessed September 18, 2015).
SOURCE: Based on illustration by the U.S. National Library of Medicine.
NOTE: DNA is a molecule that consists of a chain of nucleotides, which are composed of sugar, phosphate, and one of four bases per nucleotide: adenine, guanine, thymine, and cytosine (A, G, T, and C). The backbone of the molecule is a string of sugar and phosphate. A base—either an A, a G, a T, or a C—sticks out from each of the sugars. The two strands are held together by weak bonds between the bases: A binds with T, and G binds with C. Thus, each strand is complementary to the other.
that align amino acids into specific proteins; genes are long “sentences” of those three-letter “words” (Figure 3-3). In 1973, when Cohen and colleagues described recombinant-DNA (rDNA) techniques that allowed scientists to cut gene sequences from the DNA of one organism and splice them into the DNA of another organism (Cohen et al., 1973), the path was paved for a new approach to increase genetic diversity for use in breeding organisms, including crops: genetic engineering.
The development of GE plants was the product of convergence of several discoveries and technological developments. In addition to the development of rDNA technologies in the early 1970s, genetic engineering in plants required the ability to manipulate plant cells via tissue culture effectively and a fundamental understanding of crown gall disease biology to enable Agrobacterium-mediated gene transfer to plants.
Tissue culture is a way to maintain, grow, and manipulate cells, tissues, and organs in vitro. Its use in plants dates at least to 1902 with Haberlandt’s research in Germany (Haberlandt, 1902). Plant tissue culture was developed further in numerous laboratories in the first half of the 20th century, and Murashige and Skoog (1962) published the recipe for what has become the most used tissue-culture medium in plant biotechnology. Even though the MS (Murashige and Skoog) medium was developed for tobacco (Nicotiana tabacum), it proved effective for many plant taxa. By the time of the rDNA revolution in the 1970s, plant biologists were able to manipulate single cells and tissues of tobacco and other species in culture routinely to produce whole plants. Those developments led to the possibility of selecting and regenerating GE plants from GE cells.
Plants regenerated in tissue culture sometimes vary widely in phenotype (appearance) from the source plant and from each other, and the term somaclonal variation was established to refer collectively to such phenotypic variation (Larkin and Scowcroft, 1981). Early explanations of somaclonal variation included several types of genetic changes (mutations), but later evidence also pointed to multiple types of epigenetic changes (Neelakandan and Wang, 2012). When mutation occurs, it reduces the efficiency of obtaining useful GE plants.2 Plant biotechnologists manage that situation by producing a large number of GE parent lines or clones and selecting ones in which gene expression and phenotype are desirable. In cases in which a desirable GE line has unwanted mutations, elite germplasm is not amenable to genetic transformation, or the GE trait is desired in an array of different germplasms, the initial GE plant is crossed into plants with the desired genetic background and the backcrossing process is continued with selection for the introduced DNA until most or all genetic mutations, epigenetic changes, or undesired traits have been removed.
The crown gall story also begins in the early 1900s, when a type of plant tumor was determined to be caused by a specific bacterium, Agrobacterium tumefaciens. In the 1940s, the discovery that tumor cells retained tumorous properties in the absence of Agrobacterium led to the idea that the bacterium could cause a permanent genetic change in plant cells. The mechanism of the genetic change was elucidated in the 1970s. Agrobacterium transfers DNA
__________________
2 The rates of mutagenicity vary greatly among plant species and conditions (Jiang et al., 2011; Stroud et al., 2013).
SOURCE: National Institute of General Medical Sciences (2010).
NOTE: To express a genetic trait, information contained in DNA is copied (transcribed) into a molecule known as ribonucleic acid, or RNA. RNA specifies the synthesis of proteins. Thus, DNA carries the instructions for proteins, which are chainlike molecules (polymers) that are composed of sequences of amino acids. The genetic information is expressed when DNA (1) is transcribed to RNA (2). During transcription, a strand of DNA serves as a template for the formation of a complementary strand of messenger RNA (mRNA). Next, the messenger RNA moves from the cell nucleus to the cytoplasm, where ribosomes attach to the messenger RNA (3) and direct protein synthesis by reading the genetic code and building a chain of amino acids (4). The chain of amino acids forms a protein, which is responsible for or participates in the manifestation of one or more traits.
from a portion of a large tumor-inducing (Ti) plasmid into plant cells.3 The portion of the Ti plasmid that is transferred is known as the transfer DNA (T-DNA), and it contains genes that—when expressed in plant cells—cause tumorous growth. It also contains genes that subvert plant metabolism to benefit the bacteria. By the late 1970s, pioneering scientists found that they could remove the genes normally transferred by Agrobacterium that cause crown gall disease and replace them with genes that they wished to insert into plants cells, thus establishing the bacterium as a useful vector for plant genetic engineering. Soon they were genetically engineering plants using Agrobacterium-mediated transformation of genes cloned into the T-DNA of the Ti plasmid.
In the early 1980s, it was clear that genetic engineering could have a considerable impact on plant agriculture. A reader perusing the expert scientific commentary and review papers of that time, typified by Barton and Brill (1983) and NRC (1984), would probably conclude that researchers had an extensive list of traits that might be endowed in crops by genetic engineering and were optimistic that crop improvements would ensue rapidly. Barton and Brill predicted that improvements could be made via genetic engineering to address insect control (with the use of Bacillus thuringiensis [Bt] genes), herbicide resistance for weed control, and resistance to drought and other stresses. The final sentence in their article sums up the optimism of the era: “The future of plant genetic engineering will be exciting, as much because of the applications we cannot yet predict as because of those already expected.”
Throughout the 1980s, academic laboratories and companies set out to produce plants that could be released as commercial products. The United States approved the first GE crops for release into the environment in 1985.4 By 1988, the company Calgene had received approval from the U.S. government to field test what would eventually be known as the FLAVR SAVR™ tomato, a GE tomato that had a trait for delayed ripening. That tomato would later be the first GE crop grown for commercial sale after the 1994 growing season. In 1989, Monsanto Company received permits to field test soybean (Glycine max) that was resistant to the herbicide glyphosate and that was first sold commercially in the United States in 1996.5
__________________
3 A plasmid is a genetic structure in a bacterial cell that is physically separated from chromosomal DNA and can replicate independently.
4 The first release-into-the-environment permit (found in the U.S. Department of Agriculture database hosted at www.isb.vt.edu) was granted to the company Agracetus and included GE maize, cotton, potato, soybean, tobacco, and tomato for a trait that was undisclosed because of confidential business information.
5 Glyphosate is sold by Monsanto under the trademarked name Roundup. Soybean with the GE glyphosate-resistant trait sold by Monsanto is known as Roundup Ready soybean.
GE crop development from the 1980s to 2015 relied predominantly on the three key technologies discussed above: recombinant DNA, tissue culture, and Agrobacterium-mediated cell transformation. Another important tool, microprojectile bombardment, emerged in the latter half of the 1980s. Also known as biolistics or the gene-gun method, it was developed at least in part to increase the number of plant taxa that could be genetically engineered (Klein et al., 1987). The gene gun was invented by Sanford and colleagues at Cornell University. Various devices were engineered to accelerate micrometer-sized gold or tungsten particles, which were coated with DNA, to pierce plant cells for transformation. The biolistics device that was commercialized for plant transformation uses helium pressure to accelerate microprojectiles through a vacuum chamber to bombard plant tissue in Petri plates. Particle bombardment serves as a second reliable tool for genetic engineering, but many economically important crops that were thought to be nontransformable by Agrobacterium—such as maize—were later transformed routinely by using this bacterium (Gelvin, 2003). Almost all plant taxa (including ferns) have been shown to be amenable to Agrobacterium-mediated transformation (Muthukumar et al., 2013), although in some species only a few genotypes can be transformed efficiently.
GENETICALLY ENGINEERED CROPS IN THE EARLY 21ST CENTURY
Genetic engineering is a rapidly evolving technology. In 2015, Agrobacterium-mediated transformation described in the section above was being overtaken by new approaches (discussed in Chapter 7). This section reviews the crops and traits that had been developed and identifies where they were grown (if they were in commercial production) at the time this report was written.
Global Distribution of Genetically Engineered Crops
About 12 percent (179.7 million of 1.5 billion hectares) of global cropland produced GE crops in 2015 (FAO, 2015; James, 2015). Data for 2015 show that GE varieties were commercially available for nine food crops, three nonfood crops, and two types of flowers. GE maize and soybean were the most widely grown GE crops.
Production of GE maize has increased substantially since its first commercial release in 1996, when fewer than 300,000 hectares were planted (James, 1997). By 2006, 25.2 million hectares were in production worldwide, and the area more than doubled to 53.7 million hectares by 2015, representing one-third of all land planted to maize worldwide that year (James, 2006, 2015).
GE varieties dominated the soybean market in 2015; they were grown on about 80 percent of the 118 million hectares of soybean harvested in that year (James, 2015; USDA, 2016). As with maize, adoption of GE soybean varieties increased quickly after they were introduced in 1996. In 2001, 33 million hectares were grown globally (James, 2002); by 2015, over 92 million hectares were planted with GE varieties (James, 2015).
The seven other food crops of which GE varieties were grown in 2015 were apple (Malus domestica), canola (Brassica napus), sugar beet (Beta vulgaris), papaya (Carica papaya), potato, squash (Cucurbita pepo), and eggplant (Solanum melongena) (James, 2015). The contribution of GE varieties to the production of those crops was small, except for canola; GE varieties of canola constituted 24 percent of the 36 million hectares planted in 2015 (James, 2015).
With regard to crops that are mostly or entirely grown for nonfood uses, GE varieties of alfalfa (Medicago sativa), cotton (Gossypium hirsutum), and poplar (Populus spp.) were grown in 2015. Genetic engineering had also been used to change the color of carnations (Dianthus caryophyllus) and roses (Rosa spp.) that were sold commercially (S. Chandler, RMIT University, personal communication, December 7, 2015).
Production of GE crops in 2015 was distributed unevenly around the world (Figure 3-4). The United States produced 10 crops with GE varieties, followed by Canada with four. GE maize, soybean, and cotton were grown in many countries, whereas GE varieties of alfalfa, apple, poplar, potato, squash, and eggplant were grown in just one country each. Over 70 million of the 179.7 million hectares producing GE crops were in the United States.6 GE crops produced in Brazil, Argentina, India, and Canada accounted for another 91.3 million hectares. The remaining 17.5 million hectares were spread among 23 countries.
In 2015, an alfalfa variety with reduced lignin was also being readied for the U.S. market, and Brazil had approved GE common bean (Phaseolus vulgaris) and GE eucalyptus (Eucalyptus spp.) for commercialization. GE varieties of rice, wheat, sorghum (Sorghum bicolor), and cassava (Manihot esculenta) were in various stages of development; the same was true for banana (Musa spp.), camelina (Camelina sativa), citrus (Citrus spp.), chickpea (Cicer arietinum), cowpea (Vigna unguiculata), groundnut (Arachis hypogaea), mustard (Brassica spp.), pigeon pea (Cajanus cajan), and safflower (Carthamus tinctorius) (James, 2014). A blight-resistant American chestnut (Castanea dentata) was also in progress. Many of those crops and traits are further discussed in Chapter 8.
__________________
6 Seventy million hectares is roughly half of all U.S. cropland. Thus, about 50 percent of cropland in the United States was producing GE crops when the committee was writing its report (Fernandez-Cornejo et al., 2014).
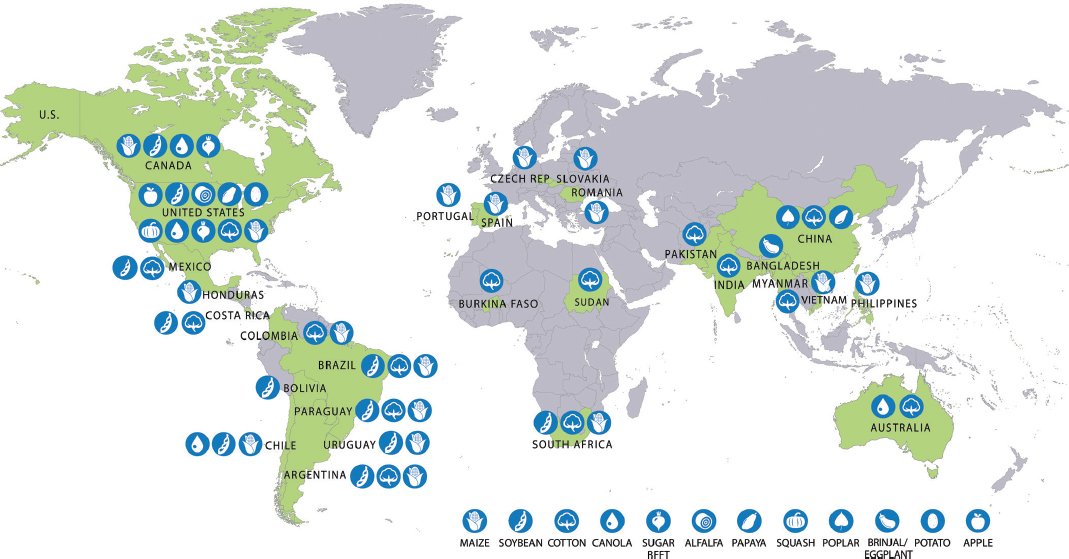
SOURCE: Adapted from James (2014, 2015).
NOTE: In addition to the crops depicted on the map, GE carnations (engineered for novel flower color) were grown in Colombia, Ecuador, and Australia and sold on wholesale cut flower markets in Canada, the United States, the European Union, Japan, Australia, Russia, and the United Arab Emirates (S. Chandler, RMIT University, personal communication, December 7, 2015; Florigene Flowers: Products. Available at http://www.florigene.com/product/. Accessed December 15, 2015). GE roses have been grown and commercially sold in Japan (S. Chandler, RMIT University, personal communication, December 7, 2015).
Genetically Engineered Traits in Commercially Produced Crops
As shown in Figure 3-4, 14 GE crops were in commercial production in 2015. However, GE crops can have one or more GE traits. For example, some varieties of soybean in the United States have been engineered to withstand one or more herbicides, whereas other varieties have been altered to produce more oleic oil (Table 3-1). GE maize varieties in the United States may be engineered to resist one or more herbicides and also contain several insecticidal proteins targeted at different species of insect pests (Box 3-1). Some maize varieties include a trait to enhance drought tolerance. Some crops are engineered to resist viruses, and others to delay ripening. Thus, describing a crop as “GE” is not informative about the purpose of the genetic alteration to the plant. This section reviews the commercialized GE traits in crops produced in 2015.
Herbicide Resistance
A herbicide-resistant (HR) trait allows a GE crop to survive application of a herbicide that would otherwise damage or kill a susceptible plant. In 2015, HR traits had been developed for nine different herbicides: eight HR traits for soybeans, six for cotton, five for maize, two for canola, two for sugar beet, and one for alfalfa (Table 3-1), but not all trait–crop combinations were in commercial production. For example, glufosinate-resistant sugar beet had been developed but was not commercially produced when the committee was writing its report. Some crop varieties that had stacked traits for resistance to two herbicides (for example, glyphosate and 2,4-D or glyphosate and dicamba) were in commercial development in 2015. However, in 1996–2015, most HR crops were engineered for resistance to only one herbicide, and the most common herbicide–HR crop combination used during that time was glyphosate with a glyphosate-resistant crop. First introduced in soybean in 1996, glyphosate resistance was also available in alfalfa, canola, cotton, maize, and sugar beet by 2015.
Insect Resistance
An insect-resistant (IR) trait incorporates insecticidal properties into a plant itself. A major example of GE insect resistance is the transfer of a gene coding for a crystalline (Cry) protein from the soil bacterium Bt to the plant (these Cry proteins are also called Bt toxins). The transferred protein is toxic to the target insect when the insect feeds on the plant. There are many kinds of Cry proteins that control various insect pests—primarily moths, beetles, and flies (Höfte and Whiteley, 1989)—and the different kinds can be stacked to protect a plant from more than one insect pest. At the time
TABLE 3-1 Genetically Engineered Traits Deregulated and Approved for Field Release in the United States as of 2015
| Crop | Crop Scientific Name | Trait | Year Approved | Developer |
|---|---|---|---|---|
| Alfalfa | Medicago sativa | Glyphosate HRa,b | 2005 |
Monsanto & Forage Genetics |
| Reduced Lignin | 2014 |
Monsanto & Forage Genetics |
||
| Apple | Malus domestica | Nonbrowning | 2015 |
Okanagan Specialty Fruits |
| Canola | Brassica napus/Brassica rapa | Oil Profile Alteredc | 1994 |
Calgene |
| Glufosinate HR | 1998 |
AgrEvo |
||
| Glyphosate HR | 1999 |
Monsanto |
||
| Cichory | Cichorium intybus | Male Sterilityc | 1997 |
Bejo |
| Cotton | Gossypium hirsutum | Bromoxynil HRc | 1994 |
Calgene |
| Bt IRd | 1995 |
Monsanto |
||
| Glyphosate HR | 1995 |
Monsanto |
||
| Sulfonylurea HR | 1996 |
DuPont |
||
| Glufosinate HR | 2003 |
Aventis |
||
| Dicamba HR | 2015 |
Monsanto |
||
| 2,4-D HR | 2015 |
Dow |
||
| Flax | Linum usitatissimum | Tolerant to Soil Residues of Sulfonylurea Herbicidec | 1999 |
University of Saskatchewan |
| Maize | Zea mays | Glufosinate HR | 1995 |
AgrEvo |
| Bt IR | 1995 |
Ciba Seeds |
||
| Male Sterilityc | 1996 |
Plant Genetic Systems |
||
| Glyphosate HR | 1997 |
Monsanto |
||
| Increased Lysinec | 2006 |
Monsanto |
||
| Imidazolinone HRc | 2009 |
Pioneer |
||
| Alpha-Amylase | 2011 |
Syngenta |
||
| Drought Tolerance | 2011 |
Monsanto |
||
| ACCasee HR | 2014 |
Dow |
||
| 2,4-D HR | 2014 |
Dow |
||
| Increased Ear Biomass | 2015 |
Monsanto |
||
| Papaya | Carica papaya | Ring Spot Virus VRf | 1996 |
Cornell University, University of Hawaii, USDA Agricultural Research Service |
| Crop | Crop Scientific Name | Trait | Year Approved | Developer |
|---|---|---|---|---|
| Plum | Prunus domestica | Plum Pox VRc | 2007 |
USDA Agricultural Research Service |
| Potato | Solanum tuberosum | Bt IRc | 1995 |
Monsanto |
| Potato Leafroll VRc | 1998 |
Monsanto |
||
| Potato Virus Y VRc | 1999 |
Monsanto |
||
| Low Acrylamide | 2014 |
Simplot Plant Sciences |
||
| Nonbrowning | 2014 |
Simplot Plant Sciences |
||
| Resistance to Late Blight Pathogen | 2015 |
Simplot Plant Sciences |
||
| Rice | Oryza sativa | Glufosinate HR | 1999 |
AgrEvo |
| Rose | Rosa spp. | Altered Flower Color | 2011 |
Florigene |
| Squash | Cucurbita pepo | Zucchini Yellow VR | 1994 |
Upjohn |
| Watermelon Mosaic VR | 1994 |
Upjohn |
||
| Cucumber Mosaic VR | 1996 |
Asgrow |
||
| Soybean | Glycine max | Glyphosate HR | 1994 |
Monsanto |
| Glufosinate HR | 1996 |
AgrEvo |
||
| High Oleic Oil | 1997 |
DuPont |
||
| Acetolactate Synthase HRc | 2008 |
Pioneer |
||
| Bt IRc | 2011 |
Monsanto |
||
| Improved Fatty Acid Profilec | 2011 |
Monsanto |
||
| Stearidonic Acid Producedc | 2012 |
Monsanto |
||
| Isoxaflutole HRc | 2013 |
Bayer and M.S. Technologies |
||
| Increased Yieldc | 2013 |
Monsanto |
||
| Imidazolinone HR | 2014 |
BASF |
||
| 2,4-D HR | 2014 |
Dow |
||
| HPPDg HRc | 2014 |
Bayer/Syngenta |
||
| Dicamba HR | 2015 |
Monsanto |
||
| Sugar beet | Beta vulgaris | Glufosinate HRc | 1998 |
AgrEvo |
| Glyphosate HR | 1998 |
Novartis & Monsanto |
||
| Tobacco | Nicotiana tabacum | Reduced nicotinec | 2002 |
Vector |
| Crop | Crop Scientific Name | Trait | Year Approved | Developer |
|---|---|---|---|---|
| Tomato | Solanum lycopersicum | Fruit Ripening Alteredc | 1992 |
Calgene |
| Fruit Polygalacturonase Level Decreasedc | 1995 |
Zeneca & Petoseed |
||
| Bt IRc | 1998 |
Monsanto |
NOTE: The table identifies the first time a trait was deregulated for a specific crop in the United States. Some deregulated trait–crop combinations have never been used in commercial production.
a HR = herbicide resistance.
b Returned to regulated status in 2007; returned to deregulated status in 2011.
c Trait–crop combination not in production in 2015.
d IR = insect resistance (different Bacillus thuringienis Cry genes inserted to encode proteins that kill specific species).
e Acetyl CoA Carboxylase inhibitor herbicide.
f VR = virus resistance.
g 4-Hydroxyphenylpyruvate dioxygenase inhibitor herbicide.
DATA SOURCE: USDA–APHIS Petitions for Determination of Nonregulated Status. Available at http://www.aphis.usda.gov/biotechnology/petitions_table_pending.shtml. Accessed December 20, 2015.
of writing this report, Bt toxins were the only form of GE insect resistance that had been commercialized. In 2015, IR varieties of cotton, eggplant, maize, poplar, and soybean were in commercial production.
Virus Resistance
Virus resistance prevents a plant from being susceptible to specific viral diseases. In the virus-resistant (VR) crops engineered as of 2015, the coat-protein gene from the targeted virus (or viruses if protection from more than one is sought) is transferred into the crop. The transgene prevents the virus from replicating successfully in the host plant. Commercially grown VR varieties of papaya were developed by Cornell University, the University of Hawaii, and the Agricultural Research Service of the U.S. Department of Agriculture and were first introduced in the state of Hawaii in 1998. VR squash production began in the United States in the late 1990s. China approved commercial production of VR sweet pepper (Capsicum annuum) in 1998, but there was no commercial production of the crop at the time this report was written.
Other Traits in Commercial Production
HR, IR, and VR traits have been in continuous production since the late 1990s. Most of the GE crops in production in 2015 had resistance to one herbicide, contain one or more IR traits, or had both HR and IR traits. However, more GE traits are being introduced each year, and many are unrelated to prevention of damage from insects or to reducing competition with weeds.
In soybean, efforts have been made to increase oxidative stability of the oil to avoid trans-fats generated through the hydrogenation process and to enhance omega-3 fatty acid content of the oil for use in both food and feed. Oils with a high percentage of oleic acid (around 80 percent) require less processing and offer a route to decreasing the concentrations of trans-fats in food products. Genetic engineering has been used to create high-oleic acid soybean through gene silencing (Buhr et al., 2002). In 2015, high-oleic acid soybean was commercially available in North America and was produced on a small number of hectares in the United States for specialty-product contracts (C. Hazel, DuPont Pioneer, personal communication, December 14, 2015).
In maize, GE traits have been developed for drought tolerance and increased alpha-amylase content. The drought-tolerant maize variety developed by Monsanto, DroughtGard™, expresses a gene that encodes cold-shock protein B (cspB) from Bacillus subtilis; under some drought conditions, cspB expression results in higher yield than that of non-GE controls
(Castiglioni et al., 2008). By introducing the alpha-amylase enzyme into the maize endosperm through genetic engineering, the company Syngenta created a maize variety in which the grain is better suited as a feedstock for ethanol production than varieties that lack the enzyme.
In 2015, nonbrowning varieties of apple and potato were sold commercially. Genetic engineering was used to silence the expression of enzymes in the polyphenol oxidase family that cause browning of the crops’ flesh after cuts or bruises. The nonbrowning trait was expected to reduce waste in apples and potatoes and to reduce the use of chemical antibrowning agents on cut apples. Six hectares of nonbrowning apple were planted in 2015, with an expected harvest date of September 2016 (N. Carter, Okanagan Specialty Fruits, personal communication, April 13, 2016).
In the GE nonbrowning potato, the gene that controls asparagine synthase production was also silenced to reduce the production of asparagine because, when potatoes are fried or baked at high temperature, asparagine breakdown results in the production of acrylamide, a potential carcinogen (Zyzak et al., 2003). Nine hundred thirty hectares of potato with GE traits for nonbrowning and low acrylamide were commercially grown in the United States in 2015 (C. Richael, Simplot Plant Sciences, personal communication, April 13, 2016).
Florigene, an Australian company, used genetic engineering to produce blue carnations and roses. The carnations are grown in Colombia, Ecuador, and Australia and shipped as cut flowers to Canada, the United States, the European Union, Japan, Australia, Russia, and the United Arab Emirates. GE roses have been grown and commercially sold in Japan (S. Chandler, RMIT University, personal communication, December 7, 2015).7
China has commercialized tomato with a GE trait for delayed ripening. However, that crop was not being produced when the committee was writing its report.
Genetically Engineered Traits Nearing Market Release
At the time of the report’s writing, several GE traits aimed at crop quality were ready to begin commercial production. GE pest-resistant varieties for some crops that had not previously had GE traits were also in development.
Simplot Plant Sciences, the company that developed the potato with GE traits for nonbrowning and low acrylamide, was in the process of commercializing a second GE potato variety as this report was being written. The second variety was engineered to resist the pathogen responsible for
__________________
7 See also Florigene Flowers: Products. Available at http://www.florigene.com/product/. Accessed December 15, 2015.
late blight—the disease best known as a proximate cause of the Irish potato famine of the 1840s—in addition to the nonbrowning and low-asparagine traits.8
Brazil approved a variety of eucalyptus that was genetically engineered for higher yields in 2015. The yield enhancement was gained through the introduction of an endoglucanase gene from the small annual plant Arabidopsis thaliana (FuturaGene, 2015). Eucalyptus is grown primarily as a source of cellulose for such products as paper, and expression of the endoglucanase gene causes more cellulose to be deposited in cell walls.
Alfalfa engineered to contain lower concentrations of lignin in secondary cell walls, a trait that makes the alfalfa easier for cows to digest, was also near commercialization in late 2015. The reduction was achieved through the partial silencing of the gene that encodes an enzyme involved in the synthesis of the monolignol building blocks of lignin. The new GE trait will be available alone or as a stack with glyphosate resistance.
Pest resistance has been engineered into common bean and plum varieties. Brazil’s government-owned research corporation, EMBRAPA, developed a GE virus-resistant bean (Faria et al., 2014) that attained approval for commercial production in 2014. Over the course of 24 years, a working group of European and U.S. scientists developed a plum (Prunus domestica) that was resistant to the plum pox virus (PPV), a serious pathogen that threatens stone fruits including plums, peaches (Prunus persica), and apricots (Prunus armeniaca) worldwide. Resistance to PPV uses co-suppression and RNA silencing (discussed more in Chapter 7). In 2015, PPV was not present in the United States, but the researchers had gained U.S. approval for the commercial production of GE plums, so they can be grown if the virus becomes a threat. Resistance had also been hybridized into many plum varieties grown in the United States to prevent plum production from being devastated if PPV emerges. VR plum has been field tested in Europe since the late 1990s. Scorza (2014) reported that European researchers were interested in submitting a request to the European Food Safety Authority for approval of GE plum because PPV is a major problem in Europe.
Genetically Engineered Traits or Crops That Have Been Discontinued or Were Never Commercialized
Many GE traits have been developed and never commercialized; others have been inserted into crops whose GE lines were never commercialized or were withdrawn from production after an initial period of commercialization. It is impossible to list every GE trait that has been developed because the traits become known only when a research entity brings a crop with a
__________________
8 More details on gene silencing and the GE potato are presented in Chapter 8.
GE trait to government regulatory authorities for approval. In this section, the committee reviews examples of GE traits and crops that were close to commercialization but were never sold or that were withdrawn from the market. Reasons have included business decisions based on nonprofitability or market failure, consumer nonpreference or social perceptions, and failure to comply with regulatory procedures.
The first commercial GE crop, the FLAVR SAVR tomato, which had delayed ripening that resulted in a longer shelf life, was originally intended for processing; however, having initially expressed interest, Campbell Soup Company decided not to use the GE tomato in its products after some members of the public expressed opposition (Vogt and Parish, 2001). The FLAVR SAVR tomato was instead planted for fresh market in 1994–1997 before being withdrawn from the market as unprofitable because it did not taste better and was more expensive than other tomatoes in the same market space (Bruening and Lyons, 2000; Martineau, 2001; Vogt and Parish, 2001).
Also in the mid-1990s, the company Zeneca marketed a GE tomato that had lower water content for use as tomato paste. The product was labeled as genetically modified. In 1996, the Safeway and Sainsbury grocery chains sold GE tomato paste under their labels in the United Kingdom. However, it was removed from the market in 1999 after sales declined following news-media reports of “biological effects . . . attributed to the process of genetic engineering” (Bruening and Lyons, 2000).
GE potatoes with IR and VR traits constitute an example of a GE crop that was withdrawn from commercial production because of governance decisions made by food retailers in the private sector and competition from other pest-control products. In 1995, Monsanto received U.S. government approval for a potato with the Cry3A (Bt) gene for the control of Colorado potato beetle (Leptinotarsa decemlineata), and 600 hectares of the IR potatoes were planted. GE potato resistant to potato leaf roll virus (Polerovirus spp.) was approved in 1998, and a variety resistant to potato virus Y was approved in 1999. The Bt trait was stacked with either the potato leaf roll virus trait or the potato virus Y trait. The area of GE potato production increased from 1995 to 1998 to about 20,000 hectares, or 3.5 percent of U.S. potato hectares (Hagedorn, 1999). However, the area planted declined sharply in 2000; the decline has been attributed to lack of acceptance by some consumers (Guenthner, 2002). In 2000, a large fast-food chain announced it would no longer purchase GE potatoes. The potato industry was not capable of segregating and testing to provide non-GE potatoes to customers (Thornton, 2003), and growers were concerned about growing a crop that their buyers would not purchase. In addition, many farmers adopted a newly introduced insecticide that controlled Colorado potato beetle and other pests rather than plant the GE variety (Nesbitt, 2005). In 2001, Monsanto closed its potato division.
Monsanto developed wheat that was resistant to glyphosate in the mid-1990s and had plans to commercialize it. However, because of lack of support from the wheat industry, the company did not take the GE wheat variety through the approval process necessary for commercialization (Stokstad, 2004). Some growers were concerned that GE wheat would be rejected by foreign markets.
The company ProdiGene was interested in using genetic engineering to produce pharmaceutical or industrial products in GE plant systems. However, it failed to comply with U.S. regulatory procedures. Not only did its product never come to market, but the company was fined for its violations (Box 3-2).
Lysine is the limiting essential amino acid in most cereal-based diets, so high lysine in maize is a trait of interest. Maize-based diets are particularly deficient in lysine because the storage protein in maize, zein, is very low in lysine. Expression of a bacterial feedback-insensitive enzyme (dihydrodipicolinate synthase) that increases lysine synthesis was used to make a GE high-lysine maize (Lucas et al., 2007), but Monsanto decided not to commercialize the product.
The evolving story of Bt eggplant in India, Bangladesh, and the Philippines illustrates complex interplays of social and legal aspects that could lead to different outcomes among these countries, all of which had previously agreed that Bt eggplant was a high-priority product for them
(Box 3-3). The case of glyphosate-resistant alfalfa, which was on the market in 2015, demonstrates the influence of legal actions on the commercial status of GE crops in the United States (Box 3-4).
EVOLUTION OF REGULATORY POLICIES FOR GENETICALLY ENGINEERED CROPS AND FOODS
The section “Governance of Genetically Engineered Crops” in Chapter 2 and the section above contain many references to regulatory oversight
or approval granted by governments for GE crops and food derived from GE crops. Why did governments decide to regulate these products and how are regulations structured? In the section below, the committee provides a brief history of why government regulations emerged for GE crops and the different ways in which governments have approached regulation of GE crops.
Policy Responses Due to Scientific and Public Concerns
As alluded to in Chapter 1, concerns about potential biosafety risks posed by genetic engineering surfaced in the scientific community almost immediately after the publication of the Cohen et al. (1973) article that described rDNA technology. Scientists attending the Gordon Conference on Nucleic Acids in 1973 called for the National Academy of Sciences to convene a study panel to develop guidelines for safe research on recombinant molecules (Singer and Soll, 1973). The 1974 report issued by the Committee on Recombinant DNA Molecules recommended that scientists
voluntarily defer conducting higher-risk research in view of the uncertainties about potential biosafety risks, pending the development of biosafety guidelines (Berg et al., 1974). That committee was concerned particularly about the potential for rDNA-modified Escherichia coli bacteria to be accidentally disseminated to laboratory workers or the broader human, animal, plant, and bacterial populations with “unpredictable effects.” The report also recommended that the National Institutes of Health (NIH) establish an advisory committee on biosafety guidelines for rDNA research and called for an international scientific conference to address the “appropriate ways to deal with the potential biohazards of recombinant DNA molecules” (Berg et al., 1974). The International Conference on Recombinant DNA Molecules was convened in February 1975 at the Asilomar Conference Center in California. The attendees developed biosafety principles that provided guidance for safe research practices with rDNA molecules in light of risks posed by the research and that allowed for the end of the voluntary research moratorium (Berg et al., 1975).
NIH was also responsive to the earlier recommendations and established the Recombinant DNA Molecular Advisory Committee (later renamed the Recombinant DNA Advisory Committee) in October 1974. Immediately after the Asilomar conference, the NIH advisory committee met to develop research guidelines, which were issued in June 1976 as Guidelines for Research Involving Recombinant DNA Molecules (NIH, 1976). The early NIH guidelines succeeded in allowing laboratory research on rDNA molecules to proceed safely. The guidelines have been modified numerous times but remain in effect as of May 2016 and focus on physical and biological containment for research based on the perceived biosafety or environmental risks of the research.
However, as research continued in the 1970s and 1980s, a number of scientists and civil society groups concerned about the potential biosafety risks associated with rDNA and about broader social and ethical issues regarding the application of the technology began to publicize their criticisms and organize opposition in the United States. As chronicled by Schurman and Munro (2010), concerns initially gained traction in a loose network of critics, including consumer, environmental, and social-justice organizations as well as groups involved in international development projects and large-scale industrialized agriculture.
Several events in the 1980s led to broader and more organized opposition. In 1980, the U.S. Supreme Court decided the case of Diamond v. Chakrabarty, upholding the patentability of living, human-made organisms. The ruling fueled concerns about the ethical implications of patenting life and the privatization of germplasm in seeds that had been traditionally viewed as a “commons” shared by all (Jasanoff, 2005). In 1983, NIH approved the first environmental release of a GE bacterium, which had
been engineered to increase the resistance of crops to frost. The decision sparked opposition from environmental and other citizen groups and generated news-media attention. Concerned groups successfully challenged NIH’s approval (Foundation on Economic Trends v. Heckler, 1985). In the mid-1980s, the development of a synthetic version of bovine somatotropin derived from GE bacteria, to be administered to cows to increase milk production, also generated opposition from a diverse coalition, including small dairy farmers and animal-welfare groups.
As time went by, European civil society groups—including farmer organizations and groups concerned with food safety, animal welfare, and the environment—amplified concerns about genetic engineering in agriculture (Schurman and Munro, 2010). Public concerns about the safety of the food supply were heightened in Europe by a series of food scares in the mid-1990s, including a major outbreak of mad cow disease.
In response to the uncertainty about how this new technology would function in the environment and to public concerns, some governments developed regulatory approaches to GE crops and to food derived from GE crops. Governments adopted different regulatory responses that depended in part on public opinion and on support and opposition by important constituencies.
Different Policy Approaches to Genetically Engineered Crops and Food
The differences in regulatory approaches among countries are discussed in Chapter 9. This section notes some salient points to provide context for later chapters.
Governmental regulatory approaches of GE crops vary in several key dimensions, including the scope of products subject to the regulatory schemes. Countries have differing statutory frameworks for making decisions that reflect the cultural traditions and risk tolerances of their citizens. Decision-makers consider input from diverse groups, which may include environmental and food-safety organizations, organic-crop farmers, large-scale farmers, animal producers, consumers, multinational agricultural companies, and many entities that are involved in the complex global food-production and food-distribution chain. As a result, it is not surprising that countries’ regulatory policy choices reflect different policy tradeoffs. (Chapter 9 provides a more detailed comparison of the regulatory systems of the United States, Canada, Brazil and the European Union.)
The scope of regulations differs among countries. Some decide the regulatory status of each product based on the process used to develop the product, that is, the regulations apply to crops made with genetic-engineering techniques but not to crops bred or produced by conventional breeding. Others focus on the potential risks associated with final products, not the process by which they are made.
Regulatory schemes also differ among countries in how the responsibilities for risk assessment and risk management are allocated. In some countries, the same agency is responsible both for conducting the risk assessment of a regulated product and for making the final approval decision on the basis of meeting a safety standard. The U.S. regulatory system is organized along those lines (Box 3-5). Other governments have separated risk assessment, which is the task of a scientific or technical body, from the final approval decision, which is given to a different government agency that can consider issues that go beyond safety concerns.
Regulatory approaches can affect how quickly GE crops are adopted by growers in different countries. Some countries adopted regulatory policies that allowed relatively quick approval of new GE crop varieties; others adopted a more cautious regulatory stance and approved relatively few new GE foods and crops. Some countries adopted regulatory systems fairly quickly; others still have not, which effectively has resulted in a ban on the import or cultivation of GE foods and crops. One author categorized first-generation regulatory systems for GE crops into four models according to their overall orientation to biotechnology (Paarlberg, 2000). Frameworks
that encouraged the development of GE crops were deemed promotional; policies that were neutral—neither encouraging nor discouraging GE crops—were termed permissive; precautionary policies tended to slow the adoption of GE crops and foods; and preventive policies were intended to block the technology. Precautionary policies are discussed further in Chapter 9 (see Box 9-2).
CONCLUSIONS
The introgression of GE traits into crops was preceded by millennia of trait introductions into domesticated crops through selection and by rapid advances in plant breeding in the 20th century. The deciphering of the genetic code in the mid-20th century, plant-breeding tools (including tissue culture), and the discovery of the properties of Agrobacterium tumefaciens made recombinant-DNA technology in plants possible. GE traits were present in 14 crops in 2015. GE varieties dominated the planted area of soybean and cotton and were planted on one-third of maize hectares and one-fourth of canola hectares in the world in 2015. However, GE varieties had not been developed for most crops, and GE crops were grown on 12 percent of the world’s cropland.
Several GE traits had been developed, but few of these were available in commercial crop varieties in 2015. Most commercially available traits in the first 20 years of GE crops were aimed at providing herbicide resistance to the crop or protecting the crop from insect damage. A few crops that had been genetically engineered to be resistant to viruses or to not turn brown when cut were also commercially available. Other types of traits, such as those conferring improved nutritional qualities or better composition for ethanol feedstock, were in commercial production, and a wider variety of traits were being readied for market release.
Approval by regulatory agencies clearly is instrumental in a GE crop’s ability to enter the marketplace. The regulatory systems of some governments are more encouraging to GE-crop commercialization than others. Regulatory systems reflect different cultural traditions, histories, and risk tolerances in the constituencies of each country.
REFERENCES
Bai, Y., and P. Lindhout. 2007 Domestication and breeding of tomatoes: What have we gained and what can we gain in the future? Annals of Botany 100:1085–1094.
Barton, K.A., and W.J. Brill. 1983. Prospects in plant genetic engineering. Science 219:671–676. Berg, P., D. Baltimore, H.W. Boyer, S.N. Cohen, R.W. Davis, D.S. Hogness, D. Nathans, R. Roblin, J.D. Watson, S. Weissman, and N.D. Zinder. 1974. Potential biohazards of recombinant DNA molecules. Science 185:303.
Berg, P., D. Baltimore, S. Brenner, R.O. Roblin, and M.F. Singer. 1975. Summary statement of the Asilomar conference on recombinant DNA molecules. Proceedings of the National Academy of Sciences of the United States of America 72:1981–1984.
Bruening, G., and J.M. Lyons. 2000. The case of the FLAVR SAVR tomato. California Agriculture 54:6–7.
Buhr, T., S. Sato, F. Ebrahim, A.Q. Xing, Y. Zhou, M. Mathiesen, B. Schweiger, A. Kinney, P. Staswick, and T. Clemente. 2002. Ribozyme termination of RNA transcripts downregulate seed fatty acid genes in transgenic soybean. Plant Journal 30:155–163.
Castiglioni, P., D. Warner, R.J. Bensen, D.C. Anstrom, J. Harrison, M. Stoecker, M. Abad, G. Kumar, S. Salvador, R. D’Ordine, S. Navarro, S. Back, M. Fernandes, J. Targolli, S. Dasgupta, C. Bonin, M.H. Luethy, and J.E. Heard. 2008. Bacterial RNA chaperones confer abiotic stress tolerance in plants and improved grain yield in maize under water-limited conditions. Plant Physiology 147:446–455.
Chauhan, C. October 27, 2014. Govt allows field trials for GM mustard, brinjal. Online. Hindustan Times. Available at http://www.hindustantimes.com/india-news/govt-allowsfield-trials-for-gm-mustard-brinjal/article1-1279197.aspx. Accessed June 12, 2015.
Choudhary, B., K.M. Nasiruddin, and K. Gaur. 2014. The Status of Commercialized Bt Brinjal in Bangladesh. Ithaca, NY: International Service for the Acquisition of Agri-biotech Applications.
Cohen, S.N., A.C.Y. Chang, H. Boyer, and R.B Helling. 1973. Construction of biologically functional bacterial plasmids in vitro. Proceedings of the National Academy of Sciences of the United States of America 70:3240–3244.
Doebley, J. 2004. The genetics of maize evolution. Annual Review of Genetics 38:37–59.
FAO (Food and Agriculture Organization). 2015. FAO Statistical Pocketbook 2015: World Food and Agriculture. Rome: FAO.
Faria, J.C., P.A.M.R. Valdisser, E.O.P.L. Nogueira, and F.J.L. Aragao. 2014. RNAi-based Bean golden mosaic virus-resistant common bean (Embrapa 5.1) shows simple inheritance for both transgene and disease resistance. Plant Breeding 133:649–653.
FDA (U.S. Department of Health and Human Services–Food and Drug Administration). 1992. Statement of Policy: Foods Derived From New Plant Varieties. Federal Register 57:22984–23005.
Fernandez-Cornejo, J., S.J. Wechsler, M. Livingston, and L. Mitchell. 2014. Genetically Engineered Crops in the United States. Washington, DC: United States Department of Agriculture–Economic Research Service.
Flint-Garcia, S.A. 2013. Genetics and consequences of crop domestication. Journal of Agricultural and Food Chemistry 61:8267–8276.
Foundation on Economic Trends, et al. v. Margaret M. Heckler, et al. 1985. U.S. Court of Appeals for the District of Columbia Circuit. 756 F.2d 143. Decided February 27, 1985. Available at http://law.justia.com/cases/federal/appellate-courts/F2/756/143/162040/. Accessed November 23, 2015.
Fuller, N.R. 2005. Image credit in National Science Foundation Press Release 05-088. Available at http://www.nsf.gov/news/news_images.jsp?cntn_id=104207&org=NSF. Accessed November 23, 2015.
FuturaGene. 2015. FuturaGene’s eucalyptus is approved for commercial use in Brazil. Available at http://www.futuragene.com/FuturaGene-eucalyptus-approved-for-commercial-use.pdf. Accessed September 23, 2015.
Geertson Farms Inc., et al. v. Mike Johanns, et al., and Monsanto Company: Memorandum and order Re: Permanent injunction. 2007. U.S. District Court for the Northern District of California. C 06-01075 CRB, Case #: 3:06-cv-01075-CRB. Decided May 3, 2007. Available at http://www.centerforfoodsafety.org/files/199_permanent_injunction_order.pdf. Accessed September 23, 2015.
Gelvin, S.B. 2003 Agrobacterium-mediated plant transformation: The biology behind the “Gene-Jockeying” tool. Microbiology and Molecular Biology Reviews 67:16–37.
Gregory, P., R.H. Potter, F.A. Shotkoski, D. Hautea, K.V. Raman, V. Vijayaraghavan, W.H. Lesser, G. Norton, and W.R. Coffman. 2008. Bioengineered crops as tools for international development: Opportunities and strategic considerations. Experimental Agriculture 44:277–299.
Guenthner, J. 2002. Consumer acceptance of genetically modified potatoes. American Journal of Potato Research 79:309–316.
Haberlandt, G. 1902. Kulturversuche mit isolierten Pflanzenzellen. Sitzungsber. Akademie de Wissenschaften Wien, Math.-Naturwissenschaften. Kl. III:69–92.
Hagedorn, C. 1999. Update on 1998 transgenic crops acreage. Virginia Cooperative Extension Crop and Soil Environmental News. Available at http://www.sites.ext.vt.edu/newsletterarchive/cses/1999-02/1999-02-02.html. Accessed September 23, 2015.
Höfte, H., and H.R. Whiteley. 1989. Insecticidal crystal proteins of Bacillus thurengiensis. Microbiological Reviews 53:242–255.
InterAksyon.com. December 13, 2015. Supreme Court Bans Development of Genetically Engineered Products. Online. InterAksyon.com. Available at http://www.interaksyon.com/article/121368/first-in-the-world--supreme-court-bans-development-of-geneticallyengineered-products. Accessed December 16, 2015.
James, C. 1997. Global Status of Transgenic Crops in 1997. Commercialized Biotech/GM Crops: 2014. Ithaca, NY: International Service for the Acquisition of Agri-biotech Applications.
James, C. 2002. Global Review of Commercialized Transgenic Crops: 2001 Feature: Bt Cotton. Ithaca, NY: International Service for the Acquisition of Agri-biotech Applications.
James, C. 2006. Global Status of Commercialized Biotech/GM Crops: 2006. Ithaca, NY: International Service for the Acquisition of Agri-biotech Applications.
James, C. 2014. Global Status of Commercialized Biotech/GM Crops: 2014. Ithaca, NY: International Service for the Acquisition of Agri-biotech Applications.
James, C. 2015. Global Status of Commercialized Biotech/GM Crops: 2015. Ithaca, NY: International Service for the Acquisition of Agri-biotech Applications.
Jasanoff, S. 2005. Designs on Nature: Science and Democracy in Europe and the United States. Princeton, NJ: Princeton University Press.
Jayaraman, K. 2010. Bt brinjal splits Indian cabinet. Nature Biotechnology 28:296.
Jiang, C., A. Mithani, X. Gan, E.J. Belfield, J.P. Klingler, J.K. Zhu, J. Ragoussis, R. Mott, and N.P. Harberd. 2011. Regenerant Arabidopsis lineages display a distinct genome-wide spectrum of mutations conferring variant phenotypes. Current Biology 21:1385–1390.
Klein, T.M., E.D. Wolf, R. Wu, and J.C. Sanford. 1987. High-velocity microprojectiles for delivering nucleic acids into living cells. Nature 327:70–73.
Larkin, P.J., and W.R. Scowcroft. 1981. Somaclonal variation—a novel source of variability from cell cultures for plant improvement. Theoretical and Applied Genetics 60:197–214.
Laursen, L. 2013. Greenpeace campaign prompts Philippine ban on Bt eggplant trials. Nature Biotechnology 31:777–778.
Lucas, D.M., M.L. Taylor, G.F. Hartnell, M.A. Nemeth, K.C. Glenn, and S.W. Davis. 2007. Broiler performance and carcass characteristics when fed diets containing lysine maize (LY038 or LY038 × MON 810), control, or conventional reference maize. Poultry Science 86:2152–2161.
Martineau, B. 2001. First Fruit: The Creation of the Flavr Savr Tomato and the Birth of Biotech Food. New York: McGraw-Hill.
Mba, C. 2013. Induced mutations unleash the potentials of plant genetic resources for food and agriculture. Agronomy 3:200–231.
Murashige, T., and F. Skoog. 1962. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiologia Plantarum 15:473–497.
Muthukumar, B., B.L. Joyce, M.P. Elless, C.N. Stewart Jr. 2013. Stable transformation of ferns using spores as targets: Pteris vittata and Ceratopteris thalictroides. Plant Physiology 163:648–658.
Neelakandan, A.K., and K. Wang. 2012. Recent progress in the understanding of tissue culture-induced genome level changes in plants and potential applications. Plant Cell Reports 31:597–620.
Nesbitt, T.C. 2005. GE Foods in the Market. Ithaca, NY: Cornell University Cooperative Extension.
National Institute of General Medical Sciences. 2010. The New Genetics. Available at https://publications.nigms.nih.gov/thenewgenetics/thenewgenetics.pdf. Accessed May 15, 2015.
NIH (National Institutes of Health). 1976. Guidelines for Research Involving Recombinant DNA Molecules. Federal Register 41:27902–27943.
NRC (National Research Council). 1984. Genetic Engineering of Plants: Agricultural Research Opportunities and Policy Concerns. Washington, DC: National Academy Press.
Ossowski, S., K. Schneeberger, J.I. Lucas-Lledo, N. Warthmann, R.M. Clark, R.G. Shaw, D. Weigel, and M. Lynch. 2010. The rate and molecular spectrum of spontaneous mutations in Arabidopsis thaliana. Science 327:92–94.
OSTP (Executive Office of the President, Office of Science and Technology Policy). 1986. Coordinated Framework for Regulation of Biotechnology. Federal Register 51:23302. Available at https://www.aphis.usda.gov/brs/fedregister/coordinated_framework.pdf. Accessed December 18, 2015.
OSTP (Executive Office of the President, Office of Science and Technology Policy). 2015. Memorandum for Heads of Food and Drug Administration, Environmental Protection Agency, and Department of Agriculture. Available at https://www.whitehouse.gov/sites/default/files/microsites/ostp/modernizing_the_reg_system_for_biotech_products_memo_final.pdf. Accessed September 25, 2015.
Paarlberg, R.L. 2000. Governing the GM Crop Revolution: Policy Choices for Developing Countries. Washington, DC: International Food Policy Research Institute.
Roychowdhury, R., and J. Tah. 2013. Mutagenesis—A potential approach for crop improvement. Pp. 149–187 in Crop Improvement: New Approaches and Modern Techniques, K.R. Hakeem, P. Ahmad, and M. Ozturk, eds. New York: Springer Science+Business Media, LLC.
Schurman, R. and W. Munro. 2010. Fighting for the Future of Food: Activists versus Agribusiness in the Struggle over Biotechnology. Minneapolis: University of Minnesota Press.
Scorza, R. 2014. Development and Regulatory Approval of Plum pox virus resistant ‘Honeysweet’ Plum. Webinar Presentation to the National Academies of Sciences, Engineering, and Medicine Committee on Genetically Engineered Crops: Past Experience and Future Prospects, November 6.
Singer, M., and Soll, D. 1973. Guidelines for DNA hybrid molecules. Science 181:1114.
Stokstad, E. 2004. Monsanto pulls the plug on genetically modified wheat. Science 304:1088–1089.
Stroud, H., B. Ding, S.A. Simon, S. Feng, M. Bellizzi, M. Pellegrini, G.L. Wang, B.C. Meyers, and S.E. Jacobsen. 2013. Plants regenerated from tissue culture contain stable epigenome changes in rice. eLife 2:e00354.
Thornton, M. 2003. The Rise and Fall of NewLeaf Potatoes. Pp. 235–243 in North American Agricultural Biotechnology Council Report 15: Science and Society at a Crossroad. Ithaca, NY: NABC.
Turner, J. 2014. Regulation of Genetically Engineered Organisms at USDA–APHIS. Presentation to the National Academy of Sciences’ Committee on Genetically Engineered Crops: Past Experience and Future Prospects, December 10, Washington, DC.
USDA (U.S. Department of Agriculture). 2016. World Agricultural Production. Foreign Agricultural Service Circular WAP 4-16. Available at http://apps.fas.usda.gov/psdonline/circulars/production.pdf. Accessed April 13, 2016.
USDA–APHIS (U.S. Department of Agriculture–Animal and Plant Health Inspection Service). 2004. USDA APHIS environmental assessment in response to permit application (04-121-01r) received from ProdiGene Inc. for field testing of genetically engineered corn, Zea mays. Available at http://www.aphis.usda.gov/brs/aphisdocs/04_12101r_ea.pdf. Accessed November 23, 2015.
USDA–APHIS (U.S. Department of Agriculture–Animal and Plant Health Inspection Service). 2005. Monsanto Co. and Forage Genetics International; Availability Determination of Nonregulated Status of Alfalfa Genetically Engineered for Tolerance to Herbicide Glyphosate. Federal Register 70:36917–36919.
USDA–APHIS (U.S. Department of Agriculture–Animal and Plant Health Inspection Service). 2007. Return to Regulated Status of Alfalfa Genetically Engineered for Tolerance to the Herbicide Glyphosate. Federal Register 72:13735–13736.
USDA–APHIS (U.S. Department of Agriculture–Animal and Plant Health Inspection Service). 2011. Determination of Regulated Status of Alfalfa Genetically Engineered for Tolerance to the Herbicide Glyphosate; Record of Decision. Federal Register 76:5780.
Vogt, D.U., and M. Parish. 2001. Food Biotechnology in the United States: Science, Regulation, and Issues. Washington, DC: Congressional Research Service.
Wang, H., A.J. Studer, Q. Zhao, R. Meeley, and J.F. Doebley. 2015. Evidence that the origin of naked kernels during maize domestication was caused by a single amino acid substitution in tga1. Genetics 200:965–974.
Wood, A. 2002. Proteins-Sigma-Aldrich Fine Chemicals signs trypsin manufacturing deal with ProdiGene. Chemical Week 164:28.
Zyzak, D.V., R.A. Sanders, M. Stojanovic, D.H. Tallmadge, B.L. Eberhart, D.K. Ewald, D.C. Gruber, T.R. Morsch, M.A. Strothers, G.P. Rizzi, and M.D. Villagran. 2003. Acrylamide formation mechanism in heated foods. Journal of Agricultural and Food Chemistry 51:4782–4787.