3
Catalytic Conversion of Methane
While the plentiful supplies of inexpensive ethane, propane, and butane from shale gas have revitalized the U.S. chemical industry, those components make up less than 25 percent gallons per thousand cubic feet (Mcf) of a typical shale gas stream (Keller, 2012), with the major component being methane. At some point, it may be desirable to have economically viable processes for converting methane into ethylene and other value-added hydrocarbons, as well as processes that are more efficient than the current industrial methods for converting methane into syngas. Such a circumstance could arise, for example, if the demand for ethane outstrips the supply, if a new process made it economical to convert methane into transportable liquids for stranded gas (i.e., methane reserves that are too small and too far from current pipelines that are often flared or burned unproductively), or if emissions policies change to prevent or penalize the current practice of flaring methane at the wellhead or that tax carbon dioxide emissions as stated by Reinhard Schomäcker, professor of technical chemistry at the Technical University of Berlin.
What would make methane conversion to ethane economically viable? Schomäcker said that any process would have to achieve a suitable added value, not waste much of the methane as byproducts, utilize low-energy separation technologies, and not have unreasonable capital costs for the process equipment. Meeting those requirements, he said, boils down to the issue of selectivity in terms of how much methane is converted to the desired product, such as ethylene, and how much byproduct has to be separated from the desired product.
METHANE TO ETHYLENE VIA OXIDATIVE COUPLING
To illustrate some of the approaches that have been or are being developed for the catalytic conversion of methane, Schomäcker discussed oxidative coupling of methane to produce ethylene. The pioneering work in this area, he noted, occurred in the 1980s (Hinsen and Baerns, 1983; Jones et al., 1984; Keller and Bhasin, 1982), and despite a great deal of effort that resulted in more than 100 publications, chemists were unable to achieve ethylene yields of much greater than 25 percent (see Figure 3-1). Throughout the 1990s, researchers developed a number of novel catalysts, including a lithium–magnesium oxide catalyst that deactivated over time (Lunsford, 1995) and a manganese–tungsten catalyst (Fang et al., 1992) that maintained stable activity for more than 100 hours without the need for regeneration. Schomäcker and his colleagues, along with dozens of other research teams worldwide, have since been able to reproduce the latter results (Simon et al., 2011).
Another milestone in the field was the development of the first detailed kinetic model for oxidative coupling of methane, in this case for a lanthanum-based catalyst (Stansch et al., 1997). This model proposed
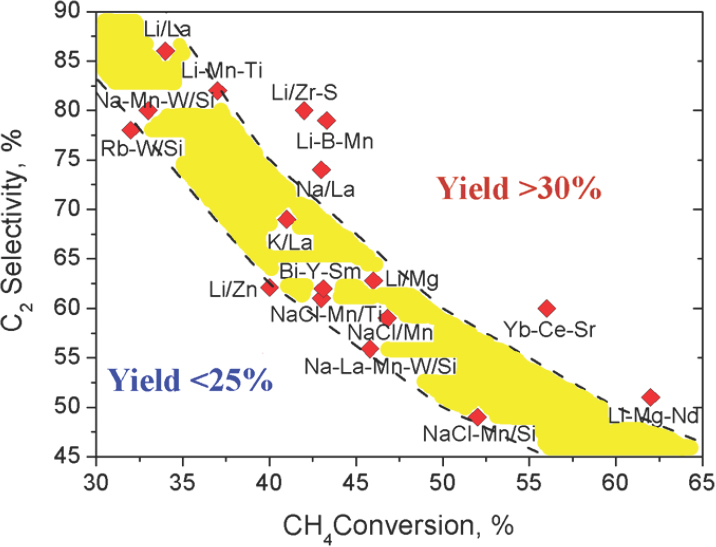
SOURCE: Zavyalova et al., 2011, Reproduced with permission by Wiley-VCH Verlag GmbH & Co. KGaA.
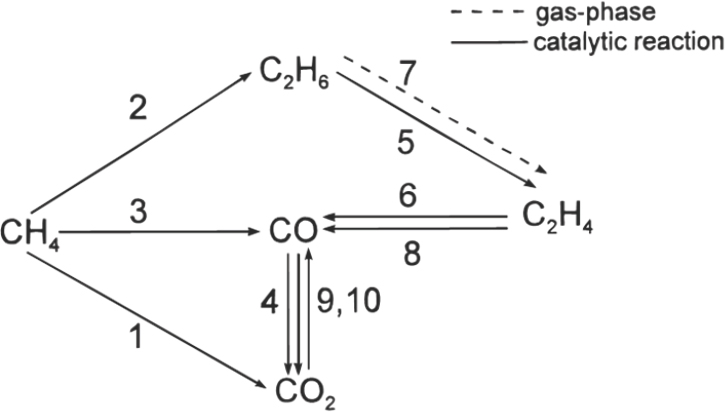
SOURCE: Stansch et al., 1997.
that important steps in the reaction occur both at the catalyst surface and in the gas phase, though it did not explain how these steps (listed as 1–8; see Figure 3-2) fit together to yield the desired product.
Step 1: CH4 + 2O2 → CO2 + 2H2O
Step 2: 2CH4 + 0.5O2 → C2H6 + H2O
Step 3: CH4 + O2 → CO + H2O + H2
Step 4: CO + 0.5O2 → CO2
Step 5: C2H6 + 0.5O2 → C2H4 + H2O
Step 6: C2H4 + 2O2 → 2CO + 2H2O
Step 7: C2H6 → C2H4 + H2
Step 8: C2H4 + 2H2O → 2CO + 4H2
Step 9: CO + H2O → CO2 + H2
Step 10: CO2 + H2 → CO + H2O
Lanthanum oxide catalysts, which numerous groups have studied, using a variety of analytical methods (Au et al., 1997; Dubois et al., 1990; Ferreira et al., 2013; Levan et al., 1993; Lin et al., 1986; Louis et al., 1993; Palmer et al., 2002; Sekine et al., 2009), have the highest activity known. Schomäcker noted that it has been easy to improve the performance of lanthanum oxide by forming lanthanum oxycarbonate or by doping it
with iron or ceria, each of which increases the selectivity of the reaction substantially, though not enough for industrial use. Researchers have even tried some unconventional approaches to boost selectivity, including going to very high temperatures in the absence of oxygen (Guo et al., 2014) or by using sulfur as a “soft” oxidant (Zhu et al., 2013).
When Schomäcker started his studies on methane oxidative coupling, he decided that rather than try to create yet another catalyst, he and his colleagues would study two existing catalysts—lithium/magnesium oxide and a sodium tungstate compound—for more detailed analysis using a variety of approaches. These studies produced a wealth of information, each piece of which provided some insight into a possible mechanism for oxidative coupling (Beck et al., 2014; Cui et al., 2013; Kwapien et al., 2014). One key finding was that the atomic structure of the catalyst had a profound effect on the outcome of the reaction and led to predictions that adding a trace metal to the magnesium oxide catalyst would improve performance. Indeed, adding a trace amount of iron to the catalyst boosted activity and selectivity and increased ethylene yield by a factor of five (Schwach et al., 2013). These studies, he explained, also revealed that the way in which the catalyst activates oxygen is the most important aspect of determining the reaction selectivity. Applying the knowledge gained from these studies, Schomäcker and his colleagues created a series of bimetallic catalysts using sodium tungstate and manganese oxide on a variety of silica support materials, the best of which proved to be a mesoporous silica material known as SBA-15 (Yildiz et al., 2014a, 2014b). He and his collaborators then tested this catalyst in a number of different reactor designs and used the data from these experiments to conduct an economic analysis estimating that, at a 15 percent rate of return, the payback period for a commercial methane to ethylene plant would be between 4 and 7 years.
HYDROCARBONS TO CHEMICALS AND FUELS VIA ENGINEERED MICROBES
In addition to the opportunities for chemical catalysis to contribute to the efficient use of the nation’s shale gas reserves, researchers are making progress harnessing the power of biological systems to convert methane and natural gas liquids to value-added products. As Lercher noted, biological approaches have the potential to lower the energy costs associated with the high temperature regimes required for methane, ethane, and propane conversion using chemical catalysis. Realizing that potential, said Greg Stephanopoulos, professor of chemical engineering at the Massachusetts Institute of Technology (MIT), would support biological systems to serve as the basic enabling technology for the 21st-century chemical industry.
Stephanopoulos outlined the currently envisioned routes for biological methane activation and conversion to chemical products and biofuels. An overall pathway of methane activation to an active intermediate CH3X and a possible assimilation route leading to the synthesis of some product like 3-hydroxybutyrate or butanol was shown. Assimilation can proceed via methanol, which is dehydrogenated to formaldehyde, and processing the latter to an intermediate metabolite of the pentose phosphate pathway. Methane activation is presently possible via anaerobic methanotrophic consortia and aerobic methanotrophs that utilize the activity of a methane monooxygenase enzyme. A challenge with the biological production of certain chemicals like methanol or butanol is the low titers in which the products are made, leading to high energy requirements for recycling huge amounts of water. However, other products like lactic acid, succinic acid, polyhydroxybutyrate (PHB), and lipids may be produced at high concentrations without issues of toxicity to the microorganisms.
Anaerobic consortia have the potential of methane activation at high efficiency, but they exhibit low rates and operate as a mixed culture whereby methane oxidation is coupled with sulfate reduction catalyzed by sulfur-reducing bacteria. No single culture of anaerobic methanotrophs has been isolated yet. Aerobic methanotrophs exhibit a higher rate of methane oxidation albeit at lower energetic efficiency. Various products have been detected to be naturally synthesized in aerobic methanotrophs, such as lipids (potential for biodiesel production) and the polymer PHB, but their concentrations are low (below 1g/L) (Kalyuzhnaya, et al., 2015; Shah et al., 1996; Strong et al., 2015). There is potential in engineering natural aerobic methanotrophs to either increase the figures of merit of naturally produced compounds such as the above, or to endow the host organisms with the pathways required for the synthesis of other products of interest. However, the biological toolkit required for the genetic modulation of these organisms remains underdeveloped, said Stephanopoulos.
On the other hand, Stephanopoulos noted, model organisms like E. coli have been engineered for the production of numerous products from carbohydrates and other substrates. These organisms could be further engineered to allow them to utilize methane, which would enable a seamless system whereby methane is activated by these model organisms, converted to an intermediate such as methanol, and the latter converted to the product of choice. Although many of the concepts for such a scheme have been worked out for other systems, formidable challenges remain in achieving the same for the activation and conversion of methane to target products. Stephanopoulos stressed that more research in this area will help develop the basic biological tools and platform strains required to realize this vision.
WORKING GROUP SESSIONS
Following Schomäcker’s presentation, the workshop participants broke into four predefined working groups, each of which explored one aspect of catalytic conversion of methane: methane to syngas, methane to ethylene, methane to aromatics, or methane to methanol. Each group, after hearing a short introductory presentation by an expert in the session topic, was asked to answer a set of questions over the course of their deliberations (see Box 3-1). Following the 2-hour discussion period, each group’s designated rapporteur summarized the group’s work to the reassembled workshop participants. An open discussion followed the four reports.
Methane to Syngas
In his introductory remarks, Jan Lerou, principal of Jan Lerou Consulting, briefly reviewed the major commercial technologies now used to convert methane into syngas, which then serves as a feedstock for ammonia, methanol, and hydrogen production. The three main technologies are
- steam reforming;
- partial oxidation via non-catalytic and catalytic processes; and
- catalytic auto-thermal reforming.
In addition, he listed two technologies that are nearing industrial use: (A) short contact time catalytic partial oxidation and (B) oxygen transfer membranes as well as two emerging technologies for converting methane into syngas: (C) chemical looping and (D) dry reforming.1
Each of these technologies has its own unique limitations and challenges, which if addressed satisfactorily would improve the economics of the processes using these technologies.
Discussion
Following the presentation, this group spent much of its time identifying four key objectives for research aimed at improvement of the available commercial processes, said Maria Flytzani-Stephanopoulos, distinguished professor and the Robert and Marcy Haber Endowed Professor in Energy Sustainability at Tufts University. These research objectives included
- identifying alternatives for the currently used air-separation unit, which adds to capital and operating costs;
- improving the carbon or thermal efficiency of these processes, which at present burn a great deal of methane to generate the necessary process heat;
- understanding how multifunctional catalyst support materials interact at the interface among materials to enable the design of better catalysts and integrate catalysts and advanced separation membranes; and
- employing process intensification, which focuses on molecular-level kinetics, thermodynamics, and heat and mass transfer, to optimize process performance, something that is happening more in Europe than in the United States at the present time.
From these objectives, this group developed the below list of research opportunities for the methane-to-syngas process:
- Develop oxygen ion- and proton-conducting ceramic membranes as dual-function materials that would replace the current energy-intensive methods used to separate oxygen from air and also serve as a catalyst support matrix.
- Determine if additive manufacturing using three-dimensional printing could enable co-printing copper or tin catalysts with reactor internals to improve heat transfer, a crucial parameter for methane-to-syngas processes.
___________________
1 A method of producing synthesis gas from the reaction of carbon dioxide and methane.
- Develop advanced heat-exchange systems and catalyst supports with novel structures.
Flytzani-Stephanopoulos also reported that the breakout group discussed chemical looping reforming, a process in which a reduced metal oxide is oxidized in one reactor and then used to partially oxidize methane to yield carbon monoxide and hydrogen while recovering the initial metal oxide form. This is a relatively new technology that shows significant promise as a more efficient means of producing syngas. Flytzani-Stephanopoulos reported, research is required, however, to improve the process itself, to develop novel materials for the reactors, and to characterize the kinetics of the process and the catalytic surface parameters that contribute to coke formation in the reactors. In particular, developing and applying methods for in situ characterization of catalyst active sites under very high temperatures typical in a chemical looping process will enable better understanding of the loss of active sites under those conditions.
Computational chemistry and experimental procedures used together have the potential, Flytzani-Stephanopoulos reported, to drive the discovery of new catalytic materials from first principles. Finally, this group felt that short-contact-time reactors have shown some promise, but there is still much to be done to achieve the level of process reliability required for an industrial process.
Methane to Ethylene
As Schomäcker had noted, oxidative coupling of methane to ethylene is technically feasible, but there are a number of features that at present make direct methane-to-ethylene processes uncompetitive commercially, a message reiterated in the introductory presentation by Bob Maughon, vice president for performance plastics and hydrocarbons research and development at The Dow Chemical Company. Maughon did note that several companies are developing novel catalytic systems for converting methane to ethylene, and one company, Siluria Technologies, is operating a demonstration plant using the catalyst it has developed.
Discussion
Following that presentation, the working group identified three specific issues that act as impediments to commercial viability, as reported by Anne Gaffney, director of process science and technology at the Idaho National Laboratory:
- The first impediment is the lack of substantial capital investment to build an industrial-scale facility for oxidative coupling compared with building an ethane cracker, even when using the first commercially available system developed by Siluria Technologies (see Figure 3-3).
- Selectivity for producing ethylene, which Schomäcker discussed, is another obstacle, particularly because poor selectivity increases the expense of separating ethylene from the reaction byproducts.
- The working group, said Gaffney, noted that the development of cost-effective and robust membrane separation technologies could significantly improve the economic competitiveness of methane
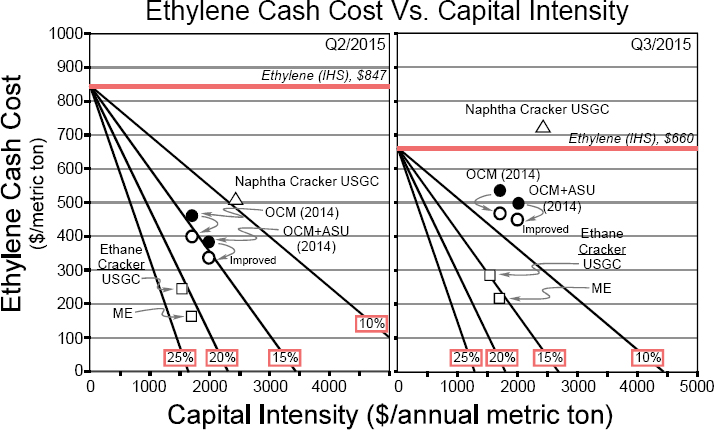
NOTE: Ethane cracking (open squares) shows the highest return on investment for both the Middle East (ME) and the U.S. Gulf Coast (USGC). Naphtha cracking (open triangle) carries a higher raw material cost and capital burden, combining to make it uneconomical relative to reported ethylene price in Q3, but not in Q2. Oxidative coupling of methane (OCM) points (circles) are intermediate, advantaged to naphtha but disadvantaged to ethane. OCM data taken from IHS report PERP 2014-07 are shown as filled circles. Return is largely unchanged by inclusion of an air separation unit (ASU) in the capital. Open circles reflect data from Linde American Institute of Chemical Engineers (AIChE) paper 207a, AIChE 2015 Spring National Meeting (Austin, Texas), April 2015. OCM points reflect public analysis of the Siluria process.
SOURCE: Maughon, 2016.
oxidative coupling to produce ethylene, though the relative low cost of ethane compared to methane today makes the economics of methane-to-ethylene conversion challenging even with technological improvements.
One way in which the direct conversion of methane to ethylene could be cost-effective would be for converting geographically isolated sources of methane into a transportable liquid. Such sources include smaller deposits of “dry” natural gas, stranded natural gas, and gas associated with oil production, all of which are currently flared, and biologically-derived methane. The challenge here would be to develop a processing technology that is economical at small scale and perhaps even transportable between these different sources.
Among the top well-established research approaches for making methane-to-ethylene conversion viable, the group listed oxidative coupling, other oxidative routes, and non-oxidative coupling that would stop at ethylene rather than proceed to produce aromatic compounds, as well as routes based on first creating syngas from methane and using that as a feedstock for ethylene production.
One of the promising but high-risk approaches being studied involves taking ethylene produced at a remote site and oligomerizing it to produce 1-hexene or 1-octene, value-added chemicals that could be readily transported. This group discussed the possibility of developing Fischer–Tropsch catalysts and processes that would favor ethylene production rather than longer-chain hydrocarbon production. Researchers are working on developing solid oxide oxygen- or proton-conducting membranes, but stability has been a problem. These membranes, Gaffney explained, can become fouled with carbonates that form from the carbon dioxide in the effluent gas stream, reducing their lifetime and efficiency. Routes to acetylene, rather than ethylene, are being explored, but coking has been a major issue. Chemical looping, which Schomäcker mentioned, holds promise if the challenge of being able to carry enough oxygen in the chemical looping agent to make the process economically viable can be solved. The group also noted work on the development of biologically mediated conversions as having some potential.
With regard to research opportunities, the group identified metal-organic frameworks as ripe for study as agents for absorbing or separating ethylene from the production stream. Electrochemical approaches to catalysis were mentioned, though the yields achieved so far have been too low to attract much interest. Research could lead to carbon-based methods for dehydrogenation, new approaches for separating olefins from paraffin, and methods that would enable the separation of dilute ethylene from product streams for low-yield processes. The group also noted that there
are many new tools available that could generate transformational results through studies of catalysts and catalytic processes. These included environmental transmission electron microscopy, atmospheric pressure X-ray photoelectron spectroscopy, and simulated moving bed chromatography, the latter which could be used to develop new separations technologies. The group discussed the potential of accelerated screening, characterization, and synthesis tools for developing improved catalysts, and of work in the area of intrinsically safe design to improve the safety and reliability of oxidation reactions. Gaffney stated that the national laboratories possess many of these tools and the expertise to use them, and there is a value with making these tools and the associated staff expertise available to the nation. Finally, this group noted that for the development and widespread use of standardized practices with regard to catalyst formation and use, reactor methodologies, and standard operating procedures are not yet the norm in this field.
Methane to Aromatics
In his introductory presentation to the third working group, Israel Wachs, the G. Whitney Snyder Professor of Chemical Engineering and director of the Operando Molecular Spectroscopy and Catalysis Research Laboratory at Lehigh University, described the mechanistic work that his group has performed to better understand the factors influencing the catalytic activity of a promising ZSM-5–supported molybdenum catalyst that achieves the dehydro-aromatization of methane to liquid aromatics, primarily benzene, and hydrogen (Gao et al., 2014, 2015). This catalyst, he said, first converts methane into ethylene and further reactions at the catalyst produce a mixture of chemicals, of which 70 to 80 percent are aromatics. The catalyst is eventually deactivated by coke formation, though it can be completely regenerated by oxidation treatment in a second reactor.
Discussion
Following this presentation, the group’s discussion, as reported by Monty Alger, director of the Pennsylvania State University’s Institute for Natural Gas Research and professor of chemical engineering at Pennsylvania State University, started by identifying two reasons for why it would be desirable to develop industrial processes for converting methane to aromatics: the substantial price spread between methane and naphtha and the increasing demand for aromatics that is starting to outstrip capacity. Because of the low cost of ethane in the United States, more ethane than naphtha is being used to produce ethylene, which produces fewer aromatics as byproducts. The group then discussed the barriers
to commercialization of the process Wachs described, starting with the energy input required to drive this endothermic reaction and the lack of reactors designed to deliver and manage the high temperature at which this reaction occurs. Other barriers the group identified included
- deactivation of the catalyst by coking;
- low methane conversion rates that result from the buildup of hydrogen;
- the capital costs associated with this process relative to that for aromatics production from petroleum; and
- the lack of cost-effective methods to remove hydrogen from the reactor and to separate benzene from naphthalene, the other major aromatic compounds produced by this reaction.
Alger added that the working group discussed the possibility that environmental regulations to limit carbon emissions could make this technology attractive compared with today’s alternatives.
With regard to additional research that could improve this process and develop other routes for converting methane into aromatics, Alger said the group noted two major impediments: the lack of sustained research funding in the United States for this type of work and the shortage of good ideas in this area. Two promising routes have been described recently—one involves a non-oxidative conversion of methane to ethylene, aromatics, and hydrogen using a lattice-confined iron catalyst (Guo et al., 2014), the other utilizing copper-based catalysts (Grundner et al., 2015)—and further research would help to characterize these systems and determine their suitability for industrial use. Additional research would also aid in verifying the results that Wachs and his colleagues obtained at high temperatures. In general, said Alger, there has been research published, but most of it has been descriptive with little mechanistic detail that could be used to develop a coherent, systematic view of the technology for converting methane to aromatics.
Much of the discussion this group had about promising but higher-risk approaches focused on combining catalysts, developing new materials, and building a mechanistic knowledge base. The group had some discussion about biocatalysis, which is not being explored currently for producing aromatics but which could have longer-term opportunities, said Alger. The group also noted the potential for the lattice-confined iron system developed in China and the possibility of designing a membrane-based reactor that could separate products from reactants and perhaps improve the yields of these processes.
The group had a long discussion on research opportunities, said Alger. These included
- rigorous high-temperature characterization during reaction;
- reproducing and understanding high-temperature results;
- high-temperature material stability and catalyst support;
- fast reactions with short-residence/contact/surface interaction time;
- catalyst/process modification to avoid coke formation;
- exploring the possibilities for hybrid-solid-molten salt catalyst;
- using predictive methods to create catalysts by design;
- developing non-ZSM-5 catalyst systems;
- identifying and studying non-oxidative chemistries; and
- conceptualizing new reactor designs and technologies.
The group also discussed commercial opportunities in the context of capital and operating costs. “The view of the group was that we are not creating new materials and products but are replacing existing materials, and therefore there are standards and upper bounds we need to be mindful of when thinking about benchmarking ideas versus existing alternatives,” Alger reported. In particular, he noted, it is important for researchers and funding agencies to recognize that the opportunity for new technologies will be bounded by other options to produce the same products and that any new technology is required to fit within an economic operating window. It will also be likely that in a zero-carbon world that there may be environmental and regulatory advantages for one technology relative to others and these advantages must be understood and quantified.
Methane to Methanol
In his introductory remarks to the fourth working group, Tobin Marks, the Vladimir N. Ipatieff Professor of Catalytic Chemistry and professor of materials science and engineering at Northwestern University, pointed out that creative catalytic chemistry must be paired with excellent engineering to develop an industrially useful process capable of supplanting the current indirect process that first converts methane to syngas and then on to methanol at a price of approximately $0.75 per gallon using Earth-abundant catalysts. The main drawback to this process, which is practiced at a huge scale, is that it requires a significant amount of heat to produce syngas and the overall process capital cost intensity. In contrast, said Marks, the dream process of directly converting methane and oxygen to methanol is exothermic, that is, it generates heat, but realizing that dream will require addressing a number of challenges, including
- managing heat and mass transfer;
- catalytic selectivity;
- product separation and purification;
- catalyst cost and supply security; and
- catalyst lifetime and regeneration.
Marks said that there have been many attempts using a variety of conditions and heterogeneous catalysts to achieve the direct conversion of methane to methanol (Alvarez-Galvan et al., 2011; Brown and Parkyns, 1991; Gesser et al., 1985; Holmen, 2009; Lunsford, 2000; Tabata et al., 2002), but any selectivity in the process was achieved at the expense of conversion and typical yields are 1 to 3 percent. Researchers at the Gas Technology Institute are reported to be developing a room-temperature, high-efficiency process to convert methane into methanol and hydrogen using metal oxide catalysts that are continuously regenerated. According to information from the Advanced Research Projects Agency-Energy (ARPA-E), this process has the potential to produce methanol at $0.24 per gallon from stranded methane or methane currently flared at the wellhead (ARPA-E, 2012).
Other approaches for direct conversion of methane to methanol have used homogeneous catalysts (Labinger and Bercaw, 2002, 2015), including functionalization of methanol using mercury-, thallium-, and platinum-based catalysts in concentrated sulfuric acid followed by reaction with water to produce methanol (Hashiguchi et al., 2012; Labinger and Bercaw, 2002, 2015; Palkovits et al., 2009; Periana et al., 1998; Soorholtz et al., 2013), and using zeolite-supported iron or copper catalysts and hydrogen peroxide as the oxidant (Hammond et al., 2012, 2013). Researchers have also explored enzymatic conversion of methane to methanol (Banerjee et al., 2015; Wang et al., 2015)
In concluding his introductory remarks, Marks said that there has been substantial progress in understanding key catalytic mechanisms and in developing new tools, including computational methods, for studying catalytic mechanisms and for predicting and screening catalysts. He also said that the materials science of heterogeneous catalysts is advancing rapidly.
Discussion
In the subsequent discussions, the working group enumerated several impediments to the commercial viability of direct methane-to-methanol conversion, including not having a direct route to offer substantial improvements over the current industrial processes based on syngas. As Karen Goldberg, the Nicole A. Boand Endowed Professor of Chemistry and director of the Center for Enabling New Technologies through
Catalysis at the University of Washington, reported, the discussion raised the point that future environmental policies could serve as a driver for the development and commercialization of smaller plants for processing stranded and flared gas using some of the technologies Marks outlined in his presentation. Current methods of managing the oxidants required for direct conversion could be improved, the group noted, and it would help to some way reduce the cost of separating oxygen from air or to develop an air-recyclable oxidant such as the process used in one variant of the Wacker reaction that recycles copper (I) to copper (II). Improved methods for separating methanol from the water used in some schemes will be helpful, too, and despite advances in mechanistic understanding, there is still room for a better fundamental understanding of the catalytic activation of the carbon–hydrogen bond. The group also noted that electrocatalytic methane activation is a new approach that highlights ways to think about entirely new concepts for catalyzing conversion of methane to methanol, and, in this arena, opportunities exist for more efficient energy production using direct methane fuel cells, but new catalytic materials are seriously lacking.
The working group then discussed some of the challenges to making current approaches viable, starting with reducing the temperature of some of the reactions and improve their selectivity. For homogeneous systems, separations can be an issue. Two overarching challenges facing catalytic conversion of methane using either heterogeneous or homogeneous catalysts include avoiding coking, in which carbonaceous deposits form on heterogeneous catalysts and thereby limit catalytic activity, and developing new ligands for homogeneous catalysts that are stable under reaction conditions. Homogeneous organometallic catalysts that have enjoyed success in major catalytic processes such as hydrogenation, metathesis, and hydroformylation typically employ ligands such as phosphines that are unstable under the conditions required for methane oxidation, which generally involve strong oxidants, strongly acidic media, and water.
Other promising but higher-risk approaches being taken included the development of movable, small-scale plants for use with stranded and flared methane, and the development of oxidants that do not need separating from the product mix. The discussion raised the question of whether it would be a better idea to convert methane to dimethyl ether, whether it would be possible to develop an oxidant that did not need separation, and if routes to methanol through syngas in an integrated process could prove viable. Another promising avenue the group noted was to get experts in heterogeneous, homogenous, biocatalytic, and electrocatalytic processes and catalyst supports together in a forum such as this workshop to generate new ideas. Goldberg said there is currently not financial support available to convene such a forum.
She concluded her report on the group’s discussions by noting some of the scientific and engineering advances that have come online and that will be useful in addressing the challenges the discussion raised. These included
- operando and ex-situ spectroscopy to probe catalyst structure and dynamics;
- new chemical and analytical techniques to probe mechanism;
- high-throughput experimentation for optimization and discovery;
- materials science of catalyst supports and plant construction materials;
- ligand supply and design;
- advances in synthesizing catalysts; and
- high-powered computational approaches for both understanding and prediction.
Additional areas of research that would benefit the field included development of new methods for synthesizing well-defined catalysts, stabilizing catalysts (especially homogeneous), and managing oxygen, both in terms of separations and for redox coupling, in a cost-efficient manner. Research that could better characterize the intermediates and transition states resulting from the interactions of methane with catalysts is another benefit.
DISCUSSION
In the ensuing open discussion, several workshop participants raised questions about the goal of eliminating the separation of oxygen from air before performing any of the catalytic oxidations identified by the working groups. Guido Pez, an independent consultant who retired from Air Products, noted that the U.S. Department of Energy funded a project that used a solid oxide-containing membrane as part of a methane oxidation scheme that did eliminate the importance for an air separation unit. Marks then asked if the methane-to-aromatics group discussed the possibility of making a specific aromatic compound or of avoiding the production of benzene. Wachs commented that there are other important issues that need to be addressed first and noted that there are many methods for taking benzene and further modifying it.
Eric Stangland from The Dow Chemical Company asked if industry needs another methane-to-methanol technology and wondered whether research funds should be spent on more pressing needs. José Santiesteban from ExxonMobil replied that industry’s perspective is that any process has to make sense from an economic viewpoint, and also consider envi-
ronmental issues and lifecycle analysis in decision making. He also said the same question could be asked for ethane-to-ethylene processes and noted that every time he hears that a particular area is mature, some development comes along that proves that idea wrong. In his opinion, research with the biggest potential for producing a breakthrough involves taking an entirely different approach to catalysis, such as the idea of combining different types of catalytic processes. Wachs added that the pulp and paper industry, the largest user of methanol as the feedstock for producing formaldehyde, strongly desires a one-step process for methane to formaldehyde or methane to methanol. Mark Barteau from the University of Michigan stressed the importance of considering the carbon budget of a process as well as the economic budget. “I think it would be a great tragedy if we had a scientific breakthrough that lowered the capital cost of a process and also lowered the carbon efficiency,” he said.
Maughon commented that if the question is about prioritizing where to spend research dollars, the answer from Dow’s perspective would be that methane-to-methanol conversion would not be a high priority, and he would guess that ExxonMobil would say the same thing. Stangland responded by saying that while it may not be Dow’s priority, methane-to-methanol conversion might be a priority for the nation as it considers how best to use the nation’s natural resources, though he agreed that methane-to-methanol conversion likely would not be a top priority given the potential for some of the other areas the working groups discussed to produce game-changing catalytic solutions for using methane to produce value-added chemicals. Santiesteban added that research prioritization should also consider what might be beneficial in the long term. “The chemical industry goes through cycles, and so we need to be ready for different situations,” said Santiesteban. “The technology we have now was not developed in 1 day. It was developed by people who had a vision and it is our responsibility to create a vision for tomorrow.”
In that vein, Alexis Bell from the University of California, Berkeley, commented that industry may take a short-term view, but it depends on researchers at the national laboratories and universities to take a longer-term view and develop the science and basic engineering that would later enable industry to implement a technology if it made sense at that time. By the same token, added Goldberg, the basic science behind methane-to-methanol conversion is providing knowledge about how to selectively activate and functionalize the carbon-hydrogen bond and how to use oxygen effectively as an oxidant in a potential industrial process. Ultimately, that knowledge may not lead to a future process for making methanol, but it could lead to processes for using methane as a feedstock to make other valuable chemicals. Alger, agreeing with Goldberg, said that history has shown that most of the great inventions resulted from research not
directly related to that invention. What is important, he said, is the cross-fertilization among fields that results in knowledge generated in one field being applied to problems in another technology area where the market is demanding a solution.
Shannon Stahl from the University of Wisconsin–Madison also commented on the importance of cross-fertilization and stressed the importance of including researchers from industry in any cross-disciplinary discussions and programs. He also suggested that the federal funding agencies consider funding a new type of program that would bring together small teams of researchers, including those from industry, to work on a focused problem as a complement to large center programs and individual investigator grants. In his opinion, this type of mid-sized team approach would provide a good return on investment and afford the opportunity to respond quickly to a research need. Alger seconded this idea and noted how little time professors have today to engage in the type of cross-disciplinary conversations this field is lacking.


















