3
Current Understanding of Stressors
INTRODUCTION
Although increased noise exposure is a concern for marine mammals, other anthropogenic activities also serve as potential stressors that can alter individual behavior and health and contribute to cumulative impacts. In general, a stressor can be defined as any causal factor or stimulus, occurring in either the animal’s internal or external environment that challenges the homeostasis of the animal. Marine mammals are exposed to a diverse set of both intrinsic and extrinsic stressors during their lifespan (see Table 3.1).
There are short-term internal stimuli that evoke myriad physiological responses occurring daily to maintain an organism near its homeostatic set points, but these are not considered stressors. However, aspects of the life cycle that result in significant changes to the set points are considered intrinsic stressors, and inherent in the life-history strategies of marine mammals are numerous features that constitute such stress. Many marine mammals are capital breeders that fast during reproduction or periods on shore. These species are intrinsically nutritionally stressed during reproduction and during migration away from foraging habitat. The amphibious lifestyle of pinnipeds requires that even income breeding species undergo food deprivation while on shore for breeding. Extended periods on shore have been associated with increases in stress hormones in numerous species (Champagne et al., 2012). Species that fast as part of their natural life history may exhibit intrinsic stress during or just after reproduction. During pregnancy, even species that do not fast will undergo significant physiological changes, including metabolic, cardiovascular, respiratory, immuno-
TABLE 3.1 Definition and Examples of Types of Stressors
| Definition | Examples | |
|---|---|---|
| Intrinsic Stressor | An internal factor or stimulus that results in a significant change to an animal’s homeostatic set points | Pregnancy, lactation, migration, molting, fasting (e.g., during the breeding season in capital breeders) |
| Extrinsic Stressor | A factor in an animal’s external environment that creates stress in an animal | Anthropogenic: Pollutants, ship strike, entanglement, noise, psychological factors (e.g., perceived threat) Natural, but potentially influenced by anthropogenic activity: Harmful algal blooms, resource limitation, predator pressure, pathogens, temperature, salinity, naturally occurring chemicals, intra- or interspecific competition |
| Ecological Driver | A biotic or abiotic feature of the environment that affects multiple components of an ecosystem directly and/or indirectly by changing exposure to a suite of extrinsic stressors | Loss of keystone or foundation species, recurring climate patterns such as El Niño, climate change |
logical, and hematological changes, in order to accommodate the growing fetus.
In addition, there are extrinsic stressors that arise from chemical, physical, or biological factors in an animal’s external environment. Extrinsic stressors may be specifically associated with anthropogenic activities (e.g., pollutants or ship strike) and include psychological factors that occur when human activities are perceived as a threat, typically a predatory threat (e.g., sonar; Isojunno et al., 2016). Extrinsic stressors may also be prompted by natural factors, although these natural factors are often influenced by anthropogenic activities to some degree (e.g., disease or resource limitation), making it difficult to classify the extrinsic stressor as unequivocally natural. Regardless of whether causal factors are purely natural or not, these stressors have potential to influence an animal’s responses to other anthropogenic stressors. In addition, how the animal responds to extrinsic stressors is dependent on its physiological capacity, which is modulated by intrinsic stressors. So long as the extrinsic stressors and intrinsic stressors do not exceed the animal’s ability to maintain organismal function (i.e., allostasis; McEwen and Wingfield, 2003), effects on health and reproduction that lead to population impacts are unlikely. Numerous studies have evaluated the impact of the various extrinsic stressors on the individual health, survival, and reproduction of marine mammal species, although these studies have been biased toward pinnipeds (reviewed by Atkinson et al., 2015). At the extreme, extrinsic stressors can result in increased mortality, demographic impacts, and even cohort failures in some marine mammal species. The cumulative effect of whatever combination of these existing intrinsic and extrinsic stressors to which an individual is exposed will influence the impact of any additional anthropogenic stressors on individuals and consequently their population-level effect.
Many extrinsic stressors can be the products of larger phenomena that are identified as ecological drivers. An ecological driver is a biotic or an abiotic feature of the environment that affects multiple components of an ecosystem directly and/or indirectly by changing exposure to a suite of extrinsic stressors. Ecological drivers may operate on multiple species at varying trophic levels and may even affect multiple ecosystems.
POTENTIAL ENVIRONMENTAL (EXTRINSIC) STRESSORS
Human activities can potentially cause mortality, injury, disturbance, and stress to marine mammals. Activities that result in immediate fatalities, such as bycatch, hunting (or other deliberate killing), and collisions with ships, will increase the population mortality rate above that caused by natural factors alone. These lethal stressors directly affect population abundance. In contrast, human activities with nonlethal effects on marine mammals may affect their behavior and physiology and lead to impacts on their health. The cumulative effect of these human activities, along with natural extrinsic stressors, on the health of individual animals may result in changes in their reproduction and survival that then affect population dynamics. In this section the committee reviews and discusses environmental stressors and their associated effects that have been reported for marine mammals. The focus is on those stressors that have been emphasized in the literature, and/or that have strong potential to interact with other stressors due to chronicity of exposure (e.g., persistent chemical contaminants to which many marine mammals are exposed over a lifetime), or the potential for a sublethal but chronic effect (e.g., permanent damage to an organ system). This should not be considered an exhaustive list of all possible environmental stressors that have potential to affect marine mammals. A comprehensive review of all potential stressors is beyond the scope of this report.
Physical Injury
Fishery Interactions
Entanglement in fishing gear represents an important source of injury and mortality in marine mammals. Bycatch mortality is estimated globally to exceed hundreds of thousands of marine mammals each year (Read et al., 2006). Bycatch occurs most frequently in association with gillnet fisheries. There is a strong spatial component to bycatch of marine mammals, with “hotspots” influenced by marine mammal density (Block et al., 2011), fishing intensity (Stewart et al., 2010), or both (Lewison et al., 2014). Spatial overlap between fisheries and marine mammals is often associated with coastal zones, shelf breaks, upwelling regions, and frontal zones (Hyrenbach et al., 2000; Scales et al., 2014). When not immediately fatal, entanglement or ingestion of fishing gear can impede the ability of marine mammals to feed and can cause injuries that eventually lead to infection and death (Wells et al., 2008; Cassoff et al., 2011; Moore and van der Hoop, 2012). Weakened animals may be more susceptible to predation (Moore and Barlow, 2013). There are also costs likely to be associated with nonlethal entanglements in terms of energy and stress (Moore and van der Hoop, 2012). The prevalence of scars on North Atlantic right whales (Eubalaena glacialis) associated with entanglements indicates the persistent and repetitive nature of this threat (Knowlton et al., 2012).
Vessel Collision
Collision with ships is a key threat to large whales (Laist et al., 2001; Thomas et al., 2016). Vessel strike also poses a risk to manatees (Runge et al., 2015) and small cetaceans in heavily populated coastal regions (e.g., Wells et al., 2008), and the risk may increase when illegal feeding has conditioned the animals to approach vessels (Donaldson
et al., 2010). Several studies have estimated quantitative relationships (i.e., dose–response relationships) between vessel speed and the lethality of collisions for large whales (Vanderlaan and Taggart, 2007; Wiley et al., 2011; Conn and Silber, 2013). Even when it is not lethal, collision with a vessel causes stress and injury, which could make individuals more susceptible to negative sequelae following exposure to subsequent stressors.
Toxic Compounds
Nonbiological Toxins
Chemical contaminants, particularly those that are persistent in the environment, are a concern for marine mammals that often occupy high trophic positions. Persistent organic pollutants (POPs), which include legacy pesticides (e.g., DDT and chlordane), legacy industrial-use chemicals (e.g., polychlorinated biphenyls [PCBs]), and emerging contaminants of concern (e.g., polybrominated diphenyl ethers and perfluorinated compounds) accumulate in fatty tissues of marine organisms and are magnified through the food chain, leading upper trophic predators to be highly exposed. High concentrations of PCBs and DDT have been reported in tissues of marine mammals in most parts of the world, particularly in coastal regions adjacent to heavy coastal development and/or industry (Ross et al., 2000; Houde et al., 2005; Kajiwara et al., 2006; Kucklick et al., 2011). These legacy POPs have been linked to a number of adverse health effects, but primary concerns relate to endocrine disruption, and specifically thyroid hormone disruption (Sormo et al., 2005; Boas et al., 2006; Tabuchi et al., 2006; Schwacke et al., 2012), reproductive impairment or developmental effects (Reijnders, 1986; Ulbrich and Stahlmann, 2004; Hall et al., 2009), and immune dysfunction or disease susceptibility (De Guise et al., 1998; Van Loveren et al., 2000; Jepson et al., 2005). Polybrominated diphenyl ethers (PBDEs), commonly used as flame retardants, are another class of POPs that have spread globally in the environment and have also been reported in a broad array of marine mammal species (Houde et al., 2009; Rotander et al., 2012). The toxicity of PBDEs has not been as thoroughly investigated in comparison to PCBs, but rodent studies have suggested developmental neurotoxicity with learning and memory impairment that can persist into adulthood, and decreased thyroid hormone production similar to the toxic effects of PCBs (Eriksson et al., 2001; Branchi et al., 2003). PBDEs can be biotransformed to hydroxylated brominated diphenyl ethers, which exhibit greater toxicity for some effect end points as compared to their parent compound, and some studies have suggested that biotransformation of naturally occurring compounds in the marine environment may be an even greater source of the hydroxylated analogues as compared to the anthropogenic flame retardants (Wiseman et al., 2011).
POPs bind to fatty tissues and as such are sequestered in the blubber of marine mammals. Concentrations are likely maintained at equilibrium, or increase with age if the exposure continues, until an event (e.g., parturition, lactation, seasonal blubber changes, or loss of prey base) prompts blubber depletion and mobilization of the sequestered contaminants (reviewed by Houde et al., 2005). Once contaminants are mobilized, they may be more likely to reach target organs and initiate mechanistic pathways for adverse health effects. Therefore, POPs have potential to affect an individual over a lifetime, depending on life events and whether or not there is continued exposure. Neonates and dependent calves or pups may be particularly susceptible due to high concentrations of POPs that are offloaded from mother to offspring through milk (Wolkers et al., 2004; Yordy et al., 2010).
Aside from POPs, other organic compounds of concern include polycyclic aromatic hydrocarbons (PAHs). PAHs exist naturally in the environment but can also be from anthropogenic sources. Crude oil, fumes, vehicle exhaust, coal, organic solvents, and wildfires are all potential sources for PAHs. Exposure may be continual, associated with runoff from impervious cover in developed coastal regions, or natural seeps that produce low-level but steady exposure. Acute events such as oil spills may produce pulses of more significant exposure. Depending on the route of exposure (inhalation/aspiration, ingestion, or direct dermal contact), PAHs can produce a broad range of health effects. Lung disease, disruption of the hypothalamic-pituitary-adrenal (HPA) axis, and altered immune response have been reported in marine mammals as well as experimental mammal species following exposure to oil (Mazet et al., 2000; Schwartz et al., 2004; Mohr et al., 2008; Schwacke et al., 2014a) or inhalation of smoke associated with wildfires (Venn-Watson et al., 2013). Although PAHs are more rapidly metabolized and do not accumulate as is the case with POPs, the toxic effects (lung disease and HPA-axis damage) may be long lasting and initiate chronic disease conditions (Smith et al., 2017). Heavy metals, particularly mercury—which has been associated with immunological and neurotoxic effects and can cause permanent damage to the brain (Kakuschke and Prange, 2007; Farina et al. 2011)—have also been widely measured in the tissues of marine mammals (Dietz et al., 1996; Wagemann et al., 1996; Weihe et al., 1996; Seixas et al., 2008). Comparison of mercury tissue concentrations with established toxicological thresholds have indicated that some Arctic marine mammal species are at risk of neurological effects (Dietz et al., 2013), and levels of mercury in Arctic regions have been increasing in recent decades (Dietz et al., 2009; Rigét et al., 2011).
Despite the vast evidence to suggest that marine mammals are exposed to anthropogenic, as well as natural, chemicals capable of producing significant toxic effects, only a few studies have actually examined the impacts on population survival or reproductive rates (e.g., Hall et al., 2006; Lane et al., 2015). Such observational assessments are inherently challenging due to the difficulty in controlling for
confounding or interacting variables, as well as the sublethal but chronic nature of chemical contaminant effects, and the difficulty of observing mortality or reproductive end points in long-lived marine mammal species, particularly cetaceans. Even fewer studies have attempted to develop quantitative relationships relating a given dose of a chemical to changes in a vital rate (e.g., reduced fecundity) and have had to rely on data from experiments with other mammalian species (e.g., Schwacke et al., 2002; Hall et al., 2006).
Biological Toxins
Marine algal toxins are produced by unicellular algae that are often present at low concentrations but that may proliferate to form dense concentrations under certain environmental conditions. When high cell concentrations form, the toxins that they produce can harm the health of marine life, and this is referred to as a harmful algal bloom (HAB). Marine mammals can be exposed to HAB toxins directly by inhalation or indirectly through food web transfer (Durbin et al. 2002), and these toxins can cause severe neurotoxic effects (reviewed by Van Dolah, 2005). Mortality and morbidity related to HAB toxins have been increasingly reported over the past several decades, and biotoxicosis has been a primary contributor to large-scale die-offs across marine mammal taxa (Van Dolah, 2005; Simeone et al., 2015). Since 1998, multiple die-offs as well as abortions and premature parturition have been reported for California sea lions (Zalophus californianus) in relation to domoic acid, a toxin produced by diatoms of the genus Pseudonitzschia (Scholin et al., 2000; Bejarano et al., 2008a). Furthermore, studies have determined that even sea lions that survive can suffer sublethal effects that could influence reproduction and longer-term survival (Gulland et al., 2002; Goldstein et al., 2008, 2009). Impacts of Pseudonitzschia blooms on marine mammal populations along the western U.S. coast have not been limited to sea lions; domoic acid has also been linked to mortalities of balaenopterids, delphinids, phocoenids, and mustelids (Van Dolah, 2005). Domoic acid has also been detected in tissues of marine mammals along the southeast U.S. coast (Schwacke et al., 2010; Twiner et al., 2011), but perhaps of greater concern in this area are the brevetoxins produced by Gulf of Mexico red tides. Brevetoxin has been implicated in multiple die-offs involving common bottlenose dolphins (Tursiops truncatus), as well as the endangered Florida manatee (Trichechus manatus latirostris) (Flewelling et al., 2005; Twiner et al., 2012; Simeone et al., 2015). Other HAB toxins, such as saxitoxin and ciguatera toxins, have been implicated in morbidity or mortality of other marine mammals, including humpback whales (Megaptera novaeangliae) and endangered monk seals (Monachus sp.) (Reyero et al., 1999; Bottein et al., 2011; summarized by Van Dolah, 2005).
Parasites and Pathogens
Parasites are ubiquitous. Parasites have the ability to cause disease and to function as pathogens. Microparasites, which include viruses, bacteria, fungi, and protozoa, multiply inside the host and are frequently associated with immune responses and development of host immunity in healthy animals. Macroparasites, which include helminths and arthropods, are larger in size and have complex life cycles that frequently involve more than one host for reproduction.
Microparasites can infect respiratory, central nervous, or other organ systems causing morbidity and mortality (e.g., Guzmán-Verri et al., 2012; Van Bressem et al., 2014; Simeone et al., 2015), and in some cases have been associated with epidemics that produce significant mortality. For example, viral pathogens of the genus Morbillivirus have been associated with severe respiratory illness and linked to large-scale die-offs of marine mammal populations worldwide (Van Bressem et al., 2014). Endemic microparasites may sporadically infect a smaller number of animals, but contribute to natural mortality as well as to widespread, low-level disease that in some cases may affect reproduction (e.g., Brucella sp.; Guzmán-Verri et al., 2012). Similarly, macroparasites may chronically infect marine mammals and contribute to low-level mortality or morbidity that reduces fitness or resilience (Simeone et al., 2015). Perrin and Powers (1980) estimated that 11-14% of natural mortality in spotted dolphins (Stenella attenuata) was attributable to the nematode Crassicauda sp. based on the prevalence of cranial lesions by age in spotted dolphins incidentally killed in the eastern tropical Pacific tuna fishery. The distribution of parasites and thus the risk of exposure and subsequent infection in marine mammals can be influenced by human activities. For example, domestic or human-managed animal populations and landscape alteration can affect terrestrial parasite distribution, and in coastal areas this can influence the risk for land-to-sea transmission. Such an influence has been supported by studies of Toxoplasma gondii transmission from terrestrial animals (feral cats and wildlife) to marine mammals in adjacent coastal waters (VanWormer et al., 2013, 2014).
Resource Limitation
Competition between marine mammals and fisheries has long been recognized (Northridge, 1984), and there is little doubt that this competition can be significant. For example, Punt and Butterworth (1995) estimated that the South African west coast population of Cape fur seals consumed some 600,000 tons of commercially valuable fish, such as Cape hake—in contrast to the average annual landings of 50,000 tons of Cape hake by South African fishing fleets. Conversely, Ford et al. (2010) discovered a strong bottom-up effect on the abundance of fish-eating killer whales in the northeastern Pacific Ocean from the availability of their
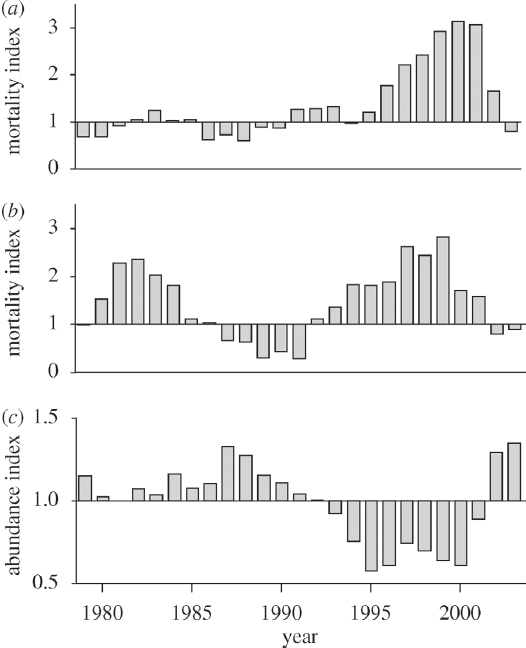
preferred prey, Chinook salmon (see Figure 3.1), although there is some uncertainty about how this interaction affects population growth (Vélez-Espino et al., 2015).
However, despite this clear connection, the systems involved are complex, and unraveling the nature and extent of the competition between marine mammals and fisheries has been challenging (Matthiopoulos et al., 2008). Fisheries may also result in a variety of indirect effects by changing the ecosystem and decreasing or increasing the abundance of potential marine mammal prey such as forage fish. Analysis challenges stem from complexities in ecosystems, such as spatial heterogeneity and multispecies interactions, which constrain the ability to clearly interpret cause and effect (Harwood, 1992; Matthiopoulos et al., 2008). Other difficulties for quantifying competition emerge from the fact that many marine mammals are generalist predators. The prey consumption of generalist predators varies with the availability of all their preferred prey species (Asseburg et al., 2006; Smout et al., 2014). As a result, more data than are usually available in field studies of marine mammals are required to realistically characterize these interactions. Thus, despite the intuitive connection between fisheries and marine mammals, there is currently no existing demonstration that resource depletion from fisheries has demographic consequences for marine mammals. Other influences of fisheries on marine mammals, such as bycatch, have been well documented.
In addition to food resources, critical marine mammal habitat can be limited by human activities. Critical habitats are areas essential to an animal’s survival, such as the islands and protected beaches that grey seals (Halichoerus grypus) need for successful breeding (Harwood, 2001). Human disturbance may reduce the ability of seals, such as Hawaiian monk seals (Monachus schauinslandi), to use critical breeding beaches (Gerrodette and Gilmartin, 1990). These habitats, and others like the seagrass beds that manatees (Trichechus manatus) require for foraging, may also become limited by environmental drivers such as sea level rise (Burns, 1997). While some marine mammals can move to find other habitats, others such as freshwater river dolphins cannot (Harwood, 2001). Ice-associated species that rely on sea ice for pupping, molting, and transportation may be particularly vulnerable to population consequences of reduction of sea ice resulting from climate change (Kovacs and Lydersen, 2008; Kovacs et al., 2011). For example, ringed seals (Phoca hispida) show a decrease in body condition, ovulation rates, and recruitment that is correlated with low ice years (Harwood et al., 2000; Ferguson et al., 2005). Likewise, in polar bears (Ursus maritimus), decreased ice cover leads to longer periods of fasting, lower reproductive rates, declining body condition and survival, and increased contact with human settlements (Stirling et al., 1999, 2004; Stirling and Parkinson, 2006). At present, few examples exist that demonstrate direct impacts of habitat limitation on marine mammal populations, but as critical habitats become more limited by ecological drivers, this type of stress may become more apparent.
As an adaptive response to reducing intraspecific competition when prey is limited, dietary specialization may occur among individuals (Tinker et al., 2008). This can result in different exposure risks to pathogens within the population. For example, sea otter feeding on abalone, a preferred prey species, had a low risk of infection by Toxoplasma gondii and Sarcocystis neurona compared to otters feeding on small marine snails, despite foraging in the same habitat (Johnson et al., 2009). Food resource limitation can therefore lead to changes in pathogen exposure and have potential adverse effects on health as a consequence of the interaction between disease and increasing prey limitation.
Perceived Threat
Frid and Dill (2002) made an important contribution to studies of disturbance in wildlife when they pointed out that anthropogenic disturbance stimuli may evoke responses similar to those evoked by predators or other threats, with which a species may have a long evolutionary history. Some
species with strong flight responses to threat may be at risk of acute lethal effects of disturbances. Cox et al. (2006) reviewed data on atypical mass strandings of beaked whales that coincided with sonar exercises and concluded that the most likely cause of these strandings involved sonar triggering a behavioral reaction that ultimately led to stranding. If sonar triggers a strong enough avoidance response to send beaked whales from their deep water habitat to water shallow enough to pose a risk of stranding, this suggests that the whales perceive the sonar as a potential threat. As mentioned in Chapter 2, mid-frequency sonar signals share some similarities with calls of killer whales, an important predator, and beaked whale responses to sonar share some similarities to responses to playback of killer whale sounds. These observations are consistent with the hypothesis that beaked whales perceive sonar as a threat, similar to the risk of predation.
Other forms of disturbance that evoke less drastic acute responses may have aggregate effects in wildlife populations. Wildlife tourism, which focuses on experiencing or interacting with wild animals, is a rapidly expanding industry (Newsome et al., 2002; Burgin and Hardiman, 2015). Although effects on marine mammal behavior have been documented, their impact at the population level is not well known (New et al., 2015). It appears that it is not only the sound produced by a whale-watching vessel that elicits a response, but the physical presence of a boat also plays a role in disturbance and the perceived threat risk. Pirotta et al. (2015a) found that the probability that bottlenose dolphins would engage in foraging activity declined by almost half in the presence of boats, but there was no relationship with the sound level. Various other short-term responses of marine mammals to boat traffic and swimmers have been reported. Well-documented examples include avoidance behavior by bottlenose dolphins (Tursiops truncatus) of swimmers (Constantine, 2001), and a reduction in resting and surface activity combined with faster swimming among southern right whales (Eubalaena australis), also in response to swimmers (Lundquist et al., 2013). Bejder et al. (2006) documented a significant reduction in the abundance of bottlenose dolphins in Shark Bay, Australia, when there were two or more wildlife tour operators compared to control sites with no tourism or when there was only one tour operator. Their findings indicated that the decline was due to a displacement of individuals, potentially those more sensitive, and a long-term shift in habitat use from disturbed sites with high vessel traffic to areas with lower activity. A study of bottlenose dolphins in Fiordland, New Zealand, also found that dolphins avoided areas where there was high tourism traffic (Lusseau et al., 2006; Lusseau and Bejder, 2007). A threshold of 68 minutes between boat interactions was identified below which dolphins switched from a short-term behavioral avoidance strategy to long-term habitat displacement. If this threshold was regularly exceeded, the population was predicted to decline as a result of a reduction in reproductive success, an increase in stillbirths, and decline in calf survival (Lusseau et al., 2006; Lusseau and Bejder, 2007). However, a recent study (Brough et al., 2016) has suggested that some of the decline in reproductive success in this population may be the result of an increase in the discharge of freshwater into the system after 2002. The Lussau and Bejder (2007) results contrast with dolphins in Sarasota Bay, Florida, where the dolphins remain even though a boat passes within 100 m every 6 minutes (Nowacek et al., 2001). One difference between these examples is that most boats in Sarasota Bay may be passing with no activity directed toward the dolphins in contrast with the tourist boat activities in Fiordland.
These studies indicate that population-level effects may be more likely to occur when individuals have small home ranges and high fidelity to sites with a high level of whale watching. In these circumstances a large number of individuals may experience repeated and long-term disturbance. In cases where individual exposure is relatively short, such as for migratory baleen whales, the effects are expected to be less. For example, Christiansen and Lusseau (2015) found that interactions between minke whales and whale-watching boats off Iceland resulted in a 42% decrease in feeding activity and an estimated 64% decrease in net energy intake. However, the aggregate exposure of individuals to whalewatching boats over the course of a summer was low (less than 450 minutes), leading to only a small decrease in female body condition that was unlikely to affect reproductive success (Christiansen and Lusseau, 2015). An examination of calving rates of humpback whales and calf survival off New England also found no evidence for negative effects of exposure to whale watching (Weinrich and Corbelli, 2009). Frameworks using individual-based models are being developed to simulate the potential effects of boat traffic and other human activities on marine mammal populations (New et al., 2013a; Pirotta et al., 2015b).
Ocean Climate and Conditions
Oceanographic and meteorological phenomena can profoundly alter characteristics of the marine environment, which, in turn, affect the distribution and resource acquisition of marine mammals. One of the strongest is the atmospheric forcing of the El Niño–Southern Oscillation (ENSO), which results in major changes in the physical structure and productivity of the North Pacific subtropical gyre (Karl et al., 1995). These changes directly impact low-latitude and coastal upwelling zones that are important habitat for marine mammals and have time-lagged effects at higher latitudes (Brinton et al., 1987). El Niño alters water temperature and structure on large spatial scales and reduces coastal upwelling. These features are important in determining habitat use and movement patterns of marine mammals (Croll et al., 2005; Doniol-Valcroze et al., 2007), altering the range and abundance of some species and concentrating individuals in areas with high productivity (Gardner and Chávez-Rosales, 2000; Benson et al., 2002). These changes in distribution
may also influence exposure to other stressors that have geospatial components. Prey limitation associated with El Niño may have severe impacts on coastal and pelagic foraging species, reducing survivorship and reproductive rates and impacting local population dynamics of cetaceans and pinnipeds (Trillmich et al., 1991; Crocker et al., 2006; Leaper et al., 2006).
Multidecadal changes in ocean climate, or regime shifts, also influence sea surface temperature, upwelling, and biological productivity (Croxall et al., 1992; Francis and Hare, 1994). These alterations that persist over longer time scales can amplify effects of ENSO variation. The Pacific Decadal Oscillation (PDO) may influence the periodicity of El Niño events, resulting in stronger cumulative impacts on individuals and populations. Warm water regimes of the PDO are associated with increased nutritional stress in Pacific marine mammals (Le Boeuf and Crocker, 2005). Similarly, a multidecadal oscillation in the climate of the North Atlantic, the North Atlantic Oscillation (NAO), influences the distribution and foraging of numerous marine mammal species and impacts reproductive rates and population dynamics (Fujiwara and Caswell, 2001; Greene and Pershing, 2004; Jiang et al., 2007). Ocean climate is thus a major driver of distribution, abundance, and reproduction of marine mammals with enormous potential to influence the way that individuals and populations respond to extrinsic stressors. However, clear linkages between ocean climate and marine mammal population trends have not been well documented. A study on southern elephant seals spanning five decades also highlighted the importance of considering density effects in combination with environmental conditions to evaluate effects on populations because these factors can interact (de Little et al., 2007).
Besides ocean climate shifts due to ENSO, PDO, or NAO, changes in global and ocean climate that result from anthropogenic climate alteration are likely to have profound impacts on marine mammals (Moore and Huntington, 2008) that will potentially interact with other stressors. Some marine mammals associated with polar ice are already showing shifts in distribution, reduced body condition, and declines in abundance and reproduction in response to declines in sea ice (Kovacs et al., 2011). However, the quality of abundance estimates varies greatly among location and species and in most cases the data currently are not sufficient for analyzing population trends (Laidre et al., 2015). For bowhead whales, the warming Arctic regions have proved beneficial. Their axial-girth-based body condition index (BCIG) is positively correlated with summer sea ice loss over the past 2.5 decades, and BCIG is significantly correlated with the duration of the melt season (George et al., 2015). Range expansions of temperate species may alter resource competition in high-latitude habitats. Long-term impacts may include alteration in oceanographic features used in foraging strategies. Changes in prey distribution and abundance may also occur as a result of disruption of ocean currents and increases in the energetic cost of calcification caused by ocean acidification (Doney et al., 2012). Ocean warming has been implicated in reports of rising disease prevalence in marine organisms, including marine mammals (Harvell et al., 2002; Lafferty et al., 2004; Burek et al., 2008; Van Bressem et al., 2009). Emerging evidence from climate change studies (Ockendon et al., 2014) suggests that indirect effects of stressors, through the disruption of interspecific interactions, may be more important than direct ones. Apparently caused largely by increased nutritification, dead zones (hypoxic areas) have increased in recent years in many coastal areas, such as the northern Gulf of Mexico (Rabalais et al., 2002; Diaz and Rosenburg, 2008). Although the influences of dead zones on marine mammals have not been well documented, reduced production and prey availability (Grimes, 2001) almost surely are detrimental to these animals.
SPATIAL AND TEMPORAL VARIATION AMONG STRESSORS
The range of extrinsic stressors to which marine mammals can potentially be exposed over a lifetime has been briefly reviewed, but to appreciate the potential for cumulative effects of these combined stressors, the spatial and temporal patterns of exposure should also be considered. The occurrence of individual stressors may show strong spatial variation, and their effects depend on the habitat used by a given marine mammal species. Even ubiquitous stressors, like anthropogenic noise and globally dispersed chemical contaminants, show variation in magnitude across geographic regions. Species that exhibit long-distance movements may be exposed to diverse stressors in disparate ecosystems, and consideration of cumulative effects must include stressors throughout this range. Although highly migratory species may be exposed to a wide range of stressors, the aggregate exposure of individuals may be low (e.g., Christiansen and Lusseau, 2015), affecting the overall impact at a population level. In contrast, species with smaller home ranges may potentially be exposed to fewer stressors, but with greater exposure times to those that occur in the region.
There is also a potential temporal component to variation in vulnerability to stressors related to life-history variation within species. For example, the need of capital breeding species to conserve energy may outweigh short-term costs of local stressors during breeding (Bishop et al., 2015). However, once breeding is completed they may be at an exceptionally low nutritional plane with high allostatic load that reduces their ability to respond to new stressors. Females with calves or pups may also be more sensitive to disturbance and perceived threats (Engelhard et al., 2002; Stamation et al., 2009). During key foraging periods, animals may be less vigilant in responding to threats, which may increase their vulnerability to other stressors such as predators. Some behavioral states also increase vulnerability to stressors. For example, during feeding North Atlantic right
whales spend much of their time just below the surface, increasing the risk of vessel collisions (Parks et al., 2012). Stressors that affect prey availability and predation risk on the feeding ground may directly impact animals’ body condition, pregnancy rate, and survival (Williams et al., 2013). Because these life-history periods are often associated with specific habitats or spatial use, managers should consider this dimension when assessing the potential impacts of the spatial component of exposure to stressors. From this perspective, chronic stressors that impact individuals across multiple life-history stages are more likely to have deleterious effects than those that impact only one life-history stage. Species or populations that are continually exposed to stressors in a particular location with a given geospatial distribution are also more likely to suffer deleterious effects than species that migrate through that location and are only periodically exposed.
The physiological and behavioral impacts of single and multiple stressors will also vary depending on the frequency of exposure. Ongoing or continuously occurring (i.e., chronic) exposure can be associated with dysregulation of endocrine and homeostatic function and therefore have negative impacts on individual fitness. Chronic activation of generalized stress responses may be an important mechanism through which cumulative impacts arise. Conversely, when exposure to a stressor is acute, occurring for a single discrete period, or intermittent, occurring repeatedly but not necessarily at frequent or regular intervals (e.g., HABs or sonar), animals may accommodate. That is, a physiological response may be invoked but normal function is then restored or a new homeostatic set point is reached. In some cases, the resulting physiological responses may be adaptive and even enhance the ability to respond to future stressors through hormesis1 (Calebrese et al., 2007). However, even if the exposure is not chronic, an alternative mechanism for cumulative impacts emerges when the adverse effect produced by the stressor persists or is irreversible (i.e., a chronic effect). For example, a permanent threshold shift in auditory sensitivity will impact behavior.
SUMMARY AND CONCLUSIONS
Numerous studies have evaluated the impact of various extrinsic stressors on the individual health, survival, or reproduction of marine mammal species. Stressors such as fishery interaction, vessel strike, HAB toxins, and pathogens can cause acute mortality. Even when there are effects that are nonfatal, they can induce sublethal effects that continue to affect the animal’s ability to maintain homeostasis and respond appropriately to other extrinsic or intrinsic stressors. The broad array of chemicals to which many marine mammals are exposed, often chronically over their lifetime, also produce sublethal physiological effects. Such effects have been documented from observational studies of marine mammals and in many cases are supported by findings from experimental studies in other mammalian species. However, linking chemical stressors to decreases in vital rates through observational assessments is inherently challenging due to the chronic nature of many exposures or effects, the complexity involved in controlling for confounding or interacting variables, and the difficulty of observing mortality or reproductive end points in long-lived marine mammal species, particularly cetaceans. These challenges extend to other stressors that induce sublethal effects. Regardless of the stressor, few studies have explicitly defined quantitative relationships between varying doses and associated mortality, reproductive, or physiological effects for marine mammals.
Finding 3.1: Numerous studies have demonstrated direct physiological effects from a broad array of extrinsic stressors in marine mammals. However, few studies have explicitly quantified the relationship between varying doses of a given stressor and the level of mortality, reproductive, or physiological effect (i.e., defined a dose–response relationship).
Ecological drivers such as ocean climate shifts act directly or indirectly through prey or other resources to induce stress on marine mammal populations. Similarly, fisheries can directly create competition for resources, or indirectly affect prey availability through ecosystem changes. Wildlife tourism or other forms of disturbance that may be perceived as a threat evoke more acute responses but may have aggregate effects. For these stressors, analysis challenges stem from complexities in ecosystems and/or difficulties in elucidating long-term shifts in behavior or habitat use, constraining the ability to clearly interpret cause and effect at the population level.
The occurrence of some stressors may show strong spatial variation. In addition, an animal’s vulnerability to stressors may vary temporally in relation to life history. Therefore, temporal and spatial variation in exposure to stressors must be considered. Ongoing or continuously occurring (i.e., chronic) exposure to a stressor can be associated with dysregulation of endocrine and homeostatic function and therefore may be an important mechanism through which a cumulative effect manifests within individuals. Even if the exposure is not chronic, an alternative mechanism for a cumulative impact emerges when the adverse effect produced by the stressor persists or is irreversible (i.e., a chronic effect).
Finding 3.2: The effects of stressors on marine mammals depend on temporal and spatial overlap in the distribution of stressors and the target organisms. Chronic exposure or a chronic effect resulting from an acute exposure provides mechanisms through which cumulative impacts may arise.
__________________
1 A phenomenon of dose–response relationships wherein a stressor that produces harmful biological effects at moderate to high doses may produce beneficial effects at low doses.








