1
Comparative Phylogeography of the Ocean Planet
BRIAN W. BOWEN,*††MICHELLE R. GAITHER,†JOSEPH D. DI BATTISTA,‡MATTHEW IACCHEI,*§KIMBERLY R. ANDREWS,||W. STEWART GRANT,#ROBERT J. TOONEN,* AND JOHN C. BRIGGS**
Understanding how geography, oceanography, and climate have ultimately shaped marine biodiversity requires aligning the distributions of genetic diversity across multiple taxa. Here, we examine phylogeographic partitions in the sea against a backdrop of biogeographic provinces defined by taxonomy, endemism, and species composition. The taxonomic identities used to define biogeographic provinces are routinely accompanied by diagnostic genetic differences between sister species, indicating interspecific concordance between biogeography and phylogeography. In cases where individual species are distributed across two or more biogeographic provinces, shifts in genotype frequencies often align with biogeographic boundaries, providing intraspecific concordance between biogeography and phylogeography. Here, we provide examples of comparative phylogeography from (i) tropical seas that host the highest marine biodiversity, (ii) temperate seas with high productiv-
__________________
* Hawai’i Institute of Marine Biology, University of Hawai’i, Kaneohe, HI 96744; †School of Biological and Biomedical Sciences, Durham University, Durham DH1 3LE, United Kingdom; ‡Department of Environment and Agriculture, Curtin University, Perth, WA 6845, Australia; §Department of Oceanography, School of Ocean and Earth Science and Technology, University of Hawai’i at Mãnoa, Honolulu, HI 96822; ||Department of Fish and Wildlife Sciences, University of Idaho, Moscow, ID 83844; #Commercial Fisheriess Division, Alaska Department of Fish and Game, Anchorage, AK 99518; and **Department of Fisheries and Wildlife, Oregon State University, Corvallis, OR 97333. ††To whom correspondence should be addressed. Email: bbowen@hawaii.edu.
ity but volatile coastlines, (iii) migratory marine fauna, and (iv) plankton that are the most abundant eukaryotes on Earth. Tropical and temperate zones both show impacts of glacial cycles, the former primarily through changing sea levels, and the latter through coastal habitat disruption. The general concordance between biogeography and phylogeography indicates that the population-level genetic divergences observed between provinces are a starting point for macroevolutionary divergences between species. However, isolation between provinces does not account for all marine biodiversity; the remainder arises through alternative pathways, such as ecological speciation and parapatric (semi-isolated) divergences within provinces and biodiversity hotspots.
Phylogeography has roots in biogeography, wherein geographic provinces are identified by concordant shifts in species composition. If the partitions defined by taxonomy are regarded as first-order approximations of evolutionary genetic separations, then continuity between biogeography and phylogeography is apparent. Marine biogeography, the study of species’ distributions and evolutionary processes in the sea, began in the mid-19th century based on taxonomic distinctions. Dana (1853) divided the surface waters of the world into several temperature zones based on the distributions of corals and crustaceans. Woodward (1851) identified a series of marine provinces based on the distributions of mollusks. Forbes (1859) made three enduring observations: (i) each biogeographic province is a center of origin for new species; (ii) these new species tend to migrate outward from the center of origin; and (iii) provinces, like species, must be traced back to their historical origins to be understood. These three fundamental contributions appeared in the same decade in which Darwin and Wallace (1858) and Darwin (1859) identified geography and natural selection as agents of evolutionary change.
It is remarkable that five essential publications in the 1850s (Woodward, 1851; Dana, 1853; Darwin and Wallace, 1858; Darwin, 1859; Forbes, 1859) set the stage for 150 years of biogeographic research. Subsequent effort was devoted to species descriptions, geographic ranges, and relationships. Evolutionary hypotheses were formulated by examining the morphology and distribution of organisms. However, not until the advent of molecular technologies in the 1970s did biogeography transition through another fundamental change (Avise et al., 1987).
A primary theme emerging from marine biogeography is concordant levels of endemism in very diverse taxa. For example, endemism in Hawai’i is 25 percent for red algae and fishes (Abbott, 1999; Randall, 2007) and 20 percent for mollusks (Kay, 1980). The Caribbean Province has 33 percent endemism for fishes (Floeter et al., 2008), 32 percent for decapod crustaceans (Boschi, 2000), and 37 percent for corals (Veron,
2000). In the Red Sea, endemism is 13 percent for fishes and polychaetes, 8 percent for echinoderms, 17 percent for ascidians, and 5.5 percent for corals (DiBattista et al., 2016b). This concordance across diverse taxonomic groups indicates unifying evolutionary processes.
Here, we demonstrate concordance between biogeographic provinces defined by taxonomy and phylogeographic clusters identified with DNA sequences. At the level of interspecific comparisons, this concordance is obvious; genetic partitions between sister species are expected. However, below this level, at the inception of speciation, it is still unclear how genetic partitions within species (defined by allele-frequency shifts and significant F-statistics) translate into species-level divergences (reciprocal monophyly and morphological distinction). Concordance between taxonomy-based biogeography and genetic-based phylogeography would indicate a continuum from population isolation to morphological divergence to evolutionary innovation. In this review, we examine comparative phylogeography, first across biogeographic provinces and second across taxonomic groups with widely divergent life histories.
A second goal is to summarize aspects of comparative phylogeography that illuminate the origins of marine biodiversity. As in terrestrial and freshwater systems, phylogeographic comparisons among species often reveal a diversity of outcomes, attributed to the idiosyncrasies of individual taxa (Toonen et al., 2011; Riddle, Chapter 2, this volume). However, the comparative approach can reveal insights unavailable from any one example (Bermingham and Moritz, 1998), as illustrated by the terrestrial biota of Hawai’i (Shaw and Gillespie, Chapter 4, this volume). Finally, illuminating the origins of new species at biodiversity hotspots and centers of endemism can illustrate conservation priorities for the ocean, the cradle of life on our beleaguered planet.
BIOGEOGRAPHIC PROVINCES
Tropical Oceans
Tropical oceans are characterized by biodiversity hotspots, including the Caribbean and the Coral Triangle (between the Philippines, Indonesia, and New Guinea) (Fig. 1.1A) and endemism hotspots, such as Hawai’i and the Red Sea on the periphery of the Indo-Pacific. The evolutionary role of biodiversity hotspots versus endemism hotspots is contentious although biodiversity hotspots are widely recognized as evolutionary incubators producing new species (Briggs, 2003; Bowen et al., 2013).
The Coral Triangle has been a stable reef habitat for tens of millions of years, and this persistence is believed to be key to the production and export of species (Pellissier et al., 2014). Pervasive signals of population
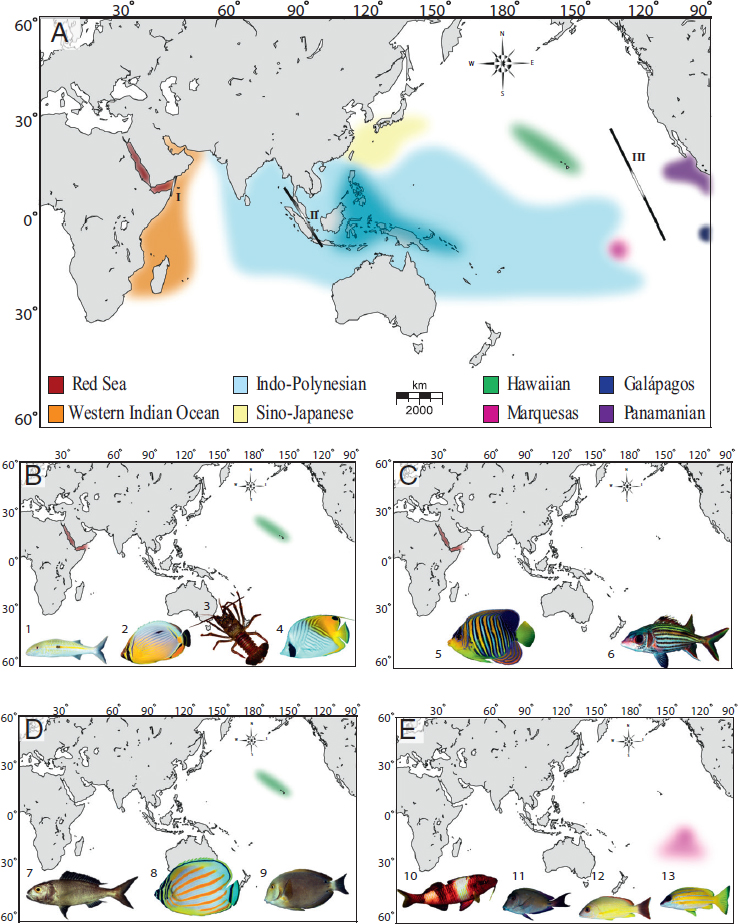
structure indicate that novel species are arising by parapatric means within the Coral Triangle, wherein partial isolation between subregions reinforces isolation along ecological gradients (Timm et al., 2008; Carpenter et al., 2010; Barber et al., 2011; Tornabene et al., 2015). Based on phylogenies of three reef fish families, Cowman and Bellwood (2013) estimate that 60 percent of Indo-Pacific reef fauna have origins in the Coral Triangle. In contrast, peripheral endemism hotspots were previously regarded as evolutionary dead ends (Alison Kay and Palumbi, 1987; Bellemain and Ricklefs, 2008), in which rare colonization events can produce endemic species, but with no further evolutionary radiations. This assumption has been challenged in recent years because phylogeographic studies show that both Hawaiian and Red Sea provinces can export novel biodiversity (DiBattista et al., 2013; Eble et al., 2015).
The dominant feature of tropical marine biogeography is the vast Indo-Polynesian Province (IPP), spanning almost half the planet (Fig. 1.1A). Concomitant with this large province are unusually large range sizes, averaging 9 million km2 for reef fishes, roughly the size of mainland China (Allen, 2008). Genetic surveys of reef organisms are generally consistent with the boundaries of the IPP, showing little genetic structure across broad areas with a few important exceptions (e.g., Indo-Pacific Barrier) (Eble et al., 2015). Schultz et al. (2008) use bathymetry profiles to demonstrate that dispersal across most of this range (Polynesia to Western Australia) requires no deep-water traverse greater than 800 km. Undoubtedly, this continuity of shallow habitat contributes to the cohesiveness of the IPP.
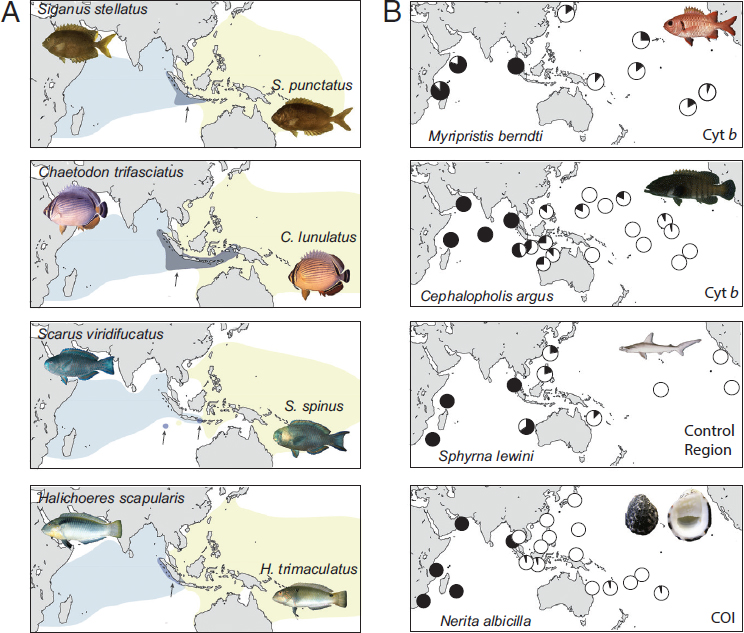
At the center of this vast province is an intermittent barrier around the Indo-Malay Archipelago, known as the Indo-Pacific Barrier (Fig. 1.1A). In the mid-Miocene (16–8 Ma), the Australian and Eurasian plates collided and reduced water flow between the Pacific and Indian Oceans (Kennett et al., 1985). During Pleistocene glacial cycles, sea level dropped as much as 130 m below present levels, further constricting connections between these ocean basins. Evidence for interruptions of gene flow can be found in the distributions of sister species, coupled with phylogeographic partitions (as defined by reciprocal monophyly or ΦST > 0.10) in green turtles (Dethmers et al., 2006), dugongs (Blair et al., 2014), and ~80 percent of surveyed reef species (Fig. 1.2) (Gaither et al., 2010; Ahti et al., 2016). Given the cyclic nature of this barrier, phylogeographic partitions driven by Pleistocene glacial fluctuations are expected to be concordant in terms of geography, but not necessarily concordant in terms of chronology.
On the eastern periphery of the enormous IPP are three isolated provinces with high endemism in reef fishes: (i) the Hawaiian Islands with 25 percent endemism (Randall, 2007), (ii) the Marquesas Islands with 13.7 percent endemism (Delrieu-Trottin et al., 2015), and (iii) Easter Island with 21.7 percent endemism (Randall and Cea, 2011). Phylogeographic studies of the first two provinces show strong concordance with biogeographic partitions (Fig. 1.1B). In Hawai’i, 11 of 16 fishes surveyed are genetically distinct from conspecifics elsewhere in the Pacific (reviewed in Gaither et al., 2011b). At the Marquesas, three of five studies reveal divergences that range from FST ≥ 0.24 at allozyme loci to reciprocal monophyly at mtDNA (Gaither et al., 2010; Szabó et al., 2014), and a RADSeq study reveals strong divergence between a Marquesan surgeonfish and a widespread sister species (Gaither et al., 2015a).
On the western side of the IPP lies the Red Sea biogeographic province, an endemism hotspot characterized by a shallow connection to the Indian Ocean and latitudinal gradients in temperature, salinity, and nutrient load (DiBattista et al., 2016a,b). Many Red Sea endemics have sister species in the adjacent Western Indian Ocean (DiBattista et al., 2016a). This interspecific pattern aligns with mtDNA partitions within species ranging from haplotype frequency shifts to reciprocal monophyly in fishes and invertebrates (table 2 in DiBattista et al., 2016a). For example, the Indo-Pacific damselfish (Dascyllus aruanus; Liu et al., 2014) and yellowstripe goatfish (Mulloidichthys flavolineatus; Fernandez-Silva et al., 2015) both demonstrate similar divisions in mtDNA sequences (ΦST > 0.65) and microsatellite genotypes (FST > 0.03). In some cases, coalescence analyses reveal that Red Sea lineages are older than those in the Indian Ocean, indicating that the former can export biodiversity to adjacent waters (DiBattista et al., 2013).
For widely distributed species, genetic divergences at peripheral locations may be the inception of speciation. The pronghorn spiny lobster, Panulirus penicillatus, with a 9-mo pelagic larval duration and a distribution across the entire tropical Indo-Pacific, illustrates genetic diversifica-
tion at both ends of its range. Iacchei et al. (2016) found fixed differences in mtDNA of East Pacific and Red Sea populations (ΦST = 0.74), corroborated by morphological differentiation in the East Pacific (George, 2005). Speciation in peripheral provinces is apparent in Thalassoma wrasses (Bernardi et al., 2004), Anampses wrasses (Hodge et al., 2012), Acanthurus surgeonfishes (Gaither et al., 2015a), Mulloidichthys goatfishes (Fernandez-Silva et al., 2015), and Montastraea corals (Gaither et al., 2016).
The East Pacific Barrier (EPB) limits the distribution of tropical species (Gaither et al., 2016), with few taxa able to maintain population connectivity across the EPB, as evidenced by the lobster P. penicillatus (Iacchei et al., 2016), and the coral Porites lobata (Baums et al., 2012; Forsman et al., 2015). However, some fishes (Lessios and Robertson, 2006) and the echinoderm Echinothrix diadema (Lessios et al., 1998) have low or insignificant ΦST values across the EPB.
Atlantic and Indo-Pacific Connections
Two geological events isolated the tropical Atlantic from the Indo-Pacific: (i) closure of the Tethys Sea ~13 Ma, brought about by the collision of Africa and Eurasia; and (ii) the rise of the Isthmus of Panama ~3.5 Ma that separated the Atlantic from the East Pacific Ocean (Lessios, 2008). For the latter, some species diverged well before the final closure, although the timing of partitions remains controversial (Marko et al., 2015) (a fruitful topic for genomic studies). Since the closure of the Tethys Sea, natural dispersal between the Atlantic and Indian Oceans has been limited to the hydrographically complex waters around southern Africa (Teske et al., 2011). A warm-water corridor here was curtailed ~2.5 Ma by the advent of modern glacial cycles and upwelling in the Benguela Current on the Atlantic side (Dwyer et al., 1995). However, the Agulhas Current on the Indian Ocean side occasionally forces warm-water gyres into the Atlantic (Hutchings et al., 2009), a potential route of colonization. Phylogeographic studies confirm sporadic dispersal along this route over the last 2.5 My, primarily from the Indian to Atlantic Ocean (Reece et al., 2010; Gaither et al., 2015b).
Summary
In conclusion: (i) Biodiversity hotspots and peripheral centers of endemism both produce and export novel evolutionary lineages. (ii) Phylogeographic partitions, as defined by mtDNA monophyly or strong population structure, align well with the biogeographic provinces defined by taxonomy. (iii) Sporadic dispersal around southern Africa is the primary avenue of colonization between Indo-Pacific and Atlantic Oceans.
Temperate and Polar Seas
Northern seas experienced greater extremes in temperature over the Pleistocene than tropical seas, and northern near-shore ecosystems were periodically eradicated by glaciers encroaching onto continental shelves, whereas interglacial warming led to colonizations and population expansions. Although phylogeographic structure generally occurs between biogeographic provinces, sub-Arctic shelf fauna have been repeatedly disrupted by glacial cycles (Marko et al., 2010). Therefore, present-day physical barriers to gene flow may not exert the same influence on phylogeographic patterns as observed in more stable tropical seas. The most notable barriers separating biogeographic domains are the large expanses of ocean waters across the North Pacific and North Atlantic.
North Pacific
Species in the temperate regions on both sides of the North Pacific show a range of evolutionary divergences that largely depend on dispersal capabilities, temperature tolerances, and climate history. Taxa at higher latitudes tend to have distributions that span the North Pacific (versus taxa at midlatitudes). For example, cold-tolerant cods (Gadus), herring (Clupea), and king crabs (Lithodes, Paralithodes) occur in both the Northwest and Northeast Pacific. Most of these trans-Pacific species show phylogeographic breaks, centered on the Aleutian Archipelago or eastern Bering Sea, that represent secondary contact zones after repeated isolations (Canino et al., 2010; Liu et al., 2012; Grant et al., 2014). In contrast, temperate fishes, invertebrates, and seaweeds at midlatitudes are generally limited to one side of the North Pacific, with closely related species on the other side. A notable exception are disjunct populations of Pacific sardines (Sardinops) in the Northwest and Northeast Pacific (Bowen and Grant, 1997).
North Atlantic
This basin is smaller than the North Pacific and has a U-shaped shoreline with Greenland, Iceland, and the Faroe Islands in midocean. Populations of fishes, invertebrates, and seaweeds show a range of genetic divergences across the North Atlantic (Árnason, 2004; Addison and Hart, 2005; McCusker and Bentzen, 2010). Conspecific populations on either side of the North Atlantic were isolated during glacial episodes, and, in some taxa, the Northwest Atlantic was extirpated and reestablished after the Last Glacial Maximum. Some populations in the Northwest Atlantic show closer genetic affiliations to the North Pacific than to the Northeast Atlantic (seagrass and sea urchins) (Olsen et al., 2004). The Baltic, North Sea, and
Mediterranean biogeographic provinces are isolated to some extent from the Atlantic by narrow straits, which often coincide with phylogeographic transitions (Johannesson and André, 2006; Patarnello et al., 2007).
Arctic Biogeographic Province
The far northern ocean has served as a pathway for dispersal between the North Atlantic and North Pacific (Vermeij, 1991). Phylogeographic and taxonomic studies reveal sister species in the North Atlantic and North Pacific, including several fishes (Grant, 1987), invertebrates (Vermeij, 1991), and seaweeds (Lindstrom, 2001). During ~20 percent of the Pleistocene, high sea levels breached the 50-m sill across the Bering Strait (Miller et al., 2005), allowing interocean dispersal as early as 6.4 Ma and again at 3.5 Ma (Marincovich and Gladenkov, 1999). More recent dispersal events have led to the co-occurrence of conspecific populations in both oceans (Carr et al., 1999).
Antarctic Biogeographic Province
The Antarctic is relatively old, ~25 My, compared with about 2.5 My for the Arctic. The result of this ancient formulation is high endemism: 88 percent in fishes (Eastman, 2005) and 42–56 percent in four invertebrate classes (Griffiths et al., 2009). The high homogeneity of taxa across this vast region is facilitated by the Antarctic Circumpolar Current, which circles the entire continent. Phylogeographic studies are consistent with a highly connected Antarctic Province, showing little (or no) population structure for two decapods (Raupach et al., 2010), one nemertean (Thornhill et al., 2008), and four ice fishes (Janko et al., 2007).
Patterns Within Biogeographic Provinces
Within the shallow-water provinces, species often share genetic breaks at specific geological features or geographical regions. Examples range from the classic study by Avise (1996) on the Carolina Province (Southeast United States), through more recent surveys of the benthic fauna along the coast of New Zealand (Ross et al., 2009), the northeastern Pacific (Kelly and Palumbi, 2010), the Coral Triangle (Carpenter et al., 2010), southern Africa (Teske et al., 2011), and Hawai’i (Toonen et al., 2011). Endemic species confined to a single province tend to show more population structure than widespread species at the same geographic scale (Carpenter et al., 2010; Tenggardjaja et al., 2014, 2016). Species that lack pelagic development generally show strong genetic structure whereas species with pelagic development are less predictable (Riginos et al., 2014; Liggins et al., 2016).
Regardless of developmental mode, ecological niche, or evolutionary relationships, species showing geographic structuring often have concordant genetic breaks, indicating that shared history or physical factors drive the observed pattern (Avise, 1996). Examination of 47 reef-associated species across the Hawaiian Archipelago reveals that multispecies trends in genetic diversity are driven by a combination of both the dominant physical, historical, and ecological features of the seascape, and ecological–genetic feedback within communities (Selkoe et al., 2016).
Species that counter these trends may be particularly informative about the process of evolution. For example, Hawaiian limpets of the genus Cellana have diversified within the archipelago along a tidal gradient that indicates ecological speciation (Bird et al., 2011). Certainly, species sharing population structure at unexpected locations within biogeographic provinces (such as Fiji in the tropical Pacific) (Drew and Barber, 2012; DiBattista et al., 2015), or other exceptions to those general trends, will provide evolutionary insights.
Summary
In conclusion: (i) Species distributions are fundamentally shaped by physiological tolerances to north–south temperature gradients in the North Pacific and North Atlantic. (ii) Glacial cycles impact phylogeography by repeatedly altering species distributions, isolating populations, and creating secondary contact zones. (iii) Shifting interactions between ocean–climate, coastal configuration, and bottom topography produce barriers to dispersal between ocean basins. (iv) Some biogeographic provinces are genetically homogeneous, with little opportunity for allopatric divergences, whereas others host heterogeneous habitats that can promote speciation along ecological boundaries.
TAXON-SPECIFIC PATTERNS
Migratory ability and historical dispersal define taxa along a continuum of evolutionary divergence. Clusters of closely related species, each confined to a single biogeographic province, are at one end of the continuum, and highly migratory megafuana are at the other end. Oceanic migrants provide special challenges to both phylogeographic studies and conservation strategies, because both must be conducted on a scale that transcends biogeographic provinces and political jurisdictions (Toonen et al., 2013). Species in the center of the continuum include temperate taxa inhabiting disjunct regions, such as antitropical taxa, sister species separated by the tropics. Comparative phylogeography of these groups
provides insights into the roles of dispersal and isolation in contributing to biodiversity.
Antitropical Taxa
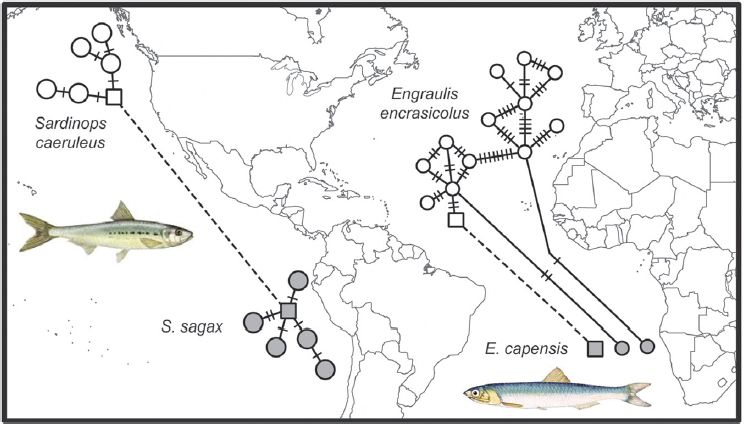
Species with disjunct distributions on both sides of the tropics provide fascinating subjects for phylogeographic study. Equatorial surface waters are lethal to these cold-adapted species, so how do they cross the tropics, and how often can this crossing be accomplished? Sister taxa of fishes on each side of the equator reveal divergences ranging from populations to distinct lineages, but without a clear pattern. For example, a single species of anchovy (Engraulis) occurs in the North Atlantic, southern Africa, and Japan, but three additional species have more restricted ranges (Grant et al., 2005; Silva et al., 2014). In contrast, a single species of sardine (Sardinops) extends from southern Africa to Australia to Chile, California, and Japan (Bowen and Grant, 1997).
Overall results show that the ability to traverse the tropics is species specific and that these events have not been limited to particular periods of global cooling. However, one possible point of concordance includes the eastern continental margins of the Atlantic (for anchovies) and the Pacific (for sardines). In both cases, colonizations across the equator have been accomplished recently, as indicated by shared mtDNA haplotypes (Fig. 1.3).
Cetaceans
Patterns of gene flow vary extensively across space and time for cetaceans, driven largely by the wide variety of life history traits (Hoelzel, 1998; Andrews, 2014). Most species exhibit limited gene flow between ocean basins, even in taxa with temperate distributions; but genetic structure within ocean basins varies substantially across species. For Mysticetes (baleen whales), patterns of gene flow are shaped by migratory pathways, with individuals typically exhibiting maternally based site fidelity to tropical breeding and temperate/Arctic feeding areas. This fidelity leads to population genetic separations between ocean basins and among breeding areas, with FST values of 0.05 to 0.1 for right whales (Carroll et al., 2015), blue whales (Torres-Florez et al., 2014), and humpback whales (Jackson et al., 2014).
In contrast, most Odontocetes (toothed whales) do not undertake large-scale migrations and often exhibit genetic structure over relatively short geographic distances due to site fidelity, resource specialization, and social structure. For example, strong fidelity to narrow ranges can result in genetically divergent populations along continuous coastlines or
between adjacent islands, as is the case for spinner dolphins (Andrews et al., 2010), Hector’s dolphins (Hamner et al., 2012), and Indo-Pacific humpback dolphins (Brown et al., 2014). Some Odontocetes have ecologically and behaviorally distinct groups (“ecotypes”), with limited gene flow even in parapatry or sympatry (Hoelzel, 1998). Several dolphin species contain genetically divergent coastal and pelagic ecotypes (Andrews et al., 2013). Killer whales have sympatric ecotypes that differ in prey type, foraging strategy, social structure, and movement (Hoelzel et al., 1998).
Sea Turtles
The seven species of sea turtles show patterns of population structure within ocean basins defined by natal homing, the habit of females (and sometimes males) to return to the vicinity of their natal beach, after decades of growth in ocean and coastal habitats. This behavior is the basis for defining regional management units (Wallace et al., 2010). On a global scale, occasional wandering provides connections between nesting populations and ocean basins. Cold-tolerant species, such as the leatherback turtle, pass freely between ocean basins (Dutton et al., 1999). Tropical species, such as the green turtle and the hawksbill turtle, make rarer connections between the Atlantic and Indo-Pacific via southern Africa (Bourjea et al., 2007; Vargas et al., 2016). Bowen and Karl (2007) note higher genetic divergences between ocean basins in tropical species, providing a signal that allopatric speciation may predominate in this group.
Pelagic Fishes
A primary phylogeographic pattern for these oceanic migrants is low to no genetic structure within ocean basins, and strong genetic structure between the Atlantic and Indo-Pacific. Some pelagic species seem to cross the Benguela Barrier (southern Africa) often enough to preclude the development of evolutionary partitions, including albacore tuna (Vinas et al., 2004; Montes et al., 2012), wahoo (Theisen et al., 2008), and the common dolphinfish (Diaz-Jaimes et al., 2010). However, these species are likely exceptions, with many large, vagile species demonstrating structured populations across this barrier, including the scalloped hammerhead shark (Duncan et al., 2006), whale shark (Castro et al., 2007), and blue marlin (Buonaccorsi et al., 2001). For tunas in particular, a recurring pattern is two mtDNA lineages: one confined to the Atlantic and an Indo-Pacific lineage that is also found in the Atlantic (table 6 in Theisen et al., 2008). This pattern indicates extended periods of isolation, punctuated by dispersal around southern Africa.
Plankton
In the oceanic pelagic zone, where all life stages are planktonic, species’ ranges are both extensive and dynamic because adult distributions are not tied to a particular benthic habitat. In turn, biogeographic provinces for the pelagic zone are based on physical and chemical properties (biogeochemical provinces) (Longhurst, 1995) rather than endemism or species assemblages. Longhurst (2007) identified ~55 biogeochemical provinces (BGCPs), nested within four biomes (Polar, Westerly Winds, Trade Winds, Coastal) across four ocean basins (Atlantic, Pacific, Indian,
Southern). Like the species they harbor, the boundaries of the BGCPs fluctuate on both seasonal and annual timescales in accordance with changing environmental conditions (Reygondeau et al., 2013). Our understanding of pelagic community composition is still nascent, but recent studies have shown concordance between BGCPs and community composition in taxa ranging from viruses (Brum et al., 2015) to phytoplankton (Alvain et al., 2005) to fishes (Reygondeau et al., 2012).
Cosmopolitan distributions in the pelagic zone initially prompted the conclusion of little to no population structure in the open ocean, a position that has eroded in recent decades (Miya and Nishida, 1997; Norris, 2000). Phylogeographic studies reveal that many cosmopolitan taxa are composed of multiple cryptic species (Miyamoto et al., 2012; Hirai et al., 2015), including some that are sympatric over part of their ranges (Andrews et al., 2014b). Populations of these cosmopolitan species are subdivided in two ways concordant with the BGCP framework: (i) by continental land masses separating ocean basins, and (ii) by habitat discontinuities in the equatorial region between subtropical gyres in the Northern and Southern Hemispheres (Goetze, 2011; Norton and Goetze, 2013; Andrews et al., 2014b). The few global-scale phylogeographic studies have been restricted to copepods, but evidence from a diversity of other taxa sampled at ocean basin scales indicates that lineages have diverged both in allopatry and sympatry at much smaller geographic distances than anticipated, with examples drawn from chaetognaths (Peijnenburg et al., 2006), euphausiids (Bucklin et al., 2007), and mollusks (Burridge et al., 2015).
These combined results indicate that population discontinuities of pelagic species are determined not by the temporal and spatial scales of dispersal, but by habitat characteristics enabling species to maintain viable populations (Norris, 2000; Peijnenburg et al., 2006). Habitat selection, rather than physical barriers, may be a primary force driving speciation in the pelagic zone (Peijnenburg and Goetze, 2013). Therefore, a biogeographic framework based on water properties is concordant with genetic partitions within species.
Summary
In conclusion: (i) Several temperate species show disjunct distributions across the tropics, indicating historical dispersals across warm-water barriers. (ii) The deepest phylogeographic separations for oceanic migrants indicate patterns of allopatric isolation between ocean basins, especially for fishes. (iii) Migratory sea turtles and cetaceans show population structure based on reproductive site fidelity. (iv) An ecological component to speciation is indicated by isolation along behavioral barriers in cetaceans, and by the presence of sympatric sister species in the plankton.
(v) Planktonic biogeographic provinces are defined by water masses that can change size and position based on oceanographic conditions. (vi) Initial plankton studies indicate concordance between biogeochemical provinces and phylogeographic partitions, particularly at the equatorial break between northern and southern subtropics.
TERRESTRIAL VS. MARINE PHYLOGEOGRAPHY
Life began in the oceans, but the field of phylogeography began with continental biota (Avise et al., 1987; Riddle, Chapter 2, this volume), and many of the insights reviewed here have precedents in terrestrial cases. The biogeographic settings have parallels between land and sea, particularly with latitudinal gradients in biodiversity and concordance between biogeographic provinces and phylogeographic partitions (Riddle, Chapter 2, this volume; Schluter, 2016). Glacial habitat disruptions in northern seas have a strong parallel in continental faunas (Hewitt, 1996; Bernatchez and Wilson, 1998). Biodiversity hotspots in Indo-Pacific reefs, forests of northern Australia, and Neotropical plant communities are all distinguished by periods of stability, habitat heterogeneity, and the ability to export species (Moritz et al., 2013; Pellissier et al., 2014; Antonelli et al., 2015). A primary difference between marine and terrestrial phylogeography is greater dispersal potential and fewer barriers in the oceans. Although a squirrel in Central Park (New York) cannot deposit progeny in Hyde Park (London), a squirrelfish is capable of dispersing on this scale (Craig et al., 2007). This difference in evolutionary processes is clear in the Hawaiian Archipelago, where rare terrestrial colonists have proliferated into dozens and hundreds of species (Shaw and Gillespie, Chapter 4, this volume) whereas marine colonists produce one or a few species (Bird et al., 2011). Therefore, the evolutionary dramas above and below the waterline have the same ingredients (isolation, selection, adaptation, speciation), but markedly different tempos and outcomes (Bowen, 2016).
CONCLUSION
Marine phylogeography encompasses half-billion-year separations and the largest habitat on the planet. Given this diversity, generalizations are few, but some are especially robust. First, phylogeography is the new incarnation of spatial biogeography (Arbogast and Kenagy, 2001). The alignment of population genetic separations and taxonomic distributions reveals that these are part of a continuum. Evolutionary partitions that could previously be described only with taxonomy are now evaluated with the genomic footprints of isolation, selection, and speciation. Second, the model of allopatric speciation that previously dominated evolutionary
thought is an incomplete fit to the dispersive aquatic medium. Phylogeography of oceanic migrants indicates a strong role for allopatric speciation, whereas heterogeneous coastal habitats provide more opportunity for sympatric/ecological divergences. Phylogeography in high latitudes is defined by shifting habitats in response to glaciation. Finally, both biodiversity hotspots and endemism hotspots are important in producing novel evolutionary lineages and may work in synergy to enhance biodiversity on the ocean planet.
ACKNOWLEDGMENTS
We thank John C. Avise and Francisco J. Ayala, co-organizers of In the Light of Evolution X: Comparative Phylogeography, an Arthur M. Sackler Colloquium of the National Academy of Sciences. For stimulating discussions, advice, and editorial prowess, we thank E. A. Hanni, P. Marko, S. A. Karl, J. Eble, L. Rocha, G. Bernardi, M. Berumen, and the B.B./R.J.T. (ToBo) laboratory. We thank T. Sinclair-Taylor, T. Lilley, and A. Cros for assistance with illustrations and editor John Avise and two anonymous reviewers for comments that improved the manuscript. The authors’ research reported here was funded by the National Science Foundation, the Seaver Institute, the University of Hawai‘i Sea Grant Program, the National Oceanic and Atmospheric Administration, the North Pacific Research Board, and the Saltonstall–Kennedy Grant Program.


















