6
Assessing Risks of Gene-Drive Modified Organisms
Advances in the molecular biology of gene drives are far outpacing research on the fate and effects of gene-drive modified organisms in the environment, as well as the development of the knowledge needed to calculate risk and describe uncertainty related to gene drives. There are many questions that need to be answered about the effects, both beneficial and harmful, that gene-drive modified organisms may have if released into the environment. For example, will the frequency of inheritance of the genetic construct remain constant from one generation to the next? What is the possibility for gene flow to non-target species? How reliable are molecular markers, such as adding a unique eye color, intended to facilitate the monitoring of gene-drive modified organisms after they have been released to the environment? What constitutes an adequate mitigation strategy for unintended, harmful effects, and how can the efficacy of such an approach be evaluated?
Although as of May 2016 many applications have been proposed, there has not yet been field tests or environmental releases of gene-drive modified organisms. Decisions will need to be made about prospective applications of gene drive research, including the direction of research, the need for public engagement, and the requirements for governance. Given the lag between this new technology’s development and experts’ understanding of its ecological implications, decision-makers’ ability to identify the potential harms for different applications and determine appropriate safeguards and mitigation strategies is somewhat limited. How can decisions be made under such conditions of uncertainty?
The answer is ecological risk assessment, the study and use of probabilistic decision-making tools to evaluate the likely benefits and potential harms of a proposed activity on the wellbeing of humans and the environment, often under conditions of uncertainty. The scientific assessment of risk is one important way in which values related to protecting and preserving human health and the environment are incorporated into decision making, particularly, when such assessments are mandated by law. This chapter focuses on why and how ecological risk assessment should be used to inform decisions around the development and application of gene-drive modified organisms, from understanding the efficacy and safety of gene drives created in the laboratory, to validating assessments in contained field trials, to assessing the risks of releasing gene-drive modified organisms into the open environment.
WHAT IS RISK?
The definition of risk varies depending on the context in which the term is used. In colloquial use, the term risk is synonymous with threat, harm, or hazard. However, in the context of ecological risk assessment, risk has a probabilistic meaning (EPA, 1992, 1998; Suter, 2007; NRC, 2009; Van den Brink et al., 2016). For the purposes of this report, the committee adopts the probabilistic definition of risk:
Risk is the probability of an effect on a specific endpoint or set of endpoints due to a specific stressor or set of stressors.
In this probabilistic definition, the stressor is any agent or actor with the potential to alter a component of the ecosystem. The effect refers to potential beneficial and harmful outcomes.
And, an endpoint is a societal, human health, or environmental value that is to be managed or protected. Endpoints reflect decisions that need to be made, and are sometimes determined by regulatory requirements. In the context of this chapter, endpoints include an ecological entity (a species, population, habitat, or ecosystem characteristic or function) and an attribute (a measurable characteristic of the entity).1 For example, endangered species of the Hawaiian honeycreeper (Case Study 3; see Chapter 3) have specific federal protections in regard to the size of their population and their habitat. In this Case Study, the gene-drive modified mosquito (Culex quinquefasciatus) that is unable to carry the malaria parasite will be introduced into the environment to reduce the incidence of avian malaria and protect the honeycreeper. The stressor in this scenario is the gene-drive modified mosquito; the effect is the replacement of wild-type mosquitoes with the gene-drive modified mosquito; and the endpoint is reducing the number of birds that die from avian malaria (see Table 6-1 for additional examples). The honeycreeper is the entity to be protected, and the increase in size of the Honeycreeper populations could be the measurable attribute.
The ability to calculate risk depends on a number of factors. First is the mathematical description of the relationships between the stressor, the environment, and the endpoint. These relationships include the distribution of the stressor in the environment, the range of probabilities that the endpoint will be exposed to the stressor, and how the stressor and the endpoint interact, including the variability in the interactions, and environmental influences on the size and distribution of changes to the endpoint.
The probabilistic definition of risk accounts for four elements:
- Probability, reflected in the probability distributions that describe the occurrence of the stressor and the resulting effects.
- Cultural values, reflected in the selected endpoints (thus a risk assessment may not encompass all possible effects that a stressor may produce in an ecosystem).
- Public engagement as a mechanism to identify and incorporate cultural values of communities, stakeholders, or other publics.
- Uncertainty, because the variability of the environmental systems, the gaps in knowledge about how these systems interact, and the challenges of accurately defining and communicating cultural values and social norms.
Given these elements, it is important for risk to be placed in a cultural framework for decision making. In many cases, cultural values are reflected in regulations that govern the decision-making process. For example, an ecological risk assessment of a fish farm will be informed by requirements of the Clean Water Act regarding the concentration of chemicals or bacteria in the water and runoff, the size of the fishery, and the concentration of mercury in the fish. Local jurisdictions may also impost other requirements, rules to protect the community from flooding and to preserve local parks, roadways, or historical sites. These regulations reflect cultural values such as citizens’ right to clean water or protected space for their homes. In the case of the Honeycreeper, a community might value the bird as its own entity while other stakeholders may value tourism related to bird watching. Both of these values could factor into the goal to reduce the burden of avian malaria on bird populations. Cultural values and preferences can be expressed as a series of criteria for the state of the system under management. Given adequate criteria, it is possible to express cultural values mathematically in the definition of endpoints.
___________________
1Environmental Protection Agency. Terminology Services. See https://ofmpub.epa.gov/sor_internet/registry/termreg/searchandretrieve/home.do [accessed April 29, 2016].
TABLE 6-1 Definitions and Examples of Risk and Related Terminology
| Risk probability of an effect on a specific endpoint due to a specific stressor |
Stressor any agent or actor with the potential to alter a component of the ecosystem |
Effect potential beneficial or harmful outcome |
Endpoint Valued characteristic of society, human health, or the environment important to decision making |
|
| Case Study 1 Aedes mosquitoes and dengue |
Probability that gene-drive modified Aedes mosquitoes will decrease new dengue infections in children by 50% | Persistence of gene-drive modified mosquito in the environment | Hybridization of gene-drive modified mosquito with other species | Decrease in incidence of new cases of human dengue infections in children |
| Case Study 5 Knapweed and biodiversity |
Probability that gene-drive modified knapweed will increase population of native plants in rangelands | Dispersal of gene-drive modified knapweed | Density of wild-type knapweed | Increase in populations of native plants |
ASSESSING ENVIRONMENTAL IMPACTS VERSUS ASSESSING RISKS
Environmental Assessment and Environmental Impact Statements Under the National Environmental Protection Act
In the United States, gene drive research will most likely be regulated under the Coordinated Framework for the Regulation of Biotechnology which assigns the primary oversight responsibilities for biotechnologies to the US Environmental Protection Agency (EPA; pesticides), the US Food and Drug Administration (FDA; animal drugs), and the US Department of Agriculture (USDA; plant pests) (see Chapter 8). To assess potential impacts of biotechnology, the agencies under the Coordinated framework must abide by the National Environmental Policy Act (NEPA; CEQ N Regulations, 40 C.F.R. § 1508.9; Box 6-1). Although NEPA has many strengths, it does not require a probabilistic assessment of potential risks. Ecological risk assessment, which is not currently required under NEPA but is used in several other regulatory frameworks, represents a more robust and appropriate framework for assessing the potential ecological harms and benefits of gene-drive modified organisms.
Processes Under the National Environmental Policy Act
Under NEPA, the two established processes for assessing impact as a component of formal decision making are environmental assessment (EA) and an environmental impact statement (EIS; see Box 6-1). An environmental assessment is a determination of whether a federal government decision to allow the introduction (field test of environmental release) of a specific biotechnology or related product has the potential to cause significant environmental effects.
EAs generally include a wide range of scientific evidence, but they do not require quantitative or probabilistic estimates of potential environmental effects. An environmental assessment is a detailed accounting of data sources, life history characteristics, and ecological information. Although EAs contain a qualitative description of uncertainty in these datasets, they do not describe quantitatively the probability of potential effects or include a quantitative uncertainty analysis. An example of an EA with some relevance to gene drives is the “Draft Environmental Assessment for Investigational Use of Aedes aegypti OX513A” (Oxitec, 2016) that Oxitec
submitted to FDA, as part of the company’s request for approval of field trials of genetically engineered mosquitoes. The draft assessment includes a section on environmental risk assessment that presents a qualitative estimate of the risk of the release of the organism in Key Haven (Monroe County), Florida, concluding that toxic or allergic effects on either animals or humans were negligible and that the effects on the ecosystem would also be negligible.
An EIS is required only if an EA determines that a proposed action will have a significant harmful impact on the environment. An EIS is generally a compendium of information on the environmental, economic, and other societal implications of the proposed activity. Like an environmental assessment, an EIS is not required to incorporate a quantitative, probabilistic analysis of the potential effects. However, an EIS includes alternative actions, including doing nothing, to permit comparative analysis of environmental and other implications across the different choices. An EIS often provides a comprehensive compilation of information about a proposed activity, including lists of stakeholders, cultural considerations, the regulatory landscape, and comments from interested citizens.
Some of the key strengths of NEPA process are that it is a standard approach required by legislation, supports the collection of large amounts of information about a proposed activity, it has clear reporting requirements, and includes provisions for public input. The NEPA process is also widely recognized by the stakeholder community. The disadvantage of the NEPA process, however, is that it is a regulatory process and not a decision science approach. Neither an EA nor an EIS requires a clear formulation of the problem that provides a quantitative cause-effect model. Analyses conducted as part of the NEPA process are not required to be probabilistic or report quantitatively on uncertainty. These gaps would make it very difficult to create testable hypothesis to conduct further research on gene-drive modified organisms and inform decision making.
The Process of Ecological Risk Assessment
Risk assessment is a process in which evidence on the probability of effects is collected, evaluated, and interpreted to estimate the probability of the sum total of effects (EPA, 1984). Risk assessment methodologies, which describe pertinent probability distributions and clearly identify critical uncertainties, are derived from many science disciplines, including decision sciences, psychology, statistics, mathematical modeling, and biomedicine. Ecological risk assessment is a related scientific process that focuses on evaluating ecological effects of exposure to one more stressors, such as invasive species, changes in land use, and infectious disease (EPA, 1992). Ecological risk assessment can be used to assess the probability of both harmful and beneficial effects. Ecological risk assessment is quantitative, deals extensively with uncertainty, and is flexible enough to evaluate processes at large spatial and temporal scales (Van den Brink et al., 2016).
Although the field of ecological risk assessment began in the late 1980s, it is not as familiar to research stakeholders or lay publics as the NEPA process (see Appendix E for a brief history of ecological risk assessment in the United States). Ecological risk assessments are not a regulatory requirement under NEPA. However, EPA conducts ecological risk assessments under other circumstances; for example, when evaluating the potential effects of pesticides on the environment or on endangered species. Examples of regulations that describe and require ecological risk assessment processes include the Federal Insecticide, Fungicide and Rodenticide Act (FIFRA), the Resource Conservation and Recovery Act (RCRA), and the Comprehensive Environmental Response, Compensation and Liability Act (CERCLA), more commonly called Superfund, and to Toxic Substances Control Act (TSCA).
In 1998, EPA issued guidelines for risk assessors and risk managers to “improve the quality and consistency” of the ecological risk assessment process (EPA, 1998). While the guidelines include approaches to assess the risks from multiple stressors and endpoints, the focus is on the risks to populations and ecosystems from toxic chemicals (Dearfield et al., 2005). In these guidelines, the ecological risk assessment begins with a planning and scoping process, which encourages risk assessors, risk managers, and stakeholders to discuss purpose, scope, and technical approaches before the risk assessment process begins (EPA, 1998; Dearfield et al., 2005). The risk assessment process itself is carried out in three phases: problem formulation, analysis, and risk characterization. Problem formation is an information-gathering phase in order to define an endpoint and an ecological entity that needs to be protected (EPA, 1998). The ecological entities to be protected are typically derived from environmental protection statutes, regulations. The analysis phase includes two key elements: characterization of effects and characterization of exposure, which provide the data needed to predict an entity's response to the expose. The risk characterization phase is when results of the analysis are used to estimate risk.
Since 1998, EPA has published other documents to update the approach to selecting endpoints and the estimation of uncertainty, and an update to incorporate ecosystem services into ecological risk assessment. In an effort to design processes specific to the needs of individual programs, there is now separate guidance available for ecological risk assessments done under FIFRA, RCRA, CERCLA, and TSCA. Despite these updates, however, EPA’s guidance for ecological risk assessment lags behind advances in the field.
A critical component in ecological risk assessment (and all risk assessments) is adequately taking into account uncertainty. Regan et al. (2002) describe two major categories of uncertainty: epistemic uncertainty and linguistic uncertainty. Epistemic uncertainty arises from a lack of knowledge about determinate facts. Epistemic uncertainty in risk assessments can arise out of variation in sampling results, variation in the quantitative relationship between an exposure and a response, and limitations in the models to describe cause and effect. Epistemic uncertainty is difficult to estimate without field data.
Linguistic uncertainty involves ambiguities in the terminology used to describe concepts such as species diversity, ecosystem health, or even “precise” or “accurate.” For example, the
term “ecosystem health” is an example of linguistic uncertainty because an ecosystem’s “health” is a normative claim regarding a characteristic (health) that it is not an inherent property of the system, but rather the meaning draws on an often unspecified value system. Minimizing linguistic uncertainty is vital in setting specifications for endpoints and communicating the results of the risk assessment to decision makers.
KEY CONSIDERATIONS FOR ECOLOGICAL RISK ASSESSMENTS OF GENE-DRIVE MODIFIED ORGANISMS
As of May 2016, no ecological risk assessments have been published for the field testing or environmental release of a gene-drive modified organism into the environment.
The 1998 EPA guidelines emphasize that a planning and scoping process should be the first step of the ecological risk assessment process (EPA, 1998). A key consideration to be discussed during the planning process for the ecological risk assessment of a gene-drive modified organism is that despite the near half century history of work, gene drive research is still at a preliminary proof-of-concept stage. For example, there are limited proof-of-concepts for gene-drive modified mosquitoes that could be used either to suppress wild-type populations (Hammond et al., 2016) or to disable their ability to carry the malaria parasite (Gantz et al. 2015). Research is under way on a gene-drive modified mouse (Campbell et al., 2015), but a proof-of-concept has not yet been published.
Many questions still remain about the efficacy and safety of gene drive technologies (see Chapters 2 through 4). Even when research for one proposed use of a gene-drive modified organisms advances, additional research, from the molecular to ecosystem level, will still need to be conducted for other proposed uses of other organisms. What is the probability that a gene drive construct will spread as intended throughout an island population of invasive rodents? What is the likelihood that a population of endangered Honeycreeper birds will recover if the release of a gene-drive modified mosquito reduces or eliminates the spread of avian malaria? What is the probability that gene-drive modified pigweed, Amaranthus palmeri, will spread to a related, non-target plant species used for food? What are the quantitative tradeoffs between pest management approaches using gene-drive modified organisms and management approaches using other methods of genetic engineering?
A third consideration is that, for some proposed applications of gene-drive modified organisms there are other strategies to address the issue. For example, there are alternative approaches to suppression of mosquito populations that could potentially be assessed as management options in a risk assessment. It may also be that a combination of a gene drive and conventional methodologies would be more effective, and at lower risk, than either approach alone—another possible consideration during planning and scoping the ecological risk assessment process.
Other key considerations about gene-drive modified organisms that will need to be accounted for in risk calculations include how the modified genetic elements move into populations, the efficiency with which the pass down from each generation to the next, and whether they are designed to affect population dynamics. Sexual reproduction between the gene-drive modified organism and the wild-type organism of the same species is required for the modified genetic element to spread in the environment, just as sharing habitat is necessary for the transmission of disease. The mere presence of the modified genetic element in other species could be considered an endpoint, for example, in risk assessment of a potential field trial on the dispersal of gene-drive modified organisms into a confined environment. Because the goal of a gene-drive modified organism is to spread, and possibly persist, in the environment, the necessary ecological risk assessment is more similar to that used for invasive species, than for environmental assessments of genetically engineered organisms.
Ecological risk assessment is equipped for the analysis of information currently available on the genetics, ecology, and potential effects of a gene-drive modified organism, and the organism’s discussed complex interactions with other species and the environment. Because of the quantitative nature of the science of ecological risk assessment, it can also be used to identify uncertainties and the additional research (data) that is needed, and can inform the development of testable hypotheses in gene drive research. In consideration of the phased testing pathway (see Figure 5-1 in Chapter 5), ecological risk assessment could also be used to inform decisions about when gene drive research should move from laboratory studies (Phase 1) to field trials (Phase 2). And similarly, it could also indicate when it would be appropriate to move from field trials (Phase 2) to staged, open releases into the environment (Phase 3). However, it is not yet clear how the values of different communities or cultures will affect the selection of endpoints or how the importance of the spread of these organisms or their sequences will be considered. The considerations described here, and others, will likely increase uncertainty in the risk assessment until more laboratory and field data are available.
What might an ecological risk assessment look like for a field test or environmental release of a gene-drive modified organism? Although the overall framework of ecological risk assessment is useful in the context of gene drives, gene-drive modified organisms have important distinguishing features that necessitate analytical tools not typically part used in conventional methods of assessing risk. Three distinguishing features are (1) a gene drive is passed on from one generation to the next at a rate greater than that described by Mendelian inheritance; (2) a gene drive construct can have effects on other parts of the organism’s genome beyond the target; and (3) gene-drive modified organisms are designed to spread, along with their effects, into the larger environment. The proposed uses of gene-drive modified organisms, by definition will be part of a system with multiple stressors and multiple interactions affecting multiple species and a number of endpoints. Because gene drives are intended to spread, gene-drive modified organisms will interact with a variety of species and they may even pass the gene drive construct to closely related individuals. The physical and ecological structure of the landscape, including the distribution of habitats and human land uses as well as elements such as predators and chemical contaminants, will influence the spread of the gene-drive modified organism. In some instances multiple releases of the gene-drive modified organism may be required to achieve the desired result. The release of reversal drives has been proposed to mitigate the unintended negative impacts of gene drives on the environment; these reversal drive constructs may also introduce their own sets of wider ecological effects.
EPA’s current framework and guidance documents for ecological risk assessment do not adequately address the assessment of multiple stressors and multiple endpoints. These standards and guidelines were based on risk assessments for single chemical stressors and their effects on a limited set of end points. The difficulty of incorporating multiple stressors into a cumulative risk assessment using these current methodologies was previously noted in the 2009 National Research Council report Science and Decisions: Advancing Risk Assessment. The inability of EPA’s framework to deal with multiple stressors combined with multiple endpoints was a driver for the development of the original relative risk model (RRM; Landis and Wiegers, 1997; Wiegers et al., 1998; Hayes and Landis, 2004).
The committee reviewed several frameworks proposed for the risk assessment of genetically modified organisms (see Appendix C). Wolt et al. (2010), for example, proposed a problem formulation process closely related to that used for pesticides under FIFRA. The methodology is built on the premise that genetically modified crops are the stressor and that they will be limited to agricultural sites. These assumed circumstances are similar to the one chemical-one environment basis of EPA’s original formulations and do not reflect the circumstances expected for many gene-drive modified organisms. In another assessment, Romeis et al. (2013) concluded that “despite the complexity of ecological systems, ecological risk assessments for genetically engineered crops do not have to be complex; they may follow the simple models used successfully for conventional chemi-
cal pesticides and biological control agents.” However, the models based on the EPA’s 1998 guidelines were not designed to account for the unique features of gene-drive modified organisms.
Van den Brink et al. (2016) provides a number of recommendations and specifications for performing ecological risk assessments in landscape-scale scenarios with multiple stressors and multiple endpoints (see Appendix E). Specifications that would likely benefit ecological risk assessments for gene-drive modified organisms include the following:
- Build a digital map of the study site that includes land use, topography, and the locations of sources, stressors, habitats, and endpoints.
- Map out regions in the landscape that have similar land uses, stressors, and management goals.
- Establish a priori the cultural values and protection goals that will determine the success of the assessment and decision-making process.
- Determine the interactions among the species and the ecological processes and functions that will be affected by the stressors.
- Construct a conceptual model that reflects the sources of stressors, the habitats, the expected effects and the impacts to the system under investigation.
- Use the conceptual model to organize all of the information that will inform the cause-effect modeling.
- Transform the cause-effect model into a quantitative structure using approaches that incorporate the dual deterministic and probabilistic nature of ecosystems.
A CONCEPTUAL CAUSE-EFFECT MODEL
An essential component of the ecological risk assessment process is developing a model that accurately portrays the relationship between stressors and endpoints, known as a cause-effect model. Cause-effect models provide a framework, based on available evidence, upon which risk calculations are built. Although much of the discussion that follows the specifications for ecological risk assessment outlined in Van den Brink et al. (2016), the cause and effect models presented here are meant to be illustrative, not prescriptive, for future efforts to conduct ecological risk assessment on gene-drive modified organisms.
Developing a cause-effect model involves three primary, interrelated steps: (1) identify a clear set of risk management questions that will be informed by the ecological risk assessment; (2) develop a detailed map of the area in question (for example, a confined field test site); and (3) construct the model and risk calculation framework.
First, identifying a clear set of management question is critical for determining the endpoints to be used in the assessment. The choice of risk management questions is heavily influenced by the relevant governance structure and publics. In many instances, the management questions are bounded by the regulations and oversight mechanisms. However, local communities and other stakeholders are critical to determining the valued components of the ecosystem in question, their relevance to human interests and well-being, and to setting risk management priorities.
Second, a detailed map of the area in question (e.g., an ecosystem or a field test site) helps to set priorities and goals for risk management. This mapping step can be summarized as “what do you care about and where is it?” Maps include a variety of place-based features that may affect endpoints such as sources of exposures, location of stressors, habitats, and differences in land use (e.g., residential, commercial, and agricultural). Maps are also useful for determining how widespread a habitat is in the area of interest, or whether particular organisms of interest are clustered within the landscape. Maps help identify features that may affect, for example, the dispersal of a gene-drive modified organism, and account for them in the risk calculation. Finally, a cause-effect model and calculation framework can be developed once the management questions
and the map are set. Figure 6-1 illustrates the basic format of a cause-effect model for ecological risk assessment. A conceptual cause-effect model for the ecological risk assessment of a gene-drive modified organism is illustrated in Figure 6-2. The format of these cause-effect models is based upon frameworks originally developed for nonindigenous species (Landis, 2003; Colnar and Landis, 2007) to include multiple stressors and multiple endpoints, and subsequently applied to other ecological contexts around the world. For example, the fundamental methodology has been used to assess the effects of contaminants, invasive species (Landis and Wiegers, 2005), forestry management practices at large spatial scales (Anderson and Landis, 2012; Ayre and Landis, 2012), and to develop conservation priorities for the tropical rivers in Northern Australia (Bartolo et al., 2012).
The cause-effect model includes five interconnected nodes: source, stressor, habitat, effects, and impacts. The source is the location of the stressor and conditions of release (i.e., the mechanism, timing, and frequency of release). The source of a gene-drive modified organism, for example, depends on whether release is part of a confined field study, part of a national control program, or perhaps due to escape caused by a failure in containment. There could be multiple release sites of the gene-drive modified organism, to account for the distribution of existing wild-type organisms in the landscape. Assuming the gene drive persists in the environment, the environment itself can be considered an additional source after the initial release.
In the context of a gene-drive modified organism, the stressor(s) can be defined by multiple factors, including the modified genetic element, the ability of the gene drive to propagate in the face of selection pressure, and the rate at which the genetic element is inherited from generation to generation. Unlike chemicals or invasive species, the ecological risk assessment of a gene-drive modified organism depends on the modified genotype in the organism and the efficiency with which the spreads to a specific wild target. In common with other stressors, there will be a focus on the survivability of the gene-drive modified organism in the wild, its transport to the sites of interest, and its persistence in the environment. There also will be numerous ecological stressors, some anthropogenic and some natural to be considered. A number of other organisms and ongoing ecological processes may alter the survival of the gene-drive modified organism, the targeted wild-type organisms, and the other organisms in the receiving environment.
The range of habitat(s) to be evaluated could potentially be as broad for the release of gene-drive modified organisms as those considered for invasive species. A number of locations and characteristics of the environment must be considered. If the gene-drive modified organism is released to reduce the number of vector organisms, then the breeding and feeding grounds need to be included. If an invasive species is being controlled, then the habitats of the target need to be included. The terrain of the landscape and the distributions of land uses and habitats will alter the exposure and, in part, govern the effects of the gene-drive modified organism and the other stressors in the environment.
Effects will largely depend on the nature of the gene drive, and will likely include changes in population sizes, predator-prey interactions, species diversity, vector densities, among other possibilities. In some instances, a gene-drive modified organism may be used to intentionally alter the composition of an ecosystem, such as by eliminating an invasive species, which is likely to change the composition of the community and energy and nutrient flows throughout the ecosystem.
The last node, impact, is the endpoints of interest. Some proposed uses of gene-drive modified organisms include reducing in the spread of human disease, controlling invasive species, and preserving endangered species. Other uses are likely been proposed as the science advances. Endpoints are shaped by human values and so will need to be derived by careful and deliberate processes of public engagement and governance. Endpoints will likely vary in location and be distributed unevenly in the receiving environment. Where endpoints may move around or vary in location, cause-effect models must reflect those spatial distributions and changes, such as in the protection of the smallmouth bass, which may move into different parts of a river system depending upon water temperature, food sources, and the need to spawn.
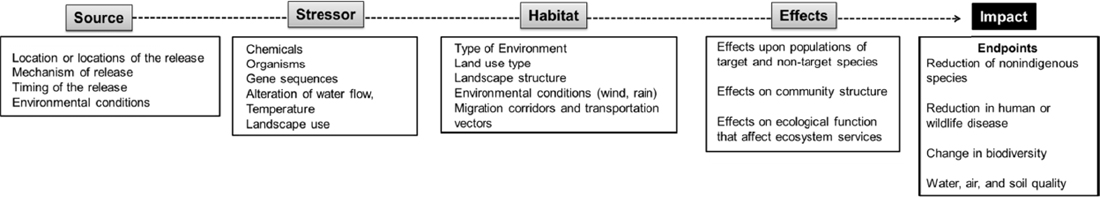
Another important dimension of the cause-effect model is a listing of confounding sources and stressors (see Figure 6-2). Confounding factors may have significant influence on estimates of risk. For example, the use of insecticides could potentially reduce or eliminate gene-drive modified insects and thus affect the potential for the modified elements to spread as intended. Farming practices, urbanization, or other alterations to the landscape may limit the ability of gene-drive modified organisms to spread or persist in the environment. Such confounding factors will need to be incorporated into the cause-effect model.
At the end of this process, the conceptual cause-effect model has been bracketed by the source of the stressor and the management goals, the endpoints, and the spatial relationships in the management area.
Building the Calculation Framework
The source–stressor–habitat–effect–impact structure of the RRM can be expressed as a Bayesian network.2 Marcot and colleagues have demonstrated the utility of Bayesian networks in conservation biology and have been pioneers in developing guidance for their use (Marcot et al., 2006; Nyberg et al., 2006; Marcot, 2012). The RRM has been modified recently to use Bayesian networks as a framework for computation and to incorporate a broad variety of evidence into the calculation of risk (Ayre and Landis, 2012; Ayre et al., 2014; Hines and Landis, 2014; Herring et al., 2015). The advantages of using Bayesian networks in ecological risk assessment have been demonstrated by Hart and Pollino (2008), Pollino et al. (2007), and Bayliss et al. (2012). Bayesian networks inherently incorporate cause-effect relationships and uncertainty and can use combinations of expert knowledge and available data (Uusitalo, 2007). Because the nodes (such as habitats and effects) in the cause-effect models are dynamic, statistical methods that account for variation in these nodes will be needed. Monte Carlo methods3 are an approach to incorporate the probability of multiple “what-if” scenarios based on those variations into an ecological risk assessment framework (EPA, 1994; Chapter 5 of Suter, 2007). This approach generates multiple estimates of risk and thus a more complete set of information for decision-makers (EPA, 1994). For example, Hayes et al. (2015) completed a hypothetical ecological risk assessment of a modified sterile male mosquito. The authors relied upon fault tree models because experimental and field data are not yet available. The statistical analysis relies upon Monte Carlo approach to address the exposure and effects combinations (see additional discussion in Appendix E).
ILLUSTRATING A CONCEPTUAL CAUSE-EFFECT MODEL USING TWO CASE STUDIES
This section describes two hypothetical examples of ecological risk assessments on how gene-drive modified organisms might be used. The first example (Case Study 1) examines the release of gene-drive modified mosquitoes to reduce the spread of dengue to humans. In this case, the goal would be to increase the proportion of the mosquito population that does not transmit disease. The second example (Case Study 4) examines the introduction of a gene-drive modified mouse for the reduction of an invasive wild mouse population that is threatening protected marine bird rookeries.
___________________
2Graphically depicted web of nodes that link cause and effect relationships using conditional probability to describe the interactions and to generate the probable outcome or outcomes (Marcot et al., 2006).
3A statistical analysis that relies on repeated sampling of probability distributions of model inputs to estimate the final probability distribution for each of the model outputs (also called Monte Carlo experiments or Monte Carlo simulations) (Burmaster and Anderson, 1994).
Control of Human Dengue (Case Study 1)
The case study on control of human dengue includes two different scenarios (see Chapter 3). First is the release of sterile male Aedes aegypti mosquitoes developed using a gene drive technique. In this case the goal is population suppression, but mosquito populations could be reestablished by dispersal into habitats where mosquito populations are reduced. The second is release of gene-drive modified Aedes aegypti that are incompetent hosts of the dengue virus. In this instance, the population of Aedes aegypti would not necessarily decline, but the gene-drive modified immunity to the dengue virus would ideally spread to other populations of Aedes aegypti by dispersal. The risk assessment process outlined here would likely be applicable to other infectious diseases of concern to humans, livestock, crops, and endangered species.
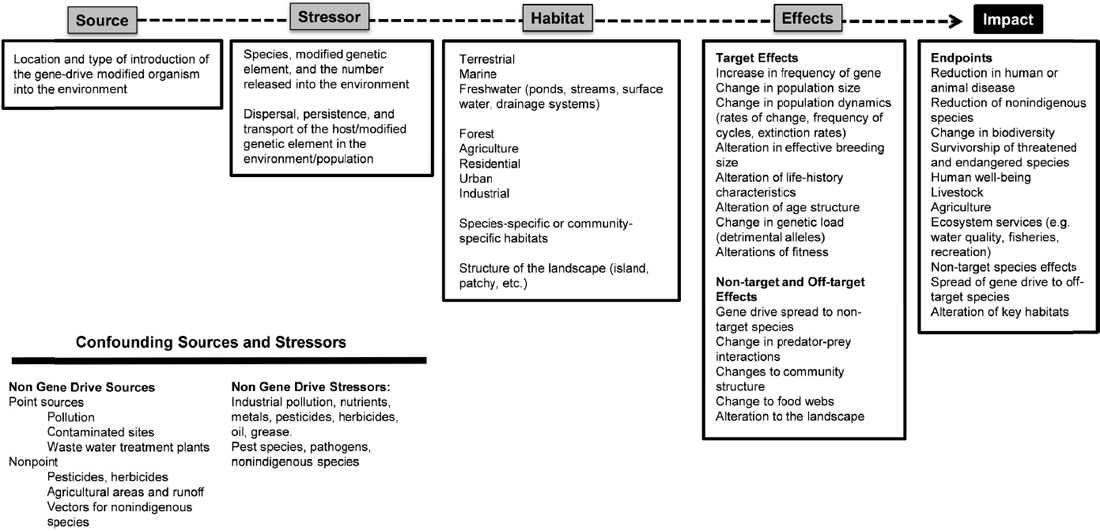
Figure 6-3 describes some of the factors to consider as part of the cause-effect pathway for the two dengue control scenarios. Such a cause-effect pathway could inform the conceptual model and eventually the probabilistic model for estimating the ecological risks of an environmental release of a gene-drive modified Aedes aegypti mosquitoes. The spatial scale of the mosquito release will be a critical factor. Because Aedes aegypti feeds, breeds, and develops in the same areas as humans, the environment for open release would likely be an urban area with high human population densities, though the mosquito can also breed in similar environments (i.e., man-made containers for water) in rural landscapes. In the case of dengue, the assumption is that release locations would be near human habitations. The source of mosquitoes carrying the gene drive includes the location of the release, the number of insects released, and the frequency of releases. Times of introduction are assumed to correspond to time periods that reflect a unique generation (i.e., when newly emerged females would be receptive to mating and therefore to gene transfer) and locations where breeding sites would be plentiful.
A number of characteristics are relevant to defining the stressor, the gene-drive modified Aedes aegypti. The genetic sequence of the mosquito suppressor drive or the sequence of the dengue anti-transmission drive is one fundamental characteristic. It is also important to consider the possibility of off-target sequences affected by the drive and their effects on survivorship and breeding.
In addition to the molecular biology of the gene drive within the organism, there will be a number of other sources of stressors in the environments where gene-drive modified mosquitoes would be released. Because the habitat is likely to be an urban environment, point-source pollution from human waste materials or water-storage containers could introduce microbiota, nutrients, pesticides, agricultural chemicals, antibiotics, herbicides, insecticides, and other substances. The gene-drive modified mosquitoes may also interact with nonindigenous mosquito species, or with other genetically modified Aedes aegypti mosquitoes, such as those introduced as population suppressors in other research trials. Some of these organisms may require tetracycline to develop successfully into adults (as in the case of Oxitec RIDL technology). It would be important to determine whether the gene-drive modified Aedes aegypti is more or less sensitive to antibiotics, insecticides, or contaminants compared to wild-type Aedes aegypti, and to consider whether the modified mosquito’s level of sensitivity to pesticides would affect the efficacy of emergency dengue control strategies, such as chemical fogging. In addition, the rates of hybridization to related sympatric mosquito species and wild-type insects would provide an indication of the spread of the gene drive to other populations and locations. Finally, because mosquitoes that host dengue can also host other viruses, potential competition between viruses may need to be considered.
In the scenario of a gene drive that would confer an inability to spread dengue, the gene drive would need to move through the native mosquito population via breeding. As such, the rate of breeding and survival must also be estimated; any physiological or other barriers to breeding could be considered stressors in this context. Experiments to define these fitness costs would need to be performed early in the research process, such as in phase I small-scale laboratory cage trials and phase II larger-scale confined field experiments.
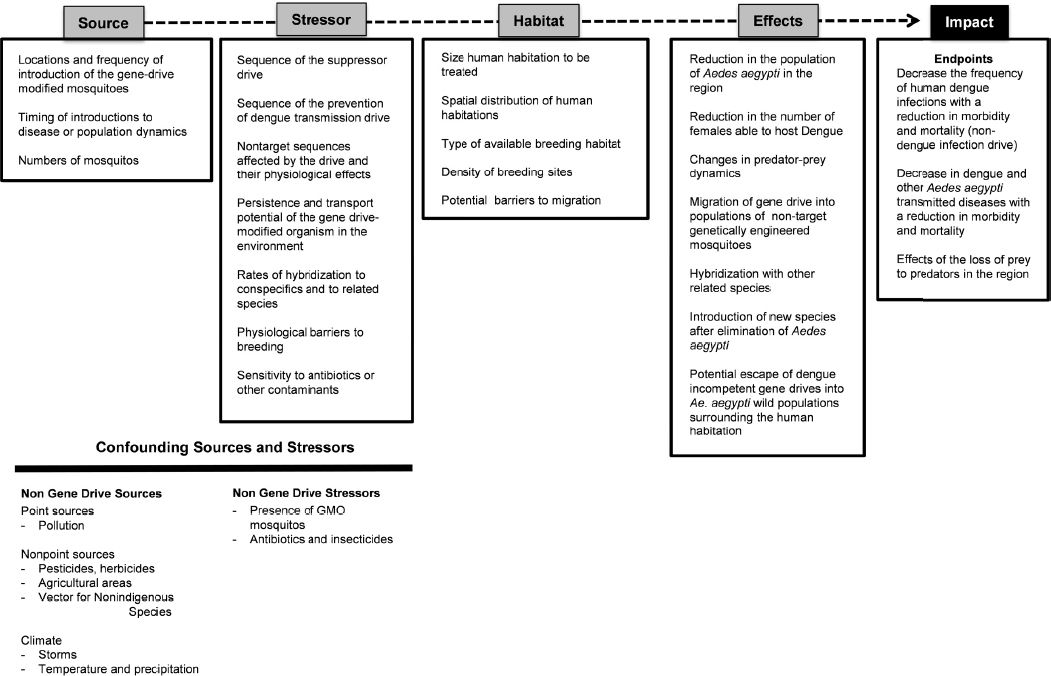
The human-centric endpoints in this case would be the decrease in the frequency of human dengue infections. This decrease should also reduce rates of morbidity and mortality associated with infection within the local human population. Since Aedes aegypti is a vector for diseases of cattle and other species, it would be expected that rates of mortality in these species would also decrease.
Lastly, the elimination of a common species might alter the niche space for other insects or organisms in the region, creating a confounding stressor that could affect the outcomes. For example, wild-type Aedes aegypti from surrounding habitat areas (such as neighborhoods where releases have not taken place) may disperse into the gene-drive modified organism’s environment. Alternatively, another mosquito species, such as Aedes albopictus, might enter or expand into the niche space formerly occupied by Aedes aegypti. Predicting the likelihood and effects of such outcomes would require information regarding the ecology of the affected region.
Eliminating Invasive Mice from Islands (Case Study 4)
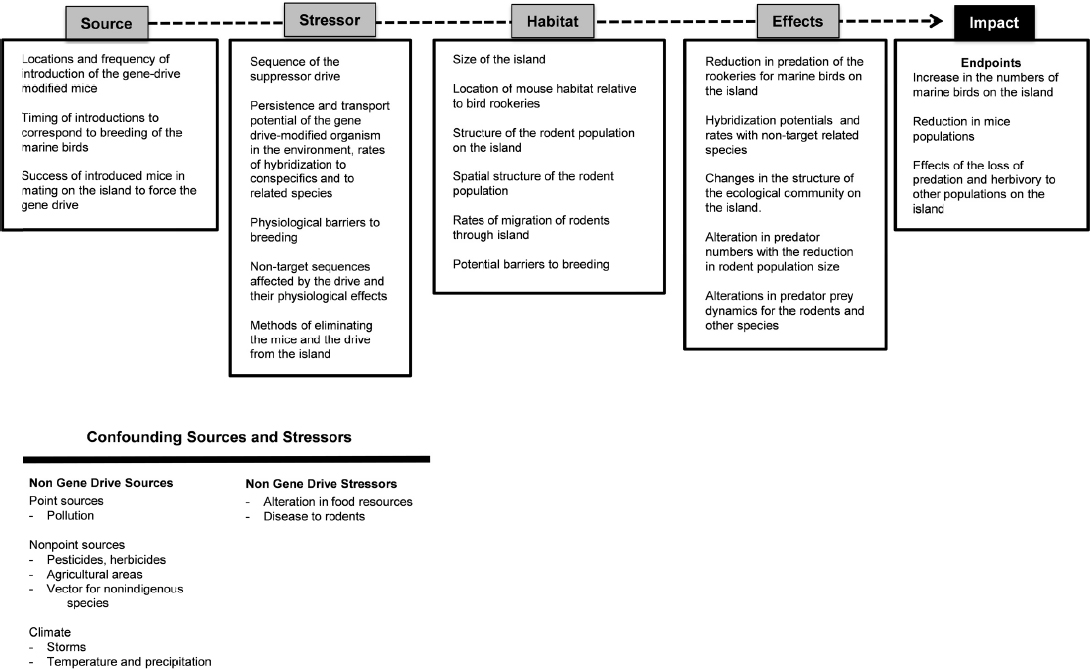
This case study involves the introduction of a gene-drive modified mouse into an island environment to suppress a non-native mouse population that is threatening protected species of marine birds (see Chapter 3). The management goal, in this case, is to improve the status of the marine bird rookery. Figure 6-4 highlights selected hypothetical elements in the cause-effect pathway for the release of a gene-drive modified mouse.
The source includes consideration of the “where, what, and when” of the introduction of the gene-drive modified mouse to the island. A quantitative estimate of the expected survival of the mice and the expectation of reproduction with the island’s extant mouse population would also be needed.
Stressors in this case include the sequence and physiological effects of the gene drive and any off-target sequences, as well as factors that influence the gene drive’s spread, such as the potential to hybridize with conspecifics and related species. The breeding structure of the mice should also be considered; mice and many other mammals exhibit dominance behavior in which only the dominant male and female are permitted to breed. Management and mitigation strategies should also be considered in the assessment of stressors, including methods to eliminate the drive if it is not successful in achieving the desired endpoints.
The habitat features of the island will determine much of the interaction between the gene drive, the rodent population, and the increase in the quality of the rookery. Is there one connected or patchy meta-population, or many sub-populations of mice on an island? What are the potential barriers to mouse breeding that would slow the rate of transmission of the gene drive? How will predators affect the population dynamics of the gene-drive modified organisms and the invasive mouse population?
Effects include the potential reduction in the rodent population along with a concordant increase in the success of the rookeries. These changes would likely have other ecological effects, such as changes to the plant and insect communities or alterations in other predator-prey interactions. The key endpoint or impact would include an increase in the number of fledgling birds.
CONCLUSIONS AND RECOMMENDATIONS
The potential for gene drives to spread throughout a population, to persist in the environment, and to cause irreversible effects on organisms and ecosystems, calls for a robust method to assess risks. Although they are widely acknowledge as valuable in other contexts, the environmental assessments and the environmental impact statements required by the National Environmental Protection Act are inappropriate tools to characterize the risks of gene-drive modified organisms. Instead, ecological risk assessment would be beneficial to gene drive research because this method can be used to estimate the probability of immediate and long-term environmental and public health harms and benefits.
Ecological risk assessments allows comparisons among alternative strategies, incorporates the concerns of relevant publics, and can be used to identify sources of uncertainty, making it well suited to inform research directions and support public policy decisions about emerging gene drive technologies. This approach could potentially be built into a structured, adaptive management process to oversee the release and management of gene-drive modified organisms in the environment. As of April 2016, no ecological risk assessment has yet been conducted for a gene-drive modified organism.
Recommendation 6-1: Researchers, regulators and other decision makers should use ecological risk assessment to estimate the probability of immediate and long-term environmental and public health effects of gene-drive modified organisms and to inform decisions about gene drive research, policy, and applications.
Two key features of ecological risk assessments are the ability to trace cause-effect pathways and the ability to quantify the probability of specific outcomes. Both of these features are strengthened by data and models based on field trials and environmental monitoring. Reliable data and robust models are particularly crucial in situations involving multiples ecological stressors and cumulative effects, as is likely to be the case in many gene drive applications.
Recommendation 6-2: To strengthen future ecological risk assessment for gene-drive modified organisms, researchers should design experimental field trials to validate or improve cause-effect pathways and further refine ecological models.
There is currently sufficient knowledge to begin constructing ecological risk assessments for some potential gene-drive modified organisms, including mosquitoes and mice. In some other cases it may be possible to extrapolate from research and risk analyses of other modified organisms and non-indigenous species. However, laboratory studies and confined field tests (or studies that mimic field tests) represent the best approaches to reduce uncertainty in an ecological risk assessment, and are likely to be of greatest use to risk assessors.
Recommendation 6-3: To facilitate appropriate interpretation of the outcomes of an ecological risk assessment, researchers and risk assessors should collaborate early and often to design studies that will provide the information needed to evaluate risks of gene drives and reduce uncertainty to the extent possible.
In the United States, the relevant guidelines and technical documents are not yet sufficient on their own to guide ecological risk assessment of gene drive technologies, because they focus predominantly on evaluating the risks to populations or ecosystems posed by toxic chemicals, and do not yet adequately address the assessment of multiple stressors and endpoints or cumulative risk. The lack of guidance from the US federal government applicable to ecological risk assessment for the gene drive research community is a critical gap.
REFERENCES
Anderson, S.A., and W.G. Landis. 2012. A pilot application of regional scale risk assessment to the forestry management of the Upper Grande Ronde watershed, Oregon. Hum Ecol. Risk Assess. 8(4):705-732.
Ayre, K.K., and W.G. Landis. 2012. A Bayesian approach to landscape ecological risk assessment applied to the Upper Grande Ronde watershed, Oregon. Hum. Ecol. Risk Assess. 18(5):946-970.
Ayre, K.K., C.A. Caldwell, J. Stinson, and W.G. Landis. 2014. Analysis of regional scale risk to whirling disease in populations of Colorado and Rio Grande cutthroat trout using Bayesian belief network model. Risk Anal. 34(9):1589-1605.
Bartolo, R.E., R.A. van Dam, and P. Bayliss. 2012. Regional ecological risk assessment for Australia's tropical rivers: Application of the relative risk model. Hum. Ecol. Risk Assess. 18(1):16-46.
Bayliss, P., R.A. van Dam, and R.E. Bartolo. 2012. Quantitative ecological risk assessment of the Magela Creek Floodplain in Kakadu National Park, Australia: Comparing point source risks from the Ranger uranium mine to diffuse landscape-scale risks. Hum. Ecol. Risk Assess. 18(1):115-151.
Burmaster, D.E., P.D. Anderson. 1994. Principles of good practice for the use of Monte Carlo techniques in human health and ecological risk assessments. Risk Anal. Aug; 14(4):477-481.
Campbell, K.J., J. Beek, C.T. Eason, A.S. Glen, J. Godwin, F. Gould, N.D. Holmes, G.R. Howald, F.M. Madden, J.B. Ponder, D.W. Threadgill, S.A. Wegmann, and G.S. Baxter. 2015. The next generation of rodent eradications: Innovative technologies and tools to improve species specificity and increase their feasibility on islands. Biol. Conserv. 185:47-58.
Colnar, A.M., and W.G. Landis. 2007. Conceptual model development for invasive species and a regional risk assessment Case Study: The European Green Crab, Carcinus maenas, at Cherry Point, Washington USA. Hum. Ecol. Risk Assess. 13(1):120-155.
Dearfield K.L., E.S. Bender, M. Kravitz, R.Wentsel, M.W. Silmak, W.H. Farland, and P. Gilman. 2005. Ecological risk assessment issues identified during the US Environmental Protection Agency’s examination of risk assessment practices. Integrated Environmental Assessment and Management 1(1):73-76.
EPA (US Environmental Protection Agency). 1984. Risk Assessment and Management: Framework for Decision Making. EPA 600/9-85-002. US Environmental Protection Agency, December 1984.
EPA. 1992. Framework for Ecological Risk Assessment. EPA/630/R-92/001. Risk Assessment Forum, US Environmental Protection Agency, Washington, DC [online]. Available at: https://www.epa.gov/sites/production/files/2014-11/documents/framework_eco_assessment.pdf [accessed April 26, 2016].
EPA. 1998. US Guidelines for Ecological Risk Assessment. EPA/630/R-95/002F. Risk Assessment Forum, U. S. Environmental Protection Agency, Washington, DC [online]. Available at: https://www.epa.gov/sites/production/files/2014-11/documents/eco_risk_assessment1998.pdf [accessed April 26, 2016].
Gantz, V.M., N. Jasinskiene, O. Tatarenkova, A. Fazekas, V.M. Macias, E. Bier, and A.A. James. 2015. Highly efficient Cas9-mediated gene drive for population modification of the malaria vector mosquito Anopheles stephensi. Proc. Natl. Acad. Sci. 112:E6736-E6743.
Hammond, A., R. Galizi, K. Kyrou, A. Simoni, C. Siniscalchi, D. Katsanos, M. Gribble, D. Baker, E. Marois, S. Russell, A. Burt, N. Windbichler, A. Crisanti, and T. Nolan. 2016. A CRISPR-Cas9 gene drive system targeting female reproduction in the malaria mosquito vector Anopheles gambiae. Nat. Biotechnol. 34(1):78-83.
Hart, B.T., and C. Pollino. 2008. Increased use of Bayesian network models will improve ecological risk assessments. Hum. Ecol. Risk Assess. 14(5):851-853.
Hayes, E.H., and W.G. Landis. 2004. Regional ecological risk assessment of a near shore marine environment: Cherry Point, WA. Hum. Ecol. Risk Assess. 10(2):299-325.
Hayes, K.R., S. Barry, N. Beebe, J.M. Dambacher, P. De Barro, S. Ferson, J. Ford, S. Foster, A. Concalves da Silva, G.R. Hosack, D. Peel, and R. Thresher. 2015. Risk Assessment for Controlling Mosquito Vectors with Engineered Nucleases, Part I: Sterile Male Construct Final Report. Technical report, CSIRO Biosecurity Flagship, Hobart, Australia, 148pp.
Herring, C.E., J. Stinson, and W.G. Landis. 2015. Evaluating non-indigenous species management in a Bayesian networks derived relative risk framework for Padilla Bay, Washington. Integr. Environ. Assess. Manag. 11(4):640-652.
Hines, E.E., and W.G. Landis. 2014. Regional risk assessment of the Puyallup River Watershed and the evaluation of low impact development in meeting management goals. Integr. Environ. Assess. Manag. 10(2):269-278.
Landis, W.G. 2003. Twenty years before and hence; Ecological risk assessment at multiple scales with multiple stressors and multiple endpoints. Hum. Ecol. Risk Assess. 9(5):1317-1326.
Landis, W.G. 2004. Ecological risk assessment conceptual model formulation for nonindigenous species. Risk Anal. 24(4):847-858.
Landis, W.G. 2007. The Exxon Valdez oil spill revisited and the dangers of normative science. Integr. Environ. Assess. Manage. 3(3):439-441.
Landis, W.G., and J.A. Wiegers. 1997. Design considerations and a suggested approach for regional and comparative ecological risk assessment. Hum. Ecol. Risk Assess. 3(3):287-297.
Landis, W.G., and J.A. Wiegers. 2005. Introduction to the regional risk assessment using the relative risk model. Pp. 11-36 in Regional Scale Ecological Risk Assessment Using the Relative Risk Model, W.G. Landis, ed. Boca Raton, FL: CRC Press.
Marcot, B.G. 2012. Metrics for evaluating performance and uncertainty of Bayesian network models. Ecol. Modell. 230:50-62.
Marcot, B.G., J.D. Steventon, G.D. Sutherland, and R.K. McCann. 2006. Guidelines for development and updating Bayesian belief networks applied to ecological modeling and conservation. Can. J. Forest Res. 36(12):3063–3074.
NRC (National Research Council). 2009. Science and Decisions: Advancing Risk Assessment. Washington, DC: The National Academies Press.
Nyberg, J.B., B.G. Marcot, and R. Sulyma. 2006. Using Bayesian belief networks in adaptive management. Can. J. Forest. Res. 36(12):3104-3116.
Oxitec. 2016. Aedes aegypti OX513A: Draft Environmental Assessment for Investigational Use of Aedes aegypti OX513A [online]. Available at: https://www.regulations.gov/#!documentDetail;D=FDA-2014-N-2235-0002 [accessed April 26, 2016].
Pollino, C.A., O. Woodberry, A. Nicholson, K. Korb, and B.T. Hart. 2007. Parameterisation and evaluation of a Bayesian network for use in an ecological risk assessment. Environ. Modell. Softw. 22(8):1140-1152.
Regan, H.M., M.C. Colyvan, and M.A. Burgman. 2002. A taxonomy and treatment of uncertainty for ecology and conservation biology. Ecol. Appl. 12(2):618-628.
Romeis, J., A. Raybould, F. Bigler, M.P. Candolfi, R.L. Hellmich, J.E. Huesing, and A.M. Shelton. 2013. Deriving criteria to select arthropod species for laboratory tests to assess the ecological risks from cultivating arthropod-resistant genetically engineered crops. Chemosphere 90(3):901–909.
Suter, G.W. 2007. Ecological Risk Assessment, 2nd Ed. Boca Rotan, FL: CRC Press.
Uusitalo, L. 2007. Advantages and challenges of Bayesian networks in environmental modeling. Ecol. Modell. 203(3-4):312-318.
Van den Brink, P.J., C.B. Choung, W. Landis, M. Mayer-Pinto, V. Pettigrove, S. Scanes, R. Smith, and J. Stauber. 2016. New approaches to the ecological risk assessment of multiple stressors. Mar. Fresh. Res. 64(4):429-439.
Wiegers, J.K., H.M. Feder, L.S. Mortensen, D.G. Shaw, V.J. Wilson, and W.G. Landis. 1998. A regional multiple stressor rank-based ecological risk assessment for the fjord of Port Valdez, AK. Hum. Ecol. Risk Assess. 4(5):1125-1173.
Wolt, J.D., P. Keese, A. Raybould, J.W. Fitzpatrick, M. Burachik, A. Gray, S.S. Olin, J. Schiemann, M. Sears, and F. Wu. 2010. Problem formulation in the environmental risk assessment for genetically modified plants. Transgenic Res. 19(3):425-436.



















