3
Particle Dynamics and Chemistry
The workshop’s second session featured three presentations on the transport, fate, and transformation of indoor PM. The session moderator, Richard Corsi of The University of Texas at Austin, explained that the discussions in this session would serve as a link between the first session on sources and the following session on exposure. He noted that mitigation strategies can have a huge effect on particle dynamics—an idea relevant to the presentation of the first speaker, Jeffrey Siegel of the University of Toronto, who provided a building-science perspective on the dynamics of particle size and concentration indoors. After Siegel’s presentation, Glenn Morrison of the Missouri University of Science and Technology discussed indoor chemistry and aerosols, and then Charles Weschler of Rutgers University described the composition of indoor PM and the influence of SVOC partitioning on that composition. An open discussion moderated by Corsi followed the three presentations.
A BUILDING SCIENCE PERSPECTIVE ON PARTICLE SIZE DYNAMICS AND INDOOR CONCENTRATION1
Jeffery Siegel focused on how a building influences particle size and the concentrations of PM indoors through its effects on particle sources and sinks and on how little is known about these effects. “Our knowledge of the
___________________
1 This section is based on the presentation by Jeffrey Siegel, a professor of civil engineering at the University of Toronto, and the statements are not endorsed or verified by the National Academies of Sciences, Engineering, and Medicine.
fundamental characteristics of buildings is insufficient to fully understand indoor aerosol exposure,” Siegel said. The main sources of indoor PM, as discussed in the previous session, include combustion and heating processes, resuspension from indoor activities, secondary organic aerosols, and penetration from outdoors, and Siegel said that research on these sources has produced a significant body of literature on the size distribution and emission rate for these sources. Cooking on both gas and electric burners, for example, has been well studied as a source of indoor PM (Wallace et al., 2008). The four main sinks in a building are deposition, portable air cleaning, ventilation and leakage, and HVAC air cleaning, Siegel said, and these, too, have been the subject of extensive research. In related research, investigators have developed size-resolved filtration efficiency curves for many types of filters (Hanley et al., 1994; Stephens and Siegel, 2013).
There are four areas in which knowledge is emerging about how buildings influence PM levels: the impact of building surfaces, the effects of HVAC systems, the heterogeneity of indoor concentrations, and the impact of non-particle constituents. Buildings have many visible and unseen surfaces, both in terms of number and variety, and surfaces interact with indoor PM and aerosols in meaningful ways, Siegel said. Researchers have developed simple, idealized models of how particles are deposited on surfaces (Lai and Nazaroff, 2000), and they have extended that work to more realistic environments containing real building materials (Afshari and Reinhold, 2008). Investigators have also measured size-resolved PM deposition rates for specific idealized conditions (Thatcher et al., 2002). Siegel considers surface deposition to be an emerging area of knowledge because it remains difficult to predict how specific particles, such as PM2.5, deposit onto a surface because of the order-of-magnitude variations for deposition rates, both modeled and measured, that have been reported in the literature. Improvements in modeling and measurements offer great promise for characterizing particulate matter accumulation on surfaces and its influence on resuspension. As Brandon Boor noted in the previous sessions, researchers have made progress in understanding the role that resuspension plays in determining indoor PM concentrations (Boor et al., 2013; Kassab et al., 2013; Mukai et al., 2009; Qian and Ferro, 2008). However, Siegel said, what is still not well characterized is the interaction between particles on a surface and the particles that then deposit on top of that initial layer and how the nature of specific materials affects resuspension.
Forced air HVAC systems are ubiquitous in the United States; they exist in nearly all commercial buildings and in 80 percent of residential buildings, creating possibilities for interactions between indoor PM and these systems, Siegel said. How the filters in a central forced system remove particles will be affected by how often the system runs and how much air goes through the filter. Leakage in such systems affects the efficiency of particle removal
and dispersal, and there can be deposition on the surfaces within the HVAC system as well as resuspension from those surfaces. HVAC systems can serve as sources of particles or of precursors to particles, such as ozone, and they alter temperature, humidity, and indoor air mixing, which can in turn affect indoor chemistry and particle formation. “If we really want to understand indoor particles, we have to understand HVAC systems,” Siegel said, “yet we are far behind in this area.”
To illustrate how little is known about key fundamental parameters of HVAC systems, Siegel said that HVAC runtimes have been measured in only 213 homes, all from the southeastern United States and only over a few days to 1 week (Cetin and Novoselac, 2015; Stephens et al., 2011; Thornburg et al., 2004). He noted that runtimes play an important role in determining how much effective recirculation of air through a filter occurs. “If runtimes are short, it does not matter what type of filter is in place because air is not going through it,” Siegel said. Runtimes matter less if the filter itself is not very good, which he said is the case in most homes. (In fact, runtimes can be quite short, and short runtimes compromise the ability to gain benefit from higher efficiency filters.) However, most of the models of runtimes and recirculation assume these to be much higher than those that actually take place in buildings.
Concerning the heterogeneity of indoor PM concentrations, Siegel said that most exposure estimates for indoor PM assume that the indoor air is mixed thoroughly because that makes the necessary calculations tractable. However, as Lynn Hildemann noted in her presentation, local concentrations near a particle source, such as a smoker or a stovetop, can be much higher than in a well-mixed environment. At the same time, the sinks in a building are also heterogeneous in terms of their effectiveness at removing PM from the air. In one experiment, for example, Siegel and a colleague put two different portable air cleaners at various places in a house, noting their distance from a particle source (Novoselac and Siegel, 2009), and found that both the location and the effectiveness of the particular device had a marked influence on indoor PM concentrations (see Figure 3-1). Proximity to a particle source also affects exposure, even at close distances, because the complicated fluid dynamics of air around a human body can affect how much is inhaled (Rim and Novoselac, 2009).
Having noted how little is known about HVAC systems, Siegel listed several other knowledge gaps that need to be filled. For example, ventilation dynamics (the AER, for example) can change dramatically over the course of a day (see Figure 3-2), but little is known about how such variations drive the levels of indoor PM. Sinks can also be dynamic, Siegel said, referring to the decreasing efficiencies of filters that occur over time (see Figure 3-3). Many HVAC filters in the United States use an electrostatic charge applied to the filter to remove particles, and this charge can decay
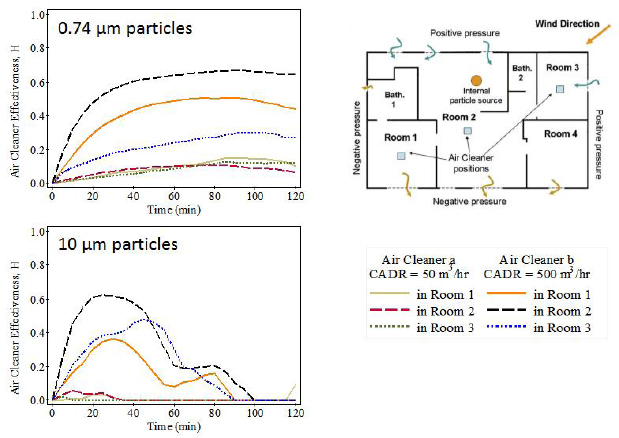
NOTE: CADR = clean air delivery rate.
SOURCES: Siegel slide 15, adapted from Novoselac and Siegel (2009) Figures 1 and 4; reprinted with permission from Elsevier.
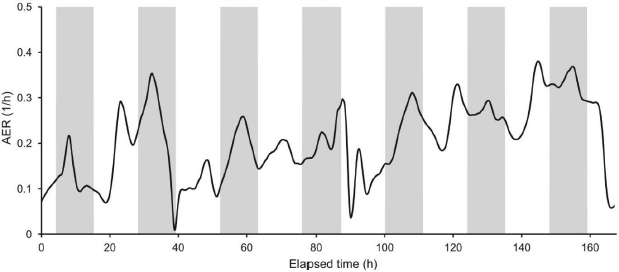
SOURCES: Siegel slide 19, from Dias Carrilho et al. (2015) Figure 4; reprinted with permission from Elsevier.
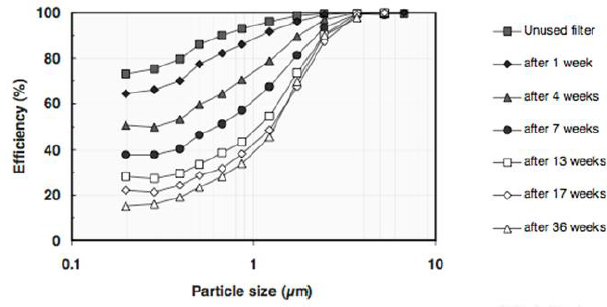
SOURCES: Siegel slide 20, from Lehtimäki et al. (2005). © 2005, ASHRAE (www.ashrae.org). Used with permission from ASHRAE (Investigation of mechanisms and operating environments that impact the filtration efficiency of charged air filtration media, RP-1189, 2005).
or be masked by particle deposition over time. “Even though this is well-identified as a problem, we do not understand what is causing this decay and why the decline is huge in some buildings and much less in other buildings,” Siegel said. As an example of the type of research that is needed, he cited recent work looking at changes in filter efficiency and pressure drop as a function of what is being deposited on the filter and the particle structures that form there (Montgomery et al., 2015). “This kind of fundamental research that lets us understand how indoor PM sinks behave is important,” Siegel added.
A final critical knowledge gap that Siegel addressed related to unseen surfaces and spaces, such as the space above drop ceilings and between walls and floors. The role that these spaces—which also include attics, crawl spaces, basements, and knee wall spaces—play as sources and sinks is largely unknown, Siegel said. Garages are perhaps the best studied of these unseen spaces as sources of indoor air pollution, but he said that he was aware of only one investigation of them that measured particle levels. “Even though we know these spaces can be important from an exposure perspective, they are largely unstudied,” he said.
As one step toward filling these knowledge gaps, Siegel suggested that the field conduct what he called a “long-form building census” which would address the key building science parameters needed to understand and mitigate exposures to particles in buildings. Conducting such a census
would not only generate knowledge but also create an opportunity for citizen science which might engage the people who work and live in buildings to pay more attention to their indoor environments.
INDOOR CHEMISTRY AND AEROSOLS2
Glenn Morrison began by noting that chemical transformations that take place in air, such as oxidation, photolysis, hydrolysis, oligomerization, and acid-base reactions, can influence aerosol levels and particulate formation. One well-studied atmospheric chemical reaction involves volatile organic compounds reacting with an oxidant, such as ozone, nitrate, or hydroxyl radical, to generate a variety of molecular products. The resulting sticky, polar molecules can serve as nuclei around which particles form, or else the polar molecules can condense onto existing particles. These particles can then agglomerate into larger masses. Together, the nuclei, condensed particles, and agglomerates are called secondary organic aerosols (SOAs). Sunlight is a major driver of this chemistry outdoors, but sunlight is less intense indoors, which potentially slows the process. Also, because of the short residence time indoors (hours or less) compared with outdoors (days), SOAs generated inside a building are “fresher” than their outdoor counterparts. Indoor surfaces play an important role in removing both SOAs and their precursors from indoor air, but they can also emit some of the precursors that eventually contribute to aerosol formation, and they can also serve as reaction sites, facilitating oxidative chemistry that can lead to SOA formation.
The indoor environment strongly influences precursor molecule levels, Morrison said. Important indoor sources of precursors include cleaning solvents, scented products, and foods. All three of these release large amounts of chemicals called terpenes, which are readily oxidized in air. Indoor oxidants levels are driven largely by outdoor ozone levels. Morrison said that there is a significant body of research showing that SOAs are generated indoors, and he cited a study showing that when an air freshener was introduced into a room with elevated ozone levels, there was a rapid increase in the concentration of submicron particles that persisted in the air for many hours (Sarwar and Corsi, 2007). A more recent study found that new particles with an average diameter of 100 nanometers can form indoors from paint solvents (Lazaridis et al., 2015).
The emerging science of indoor air chemistry is advancing on multiple
___________________
2 This section is based on the presentation by Glenn Morrison, a professor of civil, architectural, and environmental engineering at Missouri University of Science and Technology, and the statements are not endorsed or verified by the National Academies of Sciences, Engineering, and Medicine.
fronts, Morrison said. One advance is the ability to measure an increasing number of oxidized species, Morrison said, and as an example he cited a study in which the investigators were able to quantify levels of more than three dozen different compounds in aerosols formed by oxidation of solvents in household cleaners (Rossignol et al., 2013). Modeling is another area where progress is occurring, he said, with researchers adapting models developed for outdoor environments to account for the different surfaces and precursors found indoors. One such study (Carslaw et al., 2012) predicted that organic peroxides and nitrates would be the predominant compounds produced after a cleaning event but that the relative concentrations of different classes of chemicals produced in aerosols would vary depending on the amount of solvents released and the rate at which SOAs deposited on surfaces. “There is still a great deal of uncertainty in the ability to use models to be predictive of not just the aerosol mass concentration but also the composition,” Morrison said. Recent experimental work has demonstrated that changing the indoor levels of ozone and terpenes, common constituents of cleaning products, has a significant impact on the composition of the PM that forms indoors (Khurshid et al., 2016). In particular, Morrison said, the level of PM2.5-bound reactive oxygen species, which can irritate the lungs, can increase by more than four-fold when indoor levels of both ozone and terpenes are elevated.
Building operations can also influence aerosol chemistry. One recent study that Morrison cited found a strong influence of AER on peak SOA levels generated from select terpene compounds (Youssefi and Waring, 2015). “We still need to know about how buildings influence this chemistry,” he said.
Several studies have shown that ozone can react with organic molecules deposited on surfaces to produce particles in the air above those surfaces (Sleiman et al., 2010; Waring et al., 2011). Morrison and a colleague found that this chemical reaction occurs much faster than would be expected if it were happening in the air (Shu and Morrison, 2011). What this might imply, Morrison said, is that many of the products of the chemistry that take place on surfaces can be transferred to aerosols and dust that can be resuspended and inhaled.
Room occupancy is an important factor in aerosol chemistry because skin contains chemicals that react readily with ozone. Human and animal bodies constantly shed skin in the indoor environment, and these skin cells and the skin oils can adhere to indoor surfaces. As a result, surfaces in occupied rooms are coated with chemicals waiting to react with airborne ozone. “That is one reason why our indoor environments are so reactive and why indoor ozone levels are lower than those outdoors,” Morrison said. Another reason is that the humans in a room are also covered in these reactive compounds, and, in fact, research has shown that ozone levels fall
when a human enters a test chamber designed to simulate an office environment (Fadeyi et al., 2013).
While the amount of sunlight indoors is much less than outdoors, it can still be sufficient to trigger chemical reactions, Morrison said. One study, for example, found that cooking produces nitrogen dioxide as a combustion byproduct and that the nitrogen dioxide sticks to surfaces, reacts with water in the air, and produces nitrous acid (Alvarez et al., 2014). Sunlight entering a room through a window can enhance this reaction, especially when surfaces are also coated with household cleaner residues. Nitrous acid is volatile, and when released from the surface into the air it will react with sunlight to produce hydroxyl radicals, one of the oxidants that can generate SOAs. As a result, in one study, the levels of hydroxyl radical near a window were of the same order of magnitude as is found outdoors. Morrison said that hydroxyl radical is indiscriminate, reacting with a wide range of volatile organic compounds and not just with terpenes.
Morrison emphasized that the development of sensitive instruments for monitoring outdoor air composition has created a great opportunity to better understand indoor chemistry. These instruments include high-resolution aerosol mass spectrometry for analyzing PM composition, fluorescence assay by gas expansion for detecting hydroxyl radicals, cavity ring-down spectroscopy for measuring nitrogen oxides, and direct analysis in real-time mass spectrometry for the real-time characterization of surface films. “Almost none of these instruments have been used indoors until very recently,” Morrison said. One study using high-resolution aerosol mass spectrometry, for example, showed that there were hundreds—and perhaps thousands—of different compounds generated by oxidation chemistry (Romonosky et al., 2015). While it may not be possible to identify all of these compounds, Morrison suggested that this type of analysis can reveal the many factors that influence indoor chemistry.
Another area where Morrison said he expects progress to be made is in applying models of outdoor atmospheric chemistry to the indoor environment. Morrison said that the basic chemical reactions are well modeled, but researchers need to better account for the surface phenomena as well as building characteristics, occupancy, and human activity. Indoor surfaces, he reiterated, are coated with a film of organic material that can transfer material back and forth between aerosols and undergo chemical reactions. These reactions, in turn, are influenced by the acidity of the environment, which changes with human and animal activity. None of these processes are accounted for in outdoor models. Integrating building characteristics into models is challenging because of the sheer complexity and variety of building environments, Morrison added, and doing so successfully will require identifying those parameters of a building that are most important with respect to chemistry.
COMPOSITION OF INDOOR PM AND THE INFLUENCE OF SVOC PARTITIONING3
Given that buildings do a moderately good job keeping outdoor UFPs and PM10 from entering buildings, Charles Weschler said, the chemical constituents of these two classes of PM will be determined largely by chemical processes occurring indoors. Indoor UFPs are produced primarily via combustion, through gas-to-particle conversion, and via thermal desorption of SVOCs. The primary sources of indoor coarse particles include skin flakes, fibers, plastic wear particles, and soil and salt particles tracked indoors.
The chemical composition of PM2.5, however, is determined by chemistry that occurs both indoors and outdoors. Weschler said that comparing the chemicals present in indoor and outdoor PM2.5 shows indoor PM2.5 that are rich in chemicals additives used in products that are part of the indoor environment, such as phthalate plasticizers, organophosphates, brominated flame retardants, and fluorinated surfactants. By weight, indoor PM2.5 is approximately 50 percent organic carbon, with elemental carbon accounting for only 3 percent of the total particle mass. Sulfates and nitrates together account for nearly 30 percent of the weight, with ammonium ion and water together contributing about 15 percent at typical indoor relative humidities (see Figure 3-4). The total metal content in indoor PM is about 1 percent, with more than two-thirds of that being iron. Also present are zinc, vanadium, titanium, silver, copper, manganese, and chromium. Weschler explained that while small in amount, these metals may be relevant to human health given the evidence suggesting that water-soluble PM, which may be able to release those metals into the body, has a disproportionate effect on human health (Costa and Dreher, 1997).
Relative humidities above 25 percent have a measurable effect on the water content of PM2.5. Depending on the composition of the particles—in particular, the water-soluble salts and oxidized organic compounds—PM2.5 can be 10 to 40 percent water by weight when the relative humidity is between 50 and 70 percent. The water content of PM10, with its lower soluble salt and oxidized organic content, is lower than that of PM2.5. Water content is important, Weschler said, because it affects the partitioning of gases between air and particles and helps influence the chemical reactions that can occur on or within the particle, which also affects the particle composition (Lim et al., 2010). Weschler added that the water found in these particles is likely coated by an organic film (Gill et al., 1983) which can affect the transfer of gases into and out of the particle and partitioning.
___________________
3 This section is based on the presentation by Charles Weschler, an adjunct professor at Rutgers, the State University of New Jersey, and a visiting professor at the Technical University of Denmark and Tsinghua University, and the statements are not endorsed or verified by the National Academies of Sciences, Engineering, and Medicine.
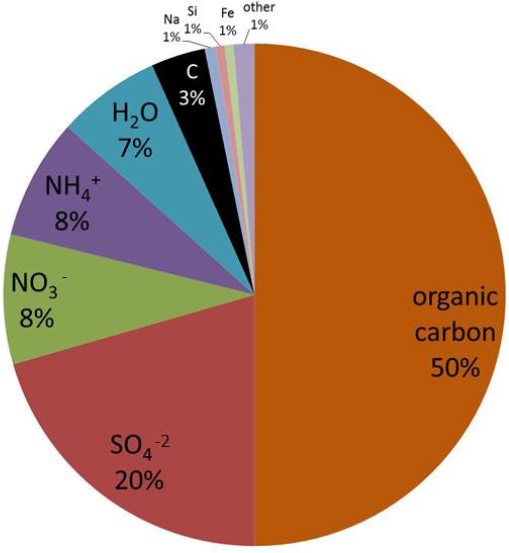
SOURCE: Weschler slide 4.
The partitioning of SVOCs and various inorganic compounds between the gas phase and the surface of airborne particles plays an important role in determining the chemical composition of particles, Weschler said. In one of the first applications of aerosol mass spectrometry to the study of indoor PM, Michael Waring and his colleagues (Johnson et al., 2016) simultaneously sampled indoor and outdoor PM on the Drexel University campus. This analysis showed that hydrocarbon-like organic aerosols made up 19 percent of indoor PM, compared to 8 percent of outdoor PM (see Figure 3-5). A large part of this increase, Weschler said, arose from the partitioning that occurs because of the much higher concentrations of SVOCs found indoors compared to outdoors. “When an outdoor particle comes indoors, it will acquire phthalates, organophosphates, and per-fluorinated surfactants from indoor air,” Weschler explained. At the same
time, the outdoor particles tend to lose polyaromatic hydrocarbons and ammonium nitrate when they move indoors. Data from the Relationship of Indoor, Outdoor, and Personal Air (RIOPA) study collected in Los Angeles, California; Houston, Texas; and Elizabeth, New Jersey, showed a similar increase in the organic content of indoor PM2.5 compared to outdoor PM2.5 (Polidori et al., 2006).
Increasing the level of SOAs affects SVOC partitioning in indoor environments by increasing both the concentration of airborne particles and the fraction of organic matter in the airborne particles, Weschler said. These increases, in turn, increase the proportion of SVOC in the particle phase versus the gas phase in a multiplicative fashion (Weschler and Nazaroff, 2008). In fact, he said, chamber experiments support this prediction (Benning et al., 2013; Chen and Hopke, 2009).
As Morrison pointed out, the occupants of a building influence the composition of indoor PM, and Weschler noted that humans shed their entire outer layer of skin every 2 to 4 weeks at a rate of 200,000 to 600,000 skin flakes per minute (30 to 90 milligrams of skin flakes per hour). Human skin is one of the very few sources of the chemical squalene in indoor environments. A 1973 study of size-fractionated indoor PM collected from a
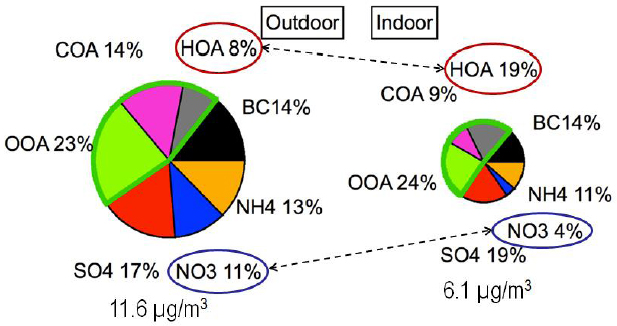
NOTE: BC = black carbon; COA = cooking organic aerosol; HOA = hydrocarbon-like organic aerosol; OOA = oxygenated organic aerosol.
SOURCE: Weschler slide 10, from Johnson et al. (2016); reprinted with permission from John Wiley & Sons, Inc. © 2016.
house, a laboratory corridor, and the London Underground found that the squalene content was between 40 and 100 micrograms per unit gram of PM (Clark and Shirley, 1973). From this number, the investigators calculated that indoor PM is about 1 percent skin flakes by weight. Weschler said that other studies conducted since then have arrived at similar values.
Occupants contribute more than just skin cells to indoor PM, he added; they also shed bacteria and fungi along with their skin cells. One study of the microbial content of indoor PM in a classroom found that the amount of bacteria in indoor PM was 80 times higher and the amount of fungi 15 times higher when the room was occupied than when the classroom was empty (Hospodsky et al., 2015). The particle mass was also nine-fold higher in the occupied classroom. This study did not determine what fraction of the microbial PM content was viable.
Indoor PM particles include thousands of organic species. “The complexity is staggering,” Weschler said, adding that little work has been done to characterize the organic molecules found in indoor PM versus outdoor PM (Heald et al., 2010). Recently, investigators have shown that the composition of PM, both indoor and outdoor, influences the uptake of gases onto the particles and the subsequent chemistry that can occur within the particle (Morgan et al., 2015). Studies have also found that particles can exist in liquid, semi-solid, and glassy phases and that multiple phases can coexist in the same particle (Koop et al., 2011). This is important, Weschler said, because water and gas partitioning depends on the phase, as does the diffusion of molecules within the particle. For example, the amount of diffusion within a particle is up to 10 million times smaller in the semi-solid phase than in the liquid phase (Hodas et al., 2015), which, he pointed out, would affect particle chemistry. Weschler said that most of the modeling work on partitioning assumes that the organic content of PM is in the liquid phase, so if this is not the case, there will be large errors in the output of these models. What is not known, he said, is if this is a serious issue for indoor PM.
Weschler said that in his view more information is needed about the chemical form and oxidation state of the metals in PM, given the important effects these have on the chemical reactivity and bioavailability of metals. One study, for example, found that 25 percent of the iron in PM from urban and rural sites in Georgia was in the Fe(II) oxide state and that 15 percent of the iron was in soluble form (Oakes et al., 2012a,b). What remains to be characterized, he said, are the identities of the ions or molecules bound (coordinated) to the iron in these particles.
Another question Weschler said he would like to see addressed concerns the timescale over which SVOCs desorb from inhaled PM and the residence time of particles in the respiratory tract, which are important factors for the potential health effects of breathing PM. Those times, he explained, will
depend on particle diameter and on the partition coefficient. His group has tried to model this process despite the various technical complications, he said. “We are fairly certain that some SVOCs in some particle size ranges make it to the alveoli, while for other SVOCs and in other size ranges, the SVOCs desorb fairly high in the respiratory tract.” Experimental studies, he added, have proved to be even more challenging than the modeling efforts.
Weschler also questioned the role that reactive oxygen species associated with PM might play in triggering oxidative stress. It is known that inhaling PM enriched in certain transition metals will induce oxidative stress, so it might be the case that having reactive oxygen species present on PM would increase the potential for harm. Studies have shown that reactive oxygen species are present in indoor air (Khurshid et al., 2016) and that they can remain active in air for many hours, with a decay half-life of 6 to 7 hours (Chen et al., 2011).
In summary, Weschler said, indoor PM is enriched in synthetic organic chemicals such as plasticizers and flame retardants, metals, and microbes from occupants, and as PM is transported from outdoors to indoors, the chemical content can change substantially. “We need to know more about the actual molecular nature of the chemicals present in indoor PM, both in terms of the transition metal complexes and the organic species,” he said. “I think a large number of people are unaware of the holes in our knowledge when it comes to the chemical composition of indoor PM.”
DISCUSSION
Corsi launched the discussion by asking the panelists to comment on the importance of unseen spaces to the topics they discussed. Siegel said that those spaces are very important and, based on energy conservation studies, are well connected to the rest of the building. As such, he said, he would like to see more research to identify how much PM is in those spaces, both airborne and deposited, and how that changes the distribution of particle size and concentration coming in from outdoors. Morrison agreed that little is known about the chemistry that occurs in interstitial spaces and said he thought that the first place to start with regard to addressing that deficit would be to collect samples using many of the new technologies used to collect outdoor PM. “Just deploying those technologies indoors will lead to a great deal of discovery,” he said. Weschler gave an example of what can be learned from studying the PM in interstitial spaces. When he worked at Bell Laboratories, he said, he and his colleagues sampled the PM that was coming from the spaces under the raised floors in telephone data centers. They found that PM was “grossly enriched” in phthalate esters, which presumably came from the plasticizers present in the PVC insulation surrounding communication cables running through the space under the floor.
Brent Stephens, noting that calculations of SVOC partitioning use the total suspended particle mass as a measure of particle concentrations, asked Weschler if the resulting estimates would be improved if the calculations accounted for the size distribution of the particles. Weschler replied that the estimates for partitioning that use total suspended particle mass are crude and provide only order of magnitude–type results. “You would certainly refine those estimates by looking at the fraction of organic matter in different size ranges,” Weschler said.
An online participant said that she had seen significant spikes in indoor PM levels associated with humidifiers, boiling water, and sometimes even showering. She asked if anyone had studied particle emissions from water. The answer, Weschler said, is yes, and those particles, sometimes referred to as “gray dust,” emerge from water-soluble salts when aerosolized water droplets evaporate. That is why it is important, he added, to use deionized water in ultrasonic humidifiers. However, Gediminas Mainelis from Rutgers University said that his group has observed that particles of unknown composition form when even the purest water is aerosolized. Morrison said that certain types of evaporative coolers also produce high particulate loads for the same reason. Siegel added that HVAC systems often generate a water aerosol for the purpose of humidification or air cleaning, but the extent to which this produces indoor PM has not been explored.
Another online participant proposed tapping into the data collected by Internet-enabled home thermostats on temperature and HVAC runtimes. Siegel said he thought this was a “great idea,” particularly if those data could be combined with information about the buildings associated with those thermostats. Privacy issues could be a concern, he said, but he expressed confidence that issue is resolvable.
Vito Ilacqua from EPA asked the panelists to suggest which parameters of the indoor environment would be most important to have more data on in order to better understand indoor PM behavior. Siegel said that acquiring data on the amount of surface area in different types of buildings, the nature of interstitial spaces, and HVAC operation parameters would be easy and inexpensive to do and simply requires making that a priority and doing it. More work is needed on advanced instrumentation to better characterize indoor PM composition, he added. Siegel also said that there are many inexpensive and easy steps that could be taken today to protect people in buildings from PM exposure without having to wait for better characterized buildings and particles. Offering one specific example of such a step, he said, “It is a no-brainer for certain people in certain indoor environments to be using better filtration or activated carbon filtration.”
Morrison agreed with Siegel that the presumption from a chemistry perspective is that indoor exposure to SOA is not good. “That is an assumption right now, and we do not know for sure what the direct health effects
are,” he said, “but if we make that assumption, it is relatively straightforward to remove ozone from indoor air, which is the main driver of SOA levels.” Morrison added that, given the difficulty in changing behaviors that lead to elevated exposures to indoor PM, it will be important to integrate mechanisms to remove the main drivers of indoor PM exposure into building design.
Lynn Hildemann asked the panelists to comment on the possible effects that humidity might play in the indoor environment. Morrison replied that there are cases where the moisture content of the air influences the chemistry on particles. Less is known, he added, about the influence of humidity on ozone uptake at the particle surface. Siegel noted that every HVAC system with an operating cooling coil has water-saturated air at some point. “High humidity is a reality in many buildings much of the time,” said Siegel.
David Young from INLOGIX asked for the panelists’ thoughts on the challenges of conducting health investigations in residences with HVAC systems equipped with ultraviolet (UV) light systems. Morrison said that while UV lights can potentially deactivate certain microorganisms, they can also produce high levels of ozone. Given what is known about ozone as a lung irritant and its ability to generate the type of reaction products—which he described in his presentation—Morrison said that he is not in favor of anything that releases ozone into the home environment. Siegel added that UV lights also contribute to the degradation of certain components and insulating materials in HVAC systems. Corsi said that he has found UV light systems in many animal shelters and has measured high ozone levels in kennels in those shelters. Mainelis added that handheld hair dryers produce high levels of ozone—as much as 10 parts per billion above the background level—within the breathing zone of the user. Morrison said that hairdryers and many other unregulated devices produce ozone unintentionally.
This page intentionally left blank.
















