4
Assessing Interactions Among Stressors
INTRODUCTION
As described in Chapters 2 and 3, marine mammals are exposed to a diverse set of extrinsic stressors during their lifespan. Understanding the way exposure to any one stressor may affect marine mammal populations is challenging; understanding the population-level consequences of exposure to multiple stressors is far more challenging. However, a key to understanding how the effects of extrinsic stressors might integrate to create cumulative effects is determining how specific stressors create responses, and evaluating the potential for interactions between the effects of these responses over the lifespan of an individual. It is important to be clear what is meant by an interaction between stressors. Gennings et al. (2005) reviewed the models that have been used to quantify toxicological interactions and defined an interaction between two chemicals as occurring when the shape of the dose–response relationship for one chemical is affected by the dose of the other chemical. The committee adopted the same definition for interactions between stressors. If the shape of the dose–response relationship of one stressor does not change in the presence of another stressor, then these stressors do not interact, and the responses are said to combine additively.
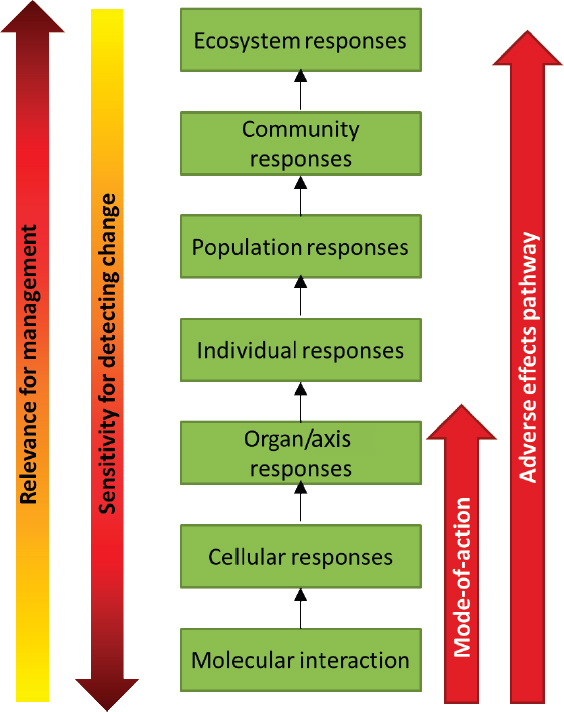
The impact of multiple extrinsic stressors can be studied at different levels of biological organization from molecular, cellular, or organ responses, to effects on the individual, to higher-order population- and community-level responses (see Figure 4.1). Accommodation, or recovery that restores normal function, may occur at any level of organization (e.g., Nichols et al., 2011). However, when the exposure to a stressor is sufficient, the response at one level will be propagated to the next level. For example, at the molecular level, changes in gene expression, enzymatic reactions, and receptor function may occur in response to a stressor; these in turn may initiate cellular responses such as differentiation, proliferation, or altered hormone synthesis. When sufficient, these cellular responses can produce an injury to an organ or disruption of an endocrine axis that eventually leads to morbidity, mortality, or reproductive failure for the individual. If sufficient individual-level responses occur, there can be impacts on populations and, ultimately, communities and ecosystems. It is at these higher levels of biological organization that responses are of greatest societal relevance and greatest concern for natural resource, coastal, and ocean management.
Although the flow of responses in Figure 4.1 is depicted as moving upward through increasingly higher levels of biological organization, responses may also be introduced at a higher level (e.g., ecosystem or community) and then initiate a cascade of responses within an individual marine mammal. The El Niño–Southern Oscillation would be an example of an ecological driver initiated at the ecosystem level, which can cause prey depletion, prompting a response at the molecular level, which then propagates upward to an individual-level response.
Unfortunately in many cases, responses at the higher levels cannot be detected until the process is so far along that the change may be catastrophic and irreversible. It is therefore important to study effects of stressors at the lower levels of biological organization. However, it is imperative to supplement the information on lower-level responses with an understanding of the linkages and processes by which such responses eventually translate into higher-level impacts. The linkages and associations of responses across different levels of biological organization are considered by ecotoxicologists when describing adverse outcome pathways (AOPs) (Ankley et al., 2010; Connon et al., 2012) and by conservation physiologists when describing biological upscaling (Cooke et al., 2014). Depending on the context, an AOP may be
considered to extend from molecular-level responses all the way through to population-, community-, or even ecosystem-level responses. Similarly, the Population Consequences of Disturbance (PCoD) model structure (New et al., 2014), which is used in Chapter 5 as the basis for a model of the population consequences of exposure to multiple stressors, describes a series of compartments and transfer functions that upscale from physiological or behavioral changes to anticipated impacts on population vital rates. The series of transfer functions between compartments from the initial physiological change to the ultimate effect on individual vital rate or population dynamics in the PCoD model is essentially equivalent to an AOP. However, for this report, the committee defines an AOP to span the molecular- to individual-level responses shown in Figure 4.1.
In practice, it is extremely difficult to detect interactions between two stressors by determining the dose–response relationship for one stressor at different dosages of the second stressor. Instead, most research has focused on detecting deviations from additivity, usually by assessing the significance of the interaction term in an analysis of variance (ANOVA) or other linear model analysis of results from a controlled factorial experiment (Folt et al., 1999), or the deviations from a null model of additive effects (e.g., Darling and Côté, 2008). However, as Greenland (2007) notes, “concepts of biologic interaction do not in general correspond to the concept of statistical interaction, because the latter is only the need for a product term in a statistical model.”
In the next section, the results of recent meta-analyses of studies of the interactions between stressor effects that have used this statistical approach are reviewed in order to assess the prevalence and nature of interactions between extrinsic stressors in marine and freshwater systems. However, as noted above, these meta-analyses only provide information on whether statistical interactions have been detected: they do not provide quantitative models of the way the stressors actually interact. In subsequent sections the committee describes how interaction effects may be quantified by considering common pathways for adverse health outcomes along which different stressors act, provides some examples of the way in which the extrinsic stressors to which marine mammals are exposed may interact, and explains how stressors might be prioritized for cumulative effects analysis. Finally, that approach is used to look at the potential causes of some unexplained declines in marine mammal populations.
STUDIES OF MULTIPLE STRESSORS: A BRIEF REVIEW
As noted in the previous section, most studies of interactions among multiple stressors test whether the effect of the stressors together is significantly different from the combined effect of each stressor acting independently. The magnitude of effect expected depends on the mathematical operation used to combine the independent effects. For example, stressor effects may be combined additively or multiplicatively depending on the nature of the response being tested. Because a multiplicative combination of stressor effects is additive on the logarithmic scale, both methods of combination are usually referred to as “additive.” The test statistics that are most commonly used are Hedges’ d, which, according to Crain et al. (2008), is “constructed similar to ANOVA where a significant interaction effect signifies deviation from the null model of additivity,” and the sum of the natural logarithms of the response ratios (lnRR) for each stressor. For the latter metric, an interaction is identified if the difference between the lnRR when both stressors are present and the sum of the lnRR values for the individual stressors is significantly greater than zero. If the combined effect of two or more stressors is greater than the combination of their individual effects, this is referred to as a synergistic interaction. If it is less than the combination of the individual effects it is referred to as an antagonistic interaction. If there is no significant difference, the cumulative effect is referred to as additive.
The complications that can arise with these simple null models are elegantly summarized by Côté et al. (2016). For
example, synergistic interactions are impossible to detect with these methods if the sum of the individual effects is greater than 100% (Folt et al., 1999). These issues can be overcome by using the “multiplicative risk model” as described by Sih et al. (1998). The predicted combined effect using the multiplicative risk model is less than the predicted effect from a simple additive model, and its use as the null model is therefore more likely to result in the detection of synergistic interactions. Further complications occur if the effect of one stressor is so large that it results in the death of most experimental animals before any other stressor can have an effect. This is referred to as “dominance” by Côté et al. (2016). It would be incorrectly identified as an antagonistic interaction using a simple additive model. Additional problems arise if the stressors under consideration have opposite effects. In these cases, the threshold for a synergistic or antagonistic effect is actually smaller than the effect of either of the stressors. Such effects have been referred to as “reversals” (Jackson et al., 2016). Finally, in some cases the combined effect of the two stressors is in the opposite direction to the effects of either of the individual stressors, a phenomenon called “mitigating synergism” by Piggott et al. (2015).
Crain et al. (2008) reviewed 171 studies that used factorial experimental designs to investigate the effects of two or more of 13 stressors on marine and coastal environments. About 90% of the experiments were done in the laboratory and three-quarters of the studies subjected single species rather than entire communities or ecosystems to the stressors. They detected synergistic interactions using Hedges’ d in 36% of the studies and antagonistic interactions in 38%. When a third stressor was added, the proportion of synergistic pairwise interactions increased from 33% to 66%. Piggott et al. (2015) reanalyzed the same data set as that used by Crain et al. (2008) to take account of comparisons in which the stressors had opposite effects and the potential for mitigating synergisms. They found fewer examples of synergistic interactions (31% versus 36%) and more examples of antagonistic interactions (43% versus 38%).
Harvey et al. (2013) analyzed 623 observations from controlled factorial studies of the cumulative effects of temperature and acidification on calcification, photosynthesis, reproduction, survival, and growth in marine organisms using lnRR as the test statistic. Their analysis found evidence for synergistic interactions between the two stressors for four of the response variables. This was the result of a greater than expected increase in photosynthesis, and a greater than expected reduction in calcification, reproduction, and survival.
Ban et al. (2014) used a parametric bootstrap approach for calculating the standard error of the interaction term in an ANOVA of the results from studies of the effects of multiple stressors on coral reefs. Their aim was to increase the statistical power of more conventional analyses, which can result in failure to detect an interaction when one is, in fact, present. They analyzed the results of 26 fully factorial studies that investigated the cumulative effect of irradiance and temperature on photosynthesis in corals and found that the mean effect size of the combined treatments was statistically indistinguishable from a purely additive model.
Jackson et al. (2016) analyzed values of Hedges’ d extracted from 286 observations of the responses of freshwater ecosystems to paired stressors in controlled factorial experiments. They found that multiple stressors exerted significant antagonistic effects on animal abundance/biomass, animal condition, animal growth/size, and animal survival.
Przeslawski et al. (2015) analyzed values of Hedges’ d extracted from the results of 104 factorial experiments that examined the cumulative effects of temperature, salinity, and pH on growth and/or survival of the embryos or larvae of marine organisms using a generalized linear mixed-effects model. They found evidence for synergistic interactions between temperature and pH in 76% of the experiments, and for synergistic interactions between temperature and salinity in 58%.
This review of meta-analyses establishes that the cumulative effects of multiple stressors may be additive, antagonistic, or synergistic in almost every setting tested. The proportion of cases providing evidence for antagonism and synergism varied substantially among studies. As a result, the prevalence of interactions between stressors in nature remains uncertain, especially because the relatively low statistical power of most of the studies (Ban et al., 2014) will have resulted in some interactions going undetected. Nonetheless, the basic conclusion that one can take from all of these studies is that there are few situations where one can confidently assume that the effects of multiple stressors are additive. Although Côté et al. (2016) have pointed out that synergies are not the most prevalent form of interaction reported in the literature, and caution about the risks of managing antagonistic interactions as if they were synergistic, they also found that “physiological response variables have so far not yielded evidence of antagonisms.” Because physiological responses are a fundamental component of most of the observed reactions of marine mammals to extrinsic stressors, this suggests that assuming the effects of individual stressors are additive may frequently lead to an underestimation of their cumulative impact.
Finding 4.1: There are few situations where one can assume that the effects of multiple stressors are simply additive, and this assumption may lead to an underestimation or overestimation of their cumulative impact.
Most of the studies of cumulative effects of multiple stressors that contributed to these reviews have used factorial designs. This leads to elegant experiments with simple analyses in situations where the conditions can be replicated and controlled. However, if the factorial design does not actually provide a dose–response relationship for each stressor–effect
pair, or for any relevant combinations of stressors, then it is of little use to management. The critical questions for managers who aim to prevent threats are “What stressor effects threaten populations or ecosystems, and what combinations of dosages of stressors elevate the effect enough to pose a risk?” Given that many anthropogenic stressors have negative effects on marine mammals, simply evaluating whether their cumulative effects may be antagonistic, additive, or synergistic does not provide the information needed to decide whether specific dosages of one or more stressors are likely to cause an effect that poses a risk to species of concern. The critical point for managers in the planning phase is to define population-level effects that need to be avoided, and then to evaluate whether the cumulative impact of a planned activity, of other activities, and of the relevant array of natural stressors poses a risk of causing the deleterious effects. After it is discovered that a population or ecosystem is in danger, then the critical issue is to evaluate what changes in stressors will provide the best reduction in risk at the least disruption of other critical human priorities. Both of these problems require assessment of dose–response relationships across the relevant range of dosages and effects. Ideally this assessment should be conducted under realistic field conditions, coupled with quantitative assessments of the interaction between all stressors that may cause the effect of concern.
Finding 4.2: The critical question for managing risk of cumulative effects is “What combinations of dosages of stressors are likely to elevate the effect enough to pose a risk to populations or ecosystems?” Once a population is found to be at risk, then the critical issue is to determine which combination of stressors could be reduced in order to bring the population or ecosystem into a more favorable state.
CUMULATIVE IMPACT SCORES
Halpern et al. (2008) used expert-derived vulnerability weights from Halpern et al. (2007) and a cumulative impact model to identify what they believed to be the greatest threats among 38 different stressors and ecological drivers at large or small spatial scales of marine ecosystems, and to identify the most threatened ecosystems. They used this method to create a global map of human impacts on marine ecosystems, and they argue further that this map can be used to allocate conservation resources for ecosystem-based management. Maxwell et al. (2013) adapted the methods of Halpern et al. (2007, 2008) and used them to estimate cumulative impacts for marine mammals and other marine predators. Here a critical review of this approach is provided.
Halpern et al. (2008) calculated cumulative impact scores IC for each 1 km2 of ocean using the following equation:
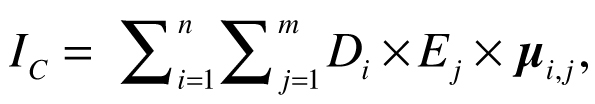
where Di is the log-transformed and normalized value of the intensity of the driver at location i, Ej is the presence or absence of ecosystem j, and µi,j is an impact weighting for each driver–ecosystem pair. Drivers were allowed to have different weights for different ecosystems, but this calculation of cumulative impact assumes the effects of the drivers are additive, with no interaction between them. Maxwell et al. (2013) estimated the cumulative impact of multiple stressors (CUI) using a similar equation:
![]()
where Di is the normalized and log-transformed value of intensity of an anthropogenic stressor at location i, Sj is the probability distribution of species j being present in a given cell, and µi,j is the impact weight, which reflects the potential effect of anthropogenic stressor i on species j. The impact weight for each stressor–species combination is calculated from expert rankings of the importance of a number of different vulnerability measures for that combination.
The determination of impact weights is a critical aspect of this approach. Halpern et al. (2007) used two numerical measures (area and recovery time) of vulnerability, and three ordinal variables (frequency, extent of ecosystem impacted, and resistance of the ecosystem to the threat). Maxwell et al. (2013) used six measures (frequency of impact, whether the impact was direct or indirect, likelihood of mortality, individual recovery time, reproductive impact, and spread of the impact across the population). These rankings are then combined into a single vulnerability score.
This kind of arbitrary tallying of ordinal scores is not uncommon in situations where, for example, a health practitioner wants a simple repeatable way to assess the cumulative risk of a series of factors for a specific adverse outcome. However, the committee thinks that the arbitrary tallying of this kind of scale requires validation. When Halpern et al. (2007) asked the experts to identify the three top threats in the ecosystems, only half of the results of the vulnerability ranking matched the judgment of the experts, indicating either that there was low confidence in the resulting rankings or that the experts suffered from perception bias.
The cumulative impact scores used by Halpern et al. (2008) and Maxwell et al. (2013) assume that cumulative effects are additive across threats within an ecosystem. As discussed above, all the reviews of the effects of multiple stressors found evidence for synergistic and antagonistic interactions, which suggests that this simple additive approach may overestimate some impacts and is likely to underestimate others. The committee recognizes the enormous amount of work that has gone into developing this approach and compiling the databases needed for its application. Determining the spatial overlap between human activities and species or ecosystems is an important first step in identifying locations where interactions between stressors
are likely to occur. However, the committee believes that a better quantitative understanding of potential exposure levels, dose–response functions, and linkages to vital rates is required to provide an adequate assessment of cumulative effects in these locations.
PREDICTING HOW MULTIPLE STRESSORS ARE LIKELY TO INTERACT
A consideration of cumulative effects has been often discussed with respect to marine mammals (Wright and Kyhn, 2015), and such effects must be considered in Environmental Assessments and Environmental Impact Studies (40 C.F.R. § 1508.7). However, in spite of the large number of factorial experiments in other taxa, no experiments have examined the cumulative effects of multiple stressors on marine mammals. Quantification of the interactions between these stressors is hindered by a limited understanding of the physiological and behavioral effects of cumulative exposure, and the logistical difficulties of measuring the impacts of this exposure on free-ranging individuals over their lifespans.
Any stressor that induces effects up to at least an individual level (e.g., mortality or reproductive impairment), whether exposure is acute, intermittent, or chronic, has the potential to contribute to a cumulative population-level impact. For example, direct lethal effects may occur as a result of acute exposure to ship strike, intermittent exposure to infectious disease outbreaks or harmful algal blooms, or to the risk of bycatch in fishing gear that is left in the water for long periods (e.g., gillnets). In most cases, the acute effects of each stressor on survival can be evaluated independently and their cumulative effect calculated using a multiplicative risk model that accounts for the fact that an individual can only be killed once.
However, it is more difficult to predict the interactions that may occur among stressors that have a chronic effect on survival and reproduction, and that therefore have the potential to generate unexpected, nonadditive effects for populations and communities. These occur when a stressor affects an individual’s homeostatic systems so that it can no longer respond appropriately to its environment, and its vulnerability to other stressors is increased. Interactions may also occur at the population level if the stressor effects result in demographic changes, for example, if mortality is preferentially focused on adult females. They may also occur at a higher level of biological organization (community or ecosystem level) if a tipping point (see Chapter 6) is reached because an ecological driver has, for example, caused a collapse in the prey base. In the rest of this section, approaches that can be used to improve understanding of potential interactions between stressor effects at the individual level are explored. The potential for interactive effects at higher levels is discussed in Chapter 6.
Insight for predicting cumulative effects at the individual level can be gained from the environmental health and ecological risk assessment communities, where scientists are grappling with the complicated issue of cumulative risk assessment for chemical mixtures. There are more than a hundred million chemical substances known to date,1 and a recent report from the Centers for Disease Control and Prevention provides data for 265 environmental chemicals that are a potential concern for human exposure.2 People, other terrestrial organisms, and marine organisms are all exposed to this plethora of potentially toxic substances to varying degrees and are most often exposed to mixtures of these chemicals chronically or repeatedly throughout their lives.
A number of different approaches have been proposed for assessing the cumulative risk for multiple chemicals. They often involve identifying a group of chemicals that can be considered collectively (EPA, 2000). One mathematical modeling approach integrates an index for chemicals that co-occur in the environment and have similar structure or mode of action in order to predict a cumulative dose (EPA, 2002; Connon et al., 2012). The index for each chemical can be based on its concentration and toxic potential; therefore, the approach is most applicable for chemicals with a well-characterized mechanism for toxicity, such as the dioxin-like compounds whose toxicity is induced through the aryl hydrocarbon receptor (Van den Berg et al., 2006). Alternative approaches have been suggested that focus on the overall physiological process, rather than mechanisms or modes of action, because there can be a multitude of underlying molecular mechanisms that contribute to a given adverse outcome. This potentially expands the array of chemicals to be considered collectively, because chemicals that have distinct modes of actions may still disrupt the same endocrine pathway or organ system and, ultimately, result in the same disease.
There are clearly limitations to the expansion of these approaches to the multitude of stressors, particularly nonchemical ones, that are of potential concern for marine mammals. However, the paradigm of using co-occurrence, and a common mechanism of action or a common outcome, may be valuable. At the molecular level, it may be possible to predict the effect of stressors that have a similar mode of action using a common dose–response relationship. The cumulative effect of these stressors will only be additive in the unlikely event that the common dose–response function is linear (see Figure 4.2).
One common assumption of ecotoxicologists is that if two or more stressors act through a common mechanism of action, then their doses can be summed to provide a cumulative dose that can then be used with a single dose–response function. Many dose–response functions are sigmoidal in shape or are otherwise nonlinear, and in these cases the sum of two doses may produce a response that is greater or less than the added responses of each stressor alone. A simple
___________________
1 See http://www.cas.org.
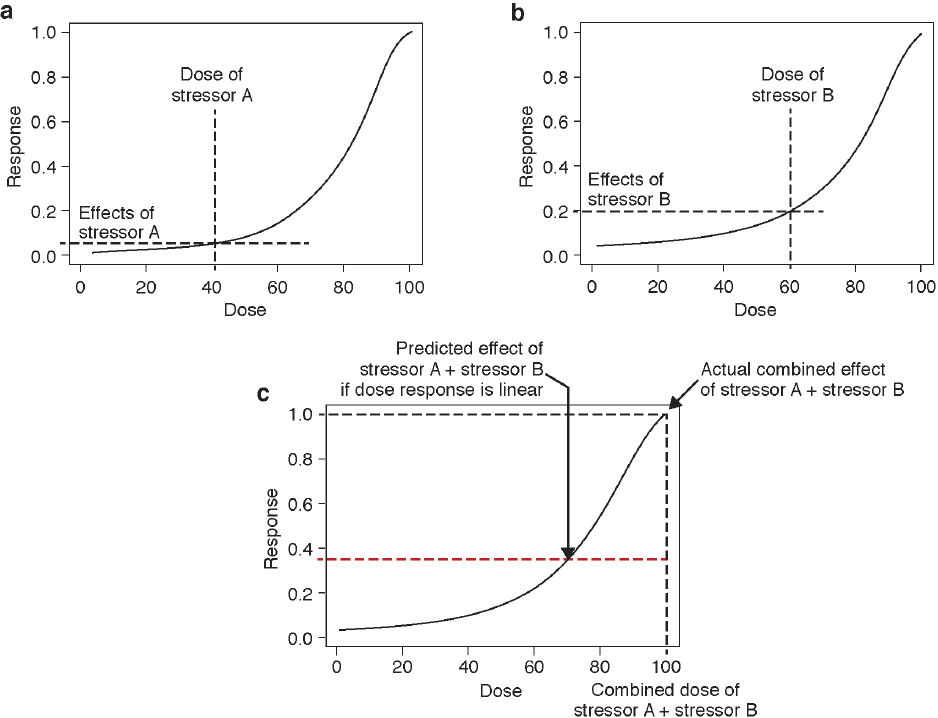
example to illustrate the complexity introduced when a dose–response function is nonlinear is discussed below.
Consider two stressors that act through a common mechanism of action. If one of these stressors is more powerful than the other, then its dosage needs to be adjusted by a metric that corrects for the difference in their relative strengths (e.g., a toxicity factor for chemical stressors). After this correction, the doses of the two stressors can be added to give a combined dosage and compared to a dose–response function (see Figure 4.2). Stressor A has an effect of 0.10 given a dose of 40 units (see Figure 4.2a), and stressor B has an effect of 0.20 given a dose of 60 units (see Figure 4.2b). If responses were additive, then the response to stressors A and B combined is expected to be 0.30. However, due to the sigmoidal shape of the dose–response function, the added doses of the two stressors (100 units) produces an effect of 1.0, more than threefold higher than the sum of the individual responses (see Figure 4.2c). Therefore, although these stressors are considered additive in terms of dosage, they produce a synergistic response. Note that this same phenomenon could also occur with aggregate exposure to a single stressor. Even for this simple situation, a prediction cannot be made of the effects of most stressors because the dose, the relative strengths of the stressors, and the dose–response functions are not known.
Similar interactions may occur at the organ system and individual levels if the stressors act through a common or connected pathway. This may occur if the stressors induce damage or provoke a physiological perturbation within the same organ system or endocrine axis, in which glands signal each other in sequence and/or with feedback loops, such as the hypothalamic-pituitary-adrenal (HPA) axis. In addition, effects via one cellular mechanism or component of an endocrine axis may impact the function of other components through shared signaling pathways. Due to this complexity, the overall physiological process or pathway for an adverse health outcome should be considered. Of primary concern are those pathways that lead to a permanent or at least long-lasting (persistent) adverse health condition, because co-occurrence of the health effects of multiple
stressors within an individual is necessary for an interaction to ensue. Alternatively, although the health effect associated with a particular exposure to a stressor could be transient, co-occurrence with other stressor effects is still likely if the exposure to the stressor is chronic.
Finding 4.3: Predicting which combinations of dosages of stressors are likely to elevate cumulative effects enough to pose a risk to populations or ecosystems will be challenging, particularly for stressors that have a chronic effect on survival and reproduction. The paradigm of using co-occurrence and a common pathway for adverse health outcomes, developed by the environmental health and ecological risk assessment communities, could be applicable for addressing this challenge.
Marine mammals are exposed to stressors that have the potential to interact as a result of chronic exposure, or because they may cause permanent or persistent health conditions. The pathways for a persistent health outcome along which each stressor may act are indicated in Table 4.1. Nonbiological toxins are divided into persistent organic pollutants (POPs), inorganic pollutants, and petroleum-associated chemicals and organic solvents, because these most often exert effects through differing pathways. Note that this table is not intended to provide an exhaustive list of all the possible sublethal effects associated with each stressor. Only the principal and previously recognized pathways are indicated, with one or more illustrative references. In addition, only direct pathways are indicated as priorities for consideration. The potential for interaction between pathways should not be disregarded. For example, although the hypothalamic-pituitarythyroid (HPT) and HPA endocrine pathways are presented separately, effects on one axis may impact the function of the other because of shared molecular substrates, enzymatic reactions, and signaling pathways (Nichols et al., 2011). Ultimately, they may impact other connected pathways, such as the immune or central nervous systems (CNS). There are strong associations in some marine mammals of contaminant burdens with suppression of sex hormones, including testosterone and estradiol. In some cases low levels of sex hormones concomitant with high POP burdens were associated with sterility or reproductive failure (Reijnders, 2003).
POTENTIAL INTERACTIONS AMONG STRESSORS
In this section the committee reviews documented or proposed interactions between stressors, focusing on interactions that occur along the same pathways for persistent health outcomes (see Table 4.1). Most of the interactions we consider are synergistic, not only because ignoring such interactions in an assessment of cumulative impacts increases the risk of underestimating those impacts, but also because Côté et al. (2016) found no evidence for antagonistic interactions involving physiological responses to stressors, such as those mediated by pathways for persistent health outcomes.
Acute Mortality
A number of the stressors listed in Table 4.1 (noise, some organic chemicals and solvents, biotoxins, microparasites, prey limitation, and predation pressure) may have direct, acute effects on survival or reproduction. In some situations where marine mammals are exposed to several of these stressors there may be little opportunity for stressor effects to interact, because individuals are likely to die from the effects of one stressor before they can be affected by any of the others. In these circumstances, as noted earlier in this chapter, treating the effects of each stressor as independent can be justified. However, it should be recognized that historical exposure to other stressors may increase an individual’s susceptibility to acute effects from a particular stressor. For example, Hall et al. (2006) showed that previous exposure to polychlorinated biphenyls (PCBs) increased the risk of death from infectious diseases in harbor porpoises. In addition, a multiplicative risk model should be used to account for the fact that individuals are unlikely to die from the effects of more than one acute stressor. Because acute effects are normally evaluated by attributing cause of death to a particular stressor, the simplest approach is to calculate the survival rate of individuals exposed to each stressor. The cumulative effect of all the stressors to which the population is exposed is then calculated by multiplying together the survival rates associated with each stressor.
Although there is little opportunity for interaction among the acute effects of different stressors, chronic effects caused by the same or other stressors can interact with acute effects if they alter individual exposure or susceptibility to the acute stressors. These interactions between acute and chronic stressor effects may be antagonistic. A classic example is the use of active sound emitters (“pingers”) to reduce the risks of cetacean bycatch in fishing gear (Dawson et al., 2013). Noise from these emitters displaces marine mammals from the area around the gear to which they are attached, thus reducing their risk of physical injury as a result of entanglement but imposing potential energetic costs.
Hypothalamic-Pituitary-Adrenal Axis
The HPA axis has a central role in coordinating an organism’s response to stress, controlling the release of glucocorticoids into circulation and moderating levels through negative feedback (Sapolsky et al., 2000). Glucocorticoid secretion is further modulated by neuronal effects of other brain structures; also gene–environment interactions in response to stressors may have long-term impacts on subsequent secretion (Alexander et al., 2009). Disruption of the HPA axis may therefore interact with the effects of other stressors, particularly if the disruption is the result of chronic
TABLE 4.1 Stressors with Potential for Chronic/Repeated Exposure or Persistent Effects, and Associated Pathways for Adverse Health Outcomes
NOTE: Publications highlighted in bold refer to studies involving marine mammals.
exposure to a persistent chemical contaminant, because of the numerous points of regulation and complexity of the involved biochemical pathways. However, an understanding of specific mechanisms for a given set of stressors would be needed to accurately predict the consequences of any resulting interactions.
The analysis provided in Table 4.1 suggests that cumulative risk associated with sound and other stressors will occur primarily through the HPA axis. While there is some evidence that the presence of ships and their accompanying sounds affect the HPA axis (Rolland et al., 2012), no studies have looked at the cumulative risk of sound and other stressors through the HPA axis. The indirect effects of sound through prey limitation and predator response are discussed in Chapter 2.
There is strong evidence that petroleum-associated chemicals can adversely affect the HPA axis, providing a potential pathway for interactions with other stressors. Studies by Mohr et al. (2008, 2010) of mink (Mustela vison) as a surrogate for sea otters (Enhydra lutris) found that exposure to fuel oil interfered with the HPA pathway, resulting in damage to the adrenal gland and an insufficient stress response when the animals were experimentally stimulated with adrenocorticotropic hormone. Polycyclic aromatic hydrocarbons (PAHs), the predominant class of chemicals in fuel oils that are linked to adverse health effects, are more rapidly metabolized (Mohr et al., 2008, 2010) than POPs. Unless there is continuing exposure to an environmental source, exposure of marine mammals to PAHs is generally more limited than to persistent organochlorines. However, the effects on the HPA pathway as a result of acute exposure from, for example, an oil spill may persist for many years. Nearly half of the live bottlenose dolphins (Tursiops truncatus) sampled from a bay within the Deepwater Horizon (DWH) oil spill footprint approximately 1 year after the massive spill had indications of insufficient production of adrenal hormones (Schwacke et al., 2014b). Adrenal insufficiency can lead to adrenal crisis and death in animals that are challenged with other stressors, such as physical injury, microparasites, or temperature extremes, to which a healthy animal would otherwise adapt. Many of the dead dolphins that were recovered in the 1.5 years post-spill had rare adrenal gland lesions, and Venn-Watson et al. (2015) suggested that a likely cause of death for these dolphins was an adrenal crisis brought on by an interaction between the effects of petroleum-associated chemicals with the HPA axis and thermal stress (a particularly cold winter in the year after the spill) or a pathogen infection. Indications of adrenal insufficiency were found in dolphins from the same bay sampled 3 to 4 years after the DWH spill (Smith et al., 2017), suggesting that injuries to the HPA axis may be long lasting.
It has been suggested that some POPs may also disrupt the HPA axis by interfering with glucocorticoid receptors or the synthesis of adrenal steroids (Martineau, 2007; Diamanti-Kandarakis et al., 2009; Harvey, 2016), but studies to support such effects are still lacking. However, there is strong evidence for an HPA axis effect for one POP: the DDT derivative o,p′-DDD, which is a well-known inhibitor of adrenal steroidogenesis and is used in the treatment of hyperadrenocorticism (chronic overproduction of glucocorticoid) in dogs (Klein and Peterson, 2010).
Permanent or persistent adverse health outcomes, including decreased glucocorticoid measures, have also been reported in survivors of toxic algal blooms (Bejarano et al., 2008b; Goldstein et al., 2008; Gulland et al., 2012), and these provide the potential synergistic interactions with other stressors. For example, sea lions exposed to domoic acid, a potent neurotoxin, from algal blooms were found to have low serum cortisol concentrations as compared to unexposed controls (Gulland et al., 2012). This effect was seen in sea lions with indication of recent exposure (domoic acid in urine or feces sample), as well as in sea lions that were assumed to have been previously exposed (undetectable domoic acid in urine or feces sample). It is unclear whether the low cortisol concentrations were due to binding of domoic acid to glutamate receptors in the endocrine glands, adrenal gland exhaustion, or other disruption of the HPA axis (see Gulland et al. [2012] for discussion). Regardless, the low cortisol suggests that these individuals were more vulnerable to the effects of other stressors (e.g., petroleum-associated chemicals, noise, and perceived threat) that affect the HPA pathway.
Hypothalamic-Pituitary-Thyroid Axis
The effects of prey limitation may interact with the effect of POPs via the HPT axis. The interference of POPs with the HPT pathway has been well established in terrestrial animals (Patrick, 2009), and there is evidence that similar HPT disruption occurs in marine mammals (Tabuchi et al., 2006; Schwacke et al., 2012). HPT disruption can produce adverse effects during critical stages of development and growth (see Zoeller et al. [2002] and Diamanti-Kandarakis et al. [2009] for review). There is strong evidence for the relationship of POP burdens to suppression of thyroid hormones in diverse species of marine mammals, including pinnipeds, cetaceans, and polar bears (Jenssen, 2006). These effects could potentially act synergistically with the effects of prey limitation, in times of nutritional stress or when animals are faced with other environmental challenges. Ford et al. (2010) suggest high POP concentrations in Pacific killer whales (Ross et al., 2000) may have acted synergistically with the effects of prey limitation, resulting in increased mortality during times of low prey abundance. Reduced prey availability would have resulted in the depletion of fat stores and could have led to mobilization of POPs sequestered in the blubber. The increase in circulating POPs could have interfered with metabolic processes. It could also have further increased suppression of immune responses that were
already being modulated by the nutritional stress, resulting in increased disease susceptibility.
Immune Pathway
Numerous researchers have suggested a potential for synergistic interactions between the effects of chemical contaminants and microparasites through the immune pathway. This is based on the well-known immunosuppressive effects of many POPs. Evidence for a greater incidence of infections in relation to POP exposure has been demonstrated in human studies (reviewed by Carpenter [2006] and Gascon et al. [2013]), and effects on immunity have been demonstrated in marine mammals using indices of immune function and/or in vitro experiments using marine mammal leukocytes (Ross et al., 1995, 1996a; De Guise et al., 1998). Exposure to POPs has been considered as a potential exacerbating factor for a number of viral epidemics, including the morbillivirus epidemics of striped dolphins in the Mediterranean in the early 1990s (Aguilar and Borrell, 1994) and common bottlenose dolphins along the Atlantic coast in the late 1980s (Kuehl et al., 1991). However, the cross-sectional nature of the studies (i.e., POP concentrations were measured simultaneously with the mortality outcome) has made it difficult to demonstrate a causal link between these stressors in wild populations because disease-related weight loss may have resulted in an increased concentration of lipophilic POPs in the remaining blubber layer (Hall et al., 1992). In order to overcome this problem, Hall et al. (2006) adopted a case-control design to analyze data from a long-term study of harbor porpoises stranded around the United Kingdom. They found an increased risk of mortality from infectious disease in animals with high tissue concentrations of POPs.
Other potential synergistic interactions mediated by the immune pathway involve petroleum-associated chemicals and microparasites. Persistent adverse health outcomes involving this pathway were reported in bottlenose dolphins following the DWH oil spill (Schwacke et al., 2014a, 2014b; Lane et al., 2015; Venn-Watson et al., 2015). The reported immune perturbations were compatible with an increased susceptibility to intracellular bacterial infections (e.g., brucellosis) that can cause reproductive failure (S. De Guise, personal communication), and in the years immediately following the spill, a higher than expected prevalence of primary bacterial pneumonia was noted in recovered dolphin carcasses (Venn-Watson et al., 2015).
The chronic effects of one pathogen may result in a synergistic interaction with the effects of other pathogens via the immune pathway. For example, morbillivirus infection may result in residual immune system perturbations. It has been shown to erase immunological memory in laboratory animals, leading to a persistent increased susceptibility to other infectious agents (de Vries et al., 2012). Impairment of cell-mediated adaptive immunity and partially upregulated humoral immune response has been reported in bottlenose dolphins with morbillivirus-positive antibody titers (Bossart et al., 2011). These perturbations could impact an animal’s ability to mount an appropriate immune response when challenged. Furthermore, opportunistic secondary infections leading to mortality following the acute phase of morbillivirus infection have been reported following a number of cetacean morbillivirus outbreaks (see Van Bressem et al. [2014] for review).
Brain/CNS Pathway
Maternal exposure to POPs, and specifically PCBs, has been linked to adverse developmental effects in human offspring, including neurological effects and reduced cognitive function (e.g., Jacobson and Jacobson, 1996; Stewart et al., 2003, 2008; reviewed by Boucher et al., 2009). Such effects would produce less fit offspring, and if similar effects occur for wild marine mammals this could clearly lead to decreased survival in the earliest life stages, if individuals are exposed to other stressors that require increased foraging proficiency or rapid avoidance responses (e.g., prey limitation, perceived threat, and noise). In addition, a recent study by Cook et al. (2015) provides evidence that hippocampal lesions caused by sublethal exposure to domoic acid linked to toxic algal blooms affect spatial memory, which potentially could impair an animal’s ability to navigate and forage. Such effects would be permanent for the individual and would likely interact with the effects of other stressors, such as prey limitation.
Animals that survive morbillivirus infection may be plagued with persistent chronic CNS infection. Chronic encephalitis was identified as a common cause of death in stranded striped dolphins (Stenella coeruleoalba) for years following a morbillivirus outbreak in the Mediterranean (Soto et al., 2011) and has also been identified in other cetacean species following morbillivirus outbreaks after the outbreak had subsided (Uchida et al., 1999; Yang et al., 2006). These chronic CNS infections could affect behavioral and physiological responses to other stressors, such as noise, particularly for deep-diving cetaceans. However, the estimated prevalence of CNS infection even following the substantial Mediterranean dolphin morbillivirus epidemic was relatively low (1-3 per 1,000 cases of infected individuals) (Soto et al., 2011) and therefore may not be a significant factor for population-level effects.
Auditory Pathway
One of the documented developmental effects of POP exposure is hearing loss, potentially mediated at least in part through the HPT axis; it involves loss of outer hair cells (Crofton et al., 2000; Lilienthal et al., 2011) and distorted development of the primary auditory cortex (Kenet et al., 2007). Such permanent conditions could result in an interaction between POP exposure and the effects of other stressors,
such as prey availability and predation pressure, mediated by the auditory pathway.
Organic solvents may also induce permanent hearing loss by damaging the outer hair cells or through effects on central auditory pathways. Studies of other mammal species (primarily rats and humans) demonstrate that the hearing frequencies affected by solvents are different from those affected by noise (reviewed by Fuente and McPherson, 2006). Furthermore, studies in rats have reported synergistic effects between some solvents and noise, demonstrating that simultaneous exposure to both produces a more severe hearing loss than the summed hearing loss produced by exposure to either agent alone (Lataye and Campo, 1997; Brandt-Lassen et al., 2000; Lataye et al., 2000; Mäkitie et al., 2003). The timing of exposure may be important as studies have also shown that the interactive effect between toluene and noise exposure was only synergistic if the exposures occurred simultaneously, or if the toluene immediately preceded the noise exposure. When the noise exposure was prior to the toluene exposure, the effects of the two stressors were independent (Johnson et al., 1990).
Interactions Across Pathways
All of the actual or potential interactions between stressor effects we have described above occur when the effects of different stressors act along the same pathway for persistent health outcomes. However, interactions may also occur across such pathways.
For example, interactions between the immune and reproductive pathways have been documented when prey is limited. The substantial metabolic cost of mounting an immune response has been well documented in diverse taxa, including mammals, birds, reptiles, and insects (Lochmiller and Deerenberg, 2000). Responses to moderate infections can lead to energetic costs as high as 55% increases in metabolic rate and 150-200% increases in the rates of glucose production. If prey is limited, animals can make allocation trade-offs between competing physiological processes. Ecological immunology theory predicts allocation trade-offs between reproductive effort and immune responses under conditions of energy limitation (Graham et al., 2011). When energy is limited, low-intensity infections may be allowed to persist if the energetic costs outweigh the benefits of clearing the infection (Sheldon and Verhulst, 1996; Martin et al., 2011). Individuals may prioritize innate immune responses over more expensive adaptive immune responses, despite greater potential for oxidative damage and autoimmunity (Downs and Dochterman, 2014).
During reproduction, nutrient limitation can force individuals to reduce their energy allocation to immune response so that they can support current reproductive effort in a way that may affect their future reproductive potential (Sheldon and Verhulst, 1996; Svensson et al., 1998). Thus, nutrient limitation may lead to impaired immune response especially during periods of reproduction. Because reproduction is associated with increased potential for pathogen exposure from conspecifics (e.g., during colonial breeding), energetic impacts on immune response can influence the survival costs of reproduction in marine mammals (Peck et al., 2016).
There is also potential for interactions between the HPA and immune pathways as a result of exposure to a range of stressors. Chronic elevation of stress hormones is known to downregulate immune response in wildlife systems (Sheldon and Verhulst, 1996; Råberg et al., 1998) through several pathways, including altering antibody responses (Fowles et al., 1993) and inhibiting lymphocyte proliferation (Rollins-Smith and Blair, 1993). Effects of glucocorticoid stress hormones are hypothesized to be an important mechanism underlying trade-offs between energy expenditure and immune response and may help to reduce the response to injury or infection during nutrient limitation (Sternberg et al., 1992; DeRijk et al., 1997).
There have been numerous efforts to examine the effect of stress hormones on immune responses in wildlife (Ricklefs and Wikelski, 2002; Acevedo-Whitehouse and Duffus, 2009; Peck et al., 2016). The few studies in marine mammals suggest that stress modulation of immune function in marine mammals is complex. Body reserves, foraging success, and the degree of plasticity in immune response may impact disease risk synergistically, through a tradeoff between immunity and starvation resistance (Brock et al., 2013a; Peck et al., 2016). Immune investment may be directly impacted by anthropogenic disturbance. Brock et al. (2013b) revealed negative associations between body condition and immune response but only in a population exposed to anthropogenic disturbance. These findings implied energetic costs to disturbance that influenced energy allocation toward fighting infection. Finally, individual components of the immune response may be impacted differentially by elevations in stress hormones and variation in body reserves in ways that differ from biomedical model species (Peck et al., 2016).
PRIORITIZING STRESSORS FOR CUMULATIVE EFFECTS ANALYSIS
As noted above, there is only limited understanding of how exposure to individual stressors may affect demographic rates or population dynamics in marine mammals. Yet most marine mammal populations are actually exposed to multiple stressors, and the committee’s review of studies of multiple stressors indicates that they are as likely to interact synergistically or antagonistically as they are to act in a simple additive way. It is necessary to find a way to understand the nature of these interactions, while recognizing that experimental investigations of the combined effect of multiple stressors on marine mammals are unlikely to be feasible or ethical. Figure 4.3 is a decision tree that can be used to identify situations in which studies of the interactions between stressors
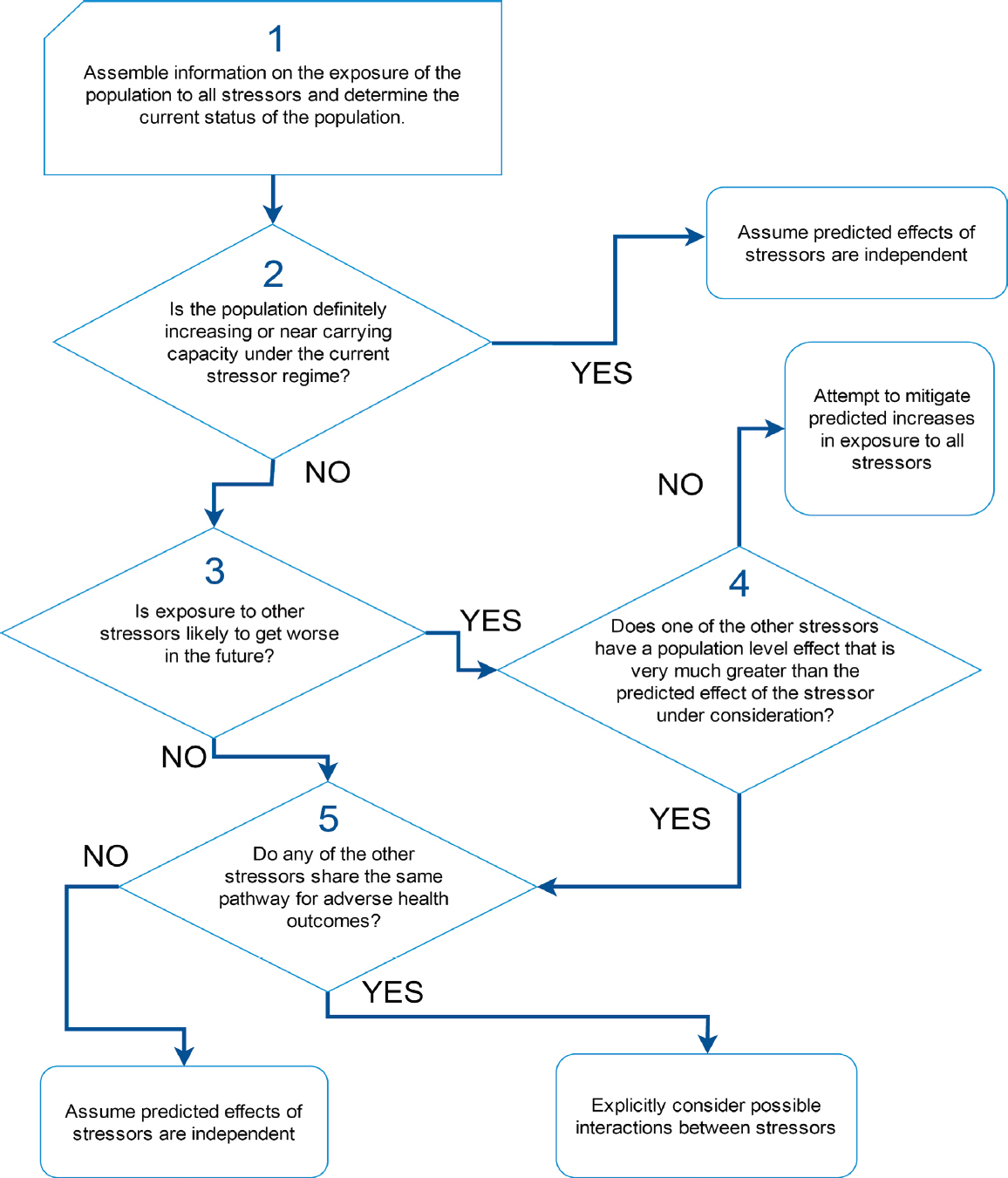
should be given high priority. It is based on the assumption that interactions are most likely to occur among stressors that share a common pathway for a persistent health outcome (Côté et al., 2016).
Step 1 in the decision process is to determine the spatial and temporal overlap between each stressor and the population of interest. Geospatial approaches, such as those described by Halpern et al. (2007) and Maxwell et al. (2013), can be used to determine this overlap, although, as noted above, these approaches do not provide a rigorous assessment of cumulative impacts. However, several issues make the estimation of exposure to multiple stressors more complicated than first meets the eye. For example, many marine mammal populations are migratory and they will therefore experience considerable temporal variation in their exposure to particular stressors. Thus, the actual duration of exposure to a stressor that is present in a particular area is limited by the amount of time the population actually spends in that area. Quantifying temporal variation in stressor presence is also important for resident populations, because the presence of a stressor may not coincide with sensitive life-history stages. In addition, prior exposure to pathogens or toxins may increase an individual’s sensitivity to additional stressors that are encountered in different locations or long after the initial exposure to the pathogen or toxin. Step 2 is to determine the current status of the population of interest (i.e., is it increasing, neither obviously decreasing nor increasing, or decreasing). Chapter 7 describes the methods that can be used to ascertain population status. If a population is definitely increasing, or if it is close to carrying capacity, it should be reasonably resilient (Taylor and DeMaster, 1993) to additional mortality caused by interactive effects between stressors. Large adverse population-level effects of these interactions are likely to be detected before the population has declined to levels of concern. In these circumstances, studies of possible synergies between stressors would not be a high priority.
Steps 3 and 4 allow the identification of situations in which the population is decreasing and the population’s exposure to stressors is expected to increase over time. If one of the existing stressors to which the population is exposed is known to have a dominant effect (Step 4), possible interactive effects should be considered for stressors that share the same pathways for adverse health outcomes as the dominant stressor. If there is no dominant stressor, efforts will likely be required to mitigate any potential increases in stressor exposure, even if there is no evidence of interaction between the stressors.
In Step 5 the other stressors to which the population is currently exposed should be reviewed to see if they share the same pathway for adverse health outcomes. If they do, then the possibility that these stressors may interact synergistically should be investigated.
When considering the way the effects of multiple stressors may be analyzed, it is important to take account of the lessons that have been learned from epidemiological studies, where confounding variables are known to give rise to spurious associations between exposure variables and effects of interest. This is particularly likely to be the case when the effects of one stressor operate along the same causal pathway as other variables. This situation may result in colinearity between stressor variables in linear models, or it may mask the indirect effects of stressors through other variables when fixed effects are assessed in an ANOVA. In these cases, analyses that are based on structural equation modeling or some other latent state modeling may better account for the causal pathways by which stressors impact physiology, behavior, health, or vital rates.
Recommendation 4.1: Situations where studies of cumulative effects should be prioritized can be identified using tools such as the decision tree developed by the committee and testing for whether pathways for adverse health outcomes are shared across stressors.
CASE STUDIES: DIFFICULTIES IN INFERRING CAUSES OF DECLINES
In this section, three case studies of marine mammal populations that have either suffered a precipitous, unexplained decline, or have failed to recover following the removal of a dominant stressor are considered. This is not a critique of the work that has been done to investigate these declines, nor is it an attempt to suggest how these populations should be managed to promote their recovery. Rather, the committee’s aim is to describe how the potential causes of the decline were initially identified, and to investigate what conclusions might have been drawn if the decision tree shown in Figure 4.3 had been used as part of this process.
Cook Inlet Beluga
The Cook Inlet (CI) beluga whale (Delphinapterus leucas) population, which is separated by the Alaska Peninsula from other beluga populations in Alaskan waters, declined from around 1,300 whales in 1979 to 367 in 1999 (Hobbs et al., 2000; see Figure 4.4). Alaskan Native subsistence harvest between 1993 and 1998 ranged from 21 in 1994 to 123 in 1996. The most reliable data come from 1995-1997, when an average of 87 whales were taken per year (Angliss and Lodge, 2002). Including this subsistence take in models of the population’s dynamics indicated that it was sufficient to account for most of the observed decline over this period. Alaskan Natives imposed a voluntary moratorium in 1999, and in 2000 the National Marine Fisheries Service (NMFS) declared the population depleted under the Marine Mammal Protection Act (65 Fed. Reg. 34590). The expectation was that with greatly reduced subsistence take the population would grow between 2% and 6% annually. Since 1999 the total subsistence harvest has been five whales, with none
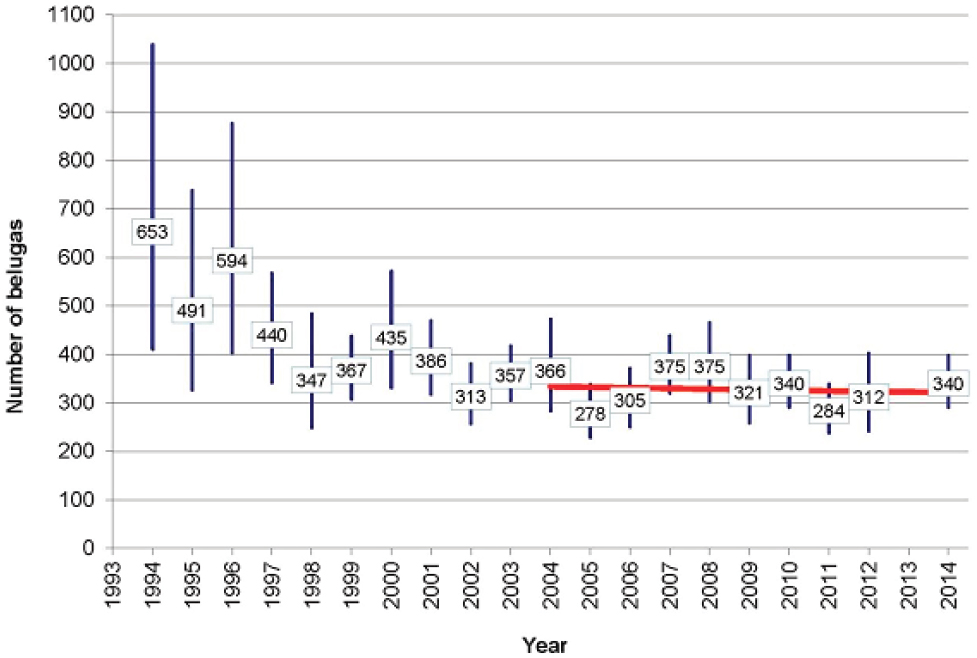
taken after 2005 (NMFS, 2015). Nonetheless, the population has shown no sign of recovery (see Figure 4.4). The most recent estimate of population size is 340 in 2014 (Shelden et al., 2015). Based on aerial surveys and satellite telemetry data, the core summer distribution of the population has contracted from more than 7,000 km2 in 1978-1979 to 2,800 km2 in 1998-2008 (Rugh et al., 2010). As a result, most of the population is concentrated in upper Cook Inlet, during the summer months. This is close to the port of Anchorage, where the population is most likely to be exposed to disturbance from human activities (NMFS, 2015). Why there has been this change of distribution is not known, although several possible reasons have been suggested (Moore et al., 2000; Shelden et al., 2003; Goetz et al., 2007).
In 2010, the NMFS established a Cook Inlet Beluga Recovery Team (CIBRT). The CIBRT drew up a list of threats which they believed “might significantly impact CI recovery” (NMFS, 2015) and used their “best professional judgment” to identify the most important threats. These threats were then ranked on the basis of their extent, frequency, trend, probability of occurrence, and potential magnitude. The 10 threats of greatest concern are listed below, with an indication (in parentheses) of which of the stressors listed in Chapter 3 might be associated with each threat:
- catastrophic events, such as an oil spill
- cumulative and synergistic effects of multiple stressors (primarily between noise, nonbiological toxins, and perceived threats)
- noise (noise, perceived threat)
- disease agents (pathogens) and harmful algal blooms (biotoxins)
- habitat loss or degradation (habitat limitation)
- reductions in prey (prey limitation)
- subsistence hunting (acute physical injury)
- unauthorized take (acute physical injury)
- pollution (nonbiological toxins)
- predation (acute physical injury, perceived threat)
Threats 1-3 were categorized as of “high relative concern,” threats 4-7 as “medium” concern, and threats 8-10 as “low” concern. The only threats for which data on beluga
morbidity and mortality exist were placed in the low- and medium-concern categories. The justification for this placement is that CI belugas generally have lower contaminant loads than belugas studied elsewhere, that killer whales (Orcinus orca) were suspected in the deaths of only three CI beluga whales in the past 17 years and that mammal-eating killer whales have not been observed in the population’s core summer range, and that the subsistence hunt is suspended until at least 2018 and would be reinstated at a low level only if it did not place the recovery of the population in jeopardy.
The draft recovery plan concluded that “disease as a factor in the deaths of CI belugas appears to be low, and there is little evidence to suggest diseases of concern are present in other mammals in the area.” It is therefore slightly surprising that disease was considered to be a threat of medium concern. However, this categorization may be because of the potential role of diseases in catastrophic events. In contrast, the draft recovery plan recognizes that “the trend of habitat loss or degradation . . . is . . . increasing over time,” but habitat degradation was only categorized as a medium concern “due to limited understanding of how . . . habitat may be altered . . . and its resilience to perturbation.” Prey limitation was also categorized as being of medium concern because “the magnitude of the impact of a reduction in prey on . . . belugas is unknown, as is the trend.”
Catastrophic events are known to strongly influence extinction risk for small populations (Morris and Doak, 2002, p. 21). Such events are particularly likely to occur when a large proportion of the population is concentrated in a small area at certain times of the year. This is one of the consequences of the contraction in the summer range of CI belugas and, as a result, many animals could be exposed to episodic stressors such as spills of petroleum-associated chemicals and solvents and outbreaks of infectious disease.
There have been no documented direct or indirect effects of noise on CI belugas, and the categorization of noise as a threat of high relative concern appears to be primarily based on “evidence from other odontocete species . . . to conclude that a high potential exists for negative impacts (of noise).” As noted in Chapter 2, evidence of the effects of noise on marine mammal populations is largely circumstantial or conjectural.
When the decision tree from Figure 4.3 is applied to the CI beluga population, one can see that the population is declining, existing stressor levels are likely to get worse in the future, there is no dominant stressor, and there are a number of stressors (noise, nonbiological toxins, microparasites, and prey limitation) that share potential pathways for adverse effects. This leads to the conclusion that efforts will be required to mitigate any potential increases in stressor exposure, even if there is no evidence of interaction between the stressors.
In summary, the initial decline of the CI beluga population can be largely explained by excessive harvesting, but the reasons why the population has failed to recover remain unknown. However, interactions between some of the many stressors to which the population is exposed may be involved in this failure. The recovery plan is primarily concerned with mitigating the threats of high and medium relative concern; this is also the recommendation that emerges from application of the decision tree in Figure 4.3. The population monitoring planned as part of the recovery plan will focus on photo-identification studies which, as we note in Chapter 7, have the potential to provide relatively precise information on many of the demographic characteristics of the population.
Collapse of Pinniped and Sea Otter Populations in the Northern North Pacific Ocean and Southern Bering Sea
Once abundant populations of harbor seals (Phoca vitulina), Steller sea lions (Eumetopias jubata), and sea otters (Enhydra lutris) have collapsed over large areas of the Gulf of Alaska, Aleutian archipelago, and southern Bering Sea during the past four or five decades (Doroff et al., 2003; NRC, 2003b; Small et al., 2008). Despite high levels of public interest in these species and legal mandates to define and assess their various stocks under the U.S. Marine Mammal Protection Act, considerable uncertainty and scientific debate remain over the patterns, causes, and consequences of these declines.
Although there is no question that these three species have declined, data on the timing and magnitude of their declines varies in quality among the species. This is largely a consequence of when the surveys were done relative to the periods of decline. For harbor seals and Steller sea lions, rigorous monitoring programs were not initiated until the 1990s after the declines had begun (NRC, 2003b; Small et al., 2008). This shortcoming is most acute for harbor seals, which were effectively unmonitored in southwestern Alaska until after the decline had run its course. Monitoring data for Steller sea lions are better in that more systematic surveys were initiated in the 1970s while the decline was ongoing (NRC, 2003b). However, few data exist from before the decline or during its early stages, thus creating uncertainty over the onset and magnitude of the decline. This shortcoming is most severe in the central and western Aleutian Islands.
While the monitoring data range from problematic to less than ideal for pinnipeds and sea otters, they are essentially nonexistent for regional stocks of small cetaceans except for killer whales. Two species are common in this area (harbor porpoise [Phocoena phocoena] and Dall’s porpoise [Phocoena dalli]), and there are a variety of rarer species (e.g., Cuvier’s beaked whale [Ziphius cavirostris], Baird’s beaked whale [Berardius bairdii], Stejneger’s beaked whale [Mesoplodon stejnegeri], beluga [Delphinapterus leucas]; possibly striped dolphin [Stenella coeruleoalba], Pacific white-sided dolphin [Lagenorhynchus obliquidens], Risso’s dolphin [Grampus griseus], false killer whale [Pseudorca crassidens]; and conceivably one or more as-yet-to-be-
described species). Part of the difficulty for monitoring these cetacean species is that they spend their entire lives in a vast oceanic environment that is difficult to access and to survey.
Except for sea otters, both the causes and consequences of the marine mammal population declines are poorly known. In the sea otter’s case, the weight of available evidence points to killer whale predation as the likely cause (Estes et al., 1998; USFWS, 2013). Ecological consequences of the sea otter collapse, which also have been reasonably well documented, include a widespread ecosystem phase shift (e.g., Selkoe et al., 2015) from a kelp-dominated to a deforested, sea urchin–dominated coastal sea floor (Estes et al., 1998) and various knock-on influences of this “trophic cascade” to other species and ecological processes (Estes et al., 2009a).
In the case of pinnipeds, there are at least four reasons for the general lack of causal understanding. A primary reason, in contrast with the sea otter decline, is that none of the systems were observed closely or carefully while the declines were in the process of occurring. Other than the declines themselves, few data exist on co-occurring patterns of changes in the abundance and distribution of other species. A second reason arises from a generally poor understanding of food web structure and dynamic process that led to spatiotemporal variation in prey in the open sea. In contrast with the sea otter’s food web, which is easy to observe and measure and can be studied experimentally, water column and oceanic food webs that sustain pinnipeds are difficult to observe and even more difficult to study experimentally. A third reason for the lack of understanding of the pinniped declines arises from the mobile nature of their predators and prey, which, when coupled with convective influences of ocean currents, produces an ecosystem in which meaningful measurements of the distribution and abundance of species must be done at large spatial scales. Finally, until the early 2000s, the pinniped declines were believed to have resulted from bottom-up forcing—detrimental impacts on survival or reproduction resulting from changes in the abundance or quality of food, which in turn were mostly thought to have resulted from changes in physical oceanography or competition with fisheries. This belief in nutritional limitation has been, and continues to be, embraced by many people in the local research and management communities, despite a general lack of evidence (NRC, 2003b). While the pervasiveness of bottom-up forcing processes in driving the sea lion declines has been questioned (Springer et al., 2003), there has been no concurrence and considerable debate over both the cause of the sea lion decline and the failure of the species to recover following various conservation and management actions (DeMaster et al., 2006; Trites et al., 2007; Wade et al., 2007, 2009; Springer et al., 2008; Estes et al., 2009b; and many others). These differing views are evident in the remarkably different perspectives and conclusions in two separate overview reports—one by the National Research Council (NRC, 2003b) and the other by the NMFS (NMFS, 2008).
This particular case study of the causal factors for the declines in sea otters and pinnipeds illustrates how the nature of evidence, together with differences in belief and scientific philosophy (i.e., one’s foundational bases for making inferences), can prevent consensus on the potential roles of even simple direct effects in marine mammal population declines. It is possible, if not likely, that sea otter and pinniped declines are the consequence of multiple stressors. However, so long as such strong debate surrounds the potential importance of the single stressors, progress in assessing the impacts of multiple stressors on marine mammals will remain an elusive goal.
Because of the lack of suitable data, it is difficult to apply the decision tree in Figure 4.3 to this case study. The two principal stressors for all species that have definitely declined appear to be food limitation, predation pressure, and (possibly) perceived threat. These do not share potential pathways for adverse effects.
Collapse of U.K. Harbor Seal Populations
U.K. populations of harbor seals are monitored on a 5-year cycle using aerial surveys of haul-out concentrations conducted during the summer molt. These surveys provided evidence of declines of around 40% between 2001 and 2006 in a number of Scottish populations (Lonergan et al., 2007). The declines have continued, with an estimated decline of 65% since 2001 in Orkney (Hanson et al., 2013), and 90% since 2002 in the Firth of Tay (Hanson et al., 2015). However, the pattern of decline has not been consistent. For example, counts in the Moray Firth declined by 50% between 1993 and 2005 (Thompson et al., 2007), probably because of the effects of deliberate killing (Matthiopoulos et al., 2014); although levels of deliberate killing have been reduced, the population has continued to fluctuate in size. Populations on the west coast of Scotland and in the southern North Sea populations have shown no obvious long-term declines (see Figure 4.5).
A workshop held in 2012 identified a long list of potential causes for these declines that included almost all of the stressors listed in Chapter 3. However, by the time a second workshop was held in 2014, this list had been narrowed down to three “key potential drivers” (Hall et al., 2015): physical injury (spiral lesions; Bexton et al., 2012), prey limitation, and biotoxins. The spiral lesions, originally attributed to collisions with ducted propellers, are now believed to be the result of predatory attacks by male grey seals (van Neer et al., 2014; Thompson et al., 2015). Deaths from these injuries may be sufficient to explain the precipitous decline of the small Firth of Tay population (Hanson et al., 2015), but it is not clear whether they can explain the decline in the much larger Orkney population. Although there is evidence that harbor seals around the United Kingdom are regularly
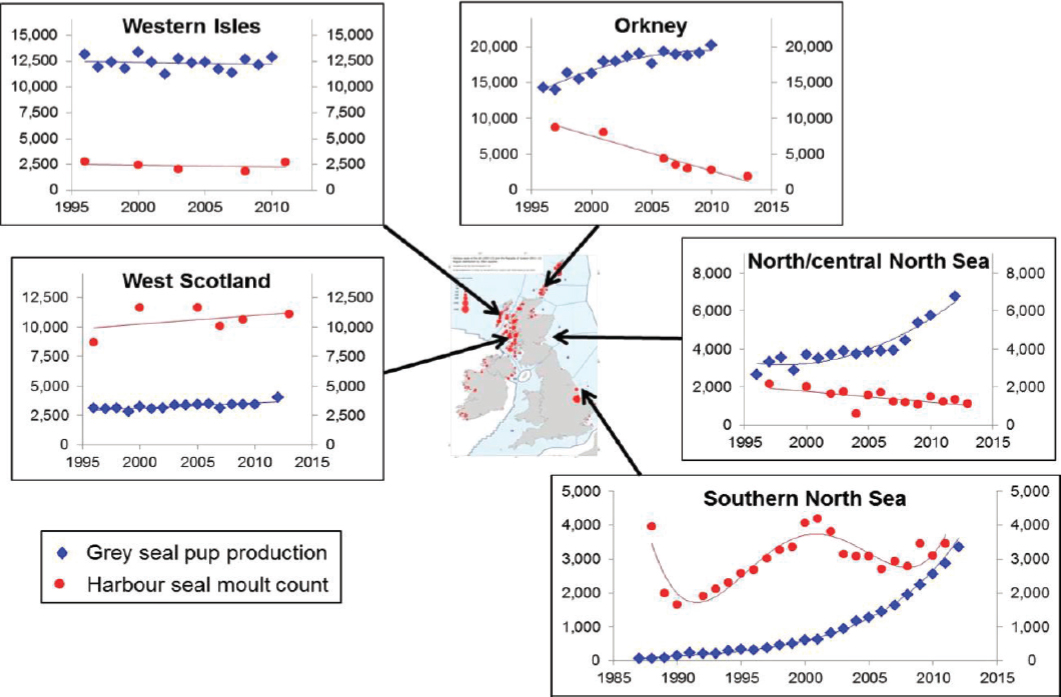
exposed to biotoxins, no deaths have actually been attributed to this cause (Jensen et al., 2015).
Application of the decision tree from Figure 4.3 indicates that the affected populations are not increasing or near carrying capacity, that some stressor levels are likely to increase (grey seal numbers, and therefore grey seal predation, are increasing, as is the incidence of toxic algal blooms in Scottish waters [Hall and Frame, 2010]), and that some of the stressors (prey limitation and biotoxins) share two pathways for adverse outcomes. There has been some preliminary work to investigate possible interactions between these stressors. Caillat and Smout (2015) modified the state-space population model developed by Matthiopoulos et al. (2014) for the Moray Firth population to include the potential effects of prey availability, grey seal numbers, and exposure to biotoxins. They used a series of logistic equations to model the potential effects of all these stressors on fecundity and pup survival. Although the logistic equation does not explicitly include an interaction term, the predicted effects of the different stressors are not additive. In fact, Caillat and Smout (2015) found that only grey seal numbers had a significant effect on pup survival, and the only stressor affecting fecundity was prey limitation. This suggests that each of these stressors had a dominant effect on one demographic rate, and that there was no interaction between their effects. This analysis was only possible because detailed information on changes in demographic rates over time were available from photo-identification studies of the Moray Firth population (Cordes, 2011).
This page intentionally left blank.


















