11
Phenotypes in Phylogeography: Species’ Traits, Environmental Variation, and Vertebrate Diversification
KELLY R. ZAMUDIO,*#RAYNA C. BELL,†‡§ AND NICHOLAS A. MASON*
Almost 30 years ago, the field of intraspecific phylogeography laid the foundation for spatially explicit and genealogically informed studies of population divergence. With new methods and markers, the focus in phylogeography shifted to previously unrecognized geographic genetic variation, thus reducing the attention paid to phenotypic variation in those same diverging lineages. Although phenotypic differences among lineages once provided the main data for studies of evolutionary change, the mechanisms shaping phenotypic differentiation and their integration with intraspecific genetic structure have been underexplored in phylogeographic studies. However, phenotypes are targets of selection and play important roles in species performance, recognition, and diversification. Here, we focus on three questions. First, how can phenotypes elucidate mechanisms underlying concordant or idiosyncratic responses of vertebrate species evolving in shared landscapes? Second, what mechanisms underlie the concordance or discordance of phenotypic and phylogeographic differentiation? Third, how can phylogeography contribute to our understanding of functional phenotypic evolution? We
__________________
* Department of Ecology and Evolutionary Biology, Cornell University, Ithaca, NY 14853; †Museum of Vertebrate Zoology, University of California, Berkeley, CA 94720; ‡Department of Integrative Biology, University of California, Berkeley, CA 94720; and §Department of Vertebrate Zoology, National Museum of Natural History, Smithsonian Institution, Washington, DC 20560. #To whom correspondence should be addressed. Email: kelly.zamudio@cornell.edu.
demonstrate that the integration of phenotypic data extends the reach of phylogeography to explain the origin and maintenance of biodiversity. Finally, we stress the importance of natural history collections as sources of high-quality phenotypic data that span temporal and spatial axes.
Phylogeography, as originally defined, focused on processes governing the spatial distribution of genealogical lineages within species (Avise et al., 1987). One of the strengths of the field at its inception was formalizing conceptual links among heredity (processes at the level of individual pedigrees), divergence at the population level, and phylogenetic relationships among species (Avise et al., 1987). This analytical framework bridged microevolutionary processes acting within populations and macroevolutionary patterns at larger spatial and temporal scales. From the earliest applications, empirical phylogeographic studies described spatial patterns of genetic diversity and inferred underlying mechanisms, thus contributing to the explanatory and predictive power of the field (Buckley, 2009). If most species show phylogeographic structure caused by landscape features that impede gene flow, then the geographic distribution of divergent lineages should coincide among species that coinhabit those landscapes. Further, phylogeographic breaks or contact zones should arise as lineages diverge allopatrically or come into secondary contact after divergence, respectively. This explicit prediction (Avise et al., 1987) resulted in a search for shared geographic patterns in genetic structure among species and the birth of comparative phylogeography (Schneider et al., 1998; Hewitt, 2000). Now, with thousands of taxon-specific phylogeographic studies published and synthesized in comparative studies (Beheregaray, 2008; Camargo et al., 2010), we have learned a tremendous amount about the geography of genetic structure both within and among species.
Phylogeography has progressed rapidly in the last three decades, with new genetic markers (Edwards et al., 2015; Garrick et al., 2015), analytical techniques (Hickerson et al., 2010), and synergies with landscape ecology and population genetics (Chan et al., 2011; Hickerson et al., 2006b; Oaks, 2014). Combined, these advances have revealed previously unrecognized genetic variation and its spatial and environmental correlates; however, phenotypic variation in those same diverging lineages has not received the same attention. Phenotypic variation among populations across a species’ range is common and often serves as the initial motivation for phylogeographic studies; however, most studies focus primarily on spatial variation in genetic lineages (Edwards et al., 2015) and their distribution relative to environmental or geographic features of the landscape. Phenotypes are targets of selection and affect the performance of organisms in variable
environments; combined, both processes contribute to diversification. Furthermore, different classes of phenotypes vary in how they impact processes such as dispersal, colonization, and persistence, thereby providing a window into the importance of various evolutionary processes in current and historical selective environments. Genetic structure of neutral genes, on the other hand, primarily reflects demographic processes (e.g., drift, expansion, changes in effective population size) that are a consequence of historical biotic and abiotic conditions during a species’ evolutionary history. Thus, a new conceptual framework that explicitly integrates quantitative analyses of phenotypic variation within a phylogeographic framework can greatly enhance our knowledge of how genetic and phenotypic divergence arise, how they are linked, and how they respond to changing ecological and evolutionary contexts.
Here we review three research areas that exemplify the benefits of integrating phenotypic and genetic datasets in vertebrate phylogeography. First, we review how species-specific traits and their interactions with the environment predict concordant or idiosyncratic phylogeographic patterns among codistributed species. Second, we examine mechanisms that underlie the spatial concordance or discordance between phenotypic and genetic diversification. Third, we consider how phylogeography contributes to our understanding of functional phenotypic variation. For each topic, we describe case studies to highlight how the integration of phenotypic and genetic evolution has contributed to long-standing questions in evolutionary biology and has advanced our understanding of biodiversity. Finally, we emphasize the importance of natural history and field collections for the successful integration of organismal phenotypes and phylogeographic studies.
SPECIES-SPECIFIC TRAITS AND IDIOSYNCRATIC PHYLOGEOGRAPHIC PATTERNS
Comparative phylogeography seeks to characterize concordant phylogeographic breaks or contact zones, biogeographic “hotspots” for understanding mechanisms shaping genetic structure within and among species with shared distributions (Rissler and Smith, 2010; Moritz et al., 2009). A common assumption of comparative phylogeography is that taxa evolving in particular landscapes respond similarly to the abiotic and biotic elements that cause genetic divergence. We know, however, that species and populations vary in tolerance, plasticity, adaptive potential, and biotic interactions, all of which mediate responses to environmental variation (Bernardo and Spotila, 2006; Ridenhour et al., 2007; Satler et al., 2016) and ultimately dictate the degree of spatial and temporal concordance in genetic structure. The early definition of phylogeographic response cat-
egories acknowledged that differences could stem from species-specific traits such as dispersal potential and life history (Avise et al., 1986, 1987). Not surprisingly, species that are exceptions to regional phylogeographic patterns have been identified in most, if not all, phylogeographic hotspots, precluding generalizations and challenging expectations for shared causes of organismal diversification.
Given that species-specific phenotypes can dictate spatial variation in population responses to environmental change, phylogeography would benefit from a more integrative and inclusive framework, one that incorporates predictions based on those phenotypes, an approach that has been termed “trait-based phylogeography” (Paz et al., 2015). A parallel example of trait-based approaches can be found in the emerging field of biodiversity and ecosystem function, which arose at the interface of community and ecosystem science (Loreau, 2010). This new framework breaks from the view of species diversity as an epiphenomenon driven by a combination of abiotic environmental factors (e.g., temperature, rainfall, soil fertility), ecosystem processes that are themselves determined by these abiotic factors (e.g., productivity, biomass and nutrient cycling), and biotic interactions among species within communities (e.g., competition and predation). Instead, this new field considers biodiversity—in particular, the identity and diversity of species—as a driver of ecosystem functioning (Loreau, 2010) and establishes causality between a species’ traits and the processes that in turn have functional consequences for ecosystems (Loreau, 2010). Our current view of biodiversity in phylogeography parallels the “old view” in ecology by considering the genetic structure of species as a consequence of abiotic conditions and the evolutionary “function” of lineage births and deaths. In other words, species themselves, and their traits, are typically not considered as functionally causal in the processes that ultimately shape them. When we consider that traits can alter an organism’s demography and interactions with the environment, we can no longer ignore the dynamic nature of these interactions and their impact on lineage diversification (He et al., 2013). Thus, this paradigm shift challenges the expectation that temporal and spatial concordance among species should be the expected pattern in comparative phylogeography (Papadopoulou and Knowles, 2015b).
Phenotypes can either promote or constrain population divergence, depending on their function and interaction with the environment. For example, phenotypes that directly affect dispersal or persistence in new environments, such as those related to locomotor efficiency, physiological tolerance, or body size, can influence the frequency of migration and gene flow among subdivided populations. Others, such as recruitment rate, life span, and time to maturity will affect population size and turnover and thus the amount of genetic variation in subdivided populations. Finally,
sexually selected phenotypes may not affect demography directly but can affect the distribution of genetic diversity indirectly via assortative mating, species recognition, and inbreeding avoidance. Variation in the distribution of phenotypes with different functions and the concordance of these phenotypes across species provide opportunities to quantify the importance of specific evolutionary processes for species inhabiting similar environments.
We are not implying that researchers have completely ignored species’ traits in interpreting phylogeographic patterns. Many studies consider dispersal capacity, environmental tolerance, and other characteristics that contribute to diversification (Wang and Summers, 2010; Bell et al., 2012; Moritz et al., 2012; Smith et al., 2014b; Paz et al., 2015) or apply predictions derived from species-specific traits in a priori hypothesis testing (Brumfield and Capparella, 1996; Brown and Knowles, 2012; He et al., 2013; Massatti and Knowles, 2014). To date, studies adopting a comparative trait-based framework typically have focused on groups of organisms evolving in and adapting to particular habitats. A comparison of four distantly related and allopatric temperate amphibian species demonstrated that population divergences are significantly lower in two desert species that breed in ephemeral habitats than in two species inhabiting mesic forested landscapes (Chan and Zamudio, 2009). The stochastic persistence of breeding ponds across years in arid habitats may select against site fidelity and favor increased dispersal and larger physiological tolerances to inhospitable environments (Chan and Zamudio, 2009). A second study in the tropics confirmed that topographic complexity and especially macro-habitat preferences had strong effects on population divergence, so that species occupying forests and topographically complex regions showed deeper phylogeographic structure (Rodríguez et al., 2015). Lower vagility across complex terrain and reliance on specific breeding habitats may lead to greater phylogeographic divergence in rainforest species. In contrast, species in more open landscapes typically use ephemeral and unpredictable breeding sites suitable for vagile generalists, possibly reducing intraspecific divergence (Rodríguez et al., 2015).
Although trait-based analyses of shared phylogeographic structure yield important correlational evidence for divergence mechanisms (Smith et al., 2014b; Paz et al., 2015), the next step in this predictive framework is to examine species-specific traits that are selected for in particular landscapes and to quantify the extent to which those traits then contribute to diversification. An important advance in this direction is the development of model-based phylogeographic methods that incorporate phenotypic variation. These efforts stem from the realization that lack of concordance in temporal and spatial clustering in codistributed taxa may not mean that taxa are not responding to a common landscape or climatic
barrier (Oaks et al., 2013); rather, discrepancies may reflect variation in ecological traits and dispersal capabilities of taxa sampled across the presumed barrier (Papadopoulou and Knowles, Chapter 8, this volume). These efforts refine expectations for spatial concordance and temporally clustered divergences by explicitly including geography and trait-based responses for each species (Massatti and Knowles, 2014). A recent study examined trait-based phylogeographic predictions using flightless beetles that coinhabit the Cycladic Plateau in the central Aegean archipelago. The species differ in body size and associate with different soil types, both traits that affect dispersal capacity and persistence in habitat patches of different sizes (Papadopoulou and Knowles, 2015b). The authors investigated the relative importance of geographic factors and species-specific traits (soil-type preference, body size) on population divergence of 13 codistributed species. They found greater support for phylogeographic concordance when the null expectation of divergence times incorporated geographic and species-specific trait data (Papadopoulou and Knowles, 2015b). Efforts such as these to inform phylogeographic inferences with relevant differences among species have great potential for improving our understanding of how landscapes and species-specific traits interact during diversification (Knowles and Alvarado-Serrano, 2010; He et al., 2013).
These empirical studies demonstrate that integrating species’ traits in phylogeographic studies can help explain the concordance (or discordance) of genealogical differentiation for species living in shared environments. This exciting prospect will greatly increase the impact of phylogeography in biodiversity science, and future studies need to consider explicitly how phenotypes can be incorporated into their predictive frameworks. Just as common responses to phylogeographic barriers became a null hypothesis early in the history of the field (Papadopoulou and Knowles, Chapter 8, this volume), an understanding of how organismal traits mediate responses to variable environments and demography will be necessary for a complete picture of the expected mode and rate of phylogeographic diversification (Papadopoulou and Knowles, 2015b). Achieving this understanding will require the development of metrics for quantifying phenotypic divergence and methods that explicitly incorporate those data in phylogeographic predictions and analyses (Knowles and Alvarado-Serrano, 2010). To be comparable, studies of geographic variation in phenotypes should strive to collect analogous data across species’ ranges. Doing so may be challenging, because phenotypes of interest will vary among systems, and each field has unique standards for replicable data, but these efforts will greatly expand the database of comparative traits for broader investigations of organismal divergence.
EVOLUTIONARY MECHANISMS LINKING GENETIC AND PHENOTYPIC DIVERSIFICATION
All lineages harbor phenotypic and genetic variation among individuals, and this variation can be geographically partitioned in different ways (Fig. 11.1 and Table 11.1). In populations that remain geographically and genetically isolated, theory predicts that phenotypic differences will become more pronounced over time as the result of both neutral and selective processes (Gould and Johnston, 1972). However, geographic variation in phenotypes does not always coincide with phylogeographic breaks (Endler, 1973). One prominent goal of phylogeography is to infer the biological mechanisms that partition genetic and phenotypic variation among populations. Recent advances in modeling (Thomé and Carstens, Chapter 7, this volume), predictive frameworks for incorporating phenotypes into phylogeography (Papadopoulou and Knowles, Chapter 8, this volume), and DNA sequencing technologies (Edwards et al., 2015)
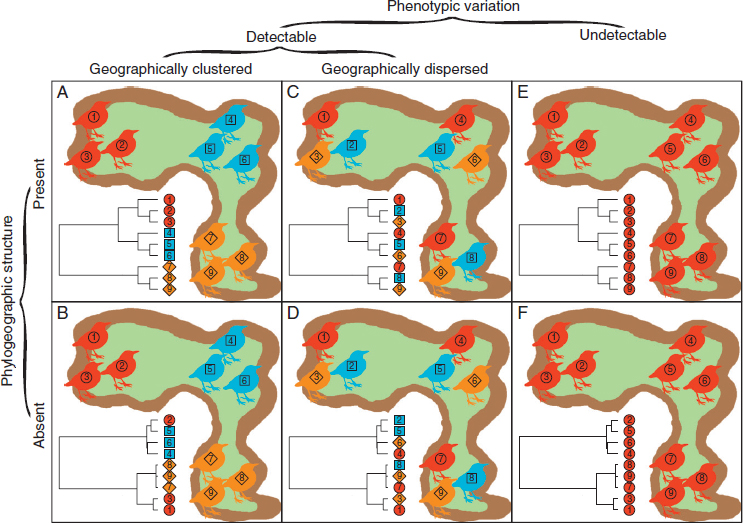
TABLE 11.1 Patterns of Phylogeographic Structure and Phenotypic Diversity in Vertebrates
| Geographic Variation in Phenotypes | Phylogeographic Structure | Biological Mechanisms or Processes | Empirical Examples |
|---|---|---|---|
| Clustered | Present | Neutral divergence, local adaptation, divergent sexual selection | Neotropical oscines (Winger and Bates, 2015); treefrogs (Warwick et al., 2015 (Waldrop et al., 2016) |
| Clustered | Absent | Rapid and recent diversification, phenotypic plasticity, gene flow | Dark-eyed juncos (McCormack et al., 2012); redpoll finches (Mason and Taylor, 2015); perch and roach (Faulks et al., 2015); spadefoot toads (Rice and Pfennig, 2010) |
| Dispersed | Present | Retention of ancestral polymorphism, convergent local adaptation, phenotypic plasticity, balancing selection | Side-blotched lizards (Corl et al., 2010); desert cichlids (Magalhaes et al., 2015); threespine sticklebacks (Colosimo et al., 2005); trout (Pearse et al., 2014); mollies (Pfenninger et al., 2015); humans (Tishkoff et al., 2007) |
| Dispersed | Absent | Convergent local adaptation, phenotypic plasticity, balancing selection, gene flow | Red crossbills (Benkman, 1996; Parchman et al., 2006); ground snakes (Cox and Davis Rabosky, 2013) |
| Undetectable | Present | Stabilizing selection, “cryptic” diversification | Sun skinks (Barley et al., 2015); black salamanders (Reilly and Wake, 2015); plain-backed thrushes (Alström et al., 2016); field voles (Paupério et al., 2012); rainforest skinks (Singhal and Moritz, 2013) |
| Undetectable | Absent | Stabilizing selection, gene flow | Straw-colored fruit bats (Peel et al., 2013) |
NOTE: For each of the patterns illustrated in Fig. 11.1, we include potential mechanisms contributing to the distribution of phenotypic and genetic variation and empirical examples.
have bolstered our ability to identify and quantify the mechanisms that generate this diversity. Here, we illustrate those biological mechanisms that favor spatial concordance (or discordance) of genetic and phenotypic variation within a phylogeographic framework.
Geographically Clustered Phenotypes with Phylogeographic Structure
Phylogeographic structure often coincides with phenotypic variation (Fig. 11.1A) (Gould and Johnston, 1972). In fact, many phylogeographic studies are initially motivated by phenotypic differences among lineages separated by putative barriers to gene flow. Phylogeographic structure is most pronounced when populations are separated by nonpermeable barriers to gene flow and/or in taxa with limited capacity for dispersal across physical barriers (Hewitt, 2000). As a corollary, those lineages with deeper phylogeographic structure likely have greater levels of phenotypic divergence, arising from longer periods of independent neutral and selective evolution (Lande, 1980). A recent study compared genetic and phenotypic differentiation between allopatric, ecologically similar sister species from eight genera of Peruvian passerine birds in cloud forests separated by the Maranon Valley, a prominent physical barrier to gene flow among central Andean taxa (Winger and Bates, 2015). Allospecies showed a positive association between genetic divergence and phenotypic differentiation, especially in plumage differentiation, a trait important for social signaling and species recognition. Empirical studies in treefrogs (Warwick et al., 2015) and butterflyfishes (Waldrop et al., 2016) indicate that although the degree of phenotypic differentiation among vertebrates may scale with the strength and duration of genetic isolation, patterns can be highly idiosyncratic among taxa. Additionally, differences in the degree of phenotypic divergence among taxa or categories of traits can represent variation in evolutionary constraints or selective pressures driving the tempo and mode of phenotypic differentiation, which in turn can shape neutral genetic connectivity among populations.
Selective pressures vary temporally and geographically, thereby potentially altering the context-dependent fitness of phenotypic variants within lineages (Wang and Bradburd, 2014). If environmental conditions favor one phenotype, then populations may diverge phenotypically and genetically through local adaptation (Kawecki and Ebert, 2004). The spatial arrangement of suitable habitat in heterogeneous landscapes, such as mosaics or clines, can also promote geographically clustered phenotypic variation (Forester et al., 2016). For example, strawberry poison dart frogs are highly polymorphic, and genetic distances among populations are more strongly associated with phenotypic differences than with geographic distances, suggesting a role for local adaptation related to preda-
tion and aposematism (Wang and Summers, 2010). Similarly, genetic isolation imposed by ecological variation also contributes to geographically clustered phenotypic and genealogical diversity in Mediterranean blue tits (Charmantier et al., 2015) and bats (Morales et al., 2016). Thus, a phylogeographic framework can reveal patterns of phenotypic and genetic variation and their regional concordance (or lack thereof), thereby elucidating roles of neutral and selective processes in lineage differentiation.
Geographically Clustered Phenotypes Without Phylogeographic Structure
In many systems, geographically clustered phenotypic variation exists without phylogeographic structure (Fig. 11.1B). Phenotypic differences can arise quickly through localized divergent selection, or, alternatively, they may seem to arise quickly because traditional molecular markers may not detect the phylogenetic structure underlying rapid diversification (Edwards and Bensch, 2009). Dark-eyed juncos, for example, exhibit pronounced phenotypic variation that is geographically clustered among subspecies that exhibit little to no phylogeographic structure (McCormack et al., 2012). This pattern likely reflects subtle phylogeographic structure resulting from recent, rapid genetic isolation or adaptive divergence with ongoing gene flow among subspecies. Fortunately, high-throughput sequencing offers increased resolution and the capacity to distinguish between incomplete lineage sorting and ongoing gene flow, thereby improving our ability to infer ongoing biological processes in these cases (Edwards et al., Chapter 9, this volume).
Alternatively, clustered phenotypic variation among populations that lack phylogeographic structure may reflect biological processes, such as phenotypic plasticity (Crispo, 2008; Pfennig et al., 2010). Phenotypic plasticity involves developmental and phenotypic responses to different environmental regimes, thereby generating phenotypic diversity without genetic differentiation. For example, redpoll finches exhibit a gradient in which longer-billed individuals with streaking occur at lower latitudes, shorter-billed individuals with little to no streaking occur at higher latitudes, and many individuals express intermediate phenotypes (Mason and Taylor, 2015). However, genome-scale SNP analyses revealed that redpolls constitute a single gene pool, regardless of their phenotype or geographic origin, so the paucity of genetic differentiation among phenotypes at a continental scale is probably not the result of recent divergence and/or insufficient molecular data (Mason and Taylor, 2015). Furthermore, polygenic patterns of gene expression are strongly correlated with continuous variation in bill shape and plumage patterns, indicating possible roles for plasticity or variation in regulatory elements in generating
and maintaining geographically clustered phenotypes (Mason and Taylor, 2015). Recent studies in perch and roach (Faulks et al., 2015) and spadefoot toads (Rice and Pfennig, 2010) similarly highlight the potential role of phenotypic plasticity in generating geographically clustered phenotypic variation without phylogeographic structure.
Distinguishing between recent, rapid bouts of adaptive genetic differentiation and phenotypic plasticity in natural populations can be difficult (Merilä and Hendry, 2014). Definitively demonstrating phenotypic plasticity requires experimental studies of captive populations or long-term longitudinal datasets, which are logistically challenging in many vertebrate, nonmodel species. Nonetheless, combining high-throughput sequencing with experimental studies to disentangle phenotypic plasticity and adaptive genetic divergence within a phylogeographic context is an exciting area of ongoing research.
Geographically Dispersed Phenotypes with Phylogeographic Structure
Many species are polymorphic with discrete or continuous phenotypic variation shared among phylogeographically structured populations (Fig. 11.1C). This pattern can be maintained through various evolutionary processes, including retention of ancestral polymorphism, balancing selection, parallel adaptation to locally variable conditions, and phenotypic plasticity. Multiple populations of side-blotched lizards in the western United States, for example, share polymorphism in throat color associated with different mating strategies that is maintained through negative frequency-dependent selection (Sinervo et al., 2000) and has persisted through multiple bouts of genetic isolation among populations (Corl et al., 2010).
Phenotypic plasticity also can contribute to geographically dispersed variation with phylogeographic structure. If plastic responses are retained among multiple populations that experience genetic isolation, then similar environmental conditions can result in shared polymorphism with phylogeographic structure. Desert cichlids restricted to the Cuatro Ciénegas valley in northern Mexico exhibit strong phylogeographic structure, and populations from each lagoon are genetically distinct from all others. However, both deep-bodied benthic morphs and slender-bodied limnetic morphs occur in each population, suggesting a role for phenotypic plasticity in generating and maintaining shared polymorphism among isolated populations (Magalhaes et al., 2015).
Parallel adaptation, or the independent evolution of similar adaptive phenotypes in different populations, is another potential mechanism for shared phenotypic variation in species with marked phylogeographic
structure. Parallel adaptations evolve under similar selective pressures, often acting on standing ancestral genetic variation (Barrett and Schluter, 2008), resulting in similar phenotypes among genetically isolated lineages (Stern, 2013). Prominent examples of parallel evolution include changes in the ectodysplasin signaling pathway in threespine sticklebacks that result in reduced armor plating in multiple, independent freshwater populations (Colosimo et al., 2005). Life history differentiation in trout (Pearse et al., 2014), adaptations to sulfidic habitats in mollies (Pfenninger et al., 2015), and lactase persistence in humans (Tishkoff et al., 2007) underscore the potential for parallel evolution of shared phenotypic variation among geographically and genetically isolated populations across a large diversity of taxa.
Geographically dispersed and shared phenotypic variation with phylogeographic structure (Fig. 11.1C) may arise through similar processes that generate geographically clustered phenotypes without phylogeographic structure (Fig. 11.1B), albeit at different temporal, spatial, and phylogenetic scales. Parallel adaptive and plastic responses to similar temporal or spatial variation in environmental conditions within the range of each lineage can generate shared polymorphisms among populations, even if they are separated by prolonged bouts of genetic isolation (Fig. 11.1C). Furthermore, rapid adaptation and phenotypic plasticity in response to regional conditions also can induce geographically clustered phenotypic variation without perceivable phylogeographic structure at larger spatial scales, especially if gene flow reduces genetic isolation among populations (Fig. 11.1B). Thus, the same adaptive and plastic processes can shape geographic and phylogenetic partitioning of phenotypic and genetic variation over space and time at different scales.
Geographically Dispersed Phenotypes Without Phylogeographic Structure
Phenotypic variation also can be dispersed and shared among populations that do not exhibit phylogeographic structure (Fig. 11.1D). Intuitively, this pattern can arise if processes that generate shared phenotypic variation (e.g., adaptations to spatial or temporal environmental heterogeneity, balancing selection, or phenotypic plasticity) occur among populations that readily exchange genes with one another. Polymorphism in red crossbills provides an empirical example: Their lower mandibles curve either left or right to facilitate feeding on conifer cones, and polymorphism is maintained by frequency-dependent selection in multiple populations connected by gene flow and thus weakly differentiated (Benkman, 1996; Parchman et al., 2006). Likewise, highly polymorphic ground snakes include various color morphs that are present in multiple, genetically
undifferentiated populations (Cox and Davis Rabosky, 2013). Although discrete forms of polymorphism are perhaps easier to identify, variation in continuous phenotypes—such as body size or limb length—is also common among populations that lack phylogeographic structure. Dispersed polymorphism among populations that lack phylogeographic structure is more likely in vagile than dispersal-limited taxa, and the maintenance of phenotypic diversity will depend on the strength of selection acting on phenotypic variants among populations connected by high levels of gene flow.
Uniform Phenotypes with Phylogeographic Structure
Many lineages exhibit phylogeographic structure with little or no detectable phenotypic variation (Fig. 11.1E). In the case of strong stabilizing selection acting on traits that characterize a species’ niche, populations will track suitable habitat as it appears and disappears over time (Wiens et al., 2010). As such, ancestral populations can subdivide and accumulate genetic differences without morphological divergence, generating “cryptic” lineages or species (Bickford et al., 2007). Philippine sun skinks, for example, exhibit deep phylogenetic splits concordant with geography but show little to no morphological variation among lineages (Barley et al., 2015). Similar patterns in black salamanders (Reilly and Wake, 2015), plain-backed thrushes (Alström et al., 2016), and field voles (Paupério et al., 2012) indicate that cryptic lineages are prevalent among vertebrates. Although cryptic lineages may not persist if previously isolated populations come into contact and exchange genes freely, speciation theory predicts that reproductive isolation—and postzygotic reproductive isolation in particular—increases with divergence time between lineages, in part through the accrual of Dobzhansky-Muller incompatibilities (Bolnick and Near, 2005). Thus, study systems in which cryptic lineages occur in secondary contact provide an opportunity to address the evolution of postzygotic reproductive isolation between cryptic populations in nature. For example, divergence time and the degree of reproductive isolation are tightly correlated across five contact zones among cryptic lineages of rainforest skinks previously isolated in glacial refugia, indicating that, even in morphologically cryptic lineages, phylogeographic splits of increasing depth represent stages along the speciation continuum (Singhal and Moritz, 2013). Lineages with limited dispersal and prominent evolutionary or developmental constraints are most likely to develop phylogeographic structure with no perceivable phenotypic diversity (Bickford et al., 2007). We expect this pattern where strong biogeographic barriers to gene flow generate genetic divergence, and the selective environments between genetically isolated populations are relatively similar over space and time.
Uniform Phenotypes Without Phylogeographic Structure
Occasionally, vertebrate taxa do not vary perceivably in phenotypes within and among populations that do not also exhibit phylogeographic structure (Fig. 11.1F). Phenotypic uniformity among populations will be more likely if gene flow homogenizes populations (Lenormand, 2002). If environmental conditions are temporally and spatially consistent, then phenotypic variation may become fixed at an optimum, such that no phenotypic variation exists across a species’ range (Kawecki and Ebert, 2004). Furthermore, if individuals consistently disperse and exchange genes with other populations, phylogeographic structure will not accumulate over time. Straw-colored fruit bats in continental Africa, for example, display no phylogeographic structure and do not vary in phenotype across their expansive distribution (Peel et al., 2013). Vagile taxa that have expanded their range rapidly from a single glacial refugium, such as the blackpoll warbler (Ralston and Kirchman, 2012), may display phenotypic uniformity and little to no phylogeographic structure among populations. A phylogeographic framework can disentangle cases in which continued gene flow homogenizes genetic and phenotypic variation among populations and cases in which historical demographic events, such as recent and/or rapid range expansions, have limited the time for phenotypic differences to accumulate (Marko and Hart, 2011).
As illustrated by the empirical and hypothetical examples discussed above (Fig. 11.1 and Table 11.1), the geographic partitioning of phenotypic and genotypic diversity is highly variable among vertebrates. Geographic partitioning of phenotypic variation across environmental gradients can occur with or without genetic differentiation, and in some cases the mechanisms for concordance (or lack thereof) are difficult to disentangle. Integrative approaches that combine high-throughput sequencing, experimental manipulations, and high-quality phenotypic datasets allow us to differentiate among biological mechanisms underlying phenotype-genotype concordance. Comparative studies adopting this framework will yield further examples of neutral divergence, local adaptation, and phenotypic and developmental plasticity, balancing selection and the prevalence of different evolutionary processes across taxa. Phylogeographic studies adopting this framework will also enhance our understanding of how rates and modes of phenotypic diversification vary among taxa. Finally, clarifying the evolutionary mechanisms underlying patterns of phenotypic and genetic diversity has implications for conserving biodiversity and for making accurate predictions of how species will respond to environmental change.
PHYLOGEOGRAPHY AND FUNCTIONAL VARIATION IN PHENOTYPES
Phylogeographic studies of functional variation in phenotypes have the potential to identify selective regimes that structure variation within and between species and ultimately shape the evolutionary history of functional traits. This approach can build on classic studies of hybrid zones and character evolution at macroevolutionary timescales by focusing on the spatial distribution of functional (or selected) traits within species. For instance, most hybrid zones result from secondary contact between populations or species that were previously allopatric; thus, the selective environment within the hybrid zone may not reflect the selective pressures that initiated divergence between parental lineages. Likewise, methods for quantifying character evolution above the species level typically do not account for within-species variation in phenotype and genotype (but see Revell and Graham Reynolds, 2012) or the range of environmental conditions across a given species’ distribution. Thus, a phylogeographic approach that encompasses phenotypic and environmental variation within species and contextualizes the demographic history of functional traits can provide exceptional insights into how organismal diversity evolves.
Identifying the role of extrinsic barriers in shaping the geographic distribution of functional phenotypic variation is an essential first step for investigating local adaptation. Thus, many studies quantify divergence at neutral genetic markers to investigate whether historical barriers coincide with the geographic distribution of variation in phenotypes such as coloration (Wang and Summers, 2010) or physiology (Moritz et al., 2012). Within this historical context, phylogeographers then can investigate whether regional environmental variation and local adaptation contribute to phenotypic divergence (e.g., Ng et al., 2013) and identify instances of parallel phenotypic evolution among phylogeographic lineages that occupy similar selective environments in different geographic areas (Richmond and Reeder, 2002; Hoekstra et al., 2005). Even in study systems with only a single evolutionary origin of the trait of interest, a broader phylogeographic framework can inform the timing and/or direction of phenotypic change between genetically differentiated lineages. For example, conspicuous coloration and toxicity vary across the range of the granular poison dart frog, and prevailing evolutionary theory contends that these traits should evolve in a correlated fashion. By reconstructing phylogeographic relationships across the species’ range, Wang (2011) demonstrated that the less conspicuous, more toxic population evolved from a more conspicuous, less toxic ancestor, thus challenging the view that conspicuousness and toxicity are tightly coupled. Finally, quantifying demographic processes, such as patterns of gene flow or changes in popu-
lation size, and the distribution of phenotypic variation among divergent lineages can provide preliminary insights as to the strength of selection on adaptive phenotypes and whether gene flow between populations introduces adaptive phenotypes to new environments. For example, in the rock pocket mouse, in which melanic pelage evolves repeatedly on dark lava flows, high levels of gene flow between neighboring populations that differ phenotypically indicate that selection for color matching is strong (Hoekstra et al., 2004, 2005). Furthermore, high rates of gene flow between melanic mice populations inhabiting neighboring lava flows suggest that on a finer spatial scale, adaptive phenotypes in these different populations have a common genetic basis (Hoekstra et al., 2005). Thus, characterizing geographic patterns of divergence at neutral genetic markers and in phenotypes contextualizes the demographic history of adaptive traits, and this evolutionary perspective then can inform in-depth investigations to identify the selective environment in which these traits evolved.
Phylogeographic studies of functional traits can identify the processes that shape adaptive variation and estimate the strength of selection acting on phenotypic variation by building links between locally adapted genotypes/phenotypes, population demography, and environmental variation in selective regimes. The challenge of this approach, however, is that it requires identifying and quantifying adaptive phenotypes, obtaining samples that encompass relevant environmental and phenotypic variation across the species’ range, and characterizing functional genetic variation underlying adaptive phenotypes as well as neutral variation to estimate population demographic history. Given that the genetic architecture of adaptive phenotypes is unknown in most nonmodel vertebrate taxa, this approach has been applied primarily in systems with extensive genomic resources (e.g., the threespine stickleback; Deagle et al., 2013) or in systems in which the links between a particular adaptive phenotype and the underlying functional genetic variation are well defined. These systems include adaptive shifts in coloration caused by variation in genes encoding the melanin pathway (Hoekstra et al., 2004), physiological adaptation to high-altitude environments caused by variation in hemoglobin subunit genes (Bulgarella et al., 2012), tetrodotoxin resistance caused by variation in skeletal muscle sodium channels (Feldman et al., 2009), and differences in adaptive immunity caused by variations in genes encoding the MHC class II subunits (Savage et al., 2015; Savage and Zamudio, 2016).
One common result of functional-trait studies is the identification of recurrent novel mutations underlying similar phenotypes (Nachman et al., 2003; Feldman et al., 2009; Savage and Zamudio, 2016). For example, mutations in the melanocortin-1 receptor gene (Mclr) are highly correlated with adaptive melanism in an Arizona population of rock pocket mice, but melanic populations in New Mexico show no association with
variation at Mclr, indicating that their dark coloration must result from changes at different genes (Hoekstra and Nachman, 2003; Nachman et al., 2003; Hoekstra et al., 2005). This finding is in contrast to traits associated with threespine sticklebacks in which the same adaptive alleles underlie multiple independent freshwater invasions on a regional scale (Fisher et al., 2012). A second theme is the role of gene flow in promoting adaptive evolution in some contexts (Hoekstra et al., 2005) and impeding adaptation in others (Savage et al., 2015; Savage and Zamudio, 2016). Finally, geographic sampling that captures environmental variation is especially valuable for understanding complex adaptive scenarios such as host–pathogen dynamics. Across its range, the lowland leopard frog exhibits population-level variation in survival after infection by the fungal pathogen Batrachochytrium dendrobatidis, which causes chytridiomycosis, a disease implicated in population declines or extinction in hundreds of amphibian species worldwide (Fisher et al., 2012). Variation in immunity loci determines susceptibility to the pathogen such that several MHC alleles are strongly associated with increased survival or susceptibility in both experimental (Savage and Zamudio, 2011) and natural settings (Savage and Zamudio, 2016). These associations are decoupled, however, for populations surrounding a thermal spring, where warm water shields frogs from developing high pathogen loads but also precludes selection from increasing the frequency of MHC survival alleles (Savage and Zamudio, 2016).
In summary, quantifying functional genetic variation within the context of the phylogeographic history of a species and across the range of environments it inhabits can reveal how regional variation in selective regimes and demographic processes drives the evolution of adaptive phenotypes. Just as phylogeography initially formalized conceptual links among heredity, population divergence, and phylogenetic relationships among species, an analytical framework that advocates genealogical and spatially explicit analyses of intraspecific functional genetic and phenotypic variation will bridge microevolutionary processes acting on individual populations and macroevolutionary patterns at larger spatial and temporal scales. These integrative and rigorous approaches have been possible only in select systems to date, but identifying the underlying genetic basis of phenotypic variation within species is becoming increasingly tractable in vertebrates. Clearly, examining functional phenotypic variation in a phylogeographic framework holds great promise for exploring links between genotypic and phenotypic diversity and adaptation across variable environments.
FIELD STUDIES AND NATURAL HISTORY COLLECTIONS: SOURCES OF PHENOTYPIC DATA
Organismal phenotypes, many of which are the target of selection and play important roles in species performance in variable environments, are important components of how we identify and categorize biodiversity. We have argued that, despite the clear benefits of integrating patterns of phenotypic evolution into phylogeographic predictions and inferences, this integration has yet to be fully realized. One reason is that high-quality phenotypic data are difficult to obtain; establishing a phenotypic database with robust sample sizes and fine-scale spatial sampling can be laborious. This challenge is solvable by relying on well-established methods in biodiversity science.
For centuries, naturalists relied solely on phenotypes to document diversity, study the relationship of organisms with their environment, and infer evolutionary change. Unaware of the genetic underpinnings of those phenotypes, early naturalists focused on explaining phenotypic diversity not only among but also within species. They did so with a large number of phenotypes, including behavior, color, morphology, life history, and ecological traits, among others. Fortunately, many of those efforts are archived in publications or are preserved in natural history collections, providing a sample of Earth’s biota that typically extends back to the 19th century, and often includes representative coverage of species’ distributions (Graham et al., 2004; Holmes et al., 2016). For some kinds of phenotypes, such as certain aspects of morphology preserved in museum specimens, natural history collections are a rich source of phenotypic data. In contrast, for phenotypes that are not easily preserved—such as behavior, ecological associations, or physiological parameters—field studies will be the ultimate source, although the metadata associated with many preserved specimens often contain important information on behavior, habitat preferences, and other ecological associations (Holmes et al., 2016).
Natural history collections also will have a large role in the analysis of more recent evolutionary change in phenotypes (Holmes et al., 2016). For some species, long-term series of collections offer a unique opportunity to infer ancestral phenotypes and how those have changed with documented changes in the environment (Ożgo and Schilthuizen, 2012). Examining these data within a phylogeographic framework provides the evolutionary context to identify rangewide dynamics of phenotypic change and may highlight regional sources of adaptive variation. Furthermore, many of these phenotypic changes can be tied to strong selection imposed by changing environments, thus providing the link between genetic and phenotypic changes under different environmental contexts. These phenotype–genotype associations provide a mechanistic basis for inferring past changes at both recent and longer evolutionary time frames
and a predictive framework for understanding how organisms will adapt to future natural and anthropogenic global change. Phenotypes, genetic structure, and environmental characteristics are intimately coupled in the processes of organismal divergence; thus advances in all three fields will enable the integrative study of divergences in natural populations.
CONCLUSIONS
We see great promise in ongoing methodological and conceptual advances that explicitly incorporate trait evolution in phylogeographic predictions and inferences. Our goal here was to highlight the many valuable avenues for future work in this area. The field of phylogeography has changed since its origin, incorporating new techniques, new analyses, and increasingly, different sources of data. Although only a fraction of extant taxa have been surveyed, the field has revealed many common patterns and mechanisms underlying diversification within broadly divergent taxonomic groups. Meanwhile, our ability to quantify genetic and phenotypic variation also has expanded; thus, the field of phylogeography now is poised for another integration, this time by incorporating data on phenotypic variation in diverging lineages, understanding the selective and genetic basis for that variation, and quantifying the role that phenotypes play in diversification. This integration has the potential to unify once again disparate fields in evolutionary biology, and address how interactions among abiotic landscape features and biological features of species shape biodiversity (Greene, 2005). This integrative framework is a powerful tool for understanding the effects of past global change on current biodiversity and for predicting the adaptive potential and resilience of species adapting to novel environments of the future.
ACKNOWLEDGMENTS
We thank J. Avise and F. Ayala for the invitation to participate in the Sackler Symposium In the Light of Evolution X: Comparative Phylogeography; and the K.R.Z., Lovette, Searle, and McGuire laboratories, A. Corl, A. Chavez, H. Greene, and two anonymous reviewers for constructive feedback on the manuscript. Our work is funded by National Science Foundation Research Grants DEB-0542848, DEB-1601072, and DEB-1309171; EPA Science to Achieve Results Fellowship F13F21201 (to N.A.M.); and a University of California Chancellor’s Postdoctoral Fellowship (to R.C.B.).




















