2
The Elimination of Hepatitis B
After analyzing the problem of hepatitis B in the United States and reviewing relevant literature, the committee concluded that eliminating the public health problem of this disease is feasible with certain conditions. This chapter lays out the committee’s logic in coming to this decision. First, it describes the epidemiology and natural history of the infection, then makes a brief statement on the committee’s conclusion regarding elimination. Next, the chapter discusses ending transmission of the virus; then it deals with preventing deaths among people already infected. The last sections lay out critical factors that would influence progress toward the elimination goal and possible barriers to this goal. A discussion of the strategies that might be employed in an elimination program is outside the scope of this report, but can be expected from phase two of this project.
EPIDEMIOLOGY OF HEPATITIS B
Hepatitis B virus (HBV) is the most prevalent cause of chronic viral hepatitis and hepatocellular carcinoma in the world. Recent estimates suggest about 250 million people have chronic HBV infection, causing about 780,000 deaths per year (Schweitzer et al., 2015; WHO, 2015b). The burden of disease is heaviest in East and Southeast Asia, sub-Saharan Africa, the Amazon Basin, and parts of Eastern Europe. In these areas, lifetime risk of HBV exposure is nearly universal and prevalence of chronic infection in adults is about 5 to 10 percent. Prevalence of chronic adult infection is generally lower in the Middle East and South Asia (about 2 to 5 percent)
and lower still (less than 1 percent) in North America, Western Europe, and Australia (Evans et al., 2014; Sobeslavsky, 1980; WHO, 2014, 2015b).
HBV is transmitted though contact with blood and bodily fluids of an infected person. Sexual contact and injection drug use drive most HBV transmission among American adults (63 and 16 percent, respectively) (Mast et al., 2006). People whose occupations put them in contact with blood or blood products are also at risk, as are household contacts of infected persons, and people travelling in endemic countries, but those routes of transmission account for only about 5 percent of cases (Mast et al., 2006). Sometimes the means of transmission is unclear. About 16 percent on newly-infected hepatitis B patients report no particular risk factors for infection (Mast et al., 2006).
Preventing transmission of HBV to vulnerable newborns and children is a pillar of all global and national HBV prevention strategies. In the United States, the Advisory Committee on Immunization Practices first recommended universal HBV vaccination in 1991 among infants (CDC, 1991). By 2013, estimated national coverage of three or more doses among children 19 to 35 months of age was 90.8 percent (95 percent confidence interval [CI]: 88.8 to 92.8 percent), and 74.2 percent (95 percent CI: 71.5 to 76.9 percent) of newborns received the first dose with three days of birth (Elam-Evans et al., 2014b). Full immunization among adolescents aged 13 to 17 years, was 93.2 percent (95 percent CI: 91.8 to 94.6 percent) in 2013 (Elam-Evans et al., 2014a). The proportion of the population that has received the vaccination and is immune will improve over time, as children gradually age.
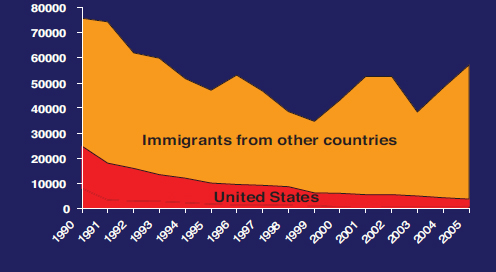
As a result, the United States has made encouraging progress against transmission of HBV, especially among children and adolescents. Reports of acute hepatitis B have declined from 8,036 cases in 2000 to 3,050 cases in 2013, a decrease largely attributable to infant and child immunization (CDC, 2013c). Acute hepatitis B is now rare in children, but remains a problem among unvaccinated adults. Adjustments for underreporting suggest an underlying incidence of acute infection at almost 20,000 cases in 2013 (CDC, 2013c). The decrease in incidence of acute hepatitis B has resulted in a decrease in new cases of chronic, domestically-acquired infection. The CDC estimates that immigration from HBV endemic countries accounts for most new chronic infections (see Figure 2-1).
Although the CDC estimates put the prevalence of chronic hepatitis B infection between 700,000 and 1.4 million, other estimates suggest more than 2 million (CDC, 2015b; Cohen et al., 2007; Kowdley et al., 2012; Wasley et al., 2010). The wide margin reflects the fact that chronic infection is often asymptomatic and borne disproportionately by immigrants from HBV-endemic countries in Asia and sub-Saharan Africa (Amiteye, 2015; Chen and Dang, 2015). Population surveys and other tools to estimate
SOURCE: Hu, 2008.
disease prevalence tend to undercount foreign-born people, who may not be proficient in English and often face barriers to accessing care (Chen and Dang, 2015; Rossi et al., 2012). It is also difficult to count the deaths attributable to hepatitis B, as its end-stage consequences (liver cirrhosis and hepatocellular carcinoma) tend to appear on death certificates without mention of the root cause (Ly et al., 2012; Manos et al., 2008).
The Hepatitis B Virus
Hepatitis B virus is a partially double-stranded, circular DNA virus surrounded by a nucleocapsid and outer envelope (Dienstag, 2008; Ganem and Prince, 2004). It replicates by reverse transcription of an RNA intermediate (Seeger et al., 1986). Measuring HBV DNA levels repeatedly over time is central to the clinical management of hepatitis B (EASL, 2012; Lok and McMahon, 2007; Terrault et al., 2016). HBV replication stimulates the host immune response, which in turn drives hepatic inflammation and progression of liver fibrosis (Bertoletti and Ferrari, 2012; Bertoletti et al., 2010). HBV DNA can integrate into the host hepatocyte genome, promoting development of hepatocellular carcinoma even without cirrhosis (Lok and McMahon, 2007; Simonetti et al., 2010; Trépo et al., 2014).
Within the nuclei of infected hepatocytes, HBV maintains a stable pool of transcriptional templates known as covalently closed circular DNA
(cccDNA). These templates are essential for viral persistence. HBV cccDNA may remain at low levels after recovery from chronic infection is thought to be responsible for HBV reactivation, however rare, during acquired immunosuppression, a phenomena discussed later in this chapter (Fattovich, 2003; Locarnini and Zoulim, 2010).
The Natural History of Hepatitis B Virus Infection
HBV infection is highly variable in both presentation and severity. Some people clear the infection spontaneously, while others suffer a lifetime of chronic complications, including hepatitis, cirrhosis, and cancer. About 70 percent of acute infections in healthy adults are asymptomatic. In the remaining 30 percent, patients have complaints such as jaundice, abdominal pain, nausea, and malaise, largely indistinguishable from other liver ailments. Severity of symptoms varies greatly, and the acute infection usually resolves without sequelae. Around 1 to 5 percent of acute infections are characterized by overwhelming liver injury leading precipitously to liver failure (called fulminant hepatitis), a likelihood increased by infection with other forms of viral hepatitis (Belongia et al., 2008; Heymann, 2014; Sako et al., 2011). Still, most HBV infection is clinically silent at the start. The serious consequences such as chronic hepatitis, fibrosis, cirrhosis, and hepatocellular carcinoma become apparent only years, even decades, later. In the meantime, people with untreated, chronic hepatitis B are a reservoir of infection to the unvaccinated (Evans et al., 2014).
People infected with HBV often carry certain antigens and antibodies in serum. Collectively these markers are called serological markers of infection. They include HBV surface antigen (HBsAg) the hallmark of active infection; HBV e antigen (HBeAg) a marker of active viral replication; HBV e antibody (anti-HBe) which reflects loss of HBeAg synthesis that can coincide with immunologic containment of infection or that can occur when viral gene mutations interfere with HBeAg synthesis; HBV core antibody (anti-HBc) a marker of past or current infection; and HBV surface antibody (anti-HBs) the marker of recovery from acute infection or immunity from vaccination (Dienstag, 2008; Lok and McMahon, 2007). Anti-HBc immunoglobulin M (IgM) appears as an antibody to the HBV core antigen during acute infection, with levels typically decreasing within 6 months despite persistence of infection (University of Washington, 2013). These markers can define different stages in clinical progression, as shown in Figure 2-2 and explained in Box 2-1.
In general, presence of HBsAg (sometimes called HBsAg positivity or written HBsAg+) indicates current viral infection; in the absence of HBsAg, the presence of anti-HBc indicates prior infection. The presence of anti-HBs indicates immunity, either natural (with anti-HBc) or vaccine-induced
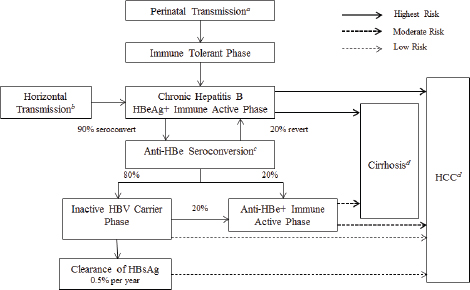
NOTES: Anti-HBe, antibody to hepatitis B e antigen; HBeAg+, hepatitis B e antigen positive; HBsAg, hepatitis B surface antigen; HBV, hepatitis B virus; HCC, hepatocellular carcinoma. a Transmission occurs in 90 percent of infants of HBsAg+/HBeAg+ mothers and 15 percent of infants of HBsAg+/anti-HBe+ mothers. b 30 percent of those infected from the age of 1-5 years and under 7 percent of those infected at the age of 6 years or older. c About 50 percent of patients by 5 years and 70 percent of patients by 10 years will seroconvert to anti-HBe. d 15-25 percent risk of premature death from cirrhosis and HCC. SOURCE: IOM, 2010.
(without anti-HBc). An anti-HBs titer of >10 mIU/ml is considered adequate for HBV immunity, though lower titers may also be protective. Table 2-1 shows patterns of different serum indicators and their clinical significance.
Predictors of Disease Progression
After exposure to HBV infection, progression from acute to chronic disease depends mainly on age and immunity. Without intervention, up to 90 percent of infants born to HBV-infected, HBeAg+ mothers become chronically infected at birth; but chronic infection develops in 5 to 20 percent of infants born to HBV-infected, HBeAg– mothers (Ko et al., 2014; Mast et al., 2006). Similarly, chronic infection develops in 90 percent of
children under five with acute HBV, but only 5 percent of acutely infected adults (Ko et al., 2014; Villeneuve, 2005).
When infection occurs in the perinatal period, babies are relatively immunologically tolerant to HBV; they generally have high circulating virus but little or no liver injury. This stage of relative immunological tolerance tends to persist for three or four decades (Schiff et al., 2012). Eventually, patients enter a relatively immunologically active phase in which liver damage emerges and persists, leading to cirrhosis and liver cancer. When acute infection occurs in healthy adults, no period of relative immunological tolerance ensues. Instead, immunologically mediated injury of virus-expressing liver cells occurs and, in all but a small proportion of patients, results in clearance of and recovery from HBV infection.
Longitudinal studies in Asia have shown that, among chronic hepatitis B patients, higher HBV DNA levels increase the risk of cirrhosis and hepatocellular carcinoma (Chen et al., 2006; Iloeje et al., 2006). Moreover,
while most chronic hepatitis B-related hepatocellular carcinomas develop after cirrhosis, about 20 percent are not preceded by cirrhosis (Trépo et al., 2014). Coinfection with hepatitis C virus (HCV) or hepatitis D virus can further accelerate liver fibrosis progression and increase the risk of hepatic decompensation and hepatocellular carcinoma in chronic infection (Crockett and Keeffe, 2005; Kushner et al., 2015). In the United States, about 5 percent of patients have chronic hepatitis B and D coinfection (Roy, 2015). The proportion of chronic hepatitis B patients coinfected with HCV is less clear, but analysis of Department of Veterans Affairs registries suggests a prevalence of 1.3 to 1.5 percent (Tyson et al., 2013).
Virus genotype also influences the course of the infection. There are at least 101 known genotypes of HBV, 8 of which are frequently studied (Locarnini, 2004). The genotypes have different geographical distributions
___________________
1 There are eight major genotypes (A through H), with two additional forms of genotype B (Bj and Ba) (Locarnini, 2004).
TABLE 2-1 Serological Markers of HBV Infection and Their Usual Significance
| IgM anti-HBc | HBeAg | ||||||||
| HBsAg | Anti-HBs | Anti-HBc | Anti-HBe | HBV DNA | |||||
| Viral Glycoprotein Coat | Antibody to HBsAg | Total Antibody to Viral Core | IgM Class Antibody to Viral Core | e Antigen, Associated with Viral Core | Antibody to HBeAg | Viral DNA | |||
| Susceptible | – | – | +/– | – | – | – | – | ||
| Natural Immunity (past infection) | – | + | + | – | Not Applicable | Not Applicable | – | ||
| Vaccine-induced Immunity | – | + | – | – | Not Applicable | Not Applicable | – | ||
| (≥10 mIU/mol considered adequate) | |||||||||
| Early Acute Infection | + | – | + | + | + | – | + | ||
| Chronic Infection | + | – | – | +/– | +/– | +/– | |||
| Presence for ≥6 months | + | ||||||||
| Resolved or Resolving Infection | – | + | + | – | – | – | – | ||
| Occult Infection | – | – | + | – | +/– | ||||
SOURCE: Evans et al., 2014.
and varying frequency of mutations associated with clinical outcomes. Because of some genotypes (and sub-genotypes) are found only in certain parts of the world, it is not always possible to study the clinical consequences of different genotypes—the patient populations are usually too different in other ways to allow for valid comparison. In places where such comparisons are possible, usually places of high endemic hepatitis B, the results are intriguing. In Taiwan, genotype C is associated with higher risk of hepatocellular carcinoma than genotype B, partly explained by the fact that genotype B is associated with higher rates of HBeAg clearance (Yang et al., 2010). Among Alaskan Natives, longitudinal studies have shown that genotypes C, D, and F are associated with higher age-specific prevalence of HBeAg compared to genotypes A and B (Livingston et al., 2007). And in sub-Saharan Africa, genotype A subtype A1 was associated with increased risk of liver cancer in younger men (McMahon, 2009).
THE FEASIBILITY OF ELIMINATING THE PUBLIC HEALTH PROBLEM OF HEPATITIS B
In general, disease elimination is a matter of reducing the basic reproductive number of the pathogen (abbreviated R0, the average number of susceptible persons infected by one infected host, given a fully susceptible population) to a value less than one, and maintaining this rate until no new infections occur. The infectious agent may still live and multiply in its host, creating what is called a reservoir of infection, so ending transmission becomes a matter of sustaining an R0 less than one until the reservoir of infection is depleted. Hepatitis B has no nonhuman reservoir and an effective vaccine. Vaccine-induced immunity to HBV is generally long-lasting with some minor age-related decline in immunogenicity. Even decades after vaccination challenge studies show strong anamnestic response with no need for a booster vaccine in otherwise healthy adults (CDC, 2012a). Among chronically infected people, antiviral drugs can reduce HBV replication to
levels that dramatically reduce liver injury, disease progression, and infectivity (Osborn and Lok, 2006). Furthermore, there are practical examples of public health programs that have proven effective at interrupting transmission (see Box 2-2). For these reasons, the committee concludes that the elimination of hepatitis B as a public health problem in the United States is feasible with certain conditions.
The conditions of the feasibility of elimination relate to some unique features of the virus. The next section will discuss how HBV can pass from mother to child, an increasingly rare but persistent problem (Ko et al., 2014). The disease is also characterized by long asymptomatic periods of chronic infection, during which time unknowing infected persons could transmit it (Anderson et al., 1992). Vaccinating all susceptible persons could prevent this transmission, but even then, there appear to be genetic factors in the host or the virus that affect the immune response (Anderson et al., 1992).
There are three main points of intervention to eliminate hepatitis B: the infection source or the infected people in the population, the susceptible hosts or those people in a population neither infected nor vaccinated, and the routes of transmission (Chen, 2010). Infection control practices such as the screening of blood, organ, and tissue donations, have done much to reduce HBV spread in the population, and would be an example of an intervention on a route of transmission. Harm reduction services (a combination of needle and syringe exchange and opiate substitution programs discussed in the next chapter) are also a known way to reduce transmission of blood-borne infection, and would be an intervention aimed at the infected people in a population (Strang et al., 2012). Similarly, though antiviral treatment can reduce viral load and therefore lower the likelihood of transmission, antiviral treatment almost never cures HBV infection (Dienstag, 2008). Patient education—simple steps such as informing HBV-infected patients why they should not share razors or toothbrushes and encouraging them to recruit their unvaccinated household members and sex partners for screening and immunization—can also help stop transmission and would be described as an intervention aimed at the susceptible hosts to the virus.
More broadly speaking, eliminating the public health problem of hepatitis B in the United States is a matter of ending transmission and preventing morbidity and mortality among people with chronic infection. This chapter discusses these two goals in turn and identifies factors critical to the success of such an endeavor. After analyzing the problem of hepatitis B in the United States, the committee concluded that control is feasible in the relatively short term. Eliminating the public health problem of hepatitis B will take more time and require considerable public will, resources, and attention to the barriers mentioned in Table 2-2.
TABLE 2-2 The Feasibility of Eliminating Hepatitis B as a Public Health Problem in the United States with Critical Factors for Success and Crosscutting Problems
| Goal | Feasibility | Critical Factors | Crosscutting Barriers | ||||||
| Ending Transmission | Perinatal | Highly feasible |
|
|
|||||
| Children | Highly feasible |
|
|||||||
| Adults | Feasible |
|
|||||||
| Reducing morbidity and mortality attributable to ongoing infection | Slowing progression to cirrhosis | Feasible |
|
||||||
| Reducing deaths | |||||||||
NOTE: cccDNA, covalently closed circular DNA; HBV, hepatitis B virus.
ENDING TRANSMISSION OF HEPATITIS B
For the most part, hepatitis B is transmitted three ways: from an infected mother to her child at birth, from direct contact with infected blood, or from unprotected sex with an infected partner. Transmission from mother to child is known as perinatal transmission; all other routes can be described as horizontal. As mentioned earlier, infection in infancy and early childhood has particularly poor prognosis. Babies born to highly viremic or HBeAg+ women have up to a 90 percent risk for chronic infection (see Figure 2-3). On the other hand, chronic infection develops in less than 5 to
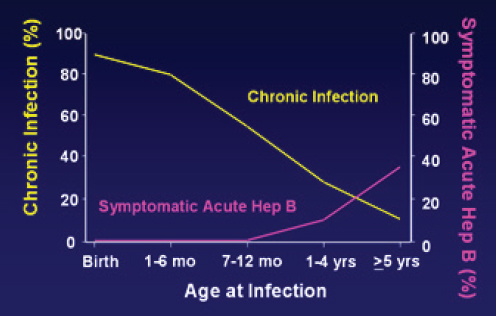
SOURCE: Morse et al., 2010.
10 percent people infected as adults, although about a third will become ill with symptoms of acute hepatitis (Liang, 2009).
Hepatitis B is unusual among carcinogens in that a vaccine exists which confers 95 percent immunity in three doses (WHO, 2015b). With perfect vaccination, hepatitis B could be eliminated in two generations (Forcione et al., 2004). In the absence of a cure, any hepatitis B elimination strategy will have to depend heavily on the vaccine’s ability to interrupt transmission. Children born to HBSAg+ mothers are a particular risk for contracting the virus, and preventing this route of transmission is entirely feasible, as, for similar reasons, is ending transmission to children. Preventing transmission among adults is more complicated, but still possible with attention to vaccine infrastructure and case detection.
Countries that have adopted universal infant and newborn hepatitis B immunization have seen marked reduction in the prevalence of hepatitis B infection and the incidence of acute hepatitis B infection in children. In China prevalence of chronic hepatitis B dropped from almost 10 percent in 1992 to 1 percent in 2006 after the vaccination campaign described in Box 2-3. Worldwide, three dose hepatitis B vaccine coverage in infants reached 82 percent in 2014, compared to one percent in 1990 (WHO, 2015a). But the birth dose of HBV vaccine, a crucial intervention for reasons discussed later in this chapter, lags behind. By WHO estimates, only 38 percent of the world’s newborns received this intervention in 2014 (WHO, 2015a). The United States could help to reduce the pool of chronically infected people in the world by supporting birth dose coverage abroad. This would also reduce the future domestic burden of chronic hepatitis B, as the majority of the chronic hepatitis B infections in the United States are imported (Weinbaum et al., 2008).
Ending Perinatal and Childhood Transmission
Barring early intervention, infants born to HBsAg+ women in the United States have an estimated 36 percent risk of contracting the virus; among women with HBV e antigen in serum, the risk rises to 90 percent (Ko et al., 2014; Smith et al., 2012; Wright, 2006). Infants who contract the virus have 90 percent risk of chronic infection (Lok and McMahon, 2007).
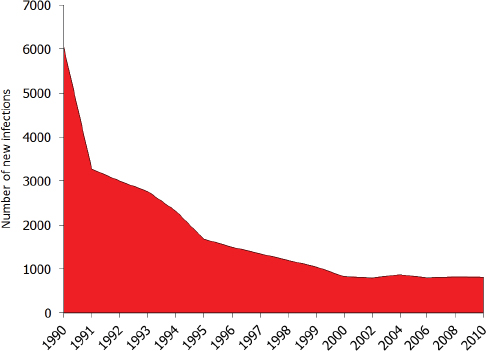
The recombinant vaccine approved in 1986 can prevent perinatal transmission of hepatitis B. In 1991 the CDC’s Advisory Committee on Immunization Practices (ACIP) recommended that all newborns receive the first dose of hepatitis B vaccine preferably before discharge from the hospital and no later than 2 months of age (CDC, 1991). The full vaccine series, completed in the first 9 to 18 months of life, confers 95 percent protection against chronic infection (WHO, 2015b). The vaccine has been immensely successful in decreasing incident infections in the United States, with the
greatest decline observed among children born since the 1991 guidelines were put into practice (see Figure 2-4). Perinatal transmission of hepatitis B is now rare in the United States, but not eliminated. Recent models suggest a frequency of 0.80-3.84 percent (Ko et al., 2014; Smith et al., 2012).
Identifying and Protecting HBV-Infected Mothers
Women with chronic hepatitis B generally have uneventful pregnancies, with no increased risk of premature delivery, fetal loss, low birth weight,
SOURCE: Ward, 2015.
congenital anomaly, or perinatal death (Park and Pan, 2014). Without immunoprophylaxis, chronic infection develops in about 40 percent of babies born to HBsAg+ women (CDC, 2015d). ACIP’s 1991 recommendations also advised routine screening of pregnant women for HBsAg, so that children of HbsAg+ women would receive a dose of the vaccine plus hepatitis B immune globulin within 12 hours of birth as post-exposure prophylaxis (CDC, 2015d). With proper prophylactic dosing, risk of chronic hepatitis B among these children dropped to between 5 and 10 percent (Tran and Keeffe, 2008).
Current national guidelines require screening all pregnant women, even those who have been vaccinated, with a TORCH titer—a serum test for toxoplasmosis and other pathogens, which usually includes hepatitis B (Ford-Jones, 1999; Mast et al., 2005; Neu et al., 2015). The American Academy of Family Physicians, the American College of Obstetricians and Gynecologists, and the American Academy of Pediatrics all support these recommendations (Lam et al., 2010). Since 1990, the Perinatal Hepatitis B
Prevention Program has expanded screening of pregnant women, and now about half of HBsAg+ women identified in pregnancy are reported to the health authorities and provided case management through this program (HHS, 2016).
High viremia increases the likelihood of perinatal transmission and immunoprophylaxis failure. Even with perfect vaccination and immune globulin dosing, about 9 or 10 percent of women with HBV DNA greater than 100 million copies/mL will pass the virus to their children (Greenup et al., 2014; Pan et al., 2012; Wiseman et al., 2009). Viremia may account for some of the 800-1000 neonates a year in the United States who become chronically infected (Ko et al., 2014). A strategy of prophylactic antiviral therapy at the start of the third trimester if the patient’s viral load is above a certain threshold might help eliminate the remaining cases of mother-to-child transmission of HBV, but the possible risks of such a strategy are not yet understood. In 2015 the American Association for the Study of Liver Disease (AASLD) made the conditional suggestion that pregnant women with a HBV viral load greater than or equal 200,000 IU receive prophylactic tenofovir in late gestation (Terrault et al., 2016). At the moment, the addition of prophylactic antivirals is not a CDC or WHO recommendation, and further data and studies are needed to determine the threshold for prophylactic antivirals in addition to immune-globulin and vaccination, as well as the duration of postpartum therapy and the risk of liver flares upon cessation of treatment.
Vaccinating Infants and Children
Timely newborn immunization is essential for stopping perinatal transmission of hepatitis B and for preventing community acquisition in childhood. In 2005, the CDC’s ACIP updated its hepatitis B vaccine recommendation, calling for universal birth dosing for all newborns, including children of HBsAg– women (Mast et al., 2005). 2014 data suggests inconsistent observance of this guideline, however. Only 64.0 percent of infants receive the vaccine within 24 hours of birth (95 percent CI: 60.9 to 67.1 percent), though state and local rates vary considerably, from 25.4 percent in Vermont (95 percent CI: 13.1 to 37.7 percent) to 81.7 percent in Alabama (95 percent CI: 68.2 to 95.2 percent) (CDC, 2014b). Nationally, about 71.0 percent of infants receive the birth dose within 2 days of birth, and 72.4 percent within 3 days (CDC, 2014b). Though cases of chronic hepatitis B in infants have declined about 75 percent since 1990, the steady, lingering infection of 800-1,000 infants is evidence for the need for better strategies to expand the birth dose and immuno-prophylaxis (CDC, 2012a; Ko et al., 2014) (see Figure 2-4).
Infants and children who miss the birth dose of vaccine can begin
SOURCE: CDC, 2013c.
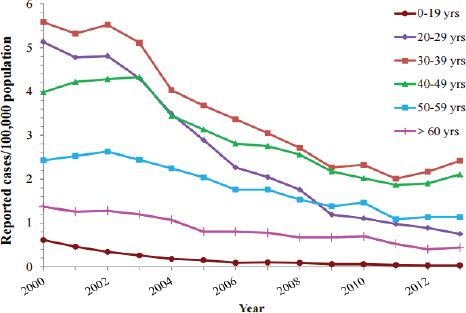
catch up vaccination at any time, with a minimum 4 week interval between the first and second doses, and a minimum 16 week interval between the first and third doses (CDC, 2015e). Childhood catch up is successful, by 2014 estimates 91.6 percent (95% CI: 89.8-93.4), of children aged 19 to 36 months were fully vaccinated against hepatitis B (Hill et al., 2015). In accordance with ACIP recommendation, 47 states now require hepatitis B immunization of children; 41 states mandate vaccination for children entering daycare, 46 states for school entry, and 39 states for middle school (Immunization Action Coalition, 2015). Widespread childhood vaccination has reduced the incidence of hepatitis B among children and adolescents to less than 1 per 100,000 from 2000 to 2013 (see Figure 2-5), a reminder that ending transmission of hepatitis B is feasible with sufficient resources and commitment (CDC, 2013c). Universal immunization of infants and children will help end transmission of HBV and protect future generations from cirrhosis and liver cancer deaths caused by chronic hepatitis B infection.
Ending Transmission in Adults
The vaccination of infants and children is clearly a national priority, cited as one of four overarching goals in the Department of Health and Hu-
SOURCE: Wasley et al., 2010.
man Services (HHS) Action Plan for the Prevention, Care, & Treatment of Viral Hepatitis (HHS, 2015a). As of 1990 federal funds were allocated to the national perinatal hepatitis B prevention program; in 1994, the Vaccines for Children program was established to provide free vaccine for children whose parents or guardians could not afford them (CDC, 2014a; Smith et al., 2012).
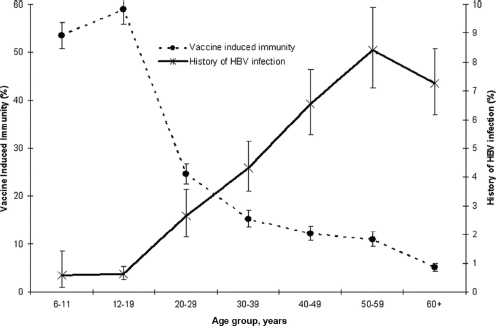
The HHS action plan cites a goal of vaccinating all adults and youths at risk for hepatitis B (HHS, 2015a). Sustaining immunity to HBV in all age groups is crucial to eliminating hepatitis B, but, as Figure 2-6 shows, the prevalence of HBV infection increases sharply after adolescence.
Vaccination of Susceptible Adults
About 95 percent of new HBV infections in the United States occur in adults (CDC, 2015f). Therefore, the CDC recommends that adults at high risk of coming into contact with HBV be immunized. Box 2-4 lists ACIP’s groups of adults recommended to receive HBV vaccination, making clear there are many adults conceivably at risk for infection, but adult vaccination coverage is low. The 2013 National Health Interview Survey conducted found adult vaccination coverage for hepatitis B was only 25 percent (95%
CI: 24.3-25.8), down 2.1 percentage points from 2012 results (Williams et al., 2015). Coverage is lower among certain minority groups; the CDC estimates that statistically significantly fewer African-American and Hispanic adults have completed the vaccine series than whites of the same age (CDC, 2013b). Even among health workers, who might be expected to have greater awareness about the importance of HBV immunization, slightly less than two-thirds are fully vaccinated (see Table 2-3).
Immunization of adults is logistically complicated; there is no good system for vaccinating people after school age. The primary responsibility for ensuring vaccination of people in high-risk groups falls on the health provider. Given the burdens already on providers discussed later in this chapter, it may be unrealistic to expect them to have the time to offer the HBV screening and vaccination to their patients, counsel them on its importance, and oversee the immunization during a brief, routine visit. Offering vaccination though organizations already in contact with people at high risk for HBV (prisons and clinics for sexually transmitted infections, for example) might be a more efficient strategy.
In Amsterdam, for example, immunization of men who have sex with men elicited a sharp decline in incidence of acute hepatitis B, despite reaching only 30 to 38 percent of the target population (van Rijckevorsel et al., 2013). The researchers attributed the decline in incidence to vaccinating a
TABLE 2-3 Estimated Proportion of Adults Aged ≥19 Years Who Received ≥3 Doses HBV Vaccine by Age Group, Race or Ethnicity,† and Other Selected Characteristics—National Health Interview Survey, United States, 2011
| Age Group or Selected Characteristic | No. in sample | % | (95% CI) | ||||||
| 19-49 years, total | 15,568 | 35.9 | (34.9-36.9) | ||||||
| 19-49 years, white | 8,256 | 37.8 | (36.5-39.2) | ||||||
| 19-49 years, black | 2,349 | 33.0 | (30.7-35.3)** | ||||||
| 19-49 years, Hispanic | 3,429 | 28.9 | (27.1-30.9)** | ||||||
| 19-49 years, Asian | 1,144 | 40.7 | (36.8-44.6) | ||||||
| 19-49 years, other | 390 | 44.1 | (38.5-49.9) | ||||||
| 19-59 years, with diabetes, overall | 1,224 | 26.9 | (23.8-30.3) | ||||||
| ≥60 years, with diabetes, overall | 1,746 | 12.4 | (10.8-14.3) | ||||||
| Health care personnel >19 years | 2564 | 63.8 | (61.4-66.2) | ||||||
NOTES: † Race or ethnicity was categorized as follows: Hispanic, black, white, Asian, and other. In this report, persons identified as Hispanic might be of any race. Persons identified as black, white, Asian, or other race are non-Hispanic. Other includes American Indian, Alaska Native, and multiple race. The five racial and ethnic categories are mutually exclusive.
** p < 0.05 by t test for comparisons with whites as the reference.
SOURCE: Adapted from CDC, 2013b.
critical mass of people most likely to transmit the virus (van Rijckevorsel et al., 2013). People at high risk for HBV infection may have relatively regular contact with staff at needle exchange sites and sexually transmitted disease clinics. Research at community health centers in Boston found 31.5 percent of patients seeking testing for HIV or sexually transmitted infection returned to the same center three times, enough to complete the HBV vaccine series (Sharfstein and Wise, 1997). Clients at a mobile needle exchange in New Haven were also amenable to vaccination. Two-thirds completed the 6-month schedule for HBV screening and vaccination (Altice et al., 2005). Jails, prisons, drug treatment centers, and clinics are all rife with missed opportunities to vaccinate for hepatitis B (Hershey et al., 2005). In the past, CDC funded state and local health departments to expand vaccination through these organizations, but these funds are now far less available (Hinman, 2005; NACCHO, 2015).
Vaccinating adults for HBV is particularly important now, as spikes in injection drug use may be leading to acute outbreaks (Iqbal et al., 2015). Incident HBV infections increased by 90 percent in Tennessee over the years from 2006 to 2011, with most new infections occurring among Caucasians in their forties and attributable to sharing injection equipment (Iqbal et al., 2015). Medical exposures may also be risk factors for acquiring HBV infection. Research among adults over 55 indicates that recently infected people have vastly increased odds of having had certain medical procedures
than matched controls (Perz et al., 2013). Compared to matched controls, cases have 2.31 greater odds of having had surgery (95 percent CI: 1.14 to 4.67 percent); 4.26 greater odds of having stayed overnight in a hospital (95 percent CI: 1.75 to 10.34 percent); 13.03 greater odds of having had dialysis (95 percent CI: 1.48 to 114.59 percent); and 23.43 greater odds of having had a blood transfusion (95 percent CI: 2.73 to 201.21 percent) (Perz et al., 2013).
The spread of HBV in medical settings is not well understood. HBV is a highly infectious virus, 50 to 100 times more infectious than HIV, and even slight lapses in infection control can result in patient-to-patient transmission (CDC, 2015c). Some infections occur in the absence of any obvious violation of infection control (Allos and Schaffner, 2007). An analysis of a 2007 case of patient-to-patient transmission at an oral surgery concluded, “the best efforts of well-meaning providers to eliminate these events will likely not completely succeed,” and advocated for universal adult vaccination as the best way to stop transmission (Allos and Schaffner, 2007, 1246).
Treatment as Prevention
Vaccination is clearly the most effective tool to prevent transmission of HBV (Mast et al., 2006). But ending transmission in adults will also require attention to the identification and treatment of chronically infected adults. The CDC recommends contact management and patient education as ways the providers and public health officials can help reduce HBV transmission (Weinbaum et al., 2008).
Eliminating transmission could also be aided by treating those with known HBV infection, a concept known as treatment as prevention. Treatment as prevention gained prominence as a way to control the spread of HIV, and was shown in a phase three clinical trials reduce sexual transmission of HIV by 96 percent (Cohen et al., 2011b; Montaner et al., 2006). Treatment as prevention is now a cornerstone of AIDS strategy around the world (ONAP, 2015; UNAIDS, 2014).
For treatment to be an effective means of prevention it should reduce viral load in bodily fluids to noninfectious levels. This reduction should be established across many populations and modes of transmission. Also, treatment should be recommended and available for everyone infected. HBV does not meet these conditions.
Nevertheless, some principles of treatment as prevention apply to HBV. Viremia in pregnant women can increase the risk of vaccination failure among children born to HBsAg+ women (del Canho et al., 1994; Ngui et al., 1998). Furthermore, treatment with antivirals has been shown to reduce perinatal transmission (Brown et al., 2016; van Zonneveld et al., 2003; Xu et al., 2009). Treatment during pregnancy has no known harmful effects
for mother or child, though the data on safety on treatment in pregnancy is limited (Brown et al., 2016).
Most research on treatment as prevention of HBV applies only to mother-to-child transmission. This is partly because HBV treatment is not recommended for all carriers. AASLD treatment guidelines recommend against antiviral therapy for adults with immune-tolerant chronic infection, despite some evidence of improved intermediate outcomes with antiviral therapy in this population (Lok et al., 2016; Terrault et al., 2016). High viremia, but normal liver enzyme levels and liver biopsy characterize the immune-tolerant phase of chronic infection. Should further research demonstrate health benefits for treating immune tolerant HBV, it would be necessary to consider whether the level of viral suppression was sufficient to reduce or eliminate transmission, and whether this treatment was cost-effective.
Given these shortcomings, treatment as prevention is not a strategy to prevent adult transmission of HBV. Patient education, vaccination, and public health management are better options. These strategies rely on early and systematic identification of chronically infected people.
The essence of public health management of HBV is the identification and immunization of susceptible people, with particular emphasis on the sex partners, household contacts, and needle sharing partners of chronically infected adults. This strategy is has only been widely applied as part of the perinatal HBV prevention program, and even then only about 26 percent of HBsAg+ women’s contacts are identified, tested, and evaluated for immunization (Weinbaum et al., 2008). Few jurisdictions have state or local programs for contact management outside the perinatal HBV program (Holmberg, 2016; Weinbaum et al., 2008). But all states and many local jurisdictions have routine partner follow up for sexually transmitted infections (Dooley, 2008). Such programs might provide a valuable infrastructure for HBV contact management.
Patient education is currently the main method used to reduce transmission of HBV to adults. This includes educating HBsAg+ people on measures they can take to reduce the risk of transmitting the virus to their contacts. Patients are also encouraged to notify their sex partners, household members, and injection drug sharing contacts so that these people can seek testing and vaccination. The actual success of patient education in reducing HBV transmission is not established, however.
PREVENTING COMPLICATIONS AND DEATHS AMONG THE CHRONICALLY INFECTED
Any discussion of disease elimination must first consider how to interrupt transmission, but this is not enough to eliminate the public health problem of hepatitis B. The 700,000 to 1.4 million HBV-infected people in the United States need appropriate care to stop the progression of the infection to cirrhosis or worse. Although there is no cure for HBV infection, antiviral treatments and careful monitoring make it reasonable to expect that no one might die from hepatitis B in the United States. This section discusses the management necessary to achieve that goal.
Slowing the Progression to Cirrhosis in Chronic HBV Infection
Action on the part of the health provider can help slow the progression of chronic hepatitis B-associated liver fibrosis and reduce the risk of cirrhosis and other liver complications. Patients in the immune-tolerant phase require ongoing monitoring for transition into the immune active phase. Such monitoring allows for early identification of possible hepatocellular carcinomas. It can also identify chronic hepatitis B patients who would benefit from antiviral treatment (Lok and McMahon, 2007; Lok et al., 2016).
It is also important to assess all chronic HBV-infected people for immunity to hepatitis A virus infection (anti-hepatitis A IgG antibody) because acute infection with hepatitis A in those with underlying chronic hepatitis B
could increase the risk of fulminant hepatitis and cause considerable patient suffering (Lok and McMahon, 2007; Vento et al., 1998). Hepatitis A vaccination is therefore recommended for all chronically infected HBV patients who are hepatitis A IgG antibody-negative (AIDSinfo, 2015). Similarly, coinfection with HCV can hasten onset of hepatic fibrosis and increase the risk of fulminant hepatitis and liver complications in chronic hepatitis B, so screening for HCV antibody is also recommended (Crockett and Keeffe, 2005; Lok and McMahon, 2007). Among those patients infected with both HBV and HCV, there is often value in antiviral treatment for the HCV infection (AASLD-IDSA, 2016). Screening for hepatitis D (delta) virus infection is recommended for hepatitis B patients with unusually severe disease and for those from countries where hepatitis D is common (Lok and McMahon, 2007). Hepatitis D only infects patients with chronic hepatitis B and may accelerate liver disease progression (Kushner et al., 2015).
Assessing alcohol intake is crucial to managing chronic hepatitis B. It is not clear what amount of alcohol hepatitis B patients can safely consume, but drinking more than 50 g (about 5 drinks) per day can accelerate progression to hepatic fibrosis; increase the risks of cirrhosis, hepatic decompensation, and hepatocellular carcinoma; and cause poor compliance with treatment (Hassan et al., 2002; Lucas et al., 2002). Providers may need to refer patients who need and want it to alcohol therapy. In the same way, over-the-counter medicines and herbal or dietary supplements can harm liver tissue already weakened by viral hepatitis. Managing these patients requires attention to their consumption of such products and, when necessary, counseling avoidance of them (Goldberg et al., 2015). Acetaminophen can be particularly toxic to the liver; doses in excess of 2 gm in a 24-hour period are discouraged for hepatitis B patients, but even this cut point is somewhat arbitrary (Lee, 2004).
Liver fibrosis is a risk factor for cirrhosis. Cirrhosis requires additional monitoring for esophageal varices and for clinical complications such as ascites, variceal hemorrhage, and hepatic encephalopathy (Benvegnù et al., 2004). While liver biopsy (a painful, risky, and expensive procedure) has been the traditional method to assess liver fibrosis, increasing evidence suggests that noninvasive analysis of serum markers and transient elastography might serve the same end (Lee et al., 2014; Leroy et al., 2014; Mayo Clinic, 2014; Myers et al., 2003; Poynard et al., 2014).
Proper medical management of hepatitis B has a clear goal of averting hepatocellular carcinoma deaths, whether by preventing the cancer or by catching it at an early stage when treatment is most effective. The AASLD guidelines suggest surveillance for hepatocellular carcinoma among certain chronic hepatitis B patients. These are Asian men over 40, Asian women over 50, African or African-Americans of any age, anyone with cirrhosis, and anyone with a family history of liver cancer (Bruix and Sherman,
2011). Caucasian patients with high HBV DNA levels and hepatic inflammation are presumed to also be at risk for hepatocellular carcinoma, although it is not clear at what age the risk becomes meaningful (Bruix and Sherman, 2011). All screening should be done with radiographic imaging, preferably abdominal ultrasound (EASL, 2012; Lok and McMahon, 2007).
Antivirals in the Treatment of Chronic Hepatitis B Virus Infection
Approved treatments of chronic hepatitis B include pegylated interferon (hereafter interferon therapy) and lamivudine, adefovir dipivoxil, telbivudine, entecavir, and tenofovir (hereafter nucleos(t)ide analogue therapy) (Terrault et al., 2016). Choosing among these treatments is complicated. Interferon, on one hand, has the advantage of finite treatment duration. It does not select resistant mutants and elicits a more durable response. At the same time, interferon is difficult for patients to tolerate and has less therapeutic success; it is also contraindicated in patients with decompensated cirrhosis (Bhattacharya and Thio, 2010; Lok et al., 2016). Tenofovir and entecavir are the currently preferred nucleos(t)ide analogue therapies because their virologic efficacy is high and risk of resistance is low (Bhattacharya and Thio, 2010; Delaney et al., 2006; Heathcote et al., 2011; Marcellin et al., 2013; Sheldon et al., 2005; Snow-Lampart et al., 2011; Terrault et al., 2016).
None of the treatments currently approved for hepatitis B cure the infection, except in a very small proportion of patients, meaning treatment does not eradicate the virus’ cccDNA from the nuclei of infected hepatocytes2 (Thio, 2009). In the absence of a cure, the goal of therapy is sustained suppression of HBV DNA to an undetectable level. This suppression reduces the likelihood of liver-related complications and the risk of horizontal transmission (Lai and Yuen, 2007; Liaw et al., 2008; Lok and McMahon, 2007; Soriano et al., 2008). Therapy can also seek to bring liver enzyme levels to normal, to induce HBeAg to anti-HBe seroconversion, to improve liver histology, and clearance of HBsAg (Terrault et al., 2016). Durable suppression of HBV replication has been shown to reverse liver fibrosis in chronic HBV infection (Calvaruso and Craxi, 2014). Still, clearance of HBsAg is unusual on nucleos(t)ide analogue therapy, and reactivation of HBV upon discontinuation of treatment is high, so once started,
___________________
2 New antiviral treatments for HBV are currently in development, including several methods targeting cccDNA (Cai et al., 2012; Cradick et al., 2010). Pharmacological elimination of cccDNA could result in virologic cure of HBV since cccDNA serves as the template for transcription of HBV RNA and for translation of HBV proteins. An alternative strategy is to prevent transcription of cccDNA to HBV RNA or to decrease the stability of HBV mRNA (Zimmerman et al., 2008). Several classes of compounds have been shown to interfere with HBV capsid assembly (Feld et al., 2007).
anti-HBV nucleos(t)ide analogue therapy is often continued indefinitely (Dore et al., 2010; Lok et al., 2016). While lifelong therapy is complicated and expensive, the risks of treatment cessation are real. Drug cessation can cause HBV flare, and risks losing the benefit accrued from prior treatment (Bellini et al., 2009; Dore et al., 2010). It is also a risk factor for hepatic decompensation (Terrault et al., 2016).
While on nucleos(t)ide analogue therapy, HBV DNA should be monitored for viral suppression every 12-24 weeks (EASL, 2012; Lok and McMahon, 2007; Terrault et al., 2016). Ideally, HBV DNA should be undetectable after 24 weeks (EASL, 2012). There is no known resistance to tenofovir so if HBV DNA is persistently detectable providers might need to inquire with their patients about treatment adherence and, if necessary, help identify strategies to support better compliance (Gordon et al., 2013; Snow-Lampart et al., 2011).
Avoiding Reactivation
Reactivation of HBV infection is an important complication of immunosuppressive treatments for cancer, organ transplant, and autoimmune diseases (Hwang and Lok, 2014). Reactivation may promote liver disease progression in patients with chronic or resolved infection (Lo Re and Schuster, 2016). HBV reactivation is characterized either by an abrupt increase in HBV DNA among HBsAg+ patients or by reappearance of serum HBV DNA in those with serological evidence of resolved infection (HBsAg-negative and anti-HBc-positive with or without anti-HBs). Reactivation is usually accompanied by elevations in liver aminotransferase levels (Hoofnagle, 2009). It is a serious complication, and one that can lead to serious acute liver injury, liver failure, and death (Lok et al., 2012).
HBV reactivation occurs because after acute infection the virus establishes cccDNA as a durable miniature chromosome within the nuclei of infected hepatocytes (Dienstag, 2008). The cccDNA may persist after recovery from chronic infection, so subsequent immunosuppression can disrupt the immune system’s ability to control the replication of the virus, and the infection becomes active again (Perrillo, 2001).
Many patients living with chronic or resolved HBV infection may at some point require immunosuppressive drug therapy for cancer, autoimmune disease, or organ transplantation, putting them at risk for reactivation. Prophylactic anti-HBV treatment with lamivudine (or better yet, entecavir or tenofovir) can greatly reduce the risk of HBV reactivation in HbsAg+ and HBsAg- and anti-HBc-positive patients on immune suppressive therapy (Loomba et al., 2008). The CDC, American Association for the Study of Liver Diseases, American Gastroenterological Association, Asian Pacific Association for the Study of the Liver, and European Association
for the Study of the Liver all recommend screening for HBV infection with HBsAg and anti-HBc in all patients receiving immunosuppressive therapy (Perrillo et al., 2015). Their rationale is that these assays are sensitive, specific, and inexpensive, and that risk-based screening methods can miss many HBV-infected patients.
Antiviral prophylaxis reduces the risk of HBV reactivation among chronic hepatitis B patients receiving immunosuppressive drug therapy for solid or liquid tumors (Dong et al., 2013; Evens et al., 2011; Paul et al., 2016). The benefits of prophylaxis for patients with resolved HBV infection who require immunosuppressive drug therapy remain less clear.
Additional study of HBV reactivation and antiviral prophylaxis is necessary. The risk of HBV reactivation with different chemotherapy regimens is not yet clear, nor is the optimal duration of antiviral prophylaxis. Immunosuppressive therapy can also be prescribed for non-malignant disorders, such as inflammatory bowel disease, rheumatoid arthritis, psoriasis, and other autoimmune conditions, but it is not clear if these treatments pose the same risk for HBV reactivation. A better understanding of these questions is necessary to avoid reactivation and prevent end-stage liver disease from HBV.
CROSSCUTTING BARRIERS TO HEPATITIS B ELIMINATION
Any progress toward the elimination of hepatitis B in the United States, either ending the transmission or improving the prognosis for people already infected, will require attention to various health system and social obstacles that keep people from being tested for HBV, prevent HBsAg+ people from accessing care, and impede the best possible provision of services.
Surveillance
Surveillance for viral hepatitis in the United States is sporadic and greatly underfunded given the scope of the epidemics and the need for accurate data to understand them. Elimination of HBV will rely on state and local jurisdictions being able to identify cases of acute and chronic HBV infection, especially among pregnant women. Identifying cases and understanding the baseline burden of disease in a community is an obvious prerequisite to spotting an outbreak; it is also essential for tracking progress toward elimination. The CDC funds comprehensive viral hepatitis surveillance in only seven jurisdictions, five states and two large cities (HHS, 2015b). The average award for these programs is $475,000; most states are unable to conduct full surveillance for HBV (CDC, 2015a).
Data from the seven comprehensive viral hepatitis surveillance sites reveals other problems with the public health infrastructure for hepatitis B. There are many serological markers of HBV (see Table 2-1), proper characterization of a patient’s status involves analysis of a panel of indicators. Inconsistencies in laboratory reporting can make it difficult to analyze a patient’s chart, a problem that only grows when multiple laboratories run tests for a single patient (Fleming et al., 2006). When the laboratory data are incomplete, the authorities cannot follow up on suspected outbreaks.
The serological maker IgM anti-HBc, described earlier in this chapter, is crucial to the identification of acute infection, as this antibody signals cases exposed in the previous six months. In people who are asymptomatic during early acute infection are not likely to seek care or be reported to the health department. The acute case definition shown in Box 2-5 also requires reporting of clinical symptoms, information health departments might not be able to obtain. Moreover, the current case classification for chronic hepatitis B requires analysis of multiple serum markers over time. Unless the health department has a highly automated disease surveillance system with synchronized laboratory reporting, tracking any one person’s results over time is exceptionally challenging. The case definition of chronic hepatitis B also depends on serological testing (see Box 2-5). Prevalence of chronic HBV infection varies widely among different state and local health departments, following the different distribution of foreign-born people and other high-risk groups. Tracking progress toward eliminating the disease will depend on good local estimates of disease burden. Increased use electronic health and laboratory records may ease case identification and advance this goal, especially if the electronic systems have a highly automated reporting function (Church et al., 2014). Hospitals and clinics also sometimes have large datasets that the health department can use to track cases and validate surveillance data (CDC, 2013a).
Public Health Case Management
Ideally, the health department surveillance group has sufficient budget and staffing to allow for case management, which includes immunization, referral to care, counseling, and clinical services. For example, when tests reveal HBsAg in women of childbearing age, the health department follows up with the woman to see if she might be pregnant, and provides intensive case management for any HBsAg+ pregnant woman and her child, a process that has helped make mother-to-child transmission of hepatitis B a rare event (Mast et al., 2005). Avoiding the 800 to 1,000 cases per year of
chronic hepatitis B in children could be advanced by better case management in all jurisdictions.
Vaccination Tracking
A strong case management system helps ensure the HBV-infected person is referred to treatment; some jurisdictions also follow up with the cases to help ensure vaccination for their close contacts. As the earlier section mentions, adult vaccination is difficult. Adult outpatient care is rife with missed opportunities for immunization in general, hepatitis B immunization in in particular (Ozisik et al., 2015; Szilagyi et al., 1993).
State immunization registries can be used to identify unvaccinated adults at risk for hepatitis B. Immunization registries are secure, population databases that collect and consolidate vaccination records from various health providers in a given area (Community Preventive Services Task Force, 2015). The systems can generate reminders when an unvaccinated patient makes an appointment; they also can provide reports on vaccination coverage within a practice or a larger geographic area. Automated immunization registries are often designed to include school and daycare immunization data, making them a useful tool to assess vaccination of children (Community Preventive Services Task Force, 2015). Registries depend on widespread enrollment and usually on active participation from providers. A 2004 CDC survey in private practices found that among 56 immunization grantees in 50 states and 6 jurisdictions, only 39 percent were actively submitting data to state or regional registries (Mast et al., 2005). By 2009, little had changed; with 38 percent of private providers participating, though variability ranged from perfect reporting in some states to only 3 percent in Hawaii (CDC, 2009). Local government support for adult registries is uneven, but there is reason to believe it is growing. By 2015, 31 jurisdictions had a law or regulation requiring some degree of reporting to immunization information systems, an almost 160 percent increase over the number of jurisdictions requiring the same in 2000 (Martin et al., 2015). Like electronic laboratory and patient record systems, immunization registries are most effective if they are designed to share information across various state and local boundaries. The ability of the information systems to work together (called interoperability) is limited in practice, however. Different vendors create widely different products, consolidating data from these systems can be impossible, and even when consistent tracking or data merging is possible, data are often labeled inconsistently, impeding aggregate analysis.
The 2009 Health Information Technology for Economic and Clinical Health Act set a national goal of meaningful use of electronic records in health (HHS, 2012). For practical purposes, the Centers for Medicare &
Medicaid Services have identified three stages of meaningful use. The first stage includes the submission of data to an immunization registry (CDC, 2012b). Participating providers establish that their electronic system can send data to public health agencies, and then to begin regular reporting. Regardless of whether the provider works in state with a requirement to report immunizations, government incentives for meaningful use of electronic systems help encourage wider use of the registries and better attention paid to them in practice.
Stigma
HBV-infected people may have a sense of shame about their condition, partly because the virus can be spread by sexual contact or injection drug use, and because liver disease in general is associated with drug and alcohol abuse. Research among cirrhotics bears this out, with younger patients reporting more stigmatizing distress over their condition than older ones (Vaughn-Sandler et al., 2014). Stigma, in turn, can cause feelings of depression and make HBV-infected people more likely to avoid medical care (Vaughn-Sandler et al., 2014). Even before patients can be brought to treatment, fear of a positive test result decreases likelihood of taking part in HBV screening (Li et al., 2012).
In the United States, most people with chronic hepatitis B come from abroad. Among East Asians, about 1 in 10 of whom have chronic hepatitis B, the stigma of the disease may be particularly severe (Philbin et al., 2012). Canadian research found that 31 percent of HBV-infected East Asians were ashamed to have HBV, and 53 percent were unwilling to discuss their illness with friends or family (Wu et al., 2009). Among Chinese immigrants in Illinois, over 20 percent believed hepatitis B infection would cause discrimination in school or at work; 36 percent felt that that people with hepatitis B bring trouble to their families (Cotler et al., 2012). Perceptions of the disease vary among different ethnic groups, but there is evidence of some consistent discrimination against HBV-infected people. When asked if they believed people avoid someone with hepatitis B, 38 percent of Vietnamese-American respondents, 55 percent of Hmong-American respondents, 47 percent of Korean-American respondents, and 70 percent of Cambodian-American respondents agreed (Maxwell et al., 2012).
It is possible to alleviate the stigma of hepatitis B, but doing so requires education and changes to social norms. As Brian McMahon of the Alaska Native Medical Center explained to the committee, “we don’t have stigma in Alaska anymore, and I think that is because people understand hepatitis B. Patients who have it understand that if they meet someone they want to have a relationship with that person can be vaccinated and they can prevent transmission. We really emphasize using barrier methods until the vaccine
series is completed, and that helps with stigma. It takes a long time, but eventually people know that they are not going to transmit the disease.” It is hard to say if reducing the stigma of HBV in Alaska was a cause or an effect of the elimination program described in Box 2-2, but it is clear that fear and silence are antithetical preventing and managing HBV.
Screening
Identifying adults infected with HBV is crucial to any elimination program. Both stopping transmission and improving prognosis for HBV-infected person depend on knowing who is infected. Though at least 700,000 to 1.4 million people in the United States have chronic hepatitis B, only about 50,000 are in treatment (Cohen et al., 2011a). With this in mind, in 2014 the US Preventive Services Task Force (USPSTF) recommended the screening of high-risk adolescents and adults, and pregnant women at first prenatal visit for HBV infection, thereby removing co-pays and co-insurance charges to people seeking this test (USPSTF, 2016).
In the United States, hepatitis B infection is greatest among people born in the HBV endemic countries of Asia and sub-Saharan Africa. More than 53,000 people immigrate to the United States every year from HBV-endemic countries (Hu et al., 2013). People born abroad are over nine times more likely than those born in the United States to be infected with chronic hepatitis B (Liu et al., 2015). The CDC therefore recommends hepatitis B screening for anyone born in country with HBsAg prevalence ≥2 percent (Weinbaum et al., 2008). Modeling exercises in Canada suggest that screening immigrants could add 1,675 productive life years to society for 250,000 people screened (Rossi et al., 2013).
Nevertheless, HBV screening is not as common as it should be. Evidence suggests that undocumented immigrants may avoid screening out a fear that a positive result, or even the interaction with the health system, could trigger deportation (Hacker et al., 2015). Other opportunities are missed for more mundane reasons. A retrospective observational study in Boston found that only 36 percent of foreign-born patients were screened for HBV infection (Waldorf et al., 2014). With this in mind, the USPSTF recommended screening for people in the risk groups shown in Box 2-6.
Improved screening for foreign-born persons would help control the progression of disease in infected individuals and prevent the further spread of infection (Petersen, 2015). Screening foreign-born individuals is a complicated proposition, however. A foreigner must live in the United States for 5 years to qualify for Medicaid; the Affordable Care Act also restricts care for temporary residents and undocumented arrivals. These restrictions mean that many people identified in screening programs may have no way to pursue treatment (Castaneda et al., 2015). The tension between the need
for screening and the onus it puts on the screener could be an obstacle to eliminating hepatitis B.
Among foreign-born people, recognized refugees are sometimes in more ready contact with the health system (Rein et al., 2010a). Some health departments screen refugees for HBV (Rein et al., 2010b). When surveyed, only 20 of these health departments were able to account for the number of refugees screened, and 13 were able to calculate the hepatitis B prevalence among refugees in their jurisdictions (Rein et al., 2010b). An analysis of their data suggest that over 2 percent of refugees in the United States have chronic hepatitis B, with prevalence among different national groups widely similar to the prevalence in their home countries (Rein et al., 2010b).
People who are already using primary care may be easier to reach. The USPSTF recommends that primary care providers screen people born places with endemic infection ≥2 percent; people whose parents were born in regions of very high HBV infection (≥8 percent); HIV+ people; people who inject drugs; men who have sex with men; and the household members or sexual partners of people with HBV infection (LeFevre, 2014). But reaching everyone at risk through primary care might not be realistic, given the demands (discussed later in this chapter) already on the primary care system. Providers are also not always aware that they should be screening. A 2008 survey among providers at federally qualified health centers serving mainly Asian-Americans and Pacific Islanders found that about two-thirds reported regularly screening patients for HBV, even though 89 percent rated hepatitis B as an “above average or huge” problem for their patients (Caballero, 2012). The limiting factor may be screening guidelines
and monitoring protocols, which only half of respondents found to be clear, and resources for treatment and referral, which only 40 percent saw as adequate (Caballero, 2012). A 2012 survey at an academic primary care practice found similar results. Approximately one-third of Asian-American patients were tested for HBV, but the primary reason for testing was usually pre-employment physicals, pregnancy or adoption requirements, and occupational exposures, not routine screening (Loo et al., 2012). And, of course, not everyone sees a primary care provider regularly or at all.
Community screening, whether in a stand-alone campaign or as part of a health fair or other community program, can be an opportunity to reach a wider audience, including many of the high-risk groups for HBV who may not otherwise have care. A survey of 55 community organizations in the United States with some involvement in HBV screening found that such screenings reached subpopulations with a 15 to 25 times greater prevalence of HBV than the United States overall (Rein et al., 2010a). Community screening poses its own challenges, however. While most community organization surveys refer HBsAg+ patients to care, only 29 percent were able to provide medical care (Rein et al., 2010a). It is difficult to link patients to care or unvaccinated people to services when the initial contact is not within the formal health system. Such contacts can be lost easily. Screening programs done through community centers may be less able to validate people’s receipt of their test results, and the screening would not generally be noted on any formal patient chart (Chen and Dang, 2015).
Enrolling and Retaining Patients in Care
Regardless of whether testing is done in community settings or through primary care, services should be linked to treatment for the patient and vaccination for his or her household contacts (HHS, 2015a). HBV infection requires ongoing, usually lifelong care. Modern medical advances mean that people should no longer die from hepatitis B, but realizing the promise of these treatments will require creative strategies to keep HBV patients in care and adhering to prescribed treatment.
Over half of the chronic hepatitis B patients in the United States are Asian-American, Native Hawaiian, or Pacific Islander, and more than half of Asian and Pacific Islanders with hepatitis B were born outside the United States (Tung, 2012). Managing health care in English can be difficult for these patients; Asian-Americans and Pacific Islanders report more problems communicating with their doctors and less positive interactions with the health system than whites (Islam et al., 2015). Lay health workers, who understand the social context and attitudes of their community, can be effective in helping reach minority patients and keep them in care (Chen and Dang, 2015; Islam et al., 2015). Although a recent systematic review
concluded that community health workers are not being sufficiently used in Asian-American and Pacific Islander communities, the authors concluded that the best strategies to employ lay health workers are not clear (Islam et al., 2015).
Though the health worker can be instrumental in screening and patient support, much of the burden of hepatitis B care lies with the managing provider. As the previous section makes clear, hepatitis B is a difficult disease to manage. A 2010 survey of San Francisco-area primary care providers found only 43 percent of respondents familiar with the AASLD guidelines for managing HBV, and about half unclear on liver cancer screening guidelines (Burman et al., 2013). A review of patient records done in conjunction with the provider survey confirmed the problems. Although about half of the records indicated some attempt at hepatocellular carcinoma screening, only a third of the screenings were done with proper imaging (Burman et al., 2013).
Most of the research documenting primary care physicians’ challenges in managing hepatitis B predate the 2014 USPSTF recommendations. This recommendation has been widely publicized (LeFevre, 2014; Mitka, 2014). It is possible that failure to implement the recommendation is a function of the limits of the primary care provider’s time. Calculations of 10 years ago (long before the USPSTF recommendations on HBV went into effect) estimated that if every recommended service were provided it would take an estimated 7.4 hours per day of the primary care provider’s time (Yarnall et al., 2003).
Electronic medical records hold promise to reduce the burden on providers, although nearly half of physicians responding to the 2014 national survey believed the electronic records impeded their efficiency (The Physicians Foundation, 2014). It is possible that electronic records, and much of the management of hepatitis B in primary care, would be best used to support team-style care. A pilot study at federally qualified health centers used electronic records to identify patients at high risk for HBV. The electronic system linked with the patients’ previous test results, and generated tools before each visit with information on managing chronic hepatitis B and counseling, prevention, and immunization for the patient’s susceptible contacts (Toyoji et al., 2015). When this information is available to team that includes a health educator, that person can take on some of the patient counseling responsibilities (Toyoji et al., 2015). The pilot program has found a 48 percent improvement in mean monthly HBV testing (Toyoji et al., 2015), consistent with earlier recommendations that that teams of health professionals might be better able to provide recommended preventative services (Ghorob and Bodenheimer, 2012; Ostbye et al., 2005).
Team based hepatitis B care has met with notable success in two practices serving a predominantly Asian-American patient base (Charles B.
Wang Community Health Center, 2011; NEMS, 2014). In San Francisco, Northeast Medical Center set a long-term goal of eliminating hepatitis B from its community (Dan, 2012). To meet that goal, the center offers free HBV screening and vaccination, thereby removing financial barriers for their uninsured patients, many of whom are Chinese immigrants (Dan, 2012). The Charles B Wang Community Health Center in New York City has similar programs; provider teams at Charles B Wang created a hepatitis B registry to link their patients’ electronic records with their records at other doctors (Dan, 2012). Hepatitis B teams at both centers include primary care physicians, case managers, health educators, and hepatologists (Dan, 2012).
Research
Hepatitis B, though not a neglected disease, would benefit from more targeted research and development. The previous section describes the problem of reactivation in patients with resolved infection, but further research is needed to identify the risk of different chemotherapy regimens as triggers for reactivation. Similarly, as much as the HBV vaccine is effective, its usefulness could be improved if it could be given in fewer or more closely timed doses. A curative treatment for the virus would clearly change the short-term likelihood of elimination. While there is evidence that such treatment may be possible, nothing of the sort is currently available (Kapoor and Kottilil, 2014; Klumpp et al., 2015).
There is also a need for implementation research in hepatitis B. While it is clear that people born abroad account for much of the adult burden of HBV, it is not clear how screening, testing, and treatment might be integrated with the immigration process or targeted services for refugee and asylee patients. These are all important gaps that would need to be better understood before elimination could be entirely feasible.
- Improved screening could help reduce complications and deaths from HBV; it could also help end transmission. Screening foreign born people would identify new infections, but restrictions on access to care could keep many of the newly identified cases from treatment.
- Screening should be accompanied by a method to enroll and retain patients in care.
- Hepatitis B is a difficult disease to manage and primary care providers are overburdened already. There is a need for better understanding of how to share patient care among teams of health personnel and make efficient use of electronic record systems.
- Research on reactivation, better vaccines, and a treatment to cure infection would facilitate HBV elimination.
REFERENCES
AASLD-IDSA (American Association for the Study of Liver Diseases-Infectious Diseases Society of America). 2016. Recommendations for testing, managing, and treating hepatitis C. http://www.hcvguidelines.org (accessed January 28, 2016).
AIDSinfo. 2015. Guidelines for prevention and treatment of opportunistic infections in HIV-infected adults and adolescents. Washington, DC: Department of Health and Human Services.
Allos, B. M., and W. Schaffner. 2007. Transmission of hepatitis B in the health care setting: The elephant in the room… or the mouse? Journal of Infectious Diseases 195(9):1245-1247.
Altice, F. L., R. D. Bruce, M. R. Walton, and M. I. Buitrago. 2005. Adherence to hepatitis B virus vaccination at syringe exchange sites. Journal of Urban Health 82(1):151-161.
Amiteye, S. 2015. African immigrants at increased risk for hepatitis B. https://blog.aids.gov/2015/04/african-immigrants-at-increased-risk-for-hepatitis-b.html (accessed February 23, 2016).
Anderson, R. M., R. M. May, and B. Anderson. 1992. The ecology and genetics of host-parasite associations. In Infectious diseases of humans: Dynamics and control. Vol. 28. Oxford: Oxford University Press.
Bellini, C., O. Keiser, J. P. Chave, J. Evison, J. Fehr, L. Kaiser, R. Weber, P. Vernazza, E. Bernasconi, A. Telenti, M. Cavassini, and Swiss H. I. V. Cohort Study. 2009. Liver enzyme elevation after lamivudine withdrawal in HIV-hepatitis B virus co-infected patients: The Swiss HIV cohort study. HIV Medicine 10(1):12-18.
Belongia, E. A., J. Costa, I. F. Gareen, J. L. Grem, J. M. Inadomi, E. R. Kern, J. A. McHugh, G. M. Petersen, M. F. Rein, M. F. Sorrell, D. B. Strader, and H. T. Trotter. 2008. NIH consensus development statement on management of hepatitis B. NIH Consens State Sci Statements 25(2):1-29.
Benvegnù, L., M. Gios, S. Boccato, and A. Alberti. 2004. Natural history of compensated viral cirrhosis: A prospective study on the incidence and hierarchy of major complications. Gut 53(5):744-749.
Bertoletti, A., and C. Ferrari. 2012. Innate and adaptive immune responses in chronic hepatitis B virus infections: Towards restoration of immune control of viral infection. Gut 61(12):1754-1764.
Bertoletti, A., M. K. Maini, and C. Ferrari. 2010. The host-pathogen interaction during HBV infection: Immunological controversies. Antiviral Therapy 15(Suppl 3):15-24.
Bhattacharya, D., and C. L. Thio. 2010. Review of hepatitis B therapeutics. Clinical Infectious Diseases 51(10):1201-1208.
Brechot, C., V. Thiers, D. Kremsdorf, B. Nalpas, S. Pol, and P. Paterlini-Brechot. 2001. Persistent hepatitis B virus infection in subjects without hepatitis B surface antigen: Clinically significant or purely “occult”? Hepatology 34(1):194-203.
Brown, R. S., Jr., B. J. McMahon, A. S. Lok, J. B. Wong, A. T. Ahmed, M. A. Mouchli, Z. Wang, L. J. Prokop, M. H. Murad, and K. Mohammed. 2016. Antiviral therapy in chronic hepatitis B viral infection during pregnancy: A systematic review and meta-analysis. Hepatology 63(1):319-333.
Bruix, J., and M. Sherman. 2011. Management of hepatocellular carcinoma: An update. Hepatology 53(3):1020-1022.
Burman, B. E., N. A. Mukhtar, B. C. Toy, T. T. Nguyen, A. H. Chen, A. Yu, P. Berman, H. Hammer, D. Chan, C. E. McCulloch, and M. Khalili. 2013. Hepatitis B management in vulnerable populations: Gaps in disease monitoring and opportunities for improved care. Digestive Diseases and Sciences 59(1):46-56.
Caballero, J. B. 2012. Hepatitis B prevention and care for Asian Americans, Native Hawaiians and Pacific Islanders at community health centers. Journal of Health Care for the Poor and Underserved 23(4):1547.
Cai, D., C. Mills, W. Yu, R. Yan, C. E. Aldrich, J. R. Saputelli, W. S. Mason, X. Xu, J. T. Guo, T. M. Block, A. Cuconati, and H. Guo. 2012. Identification of disubstituted sulfonamide compounds as specific inhibitors of hepatitis B virus covalently closed circular DNA formation. Antimicrobial Agents & Chemotherapy 56(8):4277-4288.
Calvaruso, V., and A. Craxi. 2014. Regression of fibrosis after HBV antiviral therapy. Is cirrhosis reversible? Liver International 34(Suppl 1):85-90.
Castaneda, H., S. M. Holmes, D. S. Madrigal, M. E. Young, N. Beyeler, and J. Quesada. 2015. Immigration as a social determinant of health. Annual Review of Public Health 36:375-392.
CDC (Centers for Disease Control and Prevention). 1991. Hepatitis B virus: A comprehensive strategy for eliminating transmission in the United States through universal childhood vaccination. Recommendations of the Advisory Committtee on Immunization Practices (ACIP). Morbidity and Mortality Weekly Report 40(RR-13):1-25.
CDC. 2007. Progress in hepatitis B prevention through universal infant vaccination—China, 1997-2006. Morbidity and Mortality Weekly Report 65(18):441-445.
CDC. 2008. Newborn hepatitis B vaccination coverage among children born January 2003-June 2005—United States. Morbidity and Mortality Weekly Report 57(30):825.
CDC. 2009. 2009 private provider participation table and map. http://www.cdc.gov/vaccines/programs/iis/annual-report-iisar/2009-data.html#private (accessed February 2, 2016).
CDC. 2012a. Epidemiology and prevention of vaccine-preventable diseases. 12th ed, 2nd printing ed. Washington, DC: Public Health Foundation. Pp. 115-138.
CDC. 2012b. Meaningful use and immunization information systems. http://www.cdc.gov/vaccines/programs/iis/meaningful-use (accessed February 2, 2016).
CDC. 2013a. Completeness of reporting of chronic hepatitis B and C virus infections—Michigan, 1995-2008. Morbidity and Mortality Weekly Report 62(06):99-102.
CDC. 2013b. Noninfluenza vaccination coverage among adults—United States, 2011. Morbidity and Mortality Weekly Report 62(04):66-72.
CDC. 2013c. Viral hepatitis surveillance: United States, 2013. Atlanta, GA: U.S. Centers for Disease Control and Prevention.
CDC. 2014a. About VFC. http://www.cdc.gov/vaccines/programs/vfc/about/index.html (accessed February 1, 2016).
CDC. 2014b. Estimated vaccination coverage for hepatitis B (hep B) vaccine among children from birth to age 3 days by state and immunization action plan area, National Immunization Survey (NIS), United States, 2014. http://www.cdc.gov/vaccines/imz-managers/coverage/nis/child/tables/14/tab51_hepBbirthday1-3_2014.pdf (accessed April 21, 2016).
CDC. 2015a. Full anouncement - cdc-rfa-ps13-13030401supp16 U.S. Centers for Disease Control and Prevention.
CDC. 2015b. Hepatitis B faqs for health professionals. http://www.cdc.gov/hepatitis/hbv/hbvfaq.htm#overview (accessed January 14, 2016).
CDC. 2015c. Hepatitis B FAQS for the public. http://www.cdc.gov/hepatitis/hbv/bfaq.htm (accessed February 7, 2016).
CDC. 2015d. Perinatal transmission. http://www.cdc.gov/hepatitis/hbv/perinatalxmtn.htm (accessed January 15, 2016).
CDC. 2015e. Recommended immunization schedules for persons aged 0 through 18 years, edited by Centers for Disease Control and Prevention. Atlanta, GA.
CDC. 2015f. Vaccination of adults. http://www.cdc.gov/hepatitis/hbv/vaccadults.htm (accessed January 28, 2016).
Charles B. Wang Community Health Center. 2011. Mission. http://www.cbwchc.org/mission.asp (accessed February 8, 2016).
Chen, C. J., H. I. Yang, J. Su, C. L. Jen, S. L. You, S. N. Lu, G. T. Huang, and U. H. Iloeje. 2006. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA 295(1):65-73.
Chen, D. S. 2010. Toward elimination and eradication of hepatitis B. Journal of Gastroenterology and Hepatology 25(1):19-25.
Chen, M. S., Jr., and J. Dang. 2015. Hepatitis B among Asian Americans: Prevalence, progress, and prospects for control. World Journal of Gastroenterology 21(42):11924-11930.
Church, D. R., G. A. Haney, M. Klevens, and A. DeMaria, Jr. 2014. Surveillance of viral hepatitis infections. In Concepts and methods in infectious disease surveillance, edited by N. M. M’ikanatha and J. Iskander. Hoboken, New Jersey: John Wiley & Sons. Pp. 107-121.
Cohen, C., S. D. Holmberg, B. J. McMahon, J. M. Block, C. L. Brosgart, R. G. Gish, W. T. London, and T. M. Block. 2011a. Is chronic hepatitis B being undertreated in the United States? Journal of Viral Hepatitis 18(6):377-383.
Cohen, C. A., W. T. London, A. A. Evans, J. Block, M. C. Conti, and T. Block. 2007. Underestimation of chronic hepatitis B in APIs: A call for advocacy and action. Paper read at American Public Health Association, Washington, DC, November 19, 2007.
Cohen, M. S., Y. Q. Chen, M. McCauley, T. Gamble, M. C. Hosseinipour, N. Kumarasamy, J. G. Hakim, J. Kumwenda, B. Grinsztejn, J. H. S. Pilotto, S. V. Godbole, S. Mehendale, S. Chariyalertsak, B. R. Santos, K. H. Mayer, I. F. Hoffman, S. H. Eshleman, E. PiwowarManning, L. Wang, J. Makhema, L. A. Mills, G. de Bruyn, I. Sanne, J. Eron, J. Gallant, D. Havlir, S. Swindells, H. Ribaudo, V. Elharrar, D. Burns, T. E. Taha, K. Nielsen-Saines, D. Celentano, M. Essex, and T. R. Fleming. 2011b. Prevention of HIV-1 infection with early antiretroviral therapy. New England Journal of Medicine 365(6):493-505.
Community Preventive Services Task Force. 2015. Practice brief report: Recommendation for use of immunization information systems to increase vaccination rates. Journal of Public Health Management and Practice 21(3):249-252.
Cotler, S. J., S. Cotler, H. Xie, B. J. Luc, T. J. Layden, and S. S. Wong. 2012. Characterizing hepatitis B stigma in Chinese immigrants. Journal of Viral Hepatitis 19(2):147-152.
Cradick, T. J., K. Keck, S. Bradshaw, A. C. Jamieson, and A. P. McCaffrey. 2010. Zinc-finger nucleases as a novel therapeutic strategy for targeting hepatitis B virus dnas. Molecular Therapy 18(5):947-954.
Crockett, S. D., and E. B. Keeffe. 2005. Natural history and treatment of hepatitis B virus and hepatitis C virus coinfection. Annals of Clinical Microbiology and Antimicrobials 4:1.
Cui, F., L. Li, S. C. Hadler, F. Wang, H. Zheng, Y. Chen, X. Gong, Y. J. Hutin, K. L. Cairns, and X. Liang. 2010. Factors associated with effectiveness of the first dose of hepatitis B vaccine in China: 1992–2005. Vaccine 28(37):5973-5978.
Dan, C. 2012. Addressing hepatitis B: Community health centers, partnerships and the affordable care act. Journal of Health Care for the Poor and Underserved 23(2):507.
del Canho, R., P. M. Grosheide, S. W. Schalm, R. R. de Vries, and R. A. Heijtink. 1994. Failure of neonatal hepatitis B vaccination: The role of HBV-DNA levels in hepatitis B carrier mothers and HLA antigens in neonates. Journal of Hepatology 20(4):483-486.
Delaney, W. E., A. S. Ray, H. Yang, X. Qi, S. Xiong, Y. Zhu, and M. D. Miller. 2006. Intracellular metabolism and in vitro activity of tenofovir against hepatitis B virus. Antimicrobial Agents & Chemotherapy 50(7):2471-2477.
Dienstag, J. L. 2008. Hepatitis B virus infection. New England Journal of Medicine 359(14): 1486-1500.
Dong, H. J., L. N. Ni, G. F. Sheng, H. L. Song, J. Z. Xu, and Y. Ling. 2013. Risk of hepatitis B virus (HBV) reactivation in non-Hodgkin lymphoma patients receiving rituximabchemotherapy: A meta-analysis. Journal of Clinical Virology 57(3):209-214.
Dooley, S. W. 2008. Recommendations for partner services programs for HIV infection, syphilis, gonorrhea, and chlamydial infection. Morbidity and Mortality Weekly Report 57(RR09):1-63.
Dore, G. J., V. Soriano, J. Rockstroh, B. Kupfer, E. Tedaldi, L. Peters, J. Neuhaus, M. Puoti, M. B. Klein, A. Mocroft, B. Clotet, and J. D. Lundgren. 2010. Frequent hepatitis B virus rebound among HIV-hepatitis B virus-coinfected patients following antiretroviral therapy interruption. AIDS 24(6):857-865.
EASL (European Association for the Study of the Liver). 2012. EASL clinical practice guidelines: Management of chronic hepatitis B virus infection. Journal of Hepatology 57(1):167-185.
Elam-Evans, L. D., D. Yankey, J. Jeyarajah, J. A. Singleton, R. Curtis, J. MacNeil, and S. Hariri. 2014a. National, regional, state, and selected local area vaccination coverage among adolescents aged 13-17 years—United States, 2013. Morbidity and Mortality Weekly Report 63(29):625-633.
Elam-Evans, L. D., D. Yankey, J. A. Singleton, and M. Kolasa. 2014b. National, state, and selected local area vaccination coverage among children aged 19-35 months—United States, 2013. Morbidity and Mortality Weekly Report 63(34):741-748.
Evans, A. A., C. Cohen, and T. M. Block. 2014. Hepatitis viruses: Hepatitis B and hepatitis D. In Viral infections of humans: Epidemiology and control, edited by R. A. Kaslow, L. R. Stanberry, and J. W. L. Duc. New York: Springer US. Pp. 747-764.
Evens, A. M., B. D. Jovanovic, Y. C. Su, D. W. Raisch, D. Ganger, S. M. Belknap, M. S. Dai, B. C. Chiu, B. Fintel, Y. Cheng, S. S. Chuang, M. Y. Lee, T. Y. Chen, S. F. Lin, and C. Y. Kuo. 2011. Rituximab-associated hepatitis B virus (HBV) reactivation in lymphoproliferative diseases: Meta-analysis and examination of FDA safety reports. Annals of Oncology 22(5):1170-1180.
Fattovich, G. 2003. Natural history and prognosis of hepatitis B. Seminars in Liver Disease 23(1):47-58.
Fattovich, G., F. Bortolotti, and F. Donato. 2008. Natural history of chronic hepatitis B: Special emphasis on disease progression and prognostic factors. Journal of Hepatology 48(2):335-352.
Feld, J. J., D. Colledge, V. Sozzi, R. Edwards, M. Littlejohn, and S. A. Locarnini. 2007. The phenylpropenamide derivative at-130 blocks HBV replication at the level of viral RNA packaging. Antiviral Research 76(2):168-177.
Fleming, D. T., A. Zambrowski, F. Fong, A. Lombard, L. Mercedes, C. Miller, J. Poujade, A. Roome, A. Sullivan, and L. Finelli. 2006. Surveillance programs for chronic viral hepatitis in three health departments. Public Health Reports 121(1):23-35.
Forcione, D. G., R. T. Chung, and J. L. Dienstag. 2004. Natural history of hepatitis B virus infection. In Chronic viral hepatitis, edited by R. S. Koff and G. Y. Wu. Totowa, NJ: Humana Press. P. 53.
Ford-Jones, E. L. 1999. An approach to the diagnosis of congenital infections. Paediatrics & Child Health 4(2):109-112.
Ganem, D., and A. M. Prince. 2004. Hepatitis B virus infection—natural history and clinical consequences. New England Journal of Medicine 350(11):1118-1129.
Ghorob, A., and T. Bodenheimer. 2012. Sharing the care to improve access to primary care. New England Journal of Medicine 366(21):1955-1957.
Goldberg, D. S., K. A. Forde, D. M. Carbonari, J. D. Lewis, K. B. Leidl, K. R. Reddy, K. Haynes, J. Roy, D. Sha, A. R. Marks, J. L. Schneider, B. L. Strom, D. A. Corley, and V. Lo Re, III. 2015. Population-representative incidence of drug-induced acute liver failure based on an analysis of an integrated health care system. Gastroenterology 148(7):1353-1361.
Gordon, S. C., Z. Krastev, A. Horban, J. Petersen, J. Sperl, P. Dinh, E. B. Martins, L. J. Yee, J. F. Flaherty, K. M. Kitrinos, V. K. Rustgi, and P. Marcellin. 2013. Efficacy of tenofovir disoproxil fumarate at 240 weeks in patients with chronic hepatitis B with high baseline viral load. Hepatology 58(2):505-513.
Greenup, A.-J., P. K. Tan, V. Nguyen, A. Glass, S. Davison, U. Chatterjee, S. Holdaway, D. Samarasinghe, K. Jackson, and S. A. Locarnini. 2014. Efficacy and safety of tenofovir disoproxil fumarate in pregnancy to prevent perinatal transmission of hepatitis B virus. Journal of Hepatology 61(3):502-507.
Hacker, K., M. Anies, B. L. Folb, and L. Zallman. 2015. Barriers to health care for undocumented immigrants: A literature review. Risk Management and Healthcare Policy 8:175-183.
Hassan, M. M., L. Y. Hwang, C. J. Hatten, M. Swaim, D. Li, J. L. Abbruzzese, P. Beasley, and Y. Z. Patt. 2002. Risk factors for hepatocellular carcinoma: Synergism of alcohol with viral hepatitis and diabetes mellitus. Hepatology 36(5):1206-1213.
Heathcote, E. J., P. Marcellin, M. Buti, E. Gane, R. A. De Man, Z. Krastev, G. Germanidis, S. S. Lee, R. Flisiak, K. Kaita, M. Manns, I. Kotzev, K. T chernev, P. Buggisch, F. Weilert, O. O. Kurdas, M. L. Shiffman, H. Trinh, S. Gurel, A. Snow-Lampart, K. Borroto-Esoda, E. Mondou, J. Anderson, J. Sorbel, and F. Rousseau. 2011. Three-year efficacy and safety of tenofovir disoproxil fumarate treatment for chronic hepatitis B. Gastroenterology 140(1):132-143.
Hershey, J. H., L. Schowalter, and S. B. Bailey. 2005. Public health perspective on vaccine-preventable hepatitis: Integrating hepatitis A and B vaccines into public health settings. The American Journal of Medicine 118(10):100-108.
Heymann, D. 2014. Hepatitis, viral. In Control of communicable diseases manual. 20 ed, edited by D. Heymann. Washington, DC: APHA Press.
Heyward, W. L., T. R. Bender, B. J. McMahon, D. B. Hall, D. P. Francis, A. P. Lanier, W. L. Alward, J. L. Ahtone, B. L. Murphy, and J. E. Maynard. 1985. The control of hepatitis B virus infection with vaccine in Yupik Eskimos. Demonstration of safety, immunogenicity, and efficacy under field conditions. American Journal of Epidemiology 121(6):914-923.
HHS (Department of Health and Human Services). 2012. HITECH Act enforcement interim final rule. http://www.hhs.gov/hipaa/for-professionals/special-topics/HITECHact-enforcement-interim-final-rule/index.html (accessed February 2, 2016).
HHS. 2015a. Action plan for the prevention, care, & treatment of viral hepatitis: Updated 2014-2016. Washington, DC: Department of Health and Human Services.
HHS. 2015b. Viral hepatitis—prevention and surveillance. https://www.acf.hhs.gov/hhsgrantsforecast/index.cfm?switch=grant.view&gff_grants_forecastInfoID=100000600 (accessed February 9, 2016).
HHS. 2016. Perinatal hepatitis B prevention program. https://www.healthypeople.gov/2020/data-source/perinatal-hepatitis-b-prevention-program (accessed January 15, 2016).
Hill, H. A., L. D. Elam-Evans, D. Yankey, J. A. Singleton, and M. Kolasa. 2015. National, state, and selected local area vaccination coverage among children aged 19-35 months—United States, 2014. Atlanta, GA: CDC.
Hinman, A. R. 2005. Addressing the vaccine financing conundrum. Health Affairs 24(3): 701-704.
Holmberg, S. 2016. Re: Please respond - question for IOM report, January 21, 2016.
Hoofnagle, J. H. 2009. Reactivation of hepatitis B. Hepatology 49(5 Suppl):S156-S165.
Hu, D. J. 2008. Issues related to the prevention and control of hepatitis B virus (HBV) infection in the U.S. Paper presented at IOM Roundtable on the Prevention and Control of Viral Hepatitis Infection, Washington, DC.
Hu, D. J., J. Xing, R. A. Tohme, Y. Liao, H. Pollack, J. W. Ward, and S. D. Holmberg. 2013. Hepatitis B testing and access to care among racial and ethnic minorities in selected communities across the United States, 2009-2010. Hepatology 58(3):856-862.
Hwang, J. P., and A. S. F. Lok. 2014. Management of patients with hepatitis B who require immunosuppressive therapy. Nature Reviews: Gastroenterology & Hepatology 11(4):209-219.
Iloeje, U. H., H. I. Yang, J. Su, C. L. Jen, S. L. You, and C. J. Chen. 2006. Predicting cirrhosis risk based on the level of circulating hepatitis B viral load. Gastroenterology 130(3):678-686.
Immunization Action Coalition. 2015. State information: Hepatitis B prevention mandates for daycare and K-12. http://www.immunize.org/laws/hepb.asp (accessed December 23, 2015).
IOM (Institute of Medicine). 2010. Hepatitis and liver cancer: A national strategy for prevention and control of hepatitis B and C. Washington, DC: The National Academies Press.
Iqbal, K., R. M. Klevens, M. A. Kainer, J. Baumgartner, K. Gerard, T. Poissant, K. Sweet, C. Vonderwahl, T. Knickerbocker, Y. Khudyakov, G. L. Xia, H. Roberts, and E. Teshale. 2015. Epidemiology of acute hepatitis B in the United States from population-based surveillance, 2006-2011. Clinical Infectious Diseases 61(4):584-592.
Islam, N. S., J. M. Zanowiak, L. Riley, S. K. Nadkarni, S. C. Kwon, and C. Trinh-Shevrin. 2015. Characteristics of Asian American, Native Hawaiian, and Pacific Islander community health worker programs: A systematic review. Journal of Health Care for the Poor and Underserved 26(2 Suppl):238-268.
Kapoor, R., and S. Kottilil. 2014. Strategies to eliminate HBV infection. Future Virology 9(6):565-585.
Klumpp, K., A. M. Lam, C. Lukacs, R. Vogel, S. Ren, C. Espiritu, R. Baydo, K. Atkins, J. Abendroth, G. Liao, A. Efimov, G. Hartman, and O. A. Flores. 2015. High-resolution crystal structure of a hepatitis B virus replication inhibitor bound to the viral core protein. Proceedings of the National Academy of Sciences of the United States of America 112(49):15196-15201.
Ko, S. C., L. Fan, E. A. Smith, N. Fenlon, A. K. Koneru, and T. V. Murphy. 2014. Estimated annual perinatal hepatitis B virus infections in the United States, 2000-2009. Journal of the Pediatric Infectious Diseases Society. doi: 10.1093/jpids/piu115.
Kowdley, K. V., C. C. Wang, S. Welch, H. Roberts, and C. L. Brosgart. 2012. Prevalence of chronic hepatitis B among foreign-born persons living in the United States by country of origin. Hepatology 56(2):422-433.
Kushner, T., M. Serper, and D. E. Kaplan. 2015. Delta hepatitis within the veterans affairs medical system in the United States: Prevalence, risk factors, and outcomes. Journal of Hepatology 63(3):586-592.
Lai, C. L., and M. F. Yuen. 2007. The natural history and treatment of chronic hepatitis B: A critical evaluation of standard treatment criteria and end points. Annals of Internal Medicine 147(1):58-61.
Lam, N. C., P. B. Gotsch, and R. C. Langan. 2010. Caring for pregnant women and newborns with hepatitis B or C. American Family Physician 82(10):1225-1229.
Lee, H. W., E. J. Yoo, B. K. Kim, S. U. Kim, J. Y. Park, Y. Kim do, S. H. Ahn, and K. H. Han. 2014. Prediction of development of liver-related events by transient elastography in hepatitis B patients with complete virological response on antiviral therapy. American Journal of Gastroenterology 109(8):1241-1249.
Lee, W. M. 2004. Acetaminophen and the U.S. acute liver failure study group: Lowering the risks of hepatic failure. Hepatology 40(1):6-9.
LeFevre, M. L. 2014. Screening for hepatitis B virus infection in nonpregnant adolescents and adults: U.S. Preventive Services task force recommendation statement. Annals of Internal Medicine 161(1):58-66.
Leroy, V., N. Sturm, P. Faure, C. Trocme, A. Marlu, M. N. Hilleret, F. Morel, and J. P. Zarski. 2014. Prospective evaluation of fibrotest®, fibrometer®, and hepascore® for staging liver fibrosis in chronic hepatitis B: Comparison with hepatitis C. Journal of Hepatology 61(1):28-34.
Li, D., T. Tang, M. Patterson, M. Ho, J. Heathcote, and H. Shah. 2012. The impact of hepatitis B knowledge and stigma on screening in Canadian Chinese persons. Canadian Journal of Gastroenterology 26(9):597-602.
Liang, T. J. 2009. Hepatitis B: The virus and disease. Hepatology (Baltimore, Md.) 49(5 Suppl):S13-S21.
Liang, X., S. Bi, W. Yang, L. Wang, G. Cui, F. Cui, Y. Zhang, J. Liu, X. Gong, and Y. Chen. 2009. Epidemiological serosurvey of hepatitis B in China—declining HBV prevalence due to hepatitis B vaccination. Vaccine 27(47):6550-6557.
Liaw, Y. F., N. Leung, J. H. Kao, T. Piratvisuth, E. Gane, K. H. Han, R. Guan, G. K. Lau, and S. Locarnini. 2008. Asian-Pacific consensus statement on the management of chronic hepatitis B: A 2008 update. Hepatology International 2(3):263-283.
Liu, S. J., K. Iqbal, S. Shallow, S. Speers, E. Rizzo, K. Gerard, T. Poissant, and R. M. Klevens. 2015. Characterization of chronic hepatitis B cases among foreign-born persons in six population-based surveillance sites, United States 2001-2010. Journal of Immigration and Minority Health 17(1):7-12.
Livingston, S. E., J. P. Simonetti, L. R. Bulkow, C. E. Homan, M. M. Snowball, H. H. Cagle, S. E. Negus, and B. J. McMahon. 2007. Clearance of hepatitis B e antigen in patients with chronic hepatitis B and genotypes A, B, C, D, and F. Gastroenterology 133(5):1452-1457.
Lo Re, V., III, and M. Schuster. 2016. Evaluating hepatitis B virus reactivation during solid tumor chemotherapy: Evidence to guide pretreatment hepatitis B screening and prophylaxis. Annals of Internal Medicine 164(1):64-65.
Lo Re, V., III, I. Frank, R. Gross, J. Dockter, J. M. Linnen, C. Giachetti, P. Tebas, J. Stern, M. Synnestvedt, A. R. Localio, J. R. Kostman, and B. L. Strom. 2007. Prevalence, risk factors, and outcomes for occult hepatitis B virus infection among HIV-infected patients. Journal of Acquired Immune Deficiency Syndromes 44(3):315-320.
Lo Re, V., III, B. Wertheimer, A. R. Localio, J. R. Kostman, J. Dockter, J. M. Linnen, C. Giachetti, Z. Dorey-Stein, I. Frank, B. L. Strom, and R. Gross. 2008. Incidence of transaminitis among HIV-infected patients with occult hepatitis B. Journal of clinical virology: The official publication of the Pan American Society for Clinical Virology 43(1):32-36.
Locarnini, S. 2004. Molecular virology of hepatitis B virus. Seminars in Liver Disease 24(Suppl 1):3-10.
Locarnini, S., and F. Zoulim. 2010. Molecular genetics of HBV infection. Antiviral Therapy 15(Suppl 3):3-14.
Lok, A. S., and B. J. McMahon. 2007. Chronic hepatitis B. Hepatology 45(2):507-539.
Lok, A. S., J. W. Ward, R. P. Perrillo, B. J. McMahon, and T. J. Liang. 2012. Reactivation of hepatitis B during immunosuppressive therapy: Potentially fatal yet preventable. Annals of Internal Medicine 156(10):743-745.
Lok, A. S., B. J. McMahon, R. S. Brown, Jr., J. B. Wong, A. T. Ahmed, W. Farah, J. Almasri, F. Alahdab, K. Benkhadra, M. A. Mouchli, S. Singh, E. A. Mohamed, A. M. Abu Dabrh, L. J. Prokop, Z. Wang, M. H. Murad, and K. Mohammed. 2016. Antiviral therapy for chronic hepatitis B viral infection in adults: A systematic review and meta-analysis. Hepatology 63(1):284-306.
Loo, N. M., W. Kim, J. J. Larson, M. L. Wieland, and R. Chaudhry. 2012. Hepatitis B screening in a U.S. academic primary care practice. Archives of Internal Medicine 172(19): 1517-1519.
Loomba, R., A. Rowley, R. Wesley, T. J. Liang, J. H. Hoofnagle, F. Pucino, and G. Csako. 2008. Systematic review: The effect of preventive lamivudine on hepatitis B reactivation during chemotherapy. Annals of Internal Medicine 148(7):519-528.
Lucas, G. M., K. A. Gebo, R. E. Chaisson, and R. D. Moore. 2002. Longitudinal assessment of the effects of drug and alcohol abuse on HIV-1 treatment outcomes in an urban clinic. AIDS 16(5):767-774.
Ly, K. N., J. Xing, R. M. Klevens, R. B. Jiles, J. W. Ward, and S. D. Holmberg. 2012. The increasing burden of mortality from viral hepatitis in the United States between 1999 and 2007. Annals of Internal Medicine 156(4):271-278.
Manos, M. M., W. A. Leyden, R. C. Murphy, N. A. Terrault, and B. P. Bell. 2008. Limitations of conventionally derived chronic liver disease mortality rates: Results of a comprehensive assessment. Hepatology 47(4):1150-1157.
Marcellin, P., E. Gane, M. Buti, N. Afdhal, W. Sievert, I. M. Jacobson, M. K. Washington, G. Germanidis, J. F. Flaherty, R. A. Schall, J. D. Bornstein, K. M. Kitrinos, G. M. Subramanian, J. G. McHutchison, and E. J. Heathcote. 2013. Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: A 5-year open-label follow-up study. Lancet 381(9865):468-475.
Martin, D. W., N. E. Lowery, B. Brand, R. Gold, and G. Horlick. 2015. Immunization information systems: A decade of progress in law and policy. Journal of Public Health Management and Practice 21(3):296-303.
Mast, E. E., H. S. Margolis, A. E. Fiore, E. W. Brink, S. T. Goldstein, S. A. Wang, L. A. Moyer, B. P. Bell, and M. J. Alter. 2005. A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: Recommendations of the Advisory Committee on Immunization Practices (ACIP) part 1: Immunization of infants, children, and adolescents. Atlanta, GA: Centers for Disease Control and Prevention.
Mast, E. E., C. M. Weinbaum, A. E. Fiore, M. J. Alter, B. P. Bell, L. Finelli, L. E. Rodewald, M. D. John Jr., R. S. Janssen, and J. W. Ward. 2006. A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: Recommendations of the Advisory Committee on Immunization Practices (ACIP) part ii: Immunization of adults. Morbidity and Mortality Weekly Report 55(RR16):1-25.
Maxwell, A. E., S. L. Stewart, B. A. Glenn, W. K. Wong, Y. Yasui, L. C. Chang, V. M. Taylor, T. T. Nguyen, M. S. Chen, and R. Bastani. 2012. Theoretically informed correlates of hepatitis B knowledge among four Asian groups: The health behavior framework. Asian Pacific Journal of Cancer Prevention 13(4):1687-1692.
Mayo Clinic. 2014. Liver biopsy. http://www.mayoclinic.org/tests-procedures/liver-biopsy/basics/risks/prc-20012588 (accessed February 7, 2016).
McMahon, B. J. 2009. The natural history of chronic hepatitis B virus infection. Hepatology 49(5 Suppl):S45-S55.
McMahon, B., W. Heyward, D. Ritter, R. Wainwright, E. Rhoades, E. Tower, A. Lanier, and C. Helminiak. 1987. A comprehensive programme to reduce the incidence of hepatitis B virus infection and its sequelae in Alaskan Natives. Lancet 330(8568):1134-1136.
McMahon, B. J., L. R. Bulkow, R. J. Singleton, J. Williams, M. Snowball, C. Homan, and A. J. Parkinson. 2011. Elimination of hepatocellular carcinoma and acute hepatitis B in children 25 years after a hepatitis B newborn and catch-up immunization program. Hepatology 54(3):801-807.
Mitka, M. 2014. USPSTF advises HBV screening for U.S. groups at high risk of infection. JAMA 311(10):1004.
Montaner, J. S., R. Hogg, E. Wood, T. Kerr, M. Tyndall, A. R. Levy, and P. R. Harrigan. 2006. The case for expanding access to highly active antiretroviral therapy to curb the growth of the HIV epidemic. Lancet 368(9534):531-536.
Morse, S. A., K. K. Holmes, and R. C. Ballard. 2010. Atlas of sexually transmitted diseases and AIDS. 4th ed. United Kingdom of Great Britain and Northern Ireland: Saunders.
Myers, R. P., M. H. Tainturier, V. Ratziu, A. Piton, V. Thibault, F. Imbert-Bismut, D. Messous, F. Charlotte, V. Di Martino, Y. Benhamou, and T. Poynard. 2003. Prediction of liver histological lesions with biochemical markers in patients with chronic hepatitis B. Journal of Hepatology 39(2):222-230.
NACCHO (National Association of County and City Health Officials). 2015. Local health departments prevent disease through immunization, edited by National Association of County and City Officials. Washington, DC: NACCHO.
NEMS (North East Medical Services). 2014. Key statistics. http://www.nems.org/aboutStatistics.html (accessed February 8, 2016).
Neu, N., J. Duchon, and P. Zachariah. 2015. TORCH infections. Clinics in Perinatology 42(1):77-103.
Ngui, S. L., N. J. Andrews, G. S. Underhill, J. Heptonstall, and C. G. Teo. 1998. Failed postnatal immunoprophylaxis for hepatitis B: Characteristics of maternal hepatitis B virus as risk factors. Clinical Infectious Diseases 27(1):100-106.
ONAP (Office of National AIDS Policy). 2015. National HIV/AIDS strategy for the United States: Updated to 2020. Washington, DC: The White House.
Osborn, M. K., and A. S. Lok. 2006. Antiviral options for the treatment of chronic hepatitis B. Journal of Antimicrobial Chemotherapy 57(6):1030-1034.
Ostbye, T., K. S. Yarnall, K. M. Krause, K. I. Pollak, M. Gradison, and J. L. Michener. 2005. Is there time for management of patients with chronic diseases in primary care? Annals of Family Medicine 3(3):209-214.
Ozisik, L., M. D. Tanriover, N. Calik Basaran, S. G. Oz, and S. Unal. 2015. Missed opportunities for hepatitis B vaccination among diabetic patients. Human Vaccines & Immunotherapeutics 11(12):2806-2810.
Pan, C. Q., L. J. Mi, C. Bunchorntavakul, J. Karsdon, W. M. Huang, G. Singhvi, M. G. Ghany, and K. R. Reddy. 2012. Tenofovir disoproxil fumarate for prevention of vertical transmission of hepatitis B virus infection by highly viremic pregnant women: A case series. Digestive Diseases and Sciences 57(9):2423-2429.
Papatheodoridis, G. V., S. Manolakopoulos, Y.-F. Liaw, and A. Lok. 2012. Follow-up and indications for liver biopsy in HBeAg-negative chronic hepatitis B virus infection with persistently normal ALT: A systematic review. Journal of Hepatology 57(1):196-202.
Park, J., and C. Pan. 2014. Current recommendations of managing HBV infection in preconception or pregnancy. Frontiers of Medicine 8(2):158-165.
Paul, S., A. Saxena, N. Terrin, K. Viveiros, E. M. Balk, and J. B. Wong. 2016. Hepatitis B virus reactivation and prophylaxis during solid tumor chemotherapy: A systematic review and meta-analysis. Annals of Internal Medicine 164(1):30-40.
Perrillo, R. P. 2001. Acute flares in chronic hepatitis B: The natural and unnatural history of an immunologically mediated liver disease. Gastroenterology 120(4):1009-1022.
Perrillo, R. P., P. Martin, and A. S. Lok. 2015. Preventing hepatitis B reactivation due to immunosuppressive drug treatments. JAMA 313(16):1617-1618.
Perz, J. F., S. Grytdal, S. Beck, A. M. Fireteanu, T. Poissant, E. Rizzo, K. Bornschlegel, A. Thomas, S. Balter, J. Miller, R. M. Klevens, and L. Finelli. 2013. Case-control study of hepatitis B and hepatitis C in older adults: Do healthcare exposures contribute to burden of new infections? Hepatology 57(3):917-924.
Petersen, E. 2015. Should we offer screening for hepatitis B and other infections to immigrants—legal or illegal? Journal of Travel Medicine 22(2):73-75.
Philbin, M. M., L. A. H. Erby, S. Lee, and H.-S. Juon. 2012. Hepatitis B and liver cancer among three Asian American sub-groups: A focus group inquiry. Journal of Immigrant and Minority Health/Center for Minority Public Health 14(5):858-868.
The Physicians Foundation. 2014. 2014 survey of America’s physicians. Dallas, TX.
Pita, I., A. M. Horta-Vale, H. Cardoso, and G. Macedo. 2014. Hepatitis B inactive carriers: An overlooked population? GE Portuguese Journal of Gastroenterology 21(6):241-249.
Poynard, T., J. Vergniol, Y. Ngo, J. Foucher, V. Thibault, M. Munteanu, W. Merrouche, P. Lebray, M. Rudler, O. Deckmyn, H. Perazzo, D. Thabut, V. Ratziu, and V. de Ledinghen. 2014. Staging chronic hepatitis B into seven categories, defining inactive carriers and assessing treatment impact using a fibrosis biomarker (fibrotest®) and elastography (fibroscan®). Journal of Hepatology 61(5):994-1003.
Rein, D. B., S. B. Lesesne, P. J. Leese, and C. M. Weinbaum. 2010a. Community-based hepatitis B screening programs in the United States in 2008. Journal of Viral Hepatitis 17(1):28-33.
Rein, D. B., S. B. Lesesne, A. O’Fallon, and C. M. Weinbaum. 2010b. Prevalence of hepatitis B surface antigen among refugees entering the United States between 2006 and 2008. Hepatology 51(2):431-434.
Rossi, C., I. Shrier, L. Marshall, S. Cnossen, K. Schwartzman, M. B. Klein, G. Schwarzer, and C. Greenaway. 2012. Seroprevalence of chronic hepatitis B virus infection and prior immunity in immigrants and refugees: A systematic review and meta-analysis. PLoS ONE 7(9):e44611.
Rossi, C., K. Schwartzman, O. Oxlade, M. B. Klein, and C. Greenaway. 2013. Hepatitis B screening and vaccination strategies for newly arrived adult Canadian immigrants and refugees: A cost-effectiveness analysis. PLoS ONE 8(10):e78548.
Roy, P. K. 2015. Hepatitis D. http://emedicine.medscape.com/article/178038-overview (accessed February 7, 2016).
Sako, A., H. Yasunaga, H. Horiguchi, H. Hashimoto, N. Masaki, and S. Matsuda. 2011. Acute hepatitis B in Japan: Incidence, clinical practices and health policy. Hepatology Research 41(1):39-45.
Schiff, E. R., W. C. Maddrey, and M. F. Sorrell. 2012. Schiff’s diseases of the liver. 11th ed. Oxford, UK: Wiley-Blackwell.
Schreeder, M. T., T. R. Bender, B. J. McMahon, M. R. Moser, B. L. Murphy, M. J. Sheller, W. L. Heyward, D. B. Hall, and J. E. Maynard. 1983. Prevalence of hepatitis B in selected Alaskan Eskimo villages. American Journal of Epidemiology 118(4):543-549.
Schweitzer, A., J. Horn, R. T. Mikolajczyk, G. Krause, and J. J. Ott. 2015. Estimations of worldwide prevalence of chronic hepatitis B virus infection: A systematic review of data published between 1965 and 2013. Lancet 386(10003):1546-1555.
Seeger, C., D. Ganem, and H. E. Varmus. 1986. Biochemical and genetic evidence for the hepatitis B virus replication strategy. Science 232(4749):477-484.
Sharfstein, J., and P. H. Wise. 1997. Inadequate hepatitis B vaccination of adolescents and adults at an urban community health center. Journal of the National Medical Association 89(2):86-92.
Sheldon, J., N. Camino, B. Rodes, A. Bartholomeusz, M. Kuiper, F. Tacke, M. Nunez, S. Mauss, T. Lutz, G. Klausen, S. Locarnini, and V. Soriano. 2005. Selection of hepatitis B virus polymerase mutations in HIV-coinfected patients treated with tenofovir. Antiviral Therapy 10(6):727-734.
Simonetti, J., L. Bulkow, B. J. McMahon, C. Homan, M. Snowball, S. Negus, J. Williams, and S. E. Livingston. 2010. Clearance of hepatitis B surface antigen and risk of hepatocellular carcinoma in a cohort chronically infected with hepatitis B virus. Hepatology 51(5):1531-1537.
Smith, E. A., L. Jacques-Carroll, T. Y. Walker, B. Sirotkin, and T. V. Murphy. 2012. The national perinatal hepatitis B prevention program, 1994-2008. Pediatrics 129(4):609-616.
Snow-Lampart, A., B. Chappell, M. Curtis, Y. Zhu, F. Myrick, J. Schawalder, K. Kitrinos, E. S. Svarovskaia, M. D. Miller, J. Sorbel, J. Heathcote, P. Marcellin, and K. Borroto-Esoda. 2011. No resistance to tenofovir disoproxil fumarate detected after up to 144 weeks of therapy in patients monoinfected with chronic hepatitis B virus. Hepatology 53(3):763-773.
Sobeslavsky, O. 1980. Prevalence of markers of hepatitis B virus infection in various countries: A WHO collaborative study. Bulletin of the World Health Organization 58(4):621-628.
Soriano, V., M. Puoti, M. Peters, Y. Benhamou, M. Sulkowski, F. Zoulim, S. Mauss, and J. Rockstroh. 2008. Care of HIV patients with chronic hepatitis B: Updated recommendations from the HIV-hepatitis B virus international panel. AIDS 22(12):1399-1410.
Strang, J., T. Babor, J. Caulkins, B. Fischer, D. Foxcroft, and K. Humphreys. 2012. Drug policy and the public good: Evidence for effective interventions. Lancet 379(9810):71-83.
Sweet, K. 2011a. Public health reporting and national notification for acute hepatitis B infections. Council of State and Territorial Epidemiologists: Department of Health and Human Services.
Sweet, K. 2011b. Public health reporting and national notification for chronic hepatitis B. Council of State and Territorial Epidemiologists: Department of Health and Human Services.
Szilagyi, P. G., L. E. Rodewald, S. G. Humiston, R. F. Raubertas, L. A. Cove, C. B. Doane, P. H. Lind, M. S. Tobin, K. J. Roghmann, and C. B. Hall. 1993. Missed opportunities for childhood vaccinations in office practices and the effect on vaccination status. Pediatrics 91(1):1-7.
Terrault, N. A., N. H. Bzowej, K. M. Chang, J. P. Hwang, M. M. Jonas, and M. H. Murad. 2016. AASLD guidelines for treatment of chronic hepatitis B. Hepatology 63(1):261-283.
Thio, C. L. 2009. Hepatitis B and human immunodeficiency virus coinfection. Hepatology 49(5 Suppl):S138-S145.
Torbenson, M., and D. L. Thomas. 2002. Occult hepatitis B. Lancet Infectious Diseases 2(8):479-486.
Toyoji, M., M. McKee, T. Sin, R. Weir, and C. Wang. 2015. Leveraging EHR data to assess risk and operationalize prevention services to address hepatitis B health disparities at a community health center. Paper presented at National Association of Community Health Centers Community Health Institute Annual Conference, Orlando, FL, August 22-25, 2015.
Tran, T. T., and E. B. Keeffe. 2008. Management of the pregnant hepatitis B patient. Current Hepatitis Reports 7(1):12-17.
Trépo, C., H. L. Y. Chan, and A. Lok. 2014. Hepatitis B virus infection. Lancet 384(9959): 2053-2063.
Tung, W.-C. 2012. Chronic hepatitis B among Asian and Pacific Islander Americans. Home Health Care Management & Practice 24(2):97-100.
Tyson, G. L., J. R. Kramer, Z. Duan, J. A. Davila, P. A. Richardson, and H. B. El-Serag. 2013. Prevalence and predictors of hepatitis B virus co-infection in a United States cohort of hepatitis C virus-infected patients. Hepatology (Baltimore, Md.) 58(2):538-545.
UNAIDS (Joint United Nations Programme of HIV/AIDS). 2014. 90-90-90: An ambitious treatment target to help end the AIDS epidemic. Geneva, Switzerland: United Nations.
University of Washington. 2013. Serologic response to acute HBV infection. http://depts.washington.edu/hepstudy/hepB/clindx/serology/discussion.html (accessed February 2, 2016).
USPSTF (US Preventive Services Task Force). 2016. USPSTF A and B recommendations. http://www.uspreventiveservicestaskforce.org/Page/Name/uspstf-a-and-b-recommendations (accessed February 2, 2016).
van Rijckevorsel, G., J. Whelan, M. Kretzschmar, E. Siedenburg, G. Sonder, R. Geskus, R. Coutinho, and A. van den Hoek. 2013. Targeted vaccination programme successful in reducing acute hepatitis B in men having sex with men in Amsterdam, The Netherlands. Journal of Hepatology 59(6):1177-1183.
van Zonneveld, M., A. B. van Nunen, H. G. Niesters, R. A. de Man, S. W. Schalm, and H. L. Janssen. 2003. Lamivudine treatment during pregnancy to prevent perinatal transmission of hepatitis B virus infection. Journal of Viral Hepatitis 10(4):294-297.
Vaughn-Sandler, V., C. Sherman, A. Aronsohn, and M. L. Volk. 2014. Consequences of perceived stigma among patients with cirrhosis. Digestive Diseases and Sciences 59(3): 681-686.
Vento, S., T. Garofano, C. Renzini, F. Cainelli, F. Casali, G. Ghironzi, T. Ferraro, and E. Concia. 1998. Fulminant hepatitis associated with hepatitis A virus superinfection in patients with chronic hepatitis C. New England Journal of Medicine 338(5):286-290.
Villeneuve, J. P. 2005. The natural history of chronic hepatitis B virus infection. Journal of Clinical Virology 34(Suppl 1):S139-S142.
Waldorf, B., C. Gill, and S. S. Crosby. 2014. Assessing adherence to accepted national guidelines for immigrant and refugee screening and vaccines in an urban primary care practice: A retrospective chart review. Journal of Immigration and Minorirty Health 16(5):839-845.
Ward, J. W. 2015. Placing nation on the path toward the elimination of hepatitis C. Paper presented at 1st Meeting for the Committee on the National Strategy for the Elimination of Hepatitis B and Hepatitis C, Washington, DC, November 30-December 1, 2015.
Wasley, A., D. Kruszon-Moran, W. Kuhnert, E. P. Simard, L. Finelli, G. McQuillan, and B. Bell. 2010. The prevalence of hepatitis B virus infection in the United States in the era of vaccination. Journal of Infectious Diseases 202(2):192-201.
Weinbaum, C. M., I. Williams, E. E. Mast, S. A. Wang, L. Finelli, A. Wasley, S. M. Neitzel, and J. W. Ward. 2008. Recommendations for identification and public health management of persons with chronic hepatitis B virus infection. Morbidity and Mortality Weekly Report 57(RR08):1-20.
WHO (World Health Organization). 2014. Hepatitis B fact sheet no. 204. http://www.who.int/mediacentre/factsheets/fs204/en (accessed October 23, 2015).
WHO. 2015a. Global and regional immunization profile. http://www.who.int/immunization/monitoring_surveillance/data/gs_gloprofile.pdf?ua=1 (accessed January 28, 2016).
WHO. 2015b. Hepatitis B. http://www.who.int/mediacentre/factsheets/fs204/en (accessed January 15, 2016).
Williams, W. W., P.-J. Lu, A. O’Halloran, C. B. Bridges, D. K. Kim, T. Pilishvili, C. M. Hales, and L. E. Markowitz. 2015. Vaccination coverage among adults, excluding influenza vaccination—United States, 2013. Morbidity and Mortality Weekly Report 64(4):95-102.
Wiseman, E., M. A. Fraser, S. Holden, A. Glass, B. L. Kidson, L. G. Heron, M. W. Maley, A. Ayres, S. A. Locarnini, and M. T. Levy. 2009. Perinatal transmission of hepatitis B virus: An Australian experience. Medical Journal of Australia 190(9):489-492.
Wright, T. L. 2006. Introduction to chronic hepatitis B infection. American Journal of Gastroenterology 101(S1):S1-S6.
Wu, H., C. Yim, A. Chan, M. Ho, and J. Heathcote. 2009. Sociocultural factors that potentially affect the institution of prevention and treatment strategies for hepatitis B in Chinese Canadians. Canadian Journal of Gastroenterology 23(1):31-36.
Xu, W. M., Y. T. Cui, L. Wang, H. Yang, Z. Q. Liang, X. M. Li, S. L. Zhang, F. Y. Qiao, F. Campbell, C. N. Chang, S. Gardner, and M. Atkins. 2009. Lamivudine in late pregnancy to prevent perinatal transmission of hepatitis B virus infection: A multicentre, randomized, double-blind, placebo-controlled study. Journal of Viral Hepatitis 16(2):94-103.
Yang, H. I., M. Sherman, J. Su, P. J. Chen, Y. F. Liaw, U. H. Iloeje, and C. J. Chen. 2010. Nomograms for risk of hepatocellular carcinoma in patients with chronic hepatitis B virus infection. Journal of Clinical Oncology 28(14):2437-2444.
Yarnall, K. S., K. I. Pollak, T. Ostbye, K. M. Krause, and J. L. Michener. 2003. Primary care: Is there enough time for prevention? American Journal of Public Health 93(4):635-641.
Zimmerman, K. A., K. P. Fischer, M. A. Joyce, and D. L. Tyrrell. 2008. Zinc finger proteins designed to specifically target duck hepatitis B virus covalently closed circular DNA inhibit viral transcription in tissue culture. Journal of Virology 82(16):8013-8021.


















































